Abstract
The CD4+CD25+FOXP3+ regulatory T (Treg) cells are critical for maintaining immune tolerance in healthy individuals and are reported to restrict anti-inflammatory responses and thereby promote tumor progression, suggesting them as a target in the development of antitumor immunotherapy. Forkhead box P3 (FOXP3) is a key transcription factor governing Treg lineage differentiation and their immune-suppressive function. Here, using Treg cells, as well as HEK-293T and Jurkat T cells, we report that the stability of FOXP3 is directly and positively regulated by the E3 ubiquitin ligase ring finger protein 31 (RNF31), which catalyzes the conjugation of atypical ubiquitin chains to the FOXP3 protein. We observed that shRNA-mediated RNF31 knockdown in human Treg cells decreases FOXP3 protein levels and increases levels of interferon-γ, resulting in a Th1 helper cell–like phenotype. Human Treg cells that ectopically expressed RNF31 displayed stronger immune-suppressive capacity, suggesting that RNF31 positively regulates both FOXP3 stability and Treg cell function. Moreover, we found that RNF31 is up-regulated in Treg cells that infiltrate human gastric tumor tissues compared with their counterparts residing in peripheral and normal tissue. We also found that elevated RNF31 expression in intratumoral Treg cells is associated with poor survival of gastric cancer patients, suggesting that RNF31 supports the immune-suppressive functions of Treg cells. Our results suggest that RNF31 could be a potential therapeutic target in immunity-based interventions against human gastric cancer.
Keywords: forkhead box P3 (FOXP3), T-cell, gastric cancer, immunosuppression, protein stability, human regulatory T cells, multi-monoubiquitination, Positive regulator, RNF31
Introduction
FOXP3+ regulatory T cells (Tregs)4 represent a specific subset of CD4+ T cells that are crucial for the maintenance of self-tolerance and immune homeostasis (1). The X-chromosome–encoded transcription factor FOXP3 is essential for both Treg cell development and function (2). Treg cells become instable and acquire effector T cell function by losing FOXP3 expression (3–6). Conversely, effector T cells manipulated to ectopically express FOXP3 could gain the Treg cell phenotype (2, 7). FOXP3 protein levels in Treg cells can be regulated by the balance of its expression and degradation. It has been reported that transforming growth factor-β, IL-2, or T cell receptor stimulation of T cells can up-regulate Foxp3 transcription to promote FOXP3 expression (8, 9). Our previous findings have established that FOXP3 protein could be regulated at the post-translational level (10–12). Several studies have shown that FOXP3 could be polyubiquitinated and deubiquitinated, which may impact FOXP3 protein stability and subsequent Treg cell suppressive capability (10, 11, 13). Thus, it is of paramount importance to identify potential ubiquitin ligase (E3) or deubiquitinase (DUB), which are involved in FOXP3 protein stability to explore new mechanisms underlying lineage commitment of Treg cells.
The linear ubiquitin chain assembly complex (LUBAC) is composed of three proteins: ring finger protein 31 (RNF31/HOIP), RanBP-type and C3HC4-type zinc finger containing 1 (RBCK1/HOIL-1), and SHANK-associated RH domain interacting protein (SHARPIN/SIPL1) (14, 15). RNF31 is the E3 ubiquitin protein ligase component of LUBAC, and is responsible for linear polyubiquitin chain formation. The autoinhibition of RNF31 can be released through binding to RBCK1 or SHARPIN (16). LUBAC plays a role in various cell signaling pathways by catalyzing the addition of linear polyubiquitin chains to substrates. It has been reported to be involved in innate and adaptive immune responses downstream of TLR, NLRP3, T cell receptor, B cell receptor, NOD2, and TNFR ligation (17–22). These signals involve the linear ubiquitination of ASC to facilitate NLPR3 inflammasome assembly and NEMO to strengthen canonical NF-κB activation (19, 23).
It has been demonstrated that Treg cell–specific ablation of RNF31 in mice causes severe Treg cell deficiency and lethal immune pathology, revealing an ongoing requirement of LUBAC activity for Treg cell homeostasis (24). However, whether it is critical for human Treg function and the underlying mechanism requires further exploration. In our study, we found that RNF31 was indispensable for maintaining human Treg cell function and positively regulated Treg cell suppressive capability by directly interacting and greatly stabilizing FOXP3 through catalyzing the conjugation of atypical ubiquitin chains to FOXP3. Moreover, a higher level of RNF31 in gastric tumor-infiltrated Treg cells was observed and associated with poor prognosis. Therefore, here we characterized a unique regulatory pathway for FOXP3 stability, which could be a potential drug target for anti-tumor immunotherapy.
Results
RNF31 is indispensable for human Treg cell function
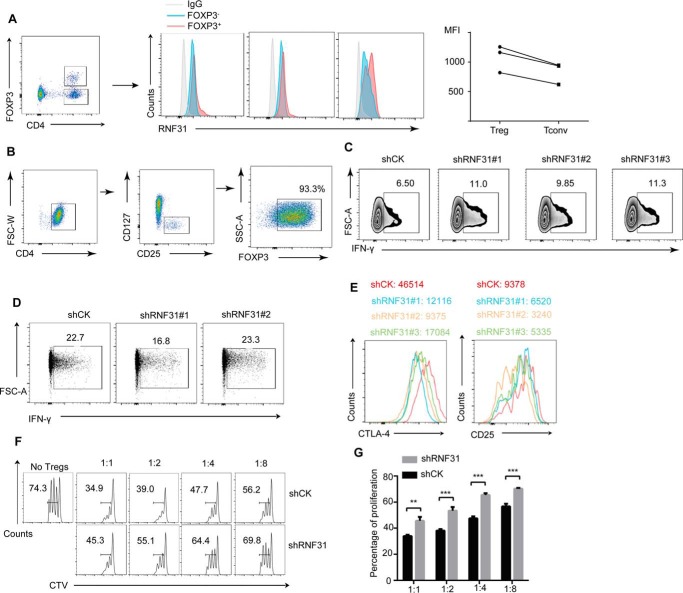
To explore the importance of RNF31 in human Treg cells, we first analyzed the expression of RNF31 in peripheral Treg cells and conventional T cells. A modestly higher mean fluorescence intensity (MFI) of RNF31 signaling was observed in Treg cells, compared with Tconv cells (Fig. 1A). We next utilized in vitro expanded human Treg cells (Fig. 1B) to test the impact of RNF31 on human Treg cell function by performing RNF31 knockdown assay, and found shRNF31-transfected human Treg cells produced higher amounts of IFN-γ (Fig. 1C), implying a Th1–like phenotype. Meanwhile, no obvious difference was observed in Tconv cells, which are negative for FOXP3 in terms of IFN-γ secretion with RNF31 knockdown, indicating RNF31 influenced IFN-γ production in a FOXP3-dependent manner (Fig. 1D). MFI of Treg cell signature markers, such as CTLA-4 and CD25, were down-regulated in human Treg cells with RNF31 knockdown (Fig. 1E). Accordingly, we examined the activity of Treg cells using an in vitro suppression assay, and found that Treg cells after RNF31 knockdown had significantly impaired suppressive capacity toward Tconv cell proliferation (Fig. 1, F and G), revealing that RNF31 is indispensable for human Treg cell function.
Figure 1.
RNF31 is indispensable for human Treg cell function. A, MFI of RNF31 expression was measured in CD4+FOXP3+ Treg cells and CD4+FOXP3− Tconv cells from PBMC of healthy donors (n = 3, *, p < 0.05). B, CD4+CD25hiCD127low (Treg cells) T cells isolated from healthy donors were stimulated with anti-CD3/CD28 beads, IL-2 (500 units) for 7 days, followed by detection of Treg purity through FOXP3 staining. C, in vitro expanded Treg cells for a knockdown assay using shRNA-bearing lentivirus, followed by detection of Treg cell function in terms of IFN-γ secretion. D, CD4+CD25lowCD127high (Tconv cells) for a knockdown assay using shRNA-bearing lentivirus, followed by detection of IFN-γ secretion. E, CTLA4 or CD25 expression were detected in in vitro expanded Treg cells transfected with shRNA-bearing lentivirus. F, in vitro suppression assay was performed in Treg cells transfected with lentivirus carrying shCK or shRNF31 constructs. G, percentage of proliferated responder T cells was assessed as shown in F (n = 3). All data represent mean ± S.D. *, p < 0.05; **, p < 0.01; ***, p < 0.01 as determined by Student's t test. NS, not significant.
RNF31 is a positive regulator for the stability of human FOXP3 protein
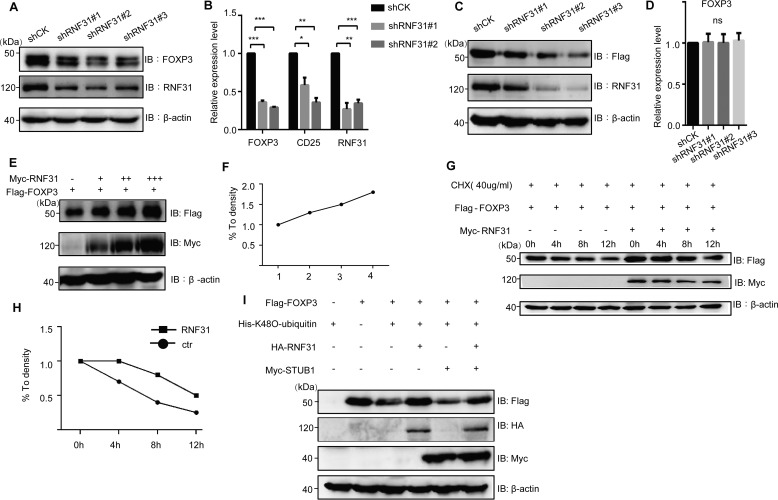
Because those markers detected above (IFN-γ, CD25, and CTLA4) were FOXP3 targeting genes (25), we further explored whether RNF31 deficiency influenced FOXP3 expression. The down-regulation of FOXP3 protein in Treg cells with RNF31 knockdown was indeed observed (Fig. 2A). Meanwhile, the FOXP3 gene was poorly transcribed in RNF31 knockdown Treg cells (Fig. 2B). Because FOXP3 transcription needs persistent FOXP3 protein expression as a positive feedback loop for the regulation of FOXP3 gene locus (26), we then established a Jurkat T cell line stably expressing FLAG-tagged FOXP3 under the control of a constitutive expressing ubiquitous promoter, to investigate how RNF31 affected FOXP3 expression. We found that there was a decreased level of FLAG-FOXP3 protein (Fig. 2C), whereas with a comparable FLAG-FOXP3 transcription in the cells with RNF31 knockdown (Fig. 2D), suggesting that RNF31 deficiency affected the post-translational modification-mediated degradation of FOXP3 protein.
Figure 2.
RNF31 is a positive regulator for the stability of human FOXP3 protein. A, RNF31 was knocked down in primary human Treg cells, and the endogenous FOXP3 level was visualized by IB. B, RNF31 was knocked down with shRNA-bearing lentiviruses in primary Treg cells, the mRNA level of CD25, FOXP3, were detected (n = 4); *, p < 0.05; **, p < 0.01; ***, p < 0.01. C, RNF31 was knocked down in the stable cell line, FLAG-FOXP3 Jurkat T cells, and the level of FLAG-FOXP3 was then detected by Western blotting. D, RNF31 was knocked down in FLAG-FOXP3 Jurkat T cells, and the mRNA level of FOXP3 was investigated (n = 2). ns, not significant. E and F, immunoblot analysis of FLAG-FOXP3 in HEK-293T cell lysate co-transfected with FLAG-FOXP3 and various doses of Myc-RNF31. G and H, immunoblot analysis for identifying the half-life of FLAG-FOXP3 in HEK-293T cells transfected with FLAG-FOXP3 and Myc-RNF31. I, immunoblot analysis for investigating the role of HA-RNF31 in Myc-STUB1–mediated protein degradation of FLAG-FOXP3 in HEK-293T cells transfected with FLAG-FOXP3, HA-RNF31, and Myc-STUB1.
To further determine whether RNF31 was required for the stability of FOXP3, we overexpressed FLAG-FOXP3 and various doses of c-Myc–tagged RNF31 and found that RNF31 enhanced FOXP3 protein stability in a dose-dependent manner (Fig. 2, E and F). Treatment of the transfected cells with cycloheximide showed the elongated half-life time on FOXP3 protein with the overexpression of RNF31 (Fig. 2, G and H), and RNF31 significantly prevented FOXP3 from STUB1-mediated protein degradation (Fig. 2I). Taken together, these results indicate that RNF31 is required for FOXP3 stability in primary human Treg cells and promotes FOXP3 stability at the post-translational level.
RNF31 interacts with FOXP3
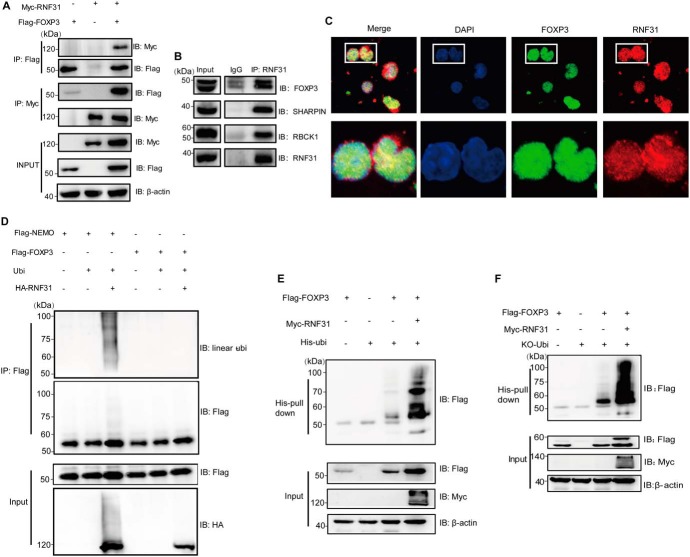
To test whether RNF31 is a direct E3 ligase of FOXP3 that is required for its stability, we first carried out a binding assay to determine whether FOXP3 could associate with RNF31. Reciprocal immunoprecipitation revealed an interaction between FOXP3 and RNF31 (Fig. 3A) as well as the other two LUBAC members (Fig. S1, A and B). In primary human Treg cells, FOXP3 immunoprecipitated with RNF31 as well as one LUBAC member RBCK1, suggestive of the endogenous interaction between FOXP3 and RNF31 (Fig. 3B, Fig. S1C). Consistently, RNF31 was also demonstrated to co-localize with FOXP3 in the nucleus of Treg cells under confocal microscopy (Fig. 3C). Through the mapping of different truncations of FOXP3, we found that the zinc finger subdomain of FOXP3 was essential for its interaction with RNF31 (Fig. S2A). Likewise, a panel of RNF31 truncation expression constructs were generated and the NZF1–NZF2 subdomain was crucial for their interaction with each other (Fig. S2B).
Figure 3.
RNF31 mediates atypical ubiquitin conjugation of FOXP3. A, Myc-RNF31 and FLAG-FOXP3 were transfected into HEK-293T cells, 48 h later, cells were harvested and lysed for co-immunoprecipitation (co-IP) as indicated. B, human CD4+CD25highCD127low expanding Treg cells were cultured with anti-CD3/CD28 Ab in the presence of IL-2. Cells were collected for endogenous co-IP and Western blotting as indicated. C, in vitro expanded human Treg cells were incubated with specific antibodies. Representative confocal images were visualized for endogenous RNF31 (red) and FOXP3 (green) with DAPI used to visualize the nuclei (blue). D, FLAG-NEMO, FLAG-FOXP3, ubiquitin, and Myc-RNF31 were transfected into HEK-293T cells, followed by immunoblot analysis of linear ubiquitinated FLAG-FOXP3 or FLAG-NEMO as indicated. E, FLAG-FOXP3, Myc-RNF31, and His-ubiquitin were transfected into HEK-293T cells, cell lysates were incubated with nickel-nitrilotriacetic acid beads, followed by immunoblot analysis of ubiquitinated FLAG-FOXP3. F, FLAG-FOXP3, Myc-RNF31, and His-ubiquitin (WT) and mutant His-ubiquitin (KO, without retention of any lysine) were transfected into HEK-293T cells, cell lysates were incubated with nickel-nitrilotriacetic acid beads, followed by immunoblot analysis of ubiquitinated FLAG-FOXP3.
RNF31 promotes FOXP3 stability through mediating atypical ubiquitin conjugation of FOXP3
Given that RNF31 is mainly reported as the E3 ligase mediating linear ubiquitination, we then explored whether RNF31 could catalyze the addition of linear polyubiquitin chains to FOXP3 with NEMO as a positive control. We observed that RNF31 could not efficiently conjugate linear polyubiquitin chains to FOXP3 (Fig. 3D). Meanwhile, we could barely observe linear polyubiquitin-conjugated FOXP3 in primary human Treg cells (Fig. S3A). Because RNF31 is also reported to catalyze the addition of nonlinear ubiquitin chains to its substrates (27), we next explored whether RNF31 modified FOXP3 in a nonlinear ubiquitination way. Accordingly, we carried out a His-ubiquitin pulldown assay in which the N-Met of ubiquitin is blocked to inhibit the formation of linear polyubiquitin chains (16), and the high level of ubiquitinated FOXP3 was observed, which could significantly stabilize the FOXP3 protein (Fig. 3E). These data suggest a nonlinear ubiquitination modification-mediated mechanism controls the stabilization of FOXP3 in cells with ectopic RNF31 expression.
We next investigated which type of ubiquitin-chain linkage was catalyzed by RNF31 on FOXP3. We transfected HEK-293T cells with plasmids expressing WT His-ubiquitin or various His-ubiquitin mutants as indicated (with all seven lysine to arginine substitutions; with only one of each the seven lysine residues remained; and with only one lysine to arginine substitution) together with RNF31. Among all the ubiquitin mutants tested, we observed that none of the seven lysine residues was essential and required for RNF31-mediated conjugation of ubiquitin chains to FOXP3 (Fig. S3B). Consequently, we proposed that RNF31 mainly catalyzed FOXP3 ubiquitination in a multi-monoubiquitination manner. And indeed it was further confirmed by using KO Ubi transfection (Fig. 3F). Of note, monoubiquitinated FOXP3 could be significantly observed (Fig. 3F).
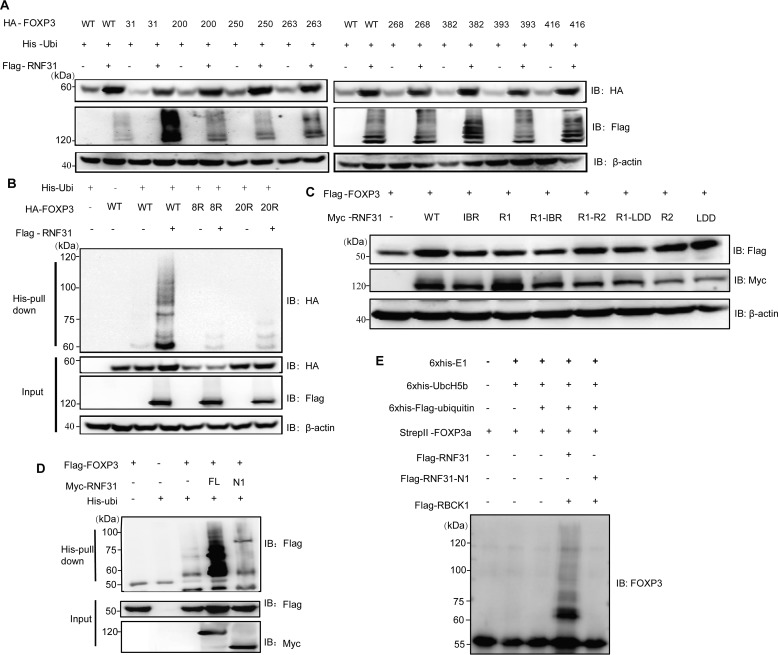
Ubiquitination is primarily reported as a sequential cascade to bind ubiquitin to lysine residues on the protein substrate via an isopeptide bond (28). FOXP3 has 20 lysine residues present at different domains. To identify the lysine residues responsible for RNF31-mediated multi-monoubiquitination, we first generated the mutant FOXP3–20R in which all of the lysine residues in FOXP3 were replaced with arginine. Then, we reintroduced individual lysine residues into FOXP3–20R to generate the single lysine mutants in which only one lysine was present (11). Co-transfection and immunoblot analysis revealed that RNF31 mediated the protein stability of FOXP3 (WT) and the eight mutants (FOXP3-K31only, K200only, K250only, K263only, K268only, K382only, K393only, K416only), but not other FOXP3 mutants (Fig. S4, Fig. 4A). To confirm the involvement of the eight lysine residues in RNF31-mediated multi-monoubiquitination and protein stability of FOXP3, we further constructed the mutant FOXP3–8R in which lysine residues at positions 31, 200, 250, 263, 268, 382, 393, and 416 were replaced with arginine. Co-transfection and a His-ubiquitin pulldown assay demonstrated that RNF31 induced protein stability and multi-monoubiquitination to FOXP3 (WT), but not mutants FOXP3–8R, as well as FOXP3–20R (Fig. 4B). Together, these data demonstrated that RNF31 catalyzed the atypical ubiquitin conjugation to FOXP3 on lysine residues 31, 200, 250, 263, 268, 382, 393, and 416.
Figure 4.
Characterization of lysine residues of FOXP3 targeted by RNF31. A, immunoblot analysis for the identification of lysine residues of FOXP3 targeted by RNF31 in the HEK-293T cell lysate transfected with FLAG-RNF31, His-ubiquitin, HA–tagged WT FOXP3, and mutant FOXP3 (with retention of one lysine residue). B, immunoblot analysis of ubiquitin chain formation on FOXP3 in HEK-293T cell lysate transfected with FLAG-RNF31, His-ubiquitin, HA–tagged WT FOXP3, and mutant FOXP3–8R/20R (with 8 lysine-to-arginine substitutions as indicated above, or with 20 lysine-to-arginine substitutions). C, immunoblot analysis for identifying RNF31 enzymatically inactive mutants in the HEK-293T cell lysate transfected with FLAG-FOXP3, Myc–tagged WT RNF31, or mutant RNF31 as indicated. D, immunoblot analysis of ubiquitin chain formation on FOXP3 in HEK-293T cell lysates transfected with FLAG-FOXP3, His-Ubiquitin, Myc–tagged WT RNF31 or N1 RNF31 truncation mutant as indicated. E, immunoblot analysis of ubiquitin chain formation on FOXP3 in a cell–free system with His6-E1 (100 ng), His6-E2 (200 ng), His6-FLAG ubiquitin (10 μg), StrepII FOXP3 (500 ng), 2× FLAG RNF31 (500 ng), 2× FLAG-N1-RNF31 (500 ng), and 2× FLAG RBCK1 (500 ng) being mixed in the reaction buffer, which was terminated by adding SDS protein loading buffer.
We next screened several reported RNF31 enzymatically inactive mutants (29), and found none of these mutants affected RNF31-mediated stability of FOXP3 (Fig. 4C). Consequently, we utilized the truncation construct N1–RNF31 whose catalytic center was deleted but the ability to interact with FOXP3 remained unaffected as the enzymatically inactive mutant. As expected, N1–RNF31 could not induce multi-monoubiquitination or enhance the FOXP3 protein level (Fig. S2B, Fig. 4D). To further verify the involvement of RNF31 in mediating FOXP3 multi-monoubiquitination, we purified StrepII-FOXP3 from CHO-K1 cell supernatant, FLAG-RNF31, N1-FLAG-RNF31, and FLAG-RBCK1 from the HEK-293T cell pellet (Fig. S5), and combined them with His-E1, His-Ubc5Hb (E2), and His-FLAG-ubiquitin, which have been reported previously for an in vitro ubiquitination assay (12). Ubiquitination, and especially monoubiquitination of FOXP3 was observed in this cell-free system (Fig. 4E). These data collectively suggested that RNF31 could directly modify FOXP3 through multi-monoubiquitination, resulting in robust FOXP3 stability.
TCR/CD28 signaling up-regulates RNF31 expression in Treg cells
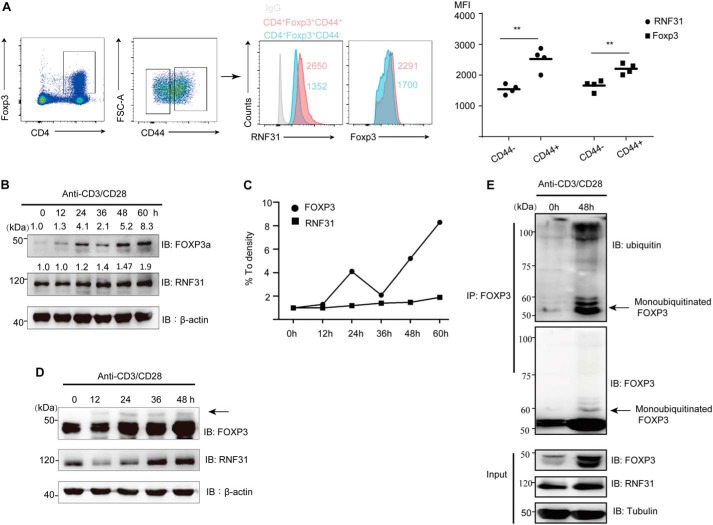
To further investigate the physiological correlation between RNF31 and FOXP3 in primary Treg cells, we compared the expression of RNF31 and Foxp3 in nonactivated naive (CD4+Foxp3+CD44low) Treg cells and activated effector/memory (CD4+Foxp3+CD44high) Treg cells from C57BL/6 mice, and found both the MFI of RNF31 and Foxp3 signaling were elevated in activated Tregs (Fig. 5A), indicating TCR/CD28 signaling may be critical for the RNF31-mediated stability of FOXP3 (30). Consistently, when we treated primary human Treg cells with anti-CD3/CD28 antibodies (Ab), we observed that the expression of RNF31 increased gradually along with TCR/CD28 activation, and FOXP3 displayed a similar trend (Fig. 5, B and C). More importantly, we found that there was an upper band about 7–8 kDa larger than FOXP3 following TCR/CD28 stimulation, which was perceived as monoubiquitinated FOXP3 (Fig. 5D). To further examine the correlation between FOXP3 stability and ubiquitination by RNF31 in primary Treg cells following TCR/CD28 activation, we treated human primary Treg cells with anti-CD3/CD28 antibodies for 48 h, and observed the increased level of both FOXP3 and RNF31 protein. Simultaneously, the ubiquitination level of FOXP3, especially monoubiquitinated FOXP3 was up-regulated (Fig. 5E). In summary, these results support that TCR/CD28 signaling up-regulates RNF31 expression and facilitates RNF31-mediated FOXP3 stability through multi-monoubiquitination in Treg cells, which can be partly responsible for the up-regulation of FOXP3 in Tregs upon TCR/CD28 activation.
Figure 5.
TCR/CD28 signaling up-regulates RNF31 expression in Treg cells. A, male C57BL/6 mice were sacrificed, lymph nodes were isolated, followed by the detection of RNF31 and Foxp3 in naive CD4+Foxp3+CD44− Treg cells and effector/memory CD4+Foxp3+CD44+ Treg cells (n = 4). **, p < 0.01. B–D, in vitro expanded human Treg cells were rested (cells were removed of anti-CD3/CD28 beads, and 100 units of IL-2 was added for sustaining survival) for 2 days and were then re-stimulated with anti-CD3/CD28 Ab (1 μg/ml) plus 500 units of IL-2 as indicated. Cells were harvested, followed by immunoblot analysis of endogenous FOXP3 and RNF31. E, rested in vitro expanded Tregs were challenged with TCR/CD28 activation as indicated, followed by immunoblot analysis for detection of endogenous RNF31, FOXP3 expression, and ubiquitination of FOXP3. The arrow in D and E indicates monoubiquitinated FOXP3.
RNF31 is positively associated with Treg cell function
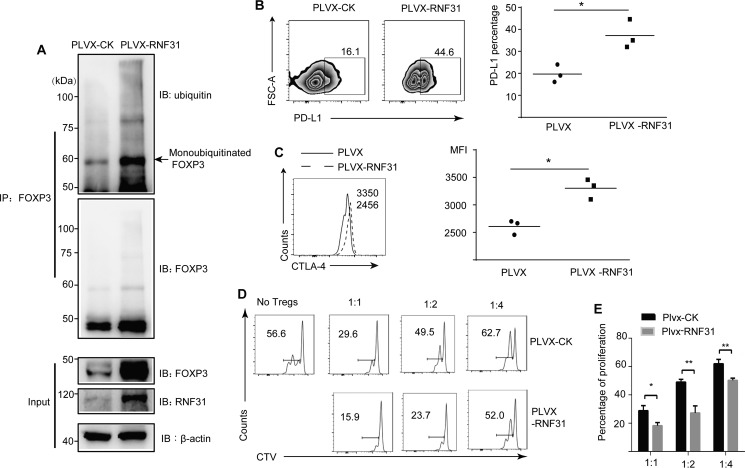
We then performed RNF31 overexpression in primary Treg cells to further investigate its role in affecting FOXP3 stability. Treg cells were transduced with lentivirus containing empty vector, or RNF31 expression constructs. In consistent with our results, FOXP3 expression was elevated in RNF31-overexpressing Treg cells, accompanied by an increased level of FOXP3 ubiquitination, especially monoubiquitinated FOXP3 (Fig. 6A). We next investigated whether RNF31 could positively regulate human Treg cell function. We observed that Treg cells overexpressing RNF31 by lentivirus displayed increased PD-L1 and CTLA-4 expression (Fig. 6, B and C). These results suggest that RNF31 positively regulate Treg cell characteristic genes. To further test the effect of RNF31 in Treg cell function, we performed an in vitro suppressive assay. Compared with empty vector-transduced cells, Treg cells overexpressing RNF31 were markedly more capable of suppressing the proliferation of conventional T cells in a cell trace violet dilution assay (Fig. 6, D and E). Taken together, these data indicate that RNF31 in primary human Treg cells results in multi-monoubiquitination–mediated stability of FOXP3, acquisition of Treg cell signature gene activation, and gain of superior Treg cell suppressive function.
Figure 6.
RNF31 is positively associated with Treg cell function. A, in vitro expanded human Treg cells were transfected with lentivirus bearing the pLVX-RNF31-IRES-GFP or pLVX-CK-IRES-GFP, followed by immunoblot analysis for detection of FOXP3 and ubiquitination of FOXP3. The arrow indicates monoubiquitinated FOXP3. B and C, Treg cell functional markers PD-L1 and CTLA4 were tested in Treg cells with overexpressed RNF31 (n = 3); *, p < 0.05. D, in vitro suppression assays were performed in Treg cells ectopically expressed RNF31 as indicated. E, percentage of proliferated responder T cells was assessed as shown (n = 2); *, p < 0.05; **, p < 0.01.
RNF31 is up-regulated in tumor-infiltrated Treg cells
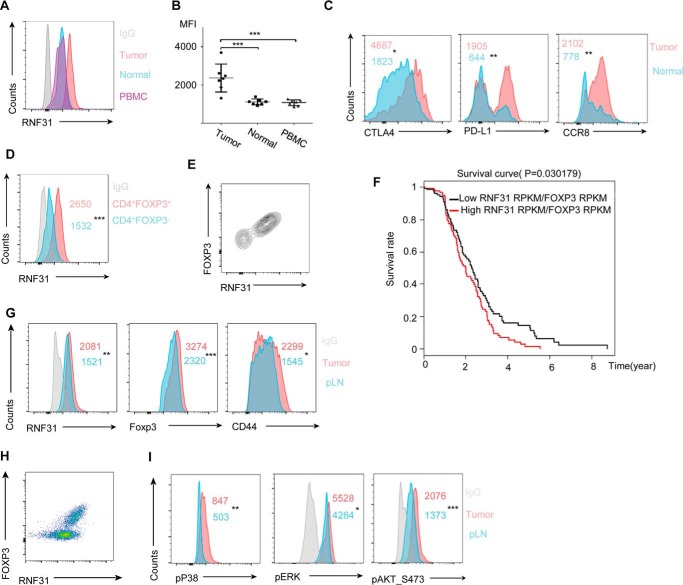
It is reported that Treg cells can facilitate tumor growth and metastasis based on the observed regression of established tumors in experimental models of Treg cell depletion (31). The proportion and function of Treg cells are shown increased within the gastric tumor microenvironment and are potent suppressors of effector cells (32). To further verify the positive role of RNF31 in Treg cells, we examined the expression of RNF31 in human gastric tumor-infiltrated Treg cells. The expression of RNF31 in the tumor-infiltrated Treg cells was elevated, compared with those from the peripheral blood and normal gastric tissues (Fig. 7, A and B). We next sought to determine the functionality of tumor-infiltrated Treg cells by investigating Treg cell signature genes and found several Treg cell functional markers including CTLA-4, PD-L1, and CCR8 were highly expressed by tumor-infiltrated Treg cells, compared with Treg cells from the normal tissues (Fig. 7C, Fig. S6A). Because RNF31 was more exclusively expressed by gastric tumor-infiltrated Treg cells than infiltrated Tconv cells (Fig. 7D, Fig. S6B) and FOXP3 in gastric tumor-infiltrated Treg cells were positively correlated with RNF31 (Fig. 7E), we then sought to determine whether RNF31 expression in Treg cells within gastric cancer correlated with disease prognosis by turning to the TCGA gastric cancer bulk tumor RNA-seq dataset (33). We observed that patients were separated into two distinct groups based on median RNF31/FOXP3 mRNA ratio. Consistent with our hypothesis, patients with a higher level of RNF31 in intratumoral Treg cells were associated with decreased survival (Fig. 7F). These data collectively indicate that targeting RNF31 might represent a promising immunotherapeutic approach for the treatment of gastric cancer.
Figure 7.
RNF31 is up-regulated in tumor-infiltrated Treg cells. A and B, MFI of RNF31 signaling was detected in CD4+FOXP3+ Treg cells of PBMC, normal gastric tissue, and gastric tumor isolated from 7 patients with gastric cancer. C, MFI of various Treg cell functional marker expression by Treg cells within gastric tumors and normal tissues as indicated (n = 6). *, p < 0.05; **, p < 0.01. D, MFI of RNF31 was detected in gastric tumor-infiltrated CD4+FOXP3− Tconv cells and CD4+FOXP3+ Treg cells from gastric tumors (n = 6); ***, p < 0.01. E, RNF31 and FOXP3 staining of total CD4+ T cells within the gastric tumor. F, disease-free survival of gastric cancer patients stratified by median tumor RNF31/FOXP3-normalized mRNA ratio. G, B16 melanoma was induced in C57BL/6 mice (n = 6), Treg cells from the tumor and peripheral lymph nodes were isolated, followed by detection of RNF31, Foxp3, and CD44 expression. *, p < 0.05; **, p < 0.01; ***, p < 0.01. H, RNF31 and FOXP3 staining of total CD4+ T cells within melonoma. I, MFI of regulators downstream of the TCR/CD28 signaling pathway as indicated (n = 6). *, p < 0.05; **, p < 0.01; ***, p < 0.001.
We next confirmed our conclusions above in the C57BL/6 mice model of melanoma (31). Male mice were subcutaneously injected with B16.F10 melanoma tumor cells, and tumors and peripheral lymph nodes were isolated for analysis on day 12 post-injection. Consistent with our results in human gastric cancer, both RNF31 and Foxp3 expression were elevated in intratumoral Treg cells (Fig. 7G, Fig. S6C), and the level of Foxp3 in intratumoral Treg cells was positively correlated with RNF31, compared with peripheral Treg cells (Fig. 7H). In the meantime, intratumoral Treg cells showed increased expression of the activation marker CD44 (Fig. 7G, Fig. S6C). Because TCR/CD28 stimulation is the driving force for priming Treg cell activation (30), we next assessed the possibility that these phenotypes were caused by the more powerful TCR/CD28 signaling. Accordingly, we checked a series of pathways downstream of TCR/CD28 signaling, and found intratumoral Treg cells showed increased AKT, extracellular signal-regulated kinase, and P38 phosphorylation (Fig. 7I, Fig. S6D) (34, 35). These data implied that Foxp3 was robustly up-regulated in tumor-infiltrated Treg cells, which was positively associated with an increased level of RNF31, whose up-regulation might be attributed to the stronger TCR/CD28 activation occurring in tumor tissues.
Discussion
It has been reported that mice with RNF31 specifically deleted in Treg cells exhibited severe immune pathology, including lymphadenopathy, lymphocytic perivascular infiltration, tissue destruction of many nonlymphoid organs, and abnormally high numbers of activated CD4+ and CD8+ T cells (24). These features reveal a requirement of RNF31 for Treg cell homeostasis. They also pointed out that RNF31 deficiency does not affect the thymic Treg cell proportions, yet results in a marked decrease in the numbers and frequency of Treg cells in the secondary lymphoid organs, and the defect was not caused by TNF-induced cell death. Their work implies that RNF31 is required for the maintenance of mature Treg cells in mice, but the underlying mechanism remains unknown.
Our work reports that RNF31 is required for maintaining human Treg lineage stability and cell function via affecting FOXP3 protein stability directly. Evidence has supported the notion how FOXP3 instability facilitates the generation of T-effector-cell–like Treg cells (10, 11). We observed that Treg cells with RNF31 knockdown acquired a Th1–like phenotype, with higher levels of IFN-γ secretion, lower levels of Treg signature gene expression, and impaired suppressive activity, which was attributed to FOXP3 instability. In the meanwhile, Treg cells overexpressing RNF31 displayed increased expression of Treg cell signature genes and stronger suppressive capacity, which was due to the enhanced FOXP3 stability. Our previous studies have reported that Treg cells can become unstable under inflammatory milieu, for instance, both TNFα and lipopolysaccharide treatment can lead to FOXP3 protein degradation (11, 36), thus resulting in Treg cell impairment. We found that RNF31 facilitated FOXP3 stability following TCR/CD28 stimulation, and this may explain the reason why RNF31 loss in mice Treg cells does not affect thymic Treg cell numbers but results in a robust decrease in Treg cell proportions and numbers from the secondary lymphoid organs, which might be due to the more intensive antigen challenge (TCR/CD28 stimulation) occurring in the peripheral, compared with the thymus.
In our work, we observed that human Treg cells knockdown of RNF31 displayed reduced FOXP3 protein and poor FOXP3 transcription as well. However, our results in the Jurkat T cell line stably expressing FLAG-tagged FOXP3 implied that RNF31 controlled FOXP3 expression at the post-translational level. We then investigated whether RNF31 could typically add linear polyubiquitin chains to FOXP3, yet we failed to observe linear polyubiquitin-conjugated FOXP3. We next turned to the atypical role of RNF31 in mediating nonlinear ubiquitination to its substrates and found RNF31 could efficiently catalyze multi-monoubiquitination to FOXP3, which significantly resulted in FOXP3 protein stability. Although we unveiled how RNF31 directly affects FOXP3 protein stability at the post-translational level, we could not rule out the possibility that RNF31 could indirectly affect FOXP3 expression at the transcriptional level because RNF31 is responsible for strengthened canonical NF-κB activation (23), which is critical for FOXP3 transcription (37). What is more, RNF31 could also affect FOXP3 transcription through the FOXP3 protein because persistent FOXP3 expression is required for constant FOXP3 transcription as a positive feedback loop for the regulation of the FOXP3 gene locus (26). Therefore, in our work, we focused on how RNF31 controlled FOXP3 expression at the post-translational level and our data suggest that RNF31 can directly target FOXP3 protein to control Treg cell lineage and functional stability.
His–pulldown analysis demonstrated that RNF31 can catalyze the atypical ubiquitin conjugation to FOXP3 on lysines 31, 200, 250, 263, 268, 382, 393, and 416. In our attempt to screen enzymatically inactive mutants for the atypical role of RNF31 in mediating FOXP3 multi-monoubiquitination, none of those reported mutants were responsible for its atypical function. It should be interesting to decipher the enzymatic core of RNF31 that is involved in mediating multi-monoubiquitination in future studies.
Treg cells are reported to restrict anti-inflammatory responses and promote tumor progression (38). Therefore, augmenting Treg cell suppressive function could facilitate tumor growth and predict poor anti-tumor immune responses. Because Treg cells within the tumor microenvironment are more capable of suppressing the proliferation and function of effector T cells (39), we then checked whether RNF31 could be correlated with the superior suppressive function of intratumoral Tregs. We observed that RNF31 expression was elevated in human gastric tumor-infiltrated Treg cells, accompanied with up-regulation of Treg functional markers, when compared with Treg cells from the normal tissues. What is more, analysis from the TCGA database demonstrates that patients with higher RNF31 in intratumoral Treg cells have poor survival, indicating that RNF31 can be a potential therapeutic target for the treatment of human gastric cancer.
We also induced a mouse melanoma model to investigate the positive role of RNF31 in intratumoral Treg cells. Consistent with the phenomenon observed in human gastric cancer, the up-regulation of RNF31 as well as Foxp3 expression was observed in Treg cells within the tumor microenvironment. Besides, the activation marker CD44 of intratumoral Treg cells was enhanced at the same time. Because TCR/CD28 stimulation is the primary driver for priming Treg cell activation (30), we further investigated the TCR signaling strength by checking a series of signaling pathways downstream. The more powerful TCR/CD28 signaling was observed in intratumoral Treg cells, which was demonstrated by increased AKT, extracellular signal-regulated kinase, and P38 phosphorylation levels. These data indicate that the stronger TCR/CD28 signaling occurred in Treg cells within the tumor microenvironment positively correlated with the enhanced RNF31 and Foxp3 protein level, which was in concert with our earlier results that TCR/CD28 stimulation increased the level of RNF31, which might contribute to the concomitant increase of FOXP3 expression. These data further correlate TCR/CD28 activation with RNF31 up-regulation and subsequent FOXP3 stability.
In summary, our work reports the indispensable and positive role of RNF31 in maintaining human Treg cell function, we also uncover the underlying mechanism of RNF31 in mediating atypical ubiquitin conjugation of FOXP3, which significantly stabilizes the FOXP3 protein at the post-translational level.
Experimental procedures
Plasmids, abs
pcDNA (optimized)-HA/FLAG–tagged FOXP3, FOXP3 truncations, FOXP3 mutants, His-tagged ubiquitin and mutants, and FLAG–tagged STUB1 were constructed as described previously (11). RBCK1, RNF31, SHARPIN, and NEMO were amplified from HEK-293T cells or human peripheral blood mononuclear cell cDNA and then cloned into the phosphatidylinositol phosphate-vectors. The following Abs were used for immunoprecipitation (IP), immunofluorescence (IF), immunoblotting (IB), and flow cytometry: mice anti-FOXP3 (Western blot, 1:2500, number 14-7979-82, ebio7979), mice anti-RNF31 (Western blot, 200 ng/ml; IF or FACS 1 μg/ml, number MAB8039, R&D875227), rabbit anti-RNF31 (Western blot, 1:1000; IF or FACS, 1:200, ab46322), rabbit anti-RBCK1 (Western blot, 1:1000, ab108479), rabbit anti-SHARPIN (Western blot, 1:1000, No. 12541S; CST, D4P5B clone), mice anti-FLAG (Western blot, 200 ng/ml, Sigma: F3165), mice anti-Myc (Western blot, 1:1000, Santa Cruz: 9E10), mice anti-linear polyubiquitin (Western blot, 1:1000, Millipore, MABS451), rabbit anti-FOXP3a (Western blot, 1:4000, ab54501), mice anti-ubiquitin (Western blot, 1:1000, Santa Cruz: P4D1), rabbit anti-pAkt (FACS, 1:200, CST, 4060S), rabbit anti-pErk1/2 (FACS, 1:200, CST, 4370S), mice IgG (CST: 5415s), rabbit IgG (CST: 2729S), HRP–anti-mice IgG (Western blot, 1:5000, Promega, W4021), HRP–anti-rabbit IgG (Western blot, 1:3000, CST, 7074S), HRP–anti-mice IgG (light chain, Western blot, 1:1000, Jackson ImmunoResearch, 115-035-174), HRP–anti-rabbit IgG (light chain, Western blot, 1:1000, Jackson ImmunoResearch, 211-002-171); goat anti-rabbit Alexa Fluor 488 (1:1000, Thermo Fisher, A-11008), goat anti-rabbit Alexa Fluor 546 (1:1000, Thermo Fisher, A-11010), goat anti-mice Alexa Fluor 488 (T1:1000, Thermo Fisher, A-11029), goat anti-mice Alexa Fluor 555 (1:1000, Thermo Fisher, A32727), and DAPI (1:1000, Invitrogen, D1306). The following antibodies were used for FACS analysis: anti-IFNγ (1:200, eBio 4S.B3), anti-IL2 (1:200, eBio MQ1–17H12), anti-FOXP3 (1:200, eBio, 236A1E7, eBio FJK-16S), anti-PDL1 (1:200, eBio 10F.9G2), anti-CD44 (1:200, MACS 130-102-982), anti-CD4 (1:200, BD RPA-T4), anti-CCR8 (1:200, BD, 566380), anti-pP38 (1:200, CST, 41666S). Data were collected on a BD Fortessa machine and analyzed using FlowJo software.
Cell culture
HEK-293T cells (ATCC) and Jurkat T cells (ATCC) were grown at 37 °C in 5% CO2 in Dulbecco's modified Eagle's medium and RPMI 1640 medium, respectively; Corning Cellgro, Thermo Fisher Scientific, Grand Island, NY) supplemented with 10% fetal bovine serum (Invitrogen, 10100147), 1% penicillin/streptomycin (Gibco, 15140122), 1% sodium pyruvate (Gibco, 11360-070), 1% minimal essential medium non-essential amino acids solution (Gibco, 11140-050).
Real-time PCR and knockdown assay primers
Total RNA was isolated using TRIzol reagent (Invitrogen) following the instructions of the manufacturer. RNA was quantified, and cDNA was reverse-transcribed with the PrimeScript RT reagent kit (TakaRa, RR037A). The cDNA samples were used at 10 ng/well in a 384-well plate and run in triplicate. PCR were set up in 10-μl volumes with SYBR Premix Ex Taq reagent (TakaRa, RR420A) on an ABI 7900HT sequence detection system. Quantification of the target mRNA expression level was normalized to actin expression. Primers for real-time PCR were used as follows: hFOXP3 forward, 5′-TCCCAGAGTTCCTCCACAAC-3′; hFOXP3 reverse, 5′-ATTGAGTGTCCGCTGCTTCT-3′; hCD25 forward, 5′-GAGACGTCCATATTTACAACAG-3′; hCD25 reverse, 5′-CCTTTGATTTCACTTGGGCTTC-3′; hIFN-γ forward, 5′-TCGCTTCCCTGTTTTAGCTGC-3′; hIFN-γ reverse, ATATTGCAGGCAGGACAACC; hβ-ACTIN forward, 5′-CTCTTCCAGCCTTCCTTCCT-3′; hβ-ACTIN reverse, 5′-CAGGGCAGTGATCTCCTTCT-3′. The short hairpin RNA target sequences related to control shRNA and RNF31 were previously described and were then conjugated to pLKO.1-GFP plasmid (40, 41).
Isolation of human Treg cells
Human PBMCs were isolated from healthy donors (Shanghai Blood Center). Human CD4+CD25hiCD127lo Treg cells and CD4+CD25loCD127hi effector T cells were sorted using a FACS ARIA II cell sorter (BD Biosciences). The purity of the isolated cells was 90–95%. In vitro expansion of Treg cells was performed in X-VIVO (Lonza) medium supplemented with 10% fetal serum (Invitrogen, 10100147), 1% GlutaMAXTM (Gibco, 35050-061), 1% sodium pyruvate (Gibco, 11360-070), and 500 units/ml of rIL-2 (R&D Systems, 202-IL) in the presence of anti-human CD3/CD28–conjugated Dynabeads (Gibco, 11132D) at a bead-to-cell ratio of 1:1. About 2 weeks later, cells can be used for functional analysis.
Immunoblot analysis and immunoprecipitation
Cells were lysed in immune precipitation assay buffer (RIPA buffer) containing 50 mm Tris/HCl (pH 7.4), 0.5% Nonidet P-40, 0.25% sodium deoxycholate, 150 mm NaCl, 1 mm EDTA with 1 mm phenylmethylsulfonyl fluoride, 1 mm Na3VO4, 1 mm NaF, and protease inhibitor (Sigma, P8340–5ML). The supernatants were immunoprecipitated with 1 μg of the indicated antibodies for 4–10 h at 4 °C, followed by adding 10 μl of protein A/G Plus-agarose (Santa Cruz Biotechnology, sc-2003) for 1–1.5 h incubation at 4 °C. The immunocomplexes were then washed 6 times with RIPA buffer containing 1 mm phenylmethylsulfonyl fluoride or 5% Tween (optional for removing nonspecific binding) and examined by Western blotting by standard procedures.
Suppressive assay
CD4+CD25−CD127+ T cells (Tconv cells) were isolated from healthy donor PBMC, and then labeled with CTV (Invitrogen, C34557), followed by different dilutions with in vitro expanded Treg cells, activated with anti-CD3/CD28 beads (Tconv: beads 4:1) for 84 h in a round bottom 96-well plate. Tconv cells were then detected for proliferation by FACS analysis.
Lentivirus transduction
The plasmids PLVX-GFP-RNF31, PLVX-GFP-CK, pLKO.1-GFP-shCK, or pLKO.1-GFP-shRNF31 were co-transfected with del8.9 and vesicular stomatitis virus-G into HEK-293T cells by calcium phosphate transfection. The viral supernatants were harvested and enriched via centrifugation. In vitro expanded human primary Treg cells were incubated with the virus supernatant, along with Polybrene (8 μg/ml, Sigma), anti-CD3/CD28 beads (Tregs: beads 10:1), rhIL-2 (500 units) overnight. After virus supernatants were removed, cells were cultured with fresh medium, 48 h later, those viable GFP-positive cells were then under investigation through FACS or cell sorting.
Immunofluorescence assay
In vitro expanded human primary Treg cells were plated onto poly-l-lysine–coated (Sigma) microscope coverslips by centrifugation and then fixed in PBS containing 4% (v/v) paraformaldehyde for 30 min at room temperature, avoiding light. After washing three times with PBS, the coverslips were incubated in blocking buffer (PBS, 3% (w/v) BSA, 0.5% Triton X-100, 10% (v/v) newborn calf serum) followed by three washings with binding buffer (PBS, 3% BSA, 0.5% Triton X-100). Samples then were incubated with primary antibodies for 1 h at room temperature diluted in binding buffer. After three washings with binding buffer, samples were incubated with secondary antibodies and DAPI nuclei stain for 1 h at room temperature in binding buffer. Then samples were washed extensively with PBS and mounted on microscope slides using Mowiol (Calbiochem). Samples were examined on a Leica TCS SP5 microscope (Leica).
Isolation of tumor-infiltrated immune cells
Human gastric cancer and mice melanoma specimens were cut into pieces on ice to about 0.2 cm diameter, then incubated in 1640 medium supplemented with 10% FBS and type IV collagenase (Sigma, C5138) solution 300 units/ml for 0.5–1 h at 37 °C. After passing through a 100-μm filter, cells were washed twice with 1× PBS prior to staining.
His–pulldown assay
His–pulldown assays were carried out as previously described (41).
In vitro ubiquitination assay
His6-E1(100 ng), His6-UbcH5b(200 ng), His6-FLAG ubiquitin (10 μg) were purified from Escherichia coli as previously described (11). StrepII FOXP3 (500 ng) purified from the supernatant of culture medium cultivating CHO-K1 cells using a Strep Tactin-Sepharose High performance kit (GE Healthcare, 29048653), 2×FLAG RNF31/RNF31-N1 (500 ng), 2×FLAG RBCK1 (500 ng) purified from HEK-293T cells with FLAG-tagged protein purification kit (Sigma, C2103) were mixed in the reaction buffer (20 mm Tris, pH 7.5, 5 mm DTT, 5 mm MgCl2, and 2 mm ATP) for 2 h, at 37 °C, then SDS was added to stop the reaction followed by Western blotting, anti-FOXP3 antibody was used to specifically detect the ubiquitination level of FOXP3.
Kaplan-Meier analysis
Based on the TCGA gastric cancer data, we used the ratio between expression of RNF31 and FOXP3 to normalize RNF31 expression for differences in tumor-infiltrated T cell densities. We found that higher expression of RNF31 was associated with a reduced disease-free survival rate (p = 0.03, log rank test).
Human samples
Samples were collected from treatment-naive adults undergoing surgery for primary gastric cancer after informed consent and approval from the Shanghai Jiaotong University School of Medicine, Renji Hospital Ethics Committee. PBMCs were obtained from patients prior to their surgical procedures.
C57BL/6 mice
Breeding mice were purchased from Jackson Laboratory. All animal protocols are approved by governmental and institutional guidelines for animal welfare.
Statistical analysis
Quantification analyses of Western blotting signals were done with ImageJ software (version 1.47, National Institute of Health). The statistical significance of the data were calculated with paired, two-sided Student's t test performed using GraphPad Prism 6. Probability values (p < 0.05) were considered as statistically significant: *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Author contributions
Fuxiang Zhu, G. Y., X. L., Fangming Zhu, R. Lin, and B. L. validation; Fuxiang Zhu, X. L., Fangming Zhu, A. Z., A. W., R. Z., Z. C., B. Z., S. F., X. Y., R. Lin, R. Liang, D. L., W. Z., Z. Z., W. G., S. Z., S. G., X. F., G. Z., and B. L. investigation; Fuxiang Zhu writing-original draft; Fuxiang Zhu, G. Y., and B. L. project administration; Fuxiang Zhu writing-review and editing; W. Z., Z. Z., G. Z., and B. L. resources; G. Z. and B. L. supervision; B. L. funding acquisition.
Supplementary Material
Acknowledgments
We thank Drs. Jia Nie, Zuojia Chen, Yangyang Li, Yue Lv, and Xuerui Luo for instructions and thank all members in our laboratory for helpful discussions.
This work was supported by National Natural Science Foundation of China NSFC 31525008, 81330072, 3187050011, 31670911, and 31370863, Science and Technology Commission of Shanghai Municipality (SMCST) Grant 14JC1406100, Shanghai Academic Research Leader Grant 16XD1403800, and National Institutes of Health NSFC collaborative Grant 81161120417. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Figs. S1–S6.
- Treg
- regulatory T cell
- LUBAC
- linear ubiquitin chain assembly complex
- IL-2
- interleukin 2
- shRNA
- short hairpin RNA
- INFγ
- interferon γ
- MFI
- mean fluorescence intensity
- Ab
- antibody
- TNFα
- tumor necrosis factor α
- IP
- immunoprecipitation
- IF
- immunofluorescence
- IB
- immunoblot
- DAPI
- 4′,6-diamidino-2-phenylindole
- PBMC
- peripheral blood mononuclear cell
- HRP
- horseradish peroxidase
- NEMO
- NF-κB essential modulator.
References
- 1. Sakaguchi S., Yamaguchi T., Nomura T., and Ono M. (2008) Regulatory T cells and immune tolerance. Cell 133, 775–787 10.1016/j.cell.2008.05.009 [DOI] [PubMed] [Google Scholar]
- 2. Hori S., Nomura T., and Sakaguchi S. (2003) Control of regulatory T cell development by the transcription factor Foxp3. Science 299, 1057–1061 10.1126/science.1079490 [DOI] [PubMed] [Google Scholar]
- 3. Zhou X., Bailey-Bucktrout S. L., Jeker L. T., Penaranda C., Martínez-Llordella M., Ashby M., Nakayama M., Rosenthal W., and Bluestone J. A. (2009) Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat. Immunol. 10, 1000–1007 10.1038/ni.1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bailey-Bucktrout S. L., Martinez-Llordella M., Zhou X., Anthony B., Rosenthal W., Luche H., Fehling H. J., and Bluestone J. A. (2013) Self-antigen-driven activation induces instability of regulatory T cells during an inflammatory autoimmune response. Immunity 39, 949–962 10.1016/j.immuni.2013.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Duarte J. H., Zelenay S., Bergman M. L., Martins A. C., and Demengeot J. (2009) Natural Treg cells spontaneously differentiate into pathogenic helper cells in lymphopenic conditions. Eur. J. Immunol. 39, 948–955 10.1002/eji.200839196 [DOI] [PubMed] [Google Scholar]
- 6. Wan Y. Y., and Flavell R. A. (2007) Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature 445, 766–770 10.1038/nature05479 [DOI] [PubMed] [Google Scholar]
- 7. Khattri R., Cox T., Yasayko S. A., and Ramsdell F. (2003) An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 4, 337–342 10.1038/ni909 [DOI] [PubMed] [Google Scholar]
- 8. Ogawa C., Tone Y., Tsuda M., Peter C., Waldmann H., and Tone M. (2014) TGF-β-mediated Foxp3 gene expression is cooperatively regulated by Stat5, Creb, and AP-1 through CNS2. J. Immunol. 192, 475–483 10.4049/jimmunol.1301892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ohkura N., Hamaguchi M., Morikawa H., Sugimura K., Tanaka A., Ito Y., Osaki M., Tanaka Y., Yamashita R., Nakano N., Huehn J., Fehling H. J., Sparwasser T., Nakai K., and Sakaguchi S. (2012) T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development. Immunity 37, 785–799 10.1016/j.immuni.2012.09.010 [DOI] [PubMed] [Google Scholar]
- 10. Li Y., Lu Y., Wang S., Han Z., Zhu F., Ni Y., Liang R., Zhang Y., Leng Q., Wei G., Shi G., Zhu R., Li D., Wang H., Zheng S. G., Xu H., Tsun A., and Li B. (2016) USP21 prevents the generation of T-helper-1-like Treg cells. Nat. Commun. 7, 13559 10.1038/ncomms13559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen Z., Barbi J., Bu S., Yang H. Y., Li Z., Gao Y., Jinasena D., Fu J., Lin F., Chen C., Zhang J., Yu N., Li X., Shan Z., Nie J., et al. (2013) The ubiquitin ligase Stub1 negatively modulates regulatory T cell suppressive activity by promoting degradation of the transcription factor Foxp3. Immunity 39, 272–285 10.1016/j.immuni.2013.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Luo X., Nie J., Wang S., Chen Z., Chen W., Li D., Hu H., and Li B. (2015) Poly (ADP-ribosyl)ation of FOXP3 protein mediated by PARP-1 protein regulates the function of regulatory T cells. J. Biol. Chem. 290, 28675–28682 10.1074/jbc.M115.661611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Loosdregt J., Fleskens V., Fu J., Brenkman A. B., Bekker C. P., Pals C. E. G. M., Meerding J., Berkers C. R., Barbi J., Gröne, Sijts A. J., Maurice M. M., Kalkhoven E., Prakken B. J., et al. (2013) Stabilization of the transcription factor Foxp3 by the deubiquitinase USP7 increases Treg-cell-suppressive capacity. Immunity 39, 259–271 10.1016/j.immuni.2013.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kirisako T., Kamei K., Murata S., Kato M., Fukumoto H., Kanie M., Sano S., Tokunaga F., Tanaka K., and Iwai K. (2006) A ubiquitin ligase complex assembles linear polyubiquitin chains. EMBO J. 25, 4877–4887 10.1038/sj.emboj.7601360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tokunaga F., Nakagawa T., Nakahara M., Saeki Y., Taniguchi M., Sakata S., Tanaka K., Nakano H., and Iwai K. (2011) SHARPIN is a component of the NF-κB-activating linear ubiquitin chain assembly complex. Nature 471, 633–636 10.1038/nature09815 [DOI] [PubMed] [Google Scholar]
- 16. Stieglitz B., Morris-Davies A. C., Koliopoulos M. G., Christodoulou E., and Rittinger K. (2012) LUBAC synthesizes linear ubiquitin chains via a thioester intermediate. EMBO Rep. 13, 840–846 10.1038/embor.2012.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zinngrebe J., Rieser E., Taraborrelli L., Peltzer N., Hartwig T., Ren H., Kovács I., Endres C., Draber P., Darding M., von Karstedt S., Lemke J., Dome B., Bergmann M., Ferguson B. J., and Walczak H. (2016) LUBAC deficiency perturbs TLR3 signaling to cause immunodeficiency and autoinflammation. J. Exp. Med. 213, 2671–2689 10.1084/jem.20160041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Damgaard R. B., Nachbur U., Yabal M., Wong W. W., Fiil B. K., Kastirr M., Rieser E., Rickard J. A., Bankovacki A., Peschel C., Ruland J., Bekker-Jensen S., Mailand N., Kaufmann T., Strasser A., et al. (2012) The ubiquitin ligase XIAP recruits LUBAC for NOD2 signaling in inflammation and innate immunity. Mol. Cell 46, 746–758 10.1016/j.molcel.2012.04.014 [DOI] [PubMed] [Google Scholar]
- 19. Rodgers M. A., Bowman J. W., Fujita H., Orazio N., Shi M., Liang Q., Amatya R., Kelly T. J., Iwai K., Ting J., and Jung J. U. (2014) The linear ubiquitin assembly complex (LUBAC) is essential for NLRP3 inflammasome activation. J. Exp. Med. 211, 1333–1347 10.1084/jem.20132486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sasaki Y., Sano S., Nakahara M., Murata S., Kometani K., Aiba Y., Sakamoto S., Watanabe Y., Tanaka K., Kurosaki T., and Iwai K. (2013) Defective immune responses in mice lacking LUBAC-mediated linear ubiquitination in B cells. EMBO J. 32, 2463–2476 10.1038/emboj.2013.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haas T. L., Emmerich C. H., Gerlach B., Schmukle A. C., Cordier S. M., Rieser E., Feltham R., Vince J., Warnken U., Wenger T., Koschny R., Komander D., Silke J., and Walczak H. (2009) Recruitment of the linear ubiquitin chain assembly complex stabilizes the TNF-R1 signaling complex and is required for TNF-mediated gene induction. Mol. Cell 36, 831–844 10.1016/j.molcel.2009.10.013 [DOI] [PubMed] [Google Scholar]
- 22. Park Y., Jin H. S., Lopez J., Lee J., Liao L., Elly C., and Liu Y. (2016) SHARPIN controls regulatory T cells by negatively modulating the T cell antigen receptor complex. Nat. Immunol. 17, 286–296 10.1038/ni.3352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fujita H., Rahighi S., Akita M., Kato R., Sasaki Y., Wakatsuki S., and Iwai K. (2014) Mechanism underlying IkappaB kinase activation mediated by the linear ubiquitin chain assembly complex. Mol. Cell. Biol. 34, 1322–1335 10.1128/MCB.01538-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Teh C. E., Lalaoui N., Jain R., Policheni A. N., Heinlein M., Alvarez-Diaz S., Sheridan J. M., Rieser E., Deuser S., Darding M., Koay H. F., Hu Y., Kupresanin F., O'Reilly L. A., Godfrey D. I., et al. (2016) Linear ubiquitin chain assembly complex coordinates late thymic T-cell differentiation and regulatory T-cell homeostasis. Nat. Commun. 7, 13353 10.1038/ncomms13353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Samstein S. R., Arvey A., Josefowicz S. Z., Peng X., Reynolds A., Sandstrom R., Neph S., Sabo P., Kim J. M., Liao W., Li M. O., Leslie C., Stamatoyannopoulos J. A., and Rudensky A. Y. (2012) Foxp3 exploits a pre-existent enhancer landscape for regulatory T cell lineage specification. Cell 151, 153–166 10.1016/j.cell.2012.06.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zheng Y., Josefowicz S., Chaudhry A., Peng X. P., Forbush K., and Rudensky A. Y. (2010) Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature 463, 808–812 10.1038/nature08750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhu J., Zhao C., Kharman-Biz A., Zhuang T., Jonsson P., Liang N., Williams C., Lin C. Y., Qiao Y., Zendehdel K., Strömblad S., Treuter E., and Dahlman-Wright K. (2014) The atypical ubiquitin ligase RNF31 stabilizes estrogen receptor α and modulates estrogen-stimulated breast cancer cell proliferation. Oncogene 33, 4340–4351 10.1038/onc.2013.573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Komander D., and Rape M. (2012) The ubiquitin code. Annu. Rev. Biochem. 81, 203–229 10.1146/annurev-biochem-060310-170328 [DOI] [PubMed] [Google Scholar]
- 29. Smit J. J., Monteferrario D., Noordermeer S. M., van Dijk W. J., Reijden B., and Sixma T. K. (2012) The E3 ligase HOIP specifies linear ubiquitin chain assembly through its RING-IBR-RING domain and the unique LDD extension. EMBO J. 31, 3833–3844 10.1038/emboj.2012.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zeng H., Chen Y., Yu M., Xue L., Gao X., Morris S. W., Wang D., and Wen R. (2008) T cell receptor-mediated activation of CD4+CD44hi T cells bypasses Bcl10: an implication of differential NF-κB dependence of naive and memory T cells during T cell receptor-mediated responses. J. Biol. Chem. 283, 24392–24399 10.1074/jbc.M802344200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Overacre-Delgoffe A. E., Chikina M., Dadey R. E., Yano H., Brunazzi E. A., Shayan G., Horne W., Moskovitz J. M., Kolls J. K., Sander C., Shuai Y., Normolle D. P., Kirkwood J. M., Ferris R. L., Delgoffe G. M., Bruno T. C., Workman C. J., and Vignali D. A. A. (2017) Interferon-γ drives Treg fragility to promote anti-tumor immunity. Cell 169, 1130–1141.e1111 10.1016/j.cell.2017.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kindlund B., Sjöling Å., Yakkala C., Adamsson J., Janzon A., Hansson L. E., Hermansson M., Janson P., Winqvist O., and Lundin S. B. (2017) CD4+ regulatory T cells in gastric cancer mucosa are proliferating and express high levels of IL-10 but little TGF-β. Gastric Cancer 20, 116–125 10.1007/s10120-015-0591-z [DOI] [PubMed] [Google Scholar]
- 33. Plitas G., Konopacki C., Wu K., Bos P. D., Morrow M., Putintseva E. V., Chudakov D. M., and Rudensky A. Y. (2016) Regulatory T cells exhibit distinct features in human breast cancer. Immunity 45, 1122–1134 10.1016/j.immuni.2016.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cho J. H., Kim H. O., Ju Y. J., Kye Y. C., Lee G. W., Lee S. W., Yun C. H., Bottini N., Webster K., Goodnow C. C., Surh C. D., King C., and Sprent J. (2016) CD45-mediated control of TCR tuning in naive and memory CD8+ T cells. Nat. Commun. 7, 13373 10.1038/ncomms13373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Courtney A. H., Lo W. L., and Weiss A. (2018) TCR signaling: mechanisms of initiation and propagation. Trends Biochem. Sci. 43, 108–123 10.1016/j.tibs.2017.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gao Y., Tang J., Chen W., Li Q., Nie J., Lin F., Wu Q., Chen Z., Gao Z., Fan H., Tsun A., Shen J., Chen G., Liu Z., Lou Z., Olsen N. J., Zheng S. G., and Li B. (2015) Inflammation negatively regulates FOXP3 and regulatory T-cell function via DBC1. Proc. Natl. Acad. Sci. U.S.A. 112, E3246–E3254 10.1073/pnas.1421463112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jana S., Jailwala P., Haribhai D., Waukau J., Glisic S., Grossman W., Mishra M., Wen R., Wang D., Williams C. B., and Ghosh S. (2009) The role of NF-κB and Smad3 in TGF-β-mediated Foxp3 expression. Eur. J. Immunol. 39, 2571–2583 10.1002/eji.200939201 [DOI] [PubMed] [Google Scholar]
- 38. Tanaka A., and Sakaguchi S. (2017) Regulatory T cells in cancer immunotherapy. Cell Res. 27, 109–118 10.1038/cr.2016.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wei T., Zhang J., Qin Y., Wu Y., Zhu L., Lu L., Tang G., and Shen Q. (2015) Increased expression of immunosuppressive molecules on intratumoral and circulating regulatory T cells in non-small-cell lung cancer patients. Am. J. Cancer Res. 5, 2190–2201 [PMC free article] [PubMed] [Google Scholar]
- 40. Dubois S. M., Alexia C., Wu Y., Leclair H. M., Leveau C., Schol E., Fest T., Tarte K., Chen Z. J., Gavard J., and Bidère N. (2014) A catalytic-independent role for the LUBAC in NF-κB activation upon antigen receptor engagement and in lymphoma cells. Blood 123, 2199–2203 10.1182/blood-2013-05-504019 [DOI] [PubMed] [Google Scholar]
- 41. Wang X., Yang J., Han L., Zhao K., Wu Q., Bao L., Li Z., Lv L., and Li B. (2015) TRAF5-mediated Lys-63-linked polyubiquitination plays an essential role in positive regulation of RORγt in promoting IL-17A expression. J. Biol. Chem. 290, 29086–29094 10.1074/jbc.M115.664573 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.