Classical Hodgkin lymphoma (cHL) is characterized by rare Hodgkin and Reed Sternberg cells (HRSC), which are embedded in an anergic inflammatory cellular microenvironment (ME).1 Constitutional and high expression of programmed cell death-protein-1 (PD-1)/PD-ligand (PD-L) pathway by HRSC indicates a role of PD-1/PD-L1 interaction in cHL.2–5
Inhibition of PD-1/PD-L1 interaction by anti-PD-1 blockade has shown impressive response rates in relapsed/refractory (r/r) cHL. Although durable responses to anti-PD-1 treatment have been documented, the majority of patients eventually relapse.4–6
To address the important, but so far unsolved issue of mechanisms of resistance to PD-1 blockade, we retrospectively assessed sequential biopsies of r/r cHL patients developing progressive disease (PD) or relapse on anti-PD-1 treatment with regard to microenvironmental changes and PD-1 as well as PD-L1 expression.
In 9 cHL patients, formalin fixed paraffin embedded biopsy specimens taken prior to anti-PD-1-treatment, and a second biopsy at relapse/progression, were available. These included 3 patients with three available biopsies (2 patients with two biopsies before and one patient with two biopsies under anti-PD-1-treatment). All patients gave their informed consent for this study which had been approved by our local ethics committee.
Biopsy specimens were stained for CD30, CD15, EBER or LMP1, CD20, and CD3 to characterize HRSC and to confirm the diagnosis using established protocols (Online Supplementary Table S1). We determined HRSC phenotypes by conventional single stainings assessed by experienced hematopathologists. Stainings for CD4 (clone 4B12, Leica), CD8 (clone C8/144B), DAKO PD-1 (clone MRQ22 Cell Marque), and PD-L1 (clone E1L3N Cell Signaling) were performed on a Leica-Bond automated stainer to characterize the ME and to quantify PD-1- and PD-L1-expression. The total number of PD-1, PD-L1, CD8 and CD4 positive microenvironmental cells was counted by 2 expert pathologists in three high-power fields (HPF, 1000-fold magnification using oil immersion) and the average value was used for further analysis (Online Supplementary Table S1). HPF were chosen with at least one HRSC in the center in order to analyze cell counts in the immediate vicinity of the neoplastic cells. RS cells and macrophages were differentiated by means of CD30- and PAX5-staining and by standardized characterization of the distinct morphology of the RS-cell and macrophage nuclei. t-tests were applied for comparison of cell counts in the HRSC vicinity in the most current biopsy before and the biopsy at relapse under anti-PD-1 treatment. P≤0.05 was considered significant.
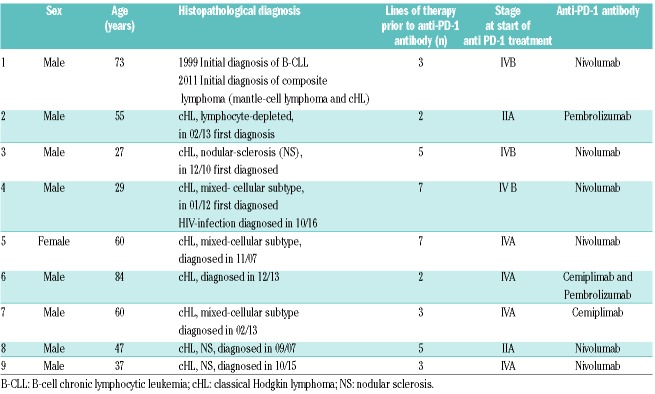
Histopathological diagnosis of cHL was confirmed in all biopsies (Table 1A). Prior to immunmodulatory checkpoint treatment (ICT), cHL subtypes were nodular sclerosis in 2, mixed cellularity in 4, lymphocyte depleted in 1, and not classifiable in 2 patients. Patients’ characteristics, including prior lines of treatment, stage at start and duration of anti-PD-1-treatment until documentation of progressive disease (PD) are summarized in detail in Tables 1A and B.
Table 1A.
Patients’ characteristics.
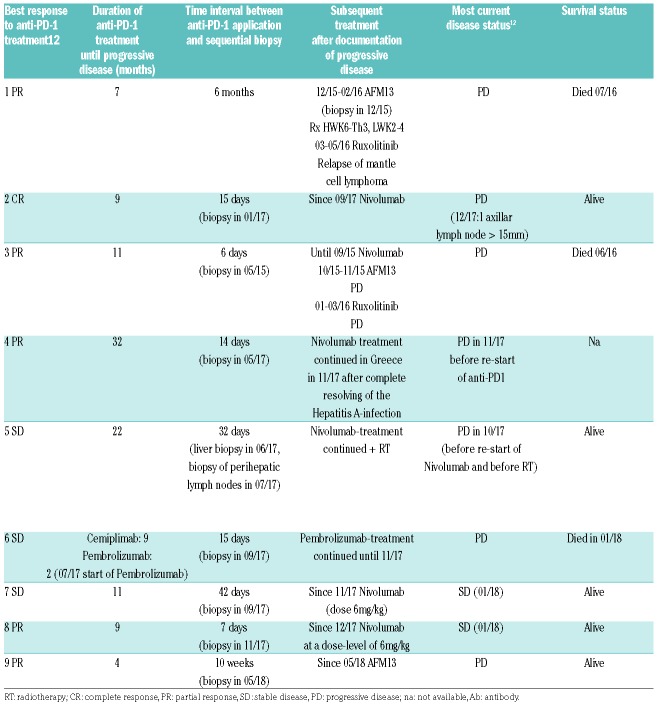
Table 1B.
Patients’ characteristics.
The time interval between the most recent application of the anti-PD-1-antibody and the biopsy at progressive disease or relapse ranged from six days to six months, with seven of eight sequential biopsies being taken within six weeks after documentation of PD.
Because of mild disease activity, anti-PD-1-treatment was continued beyond progression in 6 patients; in one patient this was combined with radiotherapy. In Patient 6, further disease progression was documented and the patient died of progressive disease. Patients 1 and 3 received subsequent treatment with AFM13 and with ruxolitinib; both patients died of progressive disease (Table 1B).7
Due to the retrospective nature of the study, available tissue (mostly core needle biopsies) was very limited and immunohistochemical analyses were incomplete in a subset of patients.
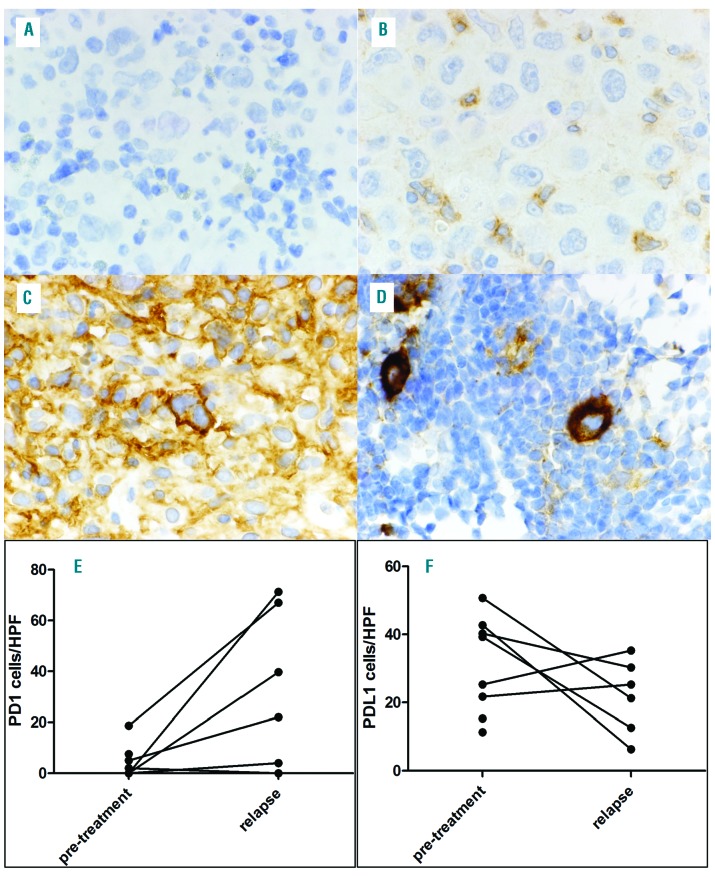
Hodgkin and Reed Sternberg cells were negative for PD-1 in all cases before or after ICT (Figure 1A). Strong expression of PD-L1 on HRSC was at least constant after anti-PD-1 treatment in 7 of 8 patients with available data (Figure 1A-D and Online Supplementary Table S1). In one patient, the expression level of PD-L1 appeared slightly reduced after anti-PD-1 treatment (Patient 4, Online Supplementary Figure S1). Thus, PD-L1 expression in HRSC seems unaffected by anti-PD-1 therapy. It is worthy of note that expression of CD30 was also strongly and abundantly positive on all HRSC at relapse, indicating that anti-PD-1 treatment does not interfere with CD30 expression (data not shown).
Figure 1.
Staining of PD-1 positive T cells and PD-L1 positive macrophages. Staining for PD-1 in Patient 2 showing an increase in PD-1-positive T cells between pre-treatment (A) and relapse (B) biopsy in close proximity to Hodgkin and Reed Sternberg cells (HRSC). Patient 2 with a marked decline in PD-L1 positive macrophages between pre-treatment (C) and relapse (D) biopsy. HRSC are strongly positive for PD-L1 at both time points. (A-D, original magnification 1000×). Comparison of PD-1-positive T-cell (E) and PD-L1-positive macrophage (F) content as cells per high-power field (HPF). Biopsies of the same patient are connected with a line.
Pairwise comparison of cell counts in the vicinity of HRSC within each individual patient revealed that the number of PD-1-positive (PD-1+) T cells trended to be higher at relapse compared to the biopsy prior to ICT [at relapse mean 25.5, standard deviation (SD) 30.3; prior to ICT mean 3.2, SD 6.5 cells per HPF; P=0.0562] (Figure 1E). In contrast, in the majority of patients, the number of PD-L1+ macrophages tended to be lower at relapse compared to the biopsy prior to ICT (at relapse: mean 21.9, SD 10.9; prior to ICT: mean 36.7, SD 11.0 cells per HPF, respectively; P=0.1138) (Figure 1F). Despite noticeable differences, statistical significance was not reached because of low case numbers. Whether the PD-1+ T cells detected at relapse represent locally residing cells that up-regulated PD-1 expression [and thus become positive by our immunohistochemistry (IHC)] or whether these cells have newly invaded the lymphoma tissue from outside remains uncertain. Interestingly, the number of CD8+ cells was low and constant between primary biopsy and relapse (data not shown), supporting the hypothesis that presentation of HLA class I neoantigens to cytotoxic T cells might not be the major axis of action of anti-PD1 blockade in cHL. Few cases were evaluable for CD4 and HLA-class staining and, therefore, no definite conclusion could be drawn (data not shown);8 however, our observation is in line with the recently published data on PD-1 expression on T-cell subsets in the ME of cHL.9 Furthermore, a recently performed analysis in paired biopsies (n=16 for PD-1 staining, n=13 for PD-L1 staining) taken at initial diagnosis and at relapse after initial polychemotherapy did not detect any significant difference in PD1+ T cells and PD-L1 expression on HRSC between primary diagnosis and relapse, and thus supports our hypothesis that the increase in PD-1+ T cells in the vicinity of HRSCs might be a mechanism of resistance specific for anti-PD-1-treatment (A. Schnitter et al., 2018, manuscript in preparation).
There are several limitations to our study, such as a small patient cohort, and a limited analysis of markers by IHC due to the scarcity of the tumor tissue. Unfortunately, the limited amount of tumor tissue did not allow assessment of 9p24.1 alterations, and immuno-histochemical staining of HLA DP/DQ/DR, HLA DM, beta-2-microglobulin (b2M) and HC10 could only be performed in 3 patients. Due to the small patient cohort and the unavailability of biopsies of a “control cohort” responding to anti-PD-1 treatment, our results are predominantly descriptive.
Nevertheless, our study reveals a first insight into the microenvironment of cHL in patients developing resistance to PD1-blockade on the tissue level. Our case series illustrates that, at relapse, the target PD-1 is present on T cells in the vicinity of HRSCs at a level easily detectable by IHC, which is not the case before treatment. We cannot conclude that more T cells would express the PD-1 receptor, as IHC is obviously not sensitive enough to detect low-level expression.9
The reason for treatment failure in cHL remains uncertain. It has been shown for malignant melanoma that loss of β2M and HLA-class I expression contributes to the development of refractoriness to PD-1 blockade.10 This mechanism is extremely unlikely in cHL, as most patients do not express HLA class I even before anti-PD-1 treatment is started due to constitutional loss of β2M in cHL.3,11 Based on our observation, we hypothesize that overexpression of the PD-1 receptor might restore the PD-L1-PD-1 axis and crosstalk between HRSC and infiltrating T cells, which is obviously needed for HRSC growth.9 This hypothesis needs to be further explored by analysis of larger patient cohorts.
Supplementary Material
Acknowledgments
The authors thank all clinicians and pathologists supporting the clinical trials and translational research program of the GHSG.
Footnotes
Funding: this project was supported by a grant of the German Cancer Aid (Deutsche Krebshilfe n. 70112502).
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Kuppers R. The biology of Hodgkin’s lymphoma. Nat Rev Cancer. 2009; 9(1):15–27. [DOI] [PubMed] [Google Scholar]
- 2.Green MR, Rodig S, Juszczynski P, et al. Constitutive AP-1 activity and EBV infection induce PD-L1 in Hodgkin lymphomas and post-transplant lymphoproliferative disorders: implications for targeted therapy. Clin Cancer Res. 2012; 18(6):1611–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roemer MG, Advani RH, Ligon AH, et al. PD-L1 and PD-L2 genetic alterations define classical Hodgkin lymphoma and predict outcome. J Clin Oncol. 2016; 34(23):2690–2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen R, Zinzani PL, Fanale MA, et al. Phase II study of the efficacy and safety of Pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. J Clin Oncol. 2017; 35(19):2125–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Younes A, Santoro A, Shipp M, et al. Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016; 17(9):1283–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armand P, Engert A, Younes A, et al. Nivolumab for relapsed/refractory classic Hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: extended follow-up of the multi-cohort single-arm phase II CheckMate 205 trial. J Clin Oncol. 2018; 36(14):1428–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rothe A, Sasse S, Topp MS, et al. A phase 1 study of the bispecific anti-CD30/CD16A antibody construct AFM13 in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2015; 125(26):4024–4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nijland M, Veenstra RN, Visser L, et al. HLA dependent immune escape mechanisms in B-cell lymphomas: Implications for immune checkpoint inhibitor therapy? Oncoimmunology. 2017; 6(4):e1295202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carey CD, Gusenleitner D, Lipschitz M, et al. Topological analysis reveals a PD-L1 associated microenvironmental niche for Reed-Sternberg cells in Hodgkin lymphoma. Blood. 2017;130(22):2420–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaretsky JM, Garcia-Diaz A, Shin DS, et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med. 2016;375(9):819–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roemer MGM, Redd RA, Cader FZ, et al. Major histocompatibility complex class II and programmed death ligand 1 expression predict outcome after programmed death 1 blockade in classic Hodgkin lymphoma. J Clin Oncol. 2018; 36(10):942–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007; 25(5):579–586. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.