Abstract
Iron recycling by macrophages is essential for erythropoiesis, but may also be relevant for iron redistribution to neighboring cells at the local tissue level. Using mice with iron retention in macrophages due to targeted inactivation of the iron exporter ferroportin, we investigated the role of macrophage iron release in hair follicle cycling and wound healing, a complex process leading to major clinical problems, if impaired. Genetic deletion of ferroportin in macrophages resulted in iron deficiency and decreased proliferation in epithelial cells, which consequently impaired hair follicle growth and caused transient alopecia. Hair loss was not related to systemic iron deficiency or anemia, thus indicating the necessity of local iron release from macrophages. Inactivation of macrophage ferroportin also led to delayed skin wound healing with defective granulation tissue formation and diminished fibroplasia. Iron retention in macrophages had no impact on the inflammatory processes accompanying wound healing, but affected stromal cell proliferation, blood and lymphatic vessel formation, and fibrogenesis. Our findings reveal that iron/ferroportin plays a largely underestimated role in macrophage trophic function in skin homeostasis and repair.
Introduction
Tissue resident macrophages play an important role both in tissue homeostasis, by supporting neighboring parenchymal cells with trophic signals and nutrients, and in tissue repair following injury.1–4 In the skin context, macrophages are critical regulators of hair follicle growth5 and cutaneous wound healing, two events with many similarities.6 Indeed, perifollicular macrophages prompt the entry of hair follicle stem cells into the anagen phase of growth,7 while selective ablation of macrophages impairs the wound healing response.8 Although wound macrophages display a mixed phenotypic and functional profile, the initial phase of an injury is characterized by the prevalence of pro-inflammatory, classically activated M1 macrophages, which are associated with the production of oxygen radicals and pro-inflammatory cytokines. Conversely, at later stages during resolution of inflammation and tissue repair, alternatively polarized M2 macrophages oriented to tissue repair and remodeling, predominate.1,9 This M1 to M2 switch is required for normal healing.2
Macrophages are also at the cross-road of iron traffic.10,11 Iron-recycling macrophages provide iron for erythropoiesis by clearing senescent erythrocytes.12 Conversely, iron sequestration by pro-inflammatory macrophages is a well-known mechanism of efficient bacteriostasis in host defense.13 In line with their different functions in homeostatic and inflammatory conditions, polarized macrophages show considerable differences in their transcriptional profiles,14 including a distinct regulation of genes related to iron metabolism.11,15 Iron retention by M1 macrophages correlates with high expression of the iron storage protein ferritin. Conversely, M2 macrophages display increased heme uptake and production of anti-inflammatory mediators via heme oxygenase-dependent heme catabolism, as well as high expression of the iron exporter ferroportin (FPN).16
A tight control of iron metabolism is needed for appropriate tissue homeostasis and healing. Excess iron, both in macrophages and in the extracellular milieu, has a deleterious effect on tissue repair,17 and heme iron has pro-oxidant and pro-inflammatory properties, so that its clearance and degradation by M2 macrophages contributes to resolution.18 However, it is also conceivable that increased iron retention in macrophages leads to lower iron availability for neighboring cells, thus compromising the trophic role of macrophages. In fact, given the necessity of iron for many essential biological functions, including cell replication,19,20 defective iron release can jeopardize iron-dependent functions essential for cutaneous homeostasis and efficient tissue restoration. Decreased iron availability could impair the growth of fibroblasts, as well as epithelial and endothelial cells during new tissue formation. Moreover, the hydroxylases necessary for efficient collagen assembly during the repair phase are iron-dependent enzymes.21
In this study, we investigated the role of macrophage iron metabolism in tissue homeostasis and repair exploiting a mouse line with iron retention in macrophages caused by targeted FPN inactivation in cells of the myeloid lineage, thus avoiding artefactual systemic iron overload and other confounding elements, such as increased local iron accumulation in other cell types. Using the skin as a model tissue, we show that macrophage-dependent FPN-mediated iron release is required for hair growth in homeostatic conditions and for efficient wound healing, a process which is essential for survival and also clinically relevant, as non-healing wounds are a major clinical problem associated with various human diseases.22,23
Methods
Animals
The crossing of mice carrying a floxed Fpn allele (Fpnfl/fl),24 provided by Dr Nancy Andrews, with mice expressing Cre under the control of the LysM promoter in the C57BL/6J background25 in order to generate mice with specific FPN-macrophage inactivation (Fpn1fl/flLysCre+/−) is described in detail in the Online Supplementary Material.
Procedures involving animals handling and care conformed with protocols approved by the Humanitas Clinical and Research Center in compliance with national (DL 116, GU suppl. 40, 18-2-1992; DL 26, GU 4-3-2014) and international law and policies (EEC Council Directive 2010/63/EU, OJ L 276/33, 22–09-2010; National Institutes of Health Guide for the Care and Use of Laboratory Animals, US National Research Council, 2011). The study was approved by the Italian Ministry of Health. All efforts were made to minimize the number of animals used and their suffering.
Statistical analysis
Results are expressed as the mean ± standard error of mean. Statistical significance between two groups was assessed by an unpaired two-tailed Mann-Whitney test or Student t test with Prism software (GraphPad). For comparison of more than two groups, data were analyzed using one-way analysis of variance (ANOVA).
Full details of the Methods are available in the Online Supplementary Material.
Results
Ferroportin deletion in macrophages causes hair follicle alterations and alopecia
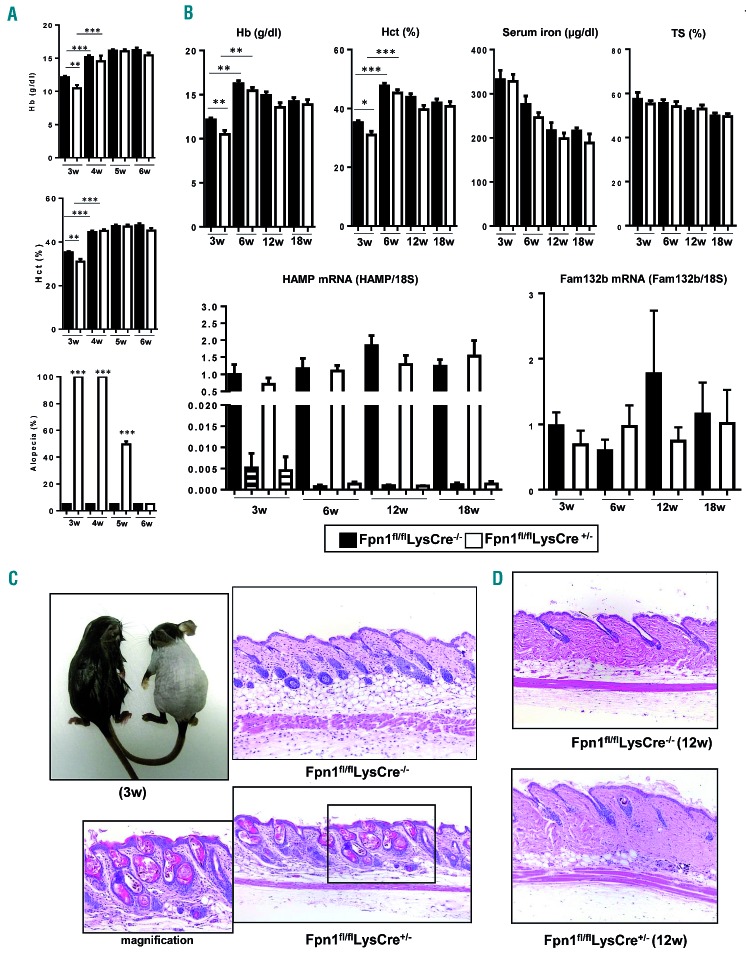
To generate mice that lack FPN in macrophages, we crossed Fpnfl/fl mice24 with LysMCre mice25 to create myeloid cell-specific FPN knockout mice. The phenotypic characterization of these Fpn1fl/flLysCre+/− mice is described in the Online Supplementary Material and illustrated in Online Supplementary Figure S1. FPN deletion in macrophages resulted in a significant decrease of hematologic parameters, such as hemoglobin level and hematocrit (Figure 1A,B), and red blood cell count, mean corpuscular volume and mean cell hemoglobin (Online Supplementary Table S1), at weaning (3 weeks after birth). Thereafter, both parameters rapidly returned to normal levels and remained almost unaltered until 18 weeks. In line with the mild anemia observed in 2-month old 129/SvEvTac mice lacking macrophage FPN,26 in weaned Fpn1fl/flLysCre+/− mice hemoglobin levels and hematocrit were lower than in Fpn1fl/flLysCre−/− mice at all time-points, although the difference never reached statistical significance (Figure 1A,B). Serum iron levels and transferrin saturation showed a tendency to decrease with age, but were never statistically different between Fpn1fl/flLysCre+/− mice and their control Fpn1fl/flLysCre−/− littermates. The hepatic expression of hepcidin (HAMP), which regulates systemic iron homeostasis by inhibiting FPN,20 showed age-related variations but was not different between Fpn1fl/flLysCre+/− mice and their control littermates. Similarly, we did not detect significant differences in skin HAMP mRNA levels, which were much lower than in the liver (Figure 1B), while hepcidin levels in skin lysates were undetectable. Accordingly, the expression of Fam132b mRNA encoding for erythroferrone, the erythroid regulator of hepcidin,27 was unchanged in both spleen and bone marrow (Figure 1B and Online Supplementary Figure S2). Fpn1fl/flLysCre+/− mice showed diffuse alopecia with sparing of the head in 100% of both male and female mice until the fourth week of age (Figure 1A,C). Histological analysis in 3-week old mice showed no differences between the two genotypes in any organ evaluated, with the exception of the increased iron accumulation in spleen and liver macrophages (Online Supplementary Figure S1) and a moderate/severe and diffuse/multifocal to coalescing dilatation of hair follicles, which contained remnants of hair shafts and keratin, and slight acanthosis of the superficial epidermis (Figure 1C). Alopecia gradually disappeared and hair re-growth was evident starting 2 weeks after weaning (Figure 1A), but minor skin alterations were still detectable in adult Fpn1fl/flLysCre+/− mice, which had a reduced number of hair follicles, multifocal areas of hair shaft rarefaction and a thin hypodermis with an apparent increase of adipose tissue (Figure 1D). Taken together, these results indicate that targeted FPN deletion in macrophages results in severe alterations of the hair follicle and transient alopecia.
Figure 1.
Transient alopecia and anemia are present in Fpn1fl/flLysCre+/− mice. (A) Hemoglobin (Hb) levels and hematocrit (Hct) in 3- to 6-week (w) old mice (mean ± SEM of 50 mice for each group; ***P<0.0001, **P<0.001). The histogram at the bottom shows the percentage alopecia at different time-points (mean ± SEM of 50 mice for each group; ***P<0.0001, **P<0.001 versus Fpn1fl/flLysCre−/−). (B) Top: Hb levels, Hct, serum iron and transferrin saturation (TS) in 3-, 6-, 12-, and 18-week old mice (mean ± SEM of 50 mice for each group; ***P<0.0001, **P<0.001, *P<0.01). Bottom: hepcidin (HAMP) expression in the liver (solid bars) and skin (striped bars) and spleen erythroferrone (Fam132b) mRNA levels of 3-, 6-, 12-, and 18-week old mice. mRNA levels were measured by quantitative real time polymerase chain reaction and normalized to the housekeeping gene 18S RNA. Data are presented as mean ± SEM of 10 mice for each group. (C) Representative appearance of 3-week old Fpn1fl/flLysCre−/− (left) and Fpn1fl/flLysCre+/− (right) mice and representative histology (dorsal area) of the same mice. Magnification 10X, in the inset 20X. (D) Representative histology of the skin (dorsal area) of adult (12-week old) mice. Tissue sections were stained with hematoxylin and eosin. Magnification: 10X.
Alopecia is not related to systemic iron deficiency
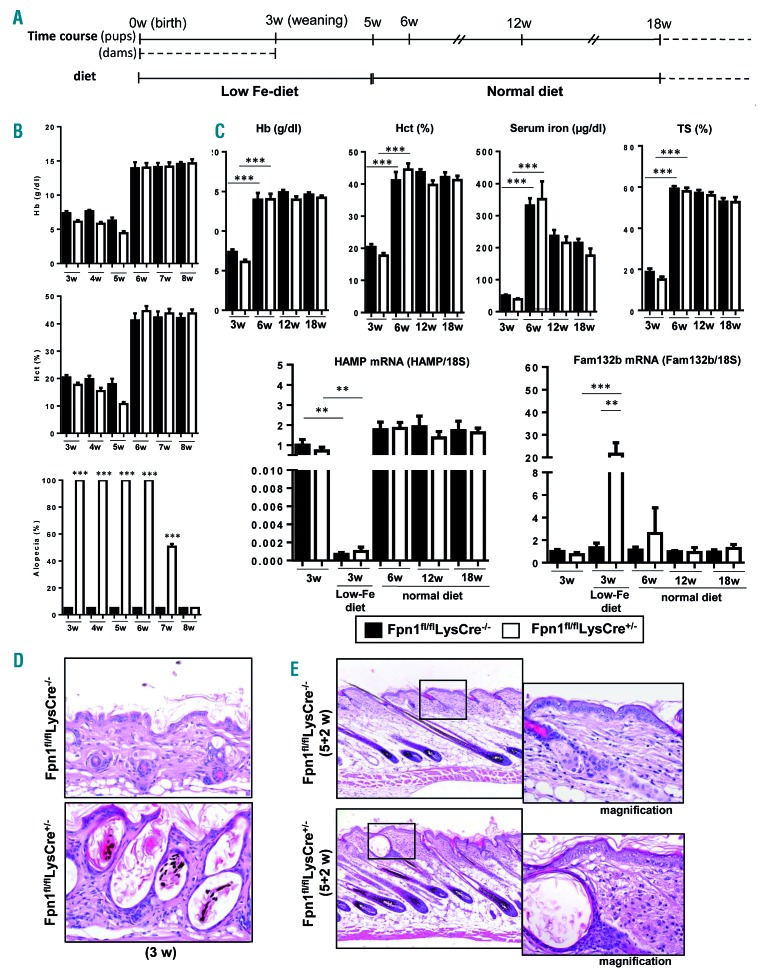
In Fpn1fl/flLysCre+/− mice hair regrowth was not complete until 3 weeks after weaning, while hemoglobin levels and hematocrit had already returned to normal after 1 week (Figure 1A) and at all time-points there was no difference in serum iron availability between Fpn1fl/flLysCre+/− and control littermates. This suggested that alopecia in Fpn1fl/flLysCre+/−mice was not a local reflection of systemic iron deficiency but a consequence of iron sequestration in skin macrophages, ultimately resulting in impaired hair follicle growth. To further assess this issue, we analyzed hematologic parameters and alopecia after exposure to low iron diet according to the protocol outlined in Figure 2A. Until week 5, both Fpn1fl/flLysCre+/− and control littermates were anemic, and hemoglobin levels and other hematologic parameters were always slightly and not significantly lower in animals lacking macrophage FPN (Figure 2B,C, Online Supplementary Table S1). Serum iron levels and transferrin saturation were lower in 3-week old Fpn1fl/flLysCre+/−mice than in control littermates, although not statistically significantly so, but returned to normal levels at 6 weeks without differences between the two strains (Figure 2C). Liver hepcidin expression was repressed by the iron-deficient diet, but was not different between Fpn1fl/flLysCre+/−mice and their control littermates (Figure 2C). HAMP mRNA levels in the skin were below the threshold of detection. A significant increase in erythroferrone expression was found in Fpn1fl/flLysCre+/− pups at 3 weeks (Figure 2C and Online Supplementary Figure S2), which is indicative of higher erythropoietic activity. After the introduction of the normal diet, both hepcidin and erythroferrone expression returned to normal levels (Figure 2C and Online Supplementary Figure S2). Mice with loss of macrophage FPN were grossly affected by diffuse alopecia of the trunk throughout the period of exposure to the low iron diet, whereas Fpn1fl/flLysCre−/− mice, despite low serum iron availability, did not develop alopecia (Figure 2B). Remarkably, alopecia did not appear in Fpn1fl/flLysCre−/− mice even after exposure to the iron-deficient diet for 11 weeks. In Fpn1fl/flLysCre+/− mice, after reintroduction of a normal diet, hemoglobin and serum iron returned to normal levels 2 weeks before the restoration of normally haired skin (Figure 2B,C), thus indicating that alopecia and hypoferremia/anemia are not associated. Histological analysis showed that in Fpn1fl/flLysCre+/− mice challenged with the low iron diet alopecia was associated with severe follicular keratosis with intraluminal accumulation of keratin and distorted hair shafts and subsequent dilation of the hair follicles. Conversely, no relevant histopathological changes were found in the haired skin of the Fpn1fl/flLysCre−/− mice maintained in the same dietary conditions (Figure 2D). Both in Fpn1fl/flLysCre+/− and Fpn1fl/flLysCre−/− mice maintained under iron deprivation conditions for 5 weeks and subsequently fed a normal diet for 2 weeks, skin histology showed that hair follicles were in the anagen stage, but in Fpn1fl/flLysCre+/− mice hair shafts did not exit the follicular ostia and follicular keratosis/dilation, increased epidermal hyperplasia and dermal inflammation were observed (Figure 2E).
Figure 2.
Alopecia in Fpn1fl/flLysCre+/− mice is not related to iron deficiency/anemia. (A) Schematic overview of the feeding protocol: pups were fed by dams kept on an iron-deficient diet for 3 weeks until weaning and then maintained on a low iron diet for another 2 weeks followed by a normal diet. (B) Hemoglobin (Hb) levels and hematocrit (Hct) in 3- to 8-week old mice (mean ± SEM of 10 mice for each group). The histogram at the bottom shows the degree of alopecia at different time-points. ***P<0.0001 versus Fpn1fl/flLysCre−/−. (C) Top: Hb levels, Hct, serum iron and transferrin saturation (TS) in 3- to 18-week old mice (mean ± SEM of 10 mice for each group; ***P<0.0001). Bottom: hepcidin (HAMP) and erythroferrone (Fam132b) mRNA levels in the liver and spleen, respectively, of 3- to 18-week old mice. Expression in 3-week old mice fed the normal diet is shown in comparison. mRNA levels were measured by quantitative real-time polymerase chain reaction and normalized to the housekeeping gene 18S RNA. Data are presented as mean ± SEM of 10 mice for each group; ***P<0.0001, **P<0.001. (D) Representative histology of the skin (dorsal area) of 3-week old Fpn1fl/flLysCre−/− and Fpn1fl/flLysCre+/− mice maintained on an iron-deficient diet. Magnification 20X. (E) Representative histology of the skin (dorsal region) after 5 weeks of an iron deficient diet plus 2 weeks of a normal diet. Tissue sections were stained with hematoxylin and eosin. Magnification: 10X; 20X in the insets.
Ferroportin deletion in macrophages leads to epithelial iron deficiency and decreased proliferation in cutaneous hair follicles
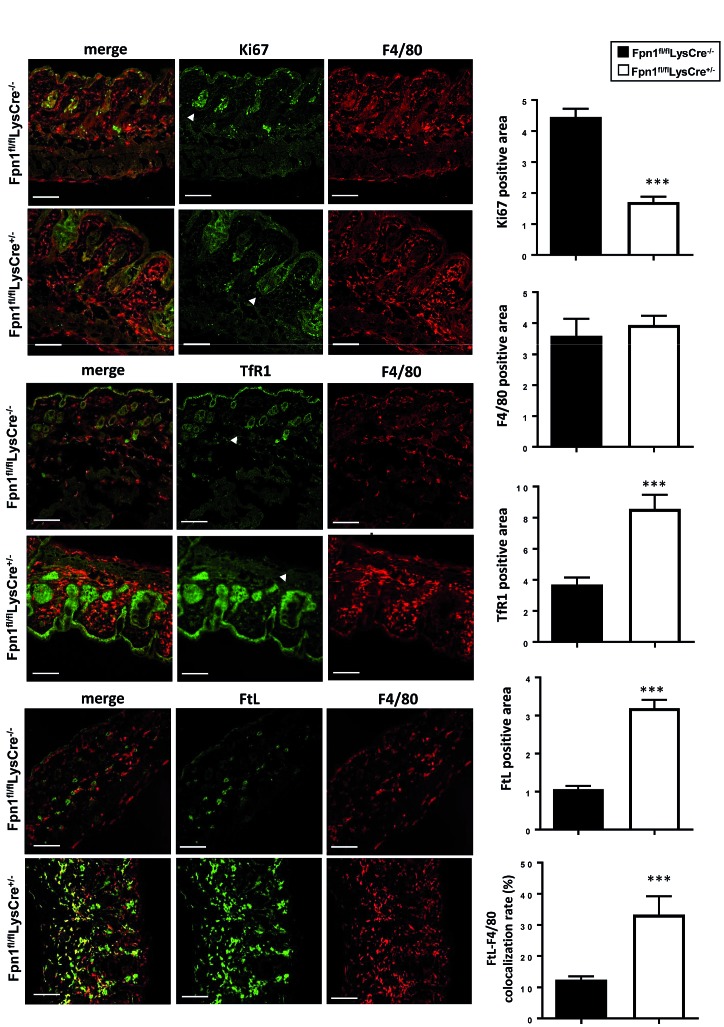
Since we showed that iron released by macrophages via FPN supports in vitro cell proliferation,15 an important role for FPN in skin macrophages could be to mediate the release of sufficient iron in the microenvironment for cell multiplication. Indeed, confocal microscopy revealed a significantly lower expression of the proliferation marker Ki67 in the epithelial cells of the hair bulbs of 3-week old Fpn1fl/flLysCre+/− mice (Figure 3). Conversely, in the same cells we found a strong increase of transferrin receptor (TfR1) expression, which is indicative of cellular iron deprivation (Figure 3). Notably, F4/80+ macrophages, which are abundant in the skin stroma but with no differences in number between Fpn1fl/flLysCre+/− and Fpn1fl/flLysCre−/− mice (Figure 3), expressed lower levels of both Ki67 and TfR1 but had an increased content of both the L and H subunits of the iron storage protein ferritin (Figure 3 and Online Supplementary Figure S3) as compared to epithelial cells. Qualitative analysis also showed that in Fpn1fl/flLysCre−/−mice ferritin is detectable only in epithelial cells (Online Supplementary Figure S3), whereas in Fpn1fl/flLysCre+/− mice ferritin expression is particularly strong in F4/80+ macrophages. These results suggest that iron retention in resident macrophages, by starving neighboring hair follicle cells of iron and hence inhibiting their proliferation, has detrimental effects on tissue homeostasis.
Figure 3.
Epithelial iron deficiency and decreased proliferation in cutaneous hair follicles of Fpn1fl/flLysCre+/− mice. Expression and localization of Ki67, F4/80, transferrin receptor (TfR1) and L ferritin subunit (FtL) in cutaneous tissue of Fpn1fl/flLysCre−/− and Fpn1fl/flLysCre+/− mice was assessed by confocal microscopy. Representative confocal microscopy images for merged signals, Ki67, TfR1, F4/80 and FtL are shown. Quantification of confocal images (5-9 fields of vision/mouse, 3 mice/group) is also reported. ***P<0.0001 versus Fpn1fl/flLysCre−/−. Arrowheads indicate hair bulbs. Bars: 100 mm. Magnification: 40X.
Ferroportin deletion in macrophages compromises wound healing
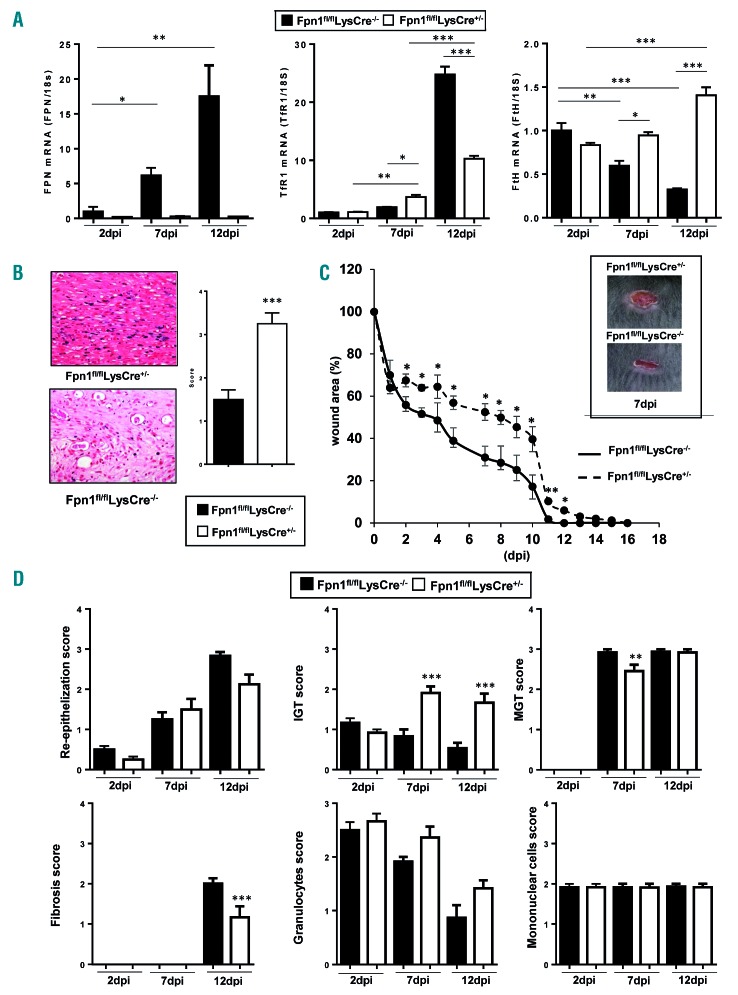
Resident macrophages support parenchymal cells with trophic signals, particularly under conditions characterized by increased cell proliferation, such as during tissue repair following injury.2 To test the role of macrophage-derived iron in this context, we investigated the wound healing process after incisional skin damage during the entire time course of repair, i.e. the early-inflammatory [2 days post injury (dpi)], middle-proliferative (7 dpi), and late-remodeling phases (12 dpi). We first investigated FPN expression in FACS-sorted macrophages from wounds; in Fpn1fl/flLysCre−/− mice FPN mRNA levels progressively increased during repair (Figure 4A), suggesting a predominant role of FPN in the late phases, whereas, as expected, FPN mRNA was always barely detectable in Fpn1fl/flLysCre+/− mice. The analysis of other iron-related genes showed a rise in the expression of TfR1, which mediates iron uptake, and a decrease of ferritin H subunit during the middle-late phase of repair in macrophages of Fpn1fl/flLysCre−/− mice, but not Fpn1fl/flLysCre+/− mice, which is evidence of iron deposition in these cells (Figure 4A). Accordingly, histological analysis showed iron accumulation in wound macrophages of Fpn1fl/flLysCre+/− mice (Figure 4B). Hepcidin-dependent FPN modulation should not play a role in wound healing, as no difference was seen in liver HAMP expression between Fpn1fl/flLysCre−/− and Fpn1fl/flLysCre+/− mice during wound repair (Online Supplementary Figure S4A) and HAMP expression in FACS-sorted macrophages was undetectable. Hepcidin levels in the wound lysate, which were much lower than in serum, were not different between Fpn1fl/flLysCre−/− and Fpn1fl/flLysCre+/− mice (Online Supplementary Figure S4B). Macroscopic analysis of wound size showed that the process of closure was considerably delayed in Fpn1fl/flLysCre+/− mice than in control littermates, with significantly wider lesions at all time points and a lag of 3-5 days at 3 dpi through 12 dpi (Figure 4C). Histological analysis performed according to the criteria described in Online Supplementary Table S2 supported this observation, as Fpn1fl/flLysCre+/− mice displayed a more prolonged inflammatory response and delayed granulation tissue formation, associated with diminished fibroplasia, whereas mononuclear cells and granulocytes were unchanged (Figure 4D).
Figure 4.
Skin repair is delayed in Fpn1fl/flLysCre+/− mice. (A) Macrophages (CD45+/CD11b+/Ly6C+/F4/80+) were sorted by FACS from wounded skin tissue of eight animals/group from Fpn1fl/flLysCre−/− and Fpn1fl/flLysCre+/− mice at 2, 7 and 12 days post-injury (dpi). Ferroportin (FPN), transferrin receptor 1 (TfR1) and H-ferritin (FtH) mRNA expression was assessed by quantitative real time polymerase chain reaction and normalized to the housekeeping gene 18S RNA. Data are presented as mean ± SEM; *P<0.01, **P<0.001, ***P<0.0001. (B) Representative histology of Perls’ Prussian blue iron staining of dorsal skin samples at 12 dpi in Fpn1fl/flLysCre−/− and Fpn1fl/flLysCre+/−mice. Magnification 40X. A semi-quantitative evaluation of Perls’ iron staining is shown on the right; n=6 for each group; ***P<0.0001. (C) Kinetic analysis of skin excisional wound areas. Values represent mean ± SEM of 24 values for each group; *P<0.01, **P<0.001 versus Fpn1fl/flLysCre−/−. One representative experiment (6 mice/group) out of four is shown. The inset shows representative macroscopic images of Fpn1fl/flLysCre−/− and Fpn1fl/flLysCre+/− mice skin wounds at 7 dpi. (D) Histological grading of wounds, based on separate evaluation of distinct features of the wound healing process, at 2, 7 and 12 dpi. IGT: immature granulation tissue, MGT: mature granulation tissue. The semiquantitative score was defined as described in Online Supplementary Table S2; n= 12 for each group; ***P<0.0001, **P<0.001 versus Fpn1fl/flLysCre−/−.
Ferroportin deletion in macrophages has no impact on leukocyte recruitment and activation in the wound
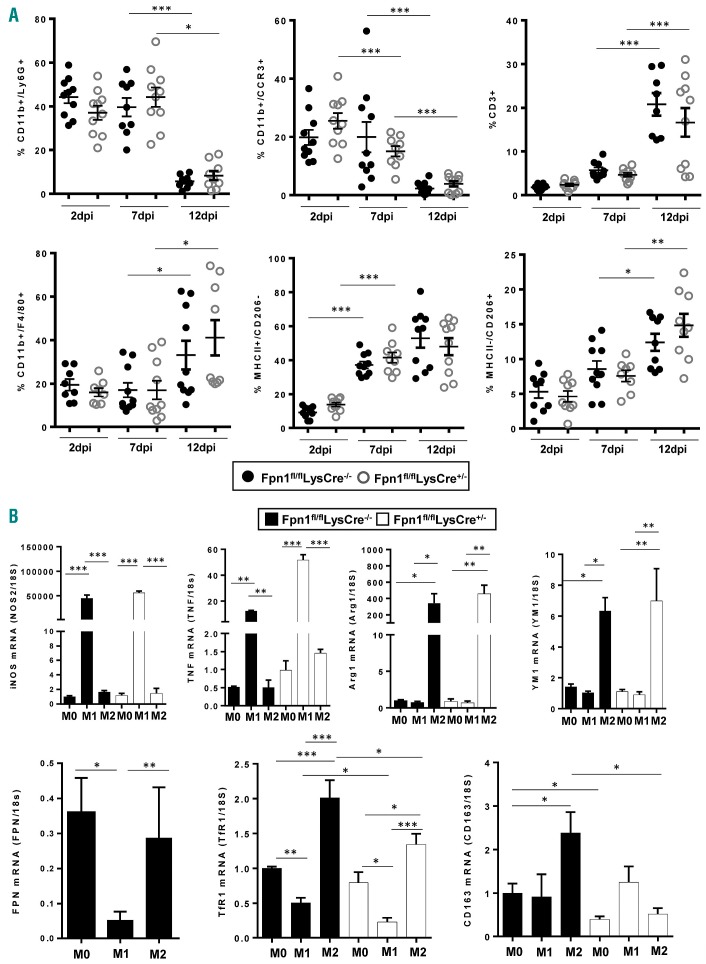
Given the role of leukocytes in tissue repair,23 we evaluated leukocyte recruitment in our experimental setting. Neutrophils (Ly6G+ cells) and eosinophils (CCR3+ cells) were abundant at 2 and 7 dpi and decreased thereafter, whereas an inverse trend was evident for T cells (CD3+ cells) and macrophages (F4/80+ cells), which increased at 12 dpi (Figure 5A). The accumulation kinetics of these cells, which are typical of skin wound healing,22 were not affect ed by the presence or absence of FPN in macrophages.
Figure 5.
Wound-infiltrating leukocytes and macrophage polarization are not altered in Fpn1fl/flLysCre+/− mice. (A) Frequencies of neutrophils (CD11b+/Ly6G+), eosinophils (CD11b+/CCR3+), T lymphocytes (CD3+), macrophages (CD11b+/F4/80+) on CD45+ cells. Frequencies of M1 (MHCII+/CD206−) and M2 (MHCII−/CD206+) polarized macrophages on total live macrophages. Dots and black lines represent single animals and the mean ± SEM, respectively; n=10 mice for each group; ***P<0.0001, **P<0.001, *P<0.01). (B) Bone marrow-derived macrophages from Fpn1fl/flLysCre−/− and Fpn1fl/flLysCre+/− mice were polarized to M1 and M2 macrophages and relative iNOS, TNFα, Arg1, YM1, FPN, TfR1 and CD163 mRNA levels were measured by quantitative real-time polymerase chain reaction at 24 h; results were normalized to the housekeeping gene 18S RNA (mean ± SEM of 12 mice for each group; ***P<0.0001, **P<0.001, *P<0.01).
Macrophages with different functional orientations have specific roles in the overlapping phases of wound repair.1,9 As iron accumulation in macrophages might favor the expression of inflammatory mediators.17,26,28,29 we evaluated the levels of inflammatory cytokines in wound lysates, but did not detect significant differences between the two mouse lines at any time-point (Online Supplementary Figure S5). Since iron accumulation in macrophages of Fpn1fl/flLysCre+/− mice could affect the polarization of these cells during the healing process,15 we investigated the distribution of the different polarized macrophages. As expected, an increase in MHCII+/CD206− M1 macrophages was detected already in the middle-proliferative phase, while a significant increase in MHCII−/CD206+ M2 macrophages was evident only in the late-remodeling phase, but no difference was found between Fpn1fl/flLysCre+/− mice and their control littermates (Figure 5A). Moreover, we evaluated the expression of polarization markers in bone marrowderived macrophages exposed in vitro to polarization stimuli, but again no difference was observed in markers for both M1 (iNOS and TNFα) and M2 (Arg1 and YM1) macrophages (Figure 5B). The expression of iron-related genes in bone marrow-derived macrophages of Fpn1fl/flLysCre−/− mice mirrored the pattern previously observed in human polarized macrophages,15 with elevated expression of FPN, TfR1 and the hemoglobin/hapto-globin complex receptor CD163 in M2 macrophages. This finding is in line with the prominent expression of FPN in macrophages during the late phase of repair, when the M2 cell infiltrate is increased (Figures 4A and 5A). Deletion of macrophage FPN resulted in lower expression of TfR1 and CD163 transcript levels in M2 macrophages (Figure 5B), possibly as a consequence of iron accumulation.
Ferroportin deletion in macrophages affects stromal cells during wound healing
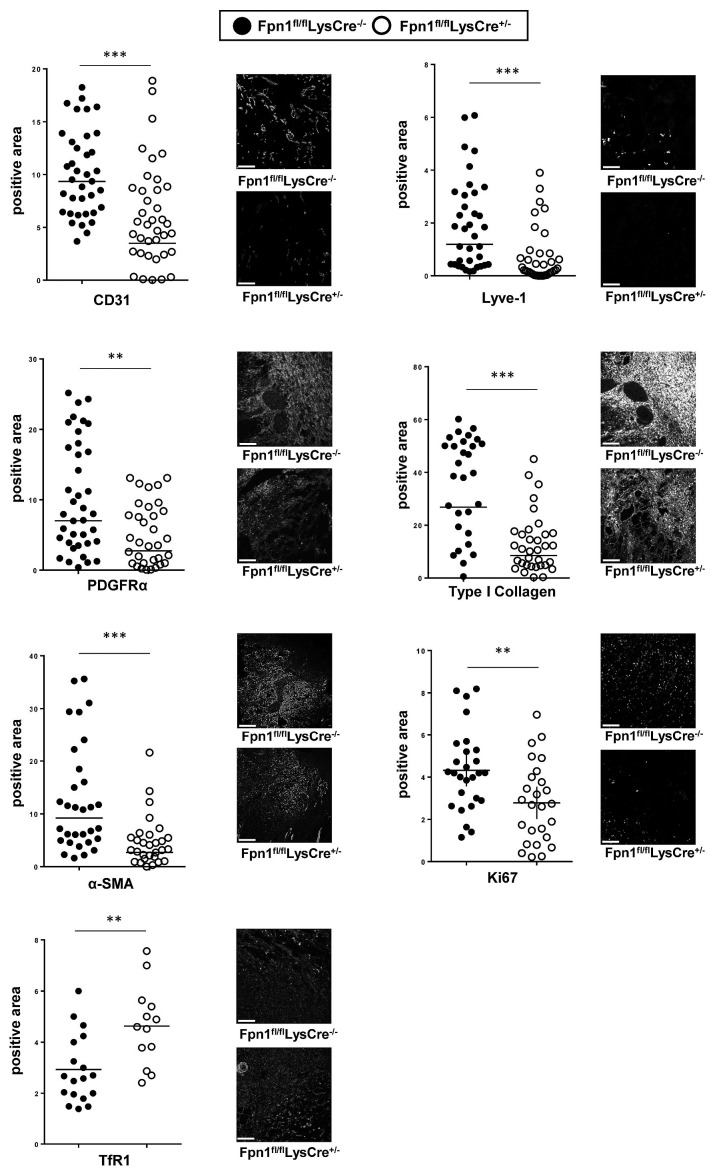
Since FPN deletion in macrophages significantly affected the wound healing process but iron accumulation in FPN-deficient macrophages did not alter the inflammatory processes associated with wound healing, we evaluated whether the defective iron release by FPN-deficient macrophages affected the biology of surrounding stromal cells in the wound tissue. Confocal analysis at 7 dpi showed reduced expression in Fpn1fl/flLysCre+/− mice, as compared to control littermates, of blood (CD31) and lymph vessel (Lyve-1) endothelium markers (Figure 6). This was accompanied by decreased expression of platelet-derived growth factor receptor-α, a marker of mesenchymal cells, and lower levels of collagen I and alpha smooth muscle actin (αSMA), which are markers of activated fibroblasts and myofibroblasts, respectively (Figure 6). Moreover, in the absence of macrophage FPN, surrounding stromal cells were iron-deficient, as indicated by upregulation of TfR1 expression, and proliferated less than control counterparts, as shown by decreased Ki67 expression (Figure 6). Taken together, these results indicate that the iron retention in macrophages caused by FPN deletion impairs blood vessel formation and stromal cell proliferation, leading to delayed skin repair.
Figure 6.
Vessel and stromal cell reduction accompanied by iron deficiency and decreased proliferation in wounds of Fpn1fl/flLysCre+/− mice. Expression of CD31, Lyve-1, collagen-1, PDFGR, αSMA, Ki67 and TfR1 after skin wounding at 7 dpi was assessed by confocal microscopy and the positive area expressed as %. Each circle represents an analysis from a single confocal image (5-9 fields of vision/mouse, 6 mice/group), ***P<0.0001, **P<0.001. Representative confocal microscopy images are shown. Bars: 100 mm. Magnification: 40X.
Discussion
The role of erythrophagocytic macrophages as a source of iron for erythropoiesis is well established.16 However, macrophages may also be involved in iron redistribution at a local tissue level, thus affecting neighboring cells. We previously showed that FPN-mediated iron release from human macrophages supports in vitro cell proliferation.15 In the present study, we showed that a steady supply of iron released by macrophage FPN is essential for tissue homeostasis in two conditions, follicular development and wound healing, which share many similarities, including fast cell growth rate.6 Our study, therefore, underlines a new iron-related function of macrophages in tissue homeostasis and regeneration, in line with the increasing recognition of these cells’ considerable functional polyvalence and trophic role, in addition to established immunological functions.30 We did not address the effect of FPN gene deletion in other myeloid cells affected in the LysM conditional model here adopted as their contribution to iron storage and release is negligible compared to that of macrophages.11,13
Our findings showing impaired hair follicle growth in mice with FPN deficiency in macrophages are in line with the report of similar hair and skin lesions in mice with altered expression of other proteins of iron metabolism,31–33 although in these other settings the presence of systemic iron deficiency/anemia did not allow the relative contributions of circulating iron versus local availability of macrophage-derived iron to be distinguished. We showed that the alopecia in mice with loss of macrophage FPN was not related to limited systemic iron availability, as evidenced by the lack of differences in serum iron availability and the similar hepatic and local hepcidin expression (Figure 1). Evidence that local iron release from macrophages, which are abundant in skin tissue (Figure 3), is more important than systemic iron levels was also provided by the persistence of alopecia after the return of normal hemoglobin and body iron levels (Figures 1 and 2), and by the absence of hair loss in hypoferremic and anemic Fpn1fl/flLysCre−/− mice (Figure 2), even when fed an iron-deficient diet for a long time. In line with the alopecia and delayed entry of the hair follicle into anagen exhibited by mice overexpressing H ferritin,34 we provide evidence that iron release from macrophages is required to sustain the rapid multiplication of hair follicle cells (Figure 3). In the absence of macrophage FPN, follicle epithelial cells are iron-deficient, as demonstrated by the increased expression of TfR1, and have a lower replication rate, as indicated by reduced Ki67 levels. The discrepancy with the lack of alopecia in a similar model of macrophage-specific FPN inactivation reported by Zhang and colleagues26 could be explained by the different iron content of the standard diet used (157 ppm versus 232, respectively) and by the different genetic backgrounds of the mice. Indeed, the role of dietary iron absorption, which is more important in mice than in humans,35 in correcting the alopecia was also indicated by the effect of switching to chow diet at weaning. Alopecia may result from insufficient iron availability caused by decreased local iron release (this study), Matriptase-dependent severe systemic iron deficiency32,33 (although the role of local FPN was not addressed in those studies) and iron sequestration in ferritin.34 The absence of alopecia in Fpn1fl/flLysCre−/− mice kept on an iron-deficient diet for almost 3 months suggests that local iron release may provide iron more directly in a paracrine fashion when circulating iron levels fall. We conclude that the essential role of macrophages in hair follicle cycling5 is not only related to their production of growth stimulators, such as Wnt,7 but also to the ability to supply the growing tissue with iron. Macrophages are part of the nurturing niche of stem cells in various tissues,36 including tumors. The results reported here in skin hair follicles raise the possibility that macrophage-dependent iron provision has a more general role in different stem cell niches.
We also report here a similar role for macrophage-derived iron during skin wound healing, a complex tissue repair process consisting of overlapping phases of inflammation and tissue remodeling in which macrophages play a key role.2 The use of mice lacking FPN selectively in cells of the myeloid lineage allowed us to define the role of macrophage iron in wound healing in the absence of the systemic iron overload and large local iron accumulation present in other models.17,28 In the present setting, the disruption of iron export from local macrophages delayed wound healing, apparently by preventing neighboring mesenchymal and stromal cells from receiving the iron supply necessary for growth/differentiation. In line with the higher FPN expression in M2 than in M1 macrophages,15,37 the lack of macrophage FPN exerts its major effects in the middle-late phase of repair when the M1 to M2 switch occurs. Indeed, in the late stages, normal M2 skin macrophages export iron through enhanced FPN expression, whereas FPN-deficient macrophages accumulate iron with concomitant induction of ferritin and repression of TfR1 (Figure 4A). The lower fibrosis score (Figure 4D) and the decreased expression of collagen-1 and αSMA (Figure 6) show that the stromal component is compromised, as the absence of macrophage FPN resulted in iron deprivation and impaired proliferation of stromal cells (Figure 6). In this context, fibroblasts may not receive enough iron, which can be among the paracrine factors secreted by M2 macrophages to favor cell multiplication.38 A detrimental effect on collagen synthesis and assembly, which require iron-dependent prolyl hydroxylases,21 or other iron-dependent functions such as dihydroxy-docosahexaenoic acid production,39 may contribute to defective repair.
Our results also show that macrophage iron is essential for the development of the vascular network during tissue healing, as both lymphatic and blood vessels were reduced (Figure 6). Although the decrease of vascular structure caused by macrophage depletion was previously ascribed to the reduced production of vascular endothelial growth factor and transforming growth factor-β,9 the latter being also involved in extracellular matrix deposition and αSMA expression,40 in our experimental model the levels of these growth factors were unchanged (Online Supplementary Figure S5). Similarly, given that hemoglobin levels of adult Fpn1fl/flLysCre+/− mice were normal, defective oxygenation as a possible factor involved in impaired vascularization can be ruled out. Therefore, our results showing reduced neovessel density, reduced granulation tissue formation and decreased fibrosis in the absence of macrophage iron release, in the face of unchanged levels of prominent angiogenic and fibrogenic factors, such as vascular endothelial growth factor and transforming growth factor-β, support the relevance of the trophic role of macrophage-derived iron in the wound milieu.
Understanding the role of iron in macrophage production of inflammatory molecules has been hampered by contradictory findings. An increased inflammatory response was found in iron-depleted macrophages,41 but not in equally iron-deficient macrophages from HFE−/−mice,42 and other studies showed that iron levels correlate positively with the synthesis of pro-inflammatory cytokines.26,43 In addition, a pro-inflammatory state has been shown in macrophages and macrophage/microglia cells exposed to heme or iron28,29 and in hemorrhagic areas within tumors.44 Similarly, decreased iron release from macrophages, associated with pro-inflammatory activation and defective M2 polarization, impaired wound healing in chronic venous leg ulcers.17 Conversely, we found that iron retention in macrophages has no impact on leukocyte recruitment and activation as well as macrophage polarization (Figure 5 and Online Supplementary Figure S5). Moreover, in vitro polarized bone marrow-derived macrophages from the two mouse lines did not show differential expression of M1 and M2 markers (Figure 5B). Therefore, in our model, iron accumulation does not exacerbate the pro-inflammatory phenotype of wound healing-associated macrophages, in keeping with a recent study showing that iron did not increase M1 polarization of RAW264.7 macrophages.45 The conflicting results may be related to the different experimental models, the heterogeneity of macrophages and the exposure to different iron sources, such as heme iron which is highly toxic.46 In the absence of FPN, macrophages from Fpn1fl/flLysCre+/− mice accumulate iron in ferritin, which increases less than 2-fold (Figure 4A), but iron deposition seems less massive than in conditions such as chronic ulcers,17 in which iron content may increase 20-fold,47 or hemolysis.29,44 In our experimental setting iron accumulation may, therefore, be insufficient to interfere with the M1/M2 switch and favor a pro-inflammatory state. A recent study demonstrated that FPN downregulation in macrophages impaired skeletal muscle regeneration after injury,48 but the effect of increased iron accumulation on the inflammatory profile of macrophages was not addressed.
In conclusion, the results of our study indicate that local macrophage FPN, by supplying iron to cells in the microenvironment, affects both the physiological context of follicular anagen and the pathophysiological context of wound healing. In its absence, stromal cells are iron-deficient and their proliferation is impaired (Figures 3 and 6). The importance of local iron recycling is underlined by the lack of changes in hepatic and skin hepcidin. A similar requirement for iron provided locally by macrophages has been described for the repair of skeletal muscle cells, in which iron retention in macrophages, by impairing myoblast proliferation, results in smaller myofibers.48 Iron should, therefore, be added to the list of trophic mediators produced locally by macrophages that stimulate the growth, differentiation and activity of neighboring parenchymal and stromal cells in order to maintain tissue homeostasis or repair.
Supplementary Material
Acknowledgments
This work was supported by grants from the Italian Association for Cancer Research (AIRC-IG 2016 #19213 to ML) and MIUR (COFIN to GC). The authors would like to thank Nancy Andrews for providing Fpnfl/fl mice, Alberto Mantovani for support and helpful comments, and Eugenio Scanziani and Camilla Recordati for their help with the histological analysis.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/104/1/47
References
- 1.Brancato SK, Albina JE. Wound macrophages as key regulators of repair: origin, phenotype, and function. Am J Pathol. 2011;178(1):19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44(3):450–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 2013;229(2):176–185. [DOI] [PubMed] [Google Scholar]
- 4.Biswas SK, Mantovani A. Orchestration of metabolism by macrophages. Cell Metab. 2012;15(4):432–437. [DOI] [PubMed] [Google Scholar]
- 5.Stenn KS, Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;81(1):449–494. [DOI] [PubMed] [Google Scholar]
- 6.Ansell DM, Kloepper JE, Thomason HA, Paus R, Hardman MJ. Exploring the “hair growth-wound healing connection”: anagen phase promotes wound re-epithelialization. J Invest Dermatol. 2011;131(2):518–528. [DOI] [PubMed] [Google Scholar]
- 7.Castellana D, Paus R, Perez-Moreno M. Macrophages contribute to the cyclic activation of adult hair follicle stem cells. PLoS Biol. 2014;12(12):e1002002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mirza R, DiPietro LA, Koh TJ. Selective and specific macrophage ablation is detrimental to wound healing in mice. Am J Pathol. 2009;175(6):2454–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lucas T, Waisman A, Ranjan R, et al. Differential roles of macrophages in diverse phases of skin repair. J Immunol. 2010;184(7):3964–3977. [DOI] [PubMed] [Google Scholar]
- 10.Recalcati S, Locati M, Cairo G. Systemic and cellular consequences of macrophage control of iron metabolism. Semin Immunol. 2012;24(6):393–398. [DOI] [PubMed] [Google Scholar]
- 11.Cairo G, Recalcati S, Mantovani A, Locati M. Iron trafficking and metabolism in macrophages: contribution to the polarized phenotype. Trends Immunol. 2011;32(6): 241–247. [DOI] [PubMed] [Google Scholar]
- 12.Soares MP, Hamza I. Macrophages and iron metabolism. Immunity. 2016;44(3):492–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ganz T, Nemeth E. Iron homeostasis in host defence and inflammation. Nat Rev Immunol. 2015;15(8):500–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177(10):7303–7311. [DOI] [PubMed] [Google Scholar]
- 15.Recalcati S, Locati M, Marini A, et al. Differential regulation of iron homeostasis during human macrophage polarized activation. Eur J Immunol. 2010;40(3):824–835. [DOI] [PubMed] [Google Scholar]
- 16.Drakesmith H, Nemeth E, Ganz T. Ironing out ferroportin. Cell Metab. 2015;22(5):777–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sindrilaru A, Peters T, Wieschalka S, et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest. 2011;121(3):985–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lundvig DM, Immenschuh S, Wagener FA. Heme oxygenase, inflammation, and fibrosis: the good, the bad, and the ugly? Front Pharmacol. 2012;3:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cairo G, Bernuzzi F, Recalcati S. A precious metal: Iron, an essential nutrient for all cells. Genes Nutr. 2006;1(1):25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muckenthaler MU, Rivella S, Hentze MW, Galy B. A red carpet for iron metabolism. Cell. 2017;168(3):344–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Markolovic S, Wilkins SE, Schofield CJ. Protein hydroxylation catalyzed by 2-oxoglutarate-dependent oxygenases. J Biol Chem. 2015;290(34):20712–20722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341(10):738–746. [DOI] [PubMed] [Google Scholar]
- 23.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453(7193):314–321. [DOI] [PubMed] [Google Scholar]
- 24.Donovan A, Lima CA, Pinkus JL, et al. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005;1(3):191–200. [DOI] [PubMed] [Google Scholar]
- 25.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Förster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8(4):265–277. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Z, Zhang F, An P, et al. Ferroportin1 deficiency in mouse macrophages impairs iron homeostasis and inflammatory responses. Blood. 2011;118(7):1912–1922. [DOI] [PubMed] [Google Scholar]
- 27.Kautz L, Jung G, Valore EV, Rivella S, Nemeth E, Ganz T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat Genet. 2014;46(7):678–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kroner A, Greenhalgh AD, Zarruk JG, Passos Dos Santos R, Gaestel M, David S. TNF and increased intracellular iron alter macrophage polarization to a detrimental M1 phenotype in the injured spinal cord. Neuron. 2014;83(5):1098–1116. [DOI] [PubMed] [Google Scholar]
- 29.Vinchi F, Costa da Silva M, Ingoglia G, et al. Hemopexin therapy reverts heme-induced proinflammatory phenotypic switching of macrophages in a mouse model of sickle cell disease. Blood. 2016;127(4):473–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496(7446):445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicolas G, Bennoun M, Porteu A, et al. Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proc Natl Acad Sci USA. 2002;99(7):4596–4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Du X, She E, Gelbart T, et al. The serine protease TMPRSS6 is required to sense iron deficiency. Science. 2008;320(5879):1088–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Folgueras AR, de Lara FM, Pendas AM, et al. Membrane-bound serine protease matriptase-2 (Tmprss6) is an essential regulator of iron homeostasis. Blood. 2008;112(6):2539–2545. [DOI] [PubMed] [Google Scholar]
- 34.Hasegawa S, Harada K, Morokoshi Y, Tsukamoto S, Furukawa T, Saga T. Growth retardation and hair loss in transgenic mice overexpressing human H-ferritin gene. Transgenic Res. 2013;22(3):651–658. [DOI] [PubMed] [Google Scholar]
- 35.Ganz T. Systemic iron homeostasis. Physiol Rev. 2013;93(4):1721–1741. [DOI] [PubMed] [Google Scholar]
- 36.Kaur S, Raggatt LJ, Batoon L, Hume DA, Levesque JP, Pettit AR. Role of bone marrow macrophages in controlling homeostasis and repair in bone and bone marrow niches. Semin Cell Dev Biol. 2017;61:12–21. [DOI] [PubMed] [Google Scholar]
- 37.Corna G, Campana L, Pignatti E, et al. Polarization dictates iron handling by inflammatory and alternatively activated macrophages. Haematologica. 2010;95(11): 1814–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ploeger DT, Hosper NA, Schipper M, Koerts JA, de Rond S, Bank RA. Cell plasticity in wound healing: paracrine factors of M1/M2 polarized macrophages influence the phenotypical state of dermal fibroblasts. Cell Commun Signal. 2013;11(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu Y, Tian H, Hong S. Novel 14,21-dihydroxy-docosahexaenoic acids: structures, formation pathways, and enhancement of wound healing. J Lipid Res. 2010;51(5):923–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127(3):526–537. [DOI] [PubMed] [Google Scholar]
- 41.Pagani A, Nai A, Corna G, et al. Low hepcidin accounts for the proinflammatory status associated with iron deficiency. Blood. 2011;118(3):736–746. [DOI] [PubMed] [Google Scholar]
- 42.Roy CN, Custodio AO, de Graaf J, et al. An Hfe-dependent pathway mediates hyposideremia in response to lipopolysac-charide-induced inflammation in mice. Nat Genet. 2004;36(5):481–485. [DOI] [PubMed] [Google Scholar]
- 43.Wang L, Johnson EE, Shi HN, Walker WA, Wessling-Resnick M, Cherayil BJ. Attenuated inflammatory responses in hemochromatosis reveal a role for iron in the regulation of macrophage cytokine translation. J Immunol. 2008;181(4):2723–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Costa da Silva M, Breckwoldt MO, Vinchi F, et al. Iron induces anti-tumor activity in tumor-associated macrophages. Front Immunol. 2017;8:1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gan ZS, Wang QQ, Li JH, Wang XL, Wang YZ, Du HH. Iron reduces M1 macrophage polarization in RAW264.7 macrophages associated with inhibition of STAT1. Mediators Inflamm. 2017;2017:8570818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gozzelino R, Jeney V, Soares MP. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol. 2010;50:323–354. [DOI] [PubMed] [Google Scholar]
- 47.Ackerman Z, Seidenbaum M, Loewenthal E, Rubinow A. Overload of iron in the skin of patients with varicose ulcers. Possible contributing role of iron accumulation in progression of the disease. Arch Dermatol. 1988;124(9):1376–1378. [PubMed] [Google Scholar]
- 48.Corna G, Caserta I, Monno A, et al. The repair of skeletal muscle requires iron recycling through macrophage ferroportin. J Immunol. 2016;197(5):1914–1925. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.