Abstract
Purpose:
Multicentric Castleman disease (MCD) is a distinct lymphoproliferative disorder characterized by inflammatory symptoms, lymphadenopathy, splenomegaly, and cytopenia. Kaposi’s sarcoma-associated herpesvirus (KSHV), also called human herpesvirus-8 (HHV-8), is the cause of virtually all cases of MCD occurring in patients with HIV infection. MCD lesions characteristically contain HHV-8-infected polyclonal IgMλ plasmablasts. A high frequency of HHV-8-related non-Hodgkin lymphoma has been reported in these patients.
Patients and methods:
We now report on three patients who presented with severe symptoms of MCD, extreme splenomegaly, and rapid expansion of B-cell lymphocytosis (44–81%) attributable to circulating HHV-8 positive plasmablasts.
Results:
The circulating plasmablastic cells shared the phenotype (IgMλ, CD19+, CD20− CD138−) of HHV-8-infected cells from MCD lesions, mimicking the leukemic phase of large B-cell lymphoma occurring in HHV-8-related MCD. These patients displayed a very high HHV-8 viral load in blood (>7 logs HHV-8 DNA copies/ml) and high levels of serum vIL-6, the viral homolog of human interleukin 6. Serum IL-6 and IL-10 were also abnormally elevated. HHV-8-infected cells were demonstrated by immunoglobulin gene rearrangement analysis, to be polyclonal and likely represent an expansion of HHV-8-infected cells similar to those found in MCD lesions.
Conclusion:
Thus, the spectrum of HHV-8-related plasmablastic lymphoproliferative disorders in patients with HIV infection is expanded to include HHV-8+ polyclonal IgMλ B-cell lymphocytosis. At onset, this lymphoproliferative disorder may mimic plasmablastic leukemia/lymphoma. Recognizing this unusual complication may have important implications in treatment decision avoiding unnecessary toxicity to the patients.
Keywords: multicentric Castleman disease, HIV, human herpesvirus-8, lymphoma
Human herpesvirus-8 (HHV-8) associated multicentric Castleman disease (MCD) is a distinct lymphoproliferative disorder associated with inflammatory symptoms and cytopenia. Spontaneous remission after a flare may occur, but rapid and fatal evolution toward multiple organ failure and hemophagocytic syndrome is still a frequent cause of death in these patients (1,2). A high incidence of aggressive lymphoma has also been reported in patients with MCD. Approximatelyone-half of these lymphomas are primary effusion lymphoma (PEL), either classical or solid PEL often associated with dual HHV-8 and EBV infection. The remainder are large cell lymphoma with plasmablastic differentiation and are associated with HHV-8 but usually not with EBV infection (3).
The HHV-8-positive cells in MCD morphologically resemble plasmablasts. They typically appear as isolated cells in the mantle zone but may coalesce to form ‘microlymphoma’ and in some cases frank HHV-8-positive plasmablastic large B-cell lymphoma. The term ‘microlymphoma’ is rather controversial as it is used for lesions suggestive of focal lymphoma but that have been shown to be polyclonal in most cases studied (4). Phenotypically, the HHV-8-positive cells from MCD lesions resemble preterminally differentiated plasmablasts which remarkably express a restricted IgMλ phenotype but have been shown to be polyclonal (4,5). Characteristically, a large proportion of these cells express viral IL-6, the viral homolog of human IL6, associated with the lytic cycle of the virus (6,7).
During MCD flares, levels of HHV-8 DNA detected in blood usually rise substantially, often up to 5–6 logs copies/mL (8). However, only a few HHV-8-positive cells, usually <1%, are detected in blood, and B-cell lymphocytosis is uncommon.
We report on three patients with symptoms indistinguishable to those of MCD flares associated with a rapid expansion of HHV-8-positive plasmablasts in the circulation mimicking the leukemic phase of a HHV-8-related plasmablastic large B-cell lymphoma. Despite this similarity, these cells were polyclonal and resemble the HHV-8-infected cells in MCD lesions.
Methods
Pathology and immunostaining
Lymph nodes and bone marrow biopsies were formalin fixed and paraffin embedded. For histology, tissues sections were stained with Hematoxylin-Eosin-Safran. Immunohistochemical stainings were performed after deparaffinization and appropriate antigen retrieval, using the Ventana NexES (Ventana Medical Systems, Tucson, AZ, USA) according to the manufacturer’s instructions. Antibodies used against B-cell markers include the following: CD20, CD138, immunoglobulin γ, μ, κ, and λ chains (DakoCytomation, Glostrup, Denmark); a CD3 polyclonal antibody was used to stain T cells (DakoCytomation). HHV-8 was detected with antibody to latent-associated nuclear antigen protein (Clone 13B10; Novocastra, Newcastle, UK). Bound antibodies were visualized by streptavidin-biotin detection as previously described (9). The presence of EBV was detected by in situ hybridization using specific EBERs probes (Inform Probes, Ventana Medical Systems).
Blood and bone marrow smears were stained with May-Grümwald-Giemsa. Immunophenotypic analysis of blood cells was performed by flow cytometry (Canto II Becton Dickinson) using antibodies to CD3, CD4, CD8, CD5, CD19, CD20, CD79a, CD23, CD38, CD138, CD45, HLA-DR, and the immunoglobulin μ, δ, γ, α, κ, and λ chains. Viral IL6 expression was assessed using the R394 monoclonal rabbit antibody as previously described (6).
Clonality determination
Peripheral blood mononuclear cells and biopsy specimen were obtained at the time of diagnosis. DNA was extracted from frozen material; DNA quality was assessed using a control gene primers set. Immunoglobulin gene rearrangement profiles were evaluated by polymerase chain reaction (PCR) using standardized BIOMED-2 protocols. Patient samples were evaluated in duplicate using the BIOMED-2 multiplex PCR tubes with multiple labeled primer sets: complete VDJ IgH PCR (FR1 and FR2), IgKappa (Vk-Jk and Kde), and IgLambda. All primers sequences and the PCR procedures were previously described in van Dongen et al.(10). Clonal, polyclonal, and other controls were included. Due to the limited size of the rearranged IgLambda junctional region, polyclonal rearrangements can exhibit an oligoclonal pattern due to the limited junctional diversity. To distinguish rearrangement patterns, we run PCR using heteroduplex PAGE analysis and GeneScanning of labeled PCR products.
HHV-8, EBV, and cytokine quantification in blood
Detection of HHV-8 and EBV DNA sequences was performed in DNA extracted from whole blood using primer sets and PCR amplification as previously described (2).
Serum vIL-6 levels were measured by ELISA as previously described using the vIL-6-specific mouse monoclonal antibody (v6 m 31.2.4) for coating (11). Serum IL-6 and IL-10 levels were measured by Quantikine ELISA (R&D Systems) according to the manufacturer’ s instructions.
Results
Case reports
Three patients with HIV infection were referred to our hospital with the classical clinical features of MCD, including severe inflammatory symptoms, massive splenomegaly, and cytopenia. Salient patient characteristics are reported in Table 1.
Table 1.
Selected clinical and biological characteristics
| At diagnosis | Patient 1 | Patient 2 | Patient 3 |
|---|---|---|---|
| Sex/Age | M/43 | M/61 | M/44 |
| Ethnicity | African | Caucasian | African |
| CD4+ cell count (×106/L) | 160 | 150 | 169 |
| Plasma HIV-RNA (copies/mL) | <20 | 963 | 5330 |
| Lymphadenopathy | + | ++ | ++ |
| Splenomegaly | 16 cm | 21 cm | 20 cm |
| Kaposi sarcoma | - | - | - |
| WBC (× 106/L) | 5800 | 2700 | 31 500 |
| Hemoglobin (g/dL) | 10.2 | 7.4 | 6.7 |
| Platelet count (×106/L) | 40 000 | 23 000 | 34 000 |
| LDH | 3.5 N | N | 10 N |
| CRP (mg/L) | 162 | 188 | 212 |
| Albumin (g/L) | 30.6 | 22 | 18.2 |
| Gammaglobulin (g/L) | 24.7 | 13.2 | 25.6 |
| Ferritin | 14 N | 9N | 13 N |
| Blood | |||
| Lymphoid cells (× 106/L) | 2400 | 1100 | 8650 |
| % B cells | 81 | 74 | 44 |
| B-cellphenotype | CD19+, 20−, 5−, 23−, 138− IgM+ IgD-lambda+ | CD19+, 20−, 5−, 23−, 138− IgM+ IgD-lambda+ | CD19+, 20−, 5−, 23−, 138− IgM+ IgD-lambda+ |
| Ig gene Rearrangement | Polyclonal | Polyclonal | Polyclonal |
| HHV-8 DNA (copies/mL) | 7.51 logs | 7.74 logs | >8.1 logs |
| EBV DNA (copies/mL) | nd | 4.68 logs | 4.71 logs |
| Bone marrow | Hemophag-ocytosis | Hemophag-ocytosis | Hemophag-ocytosis |
| Plasma cells (%) | 27 | 16 | 23 |
| Ig gene Rearrangement | nd | Polyclonal | Polyclonal |
| HHV-8 DNA (copies/mL) | >8.1 logs | 7.45 logs | nd |
| EBV-DNA (copies/mL) | 3.85 logs | 5.05 logs | nd |
| Lymph node Biopsy | nd | Castleman disease, Kaposi sarcoma | Castleman disease |
| Last follow-up (months) | 25 | 14 | 6 |
| CD4+ cell count (× 106/L) | 323 | 397 | 320 |
| Plasma HIV-RNA (copies/mL) | <20 | 99 | 820 |
| CD19+ B cells (%) | 7 | 19 | 0 |
| Blood HHV-8 DNA (copies/mL) | 2.75 logs | <2 logs | <2 logs |
WBC, white blood cells; LDH, serum lactic dehydrogenase; CRP, C reactive protein; Ig, immunoglobulin; N, normal value; nd, not done.
Patient 1, a 43-year-old man of African ancestry with asymptomatic HIV infection, had been treated for 5 months prior to admission with combined antiretroviral therapy for severe immunodeficiency. He was referred because of fever, weight loss, hepatomegaly, and splenomegaly. A bone marrow biopsy revealed plasmacytosis (27% plasma cells), presence of HHV-8-positive cells, and hemophagocytosis. High HHV-8 viral load was detected in the blood. The patient was initially treated with tocilizumab, an anti-IL6 receptor monoclonal antibody, and etoposide. The subsequent detection of large numbers of circulating HHV-8-positive plasmablastic cells with restricted IgMλ expression suggested malignant lymphoma. The patient received six cycles of R-CHVpP (rit-uximab, cyclophosphamide, doxorubicin, etoposide, prednisone) and achieved complete clinical remission. Remission is sustained 25 months after completion of treatment.
Patient 2, a 61-year-old Caucasian man diagnosed with HIV infection, had been treated for 2 months prior to admission with combined antiretroviral therapy for constitutional symptoms and low CD4+ cell count. He was referred because of fever, lymphadenopathy, rash, drowsiness, and splenomegaly. A bone marrow biopsy showed plasmacytosis (16% plasma cells) and hemophagocytosis. A lymph node biopsy revealed HHV8-associated Castleman disease and foci of Kaposi sarcoma. The detection of a large number of circulating HHV-8-positive plasmablastic cells with a restricted IgMλ expression associated with high HHV-8 viral load in the blood suggested malignant lymphoma. The patients received four cycles of R-CHVpP and achieved complete clinical remission. Remission is sustained 14 months after completion of treatment.
Patient 3, a 44-year-old man of African ancestry, was diagnosed with AIDS 4 years prior to the admission with poor compliance with therapy. Combined antiretroviral therapy was restarted 3 weeks prior to referral because of fever, weight loss, cough, lymphadenopathy, hepatomegaly, and splenomegaly. A lymph node biopsy revealed HHV8-associated Castleman disease with numerous HHV-8-positive cells. Etoposide was started with initial response followed 2 weeks later by an acute flare-up associated with massive splenomegaly and the presence of circulating HHV-8-positive plasmablastic cells with restricted expression of IgMλ. The patient received a cycle of etoposide, cyclophosphamide, and rituximab followed by three cycles of rituximab and etoposide and achieved remission. Remission is sustained 6 months after completion of treatment.
Lymph node and bone marrow biopsies
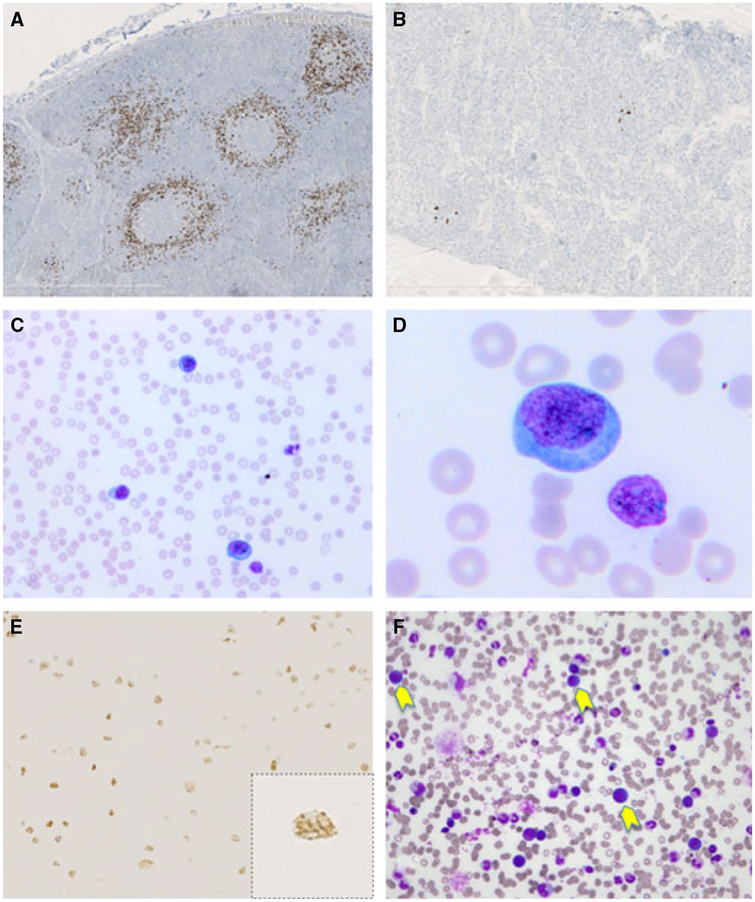
A lymph node biopsy of patient 2 showed hyalinized atrophic follicles and plasmacytosis associated with vascular hyperplasia in the interfollicular areas. HHV-8 immunostaining was positive on several isolated plasmablasts in the follicle. These cells expressed IgMλ. A pretreatment lymph node biopsy of patient 3 revealed a mixture of hyperplastic and atrophic follicles with intense plasma cell infiltration and vascular hyperplasia in the interfollicular areas; numerous HHV-8-positive cells were observed in the mantle zone area with some coalescence consistent with a diagnosis of microlymphoma (Fig. 1A). A post-treatment lymph node biopsy in this patient showed only atrophic follicles, with persistent plasma cell infiltration and interfollicular vascular hyperplasia; HHV-8-positive cells were sparse and localized in the follicles (Fig. 1B). EBV detection by EBER in situ hybridization revealed only a few positive cells, distinct from the HHV-8 positive plasmablasts.
Figure 1.
Multicentric Castleman disease and HHV-8-positive polyclonal IgMλ B-cell lymphocytosis (patient 3). The two lymph node biopsies (before and after treatment) show typical features of multicentric Castleman disease. Immunostaining with the HHV-8 LNA-1-specific monoclonal antibody shows numerous, partly coalescent HHV-8-positive plasmablastic cells in the initial biopsy (A) and only a few HHV-8-positive cells in the second post-treatment biopsy (B). The blood smear shows numerous plasmablasts (C,D); the circulating plasmablastic cells stain positive for HHV-8 with the typical nuclear dotlike staining in brown (E). Bone marrow smear reveals the presence of large plasmablastic cells (yellow arrows) (F).
Bone marrow biopsies in patients 1 and 2 revealed plasmacytosis and hemophagocytosis.
Bone marrow smears and blood lymphocytes
Bone marrow smears revealed the presence of large lymphoid cells resembling plasmablastic cells (Fig. 1F). Blood smears showed a normal distribution of lymphocytes in patients 1 and 2, but showed lymphocytosis with numerous large, plasmablastic cells in patient 3 (Fig. 1C,D). Blood samples from all three patients revealed B-cell lymphocytosis (44–81%) with the typical phenotype of the HHV-8-infected plasmablasts in MCD lesions. These cells were CD19+ and expressed IgMλ, whereas expression of CD20 and CD138 was low/absent (Fig. 2A). Immunocytochemistry confirmed that a large proportion of circulating lymphocytes were HHV-8 positive (Fig. 1E) and that a proportion of them expressed vIL6.
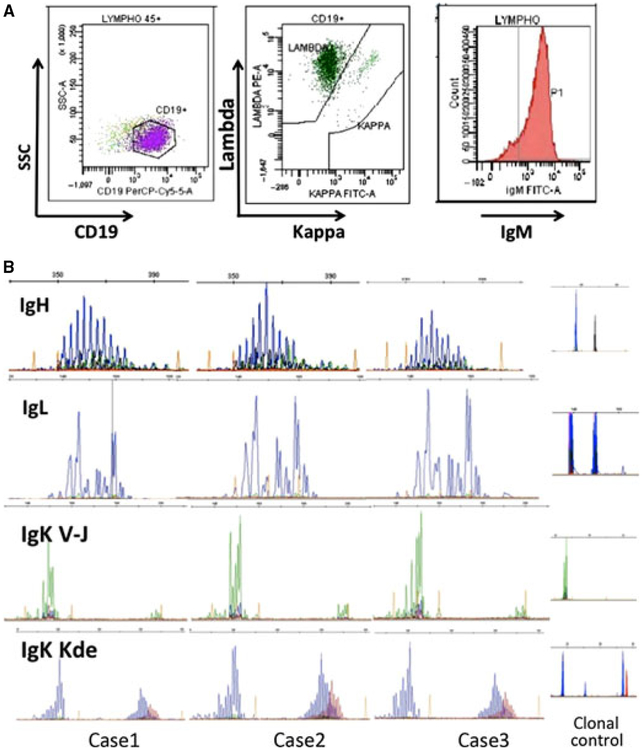
Figure 2.
Flow cytometric analysis in patient 3 shows that circulating lymphocytes are mainly CD19+B cells (81%) with high expression of surface IgM and restricted lambda phenotype (99%) (A). IgH, IgLambda, and IgKappa (VkJk and Kde) clonality profiles from the diagnostic samples suspected of Bcell clonal proliferation show a clear pattern of polyclonal rearrangements. These reproducible profiles provide evidence for a polyclonal proliferation of the monotypic IgM Lambdapositive B cells (B).
Clonality assessment of circulating B cells
GeneScan fragments analysis of all PCR products for IgH, IgLambda, and IgKappa (VkJk and Kde) showed patterns typical of polyclonal Ig chain rearrangements (Fig. 2B), providing evidence for the occurrence of a polyclonal proliferation of IgM lambda-positive cells. B-cell clonality evaluated in the lymph node biopsies of patients 2 and 3 also revealed a polyclonal pattern of immunoglobulin chain rearrangements (data not shown).
Serum cytokines quantification
Viral IL-6 was detected (range 34 128–41 197 pg/mL) in serum samples of all three patients at levels similar or higher than those detected in patients with acute MCD (8,11). Human IL-6 and IL-10 levels were abnormally elevated in serum samples from all three patients: serum IL-6 ranged between 5331 and 11 626 pg/mL (normal <20 pg/mL) and serum IL-10 ranged between 19 214 and 41 197 pg/mL (normal <10 pg/mL).
Discussion
HIV-infected patients with MCD are at a high risk of developing lymphoma, unless they are treated with rituximab (12,13). About one-half of these lymphomas are reported as HHV-8-positive/EBV-negative, large plasmablastic B-cell lymphoma. Clonality assessment by Du et al. (4) revealed polyclonality in all cases of typical MCD and in six of eight lesions considered microlymphoma. By contrast, the two plasmablastic lymphomas in the study disclosed monoclonality.
In the present series, the three patients presented with classic clinical features of severe MCD dominated by systemic inflammatory symptoms and with hemophagocytic syndrome. Rapid enlargement of the spleen was noted in all three patients. Although no lymph node biopsy was obtained from patient 1, the diagnosis of MCD was considered on the basis of typical clinical and laboratory findings associated with high HHV-8 viral load in blood. An alternate diagnosis in this patient could be KSHV-related inflammatory cytokine syndrome, a syndrome that is clinically and biologically closely related to MCD but that lacks histological evidence of MCD (11). All patients presented with marked plasmacytosis in bone marrow, a common feature of HHV8-related MCD (14). The HHV-8 viral load in the circulation was unusually high (8,15), above 7 logs HHV-8 DNA copies per mL, and was associated with numerous HHV-8-infected cells in blood, mimicking a leukemic phase of plasmablastic lymphoma. Analysis of blood lymphocytes confirmed B-cell lymphocytosis with a phenotype typical of HHV-8-infected plasmablasts observed in MCD lesions : CD19+ IgMλ cells with low or absent expression of CD20 and no expression of CD138 (16).
Because of the typical IgMλ restriction of HHV-8-infected cells in MCD lesions, phenotypic analysis failed to discriminate between a neoplastic clonal process and a polyclonal lymphoproliferative disorder. However, the combined analysis of the IgH, IgKappa genes (Vk-Jk and Kde), and IgLamb dagene rearrangements provides strong evidence that all three patients described here had a polyclonal lymphoproliferative disorder. For IgKappa analysis, both rearrangements were evaluated. As Kde rearrangements occur in IgLambda B cells, it is expected that all mature B cells should exhibit IgKappa rearrangements, whatever the light chain expressed. In case of IgLambda B-NHL, the assessment of IgLambda status does not add information relative to B-cell clonality.
These patients required immediate intensive care and treatment (17,18). Two of the patients were initially diagnosed with an aggressive form of large plasmablastic B-cell lymphoma and were successfully treated with combined immun-ochemotherapy, whereas the third patient only received rituximab and etoposide for 4 weeks. Interestingly, in the latter patient, the pretreatment lymph node biopsy showed numerous coalescent HHV-8-infected plasmablasts, whereas a second biopsy obtained 3 days after etoposide infusion showed only a few HHV-8-infected cells within Castleman pathology. The histologic change was accompanied by a rapid clinical improvement and a marked decrease in HHV-8 viral load (3 logs) in <2 weeks.
In addition to the present series, we retrospectively reviewed three other cases previously referred to us with a diagnosis of IgMλ, HHV-8-positive, EBV-negative, plasmablastic lymphoma with bone marrow involvement, but no overt lymphocytosis. All these patients disclosed a polyclonal pattern of immunoglobulin gene rearrangement in bone marrow and/or blood suggesting a very similar disease to that described herein (data not shown). They were treated with combined chemotherapy regimens and rituximab resulting in severe toxicity in at least one patient. We therefore suggest that when a diagnosis of HHV8-related large plasmablastic B-cell lymphoma is considered, the patient should be rapidly treated with a conservative approach, waiting for clonality results. Unfortunately, we failed to find other markers than clonality assessment to distinguish these patients from patients with overt plasmablastic lymphoma. In the presence of a polyclonal proliferation, a rituximab-based therapy could be proposed, just as for patients with MCD. Despite the low/absent expression of CD20 on the HHV-8-infected plasmablasts, rituximab has been shown to be pivotal in the treatment of HHV-8-associated MCD in patients with HIV infection, both in leading to persistent remission and in reducing the risk of NHL occurrence (12,13,17–20).
The pathophysiology of HHV8-related MCD remains largely unknown. One of the most puzzling issues is the IgMλ phenotypic restriction of the HHV-8-infected cells in the absence of clonal proliferation. This restriction has been demonstrated in MCD lesions and after experimental infection of human tonsillar cells (5,21). This phenotype is not observed in HHV-8-infected tumor PEL cells (22). Another puzzling issue is the pathogenesis of the characteristic severe inflammatory symptoms attributable to vIL-6, IL-6, and IL-10 (8,11). In addition, these cytokines that were found at very high levels in the serum of the three patients may also be implicated in the growth and/or differentiation of the HHV-8-infected B cells (23). A third issue is the relationship between HHV-8 latency and viral replication in the plasmablastic cells, which express features of both viral latency and replication suggesting that MCD may combine features of the virus tumorigenic potential and infectious nature (6). This complexity has already been observed in EBV-associated post-transplant lymphoproliferative disorders (PTLD) where most cells express a ‘latency’ growth program but a significant proportion of cells replicate EBV (24). In contrast to PTLD, HHV8-related MCD may occur in patients with no severe immune deficiency. In patients with HIV infection, MCD is observed with a median CD4+ T-cell count around 200 × 106/L, and all three patients from the present series had more than 150 × 106/L CD4+ T cells (13,18). In the absence of HIV infection, HHV8-related MCD appears to be very similar in clinical, biological, and pathological characteristics to that observed in patients with HIV infection (25).
Despite the hematological presentation mimicking aggressive plasmablastic leukemia/lymphoma, phenotypic and clonality analysis of circulating cells suggests that the patients described here reflect a marked expansion of the HHV-8-infected plasmablasts from MCD lesions and their release to the peripheral blood. The pathogenesis of this very unusual presentation remains unknown, but may represent an extreme phenotype of MCD, extending the spectrum of HHV8-related polyclonal lymphoproliferative disorders.
Acknowledgements
The authors thank Guislaine Carcelain and Muriel Hourseau.
Footnotes
Disclosure of conflict of interest
GT is a co-inventor on a patent describing the measurement of KSHV vIL-6. This invention was made when GT was an employee of the US Government under 45 Code of Federal Regulations Part 7. All rights, title, and interest to this patent have been assigned to the US Department of Health and Human Services. The government conveys a portion of the royalties it receives to its employee inventors under the Federal Technology Transfer Act of 1986 (P.L. 99–502). GT is supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research. The remaining authors declare no competing financial interests.
References
- 1.Oksenhendler E, Duarte M, Soulier J, Cacoub P, Welker Y, Cadranel J, Cazals-Hatem D, Autran B, Clauvel JP, Raphael M. Multicentric Castleman’s disease in HIV infection: a clinical and pathological study of 20 patients. Aids 1996;10:61–7. [PubMed] [Google Scholar]
- 2.Soulier J, Grollet L, Oksenhendler E, et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood 1995;86:1276–80. [PubMed] [Google Scholar]
- 3.Oksenhendler E, Boulanger E, Galicier L, et al. High incidence of Kaposi sarcoma-associated herpesvirus-related non-Hodgkin lymphoma in patients with HIV infection and multicentric Castleman disease. Blood 2002;99:2331–6. [DOI] [PubMed] [Google Scholar]
- 4.Du MQ, Liu H, Diss TC, Ye H, Hamoudi RA, Dupin N, Meignin V, Oksenhendler E, Boshoff C, Isaacson PG. Kaposi sarcoma-associated herpesvirus infects monotypic (IgM lambda) but polyclonal naive B cells in Castleman disease and associated lymphoproliferative disorders. Blood 2001;97:2130–6. [DOI] [PubMed] [Google Scholar]
- 5.Dupin N, Diss TL, Kellam P, Tulliez M, Du MQ, Sicard D, Weiss RA, Isaacson PG, Boshoff C. HHV-8 is associated with a plasmablastic variant of Castleman disease that is linked to HHV-8-positive plasmablastic lymphoma. Blood 2000;95:1406–12. [PubMed] [Google Scholar]
- 6.Parravicini C, Chandran B, Corbellino M, Berti E, Paulli M, Moore PS, Chang Y. Differential viral protein expression in Kaposi’s sarcoma-associated herpesvirus-infected diseases: Kaposi’s sarcoma, primary effusion lymphoma, and multicentric Castleman’s disease. Am J Pathol 2000;156:743–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aoki Y, Tosato G, Fonville TW, Pittaluga S. Serum viral interleukin-6 in AIDS-related multicentric Castleman disease. Blood 2001;97:2526–7. [DOI] [PubMed] [Google Scholar]
- 8.Oksenhendler E, Carcelain G, Aoki Y, Boulanger E, Maillard A, Clauvel JP, Agbalika F. High levels of human herpesvirus 8 viral load, human interleukin-6, interleukin-10, and C reactive protein correlate with exacerbation of multicentric castleman disease in HIV-infected patients. Blood 2000;96:2069–73. [PubMed] [Google Scholar]
- 9.Dupin N, Fisher C, Kellam P, et al. Distribution of human herpesvirus-8 latently infected cells in Kaposi’ s sarcoma, multicentric Castleman’ s disease, and primary effusion lymphoma. Proc Natl Acad Sci USA 1999;96:4546–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Dongen JJ, Langerak AW, Briiggemann M, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 concerted action BMH4-CT98–3936. Leukemia 2003;17:2257–317. [DOI] [PubMed] [Google Scholar]
- 11.Uldrick TS, Wang V, O’Mahony D, et al. An interleukin-6-related systemic inflammatory syndrome in patients coinfected with Kaposi sarcoma-associated herpesvirus and HIV but without multicentric Castleman disease. Clin Infect Dis 2010;51:350–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffmann C, Schmid H, Muller M, et al. Improved outcome with rituximab in patients with HIV-associated multicentric Castleman disease. Blood 2011;118:3499–503. [DOI] [PubMed] [Google Scholar]
- 13.Gerard L, Michot J-M, Burcheri S, et al. Rituximab decreases the risk of lymphoma in patients with HIV-associated multicentric Castleman disease. Blood 2012;119:2228–33. [DOI] [PubMed] [Google Scholar]
- 14.Venkataraman G, Uldrick TS, Aleman K, et al. Bone marrow Findings in HIV-positive patients with Kaposi sarcoma herpesvirus-associated multicentric Castleman disease. Am J Clin Pathol 2013;139:651–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tedeschi R, Marus A, Bidoli E, Simonelli C, De Paoli P. Human herpesvirus 8 DNA quantification in matched plasma and PBMCs samples of patients with HHV8-related lymphoproliferative diseases. J Clin Virol 2008;43:255–9. [DOI] [PubMed] [Google Scholar]
- 16.Chadburn A, Abdul-Nabi AM, Teruya BS, Lo AA. Lymphoid proliferations associated with human immunodeficiency virus infection. Arch Pathol Lab Med 2013;137:360–70. [DOI] [PubMed] [Google Scholar]
- 17.Oksenhendler E HIV-associated multicentric Castleman disease. Curr Opin HIV AIDS 2009;4:16–21. [DOI] [PubMed] [Google Scholar]
- 18.Bower M How i treat HIV-associated multicentric Castleman disease. Blood 2010;116:4415–21. [DOI] [PubMed] [Google Scholar]
- 19.Gerard L, Berezne A, Galicier L, et al. Prospective study of rituximab in chemotherapy-dependent human immunodeficiency virus associated multicentric Castleman’ s disease: ANRS 117 CastlemaB trial. J Clin Oncol 2007;25:3350–6. [DOI] [PubMed] [Google Scholar]
- 20.Bower M, Powles T, Williams S, et al. Brief communication: rituximab in HIV-associated multicentric Castleman disease. Ann Intern Med 2007;147:836–9. [DOI] [PubMed] [Google Scholar]
- 21.Hassman LM, Ellison TJ, Kedes DH. KSHV infects a subset of human tonsillar B cells, driving proliferation and plasmablast differentiation. J Clin Invest 2011;121:752–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Du MQ, Bacon CM, Isaacson PG. Kaposi sarcoma-associated herpesvirus/human herpesvirus 8 and lymphoproliferative disorders. J Clin Pathol 2007;60:1350–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones KD, Aoki Y, Chang Y, Moore PS, Yarchoan R, Tosato G. Involvement of interleukin-10 (IL-10) and viral IL-6 in the spontaneous growth of Kaposi’s sarcoma herpesvirus-associated infected primary effusion lymphoma cells. Blood 1999;94:2871–9. [PubMed] [Google Scholar]
- 24.Montone KT, Hodinka RL, Salhany KE, Lavi E, Rostami A, Tomaszewski JE. Identification of Epstein-Barr virus lytic activity in post-transplantation lymphoproliferative disease. Mod Pathol 1996;9:621–30. [PubMed] [Google Scholar]
- 25.Dossier A, Meignin V, Fieschi C, Boutboul D, Oksenhendler E, Galicier L. Human herpesvirus 8-related Castleman disease in the absence of HIV infection. Clin Infect Dis 2013;56: 833–42. [DOI] [PubMed] [Google Scholar]