Abstract
Aim:
We sought to design an insulin delivery method that would overcome barriers to insulin therapy and meet the needs of the users, adults with diabetes, and their health care providers (HCPs).
Methods:
We conducted focus groups and human factors studies with users to learn about their needs and requirements. We then designed an insulin delivery device, PAQ, with features that met the user’s requirements. Iterative design and human factors testing (HFT) was performed with adults with diabetes on ⩾2 injections/day and HCPs. In parallel, studies were conducted to identify an adhesive that stayed adhered for 3 days and caused minimal, if any, dermal irritation. Pilot clinical studies were then initiated.
Results:
Users want a way to administer insulin that is simple, discreet, safe, and effective. A summative HFT found the device was easy to learn and use. All participants (30/30, 100%) successfully completed the key performance measures tested. An adhesive validation study in 30 adults with diabetes found 90% of the devices remained adhered to the participant’s application site at the end of 3 days with minimal skin irritation. Data from 3 clinical studies revealed 74-75% transitioned from injectable insulin to the device with the first fixed basal rate selected, improved glycemic control, and participants’ satisfaction with the device.
Conclusion:
The collective data from the HFT, adhesive, and clinical studies demonstrated that the device provides a method of insulin delivery that overcomes barriers to injectable insulin, meets the needs of the user, and achieves glycemic control.
Keywords: adherence, continuous subcutaneous insulin infusion, type 2 diabetes, insulin pumps, insulin delivery
Type 2 diabetes mellitus (T2DM) is a progressive disorder.1 Six years following diagnosis, approximately 50% of patients with T2DM require insulin therapy.2 Over time, many adults with T2DM require basal bolus insulin therapy to reach glycemic targets.3 However, the majority of adults on insulin in the United States are not in good control.4 Adherence to and persistence with administering insulin therapy is often inadequate. Barriers to achieving adherence to insulin therapy include: the need for multiple daily injections (MDI), interference of MDI regimens with daily activities, injection pain and embarrassment, as well as just forgetting to administer injections.5 A survey of insulin delivery among adults with T2DM revealed that only 37% of adults with T2DM on <3 injections per day felt comfortable injecting themselves with insulin away from the home.6 Continuous subcutaneous insulin infusion (CSII) and bolus dosing using insulin pumps helps people with T2DM overcome many of the barriers associated with MDI therapy and might result in higher adherence to therapy. However, the complexity of existing insulin pumps often leads to discontinuation of CSII.7 Further the cost of pumps and lack of coverage by insurance companies is prohibitive to most adults with T2DM.
Given the barriers associated with traditional insulin therapy, CeQur Corporation has developed a simple CSII device called PAQ. The device is a small, discreet, wearable device that safely delivers insulin subcutaneously for up to 3 days. It has been designed to replace daily insulin injections and uses rapid-acting insulin, to deliver a preset continuous basal infusion and on-demand bolus insulin for up to 3 days (Figure 1). It is intended to be used by adults with insulin-requiring diabetes who can utilize fixed daily basal rates and can bolus dose in 2-unit increments. The device is available in one of seven preset basal doses, 16, 20, 24, 32, 40, 50, and 60 U/day, to accommodate a wide range of dosing requirements.
Figure 1.
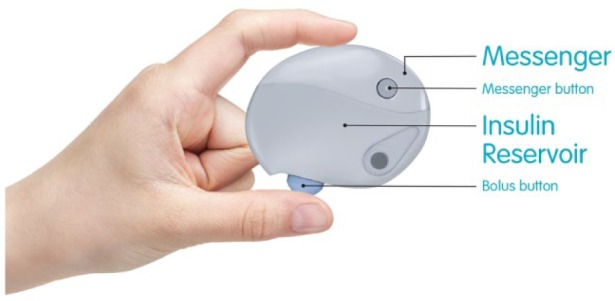
PAQ.
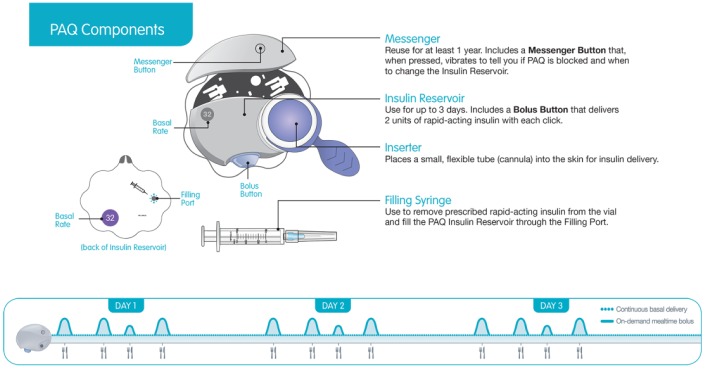
The device is composed of two parts: a disposable Insulin Reservoir with an integrated cannula Inserter and a reusable Messenger (Figure 2). The Messenger communicates to the user its battery life (each time it is attached to an Insulin Reservoir) and when the Insulin Reservoir needs to be changed (by pressing the Messenger Button).
Figure 2.
PAQ components.
The device setup and application to the body is simple. The Messenger is connected to the Insulin Reservoir, using a syringe it is filled with enough rapid-acting insulin to cover the person’s total insulin requirements for 3 days. In the next step, the device is primed and then applied to the person’s body with skin adhesive tape. Using the Inserter, a soft flexible cannula is inserted into the subcutaneous tissue and insulin is delivered from an elastomeric bladder (insulin reservoir with a capacity of 370 U) through capillary flow restrictors which control the flow rate over a 3-day period (Figure 3).
Figure 3.
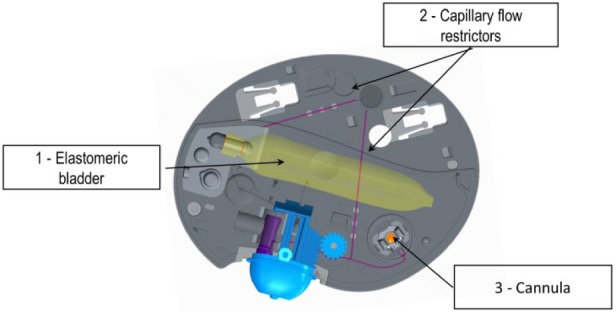
Internal components of PAQ. (1) Elastomeric bladder that drives basal flow. (2) Capillary flow restrictors that control the basal rate. (3) Teflon cannula 8 mm in length.
The user can press the Messenger button at any time to monitor the Insulin Reservoir status through vibrations. One vibration indicates the device is working, two vibrations indicates 48 hours have passed, three vibrations signals 66 hours have passed and the Insulin Reservoir needs to be replaced within 6 hours, and 4 vibrations indicates either 72 hours have passed, the Insulin Reservoir is out of insulin or the insulin fluid path is clogged. The Messenger resets each time it is disconnected from the Insulin Reservoir, for example, when the Insulin Reservoir is replaced.
The fully assembled device containing insulin, without the cannula inserter attached, measures approximately 60 mm × 77 mm × 18 mm and weighs approximately 36 g.
Needs of the User
We sought to design an alternative way to deliver insulin that would meet the needs of the users, that is, adults with T2DM on insulin and their health care providers (HCPs) who prescribe and help them to manage their diabetes. In order to determine the needs of the users, CeQur conducted focus groups, market research and human factor studies with hundreds of these users to learn about their needs and requirements (hereafter referred to as user requirements). The consensus was that users want a way to administer insulin that is simple, discreet, safe, and effective.
User Requirements for Product Design
Simple
Adults with diabetes want to eliminate the daily injection burden and want a therapy that will fit into their life. HCPs believe adherence to MDI is a challenge and want a mode of insulin delivery that is easy for people to learn and use.
Discreet
Adults with diabetes are very concerned with how noticeable their insulin treatment is. They want a mode of insulin delivery they can use without other people noticing.
Safe
HCPs are concerned with safety. They want a mode of insulin delivery that can protect the user from inadvertent bolus dosing and notify the user if the device needs to be changed.
Effective
Insulin as an effective therapy depends on a regime that is easy to follow and adhere to. Patients admitted to skipping doses, stating that injections were a pain and the majority do not like injecting themselves 3-6 times per day. HCPs also acknowledged the challenge of adherence and finding a dosing regimen that their patients can follow.
CeQur believed if they could design a device that could meet the above-mentioned user requirements for a T2 insulin delivery device, then the users would be satisfied with the device and with the use of the product and adhere to their prescribed regimen and achieve good glycemic control.
Iterative Design and Testing
Given this input the device was designed with features that met the user requirements detailed in Table 1.
Table 1.
PAQ Design Features.
| User requirements | Design feature |
|---|---|
| Simple to train, learn, and use | |
| • Setup and training | • Two components to assemble • Reservoir filled with insulin via syringe • Preassembled Inserter to place the cannula requires a single push of the hand • Basal insulin flows immediately upon cannula insertion • Bolus dosing achieved with a push of a button |
| • One type of insulin | • Uses one type of insulin—rapid acting insulin |
| • No programming | • Manufactured with preset basal rates • Bolus dose preset in 2 U increments |
| • No need for daily injections for multiple days | • Large volume reservoir accommodates 3 days of basal and bolus dosing for users with insulin needs ranging from 32 to 110 U/day; 1 injection every 3 days |
| Discreet | |
| • Not visible under clothing | • Small body worn device with flat profile and discreet grey color |
| • Quiet | • Vibrations used to communicate to the user the insulin reservoir status/flow, initiated at the user’s request |
| • Allows unnoticeable bolus dosing | • Can administer bolus insulin with a push of a button directly or over clothing |
| Safe | |
| • Protection from underdosing resulting in hyperglycemia, by device falling off the skin, cannula dislodgement and/or running out of insulin | • An optimized adhesive tape design; it can stretch with body movement keeping the cannula in place and the PAQ adhered for 3 days. • Designed with a Messenger that monitors the length of time worn, whether the flow path is clogged or the reservoir is out of insulin. The push of a button by the user provides vibratory messages which indicate whether the device needs to be changed. |
| • Protection from bolus overdosing resulting in hypoglycemia | • In order to administer a bolus dose, the user must apply a deliberate counter force to press the bolus button, thereby avoiding inadvertent pressing of the button. • Bolus button delivers a tactile response and springs back to the unpressed position after 2 U of insulin have been delivered, thus allowing the user to effectively count the number of times they have pushed the button. |
| Effective | |
| • Effective insulin delivery | Accurately provides: • ± 10% of the intended basal flow rate via CSII for up to 3 days • ± 10% of the intended bolus dose |
Human Factors Testing
During prototype development the device underwent extensive human factors testing with adults with T1DM (~10%) and T2DM on ⩾2 injections per day and HCPs. The goal of the testing was to identify potential use-related hazards associated with the setup and use of the device and to evaluate the ability of the design features to successfully mitigate the occurrence of these hazards. The early testing was conducted using a “worst case” scenario, that is, no training or demonstration of the device was provided to study participants prior to them completing the tasks required to assemble and use the device. The participants relied on the device Quick Start Guide (QSG) and Instruction for Use (IFU) manual. The rationale to this approach was to make the device as intuitive as possible for use. Following each study, changes were made as needed to the device design, QSG, IFU, and labeling materials to mitigate any identified use errors.
One of the key learnings from the testing was the importance of explaining, in layman terms, how the device worked compared to the participant’s injectable insulin therapy before the commencement of training. It was hard for participants to understand that the rapid acting insulin was going to be used for both basal and bolus insulin and that the insulin was delivered through a “plastic tube” placed into their skin. Once participants understood that the constant infusion delivered from the cannula into their skin replaced their basal/long-acting insulin, the training for the device set up and use was easy.
The culminating changes were tested in a validation study. The main objective of the study was to determine whether the device, the labeling, QSG, and IFU could be correctly, safely, and effectively used by adults with diabetes (taking at least 2 injections/day) and HCPs who would be responsible for training. The study included 30 participants: 15 adults with diabetes (T2D 80%) and 15 HCPs who specialize in diabetes management. Regarding the adults with diabetes, care was taken to identify and include adults with demographics (average age 42, range 25-60 years, 26% male), ethnicity (7% African American, 13% Pacific Islander, 40% Hispanic, and 40% Caucasian), education levels (47% high school diploma, 13% associate’s degree, 27% bachelor’s degree, and 13% master’s degree), and diabetes-related physical impairments (67% wore glasses, 13% with self-reported retinopathy/blurriness, 7% with hand impairments) that were consistent with the general population of adults with T2DM on injectable insulin therapy. All participants attended two study sessions, occurring on consecutive days. Day 1 was a training session (up to 1 hour). On day 2 the participants performed a series of unaided tasks, including identifying the proper basal rate on the packaging, setting up, filling, and applying a new device, bolusing different amounts under quiet and noisy conditions, identifying various messages, and removing a used device. Overall, the device was found to be easy to learn and use by adults with diabetes and HCPs and was very well received. All participants (30/30, 100%) successfully completed the key performance measures tested (Table 2).8
Table 2.
Key Performance Measures Tested in Human Factors Validation Study.
| Key performance measures | N = 30 |
|---|---|
| Set up tasks | |
| Able to setup PAQ | 30/30 (100%) |
| Able to fill PAQ with insulin | 30/30 (100%) |
| Able to prime (remove air) from fluid path | 30/30 (100%) |
| Able to apply and adhere device to skin | 30/30 (100%) |
| Able to deploy and insert cannula | 30/30 (100%) |
| Bolus tasks | |
| Able to deliver 8 or 16 U bolus dose (no distraction) | 30/30 (100%) |
| Able to deliver 8 or 16 U bolus dose (with distraction) | 30/30 (100%) |
| Messenger button | |
| Able to identify and press Messenger button | 30/30 (100%) |
| Able to identify the number of vibrations emitted | 30/30 (100%) |
| Able to interpret the meaning of the vibrations, and take appropriate action | 30/30 (100%) |
| Replace the reservoir after 3 days | |
| Able to remove the PAQ, disengage the Messenger component, and replace the reservoir | 30/30 (100%) |
Adhesive Studies in Healthy Volunteers
The device requires an adhesive tape to adhere it to the user’s abdomen. A series of volunteer studies were performed to identify an adhesive that would stay adhered for 3 days and cause minimal, if any, dermal irritation. Once an adhesive tape was identified that met these criteria (medical grade adhesive with >20 years of use in the market place); additional studies were conducted to evaluate the adhesive tape: shape profile, stretch orientation, method to attach the adhesive to the device, use of over-the-counter adhesive aids, and sterilization methods. The outcomes of these studies helped identify ways to optimize adherence of the device on the user’s body: a scallop shape border (rather than oval) to minimize peeling of the adhesive from the skin, a specific heat weld pattern to affix the adhesive to the device and adhesive orientation that allowed the adhesive to stretch and move with the person’s body while keeping the cannula in place, and the detrimental effect of using skin adhesives to optimize adherence of the device to the person’s skin.
Once the final design was selected, a validation study was performed. The primary objective of this study was to validate the adherence of the adhesive tape when worn by adults with T2DM. The secondary objective was to observe the occurrence and severity of dermal irritation. This study was a prospective, single-center, open-label adherence validation of the device adhesive tape over two 3-day wear periods. The first wear period was for the participants to gain experience wearing the device and to become familiar with completing the diary. The second wear period was for the validation.
Thirty volunteers enrolled in the study with a mean age of 52 years, mean body mass index (BMI) 31.7 kg/m2 and 55% male.
The adherence and dermal irritation results are summarized in Table 3. Twenty-seven out of 30 devices (90%) remained acceptably adhered to the participant’s application site at the end of the 3-day wear period. No edema or blister formation was seen. There was 1 case of mild dermal irritation at the application site and 7 participants experienced mild erythema at the application site.9
Table 3.
Adherence and Dermal Irritation Results.
| Baseline—Before PAQ application |
Day 3—PAQ removal |
|
|---|---|---|
| N = 30 Left side = 10 Right side = 19 Center = 1 |
N = 30 Left side = 10 Right side = 19 Center = 1 |
|
| Adherence to skin | ||
| 0 = full adherence to scallops loosened | N/A | 27 |
| 1 = area around cannula site loosened or device fell off | N/A | 3 |
| Erythema | ||
| 0 = None | 30 | 23 |
| 1 = Mild | 0 | 7 |
| 2 = Moderate | 0 | 0 |
| 3 = Severe | 0 | 0 |
| Edema | ||
| 0 = None | 30 | 30 |
| 1 = Mild | 0 | 0 |
| 2 = Moderate | 0 | 0 |
| 3 = Severe | 0 | 0 |
| Blister formation | ||
| 0 = None | 30 | 30 |
| 1 = Present | 0 | 0 |
| Irritation | ||
| 0 = None | 30 | 29 |
| 1 = Mild | 0 | 1 |
| 2 = Moderate | 0 | 0 |
| 3 = Severe | 0 | 0 |
Color Study
Feedback received from adults with diabetes during device development was the importance of discretion and whether the device was visible under clothing. To study this, different colors of the device were tested for detectability when worn under clothing. A white colored device and a gray colored device were placed on a body form with dark and medium skin tones. A white T-shirt was placed on the body form covering the devices. A label (A or B) was placed on top of the shirt over the devices. During the study, the labels were randomly switched, sometimes A was over the white device and other times it was over the gray device, to prevent selection bias. With the T-shirts in place, 30 adults with diabetes taking at least two injections per day of insulin (55% male, mean age 52 years with a BMI of 31.7 kg/m2) were asked to choose the least noticeable device, A or B. The majority of the participants (28/30, 93%) chose the gray device as less noticeable on both the dark and medium skin tones.10
Comparison of Basal/Bolus Insulin Delivery Methods
Given the intent was to design an alternative way to deliver insulin that would meet the needs of the users (people with T2DM and their HCPs) we compared the device to other modes of insulin delivery in the market place. The key similarities and differences of existing basal/bolus insulin delivery methods are summarized in Table 4. The key differences include the following:
Table 4.
Comparison of Basal-Bolus Delivery Methods.
| Pens and needles | PAQ® | V-Go® | OmniPod® | Paradigm Revel™ | ||
|---|---|---|---|---|---|---|
| Wearable device | Wearable device | Wearable pump—PDM for programming | Infusion set tethered to programmable pump | |||

|

|

|

|

|

|
|
|
| ||||||
| Simple | ||||||
| No. of training visits | 1 | 1a (within 60 minutes) | Data not available | 2-3b (2-3 hours/visit) | 2-3b (2-3 hours/visit) | |
| Needle sticks | ⩾2 to 9c per day | Cannula placed every 3 days | Needle placed once per day | Cannula placed once every 3 days | Cannula placed once every 3 days | |
| Insulin administered | Rapid, intermediate Long-acting Mixtures |
One—Rapid-acting | ||||
| Discreet | ||||||
| Dosing | No | Yes—worn under clothing | ||||
| Notification of insulin flow | NA | Yes—through vibration | NA | No—audible alarm | No—audible alarm | |
| Safe and effective insulin delivery | ||||||
| Mode of basal insulin delivery | Large volume injection | CSIId | CSIId | CSIId | CSIId | |
| Reservoir volume | NA | 140-330e | 76 U/day, 36 U for bolus, 20-40 basal | 85-200 | 176-300 | |
| Basal delivery | NA | 7 preset rates | 3 preset rates | Programmable rates | Programmable rates | |
| Bolus increment (units) | One injection at each meal | 2 /push of button | 2 /push of button | Programmable: 0.05, 0.1, 0.5, or 1.0 | Programmable: 0.025, 0.05, or 0.10 | |
| Satisfaction | Nof | Yesc | Yesg | Yesh | Yesi | |
| Out of insulin/occlusion detection | NA | Yes (vibration) | No | Yes (alarm) | Yes (alarm) | |
PAQ insulin delivery device summative human factors study report CQR-15008-02.
CeQur interviews with diabetes educators.
Mader J, et al. Diabet Med. 2018. Published online: June 11, 2018 (doi:10.1111/dme.13708. PMID: 29888811).
Parkner T, et al. Diabet Med. 2008;25:585-591.
Fill volume is dependent upon the amount needed over 3 days by the user.
Peyrot M, et al. Diabetes Care. 2010;33(2):240-245.
Rosenfeld C, et al. Endocr Pract. 2012;18(5):660-667.
Polonsky W, et al. Diabetes Technol Ther. 2016;18(10):664-670.
Raskin P, et al. Diabetes Care 2003;26:2598-2603; Aronson R, et al. Diabetes Technol Ther. 2015;17(Supp 1):A11.
Simple
Training time—Learning to inject insulin for the very first time for some can take quite a bit of time; however, having this knowledge facilitates training for CSII. The training required for the preset wearable insulin delivery device is much less than the programmable insulin pumps.
Number of needle sticks per day—Two to 9 injections per day versus 1 stick for cannula placement every 1 to 3 days for CSII devices.
Insulin administered—Requirement of two types of insulin (long-acting and short-acting insulin) when using injectables versus one (short acting insulin administered continuously to cover basal insulin requirements and administered as bolus to cover meals and correct hyperglycemia) for CSII devices.
Discreet
All CSII devices are discreet in that they are worn under the person’s clothing, whereas pens and needles are/can be noticeable.
Insulin flow detection—The PAQ device emits silent vibrations to notify the user if the device is out of insulin or flow is occluded, while the programmable CSII devices emits loud alarms.
Safe and Effective Insulin Delivery
Mode of basal insulin administration—Pens deliver large volume injections versus continuous subcutaneous basal infusion. Data from Parkner et al suggest a large-volume bolus of injected, delayed-action insulin absorbed more poorly than the small volume delivered through CSII.7
Reservoir volume—The PAQ and Revel have similar reservoir capacity which can accommodate up to 100-110 units/day, for up to 3 days, more if used for 2 days. However, the V-Go and OmniPod have less reservoir capacity.
Basal delivery rates—Wearable preset devices provide fixed basal rates (PAQ 7; V-Go 3) and don’t require user programming; hence no potential for programming errors.
Bolus dosing—All CSII devices have injection free bolus dose administration. V-Go is limited to 36 bolus units/day. PAQ does not have this limitation.
Satisfaction—Data from studies/retrospective reports support greater satisfaction with CSII administration versus injectable insulin delivery with pens and syringes.
Out of insulin/occlusion detection—Not all preset wearable devices have insulin flow disruption detection systems, while all programmable pumps have this feature.
Clinical Testing
Clinical studies were initiated following the human factors and adhesive tape studies. These studies have been previously reported in other journals, but merit mention in this article as they were part of the development of PAQ device.11,12 The goals of these studies were to evaluate (1) feasibility of use, (2) safety, (3) patient-reported outcomes, and (4) glycemic control.
The first of the 3 studies was reported by Mader et al.11 It was a 6-week, prospective, single-arm, pilot study that evaluated the feasibility of using the device in adults with T2DM on a stable regimen of multiple daily injections (MDI). It was a single-center, open-label, single-arm study comprising three 2-week study periods: baseline (MDI), transition from MDI to the device, and device treatment. The clinical study was performed with an early version of the device, which is similar to the current version, with one exception; the Messenger used in the study did not have the ability to sense occlusions or whether the device was running out of insulin; it did indicate how much time had passed since device activation. The primary objective of this study was to evaluate the ability of patients to correctly use the device. No optimization of insulin therapy occurred. Participants were switched from MDI to a device daily basal dose that was closest to their current dose of basal insulin. The amount of the participant’s mealtime boluses was dependent upon his or her carbohydrate intake and should have been similar to the amount they took during their Baseline Period. Twenty adults enrolled (average age 59 ± 5 years, 79% male, HbA1C 7.7 ± 0.7%, BMI 32.1 ± 5.6 kg/m2, diabetes duration 15 ± 7 years, total daily dose of insulin [TDD] 60.4 ± 19.1 U [0.63 U/kg/day]) and 18 completed the study. All (18/18) participants could set up and use the device without errors leading to serious adverse device effects. Transition to the device using the first basal dose selected was achieved in 74% of the participants. The adherence rate of the device to the users’ skin for the intended 3-day wear period was 90%. Approximately half of the participants reduced their TDD by 26% (8-23 U), 25% of participants TDD remain unchanged (dose within 10% of baseline dose) and the remainder4 had a ⩾10% increase in their TDD at the end of device treatment. A trend toward improved glycemic control was seen in the pre- and post-breakfast and bedtime self-monitored blood glucose (SMBG) values. A patient-reported outcome questionnaire revealed a high level of patient satisfaction with the device. Furthermore, results from a patient-reported outcome questionnaire showed barriers to insulin treatment were significantly reduced after the use of the device, P = .01.13
The second and third studies, which were jointly reported in one publication by Mader et al, were also pilot studies, but these studies unlike the first, had a 12-week device treatment period in order to evaluate the effect of insulin delivery by the device as measured by HbA1c.12 In addition, these studies evaluated a different population of adults with T2DM, that is, those taking at least 2 insulin injections per day, who were in poor glycemic control and required optimization of their insulin therapy. Other than these two differences the studies were conducted under a similar design as their predecessor; single-center, open-label, single-arm study comprising three study periods: 1-week baseline (MDI), transition from MDI to the device, and 12-week device treatment.
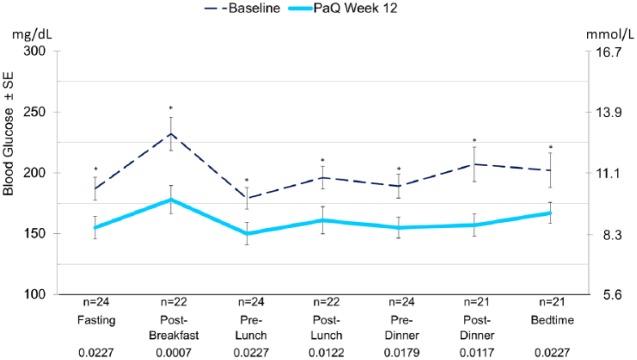
Twenty-eight adults enrolled (age 63 ± 7 years, 86% male, HbA1c 8.6 ± 1.1%, BMI 32.3 ± 4.3 kg/m2, diabetes duration 17.5 ± 8 years, TDD 58.7 ± 20.7 [0.59 U/kg]) and 24 completed these two studies. When transitioned to the device, 75% of participants continued on the first basal rate selected. After 12 weeks of using the device significant reductions from baseline were seen in HbA1c −1.5 ± 0.85 % (P < .0001) Figure 4 and all 7-point SMBG values (Figure 5). There was an increase in TDD (12.1 ± 19.5 U; P = .0058) and number of daily meal time bolus doses (0.9 ± 1.5; P = .0081). Body weight did not increase significantly from baseline. Six participants had mild to moderate catheter site reactions and one had mild skin irritation. No participant experienced severe 3rd party-assisted hypoglycemia. Furthermore, post study interviews were conducted with several of the study participants. Anecdotal comments received from these participants such as “I had freedom wearing the device,” “I had one needle stick for 3 days compared to 12,” and “I can go out to lunch and dose and no one knows” revealed the participants’ satisfaction with the device.
Figure 4.
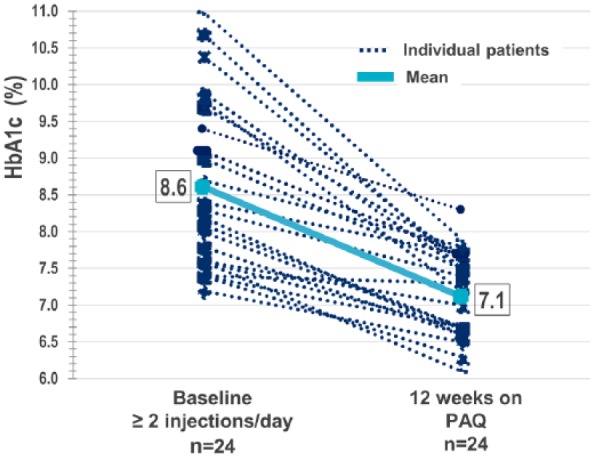
HbA1c change from baseline after 12 weeks of CSII on PAQ, per protocol population.
Figure 5.
Mean 7-point SMBG values at baseline and after 12 weeks of CSII with PAQ. Per protocol population. *P value < .05.
Affordability
While not a design specification, the device needs to be cost-effective to the health care system and affordable to people with diabetes. In order to determine if this would be the case with the device, CeQur conducted an analysis of the cost-effectiveness in the United States of a simple CSII device compared to MDI in people with T2DM not in adequate glycemic control. The UKPDS Outcomes Model was used to project long-term cost-effectiveness over 40 years, based on results from recently published studies and costs for the United States. Costs and outcomes were discounted at 3%. Cost-effectiveness was predefined in relation to per capita gross domestic product (GDP) with incremental cost-effectiveness ratios (ICERs) below 1X or
3X GDP per capita per life year gained, as “highly cost-effective” or “cost-effective,” respectively. Our analysis showed 0.11 life year gained on average and annual discounted savings on complication costs and insulin reductions of $3005. Based on projected costs and life expectancy, a simple CSII device will be highly cost-effective in the United States at a price of $13.40 per day and cost-effective at a price of $23.70. This implied an ICER at 0.56, 0.98 times per capita GDP per life year gained for the two cases, respectively. These estimates were very robust to sensitivity analyses on both reductions in HbA1c and dose effects.14 In addition, from a user perspective, out-of-pocket expenses are expected to be similar to insulin pen delivery.
Conclusion
This simple 3-day insulin delivery device aims to be an alternative insulin delivery method that overcomes the known barriers to insulin therapy, and to meet the needs of the users—be simple, discreet, safe, and effective. The collective data from the human factors, healthy volunteer, and clinical studies demonstrated that the PAQ insulin delivery device has achieved these goals. Data from the human factors testing validated the device design to show it is simple to use and safety related use errors are minimized. In addition, the device design has met the users’ need for discreetness. Data from clinical studies demonstrated that the device effectively delivers basal and bolus insulin therapy resulting in improved glycemic control by the study participants.
Footnotes
Abbreviations: BMI, body mass index; CSII, continuous subcutaneous insulin infusion; HCP, health care provider; ICER, incremental cost-effectiveness ratio; IFU, Instruction for Use; MDI, multiple daily injections; QSG, Quick Start Guide; SMBG, self-monitored blood glucose; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; TDD, total daily dose.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: LCL was an employee and is now a consultant for CeQur. JKM is a member of the advisory board for Becton-Dickinson, Boehringer Ingelheim, Eli Lilly, Medtronic, and Sanofi, and received speaker honoraria from Abbott Diabetes Care, Astra Zeneca, Eli Lilly, Nintamed, Novo Nordisk, Roche Diabetes Care, Sanofi, Servier, and Takeda. JW is an employee of CeQur.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by CeQur.
ORCID iD: Leslie C. Lilly  https://orcid.org/0000-0002-3232-0645
https://orcid.org/0000-0002-3232-0645
References
- 1. Defronzo RA. Banting lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. UK Prospective Diabetes Study Group. UK Prospective Diabetes Study 16. Overview of six years’ therapy of type 2 diabetes—a progressive disease. Diabetes. 1995;44:1249-1258. [PubMed] [Google Scholar]
- 3. Holman RR, Farmer AJ, Davies MJ, et al. Three-year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med. 2009;361:1736-1747. [DOI] [PubMed] [Google Scholar]
- 4. Selvin E, Parrinello CM, Daya N, Bergenstal RM. Trends in insulin use and diabetes control in the U.S.: 1988-1994 and 1999-2012. Diabetes Care. 2016;39:e33-e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Peyrot M, Rubin RR, Kruger DF, Travis LB. Correlates of insulin injection omission. Diabetes Care. 2010;33:240-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. dQ&A. Profiles of Insulin Delivery Among Type 2 Respondents. Report DDC-00474v1. 2016. [Google Scholar]
- 7. Parkner T, Laursen T, Vestergaard ET, et al. Insulin and glucose profiles during continuous subcutaneous insulin infusion compared with injection of a long-acting insulin in type 2 diabetes. Diabet Med. 2008;25:585-591. [DOI] [PubMed] [Google Scholar]
- 8. CeQur PAQ Insulin Delivery Device Summative Human Factors Study Report. Report USE-15005. Saratoga, CA: Interface Analysis Associates; 2015. [Google Scholar]
- 9. Validation Study in Healthy Volunteers to Assess the Performance of the Adhesive Tape. Report CQR-15008-02 R1. Marlborough, MA: CeQur Corporation; 2015. [Google Scholar]
- 10. Kuerschner C, Lilly L, Warner J. PAQ®, a simple, wearable three-day basal/bolus insulin delivery device, designed for discreetness. Adv Tech Treat Diab. 2017;19:A-104-A-105. [Google Scholar]
- 11. Mader JK, Lilly LC, Aberer F, et al. A feasibility study of a 3-day basal-bolus insulin delivery device in individuals with type 2 diabetes. Diabetes Care. 2014;37:1476-1479. [DOI] [PubMed] [Google Scholar]
- 12. Mader J, Lilly L, Aberer F, et al. Improved glycaemic control and treatment satisfaction with a simple wearable 3-day insulin delivery device in people with type 2 diabetes. Diabet Med. 2018;35:1448-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hermanns N, Lilly L, Mader J, et al. Novel simple insulin delivery device reduces barriers to insulin therapy in type 2 diabetes: results from a pilot study. J Diabetes Sci Technol. 2015;9:581-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Henricksen O, Dall M, Warner J, Parkin C. Simple CSII Is Highly Cost-Effective. Boston, MA: American Diabetes Association; 2015. [Google Scholar]