Abstract
Objectives
The present study aimed at determining the antioxidant activity, total phenols and flavonoids and to evaluate the antiproliferative activity of ethanolic extract of Matricaria recutita L. (chamomile). The antioxidant activities were measured using the 2, 2-diphenyl-1-picrylhydrazyl (DPPH) assay. The total phenolic content was measured by the Folin–Ciocalteu assay. The flavonoid content was determined using the aluminum chloride method. The MTT assay was used to estimate the antiproliferative activities against human hepatoma (HepG2) cancer cell line. We assessed the mode of action of the extract as a cancer preventive agent and reported its ability to regulate tumor angiogenesis by down regulating in a dose dependent manner the expression of some proteins involved in the process.
Results
The percentage inhibition of DPPH scavenging activity was dose-dependent ranging between (94.8% ± 0.03) at 1.50 mg/mL and (84.2% ± 0.86) at 0.15 mg/mL. It showed high polyphenols (21.4 ± 0.327 mg GAE/g) and high flavonoids content (157.9 ± 2.22 mg QE/g). Effect of extract was investigated against HepG2 cells. A dose-dependent reduction in cell viability was recorded in cells treated with the extract. The IC50 was ~ 300 µg/mL. It significantly inhibited the level of important prerequisite angiogenesis markers both in HepG2 cells and ex vivo.
Electronic supplementary material
The online version of this article (10.1186/s13104-018-3960-y) contains supplementary material, which is available to authorized users.
Keywords: Antioxidants, Anticancer, Medicinal plants, Traditional medicine
Introduction
The escalating knowledge of reactive oxygen species (ROS) has revolutionized medicine [1]. ROS contributes to cardiovascular diseases and cancer [2, 3] and could cause immune system depletion [3, 4].
A typical antioxidant donates an electron to a free radical thus neutralizing it [5] and often bind to metals [6]. Synthetic antioxidants cause negative health effects [7]. Reports have recommended to replace synthetic antioxidants with natural ones [6, 8] to control formation of free radicals [9]. The most common natural antioxidants are antioxidative enzymes [10] whereas others are well represented in different spices and herbs [11].
Chamomile is known for its as anti-inflammatory [12, 13], anti-diarrhea [14], antioxidant [14, 15], anti-cancer [16], neuro-protective [17], anti-allergic [18] and anti-microbial [19] effects. It also improves cardiac health [20].
Medicinal plants are essential to medicine [21, 22] where different preclinical studies using skin and ovarian cancer models have shown promising growth inhibitory effects [16, 23]. Similarly, chamomile was reported to induce apoptosis in cancer cells [24]. The main component of the essential oil extracted from chamomile is terpenoids α-bisabolol [25].
Main text
Methods
Plant sample and extract preparation
Dried chamomile flowers were purchased from the local market. Ten grams of the flowers were extracted with 70% ethanol and macerated for 48 h at 4 °C. The product was then filtered and concentrated under reduced pressure at 40 °C. A 30 mg/mL solution was prepared in 50% ethanol.
Total polyphenol content (TPC)
The Folin–Ciocalteu reagent was used [26]. 50% ethanol was used in the preparation of a 10% solution. Hundred µL of this solution was mixed with 200 µL of the Folin–Ciocalteu reagent and 2 mL of de-ionized water. 20% aqueous sodium carbonate (w/w, 1 mL) was added. After incubation for 1 h at room temperature, the total polyphenols were determined. Absorbance was measured at 765 nm. The total phenolic compounds were expressed in mg gallic acid equivalents (GAE) per g dry weight of plant material. Experiments were conducted in triplicates.
Free-radical scavenging activity
The 2, 2-diphenyl-1-picrylhydrazil (DPPH·) was used to measure the free radical scavenging activity [27]. 3.8 mL of 60 µg/mL methanolic solution of DPPH was mixed with 200 µL extract. This was allowed to stand for 30 min at room temperature in the dark. The absorbance was measured at 517 nm followed by the determination of the EC50 value (µg/mL). The total antioxidant activity was expressed as ascorbic acid equivalent/g dry extract. Experiments were conducted in triplicates.
Determination of total flavonoids
The aluminum chloride colorimetric method was used as described in [28]. 500 µL of the extract (600 µg/mL) was mixed with 0.1 mL of 10% (w/v) aluminum chloride, 0.1 mL of 1 M potassium acetate, 1.5 mL of methanol and 2.8 mL of distilled water. The mixture was incubated at room temperature for 30 min. The absorbance was measured at 415 nm. The mean of three readings was calculated and was expressed as mg of quercetin equivalents (QE)/g of the dry extract.
Cell culture
Human hepatoma cells (HepG2) were allowed to grow in RPMI 1640 medium (Sigma, USA) supplemented with 1 percent antibiotic cocktail and ten percent fetal bovine serum (FBS) [29]. Liver cells were keep warm at 37 °C in five percent CO2 humidified incubator then passaged every 2–3 days using 0.25% trypsin–EDTA solution.
Cytotoxicity assay
HepG2 cells were seeded at 5000 cells/well in 96-well plates (Nunc) where the tested extract was added in and its effect was assessed after 24 h. The 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay was applied to assess cell viability. Briefly, cells treated with the given extract were treated with 5 mg/mL tetrazolium MTT (Sigma, USA). The absorbance was then measured at a wavelength of 570 nm and the percentage of the viability of the treated cells was calculated using the following equation.
Assessment of morphological changes
HepG2 cells were seeded at 0.25 × 106 cells/well in 6-well plates (Nunc) and were allowed to attach overnight. They were then treated without or with increasing concentrations of chamomile for 24 h and cell morphology was monitored and analyzed.
Experimental animals
The present study was approved by the institutional (UAE University) Animal Ethics Committee (approval Reference number: A 8-15) as detailed in [30].
Rat aorta ring assay
This was carried out on rat aortic explants as described [31]. Twelve male Wistar rats were used and approximately 12–14 rings were cut out from each aorta. The detailed methodology is described in [30].
Immunofluorescence
HepG2 cells were grown and processed for immunofluorescence as previously reported in [32].
Immunoblotting
HepG2 cells were seeded at a density of 1 × 106 cells/100 mm plate and were then allowed to attach. Attached cells were treated with the given extract at the following doses (200, 400, 600, 800, 1000 µM) and for 24 h. Whole cell lysates were analyzed using 7.5% and 10% SDS polyacrylamide gel electrophoresis. Proteins were transferred onto PVDF membranes before incubation with primary antibodies (AKT, p-AKT, VEGF (Cell signalling technologies) Phospho-p44/42, Phospho-p38, MMP-9 (Abcam) and GAPDH (Abcam) as a loading control. All protein bands were detected using WesternSure Chemiluminescent Substrate (LI-COR) and C-DiGit blot scanner (LI-COR).
Results and discussion
Plant extraction
The extract was prepared by evaporation of ethanol/water and the w/w percent yield was estimated to be 13.51% based on the used solvent [33].
Total polyphenol content (TPC)
Polyphenols are aromatic secondary plant metabolites [34] that may contribute to antioxidant activity [27, 35]. The Folin–Ciocalteu reagent reacts with polyphenols [36].
The calibration curve showed linearity for gallic acid in the range of 0.5–26 µg/mL, with a correlation coefficient (R2) of 0.988. Our extract contained high polyphenols (21.4 ± 0.327 mg GAE/g). When compared to other chamomile extracts, Italian chamomile gave (2689.2 ± 15 mg GAE/100 g) which is slightly higher than ours [37] while Egyptian chamomile showed (3.7 ± 2.0 mg GAE/g) polyphenols, much less than ours [33]. Results are the average of triplicates ± SD.
DPPH radical scavenging activity
Antioxidant activity was assessed based on the ability to reduce the stable DPPH radical [27]. The DPPH radical (DPPH·) can accept an electron or hydrogen radical and form a stable diamagnetic molecule [38]. The color change of DPPH has been used to measure the radical scavenging activity [39].
The ascorbic acid calibration curve showed linearity in the range of 5–20 µg/mL, with a correlation coefficient (R2) of 0.989. The DPPH scavenging activity of our extract ranged between (94.8% ± 0.03) at 1.50 mg/mL to (84.2% ± 0.86) at 0.15 mg/mL (Additional file 1).
The EC50 value was 26.7 µg/mL. We had a significant negative correlation (R2 = 0.856, P-value < 0.05) between EC50 ((DPPH·) scavenging) and total phenolic content. This suggests that their presence contributed significantly to antioxidant activity. These findings are consistent with previous investigations [27, 35, 40]. Iranian chamomile showed very high EC50 (5.52 ± 0.15 mg/mL) compared to our chamomile indicating that it has lower antioxidant power than the extract presented here [41].
Total flavonoid contents
Flavonoids have anticancer activity [42] and contribute to antioxidant plant properties [40]. We used the aluminum chloride assay [43]. The calibration curve showed linearity in the range of 1–25 µg/mL, with a correlation coefficient (R2) of 0.999. The total flavonoids content was (157.9 ± 2.22) mg QE/g dry extract.
Antiproliferative effect of M. recutita extract against cancer cells
HCC is among the leading causes of cancer death worldwide [44]. Natural products-based biomolecules possess bioactive secondary metabolites that are the foundation of broad-spectrum integrative approach for cancer prevention and treatment [45, 46].
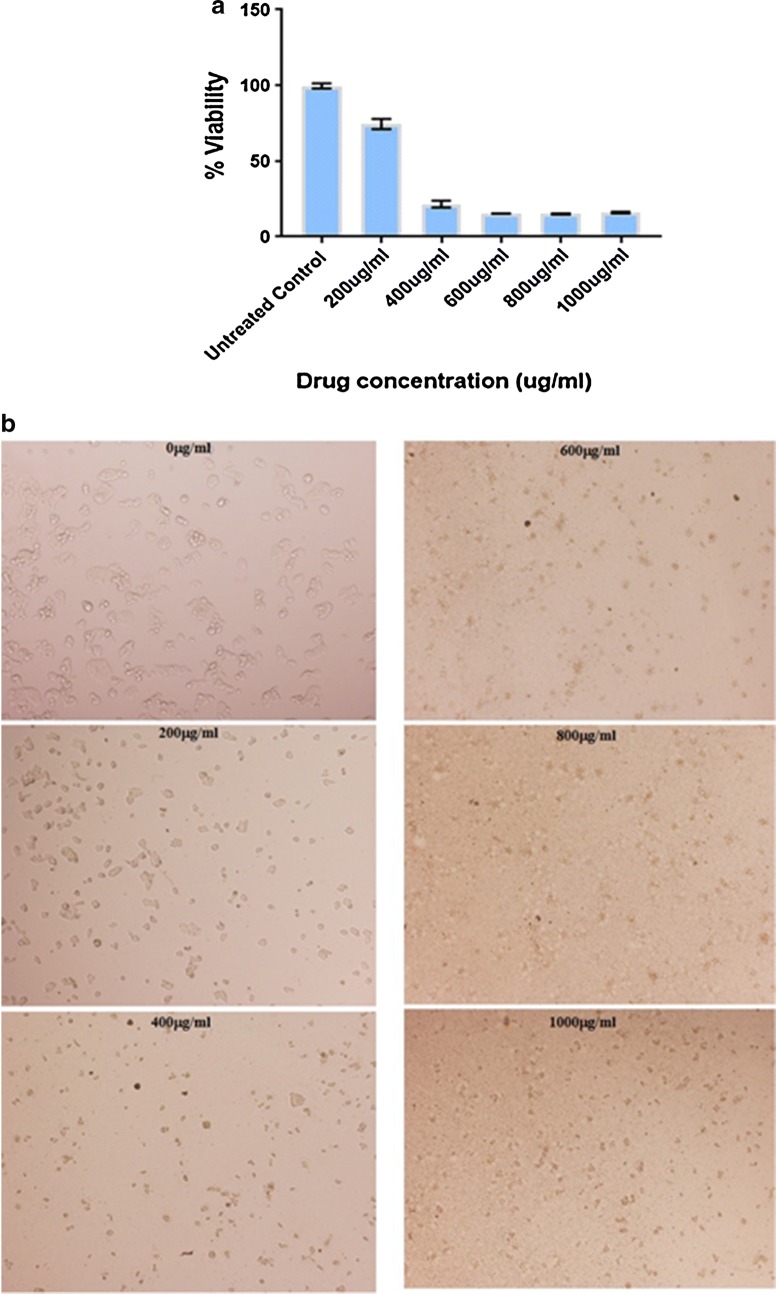
Effects of the given chamomile’s extract were investigated in vitro against HepG2 cells. A dose-dependent reduction in cell viability was evident (Fig. 1). The IC50 value was 300 µg/mL and the extract significantly enhanced the mortality of cancer cells at concentration as low as (200 µg/mL). It is worth stating here that the present extract showed no hepatotoxicity in normal porcine liver primary cells as previously reported in [47]. Chamomile-based sesquiterpenic compounds have been reported to be involved in a plethora of biological activities [48].
Fig. 1.
Assessment of the cytotoxic effects of Matricaria recutita L. extract on hepatocellular carcinoma in vitro. a MTT assay results of HepG2 cells viability after treatment with increasing concentrations of M. recutita L. for 24 h. b Assessment of morphological changes of HepG2 cells after treatment with increasing concentrations of M. recutita L. for 24 h. Cells were fixed and stained with crystal violet (scale bar = 200 μm)
Chamomile has many health promoting effects including anti-allergic and anticancer activities [49]. The composition and effects of chamomile have been studied; yet, the exact mechanism/s of its bioactivity awaits further investigations. Unlike the potent anticancer effect of the ethanolic extract of chamomile shown here, M. recutita L. infusion and plant methanol extract was selective for HCT-15 and HeLa and showed no activity against MCF-7, NCI-H460 and HepG2 [47].
Antiangiogenic activity of M. recutita extract in HepG2 cells
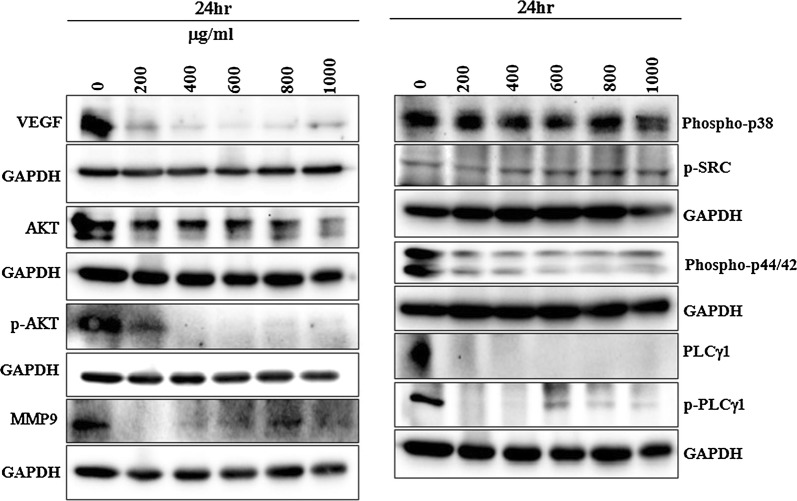
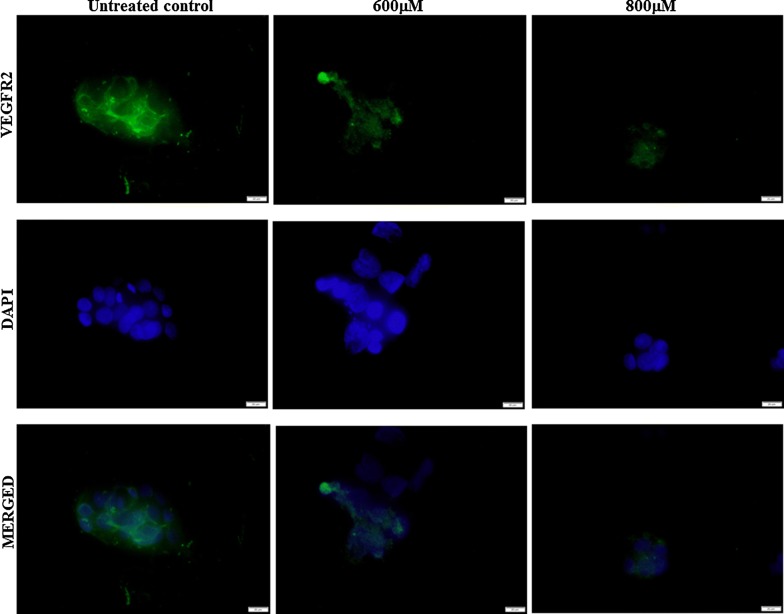
Angiogenesis is central to many physiological conditions [29]. Vascular endothelial growth factor (VEGF) is a signal protein that stimulates the process of blood vessel formation through important cellular processes of vasculogenesis and angiogenesis through receptor tyrosine kinase VEGF receptors (VEGFRs). Multiple VEGFs (VEGF-A, VEGF-B, VEGF-C and VEGF-D) interact with VEGF receptors such as VEGFR1, VEGFR2 and VEGFR3 [50]. Here, we demonstrated that an increasing dose of the present extract inhibited the protein expression of VEGF (Fig. 2) and that was consistent with an immunofluorescence assay that showed the dose dependent decrease of VEGFR2 expression (Fig. 3).
Fig. 2.
Inhibitory effect of Matricaria recutita L. extract on angiogenesis related markers. Western blot analysis of important and prerequisites markers in angiogenesis in HepG2 cells post treatment with increasing doses of chamomile for 24 h
Fig. 3.
Representative images of immunofluorescence assay of pre-treated HEPG2 cells in two doses of Matricaria recutita L. extract (600 µM and 800 µM). Cells were immunostained with antibody against VEGFR2. VEGFR-positive cells were stained green (Alexa Fluor® 488) and the nucleus stained blue (DAPI). Scale bar, 20 µm
The matrix metalloproteinases are zinc-dependent endopeptidases [51] of which MMP-9 is believed to be promoting angiogenesis [52]. The present extract dynamically down regulated the expression of MMP-9 substantiating the potential of the extract in regulating tumor angiogenesis. Protein expression analysis was performed using western blot to determine whether the present extract regulated the activity of MAPK/AKT cascades including AKT, ERK1/2 MAPK and p38 MAPK. As shown in Fig. 2, the extract substantially induced the activation of AKT and ERK1/2 MAPK and had no significant effect on phosphorylation of p38 MAPK. The tested chamomile extract inhibited the activation of AKT/ERK pathway measured by the western blot analysis of p-AKT and p-ERK1/2 (Fig. 2). Interestingly, the levels of total AKT was also down regulated at higher doses. Studies have reported that deprivation of VEGF or blockade of the VEGF signal transduction cascade with the VEGF tyrosine kinase inhibitor K787/ZK222584 resulted in a specific decrease of AKT protein level; subsequent cellular stimulation with VEGF rescued AKT stability in endothelial cells [53]. Chamomile extract used here could be a direct target for VEGF that caused the decrease of AKT protein expression and it significantly inhibited the level of VEGFR2 in HEPG2 cells representing the blockade of the VEGF signaling pathway. Similarily, Rhazya strict (Harmal) has recently been shown to possess potent antiangiogenic and antiproliferative activities in vitro [54].
Inhibitory effect of M. recutita extract on sprouting of microvessels in rat aortic explants
Cell migration and invasion are crucial in physiological processes such as angiogenesis. Similar trend was observed in aortic ring assay (Additional file 2) where treatment with chamomile reduced sprouting microvessels in a dose-dependent fashion.
Conclusions
Chamomile represents a potential in anti-cancer treatments.
Limitations
A possible limitation could be the lack of in vivo studies that was not the focus of this work but could be done in a future study.
Additional files
Additional file 1. DPPH radical scavenging activities of the tested Matricaria recutita L. extract. Description of data: The percentage inhibitions of DPPH scavenging activity of the extract.
Additional file 2. Matricaria recutita L. extract inhibits microvessels sprouting in aortic ring assay in a dose-dependent manner. (a) Representative micrographs of sprouting microvessels from aortic ring grown in the absence or presence of M. recutita L. extract with or without VEGF treatment. (b) Quantification of the number of the sprouting microvessels from aortic rings grown in the presence or absence of M. recutita L. extract with or without VEGF treatment. Description of data: Matricaria recutita L. extract inhibits microvessels sprouting in aortic ring assay in a dose-dependent manner.
Authors’ contributions
BAD and AA designed the study, analyzed the data, interpreted the results and wrote the paper; IAE, ME, CM, AAM and BA performed all the experiments and contributed to the study design and analysis. All authors read and approved the final manuscript.
Acknowledgements
This work was supported by a start-up grant (Grant 31S 215), Division of Research and Graduate Studies, UAEU and an individual research grant (Grant 31S 188), College of Science, UAEU to the PI: Dr. Bayan Al-Dabbagh.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
Ethics approval and consent to participate
All procedures were carried out according to the guidelines of Animal Ethics Committee (UAE University). The present study was submitted to the institutional animal ethics committee, “Animal Research Ethics Committee” for evaluation and was approved by the committee (approval Reference number: A 8-15) belonging to the College of Medicine and Health Sciences, UAE University.
Consent for publication
Not applicable.
Funding
This work was supported by a start-up grant (Grant 31S 215), Division of Research and Graduate Studies, UAEU and an individual research grant (Grant 31S 188), College of Science, UAEU to the PI: Dr. Bayan Al-Dabbagh.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviation
- SEM
standard error of the mean
Contributor Information
Bayan Al-Dabbagh, Phone: + 9713-7136158, Email: bayan.al-dabbagh@uaeu.ac.ae.
Ismail A. Elhaty, Email: ismailelhaty@uaeu.ac.ae
Mohamed Elhaw, Email: 201150161@uaeu.ac.ae.
Chandraprabha Murali, Email: chandra_prabha@uaeu.ac.ae.
Ameera Al Mansoori, Email: 201570229@uaeu.ac.ae.
Basma Awad, Email: 201570103@uaeu.ac.ae.
Amr Amin, Phone: +971 3 713 6519, Email: a.amin@uaeu.ac.ae.
References
- 1.Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev. 2010;4:118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aruoma OI. Free radicals, antioxidants and international nutrition. Asia Pac J Clin Nutr. 1999;8:53–63. doi: 10.1046/j.1440-6047.1999.00036.x. [DOI] [PubMed] [Google Scholar]
- 3.Gupta VK, Sharma SK. Plants as natural antioxidants. Nat Prod Radiance. 2006;5:326–334. [Google Scholar]
- 4.Magder S. Reactive oxygen species: toxic molecules or spark of life? Crit Care. 2006;10:208–216. doi: 10.1186/cc3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halliwell B. How to characterize an antioxidant: an update. Biochem Soc Symp. 1995;61:73–101. doi: 10.1042/bss0610073. [DOI] [PubMed] [Google Scholar]
- 6.Brewer MS. Natural antioxidants: sources, compounds, mechanisms of action, and potential applications. Compr Rev Food Sci Food Saf. 2011;10:221–247. doi: 10.1111/j.1541-4337.2011.00156.x. [DOI] [Google Scholar]
- 7.Ebrahimzadeh MA, Pourmorad F, Hafezi S. Antioxidant activities of Iranian corn silk. Turkish J. Biol. 2008;32:43–49. [Google Scholar]
- 8.Soobrattee MA, Neergheen VS, Luximon-Ramma A, Aruoma OI, Bahorun T. Phenolics as potential antioxidant therapeutic agents: mechanism and actions. Mutat Res. 2005;579:200–213. doi: 10.1016/j.mrfmmm.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 9.Chaudière J, Ferrari-Iliou R. Intracellular antioxidants: from chemical to biochemical mechanisms. Food Chem Toxicol. 1999;37:949–962. doi: 10.1016/S0278-6915(99)00090-3. [DOI] [PubMed] [Google Scholar]
- 10.Fridovich I. Fundamental aspects of reactive oxygen species, or what’s the matter with oxygen? Ann N Y Acad Sci. 1999;893:13–18. doi: 10.1111/j.1749-6632.1999.tb07814.x. [DOI] [PubMed] [Google Scholar]
- 11.Hinneburg I, Damien Dorman HJ, Hiltunen R. Antioxidant activities of extracts from selected culinary herbs and spices. Food Chem. 2006;97:122–129. doi: 10.1016/j.foodchem.2005.03.028. [DOI] [Google Scholar]
- 12.Carnat A, Carnat AP, Fraisse D, Ricoux L, Lamaison JL. The aromatic and polyphenolic composition of Roman camomile tea. Fitoterapia. 2004;75:32–38. doi: 10.1016/j.fitote.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Peña D, de Montes Oca N, Rojas S, Parra A, García G. Anti-inflammatory and anti-diarrheic activity of Isocarpha cubana Blake. Pharmacologyonline. 2006;3:744–749. [Google Scholar]
- 14.Sebai H, Jabri M-A, Souli A, Rtibi K, Selmi S, Tebourbi O, et al. Antidiarrheal and antioxidant activities of chamomile (Matricaria recutita L.) decoction extract in rats. J Ethnopharmacol. 2014;152:327–332. doi: 10.1016/j.jep.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 15.Zemestani M, Rafraf M, Asghari-Jafarabadi M. Chamomile tea improves glycemic indices and antioxidants status in patients with type 2 diabetes mellitus. Nutrition. 2016;32:66–72. doi: 10.1016/j.nut.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Patel D, Shukla S, Gupta S. Apigenin and cancer chemoprevention: progress, potential and promise. Int J Oncol. 2007;30:233–245. [PubMed] [Google Scholar]
- 17.Ranpariya V, Parmar S, Sheth N, Chandrashekhar V. Neuroprotective activity of Matricaria recutita against fluoride-induced stress in rats. Pharm. Biol. 2011;49:696–701. doi: 10.3109/13880209.2010.540249. [DOI] [PubMed] [Google Scholar]
- 18.Chandrashekhar V, Halagali K, Nidavani R, Shalavadi MH, Biradar BS, Biswas D, et al. Anti-allergic activity of German chamomile (Matricaria recutita L.) in mast cell mediated allergy model. J Ethnopharmacol. 2011;137:336–340. doi: 10.1016/j.jep.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 19.Silva N, Barbosa L, Seito L, Fernandes Junior A. Antimicrobial activity and phytochemical analysis of crude extracts and essential oils from medicinal plants. Nat Prod Res. 2012;26:1510–1514. doi: 10.1080/14786419.2011.564582. [DOI] [PubMed] [Google Scholar]
- 20.Gould L, Reddy CR, Gomprecht RF. Cardiac effects of chamomile tea. J Clin Pharmacol. 1973;13:475–479. doi: 10.1002/j.1552-4604.1973.tb00202.x. [DOI] [PubMed] [Google Scholar]
- 21.Koehn FE, Carter GT. The evolving role of natural products in drug discovery. Nat Rev Drug Discov. 2005;4:206–220. doi: 10.1038/nrd1657. [DOI] [PubMed] [Google Scholar]
- 22.Hadley SK, Petry JJ. Medicinal herbs: a primer for primary care. Hosp Pract. 1999;34:105–123. doi: 10.3810/hp.1999.06.151. [DOI] [PubMed] [Google Scholar]
- 23.Way T-D, Kao M-C, Lin J-K. Apigenin induces apoptosis through proteasomal degradation of HER2/neu in HER2/neu-overexpressing breast cancer cells via the phosphatidylinositol 3-kinase/Akt-dependent pathway. J Biol Chem. 2004;279:4479–4489. doi: 10.1074/jbc.M305529200. [DOI] [PubMed] [Google Scholar]
- 24.Srivastava JK, Gupta S. Antiproliferative and apoptotic effects of chamomile extract in various human cancer cells. J Agric Food Chem. 2007;55:9470–9478. doi: 10.1021/jf071953k. [DOI] [PubMed] [Google Scholar]
- 25.Srivastava JK, Shankar E, Gupta S. Chamomile: a herbal medicine of the past with bright future. Mol Med Rep. 2010;3:895–901. doi: 10.3892/mmr.2010.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singleton VL, Orthofer R, Lamuela-Raventós RM. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- 27.Lim YY, Quah EPL. Antioxidant properties of different cultivars of Portulaca oleracea. Food Chem. 2007;103:734–740. doi: 10.1016/j.foodchem.2006.09.025. [DOI] [Google Scholar]
- 28.Chang C-C, Yang M-H, Wen H-M, Chern J-C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10:178–182. [Google Scholar]
- 29.Folkman J, Long DM, Becker FF. Growth and metastasis of tumor in organ culture. Cancer. 1963;16:453–467. doi: 10.1002/1097-0142(196304)16:4<453::AID-CNCR2820160407>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 30.Al-Dabbagh B, Elhaty IA, Murali C, Madhoon AA, Amin A. Salvadora persica (Miswak): antioxidant and Promising Antiangiogenic Insights. Am J Plant Sci. 2018;09:1228–1244. doi: 10.4236/ajps.2018.96091. [DOI] [Google Scholar]
- 31.Al-Salahi OSA, Kit-Lam C, Majid AM, Al-Suede FS, Mohammed SA, Abdullah WZ, et al. Anti-angiogenic quassinoid-rich fraction from Eurycoma longifolia modulates endothelial cell function. Microvasc Res. 2013;90:30–39. doi: 10.1016/j.mvr.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Romanelli R, Petrai I, Robino G, Efsen E, Novo E, Bonacchi A, et al. Thrombopoietin stimulates migration and activates multiple signaling pathways in hepatoblastoma cells. Am J Physiol Gastrointest Liver Physiol. 2006;290:G120–G128. doi: 10.1152/ajpgi.00350.2004. [DOI] [PubMed] [Google Scholar]
- 33.Roby MHH, Sarhan MA, Selim KA-H, Khalel KI. Antioxidant and antimicrobial activities of essential oil and extracts of fennel (Foeniculum vulgare L.) and chamomile (Matricaria chamomilla L.) Ind Crops Prod. 2013;44:437–445. doi: 10.1016/j.indcrop.2012.10.012. [DOI] [Google Scholar]
- 34.Robbins RJ. Phenolic acids in foods: an overview of analytical methodology. J Agric Food Chem. 2003;51:2866–2887. doi: 10.1021/jf026182t. [DOI] [PubMed] [Google Scholar]
- 35.Wojdyło A, Oszmiański J, Czemerys R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Che. 2007;105:940–949. doi: 10.1016/j.foodchem.2007.04.038. [DOI] [Google Scholar]
- 36.Pontis JA, Costa LA, Silva SJ, Flach A. Color, phenolic and flavonoid content, and antioxidant activity of honey from Roraima, Brazil. Food Sci Technol. 2014;34:69–73. doi: 10.1590/S0101-20612014005000015. [DOI] [Google Scholar]
- 37.Formisano C, Delfine S, Oliviero F, Tenore GC, Rigano D, Senatore F. Correlation among environmental factors, chemical composition and antioxidative properties of essential oil and extracts of chamomile (Matricaria chamomilla L.) collected in Molise (South-central Italy) Ind Crops Prod. 2015;63:256–263. doi: 10.1016/j.indcrop.2014.09.042. [DOI] [Google Scholar]
- 38.Robards K, Prenzler PD, Tucker G, Swatsitang P, Glover W. Phenolic compounds and their role in oxidative processes in fruits. Food Chem. 1999;66:401–436. doi: 10.1016/S0308-8146(99)00093-X. [DOI] [Google Scholar]
- 39.Kitts DD, Wijewickreme AN, Hu C. Antioxidant properties of a North American ginseng extract. Mol Cell Biochem. 2000;203:1–10. doi: 10.1023/A:1007078414639. [DOI] [PubMed] [Google Scholar]
- 40.Shan B, Cai YZ, Sun M, Corke H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J Agric Food Chem. 2005;53:7749–7759. doi: 10.1021/jf051513y. [DOI] [PubMed] [Google Scholar]
- 41.Sazegar M, Banakar A, Bahrami N, Bahrami A, Baghbani M, Nematolahi P, et al. Determination of the antioxidant activity and stability of Chamomile (Matricaria chamomilla L.) extract in sunflower oil. World Appl Sci J. 2011;12:1500–1504. [Google Scholar]
- 42.Amin A, Mousa M. Merits of anti-cancer plants from the Arabian Gulf region. Cancer Ther. 2007;5:55–66. [Google Scholar]
- 43.Popova M, Bankova V, Butovska D, Petkov V, Nikolova-Damyanova B, Sabatini AG, et al. Validated methods for the quantification of biologically active constituents of poplar-type propolis. Phytochem Anal. 2004;15:235–240. doi: 10.1002/pca.777. [DOI] [PubMed] [Google Scholar]
- 44.Amin A, Hamza AA, Bajbouj K, Ashraf SS, Daoud S. Saffron: a potential candidate for a novel anticancer drug against hepatocellular carcinoma. Hepatology. 2011;54:857–867. doi: 10.1002/hep.24433. [DOI] [PubMed] [Google Scholar]
- 45.Al-Hrout A, Chaiboonchoe A, Khraiwesh B, Murali C, Baig B, El-Awady R, et al. Safranal induces DNA double-strand breakage and ER-stress-mediated cell death in hepatocellular carcinoma cells. Sci Rep. 2018;8(1):16951. doi: 10.1038/s41598-018-34855-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruhul Amin ARM, Karpowicz PA, Carey TE, Arbiser J, Nahta R, Chen ZG, et al. Evasion of anti-growth signaling: a key step in tumorigenesis and potential target for treatment and prophylaxis by natural compounds. Semin Cancer Biol. 2015;35:S55–S77. doi: 10.1016/j.semcancer.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guimarães R, Barros L, Dueñas M, Calhelha RC, Carvalho AM, Santos-Buelga C, et al. Infusion and decoction of wild German chamomile: bioactivity and characterization of organic acids and phenolic compounds. Food Chem. 2013;136:947–954. doi: 10.1016/j.foodchem.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 48.Cavalieri E, Mariotto S, Fabrizi C, de Prati AC, Gottardo R, Leone S, et al. α-Bisabolol, a nontoxic natural compound, strongly induces apoptosis in glioma cells. Biochem Biophys Res Commun. 2004;315:589–594. doi: 10.1016/j.bbrc.2004.01.088. [DOI] [PubMed] [Google Scholar]
- 49.Matic IZ, Juranic Z, Savikin K, Zdunic G, Nadvinski N, Godevac D. Chamomile and marigold tea: chemical characterization and evaluation of anticancer activity. Phytother Res PTR. 2013;27:852–858. doi: 10.1002/ptr.4807. [DOI] [PubMed] [Google Scholar]
- 50.Chatterjee S, Heukamp LC, Siobal M, Schöttle J, Wieczorek C, Peifer M, et al. Tumor VEGF: VEGFR2 autocrine feed-forward loop triggers angiogenesis in lung cancer. J Clin Invest. 2013;123:1732–1740. doi: 10.1172/JCI65385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuwajima A, Iwashita J, Murata J, Abe T. The histone deacetylase inhibitor butyrate inhibits melanoma cell invasion of Matrigel. Anticancer Res. 2007;27:4163–4169. [PubMed] [Google Scholar]
- 52.Sun L-C, Luo J, Mackey LV, Fuselier JA, Coy DH. A conjugate of camptothecin and a somatostatin analog against prostate cancer cell invasion via a possible signaling pathway involving PI3K/Akt, αVβ3/αVβ5 and MMP-2/-9. Cancer Lett. 2007;246:157–166. doi: 10.1016/j.canlet.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 53.Riesterer O, Zingg D, Hummerjohann J, Bodis S, Pruschy M. Degradation of PKB/Akt protein by inhibition of the VEGF receptor/mTOR pathway in endothelial cells. Oncogene. 2004;23:4624–4635. doi: 10.1038/sj.onc.1207596. [DOI] [PubMed] [Google Scholar]
- 54.Al-Dabbagh B, Elhaty IA, Al Hrout AA, Al Sakkaf R, El-Awady R, Ashraf SS, et al. Antioxidant and anticancer activities of Trigonella foenum-graecum, Cassia acutifolia and Rhazya stricta. BMC Complement Altern Med. 2018;18:240–252. doi: 10.1186/s12906-018-2285-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. DPPH radical scavenging activities of the tested Matricaria recutita L. extract. Description of data: The percentage inhibitions of DPPH scavenging activity of the extract.
Additional file 2. Matricaria recutita L. extract inhibits microvessels sprouting in aortic ring assay in a dose-dependent manner. (a) Representative micrographs of sprouting microvessels from aortic ring grown in the absence or presence of M. recutita L. extract with or without VEGF treatment. (b) Quantification of the number of the sprouting microvessels from aortic rings grown in the presence or absence of M. recutita L. extract with or without VEGF treatment. Description of data: Matricaria recutita L. extract inhibits microvessels sprouting in aortic ring assay in a dose-dependent manner.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.