Abstract
Background:
The HEART Pathway is an accelerated diagnostic protocol (ADP) designed to identify low-risk Emergency Department (ED) patients with chest pain for early discharge without stress testing or angiography. The objective of this study was to determine whether implementation of the HEART Pathway is safe (30 day death and myocardial infarction rate <1% in low-risk patients) and effective (reduces 30 day hospitalizations) in ED patients with possible acute coronary syndrome (ACS).
Methods:
A prospective pre/post study was conducted at three US sites among 8,474 adult ED patients with possible ACS. Patients included were ≥21 years old, investigated for possible ACS, and had no evidence of ST-segment elevation myocardial infarction on electrocardiography. Accrual occurred for 12 months before and after HEART Pathway implementation from November 2013- January 2016. The HEART Pathway ADP was integrated into each site’s electronic health record as an interactive clinical decision support tool. Following ADP integration, ED providers prospectively utilized the HEART Pathway to identify patients with possible ACS as low-risk (appropriate for early discharge without stress testing or angiography) or non-low-risk (appropriate for further in-hospital evaluation). The primary safety and effectiveness outcomes, death and myocardial infarction (MI) and hospitalization rates at 30 days, were determined from health records, insurance claims, and death index data.
Results:
Pre- and post-implementation cohorts included 3713 and 4761 patients, respectively. The HEART Pathway identified 30.7% as low-risk; 0.4% of these patients experienced death or MI within 30 days. Hospitalization at 30 days was reduced by 6% in the post- vs pre-implementation cohort (55.6% vs 61.6%; aOR: 0.79, 95%CI: 0.71–0.87). During the index visit more MIs were detected in the post-implementation cohort (6.6% vs 5.7%; aOR: 1.36, 95%CI: 1.12–1.65). Rates of death or MI during follow-up were similar (1.1% vs 1.3%; aOR: 0.88, 95% CI: 0.58–1.33).
Conclusions:
HEART Pathway implementation was associated with decreased hospitalizations, increased identification of index visit MIs, and a very low death and MI rate among low-risk patients. These findings support use of the HEART Pathway to identify low-risk patients that can be safely discharged without stress testing or angiography.
Clinical Trial Registration:
clinicaltrials.gov Identifier: NCT02056964
Keywords: Clinical Decision Support, Medical Decision Making, Electronic Health Records, Emergency Medicine, Acute Coronary Syndrome, Risk Stratification, Accelerated Diagnostic Protocol
Introduction
United States Emergency Departments (ED) care for 8–10 million patients with acute chest pain annually.1 To avoid missing acute coronary syndrome (ACS), providers liberally hospitalize patients with chest pain for comprehensive cardiac evaluations (serial cardiac biomarkers and stress testing or angiography). However, <10% of ED patients with chest pain are ultimately diagnosed with an ACS;2–6 with testing costing $10–13 billion annually.5, 7 While accelerated diagnostic protocols (ADPs) are designed to improve the quality and value of chest pain risk stratification, they lack sufficient prospective safety and effectiveness data. Therefore, current guidelines continue to recommend comprehensive cardiac evaluations, even for low-risk patients.7
The HEART Pathway, is an ADP, that incorporates elements of the Chronic Care Model framework (decision support and clinical information systems) by providing test ordering and disposition decision support to ED practitioners and personalized care planning for patients with acute chest pain.8–10 In prior efficacy studies, the HEART Pathway significantly increased the percent of ED patients with acute chest pain identified for early discharge and decreased objective cardiac testing (stress testing and angiography), hospital length of stay, and cost.11–14 While these studies also provided data suggesting safety, they were not adequately powered to provide tight confidence intervals around safety event rates. While a matter of debate, many believe that a successful risk stratification strategy must achieve <1% missed events among low-risk patients within 30-day follow-up.15 Our objective was to determine the safety and effectiveness of the HEART Pathway ADP by conducting an implementation study within a three-center health system.
Methods
Study Design and Oversight
We compared risk stratification of ED patients with acute chest pain before and after implementation of the HEART Pathway ADP. Participants were prospectively accrued under a waiver of informed consent from November 2013- January 2016. This study was approved by our Institutional Review Board with a waiver of informed consent and registered with clinicaltrials.gov (NCT02056964). Methods were previously described.16 The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Setting and Population
The study was done at 3 hospitals in North Carolina: Wake Forest Baptist Medical Center (WFBMC), with approximately 114000 ED visits annually; Davie Medical Center (DMC), with approximately 12000 annual ED visits; and Lexington Medical Center (LMC), with approximately 37000 annual ED visits. The target population was adult ED patients (≥21 years old) investigated for possible ACS, but without evidence of ST-segment elevation myocardial infarction (STEMI) on electrocardiography (ECG). Inclusion criteria were the same throughout the pre- and post-implementation periods. Patients with a chief complaint of chest pain and at least one troponin ordered, without evidence of a STEMI on ECG, were accrued. This included patients with known coronary artery disease (prior myocardial infarction, prior coronary revascularization, or known coronary stenosis ≥70%). In addition, patients with other complaints that were concerning for ACS were included if the provider used a study specific EHR flowsheet for possible ACS, which was available in both the Pre- and Post-cohorts.
At WFBMC and DMC, participants were accrued into the pre-implementation cohort (November 2013-October 2014) or the post-implementation cohort (February 2015-January 2016). A wash-in period (November 2014- January 2015) was used to train providers and beta-test an electronic health record (EHR)-based HEART Pathway clinical decision support tool. LMC accrued patients into the pre-implementation (January-July 2015) and post-implementation cohorts (August 2015- January 2016), with a 1-month wash-in period. Patients were accrued into each cohort based on the date of their initial ED visit; later visits for chest pain were considered recurrent care. To prevent accruing more ED repeat users/high utilizers (who often have more co-morbid conditions) into the pre-implementation cohort, patients with an ED visit for possible ACS at each site in the year before the study began (N=523) were excluded from analysis. Patients transferred within the network or visiting multiple sites were classified based on their original ED visit. For transfers, care at the receiving hospital was considered part of their index encounter.
Data Collection
Index encounter data (from initial ED presentation to discharge from the ED, observation unit, or inpatient ward) were extracted from the health system’s EHR data (Clarity-Epic Systems Corporation, Verona, WI). Pre-validated structured EHR variables or diagnoses and procedure codes (CPT, ICD9, and ICD10) were used to obtain patient demographics, past medical history, cardiovascular risk factors, comorbidities, troponin results, provider’s HEART Pathway assessments, disposition, diagnoses (including myocardial infarction), and vital status.17–21 To determine 30-day outcomes, we used the EHR for within-network return visits, insurers’ claims data, and state death index data. Claims data were available on patients insured by Blue Cross Blue Shield (BCBS) of North Carolina (the dominant insurer in the state), MedCost, and North Carolina Medicaid. We also used North Carolina State Center for Health Statistics death index data.
HEART Pathway Implementation
After the pre-implementation period concluded (during the wash-in period) the HEART Pathway ADP was fully integrated into EPIC as an interactive clinical decision support (CDS) tool. Thus, for all adult patients with chest pain and at least one troponin ordered in the post-implementation period, ED providers saw an interruptive pop-up alert for the HEART Pathway tool as a Best Practice Advisory in the EHR. In addition, during the wash-in period, the HEART Pathway tool was integrated into the study specific EHR flowsheet. This flow sheet allowed providers to manually access the HEART Pathway in patients presenting with other symptoms concerning for ACS (i.e. dyspnea, left arm pain, or jaw pain) or prior to a troponin order.
The HEART Pathway CDS tool prompted providers to answer a series of questions to prospectively risk-stratify eligible patients in real-time (patients with STEMI were excluded). Patients with known coronary artery disease, or acute ischemic changes on ECG (e.g. new t-wave inversions or ST-segment depression in contiguous leads) were immediately classified as non-low-risk for ACS and no History, ECG, Age, and Risk factor score (HEAR score) score calculation was conducted in these patients.
Among patients without STEMI, known coronary disease (CAD), or acute ischemic ECG changes, providers answered additional flow sheet questions to determine a History, ECG, Age, and Risk factor score (HEAR score); calculated based on the HEART Pathway trial algorithm (Impathiq Inc., Raleigh, NC).22 Troponin measurements were incorporated through a direct link to laboratory results. The HEART Pathway risk assessment was automatically calculated based on the HEAR score and 0 and 3-hour troponin measures.13, 23 Patients with HEAR scores of 3 or lower and without elevated troponin measures were classified as low-risk and recommended for discharge from the ED without objective cardiac testing. Patients with a HEAR score of 4 greater, an elevated troponin, known CAD, or ischemic ECG changes were classified as non-low-risk and designated for further testing and/or admission (Figure 1). During the pre-implementation period, The HEART Pathway CDS tool was not available to providers and HEAR scores were not recorded on patients with chest pain. Serum troponin was measured throughout the study period using the ADVIA Centaur platform TnI-Ultra™ assay (Siemens, Munich, Germany) or the Access AccuTnI+3 assay (Beckman Coulter, Brea, CA).
Figure 1:
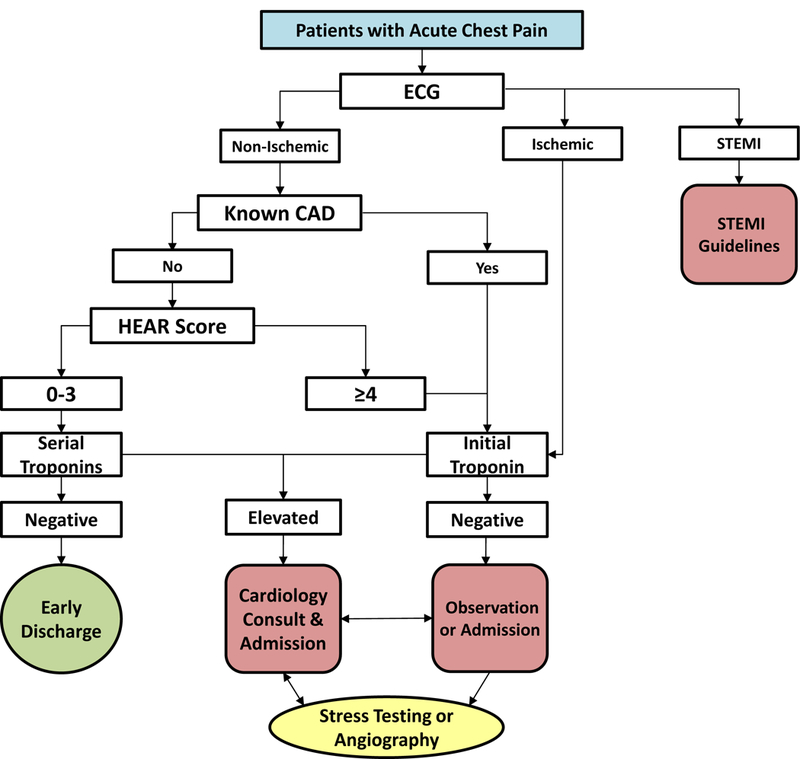
The HEART Pathway algorithm
Outcomes
The primary effectiveness outcome was hospitalization rate at 30 days (from the index visit through 30 days of follow-up). Hospitalization was defined as an inpatient admission, transfer, or observation stay (including index observation unit care). Secondary outcomes included objective cardiac testing, early discharge rates, and index visit length of stay (LOS) and ED LOS. The Objective cardiac testing rate was defined as the proportion of patients receiving stress testing, coronary CT angiography, or invasive coronary angiography. Consistent with prior studies,12, 13, 24 early discharge rate was defined as the proportion of patients discharged directly from the ED without receiving objective cardiac testing. Index visit LOS represented the time from the patient’s ED arrival to hospital discharge. ED LOS was defined as the time from ED arrival to ED discharge, transfer, or admission. In the post-implementation cohort the non-adherence rate, to the HEART Pathway’s disposition guidance was determined. Non-adherence was defined as low-risk patients receiving stress testing or hospitalization or non-low-risk patients receiving early discharge from the ED.
Primary safety outcomes were death or acute myocardial infarction (MI) during the index visit and the 30-day follow-up period. Coronary revascularization rate, a secondary endpoint, was defined as coronary artery bypass grafting, stent placement, or other percutaneous coronary intervention. MI and coronary revascularization were determined using diagnosis and procedure codes validated by prior cardiovascular trials.17–21 Major adverse cardiac events (MACE), a composite of death, MI, and revascularization, were also evaluated as a secondary endpoint.
Statistical Analysis
We anticipated a sample size of approximately 4000 in each group, allowing us to estimate safety event rates to within + 0.33% assuming an event rate of 1% (based on a large sample normal approximation to a proportion) and to detect a difference in hospitalization rate of ≥4% with 90% power at the 5% two-sided level of significance (based on a two-sample chi-square test).
We used unadjusted logistic regression to model the relationship between pre- and post- implementation periods and the rate of utilization and safety events. These models were then adjusted for potential confounders, which were selected a priori: age, sex, race, ethnicity, insurance status, enrollment site, prior known coronary artery disease, diabetes, hypertension, hyperlipidemia, chronic kidney disease, chronic obstructive pulmonary disease, cerebral vascular disease, peripheral vascular disease, cancer, smoking, body mass index (BMI), and presence of chest pain vs other symptoms concerning for ACS (EHR flowsheet use). A time effect was initially included for each time period to assess for secular trends. None of the pre-implementation cohort slopes were significantly different from zero for any index or 30 day outcome. Thus, time effects were removed from the models, so that odds ratios could be interpreted as average effects. For illustrative purposes, raw event rates were calculated for each month and regression models were used to fit one slope for the pre-implementation period and another slope for the post-implementation period.25, 26 BMI was missing for 2.9% of patients, so multivariate imputation, with replacement by predictive mean matching utilizing all predictors and outcome variables, was used to create 10 datasets with complete BMI data.27, 28 No other covariates required imputation. Logistic models were fit for each imputed dataset and results averaged across sets. Adjusted odds ratios (aOR) and 95% confidence intervals were derived for each outcome.
Post-implementation, we calculated the percentage of patients identified as low-risk and non-low risk to determine the sensitivity, specificity, and positive and negative predictive values of the HEART Pathway (and its components) for death and MI. Corresponding 95% exact binomial confidence intervals were computed. Likelihood ratios and approximate 95% CIs were calculated using the SAS macro NLEstimate. Consistent with prior studies, patients without 30-day data from the EHR, insurers, or death index were considered free of 30-day safety events.11–13, 29 Sensitivity analyses assessed the impact of missing follow-up data on safety events using multiple imputation based on several assumptions such as; patients with incomplete follow-up had the same event rate as patients with complete follow-up from the pre- and post-cohorts, or the same event rate as the pre-implementation cohort (Supplemental Table 1). Proc MI in SAS was used to generate 25 imputed datasets for each scenario, and Proc MIAnalyze was used to combine the results from the logistic regression analysis of each imputed dataset. In addition, to evaluate the completeness of EHR follow-up we determined the number of safety events detected based on insurer claims or death index data but absent in the EHR data. To assess whether differences in provider selection of patients into the pre- or post-implementation cohorts may have influenced results, a sensitivity analysis was conducted (Supplemental Tables 2 and 3), which excluded all patients accrued by use of the EHR ACS flowsheet (analyzing only patients meeting the BPA criteria of chief complaint of chest pain and troponin ordered). Pre- and post-implementation LOS outcomes were described using medians and interquartile ranges (IQR) and compared using Wilcoxon rank-sum tests. All analyses were performed using R and SAS 9.4 (Cary, NC).
Results
Patients
Over 24 months, 8474 patients were accrued (Figure 2). The cohort was 53.6% female, 28.6% African American, and 17.5% uninsured with a median age of 54. Cohort characteristics are summarized in Table 1. The death and MI rate of the cohort from index through 30 days was 6.5%, and revascularization occurred in 3.9% of patients.
Figure 2.
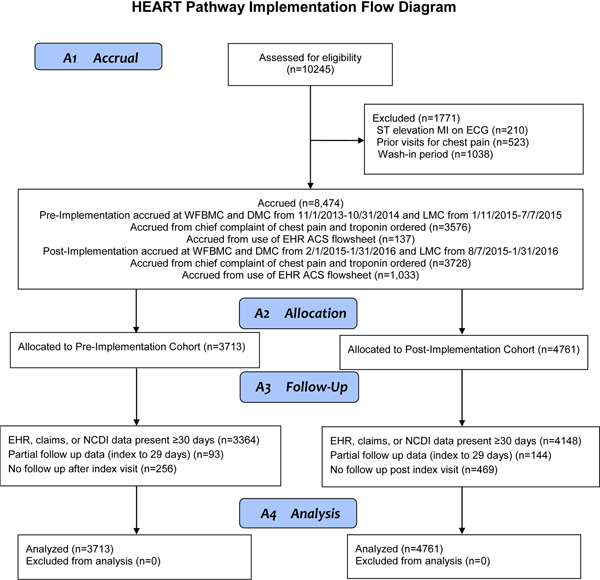
Participant flow diagram
Table 1.
Characteristics of patients in the Pre- and Post-Implementation Cohorts
| Patient Characteristics | Pre N= 3713 (%) |
Post N= 4761 (%) |
p value* |
|---|---|---|---|
| Age –median (IQR) | 54 (45, 65) | 54 (44, 66) | 0.330 |
| Female | 1965 (52.9) | 2579 (54.1) | 0.278 |
| Race | 0.038 | ||
| White or Caucasian | 2484 (66.9) | 3106 (65.2) | |
| Black or African American | 1052 (28.3) | 1371 (28.8) | |
| Other | 177 (4.8) | 284 (6.0) | |
| Ethnicity | 0.006 | ||
| Hispanic or Latino | 134 (3.6) | 230 (4.8) | |
| Site | <0.001 | ||
| WFBMC | 2720 (73.3) | 3685 (77.4) | |
| DMC | 396 (10.7) | 512 (10.8) | |
| LMC | 597 (16.1) | 564 (11.8) | |
| Insurance Status | 0.054 | ||
| Blue Cross | 790 (21.3) | 970 (20.4) | |
| MedCost | 209 (5.6) | 286 (6.0) | |
| Medicaid | 505 (13.6) | 687 (14.4) | |
| Medicare | 1189 (32.0) | 1617 (34.0) | |
| Other insurance | 321 (8.6) | 413 (8.7) | |
| Self-pay | 699 (18.8) | 788 (16.5) | |
| EHR ACS flowsheet used | 137 (3.7) | 1033 (21.7) | <0.001 |
| Risk Factors | |||
| Prior CAD | 1036 (27.9) | 1280 (26.9) | 0.297 |
| Diabetes | 1031 (27.8) | 1290 (27.1) | 0.491 |
| Hyperlipidemia | 1528 (41.2) | 1993 (41.9) | 0.512 |
| Hypertension | 2406 (64.8) | 2986 (62.7) | 0.048 |
| Smoking | 2356 (63.5) | 2878 (60.5) | 0.005 |
| BMI ≥30 | 1694 (45.6) | 2198 (46.1) | 0.302 |
| Peripheral Vascular Disease | 450 (12.1) | 635 (13.3) | 0.096 |
| Cerebrovascular Disease | 456 (12.2) | 594 (12.5) | 0.787 |
| Comorbidities | |||
| COPD | 1173 (31.6) | 1543 (32.4) | 0.424 |
| Cancer | 570 (15.4) | 746 (15.7) | 0.689 |
| Chronic Kidney Disease | 416 (11.2) | 576 (12.1) | 0.204 |
Chi-squared tests were used for categorical variables and Wilcoxon rank-sum tests were used for continuous variables. IQR= Interquartile range, WFBMC= Wake Forest Baptist Medical Center, DMC= Davie Medical Center, LMC= Lexington Medical Center. CAD= Coronary Artery Disease, BMI= Body Mass Index, COPD = Chronic Obstructive Pulmonary Disease
Safety
The HEART Pathway identified 30.7% (1461/4761) as low-risk and 53.2% (2531/4761) as non-low-risk. Another 7.0% (333/4761) had low-risk HEAR scores but lacked serial troponin measurements, and 9.2% (436/4761) had an incomplete or absent HEAR score. Among those classified as low-risk, 0.4% (6/1461; 95%CI: 0.2–0.9%) experienced death or MI from index through 30 days. Two of these events were MIs; (2/1461, 0.1%; 95%CI: 0.0–0.5%). Test characteristics of the HEART Pathway and adverse events among low-risk patients are summarized in Tables 2 and 3 respectively.
Table 2.
Test characteristics of the HEART Pathway and its components for detection of death and MI from index through 30 days.
| Sensitivity (95% CI) |
Specificity (95% CI) |
PPV (95% CI) |
NPV (95% CI) |
+LR (95% CI) |
-LR (95% CI) |
|
|---|---|---|---|---|---|---|
| HEART Pathway | 98.3% (96.3–99.4%) | 39.9% (38.3–41.5%) | 13.5% (12.2–14.9%) | 99.6% (99.1–99.9%) | 1.64 (1.59–1.68) | 0.04 (0.01–0.08) |
| HEAR Score | 83.6% (78.9–87.6%) | 43.0% (41.4–44.7%) | 11.0% (9.7–12.3% | 96.9% (95.9–97.7%) | 1.47 (1.38–1.55) | 0.38 (0.29–0.49) |
| Troponin | 91.8% (88.4–94.5) | 87.7% (86.6–88.8) | 42.6% (39.0–46.2) | 99.1% (98.7–99.4) | 7.49 (6.81–8.23) | 0.09 (0.07–0.13) |
HEART Pathway; low-risk determined by HEAR score <4, and no known CAD, and no acute ischemic ECG changes, and no troponin elevation at 0 or 3 hours. Non-low-risk determined by HEAR score ≥4, or known CAD, or an acute ischemic ECG change, or a troponin elevation at 0 or 3 hours.
HEAR Score; low risk determined by a HEAR score <4, and no known CAD, and no acute ischemic ECG changes. Non-low-risk determined by HEAR score ≥4, or known CAD, or an acute ischemic ECG change.
Troponin; low-risk determined by no troponin elevation at 0 or 3 hours. Non-low-risk determined by a troponin elevation at 0 or 3 hours.
CAD= coronary artery disease, ECG= electrocardiogram, PPV= positive predictive value, NPV=negative predictive value, +LR= positive likelihood ratio, -LR= negative likelihood ratio
Table 3.
Summary of death and MI events among patients classified as low-risk by the HEART Pathway.
| Age | Sex | Race* | Comorbidities | Site | HEAR score | 0 hr cTnI ng/ml† | 3 hr cTnI ng/ml† |
Index Visit ED Disposition | Index Cardiac Testing | Event |
|---|---|---|---|---|---|---|---|---|---|---|
| 41 | Female | W | Hypertension Hyperlipidemia Diabetes Obesity Family history of early ACS | DMC | 3 | 0.035 | 0.040 | Transfer to WFBMC for admission | Coronary angiography; multivessel disease >70% stenosis | Index visit NSTEMI; troponin peak at 0.045 ng/ml. 3-vessel CABG during follow-up period |
| 76 | Female | AA | Hypertension, autoimmune hepatitis | WFBMC | 3 | 0.007 | <0.006 | Discharged | None | Death on day 28; admitted to outside hospital for acute encephalopathy |
| 57 | Female | AA | Metastatic uterine cancer, DVT on Lovenox | WFBMC | 3 | 0.011 | 0.033 | Admitted to ICU, intubated for respiratory failure | None | Death during index hospitalization; care withdrawn |
| 73 | Male | W | COPD | WFBMC | 2 | <0.006 | <0.006 | Discharged | None | Death on day 6; Returned to ED with altered mental status from large subarachnoid hemorrhage |
| 50 | Male | AA | Hypertension, Tobacco, Cocaine abuse | WFBMC | 3 | 0.007 | 0.017 | Discharged | None | STEMI on day 12; Returned to ED with chest pain, ECG consistent with STEMI, coronary angiography with diffuse mild non-obstructive coronary artery disease (maximum stenosis = 25%) |
| 43 | Male | W | None | WFBMC | 0 | <0.006 | <0.006 | Admitted for hypoxemia and wheezing | None | Death during index visit, respiratory failure and PEA arrest |
Race; W=White, AA= African American,
cTnI at WFBMC and DMC has upper reference limit and 99th percentile value of 0.040 ng/ml and <10% coefficient of variation at 0.040 ng/ml. cTnI=cardiac troponin I, NSTEMI= non-ST segment elevation myocardial infarction, CABG= coronary artery bypass graft, DVT= deep vein thrombosis, ICU= intensive care unit, STEMI= ST segment elevation myocardial infarction, PEA= pulseless electrical activity.
During the index visit, MIs were detected in 6.6% (314/4761) of the post-implementation cohort compared to 5.7% of the pre-implementation cohort (211/3713); aOR: 1.36 (95%CI: 1.12–1.65). Index visit deaths occurred in 0.3% (15/4761) of patients in the post-implementation cohort compared to 0.2% (7/3713) pre-implementation patients; aOR: 2.01 (95%CI: 0.79–5.10). During the 30 day follow-up period (not including the index visit) death or MI rates were similar in the post-implementation cohort (1.1%, 51/4761) and pre-implementation cohort (1.3%, 50/3713); aOR: 0.88 (95%CI: 0.58–1.33). Death or MI at 30 days occurred in 0.3% (6/2046) of early discharge patients in the post-implementation cohort compared to 0.6% (8/1390) in the pre-cohort (aOR: 0.71 95%CI: 0.22–2.8).
Hospitalizations
In the post-implementation cohort, 55.6% (2649/4761) of patients were hospitalized during the index visit and 30-day follow-up, compared to 61.6% (2288/3713) in the pre-implementation cohort, a reduction of 6.0% (95% CI 3.9–8.1%) with an aOR of 0.79 (95%CI 0.71–0.87). (Figure 3)
Figure 3.
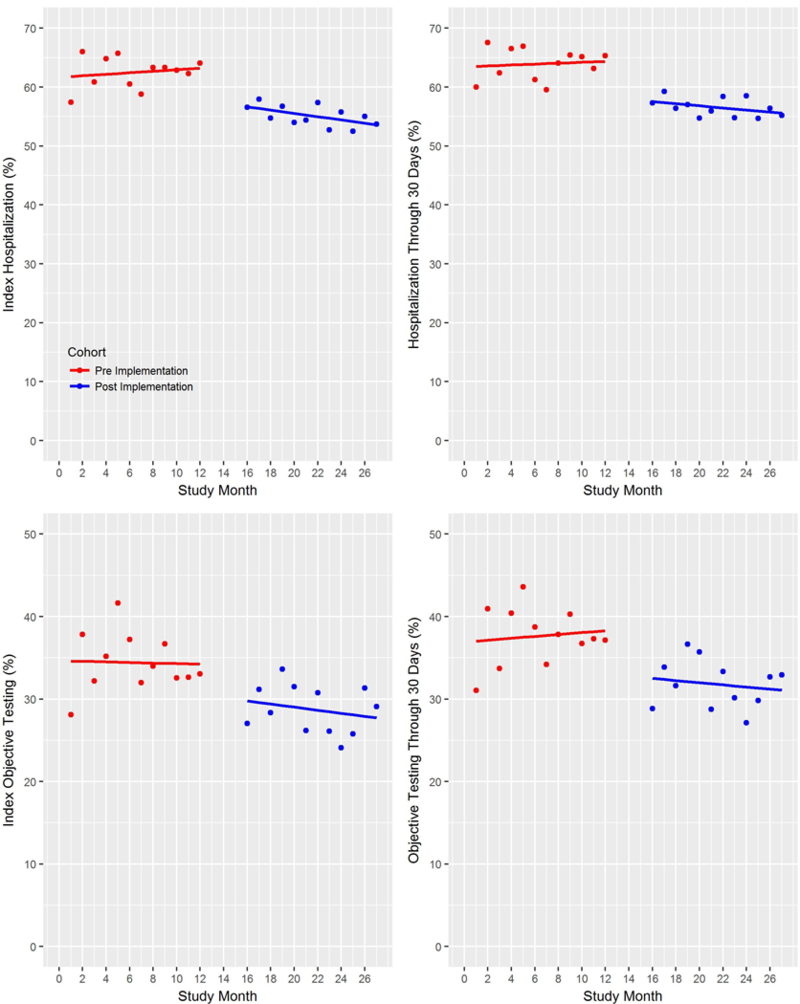
Hospitalization and objective cardiac testing rates at WFBMC and DMC sites during the index visit and through the 30-day follow-up period, with fitted regression lines. Data from LMC are excluded from this plot due to asynchronous accrual times.
Secondary Utilization Endpoints
Early discharge occurred in 43.0% (2046/4761) of the post-cohort versus 37.4% (1390/3713) in the pre-cohort; an increase of 5.6% (95%CI 3.4–7.6%) with an aOR of 1.24 (95%CI 1.12–1.37). Stress testing and angiography from index visit through 30 days was completed in 30.7% (1462/4761) of patients in the post-cohort compared to 34.5% (1281/3713) in the pre-cohort; a decrease of 3.8% (95% CI 1.8% - 5.8%) with an aOR of 0.89 (95% CI 0.81–0.99). Median index visit LOS was lower in the post-cohort compared to the pre-cohort (15.5 hours; IQR 5.2, 37.6 vs 17.6 hours; IQR 5.0, 40.5; p=0.003). However, median ED LOS was similar post- and pre-implementation (4.0 hours; IQR 2.8, 5.2 vs 3.6 hours; IQR 2.6, 5.0; p=0.15). Non-adherence to the disposition guidance of the HEART Pathway occurred in 15.6% (258/1461) of low-risk patients and 1.2% 360/2531 of non-low-risk patients. Unadjusted and adjusted models of safety and utilization endpoints are listed in Table 4. Comparison of outcomes in the post-cohort among low-risk and non-low-risk patients is summarized in Table 5.
Table 4.
Proportion of patients with events in the Pre- and Post-implementation cohorts
| Outcomes | Pre N= 3713 (%) |
Post N= 4761 (%) |
Unadjusted Odds Ratio (95% CI) |
Adjusted* Odds Ratio (95% CI) |
|---|---|---|---|---|
| Safety | ||||
| Index visit | ||||
| Death | 7 (0.2) | 15 (0.3) | 1.67 (0.68–4.11) | 2.01 (0.79–5.10) |
| MI | 211 (5.7) | 314 (6.6) | 1.17 (0.98–1.40) | 1.36 (1.12–1.65) |
| Revascularization | 119 (3.2) | 154 (3.2) | 1.01 (0.79–1.29) | 1.17 (0.90–1.52) |
| Death + MI | 217 (5.8) | 325 (6.8) | 1.18 (0.99–1.41) | 1.37 (1.13–1.66) |
| Death + MI + Revascularization | 257 (6.9) | 355 (7.5) | 1.08 (0.92–1.28) | 1.25 (1.04–1.50) |
| Follow-up period | ||||
| Death | 37 (1.0) | 24 (0.5) | 0.50 (0.30–0.84) | 0.49 (0.28–0.86) |
| MI | 18 (0.5) | 29 (0.6) | 1.26 (0.70–2.27) | 1.55 (0.85–2.83) |
| Revascularization | 25 (0.7) | 38 (0.8) | 1.19 (0.72–1.97) | 1.43 (0.85–2.42) |
| Death + MI | 50 (1.3) | 51 (1.1) | 0.79 (0.54–1.17) | 0.88 (0.58–1.33) |
| Death + MI + Revascularization | 69 (1.9) | 77 (1.6) | 0.87 (0.63–1.20) | 0.98 (0.70–1.39) |
| 30 day (Index + follow up) | ||||
| Death | 44 (1.2) | 39 (0.8) | 0.69 (0.45–1.06) | 0.73 (0.46–1.16) |
| MI | 223 (6.0) | 324 (6.8) | 1.14 (0.96–1.36) | 1.34 (1.11–1.62) |
| Revascularization | 143 (3.9) | 190 (4.0) | 1.04 (0.83–1.29) | 1.23 (0.97–1.57) |
| Death + MI | 258 (6.9) | 353 (7.4) | 1.07 (0.91–1.27) | 1.24 (1.03–1.48) |
| Death + MI + Revascularization | 303 (8.2) | 395 (8.3) | 1.02 (0.87–1.19) | 1.17 (0.99–1.39) |
|
Utilization |
||||
| Index visit | ||||
| Hospitalization | 2231 (60.1) | 2582 (54.2) | 0.79 (0.72–0.86) | 0.80 (0.72–0.88) |
| Early discharge | 1390 (37.4) | 2046 (43.0) | 1.26 (1.15–1.38) | 1.24 (1.12–1.37) |
| Objective Cardiac Testing | 1145 (30.8) | 1307 (27.5) | 0.85 (0.77–0.93) | 0.90 (0.81–0.99) |
| Follow-up period | ||||
| Hospitalization | 241 (6.5) | 271 (5.7) | 0.87 (0.73–1.04) | 0.92 (0.76–1.11) |
| Objective Cardiac Testing | 199 (5.4) | 206 (4.3) | 0.80 (0.65–0.98) | 0.86 (0.70–1.06) |
| 30 day (Index + follow up) | ||||
| Hospitalization | 2288 (61.6) | 2649 (55.6) | 0.78 (0.72–0.85) | 0.79 (0.71–0.87) |
| Objective Cardiac Testing | 1281 (34.5) | 1462 (30.7) | 0.84 (0.77–0.92) | 0.89 (0.81–0.99) |
Models adjusted for the following variables: age, gender, race, ethnicity, body mass index (BMI), emergency department location, insurance status, smoking, history of coronary artery disease, diabetes, hyperlipidemia, hypertension, cerebrovascular disease, peripheral vascular disease, chronic obstructive pulmonary disorder, chronic kidney disease, cancer (excludes non-melanoma skin cancer), and presence of chest pain vs other symptoms concerning for acute coronary syndrome.
Table 5.
Proportion of patients with events in the Post-implementation cohort based on HEART Pathway risk assessment
| Outcomes | Low-Risk N=1461 (%) |
Non-Low-Risk N=2531 (%) |
Incomplete N=769 (%) |
Percent Difference Low:Non-Low (95% CI)* |
Percent Difference Low:Incomplete (95% CI)* |
|---|---|---|---|---|---|
| Safety | |||||
| Index visit | |||||
| Death | 2 (0.1) | 12 (0.5) | 1 (0.1) | 0.3 (0.0–0.7) | 0.0 (−0.3–0.3) |
| MI | 1 (0.1) | 313 (12.4) | 0 (0) | 12.3 (11.0–13.6) | −0.1 (−0.2–0.1) |
| Revascularization | 1 (0.1) | 151 (6) | 2 (0.3) | 5.9 (5.0–6.8) | 0.2 (−0.2–0.6) |
| Death + MI | 3 (0.2) | 321 (12.7) | 1 (0.1) | 12.5 (11.2–13.8) | −0.1 (−0.4–0.3) |
| Death + MI + Revascularization | 4 (0.3) | 348 (13.7) | 3 (0.4) | 13.5 (12.1–14.8) | 0.1 (−0.4–0.6) |
| Follow-up period | |||||
| Death | 2 (0.1) | 19 (0.8) | 3 (0.4) | 0.6 (0.2–1.0) | 0.3 (−0.2–0.7) |
| MI | 1 (0.1) | 26 (1) | 2 (0.3) | 1.0 (0.5–1.4) | 0.2 (−0.2–0.6) |
| Revascularization | 1 (0.1) | 34 (1.3) | 3 (0.4) | 1.3 (0.8–1.7) | 0.3 (−0.1–0.8) |
| Death + MI | 3 (0.2) | 43 (1.7) | 5 (0.7) | 1.5 (0.9–2.0) | 0.4 (−0.2–1.1) |
| Death + MI + Revascularization | 4 (0.3) | 66 (2.6) | 7 (0.9) | 2.3 (1.7–3.0) | 0.6 (−0.1–1.4) |
| 30 day (Index + follow up) | |||||
| Death | 4 (0.3) | 31 (1.2) | 4 (0.5) | 1.0 (0.4–1.5) | 0.2 (−0.3–0.8) |
| MI | 2 (0.1) | 320 (12.6) | 2 (0.3) | 12.5 (11.2–13.8) | 0.1 (−0.3–0.5) |
| Revascularization | 2 (0.1) | 183 (7.2) | 5 (0.7) | 7.1 (6.1–8.1) | 0.5 (−0.1–1.1) |
| Death + MI | 6 (0.4) | 341 (13.5) | 6 (0.8) | 13.1 (11.7–14.4) | 0.4 (−0.3–1.1) |
| Death + MI + Revascularization | 7 (0.5) | 378 (14.9) | 10 (1.3) | 14.5 (13.0–15.9) | 0.8 (−0.1–1.7) |
|
Utilization |
|||||
| Index visit | |||||
| Hospitalization | 241 (16.5) | 2095 (82.8) | 246 (32) | 66.3 (63.9–68.7) | 15.5 (11.7–19.3) |
| Early discharge | 1203 (82.3) | 360 (14.2) | 483 (62.8) | −68.1 (−70.5−−65.7) | −19.5 (−23.5−−15.6) |
| Objective Cardiac Testing | 116 (7.9) | 1148 (45.4) | 43 (5.6) | 37.4 (35.0–39.8) | −2.3 (−4.5−−0.2) |
| Follow-up period | |||||
| Hospitalization | 43 (2.9) | 199 (7.9) | 29 (3.8) | 4.9 (3.6–6.3) | 0.8 (−0.8–2.4) |
| Objective Cardiac Testing | 43 (2.9) | 145 (5.7) | 18 (2.3) | 2.8 (1.5–4.0) | −0.6 (−2.0–0.8) |
| 30 day (Index + follow up) | |||||
| Hospitalization | 268 (18.3) | 2128 (84.1) | 253 (32.9) | 65.7 (63.3–68.2) | 14.6 (10.7–8.4) |
| Objective Cardiac Testing | 156 (10.7) | 1248 (49.3) | 58 (7.5) | 38.6 (36.1–41.1) | −3.1 (−5.6−−0.7) |
Proportions and associated 95%CI were calculated without adjustment for potential confounders.
Sensitivity Analyses
Sensitivity analyses conducted using various assumptions for patients with incomplete follow-up data did not substantively change aORs for safety outcomes (Supplemental Table 1). Analysis of the completeness of EHR follow-up, found that most safety events were captured in the EHR; with the death index and claims data identifying only 16 safety events that were not already accounted for in the EHR data. A sensitivity analysis excluding all patients accrued by use of the EHR ACS flowsheet (patients without chest pain selected by the provider) from analysis did not meaningfully change study conclusions (Supplemental Tables 2 and 3). Analyses conducted using models with fewer covariates as a precaution for overfitting did not substantively change results.
Discussion
The primary finding of this multisite implementation study is that the HEART Pathway is a safe strategy for identifying patients with acute chest pain for early discharge from the ED setting. The HEART Pathway classified 31% of ED patients with acute chest pain as low-risk; among these, only 0.4% died or had an MI at 30 days. There is some consensus that an accelerated diagnostic protocol should achieve a missed adverse event rate below 1% at 30 days.15 Our findings demonstrate that the HEART Pathway’s miss rate is well below this threshold. Furthermore, a closer evaluation of adverse events in low-risk patients (Table 3) suggests that many of the deaths were likely non-cardiac in nature, occurring in patients hospitalized for non-ACS conditions (e.g., metastatic cancer). Only two events were MIs; yielding a missed MI rate of just 0.1% for the HEART Pathway among low-risk patients.
Prior studies demonstrating the efficacy of the HEART Pathway had encouraging safety data, but were not designed to address effectiveness or powered to definitively demonstrate safety. This study estimates the adverse event rate among low-risk patients with tight 95% confidence intervals and an upper bound below 1%. Previously, a lack of sufficient prospective safety data on chest pain ADPs, such as the HEART Pathway, was a significant driver of inefficiency and over-testing. However, this study provides evidence that could change current practice patterns and guidelines: recommendations that non-invasive objective testing occur in low-risk patients may be obselete.7
The HEART Pathway identified more patients with MI during the index visit compared to the pre-implementation cohort when adjusted for potential confounding covariates. This finding suggests that the HEART Pathway not only identifies a large proportion of patients as low-risk who can be safely discharged, but also identifies patients at a higher risk of MI who may otherwise have been missed. Enhanced detection of MIs was not driven by changes in troponin assays, cut points, or measurement techniques; these remained stable throughout the study. However, increased use of serial troponin measurements after HEART Pathway implementation, or greater awareness of MI following HEART Pathway training sessions, may have increased the rate of MI detection.
Our study also demonstrates that the HEART Pathway reduced healthcare utilization. This finding is timely, given the high cost of delivering care to patients with acute chest pain and the current focus on delivering high-value care.30 While efficiency gains (reductions in hospitalizations, objective cardiac testing, and index visit LOS, and increase in early discharge rate) from the HEART Pathway were modest, when extrapolated to the 8–10 million patients with chest pain seen in a US ED annually substantial savings in healthcare resources are possible. Furthermore, even small reductions in early discharge rate, LOS, and objective cardiac testing rates can have a large impact on ED/hospital crowding and resource stewardship.31 Also, our modest reductions in utilization outcomes should be interpreted in the context of our health system’s prior experience with the HEART Pathway. Our research conducted prior to this study introduced the HEART Pathway to most of our ED providers, and some of them were informally using it during the pre-implementation period. Therefore, it is possible that “contamination” may have decreased the effect size of our intervention and hospitals, which are “naïve” to the HEART Pathway may realize larger reductions in healthcare utilization outcomes.
As the first prospective multi-site evaluation in the US of a chest pain ADP, designed to identify low-risk patients for early discharge, this study substantively adds to a growing body of literature suggesting the safety of such processes. An evaluation of a national clinical pathway in chest pain patients in New Zealand also reported a significant increase in early discharge rate while maintaining safety.32 Thus, cumulatively, there is now evidence of safe prospective use of chest pain ADPs in almost 25,000 patients.
However, a recent study evaluating use of the HEART score in the Netherlands did not find a significant increase in early discharge rate and reported a 2% MACE rate among low-risk patients.33 This may be due to several key differences between the HEART score and our HEART Pathway. First, the HEART score incorporates a single troponin measure. Although rare, patients with an elevated troponin level could have a low-risk score. Second, the HEART score can be low-risk in patients with acute ischemic changes on ECG or known CAD. The HEART Pathway CDS uses serial troponin measurements and prioritizes troponin elevation, ischemic ECG changes, and prior CAD; patients with any of these are considered non-low-risk regardless of score. Finally, the HEART score has subjective criteria and is manually calculated, which decrease its reproducibility and reliability.34, 35 The HEART Pathway CDS replaces subjective components of the HEART score with objective binary questions and uses an algorithm to determine each HEAR score component.
Limitations
Nonetheless, our study design has limitations compared to a traditional randomized design. For example, secular trends and provider maturation effects are potential threats to the validity of our results. However, event rates were fairly consistent over time (Figure 3). Using our EHR to collect events may have decreased event rates compared to traditional methods of follow-up. However, supplementing the EHR data with death index and claims data identified only 16 additional 30-day safety events. This suggests that our EHR identified most events and justifies including all patients in the analysis rather than limiting the analysis to only patients with insurance claims data. More patients were accrued into the post-implementation phase compared to the pre-implementation phase. This imbalance occurred because providers used an EHR flowsheet for patients with non-chest pain presentations more frequently once the HEART Pathway tool was available in this flowsheet. It is possible that inclusion of these patients produced a selection bias, however a sensitivity analysis excluding these patients did not significantly impact study conclusions. In addition, although our 3 sites were diverse in size and location (urban and suburban), results may not be generalizable to, or feasible in, all US health systems. However, given the size and scope of this pragmatic implementation study, our design had advantages of feasibility, cost-effectiveness, and generalizability compared to a traditional randomized trial. Some differences existed in baseline risk factors present in the pre- versus post-implementation cohorts. However, our regression analyses adjusted for these potential confounders. Also, one of our sites (LMC) did not implement the HEART Pathway on the same time schedule as the others, and it is possible that this asynchrony may have influenced our results. Finally, it is possible that safety events related to the index visit care occurred beyond the 30 day follow-up period. To address this concern, 1-year follow-up data was collected on each participant and a separate analysis of 1 year safety and utilization outcomes is planned.
Conclusions
The HEART Pathway was associated with decreased hospitalizations and death and MI rates well below 1% among low-risk patients. This study may provide a model for US health systems to provide safe and high-value care to the 8–10 million patients who present to a US ED with acute chest pain each year. Our data add to a growing body of evidence suggesting that current practice guidelines should be changed, so that stress tests or cardiac imaging are no longer recommended for most low-risk patients presenting to the ED with chest pain.
Supplementary Material
Clinical Perspective
What is new?
Among the 30.7% of patients identified by the HEART Pathway as low-risk the rate of all-cause death and myocardial infarction was 0.4%.
Implementation of the HEART Pathway was associated with increased detection of index visit myocardial infarctions; with an adjusted odd ratio of 1.36 (95%CI: 1.12–1.65).
Hospitalizations from index visit through 30 days were decreased by 6% following HEART Pathway implementation.
HEART Pathway implementation increased early discharge from the ED by 5.6%, decreased median index visit length of stay by 2.1 hours, and reduced stress testing and angiography at 30 days by 3.8%.
What are the clinical implications?
These findings demonstrate that the HEART Pathway is safe and effective at increasing early ED discharges and decreasing hospitalizations, stress testing, and index visit length of stay in patients with acute chest pain.
Given its ability to safely reduce health care utilization outcomes, the HEART Pathway may provide a model for health systems to provide safe and high-value care to patients presenting to Emergency Departments with chest pain.
Acknowledgements
Special thanks to William B. Applegate, MD, MPH, Robert F. Riley. MD, MS, Erin N. Harper, MS, Jason P. Stopyra, MD, Stephanie Elliott, Russell M. Howerton, MD, and Bob Mckee, for their assistance with and support for HEART Pathway implementation.
Funding Sources
This project was funded by the Donaghue Foundation and the Association of American Medical Colleges (AAMC). Funding sources had no role in design or conduct of this investigation. This includes no role in the collection, management, analysis, and interpretation of data nor preparation, review, or approval of the manuscript. We acknowledge the assistance of the Wake Forest Clinical and Translational Science Institute, supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through UL1TR001420 (Donald McClain, PI). The WF CTSI assisted with data extraction, study design, project management, and medical editing.
Footnotes
Disclosures
Dr. Mahler receives research funding from Abbott Point of Care, Roche Diagnostics, Siemens, he has received consulting honoraria from Roche Diagnostics, and is the Chief Medical Officer for Impathiq Inc. Dr, Mahler also receives research support from NHLBI (1 R01 HL118263–01, L30 HL120008) and PCORI. Dr. Mahler has a conflict of interest management plan in place for research, through the Conflict of Interest Office at the Wake Forest School of Medicine. Dr. Miller receives research support from Siemens, Abbott Point of Care, and 1 R01 HL118263.
References
- 1.Owens PL, Barrett ML, Gibson TB, Andrews RM, Weinick RM, Mutter RL. Emergency department care in the united states: A profile of national data sources. Ann Emerg Med. 2010;56:150–165. [DOI] [PubMed] [Google Scholar]
- 2.Pope JH, Aufderheide TP, Ruthazer R, Woolard RH, Feldman JA, Beshansky JR, Griffith JL, Selker HP. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med. 2000;342:1163–1170. [DOI] [PubMed] [Google Scholar]
- 3.Pines JM, Isserman JA, Szyld D, Dean AJ, McCusker CM, Hollander JE. The effect of physician risk tolerance and the presence of an observation unit on decision making for ed patients with chest pain. Am J Emerg Med. 2010;28:771–779. [DOI] [PubMed] [Google Scholar]
- 4.Fleischmann KE, Goldman L, Johnson PA, Krasuski RA, Bohan JS, Hartley LH, Lee TH. Critical pathways for patients with acute chest pain at low risk. J Thromb Thrombolysis. 2002;13:89–96. [DOI] [PubMed] [Google Scholar]
- 5.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics−−2011 update: A report from the american heart association. Circulation. 2011;123:e18–e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomez MA, Anderson JL, Karagounis LA, Muhlestein JB, Mooers FB. An emergency department-based protocol for rapidly ruling out myocardial ischemia reduces hospital time and expense: Results of a randomized study (romio). J Am Coll Cardiol. 1996;28:25–33. [DOI] [PubMed] [Google Scholar]
- 7.Amsterdam EA, Kirk JD, Bluemke DA, Diercks D, Farkouh ME, Garvey JL, Kontos MC, McCord J, Miller TD, Morise A, Newby LK, Ruberg FL, Scordo KA, Thompson PD. Testing of low-risk patients presenting to the emergency department with chest pain: A scientific statement from the american heart association. Circulation. 2010;122:1756–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner EH. Chronic disease management: What will it take to improve care for chronic illness? Eff Clin Pract. 1998;1:2–4. [PubMed] [Google Scholar]
- 9.Glasgow RE, Orleans CT, Wagner EH. Does the chronic care model serve also as a template for improving prevention? Milbank Q. 2001;79:579–612, iv-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness: The chronic care model, part 2. J Am Med Assoc. 2002;288:1909–1914. [DOI] [PubMed] [Google Scholar]
- 11.Mahler SA, Hiestand BC, Goff DC Jr., Hoekstra JW, Miller CD. Can the heart score safely reduce stress testing and cardiac imaging in patients at low risk for major adverse cardiac events? Crit Pathw Cardiol. 2011;10:128–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahler SA, Miller CD, Hollander JE, Nagurney JT, Birkhahn R, Singer AJ, Shapiro NI, Glynn T, Nowak R, Safdar B, Peberdy M, Counselman FL, Chandra A, Kosowsky J, Neuenschwander J, Schrock JW, Plantholt S, Diercks DB, Peacock WF. Identifying patients for early discharge: Performance of decision rules among patients with acute chest pain. Int J Cardiol. 2013;168:795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahler SA, Riley RF, Hiestand BC, Russell GB, Hoekstra JW, Lefebvre CW, Nicks BA, Cline DM, Askew KL, Elliott SB, Herrington DM, Burke GL, Miller CD. The heart pathway randomized trial: Identifying emergency department patients with acute chest pain for early discharge. Circ Cardiovasc Qual Outcomes. 2015;8:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riley RF, Miller CD, Russell GB, Harper EN, Hiestand BC, Hoekstra JW, Lefebvre CW, Nicks BA, Cline DM, Askew KL, Mahler SA. Cost analysis of the history, ecg, age, risk factors, and initial troponin (heart) pathway randomized control trial. Am J Emerg Med. 2017;35:77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Than M, Herbert M, Flaws D, Cullen L, Hess E, Hollander JE, Diercks D, Ardagh MW, Kline JA, Munro Z, Jaffe A. What is an acceptable risk of major adverse cardiac event in chest pain patients soon after discharge from the emergency department?: A clinical survey. Int J Cardiol. 2013;166:752–754. [DOI] [PubMed] [Google Scholar]
- 16.Mahler SA, Burke GL, Duncan PW, Case LD, Herrington DM, Riley RF, Wells BJ, Hiestand BC, Miller CD. Heart pathway accelerated diagnostic protocol implementation: Prospective pre-post interrupted time series design and methods. JMIR Res Protoc. 2016;5:e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in icd-9-cm and icd-10 administrative data. Med Care. 2005;43:1130–1139. [DOI] [PubMed] [Google Scholar]
- 18.Tonelli M, Wiebe N, Fortin M, Guthrie B, Hemmelgarn BR, James MT, Klarenbach SW, Lewanczuk R, Manns BJ, Ronksley P, Sargious P, Straus S, Quan H, Alberta Kidney Disease N. Methods for identifying 30 chronic conditions: Application to administrative data. BMC Med Inform Decis Mak. 2015;15:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.So L, Evans D, Quan H. Icd-10 coding algorithms for defining comorbidities of acute myocardial infarction. BMC Health Serv Res. 2006;6:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riley RF, Don CW, Powell W, Maynard C, Dean LS. Trends in coronary revascularization in the united states from 2001 to 2009: Recent declines in percutaneous coronary intervention volumes. Circ Cardiovascular quality and outcomes. 2011;4:193–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Culler SD, Kugelmass AD, Brown PP, Reynolds MR, Simon AW. Trends in coronary revascularization procedures among medicare beneficiaries between 2008 and 2012. Circulation. 2015;131:362–370; discussion 370. [DOI] [PubMed] [Google Scholar]
- 22.McCord J, Cabrera R, Lindahl B, Giannitsis E, Evans K, Nowak R, Frisoli T, Body R, Christ M, deFilippi CR, Christenson RH, Jacobsen G, Alquezar A, Panteghini M, Melki D, Plebani M, Verschuren F, French J, Bendig G, Weiser S, Mueller C, Investigators T-A. Prognostic utility of a modified heart score in chest pain patients in the emergency department. Circ Cardiovasc Qual Outcomes. 2017;10:e003617. [DOI] [PubMed] [Google Scholar]
- 23.Mahler SA, Stopyra JP, Apple FS, Riley RF, Russell GB, Hiestand BC, Hoekstra JW, Lefebvre CW, Nicks BA, Cline DM, Askew KL, Herrington DM, Burke GL, Miller CD. Use of the heart pathway with high sensitivity cardiac troponins: A secondary analysis. Clin Biochem. 2017;50:401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kashef MA, Garb J, Kugelmass A, Lotfi A. Implementation of an early discharge protocol and chest pain clinic for low-risk chest pain in the emergency department. Crit Pathw Cardiol. 2018;17:1–5. [DOI] [PubMed] [Google Scholar]
- 25.Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27:299–309. [DOI] [PubMed] [Google Scholar]
- 26.Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr. 2013;13:S38–44. [DOI] [PubMed] [Google Scholar]
- 27.Little RJA. Missing-data adjustments in large surveys. J Bus Econ Stat. 1988;6:287–296 [Google Scholar]
- 28.Moons KGM, Donders RART, Stijnen T, Harrell FE. Using the outcome for imputation of missing predictor values was preferred. J Clin Epidemiol. 2006;59:1092–1101 [DOI] [PubMed] [Google Scholar]
- 29.Hess EP, Brison RJ, Perry JJ, Calder LA, Thiruganasambandamoorthy V, Agarwal D, Sadosty AT, Silvilotti ML, Jaffe AS, Montori VM, Wells GA, Stiell IG. Development of a clinical prediction rule for 30-day cardiac events in emergency department patients with chest pain and possible acute coronary syndrome. Ann Emerg Med. 2012;59:115–125 e111. [DOI] [PubMed] [Google Scholar]
- 30.Conway Ph MFCC. The future of quality measurement for improvement and accountability. J Am Med Assoc. 2013;309:2215–2216. [DOI] [PubMed] [Google Scholar]
- 31.McHugh M, Van Dyke K, McClelland M, Moss D Improving patient flow and reducing emergency department crowding: A guide for hospitals (prepared by the health research & educational trust, an affiliate of the american hospital association, under contract 290–200-600022, task order no. 6). AHRQ publication no. 11(12)-0094. Rockville, md:. Agency for Healthcare Research and Quality; 2011; 1:1–43. [Google Scholar]
- 32.Than MP, Pickering JW, Dryden JM, Lord SJ, Aitken SA, Aldous SJ, Allan KE, Ardagh MW, Bonning JWN, Callender R, Chapman LRE, Christiansen JP, Cromhout APJ, Cullen L, Deely JM, Devlin GP, Ferrier KA, Florkowski CM, Frampton CMA, George PM, Hamilton GJ, Jaffe AS, Kerr AJ, Larkin GL, Makower RM, Matthews TJE, Parsonage WA, Peacock WF, Peckler BF, van Pelt NC, Poynton L, Richards AM, Scott AG, Simmonds MB, Smyth D, Thomas OP, To ACY, Du Toit SA, Troughton RW, Yates KM, Group IC-AI. Icare-acs (improving care processes for patients with suspected acute coronary syndrome): A study of cross-system implementation of a national clinical pathway. Circulation. 2018;137:354–363. [DOI] [PubMed] [Google Scholar]
- 33.Poldervaart JM, Reitsma JB, Backus BE, Koffijberg H, Veldkamp RF, Ten Haaf ME, Appelman Y, Mannaerts HFJ, van Dantzig JM, van den Heuvel M, El Farissi M, Rensing B, Ernst N, Dekker IMC, den Hartog FR, Oosterhof T, Lagerweij GR, Buijs EM, van Hessen MWJ, Landman MAJ, van Kimmenade RRJ, Cozijnsen L, Bucx JJJ, van Ofwegen-Hanekamp CEE, Cramer MJ, Six AJ, Doevendans PA, Hoes AW. Effect of using the heart score in patients with chest pain in the emergency department: A stepped-wedge, cluster randomized trial. Ann Intern Med. 2017;166:689–697. [DOI] [PubMed] [Google Scholar]
- 34.Mahler SA, Riley RF, Russell GB, Hiestand BC, Hoekstra JW, Lefebvre CW, Nicks BA, Cline DM, Askew KL, Bringolf J, Elliott SB, Herrington DM, Burke GL, Miller CD. Adherence to an accelerated diagnostic protocol for chest pain: Secondary analysis of the heart pathway randomized trial. Acad Emerg Med. 2016;23:70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahler SA, Riley RF, Burke GL, Hiestand BC, Miller CD. Real-time use of a chest pain risk stratification clinical decision rule: A cautionary tale. Ann Emerg Med. 2013;62:S112–S112. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


