Abstract
Objective:
Adult and adolescent studies suggest increased motivational responses to cannabis cues among regular cannabis users. However, functional magnetic resonance imaging (fMRI) studies have not explored neural activation in response to visual cannabis cues among adolescents in the United States. Gaining a better understanding of the neural circuits related to cue-elicited craving during adolescence may shed light on the neural basis for the development of problematic cannabis use that could ultimately be targeted for interventions.
Methods:
41 non-treatment-seeking youth (ages 17–21; mean age = 18.83; 46.3% female) who reported regular cannabis use underwent fMRI scanning involving a visual cannabis cue task and completed self-report and biological measures. Whole-brain activation was examined for cannabis cues compared to non-cannabis cues, and for active versus passive cannabis cues. Associations between self-reported substance use and task activation were examined.
Results:
Cannabis images were identifiable to adolescents and were rated as more rewarding than matched non-cannabis images (p < .05). Greater activation was found for the cannabis cues compared to non-cannabis cues in bilateral posterior cingulate, cuneus, fusiform, precuneus, inferior temporal and parahippocampal gyri, as well as left thalamus, medial frontal and superior frontal gyri. Cue-elicited activation was not significantly associated with self-reported cannabis use (ps > 0.05). No differences were observed for the active versus passive cue contrast.
Conclusions:
Cannabis-using youth show more activation to cannabis cues than non-cannabis cues in brain regions underlying incentive salience, reward, and visual attention. This task could be useful for future studies examining neural underpinnings of reward processes in adolescent cannabis users.
Keywords: Cannabis, Cue reactivity, fMRI, Youth, Marijuana
1. Introduction
Cannabis is the second most commonly used drug among adolescents, after alcohol, with 23% of 12th graders in the United States (U.S.) reporting cannabis use in the past month (Johnston et al., 2018). Although adolescent alcohol and tobacco use has declined in recent years, cannabis use has remained stable. It is important to gain a better understanding of the neurobiological mechanisms underlying the development of problematic use patterns among adolescents to aid development of effective treatments for adolescent cannabis use disorder (CUD).
Cue-elicited craving, or the strong desire to use a drug after being exposed to a related cue, has been a central construct to addiction research for decades (Kalivas & Volkow, 2005). According to the incentive salience model, addiction develops due to changes in neural reward systems, such that over time, with repeated drug-exposure, drug-related stimuli become highly rewarding, while natural reinforcers become less rewarding (Robinson & Berridge, 2008). Increased drug reward value is associated with drug-seeking behavior that can persist over time due to neural changes within reward and cognitive control systems following chronic drug exposure (Kalivas & Volkow, 2005; Volkow, Wang, Telang, et al., 2014). For example, recent literature suggests heightened motivational responses to cannabis cues among cannabis users (Henry, Kaye, Bryan, Hutchison, & Ito, 2014).
Typically, substance use is initiated during adolescence and young adulthood, when the brain is still developing (see Squeglia & Gray, 2016). Thus, understanding cue-elicited craving in youth may clarify how problematic cannabis use develops and help to identify neural circuits that could be targeted for interventions. Studies have shown consistent cue-elicited cannabis craving in adults (Bordnick, Copp, Traylor, et al., 2009; Field, Eastwood, Bradley, & Mogg, 2006; Filbey, Schacht, Myers, Chavez, & Hutchison, 2009; Haughey, Marshall, Schacht, Louis, & Hutchison, 2008; McRae-Clark, Carter, Price, et al., 2011; Schacht, Selling, & Hutchison, 2009; Wolfling, Flor, & Grusser, 2008). However, to date, there exists a significant gap in the literature, as only four studies have examined cannabis cue rea2ctivity in adolescents (Gray, Larowe, Watson, & Carpenter, 2011; Henry et al., 2014; Nickerson, Ravichandran, Lundahl, et al., 2011). In two studies, cannabis dependent youth showed higher skin conductance and self-reported craving following tactile cannabis cues (Gray, Larowe, & Upadhyaya, 2008). In another, 13 cannabis dependent youth (ages 14–17) showed increased event-related potentials (ERP) and higher self-reported craving following presentation of visual and tactile cannabis cues (Nickerson et al., 2011). The largest study to date examined 353 college-aged students (~18 years), and showed that cannabis use was associated with ERP reactivity to cannabis images, and was related to self-reported craving and problematic use (Henry et al., 2014).
Over the past 15 years, functional magnetic resonance imaging (fMRI) has helped to elucidate the neural substrates of drug craving. Neural cue reactivity has been related to numerous substance use indices, including addiction severity, craving, and treatment outcomes (Jasinska, Stein, Kaiser, Naumer, & Yalachkov, 2014). While several studies have examined the neural substrates of cue reactivity in alcohol, nicotine, cocaine, and opioids (Courtney, Schacht, Hutchison, Roche, & Ray, 2016), less research has explored cannabis cue reactivity. In the first published cannabis cue reactivity fMRI study, 38 frequent cannabis-using adults in the U.S. were presented with tactile cannabis and non-cannabis cues (i.e., a cannabis pipe vs. a pencil) during an fMRI scan (Filbey et al., 2009). Brain regions underlying incentive salience and reward, including the ventral tegmental area, thalamus, anterior cingulate, insula, and amygdala, showed greater activation in response to cannabis cues compared to non-cannabis cues. Activation in the orbitofrontal cortex and nucleus accumbens was positively correlated with cannabis-related problems (Filbey et al., 2009). Although the pipe cues presented in this study were consistent across participants, participants reported a wide range of modalities used to consume cannabis in their daily lives, and only 54% reported the pipe as their primary mode of consumption. A visual cue task presenting multiple modes of cannabis use could provide more varied and relevant cues. In a Dutch study, 31 frequent cannabis users, 20 sporadic users, and 21 cannabis-naïve controls underwent a visual cannabis cue fMRI task. Frequent users exhibited greater activity in reward-related areas (e.g., ventral tegmental area) during cannabis vs. non-cannabis cues, compared with sporadic users and controls. Neural response to visual images was primarily related to severity of cannabis-related problems, as opposed to quantity of use (Cousijn et al., 2013). Cue-induced activation in the left striatum predicted cannabis problem severity at 3-year follow-up in this sample (Vingerhoets, Koenders, Van Den Brink, et al., 2016). Although this was the first visual cannabis fMRI task, the cues were Dutch-specific (e.g., reflecting Dutch-branded cannabis products), thereby limiting the utility of the task among non-Dutch populations.
Two additional studies have used visual fMRI tasks, but both included treatment-seeking adults. In N = 12 treatment-seeking cannabis dependent adults, brain activation during cannabis vs. non-cannabis visual cues was greater in bilateral amygdala and hippocampus. Positive correlations were found between craving and activation in the ventral striatum and medial and lateral orbitofrontal cortex (Goldman, Szucs-Reed, Jagannathan, et al., 2013). This small sample size was demographically homogeneous (83% adult African American males who predominantly reported smoking cannabis cigars or “blunts”) and 75% were cigarette smokers, limiting generalizability of the findings. Another study subliminally presented cannabis, sexual, and aversive visual cues to adult cannabis users (N = 20; 90% African American, 60% male, 40% cigarette users). Masked cannabis vs. non-cannabis cues activated left anterior insula, bilateral ventral striatum, and left amygdala (Wetherill, Childress, Jagannathan, et al., 2014). One limitation of previous visual cue tasks is a lack of detailed information about the degree to which participants found the cannabis images rewarding (e.g., do participants like the images, do they induce feelings of wanting to use cannabis, etc.). Also, prior work has not explored differences between active and passive cannabis images (“active” cues depict cannabis being consumed, while “passive” cues only depict cannabis). One study demonstrated no differences in cue reactivity based on picture type in an alcohol-cue task (Pronk, Deursen, Beraha, Larsen, & Wiers, 2015), but this has not been explored with fMRI or in cannabis users. In summary, prior tasks either required a tactile component (Filbey et al., 2009), used cues that are specific for Dutch populations that are likely not recognizable by youth in the U.S. (Cousijn et al., 2013), or were created for a demographically homogeneous treatment-seeking adult population (Goldman et al., 2013; Wetherill et al., 2014).
The goal of the present study was to create a visual cannabis cue task using cues that were relevant to adolescents in the U.S., to obtain ratings of subjective reactions to the images, and to understand if cue type (active vs. passive) affects neural reactivity in non-treatment-seeking youth cannabis users. It was hypothesized that cannabis cues, relative to non-cannabis cues, would elicit activation in reward-related brain regions such as the orbitofrontal cortex and ventral striatum, and that activation would be positively associated with measures of cannabis dependence and quantity/frequency of cannabis use. In addition, based on previous work examining cue reactivity to alcohol cues (Pronk et al., 2015), it was hypothesized that no significant activation differences would emerge between passive and active cannabis cues. Also, cannabis cues (both active and passive) were expected to be subjectively rated by adolescent users as more rewarding than non-cannabis cues, thereby adding to the construct validity of the task.
2. Methods
2.1. Participants
Participants were 41 non-treatment-seeking youth ages 17 to 21 (mean age = 18.83; 46.3% female) who reported regular (~5 days/week) cannabis use (Table 1). Participants were recruited through media advertisements and flyers. Participants were required to be ≤21 years old and have > 52 lifetime cannabis use episodes (range of 84–2085 lifetime episodes in the sample) with at least weekly cannabis use for the past year. All participants provided informed consent. If under 18, parental consent and adolescent assent were obtained. Note that advertisements stated that the study was recruiting participants who did or did not use cannabis. The consent said that the study was ‘for teens who may or may not have used cannabis at problematic levels’ so as to not expose the child’s substance use patterns to his or her parents. The Institutional Review Board approved all protocols.
Table 1.
Subject Demographic Characteristics for 40 fMRI participants.
| n = 40* | |
|---|---|
| Characteristic | Mean (SD) or Number (%) |
| Age | 18.83 (0.96) |
| Gender (female) | 19 (47.5%) |
| Race | |
| White | 35 (87.5%) |
| Black | 4 (10%) |
| More than one race | 1 (2.5%) |
| Ethnicity | |
| Hispanic/Latino | 2 (5%) |
| Non-Hispanic/Latino | 38 (95%) |
| MacArthur Socioeconomic Scalea | 5.75 (1.7) |
| TLFB Days of Cannabis Use | 46.05 (15.8) |
| TLFB Days of Alcohol Use | 15.4 (9.2) |
| TLFB Drinks Per Drinking Day | 5.57 (2.22) |
| TLFB Total Cigarettes | 11.23 (40.7) |
| RAPI Total | 7.95 (7.8) |
| MCQ Total | 44.48 (12.3) |
| BIS Total | 19.44 (4.1) |
| BAS Drive | 11.7 (2.0) |
| BAS Reward | 17.76 (1.7) |
| BAS Fun | 13.17 (1.6) |
| STAI | 30.43 (8.9) |
| BDI Total | 6.25 (6.1) |
Note. TLFB = Timeline Followback (60 day version), RAPI = Rutgers Alcohol Problems Index, MCQ = PhenX Marijuana Craving Questionnaire, BIS = Behavioral Inhibition System, BAS = Behavioral Activation System, STAI = State Trait Anxiety Index, BDI = Beck Depression Inventory (version 2)
Second item of the MacArthur Socioeconomic Scale is reported.
1 participant was excluded from analyses due to excessive motion and all values in demographics table reflect n=40 subjects included in the imaging analyses.
Exclusion criteria included: not having a parent to consent if under age 18; MRI contraindications (e.g., braces); known prenatal alcohol (> 2 drinks on an occasion or > 4 drinks/week) or illicit drug exposure; psychoactive medications; premature birth (< 34 weeks gestation or < 5 lbs. birth weight); history of major neurological or medical disorder or head trauma (loss of consciousness > 10 min) that could affect blood oxygen level dependent (BOLD) response; current DSM-5 psychopathology (e.g., bipolar, schizophrenia); history of learning disability or pervasive developmental disorder; inadequate comprehension of English; non-correctable sensory problems; illicit substance use other than cannabis or alcohol (> 100 times); and current pregnancy.
2.2. Procedures
All individuals interested in the study underwent a rigorous telephone screening. Participants (or parents, for those under age 18) provided verbal consent for screening, and were asked about exclusionary criteria. Participants and parents were informed that all information provided was confidential within ethical and legal limits to facilitate disclosure.
Following phone screening, eligible adolescents were invited to complete a 3.5h study session. During the session they were consented by a member of the research team, were asked questions regarding demographic and background information, completed self-report measures, cognitive testing, urine drug screening, and, to reduce motion while in the scanner, underwent a mock scan session. Finally, youth completed a 30 min MRI scan, including the cannabis cue task. Breath alcohol levels were collected before all scans to ensure that no participants were acutely intoxicated during scanning procedures. Subjects were asked not to consume cannabis for at least 12 h prior to the scan, and study research assistants were instructed not to scan any subjects who appeared visibly intoxicated. If, using their clinical judgment, research assistants were concerned that a participant may be experiencing acute cannabis intoxication, they were instructed to alert the study Principal Investigator (a licensed clinical psychologist) who could assess the participant further. No participants were excluded due to acute intoxication.
2.3. Measures
2.3.1. Demographics
A clinical interview was administered to youth to collect information on sample demographics. The interview included questions about age, sex, race, ethnicity, lifetime drug use, education, maternal substance use during pregnancy, and medical history. The MacArthur Scale of Subjective Social Status (Ostrove, Adler, Kuppermann, & Washington, 2000) was administered to measure socioeconomic status. This scale is a visual analogue scale showing a 10-rung ‘social ladder’ on which participants indicate the rung on which they feel they stand in relation to 1) their community and 2) other people in the U.S.
2.3.2. Mental health
The MINI International Neuropsychiatric Interview (MINI) (Sheehan, Lecrubier, Sheehan, et al., 1998) version 7, a brief structured diagnostic interview for major Axis I disorders in DSM-5 and ICD-10, was administered by a trained researcher. Selected questions from the Diagnostic Interview Schedule for Children, version IV (DISC-IV) (Shaffer, Fisher, Lucas, Dulcan, & Schwab-Stone, 2000) were also administered to assess for the presence of disruptive disorders. The DISC-IV demonstrates acceptable reliability for common psychiatric diagnoses in children.
The Beck Depression Inventory-II (BDI-II) (Beck, Steer, & Brown, 1996), a 21-item measure of depression in the past two weeks, was administered. Internal consistency in this sample was good (α =0.88). The Spielberger State-Trait Anxiety Inventory (STAI) (Spielberger, Gorsuch, & Lushene, 1970), a 20-item measure that was used to assess state anxiety, was also administered. Internal consistency in this sample was excellent (α = 0.92).
The behavioral inhibition system and behavioral approach system (BIS/BAS) scales (Carver & White, 1994) were used to measure avoidance (BIS) and approach (BAS) sensitivities reflective of reward sensitivity personality traits. Only the BIS subscale demonstrated good internal consistency in this sample (BAS drive α = 0.76; BAS fun α = 0.37.; BAS reward α = 0.61; BIS α = 0.83).
2.3.3. Substance use assessment
The calendar-based Timeline Followback (TLFB) interview (Sobell & Sobell, 1992) was used at baseline to assess quantity and frequency of cannabis, alcohol, cigarette, and other drug use over the past 60 days. This instrument asks participants to recall all substances consumed each day over the prior 60 days. Cannabis use was assessed in terms of total number of cannabis use days (which were further quantified by number of episodes per day), drinking was assessed as total number of drinking days and drinks per day, and cigarette smoking was assessed by total number of cigarettes smoked over 60 days. Participants were allowed to use their social media accounts, personal calendars, and text exchanges to prompt their memory about substance use. The TLFB variables tested for correlations with neural activation in the present analysis were number of cannabis days in the past 60 days and number of drinking days in the past 60 days. A breathalyzer was used to confirm no acute alcohol intoxication, and urine toxicology screens were used to confirm that individuals were cannabis users.
Participants also completed the Rutgers Alcohol Problem Index (RAPI) (White & Labouvie, 1989), an 18-item measure of alcohol-related consequences. The RAPI demonstrated good internal consistency in this sample (α = 0.88). Participants also completed the Marijuana Craving Questionnaire (MCQ) measure from the PhenX toolkit (Rosenberg, 2009), which also had good internal consistency in this sample (α = 0.87).
2.4. Urine cannabinoid levels
Urine cannabinoid samples were collected at the scan visit. Urine cannabinoid (11-nor-9-carboxy-Δ9-tetrahydrocannabinol) was batch assayed in thawed frozen (−80 °C) samples using an enzyme immunoassay on an Architect Autoanalyzer (Abbot Laboratories) in the Clinical Neurobiology Laboratory at the Medical University of South Carolina. The lowest quantifiable amount was 10 ng/mL, while values above 200 ng/mL were diluted to provide a quantifiable amount. As previous research has shown that failure to account for sample dilution may lead to misinterpretation (Huestis & Cone, 1998; Lafolie, Beck, Blennow, et al., 1991), urine creatinine levels were used as an estimate of sample dilution, and normalized urine drug screen cannabinoid/creatinine ratio are reported.
2.5. Cannabis cue reactivity task
A visual fMRI cannabis cue reactivity task was designed to examine differences in activation to cannabis vs. non-cannabis images. 36 cannabis stimuli were matched by color, hue, and visual complexity to 36 non-cannabis images (i.e., each cannabis image was matched to a specific non-cannabis image on these dimensions, resulting in 36 matched cannabis/non-cannabis image pairs). The non-cannabis images were non-food objects or plants. Cannabis images were classified as depicting either active (12 images) or passive (24 images) scenes. Images were classified as active if the scene included an individual smoking or handling cannabis or paraphernalia (e.g., smoking a joint). Images were classified as passive if the scene included only paraphernalia or a cannabis plant. All images were high resolution and scaled to similar dimensions to ensure high-quality display in the MRI. After the scan, to ensure that cannabis and non-cannabis stimuli were distinguishable, participants viewed each image again and rated how much they liked and wanted each cannabis picture compared to its matched non-cannabis picture, as well as whether or not they thought each picture was cannabis-related.
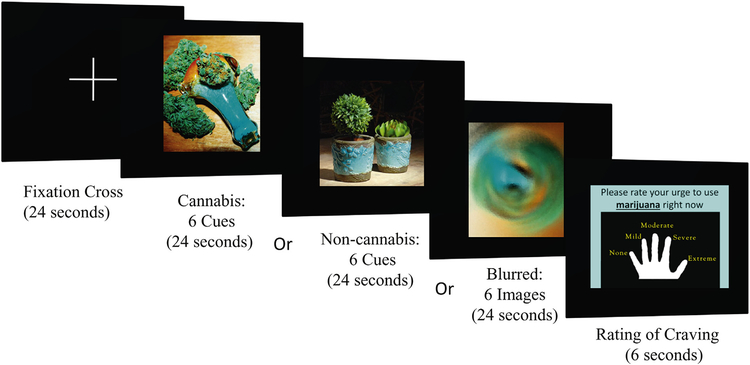
While in the scanner, participants were given instructions for completing the task (i.e., pressing the button corresponding to their urge rating, see Supplement 1). Given the simplicity of the task, no practice session was administered. During the task, participants in the scanner were shown pseudorandomly interspersed cannabis and non-cannabis images, visual control images (i.e., blurred images), and a fixation cross (Fig. 1). Blurred images and fixation trials were used as contrasts to evaluate attention and non-cannabis specific effects. Stimuli were presented in six 120-s epochs, each consisting of four 24-s blocks of an image type (one block each of cannabis, non-cannabis, control, and fixation). Each block consisted of one type of image (e.g., cannabis or non-cannabis) presented in a pseudorandom sequence. Each block was followed by a 6-s urge rating period, during which the participant rated their cannabis craving by pressing a button, allowing the hemodynamic response from the previous block to decline before the next was presented (Fig. 2).
Fig. 1.
Cannabis cue reactivity paradigm with active and passive cues. BOLD response during cannabis vs. non-cannabis cues was the main contrast of interest. Differences in activation in response to active (e.g., a person consuming cannabis; top left) vs. passive (e.g., cannabis plant; bottom left) cannabis cues were also examined. See Supplement 2 for all 36 cannabis and non-cannabis image pairs.
Fig. 2.
fMRI cannabis cue reactivity paradigm. Each task trial included a fixation cross followed by a cannabis or non-cannabis cue image presentation block, a craving rating period, and a blurred-image presentation.
2.5.1. Image acquisition, pre-processing, and analysis
Functional images were acquired with a 12-m gradient-echo, echo-planar imaging (EPI) sequence on a 3-Tesla TIM Trio Scanner (Siemens, Erlangen, Germany). Acquisition parameters were: repetition/echo time (TR/TE) = 2200/35 ms; 328 volumes; flip angle (FA) = 90°; field of view (FOV) = 192 mm; matrix = 64 × 64; voxel size = 3.00 × 3.00 mm; 37 contiguous 3-mm-thick slices). A field map was also acquired to allow geometric unwarping and cost-function masking of EPI images induced by magnetic field inhomogeneities. Using FEAT (fMRI Expert Analysis Tool) v5.98, part of FSL (FMRIB’s Software Library, Oxford) (Smith, Jenkinson, Woolrich, et al., 2004), functional images were first realigned to the middle volume to correct for head motion during the task. One subject with > 2 mm of translational/2 degress rotational movement during > 10% of TRs was excluded from analysis. Images were subsequently stripped of non-brain tissue/skull, spatially smoothed (8-mm full-width-at-half-maximum kernel), intensity normalized by the mean of all volumes, high-pass filtered (sigma = 240 s), and resampled to 2-mm isotropic voxels. Explanatory variables were created by convolving stimulus presentation timing with a double gamma hemodynamic response function in FEAT. A multiple linear regression analysis was performed to estimate the hemodynamic parameters for each explanatory variable. To identify differences in the magnitude of the BOLD signal for cannabis vs. non-cannabis images, contrast maps were created by contrasting the parameter estimates from the multiple regression for these stimuli. The opposite contrast of non-cannabis vs. cannabis images was also tested. The cannabis cue vs. fixation and non-cannabis cue vs. fixation contrasts were tested to determine whether differences observed between the cannabis and non-cannabis cues were due to activation or deactivation. Contrast maps were registered to each subject’s high-resolution anatomical image, and subsequently to the Montreal Neurological Institute (MNI) 152-subject-average template.
Higher-level analysis was conducted with FLAME (FMRIB’s Local Analysis of Mixed Effects). For analysis of cannabis vs. non-cannabis and active cannabis vs. passive cannabis activation, Z (Gaussianized T/F) statistic images were thresholded using clusters determined by z > 4.3 and a (corrected) cluster significance threshold of p = .05 (Worsley, 2001). For our exploratory analysis of correlations between cannabis vs. non-cannabis activation and self-report measures, a more lenient threshold of z > 2.3 and a (corrected) cluster significance threshold of p = .05 were used.
2.6. Region of interest (ROI) analysis
To follow up on the whole-brain exploratory analyses of cue-elicited activation and self-report measures, the average percentage change of the BOLD signal between cannabis vs. non-cannabis cues was extracted from several anatomically defined regions of interest and imported into SPSS Statistics (IBM; version 24). Specifically, we examined correlations between cannabis self-report measures and bilateral amygdala, bilateral ventral striatum and bilateral orbitofrontal cortex, given prior associations between self-report measures and cannabis cue-elicited activation in these regions (Cousijn et al., 2013; Filbey et al., 2009; Goldman et al., 2013; Vingerhoets et al., 2016; Wetherill et al., 2014). Orbitofrontal cortex and amygdala ROIs were defined using the Harvard Oxford Cortical Atlas (Desikan, Ségonne, Fischl, et al., 2006; Frazier, Chiu, Breeze, et al., 2005; Goldstein, Seidman, Makris, et al., 2007; Makris, Goldstein, Kennedy, et al., 2006). Ventral striatum ROIs were defined a priori as 6-mm-radius spheres centered at [−12, 15, −6] (left ventral striatum) and [12, 15, −6] (right ventral striatum) in MNI space, and were reverse-registered from the MNI-152 image to each subject’s anatomical image following our previous work (Schacht et al., 2011).
2.6.1. Analysis of image ratings
Participants’ ratings of each cannabis and non-cannabis image pair were examined using paired t-tests comparing participants’ ratings from 0 to 100 of how much they liked and wanted each cannabis picture compared to its matched non-cannabis picture.
3. Results
3.1. Subject characteristics
The sample was comprised of older adolescents (n=40 were incuded in imaging analysis given that one subject was dropped for excessive motion) age 17–21 (mean age = 18.83, SD = 0.96, 47.5% female). On the MacArthur Socioeconomic Ladder, participants placed themselves at an average of 5.75 (SD = 1.7) in relation to other people in the U.S. Participants exhibited minimal levels of depression (mean BDI score = 6.24, SD = 6.1), moderate levels of state anxiety (mean STAI score = 30.43, SD = 8.9), and moderate levels of behavioral avoidance (mean BIS total score = 19.44, SD = 4.1). Participant characteristics and substance use (e.g., alcohol, tobacco, and cannabis use) patterns for subjects included in imaging analysis are described in Table 1.
3.2. Cannabis consumption and urine cannabinoid levels
Total number of cannabis use episodes reported in the past seven days (calculated by summing total daily cannabis episodes reported on the TLFB for the 7 days before their visit) was positively correlated with normalized urine drug screen cannabinoid/creatinine ratio (n=40 from imaging analysis, r = 0.476, p = .002), suggesting self-reported cannabis use was consistent with biological markers of use.
3.3. Cannabis and non-cannabis image ratings
Of the 41 participants that completed the study, two did not complete the image rating portion. The subject who was dropped from the imaging analysis due to motion was included in the image rating analysis. Thus, n=39 for the image rating analysis. Most participants could correctly identify the cannabis images. Each of the 36 cannabis images was correctly identified as cannabis-related 92–100% of the time (14 images were correctly identified 100% of the time, 12 were correctly identified 97% of the time, 8 were correctly identified 95% of the time, and 2 were correctly identified 92% of the time). Each of the 36 non-cannabis images was correctly identified as not cannabis-related 92–100% of the time (24 images were correctly identified 100% of the time, 9 were correctly identified 97% of the time, 2 were correctly identified 95% of the time, and 1 was correctly identified 92% of the time).
On average, participants liked 32 of the 36 cannabis images more than the matched non-cannabis images (p < .05 for all comparisons). The average “liking” rating across all 36 cannabis images was 78.2, (SD = 13.5), compared to 55.7 (SD = 13.8) for non-cannabis images. For four pairs, there was no significant difference in liking between cannabis and non-cannabis images. Across all four, the non-cannabis images had high mean ratings [i.e., non-cannabis images were rated as highly liked (above 60 on the 100 point rating scale)], which did not appear to be driven by outlier ratings (i.e., ratings that were 3 or more standard deviations above or below mean).
For 35 out of 36 cannabis images, participants wanted to have the cannabis items more than the non-cannabis items (p < .05 for all comparisons). The average “wanting” rating across all cannabis images was 76.8, (SD = 14.8), compared to 46.6 (SD = 13.2) for non-cannabis images. For one pair, there were no significant differences in wanting between the cannabis and non-cannabis images. The non-cannabis image was given a high rating of 61 (which was not significantly different from the cannabis image rating of 66), and this was not due to outlier ratings. This pair also did not show significant differences in liking ratings.
3.4. Cannabis vs. non-cannabis activation
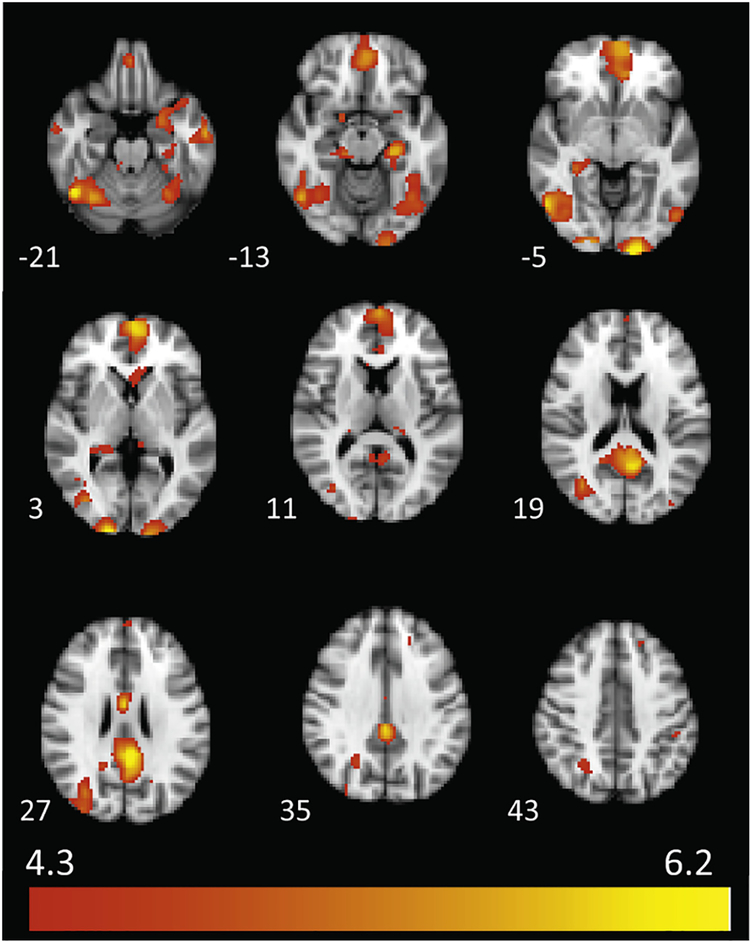
For the 40 participants with usable fMRI data, cannabis images, relative to non-cannabis images, elicited widespread activation across 19 clusters (Table 2 and Fig. 3), including bilateral fusiform gyrus, inferior temporal gyrus, cuneus, parahippocampal gyrus, and posterior cingulate, as well as left thalamus, medial frontal gyrus, middle occipital gyrus, inferior parietal lobule, superior frontal gyrus, and precuneus (ps < 05, cluster corrected). No significant activation emerged for the non-cannabis vs. cannabis contrast. In areas for which there were significant activation differences between cannabis and non-cannabis cues, there were also significant differances relative to fixation Supplementary Figs. (S1 and S2), suggesting that the observed cannabis vs. non-cannabis differences were differences in activation rather than deactivation. However, for the posterior cingulate/precuneus region, there was not significant activation for the cannabis vs. fixation contrast, suggesting that differences in the cannabis vs. non-cannabis contrast may have been driven by differences in deactivation in this region. This observation is consistent with the involvement of this region in the default mode network, which is classically deactivated during task performance.
Table 2.
Clusters of significant brain activation associated with cannabis cue vs. non-cannabis cue contrast.
| Cluster Index | Cluster size (Voxels) | Z-Max | X (mm) | Y (mm) | Z (mm) | Brain Region | Brodmann Area |
|---|---|---|---|---|---|---|---|
| 19 | 2647 | 6.22 | 46 | −56 | −22 | R Fusiform | 37 |
| 18 | 2287 | 5.98 | −6 | 60 | 0 | L Medial Frontal Gyrus | 10 |
| 17 | 1677 | 6.37 | −4 | −44 | 26 | L Posterior Cingulate/Ithsmus Cingulate | 23 |
| 16 | 856 | 5.23 | −30 | −60 | −20 | L Fusiform | 37 |
| 15 | 632 | 6.67 | −16 | −102 | −8 | L Cuneus | 18 |
| 14 | 360 | 6.48 | 18 | −102 | 2 | R Cuneus | 18 |
| 13 | 354 | 5.99 | −28 | −26 | −18 | L Parahippocampal Gyrus | 28,35,36 |
| 12 | 353 | 5.25 | 30 | −36 | −4 | R Parahippocampal Gyrus/Hippocampus | 36 |
| 11 | 286 | 5.27 | −26 | 6 | −20 | L Parahippocampal Gyrus | 34 |
| 10 | 198 | 5.59 | −60 | −10 | −20 | L Inferior Temporal Gyrus | 21 |
| 9 | 165 | 5.96 | 2 | −4 | 28 | R Posterior Cingulate Gyrus | 24 |
| 8 | 52 | 4.78 | −38 | −86 | 14 | L Middle Occipital Gyrus | 19 |
| 7 | 39 | 4.86 | 60 | −6 | −24 | R Inferior Temporal Gyrus | 20 |
| 6 | 32 | 4.54 | −46 | −36 | 42 | L Inferior Parietal Lobule | 40 |
| 5 | 27 | 4.64 | −18 | 36 | 38 | L Superior Frontal Gyrus | 9 |
| 4 | 23 | 4.56 | −14 | −28 | 10 | L Thalamus | n/a |
| 3 | 22 | 4.57 | −6 | −34 | 0 | L Thalamus | n/a |
| 2 | 22 | 5.15 | 16 | 2 | −16 | R Parahippocampal Gyrus | 34 |
| 1 | 4 | 4.53 | −20 | −64 | 26 | L Precuneus | 7 |
Note. All coordinates presented in Montreal Neurological Institute (MNI) space. For all clusters, p < .05.
Fig. 3.
Cannabis cue vs. non-cannabis cue activation. The cannabis vs. non-cannabis cue contrast elicited increased brain activation across 19 clusters (see Table 2). MNI z coordinates are indicated to the left of each slice.
3.5. Active vs. passive cannabis cue activation
No significant activation difference emerged for the active cannabis cue compared to passive cue contrast (p > 05).
3.6. Associations between cue-elicited activation and self-report measures
Cannabis cue vs. non-cannabis cue activation was not significantly associated with recent (past 60 day) cannabis use, total MCQ score, quantity of cannabis use (episodes per day), chronicity of cannabis use (lifetime cannabis use days), or past 60 day alcohol use.
3.7. Associations between cue-elicited activation in ROIs and self-report measures
After applying a Bonferroni correction to adjust for the number of tests performed, there were no significant correlations between cannabis cue-elicited activation and recent (past 60 day) cannabis use, total MCQ score, quantity of cannabis use (episodes per day), chronicity of cannabis use (lifetime cannabis use days), or past 60 day alcohol use in any of the ROIs tested.
4. Discussion
Visual cannabis cues, relative to matched non-cannabis cues, elicited greater activation in a number of brain regions among adolescent cannabis users. Unlike previous tasks, this task uses cues that are relevant to cannabis-using youth in the U.S., is more easily administered than tactile tasks, and does not show differential activation based on passive or active cannabis images. In addition, nearly all cannabis cues were rated as more rewarding than non-cannabis cues, adding to the construct validity of the task. Overall, findings suggest that this task could be useful for future examinations of the neural underpinnings of reward processes in adolescent cannabis users.
Consistent with study hypotheses, significant activation for the cannabis vs. non-cannabis cue contrast was observed in widespread brain regions including bilateral cingulate, cuneus, fusiform, inferior temporal and parahippocampal gyri, as well as left thalamus, medial frontal, and superior frontal gyri. The largest clusters of activation in the present study were right fusiform, left medial frontal, and left posterior cingulate. In general, the medial frontal cortex, which encompasses the medial frontal gyrus, is an executive function region thought to be involved in decision making and reward-learning (Nieuwenhuis, Holroyd, Mol, & Coles, 2004; Rushworth, Noonan, Boorman, Walton, & Behrens, 2011), and specifically involved in reward prediction during cue presentation (Silvetti, Castellar, Roger, & Verguts, 2014), which is consistent with the idea that the cannabis images were more rewarding to youth cannabis users compared to the non-cannabis images. Activation in the fusiform gyrus is thought to increase based on an individual’s level of expertise in the visual object they are viewing (Gauthier, Tarr, Anderson, Skudlarski, & Gore, 1999). Thus, in the present study, it is possible that youth cannabis users have some level of “expertise” in cannabis-related stimuli compared to non-cannabis-related visually matched controls. Notably, a recent meta-analysis of neuroimaging studies of drug cue reactivity also demonstrated that visual cortex activation is consistently observed in response to drug vs. neutral cues in substance dependent individuals, which is consistent with the present findings (Hanlon, Dowdle, Naselaris, Canterberry, & Cortese, 2014). The posterior cingulate is a self-referential region (Johnson et al., 2002), and has been hypothesized to play an important role in internally-directed cognition (Raichle et al., 2001), such as integrating emotion and memory in the retrieval of autobiographical memories (Addis, Wong, & Schacter, 2007; Maddock, Garrett, & Buonocore, 2001). Youth cannabis users may have emotional memories associated with cannabis, which would be consistent with the observed activation of posterior cingulate to cannabis cues in the present study.
Activation of these regions is largely consistent with limited adult and adolescent research and supports cue-elicited activation in cannabis users, particularly in regions associated with visual attention. A recent meta-analysis of neuroimaging studies on cue reactivity to drug, food, and neutral stimuli showed that drug cues (vs. natural reinforcers) are associated with distinct activation in several areas that emerged in the present study (e.g., medial frontal gyrus and posterior cingulate) (Noori, Linan, & Spanagel, 2016). Another recent review demonstrated that across 46 fMRI studies of cannabis users, frontal (63% of studies), parietal (33% of studies) and temporal regions (22% of studies) were the brain areas that most frequently showed alterations in task-based activation (Silveri, Dager, Cohen-Gilbert, & Sneider, 2016). In addition, tactile cannabis cues (vs. neutral cues) have been shown to activate the thalamus (Filbey et al., 2009). Overall, regions that emerged in the present analysis demonstrated considerable overlap with regions that have been previously associated with cue-elicited craving in substance users, and in adolescent cannabis users specifically.
While most (32 of 36) of the cannabis cues showed greater liking and wanting compared to non-cannabis cues, there were four pairs for which no such differences emerged. In all four cases, the lack of differences in liking and wanting were driven by high ratings of the non-cannabis images. These pairs had non-cannabis images that may be particularly visually appealing or interesting to participants (e.g., a firecracker, colorful pieces of pottery).
The present analysis failed to show correlations between cannabis cue related activation and measures of cannabis craving, cannabis problems, or chronicity/frequency of use. In contrast, previous studies showed correlations between visual cannabis cue-elicited activation and craving and cannabis consumption (Cousijn et al., 2013; Goldman et al., 2013; Vingerhoets et al., 2016; Wetherill et al., 2014), typically when using a ROI-based approach. For example, Goldman et al. used a mask generated from a priori hypotheses about brain regions that were expected to show activation, and showed correlations with craving, but did not examine correlations with consumption. Cousijn et al. used an ROI-based approach and found correlations between cue reactivity and craving, but not between cue reactivity and consumption. Finally, Vingerhoets et al. used an ROI-based approach and found that cue reactivity did not predict cannabis use at a 3-year follow-up, though it did predict cannabis use problem severity at follow-up. It is possible that the lack of association between cue-elicited activation and self-report measures in the present study is due to the whole brain approach being more conservative. However, follow-up ROI analyses also failed to show associations with self-report measures.
A theoretically motivated explanation for this lack of association may be that in adolescents, craving may not necessarily be a strong predictor of overall substance consumption, as has been suggested by a recent meta-analysis of laboratory studies of craving and tobacco use (Gass, Motschman, & Tiffany, 2014). Specifically, this study demonstrated a stronger relationship between craving and non-automatic drug seeking (i.e., studies that involved participants weighing contingencies and making a conscious choice about whether or not to use tobacco) than between craving and automatic seeking/consumption (i.e., studies that measured the length of time before a participant used tobacco, or simply whether or not they chose to consume tobacco, without an additional element of explicit cognitive choice). The present lack of association between cue-elicited activation and consumption may be due, at least in part, to the fact that adolescent substance use is, overall, a more opportunistic, automatic process.
Despite the challenges noted above, it remains important to have cannabis cue reactivity tasks relevant for youth, to better understand how neural processes related to cannabis misuse change over time. Broadly, cue reactivity paradigms can help to advance addiction science by better shedding light on neurobiological mechanisms underlying reward systems in addiction (Courtney et al., 2016), and how these reward systems change over time. This work also has translational utility for testing behavioral predictions and psychosocial and pharmacological interventions, and could thus inform treatment development in the future. Specifically, the present study demonstrated activation in both reward and cognitive control-related brain regions, thereby implicating interventions that target these neural systems. Testing cue reactivity before and after treatment could elucidate neural underpinnings of treatment-related change, and baseline activation patterns could be useful in predicting treatment responders. For example, cognitive bias modification (CBM) is an intervention designed to alter approach biases to substance cues (Eberl et al., 2013), and may be helpful for adolescent cannabis users given the observed activation of the medial frontal gyrus (which is involved in reward-learning) to cannabis cues in the present study. In addition, given the observed activation in the posterior cingulate, a self-referential brain region involved in inwardly-directed cognition, more cognitively focused psychosocial interventions such as cognitive behavior therapy (CBT) could be assessed pre- and post-treatment to understand how psychosocial intervention could affect neural response in these regions.
4.1. Limitations and future directions
Notably, the study was cross-sectional and lacked a control group, and thus cannot be used to inform causal hypotheses about the relationship between cannabis use and neural circuitry changes. Future studies should include a control group (i.e., non-substance-using adolescents) for comparison. Future studies should also follow-up on these findings by examining cannabis cue-elicited brain activation in adolescents over time, particularly in participants who change their cannabis use habits or become abstinent. Another limitation is due to the fact that the present study did not include collection of oral fluid samples to ensure that participants were not intoxicated during the time of the scan, or to precisely quantify recency of use. Saliva measures should be included in future work to rule out the possibility of current intoxication during study procedures. However, it should be noted that in the present study, subjects were at least asked not to consume cannabis the day of the scan, and research assistants were instructed not to scan any subjects who appeared visibly intoxicated.
Another limtation is related to the reward value of visual cues compared to other cue types; prior research has demonstrated that ol-factory alcohol cues elicit activation in reward-related brain regions, including the nucleus accumbens and ventral tegmental area (Kareken, Claus, Sabri, et al., 2004). Similarly, olfactory cues produce significant increases in craving and physiological arousal in nicotine-dependent adult smokers compared to neutral olfactory cues (Cortese, Uhde, Larowe, et al., 2015). Olfactory cannabis cues may be a stronger or more robust predictor of neural reward responding than visual cues. However, the ease of presentation of visual cues compared to olfactory cues may outweigh these benefits.
Finally, although the present sample is useful in that this age group encompasses the transition to substance use disorders (Lipari & Van Horn, 2017), it is also important to examine cue reactivity at earlier and later stages of brain development and progression of cannabis use and CUD. Future investigations should evaluate cannabis cues in younger adolescents, and among adolescents who are “lighter” cannabis users. It may also be useful to test these cues in a young adult sample (e.g., up to age 25).
5. Conclusions
This task supports examination of cannabis cue reactivity in future fMRI investigations of cannabis-using youth. Results indicate that among older adolescent cannabis users, cannabis cues elicit differential patterns of neural activation compared to non-cannabis cues in reward regions that may be targets for intervention.
Supplementary Material
HIGHLIGHTS.
Visual cannabis vs. non-cannabis cues elicit brain activation in cannabis-using youth.
Cue-associated brain regions include incentive-salience and visual attention areas.
Adolescents report cannabis cues to be more rewarding than matched non-cannabis images.
Visual cannabis cue tasks may be useful for future developmental fMRI studies.
Acknowledgements
The authors wish to acknowledge the funding sources for this study. Funding was provided by NIDA grant K12 DA031794 (Brady), NIAAA grant K23 AA025399 (Squeglia), NIAAAK99/R00 AAO21419 (Schacht) and NIH/NCATSKL2 TR001444 (Jacobus). Additional funding was also provided by the South Carolina Clinical and Translational Institute at the Medical University of South Carolina (UL1TR000062). The funding source had no role other than financial support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.addbeh.2018.09.015.
References
- Addis DR, Wong AT, & Schacter DL (2007). Remembering the past and imagining the future: Common and distinct neural substrates during event construction and elaboration. Neuropsychologia, 45(7), 1363–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A, Steer R, & Brown G (1996). Manual for the BDI-II. In. San Antonio, TX:Psychological Corporation. [Google Scholar]
- Bordnick PS, Copp HL, Traylor A, et al. (2009). Reactivity to cannabis cues in virtual reality environments. Journal of Psychoactive Drugs, 41(2), 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, & White TL (1994). Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. Journal of Personality and Social Psychology, 67(2), 319. [Google Scholar]
- Cortese BM, Uhde TW, Larowe SD, et al. (2015). Olfactory cue reactivity in nicotine-dependent adult smokers. Psychology of Addictive Behaviors, 29(1), 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney KE, Schacht JP, Hutchison K, Roche DJ, & Ray LA (2016). Neural substrates of cue reactivity: Association with treatment outcomes and relapse. Addiction Biology, 21(1), 3–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousijn J, Goudriaan AE, Ridderinkhof KR, van den Brink W, Veltman DJ, & Wiers RW (2013). Neural responses associated with cue-reactivity in frequent cannabis users. Addiction Biology, 18(3), 570–580. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, et al. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage, 31(3), 968–980. [DOI] [PubMed] [Google Scholar]
- Eberl C, Wiers RW, Pawelczack S, Rinck M, Becker ES, & Lindenmeyer J (2013). Approach bias modification in alcohol dependence: Do clinical effects replicate and for whom does it work best? Developmental Cognitive Neuroscience, 4, 38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Eastwood B, Bradley BP, & Mogg K (2006). Selective processing of cannabis cues in regular cannabis users. Drug and Alcohol Dependence, 85(1), 75–82. [DOI] [PubMed] [Google Scholar]
- Filbey FM, Schacht JP, Myers US, Chavez RS, & Hutchison KE (2009).Marijuana craving in the brain. Proceedings of the National Academy of Sciences of the United States of America, 106(31), 13016–13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier JA, Chiu S, Breeze JL, et al. (2005). Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. American Journal of Psychiatry, 162(7), 1256–1265. [DOI] [PubMed] [Google Scholar]
- Gass JC, Motschman CA, & Tiffany ST (2014). The relationship between craving and tobacco use behavior in laboratory studies: A meta-analysis. American Psychological Association. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Tarr MJ, Anderson AW, Skudlarski P, & Gore JC (1999). Activation of the middle fusiform’face area’increases with expertise in recognizing novel objects. Nature Neuroscience, 2(6), 568. [DOI] [PubMed] [Google Scholar]
- Goldman M, Szucs-Reed RP, Jagannathan K, et al. (2013). Reward-related brain response and craving correlates of marijuana cue exposure: A preliminary study in treatment-seeking marijuana-dependent subjects. Journal of Addiction Medicine, 7(1), 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Makris N, et al. (2007). Hypothalamic abnormalities in schizophrenia: Sex effects and genetic vulnerability. Biological Psychiatry, 61(8), 935–945. [DOI] [PubMed] [Google Scholar]
- Gray KM, Larowe SD, & Upadhyaya HP (2008). Cue reactivity in young marijuana smokers: A preliminary investigation. Psychology of Addictive Behaviors, 22(4), 582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray KM, Larowe SD, Watson NL, & Carpenter MJ (2011). Reactivity to in vivo marijuana cues among cannabis-dependent adolescents. Addictive Behaviors, 36(1), 140–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Dowdle LT, Naselaris T, Canterberry M, & Cortese BM (2014). Visual cortex activation to drug cues: A meta-analysis of functional neuroimaging papers in addiction and substance abuse literature. Drug and Alcohol Dependence, 143, 206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughey HM, Marshall E, Schacht JP, Louis A, & Hutchison KE (2008).Marijuana withdrawal and craving: Influence of the cannabinoid receptor 1 (CNR1) and fatty acid amide hydrolase (FAAH) genes. Addiction, 103(10), 1678–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry EA, Kaye JT, Bryan AD, Hutchison KE, & Ito TA (2014). Cannabis cue reactivity and craving among never, infrequent and heavy cannabis users. Neuropsychopharmacology, 39(5), 1214–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huestis MA, & Cone EJ (1998). Differentiating new marijuana use from residual drug excretion in occasional marijuana users. Journal of Analytical Toxicology, 22(6), 445–454. [DOI] [PubMed] [Google Scholar]
- Jasinska AJ, Stein EA, Kaiser J, Naumer MJ, & Yalachkov Y (2014). Factors modulating neural reactivity to drug cues in addiction: A survey of human neuroimaging studies. Neuroscience & Biobehavioral Reviews, 38, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, & Prigatano GP (2002). Neural correlates of self-reflection. Brain, 125(8), 1808–1814. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, & Volkow ND (2005). The neural basis of addiction: A pathology of motivation and choice. The American Journal of Psychiatry, 162(8), 1403–1413. [DOI] [PubMed] [Google Scholar]
- Kareken DA, Claus ED, Sabri M, et al. (2004). Alcohol-related olfactory cues activate the nucleus accumbens and ventral tegmental area in high-risk drinkers: Preliminary findings. Alcoholism, Clinical and Experimental Research, 28(4), 550–557. [DOI] [PubMed] [Google Scholar]
- Lafolie P, Beck O, Blennow G, et al. (1991). Importance of creatinine analyses of urine when screening for abused drugs. Clinical Chemistry, 37(11), 1927–1931. [PubMed] [Google Scholar]
- Lipari RN, & Van Horn SL (2017). Trends in substance use disorders among adults aged 18 or older. The CBHSQ Report June 29. [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, & Buonocore MH (2001). Remembering familiar people: The posterior cingulate cortex and autobiographical memory retrieval. Neuroscience, 104(3), 667–676. [DOI] [PubMed] [Google Scholar]
- Makris N, Goldstein JM, Kennedy D, et al. (2006). Decreased volume of left and total anterior insular lobule in schizophrenia. Schizophrenia Research, 83(2–3), 155–171. [DOI] [PubMed] [Google Scholar]
- McRae-Clark AL, Carter RE, Price KL, et al. (2011). Stress- and cue-elicited craving and reactivity in marijuana-dependent individuals. Psychopharmacology, 218(1), 49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, Miech RA, O’Malley PM, Bachman JG, Schulenberg JE, & Patrick ME (2018). Monitoring the Future national survey results on drug use: 1975–2017: Overview, key findings on adolescent drug use. Ann Arbor: Institute for Social Research, The University of Michigan. [Google Scholar]
- Nickerson LD, Ravichandran C, Lundahl LH, et al. (2011). Cue reactivity in cannabis-dependent adolescents. Psychology of Addictive Behaviors, 25(1), 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S, Holroyd CB, Mol N, & Coles MG (2004). Reinforcement-related brain potentials from medial frontal cortex: Origins and functional significance. Neuroscience & Biobehavioral Reviews, 28(4), 441–448. [DOI] [PubMed] [Google Scholar]
- Noori HR, Linan AC, & Spanagel R (2016). Largely overlapping neuronal substrates of reactivity to drug, gambling, food and sexual cues: A comprehensive meta-analysis. European Neuropsychopharmacology, 26(9), 1419–1430. [DOI] [PubMed] [Google Scholar]
- Ostrove JM, Adler NE, Kuppermann M, & Washington AE (2000). Objective and subjective assessments of socioeconomic status and their relationship to self-rated health in an ethnically diverse sample of pregnant women. Health Psychology, 19(6), 613–618. [DOI] [PubMed] [Google Scholar]
- Pronk T, Deursen DS, Beraha EM, Larsen H, & Wiers RW (2015). Validation of the Amsterdam Beverage Picture Set: A controlled picture set for cognitive bias measurement and modification paradigms. Alcoholism: Clinical and Experimental Research, 39(10), 2047–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, & Shulman GL (2001). A default mode of brain function. Proceedings of the National Academy of Sciences, 98(2), 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, & Berridge KC (2008). Review. The incentive sensitization theory of addiction: Some current issues. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 363(1507), 3137–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg H (2009). Clinical and laboratory assessment of the subjective experience of drug craving. Clinical Psychology Review, 29(6), 519–534. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Noonan MP, Boorman ED, Walton ME, & Behrens TE (2011). Frontal cortex and reward-guided learning and decision-making. Neuron, 70(6), 1054–1069. [DOI] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Randall PK, Li X, Henderson S, & Myrick H (2011). Stability of fMRI striatal response to alcohol cues: A hierarchical linear modeling approach. NeuroImage, 56(1), 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Selling RE, & Hutchison KE (2009). Intermediate cannabis dependence phenotypes and the FAAH C385A variant: An exploratory analysis. Psychopharmacology, 203(3), 511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, & Schwab-Stone ME (2000). NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry, 39(1), 28–38. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, et al. (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry, (59 Suppl. 20), 22–33 quiz 34–57. [PubMed] [Google Scholar]
- Silveri MM, Dager AD, Cohen-Gilbert JE, & Sneider JT (2016). Neurobiological signatures associated with alcohol and drug use in the human adolescent brain. Neuroscience & Biobehavioral Reviews, 70, 244–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvetti M, Castellar EN, Roger C, & Verguts T (2014). Reward expectation and prediction error in human medial frontal cortex: An EEG study. NeuroImage, 84, 376–382. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, et al. (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23(Suppl. 1), S208–S219. [DOI] [PubMed] [Google Scholar]
- Sobell LC, & Sobell MB (1992). Timeline follow-back. Measuring alcohol consumption(pp. 41–72). Springer. [Google Scholar]
- Spielberger CD, Gorsuch RL, & Lushene RE (1970). Manual for the state-trait anxiety inventory.
- Squeglia LM, & Gray KM (2016). Alcohol and drug use and the developing brain.Current Psychiatry Reports, 18(5), 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingerhoets W, Koenders L, Van Den Brink W, et al. (2016). Cue-induced striatal activity in frequent cannabis users independently predicts cannabis problem severity three years later. Journal of Psychopharmacology, 30(2), 152–158. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Telang F, et al. (2014). Decreased dopamine brain reactivity in marijuana abusers is associated with negative emotionality and addiction severity. Proceedings of the National Academy of Sciences, 111(30), E3149–E3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Childress AR, Jagannathan K, et al. (2014). Neural responses to subliminally presented cannabis and other emotionally evocative cues in cannabis-dependent individuals. Psychopharmacology, 231(7), 1397–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White HR, & Labouvie EW (1989). Towards the assessment of adolescent problem drinking. Journal of Studies on Alcohol and Drugs, 50(01), 30. [DOI] [PubMed] [Google Scholar]
- Wolfling K, Flor H, & Grusser SM (2008). Psychophysiological responses to drug-associated stimuli in chronic heavy cannabis use. The European Journal of Neuroscience, 27(4), 976–983. [DOI] [PubMed] [Google Scholar]
- Worsley K (2001). Statistical analysis of activation images Functional MRI: AnIntroduction to Methods, 14, 251–270. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.