Abstract
Flexible biosensors form part of a rapidly growing research field that take advantage of a multidisciplinary approach involving materials, fabrication and design strategies to be able to function at biological interfaces that may be soft, intrinsically curvy, irregular, or elastic. Numerous exciting advancements are being proposed and developed each year towards applications in healthcare, fundamental biomedical research, food safety and environmental monitoring. In order to place these developments in perspective, this review is intended to present an overview on field of flexible biosensor development. We endeavor to show how this subset of the broader field of flexible and wearable devices presents unique characteristics inherent in their design. Initially, a discussion on the structure of flexible biosensors is presented to address the critical issues specific to their design. We then summarize the different materials as substrates that can resist mechanical deformation while retaining their function of the bioreceptors and active elements. Several examples of flexible biosensors are presented based on the different environments in which they may be deployed or on the basis of targeted biological analytes. Challenges and future perspectives pertinent to the current and future stages of development are presented. Through these summaries and discussion, this review is expected to provide insights towards a systematic and fundamental understanding for the fabrication and utilization of flexible biosensors, as well as inspire and improve designs for smart and effective devices in the future.
Keywords: flexible, biosensor, epidermal sensor, implantable sensor
1. Introduction
Flexible, stretchable, and wearable sensors and biosensors for point-of-care (POC) testing form part of a broad, rapidly expanding multi-billion dollar market and research focus in the area of portable devices (IDTechEx 2017a, b, c). They represent powerful tools towards the monitoring and diagnosis of personal healthcare parameters (Karnaushenko et al. 2015; Schwartz et al. 2013; Yu et al. 2018), with additional applications in food safety (Shu et al. 2018b; Tao et al. 2012), environmental monitoring (Van Dorst et al. 2010), and veterinary settings (Sun et al. 2015; Yamakawa et al. 2014). The driving force for the advancement of such devices stems from their appeal as being able to operate by being directly attached to a system – either externally (e.g. to human skin, or even surfaces of fruits and vegetables) or internally (e.g. on soft tissue) to deliver accurate, reliable and real-time measurement of physiological parameters or biomarkers (Fernandez et al. 2015; Liu et al. 2016; Ochoa et al. 2014). In various embodiments, they can form intimate interfaces with intrinsically curved and soft surfaces, resulting in enhanced sample capture efficiency and signal transduction. Such sensors therefore possess properties including, but not limited to, mobility (implantable or wearable), light weight, biocompatibility, and ability to be noninvasive. Because flexible biosensors can locally track indicators of health vitals, infections, or disease, they are of critical value in the design of continuous and multiplexed sensing tools, as well as form components of larger customized personal health monitoring systems (Karnaushenko et al. 2015; Khan et al. 2016; Viventi et al. 2011). In comparison to traditional laboratory-based or rigid testing techniques, flexible biosensors are typically designed with the goals of not requiring complex infrastructure, while satisfying the demands of end-users for self or ambulatory testing.
In recent years, a number of excellent reviews have been published on this extensive and growing area of fundamental and applied research. From a broad perspective, these have discussed aspects of the flexible market space such as wearable devices, functional textiles, electronic skins, and flexible bio and optoelectronics (Bandodkar and Wang 2014; Benight et al. 2013; Feiner and Dvir 2018; Kim et al. 2012a; Wang et al. 2017a; Wang et al. 2017c). In the area of sensors, currently, many portable and/or wearable devices (which may not be flexible) are focused on the measurement and sensing of biophysical parameters (Heikenfeld et al. 2018).
These include temperature, pH, heart rate, blood/intraocular pressure, neural signals, and air quality (Bonato 2010; Schwartz et al. 2013). Areas of flexible electronics which include consumer products, handhelds, wearable computing, and monitoring of health vitals are also discussed (Khan et al. 2016; Rajan et al. 2018). A smaller, albeit growing subset of flexible devices lies is in the area of biochemical sensing wherein the system either comprises of a biological element for detection (e.g. an antibody, aptamer, enzyme etc.), or is used to detect specific biological or chemical analytes of interest (Ray and Joseph 2013). These can include targets such as small chemical molecules (e.g. glucose (Li et al. 2007), ascorbic acid (Cai et al. 2014), lactate (Qianwei et al. 2017), dopamine (K.P.O et al. 2018)), biomacromolecules (e.g. protein biomarkers (Kamakoti et al. 2016), DNA/RNA (Jung et al. 2014)), and even whole cells. In contrast to the measurement of physical parameters in flexible bio and optoelectronic settings, the fabrication of such biochemical devices is accompanied by unique challenges. These stem primarily from the incorporation of biorecognition elements, as well as their operational environments. Both biorecognition elements and their supports need to be optimized within specific frameworks in order to maintain transport of the target, and provide consistent signal transduction. To detect precise targets of interest at a physico-biological interface which can be soft and delicate, or have curvilinear surfaces and complex geometry, new materials and modalities of detection need to be developed or harnessed to perform a monitoring function without interrupting the normal activity of the sampling site (e.g. tissues, organs, plants) (Lee et al. 2016b; Salvatore et al. 2014; Tan et al. 2008).
Here, we present a comprehensive review that illustrate the development and function of flexible biosensors. This review intends to summarize general design principles, and recent, noteworthy systems targeting biological parameters and biomarkers. It is important to note that we will not discuss the detection/measurement of (bio) physical parameters, or the broader field of flexible electronic devices, for which the reader is referred to the excellent reviews discussed above. Our focus is on the use of diverse materials and fabrication techniques to fabricate the core components (substrates or active areas) for the detection of biological or chemical analytes. The common classes of materials that have been used to fabricate flexible biosensors are discussed regarding their advantages and selection. We discuss the environments where flexible biosensors are deployed, as well as potential targets of interest. Important details and issues in the design and fabrication of flexible biosensors are addressed to illuminate the critical features that can greatly affect their performance. Finally, the perspectives and challenges in this field are discussed. It should be clear that as a subset of the larger thematic area, such systems may share several design principles and ideas in common, or where broadly designed wearables may provide inspiration for biosensor development. Through this review, trends in the development of flexible and stretchable biosensors are expected to be illustrated, leading to new and innovative ideas.
2. The structure of flexible biosensors
As with their counterparts in “rigid” configurations, “flexible” biosensors generally have three components: (A) a substrate which forms the primary mechanical support for the entire system; (B) bioreceptor(s) specific to the analyte(s) of interest, and (C) an active material(s) to transduce the signal from the bioreceptor(s), depending on the mechanism of detection. The signals reflecting the parameter of interest are then converted, typically by software, into a readable interface by the human operator. The essential elements of the biosensors, particularly in a flexible configuration are shown in Figure 1. A brief summary of components and representative studies on mechanical flexibility are shown in Table 1. Typically, biointerfaces where flexible biosensors are designed to operate tend to be non-planar, soft, rough, or even non-stationary. Some areas of intended use are shown in Figure 2. They represent sites that are either already, or are being, targeted for the detection of specific biochemical targets. The critical design aspect is therefore that the substrate (A) needs to possess one or more of the following qualities – mechanical flexibility, bendability, stretchability, thermal/chemical stability, transparency, biocompatibility, and biodegradability (Han et al. 2017; Wang et al. 2016; Yang et al. 2017a). Note that a single system may possess one or more of these properties, and often all of them. The substrate (A) provides the fundamental exo-skeleton of the device - offering solid support, contact with the surface, holding the components together, and maintaining stable performance. Concurrently, the recognition element (B) and transduction (C) need to be integrated on the substrate, as well as be stable and amenable to the same mechanical conformations without being delaminated or removed as a result of deformation. The next section discusses some of the materials and design strategies used in flexible sensors.
Figure 1 –
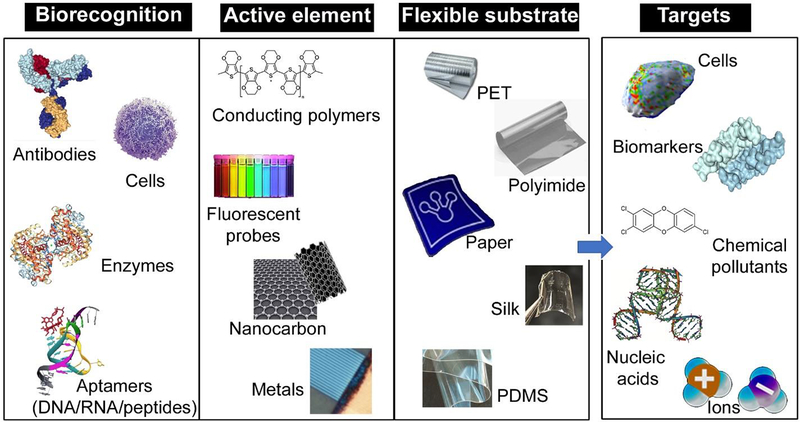
Schematic showing the essential elements of biosensors in flexible configurations.
Table 1.
Examples of flexible biosensors showing the different components and applications of the systems
| Substrate (A) | Recognition (B) | Transduction Method (C) | Target | Flexibility | Reference |
|---|---|---|---|---|---|
| PET | anti-CRP antibody | Capacitive | C-reactive protein (CRP) | Not demonstrated | (Sivashankar et al. 2015) |
| Graphite | Potentiometric | Uric acid, ascorbic acid, dopamine | 0.4% tensile strain 9.7 MPa tensile strength | (Cai et al.) | |
| Graphene oxide | Potentiometric FET | Dopamine from PC12 cells | Bending insensitive (3 cm bend radius) | (He et al.) | |
| Glucose oxidase | Potentiometric FET | Glucose | 0.01 change in signal from straight to bent (6.625 cm radius) | (Kwak et al.) | |
| Lactate oxidase | Amperometric | Lactate | Bending angle (0–180) Bending cycles (0-100) | (Labroo and Cui) | |
| Ag nanoparticles | SERS | Thiram | In-situ detection on tomato skin | (Zuo et al. 2016) | |
| Polyimide | Anti-HSA antibody | Impedimetric | Human serum albumin | Physically bend | (Chang et al. 2013) |
| Glucose oxidase | Potentiometric | Glucose | Operational above 837 μm bend radius 0.078% strain | (Rim et al.) | |
| Anti-human TNF-α / TNFS F1A antibody | Impedimetric | Tumor necrosis factor-α (TNF-α) | Not demonstrated | (Baraket et al. 2014) | |
| PEN | HIV-2 antigen | Amperometric | HIV | No change >17mm bend radius <5% change in response after 100 bend cycles | (Kwon et al.) |
| PDMS | Silver NPs | SERS | Malachite Green (MG) | no difference w/ 100° angle bend | (Kumar et al.) |
| Glucose oxidase | Amperometric | Ascorbic acid, dopamine, or glucose | Very small change w/ 90° angle bend | (Pal et al. 2018) | |
| Polyester | anti-CD4 antibodies | Optical | CD4+ T lymphocyte | Intrinsically flexible; no bending challenge | (Shafiee et al. 2015) |
| CNF | Cholesterol oxidase | Potentiometric | Cholesterol | 5% error after 90° bend | (Bajaj et al. 2016) |
| Carbon Electrode Arrays | None | Amperometric | H2O2, NADH | 180° inward folding and stretching had negligible effects on response | (Yang et al.) |
| Paper | anti-CEA antibody | Amperometric | Carcinoembryonic antigen (CEA) | No breakage after ± 180° bend tests | (Bansi et al. 2016) |
| anti-haptoglobin antibody | Colorimetric | Bovine haptoglobin | Origami foldable | (Weng et al. 2018) | |
| anti-PSA antibody | Voltammetric | prostate protein antigen | 3D origami fold | (Li et al. 2014b) | |
| Graphene | anti-E. coli antibody | Impedimetric | E. coli O157:H7 | 5% changes after 90° bend for 500 cycles | (Wang et al. 2013) |
| HBsAgbinding aptamer | Amperometric FET | hepatitis B surface antigen (HBsAg) | 5–10% change until 200 bend cycles, improved until 500 bend cycles | (Cho et al. 2018) | |
| Pt NPs | Amperometric | Hydrogen Peroxide (H2O2) | 5% decrease sensor response after 100 bending cycles | (Xiao et al.) | |
| Textile/ Fabric | Redox active molecules | Amperometric | Adrenaline, Ascorbic acid, dopamine | 7.5mm bending radius showed no change in response | (Gualandi et al.) |
| Lactate oxidase | Amperometric | Lactate | <13% when bended, <11% when twisted | (Modali et al. 2016) | |
| None | Amperometric | NADH, ferrocyanide, H2O2 | Minimal effect of stretching on NADH and ferrocyanide, improved signal from stretching for H2O2 | (Yang et al. 2010) | |
| Silk | Glucose oxidase | Amperometric | H2O2 | 30–180° bend angle and 1000 bend times; 5–25% stretch | (Liang et al. 2014) |
| Glucose oxidase | Amperometric | Ascorbic acid, dopamine, or glucose | 30° or 150 cycles bend showed slight decrease in conductivity | (Pal et al. 2016b) |
Figure 2 –
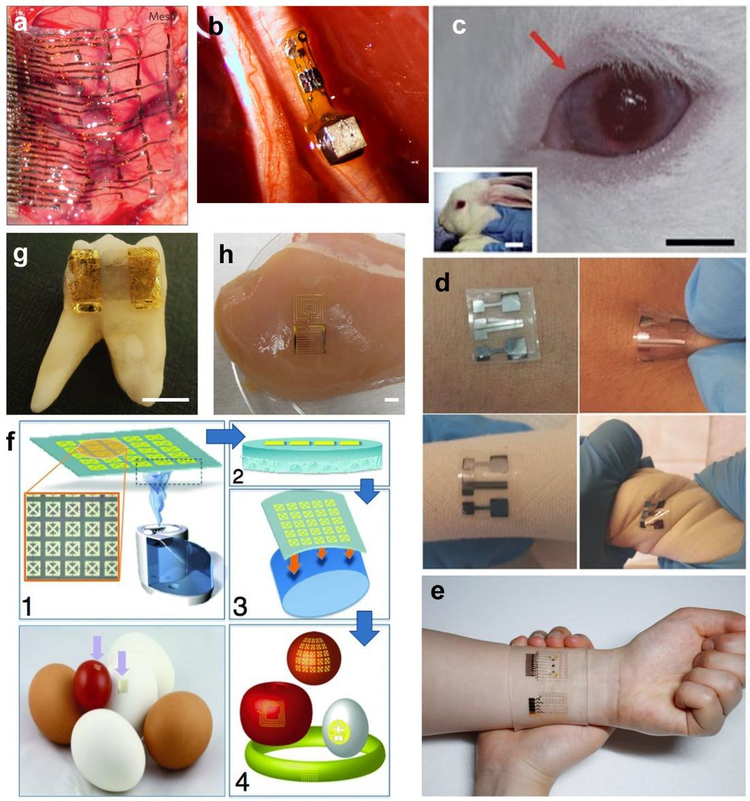
Examples of locations where flexible biosensors may be or are already being used. Clockwise from top: a) soft tissue such as the brain Adapted with permission from ref.(Kim et al. 2010). Copyright 2010 Nature Publishing Group. b) nerve fibers Adapted with permission from ref. (Seo et al. 2016). Copyright 2016 Cell Press. c) eyes/cornea (as optical implants or soft contact lenses) Adapted with permission from ref. (Kim et al. 2017). Copyright 2017 Nature Publishing Group. d, e) skin and other flexible environments, Adapted with permission from ref.(Liao et al. 2015a). Copyright 2014 Wiley. Adapted with permission from ref. (Lee et al. 2016a) Copyright 2016 Nature Publishing Group. f) surfaces of fruits and agricultural products Adapted with permission from ref. (Tao et al. 2012). Copyright 2012 Wiley. g) Enamel, h) muscle tissue. Adapted with permission from ref. (Mannoor et al. 2012). Copyright 2012 Nature Publishing Group.
3. Materials and configurations
Substrate selection forms one of the key steps of device fabrication as it provides the foundation on which to construct the biosensor. The resulting properties and functionalities are in turn, dependent on what the substrate can support (Khan et al. 2015; Windmiller and Wang 2013). By definition, mechanical flexibility is a primary characteristic, as it provides a mechanism to fit the physical dynamics of inherently non-linear, and often, non-rigid environments, thereby increasing the interaction between analytes and sensing/transducing elements. For instance, a substrate that conforms more the contours of the surface is more likely to provide a large contact area for sample collection such as body fluids. While primarily designed to broaden the scope of biomolecular detection, such devices can often also be used in combination with added functionalities (e.g. drug delivery) (Wang et al. 2016). Material selection may be initially based on the location of intended use (e.g. internal or external to the biological system). Figure 3 shows a few examples of the demands placed on the flexible devices based on their location. These can range from mechanical deformations to surface conformability. To impart characteristics of bendability, stretchability, conformability, fracture resistance, and wear resistance, two approaches are generally used: (1) select and synthesize intrinsically flexible and stretchable materials with good inherent or supplemented electronic characteristics, or (2) use rigid conductive or semi-conductive materials, and impart flexible attributes via specific geometrical or structural designs (e.g. serpentine or wave patterns) (Rogers et al. 2010; Wang et al. 2015), or pre-stressing (Zhu and Moran-Mirabal 2016)). It should be noted that while some of the materials and strategies below have been primarily reported for broader flexible devices, they constitute general design concepts that may be, or have already been adapted to flexible biosensors. Initially, we present a brief discussion on some of the characteristics of inherently flexible substrate materials.
Figure 3. Representative mechanical challenges in the design of flexible biosensors.
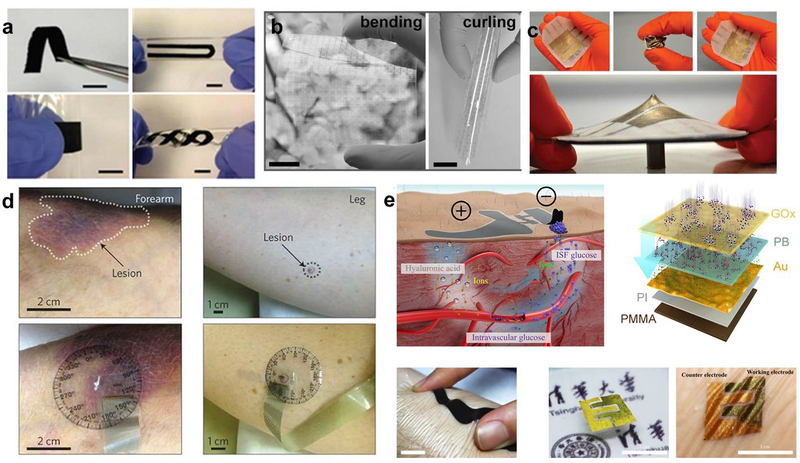
The top row reflects the idea of mechanical stress/strain (e.g. bending, stretching, rolling, twisting, curling, crumpling or even sharp indentation). a) Adapted with permission from ref. (Zhong et al. 2015). Copyright 2012 American Chemical Society. b) Adapted with permission from ref.(Yang et al. 2017b). Copyright 2012 Wiley. c) Adapted with permission from ref.(Vandeparre et al. 2013). Copyright 2013 AIP Publishing. The bottom row represents ideas of conformability and compatibility to a very delicate environment (e.g. a lesion or wound) and skin. d) Adapted with permission from ref.(Dagdeviren et al. 2015). Copyright 2015 Nature Publishing Group. e) Adapted with permission from ref.(Chen et al. 2017). Open Access.
3.1. Flexible Substrate Materials
3.1.1. Synthetic Polymers
Synthetic polymers are currently among the most commonly used substrates owing to their versatility and processability, which enable the formation of flexible architectures at low cost and high efficiency. Polyethylene terephthalate (PET), Polyethylene naphthalate (PEN), polyimide (PI), and polydimethylsiloxane (PDMS) are some of the well-studied polymers used to fabricate flexible substrates used in biosensing platforms (Lau et al. 2013; Liao et al. 2015b; Segev-Bar and Haick 2013). PET is a synthetic polyester fiber typically used in clothing, plastic containers, and as an electric insulator (Reddish 1950). Favorable characteristics including mechanical properties, inertness, thermal stability and low cost have made it a candidate to replace silicon substrates (Ahani et al. 2011). The polymer is often pressed into ultra-thin films to create optically transparent, high-contact surfaces which imparts versatility and functionality in conforming to different shapes and structures (MacDonald 2004). PEN (commercially available as Teonex®) is a synthetic polyester that has slightly better intrinsic properties (chemical and hydrolytic resistance, thermal and thermo-oxidative resistance, and ultraviolet (UV) resistance) that make it suitable as a plastic substrate (Murakami et al. 1995). PEN also provides better optical transparency and oxygen barrier giving it an edge for superior performance in optical devices. Although more rigid than PET, PEN can confer flexibility due to its intrinsic bendability (Barlow et al. 2002; Lechat et al. 2006). Both PET and PEN are often used to layer functional components such as metal coatings, metal oxides, conducting polymers, and nanoparticles to form thin structures that can easily conform to biological surfaces (Vidor et al. 2015; Zhao et al. 2007). The advancement of technologies such as screen printing has also increased their use by making it easier to incorporate fabricated ultrathin microelectrodes and other microcontact devices (Mościcki et al. 2017).
Polyimides (PI) are well known in industrial applications (one commercially available form is Kapton®) as flexible polymer fibers that can bend, fold, and be molded with relative ease. They exhibit good thermal stability, dynamic tensile strength, and excellent dielectric and structural stability. These materials are flexible and easy to manipulate, and modified to exhibit different yet unique functionalities. PIs have been modified to become photosensitive (Liaw et al. 2012) or can be metal deposited to enhance conductivity. They have also been applied in bio-electronics as substrates for neural implants, as fuel cells, and other devices by making thin film and double layer films (Xiao et al. 2008). As low cost flexible materials with high biocompatibility and efficiency, PIs are attractive substrates to form biosensors. (Lee et al. 2004).
Polydimethylsiloxane (PDMS) is a silicone-based elastomer that is well known for its use in soft lithography and microfluidics (Qin et al. 2010). PDMS is widely used in biomedical devices, incorporated into catheters, membrane oxygenators, and cartilage implants. The rapid expansion in the use of PDMS devices can be attributed to low cost, chemical inertness, thermal stability, and oxygen permeability (Mata et al. 2005). As a flexible substrate, PDMS is highly preferred due to these properties, coupled with high elasticity, low modulus, and optical transparency giving it an edge over most other polymer-based flexible substrates. As an oxygen permeable, biocompatible, and nontoxic material, it is suitable for in vitro and in vivo applications (Patrito et al. 2007). Other materials and particles can also be incorporated with PDMS to form complex systems such as lab-on-a-chip (Klemic et al. 2002) and implantable electrodes (SadAbadi et al. 2013).
3.1.2. Paper
Paper is an attractive and inexpensive semi-natural/semi-synthetic substrate to develop rapid POC diagnostics, particularly in resource constrained areas. Paper is intrinsically flexible, ubiquitous and readily available (Chinnasamy et al. 2014; Martinez et al. 2010). It is often used as a substrate for devices either by itself, or as paper-based structures. Paper substrates can support a variety of sensing modalities from optical, electrical, and electrochemical when detecting biotargets (Parolo and Merkoci 2013; Qiu et al. 2017). For example, optical transparency in paper can be obtained by decreasing the diameter of cellulose fibers from μm to nm (Bin et al. 2017). Combined with light weight and a relatively thin and porous architecture, paper can be easily formed into composites. Processing techniques including screen-printing, inkjet printing, nanopatterning, and others, have made it easier to fabricate paper-based electrodes with multilayer structures (Siegel et al. 2010). Since they absorb via capillary action, paper substrates can be used to form lateral flow assay technologies, similar to pregnancy test strips for the detection of human chorionic gonadotrophin (Choi et al. 2016a). For instance, there has been interest in developing rapid paper-assays for nucleic acid testing (Ngom et al. 2010).
While the mechanical properties of paper are easily tunable by manipulating individual cellulose fibers, it can also be fragile and easily susceptible to tearing, with a low stability in wet environments. Composites of paper with other biomaterials have been suggested to form bioactive paper. For example, cellulose fibers and nanofibers (CNFs) can be functionalized with strengthening polymers such as glyoxalated polyacrylamides (GPAM) and/or polyamideepichlorohydrin (PAE) to give a stronger and long-lasting effect of wet-strength (Pelton 2009). Since cellulose is intrinsically not degraded, strategies have been proposed to make cellulose fibers more biodegradable (Jung et al. 2015). As a synthetic material and relatively nonconductive platform, there is often a need for labeling for biomolecules, as well as doping with conducting polymers or metal oxides to provide electrochemical capabilities (Pelton 2009). Due to its poor conductivity, paper may be modified (often chemically) by pretreating to remove additives and coating with a conductive layer or other electroactive materials, and/or integrating with soft metal foils to support its application (Bin et al. 2017). Various forms of nanocellulose such as cellulose nanocrystals (CNCs), nanofibrillated cellulose (NFCs) and bacterial nanocellulose (BNCs) have also been proposed as substrates for biosensors as biological and environmentally benign materials.(Golmohammadi et al. 2017)
3.1.3. Textiles and fibers
Textiles form a broad spectrum of novel materials for flexible biosensing and can be fabricated as fibers (1D), woven, knitted, or fashioned into other non-woven (2D) structures to provide a solid support for the active sensing area (Li et al. 2014a; Stoppa and Chiolerio 2014; Windmiller and Wang 2013). These materials, including wool, cotton, or synthetics (nylon, polyester, etc.), are good candidates due to intrinsic flexibility, durability, and stability. Additionally, textile substrates can be integrated with conductive materials using various fabrication techniques. For example, fabrics have been drop coated or soaked with conductive polymers to produce conductive filaments (Ding et al. 2010). Conductive yarns can also be prepared by incorporating textiles during the process of polymerization (Akşit et al. 2009). The migrated electrical/electrochemical systems on textile substrates can fill the gap between powerful and possibly multiplexed electroanalytical devices, while satisfying demands of daily even heavy usage, with traits like light weight, convenience, integrated functions, simple operation, low-cost, and real-time display.
For use with wearable garments, stability in extended wash cycles is an added challenge. Thick-film amperometric biosensors on the elastic waistband of garments were shown using screen-printing carbon-based ink, followed by thermal curing to form textile-based carbon electrodes (TCEs) (Yang et al. 2010). These electrodes endure severe mechanical deformation without visible cracking or peeling. The electrochemical performance of the TCEs showed excellent consistency after repetitive bending or stretching, and were able to detect 0–25 mM of H2O2 and 0–100 μM of NADH towards glucose sensing and sweat monitoring. Besides conventional electrochemical biosensors, conductive textiles have been incorporated into the fabrication of transistors. A flexible OECT was fabricated by screen-printing PEDOT:PSS on the textile substrate (Gualandi et al. 2016). The OECT showed a sheet resistance ~40 Ω cm−1, lower than that of other reported PEDOT-coated yarn-based OECTs or the pristine textile (Coppede et al. 2014; Hamedi et al. 2007). An OECT based on two parallel rectangular strips working as the channel and the gate electrodes, was used to detect adrenaline, ascorbic acid, and dopamine in artificial sweat. The fabricated textile OECTs exhibited no obvious degradation of electrical performance after repetitive deformation or hand-washing cycles, demonstrating its utility as a promising platform to construct wearable biosensors for daily use.
3.1.4. Metals
Thin metallic foils have also been used as flexible substrates. While they can be useful in biosensors, their use has been more widespread in traditional electronics (Huang et al. 2011). Metal foils (from materials such as stainless steel, titanium, molybdenum, and copper) can meet the needs of high thermal stability for roll-to-roll processing down to ~0.05 mm thickness or below (Liao et al. 2012). At this level, the foils can easily bend, while still providing the superior conductivity that metal substrates are known for (Wenmin et al. 2001). In addition, flexible foil substrates are advantageous as they can be formed as large or as small as desired, while allowing for tunability in their functionality through layering or deposition of other metals (Mathew et al. 2003). Although not common in the fabrication of flexible biosensors, metallic foils have shown their potential as substrates to make solar cells and thin-film transistors (Howell et al. 2000; Park et al. 2003). Mechanically, metallic foil substrates are more durable to external bending and compression than stretching (Gleskova et al. 2002). The problems of using metallic foils as substrates in flexible biosensors are obvious. First, the production of metallic foils is still more expensive in comparison to plastic foil substrates such as PEN or PI. Moreover, the deposition of functional electronic patterns on metallic foil substrates is commonly a high temperature process. In addition to being energy and time intensive, such processes are typically not compatible with fragile bioreceptor molecules (Howell et al. 2000; Park et al. 2003). Finally, metallic foil substrates are not bio-degradable, in comparison to other alternatives for biosensing applications.
3.1.5. Bioderived materials
Bio-derived materials have received recent consideration for green and sustainable bioelectronics, owing to their characteristics of being biocompatible and biodegradable (Aizenberg and Fratzl 2009; Irimia-Vladu 2014; Muskovich and Bettinger 2012). Natural “inspiration” to design or fabricate flexible devices has also been discussed in excellent reviews (Fratzl and Barth 2009; Liu et al. 2017). Various naturally derived biomaterials have been proposed as substrates for flexible devices such as silk proteins, polysaccharides and gelatin. Silk is a naturally occurring biopolymer that is a viable supporting substrate as a film or a scaffold, or as an organic component of synthetically fabricated electronics (Jin et al. 2005; Meinel et al. 2004). It is mechanically strong as a textile, while offering excellent surface area and optical transparency as thin and ultrathin films (Jiang et al. 2007; Lawrence et al. 2008). Silk proteins can be fabricated into fibers, gels, films and sheets to meet the need of multiple biomedical applications (Altman et al. 2003). These films are mechanically flexible and tunable, as well as easily functionalized with bioreceptors (Kim et al. 2009; Lawrence et al. 2008; Li et al. 2011a). They can be combined with synthetic polymers (Liu et al. 2014), or mixed with conducting polymer inks to be used as flexible biosensors (Pal et al. 2016b). Other bio-derived materials such as chitin and chitosan have been reported for electrochemical biosensing as components, substrates, or surface modifiers for thin film electrodes (Suginta et al. 2013). The natural plant dye indigo was used to form an ambipolar organic field-effect transistor (OFET) on thin spin-coated shellac sheets. Shellac itself is a natural bio adhesive polymer composed of aliphatic and alicyclic hydroxy acids, and can be considered a natural, degradable plastic.(Irimia-Vladu et al. 2012)
3.2. Configurations
An interesting strategy to impart flexibility in devices is through the use of rational design, whereby even materials with a high moduli and low flexibility in bulk can be used.(Rogers et al. 2010) This can be in the form of using shape changes (e.g. wavy, buckled or wrinkled architectures (Benight et al. 2013; Bowden et al. 1998)) to relieve strain, or by the use of specific nanoscale architectures or designs (e.g. serpentine, helix or fractal (Fan et al. 2014; Wang et al. 2015)) to facilitate bending and/or stretchability. With the advancement of nanofabrication techniques, rigid or stiff organic or inorganic materials like silicon, metals, and quartz, can be engineered to form ultra-thin films and circuits by using extremely thin (nanoscale) structures. Techniques such as origami and kirigami for making paper art, also provide inspiration for routes to structural platforms in engineering applications. Conceptually related schemes in cutting, folding, and buckling can be used in the construction of devices such as stretchable/conformable electronics and micro/nanoscale biosensors (Ning et al. 2018). In all, these approaches permit the use of highly conductive materials such as Ag, Cu and Au which can retain their intrinsic characteristics, while being able to sustain mechanical deformation (e.g. bending, rolling, twisting) (Kim et al. 2012a; Rogers et al. 2010). While many of these configurations have been primarily used for flexible bioelectronics and biophysical sensors, to date there have been few reports in which such systems have been adapted specifically for the purposes of biosensing of chemical and biological analytes. In particular, the immobilization of biorecognition molecules in such systems has to be demonstrated. The reader is referred to a number of excellent recent reviews that discuss these designs and shapes.(Liu et al. 2017; Wang et al. 2017c)
3.3. Bioreceptors
Just like their rigid/nonflexible counterparts, most flexible biosensors may incorporate biological receptors to facilitate specific analyte detection. While label-free detection is often sought in some cases, most biosensors utilize biologically derived recognition entities coupled to transducers to allow the quantitative measurement of biochemical parameters. Enzymes, aptamers, antibodies, polysaccharides, etc. are among the various biomacromolecules used in such sensors (Figure 1, Table 1) (Luo et al. 2018; Lv et al. 2018; Mohanty and Kougianos 2006; Shu et al. 2018a). An important consideration is that the biomolecule does not delaminate or leach out from the substrate during mechanical challenge. Often, this is difficult because the ability of the bioreceptor to directly interact with the analyte of interest must be balanced by consideration of stability. The flexible substrate must therefore be modified and functionalized for the appropriate attachment to ensure functionality and longevity. These can include direct immobilization via covalent or non-covalent bonding, using different linkage chemistries (e.g. biotin-streptavidin), or dispersal within a matrix that is in turn, attached to the substrate (e.g. conductive ink) (Kergoat et al. 2012). A few representative strategies to achieve such immobilization are shown in Figure 4. Besides the inherent benefits of the material itself, compatibility with the bioreceptor is a very important factor as this dictates the sensitivity and specificity of the device as well as the magnitude of the sensor response. Typically ultrathin layers allow for simple integration of small biomolecules through chemical adsorption (Chaki and Vijayamohanan 2002). As such, enzymes are currently the most favorable bioreceptor for flexible biosensing owing to their use in electrochemical detection, strong affinity, high specificity, and low-cost (Staiano et al. 2017). In order to be well integrated with flexible substrates, bioreceptors must also have strong stability (Wang et al. 2014b). In subsequent sections, we discuss more specific bioreceptor and attachment methods used in different flexible sensors.
Figure 4. Representative strategies for the immobilization of biomolecules for flexible biosensing applications a).
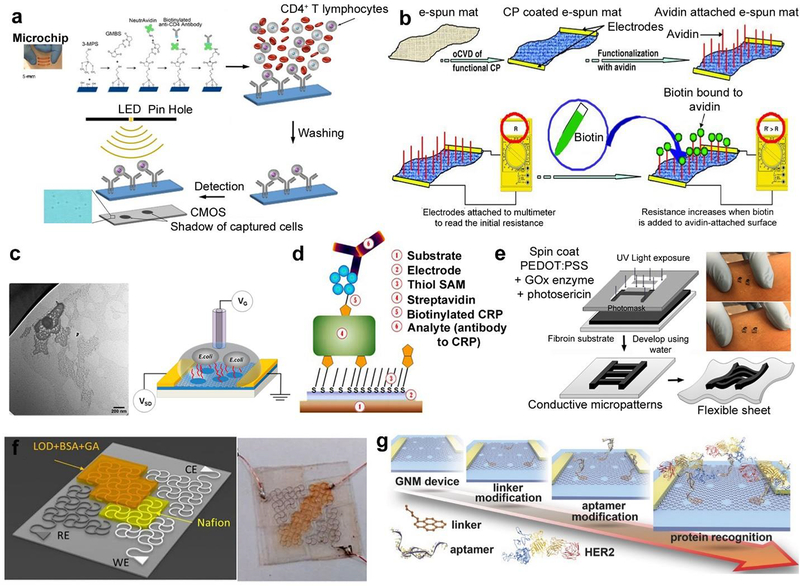
Lens-less imaging detection and counting of CD4+ T lymphocytes on polyester film-based platform with microchannels. Biotinylated anti-CD4 antibodies immobilized using NeutrAvidin on chemically activated surface. Adapted with permission from ref. (Shafiee et al. 2015). Copyright 2015 Nature Publishing Group. b) Conductive copolymer coated and avidin bound flexible electron-spun mats. Adapted with permission from ref. (Bhattacharyya et al. 2011). Copyright 2011 Wiley. c) AMP, Magainin I (GIGKFLHSAGKFGKAFVGEIMKS) covalently functionalized on hRGO, yielding a gram-negative specific biosensor Adapted with permission from ref.(Chen et al. 2014). Copyright 2014 American Chemical Society. d) biotinylated C-reactive protein (CRP) antigen and the bound analyte (anti-CRP antibody) grown on biotinylated self-assembly monolayer-covered printed gold electrodes on a paper substrate. Adapted with permission from ref. (Ihalainen et al. 2013). Copyright 2013 MDPI. e) Glucose oxidase enzyme immobilized within a PEDOT:PSS-silk sericin conducting ink, photolithographically patterned on a fibroin substrate. Adapted with permission from ref. (Pal et al. 2016a). Copyright 2016 Wiley. f) modifying silver electrode with lactate oxidase immobilized by bovine serum albumin on a PET substrate. Adapted with permission from ref. (Abrar et al. 2016). Copyright 2015 Nature Publishing Group. g) Graphene nanomesh FETs GNM FET biosensor. 1-pyrenebutanoic acid succinimidyl ester linker conjugated with the amino modified HER2-specific aptamer through the formation of an amide bond integrated on the PDMS film and attached on the human skin. Adapted with permission from ref.(Yang et al. 2017b) Copyright 2017 Wiley
3.4. Active elements (C)
In order to convert the biological event into processable or readable electronic signals, an active element is typically needed to transduce the information. In the case of a flexible biosensor, the challenge lies in connecting (large) detection devices to the sensor itself, which typically has a small footprint. Some of the common ways to generate processable electronic signals include electrochemical and to a lesser extent for flexible sensors, optical methods. Electrochemical methods have been preferred largely owing to their high sensitivity, reliability, portability, and ease of incorporation into flexible sensing schemes (for instance, fabrication of bioelectrodes). Since a discussion on the fundamental operation of such sensors is beyond the scope of this work, the reader is referred to excellent reviews on the topics.(Bandodkar and Wang 2014; Yoo and Lee 2016; Zhu et al. 2014) In this section we discuss a few emerging nanomaterials that can be integrated as part of the flexible biosensor devices to connect the substrate and the bioreceptors and offer ways to translate the biological recognition event into an electrical or optical signal.
3.4.1. Conducting Polymers
Conducting polymers (CPs) are a large family of organic materials with molecular backbones containing orbital π-conjugated systems (Heeger et al. 1987; Terje A. Skotheim 2007). The unique structure of CPs offer characteristics such as a wide range of conductivity based on doping, redox activity, and capability of mixed ionic and electronic transportation. Undoped CPs commonly show very low conductivity. However, by regulating with either p-type (oxidation) or n-type (reduction) dopants, the conductivity of CPs can be engineered across several orders of magnitude (Gerard et al. 2002; Morita et al. 1992). Moreover, the physical and chemical properties of CPs can be manipulated by doping to improve their processability. A well-known example is the conjugation of poly (3,4-ethylenedioxythiophene) (PEDOT) with poly (styrene sulfonate) (PSS) (Greco et al. 2011; Groenendaal et al. 2000) - PEDOT was initially recognized as insoluble, but after doping using the negatively charged water-soluble PSS, the resultant PEDOT:PSS can be uniformly dispersed in water. This makes the PEDOT:PSS very attractive in organic bioelectronics (Greco et al. 2011). Most CPs can physically entrap or absorb biological functional molecules or via chemical modification of functional groups such as carboxyl or amino groups, provide sites for their attachment. These approaches have allowed the coupling of biorecognition elements (enzymes, antibodies, DNA/RNA) to achieve applications in biosensing. Following development over four decades, polythiophenes, polyaniline (PANI), polypyrrole (PPy), poly (p-phenylene vinylene (PPV) as well as their derivatives are now widely used.
The polythiophene derivative-PEDOT is one of the most studied conductive polymers. Chemically or electrochemically synthesized from the 3,4-ethylenedioxythiophene (EDOT) monomer, PEDOT exhibits excellent conductivity and electronic stability in physiological environments (Groenendaal et al. 2000). PEDOT:PSS is a water-dispersible and processable polyelectrolyte system that is film-ready, highly conductive, visible light transmissive, and stable in the oxidized state. Due to its tunable conductivity, intrinsic flexibility, good biocompatibility, and low cost, PEDOT:PSS has been used as flexible polymer electrodes and organic transistors for the sensing of bacteria (He et al. 2012), biomarkers (Saurabh et al. 2016), glucose (Liao et al. 2015a; Pal et al. 2016a), and DNA/RNA (Lin et al. 2011). Recent studies focusing on PEDOT:PSS based flexible organic electrochemical transistors (OECTs) have showed impressive performance towards POC monitoring. For instance, a flexible OECT using Pt electrodes and an active layer of PEDOT:PSS on PET substrate (50 μm thick) was shown to detect glucose and uric acid in saliva samples (Liao et al. 2015a). A PEDOT:PSS based OECT sensor with an ionogel was used for the detection of lactate (Khodagholy et al. 2012).
Polyaniline (PANI) nanostructures have also been used in the development of biochemical sensors due to their electrochemical properties. PANI is a p-type semiconducting polymer with repeating units of benzenoid diamine (reduced) and quinoid diamine (oxidized), which indicates the charge carriers are primarily holes (Bhadra et al. 2008) Among the three oxidized states of PANI (leucoemeraldine, emeraldine, and pernigraniline), the emeraldine is the most conductive and stable at room temperature, and hence widely studied for biosensing applications. The sensing properties of PANI can be related to its high surface to volume ratio, and well-defined redox couples in a suitable potential range, which effectively mediate the electron transfer bridging enzymes, conductive polymer and electrodes. 2D PANI nanostructures have been reported to be more favorable in the development of flexible biosensors than 1D PANI nanostructures due to the increase in flexibility. One drawback of using PANI in biosensors is that its electronic conductivity tends to degrade significantly under physiological conditions. It is hypothesized that this is because PANI (especially emeraldine form) is normally prepared in highly protonated acidic media. Thus the conductivity of PANI can be sustained in the protonated state. Under in vivo conditions, it can become deprotonated and lose its electronic conductivity (Focke et al. 1987).
Polypyrrole (PPy) is a conductive polymer which was first been reported for the purpose of biosensing. PPy has been widely integrated into biosensors due to facile functionalization, tunable properties by different dopants during electrochemical polymerization (Schuhmann et al. 1991), simple electrochemical deposition of enzymes under mild conditions, stability, water processability, and biocompatibility. The synthesis of PPy is performed through the oxidation of pyrrole monomer which leads to the easy entrapment of various enzymes in the polymer matrix, as shown for a glucose biosensor (Wang and Musameh 2005; Weng et al. 2014). PPy has also been synthesized into different structures (nanoparticles, nanotubes, nanospheres, and core-shell nanomaterials) to improve functionalization and environmental stability. For instance, an amperometric biosensor using electrochemically deposited PPy on pyrographite or platinum electrodes followed by the adsorption of horseradish peroxidase, was used for the detection of H2O2 (Wollenberger et al. 1990).
3.4.2. Metals
Ductile metals, especially gold and silver, have long been considered as primary candidates to fabricate flexible electrodes (Gao et al. 2016; Liao et al. 2015b; Someya et al. 2005). These metals show great conductivity, strong durability to stretching and bending, and have well-established protocols to be functionalized with various bioreceptors by physiochemical modification. Thin-film metallic electrodes have also been widely used to form flexible interconnects. With special structural configurations, such as serpentine, mesh, or wavy, metallic electrodes can retain excellent electrical performance under mechanical deformation (Rogers et al. 2010; Wang et al. 2015). Commonly, metallic thin films are adhered onto the flexible substrates after the deposition of a conductive adhesive material such as chromium. The microcracks formed in the metallic thin films elongate under mechanical deformation by twisting and deflecting out of the plane, so that only small and elastic strains are distributed in the entire film network and the electrical conductivity is preserved (Lacour et al. 2006; Lacour et al. 2003).
Besides being fabricated as traditional electrodes, metallic nano- or micro structures such as gold and silver nanowires or microarrays are widely exploited as active sensing platforms. They generally have lower sheet resistance (<50 Ω−1) compared to graphene-based nanowires (several hundred Ω−1) but with similar transmittance (Luo et al. 2017). The percolating network of nanowires can be highly compliant, and can ensure the continuity of electrical conductivity under deformation. The complex metallic nanowire network provides rough surfaces with high surface-to-volume ratio, which allows it to be functionalized effectively by bio-recognition materials. A series of non-graphene 2D materials with monolayer or few-layer structures have been successfully fabricated (Ferrari et al. 2015), including transition metal oxides (TMOs), transition metal dichalcogenides (TMDCs), and transition metal chalcogenides (TMCs). The 2D form of metal oxides are particularly favored in biosensors due to their excellent electrical and mechanical properties as semiconductors. π-conjugated conductive polymers like polyaniline (PANI) and polypyrrole (PPy) have also been employed to construct the active sensing area (Gerard et al. 2002), in conjunction with non-transition metal oxides like ZnO, SnO2, and Ga2O3, which exhibit desirable conductivity and flexibility when arranged in thin layers and nanostructures (Choi et al. 2016b).
3.4.3. Carbon-based nanomaterials
Graphene and carbon nanotubes (CNTs) are among the most promising carbon-based nanomaterials for the development of flexible biosensors. Graphene, which was first introduced as a one carbon atom thick 2D nanosheet, has served as a model system with significant impact in electronics, material science, and sensing (Shavanova et al. 2016). Sensors have focused on medical applications because graphene can be fabricated into films, inks, gels, and nanoscale structures to interface with biomolecules directly or indirectly, while maintaining biocompatibility (Reina et al. 2017). The appealing versatile morphology of other forms of carbon nanomaterials (e.g. carbon nanofibers (CNFs) and C60) with proper functionalization have also offered some interesting attributes for biosensing, including high surface-to-volume ratio, mechanical flexibility, stability under different conditions, biocompatibility, and low-cost (Erol et al.; Zhang and Lieber 2016).
Graphene is a strong candidate to form flexible electrodes owing to exceptional electrical and mechanical properties, high flexibility as the supportive structural reinforcement, high transparency in the visible range, and excellent electrical conductivity with high electron carrier mobility and density (Kang et al. 2016; Reina et al. 2017). Graphene oxide (GO) and reduced graphene oxide (rGO), among various forms of graphene have also been materials of interest (Liu et al. 2012). Graphene and its derivatives can be formed by dry processes such as CVD, transfer printing (Nanda et al. 2015), or wet processes like polymerization of polyaromatic monomers (Yagmurcukardes et al. 2016), electrochemical deposition (Shu et al. 2015; Wang et al. 2017b), and physical or chemical exfoliation of graphite (Dan et al. 2009; Zhang et al. 2010). Graphene-based nanomaterials are not only affected by the preparation procedure, but also controlled by doping, dimension, layer configuration, and atomic edge termination (Dacheng and Yunqi 2010; Liu et al. 2011). For instance, bilayer graphene sheets have a tunable electronic bandgap up to 0.25 eV, whereas the bandgap of single-layer graphene is zero (Zhang et al. 2009). This small intrinsic band gap tends to restrict some of their applications (Sarkar et al. 2014).
Reduced graphene oxide (rGO) was used to fabricate a microelectrode array (MEA) on plastic substrates using nanoimprint lithography (Ng et al. 2015). The rGO/ITO MEA consisting of circular disk arrays showed great sensitivity for dopamine even without the use of mediators or functionalization of rGO. Device readouts were generally consistent regardless of mechanical deformation, long-time storage, or the presence of interferents. Graphene-based materials have been applied to fabricate transistors such as thin-film transistors (TFTs) and field-effect transistors (FETs). For instance, a transparent flexible TFTs with all-rGO micropatterns on a PET substrate were shown (He et al. 2011). The drain and source electrodes were constructed by spin-coating GO on APTES-modified PET substrate, and the channel was fabricated using microfluidic techniques with a GO thin film. After the transistors were formed, GO thin films were reduced to rGO by hydrazine vapor. The flexible all-rGO TFTs showed no obvious shift of transconductance after 5000 bending cycles or at different bending radii. The rGO channels were functionalized by 1-pyrenebutanoic acid succinimidyl ester as the linker molecules to capture fibronectin, and then successfully demonstrated to sense fibronectin.
CNTs, which can be perceived as rolled-up graphene sheets (Odom et al. 2002), include both single-walled carbon nanotubes (SWCNTs) and multi-walled carbon nanotubes (MWCNTs). Both possess similar morphology of six-carbon-ring based nanowires, which offers a highly porous complex structure when integrated into 3D networks. This porous nanostructure of CNTs brings beneficial properties including semi or metallic conductivity, high elasticity, and a large number of binding sites for active bioreceptors to anchor, which can enhance biorecognition events and lead to improved sensitivity (Le Goff et al. 2011). CNTs can be fabricated as flexible electrodes either by dry processes (direct growth of CNTs by chemical vapor deposition (CVD) (Juntae et al. 2008), dry filtration (Dong-ming et al. 2011), and transfer printing (Feng et al. 2010)), or wet processes (drop casting (Britto et al. 1996), co-deposition in a polymers-CNTs mixture (Cheung et al. 2009)) resulting in random or aligned distribution of CNTs. Their characteristics and performance are significantly affected by the fabrication techniques and distribution. A biosensor was fabricated by growing CNTs directly on a flexible polyimide substrate at low temperatures and used to quantitatively detect human serum albumin (HSA) via impedance spectroscopy (Chang et al. 2013).
3.4.3. Optical elements
Optical biosensing systems are based on the conversion of biological interactions to signals such as luminescence, fluorescence, reflectance, and absorption. While a significant number of flexible devices are based on electrochemical transduction as discussed above, optical systems offer signal delivery via light (e.g. amplitude, intensity, polarization, frequency, wavelength, and phase of the light), resulting either in visible cues of analyte discovery, and/or the ability to collect and analyze the biorecognition event non-invasively, direct, in real-time and label-free formats. Advantages include reduced interference from buffer pH or electromagnetic signals, multiplexed detection, and no requirements for electrical insulation. Optical biosensors can be of two types: direct and indirect. The former rely on the variation of optical properties of the transducers solely caused by the binding of the analyte (e.g. surface plasmon resonance (SPR), reflectometry, interferometry, and photoluminescence), whereas the latter rely on labeling agents such as fluorescent or photoluminescent quantum dots, gold or silver nanoparticles, and dyes.
The earliest flexible sensing systems involved optical fibers and waveguides, comprised of dielectric materials such as plastic or glass. Fiber based biosensors provide excellent flexibility, chemical stability, multiplexed detection, light-weight, and small size for point-of-care applications (Leung et al. 2007; Wolfbeis 2008). The sensing probe can be carefully designed at the tip of the optical fibers to evaluate the external variations in wavelength, frequency, phase, intensity, or polarization. A low-power optical transducer on plastic (PET or PEN) foil for colorimetric gas sensors was proposed. A planar optical waveguide covered with an inkjet printed ammonia sensitive film was used for gas detection (Courbat et al. 2011). An optical-fiber based flexible biosensor with replaceable SPR sensor chip was shown for the detection of water salinity (Michel et al. 2017). The design integrated components of optic-fiber polarization maintaining collimator, a micro-prism, a high-reflection mirror, and a replaceable silver nano-thin-film SPR sensor chip. This biosensor was demonstrated for sensing the salt concentration in aqueous environment with a sensitivity of 4.8 μW/ppt.
Combined with flexible substrates such as PDMS and paper, optical biosensors using labels, including organic dyes, fluorescent quantum dots (QDs), luminescent nanoparticles, and carbon dots, have emerged for disease diagnostics and POC applications (Feng et al. 2006). Using paper and other flexible substrates, fluorescein was immobilized in microfluidic channels for the capture and evaluation of CD4+ T lymphocytes in whole blood samples (Shafiee et al. 2015). Fluorescent proteins, such as green fluorescent protein (GFP), can also serve as optical labels, particularly for monitoring activity in living cells (Lippincott-Schwartz and Patterson 2003). However, organic dyes can display poor photochemical stability, susceptibility to photobleaching, and sensitivity to the environment pH. Semiconductor QDs provide a viable alternative with optical and electronic properties which are controllable based on the size and geometry (Bruchez et al. 1998; Geißler et al. 2010). Recently, fluorescent graphene QDs and carbon dots (C-dots) with high photostability and biocompatibility have been proposed. These have tunable photoluminescence properties based on size difference and are easy to functionalize (Cao et al. 2007; Kim et al. 2012c; Li et al. 2011b). Such materials have only recently begun to be integrated into transparent and flexible substrates to fabricate photonic devices for biosensing applications (Vassilakopoulou et al. 2017). The recent developments of low-power organic lightemitting diodes (OLEDs) and organic photodiodes (OPDs) are also indicative of the potential for adapting such systems for flexible biosensing (Bansal et al. 2015).
4. Fabrication strategies
Various fabrication protocols for flexible devices and biosensors are discussed throughout this review in the specific examples presented. Here, we briefly discuss a few umbrella strategies and techniques that can be used. Techniques such as inkjet printing, screen printing, and lift-off lithography have been reported for the fabrication of such biosensors. Gravure printing, and its compatibility with roll-to-roll processing, are attractive fabrication methods because of fast and large area printing characteristics. For instance, a combination of inkjet and gravure printing techniques was employed to fabricate large-area thin films over plastic surfaces as flexible biosensors for antioxidants using high-resolution interdigitated electrodes (IDEs) (Pavinatto et al. 2015).
Through various processing techniques such as chemical vapor deposition (Bhattacharyya et al. 2011), suspension coating (Xu and Wang 2009), stamping (Wang et al. 2010), drop casting (Segev-Bar et al. 2013), self-assembly (Choi et al. 2010), electrodeposition (Sun et al. 2014), inkjet printing (Xiang et al. 2016; Zahra et al. 2014), screen printing (Chou et al. 2014; Du et al. 2016), nanoparticles (Wang et al. 2010; Xu and Wang 2009; Zan et al. 2013) or one-dimensional nanostructures like nanotubes (Chang et al. 2013; Juntae et al. 2008), nanowires (Convertino et al. 2016), and nanorods (Chen et al. 2015) can be formed into thin films or sensing arrays which are used to decorate surfaces, or integrate in substrates to achieve improved performance. A pen-on-paper approach could also be used to deposit different types of nanomaterials (Au nanospheres, Au nanorods, and Ag nanospheres) (Polavarapu et al. 2014). The plasmonic nanoparticle ink was distributed by a 125 μm fountain pen nib directly onto a paper substrate. The SERS activity of three plasmonic nanoparticles and the adhesive strength between the ink and the paper substrate were tested and indicated the nanoparticles were densely packed and strongly bond to the surface of cellulose fibers. This substrate fabrication technique was used for the detection of thiabendazole, a fungicide and parasiticide, as low as 20 ppb in a 10 μL sample.
5. Application environments for flexible biosensors
In terms of their applications, flexible biosensors can be grouped according to their location of use. Figure 2 shows a few environments where such biosensors may be, or are already being used. The challenges of such interfaces are immediately in terms of being non-planar, soft and delicate, topographically rough, or even non-stationary. Devices may be created for use internally as implantable devices, or external to the body as “wearable sensors”. Flexible biosensors are also being considered for non-physiological environments such as in agriculture, or as stand-alone devices as portable diagnostic devices. Owing to the specific conditions and needs at these locations, surface characteristics, and signal to be detected, devices in these categories have characteristically different properties.
5.1. Physiological environments
Initially, we focus on physiological environments (i.e. sensors that interact directly with the human body – by either being placed within or on the body). Physiological environments emit a wide range of signals - biochemical, electrochemical, electrophysiological, mechanical, and thermal signals, from virtually all surfaces. This information can be used to inform clinicians and scientists on the state of disease and/or the progression of health (Munje et al. 2017). Flexible biosensors are primarily advantageous in physiological environments owing to the body’s soft and curvilinear surfaces. In comparison to the hard, rigid, and often bulky platforms of traditional devices, they can provide a more intimate contact between surfaces. This increases contact area between electronic transduction elements and tissue surfaces and gives us greater access to target analytes and amplify signal events (Choi et al. 2016,b; Kim et al. 2010; Yang et al. 2017a). This conformability enables the collection of information without losing sensor integrity owing to differential stresses at the interfaces (Rogers et al. 2011). Electrophysiological signals can be measured by flexible devices such as (EEG) (Cristian et al. 2011) and ECoG (Yamakawa et al. 2014) in the form of implantable electrodes that will not be discussed here. Similarly, temperature, humidity, mechanical strains and motion form some of the other parameters typically measured (Tian et al. 2014; Wang et al. 2011).
5.1.1. External to the body
The skin is the most accessible organ for biosensing in situ and represents one of the most widely applied locations for wearable and flexible biosensors. The skin can be harnessed to acquire a plethora of information for health monitoring and diagnostics, from tissue therapeutics to wound repair. In addition, the body produces a host of signals - temperature, stress, strain, pressure, and motion, which can be directly recorded on skin. Multimodal electronic skins have a huge potential for tracking temporal changes in the body [15]. As discussed above, the quantitation of these biophysical signals will be beyond the scope of this review. On the other hand, bodily fluids such as sweat and tears offer a unique route for noninvasive detection of important metabolites. This technology has been refined into wearable sensors that are lightweight, soft, flexible and tattoo-like [161]. Due to the largely planar and wide surface area of the skin, external flexible biosensors can be mechanically robust, co-planar substrates, or ultra-thin films [93]. Another benefit is that electronics on the outer layer of the skin have a greater potential to achieve continuous monitoring with miniaturized electrical components and wireless transmission.
Many physiological metabolites such as glucose, lactate, cortisol, and other small ions are secreted through sweat and tears and have feasible concentration ranges that can be detected for diagnostic purposes (Mitsubayashi et al. 1994). Biological signals can be harnessed by using sensors integrated into textiles/fibers, or in direct contact with the skin using thin films or patches (Khodagholy et al. 2012). These lab-on-a-chip technologies offer label-free detection, and are desired as they require small volumes, rapid analysis, and compatibility to many physiological environments (Weber et al. 2006). In addition, biosensors fashioned as OECTs can be integrated into textiles for low-cost and efficient sensing. A label-free and lancet-free biosensor was created to detect glucose in human sweat. Au/ZnO thin films were deposited on a porous polyamide substrate with glucose oxidase used for the assay. Impedance spectroscopy-based measurements were used to detect changes in glucose concentration from 0.01 to 200 mg/dL in human and synthetic sweat [161]. An amperometric lactate sensor for sweat was fabricated with Ag nanoparticles (AgNPs) on a flexible PET substrate. AgNPs were spray coated into crossserpentine structures and stamped in between polyurethane (PU) and PET substrate to form the biosensor. Lactate oxidase, BSA, and glutaraldehyde coated Nafion were used to functionalize the surface (Abrar et al. 2016).
Metabolites such as adrenaline and cortisol have been shown to be measured in sweat. An OECT was fabricated on a single yarn of cotton for adrenaline detection. The yarn was coated with PEDOT:PSS with Ag and Pt wires as the gate. Adrenaline is oxidized at the Pt gate, and selective response was observed with respect to other salts present in human sweat (Coppede et al. 2014). A biosensor for cortisol was formed using electrical double layer modulation as a detection mechanism. Flexible and nanoporous polyamide substrate and a ZnO thin film was deposited using Pulsed Laser Deposition with alpha-cortisol antibody as the recognition element. Cortisol was detected with a concentration range from 10–200 ng/mL. The sensor had a high sensitivity of 1 pg/mL in synthetic sweat and 1 ng/ml in human sweat (Munje et al. 2015). A “tattoo-like” temporary transfer potentiometric biosensor was created for the real-time monitoring of Na+ ion. Electrodes were screen printed onto a layered tattoo paper and insulated. PET connectors were used to couple a wireless transceiver for sensing. In vitro experiments and on-body continuous monitoring experiments were conducted and Na+ was detected in the range 30–110 mM in artificial sweat (Bandodkar et al. 2014).
5.1.2. Internal to the body
Detection of (bio) chemical analytes internal to the body is one of the biggest ongoing challenges in this field. Flexible biosensors in this category are largely specific to the surface of organs, endothelial walls of vessels, neural interfaces, and interactions with fluids inside the body. Internal bodily fluids such as blood, saliva, and cerebrospinal fluid may be used for the detection of metabolites, proteins, and biomarkers that can be useful for disease and health monitoring (Wang et al. 2016). Similarly sites of tissue repair due to a wound or surgical incisions fall under this category. Various neuro-stimulation and neural recording devices have been reported, which are beyond the scope of this work (Benfenati et al. 2012; Castagnola et al. 2015; Kim et al. 2010). While physical properties such as heart rate, temperature etc. are being externally monitored, the detection of biochemical species inside the body is largely unexplored. This is owing to challenges such as sensor design, implantability, autonomous or inbuilt power source(s), transduction and propagation of signal to an external device, and finally device retrieval or disposal at the end of functional life. Biocompatibility is a concern, as device parts must not be toxic to cells or organs upon contact. In some cases, electrodes may be coated with biocompatible and cell non-adhesive materials to achieve these requirements. Flexible devices that are placed internally must also be considered for their ability to be used for continuous monitoring, or disposable/replaceable use.
A few notable examples include a recently reported multichannel implantable flexible sensor surface modified with conductive metal–organic frameworks (MOFs) such as copper-MOF and cobalt-MOF with large surface area, high porosity, and tunable catalytic capability (Ling et al. 2018). The sensors were used to monitor nutrients such as ascorbic acid, glycine, l-tryptophan, and glucose. Sensing was shown even under deformation and a complex environment with continuous monitoring capability for 20 days. A silicon-based, bioresorbable photonic platform using thin filaments of monocrystalline silicon encapsulated by polymers as flexible, transient optical waveguides was used for accurate light delivery and sensing at targeted sites in biological systems (Bai et al. 2018). Glucose and blood oxygen saturation in live animal models was shown. Similar monocrystalline silicon nanomembranes (Si NM) were integrated with ultrathin, narrow strips of biocompatible polymers as platforms for monitoring the state of wound sites (Kim et al. 2012b). Direct integration of sensors within the mouth of patients were used to fabricate internally placed mouth-guard platforms for the detection of different analytes such as lactate (Kim et al. 2014a), salivary uric acid and glucose (Arakawa and Mitsubayashi 2017). Detection in biological fluids such as saliva and blood is discussed further below.
5.2. Analytes in biological fluids
Many personalized care and POC diagnostic devices that assess analytes from internal environments inside the body via blood samples or other biological fluids may be considered in this classification.(Wang et al. 2016) Detection using flexible devices provide significant advantages over immunoassays such as ELISA in terms of availability, cost, and efficiency (Kwon et al. 2013). Hormones, electroactive species such as hydrogen peroxide (H2O2), ascorbic acid, and dopamine are typical analytes targeted for electrochemical biosensing. In this section, we discuss some recent examples based on the specific target of interest.
5.2.1. Glucose
Detection and continuous monitoring of glucose remains one of the most important market spaces for biosensing owing to the growing population of individuals with Type 1 and 2 diabetes. Despite several commercially available products, glucose biosensors are still being improved to gain lower detection limits, increased sensitivity in biological fluids, as well as to enable continuous, non-invasive detection. Technologies that utilize flexible polymers or paper, due to their enhanced physicochemical properties, can provide portability towards such goals (Heller and Feldman 2008; Wang et al. 2016). A flexible non-enzymatic electrochemical glucose sensor based on gold nanoparticles (AuNPs)/PANI/carbon cloth (CC) integrated electrode was reported. The aniline was first deposited onto the CC flexible electrode using current polymerization. AuNPs were synthesized by electrodeposition using cyclic voltammetry. PANI enhances the electrochemical performance of the metal or metal oxide nanoparticles modified electrodes, which was also reported (Lu et al. 2014; Wang et al. 2014a). An organic electrochemical transistor (OECT) was reported for the detection of glucose and uric acid in human saliva (Liao et al. 2015a). The OECT contains a Pt electrode and a PEDOT:PSS active layer printed on a PET substrate. This study is based on the detection of H2O2 produced by an enzymatic reaction. The enzymes uricase and glucose oxidase were immobilized on the Pt electrode for the selective detection of uric acid and glucose. Selectivity of peroxide was increased by coating the Pt electrode with the conducting polymer PANI, graphene and Nafion.
Non-enzymatic glucose sensing that can directly oxidize glucose on the surface of an electrode can reduce cost and minimize complex immobilization processes in comparison to sensors that utilize glucose oxidase. Nickel is a typical electrocatalyst used in non-enzymatic glucose sensing. Ni-CNT grown on a flexible graphite foil substrate using RF sputtering was demonstrated for glucose sensing (Ryu et al. 2014). Vertically aligned CNTs were grown onto the resulting film using dc-PECVD before another layer of Ni was sputtered on top of the CNTs. CV of the electrodes showed that the Ni/VCNTs/G electrode exhibited strong peak currents, and a large surface area in alkaline solution. Amperometric sensing was used for detection of glucose. 200 bending cycles were conducted to test for a change in response, showing only a 4% drop in sensitivity, demonstrating mechanical stability with flexibility (Kim et al. 2014b).
A non-invasive In2O3 field-effect transistor (FET) conformal biosensor was reported for in situ monitoring of glucose and pH (Rim et al. 2015). A solution-processing method was employed to fabricate an ultrathin In2O3 semiconductor-based FET. The solution-processed metal oxide FET was used to overcome the problem of poor electrical performance because of the easy decomposition of nitrate-ligand-based hexa-aqua indium cation ([In(H2O)6]3+) at low annealing temperature to yield a high density In2O3 film (Rim et al. 2014). For the fabrication of the FET biosensor, the In2O3 precursor solution was first spin-coated on ultrathin polyimide (PI) substrate on a glass slide to form the channel. The interdigitated Au/Cr source and drain electrodes were then formed by photolithography. After functionalization, the fabricated In2O3/PI FETs were peeled from the glass, followed by conformable attachment to the rough surface of artificial PDMS skin, or an acrylic artificial eye for biosensing. A linear detection range of 9.0 to 5.5 and 100 μM to 4 mM was obtained for the real-time monitoring of pH and glucose, respectively. Even though the In2O3-FET had some current leakage, this work demonstrated the solution-processing method for metal oxides for the development of wearable biosensors.
5.2.2. Hydrogen Peroxide (H2O2)
H2O2 plays a key role in modulating cellular rhythms and mediators of cellular lifespan. 1D metal oxide nanostructures have been combined with 2D graphene nanosheets to form networks and large surface areas to support the loading of biorecognition elements. A template-free method of electrodepositing MnO2 nanowires on the surface of reduced graphene oxide paper (rGOP) as an electrochemical biosensing platform was shown (Xiao et al. 2012). The interconnected MnO2 nanowire networks are rich in reactive sites, while the large surface area allows the attachment of Pt nanoparticles through ultrasonic-electrodeposition. The fabricated Pt-MnO2/rGOP flexible electrodes were used for real-time, non-enzymatic detection of H2O2. The sensitivity of this sensor was retained even after 180° of bending, and the current response for 1.0 mM H2O2 maintained over 95% consistency after bending for 24 h or 100 times. A PPy nanoparticle based flexible biosensor was also developed using this strategy (Weng et al. 2014).
In this work, a printable formulation of water-dispersed pyrrole monomer and HRP or glucose oxidase (GOx) was prepared by inkjet printing on screen-printed carbon electrodes (SPCEs) with flexible PET substrates. A single layer of ethyl cellulose was then deposited to cover the PPy/enzyme/SPCE active area to prevent the leaching of enzymes. The fabricated PPy/enzyme flexible biosensors yielded stable and sensitive responses towards H2O2 and glucose with reliable repeatability and reproducibility.
A flexible and lightweight electrode based on highly dense Pt nanoparticle-decorated freestanding graphene-carbon nanotube (CNT) hybrid paper (Pt/graphene-CNT paper) was utilized for real-time monitoring of H2O2 secretion by live cells (Sun et al. 2015). The incorporation of CNTs into graphene paper prevents agglomeration between nanosheets, and imparts improved electrical conductivity, increased mechanical strength, and increased surface roughness which provides more nucleation sites for metal nanoparticles. Despite its light weight (1 mg cm−2), it has a high tensile strength. Use of rGO-CNT paper for non-enzymatic detection of peroxide showed a high sensitivity and low detection limit of 10 nM. The sensing performance was comparable or superior to other electrochemical peroxide sensors, without the common interference that normally exists in biological systems. A CVD grown graphene-based FET on a polyethylene terephthalate (PET) substrate was demonstrated for the continuous detection of hydrogen peroxide and glucose (Kwak et al. 2012).. Studies were also conducted in bent conditions of the substrate, and the results were reported to be similar to flat conditions with a slight decrease in current value.
5.2.3. Dopamine
Dopamine (DA) is an important neurotransmitter in the mammalian central nervous system. Low levels may result in disorders including Parkinson’s disease, making it an important target for detection. However, the electrochemical detection of DA in biological systems is complicated by the coexistence of interfering compounds such as ascorbic acid (AA) and uric acid (UA), oxidized at the nearly same potential at a bare electrode, resulting in an overlap of voltammetric response. Efforts have been made to address this problem by enhancing sensitivities of sensors that detect all three. In one example, graphite paper was pressed with Kapton® to create exfoliated graphite sheets (eGP) with rough surfaces for enhanced surface electrochemical activity. Using electrochemical impedance spectroscopy (EIS), the eGP exhibited a decrease in resistance and an increase in Fe(CN)63−/4− redox reaction as compared to unexfoliated graphite. CVs were performed to assess the potential for the eGP to differentiate potentials between DA, AA, and UA. The eGP was able to improve the electron transfer kinetics for all three analytes (Cai et al. 2014).
The fabrication of large-scale micropatterns of rGO on various substrates including PET was explored (He et al. 2010). The PET films were treated with aminopropyl triethoxy silane (APTES) before patterning to avoid the aggregation of rGO patterns. Patterns were created by transfer using PDMS stamps of varying width and height. These rGO FETs on the PET substrate were applied for the detection of dopamine in buffer and in living neuroendocrine PC12 cells. PC12 cells were directly cultured on top of the rGO-PET FETs to detect dopamine secretion. A flexible molecular imprinted electrochemical biosensor using Au/PPy nanowires was proposed for dopamine sensing (Huang et al. 2014). Au nanowires were first electrochemically grown on the flexible Au coated PET substrates. A mixture of dopamine and pyrrole monomer were electropolymerized on the prepared Au nanowires/PET electrodes to fabricate the Au/PPy core/shell nanowires with PPy MIP film at the surface. The fabricated MIP electrodes distinguish dopamine from other interferences with good sensitivity and retain the current intensity after days of storage, and acid and base treatment.
5.2.4. Lactate
Lactate acts as a diagnostic biomarker for conditions including heart failure, liver disease, metabolic disorders, drug toxicity, mortality in ventilated infants, etc. A flexible graphene based biosensor was reported for detecting lactate (Labroo and Cui 2013) This sensor was fabricated via transferring a graphene electrode from a rigid to a flexible substrate. Following electrode transfer, the substrate was patterned with a source and drain in addition to immobilizing a specific enzyme for lactate onto graphene. Lactate oxidase catalyzes the reaction of lactate and oxygen to pyruvate and hydrogen peroxide. When hydrogen peroxide becomes oxidized on the graphene electrode, it generates a measurable current response with a fast steady-state measuring time of 2 seconds. Additionally, the sensor could detect lactate under mechanical stress. However, sensor response to 10 μM lactate and graphene conductivity decreased by 64% and 30%, respectively, with a 45° bending angle. At a 180° bending angle, an 84% decrease in the current response and 63% decrease in graphene conductivity were observed. Reduced electron transport across the thin layer, because of the surface distortion were found to reduce conductivity and sensitivity. A similar effect was observed after increasing bending repetition. Despite graphene layer damage through increased bending and times, the sensor retained analyte sensitivity and rapid response.
A PEDOT:PSS based OECT sensor with an ionogel was used for the detection of lactate (Khodagholy et al. 2012). The ionogel component was used to replace the conventional aqueous bulk media to deliver the analyte to the electrodes. PEDOT:PSS channel and electrodes were lithographically patterned on a thin film of parylene (2 μm). A mixture of monomers (N-isopropylacrylamide and N,N-methylene-bis(acrylamide), the ionic liquid (1-Ethyl-3-methylimidazolium ethyl-sulfate), the mediator (ferrocene), the enzyme (lactate oxidase), and photo-initiator were drop-casted into a PDMS well between the channel and the gate electrode, and then photo-polymerized. The lactate in the testing samples diffused to the enzyme in the gel and ions were transferred by the mediator to the PEDOT:PSS electrodes. This flexible OECT biosensor with the ionogel solid-state electrolyte system was able to detect lactate in the relevant physiological concentration range. The ionogel system helps to preserve the enzymatic activity, thereby improving the stability and shell life of the sensor.
5.2.5. Other biomarkers
Detection of reactive oxygen species (ROS) and nitric oxide (NO) are linked to understanding pathological diseases such as cancer and neurological disorders. NO detection typically requires a close look at the cellular species that produce them in real time. To this end, an electrochemically active graphene paper was used as a conduit for cell culture to allow the monitoring of NO secreted (Xiao et al. 2013). GO paper was synthesized by mold casting on polytetrafluoroethylene (PTFE), and allowing water to evaporate from the solution to create a smooth surface with tunable thickness and volume. Au/Pt core-shell nanoparticles were then dip coated onto the surface of the graphite paper. The Au/Pt-rGO paper showed good electrochemical activity with sharp CV peaks, high surface charge, and fast charge transfer rate. Human endothelial vein cells were seeded onto the paper electrodes for electrochemical monitoring of NO secretion from live cells. Testing showed 95% viability after 6h indicating good biocompatibility between the substrate and the cells and live monitoring (Zan et al. 2013).
Tumor necrosis factor-α (TNF-α) is a key pro-inflammatory cytokine characterized by elevated circulating levels for chronic heart failure and left ventricular assisted device implantation. Most techniques for cytokine determination are expensive and time-consuming, or require highly qualified personnel. Cyclic voltammetry (CV) and EIS of the interaction of TNF-α/anti-TNF-α was conducted on functionalized gold electrodes deposited on polyimide (PI) flexible substrates (Baraket et al. 2014). Gold was deposited onto PI substrates through physical vapor deposition (PVD) with a Ti layer applied as an intermediate film for improved adhesion. After selfassembly of MHDA monolayers on the gold surface, the carboxylic acid terminal groups were activated to enable the binding of the monoclonal antibody. Sensitivity and specificity studies were performed in the presence of other cytokines. Detection of weak signals in the presence of IL-1 or IL-10 for each concentration were due to non-specific binding and interference was minimal.
A flexible FET biosensor based on 2D PANI nanostructures was reported for the detection of B-type natriuretic peptide (BNP) biomarkers (Liu et al. 2016). Electrode patterning on a PI substrate was achieved by a bilayer lithographic/ lift-off process. Conductance of the bent PANI structures were investigated, and a change of less than 20% was reported along with good restorability. Affinity biosensors using gold electrodes printed using inkjet printing on recyclable paper with different top coatings (mineral pigments, kaolin, styrene butadiene latex, talc) were demonstrated as low cost flexible sensors (Ihalainen et al. 2013). A PDMS layer was printed around the gold to stabilize any aqueous solution and prevent roll-off. Biotin/streptavidin/biotinylated C-Reactive Protein (CRP)/ anti-CRP antibody binding, detection and response were obtained using impedance analysis. The proteins were attached to the electrode via a thiol self-assembled monolayer through which supramolecular protein layers could be supported.
By immobilizing recognition elements instead of adsorbing them, paper substrates can be used as dipstick immunosensors (Hossain et al. 2009). Immobilization was performed using biocompatible sol-gel based materials to inkjet print proteins onto paper without affecting the bioactivity of the biomolecules (Harrell et al. 2004). Diglyceryl silane (DGS) and sodium silicate (SS) were used entrap acetylcholinesterase (AChE). Paper was treated with polyvinylamine (PVAm) with the SS sol coated on top. Next, the enzyme solution was entrapped on the paper and DGS sol-gel coated on top to entrap the enzymes. AChE bioactivity was evaluated as a function of enzyme concentration by adding acetylcholine. The neurotoxins paraoxon and aflatoxin B1 were used to evaluate the sensor. Colorimetric response of these neurotoxins which act as inhibitors of AChE was evaluated. AfB1 was able to inhibit 45% while paraoxon reached 25% inhibition, consistent with values found in literature.
5.2.6. Whole cell detection
Flexible biosensors for the rapid and sensitive detection of entire cells (e.g. bacteria) are desirable especially in disease diagnosis and POC monitoring. To date, a few studies using flexible biosensing platforms have been reported, primarily owing to the complexity of microbial systems and associated issues with stability. Bacterial contaminants usually have very low infective doses and therefore require sensitive detection methods (Schaad 1983). Efforts are being made to miniaturize the sensors to detect at low cell count and to ultimately achieve single cell sensing (Biran et al. 2003). These devices utilize DNA, RNA, aptamers, and antimicrobial peptides as recognition elements to identify or signify specific pathogens (Kulagina et al. 2005). An ultra-sensitive biosensor for the detection of Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) was developed using cellulose paper based on the aggregation of AuNPs (Shafiee et al. 2015). AuNPs were functionalized and bound to target pathogens. Lipopolysaccharide Binding Protein was used to conjugate AuNPs for E. coli recognition. Utilizing natural capillary effects from the internal structure of the cellulose paper, the sample transferred onto paper is uniformly distributed. The biomolecular interactions were disrupted by intermolecular interactions causing a color change. The sample (whole blood serum and peritoneal dialysis fluid) spiked by E. coli or S. aureus yield a blue color which is distinguishable from the red color of control samples. The entire quantitative analysis was performed in 10 min, and the results were acquired by a mobile app.
3D inkjet printing of a graphene FET on a flexible Kapton substrate was used for low cost, flexible, and wearable biological detection (Xiang et al. 2016). As a prototype disease target, norovirus detection with a linear response with concentrations from 0.1 to 100 μg/mL and a dynamic range of three orders of magnitude, was shown. The sensor is based on intensity changes of the AC signal with lower background noise, providing a platform for the detection of biological agents at low concentrations. A battery free graphene nanosensor capable of ultrasensitive detection of single cell bacteria was shown (Mannoor et al. 2012). Silk fibroin was used to create thin film substrates, and graphene was transfer printed to enhance the surface area by creating a monolayer for recognition and detection. Gold patterns were electrochemically deposited to form interdigitated electrodes on the graphene surface with a gold inductive coil for wireless telemetry. Naturally occurring antimicrobial peptides (AMPs) were self-assembled onto the graphene monolayer and used as recognition elements for E. coli, H. pylori and S. aureus. Detection was performed by wirelessly measuring graphene resistance over time using an RF reading device. A detection limit of a single bacterium was shown, and a linear dynamic range was created with the wireless configuration. The biosensor was tested while attached onto a bovine tooth to simulate real time detection in a bacterium rich environment. A limit of detection (LOD) at ~100 cells was observed, which is two orders of magnitudes smaller than the minimum infectious dose for H. pylori.(Mannoor et al. 2012)
A graphene derivative was produced by creating nanoscale holes in the basal layer of individual sheets via enzymatic oxidation. GO was subjected to HRP/H2O2 oxidation in PBS for 8 days, and then reduced to produce holey reduced graphene oxide (hRGO) flakes. hRGO has semiconducting properties for bacterial detection, and was covalently functionalized with magainin (an AMP), and anionic lipopolysaccharides for the detection of pathogens (Chen et al. 2014). Tween as used as a blocking buffer to block activated sites on hRGO and decrease nonspecific interaction. The device was then exposed to heat killed E. coli cells. A decrease in FET conductance was observed due to the interaction between the AMP and the bacteria which induced electron transfer to hRGO thereby decreasing the conductivity. However, these sensors were not reusable due to strong binding affinity between AMP and lipopolysaccharides from gram-negative bacteria. Another interesting work was conducted based on surface plasmon coupled emission (SPCE) to detect Mycobacterium tuberculosis (Mtb) (Mulpur et al. 2015). A thin film (~ 50 nm thick) of Ag using physical vapor deposition was formed on microscope glass slides, followed by the deposition of a C60 film (~ 10 nm) to obtain a glass/flexible Ag-C60 SPCE substrate. Rhodamine B was used as fluorophore to label the target Mtb in semen samples. From the SPCE measurements, the LOD was calculated as 20 Mtb mm−2, which is significant for the early-stage diagnosis of patients infected with tuberculosis. The observation of SPCE signals from μM concentration of fluorophores was possible using a smartphone.
5.3. Sensing in the environment
While most flexible biosensing devices have focused on the healthcare space, several possible applications exist in agriculture and the environment. The sensitive monitoring of contaminants, including chemicals, toxins and pathogens, is critical to assess and avoid risks for human and environmental health.(Van Dorst et al. 2010) A bio-inspired surface enhanced Raman spectroscopy (SERS) based flexible sensor was developed for the detection of malachite green (MG), a toxin that appears in aquaculture (Kumar et al. 2017a). This work was inspired by natural plant leaves with highly homogeneous and hydrophobic micro/nanostructures over a large surface area. The biomimetic nanostructures of SERS sensors possessing both hydrophobicity and adhesion concentrate the analyte, leading to improvement in sensor sensitivity. Microcavity/nanovoids surface structures using taro leaves as the template were negatively captured and replicated on a flexible PDMS mold. An Ag film was deposited over the microcavities to obtain plasmonic hot spots for MG detection with a linear detection range of 10−11 to 10−3 M. Even when the PDMS substrate was bent from 180o to 100o, 10−7 M of MG was detectable (Kumar et al. 2017a).
An array of passive metamaterial antennas fabricated on silk substrates were shown that could be conformally transferred and adhered to the surface of an apple. (Tao et al. 2012) This allows intimate contact of micro- and nanostructures to monitor quality control in agricultural and biological food products. Inkjet printing of functional Au components directly onto silk substrates was followed by shadow-mask transfer and casting-liftoff to fabricate split ring resonators. Adhesive gold nano-rod arrays (AgNRs) embedded in PDMS films to form robust, flexible and sensitive SERS active substrates to detect pesticides on fruit were also shown (Kumar et al. 2017b). The AgNR embedded SERS substrates were shown to be highly sensitive (detecting pesticide concentrations in the picogram range) and reproducible. Thiram pesticide solution with various concentrations (10−2 to 10−7 M) was dropped onto the surface of an apple and dried at room temperature. Samples of thiram were collected from the apple surface by AgNRs embedded PDMS substrates via “paste and peel off” method. The LOD of thiram on the flexible substrate converted to mass-to-area ratio was 2.4 ×10−9 g/cm2, which was lower than the permissible limit for apple peels (~2×10−6 g/cm2) (Kumar et al. 2017b).
A combination of inkjet and gravure printing techniques was employed to fabricate large-area thin films over plastic surfaces as flexible biosensors for antioxidants using high-resolution interdigitated electrodes (IDEs) (Pavinatto et al. 2015). Detection of phenol-based antioxidants, is of concern due to their ability to prevent oxidative stress thus retarding the aging process. Optimization of inkjet conditions facilitated production of Au IDEs with sub-100 μm features which were directly inkjet-printed on plastic substrates using a nanoparticle-based ink. Using rotogravure printing, ink containing the enzyme Tyrosinase (Tyr) was used in the active layer of the biosensors. After coating the Au IDEs, the biosensor was encapsulated with a cellulose acetate dip-coating film to avoid dissolution. The concentration of antioxidants is well below those present in wines and olive oils illustrating applications in biosensing for agricultural applications.
6. Future Perspectives
Remarkable advances have been made in the development of flexible biosensors to accommodate complex bio-interfaces. Currently, various synthetic and nature-inspired materials, architectures, and designs are all being exploited for better design of flexible biosensors. Key constraints include maintaining mechanical flexibility, adaptability to complex structures, tailorable functionalities, biocompatibility, biodegradable, low-cost, and sustainability. Understanding the role of the biorecognition element and its integration into the device is vital. In many cases, strategies developed for other forms of flexible devices are being or can be, adapted towards specific (bio) sensing. Research efforts are dedicated to make these devices balanced between flexibility and sensitivity, conformability and robustness, multiplexing while maintaining a small footprint, more integrated, yet smarter. Several scientific and engineering obstacles remain before we can fully benefit from flexible biosensors in practice.
An ongoing challenge is the fabrication of integrated devices that meet all the diverse requirements of directly interfacing with biological systems – these include not just the sensing components, but fluid delivery, power source, signal delivery units, interconnects etc. Various individual components have been demonstrated, and rapid progress is being made on integrating these aspects on a single platform. Secondly, flexibility remains a key concept in the design of such biosensors towards direct application on complex surfaces. Many prior works have demonstrated bending, stretching, or twisting as independent elements. Due to the limited understanding of device-biointerfaces, attachment and conformation still require continuous improvement. Another problem arises if one considers the need for implantation to the surface of an organ. Such systems should be soft, self-adhesive for attachment, totally organic, and resist interference or biofouling from the multitude of other analytes within the body (e.g. salts, proteins, cells etc.). Recent efforts in the development of novel biomimetic structures and biological materials that are inspired by nature could provide a potential solution to bridge flexible biosensors to clinical practice (Liu et al. 2017; Wang et al. 2017a). Through careful structural design, these biological materials can enable the enhanced adhesion and conformability for contact, as well as the protection to the active sensing layer of sensors. The integration of power sources, wireless transmitters, interconnects etc. are additional factors that are of interest. If the device is to be fully degradable, a “programmed” loss of function, or the development of transient devices is an approach (Hwang et al. 2013; Tan et al. 2016; Yin et al. 2014). Conversely, is there a need for subsequent excipient surgery to recover the device, or can it remain implanted even following an end of its useful functional life?
Besides the presence of the necessary mechanical compliance in such biosensors, one primary concern for their performance is the possibility of alteration of signal responses under physical deformation or under physiological environments. In several reports, fabricated flexible biosensors were proven to maintain effectiveness in biosensing and detection. The signal responses of these biosensors to the analytes show small shifts under the various mechanical challenges, even though the basic features of the data are sustained. However, many studies lack comprehensive characterization to illustrate the quantitative evaluation of signal stability and the accuracy of the flexible biosensors under different conditions. Few flexible biosensors have evaluated possible deterioration of signal responses under long-term influence of biological environment or during bio-degradation. Considering the complex environments for such devices (typically fluid), the stability of the device and inter-connects is also important.
Continuous monitoring of analytes continues to be an ongoing goal for such devices. A clear understanding of long-term interactions with various materials (or of their degradation products) and the body or the environment need to be investigated carefully. This involves not only using materials that are initially biocompatible without causing inflammation or damage to tissues, but have the ability to maintain biostability over functional life. This is an aspect that is still little-evaluated and requires multidisciplinary research. The ultimate goal of flexible biosensors is to bring seamless connections between humans and devices. Information regarding the health conditions, vital monitoring, disease diagnosis, drug reactions, environmental conditions etc. may be continuously and easily accessible. Flexible biosensors form vital components in the transition from “one device, one function” systems to “one device, multifunction/multi-target” systems. Smart, rational and well-organized engineering design, coupled with a fundamental knowledge of structure-function relationships forms key requirement for future flexible biosensing systems in order to accomplish necessary mechanical compliance, function, and intelligent interaction between man and machine. Opportunities therefore about in the field of materials and device design to apply flexible biosensors to dynamic and living systems.
Highlights.
Flexible biosensors involve multidisciplinary approaches towards applications in fundamental research, healthcare, food safety, and environmental monitoring.
Detection occurs at complex biointerfaces that may be soft, intrinsically curvy, irregular, or elastic, while preserving activity and sensitivity of biorecognition molecules.
State of the art is presented, along with design strategies, materials and fabrication, targets of interest, challenges, and future perspectives.
This may inspire and improve designs for smart and effective devices in the future.
Acknowledgements
The authors acknowledge the support of funding from the National Science Foundation (NSF CBET-1704435). D.O. was funded by the NIH (Grant # R25 GM089614) PREP training program in the VCU School of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting galley proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrar MA, Dong Y, Lee PK, Kim WS, 2016. Bendable electro-chemical lactate sensor printed with silver nano-particles. Sci. Rep 6, 30565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahani A, Saadati-Fard L, Sodagar AM, Boroumad FA, 2011. Engineering in Medicine and Biology Society, EMBC, 2011 Annual International Conference of the IEEE, pp. 2878–2881. IEEE. [DOI] [PubMed] [Google Scholar]
- Aizenberg J, Fratzl P, 2009. Biological and biomimetic materials. Adv. Mater 21(4), 387–388. [Google Scholar]
- Akşit AC, Onar N, Ebeoglugil MF, Birlik I, Celik E, Ozdemir Ismail, 2009. Electromagnetic and electrical properties of coated cotton fabric with barium ferrite doped polyaniline film. J. Appl. Polym. Sci 113(1), 358–366. [Google Scholar]
- Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, Lu H, Richmond J, Kaplan DL, 2003. Silk-based biomaterials. Biomaterials. 24(3), 401–416. [DOI] [PubMed] [Google Scholar]
- Arakawa T, Mitsubayashi K, 2017. Cavitas Sensors (Soft Contact Lens Type Biosensor, Mouth-Guard Type Sensor, etc.) for Daily Medicine Sensors for Everyday Life, pp. 45–65. Springer. [Google Scholar]
- Bai W, Yang H, Ma Y, Chen H, Shin J, Liu Y, Yang Q, Kandela I, Liu Z, Kang SK, 2018. Flexible Transient Optical Waveguides and Surface-Wave Biosensors Constructed from Monocrystalline Silicon. Adv. Mater 30(32), 1801584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj B, Joh HI, Jo SM, Kaur G, Sharma A, Tomar M, Gupta V, Lee S, 2016. Controllable one step copper coating on carbon nanofibers for flexible cholesterol biosensor substrates. J. Mater. Chem. B 4(2), 229–236. [DOI] [PubMed] [Google Scholar]
- Bandodkar AJ, Molinnus D, Mirza O, Guinovart T, Windmiller JR, Valdés-Ramírez G, Andrade FJ, Schöning MJ, Wang J, 2014. Epidermal tattoo potentiometric sodium sensors with wireless signal transduction for continuous non-invasive sweat monitoring. Biosens. Bioelectron 54, 603–609. [DOI] [PubMed] [Google Scholar]
- Bandodkar AJ, Wang J, 2014. Non-invasive wearable electrochemical sensors: a review. Trends Biotechnol. 32(7), 363–371. [DOI] [PubMed] [Google Scholar]
- Bansal AK, Hou S, Kulyk O, Bowman EM, Samuel ID, 2015. Wearable organic optoelectronic sensors for medicine. Adv. Mater 27(46), 7638–7644. [DOI] [PubMed] [Google Scholar]
- Bansi DM, Saurabh K, Chandra Mouli P, 2016. Nanomaterials based biosensors for cancer biomarker detection. Journal of Physics: Conference Series. 704(1), 012011. [Google Scholar]
- Baraket A, Lee M, Zine N, Yaakoubi N, Trivella MG, Elaissari A, Sigaud M, Jaffrezic-Renault N, Errachid A, 2014. A Flexible Label-Free Biosensor Sensitive and Selective to TNF- alpha : Application for Chronic Heart Failure. Sensors & Transducers. 27(5), 15. [Google Scholar]
- Barlow F, Lostetter A, Elshabini A, 2002. Low cost flex substrates for miniaturized electronic assemblies. Microelectronics Reliability. 42(7), 1091–1099. [Google Scholar]
- Benfenati V, Stahl K, Gomis-Perez C, Toffanin S, Sagnella A, Torp R, Kaplan DL, Ruani G, Omenetto FG, Zamboni R, Muccini M, 2012. Biofunctional Silk/Neuron Interfaces. Adv. Funct. Mater 22(9), 1871–1884. [Google Scholar]
- Benight SJ, Wang C, Tok JBH, Bao ZA, 2013. Stretchable and self-healing polymers and devices for electronic skin. Prog. Polym. Sci 38(12), 1961–1977. [Google Scholar]
- Bhadra S, Chattopadhyay S, Singha NK, Khastgir D, 2008. Improvement of conductivity of electrochemically synthesized polyaniline. J. Appl. Polym. Sci 108(1), 57–64. [Google Scholar]
- Bhattacharyya D, Senecal K, Marek P, Senecal A, Gleason KK, 2011. High Surface Area Flexible Chemiresistive Biosensor by Oxidative Chemical Vapor Deposition. Adv. Funct. Mater 21(22), 4328–4337. [Google Scholar]
- Bin Y, Jing Z, Tianyi K, Yu S, Tianyu L, Yat L, 2017. Paper-Based Electrodes for Flexible Energy Storage Devices. Adv. Sci 4(7), 1700107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biran I, Rissin DM, Ron EZ, Walt DR, 2003. Optical imaging fiber-based live bacterial cell array biosensor. Anal. Biochem 315(1), 106–113. [DOI] [PubMed] [Google Scholar]
- Bonato P, 2010. Wearable Sensors and Systems. IEEE Engineering in Medicine and Biology Magazine. 29(3), 25–36. [DOI] [PubMed] [Google Scholar]
- Bowden N, Brittain S, Evans AG, Hutchinson JW, Whitesides GM, 1998. Spontaneous formation of ordered structures in thin films of metals supported on an elastomeric polymer. Nature. 393(6681), 146–149. [Google Scholar]
- Britto PJ, Santhanam KSV, Ajayan PM, 1996. Carbon nanotube electrode for oxidation of dopamine. Bioelectrochem. Bioenerg 41(1), 121–125. [Google Scholar]
- Bruchez M, Moronne M, Gin P, Weiss S, Alivisatos AP, 1998. Semiconductor Nanocrystals as Fluorescent Biological Labels. Science. 281(5385), 2013–2016. [DOI] [PubMed] [Google Scholar]
- Cai W, Lai T, Du H, Ye J, 2014. Electrochemical determination of ascorbic acid, dopamine and uric acid based on an exfoliated graphite paper electrode: A high performance flexible sensor. Sens. Actuators B Chem 193 (Supplement C), 492–500. [Google Scholar]
- Cao L, Wang X, Meziani MJ, Lu F, Wang H, Luo PG, Lin Y, Harruff BA, Veca LM, Murray D, Xie S-Y, Sun Y-P, 2007. Carbon Dots for Multiphoton Bioimaging. J. Am. Chem. Soc 129(37), 11318–11319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagnola V, Descamps E, Lecestre A, Dahan L, Remaud J, Nowak LG, Bergaud C, 2015. Parylene-based flexible neural probes with PEDOT coated surface for brain stimulation and recording. Biosens. Bioelectron 67, 450–457. [DOI] [PubMed] [Google Scholar]
- Chaki NK, Vijayamohanan K, 2002. Self-assembled monolayers as a tunable platform for biosensor applications. Biosens. Bioelectron 17(1), 1–12. [DOI] [PubMed] [Google Scholar]
- Chang Y-T, Huang J-H, Tu M-C, Chang P, Yew T-R, 2013. Flexible direct-growth CNT biosensors. Biosens. Bioelectron 41, 898–902. [DOI] [PubMed] [Google Scholar]
- Chen Y, Lu S, Zhang S, Li Y, Qu Z, Chen Y, Lu B, Wang X, Feng X, 2017. Skin-like biosensor system via electrochemical channels for noninvasive blood glucose monitoring. Sci. Adv 3(12), e1701629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Michael ZP, Kotchey GP, Zhao Y, Star A, 2014. Electronic Detection of Bacteria Using Holey Reduced Graphene Oxide. ACS Appl. Mater. Inter 6(6), 3805–3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Tse WH, Chen L, Zhang J, 2015. Ag nanoparticles-decorated ZnO nanorod array on a mechanical flexible substrate with enhanced optical and antimicrobial properties. Nanoscale Res. Lett 10(1), 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung W, Chiu PL, Parajuli RR, Ma Y, Ali SR, He H, 2009. Fabrication of high performance conducting polymer nanocomposites for biosensors and flexible electronics: summary of the multiple roles of DNA dispersed and functionalized single walled carbon nanotubes. J. Mater. Chem 19(36), 6465–6480. [Google Scholar]
- Chinnasamy T, Segerink LI, Nystrand M, Gantelius J, Andersson Svahn H, 2014. Pointof-Care Vertical Flow Allergen Microarray Assay: Proof of Concept. Clin. Chem 60(9), 12091216. [DOI] [PubMed] [Google Scholar]
- Cho KH, Shin DH, Oh J, An JH, Lee JS, Jang J, 2018. Multidimensional Conductive Nanofilm-Based Flexible Aptasensor for Ultrasensitive and Selective HBsAg Detection. ACS Appl. Mater. Inter 10(34), 28412–28419. [DOI] [PubMed] [Google Scholar]
- Choi BG, Park H, Park TJ, Yang MH, Kim JS, Jang S-Y, Heo NS, Lee SY, Kong J, Hong WH, 2010. Solution Chemistry of Self-Assembled Graphene Nanohybrids for High-Performance Flexible Biosensors. ACS Nano. 4(5), 2910–2918. [DOI] [PubMed] [Google Scholar]
- Choi JR, Hu J, Tang R, Gong Y, Feng S, Ren H, Wen T, Li X, Wan Abas WAB, Pingguan-Murphy B, Xu F, 2016a. An integrated paper-based sample-to-answer biosensor for nucleic acid testing at the point of care. Lab Chip. 16(3), 611–621. [DOI] [PubMed] [Google Scholar]
- Choi S, Lee H, Ghaffari R, Hyeon T, Kim D-H, 2016b. Recent Advances in Flexible and Stretchable Bio-Electronic Devices Integrated with Nanomaterials. Adv. Mater 28(22), 4203–4218. [DOI] [PubMed] [Google Scholar]
- Chou JC, Tsai YL, Cheng TY, Liao YH, Ye GC, Yang SY, 2014. Fabrication of Arrayed Flexible Screen-Printed Glucose Biosensor Based on Microfluidic Framework. IEEE Sensors Journal. 14(1), 178–183. [Google Scholar]
- Convertino A, Mussi V, Maiolo L, 2016. Disordered array of Au covered Silicon nanowires for SERS biosensing combined with electrochemical detection. Sci. Rep 6, 25099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppede N, Tarabella G, Villani M, Calestani D, Iannotta S, Zappettini A, 2014. Human stress monitoring through an organic cotton-fiber biosensor. J. Mater. Chem. B 2(34), 5620–5626. [DOI] [PubMed] [Google Scholar]
- Courbat J, Briand D, Wollenstein J, de Rooij NF, 2011. P.olymeric foil optical waveguide with inkjet printed gas sensitive film for colorimetric sensing. Sensor Actuat. B-Chem 160(1), 910–915. [Google Scholar]
- Cristian G, Catalin DV, Siamac F, 2011. Bristle-sensors—low-cost flexible passive dry EEG electrodes for neurofeedback and BCI applications. Journal of Neural Engineering. 8(2), 025008. [DOI] [PubMed] [Google Scholar]
- Dacheng W, Yunqi L, 2010. Controllable Synthesis of Graphene and Its Applications. Adv. Mater 22(30), 3225–3241. [DOI] [PubMed] [Google Scholar]
- Dagdeviren C, Shi Y, Joe P, Ghaffari R, Balooch G, Usgaonkar K, Gur O, Tran PL, Crosby JR, Meyer M, 2015. Conformal piezoelectric systems for clinical and experimental characterization of soft tissue biomechanics. Nat. Mater 14(7), 728. [DOI] [PubMed] [Google Scholar]
- Dan Y, Lu Y, Kybert NJ, Luo Z, Johnson ATC, 2009. Intrinsic Response of Graphene Vapor Sensors. Nano Lett. 9(4), 1472–1475. [DOI] [PubMed] [Google Scholar]
- Ding Y, Invernale MA, Sotzing GA, 2010. Conductivity Trends of PEDOT-PSS Impregnated Fabric and the Effect of Conductivity on Electrochromic Textile. ACS Appl. Mater. Inter 2(6), 1588–1593. [DOI] [PubMed] [Google Scholar]
- Dong-ming S, Timmermans MY, Ying T, Nasibulin AG, Kauppinen EI, Shigeru K, Takashi M, Yutaka O, 2011. Flexible high-performance carbon nanotube integrated circuits. Nat. Nanotechnol 6(3), 156–161. [DOI] [PubMed] [Google Scholar]
- Du CX, Han L, Dong SL, Li LH, Wei Y, 2016. A novel procedure for fabricating flexible screen-printed electrodes with improved electrochemical performance. IOP Conference Series: Materials Science and Engineering. 137(1), 012060. [Google Scholar]
- Erol O, Uyan I, Hatip M, Yilmaz C, Tekinay AB, Guler MO, Recent advances in bioactive 1D and 2D carbon nanomaterials for biomedical applications. Nanomedicine: Nanotechnology, Biology and Medicine. [DOI] [PubMed] [Google Scholar]
- Fan JA, Yeo WH, Su YW, Hattori Y, Lee W, Jung SY, Zhang YH, Liu ZJ, Cheng HY, Falgout L, Bajema M, Coleman T, Gregoire D, Larsen RJ, Huang YG, Rogers JA, 2014. Fractal design concepts for stretchable electronics. Nat. Commun 5. [DOI] [PubMed] [Google Scholar]
- Feiner R, Dvir T, 2018. Tissue-electronics interfaces: from implantable devices to engineered tissues. Nat. Rev. Mater 3(1). [Google Scholar]
- Feng C, Liu K, Wu JS, Liu L, Cheng JS, Zhang Y, Sun Y, Li Q, Fan S, Jiang K, 2010. Flexible, Stretchable, Transparent Conducting Films Made from Superaligned Carbon Nanotubes. Adv. Funct. Mater 20(6), 885–891. [Google Scholar]
- Feng W, Wee Beng T, Yong Z, Xianping F, Minquan W, 2006. Luminescent nanomaterials for biological labelling. Nanotechnology. 17(1), R1. [Google Scholar]
- Fernandez RE, Lebiga E, Koklu A, Sabuncu AC, Beskok A, 2015. Flexible Bioimpedance Sensor for Label-Free Detection of Cell Viability and Biomass. IEEE Transactions on NanoBioscience. 14(7), 700–706. [DOI] [PubMed] [Google Scholar]
- Ferrari AC, Bonaccorso F, Fal’ko V, Novoselov KS, Roche S, Boggild P, Borini S, Koppens FHL, Palermo V, Pugno N, Garrido JA, Sordan R, Bianco A, Ballerini L, Prato M, Lidorikis E, Kivioja J, Marinelli C, Ryhanen T, Morpurgo A, Coleman JN, Nicolosi V, Colombo L, Fert A, Garcia-Hernandez M, Bachtold A, Schneider GF, Guinea F, Dekker C, Barbone M, Sun Z, Galiotis C, Grigorenko AN, Konstantatos G, Kis A, Katsnelson M, Vandersypen L, Loiseau A, Morandi V, Neumaier D, Treossi E, Pellegrini V, Polini M, Tredicucci A, Williams GM, Hee Hong B, Ahn J-H, Min Kim J, Zirath H, van Wees BJ, van der Zant H, Occhipinti L, Di Matteo A, Kinloch IA, Seyller T, Quesnel E, Feng X, Teo K, Rupesinghe N, Hakonen P, Neil SRT, Tannock Q, Lofwander T, Kinaret J, 2015. Science and technology roadmap for graphene, related two-dimensional crystals, and hybrid systems. Nanoscale. 7(11), 4598–4810. [DOI] [PubMed] [Google Scholar]
- Focke WW, Wnek GE, Wei Y, 1987. Influence of oxidation state, pH, and counterion on the conductivity of polyaniline. J. Phys. Chem 91(22), 5813–5818. [Google Scholar]
- Fratzl P, Barth FG, 2009. Biomaterial systems for mechanosensing and actuation. Nature. 462(7272), 442–448. [DOI] [PubMed] [Google Scholar]
- Gao W, Emaminejad S, Nyein HYY, Challa S, Chen K, Peck A, Fahad HM, Ota H, Shiraki H, Kiriya D, Lien D-H, Brooks GA, Davis RW, Javey A, 2016. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature. 529, 509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geißler D, Charbonnière LJ, Ziessel RF, Butlin NG, Löhmannsröben HG, Hildebrandt N, 2010. Quantum dot biosensors for ultrasensitive multiplexed diagnostics. Angewandte Chemie International Edition. 49(8), 1396–1401. [DOI] [PubMed] [Google Scholar]
- Gerard M, Chaubey A, Malhotra BD, 2002. Application of conducting polymers to biosensors. Biosens. Bioelectron 17(5), 345–359. [DOI] [PubMed] [Google Scholar]
- Gleskova H, Wagner S, Soboyejo W, Suo Z, 2002. Electrical response of amorphous silicon thin-film transistors under mechanical strain. J. Appl. Phys 92(10), 6224–6229. [Google Scholar]
- Golmohammadi H, Morales-Narvaez E, Naghdi T, Merkoci A, 2017. Nanocellulose in Sensing and Biosensing. Chem. Mater 29(13), 5426–5446. [Google Scholar]
- Greco F, Zucca A, Taccola S, Menciassi A, Fujie T, Haniuda H, Takeoka S, Dario P, Mattoli V, 2011. Ultra-thin conductive free-standing PEDOT/PSS nanofilms. Soft Matter. 7(22), 10642–10650. [Google Scholar]
- Groenendaal L, Jonas F, Freitag D, Pielartzik H, Reynolds JR, 2000. Poly(3,4--ethylenedioxythiophene) and Its Derivatives: Past, Present, and Future. Adv. Mater 12(7), 481–494. [Google Scholar]
- Gualandi I, Marzocchi M, Achilli A, Cavedale D, Bonfiglio A, Fraboni B, 2016. Textile Organic Electrochemical Transistors as a Platform for Wearable Biosensors. Sci. Rep 6, 33637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamedi M, Forchheimer R, Inganäs O, 2007. Towards woven logic from organic electronic fibres. Nat. Mater 6, 357. [DOI] [PubMed] [Google Scholar]
- Han S-T, Peng H, Sun Q, Venkatesh S, Chung K-S, Lau SC, Zhou Y, Roy VAL, 2017. An Overview of the Development of Flexible Sensors. Adv. Mater 29(33), 1700375. [DOI] [PubMed] [Google Scholar]
- Harrell TM, Hosticka B, Power ME, Cemke L, Hull R, Norris PM, 2004. Selective Deposition of Biocompatible Sol-Gel Materials. Journal of Sol-Gel Science and Technology. 31(1), 349–352. [Google Scholar]
- He Q, Wu S, Gao S, Cao X, Yin Z, Li H, Chen P, Zhang H, 2011. Transparent, Flexible, All-Reduced Graphene Oxide Thin Film Transistors. ACS Nano. 5(6), 5038–5044. [DOI] [PubMed] [Google Scholar]
- He QY, Sudibya HG, Yin ZY, Wu SX, Li H, Boey F, Huang W, Chen P, Zhang H, 2010. Centimeter-Long and Large-Scale Micropatterns of Reduced Graphene Oxide Films: Fabrication and Sensing Applications. ACS Nano. 4(6), 3201–3208. [DOI] [PubMed] [Google Scholar]
- He R-X, Zhang M, Tan F, Leung PHM, Zhao X-Z, Chan HLW, Yang M, Yan F, 2012. Detection of bacteria with organic electrochemical transistors. J. Mater. Chem 22(41), 22072–22076. [Google Scholar]
- Heeger AJ, Moses D, Sinclair M, 1987. Nonlinear excitations and nonlinear phenomena in conductive polymers. Synthetic Met. 17(1), 343–348. [Google Scholar]
- Heikenfeld J, Jajack A, Rogers J, Gutruf P, Tian L, Pan T, Li R, Khine M, Kim J, Wang J, Kim J, 2018. Wearable sensors: modalities, challenges, and prospects. Lab Chip. 18(2), 217–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller A, Feldman B, 2008. Electrochemical Glucose Sensors and Their Applications in Diabetes Management. Chem. Rev 108(7), 2482–2505. [DOI] [PubMed] [Google Scholar]
- Hossain SMZ, Luckham RE, Smith AM, Lebert JM, Davies LM, Pelton RH, Filipe CDM, Brennan JD, 2009. Development of a Bioactive Paper Sensor for Detection of Neurotoxins Using Piezoelectric Inkjet Printing of Sol−Gel-Derived Bioinks. Anal. Chem 81(13), 5474–5483. [DOI] [PubMed] [Google Scholar]
- Howell RS, Stewart M, Kamik SV, Saha SK, Hatalis MK, 2000. Poly-Si thin-film transistors on steel substrates. IEEE Electron. Dev. Lett 21(2), 70–72. [Google Scholar]
- Huang W-R, Chen Y-L, Lee C-Y, Chiu H-T, 2014. Fabrication of gold/polypyrrole core/shell nanowires on a flexible substrate for molecular imprinted electrochemical sensors. RSC Adv. 4(107), 62393–62398. [Google Scholar]
- Huang Y, Dong X, Liu Y, Li L-J, Chen P, 2011. Graphene-based biosensors for detection of bacteria and their metabolic activities. J. Mater. Chem 21(33), 12358–12362. [Google Scholar]
- Hwang SW, Kim DH, Tao H, Kim TI, Kim S, Yu KJ, Panilaitis B, Jeong JW, Song JK, Omenetto FG, Rogers JA, 2013. Materials and Fabrication Processes for Transient and Bioresorbable High-Performance Electronics. Adv. Funct. Mater 23(33), 4087–4093. [Google Scholar]
- IDTechEx, 2017a. Biosensors for Point-of-Care Testing: Technologies, Applications, Forecasts 2017–2027: IDTechEx. [Google Scholar]
- IDTechEx, 2017b. Stretchable and Conformal Electronics 2017–2027: IDTechEx. [Google Scholar]
- IDTechEx, 2017c. Wearable Sensors 2018–2028: Technologies, Markets & Players: IDTechEx. [Google Scholar]
- Ihalainen P, Majumdar H, Viitala T, Törngren B, Närjeoja T, Määttänen A, Sarfraz J, Härmä H, Yliperttula M, Österbacka R, Peltonen J, 2013. Application of Paper-Supported Printed Gold Electrodes for Impedimetric Immunosensor Development. Biosensors. 3(1), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irimia-Vladu M, 2014. “Green” electronics: biodegradable and biocompatible materials and devices for sustainable future Chem. Soc. Rev 43(17), 588. [DOI] [PubMed] [Google Scholar]
- Irimia-Vladu M, Glowacki ED, Troshin PA, Schwabegger G, Leonat L, Susarova DK, Krystal O, Ullah M, Kanbur Y, Bodea MA, Razumov VF, Sitter H, Bauer S, Sariciftci NS, 2012. Indigo - A Natural Pigment for High Performance Ambipolar Organic Field Effect Transistors and Circuits. Adv. Mater 24(3), 375-+. [DOI] [PubMed] [Google Scholar]
- Jiang C, Wang X, Gunawidjaja R, Lin YH, Gupta MK, Kaplan DL, Naik RR, Tsukruk VV, 2007. Mechanical Properties of Robust Ultrathin Silk Fibroin Films. Adv. Funct. Mater 17(13), 2229–2237. [Google Scholar]
- Jin HJ, Park J, Karageorgiou V, Kim UJ, Valluzzi R, Cebe P, Kaplan DL, 2005. Water-Stable Silk Films with Reduced β-Sheet Content. Adv. Funct. Mater 15(8), 1241–1247. [Google Scholar]
- Jung J, Kim SJ, Lee KW, Yoon DH, Kim Y. g., Kwak HY, Dugasani SR, Park SH, Kim HJ, 2014. Approaches to label-free flexible DNA biosensors using low-temperature solution-processed In nO thin-film transistors. Biosens. Bioelectron. 55, 99–105. [DOI] [PubMed] [Google Scholar]
- Jung YH, Chang T-H, Zhang H, Yao C, Zheng Q, Yang VW, Mi H, Kim M, Cho SJ, Park D-W, Jiang H, Lee J, Qiu Y, Zhou W, Cai Z, Gong S, Ma Z, 2015. High-performance green flexible electronics based on biodegradable cellulose nanofibril paper. Nat. Commun 6, 7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juntae K, Mihye Y, Byung Yang L, Tae Hyun K, Joohyung L, Young Min J, Seunghun H, 2008. Directed assembly of carbon nanotubes on soft substrates for use as a flexible biosensor array. Nanotechnology. 19(50), 505502. [DOI] [PubMed] [Google Scholar]
- K.P.O, M., Shown I, Chen L-C, Chen K-H, Tai Y, 2018. Flexible sensor for dopamine detection fabricated by the direct growth of α-Fe2O3 nanoparticles on carbon cloth. Appl. Surf. Sci 427(Part B), 387–395. [Google Scholar]
- Kamakoti V, Panneer Selvam A, Radha Shanmugam N, Muthukumar S, Prasad S, 2016. Flexible Molybdenum Electrodes towards Designing Affinity Based Protein Biosensors. Biosensors. 6(3), 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang P, Wang MC, Nam S, 2016. Bioelectronics with two-dimensional materials. Microelectronic Engineering. 161, 18–35. [Google Scholar]
- Karnaushenko D, Ibarlucea B, Lee S, Lin G, Baraban L, Pregl S, Melzer M, Makarov D, Weber WM, Mikolajick T, Schmidt OG, Cuniberti G, 2015. Light Weight and Flexible High-Performance Diagnostic Platform. Adv. Healthc. Mater 4(10), 1517–1525. [DOI] [PubMed] [Google Scholar]
- Kergoat L, Piro B, Berggren M, Horowitz G, Pham M-C, 2012. Advances in organic transistor-based biosensors: from organic electrochemical transistors to electrolyte-gated organic field-effect transistors. Anal. Bioanal. Chem 402(5), 1813–1826. [DOI] [PubMed] [Google Scholar]
- Khan S, Lorenzelli L, Dahiya RS, 2015. Technologies for Printing Sensors and Electronics Over Large Flexible Substrates: A Review. IEEE Sensors Journal. 15(6), 3164–3185. [Google Scholar]
- Khan Y, Ostfeld AE, Lochner CM, Pierre A, Arias AC, 2016. Monitoring of vital signs with flexible and wearable medical devices. Adv. Mater 28(22), 4373–4395. [DOI] [PubMed] [Google Scholar]
- Khodagholy D, Curto VF, Fraser KJ, Gurfinkel M, Byrne R, Diamond D, Malliaras GG, Benito-Lopez F, Owens RM, 2012. Organic electrochemical transistor incorporating an ionogel as a solid state electrolyte for lactate sensing. J. Mater. Chem 22(10), 4440–4443. [Google Scholar]
- Kim D-H, Kim Y-S, Amsden J, Panilaitis B, Kaplan DL, Omenetto FG, Zakin MR, Rogers JA, 2009. Silicon electronics on silk as a path to bioresorbable, implantable devices. Appl. Phys. Lett 95(13), 133701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Ghaffari R, Lu NS, Rogers JA, 2012a. Flexible and Stretchable Electronics for Biointegrated Devices. Annu. Rev. Biomed. Eng, Vol 14 14, 113–128. [DOI] [PubMed] [Google Scholar]
- Kim DH, Viventi J, Amsden JJ, Xiao JL, Vigeland L, Kim YS, Blanco JA, Panilaitis B, Frechette ES, Contreras D, Kaplan DL, Omenetto FG, Huang YG, Hwang KC, Zakin MR, Litt B, Rogers JA, 2010. Dissolvable films of silk fibroin for ultrathin conformal bio-integrated electronics. Nat. Mater 9(6), 511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Wang S, Keum H, Ghaffari R, Kim YS, Tao H, Panilaitis B, Li M, Kang Z, Omenetto F, 2012b. Thin, flexible sensors and actuators as ‘instrumented’surgical sutures for targeted wound monitoring and therapy. Small. 8(21), 3263–3268. [DOI] [PubMed] [Google Scholar]
- Kim J, Kim M, Lee M-S, Kim K, Ji S, Kim Y-T, Park J, Na K, Bae K-H, Kim HK, 2017. Wearable smart sensor systems integrated on soft contact lenses for wireless ocular diagnostics. Nat. Commun 8, 14997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Valdes-Ramirez G, Bandodkar AJ, Jia WZ, Martinez AG, Ramirez J, Mercier P, Wang J, 2014a. Non-invasive mouthguard biosensor for continuous salivary monitoring of metabolites. Analyst. 139(7), 1632–1636. [DOI] [PubMed] [Google Scholar]
- Kim S, Hwang SW, Kim M-K, Shin DY, Shin DH, Kim CO, Yang SB, Park JH, Hwang E, Choi S-H, Ko G, Sim S, Sone C, Choi HJ, Bae S, Hong BH, 2012c. Anomalous Behaviors of Visible Luminescence from Graphene Quantum Dots: Interplay between Size and Shape. ACS Nano. 6(9), 8203–8208. [DOI] [PubMed] [Google Scholar]
- Kim WS, Lee GJ, Ryu JH, Park K, Park HK, 2014b. A flexible, nonenzymatic glucose biosensor based on Ni-coordinated, vertically aligned carbon nanotube arrays. RSC Adv. 4(89), 48310–48316. [Google Scholar]
- Klemic KG, Klemic JF, Reed MA, Sigworth FJ, 2002. Micromolded PDMS planar electrode allows patch clamp electrical recordings from cells. Biosens. Bioelectron 17(6), 597–604. [DOI] [PubMed] [Google Scholar]
- Kulagina NV, Lassman ME, Ligler FS, Taitt CR, 2005. Antimicrobial Peptides for Detection of Bacteria in Biosensor Assays. Anal. Chem 77(19), 6504–6508. [DOI] [PubMed] [Google Scholar]
- Kumar P, Khosla R, Soni M, Deva D, Sharma SK, 2017a. A highly sensitive, flexible SERS sensor for malachite green detection based on Ag decorated microstructured PDMS substrate fabricated from Taro leaf as template. Sens. Actuators B Chem 246(Supplement C), 477–486. [Google Scholar]
- Kumar S, Goel P, Singh JP, 2017b. Flexible and robust SERS active substrates for conformal rapid detection of pesticide residues from fruits. Sens. Actuators B Chem 241, 577–583. [Google Scholar]
- Kwak YH, Choi DS, Kim YN, Kim H, Yoon DH, Ahn SS, Yang JW, Yang WS, Seo S, 2012. Flexible glucose sensor using CVD-grown graphene-based field effect transistor. Biosens. Bioelectron 37(1), 82–87. [DOI] [PubMed] [Google Scholar]
- Kwon OS, Lee SH, Park SJ, An JH, Song HS, Kim T, Oh JH, Bae J, Yoon H, Park TH, Jang J, 2013. Large-Scale Graphene Micropattern Nano-biohybrids: High-Performance Transducers for FET-Type Flexible Fluidic HIV Immunoassays. Adv. Mater 25(30), 4177–4185. [DOI] [PubMed] [Google Scholar]
- Labroo P, Cui Y, 2013. Flexible graphene bio-nanosensor for lactate. Biosens. Bioelectron 41, 852–856. [DOI] [PubMed] [Google Scholar]
- Lacour SP, Chan D, Wagner S, Li T, Suo Z, 2006. Mechanisms of reversible stretchability of thin metal films on elastomeric substrates. Appl. Phys. Lett 88(20), 204103. [Google Scholar]
- Lacour SP, Wagner S, Huang Z, Suo Z, 2003. Stretchable gold conductors on elastomeric substrates. Appl. Phys. Lett 82(15), 2404–2406. [Google Scholar]
- Lau PH, Takei K, Wang C, Ju Y, Kim J, Yu Z, Takahashi T, Cho G, Javey A, 2013. Fully Printed, High Performance Carbon Nanotube Thin-Film Transistors on Flexible Substrates. Nano Lett. 13(8), 3864–3869. [DOI] [PubMed] [Google Scholar]
- Lawrence BD, Cronin-Golomb M, Georgakoudi I, Kaplan DL, Omenetto FG, 2008. Bioactive Silk Protein Biomaterial Systems for Optical Devices. Biomacromolecules. 9(4), 1214–1220. [DOI] [PubMed] [Google Scholar]
- Le Goff A, Holzinger M, Cosnier S, 2011. Enzymatic biosensors based on SWCNT-conducting polymer electrodes. Analyst. 136(7), 1279–1287. [DOI] [PubMed] [Google Scholar]
- Lechat C, Bunsell AR, Davies P, Piant A, 2006. Mechanical behaviour of polyethylene terephthalate & polyethylene naphthalate fibres under cyclic loading. J. Mater. Sci 41(6), 17451756. [Google Scholar]
- Lee H, Choi TK, Lee YB, Cho HR, Ghaffari R, Wang L, Choi HJ, Chung TD, Lu N, Hyeon T, 2016a. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat. Nanotechnol 11(6), 566. [DOI] [PubMed] [Google Scholar]
- Lee K, Singh A, He J, Massia S, Kim B, Raupp G, 2004. Polyimide based neural implants with stiffness improvement. Sens. Actuators B Chem 102(1), 67–72. [Google Scholar]
- Lee S, Reuveny A, Reeder J, Lee S, Jin H, Liu Q, Yokota T, Sekitani T, Isoyama T, Abe Y, Suo Z, Someya T, 2016b. A transparent bending-insensitive pressure sensor. Nat. Nanotechnol 11, 472. [DOI] [PubMed] [Google Scholar]
- Leung A, Shankar PM, Mutharasan R, 2007. A review of fiber-optic biosensors. Sens. Actuators B Chem 125(2), 688–703. [Google Scholar]
- Li C, Han J, Ahn CH, 2007. Flexible biosensors on spirally rolled micro tube for cardiovascular in vivo monitoring. Biosens. Bioelectron 22(9), 1988–1993. [DOI] [PubMed] [Google Scholar]
- Li G, Liu H, Zhao H, Gao Y, Wang J, Jiang H, Boughton RI, 2011a. Chemical assembly of TiO2 and TiO2@Ag nanoparticles on silk fiber to produce multifunctional fabrics. J Colloid Interf. Sci 358(1), 307–315. [DOI] [PubMed] [Google Scholar]
- Li L, Au W. m., Ding F, Hua T, Wong KS, 2014a. Wearable electronic design: electrothermal properties of conductive knitted fabrics. Textile Research Journal. 84(5), 477–487. [Google Scholar]
- Li L, Xu J, Zheng X, Ma C, Song X, Ge S, Yu J, Yan M, 2014b. Growth of gold-manganese oxide nanostructures on a 3D origami device for glucose-oxidase label based electrochemical immunosensor. Biosens. Bioelectron 61, 76–82. [DOI] [PubMed] [Google Scholar]
- Li X, Wang H, Shimizu Y, Pyatenko A, Kawaguchi K, Koshizaki N, 2011b. Preparation of carbon quantum dots with tunable photoluminescence by rapid laser passivation in ordinary organic solvents. Chem. Commun 47(3), 932–934. [DOI] [PubMed] [Google Scholar]
- Liang B, Fang L, Hu Y, Yang G, Zhu Q, Ye X, 2014. Fabrication and application of flexible graphene silk composite film electrodes decorated with spiky Pt nanospheres. Nanoscale. 6(8), 4264–4274. [DOI] [PubMed] [Google Scholar]
- Liao C, Mak C, Zhang M, Chan HLW, Yan F, 2015a. Flexible Organic Electrochemical Transistors for Highly Selective Enzyme Biosensors and Used for Saliva Testing. Adv. Mater 27(4), 676–681. [DOI] [PubMed] [Google Scholar]
- Liao C, Zhang M, Yao MY, Hua T, Li L, Yan F, 2015b. Flexible Organic Electronics in Biology: Materials and Devices. Adv. Mater 27(46), 7493–7527. [DOI] [PubMed] [Google Scholar]
- Liao J-Y, Lei B-X, Chen H-Y, Kuang D-B, Su C-Y, 2012. Oriented hierarchical single crystalline anatase TiO2 nanowire arrays on Ti-foil substrate for efficient flexible dye-sensitized solar cells. Energy & Environmental Science. 5(2), 5750–5757. [Google Scholar]
- Liaw D-J, Wang K-L, Huang Y-C, Lee K-R, Lai J-Y, Ha C-S, 2012. Advanced polyimide materials: Syntheses, physical properties and applications. Prog. Polym. Sci 37(7), 907–974. [Google Scholar]
- Lin P, Luo X, Hsing IM, Yan F, 2011. Organic Electrochemical Transistors Integrated in Flexible Microfluidic Systems and Used for Label-Free DNA Sensing. Adv. Mater 23(35), 4035–4040. [DOI] [PubMed] [Google Scholar]
- Ling W, Liew G, Li Y, Hao Y, Pan H, Wang H, Ning B, Xu H, Huang X, 2018. Materials and Techniques for Implantable Nutrient Sensing Using Flexible Sensors Integrated with Metal–Organic Frameworks. Adv. Mater 30(23), 1800917. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Patterson GH, 2003. Development and Use of Fluorescent Protein Markers in Living Cells. Science. 300(5616), 87–91. [DOI] [PubMed] [Google Scholar]
- Liu H, Liu Y, Zhu D, 2011. Chemical doping of graphene. J. Mater. Chem 21(10), 3335–3345. [Google Scholar]
- Liu P, Zhu Y, Lee SH, Yun M, 2016. Two-dimensional polyaniline nanostructure to the development of microfluidic integrated flexible biosensors for biomarker detection. Biomed. Microdevices 18(6), 113. [DOI] [PubMed] [Google Scholar]
- Liu Y, Dong X, Chen P, 2012. Biological and chemical sensors based on graphene materials. Chem. Soc. Rev 41(6), 2283–2307. [DOI] [PubMed] [Google Scholar]
- Liu Y, Qi N, Song T, Jia M, Xia Z, Yuan Z, Yuan W, Zhang K-Q, Sun B, 2014. Highly Flexible and Lightweight Organic Solar Cells on Biocompatible Silk Fibroin. ACS Appl. Mater. Inter 6(23), 20670–20675. [DOI] [PubMed] [Google Scholar]
- Liu YQ, He K, Chen G, Leow WR, Chen XD, 2017. Nature-Inspired Structural Materials for Flexible Electronic Devices. Chem. Rev 117(20), 12893–12941. [DOI] [PubMed] [Google Scholar]
- Lu X, Ye Y, Xie Y, Song Y, Chen S, Li P, Chen L, Wang L, 2014. Copper coralloid granule/polyaniline/reduced graphene oxide nanocomposites for nonenzymatic glucose detection. Anal. Meth 6(13), 4643–4651. [Google Scholar]
- Luo M, Liu Y, Huang W, Qiao W, Zhou Y, Ye Y, Lin-Sen C, 2017. Towards Flexible Transparent Electrodes Based on Carbon and Metallic Materials. Micromachines. 8(1), 12. [Google Scholar]
- Luo Z, Zhang L, Zeng R, Su L, Tang D, 2018. Near-Infrared Light-Excited Core–Core– Shell UCNP@Au@CdS Upconversion Nanospheres for Ultrasensitive Photoelectrochemical Enzyme Immunoassay. Anal. Chem 90(15), 9568–9575. [DOI] [PubMed] [Google Scholar]
- Lv S, Zhang K, Zeng Y, Tang D, 2018. Double Photosystems-Based ‘ -Scheme’ Photoelectrochemical Sensing Mode for Ultrasensitive Detection of Disease Biomarker Accompanying Three-Dimensional DNA Walker. Anal. Chem 90(11), 7086–7093. [DOI] [PubMed] [Google Scholar]
- MacDonald WA, 2004. Engineered films for display technologies. J. Mater. Chem 14(1), 4–10. [Google Scholar]
- Mannoor MS, Tao H, Clayton JD, Sengupta A, Kaplan DL, Naik RR, Verma N, Omenetto FG, McAlpine MC, 2012. Graphene-based wireless bacteria detection on tooth enamel. Nat. Commun 3, 763. [DOI] [PubMed] [Google Scholar]
- Martinez AW, Phillips ST, Whitesides GM, Carrilho E, 2010. Diagnostics for the Developing World: Microfluidic Paper-Based Analytical Devices. Anal. Chem 82(1), 3–10. [DOI] [PubMed] [Google Scholar]
- Mata A, Fleischman AJ, Roy S, 2005. Characterization of Polydimethylsiloxane (PDMS) Properties for Biomedical Micro/Nanosystems. Biomed. Microdevices 7(4), 281–293. [DOI] [PubMed] [Google Scholar]
- Mathew X, Thompson GW, Singh VP, McClure JC, Velumani S, Mathews NR, Sebastian PJ, 2003. Development of CdTe thin films on flexible substrates—a review. Sol. Energy Mater Sol. Cells 76(3), 293–303. [Google Scholar]
- Meinel L, Karageorgiou V, Hofmann S, Fajardo R, Snyder B, Li C, Zichner L, Langer R, Vunjak Novakovic, G., Kaplan DL, 2004. Engineering bone-like tissue in vitro using human bone marrow stem cells and silk scaffolds. J. Biomed. Mater. Res. A 71A(1), 25–34. [DOI] [PubMed] [Google Scholar]
- Michel D, Xiao F, Alameh K, 2017. A compact, flexible fiber-optic Surface Plasmon Resonance sensor with changeable sensor chips. Sens. Actuators B Chem 246, 258–261. [Google Scholar]
- Mitsubayashi K, Suzuki M, Tamiya E, Karube I, 1994. Analysis of metabolites in sweat as a measure of physical condition. Anal. Chim. Acta 289(1), 27–34. [Google Scholar]
- Modali A, Vanjari SRK, Dendukuri D, 2016. Wearable Woven Electrochemical Biosensor Patch for Non-invasive Diagnostics. Electroanalysis. 28(6), 1276–1282. [Google Scholar]
- Mohanty SP, Kougianos E, 2006. Biosensors: a tutorial review. Ieee Potentials. 25(2), 35–40. [Google Scholar]
- Morita S, Zakhidov AA, Yoshino K, 1992. Doping effect of buckminsterfullerene in conducting polymer: Change of absorption spectrum and quenching of luminescene. Solid State Commun. 82(4), 249–252. [Google Scholar]
- Mościcki A, Smolarek-Nowak A, Felba J, Kinart A, 2017. Ink for Ink-Jet Printing of Electrically Conductive Structures on Flexible Substrates with Low Thermal Resistance. J. Electron. Mater 46(7), 4100–4108. [Google Scholar]
- Mulpur P, Yadavilli S, Mulpur P, Kondiparthi N, Sengupta B, Rao AM, Podila R, Kamisetti V, 2015. Flexible Ag-C60 nano-biosensors based on surface plasmon coupled emission for clinical and forensic applications. Phys. Chem. Chem. Phys 17(38), 25049–25054. [DOI] [PubMed] [Google Scholar]
- Munje RD, Muthukumar S, Panneer Selvam A, Prasad S, 2015. Flexible nanoporous tunable electrical double layer biosensors for sweat diagnostics. Sci. Rep 5, 14586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munje RD, Muthukumar S, Prasad S, 2017. Lancet-free and label-free diagnostics of glucose in sweat using Zinc Oxide based flexible bioelectronics. Sens. Actuators B Chem 238(Supplement C), 482–490. [Google Scholar]
- Murakami S, Nishikawa Y, Tsuji M, Kawaguchi A, Kohjiya S, Cakmak M, 1995. A study on the structural changes during uniaxial drawing and/or heating of poly(ethylene naphthalene-2,6-dicarboxylate) films. Polymer. 36(2), 291–297. [Google Scholar]
- Muskovich M, Bettinger CJ, 2012. Biomaterials-Based Electronics: Polymers and Interfaces for Biology and Medicine. Adv. Healthc. Mater 1(3), 248–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda SS, Papaefthymiou GC, Yi DK, 2015. Functionalization of Graphene Oxide and its Biomedical Applications. Crit. Rev. Solid State Mater. Sci 40(5), 291–315. [Google Scholar]
- Ng AMH, Kenry Teck Lim, C., Low HY, Loh KP, 2015. Highly sensitive reduced graphene oxide microelectrode array sensor. Biosens. Bioelectron 65, 265–273. [DOI] [PubMed] [Google Scholar]
- Ngom B, Guo Y, Wang X, Bi D, 2010. Development and application of lateral flow test strip technology for detection of infectious agents and chemical contaminants: a review. Anal. Bioanal. Chem 397(3), 1113–1135. [DOI] [PubMed] [Google Scholar]
- Ning X, Wang X, Zhang Y, Yu X, Choi D, Zheng N, Kim DS, Huang Y, Zhang Y, Rogers JA, 2018. Assembly of Advanced Materials into 3D Functional Structures by Methods Inspired by Origami and Kirigami: A Review. Adv. Mater. Interfaces, 1800284. [Google Scholar]
- Ochoa M, Rahimi R, Ziaie B, 2014. Flexible Sensors for Chronic Wound Management. IEEE Rev. Biomed. Eng 7, 73–86. [DOI] [PubMed] [Google Scholar]
- Odom TW, Huang J-L, Lieber CM, 2002. Single-Walled Carbon Nanotubes. Annals of the New York Academy of Sciences. 960(1), 203–215. [PubMed] [Google Scholar]
- Pal RK, Farghaly AA, Collinson MM, Kundu SC, Yadavalli VK, 2016a. Photolithographic Micropatterning of Conducting Polymers on Flexible Silk Matrices. Adv. Mater 28(7), 1406–1412. [DOI] [PubMed] [Google Scholar]
- Pal RK, Farghaly AA, Wang C, Collinson MM, Kundu SC, Yadavalli VK, 2016b. Conducting polymer-silk biocomposites for flexible and biodegradable electrochemical sensors. Biosens. Bioelectron 81, 294–302. [DOI] [PubMed] [Google Scholar]
- Pal RK, Pradhan S, Narayanan L, Yadavalli VK, 2018. Micropatterned conductive polymer biosensors on flexible PDMS films. Sens. Actuators B Chem 259, 498–504. [Google Scholar]
- Park JH, Kim DY, Ko JK, Chakrabarty K, Yi J, 2003. High temperature crystallized poly-Si on Mo substrates for TFT application. Thin Solid Films. 427(1), 303–308. [Google Scholar]
- Parolo C, Merkoci A, 2013. Paper-based nanobiosensors for diagnostics. Chem. Soc. Rev 42(2), 450–457. [DOI] [PubMed] [Google Scholar]
- Patrito N, McCague C, Norton PR, Petersen NO, 2007. Spatially Controlled Cell Adhesion via Micropatterned Surface Modification of Poly(dimethylsiloxane). Langmuir. 23(2), 715–719. [DOI] [PubMed] [Google Scholar]
- Pavinatto FJ, Paschoal CWA, Arias AC, 2015. Printed and flexible biosensor for antioxidants using interdigitated inkjetted electrodes and gravure-deposited active layer. Biosens. Bioelectron 67, 553–559. [DOI] [PubMed] [Google Scholar]
- Pelton R, 2009. Bioactive paper provides a low-cost platform for diagnostics. TrAC Trends in Anal. Chem 28(8), 925–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polavarapu L, Porta AL, Novikov SM, Coronado-Puchau M, Liz-Marzán LM, 2014. Pen-on-Paper Approach Toward the Design of Universal Surface Enhanced Raman Scattering Substrates. Small. 10(15), 3065–3071. [DOI] [PubMed] [Google Scholar]
- Qianwei C, Tai S, Xuefen S, Qincui R, Chongsheng Y, Jun Y, Hua F, Leyong Y, Dapeng W, 2017. Flexible electrochemical biosensors based on graphene nanowalls for the real-time measurement of lactate. Nanotechnology. 28(31), 315501. [DOI] [PubMed] [Google Scholar]
- Qin D, Xia Y, Whitesides GM, 2010. Soft lithography for micro- and nanoscale patterning. Nat. Protoc 5, 491. [DOI] [PubMed] [Google Scholar]
- Qiu Z, Shu J, Tang D, 2017. Bioresponsive Release System for Visual Fluorescence Detection of Carcinoembryonic Antigen from Mesoporous Silica Nanocontainers Mediated Optical Color on Quantum Dot-Enzyme-Impregnated Paper. Anal. Chem 89(9), 5152–5160. [DOI] [PubMed] [Google Scholar]
- Rajan K, Garofalo E, Chiolerio A, 2018. Wearable Intrinsically Soft, Stretchable, Flexible Devices for Memories and Computing. Sensors. 18(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray WJ, Joseph W, 2013. Wearable Electrochemical Sensors and Biosensors: A Review. Electroanalysis. 25(1), 29–46. [Google Scholar]
- Reddish W, 1950. The dielectric properties of polyethylene terephthalate (Terylene). Transactions of the Faraday Society. 46(0), 459–475. [Google Scholar]
- Reina G, Gonzalez-Dominguez JM, Criado A, Vazquez E, Bianco A, Prato M, 2017. Promises, facts and challenges for graphene in biomedical applications. Chem. Soc. Rev 46(15), 4400–4416. [DOI] [PubMed] [Google Scholar]
- Rim YS, Bae S-H, Chen H, Yang JL, Kim J, Andrews AM, Weiss PS, Yang Y, Tseng H-R, 2015. Printable Ultrathin Metal Oxide Semiconductor-Based Conformal Biosensors. ACS Nano. 9(12), 12174–12181. [DOI] [PubMed] [Google Scholar]
- Rim YS, Chen H, Kou X, Duan H-S, Zhou H, Cai M, Kim HJ, Yang Y, 2014. Boost Up Mobility of Solution-Processed Metal Oxide Thin-Film Transistors via Confining Structure on Electron Pathways. Adv. Mater 26(25), 4273–4278. [DOI] [PubMed] [Google Scholar]
- Rogers JA, Lagally MG, Nuzzo RG, 2011. Synthesis, assembly and applications of semiconductor nanomembranes. Nature. 477, 45. [DOI] [PubMed] [Google Scholar]
- Rogers JA, Someya T, Huang Y, 2010. Materials and Mechanics for Stretchable Electronics. Science. 327(5973), 1603–1607. [DOI] [PubMed] [Google Scholar]
- Ryu JH, Lee GJ, Kim WS, Lim HE, Mativenga M, Park KC, Park HK, 2014. All-Carbon Electrode Consisting of Carbon Nanotubes on Graphite Foil for Flexible Electrochemical Applications. Materials (Basel, Switzerland). 7(3), 1975–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SadAbadi H, Badilescu S, Packirisamy M, Wüthrich R, 2013. Integration of gold nanoparticles in PDMS microfluidics for lab-on-a-chip plasmonic biosensing of growth hormones. Biosens. Bioelectron 44, 77–84. [DOI] [PubMed] [Google Scholar]
- Salvatore GA, Münzenrieder N, Kinkeldei T, Petti L, Zysset C, Strebel I, Büthe L, Tröster G, 2014. Wafer-scale design of lightweight and transparent electronics that wraps around hairs. Nat. Commun 5, 2982. [DOI] [PubMed] [Google Scholar]
- Sarkar D, Liu W, Xie X, Anselmo AC, Mitragotri S, Banerjee K, 2014. MoS2 Field-Effect Transistor for Next-Generation Label-Free Biosensors. ACS Nano. 8(4), 3992–4003. [DOI] [PubMed] [Google Scholar]
- Saurabh K, Suveen K, Chandra Mouli P, Bansi DM, 2016. Conducting paper based sensor for cancer biomarker detection. Journal of Physics: Conference Series. 704(1), 012010. [Google Scholar]
- Schaad UB, 1983. Which number of infecting bacteria is of clinical relevance? Infection. 11(2), S87–S89. [DOI] [PubMed] [Google Scholar]
- Schuhmann W, Lammert R, Hämmerle M, Schmidt H-L, 1991. Electrocatalytic properties of polypyrrole in amperometric electrodes. Biosens. Bioelectron 6(8), 689–697. [Google Scholar]
- Schwartz G, Tee BCK, Mei J, Appleton AL, Kim DH, Wang H, Bao Z, 2013. Flexible polymer transistors with high pressure sensitivity for application in electronic skin and health monitoring. Nat. Commun 4, 1859. [DOI] [PubMed] [Google Scholar]
- Segev-Bar M, Haick H, 2013. Flexible sensors based on nanoparticles. ACS Nano. 7(10), 8366–8378. [DOI] [PubMed] [Google Scholar]
- Segev-Bar M, Landman A, Nir-Shapira M, Shuster G, Haick H, 2013. Tunable Touch Sensor and Combined Sensing Platform: Toward Nanoparticle-based Electronic Skin. ACS Appl. Mater. Inter 5(12), 5531–5541. [DOI] [PubMed] [Google Scholar]
- Seo D, Neely RM, Shen K, Singhal U, Alon E, Rabaey JM, Carmena JM, Maharbiz MM, 2016. Wireless recording in the peripheral nervous system with ultrasonic neural dust. Neuron. 91(3), 529–539. [DOI] [PubMed] [Google Scholar]
- Shafiee H, Asghar W, Inci F, Yuksekkaya M, Jahangir M, Zhang MH, Durmus NG, Gurkan UA, Kuritzkes DR, Demirci U, 2015. Paper and Flexible Substrates as Materials for Biosensing Platforms to Detect Multiple Biotargets. Sci. Rep 5, 8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shavanova K, Bakakina Y, Burkova I, Shtepliuk I, Viter R, Ubelis A, Beni V, Starodub N, Yakimova R, Khranovskyy V, 2016. Application of 2D Non-Graphene Materials and 2D Oxide Nanostructures for Biosensing Technology. Sensors. 16(2), 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu H, Chang G, Su J, Cao L, Huang Q, Zhang Y, Xia T, He Y, 2015. Single-step electrochemical deposition of high performance Au-graphene nanocomposites for nonenzymatic glucose sensing. Sens. Actuators B Chem 220, 331–339. [Google Scholar]
- Shu J, Qiu Z, Lv S, Zhang K, Tang D, 2018a. Plasmonic Enhancement Coupling with Defect-Engineered TiO2–x: A Mode for Sensitive Photoelectrochemical Biosensing. Anal. Chem 90(4), 2425–2429. [DOI] [PubMed] [Google Scholar]
- Shu J, Qiu Z, Tang D, 2018b. Self-Referenced Smartphone Imaging for Visual Screening of H2S Using CuxO-Polypyrrole Conductive Aerogel Doped with Graphene Oxide Framework. Anal. Chem 90(16), 9691–9694. [DOI] [PubMed] [Google Scholar]
- Siegel AC, Phillips ST, Dickey MD, Lu N, Suo Z, Whitesides GM, 2010. Foldable printed circuit boards on paper substrates. Adv. Funct. Mater 20(1), 28–35. [Google Scholar]
- Sivashankar S, Sapsanis C, Buttner U, Salama KN, 2015. Flexible low-cost cardiovascular risk marker biosensor for point-of-care applications. Electron Lett. 51(22), 1746–1748. [Google Scholar]
- Someya T, Kato Y, Sekitani T, Iba S, Noguchi Y, Murase Y, Kawaguchi H, Sakurai T, 2005. Conformable, flexible, large-area networks of pressure and thermal sensors with organic transistor active matrixes. Proc. Natl. Acad. Sci. USA 102(35), 12321–12325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staiano M, Pennacchio A, Varriale A, Capo A, Majoli A, Capacchione C, D’Auria S, 2017. Chapter Four - Enzymes as Sensors In: Thompson RB, Fierke CA (Eds.), Methods in Enzymology, pp. 115–131. Academic Press. [DOI] [PubMed] [Google Scholar]
- Stoppa M, Chiolerio A, 2014. Wearable Electronics and Smart Textiles: A Critical Review. Sensors (Basel, Switzerland). 14(7), 11957–11992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suginta W, Khunkaewla P, Schulte A, 2013. Electrochemical biosensor applications of polysaccharides chitin and chitosan. Chem. Rev 113(7), 5458–5479. [DOI] [PubMed] [Google Scholar]
- Sun H, Chao J, Zuo X, Su S, Liu X, Yuwen L, Fan C, Wang L, 2014. Gold nanoparticle-decorated MoS2 nanosheets for simultaneous detection of ascorbic acid, dopamine and uric acid. RSC Adv. 4(52), 27625–27629. [Google Scholar]
- Sun Y, He K, Zhang Z, Zhou A, Duan H, 2015. Real-time electrochemical detection of hydrogen peroxide secretion in live cells by Pt nanoparticles decorated graphene-carbon nanotube hybrid paper electrode. Biosens. Bioelectron 68, 358–364. [DOI] [PubMed] [Google Scholar]
- Tan EL, Pereles BD, Horton B, Shao R, Zourob M, Ong KG, 2008. Implantable Biosensors for Real-time Strain and Pressure Monitoring. Sensors (Basel, Switzerland). 8(10), 6396–6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan MJ, Owh C, Chee PL, Kyaw AKK, Kai D, Loh XJ, 2016. Biodegradable electronics: cornerstone for sustainable electronics and transient applications. J. Mater. Chem. C 4(24), 5531–5558. [Google Scholar]
- Tao H, Brenckle MA, Yang M, Zhang J, Liu M, Siebert SM, Averitt RD, Mannoor MS, McAlpine MC, Rogers JA, Kaplan DL, Omenetto FG, 2012. Silk-based conformal, adhesive, edible food sensors. Adv. Mater 24(8), 1067–1072. [DOI] [PubMed] [Google Scholar]
- Skotheim Terje A., J.R., 2007. Handbook of Conducting Polymers, 3rd ed. CRC Press, Boca Raton. [Google Scholar]
- Tian H, Shu Y, Cui Y-L, Mi W-T, Yang Y, Xie D, Ren T-L, 2014. Scalable fabrication of high-performance and flexible graphene strain sensors. Nanoscale. 6(2), 699–705. [DOI] [PubMed] [Google Scholar]
- Van Dorst B, Mehta J, Bekaert K, Rouah-Martin E, De Coen W, Dubruel P, Blust R, Robbens J, 2010. Recent advances in recognition elements of food and environmental biosensors: a review. Biosens. Bioelectron 226(4), 1178–1194. [DOI] [PubMed] [Google Scholar]
- Vandeparre H, Watson D, Lacour S, 2013. Extremely robust and conformable capacitive pressure sensors based on flexible polyurethane foams and stretchable metallization. Appl. Phys. Lett 103(20), 204103. [Google Scholar]
- Vassilakopoulou A, Georgakilas V, Vainos N, Koutselas I, 2017. Successful entrapment of carbon dots within flexible free-standing transparent mesoporous organic-inorganic silica hybrid films for photonic applications. J. Phys. Chem. Solids 103, 190–196. [Google Scholar]
- Vidor FF, Meyers T, Hilleringmann U, 2015. Flexible electronics: integration processes for organic and inorganic semiconductor-based thin-film transistors. Electronics. 4(3), 480–506. [Google Scholar]
- Viventi J, Kim D-H, Vigeland L, Frechette ES, Blanco JA, Kim Y-S, Avrin AE, Tiruvadi VR, Hwang S-W, Vanleer AC, Wulsin DF, Davis K, Gelber CE, Palmer L, Van der Spiegel J, Wu J, Xiao J, Huang Y, Contreras D, Rogers JA, Litt B, 2011. Flexible, foldable, actively multiplexed, high-density electrode array for mapping brain activity in vivo. Nat. Neurosci 14, 1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Musameh M, 2005. Carbon-nanotubes doped polypyrrole glucose biosensor. Anal. Chim. Acta 539(1), 209–213. [Google Scholar]
- Wang L, Chen D, Jiang K, Shen G, 2017a. New insights and perspectives into biological materials for flexible electronics. Chem. Soc. Rev 46(22), 6764–6815. [DOI] [PubMed] [Google Scholar]
- Wang L, Luo J, Yin J, Zhang H, Wu J, Shi X, Crew E, Xu Z, Rendeng Q, Lu S, Poliks M, Sammakia B, Zhong C-J, 2010. Flexible chemiresistor sensors: thin film assemblies of nanoparticles on a polyethylene terephthalate substrate. J. Mater. Chem 20(5), 907–915. [Google Scholar]
- Wang L, Ye Y, Shen Y, Wang F, Lu X, Xie Y, Chen S, Tan H, Xu F, Song Y, 2014a. Hierarchical nanocomposites of Co3O4/polyaniline nanowire arrays/reduced graphene oxide sheets for amino acid detection. Sens. Actuators B Chem 203, 864–872. [Google Scholar]
- Wang N, Lukáacs Z, Gadgil B, Damlin P, Janáky C, Kvarnström C, 2017b. Electrochemical deposition of polyviologen-reduced graphene oxide nanocomposite thin films. Electrochimica Acta. 231, 279–286. [Google Scholar]
- Wang S, Chinnasamy T, Lifson M, Inci F, Demirci U, 2016. Flexible substrate-based devices for point-of-care diagnostics. Trends Biotechnol. 34(11), 909–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Huang Y, Rogers JA, 2015. Mechanical Designs for Inorganic Stretchable Circuits in Soft Electronics. IEEE Transactions on Components, Packaging and Manufacturing Technology. 5(9), 1201–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Gu Y, Xiong Z, Cui Z, Zhang T, 2014b. Silk-Molded Flexible, Ultrasensitive, and Highly Stable Electronic Skin for Monitoring Human Physiological Signals. Adv. Mater 26(9), 1336–1342. [DOI] [PubMed] [Google Scholar]
- Wang XW, Liu Z, Zhang T, 2017c. Flexible Sensing Electronics for Wearable/Attachable Health Monitoring. Small. 13(25). [DOI] [PubMed] [Google Scholar]
- Wang Y, Ping J, Ye Z, Wu J, Ying Y, 2013. Impedimetric immunosensor based on gold nanoparticles modified graphene paper for label-free detection of Escherichia coli O157:H7. Biosens. Bioelectron 49(Supplement C), 492–498. [DOI] [PubMed] [Google Scholar]
- Wang Y, Yang R, Shi Z, Zhang L, Shi D, Wang E, Zhang G, 2011. Super-Elastic Graphene Ripples for Flexible Strain Sensors. ACS Nano. 5(5), 3645–3650. [DOI] [PubMed] [Google Scholar]
- Weber J, Kumar A, Kumar A, Bhansali S, 2006. Novel lactate and pH biosensor for skin and sweat analysis based on single walled carbon nanotubes. Sens. Actuators B Chem 117(1), 308–313. [Google Scholar]
- Weng B, Morrin A, Shepherd R, Crowley K, Killard AJ, Innis PC, Wallace GG, 2014. Wholly printed polypyrrole nanoparticle-based biosensors on flexible substrate. J. Mater. Chem. B 2(7), 793–799. [DOI] [PubMed] [Google Scholar]
- Weng X, Ahmed SR, Neethirajan S, 2018. A nanocomposite-based biosensor for bovine haptoglobin on a 3D paper-based analytical device. Sens. Actuators B Chem 265, 242–248. [Google Scholar]
- Wenmin Q, Matthias P, Wolf-Joachim F, 2001. Microfabrication of thermoelectric generators on flexible foil substrates as a power source for autonomous microsystems. J. Micromech Microeng 11(2), 146. [Google Scholar]
- Windmiller JR, Wang J, 2013. Wearable Electrochemical Sensors and Biosensors: A Review. Electroanalysis. 25(1), 29–46. [Google Scholar]
- Wolfbeis OS, 2008. Fiber-optic chemical sensors and biosensors. Anal. Chem 80(12), 4269–4283. [DOI] [PubMed] [Google Scholar]
- Wollenberger U, Bogdanovskaya V, Bobrin S, Scheller F, Tarasevich M, 1990. Enzyme Electrodes Using Bioelectrocatalytic Reduction of Hydrogen Peroxide. Anal. Lett 23(10), 1795–1808. [Google Scholar]
- Xiang L, Wang Z, Liu Z, Weigum SE, Yu Q, Chen MY, 2016. Inkjet-Printed Flexible Biosensor Based on Graphene Field Effect Transistor. IEEE Sensors Journal. 16(23), 8359–8364. [Google Scholar]
- Xiao F, Li Y, Gao H, Ge S, Duan H, 2013. Growth of coral-like PtAu-MnO2 binary nanocomposites on free-standing graphene paper for flexible nonenzymatic glucose sensors. Biosens. Bioelectron 41, 417–423. [DOI] [PubMed] [Google Scholar]
- Xiao F, Li Y, Zan X, Liao K, Xu R, Duan H, 2012. Growth of Metal–Metal Oxide Nanostructures on Freestanding Graphene Paper for Flexible Biosensors. Adv Funct Mater. 22(12), 2487–2494. [Google Scholar]
- Xiao SY, Che LF, Li XX, Wang YL, 2008. A novel fabrication process of MEMS devices on polyimide flexible substrates. Microelectronic Engineering. 85(2), 452–457. [Google Scholar]
- Xu C, Wang X, 2009. Fabrication of Flexible Metal-Nanoparticle Films Using Graphene Oxide Sheets as Substrates. Small. 5(19), 2212–2217. [DOI] [PubMed] [Google Scholar]
- Yagmurcukardes M, Peeters FM, Senger RT, Sahin H, 2016. Nanoribbons: From fundamentals to state-of-the-art applications. Appl. Phys. Rev 3(4), 041302. [Google Scholar]
- Yamakawa T, Inoue T, He Y, Fujii M, Suzuki M, Niwayama M, 2014. Development of an implantable flexible probe for simultaneous near-infrared spectroscopy and electrocorticography. IEEE transactions on bio-medical engineering. 61(2), 388–395. [DOI] [PubMed] [Google Scholar]
- Yang Y-L, Chuang M-C, Lou S-L, Wang J, 2010. Thick-film textile-based amperometric sensors and biosensors. Analyst. 135(6), 1230–1234. [DOI] [PubMed] [Google Scholar]
- Yang Y, Yang X, Tan Y, Yuan Q, 2017a. Recent progress in flexible and wearable bioelectronics based on nanomaterials. Nano Res. 10(5), 1560–1583. [Google Scholar]
- Yang Y, Yang X, Zou X, Wu S, Wan D, Cao A, Liao L, Yuan Q, Duan X, 2017b. Ultrafine Graphene Nanomesh with Large On/Off Ratio for High-Performance Flexible Biosensors. Adv. Funct. Mater 27(19), 1604096. [Google Scholar]
- Yin L, Cheng HY, Mao SM, Haasch R, Liu YH, Xie X, Hwang SW, Jain H, Kang SK, Su YW, Li R, Huang YG, Rogers JA, 2014. Dissolvable Metals for Transient Electronics. Adv. Funct. Mater 24(5), 645–658. [Google Scholar]
- Yoo SM, Lee SY, 2016. Optical Biosensors for the Detection of Pathogenic Microorganisms. Trends Biotechnol. 34(1), 7–25. [DOI] [PubMed] [Google Scholar]
- Yu Z, Cai G, Ren R, Tang D, 2018. A new enzyme immunoassay for alpha-fetoprotein in a separate setup coupling an aluminium/Prussian blue-based self-powered electrochromic display with a digital multimeter readout. Analyst. 143(13), 2992–2996. [DOI] [PubMed] [Google Scholar]
- Zahra A, Vahid M, Mansour B, Ali B, 2014. Flexible biosensor using inkjet printing of silver nanoparticles. Sensor Review. 34(4), 360–366. [Google Scholar]
- Zan X, Fang Z, Wu J, Xiao F, Huo F, Duan H, 2013. Freestanding graphene paper decorated with 2D-assembly of Au@Pt nanoparticles as flexible biosensors to monitor live cell secretion of nitric oxide. Biosens. Bioelectron 49(Supplement C), 71–78. [DOI] [PubMed] [Google Scholar]
- Zhang A, Lieber CM, 2016. Nano-Bioelectronics. Chemical Reviews. 116(1), 215–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Li X, Huang Y, Ma Y, Wan X, Chen Y, 2010. Controlled synthesis of few-layered graphene sheets on a large scale using chemical exfoliation. Carbon. 48(8), 2367–2371. [Google Scholar]
- Zhang Y, Tang T-T, Girit C, Hao Z, Martin MC, Zettl A, Crommie MF, Shen YR, Wang F, 2009. Direct observation of a widely tunable bandgap in bilayer graphene. Nature. 459, 820. [DOI] [PubMed] [Google Scholar]
- Zhao ZW, Chen XJ, Tay BK, Chen JS, Han ZJ, Khor KA, 2007. A novel amperometric biosensor based on ZnO:Co nanoclusters for biosensing glucose. Biosens. Bioelectron 23(1), 135–139. [DOI] [PubMed] [Google Scholar]
- Zhong J, Zhu H, Zhong Q, Dai J, Li W, Jang S-H, Yao Y, Henderson D, Hu Q, Hu L, Zhou J, 2015. Self-Powered Human-Interactive Transparent Nanopaper Systems. ACS Nano. 9(7), 7399–7406. [DOI] [PubMed] [Google Scholar]
- Zhu C, Yang G, Li H, Du D, Lin Y, 2014. Electrochemical sensors and biosensors based on nanomaterials and nanostructures. Anal. Chem 87(1), 230–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YJ, Moran-Mirabal J, 2016. Highly Bendable and Stretchable Electrodes Based on Micro/Nanostructured Gold Films for Flexible Sensors and Electronics. Adv. Electron. Mater 2(3). [Google Scholar]
- Zuo Z, Zhu K, Gu C, Wen Y, Cui G, Qu J, 2016. Transparent, flexible surface enhanced Raman scattering substrates based on Ag-coated structured PET (polyethylene terephthalate) for in-situ detection. Appl. Surf. Sci 379, 66–72. [Google Scholar]


