Abstract
Point-of-care devices were originally designed to allow medical testing at or near the point of care by health-care professionals. Some point-of-care devices allow medical self-testing at home but cannot fully cover the growing diagnostic needs of eHealth systems that are under development in many countries. A number of easy-to-use, network-connected diagnostic devices for self-testing are needed to allow remote monitoring of patients’ health. This Outlook highlights the essential characteristics of diagnostic devices for eHealth settings and indicates point-of-care technologies that may lead to the development of new devices. It also describes the most representative examples of simple-to-use, point-of-care devices that have been used for analysis of untreated biological samples.
Short abstract
This Outlook highlights the characteristics that diagnostic devices should have to be used in eHealth systems and discusses point-of-care technologies that may lead to new eDiagnostics.
1. Introduction
Health-care systems today may be better than ever, but are still far from ideal because of the following: (i) They consume extensive financial resources (approximately 10% of each country’s GDP is spent toward health expenditures, over 7.2 trillion USD in total in 2015).1 (ii) They are inconvenient or even inaccessible to large portions of the population, especially those in rural communities and developing countries. (iii) They struggle to detect diseases at early stages due to limited health status screening. To address these challenges, the World Health Organization (WHO) proposed the establishment of eHealth systems, and set eHealth as a top priority in 2005.
eHealth is defined as “the cost-effective and secure use of information communication technologies (ICT) in support of health and health related fields, including health-care services, health surveillance, health literature, and health education, knowledge and research”.2 The latest Global Observatory for eHealth survey of WHO (published in 2016) has shown that 73 countries already have an eHealth strategy in place,2 but most probably several years will be needed for eHealth systems to be fully operational. The three main components of eHealth systems will most likely be (a) electronic health and medical records that will enable easy and instant access to patient’s medical history as well as e-prescribing, and e-booking; (b) telehealth or telemedicine (the two terms are used interchangeably) that will allow virtual appointments between physicians and their patients, and remote monitoring of the health of the patients (even when they are at their homes); and (c) mHealth (i.e., mobileHealth) that will ease the communication between health-care providers and patients regarding patient care, emergency situations, health practices, and treatment adherence (Figure 1). Due to the fact that there is no official definition of the terms “eHealth”, “teleHealth”, “telemedicine”, and “mHealth” and very little consistency on their use in the scientific literature,3 these terms are described here based on the content that WHO has given them on its official policy documents. When eHealth systems will be fully operational, some patients will save money and time by not having to travel to health-care centers and clinics to visit a physician and perform laboratory tests; they would be able to telemeet physicians using telemedicine platforms and self-test themselves (Figure 1). Physicians will be more productive as they will be able to meet more patients; the nonattendance or no-shows rate when eHealth applications are implemented are significantly lower.4 The health-care systems might, therefore, be more patient-friendly, would operate at higher efficiency that would probably lower their cost,5,6 and result in better health outcomes.7
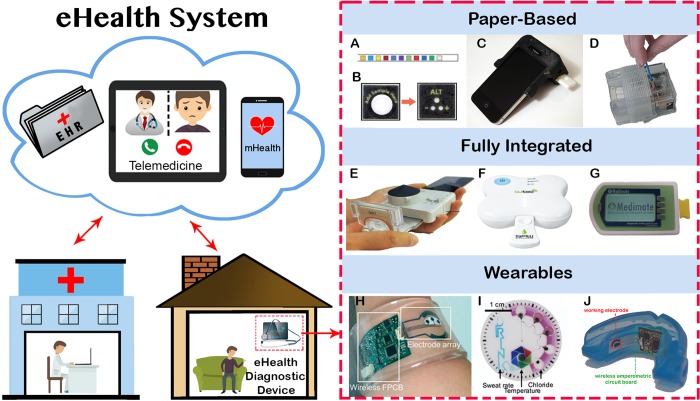
Figure 1.
Left side: overview of main components of a future eHealth system for remote examination of a patient at home using telemedicine for teleappointments between physicians and patients and eHealth diagnostic devices for remote testing. Right side: simple-to-use devices that can analyze untreated biological samples at eHealth settings. (A) Paper strip for urinalysis; (B) paper-based device for accessing liver function; (C) cellphone attachment that reads disposable lateral flow tests; (D) sample in–result out device for analyzing nasal swab to detect nucleic acids; (E) microfluidic cassette connected to a smartphone dongle for infectious diseases detection; (F) SAW (surface acoustic waves) biochip for the HIV detection in blood; (G) chip-based microfluidic device for the detection of lithium in blood; (H) wristband for real-time detection of glucose, sodium, and chloride ions in sweat; (I) sweat-monitoring patch for measuring sweat rate, temperature, and chloride ions concentration; and (J) mouthguard that integrates a biosensor and a wireless circuit board for uric acid detection in saliva. The figures were reproduced with permission from refs (93) (A), (201) (B), (112) (C), (137) (D), (199) (E), (192) (F), (195) (G), (45) (H), (59) (I), and (72) (J).
The necessary technology for most modules of eHealth applications is already available: for example, high-quality teleconferencing for teleHealth, mobile connectivity for mHealth, and advanced databases storage and analysis for electronic medical records. The technology for home-based medical testing, however, is not fully developed,8 and further research is needed to produce a range of eHealth diagnostic devices (i.e., low-cost, easy-to-use, network-connected diagnostic devices for home use) to support eHealth systems.
This Outlook aims (a) to highlight the essential characteristics of eDiagnostics; (b) to discuss POC diagnostic technologies, which allow biochemical testing in portable devices without user involvement, and after further development may lead to new eDiagnostics for self-testing; and (c) to point toward areas of future research in each POC technology that could enhance its applicability at eHealth settings. Herein, we explicitly describe examples of four POC diagnostic technologies (wearables, paper diagnostics, microcell-based sensing, and chip-based microfluidics) that have been successfully used for the analysis of untreated biological samples. Technologies of POC diagnostics whose requirements in terms of cost or infrastructure imply that they are suitable for use only in clinics, clinical laboratories, or research laboratories will not be discussed herein; we refer the reader to the scientific literature for comprehensive reviews of POC devices.9−11
2. Essential Characteristics of eDiagnostics
Any diagnostic device must be accurate and precise, and provide robust results. Diagnostic devices whose intended use is medical testing outside of clinical laboratories (e.g., at home by untrained individuals) have to receive additional clearance by local regulatory authorities. Even though each country imposes different requirements, in general, regulatory authorities require diagnostic devices for home use to be accurate, be simple-to-use by untrained individuals, and have little chance of providing misinformation or causing harm if performed incorrectly.12 To be used in eHealth systems, eDiagnostics should be capable of transmitting the test results to cloud-based systems in an automated and secure way. eDiagnostics should also have low-to-moderate cost to purchase and use; therefore, they should be fabricated by readily available materials and be easy to manufacture using conventional automated processes (e.g., roll-to-roll manufacture, 3D printing,13,14 etc.). To keep the cost per test low, eDiagnostics may consist of three elements: (i) a disposable single-use part (e.g., paper device, chip), where the chemical analysis would be performed (Figure 1); (ii) a dedicated reader or a smartphone attachment (Figure 2) that would be used for signal acquisition; and (iii) a smartphone application for signal interpretation and test results transmission to cloud databases of eHealth systems.15
Figure 2.
Examples of modified smartphones and dedicated devices for signal acquisition and data transmission to the cloud: (A) smartphone for reading lateral flow tests; (B) smartphone for bio-chemiluminescent detection on paper devices; (C) multifunctional electrochemical detector for performing various types of electrochemical techniques (e.g., chronoamperometry, pulsed voltammetry, potentiometry) using readily available electrodes; (D) updated version of device C that facilitates temperature control and electrochemical detection on paper-based devices; (E) smartphone for liquid-based colorimetric assays; and (F) surface plasma resonance biosensor installed on a smartphone. The figures were reproduced with permission from refs (111) (A), (108) (B), (180) (C), (174) (D), (179) (E), and (191) (F).
eDiagnostics should be able to perform tests in whole, untreated biological fluids, and be fully integrated (i.e., contain all the necessary reagents prestored inside them). Ideally, the devices should require absolutely minimum involvement from the user: the user only adding the sample (i.e., finger-prick blood, urine, saliva, etc.) on the device, or wearing the device. A simple extra step such as adding a drop of a solution (using a dropper) at the device may also be acceptable, if it is absolutely necessary. Additional fluid handling steps, such as mixing and pipetting solutions, may increase the complexity status of the device significantly (based on the strict criteria of regulatory authorities) and, therefore, may render the device improper for home use.
To achieve the desired level of simplicity of use, and at the same time detect a pathological condition, developers of eDiagnostics should carefully select (i) a biological fluid that could be easily obtained minimally invasively at home and contains a disease biomarker and (ii) the point-of-care diagnostic technology that could facilitate low-cost, easy-to-perform analysis of untreated biological fluids.
3. Constrains from Biological Fluid
The biological fluids that can be obtained minimally invasively at home by the patients themselves are blood, urine, interstitial fluids, saliva, tears, sweat, and breath. The delivery and availability of a biological fluid are parameters that would set specific constraints to an eDiagnostic device. For example, blood samples could be obtained using a finger-prick procedure or a dedicated blood collection device (e.g., TAP, Seventh Sense Biosystems Inc.)16−18 and delivered to a device quantitatively using a capillary tube, but the device should be able to detect the target analyte in less than 100 μL of blood (volume that can be routinely collected from a finger prick or a blood collection device)19 and perform microscale blood plasma separation inside the device. Interstitial fluids could be obtained by using a fluid collection device that incorporates microneedles20−24 or uses iontophoresis.25 Sweat and tears could be obtained noninvasively, but they could cause some distress to the patients (make them perspire or make their eyes weep). Saliva can be easily obtained using swabs, but the results may be subject to contamination from food.26 Especially sweat, a fluid that has recently attracted the attention of researchers, is available in very low volume (in the nL range for a 10 mm2 sweat area)27 and might require either sophisticated sampling devices to deliver the necessary volume quantitatively27 or wearable sensors that could stimulate, sample quantitatively, and then analyze sweat.28,29
Microscale blood plasma separation inside a portable device is quite challenging, and many scientists in academic settings develop POC devices without performing blood plasma separation;30 they test their devices with serum or plasma. Numerous passive and active blood plasma separation techniques have been developed,30−32 but a majority of them either are incompatible or could not be easily integrated within a portable device. Passive plasma separation performed by glass fibers,33 asymmetric polysulfone membranes,34 superhydrophobic plasma separators,35 or agglutinating antibodies36 are few approaches that may be suitable for POC devices. Blood plasma separation could induce in vitro hemolysis which could interfere with the analysis.37 Special care should be taken to keep in vitro hemolysis at the lowest possible levels. Blood plasma separation would be crucial for the quality of the results of devices for blood analysis; therefore, there is a great need of new materials and approaches for the reproducible and almost hemolysis-free blood plasma separation inside a low-cost portable device.
4. Wearables for eHealth Applications
Wearable devices require almost no involvement from the user and would be particularly useful for real-time monitoring of patients over specific periods of time (from minutes up to several days). The bright examples of this technology are the wearable sensors for continuous glucose monitoring (CGM) that have been recently introduced into the market and rapidly changed the landscape of glucose monitoring for patients with diabetes. Guardian Connect CGM (Medtronic Inc.),20,21 Dexcom G6 (Dexcom Inc.),21,22 and FreeStyle Libre (Abbott Diabetes Care Inc.)21,23 are commercially available CGM systems that detect glucose in interstitial fluids and consist of a microneedle that is partially inserted into the subcutaneous tissue and a wearable component that contains the electronic parts (e.g., potentiostat, wireless transmitter, etc.) for signal acquisition and wireless transmission. The devices measure interstitial glucose (using electrochemical detection and proprietary assays) and send them for storage at dedicated readers or smartphones. Eversence CGM System (Senseonics Inc.)38,39 is a small implantable CGM system that use a fluorescent assay to measure interstitial glucose for up to 90 days; the device sends the results wirelessly to an external reader. Other wearable biosensors can be shaped as patches, tattoos, or bandages or attached into mouthguards, bands, and belts to analyze sweat, saliva, or tears, etc.25
For sweat analysis, wearable patches, bands, and tattoos can facilitate electrochemical detection of biomarkers such as glucose40 and electrolytes,41−48 etc. (Figure 3). Printed electrodes can perform the electrochemical assays, and flexible electronics can perform signal acquisition and wireless results transmission to smartphones. For example, Rose et al. converted a commercial RFID chip to a wireless potentiometric sensor for the detection of sodium in sweat.49 Other research groups attached miniaturized screen-printed electrodes and flexible electronics to skin to measure (a) glucose, lactate, and alcohol using chronoamperometry;45,46,50−54 (b) pH, K+, Na+, Ca2+, Cl–, and NH4+ using potentiometry;41−47 and (c) heavy metal ions (zinc, cadmium, lead, copper, mercury) using anodic stripping voltammetry (Figure 3A).54,55 One such example is reported by Martin et al., who developed a soft epidermal microfluidic microchip device using hybridization of lithographic and screen-printed technology for continuous real-time electrochemical monitoring of glucose and lactate levels in sweat (Figure 3B).50 A single device can host several electrodes for the multiplex detection of analytes. For example, Gao et al. developed a fully integrated wearable device for sweat analysis with potentiometric sensors to detect Na+ and K+ and amperometric sensors to detect glucose and lactate (Figure 3C).53
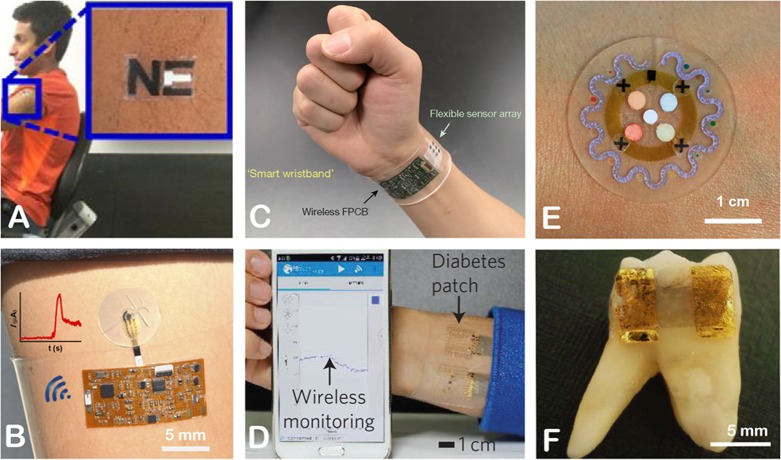
Figure 3.
Examples of wearable biosensors: (A) temporary tattoo for electrochemical sweat sensing of zinc ions; (B) sweat patch for detecting glucose and lactate using amperometry; (C) electrochemical wristband sensor for real-time monitoring of metabolites (glucose, lactate) and electrolytes (Na+, K+) in sweat; (D) diabetes patch showing real-time monitoring of sweat glucose level; (E) sweat patch for sensing temperature, glucose, lactate, chloride ions, and pH; and (F) graphene-based nanosensor attached on tooth enamel for remote monitoring of respiration and detecting bacteria in saliva. The figures were reproduced with permission from refs (55) (A), (50) (B), (53) (C), (58) (D), (60) (E), and (74) (F).
The inherent inaccessibility of sweat in sedentary individuals is an important limitation of sweat sensors. A miniaturized iontophoresis interface could be used to overcome this barrier. The iontophoresis process involves delivery of stimulating agonists to the sweat glands with the help of an electrical current for the sweat extraction. Emaminejad et al. devised a fully integrated electrochemically enhanced iontophoresis interface (using pilocarpine- and methacholine-based hydrogels as stimulating agonists) to induce sweat and integrated in a wearable sweat analysis platform for amperometric monitoring of glucose levels and potentiometric measurement of Na+ and Cl– ions (Figure 1H).45 Following a similar approach, other reports described the real-time detection of glucose, alcohol, Na+, and Cl–.45,51,56 Lipani et al. used an array of miniature pixels that contained Pt decorated graphene working electrodes to achieve transdermal amperometric glucose detection.57 The fact that interstitial fluid was collected at the small known volume of a pixel allowed a calibration-free monitoring of glucose. Lee et al. developed a wearable sweat patch for diabetes monitoring and therapy by integrating a set of electrochemical miniaturized electrochemical sensors (i.e., PEDOT-based humidity sensor, a PANI-based pH sensor, a graphene-oxide-based glucose sensor, a graphene-based temperature sensor), a (Au mesh/graphene-based) heater, and polymeric microneedles loaded with drug (Figure 3D).58 The patch was able to measure glucose and then deliver drug transcutaneously. They tested the performance of the device in diabetic mice.
Apart from electrochemical wearable biosensors, colorimetric sweat sensors have been also reported. Roger’s group devised a number of thin and soft sweat-monitoring patches for calculating sweat rates and temperature (Figure 1I)59,60 and detecting lactate, pH, chloride, and glucose levels (Figure 3E).59−62 The patches include microfluidic channels and passive valves to route the sweat from skin pores to reservoirs filled with color responsive materials for analyte quantitative analysis. A smartphone can capture and analyze an image of the patch to calculate sweat rates and concentration of analytes in sweat. The same group developed a textile-based skin patch that incorporates a passive wireless capacitive sensor for sensing the amount of sweat released from the surface of the skin.63 Mu et al. developed skin patches that contained pH test paper and anion exchangers to measure total concentration of anions in sweat.64
Smart bandages for monitoring wound healing, and mouth and eyes wearable sensors with connectivity capabilities might be also a useful technology for eHealth systems. Previous research efforts have developed smart bandages to monitor (a) uric acid in wounds using printed electrodes that perform chronoamperometric measurements;65,66 (b) pH by using PANI-based thread electrodes and potentiometric detection67,68 or a fluorescence probe and measuring fluorescence signals;69 and (c) irregular bleeding and external pressure using a capacitive sensor.70 A mouthguard support that has sensing capabilities and can send the results remotely could be used as a wearable sensor for saliva analysis (Figure 1J). For example, previous efforts incorporated printed electrodes, a miniaturized potentiostat, and a Bluetooth low-energy (BLE) transceiver on a mouthguard for monitoring glucose,71 uric acid,72 and lactate.73 Another way of analyzing saliva is by attaching a sensor onto a tooth. Mannoor et al. achieved the remote detection of pathogenic bacteria in respiration and saliva by attaching a graphene-based nanosensor on tooth enamel and wirelessly monitoring resistance changes of the nanosensor (Figure 3F).74 Borini et al. developed a thin film graphene oxide conductivity sensor for measuring humidity in breath.75 Guder et al. prepared a thin, paper-based conductivity sensor that responds to the breath’s moisture, and they managed to indirectly calculate respiration rate as the humidity of the breath changes during inhalation and exhalation cycles.76 A handful of wearable sensors have been reported for detection of glucose and lactate levels in tears by using smartly engineered contact lens amperometric biosensors.77−80
Research efforts have also been devoted to develop shirts and body suits empowered with sensing capabilities. For example, Pandian et al. have developed a SmartVest to record electrocardiogram, photoplethysmogram, body temperature, blood pressure, galvanic skin response, and heart rate by incorporating EKG electrodes, photoplethysmogram sensor, thermistor, and galvanic skin sensors into a T-shirt.81 The development of smart fabrics where sensing elements would be incorporated into the fabric (and not attached on it) is still in early stages but is expected to provide fabrics with interesting sensing capabilities. For example textile electrodes do not require careful placement of sensors, and allow skin movement without restrictions.82 Guay et al. integrated a spiral antenna, composed of a multimaterial fiber, into a cotton shirt and recorded contactless measurements of respiratory rate; the antenna geometry was changing during respiration causing changes of the antenna frequency.83 Coppedè et al. devised an electrochemical transistor inside a single cotton yarn for real-time detection of adrenaline and NaCl in sweat.84
Despite the significant advances in wearable biosensors, there are still reliability, stability, reproducibility, and biocompatibility issues that should be addressed.25,85 For example, changes in pH or temperature of sweat may influence the results of analysis (due to the influence of these factors on several enzymatic reactions); therefore, advanced calibration methods may be needed to provide accurate results. The field is also currently focused mainly on sports and military applications. Several biomarkers related to diseases (e.g., cancer, HIV, intestinal infections, cystic fibrosis, and schizophrenia, etc.) could be detected in sweat, tears, and saliva. It should be noted, however, that the concentration of several biomarkers in sweat, saliva, tears, and breath might be correlated with pathological conditions, but normal and pathological levels have not been established yet.26 After establishing these levels, eDiagnostic wearable devices could cover many diagnostic needs at eHealth systems.
5. Paper Diagnostics for eHealth Applications
Paper is a low-cost, lightweight, and flexible material that has the following four unique properties: (a) Paper has a three-dimensional, fibrous structure that acts as a microcuvette (the height of which is around 100–200 μm) where chemical reagents can be stored effectively. (b) Paper’s structure facilitates pump-free wicking of fluids. (c) Paper is fluid-permeable allowing the fabrication of multilayered devices. (d) Paper’s chemical structure (cellulose moieties) allows its functionalization to different forms (e.g., nitrocellulose, etc.) or the immobilization of biomolecules. Test strips, lateral flow tests, and paper-based analytical devices are types of diagnostic devices that are based on paper and have been widely used for biochemical, environmental, and food analysis.85−91 The terms “test strips”, “lateral flow tests”, and “paper-based analytical devices” do not have clear definitions and are sometimes used interchangeably in the scientific literature; they have, however, some design differences as could be concluded from their descriptions in the relevant sections below. Herein, we discuss mainly examples of paper devices that could analyze untreated biological samples. The results of paper devices could be read by smartphone cameras,92 smartphone attachments (Figures 1C and 2A,B), or smartphone-connected electrochemical analyzers (Figure 2C,D), analyzed in a smartphone application, and transmitted to central systems.
5.1. Test Pads
Test strips or test pads (depending on the shape of the device) are patterned pieces of paper loaded with chromogenic reagents that change color upon analyte-selective reactions. Test pads could be read and compared to a calibration chart by the naked eye, but the quality of the results would be greatly enhanced if a dedicated reader,93 a smartphone camera,94 or a smartphone accessory95 would be used for signal acquisition; signal processing and results transmission could be performed at the smartphone.
Test strips have found several applications mainly in POC urinalysis, and commercially available dipstick tests can facilitate the semiquantitative determination of several biomarkers (e.g., glucose, ketones, nitrite, protein, bilirubin, urobilinogen, erythrocytes, leukocytes, creatinine, microalbumin, etc.) in urine samples (Figure 1A).93 In research settings, among other applications, test pads have been used for the determination of blood type,96 the concentration of hemoglobin in blood,97 for breath analysis to detect lung cancer and trimethylaminuria.98−100 Besides changes in color intensity, the length of color change could be used as analytical signal. For example, Guan et al. detected the type of blood,101 Berry et al. hematocrit levels,102 and Gerold et al. K+ levels103 in untreated biological fluids by using distance-based color measurements.
Single-step sensing using colorimetric test pads would be ideal for eDiagnostic devices, but existing test pads can typically detect a limited number of analytes (mainly metabolites) at relatively high concentration levels (mM levels). For the development of the next generation of test pads, new single-step sensing approaches and probes should be developed. The interactions of analytes with micrometer-sized functionalized particles (a concept that was not so popular in conventional spectrophotometry due to scattering effects) might provide several new detection assays for test pads. For example, analyte-facilitated immunoagglutination of functionalized polymeric particles has been recently used for the single-step detection of bacteria and specific proteins; Cho et al. detected bacteria (Escherichia coli and Neisseria gonorrheae) in untreated urine104 and Lin et al. C-reactive protein in whole blood.105 Kappi et al. used the enhancement effect of biothiols at the photoreduction of silver halide particles to detect biothiols in blood plasma.106 Fluorescence-based sensing mechanisms could also be used for the single-step detection of analytes using test pads as smartphone accessories, or other low-cost readers can now measure fluorescence and bioluminescence (Figure 2B).107,108
5.2. Lateral Flow Tests
Lateral flow tests also known as lateral flow assays (LFAs) are another mature POC testing technology that has been successfully applied at point-of-care settings. The brightest example of LFAs is the human pregnancy test that has revolutionized pregnancy testing. Dozens of lateral flow tests have been commercialized for the detection of infectious diseases, cancer, cardiac diseases, illicit drugs abuse, and influenza, just to name a few.109,110 LFAs could be a core technology for a number of eDiagnostic devices, if smartphone attachments (Figures 1C and 2A)111−114 or dedicated readers115−118 read the results of analysis.
A typical lateral flow test strip is composed of three paper pads (sample pad, conjugate pad, absorbent pad) and a nitrocellulose strip; all four components are placed inside a plastic housing and partially overlap to ensure fluid’s flow. The user has to only add the biological sample (e.g., blood, urine) and a buffer solution and read the results by the naked eye after few minutes. During the analysis, an immunocomplex is formed between the target analyte (e.g., disease specific protein or antibody) and a stored biolabel (e.g., antibodies conjugated to colloidal gold or latex particles), and then it is immobilized on the test line of the strip by a capture protein that is present there. The biolabel colorates the test line to allow the visual/optical detection of the target analyte (e.g., protein, antibody, metabolite, amplified nucleic acid, RNA, etc.).90,119,120
The first generation of LFAs suffered from limited multiplex capabilities and poor sensitivity. The multiplex capabilities of LFAs have been enhanced by either incorporating multiple test strips in a single device121 or more than one test lines that exhibit same122,123 or different color121,124 in a single strip. The sensitivity of LFAs has been significantly improved by using various types of biolabels. Fluorescent dyes,117,123 carbon nanotubes,125 quantum dots,126 doubly labeled complexes,127 multifunctional nanospheres,128 upconversion nanoparticles,129 liposomes loaded with colorimetric130 or fluorescent dyes,131 photoluminescent,132 and strontium aluminate114 are a few biolabels that have been used in LFAs for the detection of disease biomarkers.
Enzyme-based,133 silver-based, or gold-based amplification schemes134 could also enhance the sensitivity of LFAs, but require additional fluidic steps that complicate the analysis. Yager and co-workers demonstrated that two-dimensional paper networks can facilitate multistep delivery of chemicals on a test line without the user performing extra steps, and used this approach to perform a signal-amplified immunoassay for the determination of malaria antigen.135,136
Yager and co-workers have also combined paper pads, lateral flow assays, and highly engineered device’s housing parts to develop fully autonomous diagnostic devices for performing immunoassays and nucleic acid detection assays. More specifically, they described an instrument-free, sample-to-result diagnostic device to detect pathogenic bacteria (ldh1 or mecA gene targets of methicillin-resistant Staphylococcus aureus) in human nasal swab specimens by performing on-board sample dilution and lysis, DNA fragmentation, DNA isothermal amplification, and DNA detection using a lateral flow assay (Figures 1D and 4A). All the necessary electronics (PCB control board, four heaters, switches, batteries), the reagents for the chemical reactions, and the buffer solutions were stored inside the device. The user had just to add the nasal swab inside the device and slide the device to start the analysis; the results were read after 60 min on the lateral flow strips of the device (Figure 1D).137 The same group reported a disposable, fully autonomous diagnostic device to detect target proteins. They detected two nucleoproteins from influenza virus A and B in nasal swab specimens. The device had all the necessary reagents and buffer solutions prestored inside its housing and performed sample lysis, protein capture, secondary labeling, rinsing, and signal amplification without user intervention. The multiplex results for infection from influenza A and B were provided in 35 min. The schematic diagram of the device is depicted in Figure 4B.138
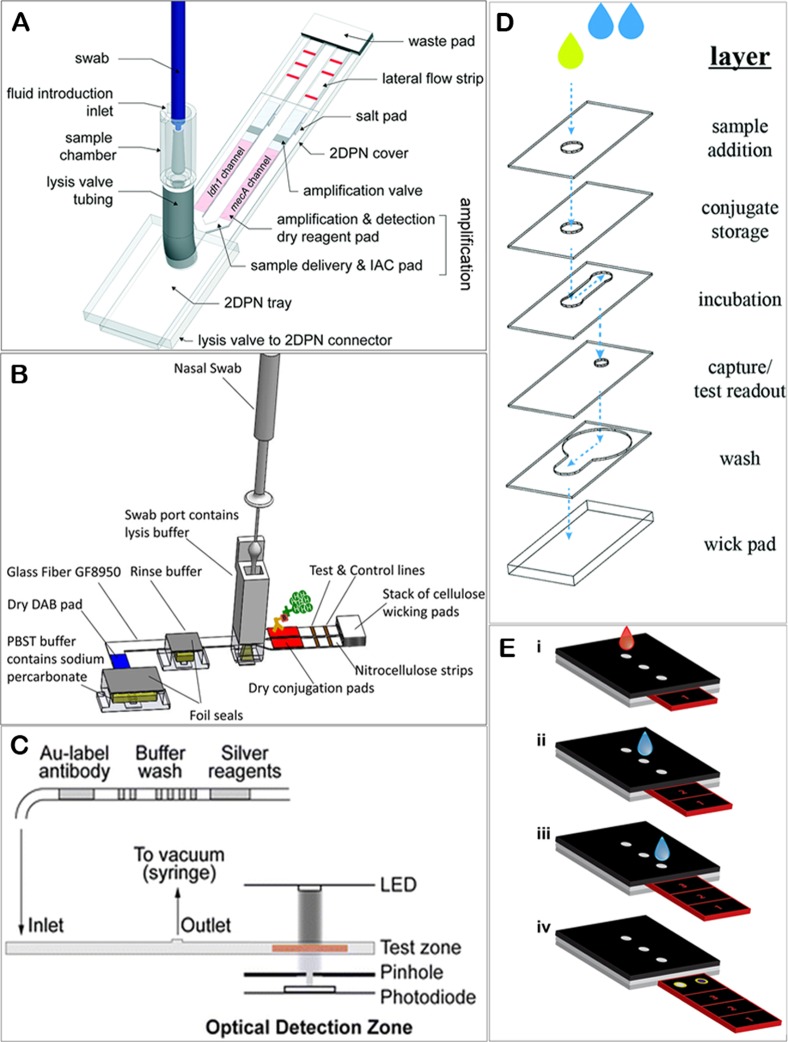
Figure 4.
(A) Diagram of a sample in–result out device for nucleic acid detection from swab specimens. A user introduces a swab into the sample chamber, and then the sample is delivered to an automated valve and is split into two physical channels followed by isothermal amplification and lateral flow detection. (B) Diagram of a sample in–result out device for protein detection from swab specimens. Nasal swab is first introduced into a swab port containing buffer followed by activating the device to release aqueous reagents into the paper network which rehydrates the stored dry reagents and then split the sample into two channels for lateral flow detection. (C) Diagram of a chip-based microfluidic device that performs signal-amplified immunoassays for multiplex detection of infectious diseases. (D) Design of a 3D-μPAD that hosts vertical and lateral pathways for performing immunoassay with visual detection. (E) Steps during analysis by using a sliding-strip 3D-μPAD. The strip is moved from position 1 to 3 along with adding the sample and water at specific time intervals. The figures were reproduced with permission from refs (137) (A), (138) (B), (199) (C), (149) (D), and (151) (E).
The quality of results of LFAs depends mainly on three factors: (a) the strong and selective binding of analyte with the capture and the reporter biorecognition elements (i.e., antigens, antibodies); (b) the strong emitting properties of biolabels; and (c) the elimination of any nonspecific binding events that result in false positive results. In the past few years significant progress has been achieved in developing approaches that can provide strong visual, optical, or fluorescent readout, but developers still depend on a trial-and-error approach to select the appropriate biorecognition elements (capture and reporter antibodies) for a target analyte and appropriate blocking reagents that eliminate false positive results. Even though the equilibrium dissociation constant (KD) that is an indicator of the strength of the specific antigen–antibody interactions has been determined (by oblique-incidence reflectivity difference139 or Biacore systems140) for most antigen–antibody pairs, these values could be used for the initial selection of possible pairs that should be further evaluated. This is because KD values measure the antigen–antibody interactions under ideal conditions (i.e., controlled immobilization of capture element in a flat chip and binding of the target analyte from standard solutions) that are different compared to those occurring during a lateral flow test (i.e., uncontrolled immobilization of biocapture elements on porous nitrocellulose or nanoparticles, target analytes partially covered by a protein corona).109 The thorough and detailed study of antigen–antibody interactions in realistic conditions and the understanding of several effects (i.e., protein corona, matrix effects due to high concentration of proteins) that may interfere with the antibody–antigen binding and result in nonspecific binding events would lead to chemical approaches that would ensure strong antigen–antibody binding and enhanced sensitivity and selectivity.
5.3. Paper-Based Analytical Devices (μPADs)
Microfluidic paper-based devices (μPADs) are another type of paper device that could find several applications in eDiagnostics. μPADs are composed of one or few stacked layers of patterned paper that expose hydrophilic channels that can store reagents and allow controlled wicking of fluids inside them.141,142 Hundreds of research efforts have described various designs, fabrication methods, approaches to add functionalities (e.g., flow rate control, flow direction control, etc.), and various detection modes (e.g., colorimetric, electrochemical, etc.)86,88,143 that could allow the development of autonomous POC devices. Similarly to other types of paper devices, if μPADs are coupled with dedicated readers or smartphones, then they could become eDiagnostic devices.
μPADs are multifunctional devices because different areas of a device can perform different functions. For example, in a 3D-μPAD, top layers could filtrate the sample; middle layers could host a chemical reaction (e.g., enzymatic reaction), and bottom layers could host the analytical detection. Several diagnostic products in the market (by the leaders of field of medical diagnostics or start-up companies) use test strips (a type of μPAD) for the detection of disease biomarkers. For example, CardioChek Home Use Analyzer test system144 (a CLIA-waived system that measured glucose, HDL/total cholesterol, triglycerides in a drop of untreated blood) uses paper-based strips to perform multistep assays for analyte detection. More specifically for cholesterol detection, (i) the blood is first filtrated through a series of filter papers that separate plasma. (ii) Plasma wicks toward a sensing pad (that contains dry reagents). (iii) Enzymatic reactions occur, which involve cholesterol and chromogenic reactions that involve one of the products of the enzymatic reaction to colorize the test pad.145 A reader (that incorporates LEDs and photodiodes) measures the color change of the pad using reflectance measurements.
In the literature, several reports describe the design and use of μPADs for biochemical analysis.86,88,143 In some of them the user only adds the sample and interprets the readout, and in others they should perform few or even many manual steps.88 Here we describe mainly examples of fully autonomous μPADs. Whitesides and co-workers developed various 3D-μPADs for the evaluation of liver function by measuring aspartate aminotransferase (AST), alkaline phosphatase (ALP), alanine aminotransferase (ALT), and total serum protein concentration in untreated blood (Figure 1B). The devices required from the user only the addition of a drop of blood and the acquisition of an image of the back of the device after 15 min;146,147 all steps of the assay (blood filtration, enzymatic modifications, and colorimetric reactions) were performed inside the device. Mace and co-workers developed 3D-μPADs for the detection of malaria and dengue fever in lysate blood and human chorionic gonadotropin (hCG) in urine by performing indirect immunoassays inside the device (gold nanoparticles were used as reporting agents) (Figure 4D).148,149 Nosrati et al. detected live and motile sperm concentrations and sperm motility by using a 3D-μPAD; the user had to only add the sperm and a buffer onto the device and take an image of the back of the device after 10 min.150 Verma et al. developed a 3D-μPAD containing a sliding strip that performed ELISA with small involvement from the user. The user should add the sample, and few drops of water on the device, and move the strip at scheduled times to certain positions (Figure 4E).151 They tested the device for the detection of C-reactive protein in blood using a three-step ELISA and colorimetric detection. Wang et al.152 and Robinson et al.153 developed foldable devices to achieve precise control over the timing of the reaction and detected β-hydroxybutyrate (BHB) and phenylalanine in blood. Several other efforts have also prepared fully autonomous paper-based analytical devices for blood analysis,154−158 and urinalysis.159−163
A number of experimental approaches that have been reported in the literature could greatly enhance the applicability and performance of μPADs if they would be incorporated in fully autonomous devices. For example, Henry and co-workers have recently developed an electrochemical μPAD by using antibody functionalized Au microwires and electrochemical impedance spectroscopy to detect virus particles that are selectively bound on the electrode during analysis. They detected West Nile particles in cell culture media at concentration levels as low as 10 particles per 50 μL sample.164 Sikes and co-workers have developed a polymer-based signal amplification method165−168 that provides time independent results and improves the ease-of-perception of the visual readout of colorimetric paper immunoassays compared to other enzymatic techniques.169,170 Connelly et al.171 developed a device composed of plastic film, magnetic slips, and paper disks that can perform nucleic acid tests in untreated fluids to detect E. coli in human plasma. All steps of the assay (cell lysis, sample preparation, isothermal DNA amplification, and detection step) were performed inside the device. The user, however, had (i) to manually add the reagents (e.g., lysis buffer, master mix, SYBR Green, etc.) into the device’s ports; (ii) to slide the moving part of the device at designated times and regions to perform the different steps; (iii) to place the device into an incubator during the amplification step; and (iv) to use a portable UV lamp and a smartphone camera to obtain the results. Bender et al. detected DNA in whole blood on a test strip using simultaneous isotachophoresis (for DNA separation), recombinase polymerase amplification (for nucleic acid amplification), and fluorescence DNA detection.172 The user also had to add the necessary reagents manually on the test strip. In principle, both approaches described by Connelly et al. and Bender et al. could be simplified significantly by storing the reagents in dry form inside an appropriately designed device; the user should then have to add only the sample and few drops of water. Li et al.173 and Tsaloglou et al.174 developed autonomous electrochemical 3D-μPADs that could detect specific DNA sequences (e.g., for the detection of hepatitis and tuberculosis). The main limitation of these approaches was that they used purified DNA as samples. If the devices would be modified to also perform DNA extraction steps, which is something doable as demonstrated by Bender et al., then these devices could analyze untreated biological samples.
Several other approaches and designs may lead to fully autonomous and operational devices; however, this needs to be demonstrated by having the relevant μPADs perform all the steps of the analysis (e.g., sample preparation, analyte detection, etc.) on board.
6. Microcell-Based Sensing for eHealth Applications
Paper devices have been widely used for decades for performing chemical measurements in the field partially because photometers, electrochemical cells, and potentiostats were bulky and expensive. Recent advances in microelectronics, LEDs, and photodiodes have allowed the development of low-cost, miniaturized readers and smartphone attachments to perform spectrophotometric (Figure 2E)108,175−179 and electrochemical measurements (Figure 2C,D)180,181 in low sample volumes. Devices that could analyze less than 100 μL of untreated samples inside a microcuvette, electrochemical microcell, or other sample holders could be called microcell devices. Due to low volume of a microcell and if the microcell is designed appropriately, then the sample may flow inside the device without applying any external force by capillary forces. HemoCue Inc. uses a microcell approach to detect hemoglobin and glucose in whole blood (using absorbance measurements),182,183 and albumin in urine (using turbidity measurements).184 Their technology is based on the storage of all the assay’s reagents in dry form inside a microcuvette. The reagents are rehydrated after the addition of the sample, and the optical measurements are performed. Several commercial available glucometers, even though this is not clearly stated, use an electrochemical microcell to measure glucose in whole blood; untreated blood flows inside a small gap (actually a microcell with volume less than around 1 μL) found in the test strips and reacts with the dry reagents and the printed electrodes found there. Other meters that have been recently developed can detect lactate, ketones, and blood coagulation using a similar approach. For example, CoaguChek XS measures prothrombin time after activation of coagulation with thromboplastin using amperometry, and then the prothrombin time is converted to International Normalized Ratio (INR).185 Zhang et al. developed a smartphone-based potentiometric biosensor for measuring salivary α-amylase. The saliva was introduced into a preloaded sensing chip by a capillary channel, followed by pressing the sensing chip to perform the potentiometric measurement.186
Impedance spectroscopy is an attractive electrochemical technique for microcell-based analysis as it does not require a label compound and could be performed in a single step (after a simple washing step). For example, Villagrasa et al. measured hematocrit levels in 50 μL of whole blood in one step by performing impedance measurements on top of screen-printed gold electrodes.187 Nidzworski et al. proposed an impedance-based method to detect anti-M1 antibodies (biomarker of influenza virus) in throat saliva or nasal samples by using functionalized boron-doped diamond electrodes.188 McMurray et al. developed an impedance biosensor based on functionalized nanoelectrodes for the detection of D-dimer protein (for diagnosis of presence of blood clot) in whole blood.189 Surface plasmon resonance (SPR) is another technique that could use a microcell-based approach to detect analytes in undiluted biological samples.190 For example, Liu et al. developed a smartphone attachment (Figure 2F) to perform surface plasmon resonance (SPR) measurements inside a capillary and monitored specifically the binding of antibodies to a functionalized sensing element.191
Recently, Turbé et al. developed a technique that could analyze an untreated biological sample in one step and detect a target analyte by monitoring phase changes of shear horizontal surface acoustic waves (SAW) caused by specific interactions between the sensing element of the device and the analyte.192 The analysis is performed on a disposable biochip and is controlled by a pocket-sized reader (Figure 1F) that is expected to cost $30. A mobile device (laptop or smartphone) was used to analyze, display, and transmit the results. The authors used this system to detect anti-HIV antibodies in 10 μL of standard solutions and an HIV-positive human blood plasma sample.
Microcell-based electrochemical and spectrophotometric assays could allow high-quality analytical assays that could support eDiagnostics. The prerequisite of microcell-based assays is, however, the effective storage of reagents inside a microcell.
7. Chip-Based Microfluidics for eHealth Applications
Since the late 80s, chip-based microfluidics has been expected to revolutionize point-of-care diagnostics. The truth is that for many years iStat (Abbott Point of Care Inc.) has been one of the very few portable devices that found their way to the market.193 The main disadvantage of chip-based microfluidics is that they typically need pumps, valves, and containers with solutions to perform the chemical analysis. To find applications in eHealth systems where there are cost and operation constraints, a chip-based microfluidic device should contain all the reagents prestored and should operate without bulky pumps. Very few research efforts have managed to develop chip-based microfluidic devices with those characteristics. Lafleur et al. developed an autonomous microfluidic device (i.e., immunoassay card) that was composed of plastic, paper-based pads (loaded with dry reagents), and membranes for plasma separation and visual detection. The user applied the sample and buffer solutions, and the pneumatic control system, which applied air pressure and vacuum, performed all the steps of the assay (blood filtration, aliquoting, dilution, IgG removal, reagent rehydration, and the multistep detection assay) automatically. The detection was facilitated via colored spots that appeared on the detection membrane. The device detected malaria antigens and Salmonella Typhi antibodies in whole blood.194 Floris et al. developed a fully autonomous chip-based microfluidic system (Figure 1G) for the detection of lithium in a drop of untreated blood.195 The system was composed of disposable microfluidic chips that were filled with a buffer and a hand-held analyzer. During analysis, lithium ions were separated from other blood ions via capillary electrophoresis and quantified by conductivity measurements.195 Sia and co-workers have developed a technology that allowed them to perform multistep signal-enhanced immunoassays (that use reporter antibodies conjugated with gold nanoparticles and a silver amplification step) with optical readout inside a microfluidic cassette without user intervention, or any external instrumentation (e.g., detector, pumps) (Figure 4C).196−200 The technology was based on cassettes that contained (a) microfluidic channels filled with up to 14 separate liquid reagents (i.e., antibodies, washing, and signal-development solutions) spatially separated by air pockets and (b) an on-board optical detection system (Figure 4C). The reagents were moved inside the cassette, and a test started once vacuum was applied (by a negative pressure chamber activated by pushing a button). A smartphone controlled the whole operation. The device could analyze 2 μL of diluted blood and provide results in 15 min (Figure 1E). The performance of the device was tested on the multiplex detection of HIV, treponemal syphilis, and nontreponemal syphilis antibodies,198 and the multiplex detection of hemoglobin and HIV antibodies using real blood samples.199
Both approaches described by Floris et al. and Sia et al. used a chip prefilled with solutions. This approach is advantageous as it eliminates the need of the use of separate liquid reagents and separate pumps for fluid handling. The continuous reduction of cost of integrated electronic systems and components, and scientific innovation in the storage of solutions inside chips could soon provide microfluidic systems with enhanced capabilities for eDiagnostics.
8. Remarks and Conclusions
In this Outlook, we described the design constrains that new eDiagnostic devices should fit and highlighted four POC technologies (i.e., wearables, paper diagnostics, microcell-based sensing, and chip-based microfluidics) that could be the core technologies of new eDiagnostics. We also briefly described examples of devices that have been successfully used for the analysis of untreated biological samples. Interestingly, out of thousands of devices that have been developed in academic laboratories for POC testing, a very small percentage of them have been used to detect analytes in untreated biological fluids using an analytical procedure that is suitable for home applications: the user only adding the sample (i.e., finger-prick blood, urine, saliva, etc.) on the device and reading the results after few minutes.
The development of a new diagnostic device is a difficult if not Herculean task.201 It is also very expensive; a study has identified that in 2010 the average cost to bring a diagnostic device (i.e., 510(k) product) from concept to market into the US is more than $30 million, and the major part of the cost was associated with the regulatory clearance of the device.202 To improve the chances of developing a technology that could transition from a research idea, to an eDiagnostic device, scientists and developers might need to consider the followings: (i) A new diagnostic device must solve a “real” problem/diagnostic need, and the existing solution to that should be inadequate or unaffordable.201 They should, therefore, carefully define the problem and justify why the new diagnostic device covers the need and leads to a better health outcome. (ii) A diagnostic need that impacts a substantial number of patients might be more prioritized than others. eDiagnostic devices for monitoring chronic diseases, metabolic disorders, and disease treatment, for detecting common viral infections (e.g., the flu, streptococcus), and for detecting biothreat agents (after bioterrorism attacks or pandemic conditions) might be needed at high volumes. (iii) The clearance from local regulatory authorities is a prerequisite that eHealth devices should achieve; therefore it would be useful if they design the devices to meet the criteria that regulatory authorities set. For example, US FDA uses seven criteria to assign the test a complexity status (waived–moderate–high); only waived complexity tests can be used outside laboratory settings (get the CLIA-waived status).12 The criteria and scoring rubric of them can be found in ref (12). Currently, scientists in the field of POC diagnostics use the “ASSURED” criteria as design guidelines.201 Not all “ASSURED” criteria, however, apply to eHealth applications: for example, the “Equipment-free” and to a lower extent the “Affordability” criteria). Under public health emergency situations (e.g., Ebola and Zika virus outbreak), regulatory authorities might follow a different expedited clearance procedure (e.g., WHO Emergency Use Assessment and Listing (EUAL) procedure)203 that accepts variations in the regular acceptable criteria, but in general these are unique cases. (iv) eDiagnostic devices should be able to analyze untreated biological samples; therefore, the performance of the devices should be evaluated using untreated samples or preferably clinical samples.204 Scientists within academia typically use buffer solutions spiked with analyte, or diluted biological fluids spiked with analyte as “samples”.204 Untreated biological samples have a very complicated chemical matrix that can severely interfere with the analysis. If researchers do not have access to real biological samples, then it would be preferable if they analyze spiked solutions of model matrixes (e.g., artificial plasma with high protein concentration around 70 mg/mL, artificial urine). (v) eDiagnostic devices should be compatible with various subsystems of eHealth systems and other diagnostic devices. (vi) The communication between eDiagnostic device and eHealth systems should be secure to ensure safety and confidentiality of the personal health records. (vii) The performance metrics (e.g., sensitivity, specificity) and definitions of each metric used during a clinical assessment of a device are different than the definitions and performance metrics (e.g., detection limit, linear dynamic range of detection) used in chemical science.204 (viii) There is a gap between proof-of-concept testing in a laboratory and a full scale clinical trial that the developers should bridge by performing initial field trials of the devices. These tasks are outside the expertise of scientists, and acquiring funding for them could be difficult (mainly because scientists are unaware of relevant funding mechanisms); however, the feedback from end users will improve chances of success.201 (ix) The “valley of death”, the phase between research and successful commercialization, could be crossed more easily by forming an interdisciplinary team (that include scientists, engineers, physicians, public health specialists, and entrepreneurs). (x) Experience and lessons drawn from others trying to move their technology from the laboratory to the hands of consumers could be very useful.201,205
The landscape of medical self-testing is changing rapidly. A decade ago, point-of-care devices were mainly used by health professionals at or near the point of care (e.g., in clinics, etc.), and very few self-testing devices (e.g., thermometers, blood pressure meters, glucometers, and pregnancy tests) were available to consumers. Within the past years, several point-of-care devices have been modified and further developed to become self-testing devices for blood oxygen, blood glucose and continuous glucose monitoring, for recording electrocardiograms,206,207 or for detecting analytes such as lactate, creatinine, cholesterol, uric acid, hemoglobin, illicit drugs, etc. Several of these self-testing devices could be called eDiagnostic devices as they can seamlessly communicate with smartphones that store, analyze, and transmit the results to physicians. Several other eDiagnostics have already got regulatory clearance and would reach the market soon. For example, Tyto Care Inc. has developed a standalone device with connectivity features that incorporates digital cameras to view the ears, throat, and skin; a microphone to listen to the heart, lungs, and abdomen; and a basal thermometer.208 Healthy.io Ltd. developed a urinalysis test kit for home use that measures 10 parameters. The kit is composed of a urinalysis test strip, a carefully designed calibration chart, and a smartphone application that use proprietary computer vision algorithms to measure the concentration of target analytes accurately in any lighting conditions using any smartphone.209 Information communication technologies (ICT) have already changed the way that people communicate, work, shop, date, and entertain, and may soon change how people receive health-care services.
Acknowledgments
The authors want to thank Austin Chen (Columbia University), Sudarshi Premawardhana (UMass Lowell), and the members of the Christodouleas group Abbas Kazi, Anna-Maria Routsi, and Melanie Conner-Myers, for useful discussions.
The authors declare no competing financial interest.
References
- Current health expenditure as a percentage of gross domestic product (GDP). http://www.who.int/gho/health_financing/health_expenditure/en/ (accessed Aug 27, 2018).
- World Health Organization. Global diffusion of eHealth: making universal health coverage achievable. Report of the third global survey on eHealth. http://www.who.int/goe/publications/global_diffusion/en/ (accessed Aug 27, 2018).
- Shaw T.; McGregor D.; Brunner M.; Keep M.; Janssen A.; Barnet S. What is eHealth (6)? Development of a conceptual model for eHealth: Qualitative study with key informants. J. Med. Internet Res. 2017, 19, e324 10.2196/jmir.8106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubrichi S.; Battistotti A.; Quaglini S. Patients’ involvement in e-health services quality assessment: a system for the automatic interpretation of SMS-based patients’ feedback. J. Biomed. Inf. 2014, 51, 41–48. 10.1016/j.jbi.2014.03.003. [DOI] [PubMed] [Google Scholar]
- de la Torre-Díez I.; López-Coronado M.; Vaca C.; Aguado J. S.; de Castro C. Cost-utility and cost-effectiveness studies of telemedicine, electronic, and mobile health systems in the literature: A systematic review. Telemed J. E Health 2015, 21, 81–85. 10.1089/tmj.2014.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein S. M.; Speedie S. M.; Potthoff S. Home telehealth improves clinical outcomes at lower cost for home healthcare. Telemed J. E Health 2006, 12, 128–136. 10.1089/tmj.2006.12.128. [DOI] [PubMed] [Google Scholar]
- Grustam A. S.; Severens J. L.; De Massari D.; Buyukkaramikli N.; Koymans R.; Vrijhoef H. J. M. Cost-effectiveness analysis in telehealth: A comparison between home telemonitoring, nurse telephone support, and usual care in chronic heart failure management. Value Health 2018, 21, 772–782. 10.1016/j.jval.2017.11.011. [DOI] [PubMed] [Google Scholar]
- Hoekstra R.; Blondeau P.; Andrade F. J. Distributed electrochemical sensors: recent advances and barriers to market adoption. Anal. Bioanal. Chem. 2018, 410, 4077–4089. 10.1007/s00216-018-1104-9. [DOI] [PubMed] [Google Scholar]
- Hu J.; Cui X.; Gong Y.; Xu X.; Gao B.; Wen T.; Lu T. J.; Xu F. Portable microfluidic and smartphone-based devices for monitoring of cardiovascular diseases at the point of care. Biotechnol. Adv. 2016, 34, 305–320. 10.1016/j.biotechadv.2016.02.008. [DOI] [PubMed] [Google Scholar]
- Nayak S.; Blumenfeld N. R.; Laksanasopin T.; Sia S. K. Point-of-care diagnostics: Recent developments in a connected age. Anal. Chem. 2017, 89, 102–123. 10.1021/acs.analchem.6b04630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J.; Dong M.; Rigatto C.; Liu Y.; Lin F. Lab-on-chip technology for chronic disease diagnosis. npj Digital Medicine 2018, 1, 7. 10.1038/s41746-017-0014-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Food and Drug Administration. CLIA Categorizations. https://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/IVDRegulatoryAssistance/ucm393229.htm#scorecard (accessed Aug 27, 2018).
- Plevniak K.; Campbell M.; Myers T.; Hodges A.; He M. 3D printed auto-mixing chip enables rapid smartphone diagnosis of anemia. Biomicrofluidics 2016, 10, 054113. 10.1063/1.4964499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross B.; Lockwood S. Y.; Spence D. M. Recent advances in analytical chemistry by 3D printing. Anal. Chem. 2017, 89, 57–70. 10.1021/acs.analchem.6b04344. [DOI] [PubMed] [Google Scholar]
- Quesada-González D.; Merkoçi A. Mobile phone-based biosensing: An emerging “diagnostic and communication” technology. Biosens. Bioelectron. 2017, 92, 549–562. 10.1016/j.bios.2016.10.062. [DOI] [PubMed] [Google Scholar]
- Blicharz T. M.; Gong P.; Bunner B. M.; Chu L. L.; Leonard K. M.; Wakefield J. A.; Williams R. E.; Dadgar M.; Tagliabue C. A.; El Khaja R.; Marlin S. L.; Haghgooie R.; Davis S. P.; Chickering D. E.; Bernstein H. Microneedle-based device for the one-step painless collection of capillary blood samples. Nat. Biomed. Eng. 2018, 2, 151–157. 10.1038/s41551-018-0194-1. [DOI] [PubMed] [Google Scholar]
- Seventh Sense Biosystems, Inc. TAP. http://www.7sbio.com/tap (accessed Oct 18, 2018).
- Schroeder L. F.; Giacherio D.; Gianchandani R.; Engoren M.; Shah N. H. Postmarket surveillance of point-of-care glucose meters through analysis of electronic medical records. Clin. Chem. 2016, 62, 716–724. 10.1373/clinchem.2015.251827. [DOI] [PubMed] [Google Scholar]
- Sauer-Budge A. F.; Brookfield S. J.; Janzen R.; McGray S.; Boardman A.; Wirz H.; Pollock N. R. A novel device for collecting and dispensing fingerstick blood for point of care testing. PLoS One 2017, 12, e0183625 10.1371/journal.pone.0183625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medtronic, Inc. The Guardian Connect CGM. https://www.medtronicdiabetes.com/products/guardian-connect-continuous-glucose-monitoring-system (accessed Aug 29, 2018).
- Dungan K.; Verma N.. Monitoring technologies–continuous glucose monitoring, mobile technology, biomarkers of glycemic control. In Endotext [Internet]; MDText.com, Inc., 2018. [PubMed] [Google Scholar]
- Dexcom, Inc. Dexcom G6. https://www.dexcom.com/g6-cgm-system (accessed Aug 29, 2018).
- Abbott diabetes care, Inc. FreeStyle Libre. https://www.freestylelibre.us/ (accessed Aug 29, 2018).
- Chang H.; Zheng M.; Yu X.; Than A.; Seeni R. Z.; Kang R.; Tian J.; Khanh D. P.; Liu L.; Chen P.; Xu C. A swellable microneedle patch to rapidly extract skin interstitial fluid for timely metabolic analysis. Adv. Mater. 2017, 29, 1702243. 10.1002/adma.201702243. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Gao W.. Wearable and flexible electronics for continuous molecular monitoring. Chem. Soc. Rev. 2018, in press. 10.1039/c7cs00730b. [DOI] [PubMed] [Google Scholar]
- Turner A. P. Biosensors: sense and sensibility. Chem. Soc. Rev. 2013, 42, 3184–96. 10.1039/c3cs35528d. [DOI] [PubMed] [Google Scholar]
- Heikenfeld J. Non-invasive analyte access and sensing through eccrine sweat: Challenges and outlook circa 2016. Electroanalysis 2016, 28, 1242–1249. 10.1002/elan.201600018. [DOI] [Google Scholar]
- Glennon T.; O’Quigley C.; McCaul M.; Matzeu G.; Beirne S.; Wallace G. G.; Stroiescu F.; O’Mahoney N.; White P.; Diamond D. ‘SWEATCH’: A wearable platform for harvesting and analysing sweat sodium content. Electroanalysis 2016, 28, 1283–1289. 10.1002/elan.201600106. [DOI] [Google Scholar]
- Liu G.; Ho C.; Slappey N.; Zhou Z.; Snelgrove S. E.; Brown M.; Grabinski A.; Guo X.; Chen Y.; Miller K.; Edwards J.; Kaya T. A wearable conductivity sensor for wireless real-time sweat monitoring. Sens. Actuators, B 2016, 227, 35–42. 10.1016/j.snb.2015.12.034. [DOI] [Google Scholar]
- Mielczarek W. S.; Obaje E. A.; Bachmann T. T.; Kersaudy-Kerhoas M. Microfluidic blood plasma separation for medical diagnostics: is it worth it?. Lab Chip 2016, 16, 3441–3448. 10.1039/C6LC00833J. [DOI] [PubMed] [Google Scholar]
- Tripathi S.; Kumar Y. B. V.; Prabhakar A.; Joshi S. S.; Agrawal A. Performance study of microfluidic devices for blood plasma separation—a designer’s perspective. J. Micromech. Microeng. 2015, 25, 084004. 10.1088/0960-1317/25/8/084004. [DOI] [Google Scholar]
- Kersaudy-Kerhoas M.; Sollier E. Micro-scale blood plasma separation: from acoustophoresis to egg-beaters. Lab Chip 2013, 13, 3323–3346. 10.1039/c3lc50432h. [DOI] [PubMed] [Google Scholar]
- Cunningham D. D. Fluidics and sample handling in clinical chemical analysis. Anal. Chim. Acta 2001, 429, 1–18. 10.1016/S0003-2670(00)01256-3. [DOI] [Google Scholar]
- Liu C.; Mauk M.; Gross R.; Bushman F. D.; Edelstein P. H.; Collman R. G.; Bau H. H. Membrane-based, sedimentation-assisted plasma separator for point-of-care applications. Anal. Chem. 2013, 85, 10463–10470. 10.1021/ac402459h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.; Liao S.-C.; Song J.; Mauk M. G.; Li X.; Wu G.; Ge D.; Greenberg R. M.; Yang S.; Bau H. H. A high-efficiency superhydrophobic plasma separator. Lab Chip 2016, 16, 553–560. 10.1039/C5LC01235J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.; Forouzan O.; Brown T. P.; Shevkoplyas S. S. Integrated separation of blood plasma from whole blood for microfluidic paper-based analytical devices. Lab Chip 2012, 12, 274–280. 10.1039/C1LC20803A. [DOI] [PubMed] [Google Scholar]
- Son J. H.; Lee S. H.; Hong S.; Park S.-m.; Lee J.; Dickey A. M.; Lee L. P. Hemolysis-free blood plasma separation. Lab Chip 2014, 14, 2287–2292. 10.1039/C4LC00149D. [DOI] [PubMed] [Google Scholar]
- Christiansen M. P.; Klaff L. J.; Brazg R.; Chang A. R.; Levy C. J.; Lam D.; Denham D. S.; Atiee G.; Bode B. W.; Walters S. J.; Kelley L.; Bailey T. S. A prospective multicenter evaluation of the accuracy of a novel implanted continuous glucose sensor: PRECISE II. Diabetes Technol. Ther. 2018, 20, 197–206. 10.1089/dia.2017.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senseonics, Inc. Eversense CGM System. https://www.eversensediabetes.com/ (accessed Aug 29, 2018).
- Kim J.; Campbell A. S.; Wang J. Wearable non-invasive epidermal glucose sensors: A review. Talanta 2018, 177, 163–170. 10.1016/j.talanta.2017.08.077. [DOI] [PubMed] [Google Scholar]
- Bandodkar A. J.; Hung V. W. S.; Jia W.; Valdés-Ramírez G.; Windmiller J. R.; Martinez A. G.; Ramírez J.; Chan G.; Kerman K.; Wang J. Tattoo-based potentiometric ion-selective sensors for epidermal pH monitoring. Analyst 2013, 138, 123–128. 10.1039/C2AN36422K. [DOI] [PubMed] [Google Scholar]
- Guinovart T.; Bandodkar A. J.; Windmiller J. R.; Andrade F. J.; Wang J. A potentiometric tattoo sensor for monitoring ammonium in sweat. Analyst 2013, 138, 7031–7038. 10.1039/c3an01672b. [DOI] [PubMed] [Google Scholar]
- Bandodkar A. J.; Molinnus D.; Mirza O.; Guinovart T.; Windmiller J. R.; Valdes-Ramirez G.; Andrade F. J.; Schoning M. J.; Wang J. Epidermal tattoo potentiometric sodium sensors with wireless signal transduction for continuous non-invasive sweat monitoring. Biosens. Bioelectron. 2014, 54, 603–609. 10.1016/j.bios.2013.11.039. [DOI] [PubMed] [Google Scholar]
- Nyein H. Y. Y.; Gao W.; Shahpar Z.; Emaminejad S.; Challa S.; Chen K.; Fahad H. M.; Tai L.-C.; Ota H.; Davis R. W. A wearable electrochemical platform for noninvasive simultaneous monitoring of Ca2+ and pH. ACS Nano 2016, 10, 7216–7224. 10.1021/acsnano.6b04005. [DOI] [PubMed] [Google Scholar]
- Emaminejad S.; Gao W.; Wu E.; Davies Z. A.; Yin Yin Nyein H.; Challa S.; Ryan S. P.; Fahad H. M.; Chen K.; Shahpar Z.; Talebi S.; Milla C.; Javey A.; Davis R. W. Autonomous sweat extraction and analysis applied to cystic fibrosis and glucose monitoring using a fully integrated wearable platform. Proc. Natl. Acad. Sci. U. S. A. 2017, 114, 4625–4630. 10.1073/pnas.1701740114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sempionatto J. R.; Nakagawa T.; Pavinatto A.; Mensah S. T.; Imani S.; Mercier P.; Wang J. Eyeglasses based wireless electrolyte and metabolite sensor platform. Lab Chip 2017, 17, 1834–1842. 10.1039/C7LC00192D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonner Z.; Wilder E.; Gaillard T.; Kasting G.; Heikenfeld J. Integrated sudomotor axon reflex sweat stimulation for continuous sweat analyte analysis with individuals at rest. Lab Chip 2017, 17, 2550–2560. 10.1039/C7LC00364A. [DOI] [PubMed] [Google Scholar]
- Economou A.; Kokkinos C.; Prodromidis M. Flexible plastic, paper and textile lab-on-a chip platforms for electrochemical biosensing. Lab Chip 2018, 18, 1812–1830. 10.1039/C8LC00025E. [DOI] [PubMed] [Google Scholar]
- Rose D. P.; Ratterman M. E.; Griffin D. K.; Hou L.; Kelley-Loughnane N.; Naik R. R.; Hagen J. A.; Papautsky I.; Heikenfeld J. C. Adhesive RFID sensor patch for monitoring of sweat electrolytes. IEEE Trans. Biomed. Eng. 2015, 62, 1457–1465. 10.1109/TBME.2014.2369991. [DOI] [PubMed] [Google Scholar]
- Martín A.; Kim J.; Kurniawan J. F.; Sempionatto J. R.; Moreto J. R.; Tang G.; Campbell A. S.; Shin A.; Lee M. Y.; Liu X. Epidermal microfluidic electrochemical detection system: Enhanced sweat sampling and metabolite detection. ACS sensors 2017, 2, 1860–1868. 10.1021/acssensors.7b00729. [DOI] [PubMed] [Google Scholar]
- Kim J.; Jeerapan I.; Imani S.; Cho T. N.; Bandodkar A.; Cinti S.; Mercier P. P.; Wang J. Noninvasive alcohol monitoring using a wearable tattoo-based iontophoretic-biosensing system. ACS Sensors 2016, 1, 1011–1019. 10.1021/acssensors.6b00356. [DOI] [Google Scholar]
- Jia W.; Bandodkar A. J.; Valdés-Ramírez G.; Windmiller J. R.; Yang Z.; Ramírez J.; Chan G.; Wang J. Electrochemical tattoo biosensors for real-time noninvasive lactate monitoring in human perspiration. Anal. Chem. 2013, 85, 6553–6560. 10.1021/ac401573r. [DOI] [PubMed] [Google Scholar]
- Gao W.; Emaminejad S.; Nyein H. Y. Y.; Challa S.; Chen K.; Peck A.; Fahad H. M.; Ota H.; Shiraki H.; Kiriya D.; Lien D.-H.; Brooks G. A.; Davis R. W.; Javey A. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 2016, 529, 509–514. 10.1038/nature16521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W.; Nyein H. Y.; Shahpar Z.; Fahad H. M.; Chen K.; Emaminejad S.; Gao Y.; Tai L.-C.; Ota H.; Wu E. Wearable microsensor array for multiplexed heavy metal monitoring of body fluids. ACS Sensors 2016, 1, 866–874. 10.1021/acssensors.6b00287. [DOI] [Google Scholar]
- Kim J.; de Araujo W. R.; Samek I. A.; Bandodkar A. J.; Jia W.; Brunetti B.; Paixão T. R. L. C.; Wang J. Wearable temporary tattoo sensor for real-time trace metal monitoring in human sweat. Electrochem. Commun. 2015, 51, 41–45. 10.1016/j.elecom.2014.11.024. [DOI] [Google Scholar]
- Chen Y.; Lu S.; Zhang S.; Li Y.; Qu Z.; Chen Y.; Lu B.; Wang X.; Feng X. Skin-like biosensor system via electrochemical channels for noninvasive blood glucose monitoring. Sci. Adv. 2017, 3, e1701629 10.1126/sciadv.1701629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipani L.; Dupont B. G. R.; Doungmene F.; Marken F.; Tyrrell R. M.; Guy R. H.; Ilie A. Non-invasive, transdermal, path-selective and specific glucose monitoring via a graphene-based platform. Nat. Nanotechnol. 2018, 13, 504–511. 10.1038/s41565-018-0112-4. [DOI] [PubMed] [Google Scholar]
- Lee H.; Choi T. K.; Lee Y. B.; Cho H. R.; Ghaffari R.; Wang L.; Choi H. J.; Chung T. D.; Lu N.; Hyeon T.; Choi S. H.; Kim D.-H. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat. Nanotechnol. 2016, 11, 566–573. 10.1038/nnano.2016.38. [DOI] [PubMed] [Google Scholar]
- Choi J.; Ghaffari R.; Baker L. B.; Rogers J. A. Skin-interfaced systems for sweat collection and analytics. Sci. Adv. 2018, 4, eaar3921 10.1126/sciadv.aar3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh A.; Kang D.; Xue Y.; Lee S.; Pielak R. M.; Kim J.; Hwang T.; Min S.; Banks A.; Bastien P.; Manco M. C.; Wang L.; Ammann K. R.; Jang K.-I.; Won P.; Han S.; Ghaffari R.; Paik U.; Slepian M. J.; Balooch G.; Huang Y.; Rogers J. A. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci. Transl. Med. 2016, 8, 366ra165. 10.1126/scitranslmed.aaf2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J.; Kang D.; Han S.; Kim S. B.; Rogers J. A. Thin, soft, skin-mounted microfluidic networks with capillary bursting valves for chrono-sampling of sweat. Adv. Healthcare Mater. 2017, 6, 1601355. 10.1002/adhm.201601355. [DOI] [PubMed] [Google Scholar]
- Kim S. B.; Zhang Y.; Won S. M.; Bandodkar A. J.; Sekine Y.; Xue Y.; Koo J.; Harshman S. W.; Martin J. A.; Park J. M.; Ray T. R.; Crawford K. E.; Lee K. T.; Choi J.; Pitsch R. L.; Grigsby C. C.; Strang A. J.; Chen Y. Y.; Xu S.; Kim J.; Koh A.; Ha J. S.; Huang Y.; Kim S. W.; Rogers J. A. Super-absorbent polymer valves and colorimetric chemistries for time-sequenced discrete sampling and chloride analysis of sweat via skin-mounted soft microfluidics. Small 2018, 14, 1703334. 10.1002/smll.201703334. [DOI] [PubMed] [Google Scholar]
- Huang X.; Liu Y.; Chen K.; Shin W.-J.; Lu C.-J.; Kong G.-W.; Patnaik D.; Lee S.-H.; Cortes J. F.; Rogers J. A. Stretchable, wireless sensors and functional substrates for epidermal characterization of sweat. Small 2014, 10, 3083–3090. 10.1002/smll.201400483. [DOI] [PubMed] [Google Scholar]
- Mu X.; Xin X.; Fan C.; Li X.; Tian X.; Xu K.-F.; Zheng Z. A paper-based skin patch for the diagnostic screening of cystic fibrosis. Chem. Commun. 2015, 51, 6365–6368. 10.1039/C5CC00717H. [DOI] [PubMed] [Google Scholar]
- Kassal P.; Kim J.; Kumar R.; de Araujo W. R.; Steinberg I. M.; Steinberg M. D.; Wang J. Smart bandage with wireless connectivity for uric acid biosensing as an indicator of wound status. Electrochem. Commun. 2015, 56, 6–10. 10.1016/j.elecom.2015.03.018. [DOI] [Google Scholar]
- Liu X.; Lillehoj P. B. Embroidered electrochemical sensors on gauze for rapid quantification of wound biomarkers. Biosens. Bioelectron. 2017, 98, 189–194. 10.1016/j.bios.2017.06.053. [DOI] [PubMed] [Google Scholar]
- Punjiya M.; Rezaei H.; Zeeshan M. A.; Sonkusale S.. A flexible pH sensing smart bandage with wireless CMOS readout for chronic wound monitoring, 2017 19th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS) 2017, June 18–22, 2017; pp 1700–1702.
- Punjiya M.; Mostafalu P.; Sonkusale S.. Smart bandages for chronic wound monitoring and on-demand drug delivery, 2017 IEEE 60th International Midwest Symposium on Circuits and Systems (MWSCAS), Aug 6–9, 2017; pp 495–498.
- Jankowska D. A.; Bannwarth M. B.; Schulenburg C.; Faccio G.; Maniura-Weber K.; Rossi R. M.; Scherer L.; Richter M.; Boesel L. F. Simultaneous detection of pH value and glucose concentrations for wound monitoring applications. Biosens. Bioelectron. 2017, 87, 312–319. 10.1016/j.bios.2016.08.072. [DOI] [PubMed] [Google Scholar]
- Farooqui M. F.; Shamim A. Low cost inkjet printed smart bandage for wireless monitoring of chronic wounds. Sci. Rep. 2016, 6, 28949. 10.1038/srep28949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa T.; Kuroki Y.; Nitta H.; Chouhan P.; Toma K.; Sawada S.-i.; Takeuchi S.; Sekita T.; Akiyoshi K.; Minakuchi S.; Mitsubayashi K. Mouthguard biosensor with telemetry system for monitoring of saliva glucose: A novel cavitas sensor. Biosens. Bioelectron. 2016, 84, 106–111. 10.1016/j.bios.2015.12.014. [DOI] [PubMed] [Google Scholar]
- Kim J.; Imani S.; de Araujo W. R.; Warchall J.; Valdes-Ramirez G.; Paixao T. R.; Mercier P. P.; Wang J. Wearable salivary uric acid mouthguard biosensor with integrated wireless electronics. Biosens. Bioelectron. 2015, 74, 1061–1068. 10.1016/j.bios.2015.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.; Valdés-Ramírez G.; Bandodkar A. J.; Jia W.; Martinez A. G.; Ramírez J.; Mercier P.; Wang J. Non-invasive mouthguard biosensor for continuous salivary monitoring of metabolites. Analyst 2014, 139, 1632–1636. 10.1039/C3AN02359A. [DOI] [PubMed] [Google Scholar]
- Mannoor M. S.; Tao H.; Clayton J. D.; Sengupta A.; Kaplan D. L.; Naik R. R.; Verma N.; Omenetto F. G.; McAlpine M. C. Graphene-based wireless bacteria detection on tooth enamel. Nat. Commun. 2012, 3, 763. 10.1038/ncomms1767. [DOI] [PubMed] [Google Scholar]
- Borini S.; White R.; Wei D.; Astley M.; Haque S.; Spigone E.; Harris N.; Kivioja J.; Ryhänen T. Ultrafast graphene oxide humidity sensors. ACS Nano 2013, 7, 11166–11173. 10.1021/nn404889b. [DOI] [PubMed] [Google Scholar]
- Güder F.; Ainla A.; Redston J.; Mosadegh B.; Glavan A.; Martin T. J.; Whitesides G. M. Paper-based electrical respiration sensor. Angew. Chem., Int. Ed. 2016, 55, 5727–5732. 10.1002/anie.201511805. [DOI] [PubMed] [Google Scholar]
- Yao H.; Shum A. J.; Cowan M.; Lahdesmaki I.; Parviz B. A. A contact lens with embedded sensor for monitoring tear glucose level. Biosens. Bioelectron. 2011, 26, 3290–6. 10.1016/j.bios.2010.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.; Kim M.; Lee M.-S.; Kim K.; Ji S.; Kim Y.-T.; Park J.; Na K.; Bae K.-H.; Kyun Kim H.; Bien F.; Young Lee C.; Park J.-U. Wearable smart sensor systems integrated on soft contact lenses for wireless ocular diagnostics. Nat. Commun. 2017, 8, 14997. 10.1038/ncomms14997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas N.; Lähdesmäki I.; Parviz B. A. A contact lens with an integrated lactate sensor. Sens. Actuators, B 2012, 162, 128–134. 10.1016/j.snb.2011.12.049. [DOI] [Google Scholar]
- Liao Y.-T.; Yao H.; Lingley A.; Parviz B.; Otis B. P. A 3-μW CMOS Glucose Sensor for Wireless Contact-Lens Tear Glucose Monitoring. IEEE J. Solid-State Circuits 2012, 47, 335–344. 10.1109/JSSC.2011.2170633. [DOI] [Google Scholar]
- Pandian P. S.; Mohanavelu K.; Safeer K. P.; Kotresh T. M.; Shakunthala D. T.; Gopal P.; Padaki V. C. Smart Vest: wearable multi-parameter remote physiological monitoring system. Med. Eng. Phys. 2008, 30, 466–477. 10.1016/j.medengphy.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Scilingo E. P.; Gemignani A.; Paradiso R.; Taccini N.; Ghelarducci B.; De Rossi D. Performance evaluation of sensing fabrics for monitoring physiological and biomechanical variables. IEEE Trans. Inf. Technol. Biomed. 2005, 9, 345–352. 10.1109/TITB.2005.854506. [DOI] [PubMed] [Google Scholar]
- Guay P.; Gorgutsa S.; LaRochelle S.; Messaddeq Y. Wearable contactless respiration sensor based on multi-material fibers integrated into textile. Sensors 2017, 17, 1050. 10.3390/s17051050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppedè N.; Tarabella G.; Villani M.; Calestani D.; Iannotta S.; Zappettini A. Human stress monitoring through an organic cotton-fiber biosensor. J. Mater. Chem. B 2014, 2, 5620–5626. 10.1039/C4TB00317A. [DOI] [PubMed] [Google Scholar]
- Heikenfeld J.; Jajack A.; Rogers J.; Gutruf P.; Tian L.; Pan T.; Li R.; Khine M.; Kim J.; Wang J.; Kim J. Wearable sensors: modalities, challenges, and prospects. Lab Chip 2018, 18, 217–248. 10.1039/C7LC00914C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cate D. M.; Adkins J. A.; Mettakoonpitak J.; Henry C. S. Recent developments in paper-based microfluidic devices. Anal. Chem. 2015, 87, 19–41. 10.1021/ac503968p. [DOI] [PubMed] [Google Scholar]
- Nery E. W.; Kubota L. T. Sensing approaches on paper-based devices: a review. Anal. Bioanal. Chem. 2013, 405, 7573–7595. 10.1007/s00216-013-6911-4. [DOI] [PubMed] [Google Scholar]
- Yetisen A. K.; Akram M. S.; Lowe C. R. Paper-based microfluidic point-of-care diagnostic devices. Lab Chip 2013, 13, 2210–2251. 10.1039/c3lc50169h. [DOI] [PubMed] [Google Scholar]
- Mettakoonpitak J.; Boehle K.; Nantaphol S.; Teengam P.; Adkins J. A.; Srisa-Art M.; Henry C. S. Electrochemistry on paper-based analytical devices: A review. Electroanalysis 2016, 28, 1420–1436. 10.1002/elan.201501143. [DOI] [Google Scholar]
- Mak W. C.; Beni V.; Turner A. P. F. Lateral-flow technology: From visual to instrumental. TrAC, Trends Anal. Chem. 2016, 79, 297–305. 10.1016/j.trac.2015.10.017. [DOI] [Google Scholar]
- Morbioli G. G.; Mazzu-Nascimento T.; Stockton A. M.; Carrilho E. Technical aspects and challenges of colorimetric detection with microfluidic paper-based analytical devices (μPADs) - A review. Anal. Chim. Acta 2017, 970, 1–22. 10.1016/j.aca.2017.03.037. [DOI] [PubMed] [Google Scholar]
- Capitán-Vallvey L. F.; López-Ruiz N.; Martínez-Olmos A.; Erenas M. M.; Palma A. J. Recent developments in computer vision-based analytical chemistry: A tutorial review. Anal. Chim. Acta 2015, 899, 23–56. 10.1016/j.aca.2015.10.009. [DOI] [PubMed] [Google Scholar]
- Lee D. S.; Jeon B. G.; Ihm C.; Park J. K.; Jung M. Y. A simple and smart telemedicine device for developing regions: a pocket-sized colorimetric reader. Lab Chip 2011, 11, 120–126. 10.1039/C0LC00209G. [DOI] [PubMed] [Google Scholar]
- Yetisen A. K.; Martinez-Hurtado J. L.; Garcia-Melendrez A.; da Cruz Vasconcellos F.; Lowe C. R. A smartphone algorithm with inter-phone repeatability for the analysis of colorimetric tests. Sens. Actuators, B 2014, 196, 156–160. 10.1016/j.snb.2014.01.077. [DOI] [Google Scholar]
- Oncescu V.; O’Dell D.; Erickson D. Smartphone based health accessory for colorimetric detection of biomarkers in sweat and saliva. Lab Chip 2013, 13, 3232–3238. 10.1039/c3lc50431j. [DOI] [PubMed] [Google Scholar]
- Li M.; Tian J.; Al-Tamimi M.; Shen W. Paper-based blood typing device that reports patient’s blood type “in writing. Angew. Chem., Int. Ed. 2012, 51, 5497–5501. 10.1002/anie.201201822. [DOI] [PubMed] [Google Scholar]
- Yang X.; Piety N. Z.; Vignes S. M.; Benton M. S.; Kanter J.; Shevkoplyas S. S. Simple paper-based test for measuring blood hemoglobin concentration in resource-limited settings. Clin. Chem. 2013, 59, 1506–1513. 10.1373/clinchem.2013.204701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone P. J.; Hammel J.; Dweik R.; Na J.; Czich C.; Laskowski D.; Mekhail T. Diagnosis of lung cancer by the analysis of exhaled breath with a colorimetric sensor array. Thorax 2007, 62, 565–568. 10.1136/thx.2006.072892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z.; Li H.; LaGasse M. K.; Suslick K. S. Rapid quantification of trimethylamine. Anal. Chem. 2016, 88, 5615–5620. 10.1021/acs.analchem.6b01170. [DOI] [PubMed] [Google Scholar]
- Mazzone P. J.; Wang X.-F.; Xu Y.; Mekhail T.; Beukemann M. C.; Na J.; Kemling J. W.; Suslick K. S.; Sasidhar M. Exhaled breath analysis with a colorimetric sensor array for the identification and characterization of lung cancer. J. Thorac. Oncol. 2012, 7, 137–142. 10.1097/JTO.0b013e318233d80f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan L.; Tian J.; Cao R.; Li M.; Cai Z.; Shen W. Barcode-like paper sensor for smartphone diagnostics: An application of blood typing. Anal. Chem. 2014, 86, 11362–11367. 10.1021/ac503300y. [DOI] [PubMed] [Google Scholar]
- Berry S. B.; Fernandes S. C.; Rajaratnam A.; DeChiara N. S.; Mace C. R. Measurement of the hematocrit using paper-based microfluidic devices. Lab Chip 2016, 16, 3689–3694. 10.1039/C6LC00895J. [DOI] [PubMed] [Google Scholar]
- Gerold C. T.; Bakker E.; Henry C. S. Selective distance-based K(+) quantification on paper-based microfluidics. Anal. Chem. 2018, 90, 4894–4900. 10.1021/acs.analchem.8b00559. [DOI] [PubMed] [Google Scholar]
- Cho S.; Park T. S.; Nahapetian T. G.; Yoon J. Y. Smartphone-based, sensitive microPAD detection of urinary tract infection and gonorrhea. Biosens. Bioelectron. 2015, 74, 601–611. 10.1016/j.bios.2015.07.014. [DOI] [PubMed] [Google Scholar]
- Lin S.-C.; Tzeng C.-Y.; Lai P.-L.; Hsu M.-Y.; Chu H.-Y.; Tseng F.-G.; Cheng C.-M. Paper-based CRP monitoring devices. Sci. Rep. 2016, 6, 38171. 10.1038/srep38171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappi F. A.; Tsogas G. Z.; Routsi A.-M.; Christodouleas D. C.; Giokas D. L. Paper-based devices for biothiols sensing using the photochemical reduction of silver halides. Anal. Chim. Acta 2018, 1036, 89–96. 10.1016/j.aca.2018.05.062. [DOI] [PubMed] [Google Scholar]
- Balsam J.; Bruck H. A.; Rasooly A.. Smartphone-based fluorescence detector for mHealth. In Mobile health technologies: Methods and protocols; Rasooly A., Herold K. E., Eds.; Springer: New York, 2015; pp 231–245. [DOI] [PubMed] [Google Scholar]
- Roda A.; Michelini E.; Cevenini L.; Calabria D.; Calabretta M. M.; Simoni P. Integrating biochemiluminescence detection on smartphones: Mobile chemistry platform for point-of-need analysis. Anal. Chem. 2014, 86, 7299–7304. 10.1021/ac502137s. [DOI] [PubMed] [Google Scholar]
- De Puig H.; Bosch I.; Gehrke L.; Hamad-Schifferli K. Challenges of the nano-bio interface in lateral flow and dipstick immunoassays. Trends Biotechnol. 2017, 35, 1169–1180. 10.1016/j.tibtech.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S.; Zapatero-Rodriguez J.; Estrela P.; O’Kennedy R. Point-of-care diagnostics in low resource settings: Present status and future role of microfluidics. Biosensors 2015, 5, 577–601. 10.3390/bios5030577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudanyali O.; Dimitrov S.; Sikora U.; Padmanabhan S.; Navruz I.; Ozcan A. Integrated rapid-diagnostic-test reader platform on a cellphone. Lab Chip 2012, 12, 2678–2686. 10.1039/c2lc40235a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You D. J.; Park T. S.; Yoon J. Y. Cell-phone-based measurement of TSH using Mie scatter optimized lateral flow assays. Biosens. Bioelectron. 2013, 40, 180–185. 10.1016/j.bios.2012.07.014. [DOI] [PubMed] [Google Scholar]
- Lee S.; Kim G.; Moon J. Performance improvement of the one-dot lateral flow immunoassay for aflatoxin B1 by using a smartphone-based reading system. Sensors 2013, 13, 5109–5116. 10.3390/s130405109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson A. S.; Raja B.; Mandadi V.; Townsend B.; Lee M.; Buell A.; Vu B.; Brgoch J.; Willson R. C. A low-cost smartphone-based platform for highly sensitive point-of-care testing with persistent luminescent phosphors. Lab Chip 2017, 17, 1051–1059. 10.1039/C6LC01167E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilavaki E.; Demosthenous A. Optimized lateral flow immunoassay reader for the detection of infectious diseases in developing countries. Sensors 2017, 17, 2673. 10.3390/s17112673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan B.; O’Dell D.; Finkelstein J. L.; Lee S.; Erickson D.; Mehta S. ironPhone: Mobile device-coupled point-of-care diagnostics for assessment of iron status by quantification of serum ferritin. Biosens. Bioelectron. 2018, 99, 115–121. 10.1016/j.bios.2017.07.038. [DOI] [PubMed] [Google Scholar]
- Lu Z.; O’Dell D.; Srinivasan B.; Rey E.; Wang R.; Vemulapati S.; Mehta S.; Erickson D. Rapid diagnostic testing platform for iron and vitamin A deficiency. Proc. Natl. Acad. Sci. U. S. A. 2017, 114, 13513–13518. 10.1073/pnas.1711464114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.; O’Dell D.; Hohenstein J.; Colt S.; Mehta S.; Erickson D. NutriPhone: a mobile platform for low-cost point-of-care quantification of vitamin B12 concentrations. Sci. Rep. 2016, 6, 28237. 10.1038/srep28237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koczula K. M.; Gallotta A. Lateral flow assays. Essays Biochem. 2016, 60, 111–120. 10.1042/EBC20150012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying N.; Ju C.; Sun X.; Li L.; Chang H.; Song G.; Li Z.; Wan J.; Dai E. Lateral flow nucleic acid biosensor for sensitive detection of microRNAs based on the dual amplification strategy of duplex-specific nuclease and hybridization chain reaction. PLoS One 2017, 12, e0185091 10.1371/journal.pone.0185091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen C.-W.; de Puig H.; Tam J. O.; Gómez-Márquez J.; Bosch I.; Hamad-Schifferli K.; Gehrke L. Multicolored silver nanoparticles for multiplexed disease diagnostics: distinguishing dengue, yellow fever, and Ebola viruses. Lab Chip 2015, 15, 1638–1641. 10.1039/C5LC00055F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch I.; de Puig H.; Hiley M.; Carré-Camps M.; Perdomo-Celis F.; Narváez C. F.; Salgado D. M.; Senthoor D.; O’Grady M.; Phillips E.; Durbin A.; Fandos D.; Miyazaki H.; Yen C.-W.; Gélvez-Ramírez M.; Warke R. V.; Ribeiro L. S.; Teixeira M. M.; Almeida R. P.; Muñóz-Medina J. E.; Ludert J. E.; Nogueira M. L.; Colombo T. E.; Terzian A. C. B.; Bozza P. T.; Calheiros A. S.; Vieira Y. R.; Barbosa-Lima G.; Vizzoni A.; Cerbino-Neto J.; Bozza F. A.; Souza T. M. L.; Trugilho M. R. O.; de Filippis A. M. B.; de Sequeira P. C.; Marques E. T. A.; Magalhaes T.; Díaz F. J.; Restrepo B. N.; Marín K.; Mattar S.; Olson D.; Asturias E. J.; Lucera M.; Singla M.; Medigeshi G. R.; de Bosch N.; Tam J.; Gómez-Márquez J.; Clavet C.; Villar L.; Hamad-Schifferli K.; Gehrke L. Rapid antigen tests for dengue virus serotypes and Zika virus in patient serum. Sci. Transl. Med. 2017, 9, eaan1589 10.1126/scitranslmed.aan1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X.; Song L.; Yang S.; Guo M.; Yuan Q.; Ge S.; Min X.; Xia N. A fast and low-cost genotyping method for hepatitis B virus based on pattern recognition in point-of-care settings. Sci. Rep. 2016, 6, 28274. 10.1038/srep28274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.; Mehta S.; Erickson D. Two-color lateral flow assay for multiplex detection of causative agents behind acute febrile illnesses. Anal. Chem. 2016, 88, 8359–8363. 10.1021/acs.analchem.6b01828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posthuma-Trumpie G. A.; Wichers J. H.; Koets M.; Berendsen L. B. J. M.; van Amerongen A. Amorphous carbon nanoparticles: a versatile label for rapid diagnostic (immuno)assays. Anal. Bioanal. Chem. 2012, 402, 593–600. 10.1007/s00216-011-5340-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z.; Wang Y.; Wang J.; Tang Z.; Pounds J. G.; Lin Y. Rapid and sensitive detection of protein biomarker using a portable fluorescence biosensor based on quantum dots and a lateral flow test strip. Anal. Chem. 2010, 82, 7008–7014. 10.1021/ac101405a. [DOI] [PubMed] [Google Scholar]
- Zhu J.; Zou N.; Zhu D.; Wang J.; Jin Q.; Zhao J.; Mao H. Simultaneous detection of high-sensitivity cardiac troponin I and myoglobin by modified sandwich lateral flow immunoassay: proof of principle. Clin. Chem. 2011, 57, 1732–8. 10.1373/clinchem.2011.171694. [DOI] [PubMed] [Google Scholar]
- Hu J.; Jiang Y.-Z.; Wu L.-L.; Wu Z.; Bi Y.; Wong G.; Qiu X.; Chen J.; Pang D.-W.; Zhang Z.-L. Dual-Signal Readout Nanospheres for Rapid Point-of-Care Detection of Ebola Virus Glycoprotein. Anal. Chem. 2017, 89, 13105–13111. 10.1021/acs.analchem.7b02222. [DOI] [PubMed] [Google Scholar]
- Jin B.; Yang Y.; He R.; Park Y. I.; Lee A.; Bai D.; Li F.; Lu T. J.; Xu F.; Lin M. Lateral flow aptamer assay integrated smartphone-based portable device for simultaneous detection of multiple targets using upconversion nanoparticles. Sens. Actuators, B 2018, 276, 48–56. 10.1016/j.snb.2018.08.074. [DOI] [Google Scholar]
- Edwards K. A.; Korff R.; Baeumner A. J.. Liposome-enhanced lateral-flow assays for clinical analyses. In Biosensors and biodetection: Methods and protocols volume 1: Optical-based detectors; Rasooly A., Prickril B., Eds.; Springer: New York, 2017; pp 407–434. [DOI] [PubMed] [Google Scholar]
- Khreich N.; Lamourette P.; Boutal H.; Devilliers K.; Créminon C.; Volland H. Detection of Staphylococcus enterotoxin B using fluorescent immunoliposomes as label for immunochromatographic testing. Anal. Biochem. 2008, 377, 182–188. 10.1016/j.ab.2008.02.032. [DOI] [PubMed] [Google Scholar]
- Morales-Narváez E.; Naghdi T.; Zor E.; Merkoçi A. Photoluminescent lateral-flow immunoassay revealed by graphene oxide: Highly sensitive paper-based pathogen detection. Anal. Chem. 2015, 87, 8573–8577. 10.1021/acs.analchem.5b02383. [DOI] [PubMed] [Google Scholar]
- Parolo C.; de la Escosura-Muñiz A.; Merkoçi A. Enhanced lateral flow immunoassay using gold nanoparticles loaded with enzymes. Biosens. Bioelectron. 2013, 40, 412–416. 10.1016/j.bios.2012.06.049. [DOI] [PubMed] [Google Scholar]
- Rodríguez M. O.; Covián L. B.; García A. C.; Blanco-López M. C. Silver and gold enhancement methods for lateral flow immunoassays. Talanta 2016, 148, 272–278. 10.1016/j.talanta.2015.10.068. [DOI] [PubMed] [Google Scholar]
- Fu E.; Liang T.; Spicar-Mihalic P.; Houghtaling J.; Ramachandran S.; Yager P. Two-dimensional paper network format that enables simple multistep assays for use in low-resource settings in the context of malaria antigen detection. Anal. Chem. 2012, 84, 4574–4579. 10.1021/ac300689s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridley G. E.; Le H.; Yager P. Highly Sensitive Immunoassay Based on Controlled Rehydration of Patterned Reagents in a 2-Dimensional Paper Network. Anal. Chem. 2014, 86, 6447–6453. 10.1021/ac500872j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafleur L. K.; Bishop J. D.; Heiniger E. K.; Gallagher R. P.; Wheeler M. D.; Kauffman P.; Zhang X.; Kline E. C.; Buser J. R.; Kumar S.; Byrnes S. A.; Vermeulen N. M. J.; Scarr N. K.; Belousov Y.; Mahoney W.; Toley B. J.; Ladd P. D.; Lutz B. R.; Yager P. A rapid, instrument-free, sample-to-result nucleic acid amplification test. Lab Chip 2016, 16, 3777–3787. 10.1039/C6LC00677A. [DOI] [PubMed] [Google Scholar]
- Huang S.; Abe K.; Bennett S.; Liang T.; Ladd P. D.; Yokobe L.; Anderson C. E.; Shah K.; Bishop J.; Purfield M.; Kauffman P. C.; Paul S.; Welch A. E.; Strelitz B.; Follmer K.; Pullar K.; Sanchez-Erebia L.; Gerth-Guyette E.; Domingo G.; Klein E.; Englund J. A.; Fu E.; Yager P. Disposable Autonomous Device for Swab-to-Result Diagnosis of Influenza. Anal. Chem. 2017, 89, 5776–5783. 10.1021/acs.analchem.6b04801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y. S.; Landry J. P.; Fei Y. Y.; Zhu X. D.; Luo J. T.; Wang X. B.; Lam K. S. Effect of fluorescently labeling protein probes on kinetics of protein–ligand reactions. Langmuir 2008, 24, 13399–13405. 10.1021/la802097z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jason-Moller L.; Murphy M.; Bruno J. Overview of biacore systems and their applications. Curr. Protoc. Protein Sci. 2006, 45, 19-13-1. 10.1002/0471140864.ps1913s45. [DOI] [PubMed] [Google Scholar]
- Martinez A. W.; Phillips S. T.; Butte M. J.; Whitesides G. M. Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew. Chem., Int. Ed. 2007, 46, 1318–1320. 10.1002/anie.200603817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A. W.; Phillips S. T.; Whitesides G. M.; Carrilho E. Diagnostics for the developing world: microfluidic paper-based analytical devices. Anal. Chem. 2010, 82, 3–10. 10.1021/ac9013989. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Noviana E.; Nguyen M. P.; Geiss B. J.; Dandy D. S.; Henry C. S. Paper-based microfluidic devices: Emerging themes and applications. Anal. Chem. 2017, 89, 71–91. 10.1021/acs.analchem.6b04581. [DOI] [PubMed] [Google Scholar]
- Polymer technology systems, Inc. CardioChek home use analyzer. http://www.ptsdiagnostics.com/cardiochek-home-use.html (accessed Aug 29, 2018).
- Oncescu V.; Mancuso M.; Erickson D. Cholesterol testing on a smartphone. Lab Chip 2014, 14, 759–763. 10.1039/C3LC51194D. [DOI] [PubMed] [Google Scholar]
- Vella S. J.; Beattie P.; Cademartiri R.; Laromaine A.; Martinez A. W.; Phillips S. T.; Mirica K. A.; Whitesides G. M. Measuring markers of liver function using a micropatterned paper device designed for blood from a fingerstick. Anal. Chem. 2012, 84, 2883–2891. 10.1021/ac203434x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock N. R.; Rolland J. P.; Kumar S.; Beattie P. D.; Jain S.; Noubary F.; Wong V. L.; Pohlmann R. A.; Ryan U. S.; Whitesides G. M. A paper-based multiplexed transaminase test for low-cost, point-of-care liver function testing. Sci. Transl. Med. 2012, 4, 152ra129. 10.1126/scitranslmed.3003981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deraney R. N.; Mace C. R.; Rolland J. P.; Schonhorn J. E. Multiplexed, patterned-paper immunoassay for detection of malaria and dengue fever. Anal. Chem. 2016, 88, 6161–6165. 10.1021/acs.analchem.6b00854. [DOI] [PubMed] [Google Scholar]
- Schonhorn J. E.; Fernandes S. C.; Rajaratnam A.; Deraney R. N.; Rolland J. P.; Mace C. R. A device architecture for three-dimensional, patterned paper immunoassays. Lab Chip 2014, 14, 4653–4658. 10.1039/C4LC00876F. [DOI] [PubMed] [Google Scholar]
- Nosrati R.; Gong M. M.; San Gabriel M. C.; Pedraza C. E.; Zini A.; Sinton D. Paper-based quantification of male fertility potential. Clin. Chem. 2016, 62, 458–465. 10.1373/clinchem.2015.250282. [DOI] [PubMed] [Google Scholar]
- Verma M. S.; Tsaloglou M.-N.; Sisley T.; Christodouleas D.; Chen A.; Milette J.; Whitesides G. M. Sliding-strip microfluidic device enables ELISA on paper. Biosens. Bioelectron. 2018, 99, 77–84. 10.1016/j.bios.2017.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. C.; Hennek J. W.; Ainla A.; Kumar A. A.; Lan W. J.; Im J.; Smith B. S.; Zhao M.; Whitesides G. M. A paper-based ″pop-up″ electrochemical device for analysis of beta-hydroxybutyrate. Anal. Chem. 2016, 88, 6326–6333. 10.1021/acs.analchem.6b00568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson R.; Wong L.; Monnat R.; Fu E. Development of a whole blood paper-based device for phenylalanine detection in the context of PKU therapy monitoring. Micromachines 2016, 7, 28. 10.3390/mi7020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong S.-G.; Lee S.-H.; Choi C.-H.; Kim J.; Lee C.-S. Toward instrument-free digital measurements: a three-dimensional microfluidic device fabricated in a single sheet of paper by double-sided printing and lamination. Lab Chip 2015, 15, 1188–1194. 10.1039/C4LC01382D. [DOI] [PubMed] [Google Scholar]
- Zhu W.-J.; Feng D.-Q.; Chen M.; Chen Z.-D.; Zhu R.; Fang H.-L.; Wang W. Bienzyme colorimetric detection of glucose with self-calibration based on tree-shaped paper strip. Sens. Actuators, B 2014, 190, 414–418. 10.1016/j.snb.2013.09.007. [DOI] [Google Scholar]
- Wang X.; Li F.; Cai Z.; Liu K.; Li J.; Zhang B.; He J. Sensitive colorimetric assay for uric acid and glucose detection based on multilayer-modified paper with smartphone as signal readout. Anal. Bioanal. Chem. 2018, 410, 2647–2655. 10.1007/s00216-018-0939-4. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Cao X.; Wang L.; Zhao X.; Zhang S.; Wang P. Printed microwells with highly stable thin-film enzyme coatings for point-of-care multiplex bioassay of blood samples. Analyst 2015, 140, 4105–4113. 10.1039/C5AN00054H. [DOI] [PubMed] [Google Scholar]
- Choi S.; Kim S.-K.; Lee G.-J.; Park H.-K. Paper-based 3D microfluidic device for multiple bioassays. Sens. Actuators, B 2015, 219, 245–250. 10.1016/j.snb.2015.05.035. [DOI] [Google Scholar]
- Sechi D.; Greer B.; Johnson J.; Hashemi N. Three-dimensional paper-based microfluidic device for assays of protein and glucose in urine. Anal. Chem. 2013, 85, 10733–10737. 10.1021/ac4014868. [DOI] [PubMed] [Google Scholar]
- Schilling K. M.; Lepore A. L.; Kurian J. A.; Martinez A. W. Fully enclosed microfluidic paper-based analytical devices. Anal. Chem. 2012, 84, 1579–1585. 10.1021/ac202837s. [DOI] [PubMed] [Google Scholar]
- Demirel G.; Babur E. Vapor-phase deposition of polymers as a simple and versatile technique to generate paper-based microfluidic platforms for bioassay applications. Analyst 2014, 139, 2326–2331. 10.1039/C4AN00022F. [DOI] [PubMed] [Google Scholar]
- Martinez A. W.; Phillips S. T.; Nie Z.; Cheng C.-M.; Carrilho E.; Wiley B. J.; Whitesides G. M. Programmable diagnostic devices made from paper and tape. Lab Chip 2010, 10, 2499–2504. 10.1039/c0lc00021c. [DOI] [PubMed] [Google Scholar]
- Talalak K.; Noiphung J.; Songjaroen T.; Chailapakul O.; Laiwattanapaisal W. A facile low-cost enzymatic paper-based assay for the determination of urine creatinine. Talanta 2015, 144, 915–921. 10.1016/j.talanta.2015.07.040. [DOI] [PubMed] [Google Scholar]
- Channon R. B.; Yang Y.; Feibelman K. M.; Geiss B. J.; Dandy D. S.; Henry C. S. Development of an electrochemical paper-based analytical device for trace detection of virus particles. Anal. Chem. 2018, 90, 7777–7783. 10.1021/acs.analchem.8b02042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaastrup K.; Sikes H. D. Using photo-initiated polymerization reactions to detect molecular recognition. Chem. Soc. Rev. 2016, 45, 532–545. 10.1039/C5CS00205B. [DOI] [PubMed] [Google Scholar]
- Badu-Tawiah A. K.; Lathwal S.; Kaastrup K.; Al-Sayah M.; Christodouleas D. C.; Smith B. S.; Whitesides G. M.; Sikes H. D. Polymerization-based signal amplification for paper-based immunoassays. Lab Chip 2015, 15, 655–659. 10.1039/C4LC01239A. [DOI] [PubMed] [Google Scholar]
- Sikes H. D.; Jenison R.; Bowman C. N. Antigen detection using polymerization-based amplification. Lab Chip 2009, 9, 653–656. 10.1039/B816198D. [DOI] [PubMed] [Google Scholar]
- Yee E. H.; Lathwal S.; Shah P. P.; Sikes H. D. Detection of biomarkers of periodontal disease in human saliva using stabilized, vertical flow immunoassays. ACS Sensors 2017, 2, 1589–1593. 10.1021/acssensors.7b00745. [DOI] [PubMed] [Google Scholar]
- Lathwal S.; Sikes H. D. Assessment of colorimetric amplification methods in a paper-based immunoassay for diagnosis of malaria. Lab Chip 2016, 16, 1374–1382. 10.1039/C6LC00058D. [DOI] [PubMed] [Google Scholar]
- Kim S.; Sikes H. D. Phenolphthalein-conjugated hydrogel formation under visible-light irradiation for reducing variability of colorimetric biodetection. ACS Appl. Bio Mater. 2018, 1, 216–220. 10.1021/acsabm.8b00148. [DOI] [PubMed] [Google Scholar]
- Connelly J. T.; Rolland J. P.; Whitesides G. M. Paper machine” for molecular diagnostics. Anal. Chem. 2015, 87, 7595–7601. 10.1021/acs.analchem.5b00411. [DOI] [PubMed] [Google Scholar]
- Bender A. T.; Borysiak M. D.; Levenson A. M.; Lillis L.; Boyle D. S.; Posner J. D. Semiquantitative nucleic acid test with simultaneous isotachophoretic extraction and amplification. Anal. Chem. 2018, 90, 7221–7229. 10.1021/acs.analchem.8b00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.; Scida K.; Crooks R. M. Detection of hepatitis B virus DNA with a paper electrochemical sensor. Anal. Chem. 2015, 87, 9009–9015. 10.1021/acs.analchem.5b02210. [DOI] [PubMed] [Google Scholar]
- Tsaloglou M.-N.; Nemiroski A.; Camci-Unal G.; Christodouleas D. C.; Murray L. P.; Connelly J. T.; Whitesides G. M. Handheld isothermal amplification and electrochemical detection of DNA in resource-limited settings. Anal. Biochem. 2018, 543, 116–121. 10.1016/j.ab.2017.11.025. [DOI] [PubMed] [Google Scholar]
- Coskun A. F.; Nagi R.; Sadeghi K.; Phillips S.; Ozcan A. Albumin testing in urine using a smart-phone. Lab Chip 2013, 13, 4231–4238. 10.1039/c3lc50785h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Q.; Luo W.; Chiang S.; Kappel T.; Mejia C.; Tseng D.; Chan R. Y. L.; Yan E.; Qi H.; Shabbir F.; Ozkan H.; Feng S.; Ozcan A. Imaging and sizing of single DNA molecules on a mobile phone. ACS Nano 2014, 8, 12725–12733. 10.1021/nn505821y. [DOI] [PubMed] [Google Scholar]
- Wei Q.; Nagi R.; Sadeghi K.; Feng S.; Yan E.; Ki S. J.; Caire R.; Tseng D.; Ozcan A. Detection and spatial mapping of mercury contamination in water samples using a smart-phone. ACS Nano 2014, 8, 1121–1129. 10.1021/nn406571t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DropSens. Portable electrochemical reader DROPSTAT. http://www.dropsens.com/en/pdfs_productos/new_brochures/dropstat.pdf (accessed Aug 29, 2018).
- Coskun A. F.; Wong J.; Khodadadi D.; Nagi R.; Tey A.; Ozcan A. A personalized food allergen testing platform on a cellphone. Lab Chip 2013, 13, 636–640. 10.1039/C2LC41152K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemiroski A.; Christodouleas D. C.; Hennek J. W.; Kumar A. A.; Maxwell E. J.; Fernández-Abedul M. T.; Whitesides G. M. Universal mobile electrochemical detector designed for use in resource-limited applications. Proc. Natl. Acad. Sci. U. S. A. 2014, 111, 11984–11989. 10.1073/pnas.1405679111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Gartia M. R.; Jiang J.; Chang T.-W.; Qian J.; Liu Y.; Liu X.; Liu G. L. Audio jack based miniaturized mobile phone electrochemical sensing platform. Sens. Actuators, B 2015, 209, 677–685. 10.1016/j.snb.2014.12.017. [DOI] [Google Scholar]
- Von Schenck H.; Falkensson M.; Lundberg B. Evaluation of ″HemoCue,″ a new device for determining hemoglobin. Clin. Chem. 1986, 32, 526–569. [PubMed] [Google Scholar]
- Ashworth L.; Gibb I.; Alberti K. G. HemoCue: evaluation of a portable photometric system for determining glucose in whole blood. Clin. Chem. 1992, 38, 1479–1482. [PubMed] [Google Scholar]
- Von Schenck H. Validation of albumin determined in urine with the HemoCue point-of-care analyser. Scand. J. Clin. Lab. Invest. 2003, 63, 119–126. 10.1080/00365510310000178. [DOI] [PubMed] [Google Scholar]
- Roche Diagnostics. CoaguChek XS. http://www.coaguchek-usa.com/coaguchek_hcp/en_US/home/products-and-solutions/coaguchek-xs-meter.html (accessed Aug 29, 2018).
- Zhang L.; Yang W.; Yang Y.; Liu H.; Gu Z. Smartphone-based point-of-care testing of salivary alpha-amylase for personal psychological measurement. Analyst 2015, 140, 7399–406. 10.1039/C5AN01664A. [DOI] [PubMed] [Google Scholar]
- Punter-Villagrasa J.; Cid J.; Paez-Aviles C.; Rodriguez-Villarreal I.; Juanola-Feliu E.; Colomer-Farrarons J.; Miribel-Catala P. L. An instantaneous low-cost point-of-care anemia detection device. Sensors 2015, 15, 4564–4577. 10.3390/s150204564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nidzworski D.; Siuzdak K.; Niedzialkowski P.; Bogdanowicz R.; Sobaszek M.; Ryl J.; Weiher P.; Sawczak M.; Wnuk E.; Goddard W. A. 3rd; Jaramillo-Botero A.; Ossowski T. A rapid-response ultrasensitive biosensor for influenza virus detection using antibody modified boron-doped diamond. Sci. Rep. 2017, 7, 15707. 10.1038/s41598-017-15806-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray A. A.; Ali Z.; Kyselovik J.; Mills C. A.; Renault N. J.; Santha H.; Strohhofer C.. A novel point of care diagnostic device: Impedimetric detection of a biomarker in whole blood, 2007 29th Annual International Conf. Proc. IEEE Engin. Med. Bio. Soc.; pp 115–118. [DOI] [PubMed]
- Masson J.-F.; Battaglia T. M.; Khairallah P.; Beaudoin S.; Booksh K. S. Quantitative measurement of cardiac markers in undiluted serum. Anal. Chem. 2007, 79, 612–619. 10.1021/ac061089f. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Liu Q.; Chen S.; Cheng F.; Wang H.; Peng W. Surface plasmon resonance biosensor based on smart phone platforms. Sci. Rep. 2015, 5, 12864. 10.1038/srep12864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turbé V.; Gray E. R.; Lawson V. E.; Nastouli E.; Brookes J. C.; Weiss R. A.; Pillay D.; Emery V. C.; Verrips C. T.; Yatsuda H.; Athey D.; McKendry R. A. Towards an ultra-rapid smartphone- connected test for infectious diseases. Sci. Rep. 2017, 7, 11971. 10.1038/s41598-017-11887-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager P. Cutting Edge: A prefilled, ready-to-use electrophoresis based lab-on-a-chip device for monitoring lithium in blood. Lab Chip 2010, 10, 1757–1757. 10.1039/c005361a. [DOI] [PubMed] [Google Scholar]
- Lafleur L.; Stevens D.; McKenzie K.; Ramachandran S.; Spicar-Mihalic P.; Singhal M.; Arjyal A.; Osborn J.; Kauffman P.; Yager P.; Lutz B. Progress toward multiplexed sample-to-result detection in low resource settings using microfluidic immunoassay cards. Lab Chip 2012, 12, 1119–1127. 10.1039/c2lc20751f. [DOI] [PubMed] [Google Scholar]
- Floris A.; Staal S.; Lenk S.; Staijen E.; Kohlheyer D.; Eijkel J.; van den Berg A. A prefilled, ready-to-use electrophoresis based lab-on-a-chip device for monitoring lithium in blood. Lab Chip 2010, 10, 1799–1806. 10.1039/c003899g. [DOI] [PubMed] [Google Scholar]
- Chin C. D.; Cheung Y. K.; Laksanasopin T.; Modena M. M.; Chin S. Y.; Sridhara A. A.; Steinmiller D.; Linder V.; Mushingantahe J.; Umviligihozo G.; Karita E.; Mwambarangwe L.; Braunstein S. L.; van de Wijgert J.; Sahabo R.; Justman J. E.; El-Sadr W.; Sia S. K. Mobile device for disease diagnosis and data tracking in resource-limited settings. Clin. Chem. 2013, 59, 629–640. 10.1373/clinchem.2012.199596. [DOI] [PubMed] [Google Scholar]
- Nayak S.; Sridhara A.; Melo R.; Richer L.; Chee N. H.; Kim J.; Linder V.; Steinmiller D.; Sia S. K.; Gomes-Solecki M. Microfluidics-based point-of-care test for serodiagnosis of Lyme disease. Sci. Rep. 2016, 6, 35069. 10.1038/srep35069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laksanasopin T.; Guo T. W.; Nayak S.; Sridhara A. A.; Xie S.; Olowookere O. O.; Cadinu P.; Meng F.; Chee N. H.; Kim J.; Chin C. D.; Munyazesa E.; Mugwaneza P.; Rai A. J.; Mugisha V.; Castro A. R.; Steinmiller D.; Linder V.; Justman J. E.; Nsanzimana S.; Sia S. K. A smartphone dongle for diagnosis of infectious diseases at the point of care. Sci. Transl. Med. 2015, 7, 273re1. 10.1126/scitranslmed.aaa0056. [DOI] [PubMed] [Google Scholar]
- Guo T.; Patnaik R.; Kuhlmann K.; Rai A. J.; Sia S. K. Smartphone dongle for simultaneous measurement of hemoglobin concentration and detection of HIV antibodies. Lab Chip 2015, 15, 3514–20. 10.1039/C5LC00609K. [DOI] [PubMed] [Google Scholar]
- Chin C. D.; Laksanasopin T.; Cheung Y. K.; Steinmiller D.; Linder V.; Parsa H.; Wang J.; Moore H.; Rouse R.; Umviligihozo G.; Karita E.; Mwambarangwe L.; Braunstein S. L.; van de Wijgert J.; Sahabo R.; Justman J. E.; El-Sadr W.; Sia S. K. Microfluidics-based diagnostics of infectious diseases in the developing world. Nat. Med. 2011, 17, 1015–1019. 10.1038/nm.2408. [DOI] [PubMed] [Google Scholar]
- Kumar A. A.; Hennek J. W.; Smith B. S.; Kumar S.; Beattie P.; Jain S.; Rolland J. P.; Stossel T. P.; Chunda-Liyoka C.; Whitesides G. M. From the bench to the field in low-cost diagnostics: Two case studies. Angew. Chem., Int. Ed. 2015, 54, 5836–5853. 10.1002/anie.201411741. [DOI] [PubMed] [Google Scholar]
- Makower J.; Meer A.; Denend L.. FDA impact on US medical technology innovation: A survey of over 200 medical technology companies. https://www.advamed.org/sites/default/files/resource/30_10_11_10_2010_Study_CAgenda_makowerreportfinal.pdf (accessed Oct 18, 2018).
- In vitro diagnostics and laboratory technology. Emergency use assessment and listing (EUAL) procedure for IVDs. http://www.who.int/diagnostics_laboratory/eual/emergency/en/ (accessed Oct 19, 2018).
- Chan W. C. W.; Udugama B.; Kadhiresan P.; Kim J.; Mubareka S.; Weiss P. S.; Parak W. J. Patients, Here Comes More Nanotechnology. ACS Nano 2016, 10, 8139–8142. 10.1021/acsnano.6b05610. [DOI] [PubMed] [Google Scholar]
- Chin C. D.; Linder V.; Sia S. K. Commercialization of microfluidic point-of-care diagnostic devices. Lab Chip 2012, 12, 2118–2134. 10.1039/c2lc21204h. [DOI] [PubMed] [Google Scholar]
- Alivecor, Inc. KardiaMobile. https://www.alivecor.com (accessed Aug 27, 2018).
- CardioComm Solutions, Inc. The HeartCheck ECG PEN. http://www.theheartcheck.com/consumer.html (accessed Aug 27, 2018).
- Tyto Care, Inc. TytoHome. https://www.tytocare.com/tytohome/ (accessed Aug 27, 2018).
- Healthy.io Ltd. Clinically approved digital urinalysis. https://healthy.io/ (accessed Aug 29, 2018).