Abstract
HIV-1 hijacks host classical cargo nuclear transportation, or nonclassical pathways by directly interacting with importin-β family proteins or nucleoporins for efficient pre-integration complex (PIC) nuclear import. Recently, an N-terminal truncated form of nucleoporin Pom121c (601–987 aa) was reported to inhibit HIV-1 replication. In contrast, we found that HIV-1 replication was significantly decreased in 293T and TZM-b1 cells with siRNA-mediated Pom121 knockdown. Quantitative PCR indicated that viral replication was impaired at the step of cDNA nuclear import. Furthermore, we found that karyopherin-β1 (KPNB1), which belongs to the importin-β family, interacts with Pom121 and is involved in Pom121-mediated PIC nuclear import. Rescue experiment indicated that the FG-repeats and the following α-helix in Pom121 are required for its role in HIV-1 PIC nuclear import. Taken together, our results showed that full-length Pom121 enables efficient PIC nuclear import, and suggested that this process may rely on KPNB1 dependent classical cargo nuclear transportation way.
Keywords: HIV-1, Pom121, Nuclear import, Pre-integration complex, Nucleoporins
1. Introduction
In eukaryotic cells, the double lipid bilayer of the nuclear envelope separates the nucleus from the cytoplasm. Transport across the nuclear envelope occurs through large multiprotein channel structures, termed nuclear pore complexes (NPCs). The structure and composition of NPCs are partly conserved between yeast and vertebrates, consisting of multiple copies of approximately 30 different nucleoporins (Nups) (Nigg, 1997; Tetenbaum-Novatt and Rout, 2010). There are two modes of nuclear transport that occur in cells: the passive diffusion of small molecules (< 40 kDa) in a signal-independent manner and the facilitated translocation of cargo molecules with molecular weights above ~ 40 kDa in a signal-dependent manner (Raveh et al., 2016; Rout et al., 2003). The presence of a nuclear localization signal (NLS) or nuclear export signal recognized by transport receptors permits transport using an energy-dependent mechanism (Chook and Suel, 2011) and allows the passage of larger molecules. Specific interactions between these transport receptors and phenylalanineglycine (FG) repeats of Nups that fill the central channel of the NPC mediate the transport of soluble cargo (Peleg and Lim, 2010; Yang, 2013), and receptor-cargo binding and release are dictated by a gradient of RanGTP across the NPC (Cook et al., 2007; Kalab et al., 2002). Under pathological conditions, including in many cancers and viral infections, the NPC is hijacked and used to transport undesired particles, or is modified to attenuate the cellular responses to disease onset (Jamali et al., 2011; Simon and Rout, 2014).
Human immunodeficiency virus (HIV) exhibits the ability to infect non-dividing cells by relying on the efficient nuclear transport of the viral pre-integration complex (PIC). The nuclear import of HIV reverse transcription products is a critical step during the life cycle of the virus (Arhel, 2010). Following nuclear import, viral complementary DNA (cDNA) is integrated into the host genome and can lead to viral replication. The basic mechanisms of nuclear import of HIV-1 PIC are apparently flexible (Bin Hamid et al., 2016). There is evidence for its import via classical pathways (binding to importin-α and then to importin-β, followed by docking to the NPC via interaction with Nups), nonclassical pathways (by directly interacting with members of the importin-β family of proteins, such as TNPO3 and importin-7), or by direct interaction with Nups (Ao et al., 2010; Fassati et al., 2003; Jayappa et al., 2012; Lee et al., 2010; Matreyek and Engelman, 2013; Ocwieja et al., 2011; Schrijvers et al., 2012; Woodward et al., 2009).
There are three vertebrate membrane Nups (Pom121, Ndc1, and gp210), and Pom121 is the least conserved of the three. It comprises a short peptide domain of approximately 30 amino acids (aa) that inserts into the endoplasmic reticulum lumen, a single transmembrane domain, and a long (> 1000 aa) polypeptide domain that extends into the cytoplasm (Funakoshi et al., 2007; Hallberg et al., 1993). Pom121 is recruited early during postmitotic nuclear assembly in mammalian cells (Bodoor et al., 1999; Daigle et al., 2001), and has been shown to be essential for nuclear envelope (NE) assembly during interphase NPC and NE biogenesis in metazoan cells (Antonin et al., 2005). Pom121 appears to be involved in a step that precedes the incorporation of Nup107–160 in membrane reorganization events leading to inner and outer nuclear membrane fusion (Mitchell et al., 2010). Recently, Saito et al. reported that the N-terminally truncated Pom121, Pom121c (aa 601–987), inhibits HIV-1 replication through a genetic screen using a cDNA expression library in a murine leukemia virus vector (Saito et al., 2017).
In the present study, we demonstrated that full-length Pom121 enables efficient nuclear import of HIV-1 PIC. Pom121 knockdown significantly decreased HIV-1 replication, whereas Pom121 upregulation increased HIV-1 replication. The quantitative polymerase chain reaction (qPCR) revealed that Pom121 promoted HIV-1 replication at the nuclear import step of PIC. Our results shed light on the novel function of Pom121 in the regulation of HIV-1 replication.
2. Materials and methods
2.1. Cells and virus
The human embryonic kidney cell line, HEK293T, and HIV-1 indicator cell line, TZM-bl, (kind gifts of Jianhua Wang, Institute Pasteur of Shanghai, China) were cultured in Dulbecco's Modidied Eagle Medium (Hyclone, Logan, UT, USA) supplemented with 10% fetal bovine serum (Hyclone) and 100 units/mL penicillin/streptomycin (Invitrogen, Carlsbad, CA, USA) in a 5% CO2 incubator at 37 °C. Single cycle luciferase reporter virus HIV-Luc stocks were generated by co-transfection of a 10 cm dish of HEK293T cells with 10 μg of pLai3ΔenvLuc2 (Yamashita and Emerman, 2004), an env-deleted and nef-inactivated HIV-1 proviral construct, and 5 μg of an expression plasmid for vesicular stomatitis virus G protein (VSV-G) as previously described (Dong et al., 2007). Replication-competent HIV-1NLAD8 was generated by transfection of HEK293T cells with 10 μg of the proviral construct pNLAD8 (Sanjana et al., 2014). Gag p24 concentrations of HIV-Luc and HIV-1NLAD8 stocks were measured using an enzyme-linked immunosorbent assay. Cells were infected with HIV-Luc or HIV-1NLAD8 virus and harvested at the indicated times. Luciferase activity of cell lysates was detected using a commercially available kit (E1483; Pro-mega, Madison, WI, USA).
2.2. Antibodies and siRNA reagents
Human Pom121 and karyopherin β1 (KPNB1) small interfering RNA (siRNA) was purchased from RiboBio Co., Ltd. (stQ0009235-1, stQ0001025-1; Guangzhou, China). The siR-Ribo™ negative control was used as control scramble siRNA. The siRNAs were transfected at a concentration of 100 nM using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's instructions. Rabbit anti-Pom121, KPNB1 were obtained from Novus Biologicals (NBP2–198890; Littleton, CO, USA), Abcam (ab459380; Cambridge, UK), respectively. The GAPDH and Flag antibodies were from Sigma-Aldrich (G9545 and F1804-1MG; St. Louis, USA). The secondary goat anti-rabbit IgG-horseradish peroxidase (HRP) antibody was purchased from Southern Biotech (4030-05; Birmingham, AL, USA).
2.3. Plasmid construction
The full-length human Pom121 isoform X1 (XP_005250783.1) contains 1249 aa. The cDNA of the N-terminal 265 aa truncated Pom121 (NP_001244119.1) was purchased from iGeneBio (Guangzhou, China). The fragment coding the N-terminal 265 aa of Pom121 was chemically synthesized and joined with the truncated Pom121 using overlap PCR. Full-length human Pom121 was cloned into the p3xFalg-CMV10 plasmid using NotI and XbaI, generating pFlag-Pom121. The FG-domain defficient Pom121dFG containing the N-terminal 834 aa was amplified from pFlag-Pom121 using the primers F, 5′-GCTTGCGGCCG CGAATTCGATGTCTCCGGCGG CTG C-3′ and R, 5′-ATCCTCTAGAGTC CTACGCAGGCTTCGAAGCAGAGTCT G-3′. The amplified DNA fragment was cloned into the NotI - and XbaI-digested p3xFlag-CMV10 vector, generating pFlag-Pom121dFG. Similarly, the C-terminal α-Helix defficient Pom121 expressing plasmid was generated using the primers F, 5′-GCTTGCGGCCGCGAATTCGATGTCTCCGGCGGCTGC-3′ and R, 5′-ATC CTCTAGAGTCCTAGGAAAATGAAAGGGCCGCCGATC-3′, generating pFlag- Pom121dHelix.
The Pom121 siRNA targeting sequence was located in the FG-domain. To generate the Pom121 expressing construct against siRNA targeting, the synonymous mutations in Pom121 siRNA targeting sequence were made. Briefiy, the primers F: 5-GCTGGCAGTGGAAGTTT CGGAATAAACGTGGCCACCCCAGGCTC-3′ and R: 5-TGGCC ACGTTTA TTCCGAAACTTCCACTGCCAGCGGGGGCTGC-3′ were used to amplify Pom121 or Pom121 dHelix, using the pFlag-Pom121 or pFlag-Pom121dHelix as template, respectively. The siRNA targeting sequence 5′-GGAGCTTTGGGATCAATGT-3′ was replaced by 5′-GAAGTTTCGGA ATAAACGT-3′ which was ialic and underlined as shown in primers. The PCR products were treated with DpnI enzyme to remove the plasmid template before gel purification. After gel purification, the PCR products were transformed to E.coli DH5α. The transformed bacterial were grown on agarose plate containing 100 μg/mL Ampicillin. The plasmids extracted from the bacterial clones on plate were further examined by DNA sequencing. The correct plasmids were named pFlag-Pom121R and pFlag-Pom121dHelix_R, respectively. For upregulation of Pom121, Pom121dFG or Pom121dHelix, 293T cells were transfected with 500 ng each of plasmid indicated above or the empty vector p3xFlag-CMV10.
2.4. Western blot and Co-Immunoprecipitation (Co-IP)
HEK293T or TZM-b1 cells were harvested at the indicated times and lysed with 5x sodium dodecyl sulfate (SDS) lysis buffer. Proteins were separated with 12% SDS-polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane. After blocking with phosphate-buffered saline (PBS) containing 0.1% Tween-20 (PBS-T) and 5% fat-free milk, membranes were incubated with the primary antibodies anti-Pom121 (1:2000), anti-KPNB1 (1:1000), or anti-GAPDH (1:2000). After washing with PBS-T three times, membranes were incubated with HRP-conjugated secondary goat anti-rabbit IgG (1:5000). Detection was performed with enhanced chemiluminescence (Pierce; Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer's instructions.
For Co-IP experiment, 5 × 106 293T cells in 10 cm dish were co-transfected with 3 μg pFlag-Pom121, or pFlag-Pom121dHelix with 3 μg KPNB1 expressing plasmid pKPNB1. The cells were harvested 48 h post transfection and washed with PBS. The cell pellet was then incubated with 1 mL IP buffer (Beyotime, China) containing PMSF protease inhibitor on ice for 30 min. The cell lysate was centrifuged at 12,000 rpm/ min for 10 min at 4 °C. The 900 μL supernatant was taken and incubated with 20uL anti-flag beads (Sigma, F2426-1mL) with shaking at 4 °C overnight. The beads were washed with three times with IP buffer next day and subjected to SDS-PAGE. After protein transfer, the poly-vinylidene fluoride membrane was probed with primary antibody for the detection of KPNB1 or Pom121.
2.5. Measurement of HIV-1 reverse transcription and nuclear import
The 293T cells (1.5 × 105 cells/well in a 24-well plate) were infected with 1.5 ng HIV-Luc virus 24 h post-Pom121 siRNA transfection. Total cellular DNA was extracted with a DNeasy Blood & Tissue kit (Qiagen, Hilden, Germany) 24 or 72 h postinfection to assess reverse transcription or nuclear import, respectively. The reverse transcription product was amplified with an LTR R-specific primer (forward; 5′-GGG AGC TCT CTG GCT AAC T-3′) and a gag-specific primer (reverse; 5′-TTA ACT GCG AAT CGT TCA C-3). For nuclear import 2-LTR circle detection, a U5-specific primer (forward; 5′-TGG GAG CTC TCT GGC TAA GA-3) and a U3-specific primer (reverse; 5′-TTG TGT GTG GTA GAT CCA TG- 3) were used. As standards for real-time PCR, serial dilutions (10–106copies) of plasmid pNLAD8 were used for the late reverse transcription reactions, and a plasmid (pSK-CJ380) containing a 2-LTR circle (Dong et al., 2007) was used for 2-LTR circle reactions. PCR reactions were performed using SYBR green real-time PCR kits (TaKaRa, China) with a QuanStudio 6 Flex real-time PCR system (Applied Bio-systems, Foster City, CA, USA) as follows: 35 cycles of 95 °C for 15 s and 60 °C for 1 min.
2.6. Statistical analysis
The data were statistically analyzed with two-tailed independent Student's t-test between two groups, and differences between multiple groups were analyzed for statistical significance by ANOVA followed by Tukey's or Bonferroni's multi-comparison post hoc test. A value of P < 0.05 was considered statistically significant.
3. Results
3.1. Pom121 supports HIV-1 replication
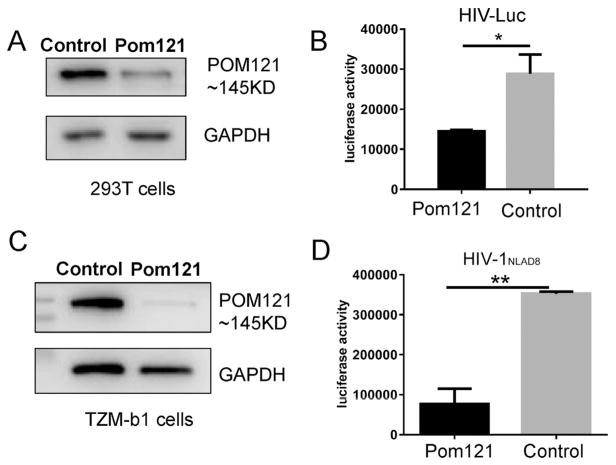
Pom121 was originally identified as an integral membrane protein in the rat liver NE and depletion of Pom121 was shown to inhibit NE formation (Antonin et al., 2005). To test the potential role of Pom121 in HIV-1 replication, Pom121 was downregulated in 293T cells with RNA interference. The 293T cells were transfected with siRNA targeting Pom121 and Pom121 protein shown at the size of 145 kDa was reduced 72 h posttransfection (Fig. 1A). Then, 293T cells with Pom121 knockdown were infected with HIV-1 using a VSV-G pseudotyped HIV-1 reporter virus (HIV-Luc), and luciferase activity was measured 3 days postinfection to assess viral infection. Interestingly, we found that viral replication was reduced 2-fold in cells with Pom121 knockdown compared with control cells (Fig. 1B). We also observed the viral replication was reduced 1.5-fold when T cell line C8166 was used (Supplementary Data 1). Furthermore, compared with control cells, viral replication was significantly decreased (4.6-fold) when replication-competent HIV-1NLAD8 was used in HeLa-derived HIV-1 reporter TZM-b1 cells with Pom121 knockdown (Fig. 1C and D). Together, these results suggested that endogenous full-length Pom121 was required for efficient HIV-1 replication.
Fig. 1.
Pom121 supports HIV-1 replication. 293T cells were seeded in 24 well plate(1.5 × 105/well) and trans-fected with Pom121 specific siRNA (100 nM). (A) The Pom121 protein level was measured by western blot 72 h post transfection; or cells were infected with HIV-Luc (1.5 ng p24/well) 24 h post transfection and (B) The luci-ferase activity in cell lysis was measured 72 h post infection. TZM-b1 cells were seeded in 24-well plates (1.0 × 105/well) and transfected with Pom121 specific siRNA in 100 nM concentration. (C) The Pom121 protein level was measured by Western blot 72 h post transfection; or cells were infected with HIV-1NLAD8 (1.8 ng p24/well) 24 h post transfection and (D) The luciferase activity in cell lysis was measured 72 h post infection. Data were presented as mean standard deviations from three independent experiments. *P < 0.05, **P < 0.01.
To further investigate the role of Pom121 in HIV-1 replication, we also increased Pom121 expression in 293T cells by transfecting a Pom121-expressing vector pFlag-Pom121. HIV-1 infection using single cycle or replication-competent viruses was performed 1 day post-transfection and viral replication was measured 3 days postinfection. However, enhanced Pom121 expression did not significantly increase HIV-1 replication in 293T or TZM-bl cells infected with HIV-Luc and HIV-1NLAD8, respectively (Supplementary data 2), suggesting that the endogenous level of Pom121 might be sufficient for efficient HIV-1 replication in these cells.
3.2. Pom121 mediates efficient HIV-1 PIC nuclear import
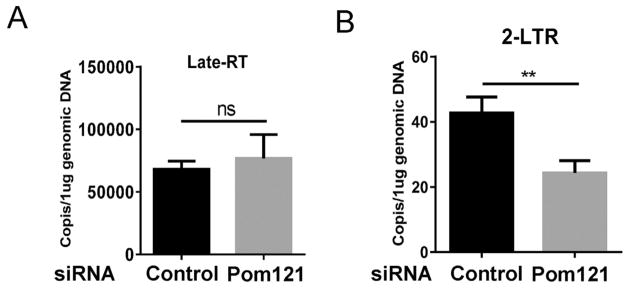
HIV-1 PIC was generated in the cytoplasm following the reverse transcription of viral RNA into viral DNA. The complex included viral components such as integrase, nucleocapsid, matrix, viral protein R, and reverse transcriptase (Sherman and Greene, 2002) as well as host cellular proteins such as lens epithelium-derived growth factor and barrier autointegration factor (Raghavendra et al., 2010). Transport of PIC into the nucleus was a prerequisite for the integration of HIV-1 DNA into cellular chromosomes. To examine in which step Pom121 affects HIV-1 replication, we measured reverse transcription and nuclear import in 293T cells with Pom121 knockdown using established qPCR assays (Dong et al., 2007). Total cellular DNA was extracted and used as a template for qPCR. Pom121 knockdown did not affect HIV-1 reverse transcription as measured by the late reverse transcription product (Fig. 2A). However, 2-LTR, an indicator of PIC nuclear import, was reduced 1.8-fold in Pom121 knockdown cells compared with control cells (Fig. 2B), indicating that Pom121 may facilitate efficient HIV-1 PIC nuclear import.
Fig. 2.
Pom121 enables efficient HIV-1 PIC nuclear import. 293T cells were seeded in 24 well plate (1.5 × 105/well) and transfected with Pom121 specific siRNA in 100 nM concentration. Cells were infected with HIV-Luc (1.5 ng p24/well) 24 h post transfection. (A) The late reverse transcription (late-RT) and (B) the 2-LTR, indicator of PIC nuclear import, were measured by Real-time PCR 24 h and 72 h post infection, respectively. Data were presented as mean standard deviations from three triplets in one experiment of three independent experiments.**P < 0.01.
3.3. Pom121 enhances PIC nuclear import via KPNB1
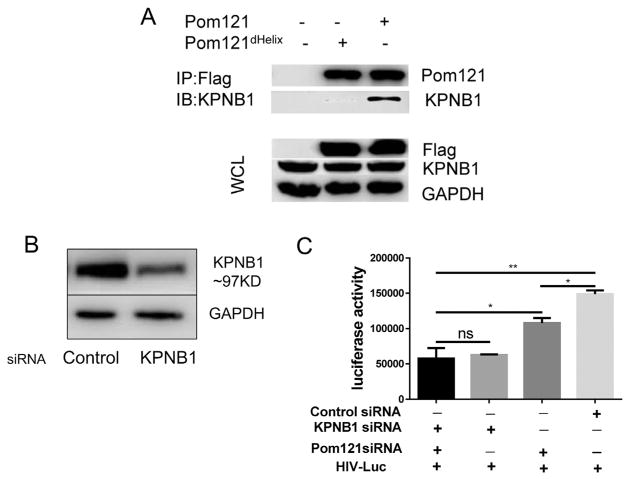
Classical import pathway cargo binds to karyopherin alpha protein through an NLS and forms a dimer. KPNB1 then binds the dimer, generating a trimeric complex, which is allowed access through the NPC (Mosammaparast and Pemberton, 2004; Stewart, 2007). It has also been reported that FG-Nups may function as an anchor downstream of KPNB1 for this transport-receptor facilitated translocation (Ma et al., 2012). Therefore, we sought to examine whether KPNB1 was also involved in PIC nuclear import. Firstly, we verified the interaction between KPNB1 and Pom121 by Co-IP assay. As shown in Fig. 3A, KPNB1 was clearly precipitated with the full length Pom121 (pFlag-Pom121) suggesting they may interact with each other as complex. Saito et al. also reported that the C-terminal α-helix of Pom121 interacts with KPNB1 and is essential for N-terminal truncated Pom121-mediated HIV-1 inhibition. We then generated the C-terminal α-helix defficient Pom121 (pFlag-Pom121dHelix). Consistent with the previous results, Flag-Pom121dHelix lost almost all of the binding ability with KPNB1 compared with the Flag-Pom121, indicating the importance of helix domain for binding (Fig. 3A). Next, knockdown of KPNB1 in 293T cells using siRNA was performed and its expression was significantly reduced (Fig. 3B). We then infected the KPNB1 knockdown cells with HIV-Luc and measured viral replication 3 days postinfection. Viral replication was decreased 1.4-fold in Pom121 knockdown cells compared with that in control cells. Interestingly, viral replication was reduced 2.4-fold when KPNB1 was knocked down. However, knockdown of both KPNB1 and Pom121 did not further reduce HIV-1 replication compared with KPNB1 knockdown alone (Fig. 3C), implying that HIV-1 PIC might also employed other FG-Nups downstream of KPNB1 for nuclear import.
Fig. 3.
KPNB1 is associated with Pom121 mediated HIV-1 PIC nuclear import. (A) KPNB1 interacted with Pom121 detected by Co-IP assay. WCL: whole cell lysate. (B) 293T cells were seeded in 24 well plate (1.5 × 105/ well) and transfected with KPNB1 specific siRNA in 100 nM concentration. The KPNB1 protein level was measured by western blot 72 h post transfection; (C) The cells were transfected with indicated siRNAs and infected with HIV-Luc (1.5 ng p24/well) 24 h post transfection. The luciferase activity in cell lysis was measured 72 h post infection. Data were presented as mean standard deviations from three independent experiments. *P < 0.05, **P < 0.01.
3.4. The Pom121 FG-domain and α-helix is required for HIV-1 PIC nuclear import
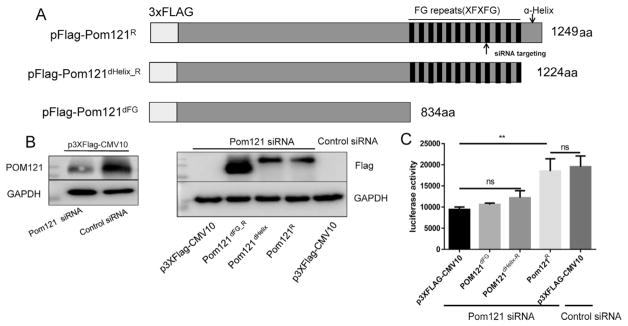
Studies have documented the importance of FG-containing Nups, including NUP153 (Matreyek et al., 2013), NUP358 (Dharan et al., 2016), and NUP98 (Di Nunzio et al., 2013) in HIV-1 PIC nuclear import. To identify whether Pom121 promoted HIV-1 PIC nuclear import through its FG-domain interacting with KPNB1 in the classical import pathway, the FG-domain defficient Pom121 (pFlag-Pom121dFG) was generated through deletion of the C terminal FG-repeat containing region (aa 835–1249; Fig. 4A). Synonymous mutations were made in Pom121dHelix_R and Pom121R constructs to resist Pom121 siRNA targeting (Fig. 4A). To reduce the endogenous level of Pom121 in cells and perform the rescue experiment, 293T cells were co-transfected with Pom121-specific siRNA and the constructs pFlag-Pom121R, pFlag-Pom121dHelix_R or Pom121dFG. Western blot showed that the endogenous Pom121 was reduced by Pom121 siRNA detected by Pom121 antibody (Fig. 4B, left panel). The expression of exogenous Pom121 was also confirmed by the anti-Flag antibody (Fig. 4B, right panel).
Fig. 4.
Pom121 FG-domain is required for HIV-1 cDNA nuclear import. (A) Schematic of pFlag-Pom121R, Pom121dHelix_R and Pom121dFG structure. The amino acids sequence XFXFG is the motif of FG repeats. The site of siRNA targeting is indicated as arrow. 293T cells were seeded in 24-well plates (1.5 × 105/well) and transfected with indicated siRNA and plasmids to regulate the cellular Pom121 expression. (B)The protein level of endogenous Pom121 or exogenous Pom121R, Pom121dHelix_R Pom121dFG was examined by Pom121 and Flag antibody 72 h post transfection, respectively. (C) The cells were infected with HIV-Luc (1.5 ng/well) 24 h post-transfection and the luciferase activity in infected cell lysis was measured 72 h post-infection. Data were presented as mean standard deviations from three independent experiments. **P < 0.01.
HIV-1 infection results indicated that overexpression of the FG-domain defficient Pom121 could not rescue viral replication. In accordance with Saito's results, the α-helix defficient Pom121dHelix_R could neither rescue viral replication. When full-length Pom121 was overexpressed, viral replication was greatly increased, but was not significantly different from that of normal cells transfected with control siRNA and p3xFlag-CMV10 (Fig. 4C). These results suggested that the FG-domain and the following α-helix domain were crucial for Pom121 to support efficient HIV-1 replication.
4. Discussion
Unlike other retroviruses, HIV-1 enters the nucleus via PIC by means of active transport through the nuclear pore (Arhel, 2010). Intensive research has been performed to better understand the mechanism regulating the nuclear import process of HIV-1. Several host factors, including import factors such as importin 7 (Fassati et al., 2003), TRN-SR2 (Christ et al., 2008; Thys et al., 2011), and importin α3 (Ao et al., 2010), as well as NUP153 (Woodward et al., 2009) and NUP3 (Ocwieja et al., 2011), have been proposed to be involved in this process. In mammals, Pom121 has been identified to anchor the NPC to the nuclear membrane (Hallberg et al., 1993). The biogenesis of NE study showed that localization of Pom121 to the inner nuclear membrane is required for an early step of interphase nuclear pore complex assembly (Funakoshi et al., 2011). In addition, NDC1 and gp210 have also been reported to be transmembrane Nups in vertebrates, which play an important role in NPC structure. Interestingly, we presented in this study for the first time evidence that HIV-1 hijacks the host transmembrane NUP Pom121 for efficient PIC nuclear import.
Recently, it was shown that N-terminally truncated Pom121c inhibits HIV-1 replication (Saito et al., 2017), which initially seems inconsistent with our results that the full-length Pom121 promotes HIV-1 replication. Nevertheless, both of these studies indicated that disruption of nucleoporin Pom121 is able to interfere HIV-1 replication. In fact, the N-terminally truncated Pom121 without the transmembrane domain alters its cellular location from the nuclear pore to the cytosol. GST pulldown assays have shown that KPNB1 is linked with Pom121c (601–987). Given that KPNB1 mediates nuclear transport through interaction with FG-Nups, it is possible that truncated Pom121c (601–987) is a dominant negative mutant that interferes with the interplay between endogenous Pom121 and KPNB1. In that case, it is not surprising to observe a reduction in HIV-1 nuclear import with expression of Pom121c (601–987aa). Our results demonstrated that Pom121 siRNA knockdown in 293T cells significantly reduced HIV replication. Moreover, detection of HIV-1 cDNA by qPCR indicated that Pom121 might be required for viral PIC nuclear import, but not for reverse transcription. One possibility is that the total nuclear pore formation is reduced by Pom121 siRNA knockdown. However, our results indicated that the nuclear pore recognized by monoantibody Mab414 didn’t change much after Pom121 knockdown(data not shown). Of note, the inhibitory effects on viral replication with Pom121 knockdown alone were not able to compensate for the reduction caused by knockdown of the upstream protein KPNB1 alone (Fig. 3). It would be interesting to further identify whether other Nups downstream of KPNB1, like Pom121, are involved in HIV-1 PIC nuclear import.
Studies have documented the importance of FG-containing Nups, including NUP153 (Matreyek et al., 2013), NUP358 (Dharan et al., 2016), and NUP98 (Di Nunzio et al., 2013), in HIV PIC nuclear import. The extensive FG repeats in FG-Nups directly bind shuttling transport receptors moving through the NPC and a capsid (CA)-dependent model has been proposed (Dharan et al., 2016). Initial CA binding to NUP358 docks the PIC to the cytoplasmic surface of the NPC. At the NPC, CA interactions with CPSF6 facilitate transport through the NPC and subsequent CA-dependent transfer to NUP153 located on the nuclear surface of the NPC. Our rescue assay indicated that Pom121 with deletion of the FG-domain did not increase siRNA-downregulated HIV nuclear import. Furthermore, KPNB1 siRNA-mediated knockdown also decreased PIC nuclear import. Together, these results supported the idea that the C-terminal FG-repeat regions of Pom121, which extend into the central channel of the NPC to join other FG-Nups, facilitate the movement of transport factors through the NPC.
In summary, our data suggested that HIV-1 may hijack Pom121 for efficient nuclear import of PIC. Knockdown of Pom121 inhibited HIV-1 replication at the nuclear import step. The Pom121-dependent increase in HIV-1 required its FG-repeats, which are important domains for KPNB1 binding in the NE transmission process. Our findings provided a new mechanism for HIV-1 PIC nuclear import that could be a potential target for viral inhibition.
Supplementary Material
Acknowledgments
This work was supported the National Natural Science Foundation of China (81101257, 31470848, 31670898 and 31470880), Open Research Fund Program of the State Key Laboratory of Virology of China (2017IOV003) and Jiangsu Provincial Innovative Research Team. LW was supported by grants (AI104483, AI120209, and GM128212) from the National Institutes of Health, USA. We would also thank Prof. Jianhua Wang at Pasteur Institute of Shanghai for great helping in the study. The authors declare that there is no conflict financial interests exist.
Appendix A. Supplementary material
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.virol.2018.06.008.
References
- Antonin W, Franz C, Haselmann U, Antony C, Mattaj IW. The integral membrane nucleoporin pom121 functionally links nuclear pore complex assembly and nuclear envelope formation. Mol Cell. 2005;17:83–92. doi: 10.1016/j.molcel.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Ao Z, Danappa Jayappa K, Wang B, Zheng Y, Kung S, Rassart E, Depping R, Kohler M, Cohen EA, Yao X. Importin alpha3 interacts with HIV-1 integrase and contributes to HIV-1 nuclear import and replication. J Virol. 2010;84:8650–8663. doi: 10.1128/JVI.00508-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arhel N. Revisiting HIV-1 uncoating. Retrovirology. 2010;7:96. doi: 10.1186/1742-4690-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bin Hamid F, Kim J, Shin CG. Cellular and viral determinants of retroviral nuclear entry. Can J Microbiol. 2016;62:1–15. doi: 10.1139/cjm-2015-0350. [DOI] [PubMed] [Google Scholar]
- Bodoor K, Shaikh S, Salina D, Raharjo WH, Bastos R, Lohka M, Burke B. Sequential recruitment of NPC proteins to the nuclear periphery at the end of mitosis. J Cell Sci. 1999;112(Pt 13):2253–2264. doi: 10.1242/jcs.112.13.2253. [DOI] [PubMed] [Google Scholar]
- Chook YM, Suel KE. Nuclear import by karyopherin-betas: recognition and inhibition. Biochim Biophys Acta. 2011;1813:1593–1606. doi: 10.1016/j.bbamcr.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ F, Thys W, De Rijck J, Gijsbers R, Albanese A, Arosio D, Emiliani S, Rain JC, Benarous R, Cereseto A, Debyser Z. Transportin-SR2 imports HIV into the nucleus. Curr Biol: CB. 2008;18:1192–1202. doi: 10.1016/j.cub.2008.07.079. [DOI] [PubMed] [Google Scholar]
- Cook A, Bono F, Jinek M, Conti E. Structural biology of nucleocytoplasmic transport. Annu Rev Biochem. 2007;76:647–671. doi: 10.1146/annurev.biochem.76.052705.161529. [DOI] [PubMed] [Google Scholar]
- Daigle N, Beaudouin J, Hartnell L, Imreh G, Hallberg E, Lippincott-Schwartz J, Ellenberg J. Nuclear pore complexes form immobile networks and have a very low turnover in live mammalian cells. J Cell Biol. 2001;154:71–84. doi: 10.1083/jcb.200101089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharan A, Talley S, Tripathi A, Mamede JI, Majetschak M, Hope TJ, Campbell EM. KIF5B and Nup358 cooperatively mediate the nuclear import of HIV-1 during Infection. PLoS Pathog. 2016;12:e1005700. doi: 10.1371/journal.ppat.1005700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Nunzio F, Fricke T, Miccio A, Valle-Casuso JC, Perez P, Souque P, Rizzi E, Severgnini M, Mavilio F, Charneau P, Diaz-Griffero F. Nup153 and Nup98 bind the HIV-1 core and contribute to the early steps of HIV-1 replication. Virology. 2013;440:8–18. doi: 10.1016/j.virol.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, Janas AM, Wang JH, Olson WJ, Wu L. Characterization of human immunodeficiency virus type 1 replication in immature and mature dendritic cells reveals dissociable cis- and trans-infection. J Virol. 2007;81:11352–11362. doi: 10.1128/JVI.01081-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassati A, Gorlich D, Harrison I, Zaytseva L, Mingot JM. Nuclear import of HIV-1 intracellular reverse transcription complexes is mediated by importin 7. EMBO J. 2003;22:3675–3685. doi: 10.1093/emboj/cdg357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funakoshi T, Clever M, Watanabe A, Imamoto N. Localization of Pom121 to the inner nuclear membrane is required for an early step of interphase nuclear pore complex assembly. Mol Biol Cell. 2011;22:1058–1069. doi: 10.1091/mbc.E10-07-0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funakoshi T, Maeshima K, Yahata K, Sugano S, Imamoto F, Imamoto N. Two distinct human POM121 genes: requirement for the formation of nuclear pore complexes. FEBS Lett. 2007;581:4910–4916. doi: 10.1016/j.febslet.2007.09.021. [DOI] [PubMed] [Google Scholar]
- Hallberg E, Wozniak RW, Blobel G. An integral membrane protein of the pore membrane domain of the nuclear envelope contains a nucleoporin-like region. J Cell Biol. 1993;122:513–521. doi: 10.1083/jcb.122.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamali T, Jamali Y, Mehrbod M, Mofrad MR. Nuclear pore complex: biochemistry and biophysics of nucleocytoplasmic transport in health and disease. Int Rev Cell Mol Biol. 2011;287:233–286. doi: 10.1016/B978-0-12-386043-9.00006-2. [DOI] [PubMed] [Google Scholar]
- Jayappa KD, Ao Z, Yao X. The HIV-1 passage from cytoplasm to nucleus: the process involving a complex exchange between the components of HIV-1 and cellular machinery to access nucleus and successful integration. Int J Biochem Mol Biol. 2012;3:70–85. [PMC free article] [PubMed] [Google Scholar]
- Kalab P, Weis K, Heald R. Visualization of a Ran-GTP gradient in interphase and mitotic Xenopus egg extracts. Science. 2002;295:2452–2456. doi: 10.1126/science.1068798. [DOI] [PubMed] [Google Scholar]
- Lee K, Ambrose Z, Martin TD, Oztop I, Mulky A, Julias JG, Vandegraaff N, Baumann JG, Wang R, Yuen W, Takemura T, Shelton K, Taniuchi I, Li Y, Sodroski J, Littman DR, Coffin JM, Hughes SH, Unutmaz D, Engelman A, KewalRamani VN. Flexible use of nuclear import pathways by HIV-1. Cell Host Microbe. 2010;7:221–233. doi: 10.1016/j.chom.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Goryaynov A, Sarma A, Yang W. Self-regulated viscous channel in the nuclear pore complex. Proc Natl Acad Sci USA. 2012;109:7326–7331. doi: 10.1073/pnas.1201724109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matreyek KA, Engelman A. Viral and cellular requirements for the nuclear entry of retroviral preintegration nucleoprotein complexes. Viruses. 2013;5:2483–2511. doi: 10.3390/v5102483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matreyek KA, Yucel SS, Li X, Engelman A. Nucleoporin NUP153 phenylalanine-glycine motifs engage a common binding pocket within the HIV-1 capsid protein to mediate lentiviral infectivity. PLoS Pathog. 2013;9:e1003693. doi: 10.1371/journal.ppat.1003693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JM, Mansfeld J, Capitanio J, Kutay U, Wozniak RW. Pom121 links two essential subcomplexes of the nuclear pore complex core to the membrane. J Cell Biol. 2010;191:505–521. doi: 10.1083/jcb.201007098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosammaparast N, Pemberton LF. Karyopherins: from nuclear-transport mediators to nuclear-function regulators. Trends Cell Biol. 2004;14:547–556. doi: 10.1016/j.tcb.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Nigg EA. Nucleocytoplasmic transport: signals, mechanisms and regulation. Nature. 1997;386:779–787. doi: 10.1038/386779a0. [DOI] [PubMed] [Google Scholar]
- Ocwieja KE, Brady TL, Ronen K, Huegel A, Roth SL, Schaller T, James LC, Towers GJ, Young JA, Chanda SK, Konig R, Malani N, Berry CC, Bushman FD. HIV integration targeting: a pathway involving Transportin-3 and the nuclear pore protein RanBP2. PLoS Pathog. 2011;7:e1001313. doi: 10.1371/journal.ppat.1001313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg O, Lim RY. Converging on the function of intrinsically disordered nucleoporins in the nuclear pore complex. Biol Chem. 2010;391:719–730. doi: 10.1515/BC.2010.092. [DOI] [PubMed] [Google Scholar]
- Raghavendra NK, Shkriabai N, Graham R, Hess S, Kvaratskhelia M, Wu L. Identification of host proteins associated with HIV-1 preintegration complexes isolated from infected CD4+ cells. Retrovirology. 2010;7:66. doi: 10.1186/1742-4690-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raveh B, Karp JM, Sparks S, Dutta K, Rout MP, Sali A, Cowburn D. Slide-and-exchange mechanism for rapid and selective transport through the nuclear pore complex. Proc Natl Acad Sci USA. 2016;113:E2489–E2497. doi: 10.1073/pnas.1522663113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rout MP, Aitchison JD, Magnasco MO, Chait BT. Virtual gating and nuclear transport: the hole picture. Trends Cell Biol. 2003;13:622–628. doi: 10.1016/j.tcb.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Saito H, Takeuchi H, Masuda T, Noda T, Yamaoka S. N-terminally truncated POM121C inhibits HIV-1 replication. PLoS One. 2017;12:e0182434. doi: 10.1371/journal.pone.0182434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjana NE, Shalem O, Zhang F. Improved vectors and genome-wide libraries for CRISPR screening. Nat Methods. 2014;11:783–784. doi: 10.1038/nmeth.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrijvers R, Vets S, De Rijck J, Malani N, Bushman FD, Debyser Z, Gijsbers R. HRP-2 determines HIV-1 integration site selection in LEDGF/p75 depleted cells. Retrovirology. 2012;9:84. doi: 10.1186/1742-4690-9-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman MP, Greene WC. Slipping through the door: HIV entry into the nucleus. Microbes Infect. 2002;4:67–73. doi: 10.1016/s1286-4579(01)01511-8. [DOI] [PubMed] [Google Scholar]
- Simon DN, Rout MP. Cancer and the nuclear pore complex. Adv Exp Med Biol. 2014;773:285–307. doi: 10.1007/978-1-4899-8032-8_13. [DOI] [PubMed] [Google Scholar]
- Stewart M. Molecular mechanism of the nuclear protein import cycle. Nature reviews Mol Cell Biol. 2007;8:195–208. doi: 10.1038/nrm2114. [DOI] [PubMed] [Google Scholar]
- Tetenbaum-Novatt J, Rout MP. The mechanism of nucleocytoplasmic transport through the nuclear pore complex. Cold Spring Harb Symp Quant Biol. 2010;75:567–584. doi: 10.1101/sqb.2010.75.033. [DOI] [PubMed] [Google Scholar]
- Thys W, De Houwer S, Demeulemeester J, Taltynov O, Vancraenenbroeck R, Gerard M, De Rijck J, Gijsbers R, Christ F, Debyser Z. Interplay between HIV entry and transportin-SR2 dependency. Retrovirology. 2011;8:7. doi: 10.1186/1742-4690-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward CL, Prakobwanakit S, Mosessian S, Chow SA. Integrase interacts with nucleoporin NUP153 to mediate the nuclear import of human immunodeficiency virus type 1. J Virol. 2009;83:6522–6533. doi: 10.1128/JVI.02061-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M, Emerman M. Capsid is a dominant determinant of retrovirus infectivity in nondividing cells. J Virol. 2004;78:5670–5678. doi: 10.1128/JVI.78.11.5670-5678.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W. Distinct, but not completely separate spatial transport routes in the nuclear pore complex. Nucleus. 2013;4:166–175. doi: 10.4161/nucl.24874. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.