Sickle-cell disease (SCA) is a hereditary disorder associated with a homozygous point mutation in the β-globin gene that leads to the production of hemoglobin S (HbS) and the development of chronic hemolytic anemia. Chronic kidney disease (CKD) is one of the most serious organ-specific complications of SCA. Proteinuria and microalbuminuria can be under-detected in SCA because of the renal concentrating defect [1]. We hypothesized that a urinary biomarker reflecting the ongoing biological process leading to kidney injury in SCA will be more specific for the early detection of CKD than proteinuria. Hemolysis contributes to the induction of the chronic inflammation in SCA that potentially contributes to the clinical manifestation of renal disease. Intravascular hemolysis leads to hemoglobinuria, which has been associated with progression of CKD [2]. We recently performed differential mass-spectrometry analysis of urine proteins in SCA patients who did not have proteinuria and microalbuminuria, and found increased levels of orosomucoid (ORM) in the samples from patients with hemoglobinuria [3]. ORM is a major acute phase protein expressed in the liver and secreted into the circulation. Tissue injury, inflammation, infection, and cancer are associated with increased ORM expression and serum concentration. ORM has been detected in urine samples from patients with a variety of renal diseases including diabetic nephropathy [4] and systemic lupus erythematosus associated renal disease [5]. Recently ORM was included in a group of urinary biomarkers for the differential diagnosis of glomerular disease [6]. However, to the best of our knowledge, urinary ORM has not been assessed in SCA patients with CKD. In the present study, ORM levels were analyzed by quantitative label-free proteomics and ELISA in SCA urine samples with hemoglobinuria and compared to urine samples without hemoglobinuria; these urine samples had normal albumin levels (< 30mg/g creatinine). ORM levels were further analyzed by ELISA in a cohort of 34 SCA patients and correlated with CKD stage. Overall our results suggest that urinary ORM may represent a non-invasive diagnostic biomarker for renal disease in SCA patients before the onset of proteinuria.
The study was approved by the institutional review board (IRB) of the University of Illinois at Chicago (UIC) and all subjects provided written informed consent prior to urine sample collection. Fifty four patients (all HbSS) were recruited, and urine samples were collected during a clinic visit when the patients were in a steady state (Supplemental Table S1). Of the 54 SCA patient samples, twenty samples (40% females) with undetectable urinary protein levels analyzed by Siemens Multistix x10SG reagent strips were selected for mass-spectrometry analysis. Creatinine (CRE, Parameter Assay kit, R&D Systems) and hemoglobin (Hgb ELISA kit, Abcam) levels were measured by ELISA kits. Samples were further separated into two groups: (i) samples with increased urinary hemoglobin (Hgb/CRE>0.8 ng/mg, N=6), and (ii) samples with low urinary hemoglobin (Hgb/CRE<0.45 ng/mg, N=14) [3]. We conducted high resolution FT-MS/MS tandem mass spectrometry of twenty samples in triplicate and a spectra analysis was perfomed with Proteome Discoverer 1.4 software, ([3] and also supplementary Methods).
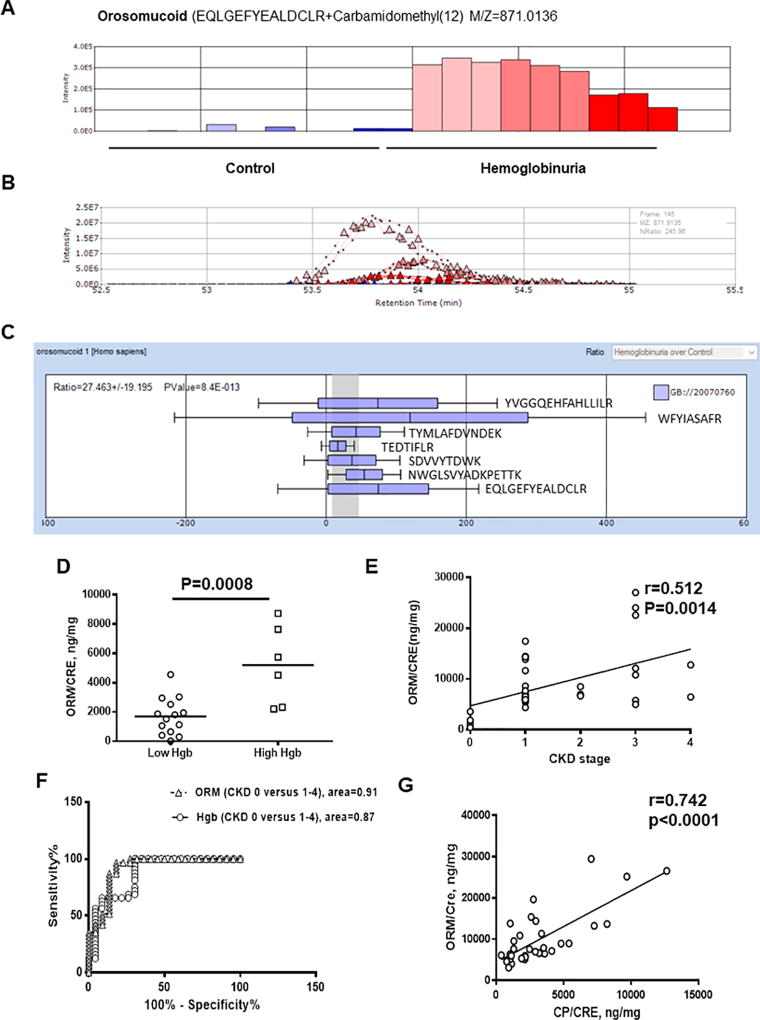
Our recent comparative proteomic analysis demonstrated increased levels of ORM in urine samples from SCA patient with Hgb/CRE >0.8 ng/mg [3]. We selected three samples from a group with Hgb/CRE >0.8 ng/mg and a group with Hgb/CRE <0.45 ng/mg based on the reproducibility among triplicates and performed label-free quantitative analysis using SIEVE 2.1 software to quantify ORM levels (Fig.1A–C, N=3 per group). Because samples that we used for proteomics analysis showed low specific gravity, reflecting a high level of urine dilution, we normalized each sample by urine CRE value using a built-in normalization tool in SEIVE 2.1. Overall, we observed good replicates (Fig.1A–B) and detected seven ORM–derived peptides (Fig. 1C). Compared to the samples with Hgb/CRE <0.45 ng/mg, ORM levels were upregulated in the samples with Hgb/CRE >0.8 ng/mg for all detected peptides (Fig.1A–C, 27.5±19.2-fold, p=8.4×10−13). Increased urine ORM levels were validated by ELISA (ORM1 ELISA kit, AssayPro) and also showed increased levels in the samples with high Hgb/CRE levels thus confirming mass spectrometry results (Fig.1D, 1697±336.9 ng/mg in control samples versus 5192±1102 ng/mg in samples with hemoglobinuria, p=8×10−4). To test whether increased urine ORM levels correlated with CKD stage, we analyzed urine ORM levels in 34 additional samples obtained from SCD patients at various stages of CKD ranging from 0 to 4/5 (stage 0 – 3 patients, stage 1 – 19 patients, stage 2 – 3 patients, stage 3 – 7 patients, and stage 4/5 – 2 patients). The estimated glomerular filtration rate (eGFR) was calculated as described [2]. Albuminuria was defined as the ratio of urinary albumin to creatinine (AL/CRE). CKD stage was defined at the time of sample collection according to the National Kidney Foundation, Kidney Disease Outcomes Quality Initiatives (K/DOQI) guidelines: stage 0 – eGFR>60 ml/min/1.73m2 and AL/CRE<30 mg/g; stage 1- eGFR>90 ml/min/1.73m2 and AL/CRE≥30 mg/g; stage 2 – eGFR 60–89 ml/min/1.73m2 and AL/CRE≥30 mg/g, stage 3 – eGFR 30 – 59 /1.73m2; stage 4 – eGFR 15 – 29 ml/min/1.73m2 and stage 5 – eGFR < 15 ml/min/1.73m2 (https://www.kidney.org/sites/default/files/docs/ckd_evaluation_classification_stratification.pdf). We observed positive correlation between increased urinary ORM/CRE levels and more advanced CKD stage (Fig.1E, N=34, r=0.51, R2=0.2627, p=0.0014). ROC analysis showed high sensitivity and specificity for urinary ORM (area under curve 91.4±4.4%; p<0.0001, sensitivity 87.1%, specificity 86.6%) similar to urinary hemoglobin (area under curve 87.0±5.2%; p<0.0001, sensitivity 68.75%, specificity 69.77%) in samples with CKD stage 1–4/5 versus CKD stage 0 (Fig.1F). Recently we identified ceruloplasmin (CP) as a potential biomarker of CKD associated with abnormal iron metabolism [3]. ORM levels correlated positively with urinary CP levels (Fig.1G, N=34, r=0.7417, R2=0.55, p<0.0001). Increased levels of urine ORM and CP have been found to be a risk predictor for diabetic nephropathy [4].
Figure 1. Urinary orosomucoid (ORM) levels correlated with progressive CKD stages.
(A–B). Label-free quantitative analysis of ORM in urine. High resolution MS spectra produced by Orbitrap MS scans were quantified by SIEVE 2.1 software. Panel A shows average intensities for EQLGEFYEALDCLR peptide are derived from integration of ion elution profiles shown in panel ORM levels detected in the samples with hemoglobinuria are shown in red colors, and in control samples – in blue colors. Panel B shows ion elution profiles for ORM peptide. Triangles indicate the time points at which MS/MS was conducted. (C). Average intensities for seven ORM peptides detected in urine samples and calculated by SIEVE 2.1. Mean and standard deviations are shown. Peptides sequences are indicated. (D) Quantification of urinary orosomucoid (ORM) performed by ELISA for 20 samples with undetected proteinuria. ORM to creatinine (CP/CRE) ratio is shown. Means are shown. Each dot represents a value obtained from individual subject. (E) Correlation analysis of ORM/CRE ratios with stages of CKD. Pearson correlation analysis was performed using GraphPad software in 34 SCD patients with different stages of CKD (0–4). (F) ROC analysis of urinary ORM/CRE and Hgb/CRE shown for urine samples from SCD patients with stage 0 CKD (23 patients) versus stage 1–4 CKD (31 patients). The analysis was performed using GraphPad software. (G) Correlation analysis of urinary ceruloplasmin (CP/CRE) and ORM/CRE ratios are shown. CP levels were measured by ELISA. Pearson correlation analysis was performed using GraphPad software.
Taken together, urinary ORM may represent a non-invasive diagnostic biomarker for renal disease in SCA patients before the onset of proteinuria and albuminuria. Further studies are needed in order to evaluate the prognostic and clinical relevance of ORM. The major limitation of our study is the relatively small size of the cross-sectional cohort. Thus our results need to be further validated in larger, longitudinal SCA patient cohorts.
Supplementary Material
Acknowledgments
This work was supported by NIH research grants P50HL118006, 1R01HL125005, 5G12MD007597 and K23HL125984.
Footnotes
CONTRIBUTORS
MJ and SN designed study, performed analysis and wrote the manuscript with the help of SLS and VRG. SLS and VRG collected samples and performed the pathological investigation. SS and NA performed ELISAs. XL performed mass-spectrometry.
References
- 1.Hatch FE, Culbertson JW, Diggs LW. Nature of the renal concentrating defect in sickle cell disease. J Clin Invest. 1967;46:336–345. doi: 10.1172/JCI105535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saraf SL, Zhang X, Kanias T, et al. Haemoglobinuria is associated with chronic kidney disease and its progression in patients with sickle cell anaemia. British journal of haematology. 2014;164:729–739. doi: 10.1111/bjh.12690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jerebtsova M, Saraf SL, Lin X, et al. Identification of ceruloplasmin as a biomarker of chronic kidney disease in urine of sickle cell disease patients by proteomic analysis. American journal of hematology. 2017 doi: 10.1002/ajh.24965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang H, Guan G, Zhang R, et al. Increased urinary excretion of orosomucoid is a risk predictor of diabetic nephropathy. Nephrology. 2009;14:332–337. doi: 10.1111/j.1440-1797.2008.01053.x. [DOI] [PubMed] [Google Scholar]
- 5.Watson L, Midgley A, Pilkington C, et al. Urinary monocyte chemoattractant protein 1 and alpha 1 acid glycoprotein as biomarkers of renal disease activity in juvenile-onset systemic lupus erythematosus. Lupus. 2012;21:496–501. doi: 10.1177/0961203311431249. [DOI] [PubMed] [Google Scholar]
- 6.Varghese SA, Powell TB, Budisavljevic MN, et al. Urine biomarkers predict the cause of glomerular disease. Journal of the American Society of Nephrology : JASN. 2007;18:913–922. doi: 10.1681/ASN.2006070767. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.