Abstract
White matter hyperintensities (WMH) are associated with cognitive decline. We aimed to identify the spatial specificity of WMH impact on cognition in non-demented, healthy elderly. We quantified WMH volume among healthy participants of a community dwelling cohort (n = 702, age range 60 – 82 years, mean age = 69.5 years, 46% female) and investigated the effects of WMH on cognition and behavior, specifically for executive function, memory, and motor speed performance. Lesion location influenced their effect on cognition and behavior: Frontal WMH in the proximity of the frontal ventricles mainly affected executive function and parieto-temporal WMH in the proximity of the posterior horns deteriorated memory, while WMH in the upper deep white matter—including the corticospinal tract—compromised motor speed performance. This study exposes the subtle and subclinical yet detrimental effects of WMH on cognition in healthy elderly, and strongly suggests a causal influence of WMH on cognition by demonstrating the spatial specificity of these effects.
Keywords: White matter hyperintensities, cerebral small vessel disease, cognitive impairment, brain atrophy, brain aging
Introduction
Rapidly aging populations are burdening societies around the globe. While healthcare costs increase with age, cognitive skills generally decline. While not every person develops clinically manifest dementia, many elderly experience some form of cognitive deterioration. Most people also exhibit white matter hyperintensities (WMH) on brain MRI.1 In this project, we studied WMH—usually a correlate of vascular changes in the aging brain—to quantify the effects on cognition.2 WMH volume increases over a lifetime, highlighting its progressive importance with advancing age.1 While the exact causal mechanisms of WMH formation remain elusive, major risk factors for the development of WMH are hypertension,3,4 smoking,5 and cardiovascular disease.6 Extensive literature describes the negative effects of WMH on cognitive functioning.7,8 However, while it is agreed that WMH have detrimental effects on cognition, there is a clinicoradiological discrepancy between the extent of the white matter disease and cognitive impairment.8 Indeed, some individuals exhibit extensive white matter disease while preserving a high level of cognitive functioning. Moreover, comprehensive meta-analyses have indicated that—among the cognitive domains—attention, executive function, and memory are specifically affected by WMH.9,10
Several studies suggest that the strategic position of the lesions is more predictive for cognitive outcome than general WMH volume. While earlier studies utilized case-control to investigate the relationship between lesion location and cognition, more recent studies have investigated localized WMH effects with ROI/voxel-based analyses.11,12
Building on this literature, the current study deploys a population-based epidemiological large-cohort study and focuses on healthy non-demented elderly participants. We quantified WMH volume and investigated the effect of total WMH volume as well as their location-specific impact on cognition. By identifying the spatial strategic effects of WMH on cognition in a large sample of healthy elderly participants, the current study elucidates the subtle relationship between WMH and subclinical cognitive decline.
Methods
Study population
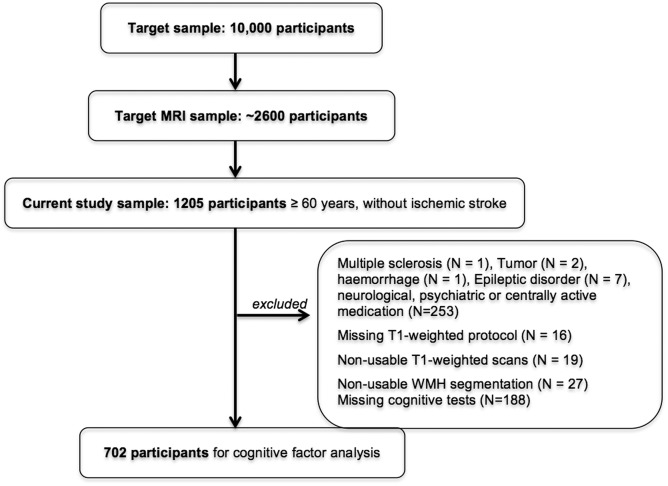
This study took place within the framework of the population-based study of the Leipzig Research Centre for Civilization Diseases (LIFE). All participants were randomly chosen and invited via the city registry office according to the study protocol from the LIFE-Adult-Study.13 After providing informed consent, all participants underwent a 3 Tesla MRI brain scan, structured interviews, neuropsychological tests, and a set of medical assessments.13 Financial compensation was provided, the study protocol was in accordance with the Declaration of Helsinki and approved by the ethics committee at the University of Leipzig. Our results are based on participants over the age of 60 years. After excluding individuals with an intake of psychoactive drugs (n = 242), neuroradiological incidental findings (n = 3), neurological diseases (n = 8), difficulties during data processing (n = 62), and missing cognitive data (n = 188), a cohort of 702 participants was included in the current study. Note that cerebral microbleeds and perivascular spaces were not taken into account. A flow chart visualizes the selection process of participants (see Figure 1) and Table 1 provides an overview of demographics of our cohort.
Figure 1.
Flow chart visualizing of the selection process of participants.
Table 1.
Demographics of the participants and distribution of visually and automatically assessed WMH volume.
| Number of subjects | 702 |
|---|---|
| Age range in years | 60–82 |
| Mean age (SD) in years | 68.49 (4.58) |
| Sex (female) in % | 46.01 |
| Fazekas rating 0, 1, 2, 3 in % | 22.2, 55.3, 21.9, 1.6 |
| Mean number of 1 mm iso-voxels WMH (SD) | 4249 (6661) |
| Mean number of 1 mm iso-voxels WM (SD) | 412,054 (43,759) |
| MMSE (SD) | 28.59 (1.28) |
SD: standard deviation; WM: white matter; WMH: white matter hyperintensity; MMSE: mini mental status examination.
MRI data acquisition
All MRI scans were performed at 3 Tesla on a MAGNETOM Verio scanner (Siemens, Erlangen, Germany). The body coil was used for RF transmission and a 32-channel head coil was used for signal reception. T1-weighted magnetization prepared rapid acquisition gradient echo (MPRAGE) and fluid-attenuated inversion recovery (FLAIR) images were acquired as part of a standardized protocol: MPRAGE (FA = 9°, TR = 2300 ms, TI = 900 ms, TE = 2.98 ms, 1 mmisotropic resolution, AT = 5.10 min); FLAIR (TR = 5000 ms, TI = 1800 ms, TE = 395 ms, 1 mmisotropic resolution, AT = 7.02 min).
Automated assessment of WMH
Prior to segmentation, all images were carefully checked by a radiologist for incidental findings. Participants with hemorrhagic, ischemic, or lacunar infarctions, or other neuroradiological incidental findings were excluded from the analysis. Lesion TOADS—a computer-based WMH segmentation algorithm that was previously validated for patients with multiple sclerosis—was used to automatically determine WMH volume on T1-weigthed MPRAGE and FLAIR images.14 As WMH differ in their pattern, intensity, and extent to those of multiple sclerosis, we adapted the algorithm to the needs of age-related WMH in our cohort in a two-step iterative process and revalidated it the algorithm on our cohort. Given the large variety of WMH, their appearance on FLAIR images had a wider range of intensities across participants. Therefore, from the first segmentation given by LesionTOADS (step 1), we renormalized the contrast of input FLAIR images to better separate WMH from healthy tissue, modeling the FLAIR intensity inside the brain as a mixture of Gaussian and outlier distributions (step 2). The intensity boundary between tissue intensity and WMH is estimated from setting the ratio of segmented WMH to brain volume as an outlier ratio, and performing the LesionTOADS step again on the re-normalized intensities. This method ensures that the relative intensities of the FLAIR in different images are similar across a large cohort of subjects. Binary WMH maps of all participants were nonlinearly co-registered to a standardized Montréal Neurological Institute (MNI) template (1 mm isometric) by applying the transformation matrix obtained from co-registering the MPRAGE of the respective participant to the template with ANTS15: a state-of-the-art registration technique.16 We afterwards carefully inspected every standardized image. This registration technique ensures a low amount of warping while aligning accurately the main morphological features of the brain (ventricles, white matter tracts, subcortex, overall shape and size of the cerebral cortex). As depicted in Figure 1, we excluded lesion segmentation of 27 participants. Reasons included erroneous skull-stripping, motion or inhomogeneity artifacts, mis-registration of the T1-weighted and FLAIR data, or incorrect warping onto the MNI template. For standard space lesion maps across participants, WMH maps were added to create a WMH frequency map with FSL.17 Brain images were visualized with the Mango viewer (http://ric.uthscsa.edu/mango/mango.html).
Cognitive assessment
Cognitive performance was assessed with a set of standard neuropsychological tests.13 Results of the Wortschatztest (a German vocabulary test), the extended version of the Consortium to Establish a Registry of Alzheimer’s Disease (CERADplus) test battery,18 the Structured Interview for Diagnosis of Dementia of Alzheimer type, as well as Multi-Infarct Dementia and Dementia of other Etiology according to DSM-III-R, DSM-IV, and ICD-10,19 were available for analysis. All subtest results were carefully checked for missing values and data recording errors.
Statistical analysis
Associations between log-transformed normalized (by WM volume) total WMH volume and cognition have been assessed using separate multiple regression models with cognitive domains as the dependent variable, and age, sex, and years of education as confounders. Significance threshold was set to α < 0.05 (one-sided) and corrected for the number of cognitive domains tested with Bonferroni correction (α < 0.0167). Significant associations are marked with an asterisk, in the results section. Statistical analyses were performed with R.20
Topographical relevance analysis of WMH for cognition
In order to identify regions where WMH occurrence is robustly related to worse performance in a specific cognitive domain, we used an ad hoc analysis routine. Here, we scaled binary WMH map (in standard space) of each participant with the standardized adjusted performance of the subject in that cognitive domain (after controlling for other domains as well as age, sex, and education based on the following formula: standardized adjusted performance of cognitive factor X = raw scores of cognitive factor X – [b1 × raw scores of cognitive factor Y + b2 × raw scores of cognitive factor Z + b3 × age + b4 × sex + b5 × education]). The sum of all scaled WMH maps for each cognitive factor were then assessed in voxel space and with a threshold (>10) to identify regions in WM, where WMH occurrence increases the probability of worse performance on each cognitive domain. Note that two participants were discarded from this analysis because of failed registration into standard space.
Results
WMH volume distribution
Characteristics of the study cohort (n = 702) are provided in Table 1. WMH volume segmented by LesionTOADS reflects the visual assessment of WMH volume represented by the visual rating (see Supplementary Figure 3(b)) and increases with age, as expected. Supplementary Figure 4 illustrates the WMH frequency distribution respectively in a characteristic lesion pattern with highest lesion frequency located adjacent to the lateral ventricles symmetrically.
WMH volume and cognition
An exploratory factor analysis was employed to assess the structure of the cognitive data and to reduce its dimensions in the cohort of 702 participants (for details see supplementary material). The factor analysis resulted in four cognitive and behavioral domains, i.e. “executive function,” “memory,” “motor speed performance,” and “visuoconstructive abilities.” The average MMSE score was 28.59 (standard deviation = 1.28). Multiple linear regression corrected for age, sex, and education showed negative, but subtle association between WMH volume and executive function (r = −0.07, p = 0.013), memory (r = −0.07, p = 0.016), and motor speed performance (r = −0.09, p = 0.006) in our cohort of non-demented, elderly participants. We did not find an association between WMH volume and visuoconstructive abilities in our sample of cognitively intact healthy elderly (r = −0.002, p = 0.34).
Topographical relevance of WMH for cognitive function
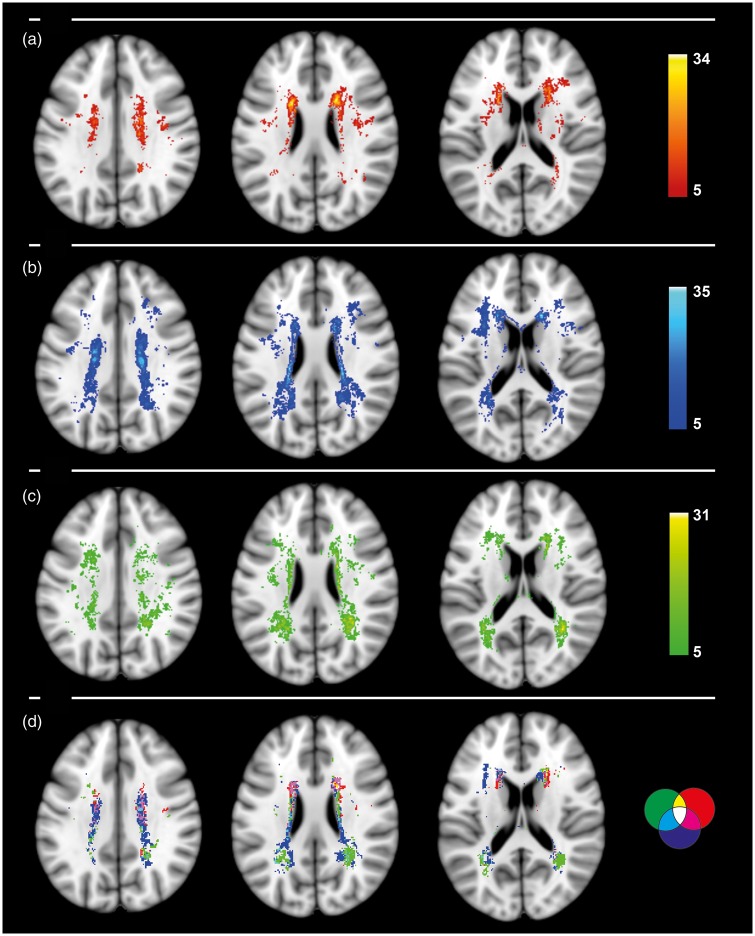
Regarding a topographical relationship between WMH and different cognitive and behavioral domains, we found that WMH in the frontal white matter adjacent to the frontal horns were much more pronounced in participants with worse performance in executive function, while participants with high cognitive performance in executive functions had less WMH in the frontal, periventricular white matter. Participants with decreased memory function had prevailing WMH in the bilateral parieto-temporal white matter junctions adjacent to the posterior horns of the lateral ventricles, while participants with higher memory performance showed less WMH in this location. Participants with worse motor speed performance showed an increased amount of WMH in the upper deep white matter—including the corticospinal tract—while participants with better motor speed performance had less lesions, respectively. All WMH locations with detrimental negative effects on cognition are bilateral with a symmetrical pattern (Figure 2). Unthresholded maps with the full range of location-specific impact on cognition are available on a meta-analysis database (http://neurovault.org/collections/66/). A separate voxel-wise lesion symptom mapping analysis (VLSM, FDR-corrected, p < 0.01) confirmed the pattern. WHM in the frontal white matter were related to negative executive function, WMH in the parieto-temporal white matter were related to negative memory function, and WMH in the upper deep white matter were related to negative motor speed performance (Supplementary Figure 6).
Figure 2.
WMH frequency maps of negative impact on cognition: thresholded above 5 on (a) executive function, (b) motor speed performance, and (c) memory; (d) all three cognitive factors thresholded above 10 with red for executive function, green for memory, and blue for motor speed performance with logical color coding of intersecting clusters.
Discussion
Based on a single sample of well-characterized non-demented elderly subjects (n = 702), we demonstrate the subtlety of the relationship between global WMH load and cognitive and behavioral functions. Furthermore, we localize the effects of WMH on cognitive and behavioral functions in the healthy elderly with a novel routine as well as with well-established VLSM. This study expands upon the existing literature, which to the largest extent has been conducted on patient data. By investigating participants from a community-dwelling large cohort study strictly focusing on healthy elderly individuals, we bring further light into the relationship between lesion location and cognitive deficits. In line with the existing literature and with a meta-analysis spanning over 30 studies investigating the relationship between WMH and cognition, we find consistent negative relations between WMH volume and executive function, memory, and motor speed performance.10 For visuoconstructive abilities, we did not find an association with WMH volume in our cohort. On the one hand, this might be due to a low sensitivity of the cognitive tests to sufficiently identify visuoconstructive differences in healthy elderly. On the other hand, visuoconstruction might in fact be spared from the detriment of WMH in healthy elderly. Note that our participants exhibited a high level of cognitive functioning (mean MMSE 28.59, SD 1.28) which emphasizes the subtlety of the WMH-effects on cognition. Furthermore, it exemplifies the clinicoradiological discrepancy between the extent of the white matter disease and cognitive impairment in healthy elderly. With respect to the clinicoradiological discrepancy, it has been suggested that WMH induce compensatory mechanisms such as changes of cerebral blood flow found in a longitudinal study,21 hinting towards an individual tolerance of WMH load before cognitive function is measurably impaired. Moreover, the incongruence between the extent of WMH volume and cognitive dysfunction could be explained by ‘cognitive reserve’—a model which conceptualizes the resilience of the individual brain to neuropathological damage by recruiting alternative neuronal capacities.22 By controlling for years of education, this study only marginally corrects for ‘cognitive reserve’.
Another general problem of MRI-based WMH studies is the lack of histopathological specification of WMH. Neuropathological studies have described heterogeneous structural correlates of WMH,8 which implies that different pathologies result in different lesions that appear similarly on FLAIR images. If different lesion etiologies have specific effects on cognition, it cannot be accounted for on FLAIR images. Furthermore, WMH are just one aspect of brain aging. While signal transmission in the white matter is crucial for cognition, other structural correlates such as gray matter have their own contribution to cognition independent of WMH volume and were not accounted for in this study.
Studies have investigated the effect of WMH location with region-based as well as with voxel-wise techniques.23 Region-based approaches with large and rather arbitrary sections were helpful to demonstrate regional differences, yet these studies were limited to uncovering localized effects beyond the predefined areas. Smith et al.,12 who employed a voxel-wise technique to disentangle the relation between WMH and cognitive deficits, have provided interesting results relating frontal WMH to executive deficits, and temporo-occipital WMH to disturbance of episodic memory in a cohort of 147 subjects with cognitive deficits ranging from dementia to normal cognition.12 Our study expands upon these findings by incorporating 702 healthy and non-demented elderly participants after rigorously excluding participants with progressive cognitive deficits and medication influencing cognition (i.e. psychoactive medication). All WMH clusters occurred in regions of high WMH density (Supplementary Figure 4) and thus reflect many subjects.
WMH clusters that related to poor executive function were predominantly located in the frontal white matter adjacent to the anterior ventricles, with peak voxels in the anterior limb of the corona radiata (anterior thalamic radiation according to the JHU White-Matter atlas), which is known to connect the prefrontal cortex with the thalamus and has high relevance for executive function.24 Given the high plausibility of relating these lesions to executive function, we conclude that our findings corroborate similar findings of previous studies.11,12 Moreover, we assume that the previous studies that did not find a relationship between executive function and frontal WMH may not have done so because of limitations such as large WM parcels and smaller sample sizes.25,26
WMH in the parieto-temporal white matter were prevailing in participants with poorer memory performance, which might be explained by WMH clusters interfering with the inferior and superior longitudinal fasciculus as well as the retrosplenial cingulum—white matter tracts which are known to be associated with memory function.27 As WMH in the deeper temporal white matter were sparse in our participants (see WMH frequency map in Supplementary Figure 4), we could not evaluate the assumed impact of these WMH for memory with sufficient power.
Motor speed performance was decreased with WMH clusters in the upper deep white matter interestingly incorporating parts of the corticospinal tract. Previous studies have assessed the disruptive characteristics of WMH on both connectivity and structural integrity.28,29 Our observations strongly support the assumption of WMH having a causal effect on cognitive deficits and further specify the location-wise impact of WMH on distinct cognitive functions.
To our knowledge, this is the first large cohort study to relate spatial specificity of WMH to subtle cognitive differences in a non-demented sample of healthy elderly from the general population.
Limitations of the study are the cross-sectional nature of the data and the lack of histopathology. With respect to the underlying pathophysiology of WMH, on the one hand, it can be safely assumed that the predominant majority of WMH in our cohort were of ischemic origin. On the other hand, given the fact that MRI findings are rather non-specific—no definite etiological assessment can be made about individual lesions in different subjects. To address the interesting question whether WMH of different origins have different effects on gray matter and cognitive function, further analysis with pathophysiological differentiation of lesions will be required for example by comparing patients with distinct lesion pathologies such as multiple sclerosis, vasculitis, etc. Combinations of innovative techniques such as quantitative MR-imaging, high-gradient diffusion weighted imaging, and MR-spectroscopy could further help to differentiate between distinct lesion etiology. A general challenge of studies with elderly participants is the non-linear registration of aged brains that often exhibit a significant amount of deformation. However, by using a recommended high-performance algorithm and through quality-checking, we assured a high standard of registration. Furthermore, while LesionTOADS is a state-of-the-art segmentation algorithm as demonstrated in the ISBI segmentation challenge,30 potential difficulties are systematic errors such as mis-segmentation of artefacts around the ventricles. While systematic false positives reduce correlations with WMH volumes, it does not affect the topographical analysis of WMH and cognitive functions. More refined WMH segmentation algorithms are under development. Another disadvantage of voxel-wise techniques to assess lesion impact is the relative scarcity of WMH in the peripheral white matter. Larger cohorts or statistical methods accounting for the structure of WM tracts might help to tackle this in future studies.
Conclusions
In non-demented, healthy elderly subjects, WMH are associated with cognitive decline. While total WMH volume is associated with deterioration in several cognitive domains, WMH location is associated with specific cognitive deficits in healthy elderly subjects.
Supplementary Material
Acknowledgements
We thank all members of the LIFE study center for conducting the LIFE-Adult-Study as well as all participants for their valuable collaboration.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is supported by the European Union, the European Regional Development Fund, and the Free State of Saxony within the framework of the excellence initiative and LIFE – Leipzig Research Center for Civilization Diseases, University of Leipzig, and by the German Research Foundation (CRC1052 Obesity mechanisms Project A01 to AV and MS). JK was funded by the Max-Planck International Research Network on Aging (MaxNetAging).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
Conceived and designed the study: LL, SKM, PLB; Acquired the data: LL, MLS, ML, AV, KA; Performed the analysis: LL, SKM; Wrote the paper: LL, PLB; Provided valuable advice during analysis and contributed writing the manuscript: SKM, JK, CJS, AVW, MLS, AV.
Supplementary material
Supplementary material for this paper can be found at the journal website: http://journals.sagepub.com/home/jcb
References
- 1.Leeuw F, De, Groot JC, De, Achten E, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry 2001; 70: 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013; 12: 822–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Longstreth WT, Manolio TA, Arnold A, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people: the cardiovascular health study. Stroke 1996; 27: 1274–1282. [DOI] [PubMed] [Google Scholar]
- 4.Dufouil C, Chalmers J, Coskun O, et al. Effects of blood pressure lowering on cerebral white matter hyperintensities in patients with stroke: the PROGRESS (Perindopril Protection Against Recurrent Stroke Study) magnetic resonance imaging substudy. Circulation 2005; 112: 1644–1650. [DOI] [PubMed] [Google Scholar]
- 5.Gons RAR, Van Norden AGW, De Laat KF, et al. Cigarette smoking is associated with reduced microstructural integrity of cerebral white matter. Brain 2011; 134: 2116–2124. [DOI] [PubMed] [Google Scholar]
- 6.Jeerakathil T, Wolf PA, Beiser A, et al. Stroke risk profile predicts white matter hyperintensity volume: the Framingham study. Stroke 2004; 35: 1857–1861. [DOI] [PubMed] [Google Scholar]
- 7.Vermeer SE, Prins ND, den Heijer T, et al. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med 2003; 348: 1215–1222. [DOI] [PubMed] [Google Scholar]
- 8.Prins ND, Scheltens P. White matter hyperintensities, cognitive impairment and dementia: an update. Nat Rev Neurol 2015; 11: 157–165. [DOI] [PubMed] [Google Scholar]
- 9.Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neutopsychology 2000; 14: 224–232. [DOI] [PubMed] [Google Scholar]
- 10.Kloppenborg RP, Nederkoorn PJ, Geerlings MI, et al. Presence and progression of white matter hyperintensities and cognition: a meta-analysis. Neurology 2014; 82: 2127–2138. [DOI] [PubMed] [Google Scholar]
- 11.Biesbroek JM, Kuijf HJ, van der Graaf Y, et al. Association between subcortical vascular lesion location and cognition: a voxel-based and tract-based lesion-symptom mapping study. The SMART-MR study. PLoS One 2013; 8: e60541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith EE, Salat DH, Jeng J, et al. Correlations between MRI white matter lesion location and executive function and episodic memory. Neurology 2011; 76: 1492–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loeffler M, Engel C, Ahnert P, et al. The LIFE-adult-study: objectives and design of a population-based cohort study with 10,000 deeply phenotyped adults in Germany. BMC Pub Health 2015; 15: 691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shiee N, Bazin P-L, Ozturk A, et al. A topology-preserving approach to the segmentation of brain images with multiple sclerosis lesions. Neuroimage 2010; 49: 1524–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Avants BB, Epstein CL, Grossman M, et al. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal 2008; 12: 26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arno Klein, Jesper Andersson BAA, et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage 2009; 46: 786–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jenkinson M, Beckmann CF, Behrens TEJ, et al. Fsl. Neuroimage 2012; 62: 782–790. [DOI] [PubMed] [Google Scholar]
- 18.Morris JC, Heyman A, Mohs RC, et al. The consortium to establish a registry for Alzheimer’s disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology 1989; 39: 1159–65. [DOI] [PubMed] [Google Scholar]
- 19.Zaudig M, Mittelhammer J, Hiller W, et al. SIDAM – a structured interview for the diagnosis of dementia of the Alzheimer type, multi-infarct dementia and dementias of other aetiology according to ICD-10 and DSM-III-R. Psychol Med 1991; 21: 225–236. [DOI] [PubMed] [Google Scholar]
- 20.R Core team. R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. ISBN 3-900051-07-0, vol. 55, pp. 275–286, www.R-project.org/ (2015, accessed 20 October 2017).
- 21.Kraut MA, Beason-Held LL, Elkins WD, et al. The impact of magnetic resonance imaging-detected white matter hyperintensities on longitudinal changes in regional cerebral blood flow. J Cereb Blood Flow Metab 2008; 28: 190–197. [DOI] [PubMed] [Google Scholar]
- 22.Pinter D, Enzinger C, Fazekas F. Cerebral small vessel disease, cognitive reserve and cognitive dysfunction. J Neurol 2015; 262: 2411–2419. [DOI] [PubMed] [Google Scholar]
- 23.Biesbroek JM, Weaver NA, Biessels GJ. Lesion location and cognitive impact of cerebral small vessel disease. Clin Sci 2017; 131: 715–728. [DOI] [PubMed] [Google Scholar]
- 24.Alvarez JA, Emory E. Executive function and the frontal lobes: a meta-analytic review. Neuropsychol Rev 2006; 16: 17–42. [DOI] [PubMed] [Google Scholar]
- 25.Marquine MJ, Attix DK, Goldstein LB, et al. Differential patterns of cognitive decline in anterior and posterior white matter hyperintensity progression. Stroke 2010; 41: 1946–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burton EJ, Kenny RA, O’Brien J, et al. White matter hyperintensities are associated with impairment of memory, attention, and global cognitive performance in older stroke patients. Stroke 2004; 35: 1270–1275. [DOI] [PubMed] [Google Scholar]
- 27.Lockhart SN, Mayda ABV, Roach AE, et al. Episodic memory function is associated with multiple measures of white matter integrity in cognitive aging. Front Hum Neurosci 2012; 6: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vernooij MW, Ikram MA, Vrooman HA, et al. White matter microstructural integrity and cognitive function in a general elderly population. Arch Gen Psychiatry 2009; 66: 545–53. [DOI] [PubMed] [Google Scholar]
- 29.Schaefer A, Quinque EM, Kipping JA, et al. Early small vessel disease affects frontoparietal and cerebellar hubs in close correlation with clinical symptoms – a resting-state fMRI study. J Cereb Blood Flow Metab 2014; 34: 1091–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carass A, Roy S, Jog A, et al. Neuroimage longitudinal multiple sclerosis lesion segmentation: resource and challenge. Neuroimage 2017; 148: 77–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.