Abstract
The current controversial discussion on the disease-specific survival of patients with human papillomavirus (HPV)-positive (+) and -negative (−) squamous cell carcinoma (SCC) of the head neck region was the motivation for the present meta-analysis. Different detection methods for HPV are available, though these often lack sensitivity. As a consequence, there may be false interpretation of HPV positivity. A bias concerning HPV status and therefore also survival rates is serving a non-durable relevance in the discussion of tailored therapies. A literature search was performed via the online database PubMed/NCBI, and data extraction and statistical analysis were conducted. A total of 139 studies published between 2004 and 2014 were evaluated in the present meta-analysis. The HPV detection methods, patient characteristics, tumor localizations and stages, as well as (neo-) adjuvant therapies and survival times were analyzed. The average incidence rates of HPV+ patients with oropharyngeal tumors were higher than those of patients with cancers of other regions of the head and neck. Upon evaluating the results of different detection methods no significant differences were identified. We have compared the HPV incidence rates of each detection method, when studies have used more than one. Regarding overall survival, the pooled adjusted hazard ratio (HR) for oropharyngeal SCC was 0.31 [95% confidence interval (CI)=0.27–0.36]. Unfortunately, only 3 equivalent studies were available on nonoropharyngeal tumors, for which the pooled adjusted HR was 1 (95% CI=0.73–1.36). Overall, the evaluation demonstrated that the survival rates reported in numerous studies were not evaluated multifactorially and important confounders were excluded from the statistics. The HPV detection methods used were often not sufficient in representing HPV positivity. In addition, oropharyngeal and oral SCCs were assessed together in the localization. The widely differing number of HPV+ patients in each of the various studies may be explained by insufficient detection methods and by a lack of localization distinction. The considerations of a tailored therapy according to HPV status should be rejected based on the present information. The previously published studies should be read critically and do not represent a basis for therapeutic decisions.
Keywords: human papillomavirus, oropharyngeal squamous cell carcinoma, detection methods, survival
Introduction
Head and neck cancer (HNC) is reportedly the sixth most common cancer diagnosed worldwide; in 2008, ~633,000 new cases were diagnosed, and ~355,000 cases resulted in mortality (1). HNC encompasses all malignant tumors of the upper aerodigestive tract, which begins at the vermilion border of the lips and extends to the beginning of the esophagus. Approximately 90% of malignant neoplasms of the head and neck are squamous cell carcinomas (SCCs), while only just >5% are adenocarcinomas (2). The incidence rate varies according to geographic region and associated risk factors of differing severity. In recent decades, a declining incidence rate, particularly for the cancers of the oral cavity, hypopharynx and larynx, has been observed as an effect of reduced tobacco consumption in industrialized countries. However, in contrast, there has been a rapid increase in the incidence rate of oropharyngeal cancers, particularly of those of the tonsils and base of the tongue, which have been associated with human papilloma virus (HPV) (3,4). In 1983, HPV was first reported in association with head and neck SCC (HNSCC) (5). The International Agency for Research on Cancer officially recognized HPV-16 infection as a risk factor for oropharyngeal SCC (OPSCC) in 2007 (6). In recent decades, the number of oropharyngeal cancers caused by HPV has risen sharply: From 1988 to 2004, it increased in the US by ≤225% (7). But not only in North America, also in Europe this observation was found (8,9). Of the 100 cancers of the pharynx, 40 are assumed to be associated with HPV (2). HPV-associated HNSCCs occur in different populations from those with cancers induced by noxious agents, and different pathogenic processes underlie their oncogenesis. In the literature, a notable discordance exists among individual studies of HPV-associated HNSCC. Estimates of the incidence rate of HPV-associated tumors also differ greatly. Furthermore, the majority of studies conclude that patients with HPV-associated HNSCC have a survival advantage. An important question regarding the selection of a therapeutic approach in the future is whether treatment that is less intense will be sufficient for HPV-associated tumors.
The objectives of the present study were to improve current understanding concerning: The incidence rate of HPV-associated tumors; the most reliable detection methods; and the survival probability of HPV+ patients.
Materials and methods
Search strategy
The meta-analysis was conducted according to the guidelines of the Cochrane Collaboration and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (10,11). For an online literature search, the PubMed database was used. To identify the primary studies, the following terms were combined: ‘HPV’ and ‘HNSCC’, ‘oral cavity’, ‘OPSCC’, ‘oropharyngeal’ or ‘tonsil’ and ‘overall survival’, ‘disease-specific survival’ or ‘treatment modalities’.
Study eligibility and data extraction
The following conditions were defined for the incorporation of studies into the meta-analysis: The object of interest had to be a patient diagnosed with HNSCC; each study had to include at least 50 patients (two exceptions - there have been ≥50 patients, but only 42 respectively 49 were available for HPV testing. These two studies were included because they seemed to be very useful, due to the fact both evaluated many parameters and also considered the kind of treatment.); HPV detection had to be conducted and explained; and the survival rates had to be described and separated into HPV+/HPV− groups. Reviews and previous meta-analyses were not eligible. The publication was not included if it was more than 10 years old (two exceptions) and if it was not written in German or English. Additionally, the publication had to be available as full text. The selected studies were analyzed with respect to the following parameters: Number of patients, HPV detection method and the number of HPV+/HPV− test results, HPV subtypes, tumor localization, treatment, mean age of HPV+/HPV− patients at the time of diagnosis, alcohol consumption of HPV+/HPV− patients, tobacco use of HPV+/HPV− patients, sex of HPV+/HPV− patients, tumor, node and metastasis (TNM) stage, country and outcome. The extracted data were collected in an Excel spreadsheet.
Statistical analysis
In the statistical evaluation, the respective minima, maxima, means and medians were determined for all extracted data sets of the above-mentioned criteria. The statistical analysis of the data to compare the detection methods and the data on the supposed survival advantage was carried out with the support of the staff of the Institute of Medical Statistics and Epidemiology of the Technical University of Munich.
For the comparison of the different detection methods the ‘Random-Effect’ model was used and the Risk Difference (RD) with the 95% CI was calculated. After the heterogeneity test, also for the statistical evaluation of the supposed survival advantage, the ‘Random-Effect’ model was used. And by using the published hazard ratios (HR) and their 95% confidence interval (CI) the pooled HR for overall survival (OS) was calculated. For data collection, statistical analysis and the preparation of tables and charts, Microsoft Excel 2011 (Microsoft Corporation, Redmond, WA, USA) and R packages (cran.r-project.org/) were used.
Results
Literature search results
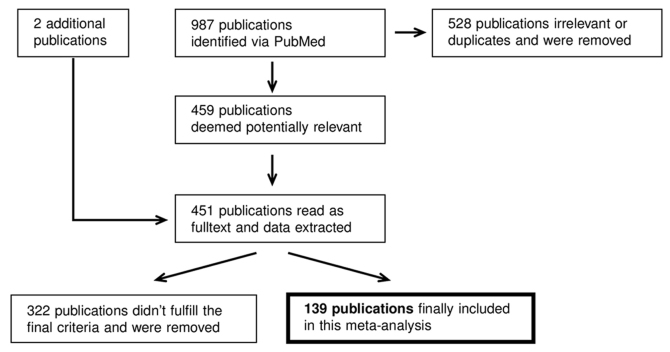
The literature search via PubMed yielded a total of 987 hits. The flow diagram in Fig. 1 illustrates how a final of 139 publications were selected. All abstracts were read to identify useful publications, and duplicates were rejected. In total, 459 publications, which were perceived as helpful, were read as full text. Among the sources of these publications, two further studies (>10 years) were identified to be useful and included in the present meta-analysis. The relevant data were extracted and transferred to an Excel spreadsheet. In 139 publications out of the 461 studies, including a total of 21,774 patients, fulfilled the final criteria. Of these studies, 80 were concerned solely with OPSCC (7,12–90), 13 with oral SCC (OSCC) (91–103) and 31 with both OPSCC and OSCC (104–134); 15 studies provided results on HNSCC in general (135–149).
Figure 1.
Schematic of the methodology employed for the literature research (Bischof C: PhD Thesis, in prep). Workflow of the data acquisition via literature research: Identification by keyword queries in PubMed, followed by removal of duplicates, inclusion of two additional older but well cited papers, then data extraction and the final dataset.
HPV prevalence
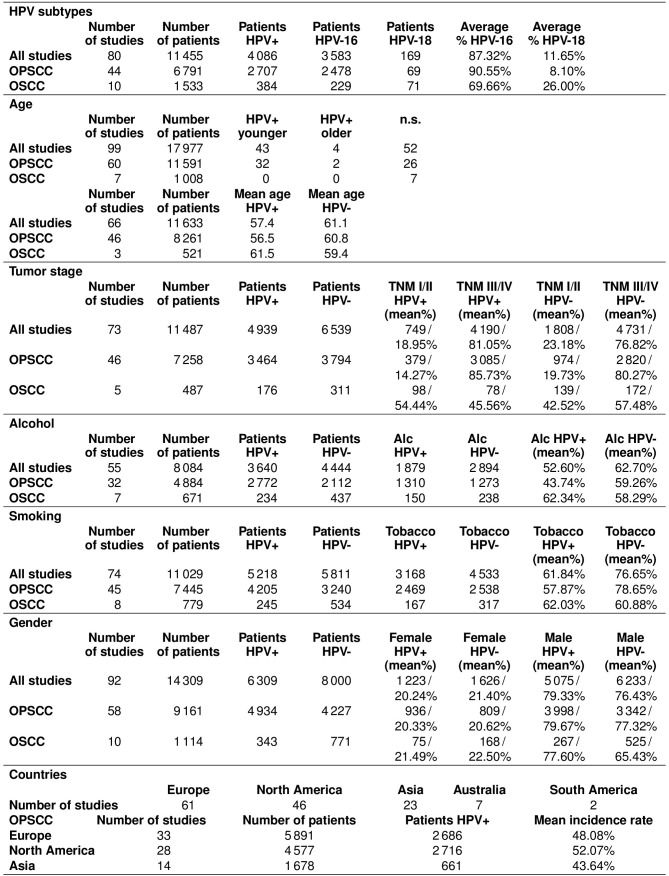
The average percentage of patients tested as HPV+ estimated from all included studies in the meta-analysis was 42.62% [95% CI=0.39–0.46]. The mean incidence rate of the 80 OPSCC studies (12,662 patients, 6,383 HPV+) was 49.85% (95% CI=0.45–0.54). By contrast, for the 13 studies on OSCC, the mean incidence rate was 27.39% (95% CI=0.18–0.36). In a further analysis, anatomic regions were divided more specifically. As the anatomical localization of the oropharynx is of special interest according to HPV positivity, this region was further divided into: Base of the tongue, tonsils and neither of these two locations [listed as other oropharyngeal SCC (OOPSCC) in Table I]. The average incidence rate of HPV+ patients with OPSCC was 50.47% (95% CI=0.47–0.54). Notably, cancers of the base of the tongue, with an average incidence of HPV+ patients of 48.61% (95% CI=0.42–0.56), and of the tonsils, with an average incidence of HPV+ patients of 55.32% (95% CI=0.50–0.61), exhibited a relatively high association with HPV infection in particular. By contrast, oropharyngeal cancer, classified as being outside of these two regions, tested as HPV+ to a much lower extent, with an average incidence of HPV positivity of 26.39% (95% CI=0.22–0.31). The average incidence rates of HPV+ patients with tumors of other head and neck regions excluding OPSCCs were generally below those for oropharyngeal tumors: For OSCC, 24.14% (95% CI=0.18–0.30), for laryngeal cancer, 20.61% (95% CI=0.140.27) and for hypopharyngeal cancer, 22.18% (95% CI=0.14–0.31). For HNSCC, the average incidence of HPV positivity was 32.93% (95% CI=0.27–0.39; Table I).
Table I.
Overview of the incidence rates.
| Variables | No. of studies | No. of patients | Patients HPV+ | Average HPV+ (%) | Minimum HPV+ (%) | Maximum HPV+ (%) | Median HPV+ (%) |
|---|---|---|---|---|---|---|---|
| All studies | 139 | 21,774 | 9,016 | 42.62 | 5.68 | 89.20 | 40.20 |
| OSCC studies | 13 | 2,044 | 495 | 27.39 | 5.68 | 64.00 | 25.09 |
| OPSCC studies | 80 | 12,662 | 6,383 | 49.85 | 11.54 | 89.20 | 51.14 |
| HNSCC | 20 | 3,433 | 1,049 | 32.93 | 11.56 | 68.63 | 29.68 |
| In HNSCC studies | |||||||
| OSCC | 40 | 3,550 | 737 | 24.14 | 0.00 | 75.68 | 21.03 |
| OPSCC | 110 | 14,230 | 7,178 | 50.47 | 3.23 | 95.00 | 50.38 |
| OOPSCC | 33 | 809 | 205 | 26.39 | 0.00 | 52.94 | 23.08 |
| Base of tongue | 34 | 1,501 | 760 | 48.61 | 1.85 | 82.26 | 50.00 |
| Tonsils | 55 | 4,316 | 2,272 | 55.32 | 4.29 | 95.00 | 57.78 |
| Larynx | 24 | 1,402 | 232 | 20.61 | 0.00 | 58.33 | 15.59 |
| Hypopharynx | 22 | 629 | 132 | 22.18 | 0.00 | 81.97 | 16.12 |
OPSCC, oropharyngeal squamous cell carcinoma; OSCC, oral squamous cell carcinoma; HNSCC, head and neck squamous cell carcinoma; OOPSCC, other oropharyngeal squamous cell carcinoma.
HPV detection
Of the studies, 95 involved polymerase chain reaction (PCR), 80 used p16 immunohistochemistry (IHC) and 30 studies employed in situ hybridization (ISH). A total of 79 studies determined HPV status with one detection method, while 60 studies used two, or all three, of the above methods. Evaluation of the different results regarding the incidence rates, if studies have used multiple detection methods, revealed no significant difference between PCR and IHC, ISH and PCR, and IHC and ISH (Fig. 2A-C).
Figure 2.
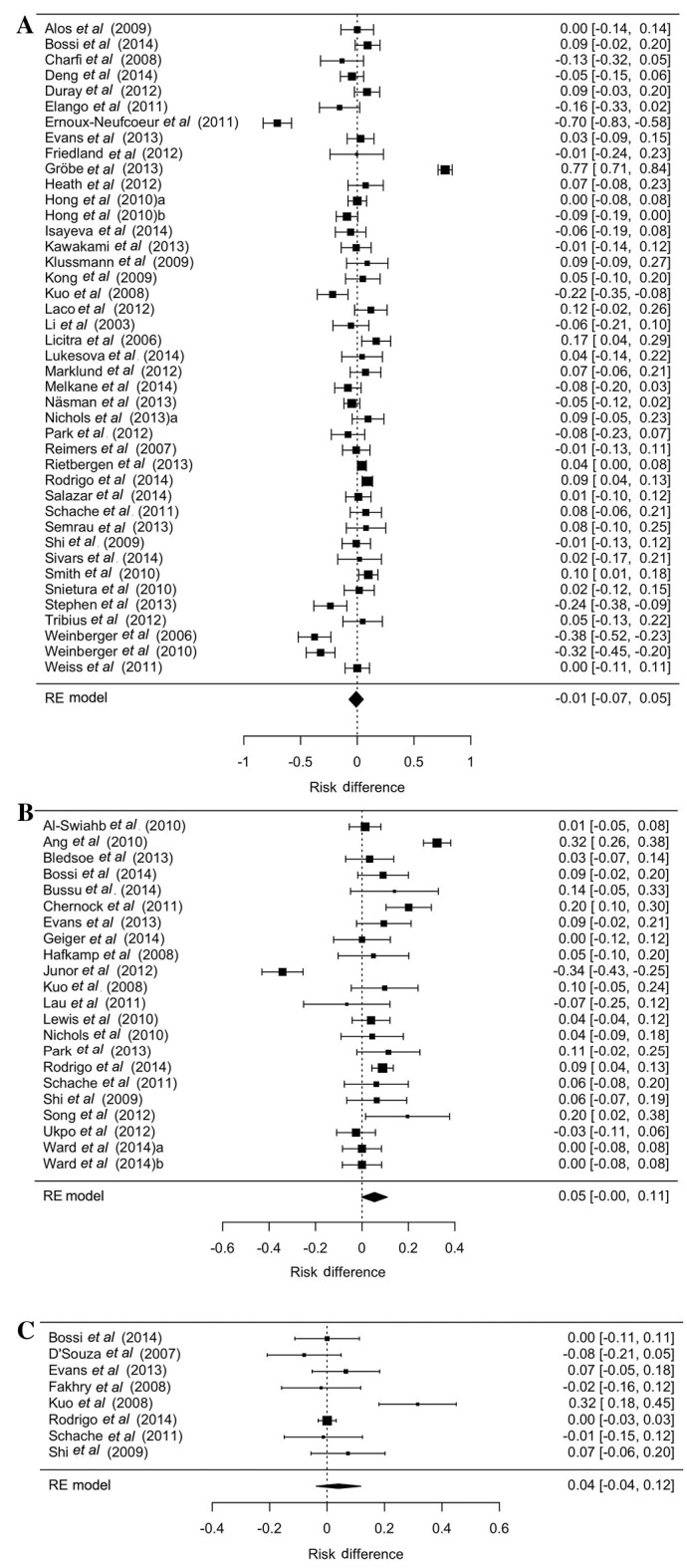
Risk differences due to HPV detection method in studies which used several HPV detection methods (Bischof C: PhD Thesis, in prep). (A) PCR and IHC; (B) ISH and PCR; and (C) IHC and ISH. By pooling the proportion of patients tested HPV-positive by the different methods the present study performed comparisons between them. HPV, human papillomavirus; PCR, polymerase chain reaction; IHC, immunohistochemistry; ISH, in situ hybridization.
HPV subtypes
A total of 80 studies (11,455 patients) provided information on the HPV subtypes. Among the 4,086 patients that tested HPV+, the two high-risk types HPV16 and 18 were the most frequently detected subtypes. In OPSCC (44 studies), HPV16 was detected on average in 90.55% (95% CI=0.87–0.94) of cases, and HPV18 was responsible for the infection on average in 8.10% (95% CI=0.05–0.11) of cases. Among the HPV-associated OSCC cases (10 studies), HPV16 and 18 were detected on average in 69.66% (95% CI=0.52–0.87) and 26% (95% CI=0.19–0.33) of the cases, respectively.
Associations with HPV profile
Data regarding other risk factors (age, TNM status, alcohol and tobacco consumption, sex) and differences between HPV+/− cases are summarized in Fig. 3. The most interesting finding was, that HPV+ patients with OPSCC were often described to be younger than HPV− patients, also the mean age was lower for HPV+ patients with OPSCC (56.5 vs. 60.8 years). But this fact could not be observed for HPV+/− patients with OSCC. Distribution of the studies according to the various countries and the mean incidence rates for OPSCC according to country are also given in Fig. 3.
Figure 3.
Data regarding HPV risk factors (Bischof C: PhD Thesis, in prep). Age: The upper panel summarizes data without detailed age information (only HPV+ patients are older/younger, no significant difference), the lower panel of the data summarizes detailed age information. Alcohol: The number of patients who described themselves as regular alcohol consumers. Smoking: The number of patients who described themselves as regular tobacco consumers. Data are presented as the n number of patients and/or the mean percentage. HPV, human papillomavirus; n.s., not significant; TNM, tumor-node-metastasis; OPSCC, oropharyngeal squamous cell carcinoma; OSCC, oral squamous cell carcinoma.
HPV and patient survival
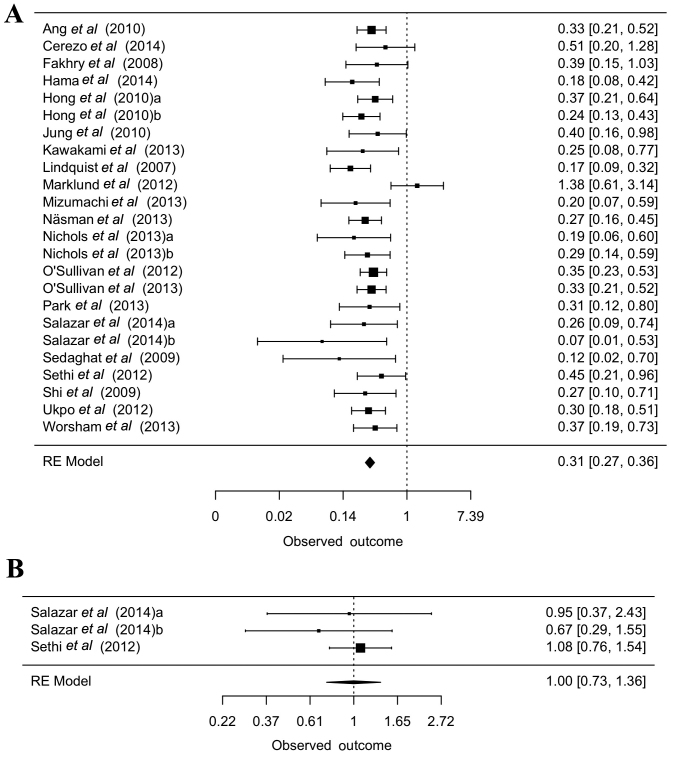
In the 135 studies providing information on patient survival, 94 reported an improved outcome for patients who tested HPV+, 5 documented a poorer survival rate, 25 could not detect any significant difference, and 11 reported a higher probability of survival only with HPV+ oropharyngeal tumors. Out of the 135 studies, 77 focused on oropharyngeal tumors with information on survival; 67 of these reported of an improved outcome in HPV+ patients, and 10 could not detect a significant difference. Of the 12 studies on OSCC with data on survival rates, 3 reported an improved outcome in HPV+ patients, 3 a poorer outcome, and 6 could not detect a significant difference. The data on the probability of survival were described in various formats, with the largest amount of comparable data being available for overall survival. The pooled adjusted hazard ratio (HR) for OPSCC was 0.31 (95% CI=0.27–0.36). Unfortunately, only 3 equivalent studies were available for nonoropharyngeal tumors, for which the pooled adjusted HR was 1 (95% CI=0.73–1.36, Fig. 4A and B).
Figure 4.
Overall survival regarding HPV positivity, revealed a better outcome for HPV+ patients. (A) oropharyngeal tumors and (B) non-oropharyngeal tumors (Bischof C: PhD Thesis, in prep). Pooled adjusted hazard ratio for the overall survival of patients with oropharyngeal squamous cell carcinoma and nonoropharyngeal tumors. The hazard ratios were given in the studies. HPV, human papillomavirus.
Discussion
In the literature, the way in which prevalence rates of HPV-associated HNSCC are described varies greatly, with the main variant factors being insufficient description and fractionation of anatomical localizations, different risk factors (attributable to the time-point and the place of study), and non-uniform detection methods. In the present meta-analysis, the average incidence rate of HPV-associated HNSCC was 42.62%. In a similar meta-analysis of 60 studies (5,046 patients), the average incidence rate was 25.9% (95% CI=0.25–0.27) (150). Another meta-analysis of 34 studies (5,681 patients) reported that 21.95% (95% CI=0.21–0.23) of HNSCCs were associated with HPV (151). The significantly higher incidence rate in the present analysis may be attributable either to i) more than half of the included studies (80 studies, 12,662 patients) only investigating OPSCC, which is known to be more frequently associated with HPV (150); ii) studies older than 10 years not being included. Analysis of the 5% of studies with the highest/lowest incidence rates of HPV-associated HNSCC revealed notable similarities: In contrast to the studies with low incidence rates, most studies with high incidence rates were from North America and involved exclusively OPSCC (15,28,31,46,49,54,79,94,97,100,112,120,136,140). The average prevalence rate in the 110 studies (14,230 patients) that provided data on tumors of the oropharynx was 50.47%; in the two meta-analyses mentioned above, the average incidence rate of HPV-associated OPSCC was 35.6% (95% CI=0.33–0.39) (150) and 41% (95% CI=0.38–0.44), respectively (151). Again, the different time periods over which the studies were conducted may be responsible for the observed differences. Many studies have reported a dramatic increase in the incidence of HPV-associated OPSCC in recent decades (8,9). However, even among the HPV-associated OPSCC cases, a relatively high level of variation exists in incidence rate (minimum: 11.54%, maximum: 89.20%) (28,49). Furthermore, analysis of the 5% of OPSCC studies with the highest/lowest incidence rates of HPV-association determined some similarities: All of the 5% of studies with the highest incidence rates were conducted in North America, whereas none of the studies with the lowest incidence rates was conducted in North America (15,22,28,31,41,49,54,73). The studies with the low incidence rates of HPV-associated HNSCC mentioned the different geographic regions (41,73), the higher prevalence of ‘classic’ risk factors and therefore a lower HPV prevalence (22,41,73), and the data collection over many years (28,73) as possible causes of the low incidence rates of HPV-associated HNSCC.
In the present analysis, OPSCCs were further divided into groups. Notably, a significantly higher HPV association for cancers of the tonsils and base of the tongue was observed, in contrast to cancers of other oropharyngeal regions. The average incidence rate of HPV-associated tumors (3,550 patients) of the 40 studies on OSCC was 24.14%, and therefore was similar to that determined in the meta-analysis by Kreimer: 23.5% (95% CI=0.22–0.25) (150). The current meta-analysis highlights the importance of a detailed description and distinction of the relevant anatomical localizations, since different HPV infection rates are obvious (8,52,53). As another factor, the time of the study appears to influence the HPV incidence rate (8,9); this may be explained by the significant decrease in smoking populations over the past decades (152). Due to the elimination of ‘classic risk factors’, including smoking and alcohol abuse, the number of cancers associated with other possible triggers increases. However, an increased HPV prevalence is also likely to serve a role; this may be attributable to changes in sexual practices and potential transmission via oral sex (24).
The lack of uniform standards for HPV detection makes it difficult to compare individual studies. Each detection method has its own advantages and disadvantages: In addition to sensitivity and specificity, the type of histological specimen, financial aspects, time available, and personal and equipment resources affect the choice of detection. Due to the various advantages and disadvantages, the most reliable HPV detection method may be a combined approach that establishes both an accurate result and a cost- and time-effective test method; for instance, the high sensitivity method of p16 IHC may be combined with the high specificity method of PCR and a chip system as described previously (153). The current analysis compared the results of the various detection methods, but no significant difference was observed. There is an urgent need for uniform standards regarding the HPV test procedure. However, the test method combination described above must first be confirmed as sufficiently accurate. Furthermore, the potential association between viral biological activity/integration into the host genome/higher viral load and higher survival rate must be clarified.
HPV16 and 18 were the most commonly detected subtypes (11,455 patients in 80 studies on subtype), with this result being consistent with other studies (150,151). Notably, HPV16 was more common in OPSCC than in OSCC (90.55 vs. 69.66%), and HPV18 was more often detected in OSCC than in OPSCC (26.00 vs. 8.10%). These observations have previously been described in other studies (8,150).
As mentioned above, the majority of studies observed a survival advantage for HPV+ HNSCC patients. It needs to be clarified whether all HPV-associated HNSCCs have this prognostic advantage, or whether only certain subsets of HPV-associated HNSCC are affected. Not only the anatomical sub-localizations but also the HPV detection method, the respective HPV subtype, the biological activity and the viral load may limit the extent of potential survival benefit. To avoid insufficient treatment, it needs to be examined more closely whether the survival benefit is independent of the applied treatment. A comparison of the individual studies is difficult, because they differ in certain key points including tumor localization, HPV detection method, the applied treatment and the endpoint of study. In concordance with other studies, a survival advantage of a 69% lower risk of fatality associated with any cause was observed for patients with HPV-associated OPSCC. For patients with non-oropharyngeal cancers, no survival benefit was identified, although only 3 comparable studies have been conducted. Many studies suggest that not all HPV-associated HNSCC have an improved prognosis, and that the anatomical localization is of prognostic importance (128,154). Also a recent study conducted at the TU Munich found no survival benefit for patients with HPV-associated OSCC (155). Among the above-mentioned five studies (HPV and patient survival) that reported of a worse outcome of patients with HPV-associated carcinomas, mainly patients with non-OPSCC were investigated (92,96,98,110,126). An interesting study by Marklund indicated that perhaps even the localization of ‘oropharynx’ is too vague, as for neither p16+ nor HPV+ patients with oropharyngeal tumors outside the tonsils and base of the tongue could prognostic benefits be observed (53). Furthermore, in another study, the survival advantage of HPV-associated OPSCC was limited to tumors of the tonsils and base of the tongue (52). Therefore, in future, the description and separation of OPSCC should be more detailed. The reason for HPV-associated cancers having an improved long-term prognosis is still not wholly clear, although several theories have been proposed. The favorable prognosis may be based on the fact that HPV-associated carcinomas have markedly fewer genetic alterations compared with carcinomas induced through noxious agents (44), and thus, an increased sensitivity to DNA-damaging processes exists (156). This observation is also in accordance with the fact that, among patients with HPV-associated cancers, fewer smokers are observed; as the probability of genetic alterations rises with each additional pack year (13). Additionally, this hypothesis is supported by the finding that HPV+ tumors with TP53 mutations have not been associated with an improved prognosis (48,157). No conclusion can be made as to whether the improved prognosis for surgically treated patients is invalid because of the small number of cases. Just as TP53 mutations can be observed less frequently in HPV-associated carcinomas (158), other relationships with specific biomarkers have been discussed, for example, the lower expression of epidermal growth factor receptor and a rarer amplification at 11q13 (159). Furthermore, combined effects of the immune response to the virus and the tumor may be responsible; specific T-cells against HPV16 E7 protein have been detected, but their role remains unclear (160). The fact that patients with HPV-associated HNSCC rarely develop secondary tumors may contribute to improved survival rates (161), although it should be noted that patients affected by HPV-associated cancers are mostly younger (13,88,139,162–165) and are less often tobacco and alcohol consumers (87,116). Certain studies claim that tobacco consumption is of prognostic significance in HPV+ and HPV− patients (13,32,33,54). However, some other studies refute this thesis, claiming the improved prognosis observed in HPV+ patients is independent of their tobacco consumption (26). Regarding comorbidities, for patients with HPV+ OPSCC and, in general, HNSCC with lower comorbidities, a significantly improved long-term survival has been observed (104). In contrast, for patients with HPV-associated OPSCC/HNSCC with higher comorbidities, no prognostic benefit has been recognized (104). This suggests that the health status of the patients should be considered (104). With respect to the detection methods, as explained above, the examination of HPV status with a combined method utilizing p16 IHC, PCR and chip analysis may be advantageous (153). However, the molecular mechanisms that underlie the presumed survival advantage have not yet been sufficiently studied. Thus, no definitive recommendations can be made for HPV detection, and further studies are still needed. Additional tests, including for TP53 mutations, amplification at 11q13, and E6 and E7 PCR may be helpful (154). In addition to the previously described factors that may influence the prognostic relevance of HPV-associated HNSCC, one important question is whether the supposed improved outcome is independent of the treatment method employed. An improved response to chemotherapy and radiotherapy has been discussed, but even for this there is disagreement; p16+ patients often underwent a more aggressive adjuvant therapy, because of their more frequent lymph node involvement and lower rate of comorbidities (14). This raises the question as to whether the prognostic benefits are attributable to the more intense treatment and not to the less aggressive tumors (14). Baumeister et al (14) also indicated that the most common causes of mortality in patients with HPV-associated carcinomas were distant metastases and the relatively late tumor onset; thus a 10-year monitoring period was suggested to be advantageous.
Regarding published studies on HPV infection in HNSCC, it should be noted that the detection methods and study cohorts may provide bias. Furthermore, the role of HPV infection in OSCC is of minor relevance, and only a minority of cases are HPV+. In OPSCC, and particularly in cancers of the tonsils and base of the tongue, HPV infection and positivity for the surrogate marker p16 may be of high relevance for survival. Therefore, p16-positivity is included in the recent World Health organization TNM classification for OPSCC. In the next few years, the predicted rising numbers of vaccination against HPV infection may serve a notable role regarding the incidence of HPV+ cancers. For details see also ‘Bischof C: PhD Thesis, in prep’.
Acknowledgements
The authors would like to thank Dr Lynne Stecher for providing support and helpful contributions to this manuscript.
Funding
No funding was received.
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
CG, CB, LS, KDW and AK designed the study. CG, CB, LS, KDW and AK conducted the study. CG and CB collected the data. CG, LS and CB analyzed the data. CG and CB interpreted the data. CG, CB, LS, KDW and AK drafted the manuscript. CG, CB, LS, KDW and AK wrote the manuscript. CG, CB, LS, KDW and AK approved the final version of the manuscript. CG, CB, LS, KDW and AK take responsibility for the integrity of the data analysis.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International journal of cancer. J Int Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Kaatsch P, Spix C, Katalinic A, Hentschel S, Luttmann S, Stegmaier C. Robert-Koch-Institut; Berlin: 2015. Cancer in Germany 2011/2012. Robert Koch Institute and the Society of Epidemiological Cancer Registries in Germany. (In German) [Google Scholar]
- 3.Licitra L, Zigon G, Gatta G, Sanchez MJ, Berrino F. Human papillomavirus in HNSCC: A European epidemiologic perspective. Hematol Oncol Clin North Am. 2008;22:1143–1153. doi: 10.1016/j.hoc.2008.10.002. vii-viii. [DOI] [PubMed] [Google Scholar]
- 4.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26:612–619. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 5.Syrjänen KJ, Pyrhönen S, Syrjänen SM, Lamberg MA. Immunohistochemical demonstration of human papilloma virus (HPV) antigens in oral squamous cell lesions. Br J Oral Surg. 1983;21:147–153. doi: 10.1016/0007-117X(83)90060-4. [DOI] [PubMed] [Google Scholar]
- 6.IARc Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 90. IARC; Lyon: 2007. World Health Organization; International Agency for Research on Cancer: Human Papillomaviruses; pp. 1–636. [Google Scholar]
- 7.Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, Jiang B, Goodman MT, Sibug-Saber M, Cozen W, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–4301. doi: 10.1200/jco.2011.29.15_suppl.5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehanna H, Beech T, Nicholson T, El-Hariry I, McConkey C, Paleri V, Roberts S. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer-systematic review and meta-analysis of trends by time and region. Head Neck. 2013;35:747–755. doi: 10.1002/hed.22015. [DOI] [PubMed] [Google Scholar]
- 9.Stein AP, Saha S, Yu M, Kimple RJ, Lambert PF. Prevalence of human papillomavirus in oropharyngeal squamous cell carcinoma in the United States across time. Chem Res Toxicol. 2014;27:462–469. doi: 10.1021/tx500034c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D1, Liberati A, Tetzlaff J, Altman DG PRISMA Group, corp-author. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 12.Al-Swiahb JN, Huang CC, Fang FM, Chuang HC, Huang HY, Luo SD, Chen CH, Chen CM, Chien CY. Prognostic impact of p16, p53, epidermal growth factor receptor, and human papillomavirus in oropharyngeal cancer in a betel nut-chewing area. Arch Otolaryngol Head Neck Surg. 2010;136:502–508. doi: 10.1001/archoto.2010.47. [DOI] [PubMed] [Google Scholar]
- 13.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, Westra WH, Chung CH, Jordan RC, Lu C, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baumeister P, Reiter M, Welz C, Becker S, Betz C, Harréus U. Surgically treated oropharyngeal cancer: Risk factors and tumor characteristics. J Cancer Res Clin Oncol. 2014;140:1011–1019. doi: 10.1007/s00432-014-1631-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bledsoe TJ, Noble AR, Hunter GK, Rybicki LA, Hoschar A, Chute DJ, Saxton JP, Greskovich JF, Adelstein DJ, Koyfman SA. Oropharyngeal squamous cell carcinoma with known human papillomavirus status treated with definitive chemoradiotherapy: Patterns of failure and toxicity outcomes. Radiat Oncol. 2013;8:174. doi: 10.1186/1748-717X-8-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bossi P, Orlandi E, Miceli R, Perrone F, Guzzo M, Mariani L, Granata R, Locati L, Fallai C, Cortelazzi B, Pilotti S, et al. Treatment-related outcome of oropharyngeal cancer patients differentiated by HPV dictated risk profile: a tertiary cancer centre series analysis. Ann Oncol. 2014;25:694–699. doi: 10.1093/annonc/mdu004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Broglie MA, Soltermann A, Rohrbach D, Haile SR, Pawlita M, Studer G, Huber GF, Moch H, Stoeckli SJ. Impact of p16, p53, smoking, and alcohol on survival in patients with oropharyngeal squamous cell carcinoma treated with primary intensity-modulated chemoradiation. Head Neck. 2013;35:1698–1706. doi: 10.1002/hed.23231. [DOI] [PubMed] [Google Scholar]
- 18.Bussu F, Sali M, Gallus R, Petrone G, Zannoni GF, Autorino R, Dinapoli N, Santangelo R, Vellone VG, Graziani C, et al. Human papillomavirus (HPV) infection in squamous cell carcinomas arising from the oropharynx: Detection of HPV DNA and p16 immunohistochemistry as diagnostic and prognostic indicators-a pilot study. Int J Radiat Oncol Biol Phys. 2014;89:1115–1120. doi: 10.1016/j.ijrobp.2014.04.044. [DOI] [PubMed] [Google Scholar]
- 19.Cerezo L, López C, de la Torre A, Suárez D, Hervás A, Ruiz A, Ballestín C, Martín M, Sandoval P. Incidence of human papillomavirus-related oropharyngeal cancer and outcomes after chemoradiation in a population of heavy smokers. Head Neck. 2014;36:782–786. doi: 10.1002/hed.23366. [DOI] [PubMed] [Google Scholar]
- 20.Charfi L, Jouffroy T, de Cremoux P, Le Peltier N, Thioux M, Fréneaux P, Point D, Girod A, Rodriguez J, Sastre-Garau X. Two types of squamous cell carcinoma of the palatine tonsil characterized by distinct etiology, molecular features and outcome. Cancer Lett. 2008;260:72–78. doi: 10.1016/j.canlet.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 21.Chernock RD, Zhang Q, El-Mofty SK, Thorstad WL, Lewis JS., Jr Human papillomavirus-related squamous cell carcinoma of the oropharynx: A comparative study in whites and African Americans. Arch Otolaryngol Head Neck Surg. 2011;137:163–169. doi: 10.1001/archoto.2010.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chien CY, Su CY, Fang FM, Huang HY, Chuang HC, Chen CM, Huang CC. Lower prevalence but favorable survival for human papillomavirus-related squamous cell carcinoma of tonsil in Taiwan. Oral Oncol. 2008;44:174–179. doi: 10.1016/j.oraloncology.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 23.Cohen MA, Weinstein GS, O'Malley BW, Jr, Feldman M, Quon H. Transoral robotic surgery and human papillomavirus status: Oncologic results. Head Neck. 2011;33:573–580. doi: 10.1002/hed.21500. [DOI] [PubMed] [Google Scholar]
- 24.D'Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, Westra WH, Gillison ML. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 25.Ernster JA, Sciotto CG, O'Brien MM, Finch JL, Robinson LJ, Willson T, Mathews M. Rising incidence of oropharyngeal cancer and the role of oncogenic human papilloma virus. Laryngoscope. 2007;117:2115–2128. doi: 10.1097/MLG.0b013e31813e5fbb. [DOI] [PubMed] [Google Scholar]
- 26.Evans M, Newcombe R, Fiander A, Powell J, Rolles M, Thavaraj S, Robinson M, Powell N. Human Papillomavirus-associated oropharyngeal cancer: An observational study of diagnosis, prevalence and prognosis in a UK population. BMC Cancer. 2013;13:220. doi: 10.1186/1471-2407-13-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fakhry C, Zhang Q, Nguyen-Tan PF, Rosenthal D, El-Naggar A, Garden AS, Soulieres D, Trotti A, Avizonis V, Ridge JA, et al. Human papillomavirus and overall survival after progression of oropharyngeal squamous cell carcinoma. J Clin Oncol. 2014;32:3365–3373. doi: 10.1200/JCO.2014.55.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fallai C, Perrone F, Licitra L, Pilotti S, Locati L, Bossi P, Orlandi E, Palazzi M, Olmi P. Oropharyngeal squamous cell carcinoma treated with radiotherapy or radiochemotherapy: Prognostic role of TP53 and HPV status. Int J Radiat Oncol Biol Phys. 2009;75:1053–1059. doi: 10.1016/j.ijrobp.2008.12.088. [DOI] [PubMed] [Google Scholar]
- 29.Fei J, Hong A, Dobbins TA, Jones D, Lee CS, Loo C, Al-Ghamdi M, Harnett GB, Clark J, O'Brien CJ, et al. Prognostic significance of vascular endothelial growth factor in squamous cell carcinomas of the tonsil in relation to human papillomavirus status and epidermal growth factor receptor. Ann Surg Oncol. 2009;16:2908–2917. doi: 10.1245/s10434-009-0579-1. [DOI] [PubMed] [Google Scholar]
- 30.Fischer CA, Kampmann M, Zlobec I, Green E, Tornillo L, Lugli A, Wolfensberger M, Terracciano LM. p16 expression in oropharyngeal cancer: its impact on staging and prognosis compared with the conventional clinical staging parameters. Ann Oncol. 2010;21:1961–1966. doi: 10.1093/annonc/mdq210. [DOI] [PubMed] [Google Scholar]
- 31.Geiger JL, Lazim AF, Walsh FJ, Foote RL, Moore EJ, Okuno SH, Olsen KD, Kasperbauer JL, Price DL, Garces YI, et al. Adjuvant chemoradiation therapy with high-dose versus weekly cisplatin for resected, locally-advanced HPV/p16-positive and negative head and neck squamous cell carcinoma. Oral Oncol. 2014;50:311–318. doi: 10.1016/j.oraloncology.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Gillison ML, Zhang Q, Jordan R, Xiao W, Westra WH, Trotti A, Spencer S, Harris J, Chung CH, Ang KK. Tobacco smoking and increased risk of death and progression for patients with p16-positive and p16-negative oropharyngeal cancer. J Clin Oncol. 2012;30:2102–2111. doi: 10.1200/JCO.2011.38.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hafkamp HC, Manni JJ, Haesevoets A, Voogd AC, Schepers M, Bot FJ, Hopman AH, Ramaekers FC, Speel EJ. Marked differences in survival rate between smokers and nonsmokers with HPV 16-associated tonsillar carcinomas. Int J Cancer. 2008;122:2656–2664. doi: 10.1002/ijc.23458. [DOI] [PubMed] [Google Scholar]
- 34.Hama T, Tokumaru Y, Fujii M, Yane K, Okami K, Kato K, Masuda M, Mineta H, Nakashima T, Sugasawa M, et al. Prevalence of human papillomavirus in oropharyngeal cancer: A multicenter study in Japan. Oncology. 2014;87:173–182. doi: 10.1159/000360991. [DOI] [PubMed] [Google Scholar]
- 35.Hannisdal K, Schjølberg A, De Angelis PM, Boysen M, Clausen OP. Human papillomavirus (HPV)-positive tonsillar carcinomas are frequent and have a favourable prognosis in males in Norway. Acta Otolaryngol. 2010;130:293–299. doi: 10.3109/00016480903071377. [DOI] [PubMed] [Google Scholar]
- 36.Hong A, Dobbins T, Lee CS, Jones D, Jackson E, Clark J, Armstrong B, Harnett G, Milross C, O'Brien C, Rose B. Relationships between epidermal growth factor receptor expression and human papillomavirus status as markers of prognosis in oropharyngeal cancer. Eur J Cancer. 2010;46:2088–2096. doi: 10.1016/j.ejca.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 37.Hong AM, Dobbins TA, Lee CS, Jones D, Harnett GB, Armstrong BK, Clark JR, Milross CG, Kim J, O'Brien CJ, et al. Human papillomavirus predicts outcome in oropharyngeal cancer in patients treated primarily with surgery or radiation therapy. Br J Cancer. 2010;103:1510–1517. doi: 10.1038/sj.bjc.6605944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang H, Zhang B, Chen W, Zhou SM, Zhang YX, Gao L, Xu ZG, Qiao YL, Tang PZ. Human papillomavirus infection and prognostic predictors in patients with oropharyngeal squamous cell carcinoma. Asian Pac J Cancer Prev. 2012;13:891–896. doi: 10.7314/APJCP.2012.13.3.891. [DOI] [PubMed] [Google Scholar]
- 39.Isayeva T, Xu J, Dai Q, Whitley AC, Bonner J, Nabell L, Spencer S, Carroll W, Jones G, Ragin C, et al. African Americans with oropharyngeal carcinoma have significantly poorer outcomes despite similar rates of human papillomavirus-mediated carcinogenesis. Hum Pathol. 2014;45:310–319. doi: 10.1016/j.humpath.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 40.Joo YH, Cho KJ, Park JO, Nam IC, Kim MS. Factors influencing the outcomes of primary surgery with postoperative radiotherapy for pN2 oropharyneal squamous cell carcinoma. Oral Oncol. 2012;48:90–94. doi: 10.1016/j.oraloncology.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 41.Jung AC, Briolat J, Millon R, de Reyniès A, Rickman D, Thomas E, Abecassis J, Clavel C, Wasylyk B. Biological and clinical relevance of transcriptionally active human papillomavirus (HPV) infection in oropharynx squamous cell carcinoma. International journal of cancer. Int J Cancer. 2010;126:1882–1894. doi: 10.1002/ijc.24911. [DOI] [PubMed] [Google Scholar]
- 42.Junor E, Kerr G, Oniscu A, Campbell S, Kouzeli I, Gourley C, Cuschieri K. Benefit of chemotherapy as part of treatment for HPV DNA-positive but p16-negative squamous cell carcinoma of the oropharynx. Br J Cancer. 2012;106:358–365. doi: 10.1038/bjc.2011.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kawakami H, Okamoto I, Terao K, Sakai K, Suzuki M, Ueda S, Tanaka K, Kuwata K, Morita Y, Ono K, et al. Human papillomavirus DNA and p16 expression in Japanese patients with oropharyngeal squamous cell carcinoma. Cancer Med. 2013;2:933–941. doi: 10.1002/cam4.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klussmann JP, Mooren JJ, Lehnen M, Claessen SM, Stenner M, Huebbers CU, Weissenborn SJ, Wedemeyer I, Preuss SF, Straetmans JM, Manni JJ. Genetic signatures of HPV-related and unrelated oropharyngeal carcinoma and their prognostic implications. Clin Cancer Res. 2009;15:1779–1786. doi: 10.1158/1078-0432.CCR-08-1463. [DOI] [PubMed] [Google Scholar]
- 45.Kuo KT, Hsiao CH, Lin CH, Kuo LT, Huang SH, Lin MC. The biomarkers of human papillomavirus infection in tonsillar squamous cell carcinoma-molecular basis and predicting favorable outcome. Mod Pathol. 2008;21:376–386. doi: 10.1038/modpathol.3800979. [DOI] [PubMed] [Google Scholar]
- 46.Lewis JS, Jr, Thorstad WL, Chernock RD, Haughey BH, Yip JH, Zhang Q, El-Mofty SK. p16 positive oropharyngeal squamous cell carcinoma:an entity with a favorable prognosis regardless of tumor HPV status. Am J Surg Pathol. 2010;34:1088–1096. doi: 10.1097/PAS.0b013e3181e84652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li W, Thompson CH, O'Brien CJ, McNeil EB, Scolyer RA, Cossart YE, Veness MJ, Walker DM, Morgan GJ, Rose BR. Human papillomavirus positivity predicts favourable outcome for squamous carcinoma of the tonsil. International journal of cancer. Int J Cancer. 2003;106:553–558. doi: 10.1002/ijc.11261. [DOI] [PubMed] [Google Scholar]
- 48.Licitra L, Perrone F, Bossi P, Suardi S, Mariani L, Artusi R, Oggionni M, Rossini C, Cantù G, Squadrelli M, et al. High-risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. J Clin Oncol. 2006;24:5630–5636. doi: 10.1200/JCO.2005.04.6136. [DOI] [PubMed] [Google Scholar]
- 49.Lin BM, Wang H, D'Souza G, Zhang Z, Fakhry C, Joseph AW, Drake VE, Sanguineti G, Westra WH, Pai SI. Long-term prognosis and risk factors among patients with HPV-associated oropharyngeal squamous cell carcinoma. Cancer. 2013;119:3462–3471. doi: 10.1002/cncr.28250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lindquist D, Ahrlund-Richter A, Tarján M, Tot T, Dalianis T. Intense CD44 expression is a negative prognostic factor in tonsillar and base of tongue cancer. Anticancer Res. 2012;32:153–161. [PubMed] [Google Scholar]
- 51.Lindquist D, Romanitan M, Hammarstedt L, Näsman A, Dahlstrand H, Lindholm J, Onelöv L, Ramqvist T, Ye W, Munck-Wikland E, et al. Human papillomavirus is a favourable prognostic factor in tonsillar cancer and its oncogenic role is supported by the expression of E6 and E7. Mol Oncol. 2007;1:350–355. doi: 10.1016/j.molonc.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ljøkjel B, Lybak S, Haave H, Olofsson J, Vintermyr OK, Aarstad HJ. The impact of HPV infection on survival in a geographically defined cohort of oropharynx squamous cell carcinoma (OPSCC) patients in whom surgical treatment has been one main treatment. Acta Otolaryngol. 2014;134:636–645. doi: 10.3109/00016489.2014.886336. [DOI] [PubMed] [Google Scholar]
- 53.Marklund L, Näsman A, Ramqvist T, Dalianis T, Munck-Wikland E, Hammarstedt L. Prevalence of human papillomavirus and survival in oropharyngeal cancer other than tonsil or base of tongue cancer. Cancer Med. 2012;1:82–88. doi: 10.1002/cam4.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maxwell JH, Kumar B, Feng FY, Worden FP, Lee JS, Eisbruch A, Wolf GT, Prince ME, Moyer JS, Teknos TN, Chepeha DB, et al. Tobacco use in human papillomavirus-positive advanced oropharynx cancer patients related to increased risk of distant metastases and tumor recurrence. Clin Cancer Res. 2010;16:1226–1235. doi: 10.1158/1078-0432.CCR-09-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Melkane AE, Auperin A, Saulnier P, Lacroix L, Vielh P, Casiraghi O, Msakni I, Drusch F, Temam S. Human papillomavirus prevalence and prognostic implication in oropharyngeal squamous cell carcinomas. Head Neck. 2014;36:257–265. doi: 10.1002/hed.23302. [DOI] [PubMed] [Google Scholar]
- 56.Mizumachi T, Kano S, Sakashita T, Hatakeyama H, Suzuki S, Homma A, Oridate N, Fukuda S. Improved survival of Japanese patients with human papillomavirus-positive oropharyngeal squamous cell carcinoma. Int J Clin Oncol. 2013;18:824–828. doi: 10.1007/s10147-012-0469-6. [DOI] [PubMed] [Google Scholar]
- 57.Näsman A, Nordfors C, Grün N, Munck-Wikland E, Ramqvist T, Marklund L, Lindquist D, Dalianis T. Absent/weak CD44 intensity and positive human papillomavirus (HPV) status in oropharyngeal squamous cell carcinoma indicates a very high survival. Cancer Med. 2013;2:507–518. doi: 10.1002/cam4.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nichols AC, Dhaliwal SS, Palma DA, Basmaji J, Chapeskie C, Dowthwaite S, Franklin JH, Fung K, Kwan K, Wehrli B, et al. Does HPV type affect outcome in oropharyngeal cancer? J Otolaryngol Head Neck Surg. 2013;42(9) doi: 10.1186/1916-0216-42-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nichols AC, Palma DA, Dhaliwal SS, Tan S, Theuer J, Chow W, Rajakumar C, Um S, Mundi N, Berk S, et al. The epidemic of human papillomavirus and oropharyngeal cancer in a Canadian population. Curr Oncol. 2013;20:212–219. doi: 10.3747/co.20.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nichols AC, Finkelstein DM, Faquin WC, Westra WH, Mroz EA, Kneuertz P, Begum S, Michaud WA, Busse PM, Clark JR, Rocco JW. Bcl2 and human papilloma virus 16 as predictors of outcome following concurrent chemoradiation for advanced oropharyngeal cancer. Clin Cancer Res. 2010;16:2138–2146. doi: 10.1158/1078-0432.CCR-09-3185. [DOI] [PubMed] [Google Scholar]
- 61.O'Sullivan B, Huang SH, Perez-Ordonez B, Massey C, Siu LL, Weinreb I, Hope A, Kim J, Bayley AJ, Cummings B, et al. Outcomes of HPV-related oropharyngeal cancer patients treated by radiotherapy alone using altered fractionation. Radiother Oncol. 2012;103:49–56. doi: 10.1016/j.radonc.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 62.O'Sullivan B, Huang SH, Siu LL, Waldron J, Zhao H, Perez-Ordonez B, Weinreb I, Kim J, Ringash J, Bayley A, et al. Deintensification candidate subgroups in human papillomavirus-related oropharyngeal cancer according to minimal risk of distant metastasis. J Clin Oncol. 2013;31:543–550. doi: 10.1200/JCO.2012.44.0164. [DOI] [PubMed] [Google Scholar]
- 63.Oguejiofor KK, Hall JS, Mani N, Douglas C, Slevin NJ, Homer J, Hall G, West CM. The prognostic significance of the biomarker p16 in oropharyngeal squamous cell carcinoma. Clin Oncol (R Coll Radiol) 2013;25:630–638. doi: 10.1016/j.clon.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 64.Park K, Cho KJ, Lee M, Yoon DH, Kim J, Kim SY, Nam SY, Choi SH, Roh JL, Han MW, et al. p16 immunohistochemistry alone is a better prognosticator in tonsil cancer than human papillomavirus in situ hybridization with or without p16 immunohistochemistry. Acta Otolaryngol. 2013;133:297–304. doi: 10.3109/00016489.2012.741327. [DOI] [PubMed] [Google Scholar]
- 65.Park WS, Ryu J, Cho KH, Choi MK, Moon SH, Yun T, Chun BS, Lee GK, Ahn HJ, Lee JH, et al. Human papillomavirus in oropharyngeal squamous cell carcinomas in Korea: Use of G1 cycle markers as new prognosticators. Head Neck. 2012;34:1408–1417. doi: 10.1002/hed.21939. [DOI] [PubMed] [Google Scholar]
- 66.Posner MR, Lorch JH, Goloubeva O, Tan M, Schumaker LM, Sarlis NJ, Haddad RI, Cullen KJ. Survival and human papillomavirus in oropharynx cancer in TAX 324: a subset analysis from an international phase III trial. Ann Oncol. 2011;22:1071–1077. doi: 10.1093/annonc/mdr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Psychogios G, Alexiou C, Agaimy A, Brunner K, Koch M, Mantsopoulos K, Tomppert A, Iro H. Epidemiology and survival of HPV-related tonsillar carcinoma. Cancer Med. 2014;3:652–659. doi: 10.1002/cam4.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Psychogios G, Mantsopoulos K, Agaimy A, Koch M, Zenk J, Waldfahrer F, Iro H. Prognostic factors in limited (T1-2, N0-1) oropharyngeal carcinoma treated with surgery ± adjuvant therapy. Head Neck. 2013;35:1752–1758. doi: 10.1002/hed.23229. [DOI] [PubMed] [Google Scholar]
- 69.Rahmati R, Dogan S, Pyke O, Palmer F, Awad M, Lee N, Kraus DH, Shah JP, Patel SG, Ganly I. Squamous cell carcinoma of the tonsil managed by conventional surgery and postoperative radiation. Head Neck. 2014;37:800–807. doi: 10.1002/hed.23679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reimers N, Kasper HU, Weissenborn SJ, Stützer H, Preuss SF, Hoffmann TK, Speel EJ, Dienes HP, Pfister HJ, Guntinas-Lichius O, Klussmann JP. Combined analysis of HPV-DNA, p16 and EGFR expression to predict prognosis in oropharyngeal cancer. Int J Cancer. 2007;120:1731–1738. doi: 10.1002/ijc.22355. [DOI] [PubMed] [Google Scholar]
- 71.Rietbergen MM, Brakenhoff RH, Bloemena E, Witte BI, Snijders PJ, Heideman DA, Boon D, Koljenovic S, Baatenburg-de Jong RJ, Leemans CR. Human papillomavirus detection and comorbidity: critical issues in selection of patients with oropharyngeal cancer for treatment De-escalation trials. Ann Oncol. 2013;24:2740–2745. doi: 10.1093/annonc/mdt319. [DOI] [PubMed] [Google Scholar]
- 72.Rischin D, Young RJ, Fisher R, Fox SB, Le QT, Peters LJ, Solomon B, Choi J, O'Sullivan B, Kenny LM, et al. Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02.02 phase III trial. J Clin Oncol. 2010;28:4142–4148. doi: 10.1200/JCO.2010.29.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rodrigo JP, Heideman DA, García-Pedrero JM, Fresno MF, Brakenhoff RH, Díaz Molina JP, Snijders PJ, Hermsen MA. Time trends in the prevalence of HPV in oropharyngeal squamous cell carcinomas in northern Spain (1990–2009) Int J Cancer. 2014;134:487–492. doi: 10.1002/ijc.28355. [DOI] [PubMed] [Google Scholar]
- 74.Schache AG, Liloglou T, Risk JM, Liloglou T, Risk JM, Filia A, Jones TM, Sheard J, Woolgar JA, Helliwell TR, Triantafyllou A, Robinson M, Sloan P, et al. Evaluation of human papilloma virus diagnostic testing in oropharyngeal squamous cell carcinoma: sensitivity, specificity, and prognostic discrimination. Clin Cancer Res. 2011;17:6262–6271. doi: 10.1158/1078-0432.CCR-11-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sedaghat AR, Zhang Z, Begum S, Palermo R, Best S, Ulmer KM, Levine M, Zinreich E, Messing BP, Gold D, et al. Prognostic significance of human papillomavirus in oropharyngeal squamous cell carcinomas. Laryngoscope. 2009;119:1542–1549. doi: 10.1002/lary.20533. [DOI] [PubMed] [Google Scholar]
- 76.Semrau R, Duerbaum H, Temming S, Huebbers C, Stenner M, Drebber U, Klussmann JP, Müller RP, Preuss SF. Prognostic impact of human papillomavirus status, survivin, and epidermal growth factor receptor expression on survival in patients treated with radiochemotherapy for very advanced nonresectable oropharyngeal cancer. Head Neck. 2013;35:1339–1344. doi: 10.1002/hed.23126. [DOI] [PubMed] [Google Scholar]
- 77.Shi W, Kato H, Perez-Ordonez B, Pintilie M, Huang S, Hui A, O'Sullivan B, Waldron J, Cummings B, Kim J, et al. Comparative prognostic value of HPV16 E6 mRNA compared with in situ hybridization for human oropharyngeal squamous carcinoma. J Clin Oncol. 2009;27:6213–6221. doi: 10.1200/JCO.2009.23.1670. [DOI] [PubMed] [Google Scholar]
- 78.Song JS, Kim MS, Park JW, Lee YS, Kang CS. Expression of human papillomavirus-related proteins and its clinical implication in tonsillar squamous cell carcinoma. Korean J Pathol. 2012;46:177–186. doi: 10.4132/KoreanJPathol.2012.46.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Song X, Sturgis EM, Huang Z, Li X, Li C, Wei Q, Li G. Potentially functional variants of p14ARF are associated with HPV-positive oropharyngeal cancer patients and survival after definitive chemoradiotherapy. Carcinogenesis. 2014;35:62–68. doi: 10.1093/carcin/bgt336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Straetmans JM, Olthof N, Mooren JJ, de Jong J, Speel EJ, Kremer B. Human papillomavirus reduces the prognostic value of nodal involvement in tonsillar squamous cell carcinomas. Laryngoscope. 2009;119:1951–1957. doi: 10.1002/lary.20593. [DOI] [PubMed] [Google Scholar]
- 81.Tural D, Eliçin O, Batur S, Arslan D, Öz B, Serdengeçti S, Uzel Ö. Human papillomavirus is independent prognostic factor on outcome of oropharyngeal squamous cell carcinoma. Tumour Biol. 2013;34:3363–3369. doi: 10.1007/s13277-013-0907-8. [DOI] [PubMed] [Google Scholar]
- 82.Ukpo OC, Flanagan JJ, Ma XJ, Luo Y, Thorstad WL, Lewis JS., Jr High-risk human papillomavirus E6/E7 mRNA detection by a novel in situ hybridization assay strongly correlates with p16 expression and patient outcomes in oropharyngeal squamous cell carcinoma. Am J Surg Pathol. 2011;35:1343–1350. doi: 10.1097/PAS.0b013e318220e59d. [DOI] [PubMed] [Google Scholar]
- 83.Ukpo OC, Pritchett CV, Lewis JE, Weaver AL, Smith DI, Moore EJ. Human papillomavirus-associated oropharyngeal squamous cell carcinomas: Primary tumor burden and survival in surgical patients. Ann Otol Rhinol Laryngol. 2009;118:368–373. doi: 10.1177/000348940911800509. [DOI] [PubMed] [Google Scholar]
- 84.Ward MJ, Mellows T, Harris S, Webb A, Patel NN, Cox HJ, Piper K, Ottensmeier CH, Thomas GJ, King EV. Staging and treatment of oropharyngeal cancer in the human papillomavirus era. Head Neck. 2015;37:1002–1013. doi: 10.1002/hed.23697. [DOI] [PubMed] [Google Scholar]
- 85.Ward MJ, Thirdborough SM, Mellows T, Riley C, Harris S, Suchak K, Webb A, Hampton C, Patel NN, Randall CJ, et al. Tumour-infiltrating lymphocytes predict for outcome in HPV-positive oropharyngeal cancer. Br J Cancer. 2014;110:489–500. doi: 10.1038/bjc.2013.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weinberger PM, Merkley MA, Khichi SS, Lee JR, Psyrri A, Jackson LL, Dynan WS. Human papillomavirus-active head and neck cancer and ethnic health disparities. Laryngoscope. 2010;120:1531–1537. doi: 10.1002/lary.20984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Weinberger PM, Yu Z, Haffty BG, Kowalski D, Harigopal M, Brandsma J, Sasaki C, Joe J, Camp RL, Rimm DL, et al. Molecular classification identifies a subset of human papillomavirus-associated oropharyngeal cancers with favorable prognosis. J Clin Oncol. 2006;24:736–747. doi: 10.1200/JCO.2004.00.3335. [DOI] [PubMed] [Google Scholar]
- 88.Worden FP, Kumar B, Lee JS, Wolf GT, Cordell KG, Taylor JM, Urba SG, Eisbruch A, Teknos TN, Chepeha DB, et al. Chemoselection as a strategy for organ preservation in advanced oropharynx cancer: Response and survival positively associated with HPV16 copy number. J Clin Oncol. 2008;26:3138–3146. doi: 10.1200/JCO.2007.12.7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Worsham MJ1, Stephen JK, Chen KM, Mahan M, Schweitzer V, Havard S, Divine G. Improved survival with HPV among African Americans with oropharyngeal cancer. Clin Cancer Res. 2013;19:2486–2492. doi: 10.1158/1078-0432.CCR-12-3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Young RJ, Rischin D, Fisher R, McArthur GA, Fox SB, Peters LJ, Corry J, Lim A, Waldeck K, Solomon B. Relationship between epidermal growth factor receptor status, p16(INK4A), and outcome in head and neck squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2011;20:1230–1237. doi: 10.1158/1055-9965.EPI-10-1262. [DOI] [PubMed] [Google Scholar]
- 91.Chen YW, Kao SY, Yang MH. Analysis of p16(INK4A) expression of oral squamous cell carcinomas in Taiwan: Prognostic correlation without relevance to betel quid consumption. J Surg Oncol. 2012;106:149–154. doi: 10.1002/jso.23067. [DOI] [PubMed] [Google Scholar]
- 92.Duray A, Descamps G, Decaestecker C, Remmelink M, Sirtaine N, Lechien J, Ernoux-Neufcoeur P, Bletard N, Somja J, Depuydt CE, et al. Human papillomavirus DNA strongly correlates with a poorer prognosis in oral cavity carcinoma. Laryngoscope. 2012;122:1558–1565. doi: 10.1002/lary.23298. [DOI] [PubMed] [Google Scholar]
- 93.Elango KJ, Suresh A, Erode EM, Subhadradevi L, Ravindran HK, Iyer SK, Iyer SK, Kuriakose MA. Role of human papilloma virus in oral tongue squamous cell carcinoma. Asian Pac J Cancer Prev. 2011;12:889–896. [PubMed] [Google Scholar]
- 94.Gröbe A, Hanken H, Kluwe L, Schöllchen M, Tribius S, Pohlenz P, Clauditz T, Grob T, Simon R, Sauter G, Heiland M, Blessmann M. Immunohistochemical analysis of p16 expression, HPV infection and its prognostic utility in oral squamous cell carcinoma. J Oral Pathol Med. 2013;42:676–681. doi: 10.1111/jop.12086. [DOI] [PubMed] [Google Scholar]
- 95.Huang SF, Li HF, Liao CT, Wang HM, Chen IH, Chang JT, Chen YJ, Cheng AJ. Association of HPV infections with second primary tumors in early-staged oral cavity cancer. Oral Dis. 2012;18:809–815. doi: 10.1111/j.1601-0825.2012.01950.x. [DOI] [PubMed] [Google Scholar]
- 96.Kozomara R, Jović N, Magić Z, Branković-Magić M, Minić V. p53 mutations and human papillomavirus infection in oral squamous cell carcinomas: correlation with overall survival. J Craniomaxillofac Surg. 2005;33:342–348. doi: 10.1016/j.jcms.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 97.Kruger M, Pabst AM, Walter C, Sagheb K, Günther C, Blatt S, Weise K, Al-Nawas B, Ziebart T. The prevalence of human papilloma virus (HPV) infections in oral squamous cell carcinomas: A retrospective analysis of 88 patients and literature overview. J Craniomaxillofac Surg. 2014;42:1506–1514. doi: 10.1016/j.jcms.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 98.Lee LA, Huang CG, Liao CT, Lee LY, Hsueh C, Chen TC, Lin CY, Fan KH, Wang HM, Huang SF, et al. Human papillomavirus-16 infection in advanced oral cavity cancer patients is related to an increased risk of distant metastases and poor survival. PLoS One. 2012;7:e40767. doi: 10.1371/journal.pone.0040767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee LA, Huang CG, Tsao KC, Liao CT, Kang CJ, Chang KP, Huang SF, Chen IH, Fang TJ, Li HY, Yang SL, et al. Increasing rates of low-risk human papillomavirus infections in patients with oral cavity squamous cell carcinoma: association with clinical outcomes. J Clin Virol. 2013;57:331–337. doi: 10.1016/j.jcv.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 100.Na, Kang HJ, Cho SY, Koh JS, Lee JK, Lee BC, Lee GH, Lee YS, Yoo HJ, Ryoo BY, Yang SH, Shim YS. EGFR mutations and human papillomavirus in squamous cell carcinoma of tongue and tonsil. Eur J Cancer. 2007;43:520–526. doi: 10.1016/j.ejca.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 101.Reuschenbach M, Kansy K, Garbe K, Vinokurova S, Flechtenmacher C, Toth C, Prigge ES, Thiele OC, Reinert S, Hoffmann J, et al. Lack of evidence of human papillomavirus-induced squamous cell carcinomas of the oral cavity in southern Germany. Oral Oncol. 2013;49:937–942. doi: 10.1016/j.oraloncology.2013.03.451. [DOI] [PubMed] [Google Scholar]
- 102.Sugiyama M, Bhawal UK, Kawamura M, Ishioka Y, Shigeishi H, Higashikawa K, Kamata N. Human papillomavirus-16 in oral squamous cell carcinoma: Clinical correlates and 5-year survival. Br J Oral Maxillofac Surg. 2007;45:116–122. doi: 10.1016/j.bjoms.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 103.Zhao D, Xu QG, Chen XM, Fan MW. Human papillomavirus as an independent predictor in oral squamous cell cancer. Int J Oral Sci. 2009;1:119–125. doi: 10.4248/IJOS.09015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ankola AA, Smith RV, Burk RD, Prystowsky MB, Sarta C, Schlecht NF. Comorbidity, human papillomavirus infection and head and neck cancer survival in an ethnically diverse population. Oral Oncol. 2013;49:911–917. doi: 10.1016/j.oraloncology.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Annertz K, Rosenquist K, Andersson G, Jacobsson H, Hansson BG, Wennerberg J. High-risk HPV and survival in patients with oral and oropharyngeal squamous cell carcinoma - 5-year follow up of a population-based study. Acta Otolaryngol. 2014;134:843–851. doi: 10.3109/00016489.2014.890289. [DOI] [PubMed] [Google Scholar]
- 106.Badaracco G, Rizzo C, Mafera B, Pichi B, Giannarelli D, Rahimi SS, Vigili MG, Venuti A. Molecular analyses and prognostic relevance of HPV in head and neck tumours. Oncol Rep. 2007;17:931–939. [PubMed] [Google Scholar]
- 107.Báez A, Almodóvar JI, Cantor A, Celestin F, Cruz-Cruz L, Fonseca S, Trinidad-Pinedo J, Vega W. High frequency of HPV16-associated head and neck squamous cell carcinoma in the Puerto Rican population. Head Neck. 2004;26:778–784. doi: 10.1002/hed.20046. [DOI] [PubMed] [Google Scholar]
- 108.Deng Z, Hasegawa M, Aoki K, Matayoshi S, Kiyuna A, Yamashita Y, Uehara T, Agena S, Maeda H, Xie M, et al. A comprehensive evaluation of human papillomavirus positive status and p16INK4a overexpression as a prognostic biomarker in head and neck squamous cell carcinoma. Int J Oncol. 2014;45:67–76. doi: 10.3892/ijo.2014.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Deng Z, Hasegawa M, Yamashita Y, Matayoshi S, Kiyuna A, Agena S, Uehara T, Maeda H, Suzuki M. Prognostic value of human papillomavirus and squamous cell carcinoma antigen in head and neck squamous cell carcinoma. Cancer Sci. 2012;103:2127–2134. doi: 10.1111/cas.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Duray A, Descamps G, Decaestecker C, Sirtaine N, Gilles A, Khalifé M, Chantrain G, Depuydt CE, Delvenne P, Saussez S. Human papillomavirus predicts the outcome following concomitant chemoradiotherapy in patients with head and neck squamous cell carcinomas. Oncol Rep. 2013;30:371–376. doi: 10.3892/or.2013.2415. [DOI] [PubMed] [Google Scholar]
- 111.Fischer CA, Zlobec I, Green E, Probst S, Storck C, Lugli A, Tornillo L, Wolfensberger M, Terracciano LM. Is the improved prognosis of p16 positive oropharyngeal squamous cell carcinoma dependent of the treatment modality? Int J Cancer. 2010;126:1256–1262. doi: 10.1002/ijc.24842. [DOI] [PubMed] [Google Scholar]
- 112.Gavid M, Pillet S, Pozzetto B, Oriol M, Dumollard JM, Timoshenko AP, Martin C, Prades JM. Human papillomavirus and head and neck squamous cell carcinomas in the South-East of France: Prevalence, viral expression, and prognostic implications. Acta Otolaryngol. 2013;133:538–543. doi: 10.3109/00016489.2012.747221. [DOI] [PubMed] [Google Scholar]
- 113.Hoffmann M, Görögh T, Gottschlich S, Lohrey C, Rittgen W, Ambrosch P, Schwarz E, Kahn T. Human papillomaviruses in head and neck cancer: 8 year-survival-analysis of 73 patients. Cancer Lett. 2005;218:199–206. doi: 10.1016/j.canlet.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 114.Joo YH, Jung CK, Sun DI, Park JO, Cho KJ, Kim MS. High-risk human papillomavirus and cervical lymph node metastasis in patients with oropharyngeal cancer. Head Neck. 2012;34:10–14. doi: 10.1002/hed.21697. [DOI] [PubMed] [Google Scholar]
- 115.Klozar J, Koslabova E, Kratochvil V, Salakova M, Tachezy R. Nodal status is not a prognostic factor in patients with HPV-positive oral/oropharyngeal tumors. J Surg Oncol. 2013;107:625–633. doi: 10.1002/jso.23292. [DOI] [PubMed] [Google Scholar]
- 116.Klozar J, Kratochvil V, Salakova M, Smahelova J, Vesela E, Hamsikova E, Betka J, Tachez R. HPV status and regional metastasis in the prognosis of oral and oropharyngeal cancer. Eur Arch Otorhinolaryngol. 2008;265(Suppl 1):S75–S82. doi: 10.1007/s00405-007-0557-9. [DOI] [PubMed] [Google Scholar]
- 117.Kong CS, Narasimhan B, Cao H, Kwok S, Erickson JP, Koong A, Pourmand N, Le QT. The relationship between human papillomavirus status and other molecular prognostic markers in head and neck squamous cell carcinomas. Int J Radiat Oncol Biol Phys. 2009;74:553–561. doi: 10.1016/j.ijrobp.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Laco J, Nekvindova J, Novakova V, Celakovsky P, Dolezalova H, Tucek L, Vosmikova H, Vosmik M, Neskudlova T, Cermakova E, et al. Biologic importance and prognostic significance of selected clinicopathological parameters in patients with oral and oropharyngeal squamous cell carcinoma, with emphasis on smoking, protein p16(INK4a) expression, and HPV status. Neoplasma. 2012;59:398–408. doi: 10.4149/neo_2012_052. [DOI] [PubMed] [Google Scholar]
- 119.Lassen P, Eriksen JG, Krogdahl A, Therkildsen MH, Ulhøi BP, Overgaard M, Specht L, Andersen E, Johansen J, Andersen LJ, et al. Danish Head and Neck Cancer Group (DAHANCA), corp-author The influence of HPV-associated p16-expression on accelerated fractionated radiotherapy in head and neck cancer: Evaluation of the randomised DAHANCA 6&7 trial. Radiother Oncol. 2011;100:49–55. doi: 10.1016/j.radonc.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 120.López RV, Levi JE, Eluf-Neto J, Koifman RJ, Koifman S, Curado MP, Michaluart-Junior P, Figueiredo DL, Saggioro FP, de Carvalho MB, et al. Human papillomavirus (HPV) 16 and the prognosis of head and neck cancer in a geographical region with a low prevalence of HPV infection. Cancer Causes Control. 2014;25:461–471. doi: 10.1007/s10552-014-0348-8. [DOI] [PubMed] [Google Scholar]
- 121.Lukesova E, Boucek J, Rotnaglova E, Salakova M, Koslabova E, Grega M, Eckschlager T, Rihova B, Prochazka B, Klozar J, et al. High level of Tregs is a positive prognostic marker in patients with HPV-positive oral and oropharyngeal squamous cell carcinomas. BioMed Res Int. 2014;2014:303929. doi: 10.1155/2014/303929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rades D, Seibold ND, Gebhard MP, Noack F, Schild SE, Thorns C. Prognostic factors (including HPV status) for irradiation of locally advanced squamous cell carcinoma of the head and neck (SCCHN) Strahlenther Onkol. 2011;187:626–632. doi: 10.1007/s00066-011-1139-8. [DOI] [PubMed] [Google Scholar]
- 123.Rautava J, Kuuskoski J, Syrjanen K, Grenman R, Syrjanen S. HPV genotypes and their prognostic significance in head and neck squamous cell carcinomas. J Clin Virol. 2012;53:116–120. doi: 10.1016/j.jcv.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 124.Ritchie JM, Smith EM, Summersgill KF, Hoffman HT, Wang D, Klussmann JP, Turek LP, Haugen TH. Human papillomavirus infection as a prognostic factor in carcinomas of the oral cavity and oropharynx. Int J Cancer. 2003;104:336–344. doi: 10.1002/ijc.10960. [DOI] [PubMed] [Google Scholar]
- 125.Rittà M, De Andrea M, Mondini M, Mazibrada J, Giordano C, Pecorari G, Garzaro M, Landolfo V, Schena M, Chiusa L, et al. Cell cycle and viral and immunologic profiles of head and neck squamous cell carcinoma as predictable variables of tumor progression. Head Neck. 2009;31:318–327. doi: 10.1002/hed.20977. [DOI] [PubMed] [Google Scholar]
- 126.Rosenquist K, Wennerberg J, Annertz K, Schildt EB, Hansson BG, Bladström A, Andersson G. Recurrence in patients with oral and oropharyngeal squamous cell carcinoma: Human papillomavirus and other risk factors. Acta Otolaryngol. 2007;127:980–987. doi: 10.1080/00016480601110162. [DOI] [PubMed] [Google Scholar]
- 127.Salazar CR, Anayannis N, Smith RV, Wang Y, Haigentz M, Jr, Garg M, Schiff BA, Kawachi N, Elman J, Belbin TJ, Prystowsky MB, Burk RD, Schlecht NF. Combined P16 and human papillomavirus testing predicts head and neck cancer survival. Int J Cancer. 2014;135:2404–2412. doi: 10.1002/ijc.28876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Salazar CR, Smith RV, Garg MK, Haigentz M, Jr, Schiff BA, Kawachi N, Anayannis N, Belbin TJ, Prystowsky MB, Burk RD, et al. Human papillomavirus-associated head and neck squamous cell carcinoma survival: A comparison by tumor site and initial treatment. Head Neck Pathol. 2014;8:77–87. doi: 10.1007/s12105-013-0486-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Settle K, Posner MR, Schumaker LM, Tan M, Suntharalingam M, Goloubeva O, Strome SE, Haddad RI, Patel SS, Cambell EV III, et al. Racial survival disparity in head and neck cancer results from low prevalence of human papillomavirus infection in black oropharyngeal cancer patients. Cancer Prev Res (Phila) 2009;2:776–781. doi: 10.1158/1940-6207.CAPR-09-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Smith EM, Rubenstein LM, Haugen TH, Pawlita M, Turek LP. Complex etiology underlies risk and survival in head and neck cancer human papillomavirus, tobacco, and alcohol: A case for multifactor disease. J Oncol. 2012;2012:571862. doi: 10.1155/2012/571862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Smith EM, Rubenstein LM, Hoffman H, Haugen TH, Turek LP. Human papillomavirus, p16 and p53 expression associated with survival of head and neck cancer. Infect Agent Cancer. 2010;5:4. doi: 10.1186/1750-9378-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Smith EM, Wang D, Rubenstein LM, Morris WA, Turek LP, Haugen TH. Association between p53 and human papillomavirus in head and neck cancer survival. Cancer Epidemiol Biomarkers Prev. 2008;17:421–427. doi: 10.1158/1055-9965.EPI-07-2597. [DOI] [PubMed] [Google Scholar]
- 133.Stephen JK, Divine G, Chen KM, Chitale D, Havard S, Worsham MJ. Significance of p16 in Site-specific HPV Positive and HPV Negative Head and Neck Squamous Cell Carcinoma. Cancer Clin Oncol. 2013;2:51–61. doi: 10.5539/cco.v2n1p51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Thibaudeau E, Fortin B, Coutlée F, Nguyen-Tan P, Weng X, Audet ML, Abboud O, Guertin L, Christopoulos A, Tabet J, et al. HPV prevalence and prognostic value in a prospective cohort of 255 patients with locally advanced HNSCC: A single-centre experience. Int J Otolaryngol. 2013;2013:437815. doi: 10.1155/2013/437815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Alos L, Moyano S, Nadal A, Alobid I, Blanch JL, Ayala E, Lloveras B, Quint W, Cardesa A, Ordi J. Human papillomaviruses are identified in a subgroup of sinonasal squamous cell carcinomas with favorable outcome. Cancer. 2009;115:2701–2709. doi: 10.1002/cncr.24309. [DOI] [PubMed] [Google Scholar]
- 136.Ernoux-Neufcoeur P, Arafa M, Decaestecker C, Duray A, Remmelink M, Leroy X, Herfs M, Somja J, Depuydt CE, Delvenne P, et al. Combined analysis of HPV DNA, p16, p21 and p53 to predict prognosis in patients with stage IV hypopharyngeal carcinoma. J Cancer Res Clin Oncol. 2011;137:173–181. doi: 10.1007/s00432-010-0871-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, Forastiere A, Gillison ML. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 138.Friedland P, Thomas A, Naran A, Amanuel B, Grieu-Iacopetta F, Carrello A, Harnett G, Meyer C, Phillips M. Human papillomavirus and gene mutations in head and neck squamous carcinomas. ANZ J Surg. 2012;82:362–366. doi: 10.1111/j.1445-2197.2011.05791.x. [DOI] [PubMed] [Google Scholar]
- 139.Heath S, Willis V, Allan K, Purdie K, Harwood C, Shields P, Simcock R, Williams T, Gilbert DC. Clinically significant human papilloma virus in squamous cell carcinoma of the head and neck in UK practice. Clin Oncol (R Coll Radiol) 2012;24:e18–23. doi: 10.1016/j.clon.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 140.Joo YH, Lee YS, Cho KJ, Park JO, Nam IC, Kim CS, Kim SY, Kim MS. Characteristics and prognostic implications of high-risk HPV-associated hypopharyngeal cancers. PLoS One. 2013;8:e78718. doi: 10.1371/journal.pone.0078718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lassen P, Eriksen JG, Hamilton-Dutoit S, Tramm T, Alsner J, Overgaard J. Effect of HPV-associated p16INK4A expression on response to radiotherapy and survival in squamous cell carcinoma of the head and neck. J Clin Oncol. 2009;27:1992–1998. doi: 10.1200/JCO.2008.20.2853. [DOI] [PubMed] [Google Scholar]
- 142.Lau HY, Brar S, Klimowicz AC, Petrillo SK, Hao D, Brockton NT, Kong CS, Lees-Miller SP, Magliocco AM. Prognostic significance of p16 in locally advanced squamous cell carcinoma of the head and neck treated with concurrent cisplatin and radiotherapy. Head Neck. 2011;33:251–256. doi: 10.1002/hed.21439. [DOI] [PubMed] [Google Scholar]
- 143.Rampias T, Pectasides E, Prasad M, Sasaki C, Gouveris P, Dimou A, Kountourakis P, Perisanidis C, Burtness B, Zaramboukas T, Rimm D, et al. Molecular profile of head and neck squamous cell carcinomas bearing p16 high phenotype. Ann Oncol. 2013;24:2124–2131. doi: 10.1093/annonc/mdt013. [DOI] [PubMed] [Google Scholar]
- 144.Sethi S, Ali-Fehmi R, Franceschi S, Struijk L, van Doorn LJ, Quint W, Albashiti B, Ibrahim M, Kato I. Characteristics and survival of head and neck cancer by HPV status: a cancer registry-based study. Int J Cancer. 2012;131:1179–1186. doi: 10.1002/ijc.26500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sivars L, Näsman A, Tertipis N, Vlastos A, Ramqvist T, Dalianis T, Munck-Wikland E, Nordemar S. Human papillomavirus and p53 expression in cancer of unknown primary in the head and neck region in relation to clinical outcome. Cancer Med. 2014;3:376–384. doi: 10.1002/cam4.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Śnietura M, Jaworska M, Pigłowski W, Goraj-Zając A, Woźniak G, and Lange D. High-risk HPV DNA status and p16 (INK4a) expression as prognostic markers in patients with squamous cell cancer of oral cavity and oropharynx. Pol J Pathol. 2010;61:133–139. [PubMed] [Google Scholar]
- 147.Stephen JK, Chen KM, Shah V, Havard S, Lu M, Schweitzer VP, Gardner G, Worsham MJ. Human papillomavirus outcomes in an access-to-care laryngeal cancer cohort. Otolaryngol Head Neck Surg. 2012;146:730–738. doi: 10.1177/0194599811434482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tribius S, Hoffmann AS, Bastrop S, Görögh T, Haag J, Röcken C, Clauditz T, Grob T, Wilczak W, Tennstedt P, et al. HPV status in patients with head and neck of carcinoma of unknown primary site: HPV, tobacco smoking, and outcome. Oral Oncol. 2012;48:1178–1184. doi: 10.1016/j.oraloncology.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 149.Weiss D, Koopmann M, Rudack C. Prevalence and impact on clinicopathological characteristics of human papillomavirus-16 DNA in cervical lymph node metastases of head and neck squamous cell carcinoma. Head Neck. 2011;33:856–862. doi: 10.1002/hed.21548. [DOI] [PubMed] [Google Scholar]
- 150.Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: A systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14:467–475. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- 151.Dayyani F, Etzel CJ, Liu M, Ho CH, Lippman SM, Tsao AS. Meta-analysis of the impact of human papillomavirus (HPV) on cancer risk and overall survival in head and neck squamous cell carcinomas (HNSCC) Head Neck Oncol. 2010;2:15. doi: 10.1186/1758-3284-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ng M, Freeman MK, Fleming TD, Robinson M, Dwyer-Lindgren L, Thomson B, Wollum A, Sanman E, Wulf S, Lopez AD, et al. Smoking prevalence and cigarette consumption in 187 countries, 1980-2012. JAMA. 2014;311:183–192. doi: 10.1001/jama.2013.284692. [DOI] [PubMed] [Google Scholar]
- 153.Adelstein DJ, Ridge JA, Gillison ML, Chaturvedi AK, D'Souza G, Gravitt PE, Westra W, Psyrri A, Kast WM, Koutsky LA, Giuliano A, et al. Head and neck squamous cell cancer and the human papillomavirus: summary of a National Cancer Institute State of the Science Meeting, November 9-10, 2008, Washington, D.C. Head Neck. 2009;31:1393–1422. doi: 10.1002/hed.21269. [DOI] [PubMed] [Google Scholar]
- 154.Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer. 2007;121:1813–1820. doi: 10.1002/ijc.22851. [DOI] [PubMed] [Google Scholar]
- 155.Götz C, Drecoll E, Straub M, Bissinger O, Wolff KD, Kolk A. Impact of HPV infection on oral squamous cell carcinoma. Oncotarget. 2016;7:76704–76712. doi: 10.18632/oncotarget.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Butz K, Geisen C, Ullmann A, Spitkovsky D, Hoppe-Seyler F. Cellular responses of HPV-positive cancer cells to genotoxic anti-cancer agents: repression of E6/E7-oncogene expression and induction of apoptosis. International journal of cancer. Journal international du cancer. 1996;68:506–513. doi: 10.1002/(SICI)1097-0215(19961115)68:4<506::AID-IJC17>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 157.Hafkamp HC, Speel EJ, Haesevoets A, et al. A subset of head and neck squamous cell carcinomas exhibits integration of HPV 16/18 DNA and overexpression of p16INK4A and p53 in the absence of mutations in p53 exons 5-8. International journal of cancer. Journal international du cancer. 2003;107:394–400. doi: 10.1002/ijc.11389. [DOI] [PubMed] [Google Scholar]
- 158.Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, Zahurak ML, Daniel RW, Viglione M, Symer DE, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 159.Ragin CC, Taioli E, Weissfeld JL, White JS, Rossie KM, Modugno F, Gollin SM. 11q13 amplification status and human papillomavirus in relation to p16 expression defines two distinct etiologies of head and neck tumours. Br J Cancer. 2006;95:1432–1438. doi: 10.1038/sj.bjc.6603394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hoffmann TK, Arsov C, Schirlau K, Bas M, Friebe-Hoffmann U, Klussmann JP, Scheckenbach K, Balz V, Bier H, Whiteside TL. T cells specific for HPV16 E7 epitopes in patients with squamous cell carcinoma of the oropharynx. Int J Cancer. 2006;118:1984–1991. doi: 10.1002/ijc.21565. [DOI] [PubMed] [Google Scholar]
- 161.Chaturvedi AK. Epidemiology and clinical aspects of HPV in head and neck cancers. Head Neck Pathol. 2012;6(Suppl 1):S16–S24. doi: 10.1007/s12105-012-0377-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.De Petrini M, Rittà M, Schena M, Chiusa L, Campisi P, Giordano C, Landolfo V, Pecorari G, Landolfo S. Head and neck squamous cell carcinoma: Role of the human papillomavirus in tumour progression. New Microbiol. 2006;29:25–33. [PubMed] [Google Scholar]
- 163.Kumar B, Cordell KG, Lee JS, Worden FP, Prince ME, Tran HH, Wolf GT, Urba SG, Chepeha DB, Teknos TN, et al. EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol. 2008;26:3128–3137. doi: 10.1200/JCO.2007.12.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mellin H, Friesland S, Lewensohn R, Dalianis T, Munck-Wikland E. Human papillomavirus (HPV) DNA in tonsillar cancer: clinical correlates, risk of relapse, and survival. International journal of cancer. Int J Cancer. 2000;89:300–304. doi: 10.1002/1097-0215(20000520)89:3<300::AID-IJC14>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 165.Schwartz SR, Yueh B, McDougall JK, Daling JR, Schwartz SM. Human papillomavirus infection and survival in oral squamous cell cancer: a population-based study. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology- Head Neck Surg. 2001;125:1–9. doi: 10.1067/mhn.2001.116979. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.