Watch a video presentation of this article
Watch the interview with the author
Abbreviations
- A1C
glycosylated hemoglobin A1C
- BCAA
branched chain amino acids
- BMI
body mass index
- CTP
Child‐Turcotte‐Pugh score
- HVPG
hepatic venous pressure gradient
- LFI
Liver Frailty Index
- MELD‐Na
Model for End‐stage Liver Disease sodium
- RCT
randomized controlled trial
- SMI
skeletal muscle index
- VO2
oxygen consumption
Frailty, the central feature of physical decline in aging and debilitating diseases, is defined as vulnerability to health stressors leading to physical dependency and death.1 Sarcopenia, anatomic loss of muscle mass, regularly accompanies frailty (Table 1). Although they are measured differently, frailty and sarcopenia are closely related concepts that share common clinical significance. They affect most patients with cirrhosis and are tightly coupled with important disturbances of ammonia metabolism that accelerate muscle injury, disability, and mortality.
Table 1.
Frailty, Sarcopenia, Malnutrition, and Cachexia
| Abnormality | Definition | Comment |
|---|---|---|
| Frailty | A syndrome defined by diminished strength, endurance, and reduced physiological function that increases vulnerability for developing physical dependency and death1 | Develops in advanced age and decades earlier in persons with chronic debilitating diseases such as cirrhosis and advanced heart, respiratory, and renal failure. |
| Sarcopenia | Loss of anatomic muscle mass4 | Commonly observed in frailty and a highly prevalent problem in cirrhosis. Measured by segmentation analysis of cross‐sectional imaging (Fig. 2) or by whole‐body bioelectrical impedance. Impedance measurements depend on the assumption that muscle mass is a constant fraction of body water, which fails in cirrhosis with fluid overload. |
| Malnutrition | A state resulting from consumption of either inadequate or excessive nutrients including calories, protein, carbohydrates, vitamins, or minerals | Frequently related to anorexia in patients with cirrhosis. Sarcopenic obesity is a state of malnutrition that regularly accompanies nonalcoholic fatty liver disease as an element of the metabolic syndrome. |
| Cachexia | Loss of lean tissue mass involving a loss of greater than 5% of body weight | Advanced starvation involving inadequate intake, absorption, or utilization of sufficient nutritional components to maintain homeostasis. |
Frailty/sarcopenia has attracted growing interest because of its robust association with adverse outcomes in cirrhosis and transplantation. We have begun to explore whether our new knowledge of frailty/sarcopenia in cirrhosis is actionable for guiding interventions to arrest its progress. Appreciation that frailty/sarcopenia is potentially modifiable or reversible is the incentive to identify these interventions.
Cirrhotic frailty/sarcopenia is a rapidly maturing new knowledge domain. As yet, it is absent from the curricula of gastroenterology and transplant hepatology fellowships and board examinations. Although it is arguably among the most prevalent and lethal complications of cirrhosis, there is no clinical practice guideline to provide evidence‐based management. Gathering, codifying, and teaching the evidence that will guide improved patient care are important new challenges.
Mechanisms of Muscle Loss in Cirrhosis
The molecular biology of cirrhotic sarcopenia was reviewed by Dasarathy and Merli.2 Muscle mass is at risk both from protein‐calorie starvation with impaired muscle protein biosynthesis and simultaneous muscle proteolysis needed for gluconeogenesis and synthesis of other proteins.
Mediators of cirrhotic sarcopenia include ammonia, a potent driver of multiple severe muscle disturbances in advanced liver disease, as well as deficient testosterone and growth hormone and increased endotoxin. As shown in Fig. 1, excess ammonia, the result of impaired hepatic ureagenesis, is delivered to muscle, where it must be detoxified by reacting with glutamate to form glutamine. The diverted glutamate is not available to support protein synthesis; moreover, the demand for glutamate impairs mitochondrial energy generation. Three additional adverse effects of ammonia include upregulation of myostatin (a powerful muscle‐wasting cytokine), increased muscle autophagy, and impaired muscle contractility.
Figure 1.
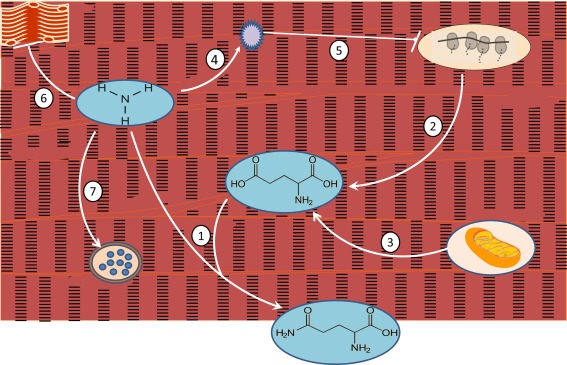
Ammonia, the key metabolic driver of cirrhotic muscle wasting. (1) Excess ammonia delivered to muscle combines with glutamic acid to form glutamine. Glutamine delivered to the gut becomes the primary ammonia excretion path in cirrhosis. (2) The increased need for glutamic acid depletes the amino acid pool needed for protein synthesis. (3) Glutamic acid formation from α‐ketoglutarate depletes that important citric acid cycle intermediate, impairing mitochondrial energy generation. (4, 5) Ammonia triggers formation and release of myostatin, a cytokine that blocks muscle protein synthesis by mammalian target of rapamycin inhibition. (6) Ammonia directly inhibits muscle contractility. (7) Ammonia stimulates muscle lysosomal autophagy.
Testosterone and growth hormone inhibit myostatin expression; their deficiency in cirrhosis may fail to overcome ammonia‐driven sarcopenia. Endotoxemia from gut barrier impairment and gut microbiome abnormalities may also drive sarcopenia via a tumor necrosis factor–dependent pathway and upregulation of autophagy.2
In nonalcoholic fatty liver disease/nonalcoholic steatohepatitis, sarcopenia may be an important cause, as well as a consequence, of the nonalcoholic fatty liver disease–specific constellation of signaling disturbances among muscle, gut, liver, and adipose tissue, amplified by the effects of physical inactivity.3
Measuring Frailty/Sarcopenia
Anatomic Sarcopenia
Muscle mass is measured using segmentation analysis of cross‐sectional images or using whole‐body bioelectrical impedance (Fig. 2A). Montano‐Loza and others4 found that anatomic sarcopenia was associated with transplant wait‐list mortality and severe transplant complications. Fat infiltration of muscle is also associated with adverse outcomes.5 A multicenter North American consortium recommended gender‐specific skeletal muscle index (SMI) cutoffs (legend of Fig. 2A) to standardize sarcopenia measurement in liver transplant candidates.6
Figure 2.
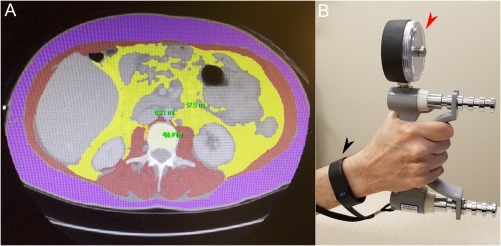
Measuring sarcopenia, physical activity, and frailty. (A) A computed tomography cross section at vertebral level L3 with muscle highlighted in red, used for calculation of the SMI as square centimeters (cm2) of muscle area divided by the square of patient height (m2).4 Gender‐specific SMI cutoffs, 50 cm2/m2 in men and 39 cm2/m2 in women, are proposed to standardize sarcopenia measurement in liver transplant candidates.6 (B) Black arrowhead: a wearable personal activity monitor. Web‐based transmission delivers step count, heart rate, calories expended, and activity intensity to a user's smartphone and their electronic medical record.8 Red arrowhead: a dynamometer for measuring grip strength, a component of the LFI.9
Measured Physical Performance Over Time
Carey et al.7 reported that distance walked over 6 minutes was strongly associated with liver transplant mortality below a cutoff of 250 m. Measuring physical activity over time with wearable monitors (Fig. 2B) gives patients a new tool for self‐assessment and provider feedback. The critical need for objective physical activity monitoring is illustrated by the large gap between patients' self‐assessed and actual activity levels, which are among the most sedentary of any patients studied.8
Frailty Testing During Clinic Visits
Rapid assessment of frailty at clinic visits requires ease of performance, reproducibility, and robust association with important outcomes, for example, transplant wait‐list mortality. Clinic frailty testing should have independent predictive value for outcomes rather than redundancy with existing parameters such as the MELD, Model for End‐stage Liver Disease sodium (MELD‐Na) score. Lai et al.,9 in a new landmark study, proposed a Liver Frailty Index (LFI) using three simple tests—grip strength (Fig. 2B), chair stands, and balance—that meets these requirements and merits wide adoption in cirrhosis care. A web‐based LFI application can be found at: http://liverfrailtyindex.ucsf.edu.9
Translating Measurement Into Action
Today, transplant centers rely on subjective clinician frailty assessments, for example, the Karnovsky scale, in evaluating physical capacity to withstand transplantation. Table 2 lists transplant care decisions we now make every day based largely on subjective judgment. If frailty/sarcopenia measurements were added to improve the precision of these decisions, there could be major consequences for patients. Transplant candidates, already hypervigilant about their MELD‐Na scores, could react adversely to the addition of frailty/sarcopenia measurements that they felt unable to predict or control. Currently, it seems reasonable to use individual patient measurements and self‐monitoring to encourage and positively reinforce performance improvement, in line with the appeal of wearable monitors for healthy persons who opt to use them for the same purpose.
Table 2.
Potential Actions Based on Frailty/Sarcopenia Measurements
| Select for liver transplant wait‐listing |
| Remove from a wait list for physical decline |
| Modify MELD‐Na‐based transplant prioritization |
| Guide clinical management of activity and nutrition care |
Potential Interventions
The eight published reports of interventions to improve frailty/sarcopenia in cirrhosis are listed in Table 3. They include supervised exercise, nutritional supplements to target ammonia‐driven sarcopenia, and androgen replacement in hypogonadal men.10, 11, 12, 13, 14, 15, 16, 17 The accelerating pace of these reports suggests that significant progress can be expected soon to support evidence‐based optimization of exercise, nutrition, and hormone/micronutrient therapy in cirrhosis.
Table 3.
Reported Interventions to Arrest or Improve Frailty and Sarcopenia in Patients With Cirrhosis
| Author (Year) | Study Type, Entry Criteriaa | Patient Numberb | Treatment Length | Exercise | Nutrition | Significant Outcomes |
|---|---|---|---|---|---|---|
| Zenith et al. (2014)10 |
RCT CTP A,B |
9 exercise 10 usual care |
8 weeks |
30+ minutes 3×/week |
Increased peak VO2, increased muscle mass, decreased fatigue | |
| Roman et al. (2014)11 |
RCT MELD ≤ 25 |
8 exercise 9 usual care |
12 weeks |
60 minutes 3×/week |
Leucine 10 g/day both groups | Increased exercise capacity and leg muscle mass, improved quality of life |
| Debette‐Gratien et al. (2015)12 |
Non‐RCT Able to exercise |
8 liver transplantation candidates | 12 weeks |
60 minutes 2×/week |
Increased VO2, increased muscle strength and 6‐minute walk distance | |
| Sinclair et al. (2016)13 |
RCT Hypogonadal men |
22 androgen transplantation 25 placebo |
12 months | Increased muscle, bone mass, bone mineral density, and exercise capacity; decreased A1C | ||
| Roman et al. (2016)14 |
RCT MELD ≤ 25 |
14 exercise 9 relaxation |
12 weeks |
60 minutes 3×/week |
Increased lean body, leg, bone mass, VO2; decreased fat mass | |
| Nishida et al. (2017)15 |
Non‐RCT CTP A |
6 women | 12 months | 140 minutes/week |
BCAA 12.45 g/day |
Increased aerobic capacity as assessed by lactate threshold |
| Berzigotti et al. (2017)16 |
Non‐RCT CTP A,B HVPG ≥ 6 BMI ≥ 26 |
50 patients | 16 weeks | 60 minutes/week | 1000 kcal/day 25% protein |
Decreased HVPG, decreased BMI, increased VO2, decreased leptin |
| Kitajima et al. (2017)17 |
Non‐RCT Albumin ≤ 3.5 |
21 patients | 48 weeks |
BCAA 12 g/day |
Stable muscle mass and decreased myosteatosis in the 11 patients who had improved albumin |
For the cited randomized trials, intervention outcomes were significant when compared with control arm outcomes. For nonrandomized trials, intervention outcomes were significant when compared with preintervention baseline data.
The patient numbers shown represent those who completed the described interventions.
Abbreviations: A1C, glycosylated hemoglobin A1C; BCAA, branched chain amino acids; BMI, body mass index; CTP, Child‐Turcotte‐Pugh score; HVPG, hepatic venous pressure gradient; RCT, randomized controlled trial; VO2, oxygen consumption.
Conclusions and Interim Recommendations
The evidence base for recognizing and treating frailty/sarcopenia in cirrhosis is rapidly expanding. Although data from the intervention studies cited earlier are encouraging, we lack the large, well‐powered patient data sets needed to recommend optimal therapy for prevention or reversal of frailty/sarcopenia.
Our current empiric recommendations for assessing and managing cirrhotic frailty/sarcopenia are listed in Table 4. Although focused on transplant candidates, they can serve other patients with cirrhosis, taking into account disease stage, comorbidities, and goals of care. The opportunity is now strong to refine and use our new appreciation of frailty/sarcopenia to improve transplant care and quality of life in all patients with cirrhosis.
Table 4.
An Empiric Standard of Care for Cirrhotic Frailty/Sarcopenia
| Component | References |
|---|---|
|
Complete a three‐domain frailty/sarcopenia assessment. 1. Anatomic: SMI from computed tomography or magnetic resonance imaging 2. Performance over time: 6‐minute walk, baseline gait speed 3. LFI baseline for future clinic visits |
4, 6, 9 |
|
Identify, treat, and monitor muscle‐specific metabolic issues. 1. Diabetes, thyroid, and male testosterone status 2. Micronutrient deficits, especially vitamin D 3. Suppression of ammonia excess |
2, 13, 15, 17 |
|
Evaluate and target motivational and performance barriers. 1. Self‐assessed physical performance capacity 2. Comorbid depression and substance use 3. Use of sedatives and narcotics |
8, 12 |
|
Prescribe and monitor a nutritional program. 1. Individual energy requirements in Kcal/day 2. Protein 1.3‐1.5 g/kg/day, including a late evening feeding 3. Accommodate diabetes, salt, and fluid constraints |
11, 15, 16, 17 |
|
Develop and execute a personalized activity program with patient engagement. 1. Identify, mitigate, and develop alternative programs to overcome specific musculoskeletal and neurological activity barriers, e.g., stationary biking, water aerobics, and calibrated resistance training bands 2. Ensure sustainability: home‐based activity, affordable, adequate time, availability of a family member, peer, or health coach 3. Balance aerobic with light resistance exercise, e.g., 80/20 4. Encourage wearable activity devices for self‐monitoring and goal setting, e.g., ≥5000 steps/day with ≥5% moderate/vigorous activity intensity |
8, 9, 10, 11, 12, 13, 14, 15, 16, 17 |
Potential conflict of interest: Nothing to report.
REFERENCES
- 1. Morley JE, Vellas B, van Kan GA, Anker SD, Bauer JM, Bernabei R, et al. Frailty consensus: a call to action. J Am Med Dir Assoc 2013;14:392‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dasarathy S, Merli M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J Hepatol 2016;65:1232‐1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bhanji RA, Narayanan P, Allen AM, Malhi H, Watt KD. Sarcopenia in hiding: the risk and consequence of underestimating muscle dysfunction in nonalcoholic steatohepatitis. Hepatology 2017;66:2055‐2065. [DOI] [PubMed] [Google Scholar]
- 4. Montano‐Loza AJ, Meza‐Junco J, Prado CM, Lieffers JR, Baracos VE, Bain VG, Sawyer MB. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol 2012;10:166‐173. [DOI] [PubMed] [Google Scholar]
- 5. Montano‐Loza AJ, Angulo P, Meza‐Junco J, Prado CM, Sawyer MB, Beaumont C, et al. Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J Cachexia Sarcopenia Muscle 2016;7:126‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carey EJ, Lai JC, Wang CW, Dasarathy S, Lobach I, Montano‐Loza AJ, Dunn MA. A multicenter study to define sarcopenia in patients with end‐stage liver disease. Liver Transpl 2017;23:625‐633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carey EJ, Steidley DE, Aqel BA, Byrne TJ, Mekeel KL, Rakela J, et al. Six‐minute walk distance predicts mortality in liver transplant candidates. Liver Transpl 2010;16:1373‐1378. [DOI] [PubMed] [Google Scholar]
- 8. Dunn MA, Josbeno DA, Schmotzer AR, Tevar AD, DiMartini AF, Landsittel DP, Delitto A. The gap between clinically assessed physical performance and objective physical activity in liver transplant candidates. Liver Transpl 2016;22:1324‐1332. [DOI] [PubMed] [Google Scholar]
- 9. Lai JC, Covinsky KE, Dodge JL, Boscardin WJ, Segev DL, Roberts JP, Feng S. Development of a novel frailty index to predict mortality in patients with end‐stage liver disease. Hepatology 2017;66:564‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zenith L, Meena N, Ramadi A, Yavari M, Harvey A, Carbonneau M, et al. Eight weeks of exercise training increases aerobic capacity and muscle mass and reduces fatigue in patients with cirrhosis. Clin Gastroenterol Hepatol 2014;12:1920‐1926. [DOI] [PubMed] [Google Scholar]
- 11. Roman E, Torrades MT, Nadal MJ, Cardenas G, Nieto JC, Vidal S, et al. Randomized pilot study: effects of an exercise programme and leucine supplementation in patients with cirrhosis. Dig Dis Sci 2014;59:1966‐1975. [DOI] [PubMed] [Google Scholar]
- 12. Debette‐Gratien M, Tabouret T, Antonini MT, Dalmay F, Carrier P, Legros R, et al. Personalized adapted physical activity before liver transplantation: acceptability and results. Transplantation 2015;99:145‐150. [DOI] [PubMed] [Google Scholar]
- 13. Sinclair M, Grossmann M, Hoermann R, Angus PW, Gow PJ. Testosterone therapy increases muscle mass in men with cirrhosis and low testosterone: a randomised controlled trial. J Hepatol 2016;65:906‐913. [DOI] [PubMed] [Google Scholar]
- 14. Roman E, Garcia‐Galceran C, Torrades T, Herrera S, Marin A, Donate M, et al. Effects of an exercise programme on functional capacity, body composition and risk of falls in patients with cirrhosis: a randomized clinical trial. PLoS One 2016;11:e0151652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nishida Y, Ide Y, Okada M, Otsuka T, Eguchi Y, Ozaki I, et al. Effects of home‐based exercise and branched‐chain amino acid supplementation on aerobic capacity and glycemic control in patients with cirrhosis. Hepatol Res 2017;47:e193‐e200. [DOI] [PubMed] [Google Scholar]
- 16. Berzigotti A, Albillos A, Villanueva C, Genesca J, Ardevol A, Augustin S, et al. Effects of an intensive lifestyle intervention program on portal hypertension in patients with cirrhosis and obesity: the SportDiet study. Hepatology 2017;65:1293‐1305. [DOI] [PubMed] [Google Scholar]
- 17. Kitajima Y, Takahashi H, Akiyama T, Murayama K, Iwane S, Kuwashiro T, et al. Supplementation with branched‐chain amino acids ameliorates hypoalbuminemia, prevents sarcopenia, and reduces fat accumulation in the skeletal muscles of patients with liver cirrhosis. J Gastroenterol 2017. 10.1007/s00535-017-1370-x [DOI] [PubMed] [Google Scholar]


