Abstract
Signaling from the postsynaptic compartment regulates multiple aspects of synaptic development and function. Syntaxin 4 (Syx4) is a plasma membrane t-SNARE that promotes the growth and plasticity of Drosophila neuromuscular junctions (NMJs) by regulating the localization of key synaptic proteins in the postsynaptic compartment. Here we describe electrophysiological analyses and report that loss of Syx4 leads to enhanced neurotransmitter release, despite a decrease in the number of active zones. We describe a requirement for postsynaptic Syx4 in regulating several presynaptic parameters, including Ca2+ cooperativity and the abundance of the presynaptic calcium channel Cacophony (Cac) at active zones. These findings indicate Syx4 negatively regulates presynaptic neurotransmitter release through a retrograde signaling mechanism from the postsynaptic compartment.
Keywords: Synaptic transmission, Synaptic plasticity, Neuromuscular junction, Drosophila
Introduction
Synapses exhibit multiple modes of plasticity, strengthening or weakening in response to activity, while also constraining such changes in strength to remain within stable physiological parameters. Alterations leading to changes in synaptic strength can occur on both sides of the synapse. Presynaptically, such changes may include the spatial organization of synaptic vesicles, active zones, or molecular components of the neurotransmitter release machinery (Kittel & Heckmann, 2016; Lazarevic, Pothula, Andres-Alonso, & Fejtova, 2013). Postsynaptically, a synapse may regulate the distribution or function of neurotransmitter receptors or other components of the postsynaptic scaffold (Chater & Goda, 2014; Choquet & Triller, 2013), or make structural changes to the postsynaptic membrane (Fu & Ip, 2017; Yin & Yuan, 2015). Transynaptic communication is critical for both pre- and postsynaptic cells to sense changes in their environment and respond with appropriate modifications.
Retrograde signaling from the postsynaptic to the presynaptic cell plays an important role in regulating the synapse. In Drosophila, key retrograde pathways that affect the morphology and function of the NMJ include TGFβ, Wnt, neurotrophins, and neuropeptides (Harris & Littleton, 2015; Menon, Carrillo, & Zinn, 2013). However, it is still poorly understood how multiple retrograde pathways are coordinated to mediate activity-dependent and homeostatic plasticity.
We previously described a role for the plasma membrane t-SNARE Syx4 in regulating the localization of key synaptic proteins in the postsynaptic compartment at the Drosophila NMJ (Harris, Zhang, Piccioli, Perrimon, & Littleton, 2016). We found that Syx4 localizes to the postsynaptic membrane and that null mutants of Syx4 exhibit reduced membrane levels of Synaptotagmin 4 (Syt4), a postsynaptic Ca2+ sensor that regulates retrograde signaling (Barber, Jorquera, Melom, & Littleton, 2009; Korkut et al., 2013; Piccioli & Littleton, 2014; Yoshihara, Adolfsen, Galle, & Littleton, 2005), and Neuroligin 1 (Nlg1), a transsynaptic adhesion molecule that regulates synaptic organization and signaling (Banerjee, Venkatesan, & Bhat, 2017; Banovic et al., 2010; Mosca, Hong, Dani, Favaloro, & Luo, 2012; Mozer & Sandstrom, 2012; Owald et al., 2012). Syx4 mutants exhibit defects in the growth and plasticity of the NMJ, including a reduction in the number of synaptic boutons, a reduction in the density of active zones, and a failure to bud new boutons in response to strong neuronal stimulation. Genetic interaction experiments indicate that Syx4 cooperates with Syt4 and Nlg1 to regulate these processes (Harris et al., 2016).
The initial investigation of Syx4 did not include analysis of synaptic transmission. Here we describe electrophysiology recordings at the NMJ to test synaptic transmission and report a surprising finding. Despite an approximate 50% reduction in the number of active zones per NMJ in Syx4 mutants, these animals exhibit synaptic enhancement, with an increase in evoked release at low stimulus frequency and no change in spontaneous release frequency or amplitude. We demonstrate that this synaptic enhancement is accompanied by presynaptic changes at active zones that affect the efficiency of neurotransmitter release. Furthermore, the enhanced presynaptic release in Syx4 mutants can be rescued by postsynaptic expression of Syx4, implying a retrograde signaling mechanism.
Materials & Methods
Drosophila stocks
All Drosophila strains were cultured on standard media at 25°C. The following stocks were used: 24B-GAL4 (BDSC 1767; Brand and Perrimon, 1993); elav-GAL4[2] (BDSC 8765; (Luo, Liao, Jan, & Jan, 1994); Cac-GFP (Matkovic et al., 2013); Syt4BA1 (Adolfsen, Saraswati, Yoshihara, & Littleton, 2004); Nlg1ex3.1 (Banovic et al., 2010); UAS-Syx4A, Syx473, Syx4PRE (Harris et al., 2016).
Electrophysiology
Wandering third instar larvae were dissected in HL3 saline (Stewart, Atwood, Renger, Wang, & Wu, 1994) for evoked release experiments and in HL3.1 saline (Feng, Ueda, & Wu, 2004) for paired-pulse facilitation experiments. The final concentration of Ca2+ is indicated in each figure legend. Recordings were taken using an AxoClamp 2B amplifier (Axon Instruments, Burlingame, CA). A recording electrode was filled with 3M KCl and inserted into muscle 6 at abdominal segments A3 or A4. A stimulating electrode filled with saline was used to stimulate the severed segmental nerve. Miniature excitatory junctional potentials (mEJPs; minis) were recorded for 2 min and 16 nerve-evoked potentials (EJPs) were recorded at 1 Hz. Analyses were performed using Clampfit 10.0 software (Molecular Devices, Sunnyvale, CA). Quantal content was determined by dividing the average EJP amplitude of a given NMJ by the average mEJP amplitude from the same NMJ. Quantal content was corrected for nonlinear summation as described in McLachlan & Martin (1981). Calcium cooperativity was determined by log-transforming corrected quantal content and calcium concentration values and finding the slope of the linear regression.
Immunostaining
Larvae were reared at 25°C and dissected at the third wandering instar stage. Larvae were dissected in HL3 solution and fixed for 10 min in 4% paraformaldehyde. Following washes in PBT (PBS containing 0.3% Triton X-100), larvae were blocked for one hour in PBT containing 2% normal goat serum, incubated overnight with primary antibody at 4°C, washed, incubated with secondary antibodies overnight at 4°C, washed, and mounted in Vectashield (Vector Laboratories) for imaging. Antibodies were as follows: anti-Brp, 1:500 (DSHB nc82; Wagh et al., 2006); DyLight 649 conjugated anti-horseradish peroxidase, 1:1000 (Jackson ImmunoResearch); rabbit anti-GFP Alexa Fluor 488, Alexa Fluor 546 goat anti-mouse, 1:400 (Life Technologies). Images were acquired with a 63× 1.4 NA oil-immersion objective (Carl Zeiss).
Quantification of confocal images
Analyses were conducted using Volocity (version 6.3) or FIJI / ImageJ (version 2.0.0-rc-32/1.49v; Schindelin et al., 2012). Measurements of Brp intensity and Cac-GFP intensity were conducted on 12 1b boutons per animal, using 1 terminal bouton and 5 adjacent non-terminal boutons, on two different branches; n refers to the number of animals analysed. Cac-GFP intensity was quantified at individual active zones by first finding Brp puncta using Thresholding and Analyze Particles in ImageJ, copying the corresponding ROIs to the Cac-GFP channel, and taking Cac intensity measurements within the same ROIs. The intensity of Cac-GFP was divided by the Brp intensity within each ROI. Brp intensity was quantified in Volocity by measuring the fluorescence intensity of Brp signal within an ROI defined by the HRP signal, and the average intensity within the ROI was divided by the average HRP intensity. All analyses were performed blind to genotype.
Statistical analysis
Statistical analyses were performed using Prism software (v. 6.0h). Statistical significance in two-way comparisons was determined by a Student’s t test, while ANOVA analysis was used when comparing more than two datasets. The p values associated with ANOVA tests were obtained from a Tukey’s post-test. When described in the text or figures, statistical comparisons are with control unless otherwise labelled and are indicated as ***p<0.005, **p<0.05, *p<0.05, ns=not significant. All mean, standard error of the mean, sample size, and p values are listed in Supplemental Table 1.
Results
To assess synaptic function in Syx4 mutant animals, we first measured nerve-evoked and spontaneous neurotransmitter release at larval NMJs. For this and all other experiments described in this study, control animals are from a precise excision line (Syx4PRE) generated during the P-element excision mutagenesis that produced the Syx473 null deletion (Harris et al., 2016). Nerve-evoked responses were collected at 1Hz stimulation in 0.3 mM extracellular Ca2+ and the mean EJP amplitude was calculated. Syx4 null mutants (Syx473) exhibited a significant increase in EJP amplitude compared to control animals (Figure 1A,B. Control, 6.4 ± 0.62 mV [n=12]; Syx473, 15.44 ± 1.7 mV [n=12], ***p<0.0001). We next attempted to rescue Syx4 mutant defects by expressing a full-length Syx4 cDNA in the Syx4 null mutant background. We expressed Syx4 cDNA with either pre- or postsynaptic-specific drivers (the pan neuronal drive elav-GAL4 and the muscle driver 24B-GAL4, respectively). When expressed in the postsynaptic cell in Syx4 mutant animals, Syx4 was able to fully rescue the Syx473 increase in evoked release (Syx473 24B-GAL4>UAS-Syx4, 6.5 ± 0.86 mV [n=10], ns p>0.999). In contrast, expressing Syx4 presynaptically in Syx4 mutant animals did not restore the evoked response to control levels (Syx473 elav-GAL4>UAS-Syx4, 14.72 ± 1.22 mV [n=10], ***p<0.0001). Simply overexpressing Syx4 in the control background, using a postsynaptic-specific driver, did not alter EJP amplitude (24B-GAL4>UAS-Syx4, 6.9 ± 0.6 mV [n=10], ns p=0.998). These findings indicate that Syx4 is required postsynaptically to regulate the amplitude of evoked potentials.
Figure 1.
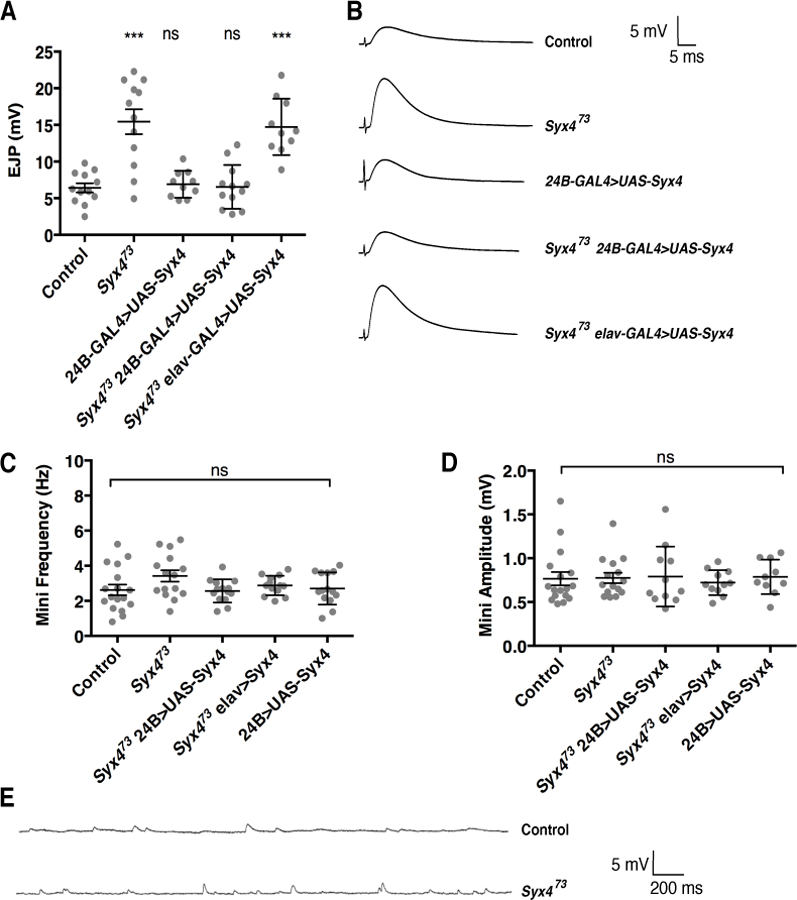
Syntaxin 4 regulates evoked release. (A) Mean nerve-evoked amplitudes (± SEM, in mV) for the indicated genotypes in HL3 saline containing 0.3 mM Ca2+. (B) Representative traces of nerve-evoked responses for the indicated genotypes. (C) Mean frequency of spontaneous (mini) release (± SEM, in Hz) for the indicated genotypes. (D) Mean amplitude of mini release (± SEM, in mV) for the indicated genotypes. (E) Representative traces of spontaneous release events for control and Syx473 mutants.
We next recorded spontaneous vesicle release to assess mEJP amplitude and frequency. We did not detect any change in mEJP parameters in Syx4 mutants or animals overexpressing Syx4 (Figure 1C–E. mEJP frequency (Hz): Control, 2.62 ± 0.30 [n=17]; Syx473, 3.42 ± 0.32 [n=15], ns p=0.172; Syx473 24B-GAL4>UAS-Syx4, 2.57 ± 0.18 [n=11], ns p>0.999; Syx473 elav-GAL4>UAS-Syx4, 2.88 ± 0.15 [n=11], ns p=0.959; 24B-GAL4>UAS-Syx4, 2.70 ± 0.25 [n=10], ns p>0.999. mEJP amplitude (mV): Control, 0.77 ± 0.08 [n=17]; Syx473, 0.78 ± 0.06, [n=15], ns p>0.999; Syx473 24B-GAL4>UAS-Syx4, 0.79 ± 0.10 [n=11], ns p=0.883; Syx473 elav-GAL4>UAS-Syx4, 0.72 ± 0.04 [n=11], ns p=0.989; 24B-GAL4>UAS-Syx4, 0.79 ± 0.06 [n=10], ns p=0.925).
We also measured input resistance and resting membrane potential (RMP) across all genotypes. RMPs were between −50 mV and −70 mV and were not different between genotypes (Supplemental Table 1. RMP (in mV): Control, −56.92 ± 1.62 [n=17]; Syx473, −57.50 ± 1.69 [n=15], ns p=0.999; Syx473 24B-GAL4>UAS-Syx4, −57.42 ± 1.67 [n=11], ns p>0.999; Syx473 elav-GAL4>UAS-Syx4, −58.10 ± 2.29 [n=11], ns p=0.991 and 24B-GAL4>UAS-Syx4, −56.70 ± 1.80 [n=10], ns p=0.999. Input resistances were between 5 MΩ and 10 MΩ and were not different between genotypes (Supplemental Table 1. Input resistance (in MΩ): Control, 6.48 ± 0.31 [n=17]; Syx473, 6.80 ± 0.29 [n=15], ns p=0.956; Syx473 24B-GAL4>UAS-Syx4, 6.73 ± 0.48 [n=11], ns p=0.986; Syx473 elav-GAL4>UAS-Syx4, 6.91 ± 0.43 [n=11], ns p=0.915 and 24B-GAL4>UAS-Syx4, 6.79 ± 0.38 [n=10], ns p=0.976.
The observations that 1) EJP amplitude is increased and 2) mini frequency is unchanged in Syx4 mutants are surprising because these animals exhibit a substantial decrease in both bouton number and active zone density, leading to an estimated 50% reduction in the number of active zones per NMJ (Harris et al., 2016). Given that glutamate receptor clusters are unaffected upon loss of Syx4 (Harris et al., 2016), and that mEJP amplitude is also unaffected (Figure 1D,E), we hypothesize that the observed increase in neurotransmission may arise from presynaptic changes leading to potentiation of active zones. To test this idea, we performed paired pulse facilitation (PPF) by measuring the ratio (P2/P1) between postsynaptic responses at interpulse intervals of 25, 50, 75, and 100 ms (Figure 2A,B). Our subsequent analysis was based only on the data from the 100 ms interpulse interval recordings, as this largest interval allowed the resting membrane potential to return to baseline between the first and second stimuli; this approach avoids differences in driving force of the second response since the genotypes tested exhibited significantly different EJP amplitudes. Control NMJs exhibited PPF as expected (P2/P1, 100 ms: 1.25 ± 0.04 [n=10]). In contrast, PPF was reduced in Syx4 mutants (P2/P1, 100 ms: 1.09 ± 0.01 [n=8], *p=0.030). These data are consistent with enhanced initial release from active zones in Syx4 mutants, such that further facilitation in response to a paired stimulus is inhibited. Expression of postsynaptic Syx4 in Syx4 mutant animals restored PPF and resulted in increased facilitation compared to controls (P2/P1, 100 ms, 1.51 ± 0.09 [n=8], **p<0.002). Similar to what was observed for the EJP amplitude phenotype, simply overexpressing Syx4 in the control genotype, using a postsynaptic-specific driver, did not alter PPF (P2/P1, 100 ms, 1.25 ± 0.03 [n=6], ns p>0.999), and presynaptic expression of Syx4 failed to rescue Syx4 mutants (P2/P1, 100 ms, 1.00 ± 0.01 [n=8], **p=0.002). Thus, Syx4 is required postsynaptically for normal PPF.
Figure 2.
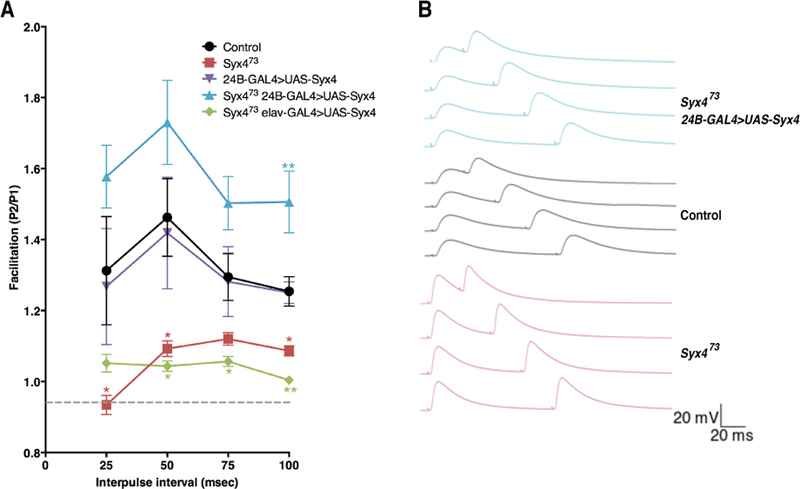
Syntaxin 4 regulates paired pulse facilitation. (A) Mean (± SEM) paired pulse ratio (the second response, P2, divided by the first response, P1) at interpulse intervals of 25, 50, 75, and 100 ms for the indicated genotypes. Recordings were performed in 0.2 mM Ca2+ in HL3.1 saline. (B) Representative traces of paired pulse facilitation. With a 100 ms interpulse interval, resting membrane potential returned to baseline before the second stimulus. Thus, the 100 ms data was used for subsequent analysis and interpretation of PPF values across genotypes.
Syx4 cooperates with both Syt4 and Nlg1 at the NMJ and together these proteins contribute to 1) synaptic morphology, as assayed by counting the number of synaptic boutons, and 2) synaptic plasticity, as assayed by measuring the ability to bud new (“ghost”) boutons in response to spaced incubations in high K+ (Harris et al., 2016). We therefore tested whether Syt4 or Nlg1 might interact with Syx4 in the context of the electrophysiology phenotypes described above, using the strategy of comparing double heterozygous combinations of null mutant alleles to single heterozygotes. We detected no significant differences in evoked amplitude or paired-pulse facilitation in Syx4/+ Syt4/+ animals or Syx4/+ Nlg1/+ animals compared to any of the single heterozygotes (Supplemental Figure 1). Both Syt4 and Nlg1 null mutants previously exhibited a decrease in evoked release at the larval NMJ (Banovic et al., 2010; Barber et al., 2009), in contrast to the increase in evoked release that occurs in Syx4 nulls. Thus, we have no compelling evidence that Syt4 or Nlg1 contributes to the synaptic facilitation observed in Syx4 mutants.
To determine the underlying mechanism of synaptic facilitation in Syx4 mutants, we measured two parameters that might affect neurotransmitter release: 1) the distribution of the presynaptic Ca2+ channel Cac, and 2) Ca2+ cooperativity of synaptic transmission. Cac encodes the pore-forming subunit of the major voltage-gated Ca2+ channel mediating neurotransmission in Drosophila (Kawasaki, Felling, & Ordway, 2000). Cac localizes to clusters associated with active zones (Kawasaki, Zou, Xu, & Ordway, 2004), and the level of Cac at individual active zones correlates with neurotransmitter release probability (Akbergenova, Zhang, Weiss-Sharabi, Cunningham, & Littleton, 2017; Gratz et al., 2018). We used a genomic cac construct labelled with GFP at the C terminus (Matkovic et al., 2013) to study the distribution of Cac in control and Syx4 mutant animals. This Cac-GFP construct is advantageous as its expression is controlled by native promoter elements (Matkovic et al., 2013); nevertheless, a caveat of this analysis is that we cannot rule out the possibility that Cac-GFP could compete with endogenous Cac for localization to active zones. We measured Cac-GFP intensity at individual active zones co-labelled with the cytomatrix protein Brp. It was previous shown that Brp size and intensity are unaffected upon loss of Syx4 (Harris et al., 2016), and we confirm that finding here (Figure 3E). Interestingly, we detected a modest increase in Cac-GFP intensity, relative to Brp fluorescence intensity, in Syx4 mutants compared to controls (Figure 3A,B,D. Control, 1.05 ± 0.05 [n=16]; Syx473, 1.33 ± 0.09 [n=16], *p=0.033). This effect is rescued by expression of Syx4 in the postsynaptic cell of Syx4 mutant animals, but not by presynaptic expression in Syx4 mutant animals, and overexpression of Syx4 in control animals has no effect (Figure 3C,D. Syx473 24B-GAL4>UAS-Syx4, 0.94 ± 0.05 [n=10], ns p=0.923; Syx473 elav-GAL4>UAS-Syx4, 1.32 ± 0.13 [n=13], *p=0.045; 24B-GAL4>UAS-Syx4, 0.93 ± 0.10 [n=12], ns p=0.864). These data indicate that Syx4 mutant active zones accumulate more Cac-GFP protein compared to controls. Assuming that Cac-GFP levels reflect an overall accumulation of Cac at active zones, this is one mechanism which could contribute to potentiation at Syx4 mutant synapses.
Figure 3.
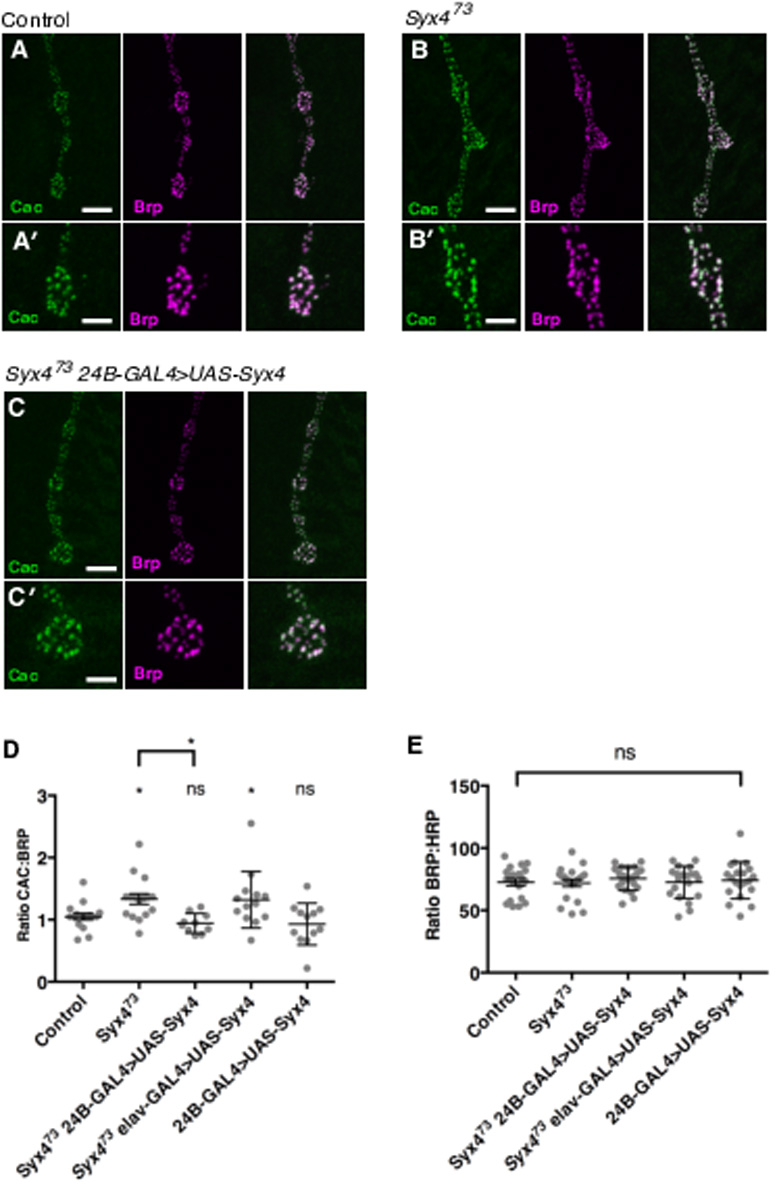
Syntaxin 4 regulates Cac levels at active zones (A–C) Representative images of Cac-GFP (green) and Brp (magenta) in control animals (A), Syx4 null mutants (B), and Syx4 null mutants expressing Syx4 in the postsynaptic cell (C). (A′–B′) Close-ups of A–C. Scale bars = 7 μm (A–C), 3.5 μm (A′–B′). (D) Mean Cac-GFP fluorescence intensity per Brp fluorescence intensity (± SEM) at individual active zones for the indicated genotypes. (E) Mean Brp fluorescence intensity per HRP fluorescence intensity (± SEM) for the indicated genotypes. Mean HRP fluorescence intensity is unchanged across the genotypes used in this study (see Supplemental Table 1).
We next tested for alterations in Ca2+ sensitivity and cooperativity by measuring the amplitude of nerve-evoked responses over a range of Ca2+ concentrations (Figure 4A,B, Supplemental Table 1). We observed a change in sensitivity to external Ca2+, indicated by a leftward shift in the EJP – Ca2+ and quantal content– Ca2+ curves (Figure 4A,B). We then estimated Ca2+ cooperativity by log-transforming the data and finding the slope of the best-fit linear regression line for non-saturating Ca2+ concentrations (Figure 4C). We measured a cooperativity coefficient of 2.64 ± 0.22 in control animals. In contrast, Syx4 mutants had a coefficient of 1.65 ± 0.20, reflecting a statistically significant reduction in the Ca2+ cooperativity of release (*p=0.039). This effect was rescued by postsynaptic (2.42 ± 0.26, ns p=0.9690), but not presynaptic (1.67 ± 0.24, *p=0.045), expression of Syx4 in Syx4 mutant animals. Overexpression of Syx4 in the postsynaptic cell in a control background did not affect Ca2+ cooperativity (2.58 ± 0.27, ns p>0.999). This increase in sensitivity to Ca2+ and decrease in Ca2+ cooperativity in Syx4 mutants is a second mechanism that may contribute to synaptic potentiation.
Figure 4.
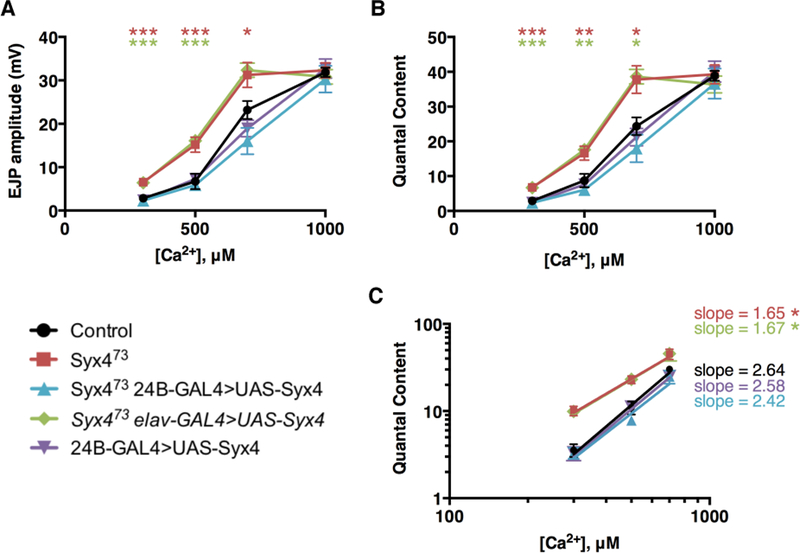
Syntaxin 4 regulates the Ca2+ cooperativity of neurotransmitter release. (A) Mean EJP amplitude (in mV, ± SEM) at various concentrations of Ca2+ (μM) for the indicated genotypes. (B) Mean corrected quantal content at various concentrations of Ca2+ (μM) for the indicated genotypes. (C) Log-log plot of mean corrected quantal content versus Ca2+ concentration. Slopes were determined from a linear regression of log-transformed data.
Discussion
Syntaxin 4 regulates multiple aspects of synaptic biology. Here we report a that loss of Syx4 leads to synaptic enhancement, a surprising finding give that Syx4 mutant synapses have significantly fewer active zones than control animals. We observed an increase in evoked release, and a reduction in paired-pulse facilitation, in Syx4 mutants. We also identified two mechanisms that are likely to contribute to the increase in neurotransmission: an increase in the levels of the presynaptic Ca2+ channel Cac at individual active zones, and a decrease in Ca2+ cooperativity. These two potentiation mechanisms could be linked – for example, an increase in Cac channels, leading to changes in Ca2+ influx and the spatial arrangement of the channels, could contribute to changes in the sensitivity of the exocytotic machinery to Ca2+. However, we cannot rule out the possibility that they are distinct phenomena. As all of these phenotypes are rescued by postsynaptic, but not presynaptic, expression of Syx4, our data indicate a retrograde signaling mechanism by which Syx4 regulates active zones.
Cac clustering at active zones is regulated by components of the active zone cytomatrix, including Brp (Kittel et al., 2006), RIM (Graf et al., 2012), RIM-binding protein (Liu et al., 2011), Fife (Bruckner et al., 2017), and Unc13 (Böhme et al., 2016). Of these, Brp has the largest effect, with an approximate 50% reduction in Cac levels at active zones of brp null mutants. Although Syx4 mutants have no obvious defects in the size or intensity of Brp clusters (Harris et al., 2016), we have not yet examined the distribution other cytomatrix proteins. One mechanism for Cac regulation downstream of Syx4 signaling could be through changes in the levels of other active zone cytomatrix components.
One possible explanation for the potentiation we observe in Syx4 mutants is that it is the result of homeostatic compensation. Many studies have described homeostatic mechanisms of potentiation and depression at the fly NMJ. In presynaptic homeostatic potentiation (PHP), perturbations that inhibit the function of postsynaptic glutamate receptors by acute pharmacological blockade (Frank, Kennedy, Goold, Marek, & Davis, 2006) or genetic loss (Petersen, Fetter, Noordermeer, Goodman, & DiAntonio, 1997) are offset by compensatory increases in neurotransmitter release. These presynaptic changes include increases in the size and intensity of Brp clusters, increases in Ca2+ influx or increases in the readily releasable vesicle pool (Goel, Li, & Dickman, 2017; Kiragasi, Wondolowski, Li, & Dickman, 2017; Müller & Davis, 2012; Weyhersmuller et al., 2011). Moderate Cac increases have also been observed in conjunction with increases in Brp during PHP (Tsurudome et al., 2010), though most studies have not reported Cac levels. Presynaptic homeostatic depression (PHD) is a distinct phenomenon in which overexpression of the vesicular glutamate transporter, resulting in more glutamate packaged per synaptic vesicle, is offset by compensatory decreases in neurotransmitter release. PHD has been shown to involve a decrease in presynaptic Ca2+ influx and a decrease in Cac levels at active zones (Gaviño, Ford, Archila, & Davis, 2015). Thus, the synapse employs multiple mechanisms during homeostatic plasticity, including regulation of Cac channels and Ca2+ influx.
An important distinction is that during homeostatic compensation the compensatory changes typically restore muscle depolarization precisely, whereas in Syx4 mutants we see a significant enhancement of neurotransmission, well beyond control levels. Nevertheless, it may be interesting to investigate whether any of the known homeostatic pathways are required for potentiation in Syx4 mutants. It is also possible that Syx4 itself is engaged in homeostatic mechanisms. For example, if Syx4 is involved in downregulating Cac channels, it could potentially participate in the PHD mechanism described by Gaviño et al (2015), which would therefore be impaired in Syx4 mutant animals. Syx4 could also interact with other retrograde pathways that affect presynaptic release probability, such as signaling through the importin Imp13, which functions postsynaptically to regulate release probability and presynaptic intracellular Ca2+ (Giagtzoglou, Lin, Haueter, & Bellen, 2009).
One intriguing observation from our study is that paired-pulse facilitation is enhanced, compared to controls, when Syx4 is expressed postsynaptically in Syx4 mutants. This is surprising since simple overexpression of Syx4 in a wildtype animal does not affect facilitation. While we do not yet understand how this enhanced facilitation arises, one possibility could be differential expression of Syx4 isoforms. All of the overexpression and rescue experiments described in this study were conducted using a full-length Syx4 cDNA (Syx4A). However, there is a second isoform of Syx4 (Syx4B), encoding a shorter N-terminus, which is redundant with the A isoform with respect to all other phenotypes characterized for Syx4 to date (Harris et al., 2016 and data not shown). It is possible that paired-pulse facilitation is particularly sensitive to the ratio of Syx4 isoforms, leading to differences in phenotype in rescued animals (where Syx4A is expressed in the null mutant background) compared to overexpression animals (where Syx4A is expressed in control background and both endogenous isoforms are present). It will be interesting to investigate this and other possible mechanisms in the future.
How Syx4-dependent signaling from the muscle leads to presynaptic changes at active zones remains an interesting open question. Syx4 regulates the membrane localization of two postsynaptic cargo molecules, Syt4 and Neuroligin 1, which cooperate to modulate the size and plasticity of the NMJ (Harris et al., 2016). However, we found no evidence that Syt4 or Nlg participate with Syx4 to regulate the Syx4 presynaptic enhancement phenotypes. Thus, it is likely that Syx4, as a postsynaptic t-SNARE, mediates the release of additional retrograde signals, and that multiple overlapping Syx4-dependent pathways are involved in establishing normal synaptic morphology, plasticity, and function.
Conclusions
We described electrophysiological analysis of animals lacking the postsynaptic t-SNARE Syx4. Syx4 mutants exhibit synaptic enhancement accompanied by presynaptic changes at active zones, including an increase in presynaptic Ca2+ channels at active zones and a decrease in Ca2+ cooperativity. All of these features are rescued by restoring postsynaptic Syx4. We conclude that retrograde pathways regulated by Syx4 inhibit active zone potentiation, and that Syx4 modulates multiple postsynaptic signaling pathways with overlapping function.
Supplementary Material
Acknowledgements
This work was supported by NSERC Discovery Grant 250078 to B.A.S. and NIH grant NS40296 to J.T.L. We thank S. Sigrist and H. Aberle for sharing reagents. We thank the Bloomington Drosophila Stock Center (NIH P40OD018537), the Drosophila Genome Resource Center (NIH 2P40OD010949–10A1), and the Developmental Studies Hybridoma Bank for providing materials used in this study.
Footnotes
The authors report no conflict of interest.
References
- Adolfsen B, Saraswati S, Yoshihara M, & Littleton JT (2004). Synaptotagmins are trafficked to distinct subcellular domains including the postsynaptic compartment. The Journal of Cell Biology, 166(2), 249–60. 10.1083/jcb.200312054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbergenova Y, Zhang YV, Weiss-Sharabi S, Cunningham KL, & Littleton JT (2017). Examining molecular determinants underlying heterogeneity of synaptic release probability using optical quantal imaging. BioRxiv, 240549. 10.1101/240549 [DOI] [Google Scholar]
- Banerjee S, Venkatesan A, & Bhat MA (2017). Neurexin, Neuroligin and Wishful Thinking coordinate synaptic cytoarchitecture and growth at neuromuscular junctions. Molecular and Cellular Neuroscience, 78, 9–24. 10.1016/J.MCN.2016.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banovic D, Khorramshahi O, Owald D, Wichmann C, Riedt T, Fouquet W, … Aberle H (2010). Drosophila neuroligin 1 promotes growth and postsynaptic differentiation at glutamatergic neuromuscular junctions. Neuron, 66(5), 724–38. 10.1016/j.neuron.2010.05.020 [DOI] [PubMed] [Google Scholar]
- Barber CF, Jorquera RA, Melom JE, & Littleton JT (2009). Postsynaptic regulation of synaptic plasticity by synaptotagmin 4 requires both C2 domains. The Journal of Cell Biology, 187(2), 295–310. 10.1083/jcb.200903098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhme MA, Beis C, Reddy-Alla S, Reynolds E, Mampell MM, Grasskamp AT, … Sigrist SJ (2016). Active zone scaffolds differentially accumulate Unc13 isoforms to tune Ca2+ channel–vesicle coupling. Nature Neuroscience, 19(10), 1311–1320. 10.1038/nn.4364 [DOI] [PubMed] [Google Scholar]
- Brand A, & Perrimon N (1993). Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development, 118(2), 401–415. Retrieved from http://dev.biologists.org/content/118/2/401.long [DOI] [PubMed] [Google Scholar]
- Bruckner JJ, Zhan H, Gratz SJ, Rao M, Ukken F, Zilberg G, & O’Connor-Giles KM (2017). Fife organizes synaptic vesicles and calcium channels for high-probability neurotransmitter release. The Journal of Cell Biology, 216(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chater TE, & Goda Y (2014). The role of AMPA receptors in postsynaptic mechanisms of synaptic plasticity. Frontiers in Cellular Neuroscience, 8, 401 10.3389/fncel.2014.00401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choquet D, & Triller A (2013). The Dynamic Synapse. Neuron, 80(3), 691–703. 10.1016/j.neuron.2013.10.013 [DOI] [PubMed] [Google Scholar]
- Feng Y, Ueda A, & Wu C-FF (2004). A modified minimal hemolymph-like solution, HL3.1, for physiological recordings at the neuromuscular junctions of normal and mutant Drosophila larvae. Journal of Neurogenetics, 18(2), 377–402. 10.1080/01677060490894522 [DOI] [PubMed] [Google Scholar]
- Frank CA, Kennedy MJ, Goold CP, Marek KW, & Davis GW (2006). Mechanisms underlying the rapid induction and sustained expression of synaptic homeostasis. Neuron, 52(4), 663–77. 10.1016/j.neuron.2006.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu AK, & Ip NY (2017). Regulation of postsynaptic signaling in structural synaptic plasticity. Current Opinion in Neurobiology, 45, 148–155. 10.1016/J.CONB.2017.05.016 [DOI] [PubMed] [Google Scholar]
- Gaviño MA, Ford KJ, Archila S, & Davis GW (2015). Homeostatic synaptic depression is achieved through a regulated decrease in presynaptic calcium channel abundance. ELife, 4 10.7554/eLife.05473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giagtzoglou N, Lin YQ, Haueter C, & Bellen HJ (2009). Importin 13 regulates neurotransmitter release at the Drosophila neuromuscular junction. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 29(17), 5628–39. 10.1523/JNEUROSCI.0794-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel P, Li X, & Dickman D (2017). Disparate Postsynaptic Induction Mechanisms Ultimately Converge to Drive the Retrograde Enhancement of Presynaptic Efficacy. Cell Reports, 21(9), 2339–2347. 10.1016/j.celrep.2017.10.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf ER, Valakh V, Wright CM, Wu C, Liu Z, Zhang YQ, & DiAntonio A (2012). RIM promotes calcium channel accumulation at active zones of the Drosophila neuromuscular junction. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 32(47), 16586–96. 10.1523/JNEUROSCI.0965-12. 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz SJ, Bruckner JJ, Hernandez RX, Khateeb K, Macleod G, & O’Connor-Giles KM (2018). Calcium channel levels at single synapses predict release probability and are upregulated in homeostatic potentiation. BioRxiv, 240051. 10.1101/240051 [DOI] [Google Scholar]
- Harris KP, & Littleton JT (2015). Transmission, Development, and Plasticity of Synapses. Genetics, 201(2), 345–75. 10.1534/genetics.115.176529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KP, Zhang YV, Piccioli ZD, Perrimon N, & Littleton JT (2016). The postsynaptic t-SNARE Syntaxin 4 controls traffic of Neuroligin 1 and Synaptotagmin 4 to regulate retrograde signaling. ELife, 5 10.7554/eLife.13881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki F, Felling R, & Ordway RW (2000). A temperature-sensitive paralytic mutant defines a primary synaptic calcium channel in Drosophila. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 20(13), 4885–9. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10864946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki F, Zou B, Xu X, & Ordway RW (2004). Active zone localization of presynaptic calcium channels encoded by the cacophony locus of Drosophila. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 24(1), 282–5. 10.1523/JNEUROSCI.3553-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiragasi B, Wondolowski J, Li Y, & Dickman DK (2017). A Presynaptic Glutamate Receptor Subunit Confers Robustness to Neurotransmission and Homeostatic Potentiation. Cell Reports, 19(13), 2694–2706. 10.1016/j.celrep.2017.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittel RJ, & Heckmann M (2016). Synaptic Vesicle Proteins and Active Zone Plasticity. Frontiers in Synaptic Neuroscience, 8, 8 10.3389/fnsyn.2016.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittel RJ, Wichmann C, Rasse TM, Fouquet W, Schmidt M, Schmid A, … Sigrist SJ (2006). Bruchpilot promotes active zone assembly, Ca2+ channel clustering, and vesicle release. Science (New York, N.Y.), 312(5776), 1051–4. 10.1126/science.1126308 [DOI] [PubMed] [Google Scholar]
- Korkut C, Li Y, Koles K, Brewer C, Ashley J, Yoshihara M, & Budnik V (2013). Regulation of postsynaptic retrograde signaling by presynaptic exosome release. Neuron, 77(6), 1039–46. 10.1016/j.neuron.2013.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarevic V, Pothula S, Andres-Alonso M, & Fejtova A (2013). Molecular mechanisms driving homeostatic plasticity of neurotransmitter release. Frontiers in Cellular Neuroscience, 7, 244 10.3389/fncel.2013.00244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KSY, Siebert M, Mertel S, Knoche E, Wegener S, Wichmann C, … Sigrist SJ (2011). RIM-binding protein, a central part of the active zone, is essential for neurotransmitter release. Science (New York, N.Y.), 334(6062), 1565–9. 10.1126/science.1212991 [DOI] [PubMed] [Google Scholar]
- Luo L, Liao YJ, Jan LY, & Jan YN (1994). Distinct morphogenetic functions of similar small GTPases: Drosophila Drac1 is involved in axonal outgrowth and myoblast fusion. Genes & Development, 8(15), 1787–802. 10.1101/gad.8.15.1787 [DOI] [PubMed] [Google Scholar]
- Matkovic T, Siebert M, Knoche E, Depner H, Mertel S, Owald D, … Sigrist SJ (2013). The Bruchpilot cytomatrix determines the size of the readily releasable pool of synaptic vesicles. The Journal of Cell Biology, 202(4), 667–83. 10.1083/jcb.201301072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan EM, & Martin AR (1981). Non‐linear summation of end‐plate potentials in the frog and mouse. The Journal of Physiology, 311(1), 307–324. 10.1113/jphysiol.1981.sp013586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon KP, Carrillo RA, & Zinn K (2013). Development and plasticity of the Drosophila larval neuromuscular junction. Wiley Interdisciplinary Reviews. Developmental Biology, 2(5), 647–70. 10.1002/wdev.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosca TJ, Hong W, Dani VS, Favaloro V, & Luo L (2012). Trans-synaptic Teneurin signalling in neuromuscular synapse organization and target choice. Nature, 484(7393), 237–41. 10.1038/nature10923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozer BA, & Sandstrom DJ (2012). Drosophila neuroligin 1 regulates synaptic growth and function in response to activity and phosphoinositide-3-kinase. Molecular and Cellular Neurosciences, 51(3–4), 89–100. 10.1016/j.mcn.2012.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M, & Davis GW (2012). Transsynaptic control of presynaptic Ca2+ influx achieves homeostatic potentiation of neurotransmitter release. Current Biology 10.1016/j.cub.2012.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owald D, Khorramshahi O, Gupta VK, Banovic D, Depner H, Fouquet W, … Sigrist SJ (2012). Cooperation of Syd-1 with Neurexin synchronizes pre- with postsynaptic assembly. Nature Neuroscience, advance on(9), 1219–26. 10.1038/nn.3183 [DOI] [PubMed] [Google Scholar]
- Petersen SA, Fetter RD, Noordermeer JN, Goodman CS, & DiAntonio A (1997). Genetic analysis of glutamate receptors in Drosophila reveals a retrograde signal regulating presynaptic transmitter release. Neuron, 19(6), 1237–1248. 10.1016/S0896-6273(00)80415-8 [DOI] [PubMed] [Google Scholar]
- Piccioli ZD, & Littleton JT (2014). Retrograde BMP signaling modulates rapid activity-dependent synaptic growth via presynaptic LIM kinase regulation of cofilin. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 34(12), 4371–81. 10.1523/JNEUROSCI.4943-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, … Cardona A (2012). Fiji: an open-source platform for biological-image analysis. Nature Methods, 9(7), 676–82. 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart BA, Atwood HL, Renger JJ, Wang J, & Wu CF (1994). Improved stability of Drosophila larval neuromuscular preparations in haemolymph-like physiological solutions. Journal of Comparative Physiology. A, Sensory, Neural, and Behavioral Physiology, 175(2), 179–91. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8071894 [DOI] [PubMed] [Google Scholar]
- Tsurudome K, Tsang K, Liao EH, Ball R, Penney J, Yang J-S, … Haghighi AP (2010). The Drosophila miR-310 cluster negatively regulates synaptic strength at the neuromuscular junction. Neuron, 68(5), 879–93. 10.1016/j.neuron.2010.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagh DA, Rasse TM, Asan E, Hofbauer A, Schwenkert I, Dürrbeck H, … Buchner E (2006). Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron, 49(6), 833–44. 10.1016/j.neuron.2006.02.008 [DOI] [PubMed] [Google Scholar]
- Weyhersmuller A, Hallermann S, Wagner N, Eilers J, Weyhersmüller A, Hallermann S, … Eilers J (2011). Rapid active zone remodeling during synaptic plasticity. Journal of Neuroscience, 31(16), 6041–6052. 10.1523/JNEUROSCI.6698-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, & Yuan Q (2015). Structural homeostasis in the nervous system: a balancing act for wiring plasticity and stability. Frontiers in Cellular Neuroscience, 8, 439 10.3389/fncel.2014.00439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara M, Adolfsen B, Galle KT, & Littleton JT (2005). Retrograde signaling by Syt 4 induces presynaptic release and synapse-specific growth. Science (New York, N.Y.), 310(5749), 858–63. 10.1126/science.1117541 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


