Burkholderia pseudomallei and Burkholderia mallei are the causative agents of melioidosis and glanders, respectively. There is no vaccine to protect against these highly pathogenic bacteria, and there is concern regarding their emergence as global public health (B. pseudomallei) and biosecurity (B. mallei) threats.
KEYWORDS: Burkholderia, glanders, humoral immunity, melioidosis, vaccines
ABSTRACT
Burkholderia pseudomallei and Burkholderia mallei are the causative agents of melioidosis and glanders, respectively. There is no vaccine to protect against these highly pathogenic bacteria, and there is concern regarding their emergence as global public health (B. pseudomallei) and biosecurity (B. mallei) threats. In this issue of mSphere, an article by Khakhum and colleagues (N. Khakhum, P. Bharaj, J. N. Myers, D. Tapia, et al., mSphere 4:e00570-18, 2019, https://doi.org/10.1128/mSphere.00570-18) describes a novel vaccination platform with excellent potential for cross-protection against both Burkholderia species. The report also highlights the importance of antibodies in immunity against these facultative intracellular organisms.
COMMENTARY
Burkholderia pseudomallei and Burkholderia mallei are closely related bacteria causing fatal infections in humans and animals. B. pseudomallei is commonly found in wet soils of countries bordering the equator and causes the global emerging tropical disease melioidosis (1–3). B. mallei is a host-adapted clone of B. pseudomallei that does not persist in the environment outside its natural equine reservoir. The organism causes the extremely contagious and incapacitating zoonosis glanders, which is a reemerging biosecurity threat closely monitored by the World Organization for Animal Health (4–6). Comparative analyses indicate that B. mallei evolved from B. pseudomallei through genomic reduction, and the genes retained by B. mallei have an average identity of 99% with B. pseudomallei orthologs (7–10). The clinical and pathological manifestations of disease caused by the organisms are also strikingly similar. In humans, infection typically occurs through punctured skin or the respiratory route, and the most common manifestations are life-threatening pneumonia and bacteremia (1, 6, 11, 12). Pathogenicity is complex and involves the coordinated expression of many virulence factors supporting extracellular and intracellular replication as well as dissemination to target organs (lungs, spleen, liver, lymph nodes) where B. pseudomallei and B. mallei form hallmark chronic lesions (13–16). Melioidosis and glanders are difficult to diagnose and require prolonged therapy with low success rates due in large part to intrinsic resistance of the organisms to antibiotics (17, 18). No vaccine exists to protect humans or animals, and there is concern regarding adversarial use given that B. mallei has previously been utilized as a biological warfare agent (6). For these reasons, the U.S. Federal Select Agent Program classifies B. pseudomallei and B. mallei as Tier 1 organisms, and the availability of medical countermeasures is considered a critical unmet need. Fortunately, the genetic, biochemical, and virulence similarities between B. pseudomallei and B. mallei, and the resemblance of the diseases they cause, suggest the feasibility of developing countermeasures that protect against both organisms.
Protection against aerosol infection is of particular interest, as it is one of the most common inoculation routes in natural cases and the most likely portal of entry for B. pseudomallei and B. mallei in the event of adversarial use. The current benchmark animal model to evaluate countermeasures is the mouse, especially the BALB/c (highly sensitive) and C57BL/6 (sensitive) strains. The model produces hallmarks of melioidosis and glanders (low infectious and lethal doses, rapid bacterial replication in the lungs, dissemination to deep tissues, and formation of chronic lesions), and infected mice produce antibodies against antigens known to be targets of the human immune response, thus demonstrating immunological parallels (19–26). A number of experimental vaccines have been tested using the model, but none achieve complete protection and sterile immunity (27–29). Best-in-class vaccines afford increased survival against lethal challenge but do not prevent persistence of the organisms; mice develop lesions with high tissue burden and succumb to chronic infection despite possessing humoral and cellular immunity against B. pseudomallei and B. mallei. This failure to eliminate infection is a major obstacle in the field and emphasizes the need to expand the current pool of Burkholderia antigens for vaccine generation and to develop efficacious vaccination platforms.
In this issue of mSphere, a study by Khakhum and colleagues (30) demonstrates that immunization of C57BL/6 mice with a novel B. pseudomallei live attenuated strain (LAS) results in remarkable protection against lethal aerosol challenge with homologous wild-type bacteria. Khakhum et al. show that LAS vaccination elicits robust humoral and cellular immune responses, provides 100% survival for a period of up to 27 days after infection with highly pathogenic B. pseudomallei strain K96243, and results in outstanding rates of bacterial clearance in the lungs, liver, and spleen (71%). Importantly, they demonstrate through depletion experiments that protection is primarily dependent on humoral immunity. Their data indicate that 16 days postchallenge, mice vaccinated with LAS and subsequently depleted of CD4+ and CD8+ T cells show 60% and 100% survival, respectively.
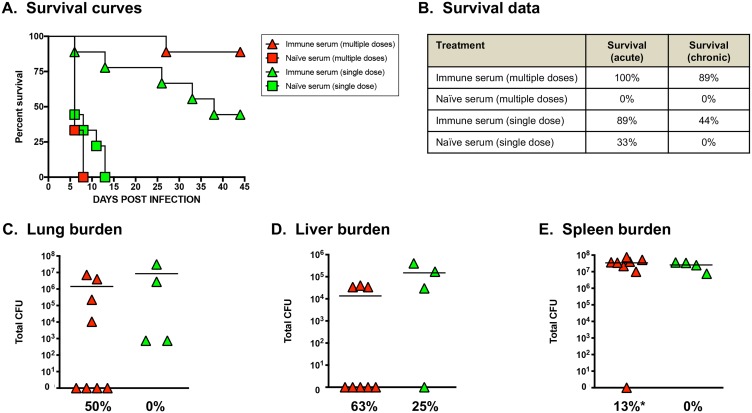
Given their ability to thrive intracellularly, it has been proposed that a vaccine for B. pseudomallei and B. mallei should primarily generate robust cellular immune responses to eliminate infected host cells and reduce the risk of chronic disease (16, 22, 28, 31–34). However, the data reported by Khakhum et al. (30) indicate that agent-specific CD4+ and CD8+ T cells play a minor role in protection. These findings are consistent with previous studies demonstrating the importance of antibodies in protection against melioidosis and glanders. For example, vaccination with the B. pseudomallei purM LAS Bp82 was shown to provide high levels of protection against lethal intranasal challenge with wild-type B. pseudomallei isolate 1026b in BALB/c and C57BL/6 mice (35). Passive transfer of immune serum (elicited by vaccination with Bp82) to BALB/c mice resulted in survival rates of ∼40%, and vaccination of mice lacking B cells with Bp82 did not protect against challenge with wild-type organisms (35). Passive transfer of immune serum elicited by vaccination with B. pseudomallei 1026b outer membrane vesicles was shown to provide 80% survival in BALB/c mice against heterologous lethal challenge with wild-type B. pseudomallei K96243 (36), and monoclonal antibodies targeting LPS passively protected BALB/c mice against lethal aerosol infection with wild-type B. mallei strain ATCC 23344 (37). In addition, hyperimmune sera from horses vaccinated with mallein extract have been successfully used to treat human patients with glanders (38–40). Published work by our group also demonstrated that passive transfer of antibodies elicited by vaccination with B. mallei ATCC 23344 batA LAS protects against lethal aerosol challenge with homologous wild-type B. mallei organisms as well as lethal exposure to multiple wild-type B. pseudomallei strains in BALB/c and C57BL/6 mice (41). Importantly, passive transfer of antibodies (elicited by vaccination with B. mallei batA LAS) results in dose-dependent, high rates of bacterial clearance from target organs (41) (Fig. 1).
FIG 1.
Passive transfer of immune serum provides protective immunity against challenge with a lethal dose of wild-type B. mallei. Groups of naive female BALB/c mice were vaccinated with B. mallei batA LAS (41) and exsanguinated 30 to 45 days postvaccination, and serum samples from these animals were pooled. Naive female BALB/c mice (7 weeks of age; n = 9 per group) were then injected intraperitoneally with 1 ml of pooled immune sera and challenged 48 h later with 5 LD50 of wild-type B. mallei ATCC 23344 bacteria via the aerosol route using a Microsprayer device (25); controls consisted of age- and weight-matched naive mice injected with 1 ml of naive serum. Some animals received only a single dose of serum prior to infection, while others also received additional doses on days 7 and 14 postchallenge. Infected animals were monitored daily for signs of illness and morbidity. (A) Kaplan-Meier survival curves. (B) Survival data during the acute (days 1 through 10 postchallenge) and chronic (days 11 through 45 postchallenge) phases of infection. (C to E) Tissues were collected from survivors, homogenized, diluted, and spread on agar plates to determine bacterial loads. Symbols represent the values for individual animals; horizontal bars show mean total CFU for each group. The values below the groups in panels C to E show the percentages of animals that cleared bacteria from tissues from the different groups. The asterisk indicates that one mouse cleared bacteria from all three tissues.
In summary, the report by Khakhum and colleagues (30) complements prior published studies and expands upon them to demonstrate that antibodies are sufficient to protect against lethal aerosol infection with B. pseudomallei and B. mallei. Future work investigating the kinetics, quality, levels, and functionality of antibody responses in mice vaccinated with highly protective LAS will help drive the melioidosis and glanders vaccine field forward and will provide a powerful platform to identify high-value Burkholderia target antigens for the development of countermeasures.
The views expressed in this article do not necessarily reflect the views of the journal or of ASM.
REFERENCES
- 1.Wiersinga WJ, Virk HS, Torres AG, Currie BJ, Peacock SJ, Dance DAB, Limmathurotsakul D. 2018. Melioidosis. Nat Rev Dis Primers 4:17107. doi: 10.1038/nrdp.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perumal Samy R, Stiles BG, Sethi G, Lim LHK. 2017. Melioidosis: clinical impact and public health threat in the tropics. PLoS Negl Trop Dis 11:e0004738. doi: 10.1371/journal.pntd.0004738. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Limmathurotsakul D, Golding N, Dance DA, Messina JP, Pigott DM, Moyes CL, Rolim DB, Bertherat E, Day NP, Peacock SJ, Hay SI. 2016. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat Microbiol 1:15008. doi: 10.1038/nmicrobiol.2015.8. [DOI] [PubMed] [Google Scholar]
- 4.Khan I, Wieler LH, Melzer F, Elschner MC, Muhammad G, Ali S, Sprague LD, Neubauer H, Saqib M. 2013. Glanders in animals: a review on epidemiology, clinical presentation, diagnosis and countermeasures. Transbound Emerg Dis 60:204–221. doi: 10.1111/j.1865-1682.2012.01342.x. [DOI] [PubMed] [Google Scholar]
- 5.Kettle AN, Wernery U. 2016. Glanders and the risk for its introduction through the international movement of horses. Equine Vet J 48:654–658. doi: 10.1111/evj.12599. [DOI] [PubMed] [Google Scholar]
- 6.Carr-Gregory B, Waag DM. 2007. Glanders, p 121–146. In Dembek ZF. (ed), Medical aspects of biological warfare. Textbooks of Military Medicine. Office of the Surgeon General, US Army, Washington, DC. [Google Scholar]
- 7.Losada L, Ronning CM, DeShazer D, Woods D, Fedorova N, Kim HS, Shabalina SA, Pearson TR, Brinkac L, Tan P, Nandi T, Crabtree J, Badger J, Beckstrom-Sternberg S, Saqib M, Schutzer SE, Keim P, Nierman WC. 2010. Continuing evolution of Burkholderia mallei through genome reduction and large-scale rearrangements. Genome Biol Evol 2:102–116. doi: 10.1093/gbe/evq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song H, Hwang J, Yi H, Ulrich RL, Yu Y, Nierman WC, Kim HS. 2010. The early stage of bacterial genome-reductive evolution in the host. PLoS Pathog 6:e1000922. doi: 10.1371/journal.ppat.1000922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holden MT, Titball RW, Peacock SJ, Cerdeno-Tarraga AM, Atkins T, Crossman LC, Pitt T, Churcher C, Mungall K, Bentley SD, Sebaihia M, Thomson NR, Bason N, Beacham IR, Brooks K, Brown KA, Brown NF, Challis GL, Cherevach I, Chillingworth T, Cronin A, Crossett B, Davis P, DeShazer D, Feltwell T, Fraser A, Hance Z, Hauser H, Holroyd S, Jagels K, Keith KE, Maddison M, Moule S, Price C, Quail MA, Rabbinowitsch E, Rutherford K, Sanders M, Simmonds M, Songsivilai S, Stevens K, Tumapa S, Vesaratchavest M, Whitehead S, Yeats C, Barrell BG, Oyston PC, Parkhill J. 2004. Genomic plasticity of the causative agent of melioidosis, Burkholderia pseudomallei. Proc Natl Acad Sci U S A 101:14240–14245. doi: 10.1073/pnas.0403302101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nierman WC, DeShazer D, Kim HS, Tettelin H, Nelson KE, Feldblyum T, Ulrich RL, Ronning CM, Brinkac LM, Daugherty SC, Davidsen TD, Deboy RT, Dimitrov G, Dodson RJ, Durkin AS, Gwinn ML, Haft DH, Khouri H, Kolonay JF, Madupu R, Mohammoud Y, Nelson WC, Radune D, Romero CM, Sarria S, Selengut J, Shamblin C, Sullivan SA, White O, Yu Y, Zafar N, Zhou L, Fraser CM. 2004. Structural flexibility in the Burkholderia mallei genome. Proc Natl Acad Sci U S A 101:14246–14251. doi: 10.1073/pnas.0403306101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Zandt KE, Greer MT, Gelhaus HC. 2013. Glanders: an overview of infection in humans. Orphanet J Rare Dis 8:131. doi: 10.1186/1750-1172-8-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiersinga WJ, Currie BJ, Peacock SJ. 2012. Melioidosis. N Engl J Med 367:1035–1044. doi: 10.1056/NEJMra1204699. [DOI] [PubMed] [Google Scholar]
- 13.Stone JK, DeShazer D, Brett PJ, Burtnick MN. 2014. Melioidosis: molecular aspects of pathogenesis. Expert Rev Anti Infect Ther 12:1487–1499. doi: 10.1586/14787210.2014.970634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galyov EE, Brett PJ, Deshazer D. 2010. Molecular insights into Burkholderia pseudomallei and Burkholderia mallei pathogenesis. Annu Rev Microbiol 64:495–517. doi: 10.1146/annurev.micro.112408.134030. [DOI] [PubMed] [Google Scholar]
- 15.David J, Bell RE, Clark GC. 2015. Mechanisms of disease: host-pathogen interactions between Burkholderia species and lung epithelial cells. Front Cell Infect Microbiol 5:80. doi: 10.3389/fcimb.2015.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hatcher CL, Muruato LA, Torres AG. 2015. Recent advances in Burkholderia mallei and B. pseudomallei research. Curr Trop Med Rep 2:62–69. doi: 10.1007/s40475-015-0042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rhodes KA, Schweizer HP. 2016. Antibiotic resistance in Burkholderia species. Drug Resist Updat 28:82–90. doi: 10.1016/j.drup.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lipsitz R, Garges S, Aurigemma R, Baccam P, Blaney DD, Cheng AC, Currie BJ, Dance DA, Gee JE, Larsen J, Limmathurotsakul D, Morrow MG, Norton R, O'Mara E, Peacock S, Pesik N, Rogers LP, Schweizer HP, Steinmetz I, Tan G, Tan P, Wiersinga WJ, Wuthiekanun V, Smith TL. 2012. Workshop on treatment of and postexposure prophylaxis for Burkholderia pseudomallei and B. mallei infection, 2010. Emerg Infect Dis 18:e2. doi: 10.3201/eid1812.120638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zimmerman SM, Michel F, Hogan RJ, Lafontaine ER. 2015. The autotransporter BpaB contributes to the virulence of Burkholderia mallei in an aerosol model of infection. PLoS One 10:e0126437. doi: 10.1371/journal.pone.0126437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Titball RW, Russell P, Cuccui J, Easton A, Haque A, Atkins T, Sarkar-Tyson M, Harley V, Wren B, Bancroft GJ. 2008. Burkholderia pseudomallei: animal models of infection. Trans R Soc Trop Med Hyg 102(Suppl 1):S111–S116. doi: 10.1016/S0035-9203(08)70026-9. [DOI] [PubMed] [Google Scholar]
- 21.Sarkar-Tyson M, Titball RW. 2010. Progress toward development of vaccines against melioidosis: a review. Clin Ther 32:1437–1445. doi: 10.1016/j.clinthera.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 22.Bondi SK, Goldberg JB. 2008. Strategies toward vaccines against Burkholderia mallei and Burkholderia pseudomallei. Expert Rev Vaccines 7:1357–1365. doi: 10.1586/14760584.7.9.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Warawa JM. 2010. Evaluation of surrogate animal models of melioidosis. Front Microbiol 1:141. doi: 10.3389/fmicb.2010.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woods DE. 2002. The use of animal infection models to study the pathogenesis of melioidosis and glanders. Trends Microbiol 10:483–484. doi: 10.1016/S0966-842X(02)02464-2. [DOI] [PubMed] [Google Scholar]
- 25.Lafontaine ER, Zimmerman SM, Shaffer TL, Michel F, Gao X, Hogan RJ. 2013. Use of a safe, reproducible, and rapid aerosol delivery method to study infection by Burkholderia pseudomallei and Burkholderia mallei in mice. PLoS One 8:e76804. doi: 10.1371/journal.pone.0076804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lafontaine ER, Balder R, Michel F, Hogan RJ. 2014. Characterization of an autotransporter adhesin protein shared by Burkholderia mallei and Burkholderia pseudomallei. BMC Microbiol 14:92. doi: 10.1186/1471-2180-14-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aschenbroich SA, Lafontaine ER, Hogan RJ. 2016. Melioidosis and glanders modulation of the innate immune system: barriers to current and future vaccine approaches. Expert Rev Vaccines 15:1163–1181. doi: 10.1586/14760584.2016.1170598. [DOI] [PubMed] [Google Scholar]
- 28.Silva EB, Dow SW. 2013. Development of Burkholderia mallei and pseudomallei vaccines. Front Cell Infect Microbiol 3:10. doi: 10.3389/fcimb.2013.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Titball RW, Burtnick MN, Bancroft GJ, Brett P. 2017. Burkholderia pseudomallei and Burkholderia mallei vaccines: are we close to clinical trials? Vaccine 35:5981–5989. doi: 10.1016/j.vaccine.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 30.Khakhum N, Bharaj P, Myers JN, Tapia D, Kilgore PB, Ross BN, Walker DH, Endsley JJ, Torres AG. 2019. Burkholderia pseudomallei ΔtonB Δhcp1 live attenuated vaccine strain elicits full protective immunity against aerosolized melioidosis infection. mSphere 4:e00570-18 10.1128/mSphere.00570-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Limmathurotsakul D, Funnell SG, Torres AG, Morici LA, Brett PJ, Dunachie S, Atkins T, Altmann DM, Bancroft G, Peacock SJ, Steering Group on Melioidosis Vaccine Development. 2015. Consensus on the development of vaccines against naturally acquired melioidosis. Emerg Infect Dis 21:e141480. doi: 10.3201/eid2106.141480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choh LC, Ong GH, Vellasamy KM, Kalaiselvam K, Kang WT, Al-Maleki AR, Mariappan V, Vadivelu J. 2013. Burkholderia vaccines: are we moving forward? Front Cell Infect Microbiol 3:5. doi: 10.3389/fcimb.2013.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel N, Conejero L, De Reynal M, Easton A, Bancroft GJ, Titball RW. 2011. Development of vaccines against Burkholderia pseudomallei. Front Microbiol 2:198. doi: 10.3389/fmicb.2011.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peacock SJ, Limmathurotsakul D, Lubell Y, Koh GC, White LJ, Day NP, Titball RW. 2012. Melioidosis vaccines: a systematic review and appraisal of the potential to exploit biodefense vaccines for public health purposes. PLoS Negl Trop Dis 6:e1488. doi: 10.1371/journal.pntd.0001488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silva EB, Goodyear A, Sutherland MD, Podnecky NL, Gonzalez-Juarrero M, Schweizer HP, Dow SW. 2013. Correlates of immune protection following cutaneous immunization with an attenuated Burkholderia pseudomallei vaccine. Infect Immun 81:4626–4634. doi: 10.1128/IAI.00915-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nieves W, Petersen H, Judy BM, Blumentritt CA, Russell-Lodrigue K, Roy CJ, Torres AG, Morici LA. 2014. A Burkholderia pseudomallei outer membrane vesicle vaccine provides protection against lethal sepsis. Clin Vaccine Immunol 21:747–754. doi: 10.1128/CVI.00119-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trevino SR, Permenter AR, England MJ, Parthasarathy N, Gibbs PH, Waag DM, Chanh TC. 2006. Monoclonal antibodies passively protect BALB/c mice against Burkholderia mallei aerosol challenge. Infect Immun 74:1958–1961. doi: 10.1128/IAI.74.3.1958-1961.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bell G. 1923. Serum treatment of glanders. Can Med Assoc J 13:200–201. [PMC free article] [PubMed] [Google Scholar]
- 39.Burgess JF. 1936. Chronic glanders. Can Med Assoc J 34:258–262. [PMC free article] [PubMed] [Google Scholar]
- 40.Watson EA. 1924. Sur la serotherapie de la morve, dans l'espece humaine en particulier. Rev Med Vet 1924:220–223. [Google Scholar]
- 41.Zimmerman SM, Dyke JS, Jelesijevic TP, Michel F, Lafontaine ER, Hogan RJ. 2017. Antibodies against in vivo-expressed antigens are sufficient to protect against lethal aerosol infection with Burkholderia mallei and Burkholderia pseudomallei. Infect Immun 85:e00102-17. doi: 10.1128/IAI.00102-17. [DOI] [PMC free article] [PubMed] [Google Scholar]