SUMMARY
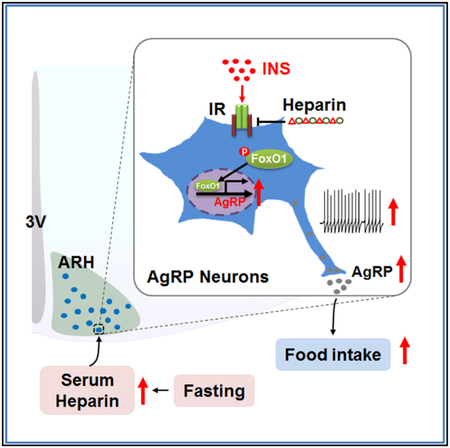
Although the widely used anticoagulant drug heparin has been shown to have many other biological functions independent of its anticoagulant role, its effects on energy homeostasis are unknown. Here, we demonstrate that heparin level is negatively associated with nutritional states and that heparin treatment increases food intake and body weight gain. By using electrophysiological, pharmacological, molecular biological, and chemogenetic approaches, we provide evidence that heparin increases food intake by stimulating AgRP neurons and increasing AgRP release. Our results support a model whereby heparin competes with insulin for insulin receptor binding on AgRP neurons, and by doing so it inhibits FoxO1 activity to promote AgRP release and feeding. Heparin may be a potential drug target for food intake regulation and body weight control.
Graphical Abstract
In Brief
Zhu et al. demonstrate that heparin competes with insulin for insulin receptor binding on AgRP neurons, and by doing so it inhibits FoxO1 activity to promote AgRP release and feeding. Heparin is identified as a potential drug target for food intake regulation and body weight control.
INTRODUCTION
Heparin, a naturally occurring mucopolysaccharide, is produced mainly by basophils and mast cells. Although heparin has been widely used as an anticoagulant and antithrombotic drug in therapeutic doses since the 1930s (McLean, 1959), endogenous heparin’s physiological function is still unclear, and it has been suggested that the main physiological role of heparin is not anticoagulant. For example, heparin is conservatively expressed in many mammalian and other vertebrate animals, including lobster (Hovingh and Linker, 1982), clam (Pejler et al., 1987), and Atlantic salmon (Flengsrud, 2016). These species do not have a blood coagulant system similar to that of mice and humans. Additionally, heparin is highly expressed in tissues that have direct contact with the outside environment, including lung, skin, and intestine, and is usually released when tissues are injured (Nader et al., 1999). It has been proposed that like inflammation, heparin release is the body’s response in vascular tissues to harmful stimuli, contributing to defense against invading bacteria or other alien materials.
Emerging evidence from both basic and clinic studies indicates that heparin may play a role in the regulation of energy homeostasis. For example, heparin has been shown to activate lipoprotein lipase and interfere with lipid metabolism in rats (Persson, 1988). Consistent with those findings, genetic analysis in cattle found that genes involved in the heparin metabolism pathway regulate lipid homeostasis (Jiang et al., 2011). Multiple clinical cases also have demonstrated that obese patients have inadequate levels of both fractionated and unfractionated heparin, suggesting a negative association between body weight and serum heparin level (Allman-Farinelli, 2011; Dager, 2010; Freeman et al., 2010). Furthermore, obese patients have low heparin sensitivity and need a high heparin infusion rate to reduce venous thromboembolism (Hurewitz et al., 2011; Myzienski et al., 2010), and BMI is strongly associated with increased rates of heparin-induced thrombocytopenia (Bloom et al., 2016). There is also some indirect evidence regarding another member of the glycosaminoglycan family, heparan sulfate, which shares a very similar structure with heparin. Both heparan sulfate and its catalytic enzyme, heparanase, have been found to regulate energy homeostasis (Karlsson-Lindahl et al., 2012; Reizes et al., 2001; Strader et al., 2004; Zcharia et al., 2004, 2009). On the basis of these observations, we speculated that heparin regulates body weight homeostasis.
In the present study, we first evaluated the physiological relevance of endogenous heparin in different nutritional states. We then investigated the effects of heparin treatment on food intake and body weight gain. Furthermore, we used electrophysiological recordings and designer receptors exclusively activated by designer drugs (DREADD) to determine whether hypothalamic neurons expressing agouti-related peptide (AgRP) are required to mediate heparin’s effects on food intake. Finally, we tested whether insulin signaling and the downstream mediator FoxO1 in AgRP neurons mediate heparin’s effect on food intake.
RESULTS
Heparin Treatment Acutely Increases Food Intake
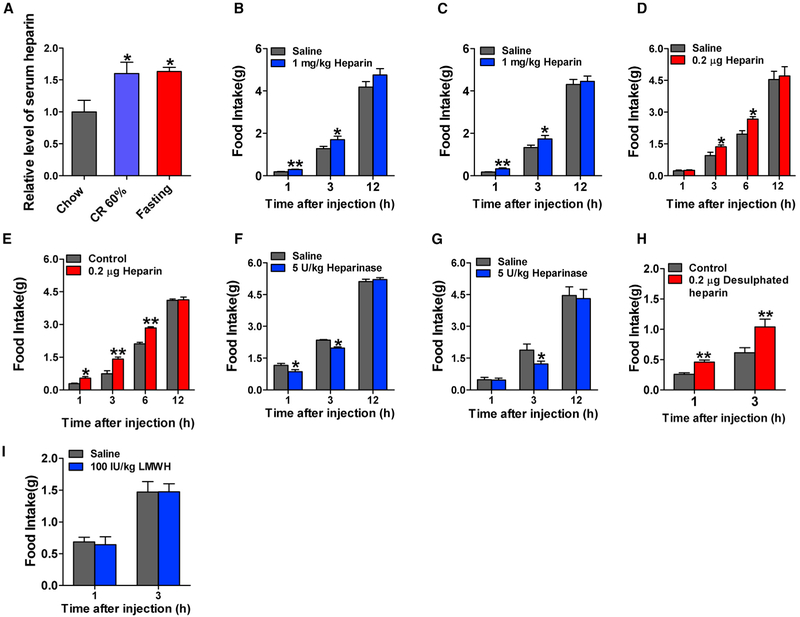
We found that serum heparin level was elevated during negative energy balance including 7-day calorie restriction (CR) or 24 hr fasting (Figure 1A). Consistent with this negative association between heparin and nutritional states, food intake was elevated after intravenous (i.v.) injection of heparin (Figure S1A) in male mice. Heparin stimulated food intake in a dose-dependent manner, and its effects reached a plateau at a dose of 1 mg/kg. The orexigenic effects of a single injection lasted for 6 hr. Similarly, peripheral intraperitoneal (i.p.) injection of heparin (1 mg/kg) increased food intake in both male and female mice 1, 3, and 12 hr after injection (Figures 1B and 1C). More interestingly, central delivery of heparin by intracerebroventricular (i.c.v.) injection also promoted food intake in the first 6 hr after injection in both male and female mice (Figures 1D and 1E), suggesting a central mechanism for orexigenic effects of heparin. Different from i.p. treatment, i.c.v. treatment with heparin did not increase food intake 1 hr after injection in male mice (Figures 1B and 1D), suggesting that a peripheral mechanism may also be involved in the orexigenic effects of heparin. Although we failed to observe any significant change in serum ghrelin after i.p. injection of heparin in male mice (Figure S1F), we found that heparin increased serum non-essential fatty acid (NEFA) and decreased glucose and insulin (Figures S1B-S1E).
Figure 1. Heparin Synthesis at Different Nutritional States and Acute Effects of Heparin.
(A) Serum heparin levels in male C57BL6/J mice fed with normal chow, fed with CR 60% diet, or fasted for 24 hr (n = 7 per group).
(B and C) Dark-cycle food intake of male (B, n = 7 or 8 per group) and female (C, n = 7 or 8 per group) C57BL6/J mice after intraperitoneal (i.p.) injection of 1 mg/kg heparin or saline.
(D and E) Dark-cycle food intake of male (D, n = 6 per group) and female (E, n = 7 or 8 per group) C57BL6/J mice after intracerebroventricular (i.c.v.) injection of 0.2 μg heparin or saline.
(F and G) Dark-cycle food intake of male (F, n = 6 or 7 per group) and female (G, n = 6 or 7 per group) C57BL6/J mice after i.p. injection of 5 U/kg heparinase or saline.
(H) Dark-cycle food intake of male C57BL6/J mice after i.c.v. injection of 0.2 μg desulfated heparin or saline (n = 6 per group).
(I) Dark-cycle food intake of female C57BL6/J mice after i.p. injection of 100 IU/kg low-molecular-weight heparin (LMWH) or saline (n = 7 or 8 per group).
Results are presented as mean ± SEM. In (A), *p ≤ 0.05 by one-way ANOVA followed by post hoc Bonferroni tests. In (B)–(I), *p ≤ 0.05and **p ≤ 0.01 by two-way ANOVA followed by post hoc Bonferroni tests. See also Figure S1.
If heparin plays a physiological role in feeding, the degradation of heparin would lead to a decrease in food intake. To directly test this possibility, the degradation of heparin was achieved by i.p. injection of heparinase, which specifically catalyzes the eliminative cleavage of polysaccharides from heparin. Food intake was reduced following i.p. injection of heparinase in both male and female mice (Figures 1F and 1G). Additionally, the orexigenic effects of two other forms of heparin, desulfated heparin and low-molecular-weight heparin (LMWH), were tested. Desulfated heparin is a heparin derivative with the 2-O and 3-O sulfate groups removed and lacks anticoagulant activity, while LMWH is depolymerized from heparin and produces more efficient anticoagulant activity. We found that i.c.v. injection of desulfated heparin significantly increased food intake (Figure 1H), whereas different doses of LMWH administered by either i.v. (Figure S1K) or i.p. (Figure 1I) injection did not affect food intake. Hence, heparin activates food intake through a central mechanism, and this effect is likely independent of its anticoagulant activity.
To further determine how i.p. injection of heparin alters the level of heparin in the circulation, we measured serum heparin levels 0, 1, 3, 6, and 12 hr after i.p. injection of 1 mg/kg heparin. We found that after i.p. administration, heparin was rapidly absorbed and efficiently entered the circulation (Figure S1L). Peak serum concentration was reached within 1 hr after administration and was 4 times higher than the physiological dose in saline-injected mice (0.772 ± 0.094 versus 0.197 ± 0.023 U/mL). Serum heparin remain elevated until 6 hr after injection. Although no concentration-effect relationship could be established, with i.p. administration, heparin’s pharmacokinetic parameters are consistent with time-response effects on food intake, both of which are significant 1 and 3 hr after injection.
Long-Term Heparin Treatment Induces Body Weight Gain in Mice
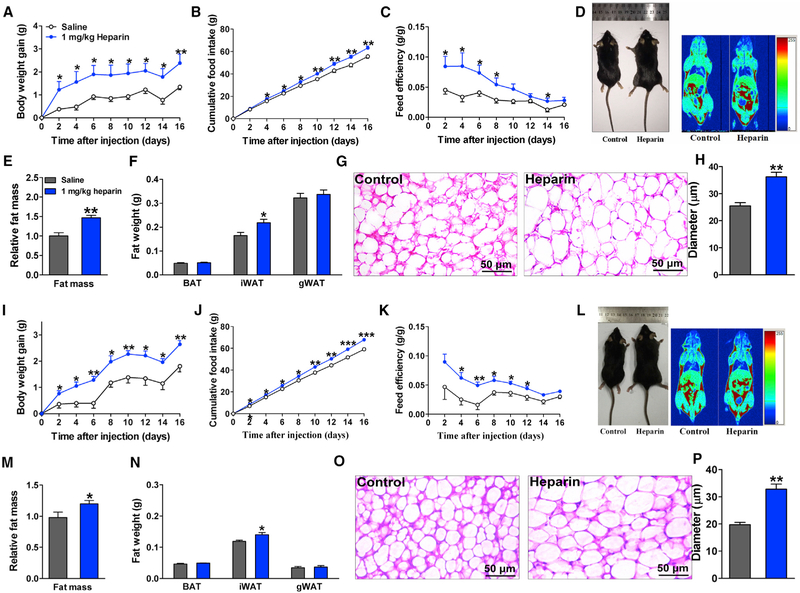
Considering the stimulatory effects of heparin on food intake, we predicted that long-term treatment with heparin would increase body weight. We found that chronic heparin treatment (i.p. for 16 days) substantially increased body weight gain in male mice fed on chow (Figures 2A and 2D). Notably, heparin-treated male mice started to gain significantly more body weight than their saline-injected controls as early as 2 days after injection, and their body weight gain remained higher during the entire 16-day period. A significant increase in fat mass was observed in heparin-treated male mice compared with saline-treated control mice (Figures 2D and 2E), which was attributed mainly to increased inguinal white adipose tissue (iWAT; Figure 2F). Consistently, the average adipocyte size of iWAT was significantly larger in heparin-treated male mice than in saline-treated control males (Figures 2G and 2H). Consistent with the acute orexigenic effects of heparin, long-term treatment with heparin also elevated cumulative food intake (Figure 2B). Energy expenditure was estimated by feed efficiency (the ratio between body weight gain and cumulative food intake). Notably, the stimulatory effects of heparin on feed efficiency indicate a decrease in energy expenditure (Figure 2C). Additionally, the effects of heparin on energy expenditure were also measured by the Comprehensive Lab Animal Monitoring System (CLAMS). A single i.p. injection of heparin significantly decreased both heat production and O2 consumption in the first 6 hr after injection in the dark cycle (Figures S1M and S1N). Thus, our results support a model whereby heparin stimulates food intake and inhibits energy expenditure to increase body weight and adiposity.
Figure 2. Long-Term Heparin Treatment Induces Body Weight Gain.
(A–C) Body weight gain (A), cumulative food intake (B), and feed efficiency (C) of male C57BL6/J mice i.p. injected with 1 mg/kg heparin or saline once or twice each day for 16 days (n = 7 or 8 per group).
(D) Representative body image (left) and quantitative magnetic resonance (QMR) (right) of male C57BL6/J mice treated with heparin or saline for 16 days.
(E) Quantification of fat mass of male C57BL6/J mice treated with heparin or saline for 16 days (n = 7 or 8 per group).
(F) Fat weight (brown adipose tissue [BAT], iWAT, and gonadal white adipose tissue [gWAT]) of male C57BL6/J mice treated with heparin or saline for 16 days (n = 7 or 8 per group).
(G and H) Representative images (G) and quantification (H) of iWAT H&E staining from male C57BL6/J mice treated with heparin or saline for 16 days (n = 5 per group).
(I–K) Body weight gain (I), cumulative food intake (J), and feed efficiency (K) of female C57BL6/J mice i.p. injected with 1 mg/kg body weight (BW) heparin or saline once or twice each day (n = 7 or 8 per group).
(L) Representative body image (left) and QMR (right) of female C57BL6/J mice treated with heparin or saline for 16 days.
(M) Quantification of fat mass of female C57BL6/J mice treated with heparin or saline for 16 days (n = 7 or 8 per group).
(N) Fat weight (BAT, iWAT, and gWAT) of female C57BL6/J mice treated with heparin or saline for 16 days (n = 7 or 8 per group).
(O and P) Representative images (O) and quantification (P) of iWAT H&E staining from female C57BL6/J mice treated with heparin or saline for 16 days (n = 5 per group).
Results are presented as mean ± SEM. In (A)–(C) and (I)–(K), *p ≤ 0.05, **p ≤ 0.01, and ***p ≤ 0.001 by two-way ANOVA followed by post hoc Bonferroni tests. In (E), (F), (H), (M), (N), and (P), *p ≤ 0.05 and **p ≤ 0.01 by non-paired Student’s t test. See also Figure S2.
Similar to heparin-treated male mice, heparin-treated female mice gained significantly more body weight (Figures 2I and 2L) and fat mass after 16 days of treatment (Figure 2M). Consistently, the size and weight of iWAT were significantly elevated after heparin treatment compared with controls (Figures 2N–2P). Additionally, increased cumulative food intake (Figure 2J) and feed efficiency (Figure 2K) were also observed after heparin treatment. Collectively, these data indicate that, similar to males, heparin treatment led to early-onset increases in body weight in female mice, associated with increased food intake and reduced energy expenditure.
Heparin Activates AgRP Neurons in the Arcuate Nucleus of the Hypothalamus
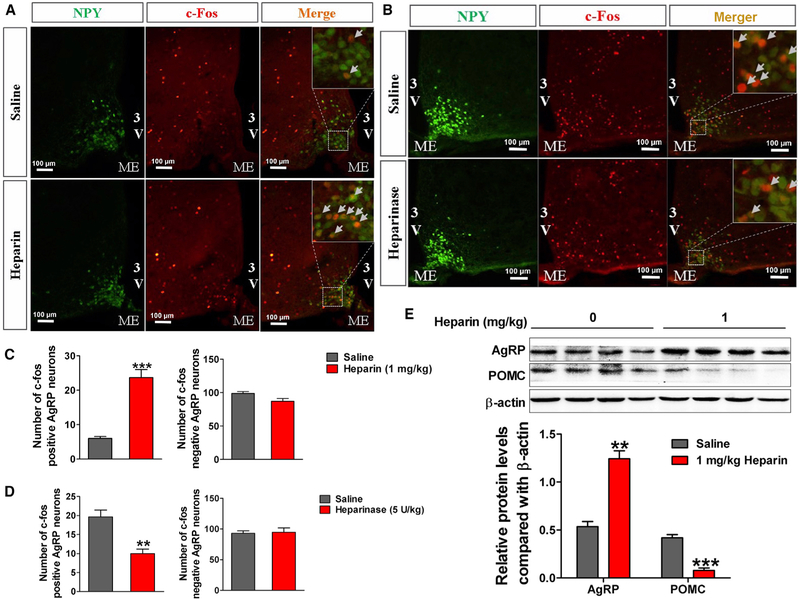
On the basis of observations that central administration of heparin induces orexigenic effects, we postulated that a central appetite-regulatory mechanism plays a role in the orexigenic effects of heparin. We asked whether AgRP neurons in the hypothalamus are regulated by heparin signals. We performed c-Fos immunofluorescence in neuropeptide Y (NPY)-GFP mice, in which NPY/AgRP neurons are labeled with GFP. We found that heparin induced an increase of c-Fos expression in NPY/AgRP neurons in the arcuate nucleus of the hypothalamus (ARH) (Figures 3A and 3C), whereas heparinase, the heparin-degrading enzyme, significantly decreased c-Fos expression in ARH NPY/AgRP neurons in fasted mice (Figures 3B and 3D). These data suggest that heparin activates AgRP neurons in the ARH and that fasting-induced activation of AgRP neurons is partially mediated by endogenous heparin. Additionally, we also observed that i.v. injection of heparin dose-dependently increased AgRP mRNA expression and decreased POMC mRNA expression in the hypothalamus (Figure S2A). Consistently, both i.v. and i.p. injection of heparin stimulated hypothalamic AgRP and NPY protein expression and inhibited hypothalamic POMC protein expression (Figures S2B and 3E). These observations indicate that heparin activates ARH AgRP neurons, the well-recognized orexigenic neuro-modulator (Aponte et al., 2011).
Figure 3. Heparin Increases AgRP Signal In Vivo.
(A and C) Representative images (A) and quantification (C) of c-Fos expression in the ARH of NPY-GFP mice 3 hr after i.p injection of 1 mg/kg heparin or saline.
(B and D) Representative images (B) and quantification (D) of c-Fos expression in the ARH of NPY-GFP mice 3 hr after i.p injection of 5 U/kg heparinase or saline.
(E) Immunoblots and quantification of AgRP and POMC protein expression in the hypothalamus of male C57BL6/J mice 3 hr after i.p injection of 1 mg/kg heparin or saline.
Results are presented as mean ± SEM. **p ≤ 0.01 and ***p ≤ 0.001 by non-paired Student’s t test.
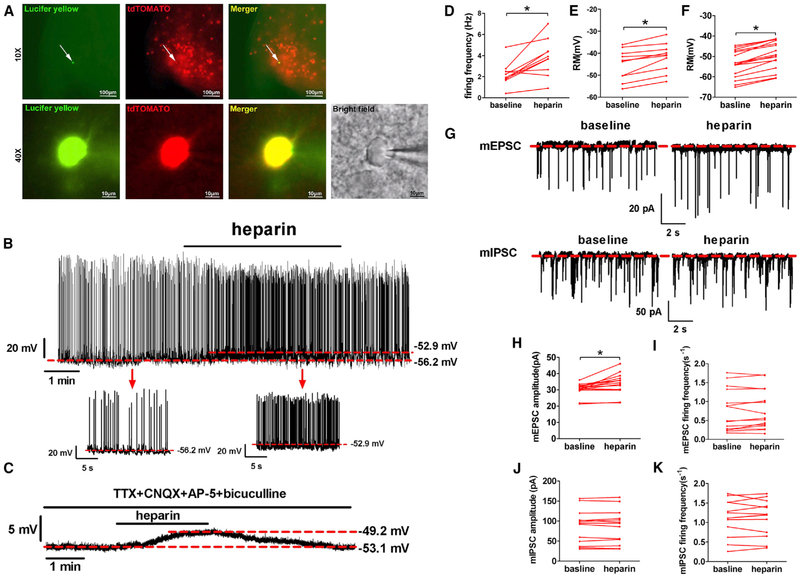
In order to support this model, we used electrophysiology to examine the effects of heparin on the neural activities of ARH AgRP neurons. To this end, we crossed the Rosa26-tdTOAMTO allele onto AgRP-Cre mice to selectively label all AgRP neurons with red tdTOMATO fluorescence. Using these mice, we recorded electrophysiological responses to heparin (100 μg/mL, 4 min) in identified ARH AgRP neurons (Figure 4A). We found that heparin caused depolarization from rest in all ten recorded AgRP neurons (−45.26 ± 2.21 versus −41.90 ± 2.06 mV, p ≤ 0.05; Figures 4B and 4E), while nine of ten recorded AgRP neurons responded to heparin with an increased firing rate (2.25 ± 0.35 versus 3.85 ± 0.55 Hz, p ≤ 0.05; Figures 4B and 4D). To block presynaptic inputs from afferent neurons, tetrodotoxin (TTX; a potent sodium channel blocker), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; a competitive glutamate AMPA receptor antagonist), 2-amino-5-phosphopentanoic acid (AP-5; a glutamate NMDA antagonist), and bicuculline (a competitive GABAA receptor antagonist) were added to the bath solution. In the presence of these blockers, heparin (100 μg/mL) treatment still depolarized all recorded AgRP neurons (−53.68 ± 6.65 versus −49.38 ± 6.83 mV, n = 16; Figures 4C and 4F), suggesting a direct stimulatory effect of heparin on AgRP neurons. This proposition was further supported by our observations on miniature excitatory postsynaptic current (mEPSC) and miniature inhibitory postsynaptic current (mIPSC) of AgRP neurons after heparin treatment. We found that heparin application increased the amplitude of spontaneous mEPSC without changing its frequency (Figures 4G–4I), suggesting a postsynaptic rather than a presynaptic mechanism of stimulation. Additionally, no AgRP neurons showed any changes of spontaneous mIPSC after heparin treatment (Figures 4G, 4J, and 4K), which is consistent with the stimulatory effects of heparin on AgRP neurons. Together, these data consistently indicate that heparin directly activates AgRP neurons in the ARH.
Figure 4. Heparin Activates AgRP Neurons Ex Vivo.
(A) Recording from a TOMATO-labeled AgRP/NPY neuron from AgRP-Cre/Rosa26-tdTOMATO mouse (top middle); lucifer yellow dye was injected into the recorded neuron for post hoc verification (top left) and merge (top right). Scale bars represent 100 μm. Low magnification of lucifer yellow (bottom left), td-TOMATO (bottom first middle), merge (bottom second middle), and bright field (bottom right). Scale bars represent 10 μm.
(B–F) Representative trace and statistic of AgRP neurons responding to heparin (100 μg/mL) in the absence (B, D, and E) or presence (C and F)of 1 μM TTX, 30 mM CNQX, 30 μM AP-5, and 50 μM bicuculline.
(G–K) Representative mEPSC and mIPSC trace (G) and statistics (H–K) of AgRP neurons responding to heparin (100 μg/mL).
Results are presented as mean ± SEM. *p ≤ 0.05 by non-paired Student’s t test.
AgRP Neurons Mediate the Orexigenic Effects of Heparin
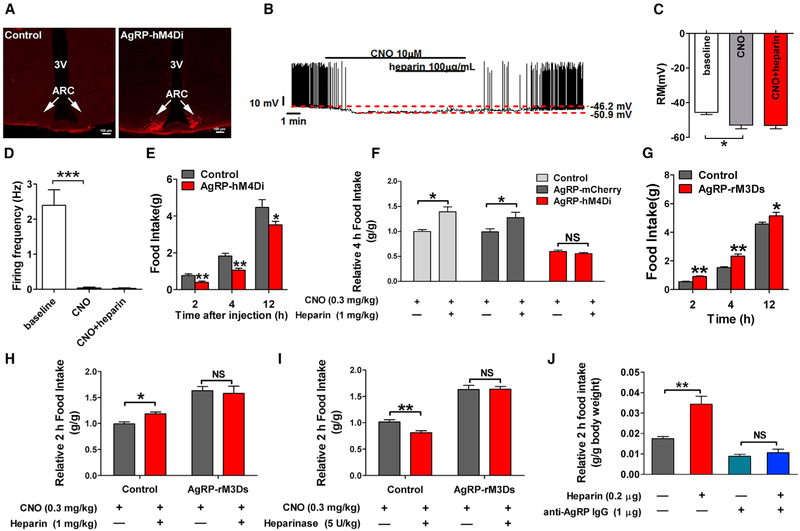
To directly test if AgRP neurons mediate the stimulatory effects of heparin on food intake, we used DREADD technology to specifically inhibit AgRP neurons in functional mice. Specifically, we generated AgRP-hM4Di mice by stereotaxically delivering Cre-dependent inhibitory DREADD virus (pAAV-hSyn-DIOhM4D[Gi]-mCherry) into the ARH regions of AgRP-Cre mice (Figure 5A). The CNO-driven activation of hM4Di hyperpolarized membrane potential and decreased the firing rate of recorded AgRP neurons and blocked heparin (100 μg/mL)-induced activation of AgRP neurons (Figures 5B–5D). CNO-mediated inhibition of AgRP neurons significantly reduced food intake compared with control mice without hM4Di expression treated with CNO (Figure 5E), consistent with previous observations on orexigenic effects of AgRP neurons (Krashes et al., 2011). Importantly, DREADD-induced inhibition of AgRP neurons totally blocked acute heparin-induced feeding (Figure 5F), suggesting a mediating role of AgRP neurons in the orexigenic effects of heparin. High-dose heparin treatment was also tested in our AgRP inhibition model. We found that a 10-fold higher heparin dose (1 mg/mL) failed to reverse the inhibitory effects of CNO on membrane potential and firing frequency of recorded AgRP neurons (Figures S3A–S3C), suggesting a dominant inhibitory effect of CNO. After CNO was washed out, the stimulatory effects of heparin (1 mg/mL) were regained, as demonstrated by an increased firing rate and an elevated membrane potential. Consistent with electrophysiological recording, the orexigenic effects of high-dose heparin (3 mg/kg) were also abolished by CNO-induced AgRP inhibition (Figure S3D).
Figure 5. AgRP Neurons Mediate Orexigenic Effects of Heparin.
(A) mCherry fluorescence after injection of inhibitory AAV-hM4Di-mCherry into the ARH of WT or AgRP-Cre mice.
(B) Representative electrophysiological response to CNO (10 μM, bath) and heparin (100 μg/mL) in ARH AgRP neurons infected with inhibitory AAV-hM4Di-mCherry.
(C and D) Statistics of resting membrane potential (C) and firing frequency (D).
(E) Effects of CNO (0.3 mg/kg, i.p.) on dark-cycle food intake measured in male WT or AgRP-Cre mice receiving inhibitory AAV-hM4Di-mCherry infection in the ARH (n = 10 or 6 per group).
(F) Effects of CNO (0.3 mg/kg) co-injected with saline or heparin (1 mg/kg) on dark-cycle food intake measured in male WT or AgRP-Cre mice receiving inhibitory AAV-hM4Di-mCherry infection in the ARC. Another group of AgRP-Cre mice were injected with AAV-DIO-mCherry to serve as another control group (n = 10, 6, or 6 per group).
(G) Effects of CNO (0.3 mg/kg, i.p.) on dark-cycle food intake measured in male WT or AgRP-Cre mice receiving AAV-rM3D(Gs)-mCherry infection in the ARH (n = 6 or 5 per group).
(H) Effects of CNO (0.3 mg/kg) co-injected with saline or heparin (1 mg/kg) on dark-cycle food intake measured in male WT or AgRP-Cre mice receiving AAV-rM3D(Gs)-mCherry infection in the ARH (n = 6 or 5 per group).
(I) Effects of CNO (0.3 mg/kg) co-injected with saline or heparinase (5 U/kg) on dark-cycle food intake measured in male WT or AgRP-Cre mice receiving AAV-rM3D(Gs)-mCherry infection in the ARH (n = 6 or 5 per group).
(J) Dark-cycle food intake of female C57BL6/J mice after i.c.v. injection of saline, 0.2 μg heparin, 1 μg anti-AgRP IgG, or heparin + anti-AgRP IgG (0.2 μg + 1 μg) (n = 7 or 8 per group).
Results are presented as mean ± SEM. *p ≤ 0.05 and **p ≤ 0.01 by one-or two-way ANOVA followed by post hoc Bonferroni tests. See also Figure S3.
To further test if AgRP release is essential for orexigenic effects of heparin, we selectively expressed a Gs-linked DREADD in the AgRP neurons (AgRP-rM3Ds). Previous studies have shown that the activation of this receptor in AgRP neurons exclusively increases the release of AgRP to stimulate food intake (Nakajima et al., 2016). Consistent with those results, we found that CNO-mediated activation of rM3Ds substantially increased food intake (Figure 5G). More important, both the orexigenic effects of heparin (Figure 5H) and the anorexigenic effects of heparinase (Figure 5I) were abolished by CNO-mediated activation of rM3Ds in AgRP neurons, indicating that the release of AgRP is required to mediate the orexigenic effects of heparin. To further support this point of view, we co-injected heparin-treated (0.2 μg, i.c.v.) mice with an anti-AgRP antibody (1 μg, i.c.v.). Strikingly, the stimulatory effects of heparin on food intake were completely blocked (Figure 5J). Taken together, our results demonstrate that AgRP neuron activation and AgRP release are required for the orexigenic effects of heparin.
Heparin Promotes Feeding by Binding to the Insulin Receptor
We used hypothalamic N38 cells as a model to explore the molecular mechanism for heparin’s actions in neurons. Consistent with previous in vivo observations, we found that in vitro 12 hr heparin treatment dose-dependently increased the mRNA and protein levels of AgRP in N38 cells (Figures S4A and S4B), while in vitro high-dose heparin treatment (100 μg/mL) time-dependently increased both the protein expression and secretion of AgRP (Figure S4C). These results indicate that heparin treatment in N38 cells exerts the same stimulatory effects on AgRP expression and secretion as in mice, which validated N38 cells as a model to study heparin’s effects on AgRP neurons.
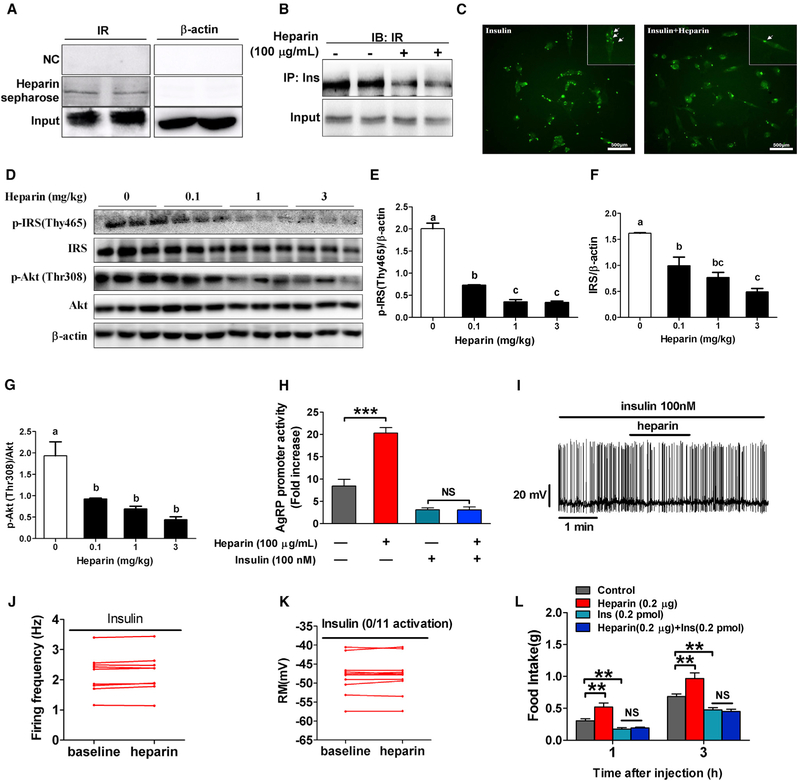
To examine how heparin interacts with the AgRP neuron membrane, we performed heparin-Sepharose chromatography to screen for the proteins or receptors that bind with heparin on the membrane. We found that heparin interacts with insulin receptor (Figure 6A), which is the primary mediating receptor for the anorexigenic effects of insulin in the brain (Brüning et al., 2000). Importantly, after heparin treatment, we found that the insulin-insulin receptor interaction was significantly reduced (Figure 6B) and that insulin binding on the membrane was also attenuated (Figure 6C), suggesting a disruption of insulin action due to heparin binding to the insulin receptor. Consistent with this view, heparin dose-dependently inhibited insulin receptor substrate 1 (IRS-1) and phosphorylation of IRS-1 and protein kinase B (Akt; insulin receptor downstream events) after 12 hr of heparin treatment (Figures S5A–S5D). A sustaining suppression of IRS-1 and phosphorylation of IRS-1 and Akt was also detected with high-dose heparin treatment (Figures S5E–S5H). More important, similar dose-dependent inhibitory effects on hypothalamic IRS-1 and phosphorylation of IRS-1 and Akt were observed in heparin-treated mice (Figures 6D–6G). These findings imply that heparin competes with insulin for insulin receptor binding and disrupts the downstream insulin signaling pathways.
Figure 6. Heparin Promotes AgRP Activity and Feeding by Competing for Insulin Binding to the Insulin Receptor.
(A) Interaction between heparin and insulin receptor (IR) in N38 cells.
(B) Heparin inhibits insulin-IR interaction in N38 cells.
(C) Representative immunocytofluorescent images of insulin binding in N38 cells cultured with vehicle or 100 μg/mL heparin for 3 hr and subsequently cultured with 100 nM insulin for 30 min.
(D–G) Immunoblots (D) and quantification of p-IRS (E), IRS (F), and p-Akt/Akt (G) protein expression in the hypothalamus of male C57BL6/J mice after i.p. injection of saline or 0.1, 1, or 3 mg/kg heparin (n = 6 per group).
(H) Relative luciferase activity driven by Agrp promoter (FOXO1 binding fragment)in N38 cells cultured with vehicle (control), 100 μg/mL heparin, 100 nM insulin, or 100 μg/mL heparin + 100 nM insulin for 3 hr (n = 6 per group).
(I-K) Representative trace (I) and statistics (J and K) of AgRP neurons responding to heparin (100 μg/mL) in the presence of 100 nM insulin.
(L) Dark-cycle food intake of female C57BL6/J mice after i.c.v. injection of saline, 0.2 μg heparin, 2 μL 100 nM insulin, or heparin + insulin (n = 5 or 6 per group). Results are presented as mean ± SEM. In (E)–(G), different letters between bars indicate p ≤ 0.05 by one-way ANOVA followed by post hoc Tukey’s tests. In (H) and (L), *p ≤ 0.05, **p ≤ 0.01, and ***p ≤ 0.001 by two-way ANOVA followed by post hoc Bonferroni tests. See also Figures S4–S6.
On the basis of previous observations that blockade of hypothalamic insulin receptors produces hyperphagia and weight gain (Messier and Teutenberg, 2005), we predicted that heparin promotes AgRP release and food intake through an insulin receptor-dependent pathway. Consistent with this view, we found that heparin significantly increased AgRP promoter activity in N38 cells, and this effect was abolished by insulin treatment (Figure 6H). Electrophysiological recording of AgRP neurons also demonstrated that the stimulatory effects of heparin (100 μg/mL) on firing rate and membrane potential were abolished by co-incubation with insulin (100 nM) (Figures 6I–6K). Importantly, our in vivo data showed that insulin diminished the increase of c-Fos expression in AgRP neurons induced by heparin (Figures S6A and S6B), suggesting a blockade of AgRP activation by insulin. This mechanism of appetite regulation is further supported by evidence that the orexigenic effect of heparin (0.2 μg, i.c.v.) in mice was blocked by co-injection of 0.2 pmol insulin (Figure 6L), while a high dose of heparin (2 μg) attenuated the anorexigenic response of insulin (Figure S5I). Hence, heparin increases food intake by competing with insulin for insulin receptor binding on AgRP neurons.
Heparin Promotes AgRP Expression and Secretion by FoxO1
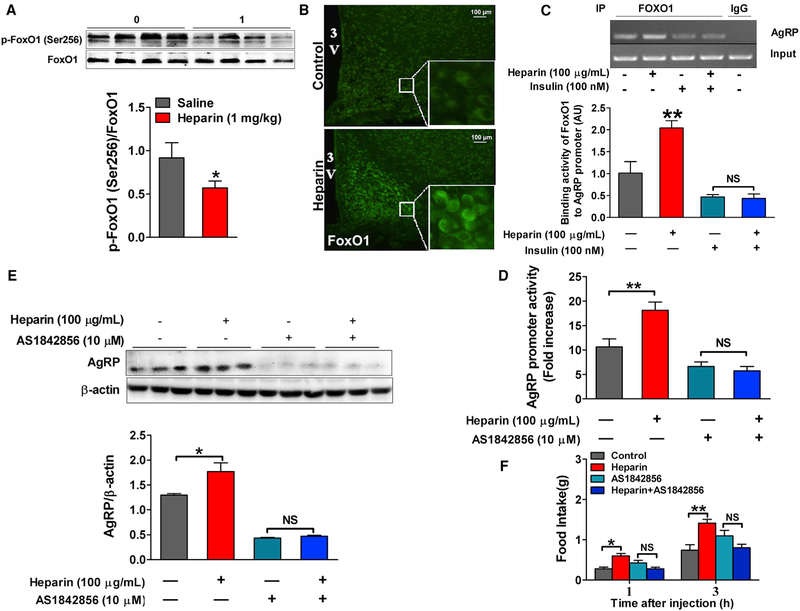
On the basis of previous reports that FoxO1 transcription factor is highly expressed in AgRP neurons and represents a key intracellular component of pathways integrating AgRP-mediated food intake and peripheral metabolic signals, including insulin and leptin (Kitamura et al., 2006; Ren et al., 2012, 2015), we examined whether FoxO1 is the intracellular mediator for stimulatory effects of heparin on AgRP neurons. We showed that heparin treatment for 3 hr inhibited phosphorylation of FoxO1 in N38 cells in a dose-dependent manner (Figure S7A). The high dose of heparin had a sustaining suppression effect on FoxO1 phosphorylation after 1 hr incubation (Figure S7B). Additionally, we also showed that heparin induced nuclear translocation of FoxO1 (Figure S7C). Consistently, long-term heparin in vivo treatment (16 days) inhibited phosphorylation and enhanced nuclei shuttling of FoxO1 in the hypothalamic neurons from wild-type (WT) mice (Figures 7A and 7B). Given that decreased phosphorylation and nuclei shuttling of FoxO1 transcriptionally activate FoxO1 (Accili and Arden, 2004), and that activation of FoxO1 increases AgRP expression (Kitamura et al., 2006), these data support the hypothesis that heparin activates FoxO1 to stimulate AgRP expression.
Figure 7. Heparin Promotes AgRP Expression and Secretion by FoxO1.
(A) Immunoblots and quantification of p-FoxO1/FoxO1 protein expression in the hypothalamus of male C57BL6/J mice after 16 days of saline or 1 mg/kg heparin i.p. injection (n = 8 per group).
(B) Representative images of FoxO1 immunofluorescent staining (green) in the hypothalamus of male C57BL6/J mice after 16 days of saline or 1 mg/kg heparin i.p. injection.
(C) Use of chromatin immunoprecipitation (ChIP) assays to detect the binding activity of FoxO1 to AgRP promoter in N38 cells cultured with vehicle, 100 μg/mL heparin, 100 nM insulin, or heparin + insulin (100 μg/mL+100 nM) for 3 hr (n = 6 per group).
(D) Relative luciferase activity driven by Agrp promoter (FOXO1 binding fragment) in N38 cells cultured with vehicle (control), 100 μg/mL heparin, 10 μM AS1842856 (FoxO1 antagonist), or 100 μg/mL heparin + 10 μM AS1842856 for 3 hr (n = 6 per group).
(E) Immunoblots and quantification of AgRP protein expression in N38 cells cultured with vehicle, 100 μg/mL heparin, 10 μM AS1842856, or 100 μg/mL heparin + 10 μM AS1842856 for 12 hr (n = 6 per group).
(F) Dark-cycle food intake of female C57BL6/J mice after i.c.v. injection of saline, 0.2 μg heparin, 20 pmol AS1842856, or heparin + AS1842856 (0.2 μg + 20 pmol) (n = 6 per group).
Results are presented as mean ± SEM. *p ≤ 0.05 and **p ≤ 0.01 by two-way ANOVA followed by post hoc Bonferroni tests. See also Figure S7.
Supporting this hypothesis, we found that the binding activity of FoxO1 on the AgRP promotor was significantly increased by heparin treatment in N38 cells, and this effect was abolished by insulin treatment (Figure 7C), suggesting insulin-dependent regulatory effects of heparin on FoxO1. Furthermore, the results of luciferase assay demonstrated that heparin significantly increased AgRP promoter activity in N38 cells, and this stimulatory effect of heparin on the AgRP promoter was totally blocked by co-treatment with AS1842856, a FoxO1 inhibitor (Figure 7D). Consistent with this, AgRP protein expression was significantly increased by heparin treatment in N38 cells, while co-treatment of AS1842856 abolished this increase induced by heparin (Figure 7E). Importantly, our in vivo data also showed that the orexigenic effect of heparin (i.p.) in mice was diminished by coinjection of AS1842856 (Figure 7F), indicating a physiological role of FoxO1 in heparin’s orexigenic effects. Taken together, our observations suggest a model whereby heparin activates FoxO1 to stimulate AgRP expression, which in turn results in increased food intake.
DISCUSSION
Although heparin has been one of the most important anticoagulant and antithrombotic drugs in clinical practice for more than 80 years (McLean, 1959), it has many other pharmacological functions, including anti-inflammatory (Young, 2008) and antimetastatic (Borsig, 2007; Mousa et al., 2006) effects and promoting fat metabolism (Inderbitzin, 1955; Losonczy and Nagy, 1984; Roth et al., 1996; Stuchlíková et al., 1967), which are independent of its anticoagulant effects. There is great potential for the development of heparin-like compounds as drugs to treat other diseases.
On the basis of previous findings in both clinical and basic studies (Allman-Farinelli, 2011; Dager, 2010; Freeman et al., 2010; Jiang et al., 2011; Persson, 1988), we predict that heparin may play an important role in the regulation of energy homeostasis. We found that negative nutritional states caused by longterm CR or short-term overnight fasting induced an increase in serum heparin level, suggesting a physiologically relevant role for heparin in energy homeostasis regulation. This is further supported by the evidence that both peripheral and central heparin treatment acutely increased food intake in both male and female mice. A central mechanism was clearly demonstrated by the strong stimulatory effect of i.c.v. heparin treatment. However, a faster orexigenic response to i.p. treatment than to i.c.v. treatment in male mice suggests that there may be a peripheral mechanism. We observed rapid decreases in blood glucose and insulin levels after i.p. heparin injection, which may be peripheral mediators for orexigenic effects of heparin. Consistent with its suppression of insulin, heparin has been well known as a lipolytic factor (Persson, 1988) that interferes with the production or secretion of lipogenic hormones such as insulin. The inhibitory effects of heparin on blood glucose were also consistently demonstrated in early clinical studies performed in diabetic patients and healthy subjects (Bozadzhieva and Markovski, 1978). Decreased blood glucose and insulin may serve as peripheral signals to promote food intake.
In contrast to heparin, heparinase, a heparin cleavage enzyme, acutely decreased food intake in both sexes. Importantly, we also demonstrate that desulfated heparin, which lacks anticoagulant activity, increases food intake, whereas LMWH, with more efficient anticoagulant activity, does not affect food intake. These results suggest that the orexigenic effects of heparin are independent of its anticoagulant activity. Notably, long-term heparin treatment in male and female mice led to a phenotype reminiscent of that observed in heparanase global knockout mice, which have increased heparan sulfate (Karlsson-Lindahl et al., 2012), with an early-onset increase in body weight due to increased fat. Increased body weight was associated with increased food intake and decreased energy expenditure, as indicated by elevated feed efficiency and heat production. Thus, our data support a model in which heparin induces body weight gain by stimulating food intake and inhibiting energy expenditure through a central mechanism.
Heparin is a large mucopolysaccharide with a molecular weight of 12,000–15,000 g/mol, which does not cross the blood-brain barrier. However, circulating heparin may reach the ventromedial part of the ARH of the hypothalamus via the leaky blood-brain barrier and elicit its physiological functions (Faouzi et al., 2007; Rodríguez et al., 2010). On the basis of previous observations that the inhibitory effects of heparanase on food intake were abolished in melanocortin 4 receptor (MC4R) global knockout mice (Karlsson-Lindahl et al., 2012), a role for the MC4R neural pathway in the regulatory effects of heparanase on food intake has been suggested. As a first step to investigate the central mechanism of the regulatory effects of heparin on food intake, we asked whether heparin signals regulate the expression of two hypothalamic neuropeptides in the ARH, AgRP and POMC, which are well known as endogenous antagonists or agonist precursors of MC4R in the brain (Biebermann et al., 2012). We found that i.p. injection of heparin significantly increased AgRP and decreased POMC protein expression in the hypothalamus. Moreover, heparin enhanced c-Fos expression of AgRP neurons when mice were fed, while heparinase attenuated fasting-induced c-Fos expression in AgRP neurons, indicating that heparin may enhance AgRP expression by activating AgRP neurons. Consistent with this view is our finding that heparin electrophysiologically increased firing frequency and membrane potential of NPY/AgRP neurons. Importantly, these effects were completely independent of presynaptic inputs, suggesting a direct stimulatory action of heparin on AgRP neurons.
Given that heparin activates AgRP neurons, and that activation of AgRP neurons increases food intake (Aponte et al., 2011; Krashes et al., 2013), we postulate that heparin increases food intake by activating AgRP neurons. This hypothesis is supported by the observation that DREADD-induced inhibition of AgRP neurons blocked the orexigenic effects of heparin. On the basis of the observation that hypothalamic AgRP expression was stimulated by heparin treatment, we predicted that AgRP is an essential mediator for the orexigenic effects of heparin. Consistent with this hypothesis, anorexigenic effects of heparinase were abolished by chemogenetic increasing of AgRP release from AgRP neurons by using Gs-linked DREADD. On the other hand, we also found that heparin failed to further increase feeding after artificial stimulation of AgRP release. Besides this chemogenetic evidence, we also pharmacologically demonstrated that stimulatory effects of heparin on food intake were diminished by anti-AgRP antibody. Taken together, the orexigenic effects of heparin are at least partially mediated by stimulating AgRP neurons to increase the release of AgRP.
As important central regulators of energy homeostasis, AgRP neurons release three different mediators, inducing the neuropeptides NPY and AgRP and the fast-acting neurotransmitter GABA to regulate feeding and other aspects of energy balance (Liu et al., 2013). Changes in AgRP neuron activity lead to alterations in the release of these three mediators. Previously, neuropeptide AgRP was shown to induce feeding over a 2 hr delayed yet prolonged period (Krashes et al., 2013). This slow-acting orexigenic effect of AgRP is in conflict with the acute feeding response (within 2 hr) induced by heparin, suggesting that another fast-acting mechanism for heparin’s orexigenic effects also exists. This acute effect of heparin may involve NPY or GABA release from AgRP neurons. Supporting this, we found that heparin acutely stimulates AgRP neurons, which may lead to alterations in the release of not only AgRP but also NPY and GABA. Additionally, i.v. injection of heparin increased not only AgRP but also NPY protein in the hypothalamus.
In order to explore the molecular mechanism of how heparin interacts with AgRP neurons to increase AgRP release, we screened for the physiologically relevant heparin-binding proteins or receptors in hypothalamic cell lines. We found that heparin interacts with insulin receptor and competes with insulin for insulin receptor binding, and by doing so, it decreases phosphorylation of downstream mediators, including IRS-1 and Akt, suggesting a disruption of the insulin signaling pathway. We also confirmed this disruption in the mouse hypothalamus after i.p. heparin treatment. Insulin, an anorectic hormone, has been shown to regulate feeding and body weight mainly through inhibition of AgRP and activation of POMC (Qiu et al., 2014; Varela and Horvath, 2012). This result prompted us to examine whether heparin promotes AgRP release and feeding by blockade of insulin receptors on AgRP neurons. We found that heparin increases the promotor activity of AgRP gene, which is consistent with the stimulatory effects of heparin on AgRP protein expression. Importantly, this increase is blocked by insulin treatment, suggesting an insulin receptor-dependent pathway. Moreover, central treatment of insulin significantly decrease food intake, as previously reported (Brown et al., 2006), and orexigenic effects of heparin were totally diminished by co-injection with insulin. Thus, our observations indicate that heparin promotes feeding by binding with insulin receptors.
FoxO1, a forkhead family member of transcription factors, represents a shared component of pathways integrating central AgRP and POMC actions on food intake and peripheral signals such as leptin and insulin (Kitamura et al., 2006; Ren et al., 2012, 2015). On the basis of these reports, we postulate that FoxO1 may also play an important role in integrating peripheral heparin signal and central AgRP-mediated feeding. Consistent with this view, we found that heparin decreased phosphorylation and increased nuclei shuttling of FoxO1 both in vitro and in vivo, suggesting a stimulatory effect on the FoxO1 signaling pathway. Because activation of FoxO1 is required for fasting-induced AgRP expression (Kitamura et al., 2006), FoxO1 may mediate heparin’s stimulatory effects on AgRP. This is further supported by the evidence that heparin increased the binding activity of FoxO1 on the AgRP promoter and that this increase was blocked by insulin, suggesting that heparin regulates FoxO1 transcriptional activity on the AgRP promoter through an insulin receptor-dependent mechanism. Moreover, the stimulatory effects of heparin on AgRP promoter activity, AgRP protein expression, and food intake were abolished by AS1842856, an antagonist of FoxO1, indicating a physiologically integrating pathway involving FoxO1 for the AgRP-dependent effects of heparin on food intake.
In conclusion, we report for the first time that heparin, previously well recognized as an anticoagulant and antithrombotic drug, plays an important role in the regulation of food intake and energy homeostasis. We further demonstrate that the orexigenic effects of heparin are driven by insulin receptor-FoxO1-mediated AgRP activation. Our findings identify heparin as a potential drug target for body weight regulation, and additional studies are warranted to assess the effects of heparin-based compounds on body weight control and energy homeostasis.
EXPERIMENTAL PROCEDURES
Animals
All groups within one experiment contained individual mice of the same strain and sex, similar in body weight and age. All mice were aged 10–18 weeks at the time when the experiments were performed. Mice were housed in a temperature-controlled environment (20°C–26°C) on a 12 hr light/12 hr dark cycle (6 a.m. and 6 p.m.). Unless otherwise stated, the mice were maintained ad libitum on standard mouse chow and water.
Metabolic Effects of Heparin
Short-term food intake was measured after i.p., i.v., ori.c.v. injection of heparin in both male and female adult mice. Long-term body weight gain and food intake were measured every other day after i.p. injection of 1 mg/kg heparin in both sexes. Body composition, fat mass, and adipocyte size were determine after 16 days of heparin treatment.
Effect of Heparin in Regulation of AgRP Neurons
Expression of c-Fos by AgRP neurons and hypothalamic AgRP mRNA and protein expression were measured after i.p. injection of 1 mg/kg heparin in male adult mice. Direct electrophysiological responses of AgRP neurons to heparin were determined ex vivo. AgRP neurons were chemogenetically inhibited or activated to determine if AgRP neurons mediate the orexigenic effects of heparin in male adult mice.
Cellular Signal Mechanisms of Heparin-Induced AgRP Activation
Heparin’s effects on insulin-insulin receptor interaction were determined in both in vitro N38 cells and invivo in the hypothalamus of male adult mice. Electrophysiological responses of AgRP neurons to heparin and insulin co-treatment were determined ex vivo. Feeding behavior was monitored after heparin (0.2 μg) and insulin (0.2 pmol) i.c.v. co-treatment in female adult mice.
Heparin’s effects on phosphorylation and nuclear translocation of FoxO1 were determined in both in vitro N38 cells and in vivo in the hypothalamus of male adult mice. Feeding behavior was monitored after heparin (0.2 μg) and AS1842856 (20 pmol, a FoxO1 inhibitor) i.c.v. co-treatment in female adult mice.
Statistics
Statistical analyses were performed using GraphPad Prism. Methods of statistical analyses were chosen on the basis of the design of each experiment and are indicated in the figure legends. Data are presented as mean ± SEM. A p value ≤ 0.05 was considered to indicate statistical significance.
Study Approval
Care of all animals and procedures at South China Agricultural University conformed to the Regulations for the Administration of Affairs Concerning Experimental Animals of the State Council of the People’s Republic of China and were approved by the Animal Subjects Committee of South China Agricultural University. Care of all animals and procedures at Baylor College of Medicine were approved by the Baylor College of Medicine Institutional Animal Care and Use Committee.
Supplementary Material
Highlights.
Heparin increases food intake and body weight in both male and female mice
Heparin promotes feeding by stimulating AgRP neurons and increasing AgRP release
Heparin competes with insulin for insulin receptor binding on AgRP neurons
Orexigenic effects of heparin are driven by FoxO1-mediated AgRP release
ACKNOWLEDGMENTS
This work was supported by grants from the National Natural Science Foundation of China (31572480 to G.S., 31472105 to Q.J.), the National Basic Research Program of China (2013CB127306 to G.S., 2013CB127304 to Q.J.), the Excellent Young Teachers Training Program Foundation of Guangdong Province to G.S., the NIH (R01DK093587 and R01DK101379 to Y.X., K99DK107008 to P.X.), the U.S. Department of Agriculture/Current Research Information System (3092-5-001-059 to Y.X.), the American Diabetes Association (1-17-PDF-138 to Y.H.), and the American Heart Association (17GRNT32960003 to Y.X.). We wish to thank Dr. Yukari Ido Kitamura and Dr. Tadahiro Kitamura for providing plasmids from Addgene, and we thank the Laboratory of Animal Center of South China Agricultural University and Baylor College of Medicine for invaluable help in mouse colony maintenance.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures and seven figures and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2017.08.049.
REFERENCES
- Accili D, and Arden KC (2004). FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell 117, 421–426. [DOI] [PubMed] [Google Scholar]
- Allman-Farinelli MA (2011). Obesity and venous thrombosis: a review. Semin. Thromb. Hemost 37, 903–907. [DOI] [PubMed] [Google Scholar]
- Aponte Y, Atasoy D, and Sternson SM (2011).AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat. Neurosci 14, 351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biebermann H, Kühnen P, Kleinau G, and Krude H (2012). The neuroendocrine circuitry controlled by POMC, MSH, and AGRP. Handb. Exp. Pharmacol (203), 47–75.. [DOI] [PubMed] [Google Scholar]
- Bloom MB, Zaw AA, Hoang DM, Mason R, Alban RF, Chung R, Melo N, Volod O, Ley EJ, and Margulies DR (2016). Body mass index strongly impacts the diagnosis and incidence of heparin-induced thrombocytopenia in the surgical intensive care unit. J. Trauma Acute Care Surg 80, 398–403. [DOI] [PubMed] [Google Scholar]
- Borsig L (2007). Antimetastatic activities of modified heparins: selectin inhibition by heparin attenuates metastasis. Semin. Thromb. Hemost 33, 540–546. [DOI] [PubMed] [Google Scholar]
- Bozadzhieva EK, and Markovski SG (1978). [Effect of heparin, heparin derivatives and heparinoids on blood sugar levels of diabetes mellitus patients]. Probl. Endokrinol. (Mosk) 24, 23–30. [PubMed] [Google Scholar]
- Brown LM, Clegg DJ, Benoit SC, and Woods SC (2006). Intraventricular insulin and leptin reduce food intake and body weight in C57BL/6J mice. Physiol. Behav 89, 687–691. [DOI] [PubMed] [Google Scholar]
- Brüuning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Müller-Wieland D, and Kahn CR (2000). Role of brain insulin receptor in control of body weight and reproduction. Science 289, 2122–2125. [DOI] [PubMed] [Google Scholar]
- Dager WE (2010). Issues in assessing and reducing the risk for venous thromboembolism. Am. J. Health Syst. Pharm 67 (10, Suppl 6), S9–S16. [DOI] [PubMed] [Google Scholar]
- Faouzi M, Leshan R, Björnholm M, Hennessey T, Jones J, and Münzberg H (2007). Differential accessibility of circulating leptin to individual hypothalamic sites. Endocrinology 148, 5414–5423. [DOI] [PubMed] [Google Scholar]
- Flengsrud R (2016). Disaccharide analysis of chondroitin and heparin from farmed Atlantic salmon. Glycoconj. J 33, 121–123. [DOI] [PubMed] [Google Scholar]
- Freeman AL, Pendleton RC, and Rondina MT (2010). Prevention of venous thromboembolism in obesity. Expert Rev. Cardiovasc. Ther 8, 1711–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovingh P, and Linker A (1982). An unusual heparan sulfate isolated from lobsters (Homarus americanus). J. Biol. Chem 257, 9840–9844. [PubMed] [Google Scholar]
- Hurewitz AN, Khan SU, Groth ML, Patrick PA, and Brand DA (2011). Dosing of unfractionated heparin in obese patients with venous thromboembolism. J. Gen. Intern. Med 26, 487–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inderbitzin T (1955). [Experiments with fat metabolism; influence of heparin and other high molecule substances]. Schweiz. Med. Wochenschr 85, 675–682. [PubMed] [Google Scholar]
- Jiang Z, Michal JJ, Wu XL, Pan Z, and MacNeil MD (2011). The heparan and heparin metabolism pathway is involved in regulation of fatty acid composition. Int. J. Biol. Sci 7, 659–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson-Lindahl L, Schmidt L, Haage D, Hansson C, Taube M, Egecioglu E, Tan YX, Admyre T, Jansson JO, Vlodavsky I, et al. (2012). Heparanase affects food intake and regulates energy balance in mice. PLoS ONE 7, e34313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Feng Y, Kitamura YI, Chua SC Jr., Xu AW, Barsh GS, Rossetti L, and Accili D (2006). Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nat. Med 12, 534–540. [DOI] [PubMed] [Google Scholar]
- Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, and Lowell BB (2011). Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Invest 121, 1424–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Shah BP, Koda S, and Lowell BB (2013). Rapid versus delayed stimulation of feeding by the endogenously released AgRP neuron mediators GABA, NPY, and AgRP. Cell Metab. 18, 588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Wang Q, Berglund ED, and Tong Q (2013). Action of neurotransmitter: a key to unlock the AgRP neuron feeding circuit. Front. Neurosci 6,200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losonczy H, and Nagy I (1984). The effects of various doses and various applications of heparin on the fat metabolism and on blood coagulation. Wien. Klin. Wochenschr 96, 885–889. [PubMed] [Google Scholar]
- McLean J (1959). The discovery of heparin. Circulation 19, 75–78. [DOI] [PubMed] [Google Scholar]
- Messier C, and Teutenberg K (2005). The role of insulin, insulin growth factor, and insulin-degrading enzyme in brain aging and Alzheimer’s disease. Neural Plast. 12,311–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousa SA, Linhardt R, Francis JL, and Amirkhosravi A (2006). Anti-metastatic effect of a non-anticoagulant low-molecular-weight heparin versus the standard low-molecular-weight heparin, enoxaparin. Thromb. Haemost 96, 816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myzienski AE, Lutz MF, and Smythe MA (2010). Unfractionated heparin dosing for venous thromboembolism in morbidly obese patients: case report and review of the literature. Pharmacotherapy 30, 324. [DOI] [PubMed] [Google Scholar]
- Nader HB, Chavante SF, dos-Santos EA, Oliveira TW, de-Paiva JF, Jeronimo SM, Medeiros GF, de-Abreu LR, Leite EL, de-Sousa-Filho JF, et al. (1999). Heparan sulfates and heparins: similar compounds performing the same functions in vertebrates and invertebrates? Braz. J. Med. Biol. Res 32, 529–538. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Cui Z, Li C, Meister J, Cui Y, Fu O, Smith AS, Jain S, Lowell BB, Krashes MJ, and Wess J (2016). Gs-coupled GPCR signalling in AgRP neurons triggers sustained increase in food intake. Nat. Commun 7, 10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pejler G, Danielsson A, Björk I, Lindahl U, Nader HB, and Dietrich CP (1987). Structure and antithrombin-binding properties of heparin isolated from the clams Anomalocardia brasiliana and Tivela mactroides. J. Biol. Chem 262, 11413–11421. [PubMed] [Google Scholar]
- Persson E (1988). Lipoprotein lipase, hepatic lipase and plasma lipolytic activity. Effects of heparin and a low molecular weight heparin fragment (Fragmin). Acta Med. Scand. Suppl 724, 1–56. [PubMed] [Google Scholar]
- Qiu J, Zhang C, Borgquist A, Nestor CC, Smith AW, Bosch MA, Ku S, Wagner EJ, Rønnekleiv OK, and Kelly MJ (2014). Insulin excites anorexigenic proopiomelanocortin neurons via activation of canonical transient receptor potential channels. Cell Metab. 19, 682–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizes O, Lincecum J, Wang Z, Goldberger O, Huang L, Kaksonen M, Ahima R, Hinkes MT, Barsh GS, Rauvala H, and Bernfield M (2001). Transgenic expression of syndecan-1 uncovers a physiological control of feeding behavior by syndecan-3. Cell 106, 105–116. [DOI] [PubMed] [Google Scholar]
- Ren H, Orozco IJ, Su Y, Suyama S, Gutiérrez-Juárez R, Horvath TL, Wardlaw SL, Plum L, Arancio O, and Accili D (2012). FoxO1 target Gpr17 activates AgRP neurons to regulate food intake. Cell 149, 1314–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H, Cook JR, Kon N, and Accili D (2015). Gpr17 in AgRP neurons regulates feeding and sensitivity to insulin and leptin. Diabetes 64, 3670–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez EM, Blázquez JL, and Guerra M (2010). The design of barriers in the hypothalamus allows the median eminence and the arcuate nucleus to enjoy private milieus: the former opens to the portal blood and the latter to the cerebrospinal fluid. Peptides 31, 757–776. [DOI] [PubMed] [Google Scholar]
- Roth B, Ekelund M, Fan BG, Ekstrom U, and Nilsson-Ehle P (1996). Effects of heparin and low molecular weight heparin on lipid transport during parenteral feeding in the rat. Acta Anaesthesiol. Scand 40, 102–111. [DOI] [PubMed] [Google Scholar]
- Strader AD, Reizes O, Woods SC, Benoit SC, and Seeley RJ (2004). Mice lacking the syndecan-3 gene are resistant to diet-induced obesity. J. Clin. Invest 114, 1354–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuchliková E, Hrůskova J, Hmza Z, Jelínková M, and Soukupová K (1967). [Fat metabolism and aging. 3. The effect of heparin on the intermediary metabolism in relation to obesity and age]. Sb. Lek 69, 154–160. [PubMed] [Google Scholar]
- Varela L, and Horvath TL (2012). Leptin and insulin pathways in POMC and AgRP neurons that modulate energy balance and glucose homeostasis. EMBO Rep. 13, 1079–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young E (2008). The anti-inflammatory effects of heparin and related compounds. Thromb. Res 122, 743–752. [DOI] [PubMed] [Google Scholar]
- Zcharia E, Metzger S, Chajek-Shaul T, Aingorn H, Elkin M, Friedmann Y, Weinstein T, Li JP, Lindahl U, and Vlodavsky I (2004). Transgenic expression of mammalian heparanase uncovers physiological functions of heparan sulfate in tissue morphogenesis, vascularization, and feeding behavior. FASEB J. 18, 252–263. [DOI] [PubMed] [Google Scholar]
- Zcharia E, Jia J, Zhang X, Baraz L, Lindahl U, Peretz T, Vlodavsky I, and Li JP (2009). Newly generated heparanase knock-out mice unravel coregulation of heparanase and matrix metalloproteinases. PLoS ONE 4, e5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.