Abstract
Hepatocellular carcinoma (HCC) is the fifth most common malignancy worldwide and the third most common cancer in Asia. HCC has heterogeneous etiologic and molecular profiles and a varied response to therapeutics. The high recurrence rate and curtailed survival in this cancer are attributed to its resistance to therapy. The ultimate goal is to develop a more effective personalized therapeutic strategy for HCC, but the first step is to develop a system for classifying the disease on the basis of molecular biomarkers. To that end, we performed mRNA and microRNA (miRNA) expression profiling in 100 HCC tissues. Clustering analysis of informative genes identified two robust subtypes, which were validated by an independent dataset. The subtype characterized by a cancer stem cell-like signature was clinically aggressive and associated with poor survival. Integrated analysis of miRNA and mRNA expression in this subtype showed that miR-148a was expressed at a significantly lower level in these tumors than in the other subtype. MiR-148a has been shown to directly suppress the expression of activin A receptor type 1 (ACVR1), a key receptor in the signaling pathway of the bone morphogenetic proteins (BMPs), which regulate many stem cell markers as well as the clinically important cytokine interleukin-8 (IL-8). Increased expression of ACVR1 and its downstream genes EPCAM, CD24, CD90, and IL-8 was associated with shorter survival in a larger cohort of 227 HCC cases. Introduction of miR-148a resulted in suppressed tumor phenotypes both in vitro and in vivo. Conclusion: We identified a clinically aggressive stem cell-like subtype of HCC that is characterized by an miR-148a-ACVR1-BMP-Wnt circuit. We propose that miR-148a may serve as a prognostic biomarker and therapeutic target for this subtype of HCC. (Hepatololgy 2015;61:574–584)
Liver cancer is the fifth most common cancer and the third most common cause of cancer death worldwide,1 and a leading cause of cancer deaths in China.2 Development of novel therapeutic strategies for hepatocellular carcinoma (HCC) is an urgent medical need, particularly for patients whose disease has a poor prognosis. The natural course and clinical outcome of HCC are heterogeneous, possibly because of the molecular heterogeneity.3 A number of studies have attempted to subclassify HCC on the basis of molecular data, but the subclasses defined in these studies often showed no association with patient survival, and molecular mechanisms underlying the subtypes were often not explained.4–6 To define the exact molecular changes in the subtypes and to identify the key molecular targets for development of new therapeutic strategies, integrated analysis of high-throughput data from multiple platforms is needed.7
MicroRNAs (miRNAs) have emerged as key regulators in gene expression networks affecting many biological processes, including cellular proliferation, differentiation, and apoptosis in liver cancer.8,9 MiR-NAs have also proven to be an important class of regulators in molecular subtypes of cancers.10 Thus far, very few studies have examined expression profiling of both mRNAs and miRNAs in tumor tissues from a large cohort of patients with HCC.
Here we report an integrated analysis of mRNA and miRNA expression profiling in 100 HCCs and a subsequent validation study that identified two distinct molecular subtypes of HCC; from this study, we identified an miRNA that might have therapeutic implications in the aggressive stem cell-like subtype of HCC.
Materials and Methods
Human Subjects (Also See Supporting Methods).
Study subjects were selected from the set of patients who underwent curative surgery for HCC at Tianjin Medical University Cancer Institute and Hospital between 2003 and 2011. Tianjin cohort1 of 100 tumor tissue samples analyzed in this study for mRNA and miRNA expression profiling was randomly selected from the patients who underwent surgery between 2003 and 2009, and Tianjin cohort2 of 197 tissue samples included in the subsequent independent validation study were selected from the patients who underwent surgery between 2004 and 2011. The gene expression data of the validation cohort (n = 225) was published earlier (accession number GSE14520).10
MiRNA and mRNA Expression Profiling.
MiRNA and mRNA expression profiling was con ducted by using Affymetrix Human Genome U133 Plus 2.0 arrays (Affymetrix, Santa Clara, CA) and GeneChip miRNA arrays (Affymetrix; including 7,815 miRNA probe sets from Sanger miRNA database V11), respectively. All of the procedures, including labeling and hybridization were conducted according to the manufacturer’s protocol and recommendations. A GeneChip scanner 3000 was used to scan the micro-array chips and the obtained images were analyzed by the GeneChip Operating Software for gene expression profiling. A GeneChip-compatible program was used for analysis of miRNA expression profiling data.
Results
Two Molecular Subtypes of HCC With Different Prognoses Identified by Consensus Clustering Analysis.
Consensus k-means unsupervised clustering of the 309 genes defined as highly variable in the 100 Tianjin cohort1 HCC samples identified two robust clusters (Supporting Fig. S1), and data were visualized according to the highly variable genes identified as significantly differentially expressed in the two clusters (Fig. 1A). To determine whether the same classification approach would yield the same two subtypes in another group of tumors, we analyzed transcriptome data from an independent set of 225 HCC (accession number GSE14520)11 by applying the same clustering approach of Tianjin cohort1 (n = 100). This analysis also yielded two distinct clusters, which were visualized using the same genes as used in Fig. 1A (Fig. 1B). The proportions of the two subtypes were similar in the two independent cohorts (Cluster 1, 26% in the Tianjin cohort1 and 33% in the validation set).
Fig. 1.
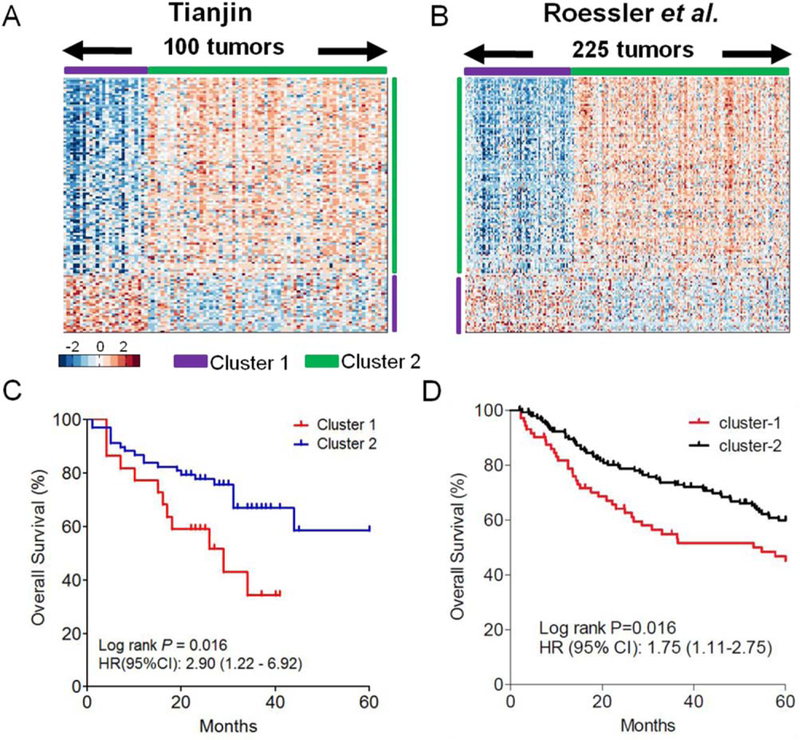
Identification of two gene expression subtypes in HCC. (A) Through clustering analysis with a list of highly varied genes generated by gene expression profiling, the 100 HCC samples were ordered on the basis of subtype prediction. Two major clusters were recapitulated by the genes that were significantly (P < 0.05) differentially expressed (2-fold or greater difference in magnitude). Some typical genes in each cluster are listed. Among the 190 differentially expressed genes, 50 were highly expressed in Cluster 1 and the other 140 were highly expressed in Cluster 2. (B) Gene order from the 100 HCC samples was maintained in the validation dataset (n = 225), a previously published cohort of HCC cases (Roessler et al.11). Cluster 1 in the validation set consisted of 75 samples and Cluster 2 of 150 samples. (C) In the initial set of 100 cases, overall survival was significantly lower for patients in Cluster 1 than for those in Cluster 2 (P = 0.016). (D) Consistent with this result, Cluster 1 in the validation dataset also had poorer survival (P = 0.016).
Kaplan-Meier survival curve analysis showed that patients in Cluster 1 of Tianjin cohort1 had poorer overall survival than those in Cluster 2 when the survival time was capped at 60 months (Fig. 1C; log rank P = 0.016, hazard ratio [HR]: 2.90, 95% confidence interval [CI]: 1.22–6.92). Similarly, Cluster 1 from the validation set also showed significantly poorer prognosis (Fig. 1D; log rank P = 0.016, HR: 1.75, 95% CI: 1.11–2.75). Thus, HCC comprises at least two molecular subtypes with distinct clinical outcomes.
Functions of the Molecular HCC Subtypes Characterized by Integrated mRNA and miRNA Analysis.
To characterize the biological functions of the two HCC transcriptome subtypes, we first performed a significance analysis of microarrays12 to identify signature genes that were specifically altered in each subtype. In all, we identified 821 genes in Cluster 1 that had a statistically significant 2-fold or greater difference in magnitude (P < 0.05) from the same genes in Cluster 2, of which 326 were down-regulated and 495 were up-regulated. The signature genes identified as overexpressed in the poor-survival subtype were significantly enriched in stem cell-related genes identified from published studies (P < 1.0E-05),6,13–15 including epithelial cell adhesion molecule (EPCAM), CD133, CD24, KRT19, KIT, and alpha-fetoprotein (AFP) (Fig. 2A). AFP has been used together with EPCAM to isolate cells with stem cell features15 and is a clinical serum biomarker of HCC.16 Other well-known markers of hepatic oval cells such as VIM, CD44, and CD90 were not included in the gene signature list because of the fold cutoff, but they exhibited significantly higher expression in Cluster 1 as well (Fig. S2). A hepatoblast subtype of HCC exhibiting stem cell-like features was identified and was likely driven by dysregulation of the AP-1 network.6 Notably, the genes comprising the AP-1 complex (i.e., FOS, JUNB, and FOSL2) and many of their reported down-stream targets were significantly up-regulated in Cluster 1 (P < 0.05, Fig. S3).
Fig. 2.
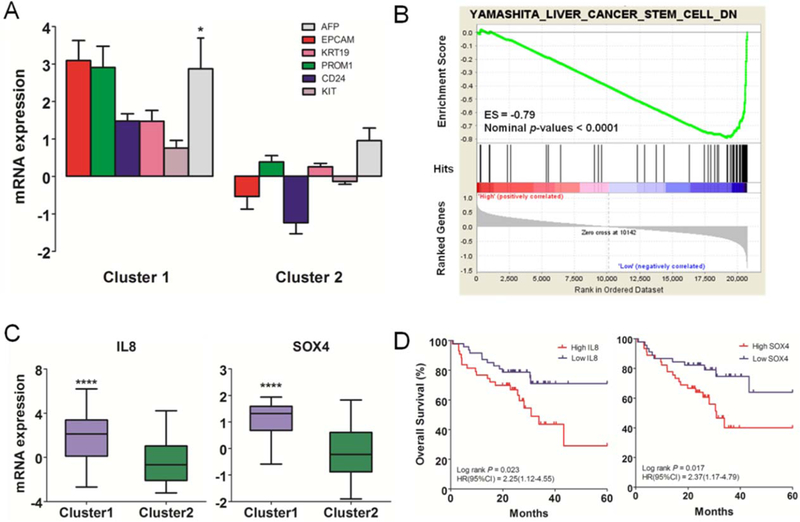
Functional annotation of gene expression subtypes. (A) Hepatic stem cell markers were enriched in the gene signature and their expression significantly higher (2-fold or greater change in magnitude) in Cluster 1. Expression of AFP, which encodes a biomarker now in clinical use, was also higher in Cluster 1. (B) Gene set enrichment analysis indicated that a set of stem cell genes specific to liver cancer was most significantly enriched in Cluster 1, with an enrichment score of −0.79 (nominal P-value < 0.0001). (C) IL-8 and SOX4 were highly expressed in Cluster 1, with a difference in magnitude of 2-fold or greater and statistical significance (P < 0.0001). (D) High levels of IL-8 and SOX4 were associated with poorer overall survival than low levels.
Next we performed gene set enrichment analysis (GSEA) of the genome-wide expression profiles to identify overrepresented gene sets. Our data identified six curated stem cell gene sets that were significantly enriched in Cluster 1, four of which were up-regulated and two were down-regulated (Fig. S4A). A leading edge analysis indicated limited overlap among these enriched sets (Fig. S4B), likely because they were derived from different cancer types. Strikingly, the down-regulated genes (YAMASHITA_LIVER_CANCER_STEM_CELL_DN) identified from the EPCAM(+) AFP(+) HCC sub-type of liver cancer, which had features of hepatic stem/progenitor cells, were most significantly enriched among the genes that were consistently down-regulated in Cluster 1, with an enrichment score of −0.79 and nominal P-value < 0.0001 (Fig. 2B). The most differentially regulated pathway was the hepatic fibrosis/hepatic stellate cell activation-related pathway in Cluster 1. Significant enrichment of both hepatic stem cell markers and stem cell gene sets prompted us to hypothesize that the HCCs in Cluster 1 might be derived from hepatic progenitor cells. On the other hand, the metabolism-related pathways were significantly overrepresented in Cluster 2 (Fig. S5). Taking together the gene signatures, GSEA results, and pathway analysis, we termed the two HCC subtypes “stem cell-like” and “metabolic,” respectively.
In addition to the stem cell markers already mentioned (Fig. 2A; Fig. S2), interleukin-8 (IL-8) and SOX4 were included in the gene signature for these HCCs, and their mRNA expression levels were significantly higher in Cluster 1 (Fig. 2C). SOX4 is known to be involved in neural progenitor cells and IL-8 is a cytokine involved in cancer metastasis.17–19 The expression levels of these two genes in the tumors were significantly correlated with overall patient survival (Fig. 2D).
Correlation of molecular subtype with clinicopathological features of the HCCs (Table S2) showed that the sub-types were not significantly associated with tumor stage or alcohol/smoking history, even though the stem cell-like subtype tended to be more invasive than the metabolic subtype. Interestingly, the patients with the stem cell-like subtype were significantly younger (P = 0.006, Table S2) and more likely to be female (P = 0.018).
MiR-148a-ACVR1 Circuitry Revealed by Integrated Analysis.
MicroRNA expression profiling of the same 100-sample set (Tianjin cohort1) provided an opportunity for integrated analysis to identify the miRNA-mRNA regulatory networks that may underlie the stem cell gene enrichment in the stem cell-like subtype. Using supervised analysis, we identified 10 miRNAs that were differentially expressed between the two transcriptome subtypes (P < 0.0001, Fig. 3A); among these, miR-148a exhibited the greatest difference and was down-regulated in the stem cell-like sub-type (P = 4.2E–7, Fig. 3A). The second most significantly differentially expressed miRNA, miR-181b, was overexpressed in the stem cell-like subtype, consistent with its known association with stem cell features.10 Integrated analysis of the down-regulated miRNAs and the signature gene expression data showed that miR-148a was significantly and negatively correlated with most of the overexpressed genes in the signatures (Fig. S6A), as compared with the other four significantly down-regulated miRNAs (Fig. S6B). Moreover, miR-148a expression was significantly correlated with patient overall survival (Fig. 3D). These results suggest that loss of miR-148a expression might be a key mechanism for up-regulation of stem cell signature genes in the poor-survival HCC subtype.
Fig. 3.

Integrative analysis of miRNA and mRNA expression profiles of the subtypes. (A) Supervised analysis of miRNA expression profiles of Cluster 1 and Cluster 2 of the initial 100 cases identified 10 differentially expressed miRNAs (statistical significance of each difference indicated in the bar graph). (B) ACVR1 mRNA expression was significantly higher in Cluster 1 (P = 0.02). (C) The ACVR1 3′-UTR has one binding site with miR-148a, as predicted from TargetScan. MHCC97H cells transfected with pmirGLO-ACVR1 and pmirGLO-ACVR1-Mut reporters, together with a miR-148a mimic or mimic negative control, miR-148a overexpression suppressed the activity of luciferase in the wild-type (P = 0.021) but not in mutant type (P = 0.596). (D) ACVR1 expression was negatively correlated with miR-148a expression (r = −0.22, P = 0.027). The 100 HCC patients were categorized into three groups based on miRNA-148a expression (low, expression <1; middle, 1 ≤ expression ≤2; high, expression >2), and overall survival in these three groups differed significantly (P = 0.03). (E) MiR-148a regulated Wnt signaling targets by down-regulating ACVR1 expression. Up-regulated genes in Cluster 1 are indicated in red, and the intensity of the red indicates the relative log2 ratio as shown in the color bar. The cyan lines and blockers represent the direction of the miRNAs, and + and − indicate positive and negative regulation of gene expression, respectively. Gray lines represent known physical interaction between genes connected.
To further evaluate the relationship between expression of miR-148a and survival in HCC, we performed quantitative reverse-transcription polymerase chain reaction (qRT-PCR) in 297 HCC tumor tissues (the 100 HCCs represented by the microarray [Tianjin cohort1] and 197 from the independent HCC cohort [Tianjin cohort2]). The baseline and clinical characteristics of both sets were similar (Table S3). In Tianjin cohort1, miR-148a expression in the stem cell-like subtype was significantly lower than in the metabolic subtype (P < 0.0001), which is consistent with the microarray data (Fig. S7). There was no significant association between miR-148a expression and baseline/ clinical characteristic except tumor size (P = 0.0147) in the 297 HCC tumor tissues (Table S4).
Of the 297 HCCs in the two Tianjin cohorts, those with low miR-148a expression were likely to be associated with significantly shorter overall survival (P = 0.0403, Fig. 4A) but not shorter recurrence-free survival (P = 0.393, Fig. 4D). Interestingly, the associations of miR-148a with overall survival and recurrencefree survival were significant only in men, and men with higher expression of miR-148a had more favorable overall and recurrence-free survival (P = 0.011 and P = 0.063, respectively; Fig. 4B,E). The associations between the expression of miR-148a and overall survival and recurrence-free survival in women were not statistically significant (P= 0.613 and P = 0.500, respectively; Fig. 4C,F). The results of univariate analysis of miR-148a expression with both overall and recurrence-free survival are summarized in Table S5.
Fig. 4.

Validation study of miR-148a expression by qRT-PCR. (A-C) The expression of miR-148a was significantly associated with overall survival in the group of patients taken as a whole (n = 297, P = 0.040, log-rank test). The association was significant in men (n = 253, P = 0.011, log-rank test) but not in women (n = 44, P = 0.613, log-rank test). (D-F) The association between miR-148a expression and recurrence-free survival was not significant in the entire group (n = 297, P = 0.393, log-rank test) or in women (n = 44, P = 0.500, log-rank test) but was marginally significant in men (n = 253, P = 0.063, log-rank test). Expression of miR-148a was calculated by use of tertile cutoffs in all patients. Red lines represent low expression, blue lines represent middle expression, and black lines represent high expression.
When we matched the 326 overexpressed genes in the stem cell-like subtype to the miR-148a conserved target genes predicted from TargetScan, only 11 genes (~3.3%) were computationally predicted to bind directly with miR-148a (Table S6), suggesting that an indirect mechanism, such as pathway regulation mediated through intermediate key node, may be at play. activin A receptor type 1 (ACVR1) expression was significantly negatively correlated with miR-148a and was significantly differentially expressed in Cluster 1 and Cluster 2 (Fig. 3B). ACVR1 is an important receptor of the bone morphogenetic protein (BMP) that is closely involved in regulation of the BMP/Wnt signaling frequently activated in stem cells, and has been reported previously to directly bind with miR-148a through its 3′-untranslated region (UTR).20 We validated this regulation by using a reporter gene assay in which the luciferase activity was decreased when cotransfection of MHCC97H cells with the pmirGLO-ACVR1 3′UTR-Luc construct and an miR-148a mimic (Fig. 3C). Consistent with this was our finding that many of the direct downstream targets of the Wnt signaling pathway (e.g., EPCAM,21 IL-8,22 and FOS23) were up-regulated in the stem cell-like subtype (Fig. 3E). Therefore, the miR-148a-ACVR1 circuit appeared to be highly active in the stem cell-like Cluster 1 of HCC.
Cancer Stem Cell Markers Are Prognostic in HCC.
The integrated genomic analysis defined a set of miR-148a-ACVR1 circuit genes, including cancer stem cell-regulatory genes and metastasis-related genes that are associated with poor survival in HCC. To determine whether these genes are indeed prognostic markers for HCC at the protein level in a larger cohort of HCC, we performed an immunohistochemical analysis on a tissue microarray representing the 227 HCC patients of the validation set. Immunostaining showed membranous and/or cytoplasmic staining for ACVR1, CD44, EPCAM, CD24, CD90, and KRT19 and nuclear and/or cytoplasmic staining for SOX4 (Fig. 5A,B; Fig. S8A). Of these HCCs, 54.1% stained positive for ACVR1, 20.4% for CD44, 40.3% for EPCAM, 59.9% for CD90, 67.1% for CD24, % for SOX4, and 9.2% for KRT19. Kaplan-Meier analysis demonstrated that positive staining for ACVR1 (P = 0.012), CD44 (P = 0.041), EPCAM (P = 0.0014), CD24 (P = 0.018), CD90 (P = 0.025), and IL-8 (P = 0.050) was associated with poor overall survival (Fig. 5C). Positive staining for ACVR1 (P = 0.050), CD44 (P = 0.035), CD24 (P = 0.040), and CD90 (P = 0.019) was associated with shorter recurrence-free survival (Fig. 5D). There was no statistically significant association between survival and expression of either SOX4 or KRT19 (Fig. S9B,C). Positive staining for EPCAM and IL-8 was not associated with recurrence-free survival (Fig. 5D).
Fig. 5.
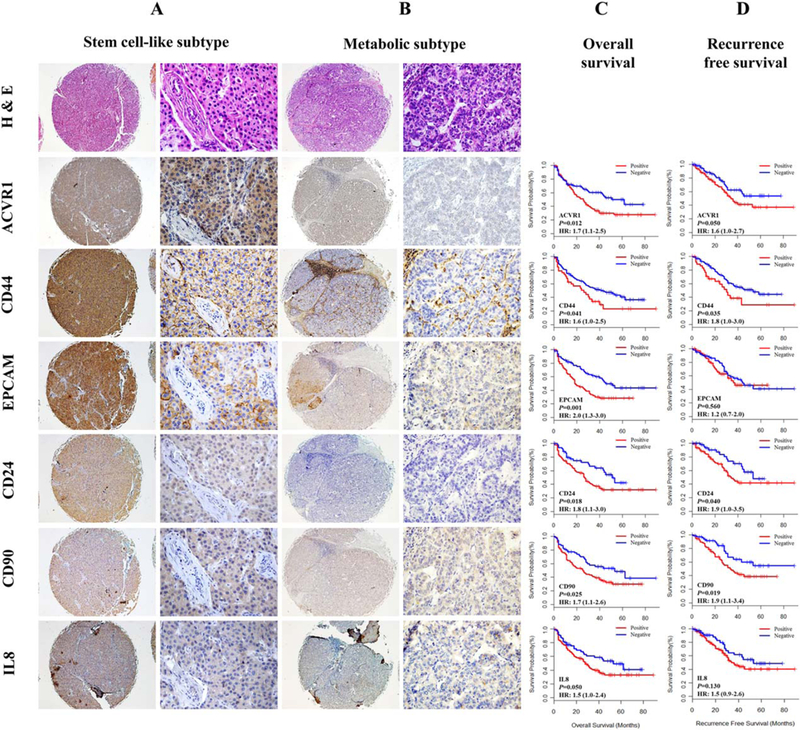
Expression of hepatic cancer stem cell-related proteins in HCC tissues related to HCC prognosis in validation study. (A,B) Hematoxylin & eosin and hepatic cancer stem cell-related protein immunostaining of stem cell-like subtype HCCs (A) and metabolic subtype HCCs (B) from the 227-case validation dataset. The staining images show high expression of ACVR1, CD44, EPCAM, CD24, CD90, and IL-8 in the stem celllike subtype HCC tissues (A) and low expression of these proteins in metabolic subtype HCC tissues (left panel: magnification ×40; right panel: magnification ×400). (C,D) Kaplan-Meier analysis of overall survival (C) and recurrence-free survival (D) according to expression of hepatic cancer stem cell-related proteins in the validation set. The expression of ACVR1, CD44, CD24, and CD90 was significantly associated with poor overall survival and recurrence-free survival. The expression of EPCAM and IL-8 was significantly associated with poor overall survival but not with recurrence-free survival. Red lines represent positive for protein markers, and blue lines represent negative for protein markers.
Overexpression of MiR-148a Suppresses Liver Cancer Cell Proliferation, Migration, and Invasion In Vitro and Inhibits Subcutaneous Growth of Liver Cancer Cells In Vivo.
To determine the effect of miR-148a overexpression on liver cancer cell properties, we treated liver cancer cells with miR-148a mimic or inhibitor. We transfected MHCC97H cells with miR-148a mimic or negative control; we found that transfection of miR-148a mimic led to inhibited cell proliferation, migration, and invasion, and there was the opposite effect when SMMC7721 cells were treated with miR-148a inhibitor or negative control (for details, see Supporting Results) (Fig. 6A–D). We next examined the effect of overexpression of miR-148a on tumor growth in BALB/C nude mice. MHCC97H cells (5.0 × 106 cells) were subcutaneously injected into both flanks of each mouse. Each mouse was treated with miR-148a mimic (1 nm/ mouse) or vehicle (negative control), and tumor volumes were measured twice a week. We found that tumor growth was significantly inhibited in the miR-148a mimic-treated group compared to the control group (P = 0.007; Fig. 6E). MiR-148a expression was significantly higher in the miR-148a mimic-treated group than in the control group (P = 0.007; Fig. 6F).
Fig. 6.
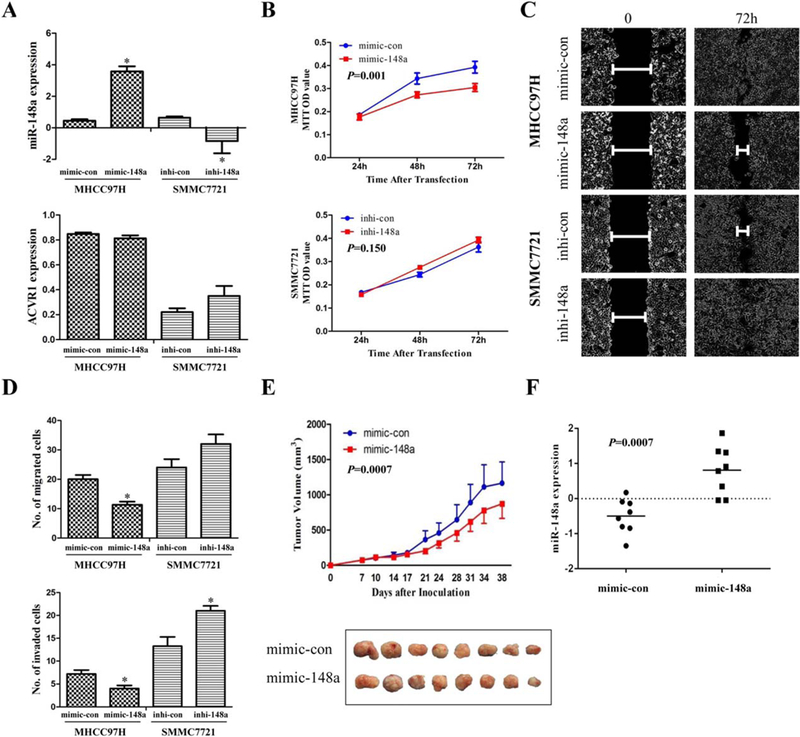
MiR-148a overexpression inhibits HCC cell proliferation, migration, and invasion in vitro and tumor growth in vivo. (A) MiR-148a expression was increased after transfection with miR-148a mimic and decreased after transfection with miR-148a inhibitor. ACVR1 expression was decreased after transfection with miR-148a mimic and increased after transfection with miR-148a inhibitor. The expression of miR-148a and ACVR1 was measured 24 hours after transfection. (B) MTT assay showed that transfection of miR-148a mimic into MHCC97H cells significantly inhibited cell proliferation compared to controls (con). (C) Wound-healing assay showed that miR-148a inhibited liver cancer cell migration. (D) Overexpression of the miR-148a mimic inhibited migration of MHCC97H cells; inhibition (inhi) of miR-148a promoted migration of SMMC7721 cells. Overexpression of miR-148a significantly inhibited migration and invasion of MHCC97H cells, and miR-148a inhibitor significantly enhanced invasion, but did not enhance migration, of SMMC7721 cells. (E) MiR-148a inhibited tumor growth in vivo. MHCC97H cells were subcutaneously injected into the flanks of nude mice, which were then injected with miR-148a mimic or negative control twice a week for 4–5 weeks. Tumor volumes at the end of this period were significantly smaller in the miR-148a-treated group than in the control group. (F) The expression level of miR-148a was significantly higher in the tumors of the miR-148a-treated group than in the control group. (*P < 0.05).
Discussion
Starting with gene expression profiling from 100 human HCC samples, we identified a clinically aggressive subtype of HCC with a cancer stem cell signature, a finding supported by the discovery of a similar cluster in an independent cohort with the same gene signature. Simultaneous profiling of the transcriptome and miRNAs gave us further insight into the key regulatory relationships in the miRNA-mRNA network that underlies the aggressive nature of this cancer stem cell-like subtype. Through integrated computational analysis coupled with experimental validation, we identified a key tumor-suppressing miRNA, miR-148a, that is attenuated in the cancer stem cell-like HCC subtype. Loss of miR-148a resulted in consistent up-regulation of a critical substrate, ACVR1, a key receptor of BMP7 and an important regulator of the BMP/Wnt signaling pathway, which is critical for cancer stem cells. BMP7 may also repress the expression of IL-8, a major cancer-related cytokine that promotes cancer growth and metastasis.24,25
Recently, it was reported that stem/progenitor cell markers such as EPCAM, JAG1, and Sox9 were enriched in severe nonalcoholic fatty liver disease (NAFLD) compared with mild NAFLD.26,27 These findings suggest that stem cell-related biological processes might be initiated long before the neoplasms emerge and that these markers may serve as bio markers for HCC risk prediction and early diagnosis.
The molecular subtyping of HCC has been reported in the literature but often without sufficient attention to the underlying mechanisms and therapeutic implications. The limitations of past studies were attributable mainly to the lack of multiplatform data and inte grated analysis. In this study, we performed integrated analysis of gene expression and miRNA expression profiles produced from the same set of 100 HCC cases. We further experimentally interrogated a key miRNA node that is responsible for regulating this stem cell-like subtype and provided evidence suggesting that miR-148a is a potential therapeutic tool for patients whose HCC is of this stem cell-like subtype. This is a clinically relevant discovery because a number of published studies have shown that HCCs that possess cancer stem cell components carry a greater risk of chemoresistance, radioresistance, and recurrence than other HCCs.19,28
Pathway analysis showed that the hepatic fibrosis/hepatic stellate cell activation-related pathway was significantly highly expressed in the stem cell-like sub-type. Hepatic stellate cells are progenitor cells that have differentiation potential and can express stem cell markers, such as CD133, Notch1, and Notch3.29 Activated hepatic stellate cells have been shown to induce the occurrence and development of liver fibrosis, and Wnt signaling pathways are up-regulated and implicated in the process.30
A number of cancer stem cell factors have been studied in depth, including EPCAM, CD24, and CD90.13,31,32 These three proteins are targets for Wnt signaling and, together with ACVR1, were shown to be associated with shorter survival in our validation studies of a larger cohort of HCC cases in a tissue microarray. Given that the liver tissue has an intrinsic capacity to renew damaged tissue, it is conceivable that abnormal activation of this process can move beyond tissue damage repair and into the tumorigenic program. Indeed, cirrhosis and hepatitis B and C viral infections, which both damage normal liver tissues, are the most common etiologic factors for HCC.
Because miRNAs are relatively stable and are naturally secreted and taken up by cells, they are consid ered a promising new class of therapeutic tool for cancer treatment. Recent studies have been found that altered miRNA expression in liver cancer stem cell subsets compared with noncancer stem cell subsets.33 We showed that expression of miR-148a was significantly lower in the stem cell-like subtype than in the metabolic subtype and was negatively correlated with ACVR1 expression. MiR-148a regulates ACVR1 protein expression by directly targeting the 30-untranslated region of its mRNA. ACVR1 is a type I receptor of BMPs and belongs to the transforming growth factor-beta superfamily. Mutation of ACVR1 has been reported in fibrodysplasia ossificans progressiva.34,35 Genetic variants of ACVR1 were associated with breast cancer36 and an anti-Mu€llerian hormone level in women with polycystic ovary syndrome.37 BMP signaling by way of ACVR1 in osteoblasts reduces canonical Wnt signaling by suppressing of Wnt inhibitors SOST and DKK1.38 Because ACVR1 activate Wnt signaling, which plays an essential role in cancer stem cells,38,39 there has been extensive effort to develop an inhibitor for ACVR1. However, no ACVR1 inhibitor has become available. The current study suggests that introduction of miR-148a might be an alternative approach.
The gene encoding miR-148a located at 7p15.2, a locus that can be silenced by hypermethylation. MiR-148a was also reported to interact with DNMT1 (DNA methyltransferase 1) in gastric cancer.40 Recent studies have reported that miR-148a promotes myogenic differentiation,41 contributes to DNA hypomethylation in lupus,42 suppresses the BMP signaling pathway in fibrodysplasia ossificans progressiva,20 and suppresses tumor cell invasion and metastasis in gastric cancer.43 MiR-148a may play a central role in HBx/ URG11-mediated HCC through modulation of β-catenin and the PTEN/AKT pathway44 and may play a role in metastasis related to HCC.45 Moreover, Zhang et al.46 reported that miR-148a suppresses the epithelial-mesenchymal transition and metastasis of hepatoma cells by targeting Met/snail signaling, and Yan et al.47 reported that miR-148a inhibits the metastasis of HCCs by blocking the epithelial-mesenchymal transition and cancer stem cell-like properties through effects on the Wnt signaling pathway mediated by direct binding with Wnt1. Gailhouste et al. reported that MiR-148a was critical for hepatic differentiation through the direct targeting of DNMT1.48,49 These reports are consistent with our results demonstrating the role of miR-148a in cancer stem cells and BMP/Wnt signaling and its relationship with HCC survival. Our in vitro and in vivo experiments showed that over-expression of miR-148a decreased liver cancer cell proliferation, migration, and invasion, and that inhibition of miR-148a expression increased proliferation, migration, and invasion. These data suggest that miR-148a could indeed suppress tumorigenesis and progression.
In summary, we identified a clinically aggressive stem cell-like subtype of HCC and found that expression of miR-148a, which is related to cancer growth and metastasis through ACVR1/BMP/Wnt signaling, was low in this subtype. Thus, this molecular classification study and integrated analysis of the miRNAmRNA circuit in HCC may lead to a novel therapeutic strategy that improves the prognosis of an aggressive subtype of HCC.
Supplementary Material
Acknowledgment:
We thank Hans Winkler of Janssen Research and Development, a Division of Janssen Pharmaceuticals, for support of the expression chip experiment. We thank Kathryn L. Hale of the Department of Scientific Publications at the University of Texas MD Anderson Cancer Center for editing the article.
Partially supported by the Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT) in China (IRT1076), the National Key Scientific and Technological Project (2011ZX09307–001-04), the National Natural Science Foundation of China (No. 81172762), China Postdoctoral Science Foundation funded Project (2012M510757), and the University of Texas MD Anderson Cancer Center Core Support Grant from the U.S. National Institutes of Health (CA016672). The tissue bank is jointly supported by the Tianjin Cancer Institute and Hospital and the U.S. National Foundation for Cancer Research. X.W.W. was supported by grants (Z01-BC 010313 and Z01-BC010876) from the Intramural Research Program of the Center for Cancer Research, the U.S. National Cancer Institute.
Abbreviations:
- ACVR1
activin A receptor type 1
- AFP
alpha-fetoprotein
- BMP
bone morphogenetic protein
- EPCAM
epithelial cell adhesion molecule
- GSEA
gene set enrichment analysis
- HCC
hepatocellular carcinoma
- IL-8
interleukin 8
- miRNA
microRNA
Footnotes
Potential conflict of interest: Nothing to report.
Author names in bold designate shared co-first authorship.
References
- 1.Ferlay J, Shin H-R, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893–2917. [DOI] [PubMed] [Google Scholar]
- 2.Chen WQ, Zheng RS, Zhang SW. Liver cancer incidence and mortality in China, 2009. Chin J Cancer 2013;32:162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llovet JM, Bustamante J, Castells A, Vilana R, Ayuso MDC, Sala M, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology 1999;29:62–67. [DOI] [PubMed] [Google Scholar]
- 4.Hoshida Y, Nijman SMB, Kobayashi M, Chan JA, Brunet J-P, Chiang DY, et al. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Res 2009;69:7385–7392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyault S, Rickman DS, de Reynies A, Balabaud C, Rebouissou S, Jeannot E, et al. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology 2007;45:42–52. [DOI] [PubMed] [Google Scholar]
- 6.Lee J-S, Heo J, Libbrecht L, Chu I-S, Kaposi-Novak P, Calvisi DF, et al. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nat Med 2006;12:410–416. [DOI] [PubMed] [Google Scholar]
- 7.Roessler S, Budhu A, Wang XW. Future of molecular profiling of human hepatocellular carcinoma. Future Oncol 2007;3:429–439. [DOI] [PubMed] [Google Scholar]
- 8.Pineau P, Volinia S, McJunkin K, Marchio A, Battiston C, Terris B, et al. miR-221 overexpression contributes to liver tumorigenesis. Proc Natl Acad Sci U S A 2010;107:264–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hou J, Lin L, Zhou W, Wang Z, Ding G, Dong Q, et al. Identifica tion of miRNomes in human liver and hepatocellular carcinoma reveals miR-199a/b-3p as therapeutic target for hepatocellular carcinoma. Cancer Cell 2011;19:232–243. [DOI] [PubMed] [Google Scholar]
- 10.Ji J, Yamashita T, Budhu A, Wang XW. Identification of microRNA 181 by genome-wide screening as a critical player in EpCAM-positive hepatic cancer stem cells. Hepatology 2009;50:472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roessler S, Jia H-L, Budhu A, Forgues M, Ye Q-H, Lee J-S, et al. A unique metastasis gene signature enables prediction of tumor relapse in early-stage hepatocellular carcinoma patients. Cancer Res 2010;70: 10202–10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A 2001;98:5116–5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terris B, Cavard C, Perret C. EpCAM, a new marker for cancer stem cells in hepatocellular carcinoma. J Hepatol 2010;52:280–281. [DOI] [PubMed] [Google Scholar]
- 14.Qian YW, Chen Y, Yang W, Fu J, Cao J, Ren YB, et al. p28(GANK) prevents degradation of Oct4 and promotes expansion of tumor-initiating cells in hepatocarcinogenesis. Gastroenterology 2012;142: 1547–1558 e1514. [DOI] [PubMed] [Google Scholar]
- 15.Liu L, Fu D, Ma Y, Shen X. The power and the promise of liver cancer stem cell markers. Stem Cells Dev 2011;20:2023–2030. [DOI] [PubMed] [Google Scholar]
- 16.Lai Q, Avolio AW, Rossi M. Role of alpha-fetoprotein in selection of patients with hepatocellular carcinoma waiting for liver transplantation: must we reconsider it? Int J Biol Markers 2011;26:153–159. [DOI] [PubMed] [Google Scholar]
- 17.Waugh DJJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res 2008;14:6735–6741. [DOI] [PubMed] [Google Scholar]
- 18.Bhattaram P, Penzo-Mendez A, Sock E, Colmenares C, Kaneko KJ, Vassilev A, et al. Organogenesis relies on SoxC transcription factors for the survival of neural and mesenchymal progenitors. Nat Commun 2010;1:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piao LS, Hur W, Kim T-K, Hong SW, Kim SW, Choi JE, et al. CD1331 liver cancer stem cells modulate radioresistance in human hepatocellular carcinoma. Cancer Lett 2012;315:129–137. [DOI] [PubMed] [Google Scholar]
- 20.Song H, Wang Q, Wen J, Liu S, Gao X, Cheng J, et al. ACVR1, a therapeutic target of fibrodysplasia ossificans progressiva, is negatively regulated by miR-148a. Int J Med Sci 2012;13:2063–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamashita T, Budhu A, Forgues M, Wang XW. Activation of hepatic stem cell marker EpCAM by Wnt/beta-catein signaling in hepatocellular carcinoma. Cancer Res 2007;67:10831–10839. [DOI] [PubMed] [Google Scholar]
- 22.Levy L, Neuveut C, Renard C, Charneau P, Buendia MA. Transcriptional activationof interleukin-8 by beta-catein-tcf4. J. Biol Chem 2002;277:42386–42393. [DOI] [PubMed] [Google Scholar]
- 23.Meyer MB, Geotsch PD, Pike JW. VDR/RXR and TCF4/beta-catein cistromes in colonic cells of colorectal tumor origin: impact on c-FOS and c-MYC gene expression. Mol Endocrinol 2012;26:37–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee YS, Choi I, Ning Y, Kim NY, Khatchadourian V, Yang D, et al. Interleukin-8 and its receptor CXCR2 in the tumour microenvironment promote colon cancer growth, progression and metastasis. Br J Cancer 2012;106:1833–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gould SE, Day M, Jones SS, Dorai H. BMP-7 regulates chemokine, cytokine, and hemodynamic gene expression in proximal tubule cells. Kidney Int 2002;61:51–60. [DOI] [PubMed] [Google Scholar]
- 26.Moylan CA, Pang H, Dellinger A, Suzuki A, Garrett ME, Guy CD, et al. Hepatic gene expression profiles differentiate presymptomatic patients with mild versus severe nonalcoholic fatty liver disease. Hepatology 2014;59:471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Baker SS, Baker RD, Zhu R, Zhu L. Systematic analysis of the gene expression in the livers of nonalcoholic steatohepatitis: implications on potential biomarkers and molecular pathological mechanism. PloS One 2012;7:e51131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lau CK, Yang ZF, Ho DW, Ng MN, Yeoh GCT, Poon RTP, et al. An Akt/hypoxia-inducible factor-1a/platelet-derived growth factor-BB autocrine loop mediates hypoxia-induced chemoresistance in liver cancer cells and tumorigenic hepatic progenitor cells. Clin Cancer Res 2009; 15:3462–3471. [DOI] [PubMed] [Google Scholar]
- 29.Reister S, Kordes C, Sawitza I, Haussinger D. The epigenetic regulation of stem cell factors in hepatic stellate cells. Stem Cells Dev 2011; 20:1687–1699. [DOI] [PubMed] [Google Scholar]
- 30.Zhang C, Wang Y, Chen H, Yang G, Wang S, Jiang M, Cong L, et al. Protective effect of the herbal medicine Ganfukang against carbon tetrachlorideinduced liver fibrosis in rats. Mol Med Rep 2013;8:954–962. [DOI] [PubMed] [Google Scholar]
- 31.Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai P, Chu PWK, et al. Significance of CD901 cancer stem cells in human liver cancer. Cancer Cell 2008;13:153–166. [DOI] [PubMed] [Google Scholar]
- 32.Lee TK, Castilho A, Cheung VC, Tang KH, Ma S, Ng IO. CD241 liver tumor-initiating cells drive self-renewal and tumor initiation through STAT3-mediated NANOG regulation. Cell Stem Cell 2011;9:50–63. [DOI] [PubMed] [Google Scholar]
- 33.Chai S, Ma S. Clinical implications of microRNAs in liver cancer stem cells. Chin J Cancer 2013;32:419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Cho TJ, Choi IH, et al. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Gen 2006;38:525–527. [DOI] [PubMed] [Google Scholar]
- 35.Nakahara Y, Katagiri T, Ogata N, Haga N. ACVR1 (587T>C) mutation in a variant form of fibrodysplasia ossificans progressiva: Second report. Am J Med Genet A 2014;164:220–224. [DOI] [PubMed] [Google Scholar]
- 36.Slattery ML, John EM, Torres-Mejia G, Herrick JS, Giuliano AR, Baumgartner KB, et al. Genetic variation in bone morphogenetic proteins and breast cancer risk in Hispanic and non-Hispanic white women: the Breast Cancer Health Disparities Study. Int J Cancer 2013;132:2928–2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kevenaar ME, Themmen AP, van Kerkwijk AJ, Valkenburg O, Uitterlinden AG, de Jong FH, et al. Variants in the ACVR1 gene are associated with AMH levels in women with polycystic ovary syndrome. Hum Reprod 2009;24:241–249. [DOI] [PubMed] [Google Scholar]
- 38.Kamiya N, Kaartinen VM, Mishina Y. Loss-of-function of ACVR1 in osteoblasts increases bone mass and activates canonical Wnt signaling through suppression of Wnt inhibitors SOST and DKK1. Biochem Biophys Res Commun 2011;414:326–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malanchi I, Peinado H, Kassen D, Hussenet T, Metzger D, Chambon P, et al. Cutaneous cancer stem cell maintenance is dependent on betacatenin signalling. Nature 2008;452:650–653. [DOI] [PubMed] [Google Scholar]
- 40.Zhu A, Xia J, Zuo J, Jin S, Zhou H, Yao L, et al. MicroRNA-148a is silenced by hypermethylation and interacts with DNA methyltransferase 1 in gastric cancer. Med Oncol 2012;29:2701–2709. [DOI] [PubMed] [Google Scholar]
- 41.Zhang J, Ying Z-z, Tang Z-l, Long L-q, Li K. MicroRNA-148a promotes myogenic differentiation by targeting the ROCK1 gene. J Biol Chem 2012;287:21093–21101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pan W, Zhu S, Yuan M, Cui H, Wang L, Luo X, et al. MicroRNA-21 and microRNA-148a contribute to DNA hypomethylation in lupus CD41 T cells by directly and indirectly targeting DNA methyltransferase 1. J Immunol 2010;184:6773–6781. [DOI] [PubMed] [Google Scholar]
- 43.Zheng B, Liang L, Wang C, Huang S, Cao X, Zha R, et al. MicroRNA-148a suppresses tumor cell invasion and metastasis by down-regulating ROCK1 in gastric cancer. Clin Cancer Res 2011;17:7574–7583. [DOI] [PubMed] [Google Scholar]
- 44.Yuan K, Lian Z, Sun B, Clayton MM, Ng IOL, Feitelson MA. Role of miR-148a in hepatitis B associated hepatocellular carcinoma. PloS One 2012;7:e35331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Budhu A, Jia H-L, Forgues M, Liu C-G, Goldstein D, Lam A, et al. Identification of metastasis-related microRNAs in hepatocellular carcinoma. Hepatology 2008;47:897–907. [DOI] [PubMed] [Google Scholar]
- 46.Zhang JP, Zeng C, Xu L, Gong J, Fang JH, Zhuang SM. MicroRNA 148a suppresses the epithelial-mesenchymal transition and metastasis of hepatoma cells by targeting Met/Snail signaling. Oncogene 2014;33: 4069–4076. [DOI] [PubMed] [Google Scholar]
- 47.Yan H, Dong X, Zhong X, Ye J, Zhou Y, Yang X, et al. Inhibitions of epithelial to mesenchymal transition and cancer stem cells-like properties are involved in miR-148a-mediated anti-metastasis of hepatocellular carcinoma. Mol Carcinog 2013. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 48.Chen Z, Ma T, Huang C, Zhang L, Xu T, Hu T, et al. MicroRNA-148a: a potential therapeutic target for cancer. Gene 2014;533:456–457. [DOI] [PubMed] [Google Scholar]
- 49.Gailhouste L, Gomez-Santos L, Hagiwara K, Hatada I, Kitagawa N, Kawaharada K, et al. miR-148a plays a pivotal role in the liver by promoting the hepatospecific phenotype and suppressing the invasiveness of transformed cells. Hepatology 2013;58:1153–1165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


