Abstract
Background:
The RPE65 gene was recently described to cause autosomal dominant retinitis pigmentosa (adRP), presenting with a phenotype resembling choroideremia. This study presents the 2-year progression of RPE65 adRP in a patient.
Methods:
This is an observational case report of one patient. The patient received a full ophthalmic examination during both visits, including diagnostic imaging such as spectral domain optical coherence tomography (SD-OCT), OCT-angiography (OCT-A), short-wave fundus autofluorescence (FAF), and fundus photography. Genetic characterization was obtained by DNA sequencing from peripheral blood lymphocytes obtained during the first visit.
Results:
RPE65 adRP phenocopied choroideremia at the initial fundoscopy. Upon the patient’s return to our clinic 2 years later, DNA sequencing revealed a heterozygous mutation in the RPE65 gene. Diagnostic imaging by SD-OCT and FAF suggested disease progression. In conjunction with clinical examination and imaging, the diagnosis was revised to autosomal dominant retinitis pigmentosa caused by RPE65.
Conclusion:
adRP due to a mutation in the gene encoding RPE65 phenocopied choroideremia. Based on our analysis of the 2-year disease progression in this patient, RPE65 adRP is mild and has a slow rate of disease progression.
Keywords: retinitis pigmentosa, autosomal dominant, RPE65, disease progression
Introduction
Retinitis pigmentosa (RP) is a group of inherited degenerative retinal diseases characterized by progressive nyctalopia and visual field constriction that may culminate in blindness.1 The vast majority of cases are inherited in Mendelian patterns, namely as autosomal dominant (30–40% of cases), autosomal recessive (50–60%), or X-linked (5–15%) inheritance. 1 Of the autosomal recessive cases, it is estimated that approximately 2% are caused by mutations in the RPE65 gene. 1, 2 RPE65 encodes for a retina-specific, 65 kDa retinol isomerase called retinal pigment epithelium-specific protein that serves as a vital component of the visual cycle, as it renews 11-cis retinal from all-trans retinol. 3 In addition to RP, recessive mutations in RPE65 are also known to cause Leber congenital amaurosis (LCA).
In 2011, Bowne et al. initially reported two Irish families with a rare case of autosomal dominant RP (adRP) of variable penetrance due to a heterozygous mutation in RPE65. 4 In 2016, Hull et al. additionally reported four patients from two different Irish families with RPE65-mediated adRP. 5 The families described in both studies presented with the heterozygous RPE65 mutation, c.1430A>G:p.Asp477Gly. Furthermore, both studies suggested that RPE65-mediated adRP phenotypically resembles choroideremia, presenting with variable penetrance as it can manifest as mild-disease or non-penetrant. 4, 5 Additional studies of this dominant mutation in mice have shown that its pathogenesis is caused by abnormally slow regeneration of the 11-cis retinal chromophore. 3
In this study, we expand on the two previous reports of RPE65-mediated adRP by presenting a new case of adRP caused by the same heterozygous c.1430A>G:p.Asp477Gly mutation in RPE65. We also use multimodal imaging to characterize the disease progression in this patient over the course of 2 years.
Case Report
A 67-year-old male was referred to our clinic for evaluation with a diagnosis of choroideremia. He was diagnosed at the age of 53 after presenting to his ophthalmologist with worsening night and peripheral vision. No significant past medical or ocular history was reported and the patient was not taking any medications at the time. Family history was significant for a sister with similar eye problems, who at the age of 52 was also diagnosed with choroideremia (Figure 1). His affected sister was not available for clinical investigation.
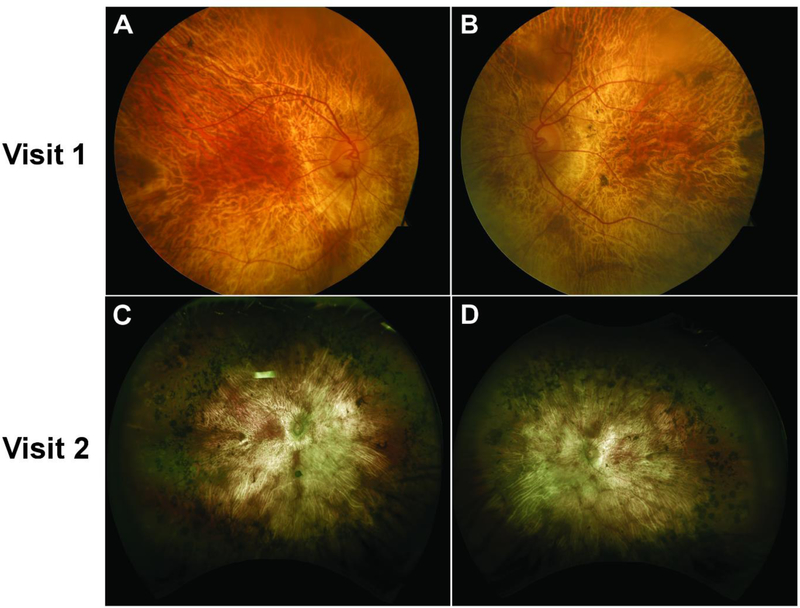
Figure 1.
Color fundus (top row) and widefield retina photography (bottom row) images showing widespread areas of chorioretinal atrophy and exposed underlying large choroidal vessels on both the right (A, C) and left (B, D) eye at visits 1 and 2. An island of spared retina on the parafoveal region was also observed bilaterally at both visits. Extensive intraretinal pigment migration in the periphery can be seen clearly in the widefield fundus images (C, D). Despite the different imaging modalities used, no significant progression is observed between the first and second visits. The difference in color and exposure is attributed to the different imaging modalities, but an island of spared retina of similar shape and size can be appreciated on both eyes at both visits.
The patient’s best corrected visual acuity (BCVA) was 20/60 and 20/125 on the right and left eye, respectively. On dilated fundus examination (DFE), there were widespread areas of chorioretinal atrophy bilaterally with the underlying larger choroidal vessels exposed. An island of spared retina on the parafoveal region was observed on both eyes, with extensive intraretinal pigment migration also found in the periphery (Figure 1A, 1B). Spectral-domain optical coherence tomography (SD-OCT) and short-wave fundus autofluorescence (FAF) (Spectralis HRA + OCT device, Heidelberg Engineering, Heidelberg, Germany) findings were in agreement with the DFE. SD-OCT showed widespread peripheral retinal atrophy with disruption of the outer nuclear layer and ellipsoid zone (EZ) (Figure 2). The EZ line was preserved only in the parafoveal area, measuring 4765 µm on the right and 910 µm on the left eye. RPE atrophy and choroidal sclerosis were extensive, as suggested by the increased transmittance of signal to the sclera. FAF showed generalized hypoautofluorescent areas corresponding to RPE atrophy bound by sharply demarcated borders. Scalloped areas of preserved retinal tissue were additionally detected in the parafoveal areas of both eyes (Figure 3). The size of these parafoveal regions of preserved retina were 12.56 mm2 in the right and 4.32 mm2 in the left eye. SD-OCT and FAF measurements were done with a measuring tool in the Spectralis software. Optical coherence tomography angiography (OCT-A) (Cirrus HD-OCT 5000, Carl Zeiss Meditec, Dublin, CA) was performed to analyze the retinal and choroidal vasculature. Images with 6×6 mm dimensions centered at the fovea were taken and the vascular layers were segmented automatically. OCT-A images showed the larger choroidal vessels from Sattler’s and Haller’s layers in areas where the overlying retinal tissue and choriocapillaries (CC) had atrophied, whereas the vasculature appeared normal in spared retinal tissue (Figure 4). Similar patterns were seen on the left eye. Overall, the clinical presentation and imaging were in agreement with the diagnosis of choroideremia. Peripheral blood lymphocytes were collected and sent for DNA sequencing to confirm the diagnosis. The thirty-one gene panel used for DNA sequencing includes the CHM gene along with other genes that commonly cause retinitis pigmentosa and LCA, including USH2A, EYS, RPE65, and RHO.
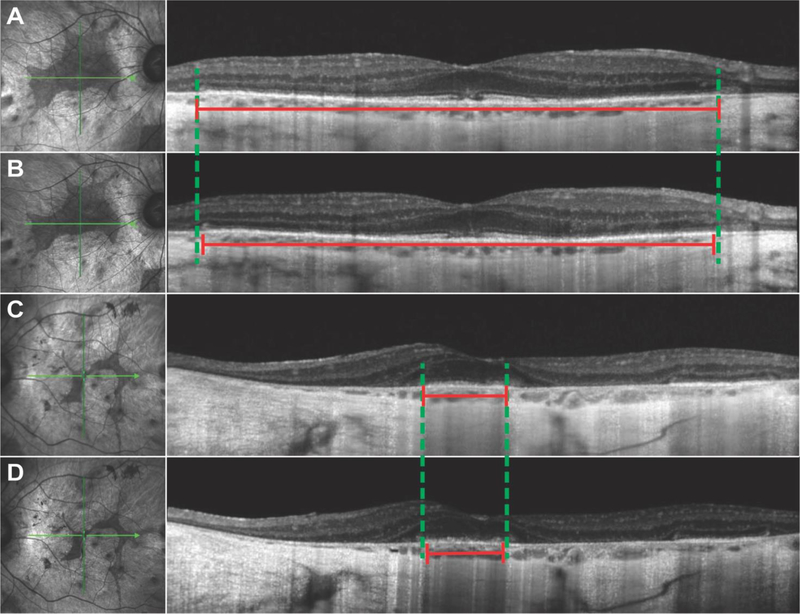
Figure 2.
SD-OCT showed widespread peripheral retinal atrophy with disruption of the outer nuclear layer and ellipsoid zone (EZ) in both the right (A, B) and left (C, D) eye. The double-headed red lines indicate the length of the EZ line, while the dashed green lines outline the borders of the EZ line according to the first visit. A small decrease in EZ line length (2% in the right, 9% in the left eye) was measured in the second visit (B, D) as compared to the first visit (A, C).
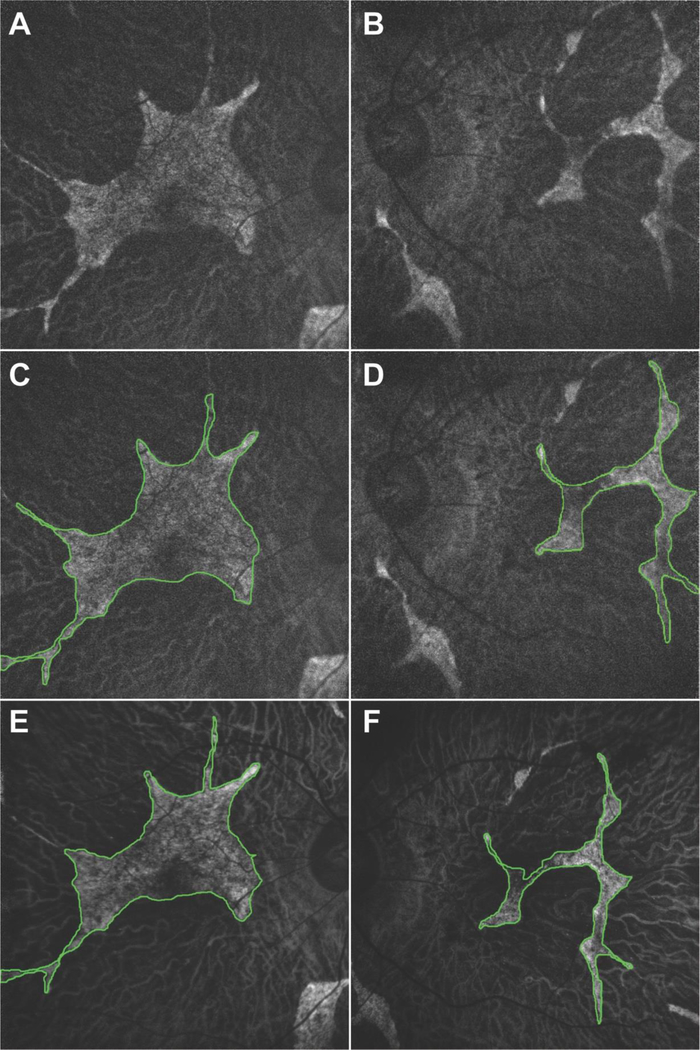
Figure 3.
Fundus autofluorescence imaging of the right (A, C, E) and left (B, D, F) eye showed generalized hypoautofluorescent areas corresponding to RPE atrophy bound by sharply demarcated borders. Areas of preserved retinal tissue were also detected on the parafoveal areas of both eyes (outlined in green). The area of preserved retinal tissue decreased in size from the first visit (A, B, C, D) to the second (E, F) visit by 17% in the right and 21% in the left eye.
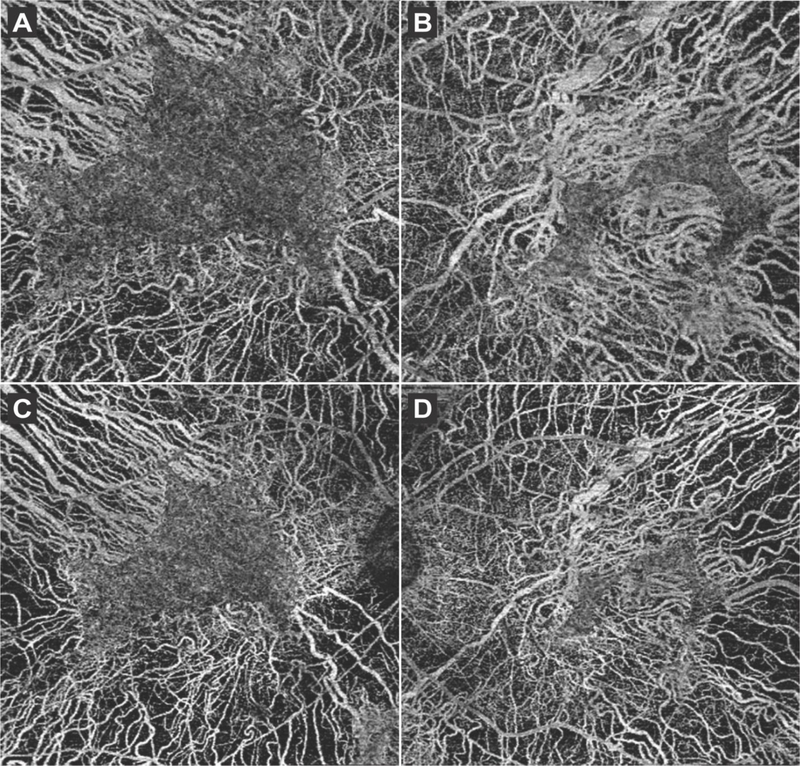
Figure 4.
6×6 mm OCT-A scans obtained in the first visit of both the right (A) and left (B) eye showed the underlying larger choroidal vessels in areas where the overlying retinal tissue and choriocapillaries (CC) had atrophied, whereas the CC vasculature appeared normal in spared retinal tissue. 8×8 mm OCT-A scans performed during the second visit (C, D) showed similar findings.
The patient returned for follow-up 2 years later due to worsening night and peripheral vision. The patient had received bilateral cataract extraction 4 months prior and his BCVA improved to 20/20 in the right and 20/50 in the left eye. DFE did not show much progression as compared to the last visit, with widespread chorioretinal atrophy, extensive intraretinal pigment migration in the periphery, and exposed underlying large choroidal vessels (Figure 1C, 1D). SD-OCT revealed mild disease progression as compared to 2 years earlier, with the EZ line decreasing in size bilaterally to 4697 µm (around a 2% decrease) in the right and 830 µm (around 9%) in the left eye. FAF also revealed that the parafoveal islands of spared tissue had decreased in size, measuring 10.49 mm2 (around 17%) and 3.42 mm2 (around 21%) in the right and left eye, respectively. OCT-A 8×8 mm images were taken to capture a wider field of view. Similar to the findings from the previous visit, retinal vasculature and CC were preserved in the islands of spared retinal tissue, while the larger choroidal vessels were seen in areas of RPE atrophy. DNA sequencing results revealed a heterozygous c.1430A>G:p.Asp477Gly mutation in the RPE65 gene, while no other variants were identified in other genes including CHM. Based on the clinical presentation and genetic results, we amended this patient’s diagnosis from choroideremia to adRP and provided appropriate genetic counseling for an autosomal dominant condition.
Discussion
Our 2-year disease progression study expands upon the currently limited information available regarding RPE65-mediated adRP. Genetic testing was crucial for identifying our patient’s correct diagnosis and the appropriate counseling needed for his condition. Based on family history, the patient’s sister suffers from similar eye problems and was also initially diagnosed with choroideremia as an X-linked carrier. However, given her symptoms and onset of disease, which resembled those of her brother, we can conclude that she in fact suffers from adRP. Based on previous studies and our own clinical experience, RPE65-mediated adRP presents with a misleading choroideremia-like phenotype, which explains why the patient and his sister were initially diagnosed with choroideremia. Choroideremia carriers, however, are mostly asymptomatic due to their mosaic phenotype caused by random X-inactivation.6 For those that are symptomatic, disease progression is milder and worsens gradually with increasing age.7 Thus, it is unlikely that a choroideremia carrier would experience the same extent of symptoms and onset of disease as the patient presented in this case.
We were unable to examine or perform DNA testing on the parents, but family history suggests that either the father carries the RPE65 mutation or the mother is an asymptomatic carrier due to non-penetrance. The father died in an accident at the age of 43 without suffering from ophthalmic symptoms. Given that both the patient and his sister began experiencing symptoms during their mid-50’s, it is possible that the father did not live long enough to begin experiencing symptoms if he indeed carried the mutation. The mother is alive with no significant ophthalmic history, so it is also possible that she is an asymptomatic carrier, especially given Hull et al.’s findings of significantly variable penetrance in patients with dominant RPE65 mutations. 5
Our study further corroborates the hypothesis that adRP caused by RPE65 arises from a common founder mutation from Ireland. All cases of RPE65-mediated adRP thus far reported in the literature and in our study are caused by the p.Asp477Gly mutation, and all patients reported with this mutation have Irish ancestry.4, 5 Although the patient in this study reports Scottish ancestry, he claims that it is possible that his ancestors migrated from Scotland to Ireland in the past.
In tracking disease progression, we monitored EZ line width on SD-OCT and the area of preserved retina on FAF from the patient’s two visits to our clinic, which were 2 years apart from each other. Studies characterizing the natural history of choroideremia have shown that quantifying the preserved retinal pigment epithelium and EZ line width on OCT and FAF is highly reproducible and that these variables are acceptable anatomic outcomes to track the progression of choroideremia over time.8, 9 Thus, given the similar phenotypes of RPE65-adRP and choroideremia, we found it appropriate to use this strategy. During the 2-year period between the patient’s visits, EZ line width decreased by 2% (68 µm) and 9% (80 µm) on the right and left eye, respectively, while the area of preserved retina on FAF decreased by 17% (2.07 mm2) on the right and 21% (0.9 mm2) on the left eye. Previous studies on RP patients, including those with autosomal dominant, recessive, and X-linked modes of inheritances, have reported more dramatic decreases of 140 µm on EZ line width per year, irrespective of mode of inheritance. 10 This decrease is approximately four times faster than what is observed in the case presented, suggesting that our case has a significantly slower rate of disease progression. Our findings are consistent with those of Hull et al., as they similarly characterized this disease as mild or non-penetrant. 5 Future studies should aim to characterize RPE65-mediated adRP disease progression in a greater number of patients, as knowledge of how the disease progresses can be crucial in designing clinical trials that test potential forms of treatment. Gene replacement trials for LCA due to RPE65 have already shown promising results, although long-term stability is not yet known.11, 12 Thus, a greater understanding of the disease progression in RPE65-mediated adRP may eventually lead to the application of genome surgery trials to patients suffering from this disease.
Acknowledgments
FUNDING
The Jonas Children’s Vision Care and Bernard & Shirlee Brown Glaucoma Laboratory are supported by the National Institutes of Health [P30EY019007, R01EY018213, R01EY024698, R01EY026682, R21AG050437], National Cancer Institute Core [5P30CA013696], the Research to Prevent Blindness (RPB) Physician-Scientist Award, unrestricted funds from RPB, New York, NY, USA. R. J. is supported by the RPB medical student eye research fellowship. S. H. T. is a member of the RD-CURE Consortium and is supported by the Tistou and Charlotte Kerstan Foundation, the Schneeweiss Stem Cell Fund, New York State [C029572], and the Gebroe Family Foundation.
Footnotes
Conflicts of interest: The authors declare that they have no conflicts of interest.
DECLARATION OF INTEREST STATEMENT
The authors report no conflicts of interest.
Informed consent: A waiver of consent was obtained as outlined by the protocol #AAAR0284 approved by the Institutional Review Board (IRB) at Columbia University.
Ethics approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet 2006;368:1795–809. [DOI] [PubMed] [Google Scholar]
- 2.Morimura H, Fishman GA, Grover SA, Fulton AB, Berson EL, Dryja TP. Mutations in the RPE65 gene in patients with autosomal recessive retinitis pigmentosa or leber congenital amaurosis. Proc Natl Acad Sci U S A 1998;95:3088–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shin Y, Moiseyev G, Chakraborty D, Ma JX. A Dominant Mutation in Rpe65, D477G, Delays Dark Adaptation and Disturbs the Visual Cycle in the Mutant Knock-In Mice. Am J Pathol 2017;187:517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowne SJ, Humphries MM, Sullivan LS, et al. A dominant mutation in RPE65 identified by whole-exome sequencing causes retinitis pigmentosa with choroidal involvement. Eur J Hum Genet 2011;19:1074–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hull S, Mukherjee R, Holder GE, Moore AT, Webster AR. The clinical features of retinal disease due to a dominant mutation in RPE65. Mol Vis 2016;22:626–35. [PMC free article] [PubMed] [Google Scholar]
- 6.Bonilha VL, Trzupek KM, Li Y, et al. Choroideremia: Analysis of the retina from a Female Symptomatic Carrier. Ophthalmic genetics 2008;29:99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coussa RG, Traboulsi EI. Choroideremia: a review of general findings and pathogenesis. Ophthalmic Genet 2012;33:57–65. [DOI] [PubMed] [Google Scholar]
- 8.Hariri AH, Velaga SB, Girach A, et al. Measurement and Reproducibility of Preserved Ellipsoid Zone Area and Preserved Retinal Pigment Epithelium Area in Eyes With Choroideremia. Am J Ophthalmol 2017;179:110–117. [DOI] [PubMed] [Google Scholar]
- 9.Heon E, Alabduljalil T, McGuigan ID, et al. Visual Function and Central Retinal Structure in Choroideremia. Invest Ophthalmol Vis Sci 2016;57:OCT377–87. [DOI] [PubMed]
- 10.Cabral T, Sengillo JD, Duong JK, et al. Retrospective Analysis of Structural Disease Progression in Retinitis Pigmentosa Utilizing Multimodal Imaging. Sci Rep 2017;7:10347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Testa F, Maguire AM, Rossi S, et al. Three-year follow-up after unilateral subretinal delivery of adeno-associated virus in patients with Leber congenital Amaurosis type 2. Ophthalmology 2013;120:1283–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bainbridge JW, Mehat MS, Sundaram V, et al. Long-term effect of gene therapy on Leber’s congenital amaurosis. N Engl J Med 2015;372:1887–97. [DOI] [PMC free article] [PubMed] [Google Scholar]