Abstract
Epidemiological studies provide strong evidence for the impact of diverse paternal life experiences on offspring neurodevelomental disease risk. While these associations are well established, the molecular mechanisms underlying these intergenerational transmissions remain elusive, though recent studies focusing on the influence of paternal experience prior to conception have implicated germ cell epigenetic programming. Any model accounting for the germline transfer of non-genetic information from sire to offspring must include certain components: 1) a vector to carry any signal from the paternal compartment to the maternal reproductive tract and future embryo; 2) a molecular signal, encoded by a paternal experience, to carry this “memory” and enact downstream responses; and 3) a target cell or tissue to receive the signal and convert it into an effect on embryonic development. In this review, we explore the field’s current understanding of the potential processes and candidate factors that may serve as these components. We specifically discuss the field’s growing appreciation for the importance of extracellular vesicles in these processes, beginning with their known role in delivering potential signals, including small RNAs, to sperm, the prototypical vector, during their post-testicular maturation. Finally we explore the possibility that paternal extracellular vesicles could themselves serve as vectors, delivering signals not only to gametes or the zygote, but also to tissues of the maternal reproductive tract to influence fetal development.
Keywords: epigenetic, paternal, extracellular vesicle, miRNA, sperm, neurodevelopment
Introduction
Neurodevelopmental and neuropsychiatric disorders exert a profound cost on society; costs that are exacerbated by current treatment modalities that are often ineffective in a significant percentage of patients. Efforts to develop more effective therapeutic interventions for these disorders have been hindered by an incomplete understanding of their etiologies, which are multifaceted and complex. Epidemiological studies provide strong evidence for the impact of parental lifetime experiences on offspring neurodevelopmental disease risk. For example, adult children of parents exposed to stressful life events, such as famine or war, are more likely to be diagnosed with a psychiatric disease (1–4). While these associations are well established, the molecular mechanisms underlying these intergenerational transmissions remain elusive, especially for paternal effects. However, recent studies focusing on the influence of preconception parental insults have implicated germ cell epigenetic programming (5–7).
Time points at which parental life experiences can shape offspring neurodevelopment are widespread, making it difficult to distinguish germline involvement from more typical modes of parental influence, including in animal models (8, 9). Differences in the quality or quantity of parental care or investment can communicate parental information to offspring. Given the importance of these interactions for offspring survival and health, the origin of outcome measures can be difficult to isolate without intrusive experimental manipulations such as crossfostering or in vitro fertilization (9). In addition, unique to the maternal experience is that maternal influences extend into the gestational compartment and postpartum periods, where changes can directly affect the programming of fetal tissues. Focusing on the transmission of paternal experiences can simplify these considerations, specifically using rodent models in which interactions between sires and their offspring can be restricted. With appropriate experimental design, the contribution of paternal experience can then be limited to factors present in semen.
Overview
At its most fundamental, intergenerational transmission of paternal experience through the germline involves a transfer of non-genetic information from sire to offspring. Any mechanism responsible for this information transfer must include certain components: 1) a vector to carry any signal from the paternal compartment to the maternal reproductive tract and future embryo; 2) a molecular signal, encoded by the paternal experience, to carry this “memory” and enact downstream responses; and 3) a target cell or tissue that can receive the molecular signal and convert it into an effect on embryonic development. In this review, we explore the field’s current understanding of the potential molecular processes and candidate factors that may serve as these components. The obvious vector for any paternal signal that will impact embryonic development is the sperm cell. We provide a general overview of sperm development, highlighting points of vulnerability to the programming effects of insults. We also explore the possibility that sperm are not the only potential vector, identifying an additional male factor in semen, extracellular vesicles (EV), that may serve this role. We then discuss candidates for the molecular signal of paternal experience carried by these vectors and ask how the signal can be initially encoded. Finally, we discuss potential targets of the signal carried in a vector, including the oocyte and tissues of the maternal reproductive tract with the ability to affect fetal development to propagate an intergenerational effect of paternal lifetime experience.
The elements of this framework are certainly not original. Many aspects of these processes have been explored in other noted excellent reviews (7, 10–12). What we hope to accomplish in this review is to present these ideas together, and to highlight exciting new directions that recent work has taken to illuminate facets and unexpected players in the intergenerational impact of paternal lifetime experience on offspring neurodevelopment.
Vector
Sperm as a vector – the challenge of the Weismann barrier
In his 1893 theory of heredity, August Weismann proposed that heritable information in multicellular organisms resided exclusively in an “immortal” germ-plasm. Central to his theory, as adapted to a modern understanding of biology, was a theoretical barrier that restricted the flow of heritable information from somatic tissues to germ cells (12). In males, very real biological manifestations of this theoretical barrier have been identified. These include the prenatal and postnatal epigenetic reprogramming events that irreversibly segregate germline from somatic cell lineages, physical and physiological barriers between the periphery and developing sperm cells, including the blood-testis barrier, and the dramatic nuclear and cellular remodeling that occurs during spermatogenesis (13). However, given that germline transmission of paternal experience is now a recognized phenomenon, these barriers are obviously not absolute. In this section we will examine these obstacles, highlighting points in development when these barriers might be diminished, or vectors that may bypass them altogether to allow the encoding of paternal experience by molecular signals, such as changes in their small RNA content.
In animals, segregation of a dedicated germline occurs early in development, a process that involves extensive reprogramming of the epigenome (8). Primordial germ cells undergo a genome-wide reduction in DNA methylation as they migrate to the gonadal ridge. Following their colonization of the embryonic gonad, mitotically-arrested spermatogonia acquire the germ cell-specific epigenetic programming that permits them to enter spermatogenesis in adulthood. We and others have hypothesized that these processes may be vulnerable to exposures that occur during prenatal development (8, 14–17). For example, we have found that male mice exposed to maternal stress specifically during early gestation exhibit a stress dysregulation phenotype as adults, and transmit this phenotype to their male offspring (18). We hypothesized that this early prenatal stress not only altered the neurodevelopment of the exposed male fetus via somatic cell effects (the F1 generation), but also affected germ cells of this fetus via epigenetic programming, leading to transmission of the stress experience through those germ cells to their offspring (the F2 generation) (18). Similarly, F1 male mice that experienced a period of in utero caloric restriction overlapping with the window of germ cell reprogramming, transmitted a metabolic phenotype to their F2 offspring, effects which were associated with changes in the DNA methylome of F1 sperm (16).
Puberty, with the coincident formation of the blood-testis/epididymis barriers and the initiation of spermatogenesis, marks the next major milestone in sperm development. These barriers along the male reproductive axis consist primarily of tight junctions between Sertoli cells in the testis or epithelial cells of the epididymis that restrict the passage of molecules and cells into the lumen where sperm are developing and maturing (19, 20). In doing so, they serve to maintain the microenvironment and protect developing sperm from autoimmune and cytotoxic factors. The importance of these barriers is supported by epidemiological studies that suggest male germ cells are more vulnerable to programming effects prior to their formation (21–23).
During spermatogenesis, immature germ cells progress from the basal compartment and pass through the blood-testis barrier, progressing from mitotic spermatogonia, to meiotic spermatocytes, to post-meiotic spermatids, and finally, to morphologically differentiated spermatozoa as they finally enter the lumen of the seminiferous tubules (8). This progression involves a series of dynamic nuclear, cytoplasmic, and morphological changes that serve as a significant barrier to the maintenance of any epigenetic signals of paternal experience that may have been encoded to this point. For example, germ cell histones and any associated posttranslational modifications are largely exchanged for protamines, resulting in a highly condensed genome and effectively halting transcriptional activity (24, 25). In addition, cytoplasmic volume is greatly reduced as it is actively extruded into residual bodies, which presumably includes much of the cell’s pre-spermatogenic transcriptome (26). However, we now appreciate that much of the influence of the environment on sperm occurs outside the course of spermatogenesis. Indeed, the post-testicular window of sperm maturation in the epididymis has been recently identified as the likely point at which the environment can alter sperm programming (6, 10, 12).
Though highly differentiated, spermatozoa in the testes are still functionally immature, lacking both motility and the ability to fertilize an ovum. Sperm are pushed by fluid motion into the caput region of the epididymis where they are exposed to critical growth factors and extracellular vesicles produced by the epididymal epithelial cells lining the tubule. Sperm are then stored in the epididymis until ejaculation when they are also exposed to factors in the ejaculate produced by the prostate and seminal vesicles (27, 28). This maturation process results in substantial changes in the lipid, protein, and RNA content of sperm. These observations raise an interesting question: how can a cell that is essentially transcriptionally and translationally inert not only survive for weeks, but also continue to undergo functional maturation during this period? The answer to this question, and perhaps also to the question of how an environmental signal of paternal experience can be dynamically encoded in sperm, appears to involve a series of interactions, in part, with the extracellular vesicles produced by the somatic tissues of the reproductive tract (12, 28, 29).
Extracellular Vesicles – bypassing the Weismann Barrier
Extracellular vesicles (EV) are small membrane bound particles produced by most, if not all, eukaryotic cells. EVs are classified primarily by their subcellular origin. Some EVs, often referred to as microvesicles (50–1000 nm in diameter), bud directly from the cell membrane. Others generated inside multivesicular bodies and released upon fusion of these compartments with the plasma membrane are generally termed exosomes (40–100 nm in diameter), though it also common to see this name altered to reflect their tissue of origin – for instance, exosomes produced in the epididymis are often called ‘epididymosomes’, while ‘prostasomes’ originate from the prostate (30). The functional relevance of these exclusive classifications remains unclear, therefore we use the more inclusive term ‘EV’ throughout this review. EVs play a very recently appreciated role in intercellular communication, having a distinct advantage over other signaling mechanisms in that they can deliver complex payloads of broad communicating factors, including proteins, lipids, and nucleic acids (30). Once reaching their select tissue target, EVs transmit signals via a number of strategies, including presenting a membrane-bound ligand to a cellular receptor, by inducing EV internalization via endocytosis, or by fusing directly to the plasma membrane, passing on membrane bound constituents and/or releasing an internal cargo to act inside a targeted cell (30). EVs are produced at high levels by the tissues of the reproductive tract and, as we will discuss, play a critical role in the intercellular signaling of these tissues with maturing sperm.
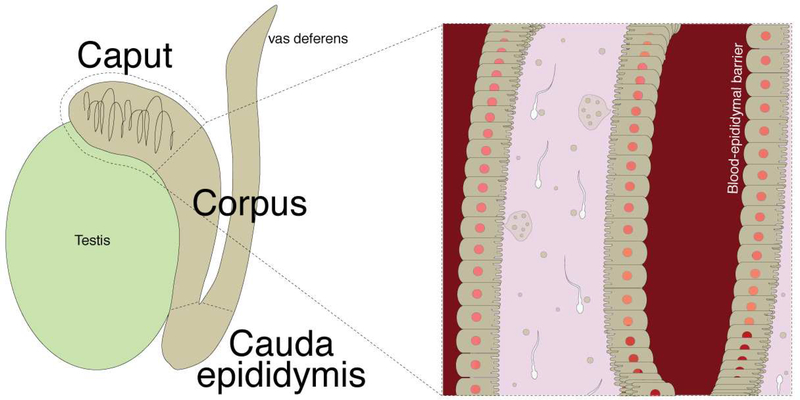
Maturing spermatozoa encounter a variety of extracellular microenvironments as they traverse the reproductive tracts (27). These microenvironments are specifically regulated by the surrounding tissues to modulate sperm development and activity. Tight junctions between the cells of the epididymal epithelium that comprise the blood-epididymal barrier maintain an environment with an electrolyte and macromolecular composition distinct from the surrounding interstitium (27). The epididymis is typically divided into three main segments: the caput, the corpus, and the cauda. Each segment forms its own microenvironment with distinct protein, lipid, and RNA profiles that align with the physiological conditions important for the sequential stages of post-testicular sperm maturation (31–34). Prevailing data suggest that most of the proteins, lipids, and small RNAs in epididymal fluid associated with post-testicular sperm maturation are transferred to sperm by EVs produced here (28). Therefore, as developing sperm exit the testis and descend through the epididymal proximal caput and corpus segments, they interact with EV-containing cargo necessary for sperm motility and oocyte recognition. In the distal caudal segment where sperm are stored prior to ejaculation, EVs cargo promote viability and maintain sperm in a quiescent state (34–36).
Recent studies have focused on the EV-mediated delivery of small RNAs, including microRNAs (miRNAs) (37). Studies in mice and bovine report that more than 80% of miRNAs contained in EVs are shared by sperm isolated from the same region of the epididymis, supporting direct interactions between secreted EVs and sperm in the epididymal lumen (32, 38). Interestingly, there was a significantly greater degree of overlap in miRNA content between these EVs and sperm than existed between the EVs and the epithelial tissue that produced them, suggesting that a population of miRNAs distinct from that of the broader intracellular compartment is specifically loaded into these secreted EVs. miRNAs are not the only small noncoding RNA conveyed to sperm by epididymal EVs. Using small RNA-sequencing, Sharma et al, found that tRNA fragments (tRFs) comprised ~80% of the small RNA content of sperm in the cauda epididymis (29). This was not the case in sperm isolated directly from the testes, suggesting that sperm gained the tRFs as they passed through the epididymis. Unsurprisingly, they also reported that epididymal EVs were the source of these small RNAs (29). Of particular relevance to this review, changes in the tRFs were identified in a screen for small RNAs in sperm affected by a low protein diet fed to male mice in an effort to identify factors associated with the intergenerational transmission of a metabolic phenotype previously characterized in this model (29). Subsequent studies showed that by manipulating the levels of these specific tRFs in the zygote, gene expression changes were produced in the offspring of protein restricted males (29). These data support the EVs as a vector by which the paternal environment and the encoding of these experiences can be transmitted to sperm for delivery to offspring (12, 39).
Importantly, EVs are specific in their targeting, where molecules on their surface promote interactions with specific adhesion proteins on the surface of the desired recipient cell. The degree of specificity appears to be so fine-tuned that some EV populations will actually target specific sub-regions of the sperm itself for the delivery of protein or lipid cargoes (40). Further, EVs isolated from the cauda epididymis transferred significantly less of their cargo to sperm isolated from the caput segment than from caudal sperm (40). This is proving to be a critical characteristic of EVs, as it has become clear that they are not restricted to acting locally, but can target tissues other than sperm at a distance (32, 41). Though we have focused here predominately on epididymal EVs, sperm also interact with secreted EVs produced by the prostate and seminal vesicles as they transit the reproductive tract. These secretions, which make up ~90% of the total volume of ejaculated semen, are also rich in EVs. EVs produced by the prostate, prostasomes, have a well characterized role in regulating sperm activities important for fertility (28). However, these EVs can also target maternal tissues, including immune cells, as we will discuss in detail later in this review (42, 43).
Signal
RNA as the signal – notes from Dad
As the paternal contribution to development is now understood to relay information about lifetime experiences, what are the known signals of transmission? Studies examining mechanisms of paternal transmission implicate sperm epigenetic marks as the substrates that convey environmental information. Currently, the known sperm epigenome includes DNA methylation, histone and protamine post-translational modifications, and as described above, the long and small noncoding RNAs. While these sperm marks appear responsive to the paternal environment, we will focus our review on sperm RNAs, as their causal and functional role in transmitting paternal lifetime exposures has been the most examined. For information on other sperm epigenetic marks, see reviews by Chan et al., Ly et al., and Miller et al. (7, 8, 44).
Mature sperm accumulate a broad range of RNAs throughout their development and maturation. Longer RNAs, including messenger and long-noncoding RNAs, are in low abundance and have been less of a focus in than small noncoding RNAs (45, 46). Small RNAs, predominately miRNAs, PIWI-associated RNAs, and tRNA-derived fragments (tRFs), have been described in the sperm content of mice, pigs, bulls, and humans (29, 46–48). The potential for external insults, such as stress or trauma, to modulate sperm small RNAs and subsequently impact fertilization and development has become an active area of investigation, including within neurodevelopment (10, 29, 49, 50).
RNA as the signal - editing Dad’s note
Some of the first studies to implicate non-genetic changes in sperm in the paternal transmission of lifetime experience examined populations living during the Swedish Famines. Well-kept records in the Overkalix region documented the births and deaths of its citizens as well as periods of nutrient abundance and scarcity. Using these records, researchers identified paternal and grand-paternal food availability during the slow-growth period prior to puberty, as predictive of mortality and cardiovascular risks in subsequent generations (21–23, 51). In other retrospective cohort studies, offspring of fathers who were Holocaust survivors had increased neuropsychiatric disease risk, including anxiety and depressive disorders, suggesting effects of trauma on the paternal germline (52). More recent prospective studies have collected semen to examine paternal sperm RNA content. For example, compared to non-smokers, male smokers had 28 consistently differentially expressed miRNA in their sperm (53). In another study where semen samples from obese versus lean men were studied, different profiles of small RNAs in their sperm were detected, effects that were partially reversed following bariatric surgeryinduced weight loss (54). What has not yet been examined in human studies, is the relevance or causal relationship between these sperm changes and any offspring outcomes.
As in humans, experience-dependent changes to sperm small RNAs have been reported in rodent models of chronic stress, dietary challenges, and substance abuse (29, 49, 50, 55–59). Stress models including maternal separation and social defeat coupled these experiences in males with depressive-like phenotypes in their offspring (60, 61). In our lab, male mice administered a chronic variable stress paradigm sired offspring with stress dysregulation as adults, with increased levels of specific sperm miRNAs as potential molecular links (50). Additionally, changes in sperm tRF content in response to both low protein and high fat diets have been identified in male rodents, as discussed above (29, 56). Microinjections of experience-altered sperm RNA into fertilized zygotes have been used to test the causal relationship between these RNA changes and offspring outcomes. Injected zygotes can be examined for the direct effects of sperm RNA or implanted into foster females to be reared and tested as adults. Such manipulations enable researchers to separate the effects of sperm RNAs from confounding factors, present in both human studies and animal models, that can also influence offspring outcomes, such as changes to paternal or maternal behaviors (62). Indeed, zygote microinjection of total sperm RNA, specific miRNAs, or specific tRFs reflective of paternal changes in these mouse models phenocopied transmission of paternal experiences (29, 49, 56, 63–65). For example, we previously demonstrated that animals resulting from zygote microinjection of the sperm miRNA altered by paternal chronic stress recapitulated the offspring stress phenotype (63). These studies demonstrate that sperm small RNA populations are sensitive to a variety of psychological and physiological perturbations and are causal mediators of offspring brain programming.
Targets of paternal RNAs – message received
Though many studies have now related paternal experiences with changes to sperm RNA content, how sperm RNA subsequently act at fertilization to alter the trajectory of offspring development remains unclear. During early embryogenesis, there are multiple players that can be both targeted by sperm RNAs and, following, influence this sensitive window of development. In this section, we discuss the major known targets of paternal RNAs delivered by either sperm or EVs present in semen, and how these events guide the trajectory of offspring development. To understand the direct effect of sperm RNAs altered by paternal exposures, the majority of studies have focused on changes to the zygote and early embryo (29, 63).
Oocyte/Zygote
Due to the relative difference in RNA levels delivered by one sperm cell (~10 fg) compared to the amount of RNA in a single oocyte (0.5–1.5 ng), the role of sperm RNA in embryogenesis has been considered questioned (66–68). However, the argument for an important role for sperm RNA was substantiated by a study where idiopathic infertility in men was correlated with a lack of specific sperm RNA (69). Further, a study in mice demonstrated that sperm treated with RNases, resulting in a 90% decrease in RNA levels, led to reduced morula-blastocyst transitions and live birth rates (70). These effects were partially rescued by supplementation with wildtype sperm RNA (70), supporting a functional and important role for sperm RNAs in embryogenesis.
Considering the important presence of sperm RNAs, what then is their contribution to development? Studies using animal models suggest that paternal RNAs are transferred to the oocyte at fertilization. For example, mRNAs present only in sperm (e.g. protamine-2) were identified specifically in hamster zygotes post-fertilization, but not in unfertilized oocytes (71). In C. elegans, breeding crosses of males with metabolically-labeled RNA and females with unlabeled RNA produced embryos with 10% labeled RNA, including mRNAs and small RNAs (72), suggesting a subset of embryonic RNA was of paternal origin. Current understanding divides sperm RNAs transferred to the oocyte into two categories: 1) those leftover from spermatogenesis, of little utility to the embryo; and 2) those important for activation of the zygotic genome and subsequent development (67, 71, 73).
Recently, more focus has been directed toward the functional roles of sperm small RNAs in embryogenesis. In particular, sperm miRNAs have been implicated in fertilization and preimplantation development. Sperm miR-34c, for example, when inhibited in the zygote, suppresses DNA synthesis and zygotic cleavage (74), suggesting this sperm miRNA plays a critical role in fertilization, despite its reported functional redundancy (75). Following fertilization, another critical stage for embryogenesis is the maternal-to-zygotic transition, wherein maternal mRNAs are degraded before zygotic transcription occurs (76). Considering the canonical function of miRNAs to degrade mRNAs, sperm miRNAs transferred to, and present in the zygote may facilitate this process. For example, male germ cell-specific knockout of Dicer1 or Drosha, two enzymes critical for processing miRNA precursors into their mature forms, produced aberrant miRNA profiles in sperm (77). Zygotes resulting from these knockout sperm had impaired maternal mRNA turnover and development (77), suggesting sperm miRNAs promote embryogenesis by facilitating the maternal-to-zygotic transition (78).
As environmental perturbations during the paternal lifetime can alter sperm miRNA populations, regulation of mRNA in the zygote may be a mechanism whereby paternal exposures influence development. To test this hypothesis, we previously developed a mouse paternal chronic stress model where a specific subset of sperm miRNAs were capable of reprogramming stress axis reactivity and hypothalamic transcription in their offspring (50, 63). Following zygote microinjection of the identified stress-altered sperm miRNAs, we examined the expression levels of the predicted maternal mRNA targets of these specific miRNAs two-cell zygotes. As expected, the majority of these predicted mRNAs were repressed (63). Interestingly, the two most downregulated transcripts were Sirt1 and Ube3a, both of which are important for mammalian development and have been implicated in neurodevelopmental and metabolic disorders in humans (79, 80).
Other small noncoding RNAs in sperm, such as tRFs, may have similar roles during embryo development. Derived from the 5’ or 3’ ends of tRNAs, tRFs can silence viral transcripts with complementary sequences and inhibit translation (81). When delivered by sperm, tRFs in the zygote repress genes associated with endogenous retroelements active in preimplantation embryos (29). For example, microinjection of sperm tRFs specifically altered by paternal high fat diets resulted in distinct transcriptomic changes at the 8-cell and blastocyst stages, with few overlapping differentially expressed genes between these stages (56). Taken together, the data suggest that diverse sperm small RNAs can directly impact gene expression in the zygote, initiating a cascade of transcriptional events that influences development during later embryonic stages, ultimately guiding towards a phenotype reflective of the paternal environment.
Cervix/Endometrium
The maternal cervix and endometrium play an important role in promoting a suitable environment for embryo implantation and development. In most mammals, coitus results in deposition of seminal fluid at the uterine ectocervix (82). As the uterine entrance, the cervix is critical for promoting immune responses, including leukocyte recruitment and inflammatory signaling to foreign pathogens and paternal antigens (82–85). This results in immune priming of the uterus, which is important for endometrial receptivity and proper placentation (86). However, in order for fertilization to occur, sperm must bypass the cervix and uterus to reach the fallopian tubes, where the oocyte awaits (87). While seminal fluid and sperm stimulate the cervical immune system, EVs found in semen, such as prostasomes, modulate the extent of the female immune response (43). For example, prostasomes directly inhibit leukocytes and contain complement proteins protecting sperm from immune targeting in the female genital tract (88–91). Therefore, EVs in semen may communicate directly with the endometrial epithelial cells, regulating the secretion of factors that promote receptivity, or indirectly through modulation of intrauterine immune priming (87).
Despite the potentially intricate interplay between EVs in seminal fluid and regions of the female reproductive tract, the mechanisms of communication have not been fully explored. In the vagina, miRNA, tRFs and mRNA present in seminal EVs were implicated in suppressing viral infections that could be transmitted to offspring at parturition (42, 92). Seminal EV RNAs, then, might also act on cells at the cervix and endometrium, though this possibility has not been well explored. As seminal EVs derive from male somatic tissues that are susceptible to environmental perturbations, paternal exposures could influence offspring development by altering semen EV content (114–116).
Conclusion
Organisms, including humans, exist in a dynamic environment. Therefore, maintaining a certain amount of developmental plasticity, permitting an organism to adapt its physiology in response to environmental cues, can confer a decidedly selective advantage. Given the complexity of our environment and our perception of these cues, the phenotypic traits induced by environmental factors, including stress and trauma, are often multifaceted. In fact, aspects of these environmentally induced traits may be viewed as deleterious in terms of human health, highlighting a common conflict in defining phenotypes as adaptive in an evolutionary vs. human health context. Research into the molecular mechanisms underlying environmentally induced traits has focused on changes in the epigenetic programming of somatic tissues. A fundamental tenet of molecular evolution, the Weismann barrier, restricts these epigenetic changes from occurring in germ cell lineages; therefore, it has generally been understood that these environmentally induced traits are not heritable. Yet, as is often the case, modern biology has identified exceptions to even this foundational theory, and intergenerational consequences of parental experience on offspring neurodevelopment continue to be documented in human epidemiological studies and animal models (5–7, 10, 12, 39).
In humans, there are many ways in which a parental trait can affect offspring development. Recent studies using animal models to specifically test the heritability of paternal acquired traits have demonstrated that the germline transmission of environmentally induced traits can occur. Future studies, therefore, should be focused on including paternal as well as maternal exposures and experiences in assessing risk for neurodevelopmental or neuropsychiatric disease. Prospective studies are especially desirable as paternal semen samples are easily obtained to facilitate the development of valuable predictive biomarkers that may be used to inform clinical decisions, including altering prenatal care and earlier interventions for at-risk children.
The process of male gametogenesis presents both barriers to and opportunities for the intergenerational germline transmission of paternal experience.
Gametogenesis begins early in embryonic development, as primordial germ cells undergo an initial wave of genomic demethylation, then acquire the germ cell-specific epigenetic programming necessary for later spermatogenesis. Beginning in puberty, waves of immature spermatogonia begin to enter spermatogenesis. In addition to undergoing meiosis, spermatogenic processes involve a series of dramatic nuclear, cytoplasmic, and morphological changes that culminate in morphologically differentiated spermatozoa. Though highly differentiated, spermatozoa in the seminiferous tubules of the testes are still functionally immature, lacking both motility and the ability to fertilize an ovum. These are among the functionalities acquired during their post-testicular maturation as they migrate through the caput, corpus, and into the caudal epididymis, where they are stored until ejaculation. Much of this maturation requires the intercellular transfer of critical factors from epididymal epithelial cells to spermatozoa via EVs, producing significant changes in the lipid, protein, and RNA content of the sperm. These interactions might also facilitate the transfer of information regarding paternal experience to future offspring. At the interphase of the blood-epididymal barrier, these epithelial cells are well placed to detect environmental stimuli/triggers and store this information via epigenetic modifications. They could encode this information in EVs, through the selective loading of bioactive cargoes such as small RNAs (signal), for transfer to sperm (vector) and, subsequently, to the oocyte (target). Alternatively the EV could serve as the vector themselves, targeting the oocyte or maternal tissues to influence the gestational environment and embryogenesis.
Acknowledgments
This work was supported by National Institutes of Health Grant Nos. MH108286, MH099910, MH104184, and ES028202 (to TLB).
Footnotes
Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yehuda R, Daskalakis NP, Lehrner A, Desarnaud F, Bader HN, Makotkine I, et al. (2014): Influences of maternal and paternal PTSD on epigenetic regulation of the glucocorticoid receptor gene in Holocaust survivor offspring. Am J Psychiatry. 171: 872–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lehrner A, Bierer LM, Passarelli V, Pratchett LC, Flory JD, Bader HN, et al. (2014): Maternal PTSD associates with greater glucocorticoid sensitivity in offspring of Holocaust survivors. Psychoneuroendocrinology. 40: 213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khashan AS, Abel KM, McNamee R, Pedersen MG, Webb RT, Baker PN, et al. (2008): Higher risk of offspring schizophrenia following antenatal maternal exposure to severe adverse life events. Arch Gen Psychiatry. 65: 146–152. [DOI] [PubMed] [Google Scholar]
- 4.van Os J, Selten JP (1998): Prenatal exposure to maternal stress and subsequent schizophrenia. The May 1940 invasion of The Netherlands. Br J Psychiatry J Ment Sci. 172: 324–326. [DOI] [PubMed] [Google Scholar]
- 5.Lane M, Robker RL, Robertson SA (2014): Parenting from before conception. Science. 345: 756–760. [DOI] [PubMed] [Google Scholar]
- 6.Rodgers AB, Bale TL (2015): Germ Cell Origins of Posttraumatic Stress Disorder Risk: The Transgenerational Impact of Parental Stress Experience. Biol Psychiatry. 78: 307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan JC, Nugent BM, Bale TL (2018): Parental Advisory: Maternal and Paternal Stress Can Impact Offspring Neurodevelopment. Biol Psychiatry. 83: 886–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ly L, Chan D, Trasler JM (2015): Developmental windows of susceptibility for epigenetic inheritance through the male germline. Semin Cell Dev Biol. 43: 96–105. [DOI] [PubMed] [Google Scholar]
- 9.Bohacek J, Mansuy IM (2017): A guide to designing germline-dependent epigenetic inheritance experiments in mammals. Nat Methods. 14: 243–249. [DOI] [PubMed] [Google Scholar]
- 10.Chen Q, Yan W, Duan E (2016): Epigenetic inheritance of acquired traits through sperm RNAs and sperm RNA modifications. Nat Rev Genet. 17: 733–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma A (2013): Transgenerational epigenetic inheritance: focus on soma to germline information transfer. Prog Biophys Mol Biol. 113: 439–446. [DOI] [PubMed] [Google Scholar]
- 12.Eaton SA, Jayasooriah N, Buckland ME, Martin DI, Cropley JE, Suter CM (2015): Roll over Weismann: extracellular vesicles in the transgenerational transmission of environmental effects. Epigenomics. 7: 1165–1171. [DOI] [PubMed] [Google Scholar]
- 13.Barkalina N, Jones C, Wood MJA, Coward K (2015): Extracellular vesicle-mediated delivery of molecular compounds into gametes and embryos: learning from nature. Hum Reprod Update. 21: 627–639. [DOI] [PubMed] [Google Scholar]
- 14.Anway MD, Cupp AS, Uzumcu M, Skinner MK (2005): Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 308: 1466–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gapp K, von Ziegler L, Tweedie-Cullen RY, Mansuy IM (2014): Early life epigenetic programming and transmission of stress-induced traits in mammals: how and when can environmental factors influence traits and their transgenerational inheritance? BioEssays News Rev Mol Cell Dev Biol. 36: 491–502. [DOI] [PubMed] [Google Scholar]
- 16.Radford EJ, Ito M, Shi H, Corish JA, Yamazawa K, Isganaitis E, et al. (2014): In utero effects. In utero undernourishment perturbs the adult sperm methylome and intergenerational metabolism. Science. 345: 1255903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunn GA, Morgan CP, Bale TL (2011): Sex-specificity in transgenerational epigenetic programming. Horm Behav. 59: 290–295. [DOI] [PubMed] [Google Scholar]
- 18.Morgan CP, Bale TL (2011): Early prenatal stress epigenetically programs dysmasculinization in second-generation offspring via the paternal lineage. J Neurosci Off J Soc Neurosci. 31: 11748–11755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mital P, Hinton BT, Dufour JM (2011): The blood-testis and blood-epididymis barriers are more than just their tight junctions. Biol Reprod. 84: 851–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stanton PG (2016): Regulation of the blood-testis barrier. Semin Cell Dev Biol. 59: 166–173. [DOI] [PubMed] [Google Scholar]
- 21.Kaati G, Bygren LO, Edvinsson S (2002): Cardiovascular and diabetes mortality determined by nutrition during parents’ and grandparents’ slow growth period. Eur J Hum Genet EJHG. 10: 682–688. [DOI] [PubMed] [Google Scholar]
- 22.Kaati G, Bygren LO, Pembrey M, Sjöström M (2007): Transgenerational response to nutrition, early life circumstances and longevity. Eur J Hum Genet EJHG. 15: 784–790. [DOI] [PubMed] [Google Scholar]
- 23.Pembrey ME, Bygren LO, Kaati G, Edvinsson S, Northstone K, Sjöström M, et al. (2006): Sex-specific, male-line transgenerational responses in humans. Eur J Hum Genet EJHG. 14: 159–166. [DOI] [PubMed] [Google Scholar]
- 24.Jodar M, Soler-Ventura A, Oliva R, Molecular Biology of Reproduction and Development Research Group (2017): Semen proteomics and male infertility. J Proteomics. 162: 125–134. [DOI] [PubMed] [Google Scholar]
- 25.Neto FTL, Bach PV, Najari BB, Li PS, Goldstein M (2016): Spermatogenesis in humans and its affecting factors. Semin Cell Dev Biol. 59: 10–26. [DOI] [PubMed] [Google Scholar]
- 26.Cooper TG (2005): Cytoplasmic droplets: the good, the bad or just confusing? Hum Reprod Oxf Engl. 20: 9–11. [DOI] [PubMed] [Google Scholar]
- 27.Sullivan R, Saez F (2013): Epididymosomes, prostasomes, and liposomes: their roles in mammalian male reproductive physiology. Reprod Camb Engl. 146: R21–35. [DOI] [PubMed] [Google Scholar]
- 28.Machtinger R, Laurent LC, Baccarelli AA (2016): Extracellular vesicles: roles in gamete maturation, fertilization and embryo implantation. Hum Reprod Update. 22: 182–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma U, Conine CC, Shea JM, Boskovic A, Derr AG, Bing XY, et al. (2016): Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science. 351: 391–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tkach M, Théry C (2016): Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell. 164: 1226–1232. [DOI] [PubMed] [Google Scholar]
- 31.Belleannée C, Calvo E, Thimon V, Cyr DG, Légaré C, Garneau L, Sullivan R (2012): Role of microRNAs in controlling gene expression in different segments of the human epididymis. PloS One. 7: e34996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Belleannée C, Calvo É, Caballero J, Sullivan R (2013): Epididymosomes convey different repertoires of microRNAs throughout the bovine epididymis. Biol Reprod. 89: 30. [DOI] [PubMed] [Google Scholar]
- 33.Dacheux J-L, Belleannée C, Guyonnet B, Labas V, Teixeira-Gomes A-P, Ecroyd H, et al. (2012): The contribution of proteomics to understanding epididymal maturation of mammalian spermatozoa. Syst Biol Reprod Med. 58: 197–210. [DOI] [PubMed] [Google Scholar]
- 34.Girouard J, Frenette G, Sullivan R (2011): Comparative proteome and lipid profiles of bovine epididymosomes collected in the intraluminal compartment of the caput and cauda epididymidis. Int J Androl. 34: e475–486. [DOI] [PubMed] [Google Scholar]
- 35.Girouard J, Frenette G, Sullivan R (2009): Compartmentalization of proteins in epididymosomes coordinates the association of epididymal proteins with the different functional structures of bovine spermatozoa. Biol Reprod. 80: 965–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frenette G, Lessard C, Sullivan R (2002): Selected proteins of “prostasome-like particles” from epididymal cauda fluid are transferred to epididymal caput spermatozoa in bull. Biol Reprod. 67: 308–313. [DOI] [PubMed] [Google Scholar]
- 37.Nixon B, Stanger SJ, Mihalas BP, Reilly JN, Anderson AL, Tyagi S, et al. (2015): The microRNA signature of mouse spermatozoa is substantially modified during epididymal maturation. Biol Reprod. 93: 91. [DOI] [PubMed] [Google Scholar]
- 38.Reilly JN, McLaughlin EA, Stanger SJ, Anderson AL, Hutcheon K, Church K, et al. (2016): Characterisation of mouse epididymosomes reveals a complex profile of microRNAs and a potential mechanism for modification of the sperm epigenome. Sci Rep. 6: 31794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma A (2017): Transgenerational epigenetics: Integrating soma to germline communication with gametic inheritance. Mech Ageing Dev. 163: 15–22. [DOI] [PubMed] [Google Scholar]
- 40.Yanagimachi R, Kamiguchi Y, Mikamo K, Suzuki F, Yanagimachi H (1985): Maturation of spermatozoa in the epididymis of the Chinese hamster. Am J Anat. 172: 317–330. [DOI] [PubMed] [Google Scholar]
- 41.Belleannée C (2015): Extracellular microRNAs from the epididymis as potential mediators of cell-to-cell communication. Asian J Androl. 17: 730–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Madison MN, Roller RJ, Okeoma CM (2014): Human semen contains exosomes with potent anti-HIV-1 activity. Retrovirology. 11: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aalberts M, Stout TAE, Stoorvogel W (2014): Prostasomes: extracellular vesicles from the prostate. Reprod Camb Engl. 147: R1–14. [DOI] [PubMed] [Google Scholar]
- 44.Miller D, Brinkworth M, Iles D (2010): Paternal DNA packaging in spermatozoa: more than the sum of its parts? DNA, histones, protamines and epigenetics. Reprod Camb Engl. 139: 287–301. [DOI] [PubMed] [Google Scholar]
- 45.Zhang X, Gao F, Fu J, Zhang P, Wang Y, Zeng X (2017): Systematic identification and characterization of long non-coding RNAs in mouse mature sperm. PloS One. 12: e0173402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kawano M, Kawaji H, Grandjean V, Kiani J, Rassoulzadegan M (2012): Novel small noncoding RNAs in mouse spermatozoa, zygotes and early embryos. PloS One. 7: e44542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krawetz SA, Kruger A, Lalancette C, Tagett R, Anton E, Draghici S, Diamond MP (2011): A survey of small RNAs in human sperm. Hum Reprod Oxf Engl. 26: 3401–3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen C, Wu H, Shen D, Wang S, Zhang L, Wang X, et al. (2017): Comparative profiling of small RNAs of pig seminal plasma and ejaculated and epididymal sperm. Reprod Camb Engl. 153: 785–796. [DOI] [PubMed] [Google Scholar]
- 49.Gapp K, Jawaid A, Sarkies P, Bohacek J, Pelczar P, Prados J, et al. (2014): Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat Neurosci. 17: 667–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL (2013): Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J Neurosci Off J Soc Neurosci. 33: 9003–9012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bygren LO, Kaati G, Edvinsson S (2001): Longevity determined by paternal ancestors’ nutrition during their slow growth period. Acta Biotheor. 49: 53–59. [DOI] [PubMed] [Google Scholar]
- 52.Yehuda R, Bell A, Bierer LM, Schmeidler J (2008): Maternal, not paternal, PTSD is related to increased risk for PTSD in offspring of Holocaust survivors. J Psychiatr Res. 42: 1104–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marczylo EL, Amoako AA, Konje JC, Gant TW, Marczylo TH (2012): Smoking induces differential miRNA expression in human spermatozoa: a potential transgenerational epigenetic concern? Epigenetics. 7: 432–439. [DOI] [PubMed] [Google Scholar]
- 54.Donkin I, Versteyhe S, Ingerslev LR, Qian K, Mechta M, Nordkap L, et al. (2016): Obesity and Bariatric Surgery Drive Epigenetic Variation of Spermatozoa in Humans. Cell Metab. 23: 369–378. [DOI] [PubMed] [Google Scholar]
- 55.Short AK, Fennell KA, Perreau VM, Fox A, O’Bryan MK, Kim JH, et al. (2016): Elevated paternal glucocorticoid exposure alters the small noncoding RNA profile in sperm and modifies anxiety and depressive phenotypes in the offspring. Transl Psychiatry. 6: e837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen Q, Yan M, Cao Z, Li X, Zhang Y, Shi J, et al. (2016): Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science. 351: 397–400. [DOI] [PubMed] [Google Scholar]
- 57.de Castro Barbosa T, Ingerslev LR, Alm PS, Versteyhe S, Massart J, Rasmussen M, et al. (2016): High-fat diet reprograms the epigenome of rat spermatozoa and transgenerationally affects metabolism of the offspring. Mol Metab. 5: 184–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fullston T, Ohlsson Teague EMC, Palmer NO, DeBlasio MJ, Mitchell M, Corbett M, et al. (2013): Paternal obesity initiates metabolic disturbances in two generations of mice with incomplete penetrance to the F2 generation and alters the transcriptional profile of testis and sperm microRNA content. FASEB J Off Publ Fed Am Soc Exp Biol. 27: 4226–4243. [DOI] [PubMed] [Google Scholar]
- 59.Rompala GR, Mounier A, Wolfe CM, Lin Q, Lefterov I, Homanics GE (2018): Heavy Chronic Intermittent Ethanol Exposure Alters Small Noncoding RNAs in Mouse Sperm and Epididymosomes. Front Genet. 9: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Franklin TB, Mansuy IM (2010): Epigenetic inheritance in mammals: evidence for the impact of adverse environmental effects. Neurobiol Dis. 39: 61–65. [DOI] [PubMed] [Google Scholar]
- 61.Dietz DM, Laplant Q, Watts EL, Hodes GE, Russo SJ, Feng J, et al. (2011): Paternal transmission of stress-induced pathologies. Biol Psychiatry. 70: 408–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Curley JP, Mashoodh R, Champagne FA (2011): Epigenetics and the origins of paternal effects. Horm Behav. 59: 306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rodgers AB, Morgan CP, Leu NA, Bale TL (2015): Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc Natl Acad Sci U S A. 112: 13699–13704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rassoulzadegan M, Grandjean V, Gounon P, Vincent S, Gillot I, Cuzin F (2006): RNAmediated non-mendelian inheritance of an epigenetic change in the mouse. Nature. 441: 469–474. [DOI] [PubMed] [Google Scholar]
- 65.Grandjean V, Fourré S, De Abreu DAF, Derieppe M-A, Remy J-J, Rassoulzadegan M (2015): RNA-mediated paternal heredity of diet-induced obesity and metabolic disorders. Sci Rep. 5: 18193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Olszańska B, Borgul A (1990): Quantitation of nanogram amounts of nucleic acids in the presence of proteins by the ethidium bromide staining technique. Acta Biochim Pol. 37: 59–63. [PubMed] [Google Scholar]
- 67.Boerke A, Dieleman SJ, Gadella BM (2007): A possible role for sperm RNA in early embryo development. Theriogenology. 68 Suppl 1: S147–155. [DOI] [PubMed] [Google Scholar]
- 68.Krawetz SA (2005): Paternal contribution: new insights and future challenges. Nat Rev Genet. 6: 633–642. [DOI] [PubMed] [Google Scholar]
- 69.Jodar M, Sendler E, Moskovtsev SI, Librach CL, Goodrich R, Swanson S, et al. (2015): Absence of sperm RNA elements correlates with idiopathic male infertility. Sci Transl Med. 7: 295re6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guo L, Chao S-B, Xiao L, Wang Z-B, Meng T-G, Li Y-Y, et al. (2017): Sperm-carried RNAs play critical roles in mouse embryonic development. Oncotarget. 8: 67394–67405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ostermeier GC, Miller D, Huntriss JD, Diamond MP, Krawetz SA (2004): Reproductive biology: delivering spermatozoan RNA to the oocyte. Nature. 429: 154. [DOI] [PubMed] [Google Scholar]
- 72.Stoeckius M, Grün D, Rajewsky N (2014): Paternal RNA contributions in the Caenorhabditis elegans zygote. EMBO J. 33: 1740–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ziyyat A, Lefèvre A (2001): Differential gene expression in pre-implantation embryos from mouse oocytes injected with round spermatids or spermatozoa. Hum Reprod Oxf Engl. 16: 1449–1456. [DOI] [PubMed] [Google Scholar]
- 74.Liu W-M, Pang RTK, Chiu PCN, Wong BPC, Lao K, Lee K-F, Yeung WSB (2012): Spermborne microRNA-34c is required for the first cleavage division in mouse. Proc Natl Acad Sci U S A. 109: 490–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu J, Bao J, Kim M, Yuan S, Tang C, Zheng H, et al. (2014): Two miRNA clusters, miR34b/c and miR-449, are essential for normal brain development, motile ciliogenesis, and spermatogenesis. Proc Natl Acad Sci U S A. 111: E2851–2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tadros W, Lipshitz HD (2009): The maternal-to-zygotic transition: a play in two acts. Dev Camb Engl. 136: 3033–3042. [DOI] [PubMed] [Google Scholar]
- 77.Yuan S, Schuster A, Tang C, Yu T, Ortogero N, Bao J, et al. (2016): Sperm-borne miRNAs and endo-siRNAs are important for fertilization and preimplantation embryonic development. Dev Camb Engl. 143: 635–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Giraldez AJ, Mishima Y, Rihel J, Grocock RJ, Van Dongen S, Inoue K, et al. (2006): Zebrafish MiR-430 promotes deadenylation and clearance of maternal mRNAs. Science. 312: 75–79. [DOI] [PubMed] [Google Scholar]
- 79.Herskovits AZ, Guarente L (2014): SIRT1 in neurodevelopment and brain senescence. Neuron. 81: 471–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Greer PL, Hanayama R, Bloodgood BL, Mardinly AR, Lipton DM, Flavell SW, et al. (2010): The Angelman Syndrome protein Ube3A regulates synapse development by ubiquitinating arc. Cell. 140: 704–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Raina M, Ibba M (2014): tRNAs as regulators of biological processes. Front Genet. 5: 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Robertson SA, Sharkey DJ (2016): Seminal fluid and fertility in women. Fertil Steril. 106: 511–519. [DOI] [PubMed] [Google Scholar]
- 83.Sharkey DJ, Tremellen KP, Jasper MJ, Gemzell-Danielsson K, Robertson SA (2012): Seminal fluid induces leukocyte recruitment and cytokine and chemokine mRNA expression in the human cervix after coitus. J Immunol Baltim Md 1950. 188: 2445–2454. [DOI] [PubMed] [Google Scholar]
- 84.Pandya IJ, Cohen J (1985): The leukocytic reaction of the human uterine cervix to spermatozoa. Fertil Steril. 43: 417–421. [DOI] [PubMed] [Google Scholar]
- 85.Bai R, Latifi Z, Kusama K, Nakamura K, Shimada M, Imakawa K (2018): Induction of immune-related gene expression by seminal exosomes in the porcine endometrium. Biochem Biophys Res Commun. 495: 1094–1101. [DOI] [PubMed] [Google Scholar]
- 86.Robertson SA, Zhang B, Chan H, Sharkey DJ, Barry SC, Fullston T, Schjenken JE (2017): MicroRNA regulation of immune events at conception. Mol Reprod Dev. 84: 914–925. [DOI] [PubMed] [Google Scholar]
- 87.Diedrich K, Fauser BCJM, Devroey P, Griesinger G, Evian Annual Reproduction (EVAR) Workshop Group (2007): The role of the endometrium and embryo in human implantation. Hum Reprod Update. 13: 365–377. [DOI] [PubMed] [Google Scholar]
- 88.Skibinski G, Kelly RW, Harkiss D, James K (1992): Immunosuppression by human seminal plasma--extracellular organelles (prostasomes) modulate activity of phagocytic cells. Am J Reprod Immunol N Y N 1989. 28: 97–103. [DOI] [PubMed] [Google Scholar]
- 89.Kelly RW, Holland P, Skibinski G, Harrison C, McMillan L, Hargreave T, James K (1991): Extracellular organelles (prostasomes) are immunosuppressive components of human semen. Clin Exp Immunol. 86: 550–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rooney IA, Atkinson JP, Krul ES, Schonfeld G, Polakoski K, Saffitz JE, Morgan BP (1993): Physiologic relevance of the membrane attack complex inhibitory protein CD59 in human seminal plasma: CD59 is present on extracellular organelles (prostasomes), binds cell membranes, and inhibits complement-mediated lysis. J Exp Med. 177: 1409–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Babiker AA, Nilsson B, Ronquist G, Carlsson L, Ekdahl KN (2005): Transfer of functional prostasomal CD59 of metastatic prostatic cancer cell origin protects cells against complement attack. The Prostate. 62: 105–114. [DOI] [PubMed] [Google Scholar]
- 92.Vojtech L, Woo S, Hughes S, Levy C, Ballweber L, Sauteraud RP, et al. (2014): Exosomes in human semen carry a distinctive repertoire of small non-coding RNAs with potential regulatory functions. Nucleic Acids Res. 42: 7290–7304. [DOI] [PMC free article] [PubMed] [Google Scholar]