Significance
“Taking a less-traveled path” is often considered an effective approach to creativity (i.e., creative thinking calls for a break from habitual thinking and associations), yet little is known about its underlying neural mechanism. In a series of four independent experiments involving electrophysiological and brain stimulation methods we provide evidence that this process is mediated by the right temporal alpha oscillations. Alpha oscillations are known to represent a process of active inhibition to suppress irrelevant information, such as inhibiting distractions during visual search. Through monitoring the brain’s electrical activity during different creativity tasks and by stimulating the right temporal brain region at the alpha frequency we show that a similar process of active inhibition is also key to creative thinking.
Keywords: alpha oscillations, creativity, active inhibition, EEG, brain stimulation
Abstract
Creative cognition requires mental exploration of remotely connected concepts while suppressing dominant ones. Across four experiments using different samples of participants, we provide evidence that right temporal alpha oscillations play a crucial role in inhibiting habitual thinking modes, thereby paving the way for accessing more remote ideas. In the first experiment, participants completed the compound remote associate task (RAT) in three separate sessions: during right temporal alpha (10 Hz) transcranial alternating current brain stimulation (tACS), left temporal alpha tACS, and sham tACS. Participants performed better under right tACS only on RAT items in which two of the three words shared misleading semantic associations. In the second experiment, we measured EEG while the participants solved RAT items with or without shared misleading associations. We observed an increase in right temporal alpha power when participants correctly solved RAT items with misleading semantic associations. The third experiment demonstrated that while solving divergent thinking tasks participants came up with more remote ideas when stimulated by right temporal alpha tACS. In the fourth experiment, we found that participants showed higher right temporal alpha power when generating more remote uses for common objects. These studies altogether indicate that right temporal alpha oscillations may support creativity by acting as a neural mechanism for an active inhibition of obvious semantic associations.
A long-standing theory of creativity postulates that the ability to come up with remote and less-expected semantic associations is a key characteristic of creative individuals (1). These semantic associations can be represented as edges between different nodes (concepts), linked through their proximity or common use (2). According to the spreading activation theory of semantic processing (3), every time we search for concepts associated with a word, we start from stronger associations to move progressively, in the order of strength of semantic associations, toward weaker or more remote ones (e.g., cat > dog > animal > pet > human > people > family). That is to say, the activation (concept’s retrieval) spreads from strongly connected nodes (concepts) to less-connected ones. Creativity requires reaching those more remote associations on the less-connected concepts. Using graph theory and an insightful analytical approach, it has been shown that highly creative individuals, compared with less-creative ones, show broader and less modular semantic networks (4, 5). Nonetheless, we do not know what the neural mechanisms are which enable to inhibit strongly connected concepts to reach the most remote ones.
A key question is how creative individuals are able to engage flexibility of thought to avoid the “most traveled paths” to get to their alternative routes and draw more remote associations. For instance, more creative individuals are shown to avoid taking obvious routes when solving creative problems (6). Further, a study showed that under low cognitive load individuals tend to explore alternative routes or more remote associations (7). The authors suggested that inhibition mediates this exploration by actively and naturally inhibiting most immediate associations, which could explain why we expand our semantic networks as we work on a problem.
Creative thinking involves searching through a clutter of associated concepts or ideas, and the presence of obvious associations is a distraction from the desired creative solution (e.g., finding unusual uses for an object or finding a remote association); such obvious but misleading associations need to be actively inhibited for producing more creative associations. Here we tested the hypothesis that alpha oscillatory activity enables us to inhibit the most obvious associations to get to more remote ideas. Considering the key role of alpha oscillations in the active inhibition of distractions in both visual search (8, 9) and working memory tasks (10), we hypothesized that this process of actively inhibiting obvious or strong associations could be mediated by an increase in alpha oscillations as it occurs when inhibiting other internal or external distractors.
We suggest that this hypothesis could potentially explain a wide range of findings with regard to the role of alpha oscillations (especially right-lateralized) in creative problem solving (11). For example, alpha power increases during both divergent (i.e., ability to come up with a large number of original ideas) and convergent (i.e., ability to come up with one appropriate correct solution) creative thinking processes under higher internal attentional demand (12). Right-lateralized alpha oscillations have also been shown to be higher during the generation of more original ideas in a divergent thinking task (13). Further, modulating frontal alpha oscillations with transcranial alternating current brain stimulation (tACS) increased performance on divergent thinking tasks (14). Finally, right-lateralized alpha oscillations increased before cognitive insight (15–17).
Here, across four experiments with independent samples, we investigated how alpha oscillations contribute to both convergent and divergent creative cognition and provided a neural mechanism linking these two distinct cognitive processes. For brain stimulation, we targeted the right temporal region due to its key role in semantic processing (18–20), integration of associated information (21), and recognizing associations between different concepts (22). The first experiment aimed at understanding the effects of the right temporal alpha (10 Hz) tACS on the remote associates task (RAT), a classical convergent thinking task, relying on the remote associations between presented cues. We predicted that right alpha tACS would improve performance on RAT items containing a shared but wrong semantic association, as these require stronger active inhibition to find the remote association. In the second experiment, by recording EEG we investigated the brain oscillatory responses to the RAT items that contained shared wrong associations compared with the ones that did not. In the third experiment, we applied tACS at the individual alpha peak frequency (IAF) over the same brain regions of Exp. 1 before, during, and after an alternative uses task, a classical divergent thinking task. We predicted that the right temporal alpha tACS would be associated with the generation of more remote ideas. In the fourth experiment, by recording EEG we investigated the IAF power during the alternative uses task. We predicted that more-remote ideas would be associated with higher individual alpha power compared with less-remote ideas. Therefore, across all four experiments our common binding hypothesis was that the right temporal alpha oscillations play a key role in creative cognition, by inhibiting the obvious semantic associations which can pave the way to more remote and creative ideas.
Experiment 1
Mednick’s RAT (1, 23) is a typical convergent thinking task which emphasizes the importance of association of remote concepts in creative cognition. In the RAT’s compound-word version (24) participants are presented with three cue words (e.g., walker/main/sweeper) and are asked to find a solution or target word which makes a compound word with each of these three words (e.g., solution is street: streetwalker/main street/street sweeper). People tend to seek the solution word by searching in the pool of semantically related words to the presented cues (25–27). However, there is a trap in this habitual thinking: When two cue words have close semantic association with a word that is not the correct solution this can get in the way of the true solution, thereby acting as an important distractor which attracts internal attention (6). For example, the two cues (ear and tone) of the RAT item ear/tone/finger share a dominant but misleading association (sound), which needs to be inhibited to reach the solution (ring). In contrary, the cues of the RAT item high/teacher/mate (solution: school) do not share any strong common association. The ability to inhibit the most obvious but misleading semantic association is therefore of particular benefit for solving difficult remote associate problems (28), and more creative individuals are found to successfully avoid most common but incorrect candidate solutions (6). However, the neural mechanism underlying this process of inhibiting the habitual, most-obvious associations and promoting the remote, less-dominant associations during creative problem solving has largely been uncharacterized.
Considering that right temporal alpha oscillations have been consistently found to be involved in the insightful solutions of these problems (15–17) and in coming up with original ideas (11, 13), we tested the role of alpha oscillations in the temporal regions (right, left, and sham). By stimulating alpha oscillations through tACS during the RAT, we tested whether alpha oscillations are involved in establishing weak or distant associations or in helping to inhibit dominant, but misleading, semantic associations. tACS can be used to modulate brain oscillations in a frequency-specific manner (e.g., ref. 29) and is a powerful tool to examine the role of cortical oscillations in human behavior by directly manipulating brain states in a controlled fashion. Considering the key role of alpha oscillations in the active inhibition of distractions (8, 30), we predicted that, rather than boosting creative problem solving in general, right temporal alpha would be specifically involved in inhibiting the most-obvious associations.
Using a large dataset of semantic associations (31, 32), we considered the RAT items as having a “shared wrong association” if two out of the three cues were strongly associated with a word which was not the solution (Materials and Methods). Thirty participants received right temporal, left temporal, and sham 10-Hz tACS in three separate sessions while solving RAT items with or without shared wrong semantic associations. We entered the proportion of correct solutions to those problems in a 2 (shared wrong association: yes vs. no) × 3 (stimulation condition: left, sham, and right tACS) within-subjects ANOVA. The results (Fig. 1A) revealed a significant effect of stimulation condition, F(2, 28) = 4.52, P = 0.015, η2 = 0.139. Importantly, we observed a significant interaction between shared wrong association and stimulation condition, F(2, 28) = 3.22, P = 0.047, η2 = 0.10, since the proportion of correct solutions was higher during right tACS compared with both sham, t(28) = 2.27, P = 0.031, Cohen’s d = 0.450, and left tACS, t(28) = 2.99, P = 0.006, Cohen’s d = 0.555, only for the RAT items with shared wrong associations; there was no significant difference between these conditions for the items without shared wrong associations (P > 0.2). There was no difference between left tACS and sham for either shared or nonshared items (P > 0.2). Unsurprisingly, there was a significant main effect for shared wrong association, F(1, 28) = 8.17, P = 0.008, η2 = 0.226, since the accuracy was expectedly higher for items which did not have a shared wrong association.
Fig. 1.
Effects of tACS on performance in the RAT. (A) Proportion of correct solutions during left, sham, and right tACS averaged over items with vs. without shared wrong semantic associations. (B) Relative efficacy index during each stimulation condition (left, sham, and right temporal tACS). (C) Relative efficacy index for each condition for items which have a shared wrong associated word between two of the cues vs. the ones that did not (without shared wrong association). (D) Relative efficacy index for items with zero, one, and two or more shared wrong associations for left (Left), sham (Middle), and right (Right) tACS stimulation. Error bars represent ±1 SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
To compare how successful the stimulation was for each of the RAT items according to their semantic associations, we calculated the relative efficacy index for each RAT item (Materials and Methods) as the difference between the proportion of correct solutions in one condition (e.g., right tACS) and the average of the proportion of correct solutions in the other two conditions (e.g., sham and left tACS). Positive (negative) values of the index indicate a larger (smaller) proportion of correct solutions under a given stimulation/sham condition in relation to the average of the other two. The mean efficacy index for each condition is presented in Fig. 1B. A repeated-measures ANOVA with stimulation condition (left tACS, sham, and right tACS) as a factor revealed that more RAT items were correctly solved during the right tACS stimulation compared with the left tACS and sham, F(2, 268) = 3.593, P = 0.029, η2 = 0.026. Further, we observed a significant linear trend in solved RAT items from left, sham, to right, F(2, 268) = 6.04, P = 0.015, η2 = 0.043, tACS. Participants correctly solved more RAT items during right than during left stimulation (P = 0.015, Cohen’s d = 0.425) and sham (P = 0.029, Cohen’s d = 0.381), but there was no difference between left tACS and sham (P = 0.612).
Next, we probed whether the items with shared wrong associations were more likely to be solved during right tACS compared with left tACS and sham, and whether this effect was stronger on items with more shared wrong associations. The relative efficacy index was analyzed in a 3 (shared wrong association: 0, 1, ≥2) × 3 (stimulation condition) mixed-design ANOVA. We observed a significant main effect of stimulation condition, F(2, 242) = 6.06, P = 0.002, η2 = 0.052, as well as a significant interaction between stimulation condition and shared wrong association, F(4, 242) = 2.57, P = 0.038, η2 = 0.041. The effectiveness of right tACS increased with the number of shared wrong associations (Fig. 1D), whereas the opposite was true for left tACS: within-subject effects for the interaction between stimulation condition and shared wrong association, F(2, 121) = 4.894, P = 0.009, η2 = 0.075. The right tACS efficacy was higher on RAT items with two or more associations, as evidenced by a large effect size: ≥2 vs. 0 shared wrong associations (Cohen’s d = 0.848, CI: 0.811–0.886), t(73) = 4.59, P < 0.001. Sham stimulation efficacy was independent of the items’ semantic associations (P > 0.3).
Experiment 2
The second experiment was designed to investigate the role of alpha oscillations in inhibiting strong misleading associations in a new group of participants. Based on the semantic analysis we performed for Exp. 1, we selected a set of 45 RAT items, which share a misleading semantic association, and another set of 45 RAT items, which do not. Of note, these two sets were matched for difficulty based on the performance accuracy in Exp. 1. We conducted an EEG study comparing IAF oscillatory power in response to RAT items containing shared versus nonshared associations. This experiment was also designed to analyze the frequency and spatial specificity of the differences between shared versus nonshared RAT items. We hypothesized that, to solve RAT items with a shared wrong association, participants would need to actively inhibit the prominent, but incorrect association to reach the desired solution. Therefore, on the neural level, we predicted that RAT items with shared wrong associations would elicit stronger right temporal IAF power compared with the nonshared RAT items. Further, correct responses to shared items were predicted to be associated with higher IAF power than incorrect responses, due to successful inhibition of the wrong association. At the behavioral level, we predicted that shared RAT items would induce a higher rate of false alarms (incorrect responses), as suggested previously (6).
Fig. 2A shows the proportion of correct solutions with or without shared wrong association; no difference between the two was observed, t(56) = −1.041, P = 0.302, Cohen’s d = 0.138, showing that the shared and nonshared categories are matched for difficulty, as expected since the two sets of items (shared and nonshared) were earlier matched for accuracy. Fig. 2B shows the proportion of incorrect solutions for the two types of RAT items; as predicted, the participants made more mistakes on items with shared wrong associations compared with nonshared (paired t test), t(56) = −3.756, P < 0.001, Cohen’s d = 0.498, suggesting that shared items induced more false alarms compared with nonshared items. Fig. 2C shows the proportion of no responses or time-out trials; a paired t test revealed that participants tended to answer more to the items with shared associations, t(56) = 3.865, P < 0.001, Cohen’s d = 0.512, which is not surprising since misleading associations might cause the participants to provide the associate word as a solution.
Fig. 2.
RAT performance accuracy. Proportion of (A) correct solutions, (B) incorrect solutions, and (C) time-outs (nonresponses) for nonshared (blue) and shared (red) items. Error bars represent ±1 SEM. ***P < 0.001.
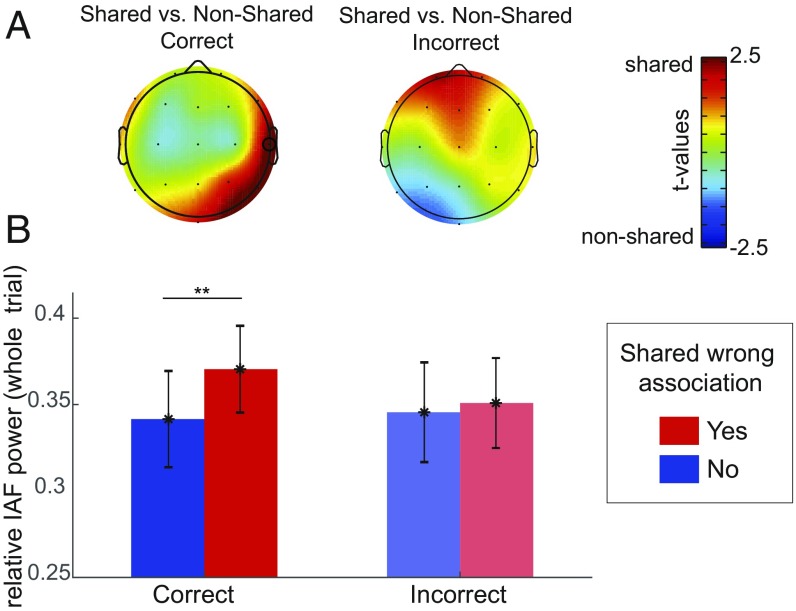
For EEG data, we compared relative power of the IAF in response to RAT with versus without shared wrong associations for correct and incorrect solutions (Fig. 3). IAF power values were analyzed by a three-way repeated measures ANOVA with shared wrong association (yes, no), accuracy (correct, incorrect), and region of interest (ROI) (right frontal, RF; left frontal, LF; right temporal, RT; left temporal, LT; right parietal, RP; left parietal, LP; and midcentral, MC) as factors. We found that IAF power was higher for shared compared with nonshared items but the effect was dependent on the ROI: interaction between shared wrong association and ROI, F(6, 246) = 3.775, P = 0.001, η2 = 0.084; and a three-way interaction between shared wrong association, ROI, and accuracy, F(6, 246) = 2.251, P = 0.039, η2 = 0.052. There was no main effect for accuracy, F(1, 41) = 2.432, P = 0.127, η2 = 0.056; or shared wrong association alone, F(1, 41) = 0.185, P = 0.669, η2 = 0.005; or interactions between the two, F(1, 41) = 0.431, P = 0.515, η2 = 0.010, indicating that the effects of shared wrong associations on alpha power was specific to the ROIs and dependent on whether the item was solved correctly. To investigate the interaction further, we compared alpha power between shared and nonshared on each of these ROIs (t maps shown in Fig. 3A). We observed that for correctly solved trials, individualized frequency alpha power was higher when the participants were solving shared compared with nonshared items: RT, t(41) = 2.685, P = 0.010, Cohen’s d = 0.416. IAF power at the right temporoparietal electrode was also higher during RAT items with shared associations, t(41) = 2.395, P = 0.021, Cohen’s d = 0.369, but not at the RP region, t(41) = 1.904, P = 0.064, Cohen’s d = 0.293 (see Materials and Methods for ROI definition).
Fig. 3.
Individual alpha power during remote associate items with and without wrong shared associations. (A) Topographical distribution of the differences represented as t values in the relative individual alpha power (IAF, log10). (B) Average relative individual alpha power (IAF, log10) at the right temporal electrode (highlighted in A) averaged over the whole trial, separately for correct and incorrect responses in RAT items with shared wrong associations (red) and without (blue). **P < 0.025.
To investigate whether this effect was specific to alpha oscillation, we conducted the same analysis on the average power over alpha band power (8–12 Hz) and also over the traditional frequency bands, including theta (4–8 Hz), beta (13–30 Hz), and gamma (30–40 Hz). The results showed that the effects were nonsignificant in other frequency bands (P > 0.05) except for the alpha frequency band, in which we observed similar effects compared with the IAF (SI Appendix, Fig. S2).
Experiment 3
Exps. 1 and 2 focused on investigating the role of alpha oscillations in inhibiting strong associations in a RAT task. However, if right temporal alpha oscillations are indeed associated with the inhibition of obvious associations in general, we expected that they would also promote more remote responses in other tasks involving creative cognition. Therefore, we conducted a third experiment to investigate the effects of right temporal tACS on the alternative uses task (34), a commonly employed measure of divergent thinking (i.e., capacity to generate a number of original ideas). A new sample of participants was asked to generate alternative uses to commonly used objects while receiving either sham, left tACS, or right tACS at their IAF (Materials and Methods) based on their resting-state EEG.
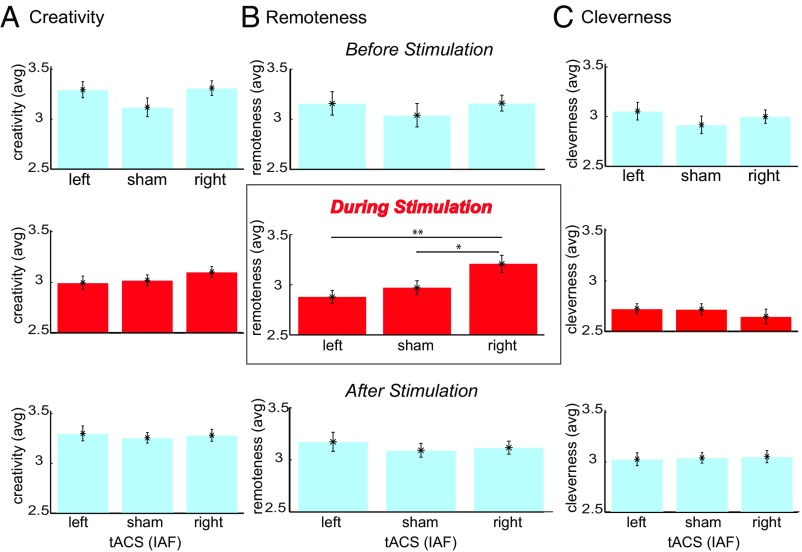
Three raters, blind to the conditions (double-blinded), rated each response for general creativity, remoteness, and cleverness. The ratings were based on items generated before, during, and after tACS (left, sham, and right IAF). We tested each period separately because the objects used during the stimulation were different (Materials and Methods). As the effects of tACS are mainly limited to the stimulation period, we expected that the effects of right IAF tACS would be significant during stimulation. For each participant, we calculated the average fluency (number of nonobvious responses) and the average ratings for general creativity, remoteness, and cleverness (Materials and Methods). Since we expected the effects to be most significant for remoteness ratings, we analyzed each rating separately by a one-way ANOVA with stimulation condition as a within-subjects factor. We predicted that the participants would come up with more remote responses during the right temporal alpha stimulation. We did not run a mixed ANOVA due to the fact that the items in the pretest and posttest were counterbalanced but the items during the stimulation were always the same (see Materials and Methods for more details).
First, we observed no significant differences between groups in the pretest for any of the measures, including fluency, F(2, 33) = 0.38, P = 0.688; general creativity, F(2, 33) = 1.66, P = 0.206; remoteness, F(2, 33) = 0.42, P = 0.663; and cleverness, F(2, 33) = 0.73, P = 0.489, suggesting no preexisting differences between groups. Second, during tACS, we observed, as predicted, a significant effect of stimulation condition on the remoteness of the uses, F(2, 33) = 5.27, P = 0.010, partial η2 = 0.24, but not on their general creativity, F(2, 33) = 0.94, P = 0.401, partial η2 = 0.054; fluency, F(2, 33) = 0.89, P = 0.421, partial η2 = 0.051; or cleverness, F(2, 33) = 0.48, P = 0.623, partial η2 = 0.028. Post hoc contrasts revealed that the right IAF tACS group came up with significantly more remote items compared with both left IAF tACS (P = 0.003, Cohen’s d = 1.3, CI = 1.18–1.39) and sham (P = 0.030, Cohen’s d = 0.92, CI = 0.82–1.03) groups (Fig. 4). There was no significant difference between sham and left IAF tACS (P = 0.385). Third, we observed that these effects vanished in the posttest period (i.e., after stimulation had ended) as there was no difference between groups in relation to the remoteness of the ideas, F(2, 33) = 0.33, P = 0.724, partial η2 = 0.019, or in any other measure including fluency, F(2, 33) = 0.80, P = 0.458, partial η2 = 0.046; general creativity, F(2, 33) = 0.129, P = 0.879, partial η2 = 0.008; and cleverness, F(2, 33) = 0.46, P = 0.955, partial η2 = 0.003.
Fig. 4.
Average ratings for AUT responses before, during, and after tACS (vs. sham). (A) Averaged creativity ratings for items before (Top, blue), during (Middle, red), and after (Bottom, blue) left, sham, and right IAF tACS. (B) The same analysis as in A for remoteness. (C) The same analysis as in A and B for the cleverness ratings. The error bars represent ±1 SEM. **P < 0.01, *P < 0.05.
Experiment 4
In Exp. 3 we demonstrated that stimulating right temporal alpha at the IAF is associated with an increase in remoteness of the responses (or ideas) generated during a divergent thinking task. Since the stimulation was delivered during the task, we tested whether IAF would be higher for more remote items. To address this question, we measured EEG while a new sample of participants generated a number of different ideas in an alternative uses task (AUT). We measured power at each participant’s IAF peak during the generation of each separate idea. All responses were judged by raters blind to the experimental conditions (Materials and Methods).
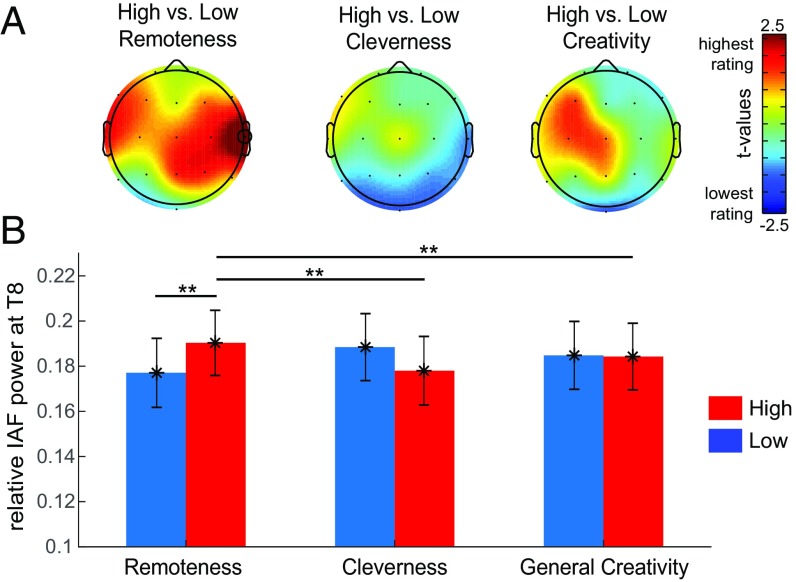
We compared IAF power on trials with average ratings above (high) or below (low) the median using a 3 (rating type: remoteness, cleverness, general creativity) × 2 (performance: high vs. low) × 7 (ROI: LF, LT, LP, ML, RF, RT, RP) within-subjects ANOVA. We observed a significant three-way interaction between rating type, performance, and ROI, F(12, 1476) = 2.030, P = 0.019, partial η2 = 0.020, since we only observed significant differences in IAF between high and low remoteness ratings. To investigate the interaction further, we ran additional 2 (performance: high vs. low) × 7 (ROI: LF, LT, LP, MC, RF, RT, RP) ANOVAs per rating type. We observed a significant interaction between performance and ROI only for remoteness ratings, F(6, 774) = 3.454, P = 0.002, partial η2 = 0.026, but not for cleverness, F(6, 774) = 1.349, P = 0.233, partial η2 = 0.010, or for general creativity, F(6, 774) = 0.738, P = 0.619, partial η2 = 0.006. The topography of the differences between high and low performance on each rating (Fig. 5) provides evidence that the differences between items with high vs. low remoteness peaked at the right temporal electrode, t(123) = 2.756, P = 0.007, Cohen’s d = 0.247. There was no statistically significant difference in IAF power between high and low performance on cleverness and general creativity in any of the ROIs (all contrasts P > 0.1). Furthermore, there was no main effect of rating performance, F(1, 123) = 0.092, P = 0.762, partial η2 = 0.001, indicating that the differences were not a result of a better performance in general. We conducted the same analysis in the traditional frequency bands (theta: 4–8 Hz, alpha: 8–12 Hz, beta:12–30 Hz, and gamma: 30–40 Hz) but observed no significant three-way interaction in any of them or a main effect of rating performance (for more details of the analysis and the topoplots of the contrasts see SI Appendix).
Fig. 5.
Differences between AUT answers rated as high vs. low in three rating dimensions. (A) Topographical distribution of the differences (paired t tests) between IAF power during the generation of ideas which were rated as high (above the median) vs. low (below the median) in remoteness, cleverness, and general creativity. (B) Mean and variability of IAF power at the right temporal electrode T8 during the generation of ideas rated as high and low in the three criteria. The error bars represent ±1 SEM. **P < 0.01.
Discussion
In this paper we provide evidence supporting the role of right temporal alpha oscillations in creative cognition. We suggest that alpha oscillations in the right temporal brain region shape inhibition of the most common or obvious associations. We presented evidence in support of this hypothesis in four separate experiments. In our first experiment, we observed that right temporal alpha tACS was most beneficial for those RAT items that required participants to override prominent but wrong candidate solutions, indicating that right temporal alpha oscillations play a critical role in the ability to override habitual, but misleading, associations. In a second experiment, we observed higher right temporal alpha power while the subjects were trying to solve RAT items with shared wrong associations. In a third experiment, we observed that the right temporal tACS at the IAF was associated with an increase in remoteness of uses in an alternative uses task, but not in cleverness or general creativity. In a fourth experiment, we observed that the participants showed higher right temporal IAF when they were generating items with higher compared with lower remoteness. Altogether, our results provided robust evidence supporting the hypothesis that right temporal alpha oscillations are involved in actively inhibiting strong semantic associations in both convergent and divergent thinking tasks. In the remainder of this discussion, we consider the principal ways our findings critically advance our understanding of the role of alpha oscillations in creative cognition, its neurophysiological mechanisms, and the limitations in our approach.
First, our findings support the hypothesis that right-lateralized alpha is a core feature of creative cognition, which might underlie our capacity to override strong semantic associations that are shaped by prior experience. Our results also support the hypothesis that exploration is mediated through active inhibition. This idea was put forward by Baror and Bar (7), who observed that when the cognitive load was high participants tended to fail in suppressing stronger semantic associations. Alpha oscillations have earlier been linked to the process of active inhibition (35): They do not merely signalize idle activity, but an energy consuming suppression process. Our study provides evidence suggesting that right temporal alpha oscillations may be critical to the inhibition of strong semantic associations.
Second, our results shed light on our understanding of both convergent and divergent creativity. Although the neuroscience of creativity has shown some inconsistent results in relation to its neural mechanisms (36), most of the EEG research on the topic showed a robust association between alpha oscillations and creativity both during task and at rest (for a review see ref. 11). This involvement with alpha oscillations is evident in a number of studies showing increases in right hemispheric alpha during creative ideation (12, 13, 37–39). For example, alpha oscillations increase over the right hemisphere during idea generation and this increase is higher for more creative ideas (13). Higher alpha oscillations are also predictive of cognitive insight (17). Furthermore, right hemispheric alpha power previous to a hint presentation in a RAT task was predictive of whether the participants would successfully use the hint to correctly solve the problem (16). These studies, though informative, have no control over how much the RAT items or specific tasks required the participants to override immediate semantic associations, yet this process is crucial in both divergent and convergent creative problem solving (40). For instance, if we need to generate alternative uses of a glass, first we must inhibit our past experience leading to think of a glass as a container. Our study demonstrates that right temporal alpha oscillations are linked with overriding these strong associations in both convergent and divergent thinking.
Third, by providing a fine-grained analysis of two well-known creativity tasks (RAT and AUT), we offer an approach for the investigation of higher-order cognition and how it links to more basic neurophysiological processes. For instance, our findings support the account that right hemispheric alpha is involved in inhibiting common or more obvious associations which might get in the way of generating nonobvious creative solutions (i.e., remote associations). We provide evidence that inhibiting wrong semantic associations can be facilitated by alpha tACS on the right, but not left, temporal area. Previous tACS work (14) showed a general effect of frontal alpha (F3, F4, and Cz) on creativity, which could be related to general top-down mechanisms necessary to complete the task rather than specific cognitive processes associated with higher originality of the responses. This is consistent with a previous EEG study (12) showing that both convergent and divergent creativity were associated with higher prefrontal alpha oscillations when these tasks are done under higher internal attentional demand. It is possible that alpha oscillatory activity could represent different processes depending on the brain regions where they occur during creative ideation.
Alpha synchronization is known to represent a process of heightened attention by blocking both external and internal distractions, which is necessary for creativity and consistent with the role of alpha oscillations in active inhibition of distractions (8, 9). Previous studies showed that higher alpha-band power is associated with the suppression of distracting information in both working memory (e.g., ref. 10) and attentional tasks (e.g., ref. 30). For creative cognition, we suggest prominent associations between two cues (i.e., wrong candidate solutions) or between an object and its common use need to be inhibited to reach more remote ones. Our findings suggest that this inhibitory process is stronger in the right temporal area, which is a key region for processing semantic associations (18, 19, 21, 22). This is relevant since here we show the role of alpha oscillations in a task-relevant area. Considering that alpha oscillations were found to coordinate the timing of the action potentials (41), it has been suggested (35) that higher alpha frequency power leads to more precise timing of neuronal activity, and therefore reflects the temporal structure for the processes controlling the access to information stored in complex knowledge systems. Selective access to higher-order information would depend on inhibiting task-irrelevant memory entries. In our study, both tasks required semantic search for remote associations that might be facilitated by sustained inhibition of stronger associations, which could be considered as task-irrelevant memories. According to Klimesh et al. (35), higher alpha amplitude in task-relevant areas promotes inhibition by silencing weakly excited cells, inducing a pulsed pattern of action potentials in cells with higher excitation level (threshold), a process which would increase the signal-to-noise ratio in the region, shaping the access to the knowledge systems. Here we speculate that the inhibition of the obvious associations requires a similar tuning of semantic association brain regions. We suggest future studies to combine EEG and fMRI to investigate how alpha oscillations shape the inhibition of the semantic association networks, as in our study we did not have enough spatial resolution to understand the anatomo-functional substrates of this process. It is important to notice that the strongest effects were observed in the individual alpha frequency which we measured based on the peak power at the right temporal region. Although the effects were similar in the traditional alpha frequency band and also pronounced when we stimulated at 10 Hz, we cannot rule out that different findings could have emerged if we had compared the conditions using the individual alpha frequency of other regions or stimulated other regions at their own individual peak frequencies.
In summary, we provided robust evidence that the right temporal alpha oscillations play a critical role in the ability to override habitual, but misleading, associations during creative problem solving. Taking a less-traveled path is often considered an effective path to creativity (i.e., creative thinking calls for a break from habitual thinking and associations), and our findings support that the underlying cognitive mechanisms are served by the temporal alpha oscillations. To understand the processes underlying the production of novel and adequate ideas, we need to break down its constituent processes, dissecting creativity as much as possible at first, and then analyzing them in context, putting them back together through careful consilience.
Materials and Methods
All participants across four experiments gave written informed consent before the beginning of each experiment. The study protocols of Exps. 1 and 3 were approved by the local ethics committee at Goldsmiths, University of London. The study protocols of Exps. 2 and 4 were approved by the local ethics committee at Queen Mary University of London. All experiments were conducted in accordance with the World Declaration of Helsinki (1964).
Experiment 1.
Participants.
Thirty (15 females) right-handed participants were recruited from the student population at Goldsmiths, University of London. Participants received course credit or monetary reimbursement at a rate of £10 per hour. Exclusion criteria were a personal or family history of epilepsy and/or neuropsychiatric disorders, pregnancy, and the presence of any metallic or medical implants. Participants were also excluded if they took any recreational drugs within the past month or consumed any alcohol within 24 h preceding each experimental session. One participant took part in another experiment on RAT before completing this study and was excluded from analysis. The final sample (n = 29) was aged between 18–46 y (24.6 ± 5.9 y, mean ± SD).
Experimental design and task.
A counterbalanced, within-participants design was adopted; participants attended three separate stimulation sessions on three different days with an intersession interval of 7 d. In each session, participants completed a computerized version of the compound word version (24) of the remote associate task (1, 23) under one of three online tACS stimulation conditions: 10-Hz RT, 10-Hz LT, and sham stimulation. Participants were blind to the condition. On each RAT trial (SI Appendix, Fig. S1A), participants were shown three cue words (e.g., line/house/palm) and had to come up with the solution word (tree), which would form a valid compound word with each of the three cue words (treeline, treehouse, palm tree). The solution word can be joined either at the beginning or end of the cue words, and the resultant compound word may be one that would be written as one word, or as two separate words (with or without a hyphen). There were 45 trials per stimulation condition (counterbalanced; see SI Appendix, Supplementary Material and Methods for details).
Semantic word association.
We extracted the word association measures based on the largest database for word associations (31, 32), available online at www.smallworldofwords.com/new/visualize/#. This database draws word associations based on a large corpus of English words (12,000 English words, with over 70,000 participants) and was built based on primed associations by asking participants to give the strongest three associated words for a given word (32). For each cue and solution word of each RAT item (i.e., triplet or triad), we checked the top 20 associated words as listed in the database. To observe if there was a shared wrong association, we looked into the first 20 associated words for each cue and found whether the cues shared a same word as top association. Subsequently, we classified the RAT items according to whether or not they shared a wrong candidate solution (yes = 59/no = 65). Two additional measures (cue-solution and solution-cue association) were also employed as a control measure (SI Appendix, Supplementary Material and Methods and Additional Analyses Experiment 1).
tACS.
tACS was delivered using a Neuroconn DC-Plus Stimulator, a constant current device (NeuroConn Ltd.). Electrodes were positioned based on the international 10–20 EEG electrode placement system, with one electrode (5 cm × 7 cm) positioned over the vertex (Cz), and the target electrode (5 cm × 5 cm) positioned over either the left (T7) or right (T8) anterior temporal lobe, depending on the stimulation condition (SI Appendix, Fig. S1B). In each session, a 10-Hz sinusoidal current (1 mA peak to peak), with a zero-degree phase offset and no dc offset, was delivered via two saline-soaked sponge-covered rubber electrodes, attached to participants’ scalps with rubber head straps. The current was ramped up and down over 10 s at the beginning and end of stimulation. In both active stimulation sessions participants received 30 min of online stimulation. For the sham condition, the stimulation was delivered for just 30 s at the start and the current was subsequently ramped down and remained off for the remainder of the session. In both active sessions, stimulation began 5 min before commencement of the experimental task and then continued for the subsequent 25 min during which participants completed the computerized RAT. Across all sessions, electrode impedance was kept below 20 kΩ throughout.
Data analysis.
We calculated the accuracy as the percentage of correct solutions for each participant in each condition. To quantify the effectiveness of a stimulation condition on individual RAT item performance, we calculated an index, termed as the relative efficacy index, which was the difference between the proportion of correct solutions for the stimulation condition (e.g., right tACS) and the average of the proportion of correct solution for the other two conditions (left tACS and sham).
Experiment 2.
Participants.
Sixty-two neurologically healthy adults (39 female) aged between 18 and 27 y (20.47 ± 0.25 y, mean ± SD) took part in this experiment. All participants were native speakers of English and right-handed (self-reported). Three participants were excluded due to technical problems (computer crashed at the end and data were not recorded) and two more due to poor performance (<10 correct responses), resulting in 57 participants used for the behavioral analysis. For the EEG analysis, five participants were further excluded due to noisy EEG recording (coughing or muscle artifacts), resulting in a total of 52 participants (5 excluded due to behavioral data and 5 due to poor EEG quality). Because we focused on the comparisons between items with versus without shared associations which were correctly responded (vs. incorrect), we included in the analysis only participants who had at least five valid trials in each condition, resulting in a total number of 42 participants. All participants received a monetary compensation of £10 per hour for their participation.
Experimental task and procedures.
The task was identical to the one in Exp. 1 (SI Appendix, Fig. S1A) except that there were 90 RAT items in total. There were 45 items with one or two shared wrong associations and 45 items with no shared associations (Experiment 1). We selected these 90 items strategically by excluding items that presented ceiling (>90% correct) or floor (<10% correct) effects to control for difficulty. Further, two categories were matched for difficulty (P > 0.05; i.e., no significant differences between the accuracy of the shared versus nonshared items based on participants’ performance in Exp. 1). The presentation order of RAT items was randomized across participants.
EEG recording and analysis.
The EEG was recorded using a Starstim 20 (Neuroelectrics) and preprocessed according to standard procedures (SI Appendix, Supplementary Material and Methods). To compute the time-frequency representation (TFR), the EEG signal (entire duration from stimulus presentation to response) was convolved with a complex Morlet wavelet on a trial-by-trial basis. The TFR was calculated from 2 to 40 Hz, in steps of 0.5 Hz, using six-cycle wavelets. The TFR values were averaged for each of the four conditions—correct shared, correct nonshared, incorrect shared, and incorrect nonshared—for each participant over the whole epoch. The IAF was calculated as the frequency with the highest power from 8 to 12 Hz at the right temporal electrode (T8). The mean IAF was 10.02 Hz (SD = 1.05).
Data analysis.
For behavioral data, we compared the proportion of correct and incorrect (false alarms) responses, reaction times, and insight ratings of each participant for shared versus nonshared items. For EEG data, we compared brain responses to shared and nonshared RAT items separately for correct and incorrect solutions; time-out trials were excluded from future analysis. Spectral power in each frequency immediately following the RAT item presentation (whole trial and also 0–1 s) was log-transformed (base 10) due to its positively skewed distribution and divided by the total (average) (2–40 Hz). Therefore, we analyzed relative power in each frequency band, theta (4–8 Hz), alpha (8–12 Hz), beta (12–30 Hz), and gamma (30–40 Hz), as well as the IAF, defined as the frequency with the highest power from 8 to 12 Hz (±2 Hz).
Experiment 3.
Participants.
Thirty-six participants aged between 19 and 35 y (23.9 ± 4.45 y, mean ± SD) took part in this study in exchange for course credit or a monetary reimbursement at £10 per hour. Participants were randomly assigned to one of the three conditions: left, sham, and right tACS. There were no differences between age and sex distribution between groups. Standard exclusion criteria were applied (the same criteria for Exps. 1 and 2).
AUT.
In this divergent thinking task (34), participants were asked to come up with unusual uses for an everyday object within a time period of 2 min per object. There were two sets, one containing four objects (set 1: tin can, newspaper, spoon, baseball cap) and another containing three objects (set 2: brick, shoe, cardboard box). The first set was used before and after the stimulation (two objects each, counterbalanced across participants), and the second set was used during the stimulation (presented in random order). Additionally, in the poststimulation period, the objects presented before the stimulation were presented again, to check for changes in performance of the new versus old objects. The order of the objects was alternated (each subsequent participant started with a different order).
Creativity ratings.
Responses were rated by three independent evaluators who were blind to the conditions and to the objectives of the experiment. We used the consensual assessment technique, CAT (42), which is considered by some as the gold-standard method for assessing creativity (43). CAT relies on intuitive ratings by two or more trained evaluators and has been successfully used to evaluate creativity in previous studies (e.g., refs. 44 and 45). Ratings of creativity have been based on the idea that creativity depends on three core factors: uncommonness, remoteness, and cleverness (46, 47). According to the three-factor definition, uncommonness relates to how unique ideas are (inversely related to their frequency), whereas remoteness refers to how far the suggested use for an object is from its common or everyday use (48). Cleverness in this context refers to how insightful, ironic, humorous, fitting, or smart a given use is. To investigate how alpha oscillatory activity relates to each of these factors, the judges provided ratings of all responses (presented in random order) on three attributes separately: (i) general creativity, how creative they felt that response was based on intuition and their own ideas of creativity; (ii) remoteness, how remote they thought that the idea was from the original use; and (iii) cleverness, how clever or appropriate the idea was. We observed a reasonable agreement between the three raters (intraclass correlation, IC) for general creativity (IC = 0.67; CI: 0.64–0.70) and remoteness (IC = 0.70; CI: 0.68–0.72) and a slightly reduced agreement on the cleverness judgments (IC = 0.56; CI: 0.50–0.62). The ratings of three judges were z-scored (all responses, per object) and averaged for analysis.
EEG and tACS protocol.
EEG was recorded before the brain stimulation session using a StarStim (Neuroelectrics) with eight channels. The EEG was recorded at a sampling frequency of 500 Hz, referenced to the arithmetic average of the left and right mastoids, high-pass-filtered at 1 Hz, and low-pass-filtered at 45 Hz. Automatic artifact rejection was applied at ±85 µV. Power was estimated in each frequency from 1 to 45 Hz in steps of 0.5 Hz using Welch’s periodogram (50% overlap). For tACS, the stimulation frequency was set at the alpha peak frequency (largest power during eyes-closed resting period, from 8 to 12 Hz) at the stimulated region (LT or RT) and the current was 1 mA (peak to peak). For the sham group, half were stimulated (only ramp up) at the IAF on the LT and the other half at the RT. The mean IAF for the left tACS group was 10.00 (SD = 0.64) and for the right tACS it was 9.99 (SD = 1.16). There was no significant difference in the IAF between the left and the right tACS groups, t(22) = 0.027, P = 0.979.
Procedures.
At the beginning, participants were instructed to keep their eyes closed for a period of 3 min while their EEGs were recorded; we estimated the IAF from this EEG recording. Subsequently, the participants responded to two practice items and carried on with the pretest task. Following the pretest, the EEG electrode corresponding to the stimulation condition (left or right) was replaced by a round rubber stimulation electrode (25 cm2) soaked in saline solution. In each session, a sinusoidal current (1 mA peak to peak) at the individual alpha peak frequency, with a zero-degree phase offset and no dc offset, was delivered via two saline-soaked sponge-covered rubber electrodes. One electrode was positioned on either T8 (RT) or T7 (LT) and the other was always positioned at Fz. During sham, half of the participants had the electrodes positioned at T8-Fz and the other half at T7-Fz. The participants were blind to the stimulation condition. The AUT started after 5 min of the start of the stimulation. The total duration of the stimulation was 25 min, during which the participants performed three AUT items and two figural creativity tasks (not analyzed in this paper). Following the stimulation, the stimulation electrode (T7 or T8) was removed, the area was cleaned, and the EEG electrodes were placed. The signal was visually inspected to ensure good quality. The EEG was recorded immediately after the signal passed this check, including 3-min eyes-closed and 3-min eyes-open (fixating on a cross on the wall) recordings. Following the EEG, the participants completed the AUT and figural creativity tasks.
Experiment 4.
Participants.
One-hundred thirty participants (67 females) aged between 18 and 32 y (21.2 ± 2.63 y, mean ± SD) took part in this experiment in exchange for course credit or a monetary reimbursement at a rate of £7.5 per hour. The exclusion criteria were the same as in the previous experiments.
AUT.
The experimental task was the same as in Exp. 3. The participants were presented with one object (e.g., a table) and were asked to generate unusual uses for it within a 2-min time period. The participants were instructed to fixate at the center of the screen while thinking and press a button to enter an idea. Once they typed and confirmed this idea, they kept generating other ideas until the 2 min were finished. In total, participants were presented with four objects (table, shoe, tin can, and umbrella) and provided an average of 25 ideas for all of the objects (SD = 11.8, range: 2–67).
EEG recording and analysis.
The EEG was recorded using a Starstim 20 (Neuroelectrics) and preprocessed according to standard procedures (SI Appendix, Supplementary Material and Methods). The IAF was estimated as with procedures identical to Exps. 2 and 3. For estimating the power spectrum during the generation of ideas in the AUT trials, we used the whole epoch, from the word presentation to the button press (to type the response). We only used those epochs which contained more than 2 s of usable data. We estimated alpha power using Welch periodogram with 1-s time windows with an overlap of 50%. The spectrum was first estimated from 4 to 40 Hz in steps of 1 Hz. The IAF was defined as earlier. The mean IAF was 9.73 (SD = 1.15). For normalization, we divided the power in the IAF adjusted band power (peak ±2 Hz) by the average power (log10) of the whole spectrum (4–40 Hz).
Creativity ratings.
As in Exp. 3, each response to the AUT was rated for general creativity, remoteness, and cleverness, on a scale from 0 (least) to 10 (most), as described in Experiment 3. Due to the large number of responses (130 participants, 4,810 responses in total), two raters rated all responses related with two objects, and other two raters another two objects. Another two raters rated the entire pool of responses. The ratings were subsequently z-scored separately, per item and per rater. This procedure resulted in a good agreement (IC) between raters for the creativity (α = 0.88), remoteness (α = 0.89), and cleverness (α = 0.84). This procedure resulted in an average of 12 ideas per condition (high and low), with the average idea (±SD) of 12.8 (SD = 6) for high and 12.6 (SD = 5.88) for low remote, 12.8 (SD = 5.9) for high and 12.7 (SD = 5.9) for low cleverness, and 12.8 for high (SD = 6) and 12.6 (SD = 5.9) for low creativity. There was no difference in the number of trials between any of the conditions (P > 0.8). However, because some participants had a low number of ideas, we conducted the main analysis using data of the participants who had a minimum of five ideas per condition (n = 124). Nonetheless, we present the analysis with all participants in SI Appendix.
Data Analysis.
We compared the whole-epoch alpha band power (IAF ± 2 Hz) during the idea generation phase (or “thinking time”), that is, when the subjects were engaged with generating ideas. For each participant, we selected the trials with ratings higher or lower than the median for each rating individually (remoteness, cleverness, and general creativity). We calculated the relative alpha power on the individual alpha frequency band (as in Exps. 2 and 3) in the low versus high rating trials of each subject.
Supplementary Material
Acknowledgments
We thank Francesca Zingales, Rawan Hassan, Leyla Al-Ashaab Mendiolea, Naml Hussain, Ellie York, and Ayesha Khan for helping to collect EEG data. We were supported by the CREAM project funded by European Commission Grant 612022. This publication reflects the views only of the authors, and the European Commission cannot be held responsible for any use which may be made of the information contained therein.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1811465115/-/DCSupplemental.
References
- 1.Mednick SA. The associative basis of the creative process. Psychol Rev. 1962;69:220–232. doi: 10.1037/h0048850. [DOI] [PubMed] [Google Scholar]
- 2.Borge-Holthoefer J, Arenas A. Semantic networks: Structure and dynamics. Entropy (Basel) 2010;12:1264–1302. [Google Scholar]
- 3.Collins AM, Loftus EF. A spreading-activation theory of semantic processing. Psychol Rev. 1975;82:407–428. [Google Scholar]
- 4.Kenett YN, Anaki D, Faust M. Investigating the structure of semantic networks in low and high creative persons. Front Hum Neurosci. 2014;8:407. doi: 10.3389/fnhum.2014.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kenett YN, et al. Flexibility of thought in high creative individuals represented by percolation analysis. Proc Natl Acad Sci USA. 2018;115:867–872. doi: 10.1073/pnas.1717362115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta N, Jang Y, Mednick SC, Huber DE. The road not taken: Creative solutions require avoidance of high-frequency responses. Psychol Sci. 2012;23:288–294. doi: 10.1177/0956797611429710. [DOI] [PubMed] [Google Scholar]
- 7.Baror S, Bar M. Associative activation and its relation to exploration and exploitation in the brain. Psychol Sci. 2016;27:776–789. doi: 10.1177/0956797616634487. [DOI] [PubMed] [Google Scholar]
- 8.Bonnefond M, Jensen O. The role of gamma and alpha oscillations for blocking out distraction. Commun Integr Biol. 2013;6:e22702. doi: 10.4161/cib.22702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haegens S, et al. Laminar profile and physiology of the α rhythm in primary visual, auditory, and somatosensory regions of neocortex. J Neurosci. 2015;35:14341–14352. doi: 10.1523/JNEUROSCI.0600-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sauseng P, et al. Brain oscillatory substrates of visual short-term memory capacity. Curr Biol. 2009;19:1846–1852. doi: 10.1016/j.cub.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 11.Fink A, Benedek M. EEG alpha power and creative ideation. Neurosci Biobehav Rev. 2014;44:111–123. doi: 10.1016/j.neubiorev.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benedek M, Bergner S, Könen T, Fink A, Neubauer AC. EEG alpha synchronization is related to top-down processing in convergent and divergent thinking. Neuropsychologia. 2011;49:3505–3511. doi: 10.1016/j.neuropsychologia.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwab D, Benedek M, Papousek I, Weiss EM, Fink A. The time-course of EEG alpha power changes in creative ideation. Front Hum Neurosci. 2014;8:310. doi: 10.3389/fnhum.2014.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lustenberger C, Boyle MR, Foulser AA, Mellin JM, Fröhlich F. Functional role of frontal alpha oscillations in creativity. Cortex. 2015;67:74–82. doi: 10.1016/j.cortex.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheth BR, Sandkühler S, Bhattacharya J. Posterior beta and anterior gamma oscillations predict cognitive insight. J Cogn Neurosci. 2009;21:1269–1279. doi: 10.1162/jocn.2009.21069. [DOI] [PubMed] [Google Scholar]
- 16.Sandkühler S, Bhattacharya J. Deconstructing insight: EEG correlates of insightful problem solving. PLoS One. 2008;3:e1459. doi: 10.1371/journal.pone.0001459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung-Beeman M, et al. Neural activity when people solve verbal problems with insight. PLoS Biol. 2004;2:E97. doi: 10.1371/journal.pbio.0020097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tranel D, Damasio H, Damasio AR. A neural basis for the retrieval of conceptual knowledge. Neuropsychologia. 1997;35:1319–1327. doi: 10.1016/s0028-3932(97)00085-7. [DOI] [PubMed] [Google Scholar]
- 19.Lambon Ralph MA, Cipolotti L, Manes F, Patterson K. Taking both sides: Do unilateral anterior temporal lobe lesions disrupt semantic memory? Brain. 2010;133:3243–3255. doi: 10.1093/brain/awq264. [DOI] [PubMed] [Google Scholar]
- 20.Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.St George M, Kutas M, Martinez A, Sereno MI. Semantic integration in reading: Engagement of the right hemisphere during discourse processing. Brain. 1999;122:1317–1325. doi: 10.1093/brain/122.7.1317. [DOI] [PubMed] [Google Scholar]
- 22.Jung-Beeman M. Bilateral brain processes for comprehending natural language. Trends Cogn Sci. 2005;9:512–518. doi: 10.1016/j.tics.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Mednick SA, Mednick MT. Examiner’s Manual: Remote Associates Test. Houghton Mifflin; Boston: 1967. [Google Scholar]
- 24.Bowden EM, Jung-Beeman M. Normative data for 144 compound remote associate problems. Behav Res Methods Instrum Comput. 2003;35:634–639. doi: 10.3758/bf03195543. [DOI] [PubMed] [Google Scholar]
- 25.Benedek M, Neubauer AC. Revisiting Mednick’s model on creativity-related differences in associative hierarchies. Evidence for a common path to uncommon thought. J Creat Behav. 2013;47:273–289. doi: 10.1002/jocb.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davelaar EJ. Semantic search in the remote associates test. Top Cogn Sci. 2015;7:494–512. doi: 10.1111/tops.12146. [DOI] [PubMed] [Google Scholar]
- 27.Smith KA, Huber DE, Vul E. Multiply-constrained semantic search in the remote associates test. Cognition. 2013;128:64–75. doi: 10.1016/j.cognition.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Klein A, Radia T. The usual and the unusual: Solving remote associates test tasks using simple statistical natural language processing based on language use. J Creat Behav. 2014;49:13–37. [Google Scholar]
- 29.Helfrich RF, et al. Entrainment of brain oscillations by transcranial alternating current stimulation. Curr Biol. 2014;24:333–339. doi: 10.1016/j.cub.2013.12.041. [DOI] [PubMed] [Google Scholar]
- 30.Kelly SP, Lalor EC, Reilly RB, Foxe JJ. Increases in alpha oscillatory power reflect an active retinotopic mechanism for distracter suppression during sustained visuospatial attention. J Neurophysiol. 2006;95:3844–3851. doi: 10.1152/jn.01234.2005. [DOI] [PubMed] [Google Scholar]
- 31.De Deyne S, Storms G. Word associations: Network and semantic properties. Behav Res Methods. 2008;40:213–231. doi: 10.3758/brm.40.1.213. [DOI] [PubMed] [Google Scholar]
- 32.De Deyne S, Navarro DJ, Storms G. Better explanations of lexical and semantic cognition using networks derived from continued rather than single-word associations. Behav Res Methods. 2013;45:480–498. doi: 10.3758/s13428-012-0260-7. [DOI] [PubMed] [Google Scholar]
- 33.Harkins SG. Mere effort as the mediator of the evaluation-performance relationship. J Pers Soc Psychol. 2006;91:436–455. doi: 10.1037/0022-3514.91.3.436. [DOI] [PubMed] [Google Scholar]
- 34.Guildford JP. The Nature of Human Intelligence. McGraw-Hill; New York: 1967. [Google Scholar]
- 35.Klimesch W, Sauseng P, Hanslmayr S. EEG alpha oscillations: The inhibition-timing hypothesis. Brain Res Brain Res Rev. 2007;53:63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 36.Dietrich A, Kanso R. A review of EEG, ERP, and neuroimaging studies of creativity and insight. Psychol Bull. 2010;136:822–848. doi: 10.1037/a0019749. [DOI] [PubMed] [Google Scholar]
- 37.Fink A, Graif B, Neubauer AC. Brain correlates underlying creative thinking: EEG alpha activity in professional vs. novice dancers. Neuroimage. 2009;46:854–862. doi: 10.1016/j.neuroimage.2009.02.036. [DOI] [PubMed] [Google Scholar]
- 38.Fink A, et al. The creative brain: Investigation of brain activity during creative problem solving by means of EEG and FMRI. Hum Brain Mapp. 2009;30:734–748. doi: 10.1002/hbm.20538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grabner RH, Fink A, Neubauer AC. Brain correlates of self-rated originality of ideas: Evidence from event-related power and phase-locking changes in the EEG. Behav Neurosci. 2007;121:224–230. doi: 10.1037/0735-7044.121.1.224. [DOI] [PubMed] [Google Scholar]
- 40.Koutstaal W, Binks J. Innovating Minds: Rethinking Creativity to Inspire Change. Oxford Univ Press; Oxford: 2015. [Google Scholar]
- 41.Haegens S, Nácher V, Luna R, Romo R, Jensen O. α-Oscillations in the monkey sensorimotor network influence discrimination performance by rhythmical inhibition of neuronal spiking. Proc Natl Acad Sci USA. 2011;108:19377–19382. doi: 10.1073/pnas.1117190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amabile TM. Social psychology of creativity: A consensual assessment technique. J Pers Soc Psychol. 1982;43:997–1013. [Google Scholar]
- 43.Baer J, McKool SS. The gold standard for assessing creativity. Int J Qual Assur Eng Technol Educ. 2014;3:81–93. [Google Scholar]
- 44.Beaty RE, et al. Robust prediction of individual creative ability from brain functional connectivity. Proc Natl Acad Sci USA. 2018;115:1087–1092. doi: 10.1073/pnas.1713532115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Benedek M, et al. Creating metaphors: The neural basis of figurative language production. Neuroimage. 2014;90:99–106. doi: 10.1016/j.neuroimage.2013.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Christensen PR, Guilford JP, Wilson RC. Relations of creative responses to working time and instructions. J Exp Psychol. 1957;53:82–88. doi: 10.1037/h0045461. [DOI] [PubMed] [Google Scholar]
- 47.Wilson RC, Guilford JP, Christensen PR. The measurement of individual differences in originality. Psychol Bull. 1953;50:362–370. doi: 10.1037/h0060857. [DOI] [PubMed] [Google Scholar]
- 48.Silvia PJ, et al. Assessing creativity with divergent thinking tasks: Exploring the reliability and validity of new subjective scoring methods. Psychol Aesthetics Creativity Arts. 2008;2:68–85. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.