Abstract
As a manifestation of their inherent plasticity, carcinoma cells undergo profound phenotypic changes during progression towards metastasis. One such phenotypic modulation is the epithelial-mesenchymal transition (EMT), an embryonically relevant process that can be reinstated by tumor cells, resulting in the acquisition of metastatic propensity, stem-like cell properties and resistance to a variety of anti-cancer therapies, including chemotherapy, radiation and some small molecule targeted therapies. Targeting of the EMT is emerging as a novel intervention against tumor progression. This review focuses on the potential use of cancer vaccine strategies targeting tumor cells that exhibit mesenchymal-like features, with an emphasis on the current status of development of vaccine platforms directed against the T-box transcription factor brachyury, a novel cancer target involved in tumor EMT, stemness and resistance to therapies. Also presented is a summary of potential mechanisms of resistance to immune-mediated attack driven by EMT and the development of novel combinatorial strategies based on the use of agents that alleviate tumor EMT for an optimized targeting of plastic tumor cells that are responsible for tumor recurrence and the establishment of therapeutic refractoriness.
Keywords: Tumor progression, Therapeutic resistance, EMT, Brachyury, Stemness, Cancer vaccines
1. INTRODUCTION
Metastatic disease, the main cause of cancer-related deaths, is driven by the ability of malignant tumors to disseminate and to colonize sites that are distant from the location of the primary mass (Hanahan & Weinberg, 2000). Frequently, tumor dissemination is also associated with refractoriness to a range of conventional anti-cancer therapies, including chemotherapy and radiation (Braun et al., 2000), and several molecular targeted therapies (Thomson et al., 2005; Thomson et al., 2008). In light of the recent advances in the field of cancer immunotherapy, an attractive therapeutic alternative to address the problem of metastatic disease is the generation of a sustained immune response directed against essential molecular drivers of tumor progression. This chapter focuses on the current status of development of immune-mediated anti-cancer interventions aimed at preventing and/or treating metastatic disease. Among the topics discussed here are (a) the role of the epithelial-mesenchymal transition (EMT) in tumor dissemination and metastasis; (b) the association of EMT with tumor stemness and resistance to multiple anti-cancer therapies; (c) how therapeutic cancer vaccines can be used to target regulators of EMT; (d) the potential for mitigation of metastatic disease by the use of combinatorial therapies of cancer vaccines and other agents that alleviate tumor EMT. While this chapter does not discuss in detail the numerous factors involved in the design and development of therapeutic cancer vaccines, comprehensive reviews on this topic can be found in the literature (Palena & Schlom, 2010; Schlom, 2012; Schlom et al., 2014).
2. TUMOR EPITHELIAL-MESENCHYMAL TRANSITION (EMT)
A. EMT AND TUMOR INVASIVENESS
In order to metastasize, cancer cells undergo a series of events known collectively as the metastatic cascade, a process that involves their detachment from the primary tumor mass and invasion into the surrounding tissues, entrance into the circulation, homing in on distant organs, and survival and proliferation at secondary sites (Nguyen & Massague, 2007; Nguyen et al., 2009). Multiple studies have now demonstrated that carcinoma cells undergo profound phenotypic changes during progression towards metastasis. These phenotypic fluctuations allow tumor cells to reversibly or irreversibly shift from (a) a relatively stationary state into an invasive one; (b) a proliferative state into a comparatively quiescent one; and (c) from being responsive to anti-tumor therapies into a refractory, non-responsive state. Altogether, these phenotypic modulations can be considered manifestations of the inherent plasticity of cancer cells, which allows the tumor to dynamically adapt and to effectively progress towards metastatic disease (Nieto, 2013).
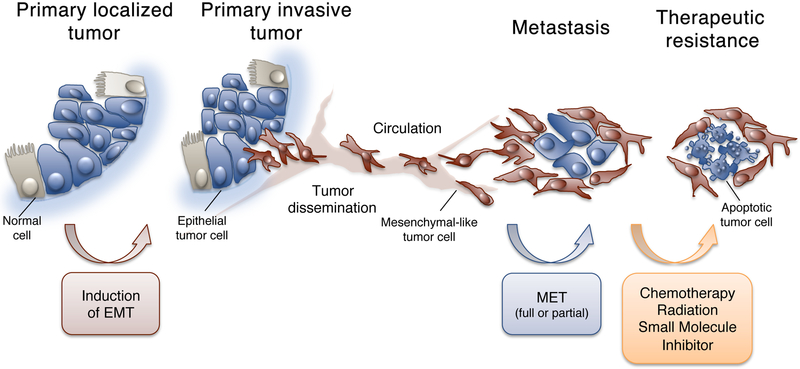
The EMT has recently gained much attention within the field of cancer research due to its potentially critical role during carcinoma progression (Kalluri & Weinberg, 2009; Thiery, 2002). While the EMT is normally involved in the morphogenetic events of embryogenesis, it is now understood that epithelial neoplasms can also aberrantly undergo a similar phenotypic switch in favor of acquiring an invasive, metastatic phenotype (Thiery, 2003; Thiery et al., 2009). During passage through an EMT, epithelial cells lose their stationary behavior, cell polarity, intercellular junctions and epithelial markers (E-cadherin, cytokeratines), and upregulate the expression of proteins normally expressed by mesenchymal cells (Fibronectin, Vimentin, N-cadherin) while acquiring motility and invasiveness, two properties that are fundamental for the initial steps of the metastatic cascade (Yang & Weinberg, 2008) (Figure 1). Like the embryogenesis-related EMT, tumor-related EMT is thought to be a reversible process, where the reacquisition of epithelial features by tumor cells may take place at the site of metastasis through a process designated as mesenchymal-epithelial transition (MET) (Figure 1) (Iwatsuki et al., 2010).
Figure 1.
Role of the EMT-MET phenomena in tumor metastasis and acquisition of therapeutic resistance. (Adapted from Palena et al., 2014a).
There are several reports that document the involvement of EMT in human carcinomas in vivo; however, the number of such studies is trivial compared to the extensive amount of data describing the occurrence and consequences of EMT in preclinical models in vitro. A recent report, for example, has shown that in breast cancer patients undergoing adjuvant therapy, recurring tumors after treatment with standard chemotherapy had an increased proportion of cells expressing EMT-associated genes, highlighting the importance of EMT in therapeutic resistance and breast cancer recurrence (Creighton et al., 2009). Another relevant report has demonstrated that circulating tumor cells (CTCs) from breast cancer patients express both mesenchymal and epithelial markers, with mesenchymal CTCs being associated with disease progression (Yu et al., 2013), thus supporting the idea that EMT may play a role in the blood-borne dissemination of breast cancer. Interestingly, the same report demonstrated that reversible shifts between the epithelial and mesenchymal phenotype of CTCs take place along cycles of treatment and disease progression, a manifestation of the plastic character of cancer cells. In line with the idea that EMT is a plastic, reversible modulation of tumor phenotype, it has been observed, for example, that epithelial E-cadherin can be reexpressed in metastatic lesions of breast cancer patients, even when the primary tumor demonstrates low levels of E-cadherin (Bukholm et al., 2000).
B. EMT AND TUMOR STEMNESS
The existence of a subpopulation of tumor cells with self-renewal and tumor-initiating properties within an individual tumor mass, designated as cancer stem cells (CSCs), has been a matter of intensive investigation in the past few years. The original understanding of the hierarchical phenomenon of stem cell division recognized in normal tissues and hematological malignancies (Chao et al., 2008; Reya et al., 2001) has now been broadened to include the idea that, in solid tumors, differentiated cancer cells could adopt stem-like properties themselves, via plastic phenotypic modulations (Scheel & Weinberg, 2011, 2012). This idea has been fueled by the detection of a link between tumor EMT and the acquisition of features normally attributable to stem cells (i.e., tumor stemness), which include the ability to initiate tumor formation, quiescence, resistance to apoptosis and metastatic propensity (Polyak & Weinberg, 2009). To cite a few examples of the association between EMT and stemness, induction of EMT in human mammary epithelial cells (HMLEs) has been shown to increase the proportion of cells bearing markers associated with stem-like cells (CD44high/CD24low) (Mani et al., 2008). In the clinical setting, the EMT-stemness association has been seen where residual breast tumor cell populations surviving post-conventional treatments were enriched in CD44+/CD24−/low cells that also exhibited mesenchymal features (Creighton et al., 2009).
It has now been demonstrated by many groups that EMT is not a twofold process but instead a dynamic modulation of phenotype that generates tumor cells with shared epithelial and mesenchymal features (Tan et al., 2014). Interestingly, some recent studies have demonstrated that these intermediate cells harbor more characteristics of stem-like cells, including resistance to cell death and ability to form spheroids, than purely epithelial or mesenchymal cells (Huang et al., 2013b; Jordan et al., 2011).
Another example of the association between tumor EMT and the establishment of a stem cell-like state comes from studies on the role of inflammatory chemokine IL-8 in tumor biology (Palena et al., 2012). In breast cancer, for example, expression of the IL-8 receptor A (IL-8RA) has been shown to be predominant among populations of breast CSCs, and the addition of IL-8 to breast cancer cell lines enhanced the number, motility and invasiveness of CSCs (Charafe-Jauffret et al., 2009; Ginestier et al., 2010). In parallel studies, it has been shown that induction of EMT in breast carcinoma cells results in a marked induction of the IL-8/IL-8R axis which, in an autocrine fashion, was able to maintain the EMT phenotype of the tumor cells (Fernando et al., 2011). Thus, the phenomena of EMT and CSCs in breast carcinomas appear to be linked by the activation of the IL-8/IL-8R axis.
C. EMT AND TUMOR RESISTANCE
A major consequence of the transition from an epithelial to a mesenchymal-like phenotype by tumor cells is the acquisition of tumor resistance to a variety of cell death-inducing signals. For example, induction of oxaliplatin-resistance in colorectal cancer cells (Yang et al., 2006), paclitaxel- or radio-resistance in ovarian cancer cells (Kajiyama et al., 2007; Kurrey et al., 2009), and gemcitabine-resistance in pancreatic carcinoma cells (Shah et al., 2007) have been all associated with a switch from an epithelial to a mesenchymal-like phenotype. Intriguingly, one point of intersection connecting the concepts of EMT, stem-like state, and therapy resistance is that of proliferative restraint. In multiple experimental model systems, the induction of tumor EMT has been demonstrated to trigger a significant inhibition of cell cycle due to the modulation of cell cycle-associated proteins, including p21 and Cyclin D1, among others (Huang et al., 2013a; Larocca et al., 2013; Mejlvang et al., 2007; Vega et al., 2004). As conventional cancer therapies such as chemotherapy and radiation require cell cycle processes to trigger apoptosis and target rapidly proliferating cells, it seems likely that inhibition of cell cycle during the EMT could allow tumor cells to initiate repair mechanisms, potentially preventing cytotoxicity (Figure 1).
In addition to inducing resistance to conventional therapeutics, acquisition of mesenchymal features by carcinoma cells has been also associated with resistance to a range of molecularly targeted therapies, including resistance to epidermal growth factor receptor (EGFR) kinase inhibitors (Byers et al., 2013; Thomson et al., 2005; Thomson et al., 2008) and acquired resistance to HER-2 directed therapy (Kim et al., 2014).
3. TARGETING OF EMT
Due to its critical role in cancer progression, targeting of EMT as a means of preventing metastasis, and, possibly, of eliminating cancer cells that would otherwise resist most currently available therapies, is being considered as an attractive therapeutic alternative to the problem of metastatic disease (Palena et al., 2011). Some of the possible approaches that are being explored to inhibit the EMT involve the use of small molecule agents that block the signaling pathways initiated by multiple cytokines, growth factors or extracellular compartment components that are responsible for the initiation and/or maintenance of EMT. These agents include, but are not limited to, specific inhibitors of the EGFR, Axl and Wnt-signaling pathways (Nantajit et al., 2014; Wilson et al., 2014; Zhang et al., 2009). Similarly, various TGF-β receptor I inhibitors have been used to revert the TGF-β-induced EMT in murine or human cancer cells in vitro (Halder et al., 2005; Peng et al., 2005). One caveat of these approaches, however, is that the signaling pathways that control EMT are typically redundant, and the blockade of one such pathway might not be efficient when other alternative signaling pathways that mediate EMT are turned on.
A. EMT TRANSCRIPTION FACTORS
A different alternative being explored to lessen the occurrence of tumor EMT is the direct targeting of the transcriptional drivers of EMT, commonly designated as “EMT transcription factors” (EMT-TFs) (Palena et al., 2014a). In general, the EMT-TFs are expressed in the early embryo, where they participate in the control of developmental EMT, and are subsequently overexpressed in tumor cells undergoing a plastic transition of phenotype. Perhaps the most studied among the EMT-TFs are those that directly repress the E-cadherin gene, including the zinc finger proteins Snail and Slug, as the event of E-cadherin loss is considered a hallmark of EMT. Both proteins have been shown to trigger EMT in multiple model systems (Bolos et al., 2003; Cano et al., 2000) and their expression has been correlated with progression in human tumors of breast and cervical origin (Blanco et al., 2002; Lee et al., 2008). Another regulator of EMT that participates directly or indirectly in the repression of E-cadherin is Twist (Yang et al., 2004), which is also increased in various types of human cancer, including breast, prostate, and cervical cancer. Twist has been correlated with poor disease outcome or more advanced disease status in various types of carcinomas (Kwok et al., 2005; Shibata et al., 2008).
A more recently described EMT-TF is the T-box transcription factor brachyury (also known as T), which is critically involved in embryonic development by promoting the formation of the posterior mesoderm via an EMT (Cunliffe & Smith, 1992; Kispert et al., 1995; Muller & Herrmann, 1997). High levels of brachyury have been demonstrated in several human tumors, including chordomas (Tirabosco et al., 2008; Vujovic et al., 2006; Yang et al., 2009), hemangioblastomas (Barresi et al., 2012), and a range of human carcinomas such as lung (Hamilton et al., 2014a; Haro et al., 2013; Roselli et al., 2012), breast (Palena et al., 2014b), colon (Kilic et al., 2011), and prostate, among others (Pinto et al., 2014), while brachyury is absent in the majority of normal tissues evaluated, with a few exceptions (Hamilton et al., 2012; Palena et al., 2007; Roselli et al., 2012). It has now been demonstrated in multiple preclinical experimental systems that brachyury promotes carcinoma cells to undergo an EMT in vitro (Fernando et al., 2010; Fernando et al., 2011; Larocca et al., 2013), characterized by loss of epithelial markers (including E-cadherin), overexpression of mesenchymal-associated proteins and gain of motility and invasiveness, while facilitating the metastatic dissemination of human tumor xenografts in vivo. In addition to its role in tumor EMT, the levels of brachyury have now been shown to positively correlate with the resistance of malignant cells to various chemotherapies and radiation (Huang et al., 2013a; Larocca et al., 2013), and several studies have demonstrated the association between robust brachyury expression and poor clinical outcome in patients with various types of carcinomas. For example, the expression of brachyury has been reported to positively correlate with tumor progression in lung (Haro et al., 2013) and breast (Palena et al., 2014b) and brachyury has been recently proposed to play a predominant role in triple negative vs. non-triple negative breast tumors (Ben-Hamo et al., 2014). In line with these observations, experiments conducted with human breast cancer cell lines showed that overexpression of brachyury drives tumor invasiveness and, at the same time, upregulates the expression of markers of stemness, including the pluripotency regulators sex determining region Y box 2 (SOX2), NANOG, and octamer-binding transcription factor 4 (OCT4), and enhances the tumor’s ability to form mammospheres in vitro, a feature associated with stem cells. Brachyury-high breast tumor cells also manifested lower cell proliferation rates in vitro and were more resistant to the cytotoxic effects of docetaxel (Palena et al., 2014b). These results are in agreement with the observation that high expression of brachyury in primary tumors of breast cancer patients treated with tamoxifen adjuvant therapy for 5 years is associated with poor prognosis, further supporting the involvement of brachyury in resistance to drug therapy.
B. ADVANTAGE OF AN IMMUNE APPROACH AGAINST EMT
The therapeutic alternative of targeting EMT-TFs as a means of alleviating EMT implies the targeting of intracellular molecules, a fact that precludes the use of monoclonal antibodies. In addition, conventional pharmacological approaches have been so far unsuccessful for the targeting of transcription factors due to their lack of a specific groove for tight binding of an inhibitor. In this context, T-cell mediated immunotherapy offers a distinct possibility. Because T cells recognize a target in the form of short peptides presented in the context of the major histocompatibility complex (MHC) on the surface of target cells, T-cell mediated immunotherapy can be used to target molecules irrespective of their cellular localization. By immunizing the patient against one of the relevant EMT-TFs. an effective T-cell immune response could be elicited that, in turn, could selectively eradicate tumor cells expressing the EMT driver of choice.
C. IMMUNOTHERAPEUTIC APPROACHES AGAINST CANCER
The aim of an immunotherapeutic approach against cancer is to elicit or to enhance a tumor-specific immune response in the host that, in turn, could mediate tumor control in the long range. There are several approaches currently under investigation to achieve this objective, including (I) vaccination of the patient against a tumor-specific antigen(s), (II) adoptive transfer of tumor-specific T cells and, (III) the blockade of immune inhibitory pathways (immune checkpoints) that mediate tumor evasion by restricting T-cell activation or effector function.
I. CANCER VACCINES
Therapeutic cancer vaccines are designed to stimulate a tumor-specific immune response by immunizing the patient against one or several tumor antigens, and may utilize one of four basic platforms: recombinant vectors, dendritic cell-based vaccines, whole tumor cell vaccines and peptide/protein vaccines (Palena & Schlom, 2010). Unlike standard cancer treatments, therapeutic cancer vaccines have shown no associated toxicities and their immunotherapeutic effect could be long-lasting. Vector-based vaccines are perhaps one of the most flexible vaccine-delivery systems; among the vectors being evaluated are poxviruses, alphaviruses, bacterial (Salmonella, Listeria) and yeast vectors. In general, the “off-the-shelf” nature of vector vaccines renders them suitable for large, multicenter randomized trials. For example, a poxviral-based vaccine (PROSTVAC) encoding for prostate-specific antigen (PSA) and a triad of costimulatory molecules (B7.1, ICAM-1, LFA-3, TRICOM) is currently being evaluated in an international, Phase III randomized clinical trial in metastatic castration-resistant prostate cancer patients (Madan et al., 2012a; Singh & Gulley, 2014). The pros and cons of each vaccine platform as well as their current status of clinical development have been extensively reviewed elsewhere (Disis, 2014; Larocca & Schlom, 2011; Schlom et al., 2014).
II. IMMUNE CHECKPOINT INHIBITION
In addition to tumor cells being poorly immunogenic, the tumor microenvironment is known to further decrease the effectiveness of anti-tumor immune responses by potentiating different immunosuppressive mechanisms (Drake et al., 2006; Igney & Krammer, 2002; Rabinovich et al., 2007). These inhibitory pathways, normally required for maintaining homeostasis within the immune system, can be functional in tumors where they could limit anti-tumor T-cell activation and effector functions. The molecules perhaps most studied in this respect are the cytotoxic T lymphocyte antigen-4 (CTLA-4) and programmed cell death-1 (PD-1) and its ligands, PDL-1 and PDL-2 (Shin & Ribas, 2015). CTLA-4 is expressed on the surface of activated T cells and delivers a negative signal upon ligation of B7–1 and B7–2; its blockade with the antibody ipilimumab has shown durable responses in patients with advance melanoma (Hodi et al., 2010). PD-1 is also expressed by activated T cells and binds PDL-1 and PDL-2 that are expressed in malignant cells; blockade of the PD-1/PD-L1 pathway has shown remarkable clinical responses in patients with advanced melanoma and some types of carcinomas, including lung cancer (Hamid et al., 2013; Hodi et al., 2010; Topalian et al., 2012a; Topalian et al., 2012b). The use of immune checkpoint inhibitors is being extended to many different cancer types and it is likely that the combination of these agents with therapeutic cancer vaccines will represent an exciting step forward in cancer treatments.
D. THE CHOICE OF TUMOR ANTIGEN
One fundamental aspect in the development of cancer vaccine approaches is the selection of the tumor antigen to be delivered to the host’s immune system. In general, tumor antigens can be classified into one of two major categories. The first category corresponds to “tumor-specific antigens,” which are molecules expressed de novo by the tumor cells, such as mutated proteins or virally-derived products in tumors driven by infectious agents (Palena & Schlom, 2010). Most antigens so far studied, however, fall within a second category of “tumor-associated antigens,” which are proteins overexpressed in tumors that could be also expressed at low levels in normal adult tissues or were expressed in early embryonic development. EMT transcription factors belong to the second category, as they are molecules normally expressed during embryo development but minimally expressed in adult tissues, and reexpressed in neoplastic cells. An additional attractive feature of EMT-TFs as targets for immunotherapy relates to their functional relevance. In general, cancer cells are able to acquire multiple mechanisms that allow them to evade immune recognition and rejection, including the loss of a targeted antigen and the establishment of antigen-negative tumor clones (DuPage et al., 2012). Because of their critical role in the establishment of a metastatic phenotype, targeting of an EMT-TF could potentially minimize the effects of antigenic loss as tumor cells that down-regulate the expression of the targeted EMT transcription factor will not be able to metastasize.
E. BRACHYURY AS A TUMOR ANTIGEN
Brachyury fulfills two major requisites for a molecule to be used as a cancer vaccine target: (a) tumor specificity, as its expression is almost exclusively confined to neoplastic tissues in the adult, and (b) immunogenicity. This last feature was first revealed when experiments conducted in vitro demonstrated that brachyury-specific cytotoxic CD8+ T cells could be expanded from the blood of cancer patients by repeated stimulation with a nonameric brachyury-derived peptide (Palena et al., 2007). The peptide, an HLA-A0201 restricted epitope (WLLPGTSTL), was identified via a binding prediction algorithm and utilized in vitro for pulsing of dendritic cells (DCs) and subsequent stimulation of autologous T cells from the blood of colorectal and ovarian cancer patients. These brachyury-specific T cells were then shown to be able to lyse carcinoma cells that present endogenously-processed epitopes of brachyury in the context of MHC class I molecules, including lung, colorectal and breast carcinoma cells (Palena et al., 2007; Palena et al., 2014b; Roselli et al., 2012). The epitope was subsequently modified by substitution of an amino acid involved in MHC-binding and the resulting agonist epitope of brachyury (WLLPGTSTV) was shown to be more efficient than the native counterpart at expanding brachyury-specific T cells from the blood of cancer patients and at lysing cancer cells expressing the native brachyury protein. The immunogenicity of this peptide was also demonstrated in vivo by vaccination of A2.1/Kb transgenic mice using peptide in adjuvant (Tucker et al., 2014).
Another demonstration of the immunogenicity of brachyury arose from observations that some patients receiving cancer vaccines directed against prostate-specific antigen (PSA) or carcinoembryonic antigen (CEA) also developed spontaneous CD8+ T-cell responses to brachyury, as measured directly from peripheral blood via the ELISPOT assay (Madan et al., 2012b). As the patients received a vaccine targeting proteins other than brachyury, it is presumed that the expansion of brachyury-specific CD8+ T cells is a manifestation of the phenomenon of antigen spreading, i.e., the generation of an immune response against tumor antigens cross-presented to the immune system following tumor destruction in response to the vaccine. These studies together demonstrated that brachyury is an immunogenic protein with the potential to serve as a target for anticancer vaccination.
Potentially, EMT-TFs other than brachyury that are known to control human tumor progression could be explored as novel tumor antigens for the targeting of metastatic disease, provided that they also meet the criteria of tumor specificity and immunogenicity.
F. THERAPEUTIC VACCINES AGAINST BRACHYURY
I. YEAST-BASED BRACHYURY VACCINE
One type of vector-based vaccine platform is a heat-killed recombinant strain of Saccharomyces cerevisiae, a nonpathogenic yeast species that efficiently activates DCs via Toll-like receptors (TLRs) and induces the production of high levels of type I cytokines, including IL-2, TNF-α, and IFN-γ (Bernstein et al., 2008; Remondo et al., 2009). Yeast vectors can be readily engineered to express one or more tumor antigens and have been well characterized in various preclinical and clinical studies. For example, a recombinant yeast-CEA vaccine has been used in vitro to expand murine and human T cells directed against the CEA protein, as well as in vivo for vaccination of mice bearing CEA-expressing tumors, resulting in anti-tumor activity (Bernstein et al., 2008; Boehm et al., 2010; Cereda et al., 2011; Remondo et al., 2009; Wansley et al., 2008). In clinical studies, heat-killed yeast vaccines have been used in multicenter trials in patients with Hepatitis C, lung and pancreatic carcinomas and in patients with metastatic CEA-expressing carcinomas (Bilusic et al., 2014; Chaft et al., 2014; Hartley et al., 2014). All of these studies demonstrated that yeast-based vaccines (a) can be injected repeatedly without inducing neutralizing responses, (b) posses excellent safety and tolerability profiles, and (c) are able to induce measurable tumor-specific immune responses.
A heat-killed recombinant yeast has been developed (designated as GI-6301) that expresses the full-length human brachyury protein. This vaccine has been initially used in preclinical studies to activate and promote the maturation of human DCs in vitro as well as to expand human brachyury-specific CD8+ and CD4+ T cells from the peripheral blood of healthy donors and cancer patients (Hamilton et al., 2013). The vaccine was also evaluated in vivo, where the administration of heat-killed brachyury-expressing yeast to mice was able to elicit brachyury-specific CD4+ and CD8+ T-cell responses that were capable of reducing tumor burden in an experimental model of brachyury-driven metastasis, in the absence of toxicity (Hamilton et al., 2013; Palena et al., 2014a). Based on these studies, a Phase I clinical trial has been initiated with the yeast-brachyury vaccine GI-6301 to treat patients with advanced carcinomas as well as chordomas (www.clinicaltrials.gov, 2013). The initial results of this Phase I trial have been reported (Heery et al., 2014; Singh et al., 2014); the yeast-brachyury vaccine was well tolerated and brachyury-specific CD8+ and/or CD4+ T-cell responses were present in the blood of some patients post- vs. pre-vaccination. These results demonstrated, for the first time in humans, that an EMT-TF could be targeted immunologically via vaccination.
II. POXVIRUS-BASED BRACHYURY VACCINE
Members of the poxvirus family, including vaccinia, modified vaccinia strain Ankara (MVA) and the avipox virus fowlpox, are being explored as cancer vaccine-delivery systems in a variety of clinical studies. Poxviruses offer several advantages as vaccine-delivery systems, including (a) the ability to accept large inserts of foreign DNA, allowing for the inclusion of multiple transgenes, (b) viral replication and transcription of the poxvirus genome takes place in the cytosol of the infected cell, thereby minimizing the risk of random insertion into the host’s DNA, and (c) the encoded transgenes can be processed and presented by both MHC-class I and II pathways, leading to activation of CD8+ and CD4+ T-cell responses. One particular recombinant poxvirus platform that has been extensively characterized both preclinically and clinically consists of a recombinant vaccinia or fowlpox virus that encodes for transgenes for one or more tumor-associated antigens (PSA, CEA, MUC-1) and the TRICOM triad of costimulatory molecules including B7.1, ICAM-1 and LFA-3. For a comprehensive review of the clinical development of poxviral-based cancer vaccines see (Madan et al., 2012a).
MVA is a highly attenuated strain of vaccinia virus that does not replicate productively in human cells, which has been administered to a large number of individuals in the final stages of the smallpox eradication campaign in Germany and Turkey (Blanchard et al., 1998; Im & Hanke, 2004; Mayr & Danner, 1978). The absence of undesired effects among vaccinated individuals, including those with immune deficiencies, makes MVA a safe, ideal poxviral vector to use in immunotherapeutic approaches against cancer (Cosma et al., 2003; Im & Hanke, 2004). Recently, an MVA-poxviral vaccine encoding human brachyury and the TRICOM molecules has been developed and its testing in a Phase I clinical trial in patients with advanced carcinomas is currently ongoing (www.clinicaltrials.gov, 2014).
To our knowledge, yeast-brachyury and MVA-brachyury-TRICOM are the first vaccines targeting a driver of the EMT that have successfully entered clinical development. Although it is still early to understand the mechanism of action of brachyury-based vaccine approaches, we speculate that the immune targeting of tumor cells undergoing brachyury-mediated EMT could have different outcomes when used at various stages of disease. If employed at an early stage of disease, the elimination of tumor cells that upregulate the expression of brachyury could prevent the establishment of metastatic lesions by eradicating tumor cells with migratory and invasive properties. At later stages of disease, however, once metastases have been established, it is also possible that a brachyury-vaccine could potentially limit the acquisition of chemo- or radioresistance by eliminating therapy-resistant, stem-like cancer cells.
Because of the variety of tumor types showing some level of brachyury expression, brachyury-based vaccines could potentially be applied towards the treatment of several types of common carcinomas. As the phenomenon of EMT, which brachyury regulates, is considered to be transient, it is important to point out that, unlike with the targeting of proteins that are constitutively expressed in cancer cells, expression of brachyury in tumors should be thought of as a dynamic and reversible event. Thus, brachyury might be restricted to a minority of tumor cells or be detectable only at a given time along tumor progression. This fact should be taken into consideration when evaluating its expression in a biopsy specimen, which would measure the level of brachyury in a specific site and at that one moment in time. In agreement with these postulates, expression of brachyury for example has been observed in a minority of cells in the primary tumor, while higher proportion of brachyury-positive tumor cells and higher levels of expression have been observed in metastatic lymph nodes or distal metastases derived from the same patient (Roselli et al., 2012).
4. OVERCOMING POTENTIAL TUMOR IMMUNE RESISTANCE
While the associations of tumor EMT with tumor escape from conventional anti-neoplastic interventions or certain small molecule targeted therapies, such as EGFR inhibitors, have been extensively documented, few reports have investigated whether tumor plasticity mediated by the EMT could also mediate resistance to immune-mediated attack, potentially contributing to tumor escape from host immune-surveillance and failure of immune-mediated rejection.
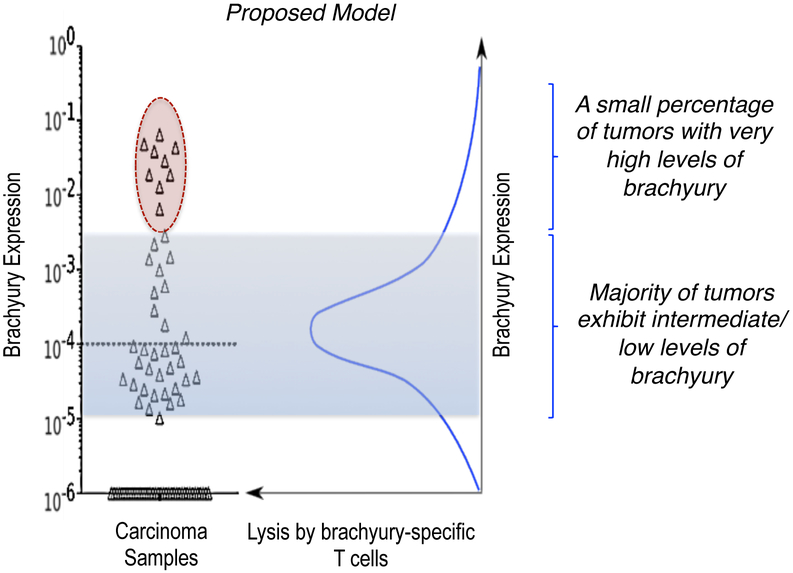
Some of the most studied mechanisms of immune evasion involve defects in any of the multiple components of the antigen-processing and/or presentation machinery, including the loss or reduction of MHC expression and the loss of antigen. However, recent reports have also started to implicate the phenomenon of EMT in the resistance of tumor cells to immune-mediated lysis. For example, it has been recently shown that the acquisition of EMT properties by tumor cells triggers the mechanism of autophagy that, in turn, protects tumor cells from cytotoxic T-cell mediated lysis (Akalay et al., 2013a; Akalay et al., 2013b). Another example of the potential negative impact of tumor EMT in immune-mediated lysis is the case of tumor cells that express very high levels of the transcription factor brachyury. The analysis of the cytotoxic response of human carcinoma cell lines with a range of brachyury expression demonstrated that the cytotoxic lysis of tumor cells with very high levels of brachyury is significantly reduced compared to that of brachyury-intermediate/low cells in response to brachyury-specific CD8+ T cells (Hamilton et al., 2014b). Interestingly, the impairment of immune effector-mediated lysis of tumor cells with very high levels of brachyury was also extended to antigen-independent lysis mediated by innate natural killer (NK) or lymphokine-activated killer (LAK) cells, as well as to lysis mediated by other antigen-specific T cells, even in the presence of normal levels of the corresponding target antigen(s). This paradoxical observation that tumor lysis diminished in the presence of very high levels of the target antigen led to the hypothesis that very high levels of brachyury, as it is observed in some tumor tissues (circle, Figure 2), could impart resistance to immune-mediated anti-tumor interventions. As seen in this proposed model, the majority of human carcinomas exhibit intermediate or low levels of brachyury and would be optimally lysed by brachyury-specific T cells, however, a small percentage of tumors with very high levels of brachyury (red circle, Figure 2) could escape immune-mediated lysis and contribute to tumor resistance to immune attack.
Figure 2.
Expression of brachyury in lung cancer tissues (triangles); shaded area indicates the range of brachyury expression (low/intermediate) corresponding to optimal lysis by brachyury-specific T cells, according to the bell-shape model shown in the right panel. Tumors with very high levels of brachyury (circle) could escape immune mediated lysis by brachyury-specific T cells. (Adapted from Roselli et al., 2012).
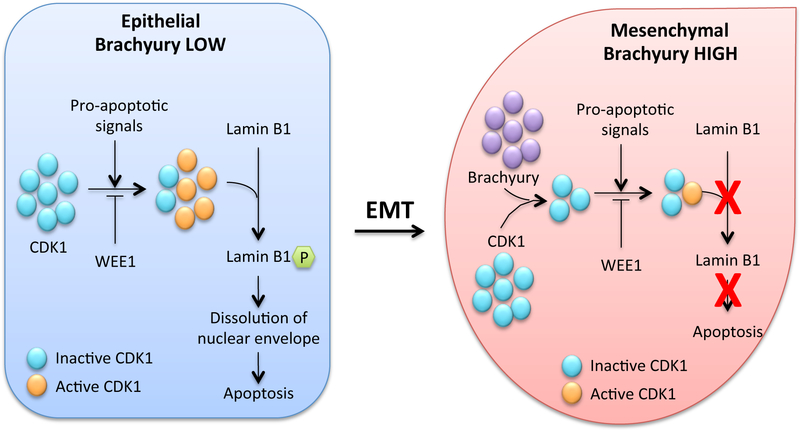
Mechanistic studies to understand the resistance of tumor cells with very high levels of brachyury showed that the defective lysis of those cells is due to inefficient caspase-dependent apoptotic death, a defect that takes place even in the presence of normal levels of fully activated effector caspases. The major apoptotic defect identified in those cells, however, was the absence of degradation of nuclear lamins due to a profound reduction on the levels of the cell cycle associated kinase CDK1 (Figure 3). The nuclear lamin, a component of the nuclear envelope, is a fibrillar mesh formed by the filament lamins A-C, which must be phosphorylated to undergo degradation by caspases during the last steps of apoptosis (McKeon et al., 1986; Rao et al., 1996). In tumor cells with very high brachyury levels, the levels of CDK1 protein are reduced and, as a consequence, lamins are not properly phosphorylated, thus precluding the degradation of the nuclear lamina during apoptosis.
Figure 3.
Schematic representation of the mechanisms of caspase-dependent lysis of brachyury-low vs. brachyury-high cells indicating that loss of CDK1 kinase in the presence of high brachyury levels results in reduced phosphorylation of nuclear lamins and reduced apoptosis. (Adapted from Hamilton et al., 2014b).
As the defective apoptosis of tumor cells with very high levels of brachyury is due to the enhanced degradation of the CDK1 protein, it has been proposed that restoration of threshold levels of CDK1 activity could allow nuclear apoptosis to proceed in those cells. This has been achieved by inhibiting the activity of the cell cycle kinase WEE1, which normally inactivates CDK1 by phosphorylating Tyr15 (Ottaviano & Gerace, 1985). A specific small molecule inhibitor of WEE1, designated as AZD1775 (previously MK1775), is currently being tested in Phase I and II clinical trials for the treatment of multiple solid tumor types in combination with chemotherapies or radiation. It has been shown in preclinical studies with human carcinoma cells that WEE1 blockade by AZD1775 is able to fully revert the resistance of brachyury-high tumor cells to caspase-dependent cell death induced by immune effector mechanisms (Hamilton et al., 2014b). This was presumably achieved by restoration of threshold levels of CDK1 activity in brachyury-high cells, which would allow the proper phosphorylation of lamins and their subsequent targeting by degradation by caspases.
It is important to point out that, as of this writing, there is no clear understanding of what mechanisms are preferred by immune effector cells (caspase-dependent vs. caspase-independent) to lyse tumor cells in vivo. It has been shown, for example, with renal cell carcinoma cells in murine preclinical studies that the lytic pathway employed by antigen-specific T cells during tumor lysis is determined by the level of MHC-class I/peptide, where the FAS lytic pathway is preferred at low peptide level, while the preference is lost at high peptide levels (Shanker et al., 2009). In particular, it has been shown that the efficient lysis of brachyury-high tumor cells could still take place if the effector cell mechanisms involved the perforin/granzyme pathway. This is consistent with the idea that during perforin/granzyme dependent apoptosis, granzymes directly cleave the nuclear lamins, thus being able to overcome the defective lamin phosphorylation that is needed for the caspase-dependent lysis (Hamilton et al., 2014b). These results also indicated that immunotherapeutic approaches able to maximize the activation of effector cells for high production of granzymes should be able to effectively eliminate tumor cells with epithelial or mesenchymal features, the latter otherwise resistant to most anti-tumor interventions.
5. CONCLUDING REMARKS
Progress in elucidating the molecular mechanisms that govern the process of metastasis, including a full understanding of the role of tumor EMT and its association with tumor stemness and resistance to therapies, will help in designing therapies better fitted at preventing and/or treating metastatic disease. Cancer vaccines able to specifically target metastatic tumor cells constitute a very attractive methodology. Unlike other modalities, vaccines may be able to generate a long-lasting anti-tumor response; until now they have demonstrated no associated toxicities, making them ideal for combination therapies, including the use of agents that alleviate tumor EMT for an optimized targeting of plastic tumor cells that are responsible for tumor recurrence and the establishment of therapeutic refractoriness.
Acknowledgments
The authors thank Debra Weingarten for editorial assistance in preparation of this chapter.
REFERENCES
- Akalay I, Janji B, Hasmim M, Noman MZ, Andre F, De Cremoux P, et al. (2013a). Epithelial-to-mesenchymal transition and autophagy induction in breast carcinoma promote escape from T-cell-mediated lysis. Cancer Res, 73, 2418–2427. [DOI] [PubMed] [Google Scholar]
- Akalay I, Janji B, Hasmim M, Noman MZ, Thiery JP, Mami-Chouaib F, et al. (2013b). EMT impairs breast carcinoma cell susceptibility to CTL-mediated lysis through autophagy induction. Autophagy, 9, 1104–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barresi V, Vitarelli E, Branca G, Antonelli M, Giangaspero F, & Barresi G (2012). Expression of brachyury in hemangioblastoma: potential use in differential diagnosis. Am J Surg Pathol, 36, 1052–1057. [DOI] [PubMed] [Google Scholar]
- Ben-Hamo R, Gidoni M, & Efroni S (2014). PhenoNet: identification of key networks associated with disease phenotype. Bioinformatics, 30, 2399–2405. [DOI] [PubMed] [Google Scholar]
- Bernstein MB, Chakraborty M, Wansley EK, Guo Z, Franzusoff A, Mostbock S, et al. (2008). Recombinant Saccharomyces cerevisiae (yeast-CEA) as a potent activator of murine dendritic cells. Vaccine, 26, 509–521. [DOI] [PubMed] [Google Scholar]
- Bilusic M, Heery CR, Arlen PM, Rauckhorst M, Apelian D, Tsang KY, et al. (2014). Phase I trial of a recombinant yeast-CEA vaccine (GI-6207) in adults with metastatic CEA-expressing carcinoma. Cancer Immunol Immunother, 63, 225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard TJ, Alcami A, Andrea P, & Smith GL (1998). Modified vaccinia virus Ankara undergoes limited replication in human cells and lacks several immunomodulatory proteins: implications for use as a human vaccine. J Gen Virol, 79 ( Pt 5), 1159–1167. [DOI] [PubMed] [Google Scholar]
- Blanco MJ, Moreno-Bueno G, Sarrio D, Locascio A, Cano A, Palacios J, et al. (2002). Correlation of Snail expression with histological grade and lymph node status in breast carcinomas. Oncogene, 21, 3241–3246. [DOI] [PubMed] [Google Scholar]
- Boehm AL, Higgins J, Franzusoff A, Schlom J, & Hodge JW (2010). Concurrent vaccination with two distinct vaccine platforms targeting the same antigen generates phenotypically and functionally distinct T-cell populations. Cancer Immunol Immunother, 59, 397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolos V, Peinado H, Perez-Moreno MA, Fraga MF, Esteller M, & Cano A (2003). The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J Cell Sci, 116, 499–511. [DOI] [PubMed] [Google Scholar]
- Braun S, Kentenich C, Janni W, Hepp F, de Waal J, Willgeroth F, et al. (2000). Lack of effect of adjuvant chemotherapy on the elimination of single dormant tumor cells in bone marrow of high-risk breast cancer patients. J Clin Oncol, 18, 80–86. [DOI] [PubMed] [Google Scholar]
- Bukholm IK, Nesland JM, & Borresen-Dale AL (2000). Re-expression of E-cadherin, alpha-catenin and beta-catenin, but not of gamma-catenin, in metastatic tissue from breast cancer patients. J Pathol, 190, 15–19. [DOI] [PubMed] [Google Scholar]
- Byers LA, Diao L, Wang J, Saintigny P, Girard L, Peyton M, et al. (2013). An epithelial-mesenchymal transition gene signature predicts resistance to EGFR and PI3K inhibitors and identifies Axl as a therapeutic target for overcoming EGFR inhibitor resistance. Clin Cancer Res, 19, 279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, et al. (2000). The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol, 2, 76–83. [DOI] [PubMed] [Google Scholar]
- Cereda V, Vergati M, Huen NY, di Bari MG, Jochems C, Intrivici C, et al. (2011). Maturation of human dendritic cells with Saccharomyces cerevisiae (yeast) reduces the number and function of regulatory T cells and enhances the ratio of antigen-specific effectors to regulatory T cells. Vaccine, 29, 4992–4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaft JE, Litvak A, Arcila ME, Patel P, D’Angelo SP, Krug LM, et al. (2014). Phase II study of the GI-4000 KRAS vaccine after curative therapy in patients with stage I-III lung adenocarcinoma harboring a KRAS G12C, G12D, or G12V mutation. Clin Lung Cancer, 15, 405–410. [DOI] [PubMed] [Google Scholar]
- Chao MP, Seita J, & Weissman IL (2008). Establishment of a normal hematopoietic and leukemia stem cell hierarchy. Cold Spring Harb Symp Quant Biol, 73, 439–449. [DOI] [PubMed] [Google Scholar]
- Charafe-Jauffret E, Ginestier C, Iovino F, Wicinski J, Cervera N, Finetti P, et al. (2009). Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res, 69, 1302–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosma A, Nagaraj R, Buhler S, Hinkula J, Busch DH, Sutter G, et al. (2003). Therapeutic vaccination with MVA-HIV-1 nef elicits Nef-specific T-helper cell responses in chronically HIV-1 infected individuals. Vaccine, 22, 21–29. [DOI] [PubMed] [Google Scholar]
- Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A, et al. (2009). Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci U S A, 106, 13820–13825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunliffe V, & Smith JC (1992). Ectopic mesoderm formation in Xenopus embryos caused by widespread expression of a Brachyury homologue. Nature, 358, 427–430. [DOI] [PubMed] [Google Scholar]
- Disis ML (2014). Mechanism of action of immunotherapy. Semin Oncol, 41 Suppl 5, S3–13. [DOI] [PubMed] [Google Scholar]
- Drake CG, Jaffee E, & Pardoll DM (2006). Mechanisms of immune evasion by tumors. Adv Immunol, 90, 51–81. [DOI] [PubMed] [Google Scholar]
- DuPage M, Mazumdar C, Schmidt LM, Cheung AF, & Jacks T (2012). Expression of tumour-specific antigens underlies cancer immunoediting. Nature, 482, 405–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando RI, Litzinger M, Trono P, Hamilton DH, Schlom J, & Palena C (2010). The T-box transcription factor Brachyury promotes epithelial-mesenchymal transition in human tumor cells. J Clin Invest, 120, 533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando RI, Castillo MD, Litzinger M, Hamilton DH, & Palena C (2011). IL-8 signaling plays a critical role in the epithelial-mesenchymal transition of human carcinoma cells. Cancer Res, 71, 5296–5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginestier C, Liu S, Diebel ME, Korkaya H, Luo M, Brown M, et al. (2010). CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J Clin Invest, 120, 485–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder SK, Beauchamp RD, & Datta PK (2005). A specific inhibitor of TGF-beta receptor kinase, SB-431542, as a potent antitumor agent for human cancers. Neoplasia, 7, 509–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. (2013). Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med, 369, 134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DH, Litzinger MT, Fernando RI, Huang B, & Palena C (2012). Cancer vaccines targeting the epithelial-mesenchymal transition: tissue distribution of brachyury and other drivers of the mesenchymal-like phenotype of carcinomas. Semin Oncol, 39, 358–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DH, Litzinger MT, Jales A, Huang B, Fernando RI, Hodge JW, et al. (2013). Immunological targeting of tumor cells undergoing an epithelial-mesenchymal transition via a recombinant brachyury-yeast vaccine. Oncotarget, 4, 1777–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DH, Fernando RI, Schlom J, & Palena C (2014a). Aberrant expression of the embryonic transcription factor brachyury in human tumors detected with a novel rabbit monoclonal antibody. Oncotarget, 6(7), 4853–4862, [Epub online], Dec 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DH, Huang B, Fernando RI, Tsang KY, & Palena C (2014b). WEE1 inhibition alleviates resistance to immune attack of tumor cells undergoing epithelial-mesenchymal transition. Cancer Res, 74, 2510–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, & Weinberg RA (2000). The hallmarks of cancer. Cell, 100, 57–70. [DOI] [PubMed] [Google Scholar]
- Haro A, Yano T, Kohno M, Yoshida T, Koga T, Okamoto T, et al. (2013). Expression of Brachyury gene is a significant prognostic factor for primary lung carcinoma. Ann Surg Oncol, 20 Suppl 3, S509–516. [DOI] [PubMed] [Google Scholar]
- Hartley ML, Bade NA, Prins PA, Ampie L, & Marshall JL (2014). Pancreatic cancer, treatment options, and GI-4000. Hum Vaccin Immunother, 10, 3347–3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heery CR, Singh H, Marte JL, Madan RA, O’Sulllivan Coyne GH, Farsaci B, et al. (2014). NCI experience using yeast–brachyury vaccine (GI-6301) in patients with advanced chordoma. J Clin Oncol 32:5 s (suppl; abstr 3081), [Google Scholar]
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. (2010). Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med, 363, 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Cohen JR, Fernando RI, Hamilton DH, Litzinger MT, Hodge JW, et al. (2013a). The embryonic transcription factor Brachyury blocks cell cycle progression and mediates tumor resistance to conventional antitumor therapies. Cell Death Dis, 4, e682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang RY, Wong MK, Tan TZ, Kuay KT, Ng AH, Chung VY, et al. (2013b). An EMT spectrum defines an anoikis-resistant and spheroidogenic intermediate mesenchymal state that is sensitive to e-cadherin restoration by a src-kinase inhibitor, saracatinib (AZD0530). Cell Death Dis, 4, e915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igney FH, & Krammer PH (2002). Immune escape of tumors: apoptosis resistance and tumor counterattack. J Leukoc Biol, 71, 907–920. [PubMed] [Google Scholar]
- Im EJ, & Hanke T (2004). MVA as a vector for vaccines against HIV-1. Expert Rev Vaccines, 3, S89–97. [DOI] [PubMed] [Google Scholar]
- Iwatsuki M, Mimori K, Yokobori T, Ishi H, Beppu T, Nakamori S, et al. (2010). Epithelial-mesenchymal transition in cancer development and its clinical significance. Cancer Sci, 101, 293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan NV, Johnson GL, & Abell AN (2011). Tracking the intermediate stages of epithelial-mesenchymal transition in epithelial stem cells and cancer. Cell Cycle, 10, 2865–2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiyama H, Shibata K, Terauchi M, Yamashita M, Ino K, Nawa A, et al. (2007). Chemoresistance to paclitaxel induces epithelial-mesenchymal transition and enhances metastatic potential for epithelial ovarian carcinoma cells. Int J Oncol, 31, 277–283. [PubMed] [Google Scholar]
- Kalluri R, & Weinberg RA (2009). The basics of epithelial-mesenchymal transition. J Clin Invest., 119, 1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilic N, Feldhaus S, Kilic E, Tennstedt P, Wicklein D, Wasielewski R, et al. (2011). Brachyury expression predicts poor prognosis at early stages of colorectal cancer. Eur J Cancer, 47, 1080–1085. [DOI] [PubMed] [Google Scholar]
- Kim HP, Han SW, Song SH, Jeong EG, Lee MY, Hwang D, et al. (2014). Testican-1-mediated epithelial-mesenchymal transition signaling confers acquired resistance to lapatinib in HER2-positive gastric cancer. Oncogene, 33, 3334–3341. [DOI] [PubMed] [Google Scholar]
- Kispert A, Koschorz B, & Herrmann BG (1995). The T protein encoded by Brachyury is a tissue-specific transcription factor. Embo J., 14, 4763–4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurrey NK, Jalgaonkar SP, Joglekar AV, Ghanate AD, Chaskar PD, Doiphode RY, et al. (2009). Snail and slug mediate radioresistance and chemoresistance by antagonizing p53-mediated apoptosis and acquiring a stem-like phenotype in ovarian cancer cells. Stem Cells, 27, 2059–2068. [DOI] [PubMed] [Google Scholar]
- Kwok WK, Ling MT, Lee TW, Lau TC, Zhou C, Zhang X, et al. (2005). Up-regulation of TWIST in prostate cancer and its implication as a therapeutic target. Cancer Res, 65, 5153–5162. [DOI] [PubMed] [Google Scholar]
- Larocca C, & Schlom J (2011). Viral vector-based therapeutic cancer vaccines. Cancer J, 17, 359–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larocca C, Cohen JR, Fernando RI, Huang B, Hamilton DH, & Palena C (2013). An autocrine loop between TGF-beta1 and the transcription factor brachyury controls the transition of human carcinoma cells into a mesenchymal phenotype. Mol Cancer Ther, 12, 1805–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MY, Chou CY, Tang MJ, & Shen MR (2008). Epithelial-mesenchymal transition in cervical cancer: correlation with tumor progression, epidermal growth factor receptor overexpression, and snail up-regulation. Clin Cancer Res, 14, 4743–4750. [DOI] [PubMed] [Google Scholar]
- Madan RA, Bilusic M, Heery C, Schlom J, & Gulley JL (2012a). Clinical evaluation of TRICOM vector therapeutic cancer vaccines. Semin Oncol, 39, 296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan RA, Mohebtash M, Arlen PM, Vergati M, Rauckhorst M, Steinberg SM, et al. (2012b). Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol, 13, 501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. (2008). The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell, 133, 704–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr A, & Danner K (1978). Vaccination against pox diseases under immunosuppressive conditions. Dev Biol Stand, 41, 225–234. [PubMed] [Google Scholar]
- McKeon FD, Kirschner MW, & Caput D (1986). Homologies in both primary and secondary structure between nuclear envelope and intermediate filament proteins. Nature, 319, 463–468. [DOI] [PubMed] [Google Scholar]
- Mejlvang J, Kriajevska M, Vandewalle C, Chernova T, Sayan AE, Berx G, et al. (2007). Direct repression of cyclin D1 by SIP1 attenuates cell cycle progression in cells undergoing an epithelial mesenchymal transition. Mol Biol Cell, 18, 4615–4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller CW, & Herrmann BG (1997). Crystallographic structure of the T domain-DNA complex of the Brachyury transcription factor. Nature., 389, 884–888. [DOI] [PubMed] [Google Scholar]
- Nantajit D, Lin D, & Li JJ (2014). The network of epithelial-mesenchymal transition: potential new targets for tumor resistance. J Cancer Res Clin Oncol, [Epub ahead of print], Oct 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen DX, & Massague J (2007). Genetic determinants of cancer metastasis. Nat Rev Genet, 8, 341–352. [DOI] [PubMed] [Google Scholar]
- Nguyen DX, Bos PD, & Massague J (2009). Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer, 9, 274–284. [DOI] [PubMed] [Google Scholar]
- Nieto MA (2013). Epithelial plasticity: a common theme in embryonic and cancer cells. Science, 342, 1234850. [DOI] [PubMed] [Google Scholar]
- Ottaviano Y, & Gerace L (1985). Phosphorylation of the nuclear lamins during interphase and mitosis. J Biol Chem, 260, 624–632. [PubMed] [Google Scholar]
- Palena C, Polev DE, Tsang KY, Fernando RI, Litzinger M, Krukovskaya LL, et al. (2007). The human T-box mesodermal transcription factor Brachyury is a candidate target for T-cell-mediated cancer immunotherapy. Clin Cancer Res, 13, 2471–2478. [DOI] [PubMed] [Google Scholar]
- Palena C, & Schlom J (2010). Vaccines against human carcinomas: strategies to improve antitumor immune responses. J Biomed Biotechnol, Epub 2010 March 16, 380697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palena C, Fernando RI, Litzinger MT, Hamilton DH, Huang B, & Schlom J (2011). Strategies to target molecules that control the acquisition of a mesenchymal-like phenotype by carcinoma cells. Exp Biol Med (Maywood), 236, 537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palena C, Hamilton DH, & Fernando RI (2012). Influence of IL-8 on the epithelial-mesenchymal transition and the tumor microenvironment. Future Oncol, 8, 713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palena C, Fernando RI, & Hamilton DH (2014a). An immunotherapeutic intervention against tumor progression: Targeting a driver of the epithelial-to-mesenchymal transition. Oncoimmunology, 3, e27220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palena C, Roselli M, Litzinger MT, Ferroni P, Costarelli L, Spila A, et al. (2014b). Overexpression of the EMT driver brachyury in breast carcinomas: association with poor prognosis. J Natl Cancer Inst, 106, pii: dju054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng SB, Yan L, Xia X, Watkins SA, Brooks HB, Beight D, et al. (2005). Kinetic characterization of novel pyrazole TGF-beta receptor I kinase inhibitors and their blockade of the epithelial-mesenchymal transition. Biochemistry, 44, 2293–2304. [DOI] [PubMed] [Google Scholar]
- Pinto F, Pertega-Gomes N, Pereira MS, Vizcaino JR, Monteiro P, Henrique RM, et al. (2014). T-box transcription factor Brachyury is associated with prostate cancer progression and aggressiveness. Clin Cancer Res, 20, 4949–4961. [DOI] [PubMed] [Google Scholar]
- Polyak K, & Weinberg RA (2009). Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer., 9, 265–273. [DOI] [PubMed] [Google Scholar]
- Rabinovich GA, Gabrilovich D, & Sotomayor EM (2007). Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol, 25, 267–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao L, Perez D, & White E (1996). Lamin proteolysis facilitates nuclear events during apoptosis. J Cell Biol, 135, 1441–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remondo C, Cereda V, Mostbock S, Sabzevari H, Franzusoff A, Schlom J, et al. (2009). Human dendritic cell maturation and activation by a heat-killed recombinant yeast (Saccharomyces cerevisiae) vector encoding carcinoembryonic antigen. Vaccine, 27, 987–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, & Weissman IL (2001). Stem cells, cancer, and cancer stem cells. Nature, 414, 105–111. [DOI] [PubMed] [Google Scholar]
- Roselli M, Fernando RI, Guadagni F, Spila A, Alessandroni J, Palmirotta R, et al. (2012). Brachyury, a driver of the epithelial-mesenchymal transition, is overexpressed in human lung tumors: an opportunity for novel interventions against lung cancer. Clin Cancer Res, 18, 3868–3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheel C, & Weinberg RA (2011). Phenotypic plasticity and epithelial-mesenchymal transitions in cancer and normal stem cells? Int J Cancer, 129, 2310–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheel C, & Weinberg RA (2012). Cancer stem cells and epithelial-mesenchymal transition: concepts and molecular links. Semin Cancer Biol, 22, 396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlom J (2012). Therapeutic cancer vaccines: current status and moving forward. J Natl Cancer Inst, 104, 599–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlom J, Hodge JW, Palena C, Tsang KY, Jochems C, Greiner JW, et al. (2014). Therapeutic cancer vaccines. Adv Cancer Res, 121, 67–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah AN, Summy JM, Zhang J, Park SI, Parikh NU, & Gallick GE (2007). Development and characterization of gemcitabine-resistant pancreatic tumor cells. Ann Surg Oncol, 14, 3629–3637. [DOI] [PubMed] [Google Scholar]
- Shanker A, Brooks AD, Jacobsen KM, Wine JW, Wiltrout RH, Yagita H, et al. (2009). Antigen presented by tumors in vivo determines the nature of CD8+ T-cell cytotoxicity. Cancer Res, 69, 6615–6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata K, Kajiyama H, Ino K, Terauchi M, Yamamoto E, Nawa A, et al. (2008). Twist expression in patients with cervical cancer is associated with poor disease outcome. Ann Oncol, 19, 81–85. [DOI] [PubMed] [Google Scholar]
- Shin DS, & Ribas A (2015). The evolution of checkpoint blockade as a cancer therapy: what’s here, what’s next? Curr Opin Immunol, 33C, 23–35. [DOI] [PubMed] [Google Scholar]
- Singh BH, & Gulley JL (2014). Therapeutic vaccines as a promising treatment modality against prostate cancer: rationale and recent advances. Ther Adv Vaccines, 2, 137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H, Heery CR, Marte JL, Farsaci B, Madan RA, G.H. OSC, et al. (2014). A phase I study of a yeast-based therapeutic cancer vaccine,GI-6301, targeting brachyury in patients with metastatic carcinoma. J Clin Oncol 32 (suppl; abstr e14026), [Google Scholar]
- Tan TZ, Miow QH, Miki Y, Noda T, Mori S, Huang RY, et al. (2014). Epithelial-mesenchymal transition spectrum quantification and its efficacy in deciphering survival and drug responses of cancer patients. EMBO Mol Med, 6, 1279–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery JP (2002). Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer, 2, 442–454. [DOI] [PubMed] [Google Scholar]
- Thiery JP (2003). Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol, 15, 740–746. [DOI] [PubMed] [Google Scholar]
- Thiery JP, Acloque H, Huang RY, & Nieto MA (2009). Epithelial-mesenchymal transitions in development and disease. Cell, 139, 871–890. [DOI] [PubMed] [Google Scholar]
- Thomson S, Buck E, Petti F, Griffin G, Brown E, Ramnarine N, et al. (2005). Epithelial to mesenchymal transition is a determinant of sensitivity of non-small-cell lung carcinoma cell lines and xenografts to epidermal growth factor receptor inhibition. Cancer Res, 65, 9455–9462. [DOI] [PubMed] [Google Scholar]
- Thomson S, Petti F, Sujka-Kwok I, Epstein D, & Haley JD (2008). Kinase switching in mesenchymal-like non-small cell lung cancer lines contributes to EGFR inhibitor resistance through pathway redundancy. Clin Exp Metastasis, 25, 843–854. [DOI] [PubMed] [Google Scholar]
- Tirabosco R, Mangham DC, Rosenberg AE, Vujovic S, Bousdras K, Pizzolitto S, et al. (2008). Brachyury expression in extra-axial skeletal and soft tissue chordomas: a marker that distinguishes chordoma from mixed tumor/myoepithelioma/parachordoma in soft tissue. Am J Surg Pathol, 32, 572–580. [DOI] [PubMed] [Google Scholar]
- Topalian SL, Drake CG, & Pardoll DM (2012a). Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol, 24, 207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. (2012b). Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med, 366, 2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker JA, Jochems C, Boyerinas B, Fallon J, Greiner JW, Palena C, et al. (2014). Identification and characterization of a cytotoxic T-lymphocyte agonist epitope of brachyury, a transcription factor involved in epithelial to mesenchymal transition and metastasis. Cancer Immunol Immunother, 63, 1307–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega S, Morales AV, Ocana OH, Valdes F, Fabregat I, & Nieto MA (2004). Snail blocks the cell cycle and confers resistance to cell death. Genes Dev, 18, 1131–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vujovic S, Henderson S, Presneau N, Odell E, Jacques TS, Tirabosco R, et al. (2006). Brachyury, a crucial regulator of notochordal development, is a novel biomarker for chordomas. J Pathol, 209, 157–165. [DOI] [PubMed] [Google Scholar]
- Wansley EK, Chakraborty M, Hance KW, Bernstein MB, Boehm AL, Guo Z, et al. (2008). Vaccination with a recombinant Saccharomyces cerevisiae expressing a tumor antigen breaks immune tolerance and elicits therapeutic antitumor responses. Clin Cancer Res, 14, 4316–4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C, Ye X, Pham T, Lin E, Chan S, McNamara E, et al. (2014). AXL inhibition sensitizes mesenchymal cancer cells to antimitotic drugs. Cancer Res, 74, 5878–5890. [DOI] [PubMed] [Google Scholar]
- http://www.clinicaltrials.gov. (2013). Open Label Study to Evaluate the Safety and Tolerability of GI-6301 a Vaccine Consisting of Whole Heat-Killed Recombitant Yeast Genetically Modified to Express Brachyury Protein in Adults With Solid Tumors. Retrieved Feb 5, 2013.
- http://www.clinicaltrials.gov. (2014). Safety and Tolerability of a Modified Vaccinia Ankara (MVA)-Based Vaccine Modified to Express Brachyury and T-cell Costimulatory Molecules (MVA-Brachyury-TRICOM). Retrieved Sep 3, 2014.
- Yang AD, Fan F, Camp ER, van Buren G, Liu W, Somcio R, et al. (2006). Chronic oxaliplatin resistance induces epithelial-to-mesenchymal transition in colorectal cancer cell lines. Clin Cancer Res, 12, 4147–4153. [DOI] [PubMed] [Google Scholar]
- Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, et al. (2004). Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell, 117, 927–939. [DOI] [PubMed] [Google Scholar]
- Yang J, & Weinberg RA (2008). Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell, 14, 818–829. [DOI] [PubMed] [Google Scholar]
- Yang XR, Ng D, Alcorta DA, Liebsch NJ, Sheridan E, Li S, et al. (2009). T (brachyury) gene duplication confers major susceptibility to familial chordoma. Nat Genet, 41, 1176–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, et al. (2013). Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science, 339, 580–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, LaFortune TA, Krishnamurthy S, Esteva FJ, Cristofanilli M, Liu P, et al. (2009). Epidermal growth factor receptor tyrosine kinase inhibitor reverses mesenchymal to epithelial phenotype and inhibits metastasis in inflammatory breast cancer. Clin Cancer Res, 15, 6639–6648. [DOI] [PMC free article] [PubMed] [Google Scholar]