Abstract
Continuous use of anticoccidial treatments against Eimeria infections has resulted in the development of drug resistance. This study aimed to evaluate the anticoccidial efficacy of a methanolic extract derived from the endemic Canary rue (Ruta pinnata) plant of the Canary Islands, Spain, against Eimeria ninakohlyakimovae using in vitro assays. Freshly unsporulated oocysts were exposed to different concentrations of R. pinnata extract and thereafter evaluated for sporulation inhibition. Additionally, anticoccidial activity was examined by testing the viability of the E. ninakohlyakimovae sporozoites and their ability to infect bovine colonic epithelial cells after incubation with different concentrations of R. pinnata plant extract. The inhibition of oocyst sporulation by the extract was both time and concentration dependent, with certain combinations affording the same levels of sporulation inhibition as formaldehyde used as positive control (P < 0.001). Moreover, concentrations >0.1 mg/mL also affected not only the viability of the sporozoites but also their cell invasion capacity (P < 0.001). Altogether, these results show that methanolic fruit extracts from R. pinnata have important anticoccidial activity against oocysts and sporozoites of Eimeria. The potential efficacy of the extracts against other animal/human parasites remains to be elucidated, and further studies are needed to better understand its mode of action against coccidian parasites.
Keywords: anticoccidials, Eimeria ninakohlyakimovae, goat, Ruta pinnata
INTRODUCTION
Caprine coccidiosis caused by the apicomplexan genus Eimeria is one of the major diseases affecting goat kids around the weaning period (Koudela and Bokova, 1998; Ruiz et al., 2006). Control of coccidiosis is currently based on management practices combined with the use of repeated administration of either prophylactic or metaphylactic anticoccidials. However, extensive use of drugs has caused the emergence of drug-resistance in Eimeria spp. strains worldwide (Peek and Landman, 2003). Moreover, no new drugs against Eimeria have been introduced into the market for many years, and international legislation increasingly favors the abolition of the use of anticoccidial/coccidiostatic drugs for livestock destined for human nutrition (Dorne et al., 2013). Among alternative methods, the use of natural bioactive plants having antiparasitic activity has been suggested as a real possibility for the control of ruminant gastrointestinal nematodes (Waller and Thamsborg, 2004; Valderrábano et al., 2010). In this context, there are also studies showing that different plant extracts have anticoccidial activity against poultry Eimeria species (Nweze et al., 2009; Michels et al., 2011; Burt et al., 2013); however, the information available in ruminants is limited and mainly refers to in vitro methods.
Within the wide spectrum of endemic species in the Canary archipelago (Bramwell, 1998; Arechavaleta et al., 2010), the Canary rue (Ruta pinnata; family Rutaceae) is well known by local people for its medical properties. Some pictures of the plant can be found in Supplementary Figures 6–8. Interestingly, ethno-veterinary medicine studies performed in Canada have shown the frequent use of Ruta species in the traditional treatment of Giardia and Toxoplasma in pigs, cats, and dogs (Lans et al., 2007). Based on this information, we aimed to evaluate the anticoccidial effect of an extract derived from the endemic Canary rue (R. pinnata) by using different in vitro assays and experimental infections with the apicomplexan protozoa Eimeria ninakohlyakimovae.
MATERIAL AND METHODS
All animal procedures conducted in this study were approved by the University of Las Palmas de Gran Canaria Animal Welfare Committee. The animals were treated according to the guidelines adopted in the European Communities Council (Directive 2010/63/EU).
Plant Material and Extracts
Mature fruits of R. pinnata (~1.5 kg) were randomly collected from wild R. pinnata plants distributed in different geographical areas of Gran Canaria, Tenerife, and La Palma (The Canary Islands, Spain: some pictures of the plant are available on the online version of the paper). After drying at room temperature in the shade, they were homogenized and the plant material was extracted with methanol (Merck) using a Soxhlet extraction system (1.5–2.0 liters of the solvent per 70 g of dried fruits). Extraction was stopped when the solvent distillation did not show any pigmentation, ca. 24 to 36 h. Then the solvent was evaporated to dryness employing a rotavapour over a warm water bath set at 40 °C. The resulting extract was kept at room temperature in the dark until required for further use.
The extract was used at different concentrations after dissolving it in DMSO (Sigma-Aldrich). All working DMSO concentrations were previously checked to demonstrate that they did not affect the viability of the E. ninakohlyakimovae stages, unsporulated oocysts or free-released sporozoites, used in the in vitro assays.
Estimation of Condensed Tannin, Polyphenol, Coumarin, and Flavonoid Contents
The extracts were cordially analyzed for condensed tannins (CT) by Professor Harley D. Naumann (Division of Plant Sciences, University of Missouri) using the acid butanol assay (Hagerman, 2011). In this method, interflavan bonds between subunits of the flavan-3-ol CT polymers were oxidatively cleaved in hot, acidic alcohol to produce colored anthocyanidins, which were read spectrophotometrically. CT were purified from the extracts with Sephadex LH-20 (Sigma-Aldrich, Saint Louis, MO) using methods adapted from Strumeyer and Malin (1975) and Naumann et al. (2014) and were used as internally derived standards in the assay.
The amount of total phenolics in extracts was determined according to the Folin–Ciocalteu assay (Julkunen-Tiitto, 1985). Samples (100 µL) were introduced into test tubes containing 8.4 mL of water and 0.5 mL of Folin–Ciocalteu’s reagent; and 1 mL of sodium carbonate (20%) were added. The tube were mixed and allowed to stand for 1 h in the darkness at room temperature. The absorbance was measured at 765 nm using a SHIMADZU 1700 UV–vis spectrophotometer. The estimation of phenolic compounds was carried out in triplicate, and the results were averaged. A calibration curve of gallic acid (GA) was prepared, and the results, determined by the regression equation of the calibration curve, were expressed as GA milligram equivalents.
Finally, the presence of coumarins and flavonoids in the extracts was estimated based on TLC on silica gel 60 PF254 + 366 plates (20 × 20 cm, 1 mm thickness, Merck Co.) by using the following mix as solvents: n-hexane-EtOAc (1:1) and n-hexane-EtOAC (3:2). After TLC, the different spots were visualized with visible spectrum light and wavelength light of 254 and 324 nm, with or without subsequent oleum treatment (sulfuric acid + glacial acetic acid + dH2O, 8/32/160) and further heating at 120 °C.
Parasite Maintenance and Preparation
The E. ninakohlyakimovae GC strain used in this investigation was initially isolated from field infections in 2006 in Gran Canaria island, Spain (Ruiz et al., 2013), and maintained by continuous passages through male Majorera goat kids for oocyst production. For harboring oocysts, goat kids were orally infected at the age of 4 wk with 2 × 105 sporulated E. ninakohlyakimovae GC oocysts. Excreted oocysts were obtained from feces collected after 2 wk post infection (p.i.) according to the methods of Jackson (1964). Briefly, the feces were washed with tap water through decreasing pore size sieves (250, 150, and 80 µm) for the elimination of debris. The resulting oocyst suspension was mixed (1:1) with a saturated sugar solution (1.5 g/L density), and transferred to rectangular plastic bowls and incubated for 2 h at room temperature (RT). Then, floating oocysts were collected by applying glass plates (25 × 25 cm) to the surface of the sugar/oocyst suspension. Attached E. ninakohlyakimovae oocysts were collected by rinsing with distilled water, concentrated by centrifugation (1,100 × g, 10 min), re-suspended in a 4% aqueous solution of potassium dichromate (w/v; Merck), and incubated in the presence of oxygen at RT to induce oocyst sporulation as described elsewhere (Hermosilla et al., 2002). Resulting sporulated E. ninakohlyakimovae GC oocysts were stored at 4 °C in a 2% aqueous solution of potassium dichromate (w/v; Merck) until further use. A total of four goat kids were employed for the maintenance of the E. ninakohlyakimovae GC strain.
To obtain unsporulated E. ninakohlyakimovae oocysts for the oocyst sporulation inhibition assay, a modified technique of Jackson (1964) was used. Freshly harvested unsporulated oocysts were partially purified by stirring oocysts (ice bath, 20 min) in a 4% sodium hypochlorite (Panreac) solution, and centrifuged (10 min, 400 × g). Further purification was attained by two centrifugation steps (20 min, 400 × g) in Percoll (Amersham) gradients of 1:60 and 1:50, respectively. Clean unsporulated oocysts were immediately transferred to tissue cell culture bottles (25 cm2, Nunc) and kept at 4 °C in PBS for no longer than 3 d after initial collection. For the sporozoite viability assay and the sporozoite invasion assay, viable E. ninakohlyakimavae sporozoites were excysted from sporulated oocysts and purified by a modified excystation protocol according to Fayer and Hammond (1967). In brief, clean oocysts purified as described above were suspended in sterile 0.02 M L-cysteine HCl/0.2 M NaHCO3 solution, and incubated in a 100% CO2 atmosphere at 37 °C for 20 h. Subsequently, sporulated oocysts were suspended in Hank’s balanced salt solution (Sigma-Aldrich) containing 0.4% (w/v) trypsin (Sigma-Aldrich) and 8% (v/v) sterile filtered caprine bile obtained from a local abattoir and were incubated under microscopic control for up to 4 h at 37 °C in a 5% CO2 atmosphere. Free-released sporozoites were washed three times (20 min, 400 × g) in tissue culture 1640 RPMI medium (Sigma-Aldrich) and thereafter re-suspended in 1640 RPMI medium (Sigma-Aldrich) until further use.
In Vitro Oocyst Sporulation Inhibition Assay
By using this assay, we aimed to investigate the capacity of the extract to inhibit the sporulation of E. ninakohlyakimovae oocysts in vitro. Routinely, 1.5 mL Eppendorf tubes were filled with a 20-µL solution of 5,000 unsporulated oocysts suspended in sterile PBS, 30 µL distilled H2O, 50 µL of 10% potassium dichromate and 100 µL of different extract (C1 to C5: 3, 1.5, 0.75, 0.18, and 0.023 mg/mL). The incubations were performed at RT over three exposure times: 30 min, 4 and 24 h. After the incubation, oocysts were washed in distilled water four times (1,500 × g, 5 min) and after the last washing step oocysts were re-suspended again in 1 mL 2% potassium dichromate, transferred into 24-multiwell tissue culture plates (Nunc) and incubated in the presence of oxygen at RT. As negative controls, similar concentrations of DMSO (Sigma-Aldrich) to those used to solve the plant extracts (from 3% to 0.023%) were used, while serial formaldehyde solutions served as positive controls (C1 to C5: 4%, 2%, 1%, 0.25%, and 0.03%). The oocyst sporulation rate was determined after 72 h at RT under microscopic analysis, and for this purpose a minimum of 100 oocysts were counted and analyzed. The assay was repeated in a different day and, in both cases, triplicates of all the concentrations assessed were performed.
Sporozoite Viability Assay
The test was used to evaluate the ability of the extract to produce irreversible damage to viable sporozoites thereby killing this parasitic stage. For this purpose, 5,000 per well viable E. ninakohlyakimovae sporozoites suspended in 100 µL sterile 1640 RPMI medium (Sigma-Aldrich) were transferred into 96-multiwell plates (Nunc) and mixed with an equal volume of different extract concentrations (C1 to C8: 10, 5, 2.5, 1.125, 0.625, 0.5, 0.1, and 0.025 mg/mL). The corresponding concentrations of DMSO (Sigma-Aldrich) were used as negative controls and heat-inactivated sporozoites (60 °C, 30 min) served as positive controls. After 3 h of incubation at RT, 100 µL of the supernatant were carefully removed and thereafter sporozoites were stained by adding 100 µL of the fluorescent dye Sytox Orange (Invitrogen) diluted in 1640 RPMI medium at 2.5 µM final concentration. After 15 min of incubation at RT, fluorescence was detected in a fluorescent microscope (Eclipse 80i, Olympus) at an excitation of 510 to 560 nm and an emission of 575 to 590 nm wavelength. All samples were run in triplicates. For the evaluation of the viability, 100 sporozoites were microscopically counted and analyzed for staining properties: Sytox Orange-unstained sporozoites were considered as ‘viable’, while stained parasites were classified as ‘dead’.
Sporozoite Cell Invasion Assay
This assay was conducted to evaluate the ability of the extract to inhibit the capacity of E. ninakohlyakimovae sporozoites to invade host cells grown as monolayer cultures. For host cell culture system, immortalized bovine colonic epithelial cells (BCEC) were seeded (100,000 cells per well) onto 12-multiwell plates (Nunc) and incubated with 1640 RMPI medium supplemented with 5% penicillin/streptomycin and 3% fetal calf serum (FCS) up to a minimum of 70 to 80% confluence. DMSO (Sigma-Aldrich) was used as negative control and as positive controls different concentrations of sulfadoxine/trimethoprim (Veterín-Diftrín 24 Intervet) (C1 to C5: 10/2, 0.1/0.02, 10−3/2 × 10−4, 10−6/2 × 10−7 and 10−9/2 × 10−10 µg/mL) were employed. Sporozoites (5 × 105) were incubated in Eppendorf tubes with different concentrations of the extract (C1 to C5: 5, 2.5, 1, 0.1, and 0.01 mg/mL), positive and negative controls. The incubations were performed during 3 h at 37 °C in 5% CO2 atmosphere. After the incubation, sporozoites were washed three times with 1640 RMPI medium (2,000 × g, 5 min) and after the last washing step the sporozoite pellet was re-suspended in 1640 RMPI (100 µL). Sporozoites receiving different treatments were added to confluent BCEC and incubated overnight at 37 °C in 5% CO2 atmosphere. The sporozoites suspension was then discarded and the medium replaced with 1640 RMPI containing 5% penicillin/streptomycin and 3% FCS. Photographs were taken of a total of 15 randomly selected spaced power vision fields per well using an optical microscope (Leica DFC 290) and an adapted camera. The invasion rate was quantified by counting the total number of non-infected BCEC and the number of BCEC carrying intracellular sporozoites.
Cytotoxicity Assays
The in vitro cytotoxicity of the plant extracts was performed on two different cell cultures: BCEC and VERO cells (African green monkey kidney cells). BCEC were cultured according to the methodology described in the inhibition cell invasion assay. VERO cells were obtained from the European Collection of Cells Cultures (ECAACC 84113001) and cultivated in RPMI 1640 culture medium (Gibco) supplemented with 2 nM l-glutamine, 500 U/mL penicillin, 50 µg/mL streptomycin, and 10% FCS (Ruiz et al., 2010). Both cell types were cultured in 24-well plates (Nunc) and, when confluent, 150 µL of the following plant extract concentrations were added: 3.125, 0.625, 0.125, and 0.0125 mg/mL. Serial dilutions of DMSO (3%, 1.5%, 0.75%, and 0.325%) were used as negative control. After an overnight incubation, 250 µL of a 2.5 µM Sytox Orange solution were added. The mix was incubated during 10 min at RT and then washed thrice with RPMI 1% penicillin/streptomycin. Finally, the fluorescence intensity was analyzed by a fluorometric reader (Ascent Fluoroskan, Labsystems) using an excitation wavelength of 530 nm and detecting at 590 nm. The results were always confirmed by microscopical observations.
Statistical Analysis
Data from the three in vitro studies and cytotoxicity test were grouped and analyzed independently for significant differences by using the nonparametric Chi-square test. Effects of the different plant extract concentrations on parasites were compared to the corresponding DMSO controls. Effective dose that produced 50% reduction (ED50) of the oocyst sporulation rate, sporozoite viability or cell invasion rate was calculated by plotting a linear regression between the Napierin logarithm (ln) of the effect (y) assessed and log transformed concentrations (x). The formula used was ln y = a + bx, where a and b are the slope of the line and the y-intercept, respectively. Differences were regarded as significant at a level of P < 0.05. For all statistical data analysis, the Sigmastat 3.1 (Windows) was here used.
RESULTS
Estimation of Condensed Tannin, Polyphenol, Coumarin, and Flavonoid Contents
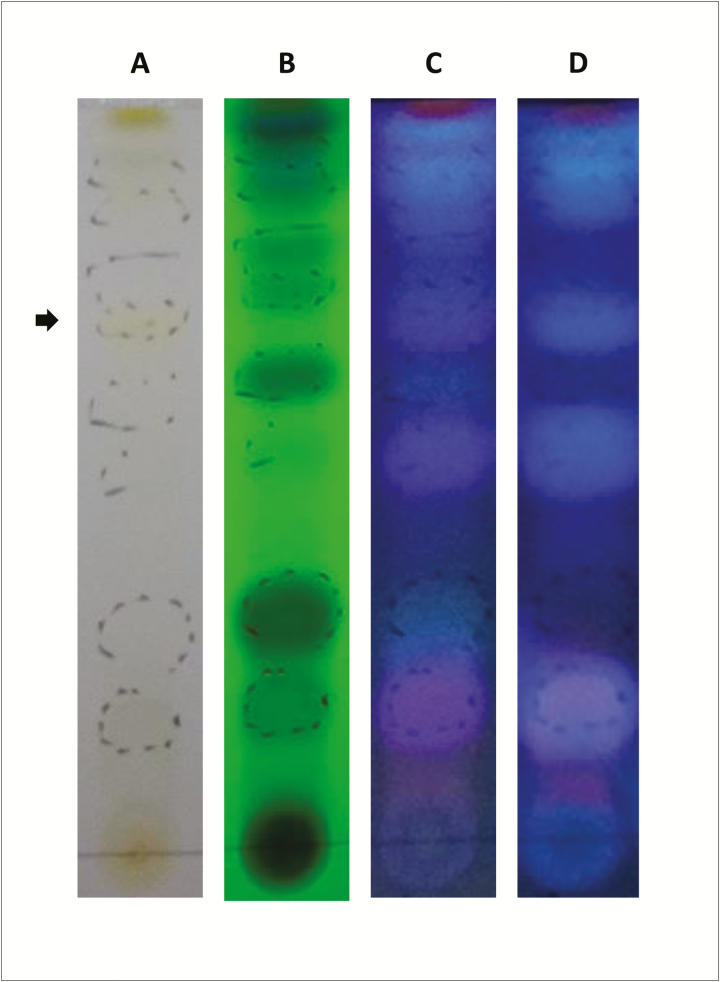
The different extracts assessed had mean values for polyphenol content of about 2 mg/mL, while no condensed tannin could be demonstrated in any of the samples evaluated. TLC showed a wide range of coumarins, specifically and strongly stained of blue color at 324 nm, mainly after oleum and heat treatment (Figure 1C and D) (Jaraíz García-Pallasar, 1972). By contrast, the only one single signal for flavonoid was visualized as a weak yellowish spot at visible spectrum light (Figure 1A, see arrow). Typical black color of the flavonoid could be also demonstrated at 254 nm, although relatively masked by the intensity of the signal of nearby compounds, e.g., coumarins (Figure 1B) (Mabry et al., 1970).
Figure 1.
TLC of methanolic extracts of R. pinnata mature fruits visualized using spectrum visible light (A) and UV light at 254 (B) nm and 324 nm (C) wavelengths. Forth line (D) represents visualization at 324 nm after after oleum and heat treatment and the arrow marks the presence of weak yellowish spot at visible spectrum light (A), which probably corresponds to a flavonoid.
In Vitro Assays
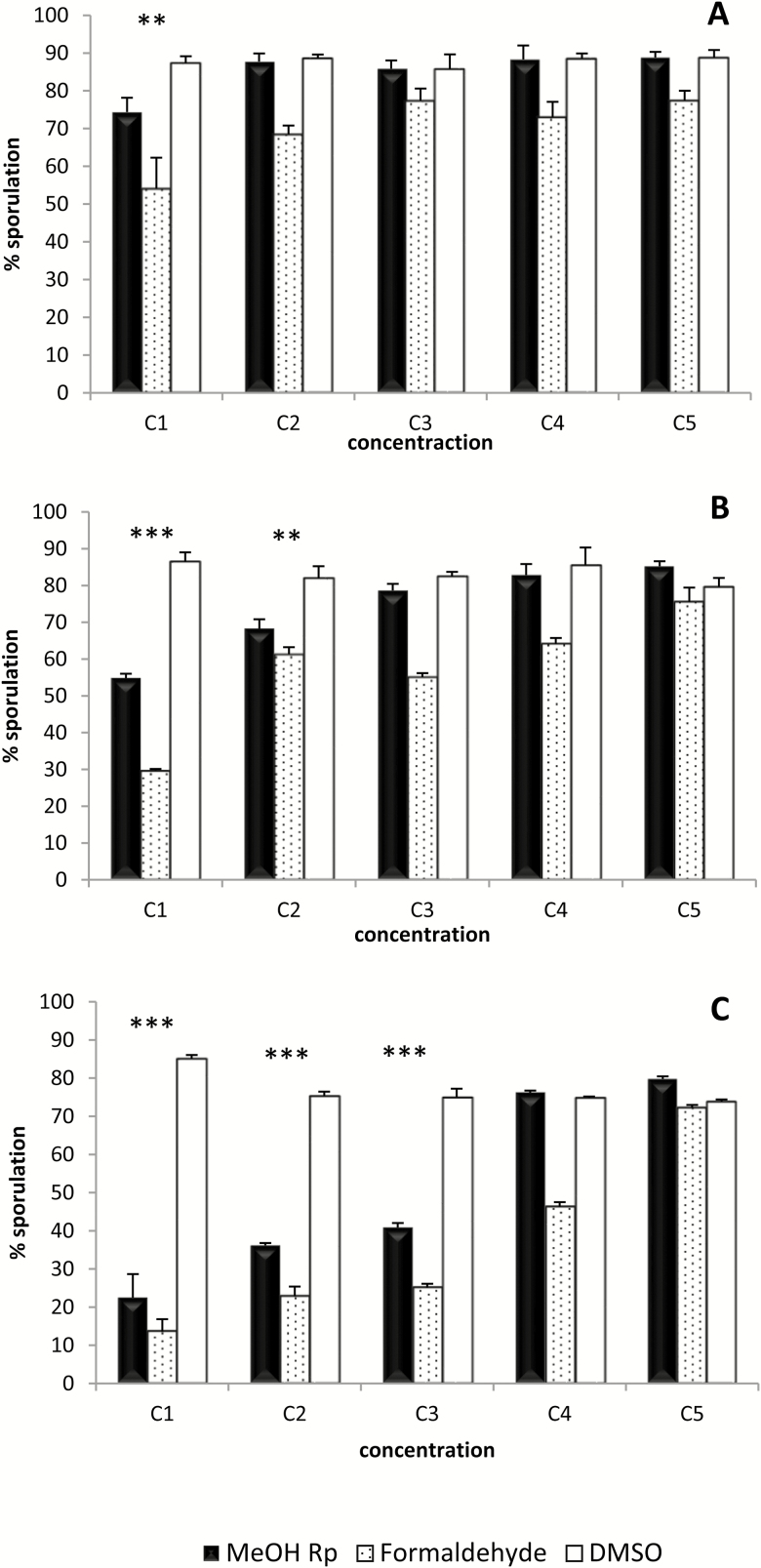
The capacity of the extract to inhibit E. ninakohlyakimovae-oocyst sporulation was both concentration and time dependent. As shown in Figure 2A, after 30 min of incubation only the highest concentration (3 mg/mL) of the extract produced a reduction in the sporulation compared to the negative control. After 4 h of incubation, both the 1.5 and 3 mg/mL concentrations resulted in a significant reduction of the sporulation rate (P < 0.01 and P < 0.001, respectively) (Figure 2B). Finally, exposure of oocysts to the extract for 24 h induced a significant inhibition of sporulation (P < 0.001) at concentrations of 0.75 mg/mL and greater (Figure 2C). Interestingly, the level of inhibition of the sporulation produced by the 3 mg/mL concentration at this incubation time was similar to that observed with the positive control (formaldehyde at a 4% dilution).
Figure 2.
Oocyst sporulation inhibition assay. Percentage of sporulation after incubation for 30 min (A), 4 h (B) and 24 h (C) with different concentrations of methanolic extracts of R. pinnata mature fruits (MeOH Rp) (C1 to C5: 3, 1.5, 0.75, 0, 0.18 and 0.023 mg/mL). DMSO at the same dilutions than for MeOH Rp (from 3% to 0.023%) was used as negative control. Serial formaldehyde solutions served as positive controls (C1 to C5: 4%, 2%, 1%, 0.25%, and 0.03%). Statistical significance for MetOH Rp vs. DMSO: P < 0.001 (***) and P < 0.01 (**).
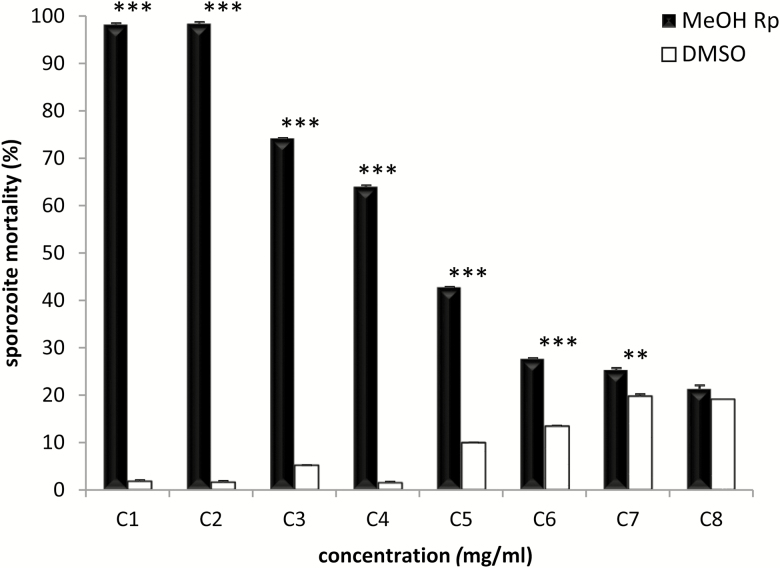
The anticoccidial effect of the extract on the viability of E. ninakohlyakimovae sporozoites was dose dependent as illustrated in Figure 3. Concentrations of 5 and 10 mg/mL of the extract produced almost 100% mortality in the sporozoites (P < 0.001), a similar result to the one obtained by the heat-inactivation treatment. Lower concentrations still produced significant killing of E. ninakohlyakimovae sporozoites (2.5, 1.125, 0.625, and 0.5 mg/mL; P < 0.001) (0.1 mg/mL; P < 0.01), with 0.84 mg/mL being the effective dose 50. Only the lowest concentration (0.025 mg/mL) used here did not resulted in increased sporozoite mortality (Figure 3).
Figure 3.
Sporozoite viability assay. Percentage of sporozoite mortality after treatment for 3 h with methanolic extracts of R. pinnata mature fruits (MeOH Rp) at different concentrations (C1 to C8: 10, 5, 2.5, 1.125, 0.625, 0.5, 0.1, and 0.025 mg/mL). The corresponding concentrations of DMSO were used as negative controls. The viability of the sporozoites was determined using the fluorescent dye Sytox Orang. Statistical significance for MetOH Rp vs. DMSO: P <0.001 (***) and P < 0.01 (**).
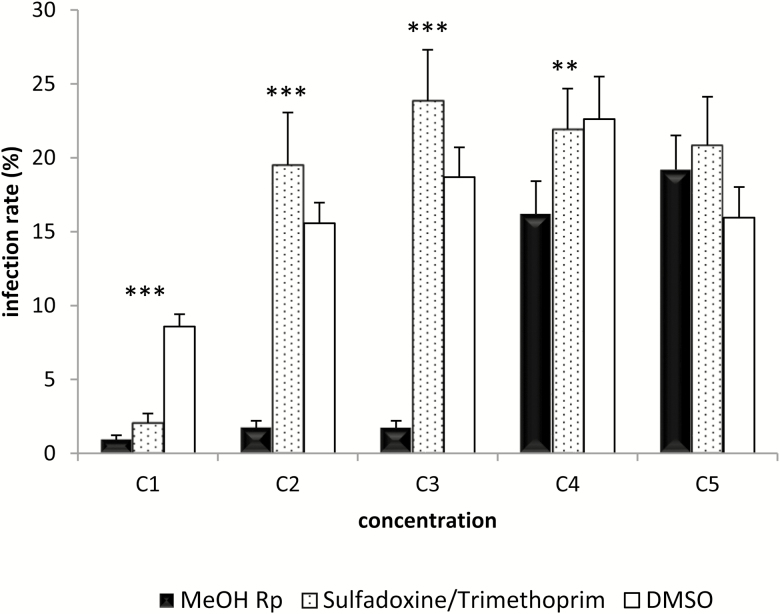
The effect of the extract on the ability of E. ninakohlyakimovae sporozoites to invade BCEC is displayed in Figure 4. The mean infection rate in negative DMSO controls cell cultures was 15%, ranging from 8.5% to 23%. Greater concentrations of methanolic extracts (3, 2.5, and 1 mg/mL) significantly reduced the infection rate of E. ninakohlyakimovae sporozoites in BCEC, with mean values of ~2% (P < 0.001). Samples incubated with 0.1 mg/mL resulted in an infection rate similar to the mean observed in positive controls, but still significantly lower than the corresponding DMSO counterpart (P < 0.01). Only the lowest concentration (0.01 mg/mL) did not produce any effect on the inhibition of host cell sporozoite invasion.
Figure 4.
Sporozoite cell invasion assay. Invasion rate on BCEC after treatment for 3 h with methanolic extracts of R. pinnata mature fruits (MeOH Rp) at different concentrations (C1 to C5: 5, 2.5, 1, 0.1, and 0.01 mg/mL). DMSO was used as negative control and as positive controls different concentrations of sulfadoxine/trimethoprim (Veterín-Diftrín 24 Intervet) (C1 to C5: 10/2, 0.1/0.02, 10−3/2 × 10−4, 10−6/2 × 10−7, and 10−9/2 × 10−10 µg/mL) were employed. The invasion rate of treated sporozoites was tested on bovine colonic epithelial cells (BCECs). Statistical significance for MetOH Rp vs. DMSO: P < 0.001 (***) and P < 0.01 (**).
Cytotoxicity Assessment
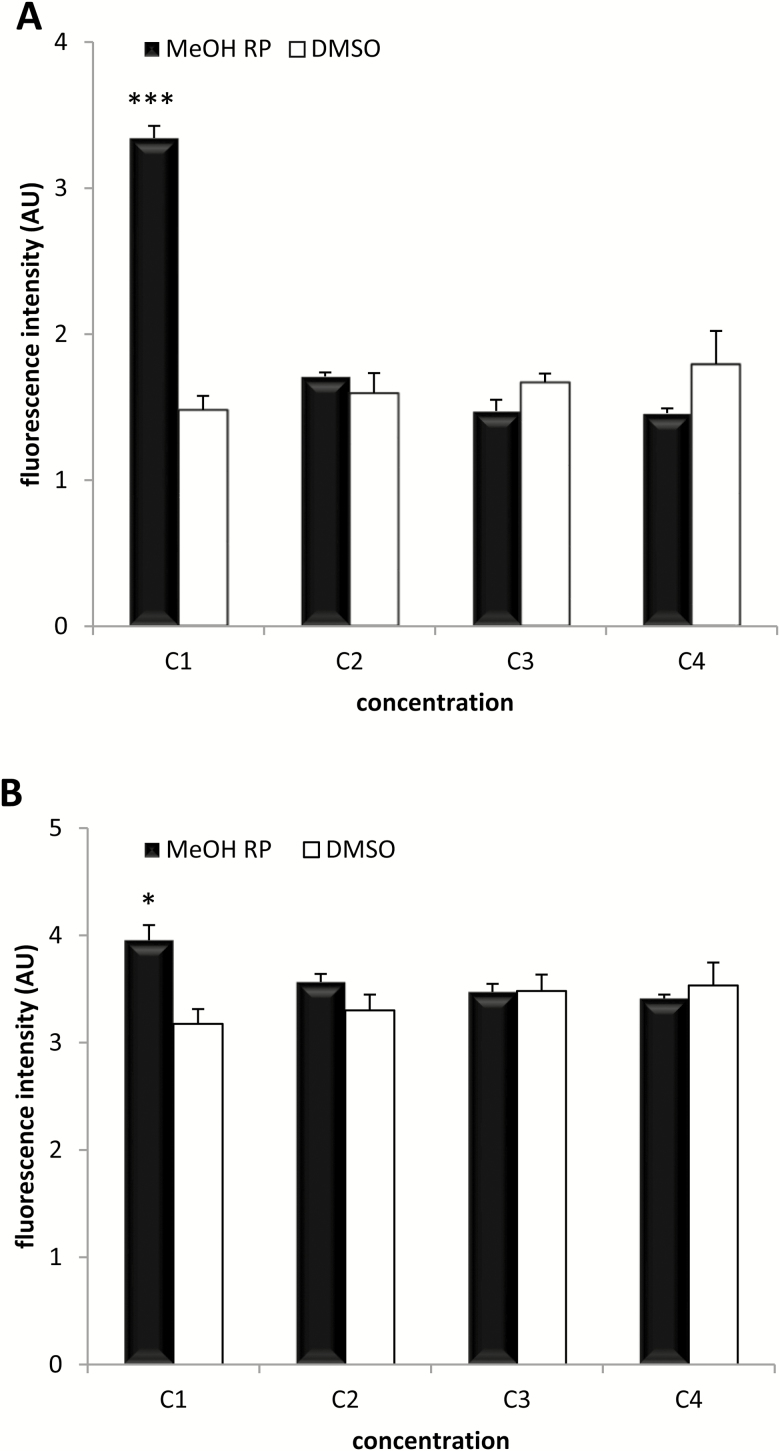
The results of the cytotoxicity assays showed that the extract used in the study did not produce relevant cell death. Only at the highest concentration of the extract (3 mg/mL) the fluorescence intensity recorded was greater than that observed in the respective negative DMSO culture, both in BCE (P < 0.001) (Figure 5A) and VERO (P < 0.05) (Figure 5B) cells. At the following extract concentrations, the fluorescence intensity values were similar to the corresponding negative controls.
Figure 5.
Cytotoxicity analysis. The cytotoxicity effect of methanolic extracts of R. pinnata mature fruits (MeOH Rp) at different concentrations (C1 to C4: 3.125, 0.625, 0.125, and 0.0125 mg/mL) after 24 h of incubation in BCECs (A) and VERO (B) cells was evaluated. DMSO was used as negative control. Fluorescence intensities are displayed in arbitrary units (AU).
DISCUSSION
In the present study, the anticoccidial activity of the endemic Canarian plant R. pinnata on E. ninakohlyakimovae oocysts and sporozoites was tested in vitro. The results demonstrate that a methanolic extract of the mature fruit from R. pinnata has marked anticoccidial properties as demonstrated by their capacity to both inhibit the sporulation of E. ninakohlyakimovae oocysts and reduce the sporozoites viability/capacity to invade epithelial cells in vitro. To the best of our knowledge, this is the first study in which the anticoccidial activity of Rutaceae has been investigated in a caprine Eimeria sp.
Alternatively to chemotherapeutic treatments, disinfectants applied to the environment to inhibit exogenous sporogony, which is an essential step for further Eimeria spp. infections, could be used to control ruminant coccidiosis as it has been suggested for poultry (Williams, 1997). Freshly Eimeria oocysts shed by infected animals are always unsporulated and undergo an exogenous asexual replication (sporogony) in the presence of oxygen and adequate temperature only after 48 h, which turns them into infectious stages containing four sporocysts with two viable sporozoites each. Only fully sporulated oocysts will be able to propagate the disease when orally ingested by other susceptible animals of the farm (Mundt et al., 2003; Daugschies and Najdrowski, 2005). Although the inhibition of oocyst sporulation by the extract after relatively short incubation periods was rather moderate, and only significant at longer incubation times (24 h), some concentrations of the extracts were actually able to induce similar inhibition rates to those observed for positive controls (4% formaldehyde). The effect of concentration and contact time on efficacy against unsporulated E. tenella oocysts has been even reported for commercial chemical disinfectants, including Ammonium hydroxide, Phenol, Zixvirox, and Eco Bio (Samaha et al., 2013). Oocysts are considered the resistant stage of coccidian parasites, and thereby able to tolerate adverse physical and chemical agents and, accordingly, McDonnell and Russell (1999) classified the oocysts as the most resistant etiological agents to disinfectants, after the prions. For this reason, moderate to high concentrations and relatively abrasive chemicals are needed for the inhibition of the oocyst sporulation. As in the present study, some other plant extracts, such as the water extracts from pine bark (Pinus radiatus), have been tested for their ability to inhibit sporogony in different Eimeria species (Molan et al., 2009). However, whether this anticoccidial activity may derive in a practical disinfection tool in field conditions deserves further and more detailed research.
The scientific relevance of the anticoccidial properties of R. pinnata also relies on its activity against the first infective stage of all Eimeria in vivo, namely the sporozoite stages. R. pinnata extracts could not only affect the sporozoite viability but also its ability to actively invade host cells, where they must develop into first generation schizonts, macroschizonts of up to 300 µm in some species (e.g., E. ninakohlyakimovae) able to produce severe damage in vivo at the mucosa of the small intestine (Dai et al., 2006; Ruiz et al., 2013). Some reports on inhibitory effects of plant extracts against avian Eimeria sporozoites have demonstrated that carvacol, curcumin, or Echinacea purpurea extracts inhibit the invasion of Madin-Darby bovine kidney cells by E. tenella sporozoites after a 2-h exposure period (Burt et al., 2013). Khalafalla et al. (2011) also demonstrated that, after 3, 6, 18, and 24 h incubation periods, curcumin had considerable effects on sporozoites morphology and viability, reducing the capacity of E. tenella sporozoites to invade cells.
The specific anticoccidial effect of R. pinnata methanolic extracts against sporozoites of E. ninakohlyakimovae is still unknown, and could not be clarified by the present study. Such effect should not necessarily be the same than the one responsible for the oocyst sporulation inhibition. Nonetheless, it seems evident that, by using the same concentrations, free sporozoites are more susceptible than oocysts when exposed to the extract. One plausible explanation for this phenomenon could be the oocyst wall, which represents a protective physical barrier for the internal sporozoites and allows E. bovis oocysts to resist up to 4.5 years in a humid environment at 4 °C (Hermosilla et al., personal communication). This could also be the explanation for the observed failure of sainfoin (Onobrychis viciifolia) phenolic extracts to inhibit sporulation of ovine Eimeria oocysts in vitro (Saratsi et al., 2012), in spite of the significant anticoccidial effect of sainfoin reported by the same authors when used as forage against Eimeria infections in lambs in vivo. By contrast, the extracts of R. pinnata used here were able to reduce the sporulation rate of E. ninakholyakimovae.
Although the exact mechanisms involved in the response of E. ninakohlyakimovae to R. pinnata extracts are yet unresolved, it could be assumed that the bioactive properties of this plant arise from its high content of plant secondary metabolites, as previously demonstrated for other plants. Natural flavonoid extracts have been shown to reduce Eimeria oocyst output, oxidative stress, and promote greater mean daily weight gains in infected lambs (Pérez-Fonseca et al., 2016) and high concentrations of CT also possess anticoccidial activities in small ruminants (Burke et al., 2013; Kommuru et al., 2014). However, these two bioactive compounds could not be the responsible of the anticocidial activity observed here, as no CT and limited flavonoids were present in the R. pinnata extract assessed. By contrast, we found relatively high concentration of polyphenols, a group of phytochemical compounds whose anticcoccidial properties have been also demonstrated in poultry. For instance, tea-based diets, which are rich in polyphenols, were effective against E. maxima (Jang et al., 2007) and proanthocyanidin, a naturally occurring polyphenolic antioxidant widely distributed in grape seed and other sources, reduced E. tenella infection as shown by gut pathology, body weight, and mortality (Wang et al., 2008). Apart from polyphenols, we consistently found different coumarins as components of the R. pinnate extracts but, surprisingly, no references in literature could be found showing the anticoccidial effects of these compounds. However, coumarins have been shown to have antiparasitic activity against other protozoa, such as Trypanosoma (Guíñez et al., 2013), Leishmania (Napolitano et al., 2004), and even the Apicomplexa Plasmodium (Moon et al., 2011). All the assumption concerning bioactive compound should nevertheless be taken with caution, as the antiparasitic activity of any plant extract might not dependent necessarily on the majoritarian compounds present in the extract (Mann et al., 1994; Li et al., 2017).
The assessment of the cytotoxicity of the extracts is commonly addressed when evaluating the antiparasitic activity of plant extracts, both in vitro and in vivo (Augustine et al., 1997; Khalafalla et al., 2011; Burt et al., 2013). Results concerning cytotoxicity obtained here revealed that the plant extracts did not induce relevant cell death on either BCEC or VERO cultures, which would be a guaranty for a safe use of R. pinnata as an anticoccidial. Although there are no data on the lack of toxicity in vivo of R. pinnata, a study on temporal and spatial variation in the diet composition of the endemic large lizard (Gallotia galloti) in Tenerife island, Spain, showed that R. pinnata fruits constitute the main ingredient of the diet, showing no cytotoxic effects to this animal (Rodriguez et al., 2008). Poikilothermic reptiles obviously differ from homeothermic ruminants in physiology and metabolic requirements, but it might be a hint for rather noncytotoxic properties of R. pinnata fruits. Besides, mostly in La Palma islands, where the natural population of R. pinnata is larger, these plants are also given to cattle when showing some gastrointestinal parasites (personal communication). Irrespectively of cytotoxicity, other side effects of the plant should be considered due to its antiprotozoal activities, particularly possible impact on beneficial/symbiotic protozoan populations within the digestive tract (e.g., rumen) (Prins, 1978; Bhatta et al., 2013).
Caprine coccidiosis in goat kids is still an important disease worldwide and there is strong evidence that control of the disease can not exclusively rely on chemical prophylactic or metaphylactic treatments but also on alternative solutions such as “green” drug discovery (phytotherapy), hygiene improvement, and/or development of immunoprophylactic strategies (Ruiz et al., 2014). This was the first attempt showing the in vitro anticoccidial effects of R. pinnata against E. ninakohlyakimovae. Further research is needed to understand the mode of action and composition of bioactive metabolites of this Rutaceae and their effect against this and other ruminant Eimeria species or even other animal/human parasites. Studies on intensive in vitro culture systems of R. pinnata plant cultivation are indispensable to solve the problem of R. pinnata-derived material availability in large quantities.
Supplementary Material
Footnotes
This study was funded by the Spanish Ministry of Science and Innovation (MICIN, project AGL2007-63415) and FEDER. The Canary Agency of Investigation, Innovation and Society of Information (ACIISI, project SolSubc2200801000244) is also here acknowledged for their financial support.
LITERATURE CITED
- Arechavaleta M., Rodríguez S., Zurita N., and García A.. 2010. Lista de especies silvestres de Canarias. Hongos, plantas y animales terrestres. Gobierno de Canarias, Santa Cruz de Tenerife, Spain. [Google Scholar]
- Augustine P. C., McNaughton J. L., Virtanen E., and Rosi L.. 1997. Effect of betaine on the growth performance of chicks inoculated with mixed cultures of avian Eimeria species and on invasion and development of Eimeria tenella and Eimeria acervulina in vitro and in vivo. Poult. Sci. 76:802–809. doi: 10.1093/ps/76.6.802 [DOI] [PubMed] [Google Scholar]
- Bhatta R., Baruah L., Saravanan M., Suresh K. P., and Sampath K. T.. 2013. Effect of medicinal and aromatic plants on rumen fermentation, protozoa population and methanogenesis in vitro. J. Anim. Physiol. Anim. Nutr. (Berl.). 97:446–456. doi: 10.1111/j.1439-0396.2012.01285.x [DOI] [PubMed] [Google Scholar]
- Bramwell D. 1998. Flora of the Canary Islands English version pocket guide. 1st ed. Rueda, Madrid, Spain. [Google Scholar]
- Burke J. M., Miller J. E., Terrill T. H., Orlik S. T., Acharya M., Garza J. J., and Mosjidis J. A.. 2013. Sericea lespdeza as an aid in the control of Emeria spp. in lambs. Vet. Parasitol. 193:39–46. doi: 10.1016/j.vetpar.2012.11.046 [DOI] [PubMed] [Google Scholar]
- Burt S. A., Tersteeg-Zijderveld M. H., Jongerius-Gortemaker B. G., Vervelde L., and Vernooij J. C.. 2013. In vitro inhibition of Eimeria tenella invasion of epithelial cells by phytochemicals. Vet. Parasitol. 191:374–378. doi: 10.1016/j.vetpar.2012.09.001 [DOI] [PubMed] [Google Scholar]
- Dai Y. B., Liu X. Y., Liu M., and Tao J. P.. 2006. Pathogenic effects of the coccidium Eimeria ninakohlyakimovae in goats. Vet. Res. Commun. 30:149–160. doi: 10.1007/s11259-006-3228-1 [DOI] [PubMed] [Google Scholar]
- Daugschies A., and Najdrowski M.. 2005. Eimeriosis in cattle: current understanding. J. Vet. Med. B Infect. Dis. Vet. Public Health 52:417–427. doi: 10.1111/j.1439-0450.2005.00894.x [DOI] [PubMed] [Google Scholar]
- Dorne J. L., Heppner C., Kass G. E., Schlatter J., Alexander J., and Fink-Gremmels J.. 2013. Special issue: risk assessment of undesirable substances in feed. Toxicol. Appl. Pharmacol. 270:185–186. doi: 10.1016/j.taap.2012.11.011 [DOI] [PubMed] [Google Scholar]
- Fayer R., and Hammond D. M.. 1967. Development of first-generation schizonts of Eimeria bovis in cultured bovine cells. J. Protozool. 14:764–772. [DOI] [PubMed] [Google Scholar]
- Guíñez R. F., Matos M. J., Vazquez-Rodriguez S., Santana L., Uriarte E., Olea-Azar C., and Maya J. D.. 2013. Synthesis and evaluation of antioxidant and trypanocidal properties of a selected series of coumarin derivatives. Future Med. Chem. 5:1911–1922. doi: 10.4155/fmc.13.147 [DOI] [PubMed] [Google Scholar]
- Hagerman A. E. 2011. Method for acid butanol assay of dried fig samples. Tannin handbook. Oxford:Miami University; Available from http://www.users.miamioh.edu/hagermae/ (Accessed 16 July 2017). [Google Scholar]
- Hermosilla C., Barbisch B., Heise A., Kowalik S., and Zahner H.. 2002. Development of Eimeria bovis in vitro: suitability of several bovine, human and porcine endothelial cell lines, bovine fetal gastrointestinal, Madin-Darby bovine kidney (MDBK) and African green monkey kidney (VERO) cells. Parasitol. Res. 88:301–307. doi:10.1007/s00436-001-0531-1 [DOI] [PubMed] [Google Scholar]
- Jackson A. R. 1964. The isolation of viable coccidial sporozoites. Parasitology 54:87–93. doi: 10.1017/S0031182000074369 [DOI] [PubMed] [Google Scholar]
- Jang S. I., Jun M. H., Lillehoj H. S., Dalloul R. A., Kong I. K., Kim S., and Min W.. 2007. Anticoccidial effect of green tea-based diets against Eimeria maxima. Vet. Parasitol. 144:172–175. doi: 10.1016/j.vetpar.2006.09.005 [DOI] [PubMed] [Google Scholar]
- Jaraíz García-Pallasar I. 1972. Cumarins of two Canarian rues [PhD thesis]. Spain: Department of Organic Chemistry, University of La Laguna. [Google Scholar]
- Julkunen-Tiitto R. 1985. Phenolic constituents in the leaves of northern willows: methods for the analysis of certain phenolics. J. Agric. Food Chem. 33:213–217. doi:10.1021/jf00062a013 [Google Scholar]
- Khalafalla R. E., Müller U., Shahiduzzaman M., Dyachenko V., Desouky A. Y., Alber G., and Daugschies A.. 2011. Effects of curcumin (diferuloylmethane) on Eimeria tenella sporozoites in vitro. Parasitol. Res. 108:879–886. doi: 10.1007/s00436-010-2129-y [DOI] [PubMed] [Google Scholar]
- Kommuru D. S., Barker T., Desai S., Burke J. M., Ramsay A., Mueller-Harvey I., Miller J. E., Mosjidis J. A., Kamisetti N., and Terrill T. H.. 2014. Use of pelleted Sericea lespedeza (Lespedeza cuneata) for natural control of coccidia and gastrointestinal nematodes in weaned goats. Vet. Parasitol. 204:191–198. doi: 10.1016/j.vetpar.2014.04.017 [DOI] [PubMed] [Google Scholar]
- Koudela B., and Bokova A.. 1998. Coccidiosis in goats in the Czech Republic. Vet. Parasitol. 76:261–267. doi:10.1016/S0304-4017(97)00147-7 [DOI] [PubMed] [Google Scholar]
- Lans C., Turner N., Khan T., and Brauer G.. 2007. Ethnoveterinary medicines used to treat endoparasites and stomach problems in pigs and pets in British Columbia, Canada. Vet. Parasitol. 148:325–340. doi: 10.1016/j.vetpar.2007.06.014 [DOI] [PubMed] [Google Scholar]
- Li X. Y., Tang H. J., Zhang L., Yang L., Li P., and Chen J.. 2017. A selective knockout method for discovery of minor active components from plant extracts: feasibility and challenges as illustrated by an application to Salvia miltiorrhiza. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 1068–1069:253–260. doi: 10.1016/j.jchromb.2017.10.011 [DOI] [PubMed] [Google Scholar]
- Mabry T. J., Markham K. R., and Thomas M. B.. 1970. The systematic identification of flavonoids. Springer-Verlag, Berlin, Heidelberg. [Google Scholar]
- Mann J., Davidson R. S., Hobbs J. B., Banthorpe D. V., and Harborne J. B.. 1994. Natural products: their chemistry and biological significance. Prentice Hall, United Kingdom. [Google Scholar]
- McDonnell G., and Russell A. D.. 1999. Antiseptics and disinfectants: activity, action, and resistance. Clin. Microbiol. Rev. 12:147–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels M. G., Bertolini L. C., Esteves A. F., Moreira P., and Franca S. C.. 2011. Anticoccidial effects of coumestans from Eclipta alba for sustainable control of Eimeria tenella parasitosis in poultry production. Vet. Parasitol. 177:55–60. doi: 10.1016/j.vetpar.2010.11.022 [DOI] [PubMed] [Google Scholar]
- Molan A. L., Liu Z., and De S.. 2009. Effect of pine bark (Pinus radiata) extracts on sporulation of coccidian oocysts. Folia Parasitol. (Praha). 56:1–5. doi:10.14411/fp.2009.001 [DOI] [PubMed] [Google Scholar]
- Moon H. I., Lee J. H., Lee Y. C., and Kim K. S.. 2011. Antiplasmodial and cytotoxic activity of coumarin derivatives from dried roots of Angelica gigas Nakai in vitro. Immunopharmacol. Immunotoxicol. 33:663–666. doi: 10.3109/08923973.2011.559248 [DOI] [PubMed] [Google Scholar]
- Mundt H. C., Daugschies A., Uebe F., and Rinke M.. 2003. Efficacy of toltrazuril against artificial infections with Eimeria bovis in calves. Parasitol. Res. 90 (Suppl. 3):S166–S167. doi: 10.1007/s00436-003-0929-z [DOI] [PubMed] [Google Scholar]
- Napolitano H. B., Silva M., Ellena J., Rodrigues B. D., Almeida A. L., Vieira P. C., Oliva G., and Thiemann O. H.. 2004. Aurapten, a coumarin with growth inhibition against Leishmania major promastigotes. Braz. J. Med. Biol. Res. 37:1847–1852. doi: /S0100-879X2004001200010 [DOI] [PubMed] [Google Scholar]
- Naumann H. D., Hagerman A. E., Lambert B. D., Muir J. P., Tedeschi L. O., and Kothmann M. M.. 2014. Molecular weight and protein-precipitating ability of condensed tannins from warm-season perennial legumes. J. Plant Interac. 9:212–219. doi:10.1080/17429145.2013.811547 [Google Scholar]
- Nweze N. E., and Obiwulu I. S.. 2009. Anticoccidial effects of Ageratum conyzoides. J. Ethnopharmacol. 122:6–9. doi: 10.1016/j.jep.2008.11.014 [DOI] [PubMed] [Google Scholar]
- Peek H. W., and Landman W. J.. 2003. Resistance to anticoccidial drugs of Dutch avian Eimeria spp. field isolates originating from 1996, 1999 and 2001. Avian Pathol. 32:391–401. doi: 10.1080/0307945031000121149 [DOI] [PubMed] [Google Scholar]
- Pérez-Fonseca A., Alcala-Canto Y., Salem A. Z., and Alberti-Navarro A. B.. 2016. Anticoccidial efficacy of naringenin and a grapefruit peel extract in growing lambs naturally-infected with Eimeria spp. Vet. Parasitol. 232:58–65. doi: 10.1016/j.vetpar.2016.11.009 [DOI] [PubMed] [Google Scholar]
- Prins R. A. 1978. Nutritional impact of intestinal drug–microbe interactions. In: Hathcock J. N., and J. Coon, editors, Nutrition and drug interrelations. Academic Press; p. 189–251. [Google Scholar]
- Rodriguez A., Nogales M., Rumeu B., and Rodríguez B.. 2008. Temporal and spatial variation in the diet of the endemic lizard Gallotia galloti in an Insular Mediterranean Scrubland. J. Herpetol. 42:213–222. doi:10.1670/07-0752.1 [Google Scholar]
- Ruiz A., Behrendt J. H., Zahner H., Hermosilla C., Pérez D., Matos L., Muñoz M. D. E. L. C., Molina J. M., and Taubert A.. 2010. Development of Eimeria ninakohlyakimovae in vitro in primary and permanent cell lines. Vet. Parasitol. 173:2–10. doi: 10.1016/j.vetpar.2010.05.023 [DOI] [PubMed] [Google Scholar]
- Ruiz A., González J. F., Rodríguez E., Martín S., Hernández Y. I., Almeida R., and Molina J. M.. 2006. Influence of climatic and management factors on Eimeria infections in goats from semi-arid zones. J. Vet. Med. B Infect. Dis. Vet. Public Health 53:399–402. doi: 10.1111/j.1439-0450.2006.00985.x [DOI] [PubMed] [Google Scholar]
- Ruiz A., Matos L., Muñoz M. C., Hermosilla C., Molina J. M., Andrada M., Rodríguez F., Pérez D., López A., Guedes A.,. et al. 2013. Isolation of an Eimeria ninakohlyakimovae field strain (Canary Islands) and analysis of its infection characteristics in goat kids. Res. Vet. Sci. 94:277–284. doi: 10.1016/j.rvsc.2012.08.003 [DOI] [PubMed] [Google Scholar]
- Ruiz A., Muñoz M. C., Molina J. M., Hermosilla C., Andrada M., Lara P., Bordón E., Pérez D., López A. M., Matos L.,. et al. 2014. Immunization with Eimeria ninakohlyakimovae-live attenuated oocysts protect goat kids from clinical coccidiosis. Vet. Parasitol. 199:8–17. doi: 10.1016/j.vetpar.2013.09.032 [DOI] [PubMed] [Google Scholar]
- Samaha H. A., Haggag Y. N., Nossair M. A., and Habib H. M.. 2013. Assessment efficiency of some chemical disinfectants commonly used against coccidian in poultry farms. Alexandria J. Vet. Sci. 39:82–90. [Google Scholar]
- Saratsis, A., I. Regos, N. Tzanidakis, N. Voutzourakis, A. Stefanakis, D. Treuter, A. Joachim, and S. Sotiraki. 2012. In vivo and in vitro efficacy of sainfoin (Onobrychis viciifolia) against Eimeria spp in lambs. Vet. Parasitol. 188:1–9. doi:10.1016/j.vetpar.2012.03.014. doi: 10.1016/j.vetpar.2012.03.014. [DOI] [PubMed] [Google Scholar]
- Strumeyer D. H., and Malin M. J.. 1975. Condensed tannins in grain sorghum: isolation, fractionation, and characterization. J. Agric. Food Chem. 23:909–914. [DOI] [PubMed] [Google Scholar]
- Valderrábano J., Calvete C., and Uriarte J.. 2010. Effect of feeding bioactive forages on infection and subsequent development of Haemonchus contortus in lamb faeces. Vet. Parasitol. 172:89–94. doi: 10.1016/j.vetpar.2010.04.018 [DOI] [PubMed] [Google Scholar]
- Waller P. J., and Thamsborg S. M.. 2004. Nematode control in ‘green’ ruminant production systems. Trends Parasitol. 20:493–497. doi: 10.1016/j.pt.2004.07.012 [DOI] [PubMed] [Google Scholar]
- Wang M. L., Suo X., Gu J. H., Zhang W. W., Fang Q., and Wang X.. 2008. Influence of grape seed proanthocyanidin extract in broiler chickens: effect on chicken coccidiosis and antioxidant status. Poult. Sci. 87:2273–2280. doi: 10.3382/ps.2008-00077 [DOI] [PubMed] [Google Scholar]
- Williams, R. B. 1997. Laboratory test of phenolic disinfectants as oocysticides against the chicken coccidium Eimeria tenella. Vet. Record. 141:447–448. doi:10.1136/vr.141.17.447. doi: 10.1136/vr.141.17.447. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.