Approximately 3-5% of children with acute lymphoblastic leukemia (ALL) present with Philadelphia chromosome (Ph+). In the pre-tyrosine kinase inhibitors (TKIs) era, Ph+ ALL was associated with a poor prognosis.1–10 The outcome improved with the use of TKIs.11–14 Results of the EsPhALL2004 study (EudraCT 2004-001647-30 and clinicaltrials.gov identifier 00287105) with the use of imatinib mesylate were published with a median follow-up time of 3.1 years;14,15 we now report on long-term outcomes and the impact of prognostic factors in this study. This update shows that event-free survival (EFS) is stable after about 4 years, and that white blood cell (WBC) count at diagnosis is the most relevant prognostic factor, in a setting where the large majority of patients receives imatinib and hematopoietic stem cell transplantation (HSCT).
Between January 2004 and December 2009, 160 Ph+ ALL patients were enrolled in the EsPhALL2004 study in the centers of 10 national study groups. Any subject aged 1-17 years, recruited into national frontline trials for ALL, who showed evidence of t(9;22)(q34;q11) translocation was eligible for the study. Ninety patients were defined as good risk (GR; prednisone good response at day 8, or ≤25% bone marrow [BM] blast cells at day 15, or <5% BM blast cells at day 21, and in complete remission (CR) at the end of induction and were randomized to receive (N=46) or not (N=44) imatinib. The remaining 70 poor risk (PR) patients were all given imatinib. Thus, 128 patients overall were given intermittent imatinib, for a total of 10 weeks if transplanted or 18 weeks if treated with chemotherapy only. In total, 130/160 patients underwent HSCT, and some received imatinib post-HSCT according to local guidelines.
EFS was calculated as the time from diagnosis to first failure, including resistance, relapse, death or second malignant neoplasm (SMN). Disease-free survival (DFS) in GR patients was defined as the time from randomization until relapse, death or SMN, whichever occurred first. Overall survival (OS) considered death as event. Observation periods were censored at the date of last contact when no event was observed. The final follow up was on June 30, 2014. DFS analysis in GR patients was done by intention to treat; a secondary analysis was done of patients as treated. EFS, DFS and OS curves were estimated according to Kaplan-Meier (with Greenwood standard error) and compared with the log-rank test. Kaplan-Meier plots for comparison of HSCT with chemotherapy alone were adjusted to account for the waiting time to transplantation. To deal with lack of proportional hazards, univariate comparison was performed at predefined time points based on log-log transformation. The impact of age and WBC count on EFS was analyzed with a Cox model, stratified by risk group (GR and PR). The cumulative incidence of relapse (CIR) and death (CID) were estimated adjusting for competing risks of other events and compared with the Gray test. All tests were two sided. All analyses were performed with SAS software (version 9.2).
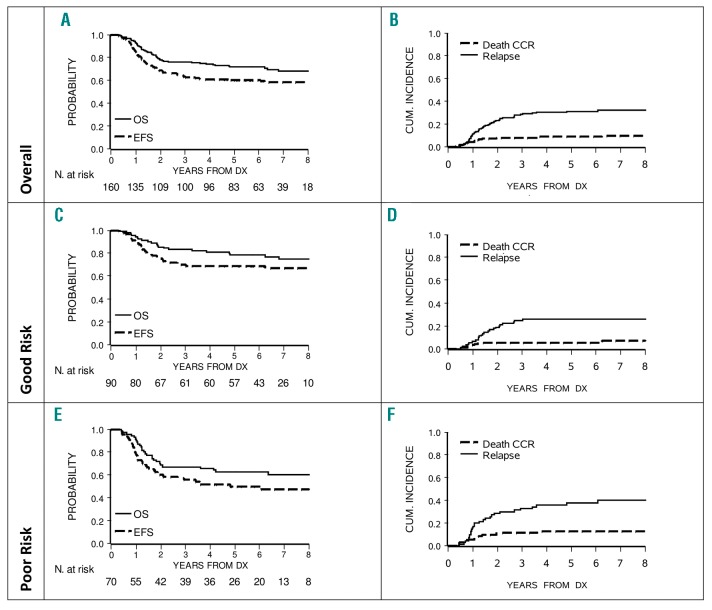
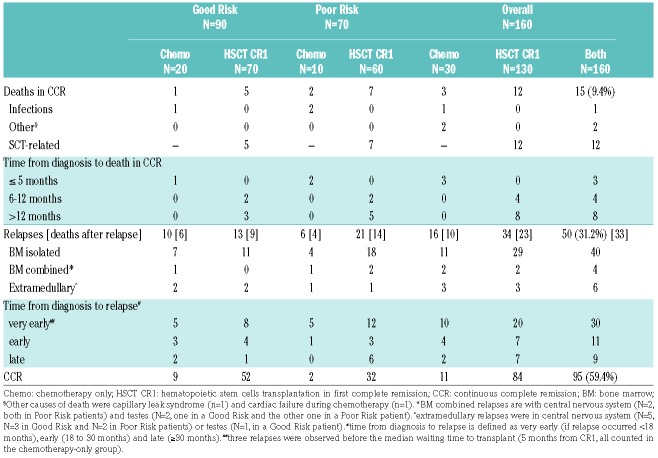
With a median follow-up time of 6.6 years (range, 0.3 – 10.5 years), the 5-year and 7-year EFS were 60.3% (SE 3.9) and 58.2% (SE 4.0). Corresponding OS figures were 71.5% (SE 3.6) and 68.2% (SE 3.9). The CIR at 5 and 7 years was 30.9% (SE 3.7) and 31.9% (SE 3.8, Figure 1, Panels A and B). Curves by risk group (Figure 1, Panels C F) showed 5-year EFS and OS of 68.5% (SE 4.9) and 78.5% (SE 4.4) in GR patients, and of 49.7% (SE 6.0) and 62.9% (SE 5.8) in PR patients. The outcome in GR patients by ITT showed a 5-year DFS in the imatinib arm of 75.5% (SE 6.4) vs. 61.4% (SE 7.3) in the no imatinib arm (P-value 0.20); the difference was higher in the analysis “as treated”, corresponding figures being 77.2% (SE 5.6) vs. 54.8% (SE 8.9, P-value=0.04). As shown in Table 1, of the 130 patients transplanted in CR1, there were 12 deaths in CCR (9.2%) and 34 relapses (26.2%), including 31 medullary and 20 very early. In the small subgroup of 30 patients treated with chemotherapy only, 6 failed within the median waiting time to transplant (5.1 months; 3 died and 3 relapsed) and 13 of the remaining 24 patients relapsed later on. Patients treated with chemotherapy had a 5-year DFS of 46.8% (SE 10.6) and a 5-year OS of 68.8% (SE 8.9). All events occurred within 3 years of diagnosis. Transplanted patients had a 5-year DFS of 66.1% (SE 4.2) and a 5-year OS of 74.2% (SE 3.9). DFS was 20% higher in HSCT (P-value at 5 years=0.07) compared to chemotherapy, while survival curves were superimposable (P-value at 5 years=0.56).
Figure 1.
Event-free survival (EFS), overall survival (OS), cumulative incidence of relapse and death of 160 Ph+ALL patients, overall (Panels A and B) and by risk group (Good Risk in Panels C and D, Poor Risk in Panels E and F). CCR: continuous complete remission.
Table 1.
Outcome by risk group and treatment performed in EsPhALL2004.
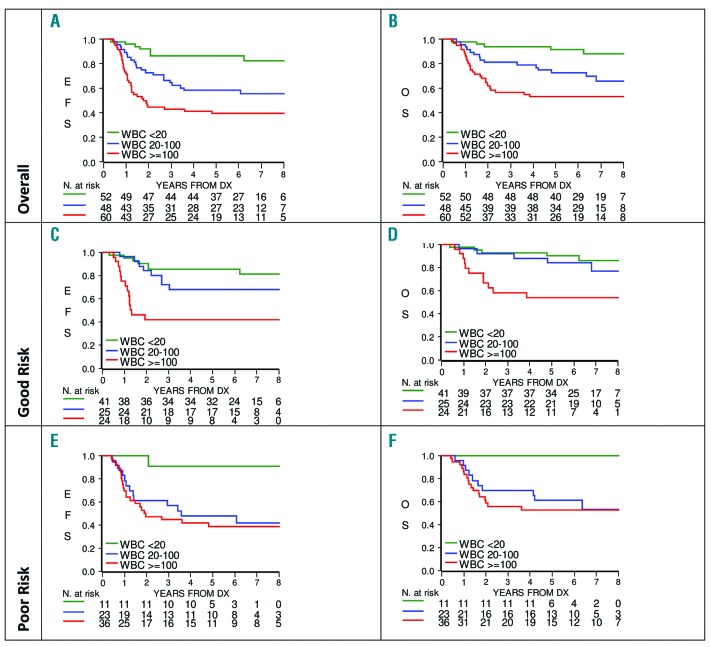
In total, 101 patients were male and 59 female; their 5-year EFS was 60.8% (SE 4.9) vs. 59.3% (SE 6.4) and 5-year OS 72.8% (SE 4.5) vs. 69.4% (SE 6.0), with non-significant differences, respectively. In the analysis by age, the outcome in the 85 patients <10 years was compared with that of 75 patients ≥10 years: their 5-year EFS was 55.9% (SE 5.4) vs. 65.1% (SE 5.5, P-value =0.22) and 5-year OS 70.1% (SE 5.0) vs. 73.0% (SE 5.2, P-value =0.63). At diagnosis, 52 patients had a WBC count of <20×109/L, 48 of 20–100×109/L and 60 of ≥100×109/L. As shown in Figure 2, their 5-year EFS was 86.3% (SE 4.8), 58.3% (SE 7.1) and 39.6% (SE 6.4; P-value <0.0001) and 5-year OS was 91.9% (SE 3.9), 72.9% (SE 6.4) and 53.2% (SE 6.5; P-value<0.0001), respectively. These differences remained statistically significant in GR patients (Figure 2, panels C and D). In PR patients, the outcome was equally unfavorable for patients with WBC count of 20–100 ×109/L and ≥100 ×109/L (Figure 2, panels E and F). A Cox regression model, stratified by risk group and including age and WBC count as covariates, revealed that WBC count was the only independent risk factor: high WBC count ≥100×109/L was associated with poor prognosis (HR 5.26, 95% CI 2.37–11.70, P-value<0.0001), as well as WBC count of 20 to 100×109/L (HR 2.90, 95% CI 1.28– 6.61, P-value=0.0111), as compared to WBC count <20×109/L. When the analysis was repeated on the sub group of 128 patients who received imatinib, results did not change substantially; HR being of 3.22 (95% CI 1.17 – 8.82) for WBC count of 20 to 100×109/L and 4.84 (95% CI 1.81 – 12.94) for WBC count ≥100×109/L. Restricting the analyses to the small subgroup of 76 patients with MRD levels available at the end of Protocol IB, WBC count ≥100×109/L was borderline significant (HR 2.85, 95% CI 1.01– 8.10, P-value=0.0490) and MRD was not significant (HR 2.09, 95% CI 0.76– 5.72 of positive vs. negative MRD, P-value=0.15). The 4-year survival after relapse was 34.2% (SE 6.7), with very similar outcome for the 34 patients who relapsed after HSCT (23 deaths) and for the 16 patients who were previously treated with chemotherapy only (10 deaths).
Figure 2.
Event-free survival (EFS) and overall survival (OS) by white blood cell count (WBC) count at diagnosis, overall (Panels A and B), in Good Risk (Panels C and D) and Poor Risk patients (Panels E and F).
The long-term follow up of the EsPhALL2004 study shows that the EFS and OS, previously reported at 4 years (EFS 61.9% and OS 72.1%), decreased slightly, with 7-year figures of 58.2% and 68.2%, respectively. After 4 years, no events occurred in the few patients who received chemotherapy only, while 3 events occurred in transplanted patients (2 relapses and 1 death in CCR due to pulmonary GvHD). Overall, these data confirm that the introduction of imatinib decreased the rate of relapses, and conveyed a marked improvement in EFS when compared to the outcome reported for Ph+ALL before the advent of TKI therapy.4 These results are in keeping with those reported for the COG studies AALL003112 and AALL062213: their 5-year OS of 81% (SE 6) and 86% (SE 5), respectively, support the use of increased exposure to imatinib (and other TKIs) and decreased need for HSCT in the next generation of studies. The comparison of outcome by treatment (chemotherapy vs. transplant) in EsPhALL2004 is very difficult, considering that 81% of patients was transplanted. Although survival was similar, a higher rate of relapse was observed in the few patients who did not undergo transplant, suggesting that intermittent and short exposure to imatinib is inadequate for patients treated with chemotherapy only. In transplanted patients, this treatment strategy was associated with low relapse rate in GR patients (18.6%) and a higher risk in PR patients (35.0%). Interestingly, a high proportion of relapses were very early (30/50). Of note, in this long-term evaluation, the most relevant unfavorable prognostic factor was high WBC count at diagnosis (≥100×109/L), while neither MRD nor age at diagnosis showed a prognostic impact in the multivariable analysis. As already reported, in EsPhALL2004 only early MRD negativity, which occurred in a small proportion of patients (10%), was associated with good prognosis.15 Interestingly, with the use of imatinib and HSCT, age is no longer a relevant prognostic factor. The main findings of this update are that EFS, DFS and OS are stable after about 4 years from diagnosis. Moreover, WBC count at diagnosis emerged as the most relevant independent prognostic factor. This observation should, however, be tested in other studies with longer exposure to TKI and fewer transplanted patients.
Supplementary Material
Acknowledgments
We thank all Centers and all patients who contributed to the study.
Footnotes
Funding: This project was partially funded by the following grants: Italian Association for Cancer Research (AIRC IG 2017 and AIRC 5×1000 Ref. 21147 to AB), TRANSCAN-2 Fondazione Regionale per la Ricerca Biomedica (to AB), MH CZ – DRO, Motol Univesity Hospital, Prague, Czech Republic, 00064203 (to JS), the “Programme Hospitalier de Recherche CliniSanté” (to VG), VS is a Margdarshi Fellow of the Wellcome-DBT India Alliance.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006; 354(2):166–178. [DOI] [PubMed] [Google Scholar]
- 2.Schrappe M, Reiter A, Zimmermann M, et al. Long-term results of four consecutive trials in childhood ALL performed by the ALL-BFM study group from 1981 to 1995. Berlin-Frankfurt-Münster. Leukemia. 2000;14(12):2205–2222. [DOI] [PubMed] [Google Scholar]
- 3.Aricò M, Valsecchi MG, Camitta B, et al. Outcome of treatment in children with Philadelphia chromosome-positive acute lymphoblastic leukemia. N Engl J Med. 2000; 342(14):998–1006. [DOI] [PubMed] [Google Scholar]
- 4.Aricò M, Schrappe M, Hunger SP, et al. Clinical outcome of children with newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia treated between 1995 and 2005. J Clin Oncol. 2010; 28(31):4755–4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schultz KR, Pullen DJ, Sather HN, et al. Risk and response-based classification of childhood B-precursor acute lymphoblastic leukemia: a combined analysis of prognostic markers from the Pediatric Oncology Group (POG) and Children’s Cancer Group (CCG). Blood. 2007; 109(3):926–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ribeiro RC, Abromowitch M, Raimondi SC, Murphy SB, Behm F, Williams DL. Clinical and biologic hallmarks of the Philadelphia chromosome in childhood acute lymphoblastic leukemia. Blood. 1987; 70(4):948–953. [PubMed] [Google Scholar]
- 7.Schrappe M, Aricò M, Harbott J, et al. Philadelphia chromosome-positive (Ph+) childhood acute lymphoblastic leukemia: good initial steroid response allows early prediction of a favorable treatment outcome. Blood. 1998; 92(8):2730–2741. [PubMed] [Google Scholar]
- 8.Burke MJ, Cao Q, Trotz B, et al. Allogeneic hematopoietic cell transplantation (allogeneic HCT) for treatment of pediatric Philadelphia chromosome-positive acute lymphoblastic leukemia (ALL). Pediatr Blood Cancer. 2009; 53(7):1289–1294. [DOI] [PubMed] [Google Scholar]
- 9.Schrappe M, Nachman J, Hunger S, et al. Educational symposium on long-term results of large prospective clinical trials for childhood acute lymphoblastic leukemia (1985–2000) Leukemia. 2010; 24(2):253–254. [DOI] [PubMed] [Google Scholar]
- 10.Bleckmann K, Schrappe M. Advances in therapy for Philadelphia-positive acute lymphoblastic leukaemia of childhood and adolescence. Br J Haematol. 2016; 172(6):855–869. [DOI] [PubMed] [Google Scholar]
- 11.Schultz KR, Bowman WP, Aledo A, et al. Improved early event-free survival with imatinib in Philadelphia chromosome-positive acute lymphoblastic leukemia: a children’s oncology group study. J Clin Oncol. 2009;27(31):5175–5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schultz KR, Carroll A, Heerema NA, et al. Long-term follow-up of imatinib in pediatric Philadelphia chromosome-positive acute lymphoblastic leukemia: Children’s Oncology Group Study AALL0031. Leukemia. 2014;28(7):1467–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slayton WB, Schultz KR, Kairalla JA, et al. Dasatinib plus intensive chemotherapy in children, adolescents, and young adults with Philadelphia chromosome-positive acute lymphoblastic leukemia: Results of Children’s Oncology Group Trial AALL0622. J Clin Oncol. 2018;36(22):2306–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biondi A, Schrappe M, De Lorenzo P, et al. Imatinib after induction for treatment of children and adolescents with Philadelphia-chromosome-positive acute lymphoblastic leukaemia (EsPhALL): a randomised, open-label, intergroup study. Lancet Oncol. 2012;13(9):936–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cazzaniga G, De Lorenzo P, Alten J, et al. Predictive value of minimal residual disease in Philadelphia-chromosome-positive acute lymphoblastic leukemia treated with imatinib in the European inter-group study of post-induction treatment of Philadelphia-chromosome- positive acute lymphoblastic leukemia, based on immunoglobulin/T-cell receptor and BCR/ABL1 methodologies. Haematologica. 2018; 103(1):107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.