Abstract
This review brings together observations on the stress-induced regulation of resilience mechanisms in body tissues. It is argued that the stresses that induce tissue resilience in mammals arise from everyday sources: sunlight, food, lack of food, hypoxia and physical stresses. At low levels, these stresses induce an organised protective response in probably all tissues; and, at some higher level, cause tissue destruction. This pattern of response to stress is well known to toxicologists, who have termed it hormesis. The phenotypes of resilience are diverse and reports of stress-induced resilience are to be found in journals of neuroscience, sports medicine, cancer, healthy ageing, dementia, parkinsonism, ophthalmology and more. This diversity makes the proposing of a general concept of induced resilience a significant task, which this review attempts. We suggest that a system of stress-induced tissue resilience has evolved to enhance the survival of animals. By analogy with acquired immunity, we term this system ‘acquired resilience’. Evidence is reviewed that acquired resilience, like acquired immunity, fades with age. This fading is, we suggest, a major component of ageing. Understanding of acquired resilience may, we argue, open pathways for the maintenance of good health in the later decades of human life.
Keywords: dose–response, preconditioning, radiation, hormesis, acquired resilience
…it was too marvellous and gave rise to skepticism
Niels Finsen,1 Nobel Laureate (1903), recalling criticism of his evidence that red light accelerated the healing of the skin lesions of smallpox.

Outline
This review brings together a range of observations on the stress-induced regulation of self-protective/self-repair mechanisms in body tissues. It is argued that the stresses that induce tissue resilience in mammals arise from several everyday sources:
sunlight (UV, visible light, infrared, X-rays);
food—the toxins of ordinary food;
lack of food: hunger and caloric restriction;
- hypoxia of a tissue caused by:
- blockage or hemorrhage of vessels supplying the tissue (ischemia),
- ischemia of distant tissues (remote ischemia),
- rapid increases in oxygen consumption (particularly exercise);
experimental or altitude hypoxia;
- physical stresses:
- heat, cold,
- mechanical damage,
- the stress of blood flow on vessel endothelium.
At low levels, these stresses induce an organized protective response in probably all tissues and, at some higher level, cause tissue destruction. This low-dose-resilience/high-dose-toxic pattern of response to stress is well known to toxicologists, who have termed it hormesis.
The phenotype of the resilience induced by low-dose stress often depends on the investigators’ interest. Studies have reported that low-dose stress induces:
The acceleration of wound healing;
Conditioning of undamaged tissue, making it resilient in the face of subsequent stress;
Slowing or stopping age-related degenerations of central nervous tissue (Parkinson, Alzheimer, macular degeneration), of connective tissue (skin aging), or of muscle (sarcopenia);
Reduction of genotoxicity (ie, protection of the genome), so reduction in the formation of cancers;
Accelerated reduction of inflammation and pain;
Supernormal function, reported in muscle and retina;
Acceleration of recovery from fatigue, reported in muscle;
Suppression of cancer, proliferation, and metastasis as well as mutagenesis.
In relation to aging, investigators have reported 2 major resilience phenomena:
Resilience fades with aging; the same stress that induces resilience in youthful tissue in age no longer induces resilience.
Resilience can be maintained into old age, by the same stresses that induce tissue resilience in the young, best studied for exercise.
As a consequence, the phenotypes of resilience include:
Reduction of morbidity, particularly in the elderly patients, contributing to greater longevity.
Because of this diversity of phenotype, reports of stress-induced resilience are to be found in journals of neuroscience, sports medicine, cancer, healthy aging, dementia, parkinsonism, ophthalmology, and more. The diversity makes the proposing of a general concept of induced resilience a significant task, which this review attempts.
The mechanisms by which low-level stress upregulates resilience have been studied intensively. Some are tissue-specific. The skin and retina, for example, have evolved skin- and retina-specific responses to daylight. By contrast, ingested plant toxins circulate through the body and induce resilience in probably all tissues, and exercise, infrared radiation, and caloric restriction also induce body-wide resilience. Further, many stressors appear to operate by common mechanisms, as their effects do not sum, and correspondingly, each can induce many, perhaps all, of the phenotypes of resilience. The “rules” of induced resilience are still being worked out.
We suggest that a system of stress-induced tissue resilience has evolved to enhance the survival of animals, which are all subject to everyday stress. By analogy with acquired immunity, in which exposure to a pathogen induces immunity, we term this system, which responds to low-level stresses by upregulated resilience-inducing pathways, “acquired resilience.” And, having in mind the fading of acquired immunity with age (immunosenescence), we consider whether a comparable fading of acquired resilience (resiliosenescence) can be identified and conclude that it can. The fading of resilience, we argue, is a major component of the cause of aging, including features of aging such as sarcopenia, cancer, slowness of wound healing, slowness in recovery from fatigue, and more. Understanding of acquired resilience may, we argue, open pathways for the maintenance of good health in the later decades of human life.
The Stresses That Induce Resilience
When the stresses known to induce tissue resilience are cataloged, they fall into groups—sunlight and other radiations, plant toxins, tissue hypoxia including the hypoxia resulting from vascular failure, respiratory dysfunction and exercise, hunger and caloric restriction, and physical stress (heat, mechanical damage, sheer stress to the vascular endothelium). These are the everyday stresses of everyday life.
The idea that some level of stress is beneficial has common currency, for example, in the saying “whatever doesn’t kill me makes me stronger.” The concept of “eustress,” or good stress, was developed by psychologists who (naturally enough) discussed it in psychological terms (eg, the study by O’Sullivan2). The term allows a useful distinction between eustress and distress, and the analogy is clear with the low-dose-tonic and high-dose-toxic phenomena of hormesis, discussed below. The forms of stress considered below arise, however, from physical, chemical, or metabolic sources and, importantly, their effects have been demonstrated in subject-blind investigations, so free of psychological influences.
Light and Other Radiations
Sunlight
The radiant energy experienced by animals arises almost entirely from the sun. The idea that sunlight has health-giving properties goes back to early traditions of medicine; histories of light as therapy can be found elsewhere.3–8 The transition from anecdotes, traditions, and clinical impressions to testable hypotheses of the curative potential of sunlight took a major step with the work of Niels Finsen1,9,10 (see also https://www.nobelprize.org/nobel_prizes/medicine/laureates/1903/) who reported healing effects of UV light for the skin lesions of tuberculosis and of red light for the skin lesions of smallpox. With Finsen’s work, recognized by the 1903 Nobel Prize, and the work of his contemporaries, the idea that sunlight can enhance the resilience of body tissues entered the peer-reviewed literature. A century and more later, thousands of peer-reviewed studies give evidence that many wavelengths within sunlight can, at appropriate low doses, induce tissue resilience.
Red–Infrared Light (600-1000 nm)
The fight against the disease smallpox had a major impact on medical science, leading to the understanding of variolation, vaccination, cross-immunity, and acquired immunity. The same fight also led, with a half-century’s delay, to some of our understanding of tissue resilience.
Physicians in Finsen’s time noted that the skin lesions of smallpox were most prominent on the arms and face, the areas most free of clothing. Daylight, physicians inferred, might be exacerbating these lesions and they prescribed darkness. Finsen and others—influenced by reports of the value of red swathing and red curtains, and by the practical need for some lighting—kept patients in filtered red light rather than darkness. In an early (1895) meta-analysis, Finsen9 reviewed reports from a dozen clinics of “the extremely favorable” effect of red light in the healing of lesions and corresponding reductions in mortality and in the scarring of survivors:
The total number of patients treated (in seven published studies) was about 70, and the method failed in only one case. It must be observed that these reports are of considerable value, as the authors as a rule were evidently exceedingly skeptical…. Some few of them have confined themselves to mere reports of the history of the cases, and have otherwise been extremely reserved in their expressions of opinion; some (Feilberg, Svendsen) have for certainty’s sake made controlling experiments; others (Oettinger, for instance) chose the most severe cases to experiment with.
Progress was delayed partly by the personal tragedy of Finsen’s early death, in 1904, and partly because advances in vaccination in the following decades did much to prevent smallpox. Those decades saw rapid advances in many areas of medicine, but the idea of light-inducible tissue resilience was not among them.
Exploration of the concept resumed in the 1960s, stimulated not by a disease but by a technical advance, the development of wavelength-specific light sources—lasers and light-emitting diodes (LEDs).5 Finsen had selected red or UV light from white light with pigmented filters; now the wavelength and energy of radiation could be engineered with precision, and their effects were explored systematically. Even so, this new phase of work began with surprise observations. In one early and influential study, Mester and colleagues11 set out (for an account see4) to test whether 694 nm laser light, shone on the shaved skin of mice, would induce cancer. No cancer formed; instead, they reported the laser radiation increased hair growth, a finding since confirmed for low-dose irradiation.12 In a second study (for an account, see the study by Gáspár13), the same group sought to use the same wavelength to destroy experimentally implanted tumors. The tumors seemed unaffected, but the irradiation accelerated the healing of the implantation wound, a finding extensively confirmed.
Since these early reports, analysis of the resilience induced by red–infrared light generated by laser sources has advanced from surprise observation to systematic laboratory studies and randomized clinical trials, on a range of tissues and with effects too numerous to be summarized readily. The terms “low-level light therapy” and “photobiomodulation” (PBM) have been adopted by the US National Library of Medicine as indexing terms for the induction of positive tissue responses using laser or LED sources (see https://www.ncbi.nlm.nih.gov/mesh/?%25term=photobiomodulation). Recent reviews describe present understanding of the impact of red–infrared light on the resilience of the skin14 and in inducing supernormal performance in muscle as well as accelerated recovery from fatigue and injury.15,16 The use of PBM as a neuroprotectant was pioneered by Eells and colleagues17 in a model of alcohol-induced degeneration of retinal photoreceptors and has been extended to the slowing of cerebral degenerations,18–24 the mitigation of the effects of traumatic brain injury,25–30 the mitigation of macular degeneration,31,32 and improvements in the outcome of stroke.33,34 The value of PBM for the mitigation of retinal damage in a range of conditions has received support from a recent meta-analysis5; the author adds caution that larger scale clinical trials are needed for a fuller understanding of the mechanisms involved.
Many studies reported trials of different dose regimes; they report consistently that PBM is effective at low doses, up to ∼10 J/cm2/d, and that increasing the daily dosage further leads to a loss of effect.4 Increasing the number of consecutive days at which a low dose is given causes a steady increase in effect, at least up to 10 days.35 But more needs to be known concerning dosage. One recent open-label, single-arm clinical study36 trialed a course of 12 doses of relatively high-intensity infrared light directed transcranially at the frontal and temporal lobes, in patients suffering mild-to-severe depression, reporting robust mitigation of depression scores, maintained for up to 55 months from a single course of treatment. Side effects were minimal; the authors suggested the outcome provided a basis for randomly controlled trials.
Mechanisms
The mechanisms of PBM have been reviewed extensively, with 2 sets of actions emerging, “direct” and “indirect.” There is strong evidence that PBM induces resilience in tissue directly irradiated and that irradiation at one site induces resilience body-wide, so indirectly.
In most studies, the tissue under study has been irradiated directly, whether cells in vitro, or skin wounds, painful joints or tooth sockets, or the retina. The brain has also been irradiated directly, either transcranially or by an optical fiber placed deep into the brain,37 or by an intranasal probe38 to reach the inferior surface of the frontal lobe. Only a minority of studies, but still many, have tested mechanisms of this direct irradiation, reviewed elsewhere.4,7,39–41 The most easily understood mechanism of direct irradiation is that the incident light is absorbed by a photoacceptor in the oxidative phosphorylation pathway of mitochondria, accelerating the production of adenosine triphosphate (ATP) in injured cells, most clearly demonstrated in vitro.42 As Hamblin and Demidova4 noted, however, there is good evidence of more-complex-to-describe actions of PBM on the tissue irradiated. The PBM may increase the production of superoxide ions, shifting the “redox state” of the cell; it may reverse the inhibition of cytochrome oxidase by nitric oxide (NO), increasing oxygen-fueled oxidative phosphorylation. Further, changes in the redox state regulate a number of transcription factors; Hamblin and Demidova identified nuclear factor (NF)-κB, p53, ARG/CREB, and HIF-like factor as regulated by redox state and therefore potentially by PBM. Finally, Hamblin and Demidova noted that some tissue responses to PBM can be described only at the cellular level—the stimulation of metabolism, migration, proliferation, and the synthesis and secretion of proteins, including powerfully trophic proteins such as FGF-2.
A still different and complex picture of the mechanisms of direct irradiation of tissue emerges when gene array technology is used. Natoli and colleagues,43 for example, examined gene regulation induced by direct PBM of retina, both uninjured retina and retina damaged by bright light (light damage or LD). Comparing normal retina with LD retina with PBM-irradiated retina with retina irradiated (conditioned) with PBM and then damaged by light, we concluded that
…PBM, given without LD, changes retinal gene expression in a significant number of entities, and that, given as a pretreatment to LD, PBM (like saffron) changes the expression of a large numbers of entities, reducing the LD-induced regulation of many and regulating many not affected by LD. PBM,…appears to regulate many intracellular pathways when given as a pretreatment…a large proportion of the entities regulated by PBM are ncRNAs, and further understanding of the protective action of PBM will require understanding to the roles of these sequences.
So, to understand the patterns of gene expression that we observed, we were obliged to distinguish between genes and noncoding RNAs (ncRNA), the latter still not well understood, and between the regulation of ncRNA and gene expression induced by PBM in unstressed (control) tissue, and the modification by PBM of the extensive changes in gene and ncRNA expression induced by light-induced damage of the retina. It is not an outcome easy to summarize, and there have been few analyses of PBM-induced gene expression in the years since to take the analysis further. An important element of the analysis is that PBM regulates many more genes and ncRNAs in injured tissue than in uninjured tissues. More generally, the description of the mechanisms of direct PBM varies with the observations made, and the response is complex, involving multiple pathways, influencing many aspects of cell function.
Realization that PBM has indirect effects came from occasional reports as early as 1989, that irradiation of a wound on one flank of an experimental animal accelerated healing on both flanks44,45; that irradiation of a crushed sciatic nerve improved the function of both nerves45; that irradiation localized to a skin wound at one point on a human arm accelerated healing several centimeters away46; that irradiating one side of the face of children undergoing immunosuppression prior to a bone marrow transplantation prevented sores forming on both sides47; that, in mice with gliomas implanted under the skin of the back, PBM directed at the abdomen inhibited tumor growth.48 These workers all sought to use nonirradiation of one side or part of the body as a control for irradiation of a wounded site. The controls did not work as expected and, when the investigators—most clearly Rochkind and colleagues45—checked why, it became clear that the effects of PBM are not confined to the tissue irradiated.
Building on these studies, the present authors tested and confirmed the indirect effect of PBM, showing that irradiation of the body of a mouse (with the head shielded) protects the substantia nigra (SNc) of the midbrain (a key locus of the neuropathology of parkinsonism) from toxin-induced damage.49–52 Further, the protection achieved by irradiation of the body was less than when both head and body were irradiated. Our interim conclusion was that PBM protects the SNc by both direct and indirect mechanisms and that—when both head and body are irradiated—their effects sum.
A separate line of evidence of the reality and mechanisms of the indirect action of PBM was developed by Oron and colleagues,53–55 who linked a series of observations. First, they observed that infrared light induces the proliferation of stem cells in vitro, extending earlier observations of PBM-induced proliferation.55 They next showed that the healing of infarcts in rat heart muscle was accelerated by the implantation of PBM-treated stem cells harvested from bone marrow54; then that PBM directed at the bone marrow was particularly effective in protecting rat heart muscle from ischemia,53 adding evidence that the protection was mediated by bone marrow–derived stem cells, migrating to or proliferating at the site of ischemia. The same authors have extended these observations, reporting that PBM directed at the bone marrow of the tibia slows the progression of Alzheimer-like pathology in the mouse18,56 and reduces scarring caused by ischemia to heart muscle in the pig.57 A third group,58 working in a mouse model of diabetic retinopathy, used a lead helmet to limit radiation to the body, showing that PBM irradiation of the body mitigated diabetes-induced changes, including leukostasis, superoxide generation, and visual performance.
The complexity of mechanisms underlying the indirect action of PBM has become evident from a line of research on subpopulations of bone marrow–derived stem cells, confirming that as Oron and colleagues have argued, resident and bone marrow–derived stem cells promote tissue regeneration and cell viability and induce angiogenesis in multiple tissues, including the nervous system, retina, and heart (see eg, the study by Muheremu et al, Bruyneel et al, Ward et al, Marichal et al, and Oner59–63). One such subpopulation recently shown to be involved in the resilience response has been dubbed “Myo/Nog cells.” Cells of this lineage were identified in the early embryo by their expression of the skeletal muscle-specific transcription factor MyoD and bone morphogenetic protein inhibitor noggin.64–66 During development, noggin released by Myo/Nog cells is critical for normal morphogenesis and skeletal muscle differentiation.64,67 In the embryo and adult, these cells also respond to injury and cell death in multiple tissues.65–70 For example, in retina damaged by excessive light or hypoxia, Myo/Nog/Nog cells accumulate in areas of cells death.65,66 If the damage to the retina is mitigated, for example, by PBM or dietary saffron (discussed below), fewer Myo/Nog cells congregate in the damaged region. A neuroprotective role for Myo/Nog cells was revealed when photoreceptor death was reduced and retinal function was improved in response to injection of brain-derived Myo/Nog cells into the vitreous humor of the eye.66 And, conversely, in the retina damaged by hypoxia, the targeted depletion of Myo/Nog cells resulted in an increase in neuronal cell death.65
This multipotency is, of course, what stems cells are about. But this evidence that Myo/Nog cells adopt new roles during the lifetime of mammals (they have been observed in mouse, rabbit, and human tissues) gives a glimpse into how difficult it may be to define the mechanism of indirect PBM in molecular terms, without knowing the underlying cellular- and organ-level mechanisms involved. Further, the multipotency may limit—certainly complicate—the use of stem cells in therapy. The expression by Myo/Nog cells of MyoD, for example, imparts the capacity to differentiate into muscle.69,71 Myofibroblast contractions can be beneficial for wound closure in skin, wherein Myo/Nog cells reside in a niche associated with hair follicles, expand in number in response to epidermal abrasion, and populate the exposed dermis within 24 hours.68 Myo/Nog cells also develop, however, into contractile myofibroblasts in the ocular lens, in response to wounding in vitro or after cataract surgery in vivo,68,70,72,73 and there the contractions produce wrinkles in the surrounding capsule that may impair vision postoperatively.69,70 A similar contractile phenomenon may occur in the retina wherein chronic stress leads to the formation of membranes containing myofibroblasts that contract and cause retinal detachment.74,75 Given their propensity to form muscle, any decision to implant or deplete Myo/Nog cells for therapeutic purposes will be dependent on knowledge of the properties of the target tissue.
But that is looking too far ahead. In the meantime, we note that the resilience response induced by PBM will have to be understood at several levels. Genome-wide analyses and analyses of specific molecular pathways will play a role, but the contribution of cell classes—like Myo/Nog cells—and responses of the whole animal will have to be analyzed, if the response of mammals to low-level stress is to be understood and deployed in therapy.
Innovations
One measure of the success of a technology is the investment made in the technology itself. The technology of “PBM” has extended from Finsen’s red filters to wavelength-specific lasers,11 to wavelength-specific LEDs,17 to delivery into the brain by optical fibres37 and intranasal probes,38 to most recently, the development of infrared-emitting cloth powered by body movement (reviewed by Tsai and Hamblin41). Not all these techniques may prove clinically useful, but there is appeal in the idea, already being tested,41 of speeding the healing of a skin wound or damaged tendon with a bandage that, powered by limb or body movement, emits an appropriate dose of infrared. This field, like all those reviewed below, is expanding rapidly, in ways difficult to anticipate.
One future innovation can be inferred from our still incomplete understanding of the indirect or remote effects of PBM, just discussed. This is the possibility that a regular dose of PBM, directed and calibrated by data yet to be complete, might be good for body-wide health, in the way we already believe that exercise and a healthy diet and intermittent hunger (all discussed below) can be. It seems an intriguing, promising idea, but an idea for some future review.
Ultraviolet Light (UV, 290-400nm)
The evidence that UV light increases the resilience of skin begins with Finsen’s9 evidence, already mentioned, that “the most refrangible rays” of visible light (blue and violet but likely including UV) promote skin healing in lupus vulgaris, an aggressive form of tuberculosis affecting the skin. The mechanism of this healing has not been identified; after Finsen’s 1903 report, tuberculosis was increasingly prevented by vaccination or cured by drugs, and Finsen’s evidence appears not to have been further analyzed.
A more familiar example is seen in the several effects of UVB light (290-320nm) on normal skin. At high intensities, UVB is toxic to the skin, downregulating immune mechanisms in the skin, inducing inflammation, DNA damage, and malignant mutations. At low daily doses, UVB conditions the skin, making it resistant to sunburn,76 and induces also the synthesis of vitamin D,76–79 which is essential for calcium absorption and bone structure and inhibits the onset of several cancers. It has not yet been established whether UV at low daily doses, such as those appropriate to induce vitamin D synthesis and adaptive responses such as mild tanning, is protective against tumors of the skin itself.
White Light (400-700 nm)
The retina
Under ideal conditions, rod photoreceptors can signal the capture of a single photon.80 This sensitivity is achieved by the amplification process of the phototransduction cascade, whereby the absorption of photons by rhodopsin in a rod outer segment leads to hyperpolarization of its axon terminal and a reduction in the release of glutamate from the terminal—the beginning of vision.
This sensitivity comes at a price, demonstrated 25 years ago by Penn and Anderson.81 They raised rats in darkness and showed that light-naive photoreceptors grow long outer segments with beautifully organized membranes, while the outer segments of photoreceptors from animals raised in more normal conditions (12 hours in darkness, 12 hours in mesopic conditions) were shorter and their membranes were damaged. But when the animals were exposed to bright daylight, light-naive photoreceptors were destroyed catastrophically, while photoreceptors with some light experience and damaged outer segments survived daylight robustly. Their work and subsequent studies (eg, the study by Liu et al82) showed that rat photoreceptors are conditioned by normal light experience to be resilient when exposed to potentially damaging levels of light. Again, low levels of stress—in this case of wavelengths found in daylight—induce resilience.
Other tissues
Although there is a long tradition of healing of body tissue by sunlight,83 few studies of broad-spectrum (white) light have appeared in the peer-reviewed literature. Several groups have, however, tested wavelengths within the white spectrum. Adamskaya and colleagues84 reported that blue light (470 nm) accelerates wound healing in a rat model, improving blood flow by inducing the release of NO; Fushimi and colleagues85 reported that red (638 nm) and green (518 nm) light accelerate wound healing and Yuan and colleagues86 observed that blue light (424 nm) protects liver and kidney tissue from ischemia–reperfusion damage. This evidence is limited but does suggest that broad-spectrum daylight, at appropriate low doses, induces tissue resilience. As a generalization, there seems to be an evolved mechanism by which skin uses the stress of everyday light to upregulate a wound healing response.
Ionizing Radiation: The Debate Over Radiation Hormesis
Interest in the biological effects of low-dose ionizing radiation has been stimulated by (at least) 2 imperatives. One was to learn as much as possible from the mass exposure of civilians to such radiation, in the nuclear bombing of 2 Japanese cities at the end of World War II. The other has been and remains to understand the risks to people exposed to low levels of radiation—patients who need radiotherapy, their radiologists, miners of radioactive materials, and engineers creating specialized forms of radiating materials.
The damaging effects of high-dose radiation are clear enough. One simplifying assumption, relied on for several decades after World War II, was that rates of tissue damage—particularly of DNA damage and its sequelae—at low doses could be extrapolated linearly from high-dose effects, for example, an increase or decrease in cancer risk of 4.5% to 7.1% per Sievert.87 This was also a safe assumption, one that responded to the fears of those subject to low-dose radiation. By the 1980s, however, the assumption was under challenge. In one early challenge,88 it was noted that, at the time of publication (1983), there were ∼1000 papers in the literature giving evidence that low-dose ionizing radiation (typically 1-50 cGy89) is not weakly toxic, as expected from the extrapolation hypothesis. Rather, it was surprisingly and distinctly beneficial to tissues. Hickey and colleagues88 argued that “the ignoring of the hormesis phenomenon seems to constitute a very serious error in modern biomedical science and in preventive medicine.” Their papers introduced the term radiation hormesis into the literature.
In 1991, Macklis and Beresford89 reviewed what was already an almost bewildering array of evidence: that cells in vitro conditioned with low-level ionizing radiation (their acronym was LLIR) are resistant to subsequent radiation at higher doses, suggesting a stress-inducible DNA repair mechanism; that LLIR induces DNA synthesis and increases antioxidant (glutathione) expression, slowing cell metabolism and increasing protection from reactive oxygen species; and that LLIR is immunostimulatory, even relatively high doses of radiation that induce a transient leukopenia and suppression of antibody production also inducing a longer period of higher-than-normal leukocyte and antibody production, associated with a resistance to transplanted tumors. They reviewed ideas that lack of exposure to LLIR may be “subtly detrimental” to cells, the way wind deprivation causes the early collapse of trees (below); that LLIR stimulates the growth and fecundity of organisms in general; and that in plants, LLIR induces a modest but measurable increase in linear growth, branching, and flowering. These authors were clearly intrigued by the diversity and the cumulative weight of the evidence then available for review, but they were also cautious, remarking that they found many individual studies “unconvincing.”
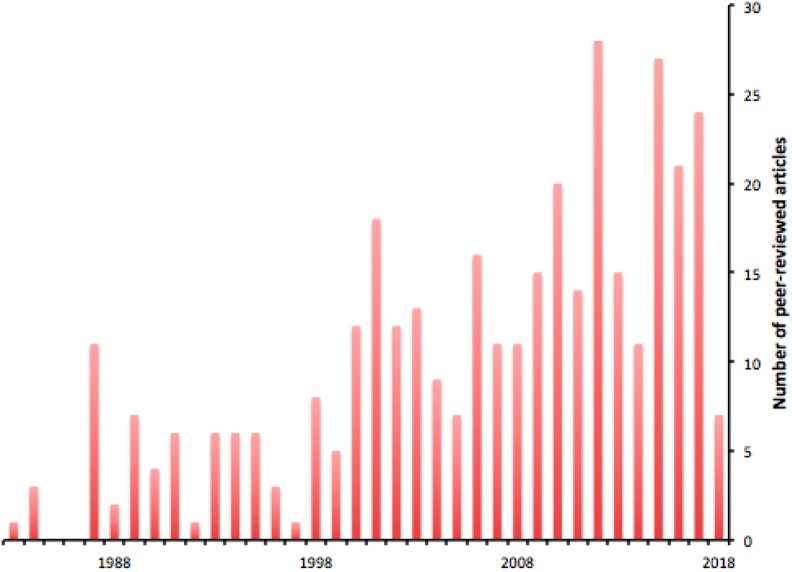
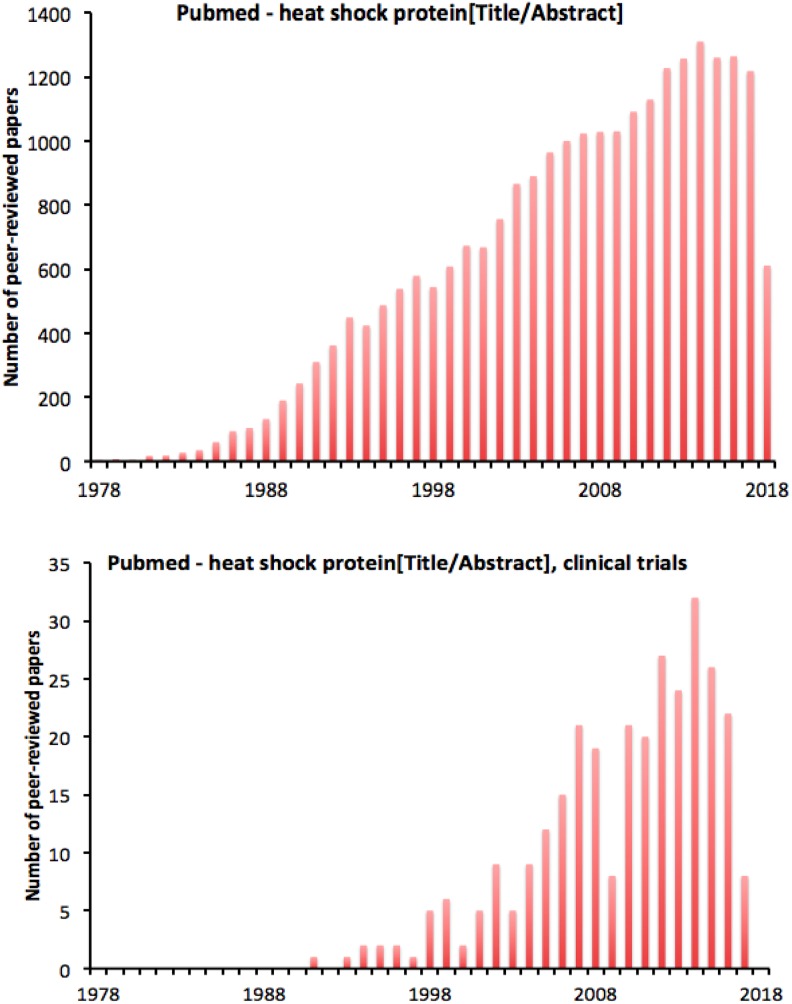
Despite this skepticism, experimental work continued (Figure 1), and reviewing the data available 2 years later, Loken and Feinendegen90 were bolder in their conclusions. The idea of radiation hormesis could not, they argued, be ignored. A further year on, Smith91 concluded that the beneficial effects of low-dose ionizing radiation had been convincingly demonstrated experimentally, but were cautious about its implications for humans, arguing that there was “no overwhelming evidence” that conditioning with low-dose irradiation reduces the occurrence of cancers.
Figure 1.
Publications per year relating to radiation hormesis, assessed by the occurrence of the term in their title or abstract; from PubMed.
By 1998, an investigator from the US Nuclear Regulatory Commission92 specifically recommended recognition of radiation hormesis as a better basis (than the linear extrapolation hypothesis) for minimizing the environmental risk of radiation. And a year later, Luckey93 noted increasing evidence that naturally occurring low-level radiation reduces cancer rates in human populations and proposed low-level ionizing therapy for the prevention of cancer, the opposite of the effect assumed from the linear extrapolation hypothesis.
The debate remains active and productive of new ideas, and of response to them, for the increasing confidence of some investigators evokes a reaction from others. Mossman94 wrote in 2001 of “deconstructing” radiation hormesis. In 2009, Jolly and Myer95 reviewed the tension between empirical findings, official policy, and boldly speculative ideas of the implications of radiation hormesis,96 and tentative views have persisted.97,98 Very recently, impatience with the long debate eventually surfaced, with reviewers declaring the linear no-threshold hypothesis to be “dead at age 89.”99
We would add only 2 comments. One is that modern animals have evolved in an environment that has always contained LLIR; there should be no surprise, at least in hindsight, if today’s genomes coded for the detection of the stress caused by such radiation and for the upregulation of a protective response. And second, the demonstration of low-level effects can be striking. Otani and colleagues,100 for example, tested whether low-dose γ-radiation makes photoreceptors in the rat retina resistant to potentially damaging levels of white light. The result was affirmative, reinforcing earlier evidence that low-level radiation is “good for us.” If it can slow neurodegenerations (itself a remarkable claim), who knows what else? More research seems certain in this important, ideas-rich area.
Food
Our nutritional requirements are usually understood to comprise carbohydrates, protein and fats, plus the vitamins and a range of minerals in small amounts. In recent decades, understanding has grown that something else in the plants we eat is good for us, reducing morbidity and delaying mortality in people with no nutritional deficiency. The idea has a background in at least 2 lines of observations. One is that a diet rich in vegetables, commonly called the “Mediterranean” diet, is associated with lower rates of cardiovascular disease and malignancies101,102; for a meta-review see Bloomfield et al.103 A vegetable-rich diet is thought of as good for our health, in the same vague way that we have long thought of moderate exercise and sunlight as being “healthful” (note 1). What is it with vegetables that can prevent diseases as severe as atherosclerosis and cancer and the neurodegenerations? The second line of observations comes from the extensive use of plants in traditional medicines, particularly the Islamic, Indian, and Chinese traditions, which seem, despite their lack of controlled trials, to deliver much.
The Resilience Induced by Certain Foods
In the past 30 years, the biomedical literature has seen the emergence of lines of studies, each focusing on the therapeutic potential of a chemical found in plants. Typically, the plant has had a long history in traditional medical practice, and the peer-reviewed literature on the compound began in the 1980s or 1990s and has grown exponentially since (Figures 1 –4).
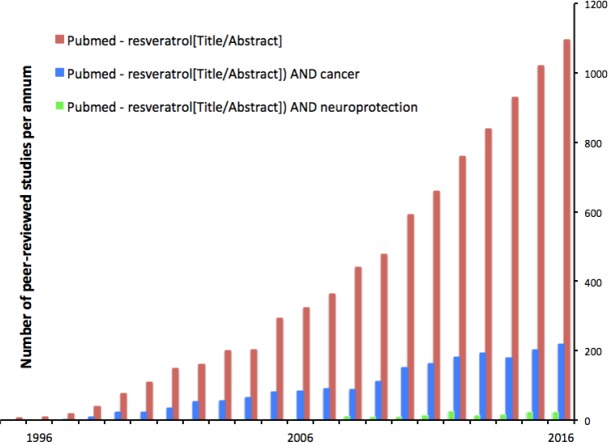
Figure 2.
Perhaps stimulated by the relatively high levels of resveratrol in plants used in Chinese traditional medicine,104–106 and in red wine, studies of resveratrol appeared in the peer-reviewed literature in the late 1980s and interest has grown rapidly since. At the time of writing, the total of papers in the PubMed database in which “resveratrol” appeared in the title or abstract was 8800. Of these, in 23% the title or abstract included “cancer” and in 1.7% included “neuroprotection.”
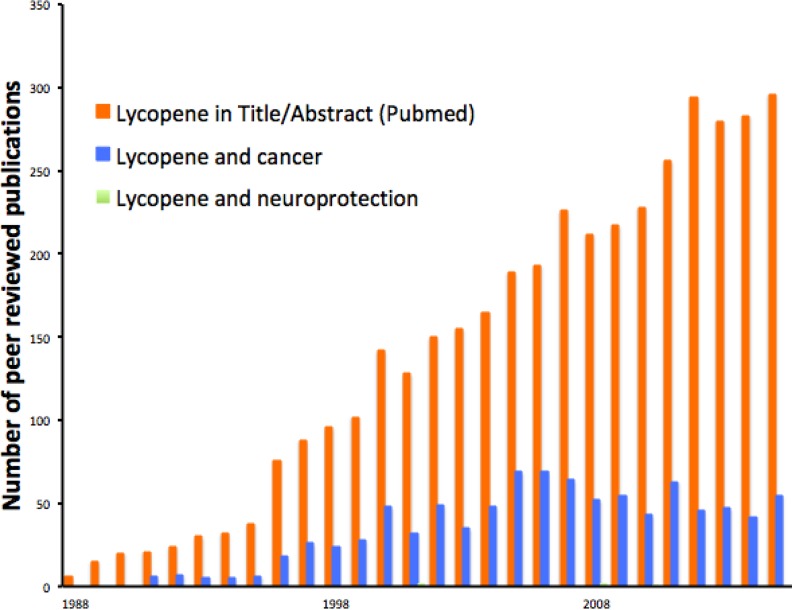
Figure 3.
The time course of peer-reviewed studies of lycopene also begins in ∼1990 and growing rapidly since. Approximately 24% of that literature concerns cancer, and a very small minority (0.1%, we found just 5 studies, not discernible on this graph) concerns neuroprotection. Overall, the number of studies on lycopene is about one-third of the number available for resveratrol.
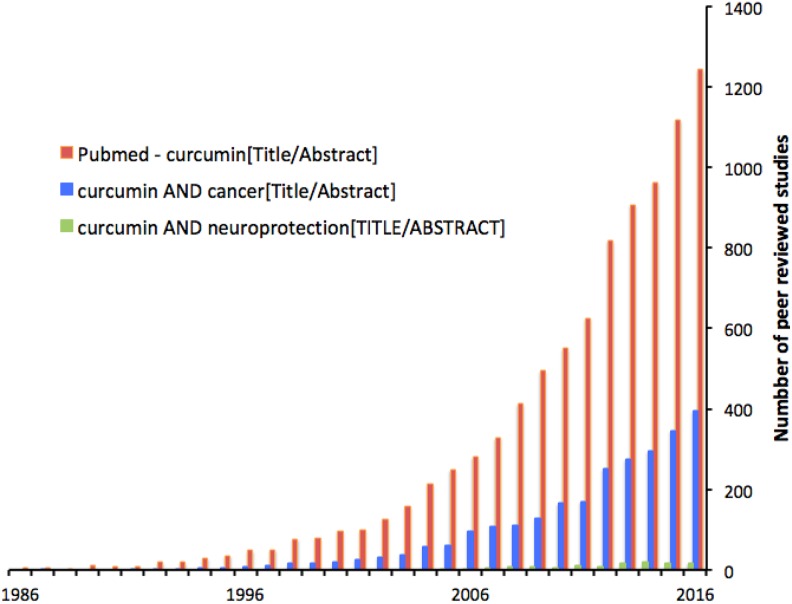
Figure 4.
Interest in curcumin has been as high as or higher than in resveratrol. Again a large minority of studies (30%) address cancer and a small minority (4%) address neuroprotection.
These chemicals are known as phytochemicals or, less cautiously, as phytotoxins—“phyto” because they have evolved in plants, “toxins” because they are demonstrably toxic to animals at higher doses. Botanists were initially unsure of their roles and gave them a noncommittal name, “secondary metabolites.” In recent decades, the roles of plant secondary metabolites have been increasingly elucidated and (reviewed in the study by Sun et al107) include the discouragement of other plants competing for resources and protection from attack by those other plants (in a process botanists call allelopathy107) and discouragement of animal predation.108 Four examples are considered below—resveratrol (evolved in red grapes and Japanese knotweed), curcumin (in turmeric), lycopene (in tomatoes and watermelon), and crocin (in saffron). Others less studied include allicin (garlic) and the catechins (green tea).109 Chemically, they are small molecules, many strongly antioxidant. Many are carotenes (crocin, lycopene, resveratrol), phenols (curcumin, catechins), or organosulfurs (allicin).
Mammals coevolved with plants and, for some plants, we have not evolved sufficient defense against or tolerance of their toxins. Ingesting small amounts of such plants makes us thoroughly ill; for us, such plants are “poisonous.” But the plants we regard as edible, and rely on for nutrition, also produce toxins and current evidence indicates that mammals (most work has been done in rodents and humans) have evolved an identifiable response to these toxins. In this response, low doses of the toxins upregulate mechanisms of tissue resilience. At high doses, all the phytotoxins are tissue destructive (Table 1), but the high-dose-toxic part of the response attracts little investigation. It is the low-dose-resilience response that is strikingly counterintuitive, therapeutically promising, and increasingly investigated.
Table 1.
Sample Reports of the Toxicity of Selected Phytochemicals.a
| Lethal Dose 50% (LD50) | Toxic Dose Low (TDLO) | |
|---|---|---|
| Resveratrol | No published data found | <2 g/d110 (human) |
| Lycopene | >3 g/kg (mouse)111 | 1.43 g/kg (human; http://www.centerchem.com/Products/DownloadFile.aspx? FileID=7345) |
| Curcumin | 5 g/kg112 (rat); ≥2 g/kg113,114 (mouse) | 12.6 g/kg (https://www.spectrumchemical.com/MSDS/TCI-C2302.pdf) |
| Saffron | >0.6 g/kg (rodents)115 | No published data found |
a The phytochemicals with protective properties are all toxic at some high dose, which varies with how it is prepared, how delivered, and how assessed. Typically, the toxicity of something for whole animals is assessed as the LD50 (the dose lethal to half the cohort tested); for humans as the TDLO (the lowest dose at which toxicity is detectable); and for cells in vitro as the IC50 (half the concentration that fully “inhibits”/kills the cells). In animals, TDLO measures have been devised to test a range of responses, including skin irritation, corneal irritation, reproductive success, and DNA damage (genotoxicity). As a generality, the tissue-protective properties of the phytochemicals discussed below (and of many others) are apparent at doses that are not toxic by any of these measures.
Resveratrol
Resveratrol is produced by many plants, including a plant known in Chinese medicine as Huzhang (in English as “Japanese knotweed”), long used for a range of cardiovascular, digestive, and metabolic complaints, and more familiar to Western culture, by red grapes, in which it concentrates in the skin and finds its way into red wine. Its presence in wine has been discussed as a happy explanation of the low rate of coronary heart disease among the French (the French paradox).116
In our reading of this literature, we were struck by the variety of morbidities—including cancer and cardiovascular disease—against which resveratrol had been tested; by the apparently uniformly positive effects of low-dose resveratrol in a wide range of nonhuman models; and by the difficulty of translating these effects to clinical trials. This difficulty is not absolute, but it seems likely that the delivery of resveratrol to human tissues will need to be improved, before the remarkable therapeutic potential suggested by now thousands of experimental studies of resveratrol is realized clinically.
The literature on the mechanisms of resveratrol’s action is diverse and difficult to summarize. One recent review summarized it this way117:
Amidst much confusion, it has become clear that resveratrol potentially has several direct targets in the cell. Although the original discovery was as a cyclooxygenase inhibitor, it has subsequently been identified as an activator of Sirt1…; an inhibitor of cAMP phosphodiesterases…; an inhibitor of the F1-ATPase…; an inhibitor of the estrogen receptor…, and a modulator of numerous other targets.
The experimental evidence that resveratrol can induce tissue resilience seems strong, in animal models and in studies in vitro. Resveratrol has been reported—these are just a few examples—to attenuate apoptosis induced in cerebrovascular endothelial cells by oxidized low-density lipoprotein (LDL) fats118; to slow diabetic retinopathy by downregulating oxidative stress and inflammation119; to inhibit the invasiveness and migration of pancreatic cancer cells120; to inhibit androgen-driven proliferation of prostate cancer cells121; to inhibit the viability and induce the death of colon cancer cells122; to induce differentiation and apoptosis in anaplastic lymphoma cells123; to maintain mitochondrial integrity; to downregulate insulin-like growth factor 1, activate SIRT1, increase the life span of yeast and mammals (reviewed in the study by Morris et al and Sun et al124,125); and to protect central nervous tissue in models of brain damage and degeneration.125 Work is beginning on the ability of resveratrol exposure to pregnant rats to improve the health of their offspring, for example, to reduce the susceptibility of offspring to toxin-induced carcinogenesis.126 And the protection provided by resveratrol against cancer is being traced, for example, to its ability at low concentrations to stabilize spindle assemblies during mitotic division of normally mitotic cells.127 The horizons of study of resveratrol, indeed of all the resilience-inducing interventions considered here, continue to broaden.
Many of these studies go to mechanisms, which are also diverse. Bitterman and Chung117 (quoted above) reviewed “controversies” concerning these mechanisms. The debates they review are real, but these debates are not about the ability of resveratrol to influence known molecular pathways. Rather, they are about the detail of that influence, whether, for example, the regulation of sirtuins can or cannot be the basis of all of resveratrol’s many known actions. The authors conclude that resveratrol is pleotropic, polypharmacological and that it regulates many target pathways. This is a recurring feature of the accounts of other resilience-inducing interventions.
Lycopene (Tomatoes and Watermelon)
Lycopene is a carotene found in red-colored fruits and vegetables, and gives their red color to tomatoes and watermelon. Many recent reviews128–134 are available of the effect of lycopene on a range of diseases.
Cancer
Prominent in these reviews and primary data studies is a line of evidence that lycopene found in tomatoes and tomato sauce reduces the incidence or limits the spread of prostate cancer, and perhaps of other cancers. Studies presented evidence, for example, that the consumption of tomatoes is inversely related to the incidence of prostate cancer,128 that dietary tomatoes or lycopene increase serum lycopene,135 and that, in vitro, lycopene promotes apoptosis and inhibits proliferation and metastasis in cell line models of prostate136 and other134 cancers; the analysis of mechanisms has begun.
The idea that phytochemicals can be “anticancer” is not new. One, called paclitaxel, has been a first-line drug for the treatment of several cancers137 since it emerged from a 1960s screen, supported by the US National Institute for Cancer, of anticancer substances. Paclitaxel at sufficient dose (in practice limited by the ability of the patient to tolerate it) stops the formation of microtubules in actively dividing cells, whether malignant or normal, resembling other chemotherapy drugs, with similarly harsh side effects. The action described here for lycopene and passim for other phytochemicals is very different; it is achieved at doses that are well below toxicity and induces a positive “cellular stress response” in normal tissues, while suppressing metastasis and proliferation in malignant cells.
By 2004, the evidence that lycopene at nontoxic doses was effective against prostate cancer had gained sufficient momentum that the US Food and Drug Administration (FDA) received 2 petitions, leading it to take a position on the matter. The FDA’s response was published in 2007.138 It was discouraging; having assessed the quality and outcomes of many studies, the authors of the response found “no credible evidence to support an association between lycopene intake and a reduced risk of prostate, lung, colorectal, gastric breast, ovarian, endometrial, or pancreatic cancer.” Similarly, they found no credible evidence of an association between tomato consumption and a reduced risk of lung, colorectal, breast, cervical, or endometrial cancer. But they did report “very limited evidence” of an association between tomato consumption and reduced risk of prostate, ovarian, gastric, and pancreatic cancers.
It was an important statement from an authoritative group, and a 2011 review of randomized control trials of the value of lycopene in prostate cancer139 was similarly discouraging. Ten years on from the FDA report, neither lycopene nor any other phytochemical has become part of mainstream management of the risk or treatment of prostate cancer.
Nevertheless, interest in the association persists in many forms. Giovannucci,140 in a rapid single author response to the FDA’s 2007 statement about lycopene, argued that the evaluation of prostate cancer outcomes in humans had been complicated by the increasing reliance on the periodic–Schiff acid test over the years reviewed by Kavanaugh and colleagues, effectively creating noise in which the signal of prostate response was lost. The same group subsequently141,142 developed evidence that lycopene exerts its effect on a subtype of prostate cancer characterized by a specific protease, partially explaining prior mixed findings. At the other end of a range of studies and reviews, a group of 180 scientists/authors143 met in 2013 and, after working in 12 teams over 2 years, published in 2015 a “broad-spectrum” review of dozens of chemical (predominantly phytochemical) interventions in a range of cancers, targeting a large range of mechanisms. Their summaries, which include but go far beyond lycopene, indicate that, for a list of 59 interventions (their Table 2), ∼1% of outcomes were “contrary,” appearing to be pro-cancer; ∼3% outcomes were “controversial,” with mixed results; 62% outcomes were “complementary” or anticancer; and 34% were “unknown,” with no clear result. Within their data, studies of lycopene followed this pattern (0, 0, 8, 3 studies in the 4 categories), as did the studies of resveratrol (0, 2, 9, 0 studies in the 4 categories) and curcumin (0, 0, 11, 0 studies in the 4 categories).
Table 2.
Recent Reviews of Phenotypes of Resilience.a
| Phenotype | Recent Reviews |
|---|---|
| Acceleration of wound healing | |
| Skin | Kuffler144 |
| Tooth sockets | Khan and Arany,145 Aoki et al146 |
| Conditioning of undamaged tissue | |
| Brain, retina, heart | Agrawal et al147 |
| Slowing/stopping tissue degeneration | |
| CNS chronic: dementia, Parkinson, AMD | de la Torre,148 Saez de Asteasu et al,149 Broadhead et al150 |
| CNS acute: stroke, TBI | Hamblin151 |
| Skin | Barolet et al14 |
| Muscle | Ziaaldini et al152 |
| Reduction of genotoxicity | Koul and Abraham153 |
| Reduction of inflammation, pain | Hamblin154 |
| Supernormal function | |
| Muscle | Ferraresi et al155 |
| Retina | Brandli and Stone156 |
| Accelerated recovery from muscle fatigue | Borsa et al,157 Pinto et al,158 Toma et al159 |
| Suppression of cancer | Block et al143 |
| The preservation/restorability of resilience in old age | Calabrese160 |
| Delay of mortality (longevity) | Huffman,161 Lopez-Luch and Navas,162 Everitt and Le Couteur163 |
Abbreviations: AMD, age-related macular degeneration; CNS, central nervous system; TBI, traumatic brain injury.
a There is some specificity to the relationship between stress and response: the effects of light on the stability of the retina have already been noted. But that specificity is limited; most tissues respond to most stresses, which is a distinguishing feature from acquired immunity. Literature references are recent, but very partial.
In summary, the debate over the effectiveness of lycopene for the prevention of prostate and other cancers, or for treatment, usually as an adjunct to medically accepted interventions, has widened—despite the 2007 FDA statement—to include a large range of phytochemicals and a large range of underlying mechanisms. It seems to be a progressive debate, dealing with issues of study organization, genetic variation within cancer types, and the complications of diagnosis and of the assessment of disease progress or regression. It is also a lively debate, made urgent by the aching need for effective treatment of still intractable cancers.
Cardiovascular health
The evidence that lycopene consumption is beneficial to cardiovascular health shares features of the lycopene and cancer data just reviewed. At the laboratory level, for example, Armoza and colleagues164 reported that the carotenoids lycopene and lutein attenuate the adhesion of inflammatory leukocytes to endothelium, identifying attenuation of NF-κB and several other molecular pathways as important in the action; Fletcher and colleagues165 reported that lycopene supplementation reduces an adhesion phenotype in peritoneal cells; in a study of human and animal endothelial cells in vitro, Lee and colleagues166 concluded that lycopene enhances barrier integrity and inhibits monocyte adhesion and migration to (inflammatory) human vascular endothelial cells by blocking activation of pro-inflammatory cytokines and expression of cell adhesion molecules and high-mobility group box 1 receptors; Zhu and colleagues167 reported that in diabetic rats, lycopene increases LDL levels and inducible nitric oxide synthase (iNOS) activity and reduces superoxide dismutase (SOD) activity, NO levels, and constitutive NOS activity, reducing endothelial cell dysfunction; and Bae and Bae168 reported, in an in vitro study of human endothelial cells, that lycopene enhances barrier integrity and inhibits leucocyte adhesion and migration to endothelial cells by blocking the activation of NF-κB, CD14, and TLR4 expression and production of tumor necrosis factor α. This sample of a wide literature suggests that there is ample proof-of-principle evidence that lycopene should enhance the health of blood vessels in humans.
In human studies, Rissanen and colleagues169 tested the hypothesis that low serum levels of lycopene are associated with an increased incidence of acute coronary events and stroke in middle-aged men. They reported that men in the lowest quartile of serum lycopene, followed over 6 years, had a 3-fold greater incidence of coronary heart disease or stroke; this confirmed an earlier report.170 Burton-Freeman and Sesson171 and Friedman172 reviewed evidence that dietary supplementation with lycopene or tomatoes is associated with a lowering of blood pressure in both normotensive and hypertensive individuals, with improvements in lipid metabolism (eg, raised high-density lipoprotein) and improvements in endothelial cell function (lower intercellular adhesion) and reductions in inflammatory responses of the endothelium; Gajendragadkar and colleagues173 reported, from a randomized controlled trial, that lycopene supplementation improves endothelial cell function, assessed by a range of tests, in patients with cardiovascular disease, although not in healthy volunteers; Wolak and Paran174 reviewed the literature on the effect of lycopene and other carotenoids on cardiovascular parameters, concluding that the effects include a decreased incidence of diabetes, lower LDL levels, improved blood pressure control, and a reduction in carotid intima–media thickness, a marker for atherosclerosis; Ried and Fakler175 undertook a meta-analysis of the protective effect of lycopene on serum cholesterol and blood pressure, concluding that the evidence is consistent that lycopene at doses of ≥25 mg/d lowers total serum cholesterol and LDL levels, as well as blood pressure. Again, this is only a sample of a wide literature, but it supports the underlying hypothesis that lycopene, a highly antioxidant carotenoid, activates a range of pathways that enhance the resilience of vascular tissue.
Neuroprotection
A small number of studies of the neuroprotective action of lycopene have appeared. In animal studies, for example, Lei and colleagues176 reported that pretreatment of rats reduced cell death and functional loss in a model of stroke, the effect involving the NF erythroid 2/heme-oxygenase pathway; Yin and colleagues177 reported that cognitive impairment induced in rats by consumption of fructose is ameliorated by lycopene administration; Prakash and Kumar178 and Yi and colleagues179 reported that lycopene reduces mitochondrial dysfunction in toxin-induced models of dementia and parkinsonism. In human studies, Karppi and colleagues180 reported that high serum levels of lycopene are associated with a decreased risk of ischemic stroke, which may of course result from the vessel-protective action of lycopene described above. On the other hand, in studies of diet and the risk of amyotrophic lateral sclerosis181 and multiple sclerosis,182 serum lycopene showed no clear association with risk. Further experimentation and observation are required, but it is possible that lycopene will prove to be as protective to the central nervous system (CNS) as resveratrol, curcumin, and saffron.
More generally, the study of the tissue-protective effects of phytochemicals is still evolving. Most studies focus understandably on one phytochemical, such as lycopene, or on one measure of tissue pathology, whether anticancer effects, or vascular integrity, or neuroprotection. We hypothesize that resilience effects of phytochemicals will prove to be broad spectrum, not specific to particular pathologies.
Curcumin (Turmeric)
Curcumin is a phenol, found in the plant turmeric. It has been used for centuries, particularly in Indian traditional medicine,183 as a spice and food coloring and as a herbal supplement with “health” properties.
At high doses, curcumin is toxic, with an LD50 in rodents of ≥2 g/kg (Table 1). The peer-reviewed literature is rich with evidence that, at low doses, curcumin is tissue protective. Many reviews are available (as for resveratrol and lycopene) of curcumin’s protective effects in a range of diseases, including several forms of cancer,134,184–188 in dementia183,189–200 and parkinsonism,201,202 in mitigating the inflammatory component of aging,203,204 and in cardiovascular disease.109,205–207 These reviews summarize hundreds of laboratory and clinical studies.
Cancer
The peer-reviewed literature (∼3000 studies) on the anticancer effects of curcumin has moved past establishing the reality of the effects to analyses of their mechanisms and to studies of how curcumin can be best configured and delivered. Curcumin has, for example, been reported to enhance the effect of cisplatin in suppressing the growth of squamous cancer cells in vitro and the suppression of xenograft tumors in vivo, mediated by the inhibition of cellular IKKβ208; to mimic the antiproliferation and cell death actions of valproic acid, by the same mechanisms (increasing Sp1 binding and the acetylation of the histones H3 and H4 in the promoter region of bax)209; to be cytotoxic to glioma cells in vitro, by regulating cell death pathways210; not to act synergistically with histone deacetylase inhibitors in their actions on cancer cell lines in vitro211; to induce apoptosis in human hepatocarcinoma cells in vitro, by disrupting the membrane potential of mitochondria and disturbing the intracellular concentration of calcium ions212; to reduce radiation-induced damage of the parotid glands in a rat model of the radiation of human head and neck cancers213; and to enhance the effect of ultrasound in the destruction of nasopharyngeal carcinoma cells in vitro.214
These consistently positive results from cell line and animal models of cancer have led to many clinical trials of curcumin related to cancer. Their results are more mixed, several authors suggesting that in humans the bioavailability of curcumin may be limited.215–217 Farzaei and colleagues218 and Maru and colleagues185 concluded that, despite extensive experimental evidence of the effectiveness of phytochemicals such as curcumin, evidence was “still lacking” from large-scale clinical trials. Responding to the problem of bioavailability, studies have been launched of the absorption, bioavailability, and metabolism of curcumin, delivered in various ways—unformulated and reformulated (with nanoparticles, liposomes, chaperone molecules) in attempts to improve bioavailability. By 2014, Pavan and colleagues186 were able to list 46 studies of reformulations of curcumin (their Table 1) designed to improve its bioavailability. Their summary is cautious:
Since ancient times, curcumin has been used in Asian countries against human ailments.…Multiple studies over the past decade have indicated the safety and efficacy of this polyphenol and have provided a solid basis for evaluating its efficacy in human clinical trials. Despite its efficacy and safety, limited curcumin bioavailability continues to be highlighted as a major concern. However in attempts to improve the bioavailability of curcumin, several strategies have been explored such as modulation of route and medium of curcumin administration, blocking of metabolic pathways by concomitant administration with other agents, and conjugation and structural modifications of curcumin…. In spite of these improvements, curcumin bioavailability, enhancement and efficacy have not gained significant attention in human studies…. Further…attempts to enhance the bioavailability, medicinal value and application of this interesting molecule…are needed.
Judged empirically (from Figure 4), however, scientists in this field are reporting on curcumin—its efficacy in in vivo and in vitro models of cancer, its safety, reformulation, and clinical value—at a steadily increasing rate. By this criterion, at least there is growing confidence in and excitement about the potential of this phytochemical to improve the treatment of still deadly diseases.
Inflammation, cardiovascular health, and neuroprotection
Although logically these are independent targets for a tissue-protective molecule, for curcumin they have often been studied together. Bala and colleagues,219 for example, reported that a curcumin-enriched diet mitigated the normal age-related increases in lipid peroxidation and lipofuscin in the rat brain and mitigated age-related decreases in the expression of antioxidant molecules and of enzymes related to ionic transport, and Sikora and colleagues203,204 have argued that chronic inflammation is a factor in age-related diseases, including the cancers, atherosclerosis, and the neuropathology of dementia. The implication is that it is the anti-inflammatory action of curcumin that underlies its anticancer, neuroprotective, and vascular-protective effects. Reviewers focusing on the role of curcumin in dementia have focused correspondingly on the anti-inflammatory and antioxidant mechanisms induced by curcumin,183,205 while others have stressed curcumin’s ability to mitigate age-related changes in protein homeostasis that lead to deposits of insoluble proteins or debris, citing Aβ, tau, or lipofuscin as examples of molecules whose deposition is both disease related and mitigated by curcumin198,201
This difficulty in separating the anti-inflammatory from the vascular-protective actions of curcumin (or any phytotoxin) presumably arises from the vascularity of the brain. We have argued elsewhere, for example,220 that age-related dementia (Alzheimer disease) is a small-vessel vascular dementia, caused by the destructive effect of the aging pulse on cerebral arterioles and capillaries. The protective effect of curcumin against Alzheimer disease could be mediated by the stabilization of proteins considered specific to the disease, by the enhancement of neurogenesis,198 by restoring redox homeostasis199 in the vulnerable neurones, or by the upregulation of antioxidant and cell survival pathways.108,206 Since these pathways are present in probably all cells, the protective effect of curcumin on the aging brain could arise from stabilization of its neural tissues or of its vasculature or of both tissues.
Challenge
Finally, the value of curcumin in all these roles has recently been subject to a robust challenge.221 Writing from the point of view of medicinal chemists, Nelson and colleagues argue that curcumin is both a PAIN and an IMP—a pan-assay interference molecule (one that interferes with other pathways) and an invalid metabolic panacea (a molecule for which wide-ranging benefits have been invalidly claimed). Their case is argued powerfully; as Finsen might have commented, the claims made for curcumin are “too marvelous,” and the explanations offered attract skepticism. Skepticism is essential to scientific method, but it is not an end in itself, and it seems to the present writers likely that curcumin and other phytochemicals may never escape these negative categories of medicinal chemistry, for there may be more going on than medicinal chemistry. Low-level toxins may induce tissue health by a distinct mechanism, as distinct for example as immunotherapy for cancer is from chemotherapy. Why did plants evolve these molecules? If they are defensive toxins, then it is not surprising that they evoke a reaction from the tissues of herbivores; it is no longer surprising, as Murugaiyah and Mattson108 have argued, that reaction has—to low doses—evolved to be self-protective. The phytochemicals may never pass muster as medicinal chemicals; they seem more likely to be stimuli to endogenous tissue-protective mechanisms evolved by animals in their struggle for survival, in some sort of long-term balance, with the plants on which we/they rely for food. It is that interaction that we, and Calabrese and Mattson and many others before us, seek to understand, seek at least to develop a conceptual framework within which this understanding can be approached.
Crocin (Saffron)
Saffron is the most legendary of the plants whose chemicals have been shown to induce tissue-protective responses in our tissues. Histories of saffron222,223 tell of its antiquity in agriculture, of the spread of its cultivation, of merchants put to death for adulterating it, of towns named after it, of recipes and medicinal preparations developed with it, and of the use of saffron as a pigment for religious robing. It was so coveted in medieval Europe to ward off the plague that it became prized booty for Mediterranean pirates and in a struggle (the “Saffron War” of 1374) between the aristocracy of the Swiss city of Basle and its merchants, who had grown powerful trading saffron after the Black Death pandemic in the middle of the 14th century.222
Saffron consists of the stigmata of the flower of Crocus sativus, a small flowering plant of the family Iridaceae, 3 stigmata in each flower, each stigma (or “thread”) weighing ∼2.5 mg. The threads must be harvested by hand (from ∼130 000 flowers to yield 1 kg), this labor making it the most expensive of spices. Their color is deep red, their aroma distinctive and rich. Major bioactive molecules, including safranal, dissolve readily into water, yielding an infusion readily ingested. The plant does not survive in the wild and has survived only because of the regard in which humans hold it and our willingness to hand-cultivate it. Although it is the stigmata (reproductive organs) of the flower that are the spice, the plant is a triploid mutant and cannot reproduce sexually. So the prized stigmata have lost their evolved function, and reproduction is by cloning of corms.
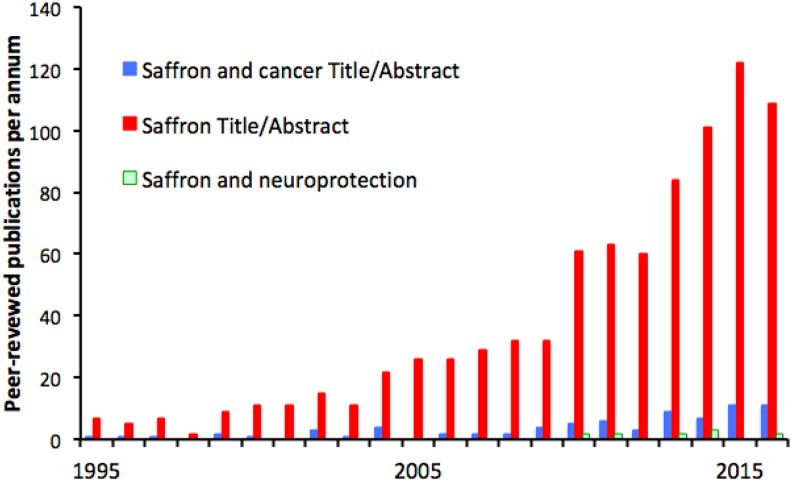
Saffron is used in many strands of traditional medicine—Islamic,224 Indian,225 and central and southern European225,226—and it was not a pure guess when scientists began to test its tissue-protective properties, now established in 2 main areas, cancer and neuroprotection. The growth of these scientific investigations has followed that of resveratrol, curcumin, and lycopene (Figure 5), but studies of saffron are fewer in number (about 2000 at time of writing, as against 10 000 for resveratrol). For several of the authors of this review, saffron was our introduction to the phytotoxins, and it was a relief when it emerged that the ability to achieve outcomes as remarkable as to slow neurodegenerations is not unique to this long-prized spice. Saffron is effective, but so is resveratrol, harvested from a notorious weed (above). The glamor of a plant is one thing; its value as a source of tissue-protective chemicals is another.
Figure 5.
Interest in saffron or its major bioactive component crocin has been less than in resveratrol, lycopene, or curcumin, but it shows the same time course, beginning in the 1980s and growing rapidly since. Again, a significant minority (9%) of the studies concern cancer and a small minority (0.9%) concern neuroprotection, but make perhaps the most audacious claims—that saffron can slow otherwise intractable degeneration of central nervous tissue.
In common with all phytochemicals, saffron at high doses causes illness; for humans, ingesting 5 g (∼2000 threads) induces intestinal bleeding, and an LD50 has been reported in rats (Table 1). At low doses, remarkable tissue-protective properties have been described for saffron in animal models, including protection of retinal photoreceptors in a LD model of macular degeneration,227,228 involving widespread changes in the expression of genes and ncRNAs43; the mitigation of DNA damage (so prevention of cancer) by the forming of specific complexes with DNA229–231; and the mitigation of toxin-induced pathology in the brain of a mouse model of parkinsonism.232
In the treatment of cancer, many saffron studies are available from cell or animal models. Among the earliest was the work of Nair and colleagues225,233 who reported that orally administered saffron extended the life of mice implanted with several forms of tumor and that in vitro a saffron extract was cytotoxic to several lines of tumor cells. The effect seemed to be mediated by disruption of DNA synthesis and to be remarkably specific to malignant cells, leaving “untransformed, normal” cells unaffected. A decade later, Abdullaev and Espinosa-Aguirre234 reviewed the growing literature, noting that saffron, or its major and most tested component crocin, is very low in toxicity but decreases lipoprotein oxidation in humans vulnerable to coronary artery disease; counters ethanol-induced loss of cognition in mice; has antihypertensive, antinociceptive, and anticonvulsant actions; protects nuclear DNA from genotoxic agents; and, as Nair and colleagues had reported, slows tumor growth in rodent models and is cytotoxic to malignant cell lines in vitro. A decade and half further on, the interest in saffron in cancer continues to grow; studies have been published of ways of increasing the bioavailability of saffron or crocin235–238; on newly discovered aspects of its mechanism—such as the suppression of multidrug resistance genes239,240; and of the value to summarizing saffron’s many therapeutic properties as “saffronomics.”241
Still lacking are major clinical trials of saffron with patients with cancer; only small trials have been reported.242 Anecdotally, it is clear that sufferers—presumably because of the urgency of their need—are including saffron among the phytochemicals with which they supplement mainstream treatment, but this use is not yet scientifically controlled; to date, it is the afflicted supplementing their mainstream treatment with low-toxicity phytochemicals with anticancer reputations, in the time of their need.
In disease or degenerations of the CNS, by contrast, clinical trials of saffron are available—small trials, some double-blind, have reported the stabilization of age-related macular degeneration (AMD) in humans,243 improvement in cognitive performance244–246 in dementia (Alzheimer disease), and relief of depression.246–249 Falsini and colleagues’ work on dietary saffron in early “dry” AMD is an example of what has been achieved in small-scale trials. Building on the pioneering experimental work of Maccarone et al in a rodent model,227 they showed in a cross-over, double-blind format that 20 mg/d saffron improved the macular ERG and visual acuity in 23 of 25 patients243; then that the improvement is maintained (if saffron is maintained in the diet) for over 12 months250; and that the protective effect of saffron is independent of the genes which regulate susceptibility to AMD.251
These are remarkable findings in neuroprotection, on a par with those reported for resveratrol, curcumin, and lycopene. Progress with all these phytochemicals is incomplete and vulnerable to the skepticism on which Finsen remarked, that it all seems too marvelous. But the pace of scientific work on these and other phytotoxins has reached that of a minor flood (Figures 2 –5), and we may have seen only its beginning.
Summary: What’s Really “Wrong” With the Western Diet?
The analyses above have implications that the present authors did not expect, when we set out to write a conventional review of what is known of certain plant chemicals, “secondary metabolites” evolved by plants to discourage predators. Instead—or as well—the analysis has led to an understanding of what is wrong with the “Western” diet. This diet, typically identified as “rich in red meat, dairy products, processed and artificially sweetened foods, and salt, with minimal intake of fruits, vegetables, fish, legumes, and whole grains”103 is not, we argue, that any component of the diet—or the combination—is bad or toxic for us. On the contrary, these analyses suggest that this diet is not toxic enough and that it lacks the toxins of the plants prominent in the Mediterranean diet, which comprises “olive oil, fruits and vegetables, whole grains and cereals, legumes, fish, and nuts; low intake of red meat, dairy products, and sweets; and moderate intake of red wine with meals.”103 It is, we suggest, the meal-after-meal exposure to these toxins that maintains the upregulation of tissue resilience. Meat, sugars, and dairy products meet our needs for protein, fats, and carbohydrates. These foods are highly nutritious but, lacking the toxins of slightly bitter vegetables, they leave our tissues less conditioned, less resilient.
There is an analogy in the resilience of trees. In the Biosphere 2 venture, trees grown inside the sphere did not experience wind. They grew well but tended to collapse before reaching maturity. Without wind, it turned out, trees do not form “stress wood” at points in their branched structure where wind normally induces the local formation of either “compression” wood or “tensile” wood. The story of wind stress and a tree’s response to it is more complex than this,252 but, fundamentally, it is that trees use the stress of wind to induce the production of tougher wood, at locations that bear the stress. Without wind, still-young trees collapse under their own weight (http://awesci.com/the-role-of-wind-in-a-trees-life/). The analogy seems strong with still-young humans maturing without daily exposure to everyday stresses—the plant toxins but also daylight (above), exercise, and intermittent hunger (below) —and “collapsing” with early morbidity and mortality.
And one implication of this suggestion is that we can hew to the diet with which we are comfortable, but we should consider supplementing it with the phytotoxins. The experiment has already been done, for one phytotoxin (resveratrol253), though interpreted slightly differently. The “goodness” of the Mediterranean diet is that it daily delivers low doses of a range of poisons; who would have thought?
And one answer to this last, usually rhetorical question is that botanists might well have thought of this reaction of animals to plants. For plant biologists have shown that modern plants are the survivors of a long competition for resources between plants, each evolving toxins to discourage competitor plants and each evolving mechanisms to evade or counter the others’ toxins. Botanists refer to this battle between plants as allelopathy. The toxins that plants produce that discourage animal predators are an extension of the same battle, in which plants do things that at first learning seems extraordinary. As one example, many plants produce cyanide as a toxin, packaged with a sugar (as a cyanogenic glycoside) to prevent the cyanide killing the plant itself. The plant also produces a cyanoglycosidase, packaged in the same leaf but separately. When a herbivore detaches and chews the leaf, the enzyme and substrate are brought together, cyanide is released, and the animal is, well, discouraged.254
Humans can and do choose our foods, and we naturally choose and cultivate plants that are productive, palatable, nutritious, and less dramatically toxic than the cyanogenic. Still, many of the plants we rely on as food produce toxins, and the response of animals to their “attack” has been at least 2-fold—to evolve metabolic pathways which can rapidly detoxify the toxins, making the animal “tolerant” to them, and second—and this is the message of this long section on phytochemicals—is to evolve a general mechanism of resilience, in which the toxins at low dose induce pathways of cellular resilience, the “adaptive cellular stress response” emphasized by Mattson and Calabrese.108,109,255 Which is a long way of saying that vegetables are good for us in ways we have not always understood.
The Resilience Induced by Lack of Food (Caloric Restriction, Hunger)
It is a recurring feature of the bodies of literature brought together in this review that they begin with surprises, then doubts and dismissal, then reassertion of the major claims. We could find no evidence of it, but it must surely have been a surprise when investigators first—the work goes back at least to the 1930s—observed that animals deprived of food were freer of disease and lived longer. The struggle for continuity and sufficiency of food supply had shaped animal and human behavior and conflict. Why would restricting animals to 80% of their ad libitum diet—for humans, the 5/2 diet, for example—possibly be good for us? That caloric restriction, carefully done, produces healthful outcomes is no longer debated; in recent reviews, for example,256 the debate has moved on to mechanisms and doses, especially as between chronic and intermittent restriction, and the interaction between daytime fasting and the natural period of overnight fasting, during sleep.257
The literature on caloric restriction—the deliberate reduction of food intake to ∼80% of ad libitum consumption—is diverse and rich with ideas. Walford et al258 report a simple-to-describe experiment in the eco-research station called Biosphere 2, located in Arizona. In 1992, 8 scientists entered the closed ecosystem, committed to 2 years of active experiments on isolated team living, which involved a commitment to a self-grown, nutrient-adequate diet of ∼2000 kcal/d. Over the 2 years, during which they maintained “excellent health and a…high level of physical and mental activity,” they experienced falls of body weight (∼17%, leveling out after 8 months), blood pressure (∼25%), blood levels of sugar (21%), insulin (42%), and cholesterol (30%), and many other healthful changes that had been associated with caloric restriction in animals over the preceding century, and since confirmed in humans.259 It is salutary, however, to read accounts of group dynamics among the Biosphere 2 crew, accounts that are part of a more general literature on isolated teams. Writing in 2015, Nelson and colleagues260 noted that “food was a prime concern inside Biosphere 2,” the concern arising partly from the time and energy involved in producing it (∼36% of the Biospherians’ labor), partly from the pressure to maintain that labor (“if we want it we have to grow it”) and partly because the crew was “unused to dealing with hunger.” At the tissue level, daily caloric restriction made the team members lean and mission effective, but, at the psychological level, daily hunger and concern about food supply seem to have made it difficult to optimize group dynamics for a long and complex mission.
The stress of chronic or intermittent hunger has, we argue, been part of the experience of mammals for so long that we have evolved pathways that use the stress to make the body resistant to cardiovascular and metabolic disease, reducing morbidity and delaying mortality. It is of interest that humans seem not to have evolved ways of extending that resilience to our complex psychology; perhaps that element of evolution lies ahead for Homo sapiens. If so, the justification for the adjective sapiens will be strengthened. Following that thought a decade after the Biospherians reported in 2002, Blagosklonny261 speculated that the Slavic folklore figure Koschei the Immortal—old, lean-as-a-skeleton, combative, magically resilient in the face of injury directed at him because of his relentless anger and ill-humor—may have been a caricature of men who survive the stress of prolonged hunger, reinforced (the author speculated) by a hunger mimetic (perhaps rapamycin), into late age.
In the intervening years and still, the scientific study of caloric restriction has increased its pace (Figure 6), and some researchers have concluded that caloric restriction is the most effective intervention known for the slowing of aging.262 In those years, the effect of caloric restriction on atherosclerosis was confirmed by Fontana and colleagues263; Kyriazis264 suggested that caloric restriction can best be understood and deployed as a “hormetic strategy” to combat aging; and Martin and colleagues265 reviewed the evidence that both caloric restriction and a variant hunger strategy—that of “intermittent fasting,” something like the 5/2 diet presently fashionable—are effective neuroprotectants, protecting the brain from age-related changes.
Figure 6.
The study of caloric restriction can be traced back to the 1940s. It continues to increase, year by year.
Some investigators, perhaps alarmed at the prospect of millions of people dieting to a lean, long-lasting but angry-and-mean old age, have asked whether other interventions could mimic caloric restriction, with a gentler outcome. Prominent among the mimetics investigated are phytochemicals already discussed (resveratrol, curcumin266–268) and more focused drugs, such as metformin and rapamycin.266 Logically, it is a moot point whether one might test resveratrol or any plant toxin as a mimic of caloric restriction, or vice versa; understanding of this interchangeability is evident in recent reviews.262,265 The more general point, already touched on above, is that everyday stresses, like light and plant toxins and hunger all, at low doses, upregulate endogenous resilience pathways, and likely the same pathways.
Independent Variables in What We Eat: Hunger Versus Balanced Versus Toxic, Resilience-Inducing Supplements
Assuming plenitude, meaning no long-term lack of food or vitamins or the required trace elements, several issues are involved in the impact of what we ingest on health:
One is caloric restriction and its documented impact on morbidity and mortality. Given the arguments proposed above, we suggest that caloric restriction has its impact because the metabolic stress of the restriction upregulates resilience mechanisms.
A second is the concept of a balanced diet: holding caloric intake constant and supplying vitamins and trace elements—what balance of fats, carbohydrates, and protein produces least morbidity and greatest longevity? Studies on this balance in a range of species, including humans, suggest a particular balance—low (10%) protein and high carbohydrate.269
A third is the level of resilience-inducing toxins present in the diet, as discussed above. This level can be increased by choosing toxin-delivering foods (the Mediterranean diet) or with supplements, without affecting either the level of hunger or the protein/carbohydrate/fat balance.
There may be more such variables to consider, but these 3 seem important, and independent of each other, in analyzing the impact of diet on morbidity and mortality.
Lack of Oxygen: Ischemia, Remote Ischemia, Exercise-Induced Ischemia, Hypoxia
Mammals require a constant supply of oxygen. There is a technical term for it—we are “obligate aerobes.” Our tissues can and do produce quantities of ATP from glucose without oxygen, using the anaerobic glycolysis pathways inherited from bacteria. The power of the oxidative phosphorylation pathways is that they have evolved to act in series with glycolysis, combining one of the end products of glycolysis (lactic acid) with oxygen, to produce ∼10-fold more ATP from the same glucose, and hence from the same meal.
This greater yield of ATP from oxidative metabolic pathways fuels the warmth and vigor of mammals, but the price for this vigor is our utter dependence on inhaled oxygen. The oxygen is delivered to our tissues via the pulmonary and systemic circulations, but the delivery is what logistics engineers call “just in time”. None of our tissues has evolved a way to store oxygen (they have evolved a way to store glucose, as glycogen) and the brain, the most oxygen demanding and oxygen sensitive of our organs, starts to fail (we lose consciousness) within seconds of strangulation. So the obligation of obligate aerobes is to prevent—and if it cannot be prevented to survive—a failure of oxygen supply and therefore any failure of blood supply (ischemia).
Local Ischemia Induces Local Resilience
Our tissues can become short of oxygen—hypoxic—in several ways. The most common are a blockage in the artery bringing blood to them—ischemia, or a breach of the artery wall—a hemorrhage. The tissue normally supplied by the artery lacks a supply of oxygenated blood; it is said to be ischemic. Prolonged ischemia causes death of the tissue affected, but if the ischemia is partial or brief, the tissue survives and reacts in self-protective ways.
The reports of Murry and colleagues270,271 pioneered understanding of the protective effect of brief ischemia. Their model was the occlusion of one coronary artery (the circumflex) of the dog heart for brief (usually 5 minutes) periods of ischemia, separated by 5-minute reperfusion, simulating the transient ischemia believed to cause angina pectoris; they also used single, longer (30 or 180 minutes) periods, simulating a full-blown heart attack.
Using a series of shorter periods, these investigators tested271 whether the condition of the ischemic muscle deteriorated from the one 5-minute period of ischemia to the next; would the effect be cumulative, resulting in muscle death? It was surely a surprise when they noted that, while an initial brief period of ischemia significantly compromised the muscle, reducing its production of ATP and clearance of metabolites, subsequent brief periods had little added effect. The muscle somehow changed its state, stabilizing performance in the face of repeated brief ischemic episodes, and no infarct formed.
From this limited observation, much has followed, for it implied that sublethal ischemia was inducing resilience in the muscle. To test this implication, the same investigators tested whether the muscle was “conditioned” by brief (5 minutes) occlusions, after which they applied a more sustained (40 minutes) occlusion, long enough—without conditioning—to kill muscle and result in an infarct. Conditioning with several periods of sublethal ischemia reduced the size of infarct caused by the 40-minute occlusion dramatically (75%). And when the “test” occlusion was increased from 40 to 180 minutes, the preconditioning did not reduce infarct size; the protective effect seemed overwhelmed. These observations established that sublethal ischemia can be very—but not infinitely—protective and raised many questions, tackled in subsequent decades by several groups.271,272 And there were clinical implications, which these early authors foresaw:
…myocardial infarction often is preceded by multiple episodes of angina pectoris. It is possible…that patients who experience repeated episodes of angina may similarly precondition their myocardium…(and) alter the time course of cell death after the onset of a sustained coronary occlusion. If this is true, then the onset and early progression of cell death may be slower in many patients than the results of animal studies have suggested…. A slower progression of cell death implies a longer window of time in which it might be possible to salvage myocardium via reperfusion, e.g. with thrombolytic therapy or coronary angioplasty.270
This prediction, that angina pectoris will prove to be protective against a heart attack, has been confirmed many times and its limits explored.273–276 The idea has been extended to other organs examined both experimentally277 and clinically. For example, transient ischemia of the brain protects the brain against ischemic stroke,278 and transient ischemia of the retina protects the retina against more sustained ischemia.279 All these authors comment, in various ways, that the protection they observe indicates the presence of endogenous, ischemia-inducible protective mechanisms in the tissue under study.
At the molecular level, studies of the mechanisms of ischemic conditioning have been reported for 2 decades. Early in this period, Barone and colleagues277 reported that, during conditioning ischemia of rat brain, the expressions of interleukin-1 receptor antagonist messenger RNA (mRNA) and protein were increased and the expression of c-fos was reduced. Li and colleagues280 examined the effect of sublethal ischemia to the retina on the expression of heat shock protein (HSP)-27, HSP-70, and HSP-90, reporting a marked (2-fold) increase in the expression of the mRNA and protein of HSP-27. Kawahara and colleagues281 undertook a “genome-wide” microarray analysis of ischemic conditioning in the rat brain, with several experimental groups. The authors noted that protective ischemic conditioning was associated with the upregulation of transcription factors, including c-Fos, Hsp-70, and MAP kinase-related genes, and neuronal death was associated with upregulation of proapoptotic genes and downregulation of genes implicated in survival mechanisms, which they identified as including the MKKPI4 kinase and DAG/PKC pathways. At a more general level, they noted that, following sublethal ischemia, 246 of the 8799 genes available in their arrays were upregulated and another 213 downregulated. Viewed in this broad way, the molecular response of brain tissue to sublethal ischemia is complex, involving several pathways, and difficult to summarize. Writing in 2011, Morris and colleagues124 focused on one family of genes, the sirtuins, and emphasized evidence that these are involved in the tissue-protective effects of sublethal ischemia and also of resveratrol (reviewed above) and of caloric restriction (also reviewed above). Their analysis particularly notes the role of sirtuins in maintaining mitochondrial function, influencing cell metabolism and survival/death, the evidence deriving from work on species from yeast to mammals, and emphasizes how several forms of stress can activate a particular (key) family of genes. Brooks and Andrews’ 2013 review272 of the mechanisms of ischemic conditioning of heart muscle had a different emphasis. Their description highlights the release of cytokines (bradykinin, adenosine, and opioid peptides) from heart muscle made briefly ischemic and their role in triggering a “cascade” of intracellular pathways via receptor activation of membrane-bound G-proteins. Proteins generated by these pathways converge on the inner mitochondrial membrane where they reduce the generation of reactive oxygen species. They also summarized pathways that induce a period of delayed (by 12-24 hours) protection of heart muscle following brief ischemia. The same endogenous cytokines that mediate the short-term protective response activate nuclear transcription factors (they identify NF-κB, AP-1, and HIP-1a), triggering the synthesis of known mediators of tissue protection (iNOS, COX-2, aldose reductase, HSP, and Mn-SOD). It is this process of protein synthesis that, these authors suggest, delays the protection provided by these pathways. The sirtuins were not mentioned in this analysis.
As with PBM and the phytotoxins, it would seem that the mechanisms of ischemic conditioning are multiple and difficult to summarize. Among the factors common to them are that resilience is induced by low levels of stress and that higher levels of the same stress are tissue destructive.
“Remote” Ischemia Induces Resilience at a Distance
Ischemic conditioning does not provide an attractive path to therapy; there has been no move to induce brief ischemia in a threatened organ, in the heart, for example, to protect it from later damage. A pathway to therapy did seem to emerge when investigators282 noted that ischemia of part of the heart (brief closure of one coronary artery) induced a protective response throughout the heart. An unsuspected mechanism was spreading the benefit from a patch of ischemic heart muscle to the whole heart. How far could this benefit of brief ischemia spread?
This founding observation of “remote ischemic conditioning”282 (RIC) has been confirmed and expanded (Figure 7) in many configurations. In the laboratory and in small clinical trials, brief ischemia of one limb, for example, has been shown to protect the brain,283 lung,284 kidneys, liver, skin flaps, intestines,285,286 and retina,287 as well as the heart, from subsequent damage. Further, the protection provided by remote (usually limb) ischemia functions against not only ischemia but against other forms of damage—for example, protecting retinal neurones from axon damage288 and LD,289 as well as from ischemia.287 Further, RIC is not specific to a particular tissue; in small clinical trials, brief ischemia to a limb has been reported to be protective to the heart, lung, and brain290; the protective pathways are present in the genome of every cell and therefore every tissue. Still further, the effect of RIC on other tissues may go beyond protection. Brandli and colleagues156 reported, for example, that brief limb ischemia causes a 10% to 15% supernormality in the electroretinogram. The phenotypes of stress-induced tissue resilience are discussed below; stress-induced supernormal function has been described for muscle as well as retina.
Figure 7.
Evidence of the growing interest in remote ischemic conditioning.
This evidence that “remote” (usually limb) ischemia induces body-wide resilience and even supernormal performance has led (as with other stressors) to a search for mechanisms, driven partly by the need to understand, and partly by the need to be sure that the effects are identifiable at the molecular level, for they seem “too marvelous” in vivo. As with light and the phytochemicals and hunger (above) and exercise (below), the search has yielded a rich range of candidates. In reviewing this profusion, Pickard and colleagues286 noted that the pathways of RIC must involve a reaction in the tissue-made ischemic, then pathways—neural and/or humoral—connecting the ischemic tissue to tissues in which protection is assessed, and finally a reaction (more pathways) in the tissues made resilient. In the case of limb ischemia-induced cardioprotection, they summarized evidence that adenosine released by ischemic muscle activates small-diameter sensory fibers serving the muscle in a spinal reflex, for which the output pathway may be parasympathetic supply to the heart. And they noted evidence that the cardioprotective effect is partly or wholly mediated by blood-borne factors, including opioids, cannabinoids, and transcription factors such as HIF-1α. In more recent studies, discussed above in the context of the remote effects of PBM and reviewed by Kim et al,52 a third mechanism of remote conditioning is emerging—the mobilization of bone marrow–derived “stem cells”53 or Myo/Nog cells. There is no lack of candidate mechanisms; all deserve further investigation.
In humans, recent clinical trials give evidence that RIC can improve neurological outcomes in stroke291 and aneurysmal subarachnoid hemorrhage292; can improve the microcirculation of skin flaps used in reconstructive surgery293; can protect heart, lung, and liver function during heart valve surgery294; and can reduce infarct size in myocardial infarction (heart attack).295
Results of trials have not been uniformly positive, however. Although no investigators have reported RIC to be damaging, in several trials and meta-analyses, investigators have reported, for example, that RIC had no beneficial effect in heart attack296 or heart valve surgery297 and failed to improve kidney function after transplantation.298 In a large-scale and recent meta-analysis, Benstoem and colleagues299 concluded that no beneficial effect of RIC survived the analysis, which included many protocols of RIC, many analyses, many outcome measures, and many apparently promising smaller studies. The promise of RIC seemed elusive, too open to observer bias, small numbers of trial participants, and inadequate statistical testing.
This contrast between the promise of laboratory studies and small clinical trials, on the one hand, and lack of promise indicated by meta-analyses of the clinical trials, on the other, has not slowed interest in RIC (Figure 7). Investigators have approached the disappointment of the larger analyses in 2 ways: (1) They have searched for details of pathway regulation by RIC. Nikkola and colleagues,300 for example, compared whole-blood transcriptomes using RNA sequencing and genome-wide DNA methylomes before and after RIC, identifying >130 differentially expressed genes and almost 3500 differentially methylated CpG sites, which overlapped with >100 of the differentially expressed genes. Further, the differentially expressed and methylated genes formed part a tightly coexpressed group of genes related to cell cycle pathways and inflammatory responses. The outcome gives “hard evidence” mechanistic support for the positive clinical outcomes. And (2) Investigators have begun or encouraged299,301 the search for “confounding factors.” Rather than accept the “no real effect” neutrality of meta-analyses, they have begun to search for reasons why large-scale analyses are leading to conclusions of “no effect.” The most common explanation proffered by meta-analysts for their negative conclusions has been that the pioneering studies were biased or underpowered. In response, the pioneering researchers are beginning to ask whether the larger analyses might (or not) have a tendency to bury the promise of the smaller studies in unidentified heterogeneity.
Is Hypoxia the Key Stress of Ischemia-Induced Tissue Protection?
Hypoxia can and does occur without ischemia and, if severe and prolonged, is quickly fatal. The question arises and has been addressed, whether brief or partial hypoxia without ischemia can induce tissue resilience like that induced by low-dose ischemia and remote ischemia. The answer to the question is affirmative. For example, Zhu and colleagues reported in work on rats that sublethal hypoxia (11% inspired O2), without any blockage to blood flow, induces the same protection of the retina as sublethal ischemia.259 Barrington and colleagues302 specifically compared ischemic with hypoxic stress in healthy men, noting essentially similar upregulation of HSPs. A recent review of the value of hypoxic conditioning for the protection of the CNS303 traced many studies of this issue. In animal studies, for example, hypoxia in adult rats increased hippocampal neurogenesis304 and, given after experimentally induced stroke, increased hippocampal neurogenesis and mitigated memory loss.305–307 Hypoxic preconditioning (exposure to hypoxia before an induced experimental stroke) also mitigated structural loss,308–310 reducing the size of the infarct by as much as 50%. These effects involved, or at least were associated with, regulation of a number of pathways, including the expression of HIF-1α and its target genes (erythropoietin, vascular endothelial-like growth factor).309,311–313
Two forms of hypoxia have been well studied clinically. In humans suffering the hypoxia of sleep apnea, Lavie and Lavie314 reported that, while severe apnea in humans was associated with higher morbidity and earlier mortality, chronic “mild” apnea, causing correspondingly mild hypoxia, was associated with reduced morbidity and delayed mortality, less than in apnea-free controls. In the second form, more often regarded as an adventure than an experiment, humans voluntarily subject themselves to hypoxia as passengers or crew on airlines or as athletes training at high altitude. The outcome of these latter situations has been much studied but, as far as we can tell, not with the idea of hypoxia-induced tissue resilience in mind. Airlines are concerned for the immediate welfare of passengers, and athletes and their coaches seek enhanced performance in imminent competition. There is evidence of overall reduced morbidity and delayed mortality in long-term air crew,315–317 compared to general populations, despite raised incidences of death rates caused by flight accidents and some cancers. All studies noted flight crew-specific factors, including the health requirements for admission to crew status, irregular working hours, greater exposure to exhaust fumes, and incident radiation, but not the possible resilience effects of repeated exposure to mild hypoxia. Perhaps the clearest observation was that of Linnersjo and colleagues’ report of lower incidence of myocardial infarction and mortality during the pilots’ flying career and beyond. This literature is limited, but the number of people who experience the mild hypoxia of airline flight (most flights are pressurized to the equivalent of 8000 ft altitude, at which the partial pressure of oxygen is 25% less than at sea level) is enormous, approximately 3 million per day. There may be much still to be learned from this daily “experiment.”
Summarizing, there is limited but so far uncontradicted evidence that mild hypoxia induces protective effects comparable to those induced by ischemia; hypoxia may be the key resilience-inducing element of ischemia.
Excess Demand: The Resilience Induced by Exercise
The understanding that exercise is healthful goes back millennia, to the writings of Hippocrates in the Western tradition and of Susruta in the Indian tradition. Histories of the understanding can be traced from elsewhere318–320; the literature is considerable. By contrast, understanding of the mechanisms of that healthfulness, and of why the mechanisms evolved as they have, is recent and incomplete. One way of understanding both the mechanisms and their evolution is to view exercise as an everyday stress that induces hypoxia in muscle, activating the mechanisms identified in experimental work on direct and remote ischemia of muscle and on hypoxia (above).
The recent literature on exercise has shown the same rapidly increasing pattern seen for light and food—a year-after-year growth since the 1990s (Figures 8 and 9). In these studies, hypoxia in muscle is rarely measured; instead, the level of exercise is measured or the accumulation of the products of anaerobic glycolysis assessed, for example, as lactate levels in blood. The phenomenon already discussed of RIC suggests that the benefits of exercise are mimicked by making one limb ischemic, that the oxygen debt induced by increased demand is the key element in exercise-induced tissue resilience. Put more simply, exercise is good for us because it makes our skeletal muscle hypoxic, and the muscles respond by releasing identifiable “myokines,”321,322 whose actions include the upregulation of endogenous mechanisms of tissue resilience.
Figure 8.
The growth in studies published on the relationship of exercise to chemotherapy or radiotherapy.
Figure 9.
Recent growth in published studies on the impact on exercise on cognition and on Alzheimer’s disease.
Exercise and Cancer
A great deal of interest has centered on the value of exercise in the treatment and management of cancer, with hundreds of studies and reviews published, at a steadily growing rate (Figure 8). There seems to be wide agreement that exercise can improve the quality of life and the psychological and physical strength of patients with cancer undergoing therapy323–325 and can counter the side effects of therapy, for example, of androgen deprivation therapy in men with prostate cancer.326 A smaller number of studies report evidence that exercise can prevent onset or recurrence of cancers (eg, Adraskela et al and Friedenreich et al327,328). Understanding of the mechanisms of exercise-induced resilience to cancer has been approached in several ways. One intriguing line of work has suggested that exercise upregulates the immune and anti-inflammatory systems, mobilizing natural killer cells,329,330 recruiting the anticancer potential of these systems. At the molecular level, Coyle and colleagues331 have reported evidence that exercise in women reduces promoter hypermethylation of tumor suppressor genes, such as APC, in nonmalignant breast tissue; hypermethylation of the promoter is considered to suppress expression of the gene, so making the formation of a tumor more likely. Sanchis-Gomar and colleagues332 have suggested, more generally, that the anticancer effects of exercise may be exerted by epigenetic modulation, that is, by regulating expression of the genome through methylation of genes, and they borrowed the term “eustress” to denote stress that induces healthful epigenetic changes. Other authors333 influenced by knowledge that—for many cancers—age is a major risk factor and by growing evidence that the immune system is an effective defense against cancer, have reviewed studies that show that exercise slows immunosenescence, the slow loss of immune function associated with age. At least some of the anticancer effect of exercise, these studies suggest, may be exerted via the maintenance of immune function.
Exercise and Cognition
Exercise (voluntary wheel running) mitigates the loss of hippocampal neurogenesis and of maze learning in aging wild-type mice.334 Exercise, when trialed in mouse models of Alzheimer disease—usually transgenic strains that develop the Aβ and tau pathology of that condition—has been shown to decelerate that pathology335–337 and to mitigate the associated cognitive loss and loss of transmitter-related enzymes. The effect is robust, reliable, and, measured as the level of soluble Aβ in the hippocampus, is dose dependent.338 The effect has provided a starting point for further, striking observations. In one follow-on observation, for example, Herring and colleagues339 reported that exercise in the pregnant mother slows the development of the Aβ plaque pathology of her progeny, suggesting epigenetic transmission of the effect for at least one generation (and another challenge for human mothers determined to have the perfect baby). Fragoso and colleagues340 provided a confirming observation that maternal exercise during pregnancy in rats attenuates the damage to the fetal brain caused by malnutrition.
For reasons argued elsewhere,220 we suggest that, in these transgenic models of dementia, exercise may act by preserving the structural integrity of cerebral vessels, which are weakened by the transgenes. These transgenes are derived from human mutations that cause early-onset dementia and early-onset stroke (see, eg, Figure 2 in the study by Kumar-Singh et al341). The importance of vessel integrity in the exercise-induced mitigation of cognition is supported by the observation that the gene most powerful in regulating susceptibility of age-related dementia in humans (APOE) is critical for the effect of exercise in protecting the integrity of the “neurovascular unit.”342 And several studies, reviewed by Rzechorzek et al,343 have directly linked exercise to the preservation of the integrity of cerebral vessels.
The above observations of the protective effect of exercise on cognition in rodents have been translated to humans. Several authors have reported that moderate exercise (eg, two 20-minute bouts of cycling per week344) improves cognitive performance in patients with mild cognitive impairment, an improvement that was enhanced by cognitive enrichment and faded if the exercise was not persisted with. By 2017, Saez de Asteasu and colleagues149 were able to review 26 randomized control trials, testing the effects of exercise on cognition, but now seeking to identify which forms of exercise were more or less effective. Searching for mechanisms, Dinoff and colleagues345 undertook a meta-analysis of 55 published studies of the effects on exercise on blood levels of brain-derived neurotrophic factor (BDNF), confirming that exercise induces increases in plasma levels, in a dose-related relationship. BDNF is one of the “myokines” (molecules released by exercising muscle into the bloodstream) and one of the most prominent factors identified in studies of the mechanisms of resilience induced by exercise and the other interventions considered here.24,178,289,346–349 In a meta-analysis of 35 studies of humans with chronic diseases, Cai and colleagues350 reported that their analysis suggests that “exercise interventions positively influence cognitive function…independent of the type of disease (and)…of the type, frequency and intensity of the…intervention.”
Exercise and Aging
The literature on exercise and aging is fast-growing, raising the questions whether resilience fades with aging and whether that fading can be mitigated.
Resiliosenescence?
Frailty—the opposite of resilience—is the common experience of aging humans. Our skin tears more easily and heals more slowly, our muscles weaken, we bounce back less readily from falls and disease. Intellectually, though we may long maintain cognitive performance, our “cognitive margin” shrinks; in a high fever, the old become delirious more easily and recover more slowly. The examples are too many to allow documentation, but several theories of aging are currently debated— “rate of living” or “oxidative damage”259 or the shortening of telomeres.
So far, the evidence is clear, as far as it goes. The low-stress-resilience response—and therefore resilience—fade with age and the fading can be slowed or stopped with low-level stress. Power and colleagues351,352 reported that the number of motor units in muscle (they examined the tibialis anterior muscle of the leg) declines with age and that the decline is less in “masters” running champions, so maintaining muscle strength closer to youthful levels. Soto and colleagues342 reported that in mice the structural integrity of cerebral vessels declines with age (with loss of basement membrane and pericytes and breakdown of the blood–brain barrier), and that the decline is mitigated by long-term aerobic exercise, and further that the mitigation is dependent on the APOE gene, known to be an important regulator of age-related dementia in humans. Rzechorzek and colleagues343 reported that the pial circulation becomes “rarified” and the severity of stroke increases in aging mice and that both age-related changes are mitigated by aerobic exercise. Dimauro and colleagues353 reported that regular exercise in human patients suffering type 2 diabetes mitigated the shortening of telomeres and other measures of DNA damage in leukocytes taken from their blood and reduced levels of apoptotic cell death in their lymphocytes. The observation that exercise mitigates the damage to DNA associated with age—telomere shortening and oxidative damage—has enjoyed considerable recent confirmation.354–356 Correspondingly, one of the intriguing resilience responses of the heart—angina-induced protection against infarction—is lost in the elderly patients, except in a subcohort that exercised regularly.273,274
These specific observations for aging and exercise, taken together with the evidence that caloric restriction slows aspects of aging (above), that saffron as a dietary supplement and PBM can slow, even partially reverse the age-related degeneration of the macula region of the retina,31,243 suggest that loss of resilience is a factor, not previously recognized but of major importance, in the biology of aging. Put another way, when one catalogs the interventions known from rigorous studies to slow the gathering frailties of age, they include not only exercise and caloric restriction (most clearly) but also plant toxins and PBM—stresses that induce the more acute resilience responses discussed above. Resilience, like acquired immunity, fades with age and the loss can be mitigated by the daily low-level stress of exercise or (at least for resilience) by a slightly toxic diet or caloric restriction or PBM.
Physical Stresses
One further group of stresses needs to be considered—the physical/ mechanical stresses of temperature, external abrasion, and even (and especially) the stresses of pulsatile blood flow on our blood vessels.
Heat
The literature on the effect of low-level heat stress on animal tissues is very considerable. It is older than the literatures on phytochemicals and seems more mature: Rates of publication been very high but have plateaued or even fallen in recent years (Figure 10). The move to clinical trials has commenced, though it has been late in coming; there has presumably been hesitation to use heat to condition patients for surgery or to slow degenerations. It was the study of the response of tissues to moderate heat (for mammalian tissues up to 42°C) that pioneered the identification of a major mechanism of stress-induced resilience, the HSPs, and the transcription factor that regulates their expression (heat shock factor 1).
Figure 10.
Publications (above) and reports of clinical trials (below) mentioning heat-shock proteins in their title or abstract, from PubMed at May 2018.
As more was learned about HSPs, it became evident that, although they were first named after their upregulation by low-level heat stress on cells in culture or in nonmammalian organisms, they are highly conserved from yeast to mammals.357,358 Further, they are upregulated, not just by heat, by a range of stresses and—arguably—had been better dubbed “stress shock” proteins.359 Their tissue-protective effects derive from the effectiveness of HSPs as chaperones for proteins, ensuring the correct folding of proteins as they are formed, the repair of damaged proteins, and the stability of proteins already formed.357,359 They have been recognized in many studies as playing a role in the tissue-protective effects of several of the interventions considered above: plant toxins,360–362 caloric restriction,363,364 and hypoxia and exercise.365,366 Further, the failure of the heat stress response (ie, the failure of cells to upregulate their expression of HSPs in response to stress) appears to be a factor in aging367 and therefore in the trend already reviewed above for tissue resilience to fade with age.
Reinforcing the idea that HSPs are important in tissue resilience generally, they are reported to be effective in slowing muscular dystrophy in a rat model,368,369 in slowing the development of cortical pathology in a rodent model of dementia,370,371 in mitigating the tissue pathologies of diabetes,372 in protecting the failing heart,373 in reducing neuroinflammatory markers in astrocytes, and in conditioning of cardiomyocytes374 and fibroblasts375 in vitro. In short, HSPs are upregulated by a range of stresses and are protective to a range of tissues, ranges comparable those of the inducers of resilience and of tissues protected, considered in previous sections.
Cold
The question whether moderate levels of cold also induce resilience in body tissues remains unsettled. Cold-shock proteins were first described in plants,376 in work that focused on a comparison between plant responses to heat and cold; it was concluded that the response to cold was distinctive, with no homology to the response to heat. Cold-shock proteins were identified in mammals a decade later,377 including a cold-specific CIRP (cold-induced RNA-binding protein). Fujita and colleagues suggested that CIRP, like HSPs, acts as a protein chaperone, maintaining, in this case, the stability of RNAs in the face of stress. Al-Fageeh and Smales378 reviewed detailed molecular mechanisms of the mammalian response to cold. Recent reviews and reports suggest, however, that the protective effect of cold, at least for the oft-studied traumatized CNS, arises not from an upregulation of endogenous protective pathways (such as HSP expression379) but from a downregulation of metabolic demand.380
In recent years, several studies have addressed with the question whether cold can condition tissues to a subsequent stress. The evidence seems mixed on this point. Cold acclimatization of humans was reported, for example, not to mitigate cognitive loss to subsequent exposure to cold.381 On the other hand, Qin and colleagues382 reported that “mild” hypothermic preconditioning of liver cells (exposure to 26°C for 3 × 10 minutes) preserved the viability of the cells, after they were cooled to 4°C for storage. Overall, work on cold conditioning is at a relatively preliminary stage.
Mechanical Injury: The CNS and the Vasculature
Evidence has been observed of resilience induced by mechanical damage in the CNS. Wen and colleagues349 reported a finding that was surprising at the time. They were studying the ability of growth factors injected into the vitreous humor of the rat eye to slow an inherited photoreceptor degeneration; they were pioneering then-new approaches to mitigating retinitis pigmentosa. As a control for the mechanical effect of the injection, they observed that simply inserting a needle (not loaded with a growth factor) through the retina into the vitreous humor slowed the death of photoreceptors for over 1 mm around the point of penetration of the retina. They associated this protection of photoreceptors with the upregulation of particular growth factors (CNTF and bFGF). This observation influenced Purushothuman and colleagues383 to examine the status of cerebral cortical tissue surrounding a needlestick lesion in the healthy, young brain. The lesion was made to test whether the small associated hemorrhages led to the formation of Aβ-rich plaques, like senile plaques in the human brain. They did, but in the regions flanking the needlestick track (up to 1 mm away), these authors reported a transient upregulation of the expression of Aβ intraneuronally, of oxidative damage to nuclear DNA, and of hyperphosphorylation of the cytoskeletal protein tau, but no neuronal death. They suggested that, for several days after the needlestick injury, nearby cortical tissue upregulates endogenous protective pathways, in which Aβ plays a protective role. These observations have implications for the understanding of the degenerating CNS; they imply, for example, that each plaque in the dementing human brain may be surrounded by a sphere of resilient tissue, its resilience explaining the year-long course of dementia in many patients.383 These observations on the CNS remain few, however, and raise questions. Within the heart, for example, localized ischemic stress induces resilience throughout the heart,282 whereas in the brain and retina, the spread of resilience from a site of damage appears to be in the order of a millimeter. In both tissues (CNS and heart), protection spreads from a site of stress, but over very different distances; factors determining the spread deserve investigation.
By contrast, very considerable work has gone into identifying the response of the vascular endothelium, especially of arteries, to another mechanical stress—the shear stress created by the flow of blood over the endothelium. Recent studies have sought to identify how the endothelial cells sense the shear stress of blood flow384; to define mechanosensitive networks involved,385,386 especially where flow is “complex” and atherosclerotic plaques are likely to form387; and to follow the involvement of HSPs in shear-induced responses of endothelial cells.388
Despite the richness of this literature, however, a direct link from shear stress on endothelial cells to HSP expression to the resilience of blood vessels has not been defined, although the work of Adams and colleagues389,390 comes close to demonstrating it. Working in a pig model of ventricular fibrillation, they exposed the anesthetized animal to mechanical head-to-foot shaking, at frequencies designed to create a sinusoidal shear stress on endothelial cells of blood vessels, additional to the pulse. They applied this shaking prior to inducing ventricular fibrillation and then recovery from fibrillation (mimicking a heart attack and recovery). The preconditioning shake improved the viability of the myocardium after the experimental fibrillation and defibrillation. This approach remains at the experimental stage, but it does contribute one step in the link from shear stress to pathway upregulation to tissue resilience.
Understanding Stress
Steps and Missteps in Understanding Hormesis
Concepts can be defined. A recent definition of hormesis can found elsewhere,108,391 where Mattson proposed that hormesis is a term used by toxicologists:
…to refer to a biphasic dose-response to an environmental agent characterized by a low dose stimulation or beneficial effect and a high dose inhibitory or toxic effect.
Or, more briefly, Calabrese and Baldwin392 described hormesis as:
…a dose-response relationship phenomenon characterized by low-dose stimulation and high-dose inhibition
Concepts are perhaps best understood, however, by their history, controversies, and explanatory power. The idea of hormesis is sometimes traced to the Swiss scientist, physician, and astrologer Paracelsus (1493-1541), whose writings, in many fields, are best known today for one maxim—
Alle Dinge sind Gift, und nichts ist ohne Gift, allein die Dosis macht dass ein Ding kein Gift ist.393 (All things are poison, and nothing is without poison, the dosage alone makes it that a thing is not a poison)
—which he formulated to defend his use of inorganic chemicals in the treatment of his patients. Historians of toxicology (eg, the study by Borzelleca394) regard Paracelsus as a founder of the field, because he drew attention, early in the Renaissance, to the importance of dose–response relationships. Paracelsus’ maxim, though influential, does not capture the most intriguing feature of the low-dose zone of dose–response relationships—that many “Dinge” regarded as toxins not only lose their toxicity at low doses but are tonics, evoking positive tissue responses. This realization came in the late 19th century, formulated as the Arndt-Schulz rule. In Wikipedia, the rule is stated as:
For every substance, small doses stimulate, moderate doses inhibit, large doses kill.
In a medical dictionary (http://medical-dictionary.thefreedictionary.com/Arndt-Schulz+law), it is stated as:
…weak stimuli accelerate physiologic activity, medium stimuli inhibit physiologic activity, and strong stimuli halt physiologic activity.
And from there followed a series of missteps, which must be understood, lest we extend the series. One early misstep has been laid at the feet of Hugo Schulz, because he saw low-dose-positive responses as the explanation of the then-popular doctrine of homeopathy and pursued the idea through his career. A second misstep was, Calabrese has argued,395 an overreaction to Schulz’ confidence in the efficacy of extreme low doses; it was to accept Paracelsus’ view that dosage is of major importance, yet ignore the low-dose-positive phenomena, because of their association with homeopathy. Interest in the low-dose-positive zone of the dose–response curve of toxins was, Calabrese argues, set aside for decades, until the idea was picked up again, in the middle of the 20th century396 in a nonmedical context.
This set-aside was not a trivial omission. Stress is commonly assumed to be a cause of ill-health, not health. On this assumption, epidemiologists have long extrapolated rates of cancer induction in healthy tissue by low doses of known carcinogens from high-dose effects. Recently, however, this “linear no-threshold” model has been declared dead99; evidence has been gathered that low-dose exposure to radiation is protective against cancer397 and neurodegeneration,100 and some have argued that low-dose radiation could be employed to prevent cancer.398 And conversely, drugs used to kill cancer cells in vivo (chemotherapy drugs) at a high dose (usually the highest dose that doesn’t kill the patient) have the opposite effect at low dose, encouraging the viability/proliferation of cancer cells.399 Current approaches to both carcinogens and chemotherapy still, Calabrese argues, too often ignore the implications of these low-dose effects. The practical question arises, for example, whether, as a chemotherapy drug is cleared from the body at the end of each cycle of chemotherapy, there is a period during which the drug at low concentration partially reverses its good work of killing the tumor. The question may or may not change the practice of chemotherapy, but it deserves an answer.
The story of the rerecognition of low-dose-tonic responses is of interest. Southam and Ehrlich396 described the effect of an extract from the wood of the Western red cedar tree on invasive fungi. It was Southam’s research project in forestry, done in the midst of World War II (Figure 11). Southam later studied medicine and published in the area of antibiotics and bacteria (for a very readable account, see http://dose-response.org/chester-m-southam/). Southam’s data showed that a hot water extract of the wood is toxic to the fungi, but when he diluted the extract successively, to construct a dose–response curve (as Paracelsus would have recommended), low concentrations of the same extract stimulated growth of the fungi. So, the tree had evolved a chemical to deal with the fungi and the fungi had evolved a mechanism to use low doses of the toxin to upregulate their reproductive pathways. Southam and Ehrlich396 did not argue this evolutionary interaction—that idea came later. But they did suggest a new term:
The term hormesis (adj. hormetic) is proposed to designate such a stimulatory effect of sub-inhibitory concentrations of any toxic substance of any organism.
Figure 11.
From Southam’s research thesis (1941), the origin of the term hormesis (http://dose-response.org/wp-content/uploads/2002/01/SouthamThesis1941.pdf). The change of “toxicotrophism” to “hormesis” is, of course, Southam’s.
The interaction between the tree and the predatory fungi seems comparable to the interaction between vegetables and predatory animals, in which animals have evolved a dual response. One response, as Mattson and colleagues108,400 have pointed out, is to evolve toxin-metabolizing pathways to clear the toxin quickly. The second is to evolve pathways that use the toxins to upregulate resilience pathways, so that low doses of the toxins act as tonics. The first of these responses has long been recognized and termed “tolerance.” It is the second response that Arndt and Schulz observed in the late 19th century and Southam, in the mid-20th century, called hormesis. The response is modest and counterintuitive and readily misinterpreted. The reader can assess the still slow recognition of its importance in the accounts given above of the resilience induced at low doses by sunlight, phytochemicals, ischemia and exercise, caloric restriction, and mechanical or thermal damage to tissues, that is, by the stresses of everyday life.
As so often in the recognition of biological systems, evolution provides the conceptual framework for understanding. Organisms that adapt survive; the adaptation between plants and animals is typically interactive108 and, when we understand the interaction, we also understand another mechanism evolved by animals to survive, and we can make the stresses tools in our struggle for individual health and longevity.
The concepts of dose–response and hormesis will likely continue to evolve. Sanchis-Gomar and colleagues332 have recently restated the idea of hormesis as “eustress,” a level of stress that, through epigenetic mechanisms, induces healthful responses body-wide; the term eustress came (as the authors acknowledge) from earlier workers. Their contribution was in the context of the beneficial effects of moderate exercise on mortality and many forms of morbidity. They did not mention hormesis or its history as an idea. Murugaiyah and Mattson108 recently argued (and see above) that the interaction between plants and herbivores has evolved through several steps, shown in their Figure 1, each “side” in the struggle developing new weapons during long battles. In terms of evolution, the process is adaptive, the plants evolving successive ways to deter and herbivores evolving successive ways to evade the deterrence. These authors argued that hormesis plays a central role in evolution and, conversely, that evolutionary pressures have had a central role in the development of hormesis.
More generally, Murugaiyah and Mattson108 have argued that the concept of hormesis applies not just to toxins but to other stresses. They identify exercise and caloric restriction and make out a strong case for their role in eliciting “positive” tissue responses. We here extend the idea to include ischemia and remote ischemia, sunlight (especially PBM), and mechanical or thermal damage, so to the several classes of everyday stress mentioned at the beginning of this review.
The Explanatory Power of a Concept
Good new ideas, it has been said, rapidly seem obvious. Hormesis, to those of us writing in the area, has that quality. That the idea has long been partly understood, partly misunderstood, too long ignored becomes apparent when its explanatory power is examined in specific examples.
Hair Removal and Hair Growth
Hair management is not the most serious of medical issues but—for cosmetic reasons—folk seek both to eliminate hair and to encourage its growth, and use light energy, in many cases of the same wavelength, for both purposes. At high energy, laser-sourced red–infrared energy will destroy a hair follicle permanently; the technique is long established. Conversely, low-energy laser-sourced irradiation of the skin promotes hair growth.11
Light Conditioning and LD to the Retina
The complex relationship between light and the vulnerability of rod and cone photoreceptors to damage (discussed previously) is also best understood in terms of hormesis. As noted above (in section “White Light”), even low levels of light damage the outer segments of light-naive rods, which become shorter, less sensitive, their beautifully folded membranes becoming disrupted and irregular. Yet, without some level of light experience and damage, the photoreceptors are devastated by bright daylight.81 The normal state of photoreceptors is that they are both damaged and made resilient by normal exposure to light.
Aβ, Alzheimer Disease, and an Insight Into Neuroprotection
This is an issue of major clinical importance. For some decades, the most widely accepted view of the cause of age-related dementia has been the amyloid cascade hypothesis, which posits the cause in proteinopathies, abnormalities of 2 molecules expressed prominently in the brain, the peptide Aβ, and the protein tau.401–403 In this understanding, a “chronic imbalance in the production and clearance of Aβ” as the brain ages results in the accumulation of various forms of the peptide and eventually in its deposition in an insoluble form, demonstrably present in senile plaques. Although the monomeric peptide and deposited insoluble forms appear not to be toxic, intermediate, oligomeric forms are considered toxic to the membranes of surrounding neurones, damaging synapses and killing neurones.404 The damage spreads until the brain is riddled with sites of deposition of the peptide, the plaques that Alzheimer and his contemporaries observed, and of hyperphosphorylated forms of tau, a protein that forms part of the internal skeleton of neurones. Further, “all of the genetic events currently known to predispose to the development of AD act to alter the economy of Aβ in brain tissue.”404 Mutations that cause familial forms of the dementia are all found in APP, the gene that generates the precursor protein APP, from which the peptide Aβ is excised, or in the enzymes that perform the excision. Further, APP is found on chromosome 21 and a trisomy of 21 causes a syndrome (Downs) in which, inter alia, the same brain pathology is found. The amyloid cascade hypothesis remains widely debated,405 and clinical trials of antiamyloid drugs continue to attract investment, if not success.
In more recent years, others have predicted406 and then reported evidence407,408 that, when attention was given to the effects of lower doses, Aβ is neurotrophic, enhancing synapse formation and memory-related LTPs, upregulating antioxidant mechanisms, and inhibiting microbial infections.409 From this work, the long-elusive physiological role of Aβ (Why would the brain have evolved to secrete a self-destructive molecule?) seems to be emerging: It is a self-protective molecule, upregulated by stress. Other questions remained. It was already known that the constitutive expression of Aβ is higher in the brain than in other organs410 and that its expression in the brain is upregulated from this relatively constitutive high level by stress.405,411 So, what is special about brain tissue that has led to the evolution to this brain-prominent form of stress-inducible self-protection? Kuo and colleagues described the preferential binding of hemoglobin to Aβ.412,413 This binding, which may414 be a step in the extracellular deposition of Aβ after hemorrhage to form the Aβ+ plaques prominent in the aging brain, may also serve to reduce the toxicity of hemoglobin to central nervous tissue.412 The idea and the evidence that each plaque forms at a site of hemorrhage from a small cerebral vessel were developed by Cullen and colleagues.415,416
In short, as the brain evolved to greater complexity in longer lived species such as humans, requiring greater rates of cerebral blood flow, the risk of hemorrhage from cerebral vessels, with their potential to spill hemoglobin into the neuropil, rose toward inevitability. The hypoxia-induced expression of Aβ by central nervous tissue may have evolved to protect the brain from the toxicity of the protein (hemoglobin) that evolved to deliver oxygen to it.
Attention to the low-dose end of the relationship between the concentration of Aβ and its impact on brain tissue has thus contributed to new understanding of the cause of age-related dementia and of the function of the much studied, ill understood Aβ peptide. Aβ may be not the prime driver of the dementia (as in the amyloid cascade hypothesis) but a neuroprotective molecule secreted by neural cells for self-protection. We have recently220 reviewed the evidence that aging of the vasculature (hardening of the great arteries) is the factor that drives small-vessel hemorrhage in the aging brain, generating the pathology reported by Alzheimer and the associated dementia.
Sleep Apnea, Preinfarction Angina, Prestroke Transient Ischemic Attacks, and Peripheral Vascular Disease
It is not difficult to add to this list of paradoxes explained. The list includes (all are reviewed above) the protective effects of mild sleep apnea, of angina pectoris against a subsequent heart attack, and of transient ischemic strokes against a subsequent stroke; the long-held views that daylight and exercise and vegetable-rich diets are “good” for us; the evidence that low-level hunger reduces morbidity and delays mortality; the ability of small doses of certain plant chemicals to mitigate neurodegenerations; and the ability of low-level γ-rays to protect tissue irradiated at low doses. All were surprises, counterintuitive, “too marvelous, inviting skepticism.” It is perhaps time to put aside the skepticism that surprise induces in us and take seriously, as Calabrese400 and Mattson have pleaded,391 the broad idea that the mammalian body has evolved mechanisms that use everyday stress to upregulate mechanisms of tissue resilience.
Detaching and Naming the Low-Dose-Resilience Response
Having acknowledged the explanatory power of hormesis, we now identify a problem. The term hormesis is used to denote, or is defined as, a dose–response relationship, said to have a U- or J-shape or an inverted U- or J-shape, depending on the sign given to the ordinate, as, for example, in Figure 1 in the study by Calabrese et al.417 The problem is that the use of such graphs, so important in toxicology, rests on an assumption that the change in response between low-dose stress and high-dose stress is quantitative: more cells in the dish than in a control, or fewer; more fibers in the muscle treated, or fewer; more occurrences or recurrences of cancer, or fewer.
In our view, the reality (and with it much excitement) is that the change in tissue response with the dose of stress is qualitative, from the upregulation of complex, evolved resilience pathways at low doses to chaotic tissue destruction at high doses. The use of a graph tends to hide the qualitative nature of this change, which is as great as the difference between immunity to a disease and the disease itself. We suggest that the next step in this much-thought-about field is to detach the low- and high-stress responses conceptually. Put another way, the high-stress-makes-a-toxin idea was the point of Paracelsus’ maxim—everything is toxic at some dose. The low-stress-can-induce-resilience idea goes back to Arndt and Schultz. They are different phenomena; they do not belong on the same axis of a graph, though the present writers understand why they have so often been portrayed this way. The problem was identified by Kitchin,418 who expressed the need to find a single mechanism to explain both low- and high-dose responses; once it is accepted that the responses differ qualitatively, the need for a single mechanism goes away. And several authors, despite the above quoted definitions of hormesis, have applied the name just to the low-dose response. This field is expanding rapidly and shifts in the use of names are a healthy sign of that growth.
This review has focused on the low-stress-resilience response that arises, as others have suggested, from an evolved system of stress-inducible pathways. The response evoked by low-level stress has a name, indeed several depending on the experimental or clinical context417; the system deserves a name.
Choosing the Name
For the low-stress-resilience response, a valuable consensus review of nomenclature has been provided by Calabrese and colleagues.417 We sought a name, not for the response, but for the underlying evolved system of cellular and molecular pathways, ideally a name that captures the evolutionary value of the system to the organism. We also wanted a name that was heuristically open; we have explored elsewhere the heuristic issues involved in naming biological phenomena. They are considerable419 and interact with understanding of scientific method.
By analogy with acquired immunity, we suggest the term acquired resilience. Several related, novel questions can then be asked and tested in terms of “acquired resilience,” including—What are the phenotypes of acquired resilience? Does acquired resilience fade with aging? Is there a phenomenon of resiliosenescence, comparable to immunosenescence? Is resiliosenescence part of the cause of aging? If so, can resiliosenescence—like immunosenescence—be slowed, even reversed? How is acquired resilience distinct from the better known acquired immunity? In complex organisms such as mammals, which show evidence of both acquired immunity and acquired resilience, do the 2 systems interact? Is there a destructive form of acquired resilience, comparable to autoimmunity?
Below, and briefly, each of these questions is addressed.
The Phenotypes of Acquired Resilience
The phenotypes of resilience are summarized in Table 2, to minimize repetition of material already reviewed. The phenotypes vary with the experimental model or hypothesis of the investigators. When investigators are interested in the healing of a skin wound or a tooth socket, the phenotype is accelerated healing. If their interest is in retinal degeneration, the phenotype is improved retinal function and structure; if in sarcopenia, the phenotype is improved muscle strength and muscle fiber number; if in the cellular processes of cancer, the phenotype is prevention of the proliferation or metastasis of cancer cells; if in the treatment of cancer, the phenotype is improved quality of life and longer remission; if in rejuvenation, the phenotype may be smoother, less fragile skin. The point of the summary is that probably all tissues are capable of a resilience response and that probably all of the stresses considered previously can elicit each response (that has been shown only partially).
What Happens to Acquired Resilience With Age? Is Resiliosenescence Part of Aging?
Age-related frailty is plain in our elders; and for those of us who have survived to be elders, then in ourselves. The evidence that a fading of acquired resilience underlies the frailty of age is limited but clear enough. Documented examples, already quoted above, include the loss of the angina-induced protection from coronary occlusion,273,275 the loss of transient-ischemia-induced protection from stroke,278 the loss of ischemia-induced protection of the retina,420 and the loss of caloric-restriction-induced protection of the heart.160 It seems probable, but strictly has yet to be shown, that all forms of resilience induction, by low-level stresses to the resilience phenotypes summarized in Table 2, fade with age.
Can Resiliosenescence be Stopped?
There is evidence, already noted above, that resiliosenescence can be slowed/stopped by exercise and caloric restriction. The question whether other everyday stresses (hypoxia/ischemia radiation, plant toxins) can also reverse resiliosenescence has been less well examined. It is likely that in the near future the question will be addressed and, for the many who reach the late decades of human lifespan, a regime of exercise, caloric restriction, and dietary supplements will emerge, to optimize these late years. How welcome such lifestyle management can be made remains to be seen. How much of our available energy will be needed to keep us healthy? Will it require a month each year on the exercise bicycle to give us another month of healthy life? Or will the reward be substantially greater?
The problem is, of course, evolutionary. The youthful body is organized by adaptive pressures; aging is the breakdown of that organization. Humans, committed emotionally to delaying death, put enormous resources into keeping healthy long past child-bearing and child-rearing, defying our irrelevance to evolution. How deep into old age will persist our willingness to exercise, go hungry, eat bitter foods, and manage our exposure to sunlight? Will elderly patients be able to claim that good health as grandparents or grand-aunts and grand-uncles adds to the success of child-rearing, thus maintaining an evolutionary role for old? Much remains to be learned.
Do Acquired Resilience and Acquired Immunity Interact?
If 2 distinct systems of tissue maintenance are functioning in the mammalian body, each evolved and organized, the question can be asked—do they interact in any way? One interaction is that both exercise and caloric restriction “hold back the clock” on both immunosenescence and resiliosenescence. There is no present understanding of the mechanism of this stress-induced preservation in the aged of the ability of both the immune and resilience systems to respond to the inducers that were once effective in youth. Again, much remains to be understood.
Is There a Damaging Form of Acquired Resilience (Comparable to Autoimmunity)?
Finally, we note that there appear to be few forms of resilience that are damaging, as autoimmunity can be damaging to the otherwise healthy organism. At the molecular level, Gargini and colleagues421 reported that the upregulation of the growth factor FGF-2 in retinal photoreceptors associated with stress-induced resistance to LD also increased photoreceptor sensitivity but reduced transmission of the photoreceptor response to inner retina, resulting in a limited reduction of retinal output. And at the psychological level (discussed above), Nelson and colleagues260 discussed the impact of caloric restriction on the psychology of a team isolated in the Biosphere 2 venture. Caloric restriction and the effort of growing their food made the team leaner and healthier but did not optimize group dynamics for a long mission. With those exceptions, it seems so far that activation of the pathways of acquired resilience does not damage the function or integrity of body tissues.
Acquired Resilience, Acquired Immunity: A Brief Comparison
It is useful, to emphasize that acquired resilience is a distinct system of tissue protection, to compare it with acquired immunity. Among the similarities, both systems are activated by an encounter with an environmental challenge—a pathogen or a stress; both can prevent or slow major diseases—cancer, for example; the ability to acquire both fades with age—immunosenescence and resiliosenescence; and both can be maintained into old age by the stresses that induce resilience earlier in life.
Among the differences are the inducing challenges. The resilience response is induced by physical or metabolic stresses; the immune response by biologically active pathogens. Their mechanisms also differ. The principal mechanisms of acquired immunity are humoral (the production of antibodies to a foreign antigen) and cellular (the proliferation of killer lymphocytes to kill cells carrying a foreign antigen). The mechanisms of acquired resilience include the mobilization of bone marrow–derived cells that enhance the healing of wounds, the absorption of infrared light to upregulate mitochondrial function in damaged cells, and the release of trophic cytokines by ischemic muscle. Further, acquired immunity is much more specific. Although there is some evidence of cross-immunity (dairy maids do not get smallpox—the founding observation of acquired immunity), vaccination against polio is specific to polio, and vaccination against this winter’s ‘flu may not work against next winter’s. By contrast, the resilience induced by exercise, intermittent hunger, hypoxia, plant toxins, and radiation affects the whole organism, in a range of phenotypes. And finally, as just noted, there is only limited evidence of a phenomenon of “autoresilience,” analogous to autoimmunity. Resilience mechanisms act to stabilize the structure and preserve the function of the tissues of the host animal, while the mechanisms of immunity are those of counterattack, aimed at foreign antigens, killing pathogen-infected cells, but capable of running out of control and killing healthy cells, even the whole animal. That difference between defense (resilience) and counterattack (immunity) is perhaps the fundamental difference between the 2 systems, both evolved to protect the organism.
Summary and Conclusions
This review proposes the recognition of an evolved system of cellular mechanisms in mammals that underlies the low-dose-resilience response—the ability of organisms to use everyday stresses as stimuli to upregulate endogenous mechanisms that increase the resilience of tissues. We suggest terming this evolved system “acquired resilience,” by analogy with acquired immunity. Acquired resilience is distinctive in that its cellular mechanisms are not (like those of acquired immunity) mechanisms of attack, countering invading pathogens very specifically but capable of exceeding their targets and destroying the host animal (autoimmunity). The mechanisms of resilience appear to be ones of pure defense; as a consequence, there is little evidence of a phenomenon of autoresilience, comparable to autoimmunity. One feature common to acquired immunity and acquired resilience is that both fade with age and that both can be extended into old age by everyday stresses such as exercise and caloric restriction, and perhaps more. This extension is the basis of long-accepted but partly understood views that moderate exercise and certain “healthy” diets are “good for us,” decreasing morbidity in the aging human and delaying mortality.
Understanding of acquired resilience, and of the parameters of the low-stress-resilience response that underlies it, is already providing therapeutic tools for medical conditions as serious as heart attack, stroke, and the age-related neurodegenerations (including dementia, parkinsonism, and macular degeneration). Much remains to be learned, but the outlines of the system, and its emergence through ideas of dose–response relationships and hormesis, so from the work of Paracelsus during the Renaissance, to Arndt and Schulz in the 19th century, to more recent investigators such as Southam last century and Calabrese and Mattson in this century, can now be discerned. It seems likely to become a reference point in many aspects of medicine, from prescribing a healthy diet to the management of cancer to understanding what is required for healthy aging.
Note
How long have we believed exercise to be good for us? Even if we ignore ancient treatises, then at least 2 centuries: Jane Austen used the term “healthful” in Pride and Prejudice (1813, Chapter 28): “To work in the garden was one of his (Charlotte’s vexing husband Mr Collins’) most respectable pleasures; and Elizabeth admired the command of countenance with which Charlotte talked of the healthfulness of the exercise, and owned she encouraged it as much as possible.” The phrase to take a “constitutional” walk also dates to the early 19th century (1829, according to Merriam Webster’s Dictionary).
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: J.S. is a director of CSCM Pty Ltd. R.M. has received consultancy fees over the past 3 years from Australian Doctor Eduction. M.G.W. is coinventor on the patent: Compositions and Methods for the Treatment of Lens Fibrotic Disease WO2010065920 A1, licensed by Genisphere, LLC. S.B. holds a nonremunerative relationship with Hortus Novus s.r.l.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: J.S. acknowledges with gratitude the support of the Sir Zelman Cowen Universities Fund and the Lord Mayor’s Charitable Fund (Melbourne). J.E. acknowledges N H/NEI R43-EY025892 (Eells, Co-PI, Tedford Co-PI; The Development of Photobiomodulation for the Treatment of Age-Related Macular Degeneration. 09/01/15-8/31/18). M.G.W. acknowledges support from the Sharpe-Strumia Research Foundation, the March of Dimes; the W.W. Smith Foundations; and the National Institutes of Health: NIH RO1 HD043157-01, 2003-2007, Origin and Fate of Myogenic Stem Cells; and NIH RO1 AR052326-04, 2008-2011, Directing the Fate of Cells to Myogenic Lineages.
References
- 1. Finsen N. The red-light treatment of smallpox. JAMA. 1903;XLI(20):1207–1208. [Google Scholar]
- 2. O’Sullivan G. The relationship between hope, eustress, self-efficacy, and life satisfaction among undergraduates. Soc Indic Res. 2010;101(1):155–172. [Google Scholar]
- 3. Roelandts R. The history of phototherapy: something new under the sun? J Am Acad Dermatol. 2002;46(6):926–930. [DOI] [PubMed] [Google Scholar]
- 4. Hamblin M, Demidova N. Mechanisms of low-light therapy In: Hamblin M, Waynant R, Anders J, eds. Mechanisms for Low-Light Therapy. SPIE Proceedings; 2006:1–12. [Google Scholar]
- 5. Geneva II. Photobiomodulation for the treatment of retinal diseases: a review. Int J Ophthalmol. 2016;9(1):145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moskvin S. Low-level laser therapy in Russia: history, science and practice. J Lasers Med Sci. 2017;8(2):56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hamblin MR. Photobiomodulation or low-level laser therapy. J Biophotonics. 2016;9(11-12):1122–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hamblin M. History of low-level laser (light) therapy In: Hamblin M, Pires de Sousa MV, agrawal A, eds. Handbook of Low-Level Laser Therapy. Singapore, Singapore: Pan Stanford; 2017:17–34. [Google Scholar]
- 9. Finsen N. The red light treatment of smallpox. Br Med J. 1895;2(1823):1412–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Finsen N. Remarks on the red-light treatment of smallpox. Br Med J. 1903;1(2214):1297–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mester E, Szende B, Gartner P. The effect of laser beams on the growth of hair in mice. Radiobiol Radiother (Berl). 1968;9(5):621–626. [PubMed] [Google Scholar]
- 12. Zarei M, Wikramanayake TC, Falto-Aizpurua L, Schachner LA, Jimenez JJ. Low level laser therapy and hair regrowth: an evidence-based review. Lasers Med Sci. 2016;31(2):363–371. [DOI] [PubMed] [Google Scholar]
- 13. Gáspár L. Professor Endre Mester, the father of photobiomodulation. J Laser Dent. 2009;17(3):146–148. [Google Scholar]
- 14. Barolet D, Christiaens F, Hamblin MR. Infrared and skin: friend or foe. J Photochem Photobiol B. 2016;155:78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ferraresi C, Hamblin MR, Parizotto NA. Low-level laser (light) therapy (LLLT) on muscle tissue: performance, fatigue and repair benefited by the power of light. Photonics Lasers Med. 2012;1(4):267–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Leal-Junior EC, Vanin AA, Miranda EF, de Carvalho Pde T, Dal Corso S, Bjordal JM. Effect of phototherapy (low-level laser therapy and light-emitting diode therapy) on exercise performance and markers of exercise recovery: a systematic review with meta-analysis. Lasers Med Sci. 2015;30(2):925–939. [DOI] [PubMed] [Google Scholar]
- 17. Eells JT, Henry MM, Summerfelt P, et al. Therapeutic photobiomodulation for methanol-induced retinal toxicity. Proc Natl Acad Sci U S A. 2003;100(6):3439–3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Farfara D, Tuby H, Trudler D, et al. Low-level laser therapy ameliorates disease progression in a mouse model of Alzheimer’s disease. J Mol Neurosci. 2015;55(2):430–436. [DOI] [PubMed] [Google Scholar]
- 19. Purushothuman S, Johnstone DM, Nandasena C, et al. Near infrared light mitigates cerebellar pathology in transgenic mouse models of dementia. Neurosci Lett. 2015;591:155–159. [DOI] [PubMed] [Google Scholar]
- 20. Purushothuman S, Johnstone DM, Nandasena C, Mitrofanis J, Stone J. Photobiomodulation with near infrared light mitigates Alzheimer’s disease-related pathology in cerebral cortex – evidence from two transgenic mouse models. Alzheimers Res Ther. 2014;6(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Johnstone D, Moro C, Stone J, Benabid AL, Mitrofanis J. Turning on lights to stop neurodegeneration: the potential of of near infrared light therapy in Alzheimer’s and Parkinson’s disease. Front Neurosci. 2016;9:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Darlot F, Moro C, El Massri N, et al. Near-infrared light is neuroprotective in a monkey model of Parkinson disease. Ann Neurol. 2016;79(1):59–75. [DOI] [PubMed] [Google Scholar]
- 23. Cassano P, Petrie SR, Hamblin MR, Henderson TA, Iosifescu DV. Review of transcranial photobiomodulation for major depressive disorder: targeting brain metabolism, inflammation, oxidative stress, and neurogenesis. Neurophotonics. 2016;3(3):031404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ghanbari A, Ghareghani M, Zibara K, Delaviz H, Ebadi E, Jahantab MH. Light-emitting diode (LED) therapy improves occipital cortex damage by decreasing apoptosis and increasing BDNF-expressing cells in methanol-induced toxicity in rats. Biomed Pharmacother. 2017;89:1320–1330. [DOI] [PubMed] [Google Scholar]
- 25. Hamblin MR. Shining light on the head: photobiomodulation for brain disorders. BBA Clin. 2016;6:113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Naeser MA, Hamblin MR. Traumatic brain injury: a major medical problem that could be treated using transcranial, red/near-infrared led photobiomodulation. Photomed Laser Surg. 2015;33(9):443–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Naeser MA, Martin PI, Ho MD, et al. Transcranial, red/near-infrared light-emitting diode therapy to improve cognition in chronic traumatic brain injury. Photomed Laser Surg. 2016;34(12):610–626. [DOI] [PubMed] [Google Scholar]
- 28. Naeser MA, Zafonte R, Krengel MH, et al. Significant improvements in cognitive performance post-transcranial, red/near-infrared light-emitting diode treatments in chronic, mild traumatic brain injury: open-protocol study. J Neurotrauma. 2014;31(11):1008–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thunshelle C, Hamblin MR. Transcranial low-level laser (light) therapy for brain injury. Photomed Laser Surg. 2016;34(12):587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xuan W, Huang L, Hamblin MR. Repeated transcranial low-level laser therapy for traumatic brain injury in mice: biphasic dose response and long-term treatment outcome. J Biophotonics. 2016;9(11-12):1263–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ivandic BT, Ivandic T. Low-level laser therapy improves vision in patients with age-related macular degeneration. Photomed Laser Surg. 2008;26(3):241–245. [DOI] [PubMed] [Google Scholar]
- 32. Koev K, Avramov L, Borissova E. Six years follow-up of low-levellaser therapy (LLLT) in patients with age-relaed macular degeneeration. Int J Adv Res. 2017;5(9):1428–1432. [Google Scholar]
- 33. Oron A, Oron U, Chen J, et al. Low-level laser therapy applied transcranially to rats after induction of stroke significantly reduces long-term neurological deficits. Stroke. 2006;37(10):2620–2624. [DOI] [PubMed] [Google Scholar]
- 34. Lampl Y, Zivin JA, Fisher M, et al. Infrared laser therapy for ischemic stroke: a new treatment strategy: results of the NeuroThera Effectiveness and Safety Trial-1 (NEST-1). Stroke. 2007;38(6):1843–1849. [DOI] [PubMed] [Google Scholar]
- 35. Di Marco F, Romeo S, Nandasena C, et al. The time course of action of two neuroprotectants, dietary saffron and photobiomodulation, assessed in the rat retina. Am J Neurodegener Dis. 2013;2(3):208–220. [PMC free article] [PubMed] [Google Scholar]
- 36. Henderson TA, Morries LD. Multi-watt near-infrared phototherapy for the treatment of comorbid depression: an open-label single-arm study. Front Psychiatry. 2017;8:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moro C, Massri NE, Torres N, et al. Photobiomodulation inside the brain: a novel method of applying near-infrared light intracranially and its impact on dopaminergic cell survival in MPTP-treated mice. J Neurosurg. 2014;120(3):670–683. [DOI] [PubMed] [Google Scholar]
- 38. Saltmarche AE, Naeser MA, Ho KF, Hamblin MR, Lim L. Significant improvement in cognition in mild to moderately severe dementia cases treated with transcranial plus intranasal photobiomodulation: case series report. Photomed Laser Surg. 2017;35(8):432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Desmet KD, Paz DA, Corry JJ, et al. Clinical and experimental applications of NIR-LED photobiomodulation. Photomed Laser Surg. 2006;24(2):121–128. [DOI] [PubMed] [Google Scholar]
- 40. Eells J, DeSmet K, Kirk D, et al. Photobiomodulation for the treatment of retinal injury and retinal degenerative diseases In: Waynant R, Tata D, eds. Proceeding of Light-Assisted Tissue Regeneration and Therapy Conference. Vol I New York, NY: Springer; 2008:39–51. [Google Scholar]
- 41. Tsai SR, Hamblin MR. Biological effects and medical applications of infrared radiation. J Photochem Photobiol B. 2017;170:197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wong-Riley MT, Liang HL, Eells JT, et al. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. J Biol Chem. 2005;280(6):4761–4771. [DOI] [PubMed] [Google Scholar]
- 43. Natoli R, Zhu Y, Valter K, Bisti S, Eells J, Stone J. Gene and noncoding RNA regulation underlying photoreceptor protection: microarray study of dietary antioxidant saffron and photobiomodulation in rat retina. Mol Vis. 2010;16:1801–1822. [PMC free article] [PubMed] [Google Scholar]
- 44. Braverman B, McCarthy RJ, Ivankovich AD, Forde DE, Overfield M, Bapna MS. Effect of helium–neon and infrared laser irradiation on wound healing in rabbits. Lasers Surg Med. 1989;9(1):50–58. [DOI] [PubMed] [Google Scholar]
- 45. Rochkind S, Rousso M, Nissan M, Villarreal M, Barr-Nea L, Rees DG. Systemic effects of low-power laser irradiation on the peripheral and central nervous system, cutaneous wounds, and burns. Lasers Surg Med. 1989;9(2):174–182. [DOI] [PubMed] [Google Scholar]
- 46. Hopkins JT, McLoda TA, Seegmiller JG, David Baxter G. Low-level laser therapy facilitates superficial wound healing in humans: a triple-blind, sham-controlled study. J Athl Train. 2004;39(3):223–229. [PMC free article] [PubMed] [Google Scholar]
- 47. Whelan HT, Connelly JF, Hodgson BD, et al. NASA light-emitting diodes for the prevention of oral mucositis in pediatric bone marrow transplant patients. J Clin Laser Med Surg. 2002;20(6):319–324. [DOI] [PubMed] [Google Scholar]
- 48. Abe M, Fujisawa K, Suzuki H, Sugimoto T, Kanno T. Role of 830 nm low reactive level laser on the growth of an implanted glioma in mice. Keio J Med. 1993;42(4):177–179. [DOI] [PubMed] [Google Scholar]
- 49. Stone J, Johnstone D, Mitrofanis J. The helmet experiment in Parkinson’s disease: an observation of the mechanism of action of near infra-red light In: Laakso L, ed. Proceedings of the 9th World Association for Laser Therapy Congress. Bologna, Italy: Medimond s.r.l; 2013:3. [Google Scholar]
- 50. Johnstone DM, Mitrofanis J, Stone J. Targeting the body to protect the brain: inducing neuroprotection with remotely-applied near infrared light. Neural Regen Res. 2015;10(3):349–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Johnstone DM, El Massri N, Moro C, et al. Indirect application of near infrared light induces neuroprotection in a mouse model of parkinsonism – an abscopal neuroprotective effect. Neuroscience. 2014;274:93–101. [DOI] [PubMed] [Google Scholar]
- 52. Kim B, Brandli A, Mitrofanis J, Stone J, Purushothuman S, Johnstone DM. Remote tissue conditioning – an emerging approach for inducing body-wide protection against diseases of ageing. Ageing Res Rev. 2017;37:69–78. [DOI] [PubMed] [Google Scholar]
- 53. Tuby H, Maltz L, Oron U. Induction of autologous mesenchymal stem cells in the bone marrow by low-level laser therapy has profound beneficial effects on the infarcted rat heart. Lasers Surg Med. 2011;43(5):401–409. [DOI] [PubMed] [Google Scholar]
- 54. Tuby H, Maltz L, Oron U. Implantation of low-level laser irradiated mesenchymal stem cells into the infarcted rat heart is associated with reduction in infarct size and enhanced angiogenesis. Photomed Laser Surg. 2009;27(2):227–233. [DOI] [PubMed] [Google Scholar]
- 55. Tuby H, Maltz L, Oron U. Low-level laser irradiation (LLLI) promotes proliferation of mesenchymal and cardiac stem cells in culture. Lasers Surg Med. 2007;39(4):373–378. [DOI] [PubMed] [Google Scholar]
- 56. Oron A, Oron U. Low-level laser therapy to the bone marrow ameliorates neurodegenerative disease progression in a mouse model of Alzheimer’s disease: a minireview. Photomed Laser Surg. 2016;34(12):627–630. [DOI] [PubMed] [Google Scholar]
- 57. Blatt A, Elbaz-Greener GA, Tuby H, et al. Low-level laser therapy to the bone marrow reduces scarring and improves heart function post-acute myocardial infarction in the pig. Photomed Laser Surg. 2016;34(11):516–524. [DOI] [PubMed] [Google Scholar]
- 58. Saliba A, Du Y, Liu H, et al. Photobiomodulation mitigates diabetes-induced retinopathy by direct and indirect mechanisms: evidence from intervention studies in pigmented mice. PLoS One. 2015;10(10):e0139003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Muheremu A, Peng J, Ao Q. Stem cell based therapies for spinal cord injury. Tissue Cell. 2016;48(4):328–333. [DOI] [PubMed] [Google Scholar]
- 60. Bruyneel AA, Sehgal A, Malandraki-Miller S, Carr C. Stem cell therapy for the heart: blind alley or magic bullet? J Cardiovasc Transl Res. 2016;9(5-6):405–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ward MR, Abadeh A, Connelly KA. Concise review: rational use of mesenchymal stem cells in the treatment of ischemic heart disease [published online ahead of print April 17, 2018]. Stem Cells Transl Med. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Marichal N, Reali C, Trujillo-Cenoz O, Russo RE. Spinal cord stem cells in their microenvironment: the ependyma as a stem cell niche. Adv Exp Med Biol. 2017;1041:55–79. [DOI] [PubMed] [Google Scholar]
- 63. Oner A. Stem cell treatment in retinal diseases: recent developments. Turk J Ophthalmol. 2018;48(1):33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gerhart J, Elder J, Neely C, et al. MyoD-positive epiblast cells regulate skeletal muscle differentiation in the embryo. J Cell Biol. 2006;175(2):283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bravo-Nuevo A, Brandli A, Gerhart J, et al. Neuroprotective effect of myo/nog cells in the stressed retina. Exp Eye Res. 2016;146:22–25. [DOI] [PubMed] [Google Scholar]
- 66. Brandli A, Gerhart J, Sutera CK, et al. Role of Myo/Nog cells in neuroprotection: evidence from the light damaged retina. PLoS One. 2017;12(1):e0169744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gerhart J, Scheinfeld VL, Milito T, et al. Myo/Nog cell regulation of bone morphogenetic protein signaling in the blastocyst is essential for normal morphogenesis and striated muscle lineage specification. Dev Biol. 2011;359(1):12–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gerhart J, Hayes C, Scheinfeld V, Chernick M, Gilmour S, George-Weinstein M. Myo/Nog cells in normal, wounded and tumor-bearing skin. Exp Dermatol. 2012;21(6):466–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gerhart J, Greenbaum M, Scheinfeld V, et al. Myo/Nog cells: targets for preventing the accumulation of skeletal muscle-like cells in the human lens. PLoS One. 2014;9(4):e95262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gerhart J, Withers C, Gerhart C, et al. Myo/Nog cells are present in the ciliary processes, on the zonule of Zinn and posterior capsule of the lens following cataract surgery. Exp Eye Res. 2018;171:101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gerhart J, Neely C, Stewart B, et al. Epiblast cells that express myod recruit pluripotent cells to the skeletal muscle lineage. J Cell Biol. 2004;164(5):739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gerhart J, Greenbaum M, Casta L, et al. Antibody-conjugated, DNA-based nanocarriers intercalated with doxorubicin eliminate myofibroblasts in explants of human lens tissue. J Pharmacol Exp Ther. 2017;361(1):60–67. [DOI] [PubMed] [Google Scholar]
- 73. Walker JL, Zhai N, Zhang L, et al. Unique precursors for the mesenchymal cells involved in injury response and fibrosis. Proc Natl Acad Sci U S A. 2010;107(31):13730–13735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Guidry C. The role of Muller cells in fibrocontractive retinal disorders. Prog Retin Eye Res. 2005;24(1):75–86. [DOI] [PubMed] [Google Scholar]
- 75. Leiderman YI, Miller JW. Proliferative vitreoretinopathy: pathobiology and therapeutic targets. Semin Ophthalmol. 2009;24(2):62–69. [DOI] [PubMed] [Google Scholar]
- 76. Mason RS, Reichrath J. Sunlight vitamin D and skin cancer. Anticancer Agents Med Chem. 2013;13(1):83–97. [PubMed] [Google Scholar]
- 77. Deluca H. Historical overview of vitamin D In: Feldman D, Pike J, Adams J, eds. Vitamin D. 3rd ed Cambridge, MA: Academic Press; 2011:3–12. [Google Scholar]
- 78. Tongkao-On W, Carter S, Reeve VE, et al. CYP11A1 in skin: an alternative route to photoprotection by vitamin D compounds. J Steroid Biochem Mol Biol. 2015;148:72–78. [DOI] [PubMed] [Google Scholar]
- 79. McCarthy B, Dixon KM, Halliday GM, Reeve VE, Mason RS. The vitamin D saga: breaking dawn. Immun Endoc Metab Agents Med Chem. 2014;14(3):137–151. [Google Scholar]
- 80. Berntson A, Smith RG, Taylor WR. Transmission of single photon signals through a binary synapse in the mammalian retina. Vis Neurosci. 2004;21(5):693–702. [DOI] [PubMed] [Google Scholar]
- 81. Penn J, Anderson R. Effects of light history on the rat retina. Prog Ret Res. 1991;11:75–98. [Google Scholar]
- 82. Liu C, Peng M, Laties AM, Wen R. Preconditioning with bright light evokes a protective response against light damage in the rat retina. J Neurosci. 1998;18(4):1337–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Roelandts R. Phototherapy of photodermatoses. J Dermatolog Treat. 2002;13(4):157–160. [DOI] [PubMed] [Google Scholar]
- 84. Adamskaya N, Dungel P, Mittermayr R, et al. Light therapy by blue LED improves wound healing in an excision model in rats. Injury. 2011;42(9):917–921. [DOI] [PubMed] [Google Scholar]
- 85. Fushimi T, Inui S, Nakajima T, Ogasawara M, Hosokawa K, Itami S. Green light emitting diodes accelerate wound healing: characterization of the effect and its molecular basis in vitro and in vivo. Wound Repair Regen. 2012;20(2):226–235. [DOI] [PubMed] [Google Scholar]
- 86. Yuan D, Collage RD, Huang H, et al. Blue light reduces organ injury from ischemia and reperfusion. Proc Natl Acad Sci U S A. 2016;113(19):5239–5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Balaram P, Mani KS. Low dose radiation—a curse or a boon? Natl Med J India. 1994;7(4):169–172. [PubMed] [Google Scholar]
- 88. Hickey RJ, Bowers EJ, Clelland RC. Radiation hormesis, public health, and public policy: a commentary. Health Phys. 1983;44(3):207–219. [DOI] [PubMed] [Google Scholar]
- 89. Macklis RM, Beresford B. Radiation hormesis. J Nucl Med. 1991;32(2):350–359. [PubMed] [Google Scholar]
- 90. Loken MK, Feinendegen LE. Radiation hormesis. Its emerging significance in medical practice. Invest Radiol. 1993;28(5):446–450. [PubMed] [Google Scholar]
- 91. Smith H. Radiation hormesis in relation to radiation protection. Chin Med J (Engl). 1994;107(8):615–623. [PubMed] [Google Scholar]
- 92. Pollycove M. Nonlinearity of radiation health effects. Environ Health Perspect. 1998;106(suppl 1):363–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Luckey TD. Nurture with ionizing radiation: a provocative hypothesis. Nutr Cancer. 1999;34(1):1–11. [DOI] [PubMed] [Google Scholar]
- 94. Mossman KL. Deconstructing radiation hormesis. Health Phys. 2001;80(3):263–269. [DOI] [PubMed] [Google Scholar]
- 95. Jolly D, Meyer J. A brief review of radiation hormesis. Australas Phys Eng Sci Med. 2009;32(4):180–187. [DOI] [PubMed] [Google Scholar]
- 96. Jaworowski Z. Radiation hormesis—a remedy for fear. Hum Exp Toxicol. 2010;29(4):263–270. [DOI] [PubMed] [Google Scholar]
- 97. Ma S, Kong B, Liu B, Liu X. Biological effects of low-dose radiation from computed tomography scanning. Int J Radiat Biol. 2013;89(5):326–333. [DOI] [PubMed] [Google Scholar]
- 98. Morgan WF, Bair WJ. Issues in low dose radiation biology: the controversy continues. A perspective. Radiat Res. 2013;179(5):501–510. [DOI] [PubMed] [Google Scholar]
- 99. Calabrese EJ. Obituary notice: LNT dead at 89 years, a life in the spotlight. Environ Res. 2017;155:276–278. [DOI] [PubMed] [Google Scholar]
- 100. Otani A, Kojima H, Guo C, Oishi A, Yoshimura N. Low-dose-rate, low-dose irradiation delays neurodegeneration in a model of retinitis pigmentosa. Am J Pathol. 2012;180(1):328–336. [DOI] [PubMed] [Google Scholar]
- 101. Serra-Majem L, Roman B, Estruch R. Scientific evidence of interventions using the Mediterranean diet: a systematic review. Nutr Rev. 2006;64(2 pt 2):S27–S47. [DOI] [PubMed] [Google Scholar]
- 102. de Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation. 1999;99(6):779–785. [DOI] [PubMed] [Google Scholar]
- 103. Bloomfield HE, Kane R, Koeller E, Greer N, MacDonald R, Wilt T. Benefits and Harms of the Mediterranean Diet Compared to Other Diets. Washington, DC: Department of Veterans Affairs; 2015. [PubMed] [Google Scholar]
- 104. Sun D, Yue Q, Guo W, et al. Neuroprotection of resveratrol against neurotoxicity induced by methamphetamine in mouse mesencephalic dopaminergic neurons. Biofactors. 2015;41(4):252–260. [DOI] [PubMed] [Google Scholar]
- 105. Yang T, Wang L, Zhu M, Zhang L, Yan L. Properties and molecular mechanisms of resveratrol: a review. Pharmazie. 2015;70(8):501–506. [PubMed] [Google Scholar]
- 106. Jing X, Cheng W, Wang S, Li P, He L. Resveratrol induces cell cycle arrest in human gastric cancer MGC803 cells via the PTEN-regulated PI3K/Akt signaling pathway. Oncol Rep. 2016;35(1):472–478. [DOI] [PubMed] [Google Scholar]
- 107. Inderjit Wardle DA, Karban R, Callaway RM. The ecosystem and evolutionary contexts of allelopathy. Trends Ecol Evol. 2011;26(12):655–662. [DOI] [PubMed] [Google Scholar]
- 108. Murugaiyah V, Mattson MP. Neurohormetic phytochemicals: an evolutionary-bioenergetic perspective. Neurochem Int. 2015;89:271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Mattson MP, Cheng A. Neurohormetic phytochemicals: low-dose toxins that induce adaptive neuronal stress responses. Trends Neurosci. 2006;29(11):632–639. [DOI] [PubMed] [Google Scholar]
- 110. Turner RS, Thomas RG, Craft S, et al. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology. 2015;85(16):1383–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Milani C, Maccari M, Mosconi P. Action of lycopene in the experimental gastric ulcer. Pharmacology. 1970;4(6):334–340. [DOI] [PubMed] [Google Scholar]
- 112. Wahlstrom B, Blennow G. A study on the fate of curcumin in the rat. Acta Pharmacol Toxicol (Copenh). 1978;43(2):86–92. [DOI] [PubMed] [Google Scholar]
- 113. Srimal RC, Dhawan BN. Pharmacology of diferuloyl methane (curcumin), a non-steroidal anti-inflammatory agent. J Pharm Pharmacol. 1973;25(6):447–452. [DOI] [PubMed] [Google Scholar]
- 114. Kohli K, Ali J, Ansari M, Raheman A. Curcumin: a natural antiinflammatory agent. Indian J Pharmacol. 2005;37(3):141–147. [Google Scholar]
- 115. World Health Organization. WHO Monographs on Selected Medicinal Plants. Vol. 3 Geneva, Switzerland: WHO Press; 2007. [Google Scholar]
- 116. Biagi M, Bertelli AA. Wine, alcohol and pills: what future for the French paradox? Life Sci. 2015;131:19–22. [DOI] [PubMed] [Google Scholar]
- 117. Bitterman JL, Chung JH. Metabolic effects of resveratrol: addressing the controversies. Cell Mol Life Sci. 2015;72(8):1473–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Chang HC, Chen TG, Tai YT, Chen TL, Chiu WT, Chen RM. Resveratrol attenuates oxidized LDL-evoked Lox-1 signaling and consequently protects against apoptotic insults to cerebrovascular endothelial cells. J Cereb Blood Flow Metab. 2011;31(3):842–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Chang CC, Chang CY, Wu YT, Huang JP, Yen TH, Hung LM. Resveratrol retards progression of diabetic nephropathy through modulations of oxidative stress, proinflammatory cytokines, and AMP-activated protein kinase. J Biomed Sci. 2011;18(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Cao L, Chen X, Xiao X, Ma Q, Li W. Resveratrol inhibits hyperglycemia-driven ROS-induced invasion and migration of pancreatic cancer cells via suppression of the ERK and p38 MAPK signaling pathways. Int J Oncol. 2016;49(2):735–743. [DOI] [PubMed] [Google Scholar]
- 121. Chakraborty S, Kumar A, Butt NA, et al. Molecular insight into the differential anti-androgenic activity of resveratrol and its natural analogs: in silico approach to understand biological actions. Mol Biosyst. 2016;12(5):1702–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Feng M, Zhong LX, Zhan ZY, Huang ZH, Xiong JP. Resveratrol treatment inhibits proliferation of and induces apoptosis in human colon cancer cells. Med Sci Monit. 2016;22:1101–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Ko YC, Chang CL, Chien HF, Wu CH, Lin LI. Resveratrol enhances the expression of death receptor Fas/CD95 and induces differentiation and apoptosis in anaplastic large-cell lymphoma cells. Cancer Lett. 2011;309(1):46–53. [DOI] [PubMed] [Google Scholar]
- 124. Morris KC, Lin HW, Thompson JW, Perez-Pinzon MA. Pathways for ischemic cytoprotection: role of sirtuins in caloric restriction, resveratrol, and ischemic preconditioning. J Cereb Blood Flow Metab. 2011;31(4):1003–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Sun AY, Wang Q, Simonyi A, Sun GY. Resveratrol as a therapeutic agent for neurodegenerative diseases. Mol Neurobiol. 2010;41(2-3):375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. de Lima ESTC, da Silveira LTR, Fragoso MF, et al. Maternal resveratrol treatment reduces the risk of mammary carcinogenesis in female offspring prenatally exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Horm Cancer. 2017;8(5-6):286–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Guo X, Ni J, Dai X, et al. Biphasic regulation of spindle assembly checkpoint by low and high concentrations of resveratrol leads to the opposite effect on chromosomal instability. Mutat Res. 2018;825:19–30. [DOI] [PubMed] [Google Scholar]
- 128. Peisch SF, Van Blarigan EL, Chan JM, Stampfer MJ, Kenfield SA. Prostate cancer progression and mortality: a review of diet and lifestyle factors. World J Urol. 2016;35(6):867–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Paur I, Lilleby W, Bohn SK, et al. Tomato-based randomized controlled trial in prostate cancer patients: effect on PSA. Clin Nutr. 2016;36(3):672–679. [DOI] [PubMed] [Google Scholar]
- 130. Wang Y, Jacobs EJ, Newton CC, McCullough ML. Lycopene, tomato products and prostate cancer-specific mortality among men diagnosed with nonmetastatic prostate cancer in the Cancer Prevention Study II Nutrition Cohort. Int J Cancer. 2016;138(12):2846–2855. [DOI] [PubMed] [Google Scholar]
- 131. Key TJ, Appleby PN, Travis RC, et al. Carotenoids, retinol, tocopherols, and prostate cancer risk: pooled analysis of 15 studies. Am J Clin Nutr. 2015;102(5):1142–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Hackshaw-McGeagh LE, Perry RE, Leach VA, et al. A systematic review of dietary, nutritional, and physical activity interventions for the prevention of prostate cancer progression and mortality. Cancer Causes Control. 2015;26(11):1521–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Ono M, Takeshima M, Nakano S. Mechanism of the anticancer effect of lycopene (tetraterpenoids). Enzymes. 2015;37:139–166. [DOI] [PubMed] [Google Scholar]
- 134. Kotecha R, Takami A, Espinoza JL. Dietary phytochemicals and cancer chemoprevention: a review of the clinical evidence. Oncotarget. 2016;7(32):52517–52529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Grainger EM, Hadley CW, Moran NE, et al. A comparison of plasma and prostate lycopene in response to typical servings of tomato soup, sauce or juice in men before prostatectomy. Br J Nutr. 2015;114(4):596–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Li D, Chen L, Zhao W, Hao J, An R. MicroRNA-let-7f-1 is induced by lycopene and inhibits cell proliferation and triggers apoptosis in prostate cancer. Mol Med Rep. 2016;13(3):2708–2714. [DOI] [PubMed] [Google Scholar]
- 137. Stage TB, Bergmann TK, Kroetz DL. Clinical pharmacokinetics of paclitaxel monotherapy: an updated literature review. Clin Pharmacokinet. 2018;57(1):7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Kavanaugh CJ, Trumbo PR, Ellwood KC. The U.S. Food and Drug Administration’s evidence-based review for qualified health claims: tomatoes, lycopene, and cancer. J Natl Cancer Inst. 2007;99(14):1074–1085. [DOI] [PubMed] [Google Scholar]
- 139. Ilic D, Forbes KM, Hassed C. Lycopene for the prevention of prostate cancer. Cochrane Database Syst Rev. 2011;(11):CD008007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Giovannucci E. Does prostate-specific antigen screening influence the results of studies of tomatoes, lycopene, and prostate cancer risk? J Natl Cancer Inst. 2007;99(14):1060–1062. [DOI] [PubMed] [Google Scholar]
- 141. Giovannucci E. Commentary: serum lycopene and prostate cancer progression: a re-consideration of findings from the prostate cancer prevention trial. Cancer Causes Control. 2011;22(7):1055–1059. [DOI] [PubMed] [Google Scholar]
- 142. Graff RE, Pettersson A, Lis RT, et al. Dietary lycopene intake and risk of prostate cancer defined by ERG protein expression. Am J Clin Nutr. 2016;103(3):851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Block KI, Gyllenhaal C, Lowe L, et al. Designing a broad-spectrum integrative approach for cancer prevention and treatment. Semin Cancer Biol. 2015;35:S276–S304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Kuffler DP. Photobiomodulation in promoting wound healing: a review. Regen Med. 2016;11(1):107–122. [DOI] [PubMed] [Google Scholar]
- 145. Khan I, Arany P. Biophysical approaches for oral wound healing: emphasis on photobiomodulation. Adv Wound Care (New Rochelle). 2015;4(12):724–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Aoki A, Mizutani K, Schwarz F, et al. Periodontal and peri-implant wound healing following laser therapy. Periodontol 2000. 2015;68(1):217–269. [DOI] [PubMed] [Google Scholar]
- 147. Agrawal T, Gupta GK, Rai V, Carroll JD, Hamblin MR. Pre-conditioning with low-level laser (light) therapy: light before the storm. Dose Response. 2014;12(4):619–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. de la Torre JC. Treating cognitive impairment with transcranial low level laser therapy. J Photochem Photobiol B. 2017;168:149–155. [DOI] [PubMed] [Google Scholar]
- 149. Saez de Asteasu ML, Martinez-Velilla N, Zambom-Ferraresi F, Casas-Herrero A, Izquierdo M. Role of physical exercise on cognitive function in healthy older adults: a systematic review of randomized clinical trials. Ageing Res Rev. 2017;37:117–134. [DOI] [PubMed] [Google Scholar]
- 150. Broadhead GK, Chang A, Grigg J, McCluskey P. Efficacy and safety of saffron supplementation: current clinical findings. Crit Rev Food Sci Nutr. 2016;56(16):2767–2776. [DOI] [PubMed] [Google Scholar]
- 151. Hamblin MR. Photobiomodulation for traumatic brain injury and stroke. J Neurosci Res. 2018;96(4):731–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Ziaaldini MM, Marzetti E, Picca A, Murlasits Z. Biochemical pathways of sarcopenia and their modulation by physical exercise: a narrative review. Front Med (Lausanne). 2017;4:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Koul A, Abraham SK. Efficacy of crocin and safranal as protective agents against genotoxic stress induced by gamma radiation, urethane and procarbazine in mice. Hum Exp Toxicol. 2018;37(1):13–20. [DOI] [PubMed] [Google Scholar]
- 154. Hamblin MR. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017;4(3):337–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Ferraresi C, Huang YY, Hamblin MR. Photobiomodulation in human muscle tissue: an advantage in sports performance? J Biophotonics. 2016;9(11-12):1273–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Brandli A, Stone J. Remote ischemia influences the responsiveness of the retina: observations in the rat. Invest Ophthalmol Vis Sci. 2014;55(4):2088–2096. [DOI] [PubMed] [Google Scholar]
- 157. Borsa PA, Larkin KA, True JM. Does phototherapy enhance skeletal muscle contractile function and postexercise recovery? A systematic review. J Athl Train. 2013;48(1):57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Pinto HD, Vanin AA, Miranda EF, et al. Photobiomodulation therapy improves performance and accelerates recovery of high-level rugby players in field test: a randomized, crossover, double-blind, placebo-controlled clinical study. J Strength Cond Res. 2016;30(12):3329–3338. [DOI] [PubMed] [Google Scholar]
- 159. Toma RL, Oliveira MX, Renno ACM, Laakso EL. Photobiomodulation (PBM) therapy at 904 nm mitigates effects of exercise-induced skeletal muscle fatigue in young women. Lasers Med Sci. 2018:33(6):1197–1205. [DOI] [PubMed] [Google Scholar]
- 160. Calabrese EJ. Pre- and post-conditioning hormesis in elderly mice, rats, and humans: its loss and restoration. Biogerontology. 2016;17(4):681–702. [DOI] [PubMed] [Google Scholar]
- 161. Huffman DM. Exercise as a calorie restriction mimetic: implications for improving healthy aging and longevity. Interdiscip Top Gerontol. 2010;37:157–174. [DOI] [PubMed] [Google Scholar]
- 162. Lopez-Lluch G, Navas P. Calorie restriction as an intervention in ageing. J Physiol. 2016;594(8):2043–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Everitt AV, Le Couteur DG. Life extension by calorie restriction in humans. Ann N Y Acad Sci. 2007;1114:428–433. [DOI] [PubMed] [Google Scholar]
- 164. Armoza A, Haim Y, Bashiri A, Wolak T, Paran E. Tomato extract and the carotenoids lycopene and lutein improve endothelial function and attenuate inflammatory NF-kappaB signaling in endothelial cells. J Hypertens. 2013;31(3):521–529; discussion 529. [DOI] [PubMed] [Google Scholar]
- 165. Fletcher NM, Awonuga AO, Saed MG, Abu-Soud HM, Diamond MP, Saed GM. Lycopene, a powerful antioxidant, significantly reduces the development of the adhesion phenotype. Syst Biol Reprod Med. 2014;60(1):14–20. [DOI] [PubMed] [Google Scholar]
- 166. Lee W, Ku SK, Bae JW, Bae JS. Inhibitory effects of lycopene on HMGB1-mediated pro-inflammatory responses in both cellular and animal models. Food Chem Toxicol. 2012;50(6):1826–1833. [DOI] [PubMed] [Google Scholar]
- 167. Zhu J, Wang CG, Xu YG. Lycopene attenuates endothelial dysfunction in streptozotocin-induced diabetic rats by reducing oxidative stress. Pharm Biol. 2011;49(11):1144–1149. [DOI] [PubMed] [Google Scholar]
- 168. Bae JW, Bae JS. Barrier protective effects of lycopene in human endothelial cells. Inflamm Res. 2011;60(8):751–758. [DOI] [PubMed] [Google Scholar]
- 169. Rissanen TH, Voutilainen S, Nyyssonen K, et al. Low serum lycopene concentration is associated with an excess incidence of acute coronary events and stroke: the Kuopio Ischaemic Heart Disease Risk Factor Study. Br J Nutr. 2001;85(6):749–754. [DOI] [PubMed] [Google Scholar]
- 170. Karppi J, Laukkanen JA, Makikallio TH, Kurl S. Low serum lycopene and beta-carotene increase risk of acute myocardial infarction in men. Eur J Public Health. 2012;22(6):835–840. [DOI] [PubMed] [Google Scholar]
- 171. Burton-Freeman B, Sesso HD. Whole food versus supplement: comparing the clinical evidence of tomato intake and lycopene supplementation on cardiovascular risk factors. Adv Nutr. 2014;5(5):457–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Friedman M. Anticarcinogenic, cardioprotective, and other health benefits of tomato compounds lycopene, alpha-tomatine, and tomatidine in pure form and in fresh and processed tomatoes. J Agric Food Chem. 2013;61(40):9534–9550. [DOI] [PubMed] [Google Scholar]
- 173. Gajendragadkar PR, Hubsch A, Maki-Petaja KM, Serg M, Wilkinson IB, Cheriyan J. Effects of oral lycopene supplementation on vascular function in patients with cardiovascular disease and healthy volunteers: a randomised controlled trial. PLoS One. 2014;9(6):e99070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Wolak T, Paran E. Can carotenoids attenuate vascular aging? Vascul Pharmacol. 2013;59(3-4):63–66. [DOI] [PubMed] [Google Scholar]
- 175. Ried K, Fakler P. Protective effect of lycopene on serum cholesterol and blood pressure: meta-analyses of intervention trials. Maturitas. 2011;68(4):299–310. [DOI] [PubMed] [Google Scholar]
- 176. Lei X, Lei L, Zhang Z, Cheng Y. Neuroprotective effects of lycopene pretreatment on transient global cerebral ischemiareperfusion in rats: the role of the Nrf2/HO1 signaling pathway. Mol Med Rep. 2016;13(1):412–418. [DOI] [PubMed] [Google Scholar]
- 177. Yin Q, Ma Y, Hong Y, et al. Lycopene attenuates insulin signaling deficits, oxidative stress, neuroinflammation, and cognitive impairment in fructose-drinking insulin resistant rats. Neuropharmacology. 2014;86:389–396. [DOI] [PubMed] [Google Scholar]
- 178. Prakash A, Kumar A. Implicating the role of lycopene in restoration of mitochondrial enzymes and BDNF levels in beta-amyloid induced Alzheimers disease. Eur J Pharmacol. 2014;741:104–111. [DOI] [PubMed] [Google Scholar]
- 179. Yi F, He X, Wang D. Lycopene protects against MPP(+)-induced cytotoxicity by maintaining mitochondrial function in SH-SY5Y cells. Neurochem Res. 2013;38(8):1747–1757. [DOI] [PubMed] [Google Scholar]
- 180. Karppi J, Laukkanen JA, Sivenius J, Ronkainen K, Kurl S. Serum lycopene decreases the risk of stroke in men: a population-based follow-up study. Neurology. 2012;79(15):1540–1547. [DOI] [PubMed] [Google Scholar]
- 181. Veldink JH, Kalmijn S, Groeneveld GJ, et al. Intake of polyunsaturated fatty acids and vitamin E reduces the risk of developing amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2007;78(4):367–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Zhang SM, Hernan MA, Olek MJ, Spiegelman D, Willett WC, Ascherio A. Intakes of carotenoids, vitamin C, and vitamin E and MS risk among two large cohorts of women. Neurology. 2001;57(1):75–80. [DOI] [PubMed] [Google Scholar]
- 183. Mishra S, Palanivelu K. The effect of curcumin (turmeric) on Alzheimer’s disease: an overview. Ann Indian Acad Neurol. 2008;11(1):13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Ferrucci V, Boffa I, De Masi G, Zollo M. Natural compounds for pediatric cancer treatment. Naunyn Schmiedebergs Arch Pharmacol. 2015;389(2):131–149. [DOI] [PubMed] [Google Scholar]
- 185. Maru GB, Hudlikar RR, Kumar G, Gandhi K, Mahimkar MB. Understanding the molecular mechanisms of cancer prevention by dietary phytochemicals: from experimental models to clinical trials. World J Biol Chem. 2016;7(1):88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Pavan AR, Silva GD, Jornada DH, et al. Unraveling the anticancer effect of curcumin and resveratrol. Nutrients. 2016;8(11):E628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Singh AK, Sharma N, Ghosh M, Park YH, Jeong DK. Emerging importance of dietary phytochemicals in fight against cancer: role in targeting cancer stem cells. Crit Rev Food Sci Nutr. 2017;57(16):3449–3463. [DOI] [PubMed] [Google Scholar]
- 188. Yarla NS, Bishayee A, Sethi G, et al. Targeting arachidonic acid pathway by natural products for cancer prevention and therapy. Semin Cancer Biol. 2016;40-44:48–81. [DOI] [PubMed] [Google Scholar]
- 189. Grundman M, Grundman M, Delaney P. Antioxidant strategies for Alzheimer’s disease. Proc Nutr Soc. 2002;61(2):191–202. [DOI] [PubMed] [Google Scholar]
- 190. Ahmed T, Gilani AH. Therapeutic potential of turmeric in Alzheimer’s disease: curcumin or curcuminoids? Phytother Res. 2014;28(4):517–525. [DOI] [PubMed] [Google Scholar]
- 191. Calcul L, Zhang B, Jinwal UK, Dickey CA, Baker BJ. Natural products as a rich source of tau-targeting drugs for Alzheimer’s disease. Future Med Chem. 2012;4(13):1751–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Candore G, Bulati M, Caruso C, et al. Inflammation, cytokines, immune response, apolipoprotein E, cholesterol, and oxidative stress in Alzheimer disease: therapeutic implications. Rejuvenation Res. 2010;13(2-3):301–313. [DOI] [PubMed] [Google Scholar]
- 193. Chin D, Huebbe P, Pallauf K, Rimbach G. Neuroprotective properties of curcumin in Alzheimer’s disease – merits and limitations. Curr Med Chem. 2013;20(32):3955–3985. [DOI] [PubMed] [Google Scholar]
- 194. Goozee KG, Shah TM, Sohrabi HR, et al. Examining the potential clinical value of curcumin in the prevention and diagnosis of Alzheimer’s disease. Br J Nutr. 2016;115(3):449–465. [DOI] [PubMed] [Google Scholar]
- 195. Jayasena T, Poljak A, Smythe G, Braidy N, Munch G, Sachdev P. The role of polyphenols in the modulation of sirtuins and other pathways involved in Alzheimer’s disease. Ageing Res Rev. 2013;12(4):867–883. [DOI] [PubMed] [Google Scholar]
- 196. Kim MH, Kim SH, Yang WM. Mechanisms of action of phytochemicals from medicinal herbs in the treatment of Alzheimer’s disease. Planta Med. 2014;80(15):1249–1258. [DOI] [PubMed] [Google Scholar]
- 197. Morales I, Guzman-Martinez L, Cerda-Troncoso C, Farias GA, Maccioni RB. Neuroinflammation in the pathogenesis of Alzheimer’s disease. A rational framework for the search of novel therapeutic approaches. Front Cell Neurosci. 2014;8:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Pluta R, Bogucka-Kocka A, Ulamek-Koziol M, et al. Neurogenesis and neuroprotection in postischemic brain neurodegeneration with Alzheimer phenotype: is there a role for curcumin? Folia Neuropathol. 2015;53(2):89–99. [DOI] [PubMed] [Google Scholar]
- 199. Racchi M, Uberti D, Govoni S, et al. Alzheimer’s disease: new diagnostic and therapeutic tools. Immun Ageing. 2008;5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Villaflores OB, Chen YJ, Chen CP, Yeh JM, Wu TY. Curcuminoids and resveratrol as anti-Alzheimer agents. Taiwan J Obstet Gynecol. 2012;51(4):515–525. [DOI] [PubMed] [Google Scholar]
- 201. Calabrese V, Cornelius C, Mancuso C, et al. Cellular stress response: a novel target for chemoprevention and nutritional neuroprotection in aging, neurodegenerative disorders and longevity. Neurochem Res. 2008;33(12):2444–2471. [DOI] [PubMed] [Google Scholar]
- 202. Ono K, Hirohata M, Yamada M. Alpha-synuclein assembly as a therapeutic target of Parkinson’s disease and related disorders. Curr Pharm Des. 2008;14(30):3247–3266. [DOI] [PubMed] [Google Scholar]
- 203. Sikora E, Bielak-Zmijewska A, Mosieniak G, Piwocka K. The promise of slow down ageing may come from curcumin. Curr Pharm Des. 2010;16(7):884–892. [DOI] [PubMed] [Google Scholar]
- 204. Sikora E, Scapagnini G, Barbagallo M. Curcumin, inflammation, ageing and age-related diseases. Immun Ageing. 2010;7(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Craggs L, Kalaria RN. Revisiting dietary antioxidants, neurodegeneration and dementia. Neuroreport. 2011;22(1):1–3. [DOI] [PubMed] [Google Scholar]
- 206. Mattson MP, Son TG, Camandola S. Viewpoint: mechanisms of action and therapeutic potential of neurohormetic phytochemicals. Dose Response. 2007;5(3):174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Tian M, Zhang X, Wang L, Li Y. Curcumin induces ABCA1 expression and apolipoprotein A-I-mediated cholesterol transmembrane in the chronic cerebral hypoperfusion aging rats. Am J Chin Med. 2013;41(5):1027–1042. [DOI] [PubMed] [Google Scholar]
- 208. Duarte VM, Han E, Veena MS, et al. Curcumin enhances the effect of cisplatin in suppression of head and neck squamous cell carcinoma via inhibition of IKKbeta protein of the NFkappaB pathway. Mol Cancer Ther. 2010;9(10):2665–2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Chen J, Wang G, Wang L, Kang J, Wang J. Curcumin p38-dependently enhances the anticancer activity of valproic acid in human leukemia cells. Eur J Pharm Sci. 2010;41(2):210–218. [DOI] [PubMed] [Google Scholar]
- 210. Su CC, Wang MJ, Chiu TL. The anti-cancer efficacy of curcumin scrutinized through core signaling pathways in glioblastoma. Int J Mol Med. 2010;26(2):217–224. [DOI] [PubMed] [Google Scholar]
- 211. Zhao JY, Lu N, Yan Z, Wang N. SAHA and curcumin combinations co-enhance histone acetylation in human cancer cells but operate antagonistically in exerting cytotoxic effects. J Asian Nat Prod Res. 2010;12(5):335–348. [DOI] [PubMed] [Google Scholar]
- 212. Wang M, Ruan Y, Chen Q, Li S, Wang Q, Cai J. Curcumin induced HepG2 cell apoptosis-associated mitochondrial membrane potential and intracellular free Ca(2+) concentration. Eur J Pharmacol. 2011;650(1):41–47. [DOI] [PubMed] [Google Scholar]
- 213. Lopez-Jornet P, Gomez-Garcia F, Garcia Carrillo N, Valle-Rodriguez E, Xerafin A, Vicente-Ortega V. Radioprotective effects of lycopene and curcumin during local irradiation of parotid glands in Sprague Dawley rats. Br J Oral Maxillofac Surg. 2016;54(3):275–279. [DOI] [PubMed] [Google Scholar]
- 214. Wang X, Xia X, Xu C, et al. Ultrasound-induced cell death of nasopharyngeal carcinoma cells in the presence of curcumin. Integr Cancer Ther. 2011;10(1):70–76. [DOI] [PubMed] [Google Scholar]
- 215. Bar-Sela G, Epelbaum R, Schaffer M. Curcumin as an anti-cancer agent: review of the gap between basic and clinical applications. Curr Med Chem. 2010;17(3):190–197. [DOI] [PubMed] [Google Scholar]
- 216. Prasad S, Tyagi AK, Aggarwal BB. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: the golden pigment from golden spice. Cancer Res Treat. 2014;46(1):2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Sharma RA, McLelland HR, Hill KA, et al. Pharmacodynamic and pharmacokinetic study of oral curcuma extract in patients with colorectal cancer. Clin Cancer Res. 2001;7(7):1894–1900. [PubMed] [Google Scholar]
- 218. Farzaei MH, Bahramsoltani R, Rahimi R. Phytochemicals as adjunctive with conventional anticancer therapies. Curr Pharm Des. 2016;22(27):4201–4218. [DOI] [PubMed] [Google Scholar]
- 219. Bala K, Tripathy BC, Sharma D. Neuroprotective and anti-ageing effects of curcumin in aged rat brain regions. Biogerontology. 2006;7(2):81–89. [DOI] [PubMed] [Google Scholar]
- 220. Stone J, Johnstone DM, Mitrofanis J, O’Rourke M. The mechanical cause of age-related dementia (Alzheimer’s disease): the brain is destroyed by the pulse. J Alzheimers Dis. 2015;44(2):355–373. [DOI] [PubMed] [Google Scholar]
- 221. Nelson KM, Dahlin JL, Bisson J, Graham J, Pauli GF, Walters MA. The essential medicinal chemistry of curcumin. J Med Chem. 2017;60(5):1620–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Willard P. The Secrets of Saffron: The Vagabond Life of the World’s Most Seductive Spice. Boston, MA: Beacon Press; 2001. [Google Scholar]
- 223. Humphries J. The Essential Saffron Companion. Berkeley, CA: Ten Speed Press; 1998. [Google Scholar]
- 224. Javadi B, Sahebkar A, Emami SA. A survey on saffron in major islamic traditional medicine books. Iran J Basic Med Sci. 2013;16(1):1–11. [PMC free article] [PubMed] [Google Scholar]
- 225. Nair SC, Pannikar B, Panikkar KR. Antitumour activity of saffron (Crocus sativus). Cancer Lett. 1991;57(2):109–114. [DOI] [PubMed] [Google Scholar]
- 226. Giaccio M. Crocetin from saffron: an active component of an ancient spice. Crit Rev Food Sci Nutr. 2004;44(3):155–172. [DOI] [PubMed] [Google Scholar]
- 227. Maccarone R, Di Marco S, Bisti S. Saffron supplement maintains morphology and function after exposure to damaging light in mammalian retina. Invest Ophthalmol Vis Sci. 2008;49(3):1254–1261. [DOI] [PubMed] [Google Scholar]
- 228. Di Marco F, Di Paolo M, Romeo S, et al. Combining neuroprotectants in a model of retinal degeneration: no additive benefit. PLoS One. 2014;9(6):e100389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Abdullaev FI, Riveron-Negrete L, Caballero-Ortega H, et al. Use of in vitro assays to assess the potential antigenotoxic and cytotoxic effects of saffron (Crocus sativus L.). Toxicol In Vitro. 2003;17(5-6):731–736. [DOI] [PubMed] [Google Scholar]
- 230. Kanakis CD, Tarantilis PA, Pappas C, Bariyanga J, Tajmir-Riahi HA, Polissiou MG. An overview of structural features of DNA and RNA complexes with saffron compounds: models and antioxidant activity. J Photochem Photobiol B. 2009;95(3):204–212. [DOI] [PubMed] [Google Scholar]
- 231. Premkumar K, Thirunavukkarasu C, Abraham SK, Santhiya ST, Ramesh A. Protective effect of saffron (Crocus sativus L.) aqueous extract against genetic damage induced by anti-tumor agents in mice. Hum Exp Toxicol. 2006;25(2):79–84. [DOI] [PubMed] [Google Scholar]
- 232. Purushothuman S, Nandasena C, Peoples CL, et al. Saffron pre-treatment offers neuroprotection to Nigral and retinal dopaminergic cells of MPTP-treated mice. J Parkinsons Dis. 2013;3(1):77–83. [DOI] [PubMed] [Google Scholar]
- 233. Nair SC, Kurumboor SK, Hasegawa JH. Saffron chemoprevention in biology and medicine: a review. Cancer Biother. 1995;10(4):257–264. [DOI] [PubMed] [Google Scholar]
- 234. Abdullaev FI, Espinosa-Aguirre JJ. Biomedical properties of saffron and its potential use in cancer therapy and chemoprevention trials. Cancer Detect Prev. 2004;28(6):426–432. [DOI] [PubMed] [Google Scholar]
- 235. El-Kharrag R, Amin A, Hisaindee S, Greish Y, Karam SM. Development of a therapeutic model of precancerous liver using crocin-coated magnetite nanoparticles. Int J Oncol. 2017;50(1):212–222. [DOI] [PubMed] [Google Scholar]
- 236. Malaekeh-Nikouei B, Mousavi SH, Shahsavand S, Mehri S, Nassirli H, Moallem SA. Assessment of cytotoxic properties of safranal and nanoliposomal safranal in various cancer cell lines. Phytother Res. 2013;27(12):1868–1873. [DOI] [PubMed] [Google Scholar]
- 237. Rastgoo M, Hosseinzadeh H, Alavizadeh H, Abbasi A, Ayati Z, Jaafari MR. Antitumor activity of PEGylated nanoliposomes containing crocin in mice bearing C26 colon carcinoma. Planta Med. 2013;79(6):447–451. [DOI] [PubMed] [Google Scholar]
- 238. Mousavi SH, Moallem SA, Mehri S, Shahsavand S, Nassirli H, Malaekeh-Nikouei B. Improvement of cytotoxic and apoptogenic properties of crocin in cancer cell lines by its nanoliposomal form. Pharm Biol. 2011;49(10):1039–1045. [DOI] [PubMed] [Google Scholar]
- 239. Mahdizadeh S, Karimi G, Behravan J, Arabzadeh S, Lage H, Kalalinia F. Crocin suppresses multidrug resistance in MRP overexpressing ovarian cancer cell line. Daru. 2016;24(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Eid SY, El-Readi MZ, Wink M. Carotenoids reverse multidrug resistance in cancer cells by interfering with ABC-transporters. Phytomedicine. 2012;19(11):977–987. [DOI] [PubMed] [Google Scholar]
- 241. Panchangam SS, Vahedi M, Megha MJ, et al. Saffron ‘omics’: the challenges of integrating omic technologies. Avicenna J Phytomed. 2016;6(6):604–620. [PMC free article] [PubMed] [Google Scholar]
- 242. Hosseini A, Mousavi SH, Ghanbari A, et al. Effect of saffron on liver metastases in patients suffering from cancers with liver metastases: a randomized, double blind, placebo-controlled clinical trial. Avicenna J Phytomed. 2015;5(5):434–440. [PMC free article] [PubMed] [Google Scholar]
- 243. Falsini B, Piccardi M, Minnella A, et al. Saffron supplementation improves retinal flicker sensitivity in early age-related macular degeneration. Invest Ophthalmol Vis Sci. 2010;51(12):6118–6124. [DOI] [PubMed] [Google Scholar]
- 244. Akhondzadeh S, Sabet MS, Harirchian MH, et al. Original article. Saffron in the treatment of patients with mild to moderate Alzheimer’s disease: a 16-week, randomized and placebo-controlled trial. J Clin Pharm Ther. 2010;35(5):581–588. [DOI] [PubMed] [Google Scholar]
- 245. Akhondzadeh S, Shafiee Sabet M, Harirchian MH, et al. A 22-week, multicenter, randomized, double-blind controlled trial of Crocus sativus in the treatment of mild-to-moderate Alzheimer’s disease. Psychopharmacology (Berl). 2009;207(4):637–643. [DOI] [PubMed] [Google Scholar]
- 246. Akhondzadeh S, Tahmacebi-Pour N, Noorbala AA, et al. Crocus sativus L. in the treatment of mild to moderate depression: a double-blind, randomized and placebo-controlled trial. Phytother Res. 2005;19(2):148–151. [DOI] [PubMed] [Google Scholar]
- 247. Hausenblas HA, Heekin K, Mutchie HL, Anton S. A systematic review of randomized controlled trials examining the effectiveness of saffron (Crocus sativus L.) on psychological and behavioral outcomes. J Integr Med. 2015;13(4):231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Noorbala AA, Akhondzadeh S, Tahmacebi-Pour N, Jamshidi AH. Hydro-alcoholic extract of Crocus sativus L. versus fluoxetine in the treatment of mild to moderate depression: a double-blind, randomized pilot trial. J Ethnopharmacol. 2005;97(2):281–284. [DOI] [PubMed] [Google Scholar]
- 249. Schmidt M, Betti G, Hensel A. Saffron in phytotherapy: pharmacology and clinical uses. Wien Med Wochenschr. 2007;157(13-14):315–319. [DOI] [PubMed] [Google Scholar]
- 250. Piccardi M, Marangoni D, Minnella AM, et al. A longitudinal follow-up study of saffron supplementation in early age-related macular degeneration: sustained benefits to central retinal function. Evid Based Complement Alternat Med. 2012;2012:429124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Marangoni D, Falsini B, Piccardi M, et al. Functional effect of saffron supplementation and risk genotypes in early age-related macular degeneration: a preliminary report. J Transl Med. 2013;11:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Gril J, Jullien D, Bardet J, Yamamoto H. Tree growth stress and related problems. J Wood Sci. 2017;63(5):411–432. [Google Scholar]
- 253. Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444(7117):337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Dunstan WR, Henry TA. The nature and origin of the poison of Lotus arabicus. Proc R Soc Lond. 1901;68(442-450):374–378. [Google Scholar]
- 255. Calabrese EJ. Preconditioning is hormesis, part II: how the conditioning dose mediates protection: dose optimization within temporal and mechanistic frameworks. Pharmacol Res. 2016;110:265–275. [DOI] [PubMed] [Google Scholar]
- 256. Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2017;39:46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Longo VD, Panda S. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab. 2016;23(6):1048–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Walford RL, Mock D, Verdery R, MacCallum T. Calorie restriction in biosphere 2: alterations in physiologic, hematologic, hormonal, and biochemical parameters in humans restricted for a 2-year period. J Gerontol A Biol Sci Med Sci. 2002;57(6):B211–B224. [DOI] [PubMed] [Google Scholar]
- 259. Redman LM, Smith SR, Burton JH, Martin CK, Il’yasova D, Ravussin E. Metabolic slowing and reduced oxidative damage with sustained caloric restriction support the rate of living and oxidative damage theories of aging. Cell Metab. 2018;27(4):805–815.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Nelson M, Gray K, Allen JP. Group dynamics challenges: insights from biosphere 2 experiments. Life Sci Space Res (Amst). 2015;6:79–86. [DOI] [PubMed] [Google Scholar]
- 261. Blagosklonny MV. Koschei the immortal and anti-aging drugs. Cell Death Dis. 2014;5:e1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Roth GS, Ingram DK. Manipulation of health span and function by dietary caloric restriction mimetics. Ann N Y Acad Sci. 2016;1363(1):5–10. [DOI] [PubMed] [Google Scholar]
- 263. Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci U S A. 2004;101(17):6659–6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Kyriazis M. Clinical anti-aging hormetic strategies. Rejuvenation Res. 2005;8(2):96–100. [DOI] [PubMed] [Google Scholar]
- 265. Martin B, Mattson MP, Maudsley S. Caloric restriction and intermittent fasting: two potential diets for successful brain aging. Ageing Res Rev. 2006;5(3):332–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Handschin C. Caloric restriction and exercise “mimetics”: ready for prime time? Pharmacol Res. 2016;103:158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Ingram DK, Roth GS. Calorie restriction mimetics: can you have your cake and eat it, too? Ageing Res Rev. 2015;20:46–62. [DOI] [PubMed] [Google Scholar]
- 268. Timmers S, Konings E, Bilet L, et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14(5):612–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Le Couteur DG, Solon-Biet S, Wahl D, et al. New horizons: dietary protein, ageing and the okinawan ratio. Age Ageing. 2016;45(4):443–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74(5):1124–1136. [DOI] [PubMed] [Google Scholar]
- 271. Reimer KA, Murry CE, Jennings RB. Cardiac adaptation to ischemia. Ischemic preconditioning increases myocardial tolerance to subsequent ischemic episodes. Circulation. 1990;82(6):2266–2268. [DOI] [PubMed] [Google Scholar]
- 272. Brooks MJ, Andrews DT. Molecular mechanisms of ischemic conditioning: translation into patient outcomes. Future Cardiol. 2013;9(4):549–568. [DOI] [PubMed] [Google Scholar]
- 273. Abete P, Ferrara N, Cacciatore F, et al. Angina-induced protection against myocardial infarction in adult and elderly patients: a loss of preconditioning mechanism in the aging heart? J Am Coll Cardiol. 1997;30(4):947–954. [DOI] [PubMed] [Google Scholar]
- 274. Abete P, Ferrara N, Cacciatore F, et al. High level of physical activity preserves the cardioprotective effect of preinfarction angina in elderly patients. J Am Coll Cardiol. 2001;38(5):1357–1365. [DOI] [PubMed] [Google Scholar]
- 275. Abete P, Napoli C, Rengo F. Loss of cardioprotective effects of preinfarction angina in elderly but not in adult patients. Am J Cardiol. 2001;88(6):721. [DOI] [PubMed] [Google Scholar]
- 276. Kloner RA, Jennings RB. Consequences of brief ischemia: stunning, preconditioning, and their clinical implications: part 2. Circulation. 2001;104(25):3158–3167. [DOI] [PubMed] [Google Scholar]
- 277. Barone FC, White RF, Spera PA, et al. Ischemic preconditioning and brain tolerance: temporal histological and functional outcomes, protein synthesis requirement, and interleukin-1 receptor antagonist and early gene expression. Stroke. 1998;29(9):1937–1950; discussion 1950-1931. [DOI] [PubMed] [Google Scholar]
- 278. Wegener S, Gottschalk B, Jovanovic V, et al. Transient ischemic attacks before ischemic stroke: preconditioning the human brain? A multicenter magnetic resonance imaging study. Stroke. 2004;35(3):616–621. [DOI] [PubMed] [Google Scholar]
- 279. Zhu Y, Ohlemiller KK, McMahan BK, Gidday JM. Mouse models of retinal ischemic tolerance. Invest Ophthalmol Vis Sci. 2002;43(6):1903–1911. [PubMed] [Google Scholar]
- 280. Li Y, Roth S, Laser M, Ma JX, Crosson CE. Retinal preconditioning and the induction of heat-shock protein 27. Invest Ophthalmol Vis Sci. 2003;44(3):1299–1304. [DOI] [PubMed] [Google Scholar]
- 281. Kawahara N, Wang Y, Mukasa A, et al. Genome-wide gene expression analysis for induced ischemic tolerance and delayed neuronal death following transient global ischemia in rats. J Cereb Blood Flow Metab. 2004;24(2):212–223. [DOI] [PubMed] [Google Scholar]
- 282. Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P. Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation. 1993;87(3):893–899. [DOI] [PubMed] [Google Scholar]
- 283. Jensen HA, Loukogeorgakis S, Yannopoulos F, et al. Remote ischemic preconditioning protects the brain against injury after hypothermic circulatory arrest. Circulation. 2011;123(7):714–721. [DOI] [PubMed] [Google Scholar]
- 284. Harkin DW, Barros D’Sa AA, McCallion K, Hoper M, Campbell FC. Ischemic preconditioning before lower limb ischemia–reperfusion protects against acute lung injury. J Vasc Surg. 2002;35(6):1264–1273. [DOI] [PubMed] [Google Scholar]
- 285. Candilio L, Malik A, Hausenloy DJ. Protection of organs other than the heart by remote ischemic conditioning. J Cardiovasc Med (Hagerstown). 2013;14(3):193–205. [DOI] [PubMed] [Google Scholar]
- 286. Pickard JM, Botker HE, Crimi G, et al. Remote ischemic conditioning: from experimental observation to clinical application: report from the 8th Biennial Hatter Cardiovascular Institute Workshop. Basic Res Cardiol. 2015;110(1):453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Zhang X, Jizhang Y, Xu X, et al. Protective effects of remote ischemic conditioning against ischemia/reperfusion-induced retinal injury in rats. Vis Neurosci. 2014;31(3):245–252. [DOI] [PubMed] [Google Scholar]
- 288. Liu X, Sha O, Cho EY. Remote ischemic postconditioning promotes the survival of retinal ganglion cells after optic nerve injury. J Mol Neurosci. 2013;51(3):639–646. [DOI] [PubMed] [Google Scholar]
- 289. Brandli A, Johnstone DM, Stone J. Remote ischemic preconditioning protects retinal photoreceptors: evidence from a rat model of light-induced photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2016;57(13):5302–5313. [DOI] [PubMed] [Google Scholar]
- 290. Meng R, Asmaro K, Meng L, et al. Upper limb ischemic preconditioning prevents recurrent stroke in intracranial arterial stenosis. Neurology. 2012;79(18):1853–1861. [DOI] [PubMed] [Google Scholar]
- 291. England TJ, Hedstrom A, O’Sullivan S, et al. RECAST (Remote Ischemic Conditioning After Stroke Trial): a pilot randomized placebo controlled phase II trial in acute ischemic stroke. Stroke. 2017;48(5):1412–1415. [DOI] [PubMed] [Google Scholar]
- 292. Laiwalla AN, Ooi YC, Liou R, Gonzalez NR. Matched cohort analysis of the effects of limb remote ischemic conditioning in patients with aneurysmal subarachnoid hemorrhage. Transl Stroke Res. 2016;7(1):42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Kolbenschlag J, Sogorski A, Kapalschinski N, et al. Remote ischemic conditioning improves blood flow and oxygen saturation in pedicled and free surgical flaps. Plast Reconstr Surg. 2016;138(5):1089–1097. [DOI] [PubMed] [Google Scholar]
- 294. Hu Q, Luo W, Huang L, Huang R, Chen R, Gao Y. Multiorgan protection of remote ischemic perconditioning in valve replacement surgery. J Surg Res. 2016;200(1):13–20. [DOI] [PubMed] [Google Scholar]
- 295. White SK, Frohlich GM, Sado DM, et al. Remote ischemic conditioning reduces myocardial infarct size and edema in patients with ST-segment elevation myocardial infarction. JACC Cardiovasc Interv. 2015;8(1 Pt B):178–188. [DOI] [PubMed] [Google Scholar]
- 296. Verouhis D, Sorensson P, Gourine A, et al. Effect of remote ischemic conditioning on infarct size in patients with anterior ST-elevation myocardial infarction. Am Heart J. 2016;181:66–73. [DOI] [PubMed] [Google Scholar]
- 297. Pinaud F, Corbeau JJ, Baufreton C, et al. Remote ischemic preconditioning in aortic valve surgery: results of a randomized controlled study. J Cardiol. 2016;67(1):36–41. [DOI] [PubMed] [Google Scholar]
- 298. Nicholson ML, Pattenden CJ, Barlow AD, Hunter JP, Lee G, Hosgood SA. A double blind randomized clinical trial of remote ischemic conditioning in live donor renal transplantation. Medicine (Baltimore). 2015;94(31):e1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Benstoem C, Stoppe C, Liakopoulos OJ, et al. Remote ischaemic preconditioning for coronary artery bypass grafting (with or without valve surgery). Cochrane Database Syst Rev. 2017;(5):CD011719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Nikkola E, Laiwalla A, Ko A, et al. Remote ischemic conditioning alters methylation and expression of cell cycle genes in aneurysmal subarachnoid hemorrhage. Stroke. 2015;46(9):2445–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Kleinbongard P, Skyschally A, Heusch G. Cardioprotection by remote ischemic conditioning and its signal transduction. Pflugers Arch. 2017;469(2):159–181. [DOI] [PubMed] [Google Scholar]
- 302. Barrington JH, Chrismas BCR, Gibson OR, et al. Hypoxic air inhalation and ischemia interventions both elicit preconditioning which attenuate subsequent cellular stress in vivo following blood flow occlusion and reperfusion. Front Physiol. 2017;8:560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Baillieul S, Chacaroun S, Doutreleau S, Detante O, Pepin JL, Verges S. Hypoxic conditioning and the central nervous system: a new therapeutic opportunity for brain and spinal cord injuries? Exp Biol Med (Maywood). 2017;242(11):1198–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Zhu LL, Zhao T, Li HS, et al. Neurogenesis in the adult rat brain after intermittent hypoxia. Brain Res. 2005;1055(1-2):1–6. [DOI] [PubMed] [Google Scholar]
- 305. Tsai YW, Yang YR, Wang PS, Wang RY. Intermittent hypoxia after transient focal ischemia induces hippocampal neurogenesis and c-Fos expression and reverses spatial memory deficits in rats. PLoS One. 2011;6(8):e24001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Qiao Y, Liu Z, Yan X, Luo C. Effect of intermittent hypoxia on neuro-functional recovery post brain ischemia in mice. J Mol Neurosci. 2015;55(4):923–930. [DOI] [PubMed] [Google Scholar]
- 307. Leconte C, Tixier E, Freret T, et al. Delayed hypoxic postconditioning protects against cerebral ischemia in the mouse. Stroke. 2009;40(10):3349–3355. [DOI] [PubMed] [Google Scholar]
- 308. Miller BA, Perez RS, Shah AR, Gonzales ER, Park TS, Gidday JM. Cerebral protection by hypoxic preconditioning in a murine model of focal ischemia–reperfusion. Neuroreport. 2001;12(8):1663–1669. [DOI] [PubMed] [Google Scholar]
- 309. Bernaudin M, Nedelec AS, Divoux D, MacKenzie ET, Petit E, Schumann-Bard P. Normobaric hypoxia induces tolerance to focal permanent cerebral ischemia in association with an increased expression of hypoxia-inducible factor-1 and its target genes, erythropoietin and VEGF, in the adult mouse brain. J Cereb Blood Flow Metab. 2002;22(4):393–403. [DOI] [PubMed] [Google Scholar]
- 310. Lin AM, Dung SW, Chen CF, Chen WH, Ho LT. Hypoxic preconditioning prevents cortical infarction by transient focal ischemia–reperfusion. Ann N Y Acad Sci. 2003;993:168–178; discussion 195-166. [DOI] [PubMed] [Google Scholar]
- 311. Bernaudin M, Marti HH, Roussel S, et al. A potential role for erythropoietin in focal permanent cerebral ischemia in mice. J Cereb Blood Flow Metab. 1999;19(6):643–651. [DOI] [PubMed] [Google Scholar]
- 312. Ruscher K, Freyer D, Karsch M, et al. Erythropoietin is a paracrine mediator of ischemic tolerance in the brain: evidence from an in vitro model. J Neurosci. 2002;22(23):10291–10301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Taie S, Ono J, Iwanaga Y, et al. Hypoxia-inducible factor-1 alpha has a key role in hypoxic preconditioning. J Clin Neurosci. 2009;16(8):1056–1060. [DOI] [PubMed] [Google Scholar]
- 314. Lavie P, Lavie L. Unexpected survival advantage in elderly people with moderate sleep apnoea. J Sleep Res. 2009;18(4):397–403. [DOI] [PubMed] [Google Scholar]
- 315. Band PR, Le ND, Fang R, et al. Cohort study of air Canada pilots: mortality, cancer incidence, and leukemia risk. Am J Epidemiol. 1996;143(2):137–143. [DOI] [PubMed] [Google Scholar]
- 316. Paridou A, Velonakis E, Langner I, Zeeb H, Blettner M, Tzonou A. Mortality among pilots and cabin crew in Greece, 1960–1997. Int J Epidemiol. 2003;32(2):244–247. [DOI] [PubMed] [Google Scholar]
- 317. Linnersjo A, Brodin LA, Andersson C, Alfredsson L, Hammar N. Low mortality and myocardial infarction incidence among flying personnel during working career and beyond. Scand J Work Environ Health. 2011;37(3):219–226. [DOI] [PubMed] [Google Scholar]
- 318. Berryman JW. Motion and rest: galen on exercise and health. Lancet. 2012;380(9838):210–211. [DOI] [PubMed] [Google Scholar]
- 319. Tipton CM. The history of “Exercise Is Medicine” in ancient civilizations. Adv Physiol Educ. 2014;38(2):109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Tipton CM. Susruta of India, an unrecognized contributor to the history of exercise physiology. J Appl Physiol (1985). 2008;104(6):1553–1556. [DOI] [PubMed] [Google Scholar]
- 321. Huh JY. The role of exercise-induced myokines in regulating metabolism. Arch Pharm Res. 2018;41(1):14–29. [DOI] [PubMed] [Google Scholar]
- 322. So B, Kim HJ, Kim J, Song W. Exercise-induced myokines in health and metabolic diseases. Integr Med Res. 2014;3(4):172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Newton RU, Galvao DA. Exercise in prevention and management of cancer. Curr Treat Opt Oncol. 2008;9(2-3):135–146. [DOI] [PubMed] [Google Scholar]
- 324. Vashistha V, Singh B, Kaur S, Prokop LJ, Kaushik D. The effects of exercise on fatigue, quality of life, and psychological function for men with prostate cancer: systematic review and meta-analyses. Eur Urol Focus. 2016;2(3):284–295. [DOI] [PubMed] [Google Scholar]
- 325. Baguley BJ, Bolam KA, Wright ORL, Skinner TL. The effect of nutrition therapy and exercise on cancer-related fatigue and quality of life in men with prostate cancer: a systematic review. Nutrients. 2017;9(9):E1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326. Yunfeng G, Weiyang H, Xueyang H, Yilong H, Xin G. Exercise overcome adverse effects among prostate cancer patients receiving androgen deprivation therapy: an update meta-analysis. Medicine (Baltimore). 2017;96(27):e7368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Adraskela K, Veisaki E, Koutsilieris M, Philippou A. Physical exercise positively influences breast cancer evolution. Clin Breast Cancer. 2017;17(6):408–417. [DOI] [PubMed] [Google Scholar]
- 328. Friedenreich CM, Shaw E, Neilson HK, Brenner DR. Epidemiology and biology of physical activity and cancer recurrence. J Mol Med (Berl). 2017;95(10):1029–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Pedersen L, Idorn M, Olofsson GH, et al. Voluntary running suppresses tumor growth through epinephrine- and IL-6-dependent NK cell mobilization and redistribution. Cell Metab. 2016;23(3):554–562. [DOI] [PubMed] [Google Scholar]
- 330. Schmidt T, van Mackelenbergh M, Wesch D, Mundhenke C. Physical activity influences the immune system of breast cancer patients. J Cancer Res Ther. 2017;13(3):392–398. [DOI] [PubMed] [Google Scholar]
- 331. Coyle YM, Xie XJ, Lewis CM, Bu D, Milchgrub S, Euhus DM. Role of physical activity in modulating breast cancer risk as defined by APC and RASSF1A promoter hypermethylation in nonmalignant breast tissue. Cancer Epidemiol Biomarkers Prev. 2007;16(2):192–196. [DOI] [PubMed] [Google Scholar]
- 332. Sanchis-Gomar F, Garcia-Gimenez JL, Perez-Quilis C, Gomez-Cabrera MC, Pallardo FV, Lippi G. Physical exercise as an epigenetic modulator: eustress, the “positive stress” as an effector of gene expression. J Strength Cond Res. 2012;26(12):3469–3472. [DOI] [PubMed] [Google Scholar]
- 333. Turner JE, Brum PC. Does regular exercise counter T cell immunosenescence reducing the risk of developing cancer and promoting successful treatment of malignancies? Oxid Med Cell Longev. 2017;2017:4234765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25(38):8680–8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Belarbi K, Burnouf S, Fernandez-Gomez FJ, et al. Beneficial effects of exercise in a transgenic mouse model of Alzheimer’s disease-like tau pathology. Neurobiol Dis. 2011;43(2):486–494. [DOI] [PubMed] [Google Scholar]
- 336. Garcia-Mesa Y, Gimenez-Llort L, Lopez LC, et al. Melatonin plus physical exercise are highly neuroprotective in the 3xTg-AD mouse. Neurobiol Aging. 2012;33(6):1124 e1113–1124 e1129. [DOI] [PubMed] [Google Scholar]
- 337. Garcia-Mesa Y, Lopez-Ramos JC, Gimenez-Llort L, et al. Physical exercise protects against Alzheimer’s disease in 3xTg-AD mice. J Alzheimers Dis. 2011;24(3):421–454. [DOI] [PubMed] [Google Scholar]
- 338. Moore KM, Girens RE, Larson SK, et al. A spectrum of exercise training reduces soluble Abeta in a dose-dependent manner in a mouse model of Alzheimer’s disease. Neurobiol Dis. 2016;85:218–224. [DOI] [PubMed] [Google Scholar]
- 339. Herring A, Donath A, Yarmolenko M, et al. Exercise during pregnancy mitigates Alzheimer-like pathology in mouse offspring. FASEB J. 2012;26(1):117–128. [DOI] [PubMed] [Google Scholar]
- 340. Fragoso J, Lira AO, Chagas GS, et al. Maternal voluntary physical activity attenuates delayed neurodevelopment in malnourished rats. Exp Physiol. 2017;102(11):1486–1499. [DOI] [PubMed] [Google Scholar]
- 341. Kumar-Singh S, Cras P, Wang R, et al. Dense-core senile plaques in the Flemish variant of Alzheimer’s disease are vasocentric. Am J Pathol. 2002;161(2):507–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342. Soto I, Graham LC, Richter HJ, et al. APOE stabilization by exercise prevents aging neurovascular dysfunction and complement induction. PLoS Biol. 2015;13(10):e1002279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343. Rzechorzek W, Zhang H, Buckley BK, Hua K, Pomp D, Faber JE. Aerobic exercise prevents rarefaction of pial collaterals and increased stroke severity that occur with aging. J Cereb Blood Flow Metab. 2017;37(11):3544–3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Sacco G, Caillaud C, Ben Sadoun G, Robert P, David R, Brisswalter J. Exercise plus cognitive performance over and above exercise alone in subjects with mild cognitive impairment. J Alzheimers Dis. 2015;50(1):19–25. [DOI] [PubMed] [Google Scholar]
- 345. Dinoff A, Herrmann N, Swardfager W, Lanctot KL. The effect of acute exercise on blood concentrations of brain-derived neurotrophic factor in healthy adults: a meta-analysis. Eur J Neurosci. 2017;46(1):1635–1646. [DOI] [PubMed] [Google Scholar]
- 346. Beilharz JE, Maniam J, Morris MJ. Diet-induced cognitive deficits: the role of fat and sugar, potential mechanisms and nutritional interventions. Nutrients. 2015;7(8):6719–6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347. Marosi K, Mattson MP. BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol Metab. 2014;25(2):89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348. Meng C, He Z, Xing D. Low-level laser therapy rescues dendrite atrophy via upregulating BDNF expression: implications for Alzheimer’s disease. J Neurosci. 2013;33(33):13505–13517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349. Wen R, Song Y, Cheng T, et al. Injury-induced upregulation of bFGF and CNTF mRNAS in the rat retina. J Neurosci. 1995;15(11):7377–7385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350. Cai H, Li G, Hua S, Liu Y, Chen L. Effect of exercise on cognitive function in chronic disease patients: a meta-analysis and systematic review of randomized controlled trials. Clin Interv Aging. 2017;12:773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351. Power GA, Allen MD, Gilmore KJ, et al. Motor unit number and transmission stability in octogenarian world class athletes: can age-related deficits be outrun? J Appl Physiol (1985). 2016;121(4):1013–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352. Power GA, Dalton BH, Behm DG, Vandervoort AA, Doherty TJ, Rice CL. Motor unit number estimates in masters runners: use it or lose it? Med Sci Sports Exerc. 2010;42(9):1644–1650. [DOI] [PubMed] [Google Scholar]
- 353. Dimauro I, Sgura A, Pittaluga M, et al. Regular exercise participation improves genomic stability in diabetic patients: an exploratory study to analyse telomere length and DNA damage. Sci Rep. 2017;7(1):4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Cash SW, Beresford SA, Vaughan TL, et al. Recent physical activity in relation to DNA damage and repair using the comet assay. J Phys Act Health. 2014;11(4):770–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. Schmidt RH, Nickerson JM, Boatright JH. Exercise as gene therapy: BDNF and DNA damage repair. Asia Pac J Ophthalmol (Phila). 2016;5(4):309–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356. Davison GW. Exercise and oxidative damage in nucleoid DNA quantified using single cell gel electrophoresis: present and future application. Front Physiol. 2016;7:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Akerfelt M, Trouillet D, Mezger V, Sistonen L. Heat shock factors at a crossroad between stress and development. Ann N Y Acad Sci. 2007;1113:15–27. [DOI] [PubMed] [Google Scholar]
- 358. Bellini S, Barutta F, Mastrocola R, Imperatore L, Bruno G, Gruden G. Heat shock proteins in vascular diabetic complications: review and future perspective. Int J Mol Sci. 2017;18(12):pii: E2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Benjamin IJ, McMillan DR. Stress (heat shock) proteins: molecular chaperones in cardiovascular biology and disease. Circ Res. 1998;83(2):117–132. [DOI] [PubMed] [Google Scholar]
- 360. Han S, Choi JR, Soon Shin K, Kang SJ. Resveratrol upregulated heat shock proteins and extended the survival of G93A-SOD1 mice. Brain Res. 2012;1483:112–117. [DOI] [PubMed] [Google Scholar]
- 361. Sahin K, Orhan C, Akdemir F, Tuzcu M, Iben C, Sahin N. Resveratrol protects quail hepatocytes against heat stress: modulation of the Nrf2 transcription factor and heat shock proteins. J Anim Physiol Anim Nutr (Berl). 2012;96(1):66–74. [DOI] [PubMed] [Google Scholar]
- 362. Gounden S, Chuturgoon A. Curcumin upregulates antioxidant defense, lon protease, and heat-shock protein 70 under hyperglycemic conditions in human hepatoma cells. J Med Food. 2017;20(5):465–473. [DOI] [PubMed] [Google Scholar]
- 363. Hanzen S, Vielfort K, Yang J, et al. Lifespan control by redox-dependent recruitment of chaperones to misfolded proteins. Cell. 2016;166(1):140–151. [DOI] [PubMed] [Google Scholar]
- 364. Yang L, Licastro D, Cava E, et al. Long-term calorie restriction enhances cellular quality-control processes in human skeletal muscle. Cell Rep. 2016;14(3):422–428. [DOI] [PubMed] [Google Scholar]
- 365. Archer AE, Von Schulze AT, Geiger PC. Exercise, heat shock proteins and insulin resistance. Philos Trans R Soc Lond B Biol Sci. 2018;373(1738):pii: 20160529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366. Borges JP, da Silva Verdoorn K. Cardiac ischemia/reperfusion injury: the beneficial effects of exercise. Adv Exp Med Biol. 2017;999:155–179. [DOI] [PubMed] [Google Scholar]
- 367. Anckar J, Sistonen L. Regulation of HSF1 function in the heat stress response: implications in aging and disease. Annu Rev Biochem. 2011;80:1089–1115. [DOI] [PubMed] [Google Scholar]
- 368. Kataoka H, Nakano J, Kondo Y, et al. The influence of aging on the effectiveness of heat stress in preventing disuse muscle atrophy. Physiol Int. 2017;104(4):316–328. [DOI] [PubMed] [Google Scholar]
- 369. Thakur SS, Swiderski K, Ryall JG, Lynch GS. Therapeutic potential of heat shock protein induction for muscular dystrophy and other muscle wasting conditions. Philos Trans R Soc Lond B Biol Sci. 2018;373(1738):pii: 20160528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370. Sun Y, Zhang JR, Chen S. Suppression of Alzheimer’s disease-related phenotypes by the heat shock protein 70 inducer, geranylgeranylacetone, in APP/PS1 transgenic mice via the ERK/p38 MAPK signaling pathway. Exp Ther Med. 2017;14(6):5267–5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. Lu RC, Tan MS, Wang H, Xie AM, Yu JT, Tan L. Heat shock protein 70 in Alzheimer’s disease. Biomed Res Int. 2014;2014:435203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372. Henstridge DC, Whitham M, Febbraio MA. Chaperoning to the metabolic party: the emerging therapeutic role of heat-shock proteins in obesity and type 2 diabetes. Mol Metab. 2014;3(8):781–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373. Ranek MJ, Stachowski MJ, Kirk JA, Willis MS. The role of heat shock proteins and co-chaperones in heart failure. Philos Trans R Soc Lond B Biol Sci. 2018;373(1738):pii: 20160530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374. Tu RH, Li QJ, Huang Z, et al. Novel functional role of heat shock protein 90 in mitochondrial connexin 43-mediated hypoxic postconditioning. Cell Physiol Biochem. 2017;44(3):982–997. [DOI] [PubMed] [Google Scholar]
- 375. Rattan SI. Hormetic modulation of aging and longevity by mild heat stress. Dose Response. 2006;3(4):533–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376. Ougham HJ, Howarth CJ. Temperature shock proteins in plants. Symp Soc Exp Biol. 1988;42:259–280. [PubMed] [Google Scholar]
- 377. Fujita J. Cold shock response in mammalian cells. J Mol Microbiol Biotechnol. 1999;1(2):243–255. [PubMed] [Google Scholar]
- 378. Al-Fageeh MB, Smales CM. Control and regulation of the cellular responses to cold shock: the responses in yeast and mammalian systems. Biochem J. 2006;397(2):247–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379. Lee BS, Jung E, Lee Y, Chung SH. Hypothermia decreased the expression of heat shock proteins in neonatal rat model of hypoxic ischemic encephalopathy. Cell Stress Chaperones. 2017;22(3):409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380. Lee JH, Zhang J, Yu SP. Neuroprotective mechanisms and translational potential of therapeutic hypothermia in the treatment of ischemic stroke. Neural Regen Res. 2017;12(3):341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381. Jones DM, Bailey SP, Roelands B, Buono MJ, Meeusen R. Cold acclimation and cognitive performance: a review. Auton Neurosci. 2017;208:36–42. [DOI] [PubMed] [Google Scholar]
- 382. Qin J, Mai Y, Li Y, Jiang Z, Gao Y. Effect of mild hypothermia preconditioning against low temperature (4 degrees C) induced rat liver cell injury in vitro. PLoS One. 2017;12(4):e0176652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383. Purushothuman S, Stone J. The reaction of cerebral cortex to a nearby lesion: damage, survival, self-protection. Brain Res. 2015;1601:52–63. [DOI] [PubMed] [Google Scholar]
- 384. Bryan MT, Duckles H, Feng S, et al. Mechanoresponsive networks controlling vascular inflammation. Arterioscler Thromb Vasc Biol. 2014;34(10):2199–2205. [DOI] [PubMed] [Google Scholar]
- 385. Baratchi S, Khoshmanesh K, Woodman OL, Potocnik S, Peter K, McIntyre P. Molecular sensors of blood flow in endothelial cells. Trends Mol Med. 2017;23(9):850–868. [DOI] [PubMed] [Google Scholar]
- 386. Simmons RD, Kumar S, Jo H. The role of endothelial mechanosensitive genes in atherosclerosis and omics approaches. Arch Biochem Biophys. 2016;591:111–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387. Davies PF. Endothelial transcriptome profiles in vivo in complex arterial flow fields. Ann Biomed Eng. 2008;36(4):563–570. [DOI] [PubMed] [Google Scholar]
- 388. Hochleitner BW, Hochleitner EO, Obrist P, et al. Fluid shear stress induces heat shock protein 60 expression in endothelial cells in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2000;20(3):617–623. [DOI] [PubMed] [Google Scholar]
- 389. Adams JA, Wu H, Bassuk JA, et al. Periodic acceleration (pGz) prior to whole body ischemia reperfusion injury provides early cardioprotective preconditioning. Life Sci. 2010;86(19-20):707–715. [DOI] [PubMed] [Google Scholar]
- 390. Bassuk JI, Wu H, Arias J, Kurlansky P, Adams JA. Whole body periodic acceleration (pGz) improves survival and allows for resuscitation in a model of severe hemorrhagic shock in pigs. J Surg Res. 2010;164(2):e281–e289. [DOI] [PubMed] [Google Scholar]
- 391. Mattson MP, Calabrese C. Hormesis: A Revolution in Biology, Toxicology and Medicine. New York, NY: Springer; 2009. [Google Scholar]
- 392. Calabrese EJ, Baldwin LA. Hormesis: the dose–response revolution. Annu Rev Pharmacol Toxicol. 2003;43:175–197. [DOI] [PubMed] [Google Scholar]
- 393. Paracelsus. Die dritte defension wegen des schreibens der neuen rezepte, 1965. Vol Septem Defensiones: Darmstadt 1965;1538:510. [Google Scholar]
- 394. Borzelleca JF. Paracelsus: herald of modern toxicology. Toxicol Sci. 2000;53(1):2–4. [DOI] [PubMed] [Google Scholar]
- 395. Calabrese EJ. Historical blunders: how toxicology got the dose–response relationship half right. Cell Mol Biol (Noisy-le-grand). 2005;51(7):643–654. [PubMed] [Google Scholar]
- 396. Southam CM, Ehrlich J. Effects of extract of western red-cedar heartwood on certain wood-decaying fungi in culture. Phytopathology. 1943;33:517–524. [Google Scholar]
- 397. Sutou S. The 10th Anniversary of the Publication of Genes and Environment: memoir of establishing the Japanese Environmental Mutagen Society and a proposal for a new collaborative study on mutagenic hormesis. Genes Environ. 2017;39:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398. Doss M. Evidence supporting radiation hormesis in atomic bomb survivor cancer mortality data. Dose Response. 2012;10(4):584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399. Calabrese EJ. Cancer biology and hormesis: human tumor cell lines commonly display hormetic (biphasic) dose responses. Crit Rev Toxicol. 2005;35(6):463–582. [DOI] [PubMed] [Google Scholar]
- 400. Mattson MP, Calabrese EJ. Hormesis: a Revolution in Biology, Toxicology and Medicine. New York, NY: Springer; 2010. [Google Scholar]
- 401. Selkoe DJ. Amyloid beta-protein precursor: new clues to the genesis of Alzheimer’s disease. Curr Opin Neurobiol. 1994;4(5):708–716. [DOI] [PubMed] [Google Scholar]
- 402. Yankner BA. New clues to Alzheimer’s disease: unraveling the roles of amyloid and tau. Nat Med. 1996;2(8):850–852. [DOI] [PubMed] [Google Scholar]
- 403. Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol Sci. 1991;12(10):383–388. [DOI] [PubMed] [Google Scholar]
- 404. Selkoe DJ. Toward a comprehensive theory for Alzheimer’s disease. Hypothesis: Alzheimer’s disease is caused by the cerebral accumulation and cytotoxicity of amyloid beta-protein. Ann N Y Acad Sci. 2000;924:17–25. [DOI] [PubMed] [Google Scholar]
- 405. Rottkamp CA, Atwood CS, Joseph JA, Nunomura A, Perry G, Smith MA. The state versus amyloid-beta: the trial of the most wanted criminal in Alzheimer disease. Peptides. 2002;23(7):1333–1341. [DOI] [PubMed] [Google Scholar]
- 406. Lee HG, Casadesus G, Zhu X, Takeda A, Perry G, Smith MA. Challenging the amyloid cascade hypothesis: senile plaques and amyloid-beta as protective adaptations to Alzheimer disease. Ann N Y Acad Sci. 2004;1019:1–4. [DOI] [PubMed] [Google Scholar]
- 407. Puzzo D, Privitera L, Palmeri A. Hormetic effect of amyloid-beta peptide in synaptic plasticity and memory. Neurobiol Aging. 2012;33(7):1484 e1415–1484 e1424. [DOI] [PubMed] [Google Scholar]
- 408. Morley JE, Farr SA. Hormesis and amyloid-beta protein: physiology or pathology? J Alzheimers Dis. 2012;29(3):487–492. [DOI] [PubMed] [Google Scholar]
- 409. Soscia SJ, Kirby JE, Washicosky KJ, et al. The Alzheimer’s disease-associated amyloid beta-protein is an antimicrobial peptide. PLoS One. 2010;5(3):e9505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410. Mita S, Schon EA, Herbert J. Widespread expression of amyloid beta-protein precursor gene in rat brain. Am J Pathol. 1989;134(6):1253–1261. [PMC free article] [PubMed] [Google Scholar]
- 411. Green KN, Boyle JP, Peers C. Hypoxia potentiates exocytosis and Ca2+ channels in PC12 cells via increased amyloid beta peptide formation and reactive oxygen species generation. J Physiol. 2002;541(pt 3):1013–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412. Chuang JY, Lee CW, Shih YH, Yang T, Yu L, Kuo YM. Interactions between amyloid-beta and hemoglobin: implications for amyloid plaque formation in Alzheimer’s disease. PLoS One. 2012;7(3):e33120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413. Wu CW, Liao PC, Yu L, et al. Hemoglobin promotes Abeta oligomer formation and localizes in neurons and amyloid deposits. Neurobiol Dis. 2004;17(3):367–377. [DOI] [PubMed] [Google Scholar]
- 414. Stone J. What initiates the formation of senile plaques? The origin of Alzheimer-like dementias in capillary haemorrhages. Med Hypo. 2008;71(3):347–359. [DOI] [PubMed] [Google Scholar]
- 415. Cullen KM, Kocsi Z, Stone J. Pericapillary haem-rich deposits: evidence for microhaemorrhages in aging human cerebral cortex. J Cereb Blood Flow Metab. 2005;25(12):1656–1667. [DOI] [PubMed] [Google Scholar]
- 416. Cullen KM, Kocsi Z, Stone J. Microvascular pathology in the aging human brain: evidence that senile plaques are sites of microhaemorrhages. Neurobiol Aging. 2006;27(12):1786–1796. [DOI] [PubMed] [Google Scholar]
- 417. Calabrese EJ, Bachmann KA, Bailer AJ, et al. Biological stress response terminology: integrating the concepts of adaptive response and preconditioning stress within a hormetic dose–response framework. Toxicol Appl Pharmacol. 2007;222(1):122–128. [DOI] [PubMed] [Google Scholar]
- 418. Kitchin KT. Defining, explaining and understanding hormesis. Hum Exp Toxicol. 2002;21(2):105–106; discussion 113-104. [DOI] [PubMed] [Google Scholar]
- 419. Rowe M, Stone J. The naming of neurones: the classification and naming of cat retinal ganglion cells. Brain Behav Evol. 1977;14:185–216. [DOI] [PubMed] [Google Scholar]
- 420. Kong YX, van Bergen N, Bui BV, et al. Impact of aging and diet restriction on retinal function during and after acute intraocular pressure injury. Neurobiol Aging. 2012;33(6):1126 e1115–1126 e1125. [DOI] [PubMed] [Google Scholar]
- 421. Gargini C, Belfiore MS, Bisti S, Cervetto L, Valter K, Stone J. The impact of basic fibroblast growth factor on photoreceptor function and morphology. Invest Ophthalmol Vis Sci. 1999;40(9):2088–2099. [PubMed] [Google Scholar]