Abstract
Only few studies document longer periods of fasting in large cohorts including non-obese participants. The aim of this study was to document prospectively the safety and any changes in basic health and well-being indicators during Buchinger periodic fasting within a specialised clinic. In a one-year observational study 1422 subjects participated in a fasting program consisting of fasting periods of between 4 and 21 days. Subjects were grouped in fasting period lengths of 5, 10, 15 and 20±2 days. The participants fasted according to the Buchinger guidelines with a daily caloric intake of 200–250 kcal accompanied by a moderate-intensity lifestyle program. Clinical parameters as well as adverse effects and well-being were documented daily. Blood examinations before and at the end of the fasting period complemented the pre-post analysis using mixed-effects linear models. Significant reductions in weight, abdominal circumference and blood pressure were observed in the whole group (each p<0.001). A beneficial modulating effect of fasting on blood lipids, glucoregulation and further general health-related blood parameters was shown. In all groups, fasting led to a decrease in blood glucose levels to low norm range and to an increase in ketone bodies levels (each p<0.001), documenting the metabolic switch. An increase in physical and emotional well-being (each p<0.001) and an absence of hunger feeling in 93.2% of the subjects supported the feasibility of prolonged fasting. Among the 404 subjects with pre-existing health-complaints, 341 (84.4%) reported an improvement. Adverse effects were reported in less than 1% of the participants. The results from 1422 subjects showed for the first time that Buchinger periodic fasting lasting from 4 to 21 days is safe and well tolerated. It led to enhancement of emotional and physical well-being and improvements in relevant cardiovascular and general risk factors, as well as subjective health complaints.
Introduction
There are a growing number of recent publications on intermittent fasting (IF), generally lasting 16 to 48 hours, and calorie restriction (CR). Periodic fasting (PF), recently defined as lasting from 2 to as many as 21 or more days, is less studied in humans, especially for periods longer than 4 days [1, 2]. Results show that lifespan and healthspan are prolonged in several animal models by fasting and CR [2–7] and that parameters of age-related diseases are improved in humans [5, 8, 9]. Fasting leads to pronounced metabolic changes: The shift from carbohydrates and glucose to fatty acids and ketones as the major cellular fuel source for body and brain seems to play a key role. It has recently been referred to as intermittent metabolic switching (IMS) and glucose-to-ketone (G-to-K) switch. The reverse step–ketone-to-glucose (K-to-G) switch–happens upon refeeding [2]. The G-to-K switch includes reduction in blood glucose, insulin and IGF-1 levels, depletion or reduction of glycogen stores, and an increase in lipolysis and ketogenesis [2, 5, 10]. Fasting has been shown to induce differential cellular stress resistance [11] and autophagy [12, 13], as well as triggering the synthesis of detoxification enzymes [9, 14]. Fasting seems to modify the intestinal microbiome [9, 15]. It also leads to changes in the intestinal mucosal walls in rats [16] and to pronounced neuroendocrine adaptation processes [2, 17]. Finally, in the K-to-G switch, fasting has been found to activate stem cells and multiple system regeneration in the refeeding period [4, 18, 19] and to increase the mitochondrial biogenesis in neurons and other body cells [2, 9].
Long periods of fasting, lasting several days to several weeks, are physiologic, e.g. during seasons of low sun exposure, and are still part of the life of most animals [20, 21] as well as of humans living without food conservation technologies [22].
Fasting periods with various patterns are found in most religions [23]. For instance, Ramadan intermittent fasting was linked with improvements in cardiometabolic risk factors [24]. Furthermore, morbid obesity and associated diseases were treated in the 1960s with long periods of fasting that were termed the “zero calorie diet” [25, 26]. In exceptional circumstances these periods could last up to 249 days or more [27, 28]. A medical program of periodic fasting developed by the German physician Otto Buchinger to treat obesity and metabolic and inflammatory pathologies is well-known in central Europe [29–31]. The therapeutic effects of Buchinger periodic fasting are documented in small studies on overweight [32], blood pressure [33], metabolic syndrome [34], fibromyalgia [35], chronic pain syndromes [36] and the enhancement of quality of life [37]. The effects of repeated cycles of Buchinger fasting have also been reported [38, 39].
We are not aware of large studies on PF including normal weight or moderately obese subjects and focused on safety and tolerability. In the present observational study, we documented prospectively the safety, general health-related outcomes and well-being of 1422 subjects. They fasted for periods between 4 to 21 days under medical supervision according to the Buchinger fasting program, as described in peer-reviewed guidelines [31]. The fasting took place in a facility specialized in therapeutic fasting, the Buchinger Wilhelmi clinic (BWC) in Germany. The protocol involved daily clinical monitoring, intake of 2–3 L of water per day and 250 kcal of food, as well as a multi-disciplinary program including health education and physical activity.
The aim of this study was to assess for the first time prospectively the safety, therapeutic efficiency and effects on well-being of Buchinger periodic fasting in a large cohort.
Materials and methods
Ethics
This observational and prospective study was approved by the medical council of Baden-Württemberg and the Ethics Committee of the Charité-University Medical Center, Berlin (application number: EA4/054/15) on 5 May 2015. The study protocol was registered in the German Clinical Trials Register (DRKS-ID: DRKS00010111). The authors confirm that all ongoing and related trials for this intervention are registered. At the time of obtaining the ethical approval by the German authorities it was not mandatory to register an observational study. Only randomized clinical trials were clearly recommended to register. Nevertheless, we decided to register this observational study (on 3 June 2016).
Participants were enrolled after giving their written informed consent between 1 January and 31 December 2016. The study was conducted in the BWC in Überlingen (Germany) in accordance with the principles of the Declaration of Helsinki. The follow-up was completed between 26 January 2016 and 18 December 2017.
Participants
Our study on Buchinger fasting during periods of 4 to 21 days included 1422 subjects. They were selected out of a total of 3929 subjects who were admitted to the BWC and fulfilled the following criteria: they had a clinic stay of at least 10 nights and signed the informed written consent at the beginning of the inpatient stay after confirming that they would not participate in another study. Subjects were aged between 18–99 years and had no predefined contraindication to Buchinger fasting (e.g. cachexia, anorexia nervosa, advanced kidney, liver or cerebrovascular insufficiency, dementia or other severely debilitating cognitive disease and no existing pregnancy or lactation period) [31]. Furthermore, blood must have been collected on the precise days defined in the protocol (S2 and S3 Text). We excluded subjects who were prescribed other diets than fasting according to predefined criteria as well as those who could not follow the study procedures due to an inability to speak German, English or French. A flow chart reflecting the selection procedure is given in Fig 1.
Fig 1. Flow chart of the selection procedure of study participants.
The subjects came voluntarily to the BWC for preventive or therapeutic reasons. They selected established programs of 5, 10, 15 and 20 fasting days, which are reflected in the 4 groups (F5d, F10d, F15d, F20d). The main personal intentions for the fasting intervention were reduction of cardiovascular risk factors, weight loss in case of obesity and relief of general health problems such as inflammatory diseases, distress and exhaustion. In case of prescribed drug intake, the dosage was adapted during the stay by the 8 physicians of the clinic, who examined all participants 2 to 3 times per week. The subjects had a wide diversity of national and cultural backgrounds. The majority of them came from upper social classes and had high education levels.
Fasting program
All subjects fasted according to the guidelines of the Buchinger fasting therapy [31] under daily supervision of nurses and specialized physicians. On the day before the beginning of the fast, the participants were given a 600 kcal vegetarian diet divided into 3 meals of either rice and vegetables or fruits, according to individual preference. To initiate the fasting period, the intestinal tract was emptied through the intake of a laxative (20–40 g NaSO4 in 500 ml water). During fasting all subjects were asked to drink 3 L of water or non-caloric herbal teas daily with an optional portion of 20 g honey. Additionally, an organic freshly squeezed fruit or vegetable juice (250 ml) was served at noon and a vegetable soup (250 ml) in the evening, leading to an average total calorie intake of 200–250 kcal and 25–35 g of carbohydrates per day. At the beginning of the fasting period the subjects entered a program of light physical exercise alternating with rest and individual mild non-physical treatments like hydrotherapy or physiotherapy. The exercise program consisted of light to moderate intensity outdoor walks and group gymnastics. The whole program was led by certified trainers. During the fasting period an enema or, if preferred by the patient, a mild laxative was applied every second day in order to remove intestinal remnants and desquamated mucosal cells. On the last day of fasting, food was stepwise reintroduced during an average of 4 days, with an ovo-lacto-vegetarian organic diet progressively increasing from 800 to 1600 kcal/day.
Measurements
To document safety as well as health benefits and well-being during a prolonged periodic fasting program, we performed the predefined following measurements at baseline (pre-) and at the completion of fasting (post-). Before starting the fast all subjects went through a thorough physical examination and their medical history was documented.
Well-being, ketone bodies, mild symptoms and any changes in major health complaints were self-reported under supervision. The results were daily noted in a questionnaire (S4, S5, S6 Text). A total of 1311 subjects out of the 1422 returned the completed questionnaire.
Weight, abdominal circumference, blood pressure and pulse
Clinical data were collected by the physicians. Trained nurses documented every morning according to a standardized protocol the body weight of the participants wearing standard clothing (Seca 704, Seca, Hamburg, Germany). Blood pressure and pulse were measured after a pause, once at the non-dominant arm in sitting position (upper arm blood pressure monitor, boso Carat professional, BOSCH + SOHN GmbH u. Co. KG, Jungingen, Germany). Height was assessed with seca 285 (Seca, Hamburg, Germany) and abdominal circumference was determined with a measuring tape mid-way between the lowest rib and the iliac crest (openmindz GmbH, Heidelberg, Germany).
Well-being
To evaluate well-being, the participants self-reported daily their physical (PWB) and emotional well-being (EWB) on numeric rating scales from 0 (very bad) to 10 (excellent), under nurses’ supervision. The aim was to document the tolerability of the fasting program.
In a pre-study sample, we evaluated the acceptance of validated questionnaires to assess well-being within the patient population of the BWC, but found that they were regarded as too time-consuming in comparison with the numeric rating scales. To avoid drop-out and missing data, we therefore decided to use numeric rating scales.
Ketone bodies
The subjects self-measured the semi-quantitative concentration of ketone bodies in the first morning urine using Ketostix (Bayer AG, Leverkusen, Germany), which reacts according to the concentration of acetoacetic acid.
Mild symptoms and adverse effects
The Buchinger periodic fasting program was continuously monitored for safety and supervised by the medical staff: mild symptoms were reported daily by means of a multiple choice questionnaire, completed by the subjects under the supervision of nurses. This questionnaire listed the 19 most frequent mild symptoms that are observed in BWC and mentioned in the guidelines of the Buchinger fasting therapy [31]. We considered a mild symptom as being relevant when it was mentioned at least 3 times. In addition to the listed symptoms, the medical staff reported further mild symptoms that we categorized as “observed symptoms”. Furthermore, occasional adverse effects (AE) were documented by the physicians.
Major health complaint
A self-evaluation of health status was undertaken at the end of the stay: the subjects were asked to self-rate any changes in their major health complaint (in cases in which they indicated one at the begin of the fast) during the fasting intervention on a visual numeric scale from 0 (much worse) to 7 (much better).
Blood analysis
A blood analysis was taken according to international methods (see below): (lipid parameters: total cholesterol [TC], triglycerides [TG], high-density lipoprotein [HDL-C], low-density cholesterol [LDL], LDL-C/HDL-C ratio [LDL/HDL-ratio]; glycaemia: blood glucose and glycated haemoglobin [HbA1c]; blood count: leukocytes, erythrocytes, haemoglobin, haematocrit, mean cell volume [MCV], mean corpuscular haemoglobin [MCH], mean corpuscular haemoglobin concentration [MCHC], thrombocytes; coagulation: international normalized ratio [INR], Quick, partial thromboplastin time [PTT]; liver function: serum glutamic oxaloacetic transaminase [GOT], serum glutamate pyruvate transaminase [GPT], serum gamma-glutamyl transferase [GGT], alkaline phosphatase [AP]; inflammatory biomarkers: C-reactive protein [CRP], erythrocyte sedimentation rate [ESR] after 1 and 2 hours; renal function: uric acid, urea and creatinine and electrolytes: sodium [Na], potassium [K], calcium [Ca], magnesium [Mg]).
Laboratory examinations
Blood samples were collected twice, namely before the start of the fasting (thereafter referred as baseline values) and at the end of the fasting period. They were collected by trained medical-technical assistants between 7.30 and 9.30 am and drawn into EDTA (S-Monovette 2.7 ml K3 EDTA), citrate (S-Monovette 3 ml 9NC, Citrate 3.2% [1:10]) and blood sedimentation tubes (S-Sedivette 3.5 ml 4NC, ESR/Citrate Buffer [1:5]), that were shaken gently after filling. Additionally, serum tubes including serum gel with clotting activator (S-Monovette 9 ml Z-Gel) were used and stored upright for 30 min until coagulation, with subsequent centrifugation at 3920 g (5000 rpm) for 10 min at room temperature. All tubes were manufactured by Sarsteadt AG & Co. (Nürnbrecht, Germany).
The ESR was assessed within a period of 4 hours after blood collection and determined after 1 and 2 hours of blood sedimentation. All further analyses were performed at MVZ Labor Ravensburg, according to the manufacturer’s instruction, in a fully- automated laboratory. Blood cell count (leukocytes, erythrocytes, haemoglobin, MCV, MCH, MCHC, thrombocytes) was measured using the blood analyser Sysmex XN-9000 (Sysmex Europe GmbH, Norderstedt, Germany). Coagulation parameters (INR, Quick, PTT) were assessed on ACL Top (Werfen, Kirchheim, Germany). The liver enzymes (GOT, GPT, GGT, AP), kidney parameters (urea, creatinine, uric acid), lipid parameters (TC, TG, HDL-C, LDL-C, LDL/HDL ratio), electrolytes (Na, K, Ca), glucose and CRP were analysed with ADVIA 2400 (Siemens Healthcare GmbH, Erlangen, Germany). The HbA1c was assessed with TOSOHTM (Bio-Rad Laboratories GmbH, München, Germany) and Mg with ICP-MS 7700x series (Agilent, Waldbronn, Germany).
Data and statistical analysis
Participants were divided into four groups according to the duration of their fasting period (Fig 1): F5d underwent a fasting period of 5±2 days, with an average of 5.4 (n = 695), F10d underwent a fasting period of 10±2 days, with an average of 8.6 (n = 530), F15d underwent a fasting period of 15±2 days, with an average of 14.1 (n = 196) and F20d underwent a fasting period of 20±2 days, with an average of 20.1 (n = 37). Between-group differences at baseline were tested using a one-way ANOVA test followed by Tukey’s post-hoc tests.
We tested the effect of fasting, while taking into account the sex and fasting duration group effects, by using a multistep parsimonious statistical approach. First, for each outcome the effect of fasting was assessed by using mixed linear models taking repeated measurements among subjects into account, with fasting intervention, fasting duration group, sex, fasting duration group-by-fasting-intervention, fasting intervention-by-sex, sex-by-fasting duration group and baseline values of the outcome (pre-fasting) as fixed effects. For each outcome, the covariance structures was selected among three (compound symmetry (CS), autoregressive (AR(1)) and variance components (VC)) using the Bayesian information criteria (BIC). In a last step the interaction effects that were not significant, were removed from the model to obtain a more parsimonious model. To simplify the presentation of the results, sex differences are presented in figures only when the fasting-intervention-by-sex effect was significant. To take into account the multiple tests performed on this dataset, significance was set at a conservative level of p<0.01.
Data are shown as mean±standard error of the mean (SEM), if not indicated otherwise. Statistical analyses were performed with SAS version 9.4 (SAS Institute, Cary, USA). Graphs were generated using GraphPad Prism version 6 for Windows (GraphPad Software, La Jolla California USA).
Results
General parameters
The baseline characteristics of the 1422 adult participants are shown in Table 1. Mean age was 55.4±0.4 with 59.1% women and 40.9% men. In total 63.4% of the subjects were non-obese (BMI<30). Grade I obesity (30≤BMI<35) was present in 19.5% and grade II or higher (BMI≥35) in 10.3%. Subjects who chose to fast on average for 20 days (F20d) had the highest baseline body mass index (BMI), the highest abdominal circumference (waist), and largest weight reduction (-8.6±0.3 kg) (each p<0.001). Men had a higher mean BMI at baseline (Table 1).
Table 1. Baseline characteristics of the subjects.
| All | F5d | F10d | F15d | F20d | |
|---|---|---|---|---|---|
| Days (d) | 5±2 | 10±2 | 15±2 | 20±2 | |
| Subjects, n (%) | 1422 (100.0) | 659 (46.3) | 530 (37.3) | 196 (13.8) | 37 (2.6) |
| Men (%) | 581 (40.9) | 278 (42.2) | 214 (40.4) | 76 (38.8) | 13 (35.1) |
| Women (%) | 841 (59.1) | 381 (57.8) | 316 (59.6) | 120 (61.2) | 24 (64.9) |
| Age, years | 55.4±0.4 | 54.2±0.5 b,c | 56.3±0.6 a | 56.4±0.9 a | 56.4±2.3 |
| Fasting length (days) | 8.2±0.1 | 5.4±0.0 b,c,d | 8.6±0.0 a,c,d | 14.1±0.1 a,b,d | 20.1±0.2 a,b,c |
| Waist, cm | 94.0±0.4 | 91.3±0.6 b,c,d | 94.8±0.7 a,c,d | 98.3±1.2 a,b,d | 106.3±2.8 a,b,c |
| Weight, kg | 82.0±0.5 | 79.3±0.8 b,c,d | 82.7±0.9 a,c,d | 86.6±1.6 a,b,d | 96.7±4.0 a,b,c |
| BMI, kg/m2 | 28.2±0.2 | 27.2±0.2 b,c,d | 28.5±0.3 a,c,d | 29.7±0.4 a,b,d | 33.6±1.1 a,b,c |
| BMI<25, n (%) | 404 (28.4) | 227 (56.2) | 133 (32.9) | 41 (10.1) | 3 (0.7) |
| 25≤BMI<30, n (%) | 497 (35.0) | 232 (46.7) | 199 (40.0) | 61 (12.3) | 5 (1.0) |
| BMI≥30, n (%) | 425 (29.9) | 155 (36.5) | 160 (37.6) | 84 (19.8) | 26 (6.1) |
| BMI men, kg/m2 | 30.0±0.2 | 29.2±0.3 | 30.3±0.3 | 31.3±0.7 | 34.0±1.5 |
| BMI<25, n (%) | 74 (12.7) | 46 (62.2) | 18 (24.3) | 10 (13.5) | 0 (0.0) |
| 25≤BMI<30, n (%) | 231 (39.8) | 117 (50.6) | 92 (39.8) | 20 (8.7) | 2 (0.9) |
| BMI≥30, n (%) | 230 (39.6) | 94 (40.9) | 87 (37.8) | 40 (17.4) | 9 (3.9) |
| BMI women, kg/m2 | 27.0±0.2 | 25.7±0.2 | 27.3±0.3 | 28.7±0.5 | 33.3±1.4 |
| BMI<25, n (%) | 330 (39.2) | 181 (54.8) | 115 (34.8) | 31 (9.4) | 3 (0.9) |
| 25≤BMI<30, n (%) | 266 (31.6) | 115 (43.2) | 107 (40.2) | 41 (15.4) | 3 (1.1) |
| BMI≥30, n (%) | 195 (23.2) | 61 (31.3) | 73 (37.4) | 44 (22.6) | 17 (8.7) |
Subjects were divided into 4 groups according to the fasting lengths: 5, 10, 15 and 20±2 days. Significant differences between the groups are indicated as
a, p<0.05 versus F5d
b, p<0.05 versus F10d
c, p<0.05 versus F15d
d, p<0.05 versus F20d. BMI, body mass index. Data are presented as mean±SEM.
Weight, abdominal circumference and blood pressure
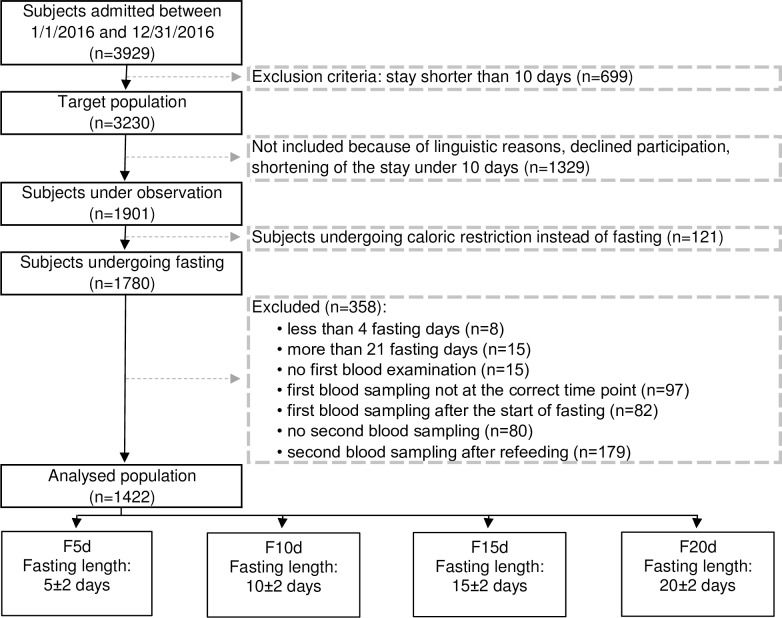
As expected, weight and BMI showed a significant decrease (fasting intervention: p<0.001) in all 4 groups (S1 Table). The weight loss increased with the fasting period length and varied between 3.2±0.0 kg for F5d and 8.6±0.3 kg for F20d (fasting duration group-by-fasting intervention: p<0.001). Abdominal circumference also decreased significantly (fasting intervention: p<0.001). The reduction varied between 4.6±0.1 cm for F5d and 8.8±0.8 cm for F20d (fasting duration group-by-fasting intervention: p<0.001). Weight and abdominal circumference reduction were significantly higher (fasting-intervention-by-sex: each p<0.001) in men in all groups (Fig 2A and 2B), compared with women.
Fig 2.
Changes in weight (A) and abdominal circumference (B) according to the length of fast and gender.
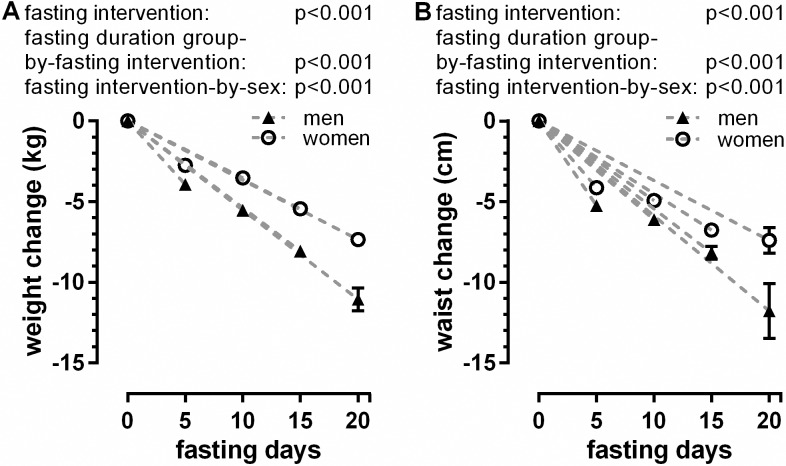
Baseline values of systolic blood pressure (SBP) and diastolic blood pressure (DBP) were higher in the groups fasting longer (Fig 3A and 3B). The mean values for the whole cohort decreased significantly from 131.6±0.7 to 120.7±0.4 for SBP (fasting intervention: p<0.001) and from 83.7±0.4 to 77.9±0.3 for DBP (fasting intervention: p<0.001). The reduction of SBP and DBP was greater in the groups who fasted longer (fasting duration group-by-fasting intervention: each p<0.001) without gender difference (Fig 3A and 3B), stabilizing for the whole cohort around 120/78 mm Hg (S1 Table). We did not observe significant changes in heart rate during fasting in the whole group (S1 Table).
Fig 3.
Changes in systolic (A) and diastolic blood pressure (B) according to the fasting length.
Well-being
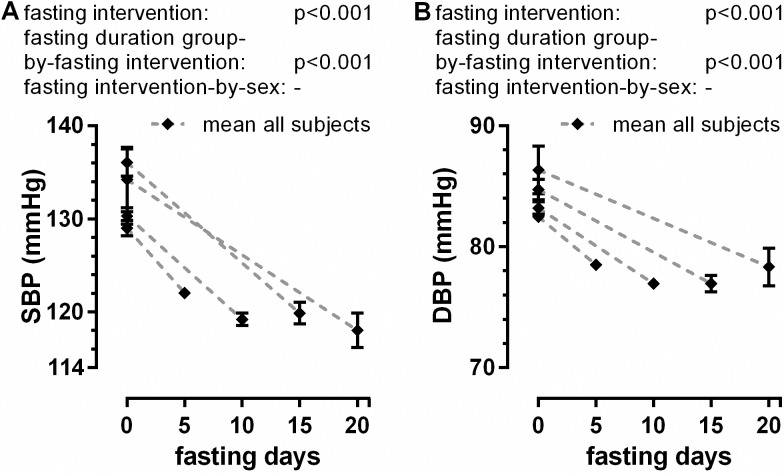
Baseline values of emotional well-being (EWB) and physical well-being (PWB) were lower in the groups that fasted longer. This suggests that subjects choosing longer fasting periods had lower emotional and physical self-ratings at baseline than the ones who selected shorter periods of fast. EWB as well as PWB were both significantly enhanced in the course of the fast (fasting intervention: each p<0.001) (S1 Table). There is no difference between genders for those parameters (Fig 4A and 4B). All groups reached similar increased values of well-being at the end of their stay.
Fig 4.
Changes in emotional (A) and physical well-being (B) during fasting. Self-recorded data of a 0–10 numeric rating scale for a total of 1074 volunteers are shown.
Ketone bodies
Acetoacetic acid, reflecting ketosis, increased significantly from baseline to the end of fast (fasting intervention: p<0.001), suggesting a plateau value reached after 5 days. Men had higher scores of acetoacetic acid than women (fasting intervention-by-sex: p<0.001) (S1 Table).
Mild symptoms and adverse effects
The safety of the Buchinger fasting program was assessed by collecting daily all self-reported and observed mild symptoms (Table 2). Of the 1311 participants who returned the filled questionnaire, 0.35% reported muscular cramp, which was the least frequent mild symptom, and 14.94% sleep disturbances, which was the most frequent mild symptom. As shown in S1 Fig. the incidence of mild symptoms like muscle pain, sleep disturbances, headaches, and hunger occurred mainly in the first days of the fast.
Table 2. Mild symptoms and adverse effects (AE).
| Mild symptoms (self-reported) | n | % | Mild symptoms (observed) | n | % | |
|---|---|---|---|---|---|---|
| Sleep Disturbance | 169 | 14.94 | Dizziness | 2 | 0.14 | |
| Fatigue | 155 | 13.70 | Eczema | 2 | 0.14 | |
| Dry Mouth | 100 | 8.84 | Bleeding gums | 1 | 0.07 | |
| Back Pain | 84 | 7.43 | Hyperventilation | 1 | 0.07 | |
| Hunger | 77 | 6.81 | Outbreak of infection | 1 | 0.07 | |
| Bad Breath | 61 | 5.39 | Pleuropneumonia | 1 | 0.07 | |
| Headache | 61 | 5.39 | Tetany | 1 | 0.07 | |
| Muscle Pain | 49 | 4.33 | Visual disorder | 1 | 0.07 | |
| Abdominal Bloating | 47 | 4.16 | ||||
| Diarrhoea | 38 | 3.36 | ||||
| Sensitivity to Cold | 33 | 2.92 | AE | n | % | |
| Cravings | 29 | 2.56 | Cardiac arrhythmia | 3 | 0.21 | |
| Vertigo | 28 | 2.48 | Hyponatremia | 3 | 0.21 | |
| Blurred Vision | 23 | 2.03 | Hospitalisation | 2 | 0.14 | |
| Restless Legs | 23 | 2.03 | Hypoglycaemia | 2 | 0.14 | |
| Skin Rash | 19 | 1.68 | Hypokalaemia | 1 | 0.07 | |
| Nausea | 13 | 1.15 | Gout | 1 | 0.07 | |
| Palpitation | 13 | 1.15 | Vomiting | 1 | 0.07 | |
| Dyspepsia | 12 | 1.06 | Spasmodic abdominal pain | 1 | 0.07 | |
| Muscular cramp | 4 | 0.35 | ||||
Out of the total of 1422 a group of 1311 subjects completed and returned the daily questionnaire to self-record mild symptoms. In contrast to the observed symptoms and AE, self-reported mild symptoms were recorded if a particular symptom was experienced by the same person more than 3 times during the fasting period. The observed symptoms and AE were documented during the daily nurse visit and/or the medical visit for all subjects. The same subject could mention more than one symptom or AE.
No fatalities or permanent adverse effects were observed. Two subjects had to be admitted to hospital. A 75-year old man with known coronary artery disease had on the 9th fasting day a non-ST segment elevation myocardial infarction and received uncomplicated percutaneous coronary intervention. After 3 days in the hospital he returned to BWC. The second case was a 67-year old woman who had a one-day hospitalisation because of vomiting with dizziness and diarrhoea on the 4th fasting day. After returning to BWC she received an 800 kcal/d diet. The other AE were transitory and did not lead to an interruption of the fasting therapy. AE (Table 2) such as cardiac arrhythmia were low-grade, transitory and could be treated uncomplicatedly without stopping the fasting. The same applied to transitory hypoglycaemia. We also observed one case of gout attack in a patient treated previous to the fasting with allopurinol for hyperuricemia and frequent gout attacks. He was able to be symptomatically treated and went on fasting.
Major health complaint
To document the effects of the Buchinger fasting program on their health we asked the participants to self-report if they had a major health complaint before the fasting and how this condition had been influenced by the fast. A group of 404 subjects out of the 1311 who returned the self-report (S2 Fig) mentioned having a major health complaint previous to the fasting. They were asked to evaluate the changes after the fasting. In 84.4% of the 404 subjects the major health complaint had much improved, 8.7% reported that it remained unchanged and 6.9% reported a worsening.
Blood lipids and glycaemia
We assessed the impact of the Buchinger fasting program on metabolism by analysing several blood parameters (S2 Table).
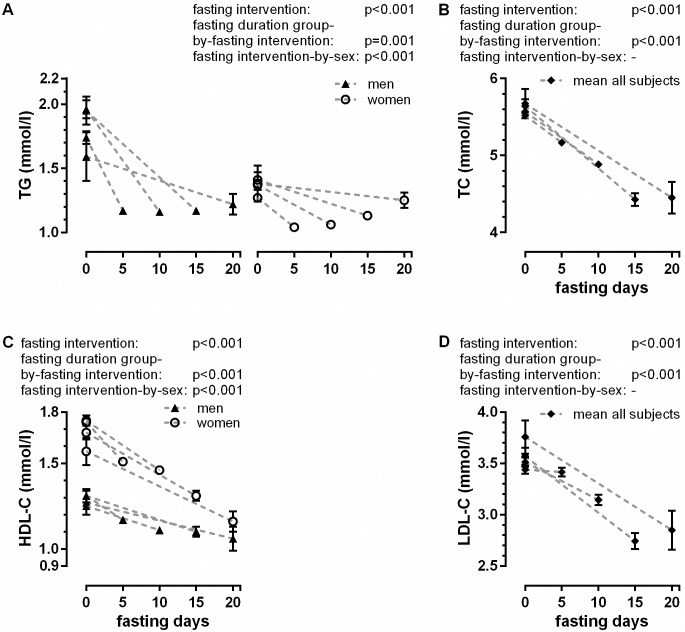
The lipid values are indicated in S2 Table and Fig 5. At baseline, TG values of women were lower than the values of men. Fasting reduced TG levels by 0.44 mmol/L on average (fasting intervention: p<0.001) (S2 Table). TG levels at the end of the fasting were similar in all groups, suggesting a floor effect (Fig 5A). The decrease in TC was significant (fasting intervention: p<0.001) and higher in the groups who fasted for longer (fasting duration group-by-fasting intervention: p<0.001). Fig 5B indicates that F15d and F20d had similar post-values. There was no difference in TC changes during fasting between men and women. Baseline HDL-C values were higher in women (Fig 5C). HDL-C decreased significantly (fasting intervention: p<0.001). The reduction was higher in the groups that fasted longer (fasting duration group-by-fasting intervention: p<0.001) and in women compared to men (fasting intervention-by-sex: p<0.001). LDL-C decreased significantly (fasting intervention: p<0.001) (Fig 5D) and again the decrease was higher in the groups that fasted longer (fasting duration group-by-fasting intervention: p<0.001). Gender differences for LDL-C were not significant. The LDL/HDL ratio was not influenced by fasting.
Fig 5. Fasting-induced changes in lipid metabolism.
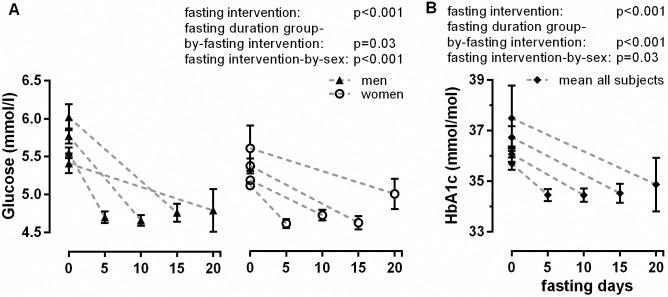
The blood glucose parameters are given in S2 Table and Fig 6. Baseline values for glucose were higher in men compared to women (Fig 6). The glucose values decreased significantly (fasting intervention: p<0.001) without differences between the fasting period lengths and stabilized at an average of 4.7 mmol/L (S2 Table). Fig 6B shows the significant decrease in HbA1c (fasting intervention: p<0.001), which varied between a decrease of 1.2±0.1 for F5d and 2.6±0.5 mmol/mol for F20d (fasting duration group-by-fasting intervention: p<0.001).
Fig 6.
Changes of blood glucose (A) and glycated haemoglobin (HbA1c) (B). The panel A was split for gender to increase the readability of the figure.
Blood count
Table 3 shows the impact of fasting on blood count. Leucocytes decreased significantly in all groups (fasting intervention: p<0.001) with stronger reduction in the groups who fasted longer (fasting duration group-by-fasting intervention: p<0.001) and without statistical significance between men and women. Erythrocytes showed an increase (fasting intervention: p<0.001) of an average of 0.06 x106/μl in all groups and steadied at around 4.82x106/μl. Haemoglobin showed also an increase (fasting intervention: p<0.001) of about 0.1 mmol/L that was independent of the fasting length. Haematocrit was not influenced by fasting. Thrombocytes showed a significant reduction (fasting intervention: p<0.001) during fasting by a mean of 6.6±0.7x103/μl, with gender difference (fasting intervention-by-sex: p<0.001) and influence of the fasting length (fasting duration group-by-fasting intervention: p<0.001).
Table 3. Blood cells pre- and post-fasting.
| All (n = 1422) |
F5d 5±2 d |
F10d 10±2 d |
F15d 15±2 d |
F20d 20±2 d |
p-values | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pre | post | pre | post | pre | post | Pre | post | pre | post | fasting inter- vention |
fasting dura- tion group |
sex | fasting dura- tion group-by-fasting inter- vention |
fasting inter- vention-by-sex |
|
| Leukocytes, 103/μl | 5.9±0.0 | 5.4±0.0 | 5.9±0.1 | 5.5±0.1 | 6.0±0.1 | 5.4±0.1 | 6.0±0.1 | 5.0±0.1 | 5.7±0.3 | 4.7±0.2 | <0.001 | <0.001 | 0.18 | <0.001 | ‒ |
| Erythrocytes, 106/μl | 4.76±0.01 | 4.82±0.01 | 4.76±0.02 | 4.82±0.02 | 4.76±0.02 | 4.81±0.02 | 4.74±0.03 | 4.81±0.03 | 4.74±0.07 | 4.86±0.07 | <0.001 | 0.96 | <0.001 | ‒ | 0.001 |
| Haemoglobin, mmol/L | 8.9±0.0 | 9.0±0.0 | 8.9±0.0 | 9.0±0.0 | 8.9±0.0 | 9.0±0.0 | 8.9±0.1 | 9.0±0.0 | 8.7±0.1 | 8.9±0.1 | <0.001 | 0.72 | 0.33 | ‒ | <0.001 |
| Haematocrit, % | 42.2±0.1 | 42.3±0.1 | 42.2±0.1 | 42.3±0.1 | 42.3±0.1 | 42.3±0.1 | 42.2±0.2 | 42.3±0.2 | 41.8±0.5 | 42.2±0.5 | 0.74 | 0.83 | 0.06 | ‒ | 0.02 |
| MCV, fl | 89.0±0.1 | 88.0±0.1 | 88.8±0.2 | 87.9±0.2 | 89.1±0.2 | 88.0±0.2 | 89.3±0.3 | 88.0±0.3 | 88.5±0.9 | 87.0±0.8 | <0.001 | 0.001 | <0.001 | 0.001 | <0.001 |
| MCH, pg | 30.1±0.1 | 30.1±0.1 | 30.1±0.1 | 30.1±0.1 | 30.2±0.1 | 30.2±0.1 | 30.2±0.1 | 30.1±0.1 | 29.7±0.3 | 29.7±0.3 | 0.38 | 0.12 | 0.82 | 0.09 | ‒ |
| MCHC, g/dl | 33.9±0.0 | 34.2±0.0 | 33.9±0.0 | 34.2±0.0 | 33.9±0.0 | 34.3±0.0 | 33.8±0.1 | 34.2±0.1 | 33.6±0.2 | 34.0±0.1 | <0.001 | 0.80 | 0.11 | ‒ | 0.004 |
| Thrombocytes, 103/μl | 244.1±1.5 | 237.5±1.5 | 242.6±2.2 | 239.2±2.2 | 245.9±2.5 | 238.9±2.6 | 243.9±3.9 | 230.6±4.1 | 245.6±11.4 | 224.4±11.4 | <0.001 | <0.001 | 0.03 | <0.001 | <0.001 |
Values are shown as mean±SEM for all of the groups with different fasting lengths. P-values were calculated for the effects of fasting intervention as well as the effects of the fasting length (fasting duration group) and gender (sex). Interactions between fasting intervention by fasting duration group (fasting duration group-by-fasting intervention) and fasting intervention by gender (fasting intervention-by-sex) are shown.
MCV, mean cell volume; MCH, mean corpuscular haemoglobin; MCHC, mean corpuscular haemoglobin concentration.
Coagulation
Table 4 shows changes in blood coagulation parameters, liver function, inflammatory parameters, kidney function and electrolytes. INR and PTT increased (fasting intervention: each p<0.001) and Quick value decreased (fasting intervention: p<0.001) significantly during fasting. The fasting period length had a significant influence on the coagulation parameters (fasting duration group-by-fasting intervention: each p<0.001) and more pronounced changes were observed in groups of longer fasting periods.
Table 4. Blood parameters pre- and post-fasting.
| All (n = 1422) |
F5d 5±2 d |
F10d 10±2 d |
F15d 15±2 d |
F20d 20±2 d |
p-values | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pre | post | pre | post | pre | post | Pre | post | pre | post | fasting inter- vention |
fasting dura- tion group |
sex | fasting dura- tion group-by-fasting inter- vention |
fasting inter- vention-by-sex |
|
| INR | 0.99±0.00 | 1.08±0.00 | 0.98±0.00 | 1.06±0.00 | 0.99±0.01 | 1.09±0.01 | 0.99±0.02 | 1.11±0.02 | 0.98±0.01 | 1.10±0.02 | <0.001 | <0.001 | 0.84 | <0.001 | ‒ |
| Quick, % | 104.3±0.3 | 91.4±0.3 | 104.2±0.5 | 92.9±0.4 | 104.0±0.6 | 90.6±0.5 | 105.6±1.0 | 89.1±0.9 | 104.1±1.2 | 88.3±1.9 | <0.001 | <0.001 | 0.33 | <0.001 | 0.005 |
| PTT, sec | 31.1±0.1 | 32.7±0.1 | 31.1±0.1 | 32.4±0.1 | 31.0±0.1 | 32.8±0.2 | 31.2±0.2 | 33.7±0.3 | 31.4±0.4 | 32.9±0.5 | <0.001 | <0.001 | 0.88 | <0.001 | ‒ |
| GOT, μkat/L | 0.4±0.0 | 0.6±0.0 | 0.4±0.0 | 0.6±0.0 | 0.4±0.0 | 0.6±0.0 | 0.4±0.0 | 0.6±0.0 | 0.4±0.0 | 0.7±0.0 | <0.001 | 0.73 | 0.007 | ‒ | <0.001 |
| GPT, μkat/L | 0.5±0.0 | 0.7±0.0 | 0.5±0.0 | 0.6±0.0 | 0.5±0.0 | 0.7±0.0 | 0.5±0.0 | 0.7±0.0 | 0.6±0.0 | 0.8±0.1 | <0.001 | 0.07 | 0.65 | 0.10 | ‒ |
| GGT, μkat/L | 0.6±0.0 | 0.4±0.0 | 0.5±0.0 | 0.4±0.0 | 0.6±0.1 | 0.4±0.0 | 0.6±0.1 | 0.4±0.0 | 0.5±0.1 | 0.4±0.01 | <0.001 | <0.001 | 0.18 | <0.001 | <0.001 |
| AP, μkat/L | 1.1±0.0 | 1.0±0.0 | 1.0±0.0 | 1.0±0.0 | 1.1±0.0 | 1.1±0.0 | 1.1±0.0 | 1.0±0.0 | 1.2±0.1 | 1.1±0.1 | <0.001 | <0.001 | 0.003 | <0.001 | 0.002 |
| CRP, mg/L | 2.85±0.14 | 4.30±0.20 | 2.49±0.19 | 4.11±0.27 | 3.02±0.28 | 4.35±0.36 | 3.37±0.32 | 4.74±0.63 | 4.08±0.67 | 4.67±0.82 | 0.001 | 0.97 | 0.99 | 0.81 | 0.53 |
| ESR 1h | 11.6±0.2 | 11.4±0.2 | 10.9±0.3 | 11.7±0.3 | 11.8±0.4 | 11.5±0.4 | 12.8±0.7 | 10.6±0.6 | 15.1±1.6 | 10.2±1.4 | <0.001 | <0.001 | 0.002 | <0.001 | <0.001 |
| ESR 2h | 21.7±0.4 | 21.3±0.4 | 20.4±0.5 | 21.8±0.5 | 22.1±0.6 | 21.4±0.6 | 23.9±1.1 | 19.7±1.0 | 28.2±2.8 | 20.1±2.4 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Uric acid, μmol/L | 338.1±2.3 | 495.2±4.4 | 334.0±3.3 | 481.1±6.0 | 339.2±3.8 | 505.5±7.5 | 345.3±6.4 | 513.0±13.5 | 355.9±12.8 | 505.4±30.6 | <0.001 | 0.01 | <0.001 | 0.01 | <0.001 |
| Urea, mmol/L | 4.7±0.0 | 3.1±0.0 | 4.6±0.1 | 3.3±0.1 | 4.7±0.1 | 3.0±0.1 | 4.7±0.1 | 2.7±0.1 | 5.1±0.3 | 2.8±0.3 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Creatinine, μmol/L | 71.92±0.40 | 76.43±0.54 | 72.53±0.58 | 76.45±0.76 | 71.88±0.68 | 76.88±1.00 | 69.86±0.98 | 74.98±1.21 | 72.27±2.53 | 77.17±3.13 | <0.001 | 0.34 | <0.001 | ‒ | <0.001 |
| Na,mmol/L | 140.1±0.1 | 138.7±0.1 | 140.0±0.1 | 138.4±0.1 | 140.1±0.1 | 138.8±0.1 | 139.8±0.3 | 139.2±0.2 | 141.0±0.33 | 139.9±0.4 | <0.001 | <0.001 | 0.37 | 0.003 | ‒ |
| K, mmol/L | 4.4±0.0 | 4.4±0.0 | 4.4±0.0 | 4.4±0.0 | 4.4±0.0 | 4.4±0.0 | 4.3±0.0 | 4.4±0.0 | 4.4±0.1 | 4.4±0.1 | 0.001 | 0.74 | <0.001 | ‒ | 0.008 |
| Ca, mmol/L | 2.32±0.00 | 2.38±0.00 | 2.33±0.00 | 2.36±0.00 | 2.32±0.00 | 2.39±0.00 | 2.32±0.01 | 2.39±0.01 | 2.33±0.02 | 2.40±0.02 | <0.001 | <0.001 | 0.14 | <0.001 | ‒ |
| Mg, mmol/L | 0.86±0.00 | 0.87±0.00 | 0.87±0.00 | 0.89±0.00 | 0.87±0.00 | 0.87±0.00 | 0.85±0.00 | 0.86±0.01 | 0.86±0.01 | 0.85±0.01 | 0.09 | <0.001 | <0.001 | <0.001 | <0.001 |
Values are shown as mean±SEM for all of the groups with different fasting lengths. P-values were calculated for the effects of fasting (fasting intervention) as well as the effects of the fasting length (fasting duration group) and gender (sex). Interactions between fasting intervention by fasting duration group (fasting duration group-by-fasting intervention) and fasting intervention by gender (fasting intervention-by-sex) are shown.
INR, international normalized ratio; PTT, partial thromboplastin time; GOT, serum glutamic oxaloacetic transaminase; GPT, serum glutamate pyruvate transaminase; GGT, serum gamma-glutamyl transferase; AP, alkaline phosphatase; CRP, C-reactive protein; ESR 1h, erythrocyte sedimentation rate after 1 hour; ESR 2h, erythrocyte sedimentation rate after 2 hours; Na, sodium; K, potassium; Ca, calcium, Mg, magnesium
Liver function
Regarding liver function, GOT and GPT levels rose significantly during the course of the fast (fasting intervention: each p<0.001) without difference between groups. The values at baseline and at the end remained within norm ranges (<0.8 μkat/L) increasing for GOT in average from 0.4 to 0.6 μkat/L and GPT from 0.5 to 0.7 μkat/L. GGT levels decreased significantly from a mean of 0.6 to 0.4 μkat/L (fasting intervention: p<0.001). AP showed a significant decrease (fasting intervention: p<0.001), more pronounced in the groups that fasted longer (fasting duration group-by-fasting intervention: p<0.001), with a slight dependence on gender (fasting intervention-by-sex: p = 0.002). The mean values before and after the fast were all in the norm range.
Inflammatory biomarkers
The inflammatory biomarkers CRP and ESR were analysed. The mean values at baseline and at the end were all in norm range (<5.0 mg/L). CRP raised significantly during fasting (fasting intervention: p<0.001). There was no difference between groups and gender. ESR after 1 and 2 hours decreased significantly (fasting intervention: each p<0.001) and the groups with longer fasting periods displayed stronger reductions (fasting duration group-by-fasting intervention: each p<0.001).
Renal function and uric acid
Uric acid levels, as well as renal function as reflected by urea and creatinine values, are given in Table 4. A significant increase of blood uric acid was observed (338.1±2.3 to 495.2±4.4 μmol/L) (fasting intervention: p<0.001). The highest uric acid level was measured in F15d. Urea concentrations decreased significantly in all groups (fasting intervention: p<0.001), but the decrease was stronger in the groups with longer fasting periods (fasting duration group-by-fasting intervention: p<0.001). Creatinine levels increased significantly (fasting intervention: p<0.001) with differences between the sexes (fasting intervention-by-sex: p<0.001) but without differences between groups.
Electrolytes
Sodium concentrations remained in norm range but showed a significant reduction (fasting intervention: p<0.001) from a mean of 140.1±0.1 to 138.7±0.1 mmol/L. Calcium levels increased significantly (fasting intervention: p<0.001), with an effect of groups (fasting duration group-by-fasting intervention: p<0.001) but not of gender. Potassium showed a reduction during fasting (fasting intervention: p = 0.001) and magnesium levels remained stable. All values pre- and post-fasting remained in norm range.
Discussion
The present prospective observational study systematically investigated for the first time the effects of long periods of Buchinger fasting within a specialized clinic in a cohort of 1422 subjects. The results showed that this type of fasting from 4 to 21 days is safe and well tolerated. Furthermore, it led to enhancement of emotional and physical well-being and improvement of relevant cardiovascular risk factors and subjective health complaints.
Fasting resulted as expected in marked weight loss with reduction of abdominal circumference, which was more pronounced in the groups who fasted longer. Thus it can be assumed that preferentially visceral adipose tissue was reduced with weight loss [40]. Of note, no particular rebound in weight gain has been shown after repeated cycles of Buchinger fasting in a previous study [38, 39].
Blood pressure showed a significant decrease, whereby mean values did not fall below the lower norm range, indicating a floor effect. Accordingly, serious hypotensive complications were not reported. Blood pressure reduction might be triggered by factors such as the increase of parasympathetic activity due to the release of brain-derived neurotrophic factor (BDNF) [2, 41, 42], increased renal Na excretion [43] and enhanced receptor sensitivity of natriuretic peptides and insulin [44]. Earlier studies on zero calorie diets and very-low-calorie diets (VLCD) [45–47]–and more recently in smaller studies on Buchinger fasting [33, 34] and water fasting [48]–also described this blood pressure-reducing effect.
We further found decreases in blood lipid levels following the fasting periods: TG levels as well as TC and LDL-C decreased significantly in all groups. Glucose levels and HbA1c decreased also significantly which points out to a positive effect of fasting on glucoregulation. It was also found in two previous smaller studies using Buchinger fasting [49, 50].
Altogether, the positive impact of periodic Buchinger fasting on the above mentioned parameters suggests a general cardioprotective effect that has also been shown in IF [51].
The continuous increase in emotional as well as physical well-being was evident across all groups of different fasting period lengths. This is an important component to increase compliance and has been reported in earlier studies based on daily mood ratings [26, 52, 53]. Weight loss, especially in obese subjects, is linked with mood improvement in many studies [54]. The G-to-K switch has been shown in IF to lead to enhanced performance in cognition, mood, motor and autonomic nervous system function [2, 55]. In IF and CR this is linked to the release of BDNF [51, 56]. BDNF, associated with neurogenesis and neuron protection, enhances the growth and survival of serotonin neurons [51, 57]. Furthermore, the reduction of insulin and leptin levels appears to act on the hypothalamic-pituitary-adrenal axis, thereby impacting mood positively [58]. Endogenous opioid (β-endorphin) could also contribute to well-being, as documented in a 10-day fasting trial in men [59]. In our study, the reported improvement of a major health complaint, often accompanied by pain relief, could possibly contribute to the increase of well-being. Moreover, it appears likely that a successful completion of a longer fasting period improves the feeling of self-efficacy, thereby enhancing subjective well-being [37]. It has been reported that short fasting periods of two days, as well as alternate-day fasting, are associated with the feeling of hunger [60–62]. This seems to be an obstacle to patient compliance [61, 62]. In contrast, hunger was not reported by 93% of the subjects in our study, which often surprised them positively. This possibly contributed to enhanced well-being.
As expected, we observed a significant increase in urinary ketone bodies excretion up to a maximum level that was similar in all groups. This suggests that in 5 days a plateau was reached. Ketosis and IMS was achieved, although Buchinger fasting provides small quantities of fruit juices and some honey, providing up to 25–35 g carbohydrates/day. Experimental research points to ketosis as the trigger of beneficial effects on brain and neurological diseases [2]. Daily time-restricted feeding causing IMS ameliorates anxiety-like behaviour in mice [63]. IMS enhances also structural and functional synaptic plasticity, cognition and neuronal stress response [64].
In our cohort, no fatal or life-threatening event occurred. Self-reported mild symptoms were observed mainly in the first days and disappeared either spontaneously or with natural remedies. Adverse effects were observed in 0.7% of subjects. Only two subjects had to interrupt the fasting. Adverse effects have also been mentioned in other studies [34, 65].
A recent publication that analysed retrospectively water-only fasting data found relatively more adverse effects, e. g. nausea, presyncope, dyspepsia, vomiting and palpitations [66], which we observed only in single cases. The use of different methodologies to assess and characterise AE limits the comparison with our study, which was prospective. Nevertheless, it could be possible that the supplementation with juices and soups, which slows down initial protein catabolism [67], enhances tolerability by modulating the onset of ketosis.
As already mentioned, we observed a subjective improvement in 85% of cases of a major health complaint. This documents within the limitations of a non-confirmatory study design the therapeutic effectiveness of Buchinger periodic fasting.
To assess further therapeutic effects of fasting, as well as the safety of this procedure, we performed extended laboratory analysis. Within the blood count leukocytes and thrombocytes decreased significantly but not below norm range. In humans after CR as well as in mice fed with cycles of a low calorie fasting mimicking diet, a drop in leukocytes count was followed by an increase in hematopoietic, mesenchymal stem and progenitor cells in the bone marrow. This was associated with the regeneration of all blood cell types and haemoglobin upon refeeding [4, 19]. We cannot extrapolate from our data whether this regeneration applies also to fasting in humans.
The increase in INR values was significant and more marked in the groups who fasted longer. Bleeding time (PTT) was also increased. The increase in INR is well-known [68, 69] and can be relevant for anticoagulated patients. During fasting they should be monitored and their medication adapted.
GOT and GPT levels showed a significant increase within the norm range. GGT instead, dropped significantly and stronger in the groups who fasted longer. A zero-calorie diet in 88 obese people for a duration of up to 35 days showed an increased activity of the GOT and GPT, with a peak in the 3rd fasting week [25]. This moderate increase was explained by the enhanced transamination processes in the course of fasting. An initial overload of liver detoxifying activity could be postulated and does not seem to have been deleterious effects, since GGT levels decreased and physical well-being increased steadily.
CRP values for all groups showed a significant increase within the norm range. A similar mild CRP increase was explained by the transient increase in circulating levels of catecholamines [34]. In a recent study, the modulation of CRP levels was linked to changes in lipid profiles and associated to cardiovascular outcomes [70]. ESR after 1 h and 2 h decreased significantly. Periodic fasting has been shown to clinically improve symptoms of rheumatoid arthritis, suggesting decreased inflammatory processes [71]. IF boosts levels of antioxidants and reduces levels of pro-inflammatory cytokines TNF-α, IL-1β and IL-6 [72]. Serum markers of oxidative damage and inflammation are reduced in asthma patients maintained on an alternate day fast [62]. Moreover, the reduction of abdominal circumference which was significant in our study is also associated with a decrease in pro-inflammatory activity [73].
We observed an increase of uric acid in all groups, with a lower value in F20d than in F15d, suggesting that the peak of uric acid concentration was overwhelmed after 15 days. This corresponds to previous observations from a zero-calorie diet [45, 74]. The increase of uric acid concentrations that exceeded the norm range, interestingly caused only one incidence of gout attack in a 72-year-old obese man, treated for hyperuricemia and gout previous to fasting.
The increased concentration of blood uric acid is due to a slight initial increase in protein catabolism, but above all is related to retention caused by competitive tubular secretion with ketone bodies. The latter are preferentially secreted during fasting.
Uric acid has a known antioxidant activity and is a potent scavenger of free radicals in blood plasma [75, 76]. Given that fasting is the product of a long evolutionary survival strategy, the antioxidant power of uric acid should be taken into consideration.
A significant reduction in urea as well as a significant increase in creatinine was observed, both remaining in norm range. This has been previously demonstrated in obese persons undergoing prolonged periods of a zero-calorie diet [45].
We did not observe any renal dysfunction in our cohort such as has been described in cases of extracellular hypovolemia [77]. This is probably explained by the fact that the subjects were asked to drink 3 L of water per day.
Sodium, potassium, calcium and magnesium were in norm range at the beginning and the end of the fast. They remained stable despite a slight elevation in calcium and slight decrease in sodium levels. Enhanced natriuresis has been described in association with ketosuria in the first phase of fasting, diminishing when ammonium, a metabolite of kidney gluconeogenesis, replaces sodium as accompanying cation [43, 47]. We registered six cases of mild hyponatremia in the course of the fast, with the lowest sodium level of 127 mmol/L. They were all non-serious and were normalized by the administration of sodium chloride.
There are some limitations related to our study. First, this was an observational cohort study with its well-known restrictions regarding interpretation of efficacy. Second, our findings are specific for BWC and may not be applicable for other institutions specialised in fasting, or for persons fasting without medical supervision, or outside of facilities specialized in fasting treatments. Third, data assessment and data entry was not blinded and was performed by the staff of the BWC.
Conclusions
In conclusion, this one-year observational study demonstrates the safety of a periodic Buchinger fast of between 4 and 21 days, as well as its beneficial effects on health and well-being. Periodic fasting led to marked weight loss and improvements in several cardiovascular risk factors, such as overweight, abdominal circumference and blood pressure. It was accompanied by normalization of numerous blood parameters and led to pronounced improvement of the major health complaint in most participants. Importantly, periodic Buchinger fasting was not linked to relevant perception of hunger. On the contrary, it was subjectively experienced as enjoyable, which is an important factor for compliance.
Further studies should evaluate the long-term specific health-related preventive and therapeutic effects of periodic fasting.
Supporting information
(PDF)
(PDF)
Changes in weight and abdominal circumference according to the length of fast and gender.
(XLSX)
Changes in systolic and diastolic blood pressure according to the fasting length.
(XLSX)
Effect of fasting on clinical parameters, well-being and ketosis.
(XLSX)
(PDF)
A subgroup of 404 subjects indicated to have a major health complaint previous to the fasting.
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
We are grateful to Birgit Maser and the Medical Center, as well as Dr Eva Lischka, Dr Norbert Lischka, Siegfried Bäumler, Martine van Houten, Dr Andrea Siegler, Dr Henning Wittrock and the whole staff of BWC. We thank Dr Diethard Müller and his colleagues at MVZ Labor Ravensburg for the support and laboratory analysis. We are also very grateful to Prof Chantal Simon for her continuous support and the native speaker Claire Robinson for proofreading our manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The study was financed by Amplius GmbH, Überlingen, Germany. This company has the task to develop a research department for the Buchinger Wilhelmi Clinics Überlingen and Marbella who are the funders. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
References
- 1.Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing research reviews. 2017;39:46–58. 10.1016/j.arr.2016.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mattson MP, Moehl K, Ghena N, Schmaedick M, Cheng A. Intermittent metabolic switching, neuroplasticity and brain health. Nature Reviews Neuroscience. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamada Y, Kemnitz JW, Weindruch R, Anderson RM, Schoeller DA, Colman RJ. Caloric Restriction and Healthy Life Span: Frail Phenotype of Nonhuman Primates in the Wisconsin National Primate Research Center Caloric Restriction Study. The Journals of Gerontology: Series A. 2018;73(3):273–8. 10.1093/gerona/glx059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandhorst S, Choi IY, Wei M, Cheng CW, Sedrakyan S, Navarrete G, et al. A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and healthspan. Cell metabolism. 2015;22(1):86–99. 10.1016/j.cmet.2015.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Longo VD, Mattson MP. Fasting: molecular mechanisms and clinical applications. Cell metabolism. 2014;19(2):181–92. 10.1016/j.cmet.2013.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun S, Hanzawa F, Umeki M, Ikeda S, Mochizuki S, Oda H. Time-restricted feeding suppresses excess sucrose-induced plasma and liver lipid accumulation in rats. PloS one. 2018;13(8):e0201261 10.1371/journal.pone.0201261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lettieri-Barbato D, Cannata SM, Casagrande V, Ciriolo MR, Aquilano K. Time-controlled fasting prevents aging-like mitochondrial changes induced by persistent dietary fat overload in skeletal muscle. PloS one. 2018;13(5):e0195912 10.1371/journal.pone.0195912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walford RL, Mock D, Verdery R, MacCallum T. Calorie restriction in biosphere 2: alterations in physiologic, hematologic, hormonal, and biochemical parameters in humans restricted for a 2-year period. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2002;57(6):B211–B24. [DOI] [PubMed] [Google Scholar]
- 9.Fontana L, Partridge L. Promoting Health and Longevity through Diet: From Model Organisms to Humans. Cell. 2015;161(1):106–18. 10.1016/j.cell.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anton SD, Moehl K, Donahoo WT, Marosi K, Lee SA, Mainous AG, et al. Flipping the Metabolic Switch: Understanding and Applying the Health Benefits of Fasting. Obesity. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raffaghello L, Lee C, Safdie FM, Wei M, Madia F, Bianchi G, et al. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proceedings of the National Academy of Sciences. 2008;105(24):8215–20. 10.1073/pnas.0708100105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.López-Otín C, Galluzzi L, Freije JMP, Madeo F, Kroemer G. Metabolic Control of Longevity. Cell. 2016;166(4):802–21. 10.1016/j.cell.2016.07.031 [DOI] [PubMed] [Google Scholar]
- 13.Alirezaei M, Kemball CC, Flynn CT, Wood MR, Whitton JL, Kiosses WB. Short-term fasting induces profound neuronal autophagy. Autophagy. 2010;6(6):702–10. 10.4161/auto.6.6.12376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee C, Raffaghello L, Longo VD. Starvation, detoxification, and multidrug resistance in cancer therapy. Drug Resistance Updates. 2012;15(1):114–22. 10.1016/j.drup.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Remely M, Hippe B, Geretschlaeger I, Stegmayer S, Hoefinger I, Haslberger A. Increased gut microbiota diversity and abundance of Faecalibacterium prausnitzii and Akkermansia after fasting: a pilot study. Wiener klinische Wochenschrift. 2015;127(9–10):394–8. 10.1007/s00508-015-0755-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Habold C, Reichardt F, Foltzer-Jourdainne C, Lignot J-H. Morphological changes of the rat intestinal lining in relation to body stores depletion during fasting and after refeeding. Pflügers Archiv-European Journal of Physiology. 2007;455(2):323–32. 10.1007/s00424-007-0289-0 [DOI] [PubMed] [Google Scholar]
- 17.Fontana L, Partridge L, Longo VD. Dietary Restriction, Growth Factors and Aging: from yeast to humans. Science (New York, NY). 2010;328(5976):321–6. 10.1126/science.1172539 PMC3607354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brandhorst S, Harputlugil E, Mitchell JR, Longo VD. Protective effects of short-term dietary restriction in surgical stress and chemotherapy. Ageing Research Reviews. 2017;39:68–77. 10.1016/j.arr.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng C-W, Adams GB, Perin L, Wei M, Zhou X, Lam BS, et al. Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression. Cell stem cell. 2014;14(6):810–23. 10.1016/j.stem.2014.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bertile F, Fouillen L, Wasselin T, Maes P, Le Maho Y, Van Dorsselaer A, et al. The Safety Limits Of An Extended Fast: Lessons from a Non-Model Organism. Scientific reports. 2016;6:39008 10.1038/srep39008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCue MD. Comparative physiology of fasting, starvation, and food limitation: Springer; 2012. [Google Scholar]
- 22.Prentice AM, Whitehead RG, Roberts SB, Paul AA. Long-term energy balance in child-bearing Gambian women. The American journal of clinical nutrition. 1981;34(12):2790–9. 10.1093/ajcn/34.12.2790 [DOI] [PubMed] [Google Scholar]
- 23.Persynaki A, Karras S, Pichard C. Unraveling the metabolic health benefits of fasting related to religious beliefs: A narrative review. Nutrition. 2017;35:14–20. 10.1016/j.nut.2016.10.005 [DOI] [PubMed] [Google Scholar]
- 24.Ajabnoor GM, Bahijri S, Shaik NA, Borai A, Alamoudi AA, Al-Aama JY, et al. Ramadan fasting in Saudi Arabia is associated with altered expression of CLOCK, DUSP and IL-1alpha genes, as well as changes in cardiometabolic risk factors. PloS one. 2017;12(4):e0174342 10.1371/journal.pone.0174342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ditschuneit H, Faulhaber J, Beil I, Pfeiffer E. Metabolic changes in zero-diet. Der Internist. 1970;11(5):176 [PubMed] [Google Scholar]
- 26.Runcie J, Hilditch T. Energy provision, tissue utilization, and weight loss in prolonged starvation. Br Med J. 1974;2(5915):352–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomson T, Runcie J, Miller V. Treatment of obesity by total fasting for up to 249 days. Lancet. 1966;2:992–6. [DOI] [PubMed] [Google Scholar]
- 28.Stewart W, Fleming LW. Features of a successful therapeutic fast of 382 days´ duration. Postgraduate medical journal. 1973;49(569):203–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Toledo FW, Hohler H. Therapeutic Fasting: The Buchinger Amplius Method: The Buchinger Amplius Method: Thieme; 2011. [Google Scholar]
- 30.Buchinger O. Das Heilfasten und seine Hilfsmethoden als biologischer Weg: Georg Thieme Verlag; 2005. [Google Scholar]
- 31.Wilhelmi de Toledo F, Buchinger A, Burggrabe H, Hölz G, Kuhn C, Lischka E, et al. Fasting therapy-an expert panel update of the 2002 consensus guidelines. Forschende Komplementärmedizin/Research in Complementary Medicine. 2013;20(6):434–43. 10.1159/000357602 [DOI] [PubMed] [Google Scholar]
- 32.Wilhelmi de Toledo F FR, Hebisch D., Kuhn C., Platzer G., Schrag S. The Klinik Buchinger Program For The Treatment of Obesity. Obesity in Europe 1993 / Proceedings of the 5th European congress on obesity, Ulm. 1994:1–9.
- 33.Müller H, Wilhelmi de Toledo F, Schuck P, Resch K. Blutdrucksenkung durch Fasten bei adipösen und nichtadipösen Hypertonikern. Perfusion. 2001;14:108–12. [Google Scholar]
- 34.Li C, Ostermann T, Hardt M, Lüdtke R, Broecker-Preuss M, Dobos G, et al. Metabolic and psychological response to 7-day fasting in obese patients with and without metabolic syndrome. Complementary Medicine Research. 2013;20(6):413–20. [DOI] [PubMed] [Google Scholar]
- 35.Michalsen A, Li C, Kaiser K, Lüdtke R, Meier L, Stange R, et al. I n-patient treatment of fibromyalgia: a controlled nonrandomized comparison of conventional medicine versus integrative medicine including fasting therapy. Evidence-based complementary and alternative medicine. 2013;2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michalsen A. Prolonged fasting as a method of mood enhancement in chronic pain syndromes: a review of clinical evidence and mechanisms. Current pain and headache reports. 2010;14(2):80–7. 10.1007/s11916-010-0104-z [DOI] [PubMed] [Google Scholar]
- 37.Michalsen A, Grossman P, Lehmann N, Knoblauch NT, Paul A, Moebus S, et al. Psychological and quality-of-life outcomes from a comprehensive stress reduction and lifestyle program in patients with coronary artery disease: results of a randomized trial. Psychotherapy and psychosomatics. 2005;74(6):344–52. 10.1159/000087781 [DOI] [PubMed] [Google Scholar]
- 38.Brubacher D, Jordan P, Wilhelmi de Toledo F, Brubacher G. Prediction of Weight Development on a 250 kcal/day Diet by a Simple Two-Compartment Model. Aktuelle Ernährungsmedizin. 1998;23(6):293–8. [Google Scholar]
- 39.Brubacher D, Jordan P, Wilhelmi-de Toledo F, Brubacher G. Relationship between the rate of weight loss in a low caloric diet (250 kcal/day) and age, body mass index, gender, and number of fasting cycles Aktuelle Ernaehrungsmedizin; (Germany: ). 1999. [Google Scholar]
- 40.Han TS, van Leer EM, Seidell JC, Lean MEJ. Waist circumference action levels in the identification of cardiovascular risk factors: prevalence study in a random sample. BMJ. 1995;311(7017):1401–5. 10.1136/bmj.311.7017.1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wan R, Weigand LA, Bateman R, Griffioen K, Mendelowitz D, Mattson MP. Evidence that BDNF regulates heart rate by a mechanism involving increased brainstem parasympathetic neuron excitability. Journal of neurochemistry. 2014;129(4):573–80. 10.1111/jnc.12656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mager DE, Wan R, Brown M, Cheng A, Wareski P, Abernethy DR, et al. Caloric restriction and intermittent fasting alter spectral measures of heart rate and blood pressure variability in rats. The FASEB Journal. 2006;20(6):631–7. 10.1096/fj.05-5263com [DOI] [PubMed] [Google Scholar]
- 43.Kolanowski J, Bodson A, Desmecht P, Bemelmans S, Stein F, Crabbe J. On the relationship between ketonuria and natriuresis during fasting and upon refeeding in obese patients. European journal of clinical investigation. 1978;8(5):277–82. [DOI] [PubMed] [Google Scholar]
- 44.Spark RF, Arky RA, Boulter PR, Saudek CD, O'Brian JT. Renin, aldosterone and glucagon in the natriuresis of fasting. New England Journal of Medicine. 1975;292(25):1335–40. 10.1056/NEJM197506192922506 [DOI] [PubMed] [Google Scholar]
- 45.Wechsler JG. Fastentherapie der Adipositas: G Thieme; 1983. [Google Scholar]
- 46.Wadden TA, Foster GD, Letizia KA, Stunkard AJ. A multicenter evaluation of a proprietary weight reduction program for the treatment of marked obesity. Archives of Internal Medicine. 1992;152(5):961–6. [PubMed] [Google Scholar]
- 47.Kerndt PR, Naughton JL, Driscoll CE, Loxterkamp DA. Fasting: the history, pathophysiology and complications. Western Journal of Medicine. 1982;137(5):379–99. [PMC free article] [PubMed] [Google Scholar]
- 48.Goldhamer A, Lisle D, Parpia B, Anderson SV, Campbell TC. Medically supervised water-only fasting in the treatment of hypertension. Journal of manipulative and physiological therapeutics. 2001;24(5):335–9. 10.1067/mmt.2001.115263 [DOI] [PubMed] [Google Scholar]
- 49.Stange R, Pflugbeil C, Michalsen A, Uehleke B. Therapeutic Fasting in Patients with Metabolic Syndrome and Impaired Insulin Resistance. Complementary Medicine Research. 2013;20(6):421–6. [DOI] [PubMed] [Google Scholar]
- 50.Li C, Sadraie B, Steckhan N, Kessler C, Stange R, Jeitler M, et al. Effects of a one-week fasting therapy in patients with type-2 diabetes mellitus and metabolic syndrome–A randomized controlled explorative study. Experimental and Clinical Endocrinology & Diabetes. 2017;125(09):618–24. [DOI] [PubMed] [Google Scholar]
- 51.Mattson MP, Wan R. Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems. The Journal of nutritional biochemistry. 2005;16(3):129–37. 10.1016/j.jnutbio.2004.12.007 [DOI] [PubMed] [Google Scholar]
- 52.Michalsen A, Kuhlmann MK, Lüdtke R, Bäcker M, Langhorst J, Dobos GJ. Prolonged fasting in patients with chronic pain syndromes leads to late mood-enhancement not related to weight loss and fasting-induced leptin depletion. Nutritional neuroscience. 2006:1–6. 10.1080/10284150600627128 [DOI] [PubMed] [Google Scholar]
- 53.Michalsen A, Schneider S, Rodenbeck A, Lüdtke R, Huether G, Dobos G. The short-term effects of fasting on the neuroendocrine system in patients with chronic pain syndromes. Nutritional neuroscience. 2003;6(1):11–8. 10.1080/1028415021000042811 [DOI] [PubMed] [Google Scholar]
- 54.Faulconbridge LF, Wadden TA, Rubin RR, Wing RR, Walkup MP, Fabricatore AN, et al. One‐year changes in symptoms of depression and weight in overweight/obese individuals with type 2 diabetes in the look AHEAD study. Obesity. 2012;20(4):783–93. 10.1038/oby.2011.315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cahill GF Jr, Veech RL. Ketoacids? Good medicine? Transactions of the american clinical and climatological association. 2003;114:149 [PMC free article] [PubMed] [Google Scholar]
- 56.Marosi K, Kim SW, Moehl K, Scheibye‐Knudsen M, Cheng A, Cutler R, et al. 3‐hydroxybutyrate regulates energy metabolism and induces BDNF expression in cerebral cortical neurons. Journal of neurochemistry. 2016;139(5):769–81. 10.1111/jnc.13868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mattson MP, Maudsley S, Martin B. BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends in neurosciences. 2004;27(10):589–94. 10.1016/j.tins.2004.08.001 [DOI] [PubMed] [Google Scholar]
- 58.Fond G, Macgregor A, Leboyer M, Michalsen A. Fasting in mood disorders: neurobiology and effectiveness. A review of the literature. Psychiatry research. 2013;209(3):253–8. 10.1016/j.psychres.2012.12.018 [DOI] [PubMed] [Google Scholar]
- 59.Komaki G, Tamai H, Sumioki H, Mori T, Kobayashi N, Mori K, et al. Plasma beta-endorphin during fasting in man. Hormone Research in Paediatrics. 1990;33(6):239–43. [DOI] [PubMed] [Google Scholar]
- 60.Solianik R, Sujeta A. Two-day fasting evokes stress, but does not affect mood, brain activity, cognitive, psychomotor, and motor performance in overweight women. Behavioural brain research. 2018;338:166–72. 10.1016/j.bbr.2017.10.028 [DOI] [PubMed] [Google Scholar]
- 61.Heilbronn LK, Civitarese AE, Bogacka I, Smith SR, Hulver M, Ravussin E. Glucose tolerance and skeletal muscle gene expression in response to alternate day fasting. Obesity. 2005;13(3):574–81. [DOI] [PubMed] [Google Scholar]
- 62.Johnson JB, Summer W, Cutler RG, Martin B, Hyun D-H, Dixit VD, et al. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radical Biology and Medicine. 2007;42(5):665–74. 10.1016/j.freeradbiomed.2006.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Parikh I, Guo J, Chuang K-H, Zhong Y, Rempe RG, Hoffman JD, et al. Caloric restriction preserves memory and reduces anxiety of aging mice with early enhancement of neurovascular functions. Aging (Albany NY). 2016;8(11):2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stillman CM, Cohen J, Lehman ME, Erickson KI. Mediators of physical activity on neurocognitive function: a review at multiple levels of analysis. Frontiers in human neuroscience. 2016;10:626 10.3389/fnhum.2016.00626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mojto V, Gvozdjakova A, Kucharska J, Rausova Z, Vancova O, Valuch J. Effects of complete water fasting and regeneration diet on kidney function, oxidative stress and antioxidants. Bratislavske lekarske listy. 2018;119(2):107–11. 10.4149/BLL_2018_020 [DOI] [PubMed] [Google Scholar]
- 66.Finnell JS, Saul BC, Goldhamer AC, Myers TR. Is fasting safe? A chart review of adverse events during medically supervised, water-only fasting. BMC complementary and alternative medicine. 2018;18(1):67 10.1186/s12906-018-2136-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cahill GF Jr. Starvation in man. New England Journal of Medicine. 1970;282(12):668–75. 10.1056/NEJM197003192821209 [DOI] [PubMed] [Google Scholar]
- 68.Huber R, Nauck M, Basler N, Haas B, Mattern M, Lüdtke R, et al. Effects of subtotal fasting on plasmatic coagulation, fibrinolytic status and platelet activation: a controlled pilot study in healthy subjects. Nutrition, metabolism and cardiovascular diseases. 2005;15(3):212–8. 10.1016/j.numecd.2004.12.003 [DOI] [PubMed] [Google Scholar]
- 69.Drinda S, Wilhelmi de Toledo F. Heilfasten erhöht die «International Normalized Ratio» bei Gesunden stärker als eine ovo-lacto-vegetarische Diät. Schweizerische Zeitschrift für Ganzheitsmedizin/Swiss Journal of Integrative Medicine. 2012;24(1):46–9. 10.1159/000335953 [DOI] [Google Scholar]
- 70.Ruscica M, Ferri N, Macchi C, Corsini A, Sirtori C. Lipid lowering drugs and inflammatory changes: an impact on cardiovascular outcomes? Annals of medicine. 2018:1–24. [DOI] [PubMed] [Google Scholar]
- 71.Müller H, de Toledo FW, Resch K-L. Fasting followed by vegetarian diet in patients with rheumatoid arthritis: a systematic review. Scandinavian journal of rheumatology. 2001;30(1):1–10. [DOI] [PubMed] [Google Scholar]
- 72.Arumugam T, Phillips T, Cheng A, Morrell C, Mattson M, Wan R. Age and energy intake interact to modify cell stress pathways and stroke outcome. Annals of Neurology. 2010;67(1):41–52. 10.1002/ana.21798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Silva DC, Costa LO, Vasconcelos AA, Cerqueira JC, Fantato D, Torres DC, et al. Waist circumference and menopausal status are independent predictors of endothelial low-grade inflammation. Endocrine Research. 2014;39(1):22–5. 10.3109/07435800.2013.797431 [DOI] [PubMed] [Google Scholar]
- 74.Lennox WG. A study of the retention of uric acid during fasting. Journal of Biological Chemistry. 1925;66(2):521–72. [Google Scholar]
- 75.Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant-and radical-caused aging and cancer: a hypothesis. Proceedings of the National Academy of Sciences. 1981;78(11):6858–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Becker BF. Towards the physiological function of uric acid. Free Radical Biology and Medicine. 1993;14(6):615–31. [DOI] [PubMed] [Google Scholar]
- 77.Padova J, Bendersky G. Hyperuricemia in diabetic ketoacidosis. New England Journal of Medicine. 1962;267(11):530–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
Changes in weight and abdominal circumference according to the length of fast and gender.
(XLSX)
Changes in systolic and diastolic blood pressure according to the fasting length.
(XLSX)
Effect of fasting on clinical parameters, well-being and ketosis.
(XLSX)
(PDF)
A subgroup of 404 subjects indicated to have a major health complaint previous to the fasting.
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.