Abstract
Cachexia, the unintentional loss of body weight, is prevalent in many cancer types, and the associated skeletal muscle mass depletion increases patient morbidity and mortality. While anorexia can be present, cachexia is not reversible with nutritional therapies alone. Pharmacological agents have been proposed to treat this condition, but there are currently no approved treatments. Nonetheless, the hallmark characteristics associated with cancer cachexia remain viable foundations for future therapies. Regular physical activity holds a promising future as a nonpharmacological alternative to improve patient survival through cachexia prevention. Evidence suggests exercise training is beneficial during cancer treatment and survival. However, the mechanistic examination of cachectic skeletal muscle’s response to exercise is both needed and justified. The primary objective of this review is to discuss the role of exercise for the prevention and treatment of cancer-associated muscle wasting. Initially, we provide an overview of systemic alterations induced by cancer and their role in the regulation of wasting processes during cachexia progression. We then discuss how exercise could alter disrupted regulatory pathways related to growth and metabolism during cancer-induced muscle atrophy. Last, we outline current exercise prescription guidelines and how exercise could be a potential behavioral therapy to curtail cachexia development in cancer patients.
Keywords: cancer cachexia, systemic alterations, skeletal muscle, exercise
‘Given the importance of muscle mass in relation to cancer mortality, therapies that preserve muscle mass and function may be effective for improving cancer patient survival.’
Cancer cachexia is a complex metabolic wasting syndrome characterized by the unintentional loss of skeletal muscle mass (with or without fat loss), which cannot be reversed by standard nutritional treatment and leads to progressive functional impairment.1,2 Cachexia accounts for approximately 20% to 40% of all cancer-related deaths, and it is directly associated with cancer patient morbidity and mortality.3 While cachexia can occur with many cancer types, it is most prevalent in pancreatic, lung, colorectal, and gastrointestinal cancers.4 Consequences of cachexia can include reduced anticancer therapy tolerance, increased susceptibility to treatment toxicity, and decreased patient quantity and quality of life.2,5,6 The underlying mechanisms associated with cachexia development are complex and the degree of wasting can vary with cancer stage and progression. Thus, the difficulty in treating cancer cachexia parallels the condition’s multifactorial nature, which serves to directly impede the development of approved treatments.
While previous diagnostic criteria have been established for cachexia across several chronic diseases,2 cancer-specific criteria include >5% weight loss over the past 6 months or >2% weight loss in patients that have demonstrated body mass index (BMI) <20 kg/m2 or skeletal muscle mass depletion.1 The cachectic patient can also be assessed and characterized by anorexia or reduced food intake, decrements in muscle function, and psychosocial impairment.1,7,8 The progression of cachexia has been described as a continuum spanning the categories of pre-cachexia, cachexia, and refractory cachexia.1,9 Progression to a more severe cachectic phenotype can be driven by factors such as systemic catabolism, increased energy expenditure, insulin resistance, anemia, and hypogonadism.1,2 These alterations can promote a hypermetabolic and hypercatabolic state, which contributes to the wasting phenotype.9 However, the classification of cachectic cancer patients is further complicated by the heterogeneity of symptoms, and this variability remains a weakness for interpreting the present body of clinical research examining the drivers of cancer cachexia. Clearly, this field will benefit from more stringent and physiological relevant classification criteria.
The progressive loss of skeletal muscle alters metabolic health and physical function,10 which impairs a cancer patient’s quantity and quality of life. Given the importance of muscle mass in relation to cancer mortality,5 therapies that preserve muscle mass and function may be effective for improving cancer patient survival. While there is rapidly mounting evidence for the positive effects of physical activity and exercise on cancer patient survival, far less is understood about exercise’s ability to prevent or treat cancer cachexia. The primary objective of this review is to discuss physical activity and exercise’s role in the prevention and treatment of muscle wasting associated with cancer. Initially, we describe systemic alterations associated with the cancer environment and their contribution to cachexia-induced wasting. We then discuss disrupted signaling pathways linked to muscle atrophy. Next, we highlight how physical activity and exercise could alter wasting processes in cancer patients. Last, we outline current exercise prescription guidelines for cancer patients and give perspective for future exercise investigations in cachectic cancer patients. Throughout the review we provide evidence from both human cancer patients and preclinical models when feasible.
Disruptions to Systemic Homeostasis Induced by Cancer Cachexia
Numerous systemic disorders have been associated with cachexia development.1,2 With cancer, the progression from the pre-cachexia to refractory cachexia has been linked to tumor burden or type, increased systemic inflammation, and a decline in nutritional status.1,9 Other systemic disruptions that can occur with cancer cachexia progression include insulin resistance, anemia, and hypogonadism.1,2 These systemic alterations can be used in the assessment of the patient and may provide a foundation for an individualized treatment based on the patient’s characteristics. However, a current formidable gap in our understanding of cancer cachexia regulation involves the determination of systemic disruptions that drive cachexia progression, versus changes that are just associated with the condition. This knowledge gap is further complicated by the heterogeneity of cancer and the specific systemic disruptions that are provoked within individual cancer patients. For example, the age, sex, BMI, and initial fitness level could significantly affect the development of the cachectic environment in the cancer patient. While the complexity of this multifactorial condition has impeded the development of a one size fits all therapy, there remains strong optimism that a better understanding of cancer-induced systemic disruptions will provide a physiological signature that can lead to effective therapies that prevent or treat cachexia. Therefore, several systemic disruptions that have been identified as therapeutic targets are highlighted in this section.
Systemic Inflammation
Systemic inflammation is present in many wasting conditions, and the associated pro-inflammatory cytokines have been used clinically during patient assessment and widely investigated as potential drivers of cancer cachexia development.11-14 The source of elevated circulating cytokines has been attributed to both the tumor and to tumor-host immune interactions.11 The chronic elevation of inflammatory cytokines has well described actions on cellular functions in liver, adipose, and skeletal muscle.15,16 Pro-inflammatory cytokines such as intereleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) have been implicated in cancer cachexia development in both humans and preclinical models.15,17-21 Elevated plasma TNF-α is present in several human cancers20,22 and has been associated with anorexia, enhanced lipolysis, and muscle atrophy in tumor bearing rodents.23-25 While inhibiting TNF-α has demonstrated favorable results in some preclinical cancer models,24-26 anti-TNF-α treatments in clinical trials have not prevented weight loss in cancer patients.27,28 IL-6 also has established roles in systemic tissue dysregulation. Systemically elevated IL-6 can induce liver growth and insulin resistance,29,30 activate adipose tissue lipolysis,31 and disrupt muscle protein turnover.32-34 IL-6 has been associated with cachexia development across several preclinical mouse models,14,34-36 and plasma levels are inversely related to survival in metastatic lung cancer patients.14 Treatment with an anti-IL-6 receptor neutralizing antibody (tocilizumab) improved indices of cachexia progression in lung cancer patients.37,38 While significant advances have been made in determining the role of specific cytokines in the regulation of muscle wasting and cachexia progression, it is likely that cachexia development and progression results in part from the release of multiple cytokines.
Liver Dysfunction
Due to critical regulatory roles in systemic metabolism and metabolic function, there is a strong rationale for examining liver dysfunction as a potential driver of cancer cachexia. Specifically, liver regulation of physiological processes related to glucose, amino acid and lipid metabolism, hormone synthesis (eg, insulin-like growth factor-1), and metabolite detoxification39 could all promote cachexia. Indeed, the liver’s high metabolic activity significantly contributes (~20%) to whole-body resting energy expenditure.40,41 Despite reductions in physical activity, energy expenditure is increased in cachectic cancer patients.42,43 Hepatomegaly can be observed in both cancer patients and preclinical models,44-46 and it has been associated with increased energy expenditure in colorectal cancer patients.46 Interestingly, IL-6 has been implicated in this regulation, as chronically elevated IL-6 can induce liver growth independent of cancer in mice.29 Additionally, enhanced liver inflammatory signaling has been associated with an increased acute phase protein response in cancer patients and preclinical models.43-45,47,48 It has been suggested that the breakdown of less metabolically active tissues (eg, adipose and muscle) provides a substrate source for gluconeogenesis to support tumor metabolism and liver acute phase protein synthesis,16,49 which contributes to the hypermetabolic state. Collectively, these data suggest that liver metabolic function may be a central regulator of wasting process, and targeted treatments may prevent cachexia progression.
Adipose Tissue Dysfunction
The loss and dysfunction of adipose tissue is an established component of cancer-induced wasting.22,50 For many years adipose tissue’s potential to regulate the progression of cachexia was not fully appreciated, and the importance deemed secondary to lean tissue regulation. However, recent advances in adipose tissue biology and physiology have provided an excellent premise to investigate a more active role of adipose tissue in the regulation of cachexia. While there is emerging evidence for examining adipose tissue’s endocrine function as the source of adipokines that regulate whole-body metabolism and appetite,51 adipose tissue dysregulation of lipolysis and lipid storage has been actively examined with cancer cachexia. White adipose tissue depletion can occur through activated hormone-sensitive lipase, decreased lipoprotein lipase activity, and reduced de novo lipogenesis.52,53 Thus, enhanced lipolysis coupled with impaired lipid storage can elevate circulating free fatty acids and create a systemic environment that generates metabolic stress in numerous tissues, including skeletal muscle. This condition has been termed lipotoxicity.31 In cachectic cancer patients, BMI has been negatively correlated to both adipose triglyceride lipase and hormone-sensitive lipase activities.54 Furthermore, intramyocellular lipids have been observed in cachectic cancer patients.55,56 Excess skeletal muscle lipid exposure and storage can also contribute to insulin resistance and mitochondrial dysfunction.31 Collectively, these studies demonstrate adipose tissue wasting may be an early event that has a role in the initiation of cancer cachexia,54,57 and the prevention of inflammation-induced white adipose tissue loss may serve as a viable therapeutic target for preventing the progression of cachexia.
Hypogonadism
Hypogonadism in both males and females is often associated with cachexia progression.2 Although a decline in sex hormones has demonstrated effects on musculoskeletal homeostasis and metabolism,58 there are significant gaps in our understanding of hypogonadism’s regulatory role in cancer-induced wasting. To this end, the sex-specific responses and regulation are significantly understudied in the female. Indeed, sex differences in cachexia susceptibility have been reported in both cancer patients and preclinical models.13,59,60 While not reported with all cancer types, low total or bioavailable testosterone has been reported in male cancer patients with and without weight loss.12,20,47,61-63 Low testosterone has also been associated with decreased survival in hypogonadal male pancreatic cancer patients.47 Similarly, circulating testosterone is reduced during the progression of cachexia and is correlated with muscle mass loss in tumor bearing mice.64 Primary and secondary hypogonadism can occur in cancer patients, and several factors associated with the pathogenesis include systemic inflammation, hormones (eg, leptin, ghrelin), opioid use, and chemotherapy.12,61,62 While testosterone replacement can improve muscle mass and strength in healthy adults,65 this effect has not been established with cancer cachexia. However, a phase I, double-blinded randomized control trial (NCT00878995) has recently been completed; the results have yet to be disseminated. While androgens have established anabolic effects in muscle, the lack of tissue specificity and associated side effects has limited their use to treat cancer cachexia. Future studies evaluating selective androgen receptor modulators, which demonstrate robust anabolic activity and high tissue specificity,66 may provide a safer and more efficacious treatment alternative.
Muscle hormone sensitivity can be regulated by muscle androgen and estrogen receptor expression (AR and ER, respectively).67 AR expression is decreased in cachectic mouse skeletal muscle,64 but this reduction has not been established in human cancer patients. There is evidence suggesting that estrogen and the corresponding intracellular signaling pathways can modulate wasting susceptibility in both human cancer patients and preclinical cancer models. Several studies have found that male cancer patients are more susceptible to cachexia and demonstrate increased overall mortality when compared to females.13,68,69 Furthermore, female tumor bearing mice were protected against cancer-induced cardiac atrophy, and inhibiting estrogen signaling through the ER could recapitulate the male wasting phenotype.59 The cardioprotective effects of estrogen may be related to reduced oxidative stress or activation of cell survival pathways70; however, this has not been investigated during cancer cachexia. Nonetheless, cachexia severity in female tumor bearing mice has also been associated with acyclicity (eg, loss of estrous cycle).71 The protective effects of estrogen on cardiac and skeletal muscle atrophy may be related to inflammatory signaling, as we have previously found that ovariectomy increased muscle sensitivity to IL-6.60 Additional investigations that serve to improve our mechanistic understanding of circulating sex hormones in the regulation of wasting susceptibility are clearly warranted in both sexes.
Anemia
Anemia, a condition of reduced red blood cell quantity (hemoglobin level <12 g/dL), is common in cancer patients and can contribute to weight loss, reduced exercise capacity, and altered energy balance.72 The prevalence and severity of anemia can be influenced by tumor burden and stage, which can adversely affect quality of life and increase mortality.73,74 Additionally, the presence of anemia may also have detrimental effects on disease and cachexia progression. Anemia can be caused by hemolysis, blood loss, or impaired red blood cell production, which can be influenced by both cancer and anticancer treatment.75 While the mechanisms causing anemia with cancer cachexia are not well understood, increasing erythropoietin levels pharmacologically can improve hemoglobin levels and quality of life.76 Given the potential impact of anemia on survival and quality of life, anemia treatment has been examined in both cancer patients and preclinical models. Treatment options for anemia include blood transfusion, iron supplementation, and erythropoiesis stimulating agents.77,78 Erythropoiesis stimulating agents such as recombinant human erythropoietin improved hemoglobin levels, maintained exercise capacity, decreased transfusion requirements, and increased functional ability in cancer patients.79,80 Collectively, these studies demonstrate the importance of treating and accounting for the effects of anemia in the cancer patient. Further research is necessary to determine if long-term treatments to counteract anemia are sustainable and to improve our understanding of mechanisms inducing the anemic condition.
Physical Inactivity and Muscle Dysfunction
Physical inactivity has established roles in the pathogenesis of many chronic diseases.81 However, the significant progress that has been made in our understanding of physical activity’s impact on health status and the prevention of cancer has not carried forward to its contribution in cancer cachexia progression. Physical inactivity has been documented in both cachectic cancer patients and preclinical cachexia models. While basal metabolic rate is increased in cachectic lung cancer patients, total energy expenditure and physical activity levels are drastically reduced compared to moderately active healthy individuals.82 Moreover, cachectic pancreatic cancer patients have comparable physical activity levels to individuals with spinal cord injury or cerebral palsy patients.83 Preclinical models have also demonstrated reduced voluntary physical activity during late-stage cachexia.36,84 Interestingly, physical inactivity can also occur prior to weight loss, which highlights a potential role for activity in wasting regulation during cachexia progression.85 Given that physical inactivity and decreased muscle use can promote systemic metabolic dysfunction and muscle atrophy,81 future studies are needed to determine if physical inactivity contributes to disrupted systemic metabolism and muscle wasting during cancer cachexia progression.
Muscular strength and exercise capacity are reduced during cancer cachexia. Isometric knee extensor strength (absolute and relative) is reduced in cachectic gastrointestinal cancer patients compared to both healthy individuals and non-cachectic cancer patients.13 Additionally, exercise capacity (peak volume oxygen [VO2] consumed) is decreased in colorectal cancer patients.86 Similarly, voluntary muscle strength and exercise capacity are also reduced in preclinical models.84,85,87,88 Moreover, muscle contractile properties related to force production and fatigue resistance, measured in situ and ex vivo, are also disrupted during cancer cachexia.87,89,90 Collectively, these studies support the concept that physical inactivity is present during cachexia progression and may contribute to muscle atrophy and dysfunction during cancer cachexia.
Mechanisms of Muscle Wasting and Cachexia
Muscle mass and metabolic function are critical for health.10 While cachexia results in systemic metabolic dysfunction, skeletal muscle mass and function have an established role in cancer patient morbidity and mortality. Thus, a mechanistic understanding of muscle wasting regulation is imperative for the development of preventative and therapeutic strategies for cancer cachexia treatment. Muscle mass reflects an intricate balance between the rates of protein synthesis and breakdown, termed protein turnover.91,92 While cellular proteins are in constant state of turnover to maintain protein homeostasis (proteostasis) and function, these processes are disrupted during cancer cachexia. Cancer-induced muscle wasting is accompanied by protein breakdown activation and protein synthesis suppression.93-95 Additionally, muscle oxidative capacity, which can influence cellular processes related to growth and metabolism, is disrupted during cachexia progression.96 The following section will highlight key signaling pathways altered by the cachectic environment during cancer.
Protein Synthesis
Skeletal muscle protein synthesis can be regulated by nutrients, physical activity level, and inflammation. Protein kinase B (PKB, also known as Akt) and the mechanistic target of rapamycin complex 1 (mTORC1) have established roles for the integration of anabolic signaling initiated by growth factors, nutrients, and mechanical loading to regulate protein synthesis.91,92,97 Growth factors such as insulin and insulin-like growth factor-1 (IGF-1) stimulate mTORC1 signaling through the activation of Akt.91,92 Binding of insulin/IGF-1 to its respective cell surface receptor initiates tyrosine kinase activity and phosphoinositide 3-kinase (PI3K)-dependent activation of Akt through phosphoinositide-dependent kinase 1 (PDK1). The rapamycin-insensitive mTOR complex 2 (mTORC2) has also been implicated in growth factor signaling through the Akt phosphorylation.98 Subsequent downstream targets of activated Akt, which can control muscle protein synthesis, include tuberous sclerosis 2 (TSC2), glycogen synthase kinase-3β (GSK-3β), and proline-rich Akt substrate 40 kDa (PRAS40).91,92 mTORC1 activation by Akt-dependent (eg, insulin) and Akt-independent (eg, mechanical loading, nutrition) processes promotes protein synthesis by phosphorylating the eukaryotic initiation factor 4E (4E-BP1) and the p70 ribosomal S6 kinase (S6K1). The hyperphosphorylation of 4E-BP1 prevents binding to eukaryotic initiation factor 4E (eIF4E) and the formation of 4E-BP1-eIF4E complex, resulting in the assembly of the eIF4F complex and translation initiation.91 In addition, S6K1 activation by mTORC1 has been implicated in cap-dependent translation, translation elongation, and ribosomal biogenesis.97,99,100 Several components of Akt/mTORC1 signaling and muscle protein synthesis are disrupted during cancer cachexia.
While suppressed protein synthesis has established roles in many wasting conditions, gaps remain in our understanding of suppressed muscle protein synthesis during cancer cachexia.23,101 Disrupted basal and postprandial protein synthesis regulation could contribute to muscle loss in cachectic cancer patients.102 While results are equivocal, there is evidence that basal muscle protein synthesis may not be altered in cancer patients.103,104 However, these studies have limitations related to the patient population, the type and stage of the cancer, and the degree and duration of the cachectic phenotype. Nonetheless, several studies have reported an impaired muscle protein synthesis response to feeding (eg, anabolic resistance) during cancer.105,106 While anabolic resistance has been hypothesized to play a role in age-related sarcopenia,107 further research is required to determine if anabolic resistance contributes to muscle mass loss in cachectic cancer patients.102,108 Basal protein synthesis is reduced in preclinical models of cachexia34,94,95 and is associated with disrupted Akt/mTORC1 signaling during cachexia progression. Muscle protein synthesis is suppressed during the initial stages of weight loss (<5% loss) and is further reduced with cachexia progression in tumor bearing mice.95 The suppression of muscle protein synthesis corresponds to reduced muscle IGF-1 expression and mTORC1 signaling.95 Collectively, these studies highlight a role for disrupted protein synthesis regulation throughout cachexia progression, which may serve as a potential therapeutic target to treat or prevent cancer cachexia.
Protein Breakdown
Accelerated protein breakdown has established roles in skeletal muscle atrophy. The 2 main proteolytic systems regulating atrophy include the ubiquitin-proteasome system (UPS) and the autophagy-lysosomal system. The degradation of protein via the UPS is a multistep process that requires a series of ATP-dependent enzymatic steps (E1, activation; E2, conjugation; E3, ligation) that conjugate ubiquitin to intracellular proteins, which are then degraded by the proteasome.109,110 The digested peptides can then be used for cellular processes related to gluconeogenesis, protein synthesis, or energy production dependent on organismal energy demands.111 In wasting conditions, 2 muscle-specific E3 ubiquitin-conjugating enzymes, Atrogin-1 (also known as MAFbx) and MuRF-1, are thought to be critical for the breakdown of muscle proteins.112 The bulk of intracellular muscle proteins, particularly myofibrillar components, are degraded by the UPS during cancer cachexia.109 In contrast, cellular organelles can also be removed through the nonselective autophagy-lysosomal system, which is an ATP-independent process. Indeed, the autophagy-lysosomal system plays a critical role in the breakdown of cellular components during normal homeostasis and in response to various stimuli such as nutrient deprivation and muscle contraction.113 Several forms of autophagy can occur in various cell types. During macroautophagy, cytosolic components (eg, organelles, glycogen) are sequestered within double-membrane vesicles (autophagosomes), which are then fused to the lysosome and the contents are degraded by hydrolases via the autolysosome.113,114 The selective removal of mitochondria, termed mitophagy, has also been shown to play an important role in muscle homeostasis in healthy and diseased states. Thus, disrupted protein breakdown could negatively affect muscle homeostasis.
It is well established that protein breakdown is elevated during cancer cachexia, which results from both the activation of the ubiquitin proteasome and lysosomal-autophagy systems in both human and preclinical models.115-117 In human cancer patients, there is evidence that tumor-derived factors and cytokines can induce autophagy in systemic tissues such as skeletal muscle.116 During the initiation of cachexia, ATP-dependent processes drive protein breakdown, while both ATP-dependent and ATP-independent processes are activated during late-stage cachexia in tumor bearing mice.95 Total muscle ubiquitination, E3 ligase expression, and proteasomal subunit expression are induced during the initiation of cachexia, and are further increased during cachexia progression.95 Autophagy-related protein expression is also increased during late-stage cachexia across multiple preclinical models.95,118 These findings highlight differential expression related to the ubiquitin and lysosomal-autophagy systems during the progression of cachexia. Autophagy flux, which may more accurately reflect activity, is also increased in cancer patients and preclinical models.95,118,119 Further research is needed to determine the precise signaling pathways regulating the autophagy response in skeletal muscle. Nonetheless, the overt activation of these 2 proteolytic pathways can negatively affect muscle size, metabolic and contractile function, and substrate utilization during cancer cachexia.
Mitochondrial Function/Oxidative Capacity Regulation
Mitochondria oxidative capacity and function are important for the maintenance of muscle mass and metabolic function. The preservation of healthy mitochondria, which can directly affect muscle oxidative capacity, involves the coordinated processes related to biogenesis, dynamics, and mitophagy.120 Disruptions to any of these processes can alter mitochondrial structure and function and lead to impairments in muscle metabolism and contractile function. Mitochondrial biogenesis is responsible for the generation of newly synthesized mitochondria, and this highly regulated process is influenced by the peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), a transcriptional coactivator that regulates genes involved in mitochondrial and energy metabolism.96 Mitochondrial dynamics, comprising fission and fusion processes, helps maintain the mitochondrial network and overall homeostasis. Fission involves the division of mitochondria and results in fragmentation, whereas fusion combines mitochondria and leads to network elongation.121 Mitophagy, the autophagic removal of damaged or dysfunctional mitochondria, helps maintain a healthy mitochondrial network. Disruption to any of these processes can lead to altered tissue function and systemic metabolic function.
Mitochondria oxidative capacity and function are important for the maintenance of muscle mass.96 While altered oxidative capacity is commonly observed in preclinical models, this has not been uniformly found in cachectic cancer patients.122 Nonetheless, several aspects of mitochondrial quality and function related to biogenesis, dynamics, and mitophagy are altered during the progression of cachexia.32 Preclinical cachexia models clearly demonstrate disrupted skeletal muscle oxidative metabolism during the progression of cachexia,32,123 and the loss of muscle oxidative capacity parallels skeletal muscle mass atrophy and disrupted protein turnover.32,35 The suppression of PGC-1α precedes the loss of mitochondrial content (mtDNA) and protein expression (cytochrome C and COXIV) during the progression of cachexia.32,123 Moreover, reductions in mitochondrial fusion proteins Mfn-1 and -2 (Mfn1/2) precede mitochondria loss during the initial stages of cachexia. In contrast, the induction of Fis1 occurs during late-stage cachexia.32,123 Therefore, treatments targeting mitochondrial quality and function may improve muscle mass and function during cancer cachexia progression.
Systemic and Muscle Alterations Targeted by Exercise
Exercise and physical activity are nonpharmacological treatments that improve indices of muscle strength and metabolic function in healthy and diseased individuals.124,125 A single bout of physical activity/exercise induces muscle molecular signaling pathways linked to energy metabolism that have documented effects on metabolic health. Furthermore, repeated bouts of activity (eg, training) can stimulate beneficial metabolic adaptations. These acute responses and chronic adaptations to exercise provide optimism for investigating their utility for improving muscle metabolic function in the cancer patient. Treadmill exercise alone or in combination with nutritional treatments can reduce tumor growth and improve muscle mass in rodents.126-128 Related to resistance exercise, rodent models of overload hypertrophy and stimulated eccentric contractions have demonstrated preserved muscle mass whether it is performed prior to or at tumor-implantation,129-132 and even after significant weight loss has occurred.133 Interestingly, it appears these improvements can occur despite the presence of a systemic cachectic environment.133 Thus, preclinical studies collectively demonstrate that both treadmill and resistance exercise may be a potential treatment to augment muscle wasting during cachexia development and progression. However, whether cachectic skeletal muscle retains the anabolic and metabolic capacity to respond to different types of muscle contraction requires further investigation. The following section will highlight evidence for exercise to alter the systemic and muscle environment during cancer cachexia (Table 1).
Table 1.
Potential Skeletal Muscle Adaptations to Exercise Training During Cancer Cachexia.
| Muscle Property | Cancer Cachexia | Training Adaptation |
|---|---|---|
| Mass | Decreased | Increased |
| Myofiber CSA | Decreased | Increased |
| Strength | Decreased | Increased |
| Mass regulation | ||
| Protein synthesis | Decreased | Increased |
| Protein breakdown | Increased | Decreased |
| Oxidative capacity | Decreased | Increased |
Abbreviation: CSA, cross-sectional area.
Systemic Inflammation
The elevated pro-inflammatory cytokine environment associated with cancer cachexia is a potential target for physical activity and exercise. The anti-inflammatory effects of exercise have been documented in healthy adults and those with chronic disease. Exercise can regulate several mediators of inflammation including visceral adiposity, production and secretion of anti-inflammatory cytokines from contracting muscle (termed myokines), and altered monocyte and macrophage receptor expression.134 Treadmill exercise could improve survival, and it prevented muscle mass loss in a metastatic tumor model.135 Moreover, exercise training during systemic IL-6 overexpression can prevent body weight and muscle mass loss in tumor bearing mice.35 These data further suggest that aerobic exercise training prior to and during cachexia progression can attenuate muscle mass and strength loss despite elevated systemic inflammation. Interestingly, while cancer patients demonstrate elevated systemic inflammation, IL-6 is increased following a single bout of exercise and has been implicated in mediating the anti-inflammatory effects of exercise. IL-6 can stimulate the production of the anti-inflammatory cytokines IL-1 receptor agonist (IL-1ra) and IL-10,136 and inhibit the production of the pro-inflammatory cytokine TNF-α.137 Related to exercise, the transient induction of these 2 cytokines by exercise is preceded by IL-6.138 Given the role of physical activity and myokines in systemic metabolism, future research is needed to understand the differential responses to acute and chronic exposure to inflammatory cytokines.
Hypogonadism
Low testosterone is associated with reduced muscle mass and function12,64 and significantly contributes to decreased survival during cancer cachexia.47 Exercise has the potential to improve circulating sex hormones or muscle receptor expression. Interestingly, while exercise can regulate the systemic environment, which could also influence circulating sex hormones, muscle loading can increase muscle AR expression independent to changes in systemic hormones in both humans and rodents.139-141 Mechanical loading and muscle growth are accompanied by increased AR concentration in rodents.140,141 In healthy individuals, changes in muscle AR protein content correlated with resistance training induced muscle hypertrophy.139 While speculative, these studies suggest that loading may increase muscle’s sensitivity to circulating androgens and/or nongenomic receptor actions may cooperate with mechano-signaling pathways to improve mass regulation. Future studies are required to determine the precise molecular and functional consequences of increased AR on protein accretion in healthy and wasting skeletal muscle. Interestingly, recent findings suggest that the systemic hormone responses to acute resistance exercise may not be the key determinant for the hypertrophic growth response following exercise training,139,142 which could have significant clinical and functional implications in hypogonadal patients or those undergoing androgen deprivation therapy (eg, prostate cancer). In support of this, eccentric contraction-induced muscle protein synthesis is not altered in castrated mice,143 and castration does not attenuate long-term overload-induced muscle growth in rats.144 Related to cancer, resistance exercise training can improve muscle mass and function in prostate cancer patients receiving androgen deprivation therapy.145 Collectively, these studies suggest that muscle loading/contraction can positively regulate muscle mass and function independent to circulating androgen levels, which could have significant implications in the treatment of cancer cachexia.
Anemia
Anemia is commonly observed in cancer patients and is tightly associated with reduced exercise capacity and increased fatigue.146 In healthy individuals, exercise training increases total hemoglobin and red blood cell mass by stimulating erythropoiesis, which can enhance oxygen delivery to peripheral tissues.147 While exercise can improve functional capacity, and decrease fatigue in cancer patients, whether this is related to improved erythropoiesis and red cell mass has not been mechanistically determined. Related to cancer cachexia, whether exercise is beneficial when anemia is present has been questioned, and it has been suggested that rectifying anemia prior to exercise training may be advantageous.148 In this context, exercise training could be detrimental and potentially reduce survival in exercise intolerant patients. Nonetheless, erythropoiesis stimulating agents and exercise training have been investigated in both cancer patients and preclinical models. Interestingly, while combined treatments can improve indices of oxidative capacity, EPO and exercise can independently improve distinct processes related to improved physical function (eg, anemia and muscle strength).149 Further research is needed to determine the distinct molecular mechanisms associated with improved outcomes related to exercise and combined therapies in cancer patients. Additionally, the appropriate mode, intensity, and frequency of exercise training in different types of anemia need to be established.
Muscle Protein Turnover
In healthy adults, a single bout of exercise can stimulate protein synthesis in various cellular fractions (eg, myofibrillar vs mitochondrial) and can remain elevated for several hours following contraction.150-153 The duration and extent of this induction can be affected by variables such as the exercise type (endurance vs resistance), intensity and workload of muscle contractions, and the nutritional status during the postexercise recovery.151,152,154 The muscle protein synthesis induction by muscle contraction or mechanical signaling corresponds to the activation of mTORC1 signaling in both humans and rodents.151,155-157 Given that exercise can induce protein synthesis for many hours, the potential benefits of exercise for cachectic cancer patients will likely occur in the hours post exercise and coincides with enhanced nutritional responsiveness. Preclinical studies have demonstrated that cachectic muscle retains the capacity to hypertrophy after overload.131,132 The ability of muscle contraction to induce protein synthesis has significant ramifications for cancer cachexia in which Akt/mTORC1 signaling may be disrupted.34,95 Treadmill training during systemic IL-6 overexpression improved mTORC1 signaling in tumor bearing mice.34 Furthermore, a single bout of resistance exercise combined with protein ingestion stimulated muscle protein synthesis in prostate cancer patients on androgen deprivation.158 Given that muscle contraction can upregulate muscle IGF-1 expression, further work is needed to determine if this induction may have critical anabolic or anti-catabolic roles that extend beyond the acute exercise response.
The breakdown of damaged or dysfunctional proteins and organelles is required to maintain normal homeostasis. The UPS and the autophagy-lysosomal system have regulatory roles in skeletal muscle wasting with cancer.110,159,160 While the targeting of aberrant protein degradation processes has been suggested as a potential treatment for cachexia,159 it is conceivable that inhibiting protein degradation processes may be detrimental to systemic metabolism and muscle function. Additionally, damaged proteins induced by contraction or mechanical loading must be removed for the successful restoration of muscle function. Indeed, muscle protein breakdown is also stimulated by both acute endurance and resistance exercise.161-163 While acute exercise can accelerate protein turnover and may serve to replace damaged proteins, this has yet to be examined during cancer cachexia. In contrast, exercise training can decrease indices of protein breakdown in muscle, which may be related to improved protein quality in previously cachectic muscle. Indeed, voluntary wheel running decreased muscle E3 ligase mRNA expression and autophagy protein expression in tumor bearing mice.118 Additionally, treadmill exercise prior to tumor inoculation decreased muscle protein breakdown in rats.128 While exercise-induced improvements have not been observed in all preclinical studies,164 this could be related to variables such as tumor type or exercise intensity. Nonetheless, a primary benefit of exercise on skeletal muscle appears to be the stimulation of protein turnover, thereby improving overall muscle metabolism and function. Additional studies are required to determine if these training adaptations can occur in the cachectic cancer patient.
Muscle Oxidative Metabolism
Endurance training is associated with increased mitochondrial density, capillary supply, and key metabolic enzymes in muscle.165 Repeated exercise induces the expression of several mitochondrial proteins such as PGC-1α, mitochondrial transcription factor A (TFAM), and nuclear respiratory factor (NRF), which can lead to improved muscle oxidative capacity.166 Furthermore, a single bout of exercise rapidly induces PGC-1α expression and mitochondrial-associated gene transcription.167,168 Endurance exercise can also positively regulate mitochondrial dynamics through Mfn1/2 and Fis1 mRNA expression.169 Emerging evidence also suggests that resistance exercise can improve mitochondrial quality and function. mTORC1 associated with Yin Yang 1 (YY1) and PGC-1α signaling can increase mitochondrial gene expression and oxidative function.170 Resistance exercise also induces muscle PGC-1α protein expression.171 Furthermore, improved mitochondrial respiratory capacity and mitochondrial complex protein expression have been observed in hypertrophic muscle following 12 weeks of resistance exercise training in humans.172 Damaged or dysfunctional mitochondria removal has also been identified as an important exercise benefit.120 Indeed, treadmill exercise can prevent IL-6 dysregulation of mitochondrial biogenesis and dynamics in tumor bearing mice initiating cachexia.32 However, severe cachexia can impair the induction of mitochondrial biogenesis signaling after a single bout of concentric muscle contractions.84 Interestingly, repeated bouts of eccentric muscle contraction improved muscle succinate dehydrogenase enzyme activity after the initiation of cachexia,133 which highlights a potential role for resistance exercise in atrophic muscle. While endurance and resistance exercise can alter specific aspects of mitochondrial quality control, further research is needed to determine the impact of these changes for the prevention of muscle wasting, and if cachectic muscle oxidative metabolism retains the plasticity to respond to exercise and muscle contraction.
Exercise Prescription for Cancer Cachexia
American College of Sports Medicine (ACSM) Exercise Prescription for Cancer Survivors
Physical activity is an established modifiable risk factor that is associated with disease occurrence and all-cause mortality.173 Given the importance of physical activity for a cancer survivor’s quantity and quality of life,174 the ACSM has developed exercise guidelines for the cancer patient.175,176 These guidelines are an extension of the US Department of Health and Human Services (US DHHS) Physical Activity Guidelines for Americans.177 The US DHHS guidelines provided generalized recommendations (eg, avoid inactivity) for individuals with chronic conditions such as cancer,177 and therefore the current ACSM recommendations for cancer patients have considerable overlap with the guidelines for healthy individuals (Table 2), which incorporate aerobic, resistance, and flexibility activities. Individuals are encouraged to perform aerobic exercise 3 to 5 days a week at a moderate (150 minutes) to vigorous (75 minutes) intensity, muscle strengthening exercises (eg, resistance training) at least 2 days a week, and incorporate stretching of major muscle groups on days other activities are performed.175,177 Additionally, the ACSM guidelines account for cancer-specific issues (eg, contraindications to exercise, injury risk, etc), and it is highly recommended that exercise programs are adapted based on the individual’s health status, treatment history, and anticipated disease trajectory.175 For example, lower exercise volume/intensity and slower exercise progression may be required depending on cancer type or the individual’s work capacity. Nonetheless, provided the patient has no contraindications and can properly perform exercise, cancer patients are recommended the same physical activity/exercise guidelines as healthy individuals.175,177,178 While the field of “exercise-oncology” is fairly new, these guidelines provide a rationale goal for cancer patients to engage in a structured physical activity and exercise program. Further research is necessary to determine the effectiveness of these recommendations for the prevention of cancer cachexia or the restoration of muscle mass in the wasting patient.
Table 2.
ACSM Exercise Guidelines for Cancer Survivorsa.
| Aerobic | Resistance | Flexibility |
|---|---|---|
| • 150 min/week of moderate-intensity activity, or | • Muscle strengthening activities at moderate intensity >2 days/week for each muscle group | • Stretch major muscle groups and tendons on days other activities are performed |
| • 75 min/week of vigorous-intensity activity, or | ||
| • Equivalent combination |
Abbreviation: ACSM, American College of Sports Medicine.
The guidelines were adapted from the US Department of Health and Human Services (US DHHS) Physical Activity Guidelines for Americans.177 In general, cancer patients are recommended a similar exercise prescription as healthy individuals provided the individual has no contraindications to exercise or a limited work capacity. However, additional supervision may be required depending on the health status or functional impairments (eg, cachexia severity, cancer type, cancer stage, etc). Lighter intensities and slower progression to greater volume/intensities are recommended.
Current Trials and Future Recommendations
The current body of research examining cancer cachexia suggests that a multimodal intervention, which encompasses exercise, nutritional, and pharmacological strategies, will likely be required to combat the condition.179 While exercise has generally shown to be beneficial for the cancer patient, it is currently not established if similar benefits can be achieved in a wasting cancer patient with a limited work capacity. To date there has only been 2 phase II randomized control trials utilizing exercise within a multimodal treatment regimen of cachectic cancer patients.179,180 An ongoing clinical trial (NCT02330926) and a second completed clinical trial (NCT01419145) have reported the high feasibility of exercise training in advanced-stage cancer patients.180 While the study interpretations were limited by being statistically underpowered, this multimodal trial also suggested improved body weight and arm muscle mass in the treatment group.180 Additionally, a phase III randomized control trial is currently underway to determine if exercise can prevent/attenuate cachexia in cancer patients (EudraCT 2013-002282-19). The results from these studies should significantly enhance our understanding of exercise’s effects on clinically relevant outcomes (eg, muscle mass, survival) in cachectic cancer patients. It appears that both aerobic and resistance exercise programs may be beneficial for the cachectic cancer patient. Since cancer patients will likely participate in combined exercise programs, this should be investigated in future preclinical studies. Given the heterogeneity of cancer types, treatment regimens, and physical limitations during cachexia progression, an individualized exercise prescription tailored to clinically relevant outcomes has been recommended.181 While the exact exercise prescription will not be identical for all cachectic cancer patients, future training studies should emphasize both aerobic and resistance exercise components and account for the patient’s limitations. Nonetheless, a multimodal therapy (eg, exercise, nutrition, cancer treatment) will likely be needed to maximally prevent cachexia and improve a cancer patient’s quantity and quality of life.
Conclusion
While the successful treatment for the cachectic cancer patient remains elusive, common systemic disruptions with cancer remain viable therapeutic targets to both prevent and treat the cachectic condition. Furthermore, targeting skeletal muscle has the potential to reduce systemic wasting and improve overall metabolism and physical function. Muscle metabolic health and mass are dramatically affected by physical activity and exercise. Physical activity and exercise are already thought to be beneficial during cancer treatment and survival and hold clear potential as a nonpharmacological treatment for muscle wasting conditions. While successful randomized controlled trials in cachectic cancer patients are lacking, emerging evidence from preclinical cancer models suggest an interaction between increased physical activity/exercise and improvements to muscle mass, metabolism, and function (Figure 1). However, these treatments may be specific to the type of cancer and will likely need to be used in a multimodal format. Further research is warranted to determine the mechanistic basis of these improvements and if these benefits can be achieved in the cachectic cancer patient.
Figure 1.
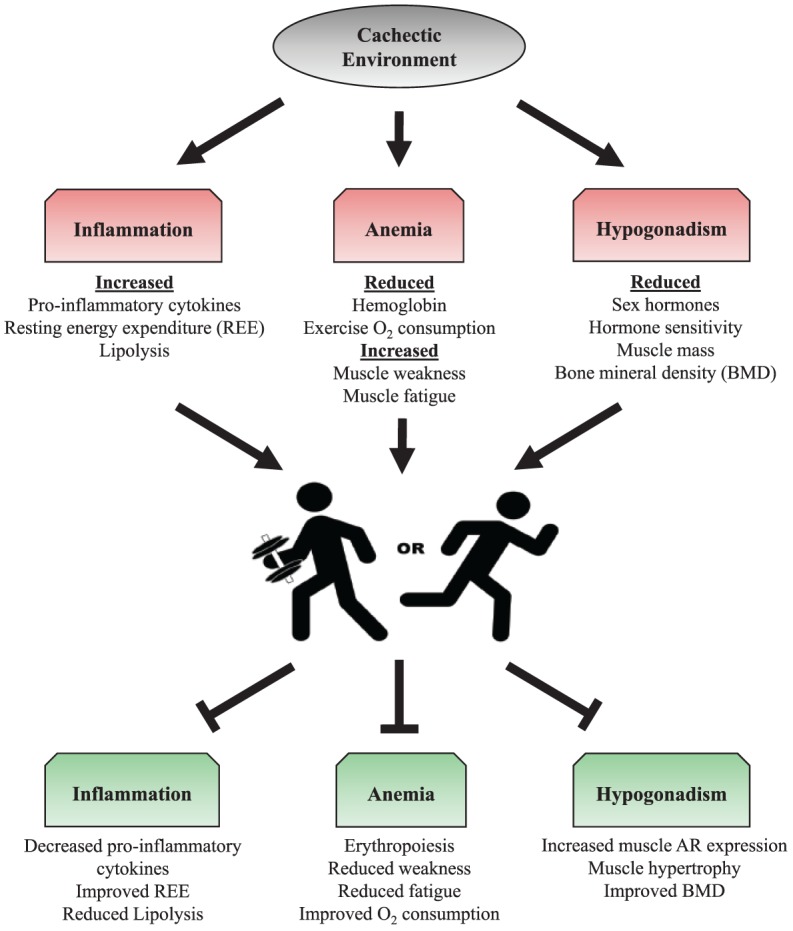
Potential exercise training alterations to the systemic environment during cancer cachexia. Systemic disruptions (eg, inflammation, anemia, hypogonadism) have been identified as therapeutic targets of cancer cachexia. Resistance and aerobic exercise training can positively influence these characteristics, and therefore may be a potential target to increase patient survival. Abbreviations: REE, resting energy expenditure; O2, oxygen; BMD, bone mineral density; AR, androgen receptor.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Institutes of Health Grant # R01-CA121249 from the National Cancer Institute (JAC), National Institutes of Health Grant # P20 RR-017698 from the National Center for Research (JAC), SPARC Graduate Research Grant from the Office of the Vice President for Research at the University of South Carolina (JPH), and an ACSM Foundation Research Grant from the American College of Sports Medicine Foundation (JPH).
References
- 1. Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489-495. [DOI] [PubMed] [Google Scholar]
- 2. Evans WJ, Morley JE, Argilés J, et al. Cachexia: a new definition. Clin Nutr. 2008;27:793-799. [DOI] [PubMed] [Google Scholar]
- 3. Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev. 2009;89:381-410. [DOI] [PubMed] [Google Scholar]
- 4. Dodson S, Baracos VE, Jatoi A, et al. Muscle wasting in cancer cachexia: clinical implications, diagnosis, and emerging treatment strategies. Annu Rev Med. 2011;62:265-279. [DOI] [PubMed] [Google Scholar]
- 5. Jung HW, Kim JW, Kim JY, et al. Effect of muscle mass on toxicity and survival in patients with colon cancer undergoing adjuvant chemotherapy. Support Care Cancer. 2015;23:687-694. [DOI] [PubMed] [Google Scholar]
- 6. Barret M, Antoun S, Dalban C, et al. Sarcopenia is linked to treatment toxicity in patients with metastatic colorectal cancer. Nutr Cancer. 2014;66:583-589. [DOI] [PubMed] [Google Scholar]
- 7. Couch ME, Dittus K, Toth MJ, et al. Cancer cachexia update in head and neck cancer: pathophysiology and treatment. Head Neck. 2015;37:1057-1072. [DOI] [PubMed] [Google Scholar]
- 8. Fearon KC, Voss AC, Hustead DS; Cancer Cachexia Study Group. Definition of cancer cachexia: effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am J Clin Nutr. 2006;83:1345-1350. [DOI] [PubMed] [Google Scholar]
- 9. Vaughan VC, Martin P, Lewandowski PA. Cancer cachexia: impact, mechanisms, and emerging treatments. J Cachexia Sarcopenia Muscle. 2013;4:95-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006;84:475-482. [DOI] [PubMed] [Google Scholar]
- 11. Argiles JM, Lopez-Soriano FJ, Busquets S. Counteracting inflammation: a promising therapy in cachexia. Crit Rev Oncog. 2012;17:253-262. [DOI] [PubMed] [Google Scholar]
- 12. Burney BO, Hayes TG, Smiechowska J, et al. Low testosterone levels and increased inflammatory markers in patients with cancer and relationship with cachexia. J Clin Endocrinol Metab. 2012;97:E700-E709. [DOI] [PubMed] [Google Scholar]
- 13. Stephens NA, Gray C, MacDonald AJ, et al. Sexual dimorphism modulates the impact of cancer cachexia on lower limb muscle mass and function. Clin Nutr. 2012;31:499-505. [DOI] [PubMed] [Google Scholar]
- 14. Ando K, Takahashi F, Kato M, et al. Tocilizumab, a proposed therapy for the cachexia of interleukin6-expressing lung cancer. PLoS One. 2014;9:e102436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Narsale AA, Carson JA. Role of interleukin-6 in cachexia: therapeutic implications. Curr Opin Support Palliat Care. 2014;8:321-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tisdale MJ. Cachexia in cancer patients. Nat Rev Cancer. 2002;2:862-871. [DOI] [PubMed] [Google Scholar]
- 17. Onesti JK, Guttridge DC. Inflammation based regulation of cancer cachexia. Biomed Res Int. 2014;2014:168407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carson JA, Baltgalvis KA. Interleukin 6 as a key regulator of muscle mass during cachexia. Exerc Sport Sci Rev. 2010;38:168-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Strassmann G, Fong M, Kenney JS, Jacob CO. Evidence for the involvement of interleukin 6 in experimental cancer cachexia. J Clin Invest. 1992;89:1681-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bilir C, Engin H, Can M, Temi YB, Demirtas D. The prognostic role of inflammation and hormones in patients with metastatic cancer with cachexia. Med Oncol. 2015;32:56. [DOI] [PubMed] [Google Scholar]
- 21. Carbó N, Busquets S, van Royen M, Alvarez B, López-Soriano FJ, Argilés JM. TNF-alpha is involved in activating DNA fragmentation in skeletal muscle. Br J Cancer. 2002;86:1012-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alves MJ, Figuerêdo RG, Azevedo FF, et al. Adipose tissue fibrosis in human cancer cachexia: the role of TGFβ pathway. BMC Cancer. 2017;17:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Llovera M, Garcia-Martínez C, López-Soriano J, et al. Protein turnover in skeletal muscle of tumour-bearing transgenic mice overexpressing the soluble TNF receptor-1. Cancer Lett. 1998;130:19-27. [DOI] [PubMed] [Google Scholar]
- 24. López-Soriano J, Llovera M, Carbó N, Garcia-Martinez C, López-Soriano FJ, Argiles JM. Lipid metabolism in tumour-bearing mice: studies with knockout mice for tumour necrosis factor receptor 1 protein. Mol Cell Endocrinol. 1997;132:93-99. [DOI] [PubMed] [Google Scholar]
- 25. Costelli P, Carbó N, Tessitore L, et al. Tumor necrosis factor-alpha mediates changes in tissue protein turnover in a rat cancer cachexia model. J Clin Invest. 1993;92:2783-2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Llovera M, Carbo N, Garcia-Martinez C, et al. Anti-TNF treatment reverts increased muscle ubiquitin gene expression in tumour-bearing rats. Biochem Biophys Res Commun. 1996;221:653-655. [DOI] [PubMed] [Google Scholar]
- 27. Jatoi A, Dakhil SR, Nguyen PL, et al. A placebo-controlled double blind trial of etanercept for the cancer anorexia/weight loss syndrome: results from N00C1 from the North Central cancer treatment group. Cancer. 2007;110:1396-1403. [DOI] [PubMed] [Google Scholar]
- 28. Jatoi A, Ritter HL, Dueck A, et al. A placebo-controlled, double-blind trial of infliximab for cancer-associated weight loss in elderly and/or poor performance non-small cell lung cancer patients (N01C9). Lung Cancer. 2010;68:234-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zimmers TA, McKillop IH, Pierce RH, Yoo JY, Koniaris LG. Massive liver growth in mice induced by systemic interleukin 6 administration. Hepatology. 2003;38:326-334. [DOI] [PubMed] [Google Scholar]
- 30. Senn JJ, Klover PJ, Nowak IA, et al. Suppressor of cytokine signaling-3 (SOCS-3), a potential mediator of interleukin-6-dependent insulin resistance in hepatocytes. J Biol Chem. 2003;278:13740-13746. [DOI] [PubMed] [Google Scholar]
- 31. Das SK, Hoefler G. The role of triglyceride lipases in cancer associated cachexia. Trends Mol Med. 2013;19:292-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. White JP, Puppa MJ, Sato S, et al. IL-6 regulation on skeletal muscle mitochondrial remodeling during cancer cachexia in the ApcMin/+ mouse. Skelet Muscle. 2012;2:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bonetto A, Aydogdu T, Jin X, et al. JAK/STAT3 pathway inhibition blocks skeletal muscle wasting downstream of IL-6 and in experimental cancer cachexia. Am J Physiol Endocrinol Metab. 2012;303:E410-E421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. White JP, Puppa MJ, Gao S, Sato S, Welle SL, Carson JA. Muscle mTORC1 suppression by IL-6 during cancer cachexia: a role for AMPK. Am J Physiol Endocrinol Metab. 2013;304:E1042-E1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Puppa MJ, White JP, Velázquez KT, et al. The effect of exercise on IL-6-induced cachexia in the Apc (Min/+) mouse. J Cachexia Sarcopenia Muscle. 2012;3:117-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Toledo M, Penna F, Busquets S, López-Soriano FJ, Argilés JM. Distinct behaviour of sorafenib in experimental cachexia-inducing tumours: the role of STAT3. PLoS One. 2014;9:e113931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ando K, Takahashi F, Motojima S, et al. Possible role for tocilizumab, an anti-interleukin-6 receptor antibody, in treating cancer cachexia. J Clin Oncol. 2013;31:e69-e72. [DOI] [PubMed] [Google Scholar]
- 38. Hirata H, Tetsumoto S, Kijima T, et al. Favorable responses to tocilizumab in two patients with cancer-related cachexia. J Pain Symptom Manage. 2013;46:e9-e13. [DOI] [PubMed] [Google Scholar]
- 39. Reinke H, Asher G. Circadian clock control of liver metabolic functions. Gastroenterology. 2016;150:574-580. [DOI] [PubMed] [Google Scholar]
- 40. Wang Z, Ying Z, Bosy-Westphal A, et al. Specific metabolic rates of major organs and tissues across adulthood: evaluation by mechanistic model of resting energy expenditure. Am J Clin Nutr. 2010;92:1369-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gallagher D, Belmonte D, Deurenberg P, et al. Organ-tissue mass measurement allows modeling of REE and metabolically active tissue mass. Am J Physiol. 1998;275(2 pt 1):E249-E258. [DOI] [PubMed] [Google Scholar]
- 42. Fredrix EW, Soeters PB, Wouters EF, Deerenberg IM, von Meyenfeldt MF, Saris WH. Energy balance in relation to cancer cachexia. Clin Nutr. 1990;9:319-324. [DOI] [PubMed] [Google Scholar]
- 43. Falconer JS, Fearon KC, Plester CE, Ross JA, Carter DC. Cytokines, the acute-phase response, and resting energy expenditure in cachectic patients with pancreatic cancer. Ann Surg. 1994;219:325-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bonetto A, Aydogdu T, Kunzevitzky N, et al. STAT3 activation in skeletal muscle links muscle wasting and the acute phase response in cancer cachexia. PLoS One. 2011;6:e22538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Narsale AA, Enos RT, Puppa MJ, et al. Liver inflammation and metabolic signaling in ApcMin/+ mice: the role of cachexia progression. PLoS One. 2015;10:e0119888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lieffers JR, Mourtzakis M, Hall KD, McCargar LJ, Prado CM, Baracos VE. A viscerally driven cachexia syndrome in patients with advanced colorectal cancer: contributions of organ and tumor mass to whole-body energy demands. Am J Clin Nutr. 2009;89:1173-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Skipworth RJ, Moses AG, Sangster K, et al. Interaction of gonadal status with systemic inflammation and opioid use in determining nutritional status and prognosis in advanced pancreatic cancer. Support Care Cancer. 2011;19:391-401. [DOI] [PubMed] [Google Scholar]
- 48. Scott HR, McMillan DC, Crilly A, McArdle CS, Milroy R. The relationship between weight loss and interleukin 6 in non-small-cell lung cancer. Br J Cancer. 1996;73:1560-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tisdale MJ. Molecular pathways leading to cancer cachexia. Physiology (Bethesda). 2005;20:340-348. [DOI] [PubMed] [Google Scholar]
- 50. Yamashita AS, das Neves RX, Rosa-Neto JC, et al. White adipose tissue IFN-γ expression and signalling along the progression of rodent cancer cachexia. Cytokine. 2017;89:122-126. [DOI] [PubMed] [Google Scholar]
- 51. Stanford KI, Goodyear LJ. Muscle-adipose tissue cross talk [published online May 15, 2017]. Cold Spring Harb Perspect Med. doi: 10.1101/cshperspect.a029801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Argilés JM, Busquets S, Stemmler B, López-Soriano FJ. Cancer cachexia: understanding the molecular basis. Nat Rev Cancer. 2014;14:754-762. [DOI] [PubMed] [Google Scholar]
- 53. Argilés JM, López-Soriano J, Almendro V, Busquets S, López-Soriano FJ. Cross-talk between skeletal muscle and adipose tissue: a link with obesity? Med Res Rev. 2005;25:49-65. [DOI] [PubMed] [Google Scholar]
- 54. Das SK, Eder S, Schauer S, et al. Adipose triglyceride lipase contributes to cancer-associated cachexia. Science. 2011;333:233-238. [DOI] [PubMed] [Google Scholar]
- 55. Stephens NA, Skipworth RJ, Macdonald AJ, Greig CA, Ross JA, Fearon KC. Intramyocellular lipid droplets increase with progression of cachexia in cancer patients. J Cachexia Sarcopenia Muscle. 2011;2:111-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Weber MA, Krakowski-Roosen H, Schroder L, et al. Morphology, metabolism, microcirculation, and strength of skeletal muscles in cancer-related cachexia. Acta Oncol. 2009;48:116-124. [DOI] [PubMed] [Google Scholar]
- 57. Agustsson T, Wikrantz P, Rydén M, Brismar T, Isaksson B. Adipose tissue volume is decreased in recently diagnosed cancer patients with cachexia. Nutrition. 2012;28:851-855. [DOI] [PubMed] [Google Scholar]
- 58. Carson JA, Manolagas SC. Effects of sex steroids on bones and muscles: similarities, parallels, and putative interactions in health and disease. Bone. 2015;80:67-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cosper PF, Leinwand LA. Cancer causes cardiac atrophy and autophagy in a sexually dimorphic manner. Cancer Res. 2011;71:1710-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hetzler KL, Hardee JP, Puppa MJ, et al. Sex differences in the relationship of IL-6 signaling to cancer cachexia progression. Biochim Biophys Acta. 2015;1852:816-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Burney BO, Garcia JM. Hypogonadism in male cancer patients. J Cachexia Sarcopenia Muscle. 2012;3:149-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Garcia JM, Li H, Mann D, et al. Hypogonadism in male patients with cancer. Cancer. 2006;106:2583-2591. [DOI] [PubMed] [Google Scholar]
- 63. Simons JP, Schols AM, Buurman WA, Wouters EF. Weight loss and low body cell mass in males with lung cancer: relationship with systemic inflammation, acute-phase response, resting energy expenditure, and catabolic and anabolic hormones. Clin Sci (Lond). 1999;97:215-223. [PubMed] [Google Scholar]
- 64. White JP, Puppa MJ, Narsale A, Carson JA. Characterization of the male ApcMin/+ mouse as a hypogonadism model related to cancer cachexia. Biol Open. 2013;2:1346-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bhasin S, Storer TW, Berman N, et al. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med. 1996;335:1-7. [DOI] [PubMed] [Google Scholar]
- 66. Bhasin S, Calof OM, Storer TW, et al. Drug insight: testosterone and selective androgen receptor modulators as anabolic therapies for chronic illness and aging. Nat Clin Pract Endocrinol Metab. 2006;2:146-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Dubois V, Laurent M, Boonen S, Vanderschueren D, Claessens F. Androgens and skeletal muscle: cellular and molecular action mechanisms underlying the anabolic actions. Cell Mol Life Sci. 2012;69:1651-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hendifar A, Yang D, Lenz F, et al. Gender disparities in metastatic colorectal cancer survival. Clin Cancer Res. 2009;15:6391-6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Palomares MR, Sayre JW, Shekar KC, Lillington LM, Chlebowski RT. Gender influence on weight-loss pattern and survival of nonsmall cell lung carcinoma patients. Cancer. 1996;78:2119-2126. [DOI] [PubMed] [Google Scholar]
- 70. Murphy E. Estrogen signaling and cardiovascular disease. Circ Res. 2011;109:687-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hetzler KL, Hardee JP, LaVoie HA, Murphy EA, Carson JA. Ovarian function’s role during cancer cachexia progression in the female mouse. Am J Physiol Endocrinol Metab. 2017;312:E447-E459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bruera E, Sweeney C. Cachexia and asthenia in cancer patients. Lancet Oncol. 2000;1:138-147. [DOI] [PubMed] [Google Scholar]
- 73. Knight K, Wade S, Balducci L. Prevalence and outcomes of anemia in cancer: a systematic review of the literature. Am J Med. 2004;116(suppl 7A):11S-26S. [DOI] [PubMed] [Google Scholar]
- 74. Caro JJ, Salas M, Ward A, Goss G. Anemia as an independent prognostic factor for survival in patients with cancer: a systemic, quantitative review. Cancer. 2001;91:2214-2221. [PubMed] [Google Scholar]
- 75. Spivak JL. Cancer-related anemia: its causes and characteristics. Semin Oncol. 1994;21(2 suppl 3):3-8. [PubMed] [Google Scholar]
- 76. Glaspy J, Degos L, Dicato M, Demetri GD. Comparable efficacy of epoetin alfa for anemic cancer patients receiving platinum- and nonplatinum-based chemotherapy: a retrospective subanalysis of two large, community-based trials. Oncologist. 2002;7:126-135. [DOI] [PubMed] [Google Scholar]
- 77. Henry DH. Optimizing the treatment of anemia in cancer patients. The role of a new erythropoietic agent. Oncology (Williston Park). 2002;16(10 suppl 11):9-12. [PubMed] [Google Scholar]
- 78. Milano M, Schneider M. EPO in cancer anemia: benefits and potential risks. Crit Rev Oncol Hematol. 2007;62:119-125. [DOI] [PubMed] [Google Scholar]
- 79. Shasha D, George MJ, Harrison LB. Once-weekly dosing of epoetin-alpha increases hemoglobin and improves quality of life in anemic cancer patients receiving radiation therapy either concomitantly or sequentially with chemotherapy. Cancer. 2003;98:1072-1079. [DOI] [PubMed] [Google Scholar]
- 80. Daneryd P, Svanberg E, Körner U, et al. Protection of metabolic and exercise capacity in unselected weight-losing cancer patients following treatment with recombinant erythropoietin: a randomized prospective study. Cancer Res. 1998;58:5374-5379. [PubMed] [Google Scholar]
- 81. Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol. 2012;2:1143-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gibney E, Elia M, Jebb SA, Murgatroyd P, Jennings G. Total energy expenditure in patients with small-cell lung cancer: results of a validated study using the bicarbonate-urea method. Metabolism. 1997;46:1412-1417. [DOI] [PubMed] [Google Scholar]
- 83. Moses AW, Slater C, Preston T, Barber MD, Fearon KC. Reduced total energy expenditure and physical activity in cachectic patients with pancreatic cancer can be modulated by an energy and protein dense oral supplement enriched with n-3 fatty acids. Br J Cancer. 2004;90:996-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Puppa MJ, Murphy EA, Fayad R, Hand GA, Carson JA. Cachectic skeletal muscle response to a novel bout of low-frequency stimulation. J Appl Physiol (1985). 2014;116:1078-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Baltgalvis KA, Berger FG, Peña MM, Mark Davis J, White JP, Carson JA. Activity level, apoptosis, and development of cachexia in Apc(Min/+) mice. J Appl Physiol (1985). 2010;109:1155-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Cramer L, Hildebrandt B, Kung T, et al. Cardiovascular function and predictors of exercise capacity in patients with colorectal cancer. J Am Coll Cardiol. 2014;64:1310-1319. [DOI] [PubMed] [Google Scholar]
- 87. Murphy KT, Chee A, Trieu J, Naim T, Lynch GS. Importance of functional and metabolic impairments in the characterization of the C-26 murine model of cancer cachexia. Dis Model Mech. 2012;5:533-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Murphy KT, Struk A, Malcontenti-Wilson C, Christophi C, Lynch GS. Physiological characterization of a mouse model of cachexia in colorectal liver metastases. Am J Physiol Regul Integr Comp Physiol. 2013;304:R854-R864. [DOI] [PubMed] [Google Scholar]
- 89. Roberts BM, Frye GS, Ahn B, Ferreira LF, Judge AR. Cancer cachexia decreases specific force and accelerates fatigue in limb muscle. Biochem Biophys Res Commun. 2013;435:488-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Aulino P, Berardi E, Cardillo VM, et al. Molecular, cellular and physiological characterization of the cancer cachexia-inducing C26 colon carcinoma in mouse. BMC Cancer. 2010;10:363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kimball SR, Farrell PA, Jefferson LS. Invited review: role of insulin in translational control of protein synthesis in skeletal muscle by amino acids or exercise. J Appl Physiol (1985). 2002;93:1168-1180. [DOI] [PubMed] [Google Scholar]
- 92. Schiaffino S, Mammucari C. Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: insights from genetic models. Skelet Muscle. 2011;1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Samuels SE, Knowles AL, Tilignac T, Debiton E, Madelmont JC, Attaix D. Higher skeletal muscle protein synthesis and lower breakdown after chemotherapy in cachectic mice. Am J Physiol Regul Integr Comp Physiol. 2001;281:R133-R139. [DOI] [PubMed] [Google Scholar]
- 94. Smith KL, Tisdale MJ. Increased protein degradation and decreased protein synthesis in skeletal muscle during cancer cachexia. Br J Cancer. 1993;67:680-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. White JP, Baynes JW, Welle SL, et al. The regulation of skeletal muscle protein turnover during the progression of cancer cachexia in the Apc(Min/+) mouse. PLoS One. 2011;6:e24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Carson JA, Hardee JP, VanderVeen BN. The emerging role of skeletal muscle oxidative metabolism as a biological target and cellular regulator of cancer-induced muscle wasting. Semin Cell Dev Biol. 2016;54:53-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122(pt 20):3589-3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Fayard E, Tintignac LA, Baudry A, Hemmings BA. Protein kinase B/Akt at a glance. J Cell Sci. 2005;118(pt 24):5675-5678. [DOI] [PubMed] [Google Scholar]
- 99. Bentzinger CF, Lin S, Romanino K, et al. Differential response of skeletal muscles to mTORC1 signaling during atrophy and hypertrophy. Skelet Muscle. 2013;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bentzinger CF, Romanino K, Cloëtta D, et al. Skeletal muscle-specific ablation of raptor, but not of rictor, causes metabolic changes and results in muscle dystrophy. Cell Metab. 2008;8:411-424. [DOI] [PubMed] [Google Scholar]
- 101. Combaret L, Rallière C, Taillandier D, Tanaka K, Attaix D. Manipulation of the ubiquitin-proteasome pathway in cachexia: pentoxifylline suppresses the activation of 20S and 26S proteasomes in muscles from tumor-bearing rats. Mol Biol Rep. 1999;26:95-101. [DOI] [PubMed] [Google Scholar]
- 102. Horstman AM, Olde Damink SW, Schols AM, van Loon LJ. Is cancer cachexia attributed to impairments in basal or postprandial muscle protein metabolism? Nutrients. 2016;8(8). doi: 10.3390/nu8080499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Dillon EL, Basra G, Horstman AM, et al. Cancer cachexia and anabolic interventions: a case report. J Cachexia Sarcopenia Muscle. 2012;3:253-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Dillon EL, Volpi E, Wolfe RR, et al. Amino acid metabolism and inflammatory burden in ovarian cancer patients undergoing intense oncological therapy. Clin Nutr. 2007;26:736-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Williams JP, Phillips BE, Smith K, et al. Effect of tumor burden and subsequent surgical resection on skeletal muscle mass and protein turnover in colorectal cancer patients. Am J Clin Nutr. 2012;96:1064-1070. [DOI] [PubMed] [Google Scholar]
- 106. Deutz NE, Safar A, Schutzler S, et al. Muscle protein synthesis in cancer patients can be stimulated with a specially formulated medical food. Clin Nutr. 2011;30:759-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Breen L, Phillips SM. Skeletal muscle protein metabolism in the elderly: interventions to counteract the “anabolic resistance” of ageing. Nutr Metab (Lond). 2011;8:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Horstman AM, Sheffield-Moore M. Nutritional/metabolic response in older cancer patients. Nutrition. 2015;31:605-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Lecker SH, Solomon V, Mitch WE, Goldberg AL. Muscle protein breakdown and the critical role of the ubiquitin-proteasome pathway in normal and disease states. J Nutr. 1999;129(1S suppl):227S-237S. [DOI] [PubMed] [Google Scholar]
- 110. Sandri M. Protein breakdown in muscle wasting: role of autophagy-lysosome and ubiquitin-proteasome. Int J Biochem Cell Biol. 2013;45:2121-2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Attaix D, Ventadour S, Codran A, Béchet D, Taillandier D, Combaret L. The ubiquitin-proteasome system and skeletal muscle wasting. Essays Biochem. 2005;41:173-186. [DOI] [PubMed] [Google Scholar]
- 112. Bodine SC, Latres E, Baumhueter S, et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704-1708. [DOI] [PubMed] [Google Scholar]
- 113. Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Eskelinen EL, Saftig P. Autophagy: a lysosomal degradation pathway with a central role in health and disease. Biochim Biophys Acta. 2009;1793:664-673. [DOI] [PubMed] [Google Scholar]
- 115. Ham DJ, Murphy KT, Chee A, Lynch GS, Koopman R. Glycine administration attenuates skeletal muscle wasting in a mouse model of cancer cachexia. Clin Nutr. 2014;33:448-458. [DOI] [PubMed] [Google Scholar]
- 116. Pettersen K, Andersen S, Degen S, et al. Cancer cachexia associates with a systemic autophagy-inducing activity mimicked by cancer cell-derived IL-6 trans-signaling. Sci Rep. 2017;7:2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Aversa Z, Pin F, Lucia S, et al. Autophagy is induced in the skeletal muscle of cachectic cancer patients. Sci Rep. 2016;6:30340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Pigna E, Berardi E, Aulino P, et al. Aerobic exercise and pharmacological treatments counteract cachexia by modulating autophagy in colon cancer. Sci Rep. 2016;6:26991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Stephens NA, Skipworth RJ, Gallagher IJ, et al. Evaluating potential biomarkers of cachexia and survival in skeletal muscle of upper gastrointestinal cancer patients. J Cachexia Sarcopenia Muscle. 2015;6:53-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Yan Z, Lira VA, Greene NP. Exercise training-induced regulation of mitochondrial quality. Exerc Sport Sci Rev. 2012;40:159-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Chan DC. Fusion and fission: interlinked processes critical for mitochondrial health. Annu Rev Genet. 2012;46:265-287. [DOI] [PubMed] [Google Scholar]
- 122. Op den Kamp CM, Gosker HR, Lagarde S, et al. Preserved muscle oxidative metabolic phenotype in newly diagnosed non-small cell lung cancer cachexia. J Cachexia Sarcopenia Muscle. 2015;6:164-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. White JP, Baltgalvis KA, Puppa MJ, Sato S, Baynes JW, Carson JA. Muscle oxidative capacity during IL-6-dependent cancer cachexia. Am J Physiol Regul Integr Comp Physiol. 2011;300:R201-R211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Zinna EM, Yarasheski KE. Exercise treatment to counteract protein wasting of chronic diseases. Curr Opin Clin Nutr Metab Care. 2003;6:87-93. [DOI] [PubMed] [Google Scholar]
- 125. Hurley BF, Hanson ED, Sheaff AK. Strength training as a countermeasure to aging muscle and chronic disease. Sports Med. 2011;41:289-306. [DOI] [PubMed] [Google Scholar]
- 126. Deuster PA, Morrison SD, Ahrens RA. Endurance exercise modifies cachexia of tumor growth in rats. Med Sci Sports Exerc. 1985;17:385-392. [PubMed] [Google Scholar]
- 127. Penna F, Busquets S, Pin F, et al. Combined approach to counteract experimental cancer cachexia: eicosapentaenoic acid and training exercise. J Cachexia Sarcopenia Muscle. 2011;2:95-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Salomão EM, Toneto AT, Silva GO, Gomes-Marcondes MC. Physical exercise and a leucine-rich diet modulate the muscle protein metabolism in Walker tumor-bearing rats. Nutr Cancer. 2010;62:1095-1104. [DOI] [PubMed] [Google Scholar]
- 129. al-Majid S, McCarthy DO. Resistance exercise training attenuates wasting of the extensor digitorum longus muscle in mice bearing the colon-26 adenocarcinoma. Biol Res Nurs. 2001;2:155-166. [DOI] [PubMed] [Google Scholar]
- 130. Jaweed MM, Herbison GJ, Miller EE, Ditunno JF. Compensatory hypertrophy of the soleus in tumor-bearing rats. J Neurol Sci. 1983;61:171-179. [DOI] [PubMed] [Google Scholar]
- 131. Norton JA, Lowry SF, Brennan MF. Effect of work-induced hypertrophy on skeletal muscle of tumor- and nontumor-bearing rats. J Appl Physiol Respir Environ Exerc Physiol. 1979;46:654-657. [DOI] [PubMed] [Google Scholar]
- 132. Otis JS, Lees SJ, Williams JH. Functional overload attenuates plantaris atrophy in tumor-bearing rats. BMC Cancer. 2007;7:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Hardee JP, Mangum JE, Gao S, et al. Eccentric contraction-induced myofiber growth in tumor-bearing mice. J Appl Physiol (1985). 2016;120:29-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11:607-615. [DOI] [PubMed] [Google Scholar]
- 135. Jee H, Chang JE, Yang EJ. Positive prehabilitative effect of intense treadmill exercise for ameliorating cancer cachexia symptoms in a mouse model. J Cancer. 2016;7:2378-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Steensberg A, Fischer CP, Keller C, Møller K, Pedersen BK. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am J Physiol Endocrinol Metab. 2003;285:E433-E437. [DOI] [PubMed] [Google Scholar]
- 137. Mizuhara H, O’Neill E, Seki N, et al. T cell activation-associated hepatic injury: mediation by tumor necrosis factors and protection by interleukin 6. J Exp Med. 1994;179:1529-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol (1985). 2005;98:1154-1162. [DOI] [PubMed] [Google Scholar]
- 139. Mitchell CJ, Churchward-Venne TA, Bellamy L, Parise G, Baker SK, Phillips SM. Muscular and systemic correlates of resistance training-induced muscle hypertrophy. PLoS One. 2013;8:e78636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Lee WJ, Thompson RW, McClung JM, Carson JA. Regulation of androgen receptor expression at the onset of functional overload in rat plantaris muscle. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1076-R1085. [DOI] [PubMed] [Google Scholar]
- 141. Lee WJ, McClung J, Hand GA, Carson JA. Overload-induced androgen receptor expression in the aged rat hindlimb receiving nandrolone decanoate. J Appl Physiol (1985). 2003;94:1153-1161. [DOI] [PubMed] [Google Scholar]
- 142. West DW, Phillips SM. Associations of exercise-induced hormone profiles and gains in strength and hypertrophy in a large cohort after weight training. Eur J Appl Physiol. 2012;112:2693-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Steiner JL, Fukuda DH, Rossetti ML, Hoffman JR, Gordon BS. Castration alters protein balance after high-frequency muscle contraction. J Appl Physiol (1985). 2017;122:264-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Max SR, Rance NE. No effect of sex steroids on compensatory muscle hypertrophy. J Appl Physiol Respir Environ Exerc Physiol. 1984;56:1589-1593. [DOI] [PubMed] [Google Scholar]
- 145. Segal RJ, Reid RD, Courneya KS, et al. Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. J Clin Oncol. 2003;21:1653-1659. [DOI] [PubMed] [Google Scholar]
- 146. Groopman JE, Itri LM. Chemotherapy-induced anemia in adults: incidence and treatment. J Natl Cancer Inst. 1999;91:1616-1634. [DOI] [PubMed] [Google Scholar]
- 147. Mairbäurl H. Red blood cells in sports: effects of exercise and training on oxygen supply by red blood cells. Front Physiol. 2013;4:332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Argilés JM, Busquets S, López-Soriano FJ, Costelli P, Penna F. Are there any benefits of exercise training in cancer cachexia? J Cachexia Sarcopenia Muscle. 2012;3:73-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Pin F, Busquets S, Toledo M, et al. Combination of exercise training and erythropoietin prevents cancer-induced muscle alterations. Oncotarget. 2015;6:43202-43215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Philp A, Schenk S, Perez-Schindler J, et al. Rapamycin does not prevent increases in myofibrillar or mitochondrial protein synthesis following endurance exercise. J Physiol. 2015;593:4275-4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Wilkinson SB, Phillips SM, Atherton PJ, et al. Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J Physiol. 2008;586:3701-3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Atherton PJ, Smith K. Muscle protein synthesis in response to nutrition and exercise. J Physiol. 2012;590:1049-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Cuthbertson DJ, Babraj J, Smith K, et al. Anabolic signaling and protein synthesis in human skeletal muscle after dynamic shortening or lengthening exercise. Am J Physiol Endocrinol Metab. 2006;290:E731-E738. [DOI] [PubMed] [Google Scholar]
- 154. Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013;17:162-184. [DOI] [PubMed] [Google Scholar]
- 155. Witkowski S, Lovering RM, Spangenburg EE. High-frequency electrically stimulated skeletal muscle contractions increase p70s6k phosphorylation independent of known IGF-I sensitive signaling pathways. FEBS Lett. 2010;584:2891-2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Spangenburg EE, Le Roith D, Ward CW, Bodine SC. A functional insulin-like growth factor receptor is not necessary for load-induced skeletal muscle hypertrophy. J Physiol. 2008;586:283-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Hamilton DL, Philp A, MacKenzie MG, Baar K. A limited role for PI(3,4,5)P3 regulation in controlling skeletal muscle mass in response to resistance exercise. PLoS One. 2010;5:e11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Hanson ED, Nelson AR, West DW, et al. Attenuation of resting but not load-mediated protein synthesis in prostate cancer patients on androgen deprivation. J Clin Endocrinol Metab. 2017;102:1076-1083. [DOI] [PubMed] [Google Scholar]
- 159. Cohen S, Nathan JA, Goldberg AL. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat Rev Drug Discov. 2015;14:58-74. [DOI] [PubMed] [Google Scholar]
- 160. Bonaldo P, Sandri M. Cellular and molecular mechanisms of muscle atrophy. Dis Model Mech. 2013;6:25-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Wolfe RR. Skeletal muscle protein metabolism and resistance exercise. J Nutr. 2006;136:525S-528S. [DOI] [PubMed] [Google Scholar]
- 162. He C, Bassik MC, Moresi V, et al. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012;481:511-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Vainshtein A, Tryon LD, Pauly M, Hood DA. Role of PGC-1α during acute exercise-induced autophagy and mitophagy in skeletal muscle. Am J Physiol Cell Physiol. 2015;308:C710-C719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Khamoui AV, Park BS, Kim DH, et al. Aerobic and resistance training dependent skeletal muscle plasticity in the colon-26 murine model of cancer cachexia. Metabolism. 2016;65:685-698. [DOI] [PubMed] [Google Scholar]
- 165. Holloszy JO, Booth FW. Biochemical adaptations to endurance exercise in muscle. Annu Rev Physiol. 1976;38:273-291. [DOI] [PubMed] [Google Scholar]
- 166. Gordon JW, Rungi AA, Inagaki H, Hood DA. Effects of contractile activity on mitochondrial transcription factor A expression in skeletal muscle. J Appl Physiol (1985). 2001;90:389-396. [DOI] [PubMed] [Google Scholar]
- 167. Baar K, Wende AR, Jones TE, et al. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J. 2002;16:1879-1886. [DOI] [PubMed] [Google Scholar]
- 168. Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the PGC-1alpha gene in human skeletal muscle. J Physiol. 2003;546(pt 3):851-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Ding H, Jiang N, Liu H, et al. Response of mitochondrial fusion and fission protein gene expression to exercise in rat skeletal muscle. Biochim Biophys Acta. 2010;1800:250-256. [DOI] [PubMed] [Google Scholar]
- 170. Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, Puigserver P. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature. 2007;450:736-740. [DOI] [PubMed] [Google Scholar]
- 171. Ydfors M, Fischer H, Mascher H, Blomstrand E, Norrbom J, Gustafsson T. The truncated splice variants, NT-PGC-1α and PGC-1α4, increase with both endurance and resistance exercise in human skeletal muscle. Physiol Rep. 2013;1:e00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Porter C, Reidy PT, Bhattarai N, Sidossis LS, Rasmussen BB. Resistance exercise training alters mitochondrial function in human skeletal muscle. Med Sci Sports Exerc. 2015;47:1922-1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Blair SN, Kohl HW, 3rd, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989;262:2395-2401. [DOI] [PubMed] [Google Scholar]
- 174. Lee IM, Shiroma EJ, Lobelo F, et al. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012;380:219-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42:1409-1426. [DOI] [PubMed] [Google Scholar]
- 176. Kushi LH, Doyle C, McCullough M, et al. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2012;62:30-67. [DOI] [PubMed] [Google Scholar]
- 177. Physical Activity Guidelines Advisory Committee Members. Physical Activity Guidelines Advisory Committee Report, 2008. Washington, DC: US Department of Health and Human Services; 2008:A1-H14. [Google Scholar]
- 178. Rajarajeswaran P, Vishnupriya R. Exercise in cancer. Indian J Med Paediatr Oncol. 2009;30:61-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Anderson LJ, Albrecht ED, Garcia JM. Update on management of cancer-related cachexia. Curr Oncol Rep. 2017;19:3. [DOI] [PubMed] [Google Scholar]
- 180. Solheim TS, Laird BJA, Balstad TR, et al. A randomized phase II feasibility trial of a multimodal intervention for the management of cachexia in lung and pancreatic cancer [published online June 14, 2017]. J Cachexia Sarcopenia Muscle. doi: 10.1002/jcsm.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Sasso JP, Eves ND, Christensen JF, Koelwyn GJ, Scott J, Jones LW. A framework for prescription in exercise-oncology research. J Cachexia Sarcopenia Muscle. 2015;6:115-124. [DOI] [PMC free article] [PubMed] [Google Scholar]


