Abstract
To screen the tens of thousands of chemicals for which no toxicity data currently exists, it is necessary to move from in vivo rodent models to alternative models, such as zebrafish. Here, we used dechorionated Tropical 5D wild-type zebrafish embryos to screen a 91-compound library provided by the National Toxicology Program (NTP) for developmental toxicity. This library contained 86 unique chemicals that included negative controls, flame retardants, polycyclic aromatic hydrocarbons (PAHs), drugs, industrial chemicals, and pesticides. Fish were exposed to 5 concentrations of each chemical or an equal amount of vehicle (0.5% DMSO) in embryo medium from 6 h post-fertilization (hpf) to 5 days post-fertilization (dpf). Fish were examined daily for mortality and teratogenic effects and photomotor behavior was assessed at 4 and 5 dpf. Of the 5 negative control compounds in the library, none caused mortality/teratogenesis, but two altered behavior. Chemicals provided in duplicate produced similar outcomes. Overall, 13 compounds caused mortality/teratology but not behavioral abnormalities, 24 only affected behavior, and 18 altered both endpoints, with behavior affected at concentrations that did not cause mortality/teratology (55/86 hits). Of the compounds that affected behavior, 52% caused behavioral abnormalities at either 4 or 5 dpf. Compounds within the same functional group caused different behavioral abnormalities, while similar behavioral patterns were caused by compounds from different groups. Our data suggest that behavior is a sensitive endpoint for developmental toxicity screening that integrates multiple modes of toxic action and is influenced by the age of the larval fish at the time of testing.
Keywords: development, photomotor behavior, toxicity screen, teratology, Tox21, zebrafish larvae
This article is published as part of the NTP Neurotoxicology Screening Strategies Initiative.
There is a well-recognized need to move from time- and cost-intensive toxicity testing in rodent models to alternative models amenable to high(er) throughput screening in order to test the tens of thousands of chemicals for which there currently exists no or limited toxicity data (Collins et al., 2008). Since inception of the “Toxicology Testing in the 21st Century”, or “Tox21” program (www.tox21.gov) almost a decade ago, thousands of chemicals have been tested using high throughput in vitro screens based on known molecular modes of toxic action, with the goal of prioritizing chemicals for further targeted testing. However, interpreting these data in the context of risk to human health has been challenging, in part, because these in vitro systems do not recapitulate many of the complex cell-cell and cell-matrix interactions or systemic influences that significantly influence toxic outcomes in the intact organism (Lein et al., 2005). This is particularly problematic in the context of developmental neurotoxicity, which has not been well represented in the Tox21 screening platforms. In vitro models of the developing nervous system miss not only significant interactions, but also key differences, between cell types, brain regions, and developmental stages that influence developmental neurotoxicity (Lein et al., 2005).
Simple systems-based models, such as the nematode, planarian flatworm, fruit fly, and zebrafish, have emerged as alternative models that enable medium-to-high throughput assessment while retaining the biological complexity needed to anchor molecular and cellular effects to perturbations of structure, function, and behavior (Avila et al., 2012; Lein et al., 2005; Rand, 2010; Truong et al., 2014). These organisms are easy to breed, have a short developmental time, are relatively inexpensive to maintain, and are genetically tractable. Key to their use in developmental neurotoxicity testing, the fundamental processes of neurodevelopment in these simpler model organisms are homologous to those in humans, and they express orthologues of human genes known to be important in neurodevelopmental disorders (Gilbert and Barresi, 2016). Although each model has its unique advantages, the zebrafish (Danio rerio) exhibits a number of characteristics that have contributed to its emergence as a powerful model for chemical screening, and in particular for developmental neurotoxicity testing (Bailey et al., 2013; Garcia et al., 2016; Truong et al., 2014). These characteristics include: (1) zebrafish larvae develop externally and are transparent, which facilitates morphological assessments of developing internal structures in living animals (Kimmel et al., 1995; Truong et al., 2014); (2) gene regulation in zebrafish is similar to that of humans, and there is significant physiological and genetic homology between the zebrafish and human genome (Howe et al., 2013); (3) the overall organization of the fish brain is highly homologous to the human brain (Wullimann and Mueller, 2004); (4) anatomic substrates of cognitive behavior are conserved between fish and other vertebrates (Rodriguez et al., 2002a,b); and (5) zebrafish express neurochemical phenotypes similar to those of humans (Miller et al., 2018).
As part of a larger initiative sponsored by the National Institute of Environmental Health Sciences (NIEHS) to evaluate concordance of screening data across diverse alternative models of developmental neurotoxicity, we used larval zebrafish to screen a 91-compound library provided by the National Toxicology Program (NTP). This library consisted of known and suspected developmental neurotoxicants that were subdivided into 6 chemical sets: pesticides, drugs, flame retardants, polycyclic aromatic hydrocarbons (PAHs), industrial chemicals, and negative controls. Dechorionated zebrafish were statically exposed to these compounds beginning 6 h post-fertilization (hpf) through 5 days post-fertilization (dpf), and assessed for mortality, teratogenic outcomes, and photomotor behavior. Every fish was examined for mortality and teratology every 24 h throughout the exposure period, and tested in the photomotor assay at both 4 and 5 dpf. The latter is in contrast to the more common practice of assessing photomotor activity at either 4 dpf (Jin et al., 2015; Liu et al., 2018; Zhang et al., 2017) or 5 dpf (Chen et al., 2012; Noyes et al., 2015; Olivares et al., 2016; Sun et al., 2016; Truong et al., 2014). The results from these studies suggest that behavior is a sensitive endpoint for developmental toxicity screening that likely reflects multiple modes of toxic action and is influenced by the age of the larval fish at the time of testing.
MATERIALS AND METHODS
Zebrafish husbandry and spawning
Fish husbandry, spawning, and all experiments using fish were performed in accordance with protocols approved by the University of California-Davis Institutional Animal Care and Use Committee (IACUC). Tropical 5D wild-type adult zebrafish were obtained from the Sinnhuber Aquatic Research Laboratory (SARL) at Oregon State University (Corvallis, Oregon) and subsequent generations were raised at UC Davis. Adult zebrafish were maintained under standard laboratory conditions under a 14 h light (∼850 lux): 10 h dark cycle (Harper and Lawrence, 2011) in a standalone aquatic flow-through system (Aquaneering, San Diego, California) at a density of approximately 8 fish per liter. Water used in the system (referred to as “system fish water”) was deionized water that was further purified by reverse osmosis (Reverse Osmosis System Model AAA-1005, Applied Membranes Inc., California). System fish water was maintained at 28.5°C ± 0.5°C, and supplemented with 20 g/l NaHCO3 to maintain a pH of 7.5 ± 0.3 and 40 g/l sea salt solution (Instant Ocean) to maintain conductivity at 700 ± 100 µS. Adult fish were fed twice daily with commercially available GEMMA Micro 500 (Skretting, Salt Lake City, Utah).
Adult zebrafish were set up for sex-separated spawning in groups of 5–7 fish per spawning tank (Aquaneering, San Diego, California) overnight in system fish water. Gates were pulled in the morning directly after the lights turned on. After 30–45 min, the embryos were collected in petri dishes with fish water. At the 2–4 cell stage (age-matched), embryos were randomized to insure genetic diversity and transferred to new petri dishes. Embryos were then kept in an incubator at 28.5°C until enzymatic removal of their chorion at 4 hpf.
Compounds
The 91-compound library screened in this study was provided by the NIEHS NTP (Research Triangle Park, North Carolina). Table 1 lists the compounds along with their respective identification numbers (ID), substance class, suppliers, purity, and exposure concentrations. Two compounds were provided in duplicate (4 out of 91), each with a unique ID, and one compound was eliminated from the library because of misclassification by NIEHS staff. Thus, the library contained 86 unique compounds. Compounds were provided in clear plastic vials as stocks at 20 mM or lower in 100% dimethyl sulfoxide (DMSO). Upon receipt in the laboratory, working stock solutions were prepared in 100% DMSO at 200 times the highest concentration used for testing and stored in amber glass vials at 4°C. Immediately prior to exposure, stocks were diluted in embryo medium (Westerfield and ZFIN, 2000) to 2× the final concentration via serial dilution in 15 ml Falcon conical tubes (Fisher Scientific, Hampton, New Hampshire). Most of the compounds were tested at 0.3, 1, 3, 10, and 30 µM. If compounds were provided at concentrations <20 mM due to solubility issues, the upper end of the concentration range tested was decreased, but the same dilution scheme was used. Four chemicals—6-hydroxydopamine hydrochloride, benzo(a)pyrene, benzo(b)fluoranthene, and benzo(e)pyrene)—precipitated out of solution by 1 dpf in wells with the highest concentration; however, the exposures were not terminated because the chemical appeared to remain in solution at concentrations <30 µM. Because some compounds were not tolerated by the fish at the concentrations initially tested, experiments were repeated using lower concentrations.
Table 1.
Screened Compound Library
| Category | Compound | ID | Supplier | Purity (%) | Concentrations Tested (µM) |
|---|---|---|---|---|---|
| Drug | 1-Methyl-4-phenylpyridinium iodide | A2 | Sigma-Aldrich | 99.8 | 0.3, 1, 3, 10, 30 |
| 5-Fluorouracil | B1 | Sigma-Aldrich | 99 | 0.3, 1, 3, 10, 30 | |
| 6-Hydroxydopamine hydrochloride | B2 | Sigma-Aldrich | 100.9 | 0.3, 1, 3, 10, 30 | |
| 6-Propyl-2-thiouracil | B3 | Sigma-Aldrich | 100 | 0.3, 1, 3, 10, 30 | |
| Amoxicillin | B11 | Sigma-Aldrich | ∼85.2a | 0.3, 1, 3, 10, 30 | |
| Berberine chloride | C8 | Sigma-Aldrich | ∼84.3a | 0.3, 1, 3, 10, 30 | |
| Colchicine | D8 | Sigma-Aldrich | 97 | 0.3, 1, 3, 10, 30 | |
| Diazepam | D12 | Spectrum Chemical Mfg. Corp. | 99.3 | 0.3, 1, 3, 10, 30 | |
| Diethylstilbestrol (DES) | E5 | Sigma-Aldrich | 99.6 | 0.3, 1, 3, 10, 30 | |
| Estradiol | E6 | Spectrum Chemical Mfg. Corp. | ∼95.6a | 0.3, 1, 3, 10, 30 | |
| Hexachlorophene | E10 | Sigma-Aldrich | 99.9 | 0.03, 0.1, 0.3, 1, 3 | |
| Hydroxyurea | E11 | Sigma-Aldrich | 100 | 0.3, 1, 3, 10, 30 | |
| Phenobarbital | F11 | Spectrum Chemical Mfg. Corp. | 100 | 0.3, 1, 3, 10, 30 | |
| Phenobarbital sodium salt | F12 | Ganes Chemicals Inc. | ∼95.9a | 0.3, 1, 3, 10, 30 | |
| Tetraethylthiuram disulfide | G7 | Sigma-Aldrich | 99.7 | 0.003, 0.01, 0.03, 0.1, 0.3 | |
| Thalidomide | G8 | Sigma-Aldrich | 100 | 0.3, 1, 3, 10, 30 | |
| Valinomycin | H1 | Cayman Chemical Company | 100.00 | 0.003, 0.01, 0.03, 0.1, 0.3 | |
| Valproic acid sodium salt | H2 | Sigma-Aldrich | 100 | 0.3, 1, 3, 10, 30 | |
| Flame retardant | 2-Ethylhexyl diphenyl phosphate (EHDP) | A3 | TCI America | 92.8 | 0.3, 1, 3, 10, 30 |
| 2,2',4,4',5,5'-Hexabromodiphenyl ether (BDE-153) | A7 | Battelle Memorial Institute (via Cerilliant Corp.) | Unknown | 0.1, 0.3, 1, 3, 10 | |
| 2,2',4,4',5-Pentabromodiphenyl ether (BDE-99) | A6 | Cerilliant Corp. | 96 | 0.3, 1, 3, 10, 30 | |
| 2,2',4,4'-Tetrabromodiphenyl ether (BDE-47) | A8 | Cerilliant Corp. | 98 | 0.3, 1, 3, 10, 30 | |
| 2-Ethylhexyl-2,3,4,5-tetrabromobenzoate (TBB) | A4 | Toronto Research Chemicals Inc. | 95 | 0.3, 1, 3, 10, 30 | |
| 3,3′,5,5′-Tetrabromobisphenol A (TBBPA) | A11 | Albemarle Corporation via MRIGlobal | Unknown | 0.03, 0.1, 0.3, 1, 3 | |
| Bis(2-ethylhexyl) 3,4,5,6-tetrabromophthalate (TBPH) | C9 | Toronto Research Chemicals Inc. | 97 | 0.3, 1, 3, 10, 30 | |
| Firemaster 550 | E7 | Chemtura Corporation | Not listed | 0.3, 1, 3, 10, 30 | |
| Isodecyl diphenyl phosphate | E12 | Bayville Chemical Supply Company, Inc. | ≥95 | 0.3, 1, 3, 10, 30 | |
| Phenol, isopropylated, phosphate (3:1) (IPPP) | G1 | Amfinecom Inc. | Not listed | 0.3, 1, 3, 10, 30 | |
| tert-Butylphenyl diphenyl phosphate | G6 | MRIGlobal via Ubichem PLC | Unknown | 0.3, 1, 3, 10, 30 | |
| Tricresyl phosphate (TCP) | G10 | Sigma-Aldrich | 98.6 | 0.3, 1, 3, 10, 30 | |
| Triphenyl phosphate (TPhP) | G11, H7 | Sigma-Aldrich | 99.9 | 0.3, 1, 3, 10, 30 | |
| Tris(2-chloroethyl) phosphate (TCEP) | G12 | Sigma-Aldrich | 98.8 | 0.3, 1, 3, 10, 30 | |
| tris(Chloropropyl) phosphate (TCPP) | H3 | Albemarle Corporation via MRIGlobal | Unknown | 0.3, 1, 3, 10, 30 | |
| Industrial | 1-Ethyl-3-methylimidazolium diethylphosphate | A1 | Sigma-Aldrich | 99.5 | 0.3, 1, 3, 10, 30 |
| 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) | A9 | MRIGlobal | ∼98 | 0.000001, 0.000003, 0.00001, 0.00003, 0.0001 | |
| 2-Methoxyethanol | A5 | Sigma-Aldrich | 99.94 | 0.3, 1, 3, 10, 30 | |
| 3,3'-Iminodipropionitrile (IDPN) | A10 | TCI America | 99.6 | 0.3, 1, 3, 10, 30 | |
| Acetic acid, manganese (2+) salt | B7 | Sigma-Aldrich | ∼101.7b | 0.3, 1, 3, 10, 30 | |
| Acrylamide | B9 | Sigma-Aldrich | 99.6 | 0.3, 1, 3, 10, 30 | |
| Auramine O | C1 | Sigma-Aldrich | ∼92.5a | 0.3, 1, 3, 10, 30 | |
| Bisphenol A (BPA) | C11 | Sigma-Aldrich | 99.7 | 0.3, 1, 3, 10, 30 | |
| Bisphenol AF (BPAF) | C12 | Sigma-Aldrich | 99.9 | 0.3, 1, 3, 10, 30 | |
| Bisphenol S (BPS) | D1 | Sigma-Aldrich | 99.9 | 0.3, 1, 3, 10, 30 | |
| Di(2-ethylhexyl) phthalate (DEHP) | D11 | NTP | Unknown | 0.3, 1, 3, 10, 30 | |
| Lead (II) acetate trihydrate | F2 | Pfaltz & Bauer, Inc. | Not Listed | 0.3, 1, 3, 10, 30 | |
| Manganese, tricarbonyl[(1,2,3,4,5-.eta.)-1-methyl-2, 4-cyclopentadien-1-yl] | F4 | Alfa Aesar | ∼96.9b | 0.3, 1, 3, 10, 30 | |
| n-Hexane | F6 | Sigma-Aldrich | 99.3 | 0.3, 1, 3, 10, 30 | |
| Toluene | G9 | Sigma-Aldrich | 99.94 | 0.3, 1, 3, 10, 30 | |
| Polycyclic aromatic hydrocarbon | 4-H-Cyclopenta(d,e,f)phenanthrene | A12 | Sigma-Aldrich | 99.3 | 0.3, 1, 3, 10, 30 |
| Acenaphthene | B4 | Sigma-Aldrich | 99.9 | 0.3, 1, 3, 10, 30 | |
| Acenaphthylene | B5 | Sigma-Aldrich | 99.7 | 0.3, 1, 3, 10, 30 | |
| Anthracene | B12 | Sigma-Aldrich | 99.1 | 0.3, 1, 3, 10, 30 | |
| Benz(a)anthracene | C2 | Sigma-Aldrich | 99.2 | 0.3, 1, 3, 10, 30 | |
| Benzo(a)pyrene | C3 | Sigma-Aldrich | 97 | 0.3, 1, 3, 10, 30 | |
| Benzo(b)fluoranthene | C4 | Sigma-Aldrich | 98.7 | 0.3, 1, 3, 10, 30 | |
| Benzo(e)pyrene | C5 | Sigma-Aldrich | 99.7 | 0.3, 1, 3, 10, 30 | |
| Benzo(k)fluoranthene | C6 | Sigma-Aldrich | 100.0 | 0.3, 1, 3, 10, 30 | |
| Benzo[g,h,i]perylene | C7 | Sigma-Aldrich | 98 | 0.01, 0.03, 0.1, 0.3, 1 | |
| Chrysene | D7 | Supelco via Sigma-Aldrich | 99.9 | 0.1, 0.3, 1, 3, 10 | |
| Dibenz(a,h)anthracene | E1 | Supelco via Sigma-Aldrich | 99.9 | 0.1, 0.3, 1, 3, 10 | |
| Dibenz[a,c]anthracene | E2 | MRIGlobal | >99 | 0.3, 1, 3, 10, 30 | |
| Fluorene | E8 | Sigma-Aldrich | 99.9 | 0.3, 1, 3, 10, 30 | |
| Naphthalene | F7 | Sigma-Aldrich | 99.8 | 0.3, 1, 3, 10, 30 | |
| Phenanthrene | F10 | Sigma-Aldrich | 99.6 | 0.3, 1, 3, 10, 30 | |
| Pyrene | G2 | Sigma-Aldrich | 98.30 | 0.3, 1, 3, 10, 30 | |
| Pesticide | Aldicarb | B10 | Sigma-Aldrich | 99.9 | 0.3, 1, 3, 10, 30 |
| Bis(tributyltin)oxide (TBTO) | C10 | Sigma-Aldrich | 97.9 | 0.003, 0.01, 0.03, 0.1, 0.3 | |
| Captan | D3 | Sigma-Aldrich | 99.7 | 0.3, 1, 3, 10, 30 | |
| Carbamic acid, butyl-, 3-iodo-2-propynyl ester | D4 | Sigma-Aldrich | 99.4 | 0.03, 0.1, 0.3, 1, 3 | |
| Carbaryl | D5 | Sigma-Aldrich | 97 | 0.3, 1, 3, 10, 30 | |
| Chlorpyrifos (Dursban) | D6 | Toronto Research Chemicals Inc. | 98 | 0.3, 1, 3, 10, 30 | |
| Deltamethrin | D10, H4 | Chem Service, Inc. | 99.5 | 0.003, 0.01, 0.03, 0.1, 0.3 | |
| Dichlorodiphenyltrichloroethane (DDT) | E3 | Sigma-Aldrich | 99.3 | 0.3, 1, 3, 10, 30 | |
| Dieldrin | E4 | Sigma-Aldrich | 92.9 | 0.3, 1, 3, 10, 30 | |
| Heptachlor | E9 | Radian International LLC | 99 | 0.3, 1, 3, 10, 30 | |
| Lindane | F3 | Sigma-Aldrich | 99 | 0.3, 1, 3, 10, 30 | |
| Methyl mercuric (II) chloride | F5, H5 | Sigma-Aldrich | ∼94.2b | 0.003, 0.01, 0.03, 0.1, 0.3 | |
| Parathion | F8 | Chem Service, Inc. | 99.2 | 0.3, 1, 3, 10, 30 | |
| Permethrin | F9 | Chem Service, Inc. | 46.1% cis, 53.2% trans | 0.3, 1, 3, 10, 30 | |
| Rotenone | G3 | Sigma-Aldrich | 98 | 0.003, 0.01, 0.03, 0.1, 0.3 | |
| Tebuconazole | G5 | Bayer Corporation | 97.5 | 0.3, 1, 3, 10, 30 | |
| Negative | Acetaminophen (4-hydroxyacetanilide) | B6 | Sigma-Aldrich | 98.7 | 0.3, 1, 3, 10, 30 |
| Acetylsalicylic acid | B8 | Sigma-Aldrich | 99.9 | 0.3, 1, 3, 10, 30 | |
| d-Glucitol | D9 | Sigma-Aldrich | 99.7 | 0.3, 1, 3, 10, 30 | |
| l-Ascorbic acid | F1 | Sigma-Aldrich | 100.29 | 0.3, 1, 3, 10, 30 | |
| Saccharin sodium salt hydrate | G4, H6 | Sigma-Aldrich | ∼86.97a | 0.3, 1, 3, 10, 30 |
Note: Blank DMSO (100% purity) was provided.
Note: Argon gas headspace added to chemical vials prior to storage/shipment.
Vial type: polypropylene, 0.75 ml alphanumeric screwcap tubes, 96-vial plate rack, polypropylene screw cap with silicon o-ring.
Purity corrected for water content.
Purity calculated from element analysis.
Dechorionation, embryo plating, and compound exposures
Embryos were dechorionated as described previously (Truong et al., 2011). Approximately 1000 embryos were treated at a time with 50 µl of 63.6 mg/ml (∼11.12 U) protease from Streptomyces griseus (Pronase E, ≥3.5 U/mg, P5147 Sigma-Aldrich, St. Louis, Missouri) in 25 ml system fish water in glass dishes (10 cm) with gentle agitation for a maximum of 6 min or until the first embryo was observed to be dechorionated. Embryos were then washed with 2 l of warm system water to remove chorions and dilute the protease. At 5 hpf, dechorionated embryos were randomly placed into individual wells of 96-well plates containing 100 µl embryo medium at a density of 1 embryo per well. At 6 hpf, 100 µl of a 2× concentration of compound or vehicle (DMSO) was added to each well. Final DMSO concentrations were 0.5% (vol/vol) for all exposures. Wells were covered with Parafilm M (Bemis, North America, Neenah, Wisconsin) then covered with the plate lid to prevent evaporation. Plates were maintained in an incubator at 28.5°C with a 14 h light (∼300 lux)/10 h dark cycle. Fish were statically exposed until 5 dpf. Negative controls were exposed to embryo medium as sentinels for monitoring individual spawn health.
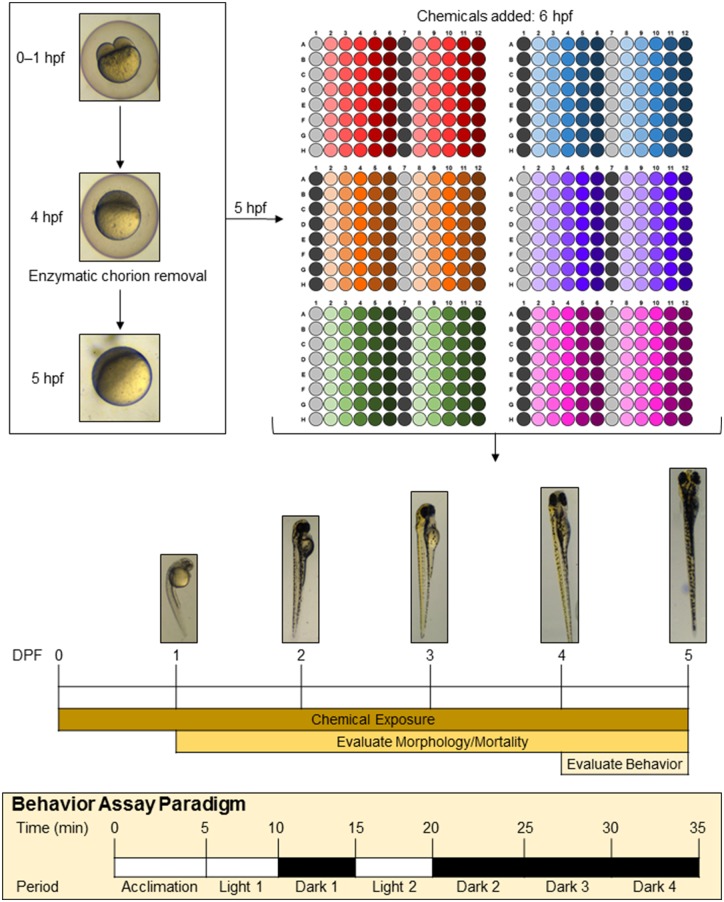
All compounds were tested for both mortality/teratology and photomotor behavior (described below) in duplicate experiments (two independent experiments conducted on independent days using fish from independent spawns). For each experiment, 16 fish were tested per concentration of each compound, thus the total sample size for each experimental condition was 32 fish. DMSO control fish (n = 48 per experiment) were distributed over 6 plates from the same spawn, totaling 96 control fish. A schematic of the experimental design is depicted in Figure 1.
Figure 1.
Experimental design. Each experiment started with age-matched embryos that were collected at the 2–4 cell stage and stored in system water in an incubator at 28.5°C ± 0.5°C until enzymatic chorion removal at 4 hpf. At 5 hpf, dechorionated embryos were transferred into six 96-well plates containing embryo medium. At 6 hpf, embryos were exposed to vehicle control (0.5% DMSO) or one of the compounds contained in the NTP 91-compound library at one of 5 increasing concentrations. Vehicle controls (gray) and internal negative control (dark gray) were loaded in column 1 or 7. Unique compounds tested are represented by wells colored in red, blue, orange, purple, green, and pink, where light to dark shades indicate increasing in concentration (columns 2–6 and 8–12). Thus, each experiment included 16 biological replicates for each concentration of each compound. All compounds were tested in two replicate experiments. Plates were maintained at 28.5°C ± 0.5°C under a 14 h light/10 h dark cycle until 5 dpf. Exposure solutions were not changed throughout the exposure period. Each day throughout the exposure period, mortality and teratology were assessed, and at 4 and 5 dpf, photomotor behavior was assessed. The behavior assay consisted of a 5 min acclimation period in the light followed sequentially by a 5 min light cycle (L1), a 5 min dark cycle (D1), a 5 min light cycle (L2), and 3 consecutive 5 min dark cycles (D2, D3, and D4).
Mortality/teratology endpoint
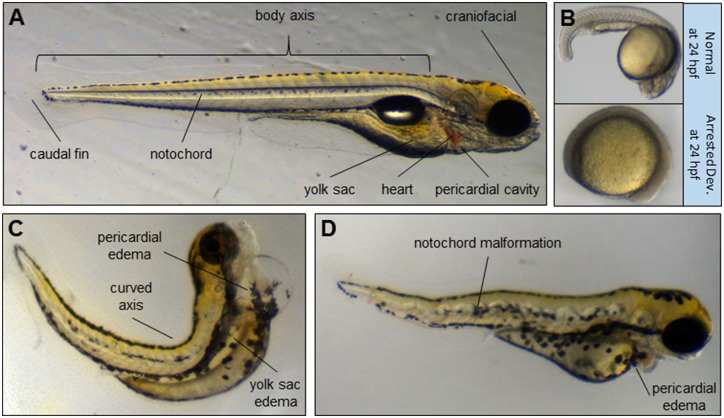
At 1, 2, 3, 4, and 5 dpf, fish were examined for mortality and developmental malformations. Fish were examined by removing the lid and Parafilm from the plate before manually scoring malformations and mortality by eye using an Olympus Stereo Microscope Model SZ61 (Olympus, Japan) up to 4.5× magnification. Traditional endpoints of zebrafish teratology were scored, including arrested development, yolk sac edema, pericardial edema, red heart, deformities of the body axis, notochord aberrations, and malformations of craniofacial structures or the caudal fin (Figure 2). Morphological assessments were quantified using a binary scale of either normal or abnormal morphology. Two researchers were trained on internally validated screening criteria prior to assessing teratological endpoints and a subset of plates were scored by both researchers as a quality check. Researchers were blinded to specific chemicals but not to controls. For each exposure, the number of fish that were dead was divided by the total number of biological replicates within each group (n = 32). Fish that exhibited a teratogenic effect were divided by the number of viable fish within each group. If the incidence of mortality or of any individual teratogenic endpoint within any exposure group was more than 30%, the compound was considered a hit. The OECD TG 210 guidelines recommend a threshold of 25%–30% mortality as a quality control measure for chemical exposures; therefore, all mortality and/or teratology endpoints above this threshold were considered hits. The incidence of teratogenic hits in DMSO control fish across all the studies described herein is provided in Supplementary Figure 1.
Figure 2.
Representative images of teratogenic endpoints assessed for mortality/teratology. A, Healthy zebrafish larva at 5 dpf with anatomic points of interest identified. B, A normal zebrafish embryo at 1 dpf and an abnormal embryo at 1 dpf with development arrested at a stage corresponding to approximately 11 hpf. C, Zebrafish larva at 5 dpf with body axis malformation, yolk sac edema, and pericardial edema. D, Zebrafish larva at 3 dpf illustrating notochord malformation.
Photomotor behavior
Photomotor behavior was assessed at 4 and 5 dpf using the DanioVision system (Noldus, Leesburg, Virginia). Behavior tests were conducted in the same 96-well plates used to expose zebrafish and only live larvae with no visible anatomic malformations were included in behavioral analyses. Temperature was maintained at 28.5°C ± 0.5°C using the Noldus temperature control unit. The testing paradigm consisted of a 10 min light period (∼1900 lux), which included a 5 min acclimation period and 5 min baseline swimming (L1), followed sequentially by a 5 min dark period (∼0 lux) to stimulate swimming behavior (D1), a 5 min light period (∼1900 lux) to provoke freezing behavior (L2), and a 15 min dark period (∼0 lux) to initially increase swimming behavior, and then record subsequent acclimation to the dark conditions (D2, D3, D4, 5 min each). Larval movement was recorded using a GigE camera (Noldus) with infrared filter, and tracked using EthoVisionXT software (Noldus). Larval movement in mm swam per fish was exported in 1 min bins into Excel 2016 (Microsoft, Albuquerque, New Mexico) for analysis.
The total distance swam in mm for each min during the behavioral test was pooled for all fish within the same exposure group at each time point (4 and 5 dpf), and behavioral data from the two experiments were combined. Distance swam was plotted for each min of the behavioral test, and the resulting curves analyzed with respect to the degree of similarity between any given exposure group and the DMSO control within the same spawn. Curve similarities were analyzed by calculating the residuals of all individual fish against a curve fit from the data obtained for the DMSO control for each individual cycle (L1, D1, L2, D2, D3, and D4) using the following equation:
| (1) |
where YFit,k, corresponds to the movement (distance swam) at minute k for the fit function of the DMSO control within the same spawn; Yk, to the movement of an individual fish at minute k; and Diffcycle, to the result of equation 1 for a given light or dark cycle. Equation 1 is similar to the sum of squared errors (SSE), with the exception of not squaring the difference in order to maintain the mathematical sign (positive or negative). Results of equation 1 were calculated for each fish and saved in an Excel file. The values of the exposed fish for each cycle were then compared with those of DMSO controls using a Kruskal-Wallis test (data was determined to be not normally distributed) with a Bonferroni post hoc test, with p < .05 set as significant. p-values were saved in the same Excel file. Only exposures in which at least 22 out of 32 fish (70%) were healthy were included in the statistical analyses (eg, n ≥ 22 per exposure for behavioral analyses).
Categorization of behavioral abnormalities
Behavioral abnormalities were defined as photomotor behavior that differed significantly from that observed in DMSO control larvae from the same spawn (see Behavioral analysis in the preceding section for description of how significant differences were identified). The variability in photomotor behavior of DMSO control fish between replicates within an experiment and between spawns is described in Supplementary Figure 2. The different types of behavioral abnormalities observed in the screen were categorized as: (1) hypoactivity/hyperactivity behaviors in which significantly decreased/increased movement was detected in at least one dark cycle; (2) constant movement, larvae did not react to changes in lighting conditions and displayed constant locomotor activity throughout the 35 min assay; (3) altered reactions to lighting changes in which peak movement in response to changes in lighting was significantly different from that of DMSO control larvae; and (4) no movement, activity was significantly decreased relative to vehicle controls in D1, D2, and D3, and less than 25 mm total distance per min bin.
RESULTS
Mortality/Teratology
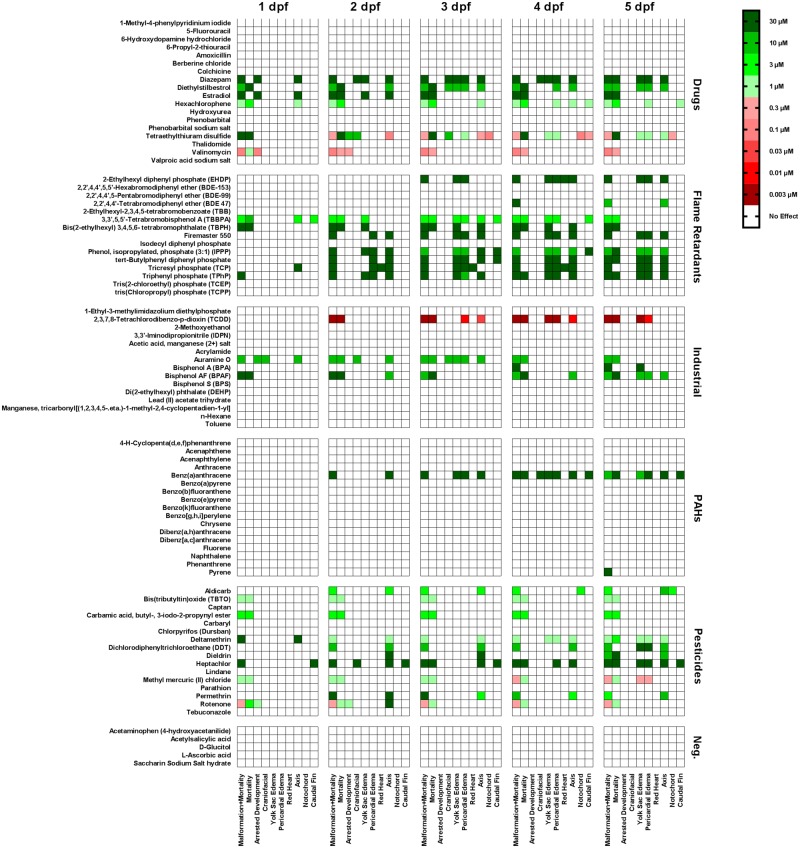
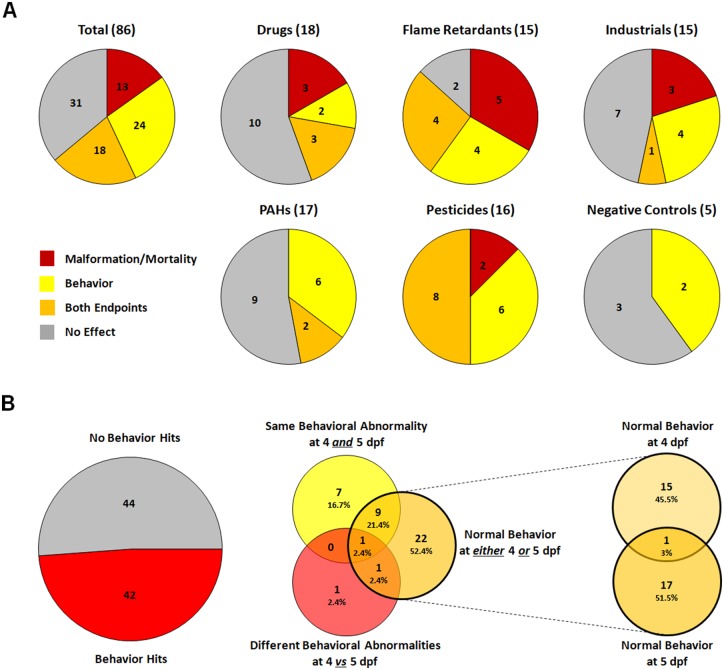
Fish were examined daily from 1 to 5 dpf for mortality and teratogenic effects (Figs. 1 and 2). Positive hits were defined as compounds that increased mortality or the occurrence of malformations by ≥30%. Among DMSO control fish, the incidence of mortality was 4.5% while the incidence of malformations was 1.5% across all 5 time points. The lowest concentration at which an individual compound was scored as a hit for any mortality/teratology endpoint (lowest observed adverse effect level) is indicated in Figure 3. None of the negative controls significantly increased mortality or caused teratogenic effects at any of the concentrations tested. Of the remaining test compounds, approximately 24% were scored as hits for mortality, and approximately 34% as hits for teratology. In general, the number of hits for mortality or teratology increased with increasing exposure time.
Figure 3.
Results of mortality/teratology endpoints. Heatmap summarizing the results of mortality/teratology (arrested development, craniofacial malformations, yolk sac edema, pericardial edema, red heart, body axis malformations, notochord malformation, and caudal fin malformations). An exposure was considered a “hit” for a specific endpoint (listed along the bottom of the heatmap) if the percentage of dead fish or fish displaying a specific malformation was greater than 30%. For compounds that caused significant mortality and/or teratology, the lowest concentration (as indicated by the color code shown in the upper right corner) that generated a hit is indicated in the heatmap (n = 32 larvae from 2 independent spawns per exposure).
Comparing effects across the different chemical sets, the greatest incidence of mortality and teratology was observed among the pesticides (63%), flame retardants (60%), and drugs (33%), while only a few industrial chemicals and one PAH were scored as hits. For the majority of the compounds identified as hits, significant mortality or teratogenicity was observed only at the high end of the concentration range (1–30 µM). One exception was the industrial chemical, TCDD, which caused mortality at a concentration as low as 0.003 µM, making it the most lethal compound in the library. In the drug category, the most potent compound was tetraethylthriuram disulfide, which caused notochord and axis malformations in the absence of significant mortality at a concentration of 0.1 µM. Among the pesticides, methyl mercuric chloride caused pericardial edema at 0.3 µM.
Photomotor Behavior
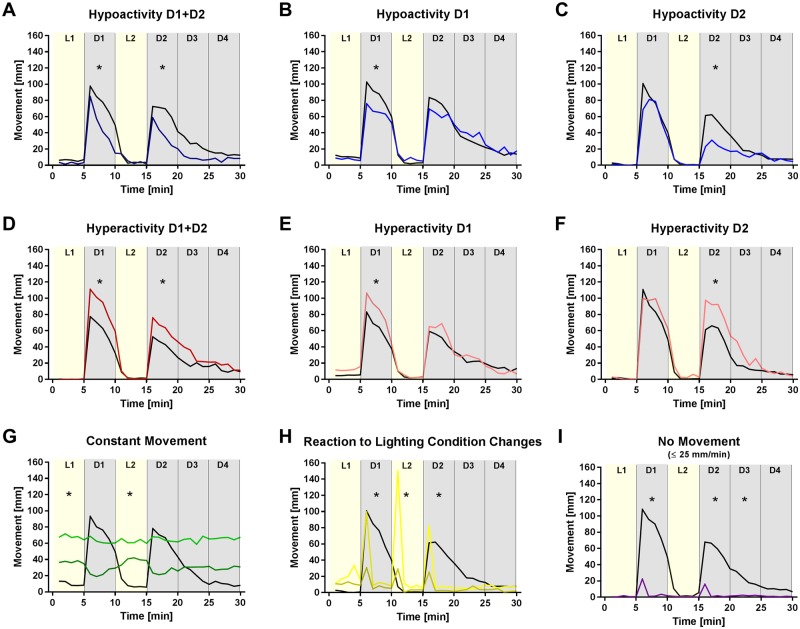
Photomotor behavior displayed by zebrafish larvae in response to sudden changes between light and dark conditions is used to detect changes in nervous system development or function (Emran et al., 2008). Abrupt change from dark to light causes larval zebrafish to decrease or stop swimming, whereas sudden change from light to dark triggers increased locomotion, which gradually subsides as larvae habituate to dark conditions (MacPhail et al., 2009). Consistent with these published data, DMSO control larvae exhibited little to no movement during light cycles, but were active during dark cycles, exhibiting peak movement (distance swam) of 80–110 mm during the first min of D1 (Figure 4). After the first min, fish acclimated to the dark and movement (distance swam) decreased with increasing time in the dark cycle; moreover, the peak distance swam decreased in subsequent dark cycles (Figure 4).
Figure 4.
Types of observed photomotor behavior abnormalities. Representative graphs illustrating the different types of behavioral abnormalities observed in exposed zebrafish larvae. Graphs plot the average total distance swam (movement) for each min of the different cycles of the photomotor behavior assay. DMSO controls are shown in black (n = 85–93). The behavioral abnormalities were classified as: (A–C) hypoactivity (n = 28–31); (D–F) hyperactivity (n = 30); (G) constant movement (n = 22–32); (H) altered reaction to lighting changes (n = 29–31); and (I) no movement (n = 25). * indicates the cycle(s) in which exposure caused a significant (p < .05) difference in behavior compared with the DMSO controls within the same spawn.
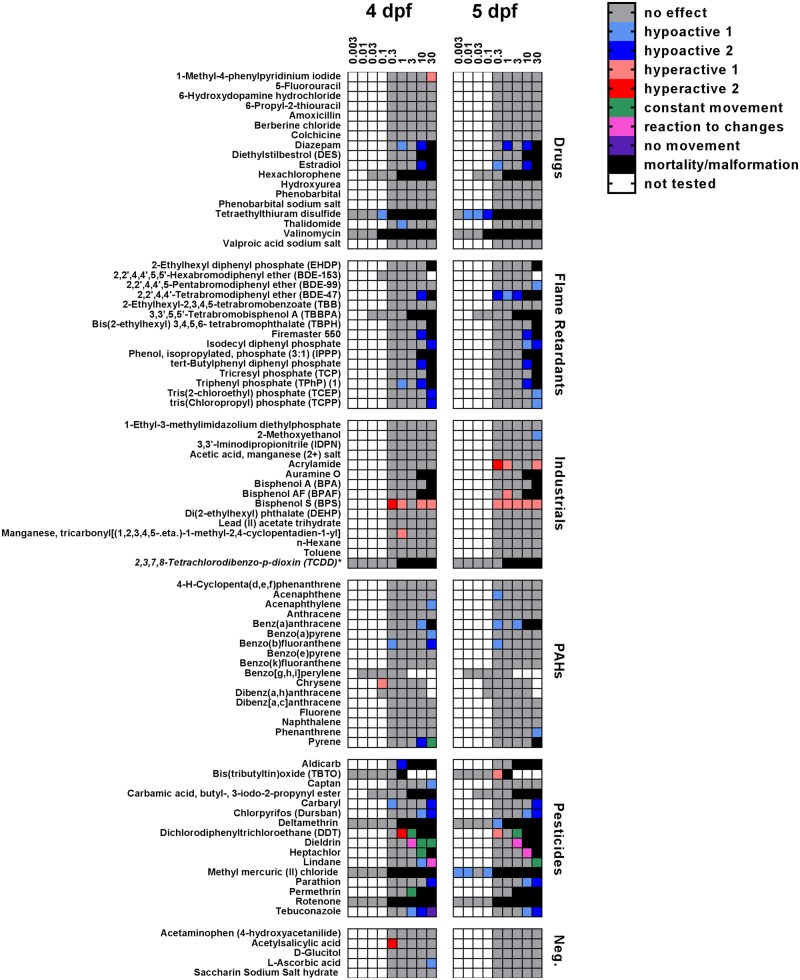
Compounds perturbed photomotor behavior in varying ways that were categorized into behavioral phenotypes: (1) hypoactivity (Figs. 4A–C); (2) hyperactivity (Figs. 4D–F); (3) constant movement regardless of changing light conditions (Figure 4G); (4) altered reaction to changes in light conditions relative to DMSO controls (Figure 4H); and (5) minimal movement throughout the 35 min test (Figure 4I). Hits were identified in each chemical set, including the negative controls (Figure 5). The greatest incidence of behavioral hits was observed among the pesticides (88%), followed by the flame retardants (53%), the PAHs (47%), the industrial chemicals (33%), and the drugs (28%). Two of the five negative controls, acetylsalicylic acid (0.3 µM) and l-ascorbic acid (30 µM), caused hyperactivity and hypoactivity, respectively, on 4 dpf, but had no effect on photomotor behavior at 5 dpf.
Figure 5.
Summary of photomotor behavior results. Heatmap summarizing photomotor behavior at 4 and 5 dpf (n = 22–32 larvae from 2 independent spawns per exposure). White squares, concentrations that were not tested; gray squares, concentrations that had no effect; black squares, concentrations that were excluded from behavioral analyzes because of a high incidence of mortality and/or teratology. Hypoactivity 1 and hyperactivity 1 refer to compounds that caused the behavioral abnormality in either D1 or D2 cycle; hypoactivity 2 and hyperactivity 2, compounds that caused the behavioral abnormality in both D1 and D2 cycles *The chemical TCDD was tested for behavioral effects at a lower concentration range (0.00001–0.1 µM) than other compounds in the library due to a high mortality/malformation rate at higher concentrations.
In general, drugs, flame retardants, and PAHs caused hypoactivity, while the industrial chemicals caused hyperactivity. Pesticides caused multiple types of abnormal photomotor behavior, including hypoactivity, hyperactivity, altered reaction in response to light changes, or constant movement. Larvae exposed to the highest concentration of tebuconazole (30 µM) lacked locomotor activity at 4 dpf. Many compounds altered photomotor behavior on only one of the 2 days tested, although the affected day (4 dpf vs 5 dpf) varied across compounds even within the same chemical set. For example, among the PAHs, acenaphthylene and benzo(a)pyrene significantly altered behavior at 4 dpf but not at 5 dpf, whereas acenaphthene had no effect on behavior at 4 dpf, but caused significant hypoactivity at 5 dpf (Figure 5). In few instances, different concentrations of the same compound resulted in completely different behavioral outcomes. For example, the pesticide lindane caused hypoactivity at 10 µM, but altered reactions to changes in light conditions at 30 µM, while the PAH pyrene caused hypoactivity at 10 µM but constant movement across changing light conditions at 30 µM (Figure 5). There were a large number of compounds that had no effect on photomotor behavior at the lower end of the concentration range, but caused significant mortality or teratogenic effects at higher concentrations, which precluded behavioral assessment.
Comparison of Behavioral Effects at 4 dpf versus 5 dpf
Forty-two of the 86 unique compounds affected behavior on at least one of the testing days while 44 compounds did not elicit behavioral abnormalities at either 4 or 5 dpf (Figure 6B). The 42 behavior hits were grouped into 3 categories: (1) those that caused the same behavioral abnormality at 4 and 5 dpf; (2) those that caused different behavioral abnormalities at 4 versus 5 dpf; and (3) those that altered behavior at either 4 or 5 dpf but not both (eg, normal behavior was exhibited on one of the 2 days). In some instances, different concentrations of the same compound caused different behavioral patterns on the same testing day; these compounds are listed in more than one group. One compound, permethrin, altered behavior (constant movement) at only one concentration (3 µM) at 4 dpf, while at 5 dpf >30% of the permethrin-exposed fish did not survive. Therefore, permethrin is not listed in any of the three groups (which is why Figure 6B shows only 41 behavior hits). At 30 µM, lindane caused different behavioral abnormalities on 4 dpf (reaction to changes) versus 5 dpf (constant movement), while fish exposed to lindane at 10 µM exhibited hypoactive behavior at 4 dpf and normal behavior at 5 dpf. Tebuconazole affected behavior at 3 different concentrations with different patterns exhibited by each concentration group across the 2 testing days (3 µM, hypoactive-normal; 10 µM, hypoactive-hypoactive; 30 µM, no movement-hypoactive). Of the 33 compounds that had no effects on behavior (eg, “normal” behavior) at either 4 or 5 dpf, 15 had no effects on behavior at 4 dpf but caused behavioral abnormalities at 5 dpf, while 17 caused behavioral abnormalities at 4 dpf but not at 5 dpf. At 0.3 µM, DDT had no effect on photomotor behavior at 4 dpf, but caused hyperactivity at 5 dpf, whereas at 1 µM, DDT caused hyperactivity at 4 dpf, but had no effect on behavior at 5 dpf.
Figure 6.
Summary of hits. A, Pie graphs illustrating the total number of compounds within each chemical set that caused only mortality/teratology (red), only behavioral abnormalities (yellow), were hits for both mortality/teratology and behavior (orange) or were negative for both endpoints (gray). The number in parentheses next to the name of each chemical set indicates the total number of compounds in the set. B, Venn diagrams illustrating the total number of compounds that caused behavioral abnormalities at either time point, with further breakdown as to the number that caused the same behavioral abnormality at both 4 and 5 dpf (yellow) versus the number that caused different behavioral abnormalities at 4 versus 5 dpf (red) versus the number that did not alter normal behavior at either 4 or 5 dpf (orange). Any individual compound could belong to more than one behavioral category if different concentrations caused different outcomes.
To determine whether daily removal of the lid for teratological testing or behavior testing at 4 dpf altered photomotor behavior at 5 dpf, we exposed fish from the same spawn to 0.1% DMSO from 6 hpf to 5 dpf. A third of the fish (n = 16) were handled per our standard protocol, eg, plate lids were removed daily and photomotor assays were conducted at 4 and 5 dpf. Another third were tested in the photomotor behavior assay at 4 and 5 dpf but lids were not removed from the plates during the 5 days exposure period. The last third were handled identically to the control with respect to removing the plate lid daily, but behavior was only conducted at 5 dpf. We found no significant differences (p = .5967) between the 3 groups in photomotor response at 5 dpf (Supplementary Figure 3). Therefore, plate handling does not significantly alter behavior during light/dark cycles and repeated behavior testing on 4 and 5 dpf should not influence the hit count in the screen.
Summary of Hits
Of the 86 unique compounds, 13 were hits only for mortality/teratology, 24 only altered photomotor behavior, 18 were hits for both mortality/teratology and photomotor behavior, and 31 had no effect on either endpoint (Figure 6A). The 2 compounds provided as duplicates in the 91-compound library produced similar results in our screen. More than half of the drugs and PAHs were negative for both mortality/teratology and behavior endpoints; in contrast, all of the pesticides were hits for at least one of the 2 endpoints. Of the 18 drugs, 3 only caused mortality/teratology, 2 only altered behavior, while 3 altered both mortality/teratology and behavior endpoints. Two PAHs were hits for both mortality/teratology and behavior, while 8 interfered with photomotor behavior, but had no effect on mortality/teratology. None of the PAHs were hits only for mortality/teratology. Most flame retardants (13 out of 15 compounds) affected at least one endpoint with almost equal distribution between those that were hits only in mortality/teratology (5 compounds) versus those that only altered photomotor behavior (4 compounds) versus those that were hits for both (4 compounds). Most of the 15 industrial chemicals were hits for either mortality/teratology (3 compounds) or behavior (4 compounds) with only one industrial chemical identified as a hit for both. Half of the pesticides were hits for both mortality/teratology and behavior, while 6 caused only behavior abnormalities and 2 only affected mortality/teratology. None of the negative controls caused mortality or were teratogenic; however, 2 altered photomotor behavior at 4 dpf, but not 5 dpf.
In addition to comparing hits across the sets of test compounds, we assessed whether the 2D compound structural similarities correlated with patterns of hits. NCBI’s PubChem BioAssay Structural Clustering Tool clusters substructural fingerprints with a single linkage algorithm. Post-analysis data specifies a unique 2D Tanimoto similarity score. Substructural significance is identified as similarity scores ≥0.68 (≥95% confidence interval) (Kim et al., 2012). Although multiple clusters met the 0.68 significance threshold, only 2 clusters resulted in similar developmental toxicity profiles (see Supplementary Figure 4). Deltamethrin and permethrin, both pyrethroid pesticides, similarly altered mortality/teratology and behavior. Diazepam (drug) and auramine O (industrial) had similar effects on mortality/teratology and behavior endpoints. Although all PAHs significantly clustered substructurally, no common toxicity endpoints were observed.
DISCUSSION
Overall, our screen of the NTP 91-compound library for mortality/teratology and adverse effects on photomotor behavior in a larval zebrafish model identified approximately 15% (13 compounds) that only caused mortality or were teratogenic, 28% (24 compounds) that only caused aberrant photomotor behavior, and 21% (18 compounds) that were hits for both mortality/teratology and behavior. Over 36% (31 compounds) of the 86 unique compounds had no effect on either endpoint. Whether these compounds are true negatives or whether the lack of toxic effect is due to toxicokinetic (reduced bioavailability due to minimal uptake of the compound, lack of metabolic activation or photoinactivation of the compound) or toxicodynamic (deficient target expression) differences in developing zebrafish versus mammalian models remains to be determined. As expected, TCDD was by far the most lethal compound at a concentration as low as 3 nM, which is similar to lethal concentrations reported for zebrafish by others (Lanham et al., 2012; Prasch et al., 2003). Pesticides were the most toxic group, with every compound in this group causing mortality/teratogenic effects, behavioral abnormalities, or both.
This screen revealed interesting chemical set- and compound-specific behavioral effects in larval zebrafish. For example, the behavioral effects observed in larvae exposed to flame retardants and industrial chemicals were common across hits within each chemical set, with flame retardants causing hypoactivity and industrial chemicals causing hyperactivity. In contrast, the behavioral effects caused by pesticides varied between compounds within the set, consistent with known differences in the modes of action by which these pesticides cause developmental neurotoxicity. Many compounds from multiple chemical sets exhibited nonmonotonic dose-response relationships, wherein behavioral effects were observed at lower but not higher concentrations. Similar nonmonotonic dose-response relationships were reported in a screen of this same compound library using freshwater planarian Dugesia japonica (Zhang et al., 2018). In addition, this study found a number of compounds that elicited behavioral abnormalities at either 4 or 5 dpf, but not both. What this means toxicologically is not clear. About half of these chemicals altered behavior at 5 but not 4 dpf, which may reflect bioaccumulation over time to reach toxic tissue levels. With regards to the chemicals that had behavioral effects only at 4 dpf, it is possible that this represents a tipping point, at which the system switches from recovery to nonrecovery trajectories (Frank et al., 2018). However, for none of the compounds in this group was there evidence of mortality/teratology at 5 dpf, so this seems to be an unlikely explanation. More likely is that the expression of critical xenobiotic metabolizing enzymes and/or molecular targets is developmentally regulated to switch on or off between 4 and 5 dpf. Nonetheless, this observation suggests the importance of screening behavioral outcomes at multiple developmental stages.
Challenges
Traditional approaches, such as ANOVA or Student’s t test of, eg, area under the curve are not suitable for analyzing photomotor behavior because of the variability of zebrafish behavior within and between experiments (Liu et al., 2017) (and see Supplementary Material, pages 3–5). Alternative approaches that consider both distance swam (or movement) as a function of time using 2-way ANOVA are problematic because: (1) behavioral profiles are often not normally distributed; and (2) adjusted p-values derived by post hoc tests are dependent on the number of groups. Therefore, alternative approaches, such as generalized mixed models are recommended (Liu et al., 2017). However, such model approaches require knowledge of the significant variables, which can, for example, be assessed using the Hotelling’s T-squared test (Liu et al., 2017). This makes these model approaches significantly more time consuming than traditional approaches. Therefore, we present an alternative approach in which we compare behavior curves of control versus exposed fish with respect to similarity. This allows analysis of data with a nonnormal distribution, and requires no a priori knowledge of the behavioral readout. These two factors enable high-throughput analysis. Which approach is the most sensitive and robust has yet to be determined (Liu et. al., 2017, Zhang et. al., 2017).
A factor that significantly influences reproducibility in this model is the viability of individual spawns. The influence of spawn variability was mitigated by discarding plates with mortality/teratology hits of ≥20% in the DMSO controls. Using this criterion, 12 plates (corresponding to 2 spawns) out of a total of 216 plates (corresponding to 36 spawns) were discarded. Thus, ∼6% of the plates that were set up did not meet our criterion for acceptable viability. Plate effects represent another potential source of variability. Precautions taken to minimize plate effects included using 96-well plates with identical lot numbers throughout the entire study and distributing exposure solutions in alternating columns on the plate to minimize edge effects.
Negative Controls
The NTP 91-compound library included 5 negative controls (acetaminophen, acetylsalicylic acid, d-glucitol, l-ascorbic acid, and saccharin sodium salt hydrate) presumed not to be developmentally neurotoxic. Although none of the negative controls caused mortality or teratogenic effects at the concentrations tested, 2 did significantly alter photomotor behavior. At the lowest concentration tested, 0.3 µM, acetylsalicylic acid increased locomotor activity at 4 dpf, whereas at the highest concentration tested, 30 µM, l-ascorbic acid decreased locomotor activity at 4 dpf. Neither compound had behavioral effects at 5 dpf. Consistent with these findings, adverse effects on locomotor activity has been reported in larval zebrafish exposed to acetylsalicylic acid and l-ascorbic acid (Hagstrom et al., 2018), although these effects were observed at 5 dpf. Although neither the effective concentration nor type of behavioral effect were described in the Hagstrom et al. study, the fact that 2 different groups have identified these compounds as hits suggest that developing zebrafish are abnormally sensitive to acetylsalicylic acid and l-ascorbic acid. Thus, these compounds may not be useful as negative controls in developmental neurotoxicity screens that use zebrafish larvae.
Comparison of Hits to Existing Zebrafish Data
Several of the compounds in the NTP 91-compound library have been extensively studied in larval, juvenile, and adult zebrafish for effects on morphological, behavioral, and molecular endpoints. An important consideration in comparing results across these studies is the criteria used to define positive hits. Mortality and teratology are usually comparable across studies because these endpoints are usually collected in a binary matter. However, analysis of zebrafish behavior is far more variable between laboratories. Nonetheless, consistent with previous reports that TCDD is teratogenic (Carney et al., 2006) and causes abnormal behavior in larval zebrafish (Garcia et al., 2018), we detected TCDD as a hit for both endpoints. Similarly, in accordance with Noyes et al. (2015), we identified BDE-99, BDE-47, BPDP, TPhP, TCEP, and TCPP as causing hypoactivity in the photomotor behavior assay at 5 dpf. In contrast, toluene has been reported to cause pericardial edema accompanied by axis deformities in developing zebrafish larvae (George et al., 2011), but we observed neither teratogenic effects nor behavioral abnormalities in larval zebrafish exposed to toluene. This discrepancy may reflect differences in absorption between dishes used for exposures and/or concentration ranges tested, both of which affect toxicokinetics.
The same NTP compound library screened here has also been tested in larval zebrafish of the same strain by another group using very similar endpoints, specifically mortality/teratology and photomotor behavior at 1 and 5 dpf (Hagstrom et al., 2018). Relative to our observation of 55/86 hits, Hagstrom et al. reported 86/87 hits. One likely explanation for this discrepancy is that the maximum concentration we tested was 30 µM; in contrast, Hagstrom et al. tested up to 67 µM. Second, we maintained fish on a light/dark cycle whereas Hagstrom et al. maintained fish in the dark between 1 and 5 dpf. Variables impacted by this procedural difference include: (1) half-life of photosensitive compounds, with potentially decreased levels/toxicity in our screen vs. the Hagstrom screen; and (2) differing circadian rhythms in the fish in the 2 studies, resulting in differential xenobiotic metabolism or expression of molecular targets.
Comparison to Other Models
A significant goal of the NIEHS initiative is to identify alternative models that are predictive of adverse outcomes in mammalian models, particularly humans. A comprehensive analysis of the concordance of our screening data with available human and rodent data is the focus of another article in this special issue. However, our results are consistent with previous data in which 10 (chlorpyrifos, lindane, TBTO, deltamethrin, dieldrin, heptachlor, methyl mercuric chloride, parathion, permethrin, and tebuconazole) of the 16 pesticides in the library were shown to alter behavior and/or brain morphology in rodents and humans (Mundy et al., 2015). Our screen also successfully identified polybrominated diphenyl ethers and organophosphate flame retardants as DNT compounds, which have previously been described to cause developmental neurotoxicity in mammalian models (Aschner et al., 2017; Behl et al., 2015; Mundy et al., 2015).
Several compounds identified as reference compounds for developmental neurotoxicity (Aschner et al., 2017) were negative behavior hits in our screen, including lead acetate trihydrate, valproic acid sodium salt, and toluene. However, our data are not at odds with the zebrafish literature. Zebrafish embryo media contains sulfates that are known to precipitate with lead, thereby decreasing aqueous exposures (Marani et al., 1995). Although teratogenicity or behavioral changes have been reported in zebrafish exposed to valproic acid sodium salt at concentrations >30 µM (Gurvich et al., 2005; Herrmann, 1993; Li et al., 2009; Selderslaghs et al., 2009; Zellner et al., 2011), consistent with our observations, other studies have not observed dysmorphologies or altered photomotor behavior at valproate concentrations ≤30 µM (Cowden et al., 2012; Isenberg et al., 2007).
In conclusion, the development of screening platforms that efficiently and accurately assess the potential risk of developmental neurotoxicity associated with compounds in the human chemosphere are critical to effective risk management in today’s chemically complex landscape. The ability of the zebrafish platform to detect developmental neurotoxicity is influenced by toxicokinetic and toxicodynamic factors, which are influenced by the developmental stage of the zebrafish, and the inherent physiochemical properties of the compounds. As indicated by our screen of photomotor behavior at 4 versus 5 dpf, developmental stage also significantly influences outcome of this assay.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Galen W. Miller (University of California-Davis) for setting up the zebrafish facility in the Lein laboratory and for assisting with the experimental design. We also thank Madison Koch and Kristina Bischak (both students at the University of California-Davis) for their help in performing these experiments. The authors declare no conflicts of interest.
FUNDING
This work was supported by the National Institute of Environmental Health Sciences (grant numbers P01 ES011269 and P30 ES023513) and by the United States Environmental Protection Agency (grant number RD83543201). The contents of this work do not necessarily represent the official views of the NIEHS or USEPA; the NIEHS and USEPA do not endorse the purchase of any commercial products or services mentioned in the publication. The sponsors were not involved in the study design, the collection, analysis, and interpretation of data, in the writing of the report or in the decision to submit the article for publication.
REFERENCES
- Aschner M., Ceccatelli S., Daneshian M., Fritsche E., Hasiwa N., Hartung T., Hogberg H. T., Leist M., Li A., Mundi W. R., et al. (2017). Reference compounds for alternative test methods to indicate developmental neurotoxicity (DNT) potential of chemicals: Example lists and criteria for their selection and use. ALTEX 34, 49–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila D., Helmcke K., Aschner M. (2012). The Caenorhabiditis elegans model as a reliable tool in neurotoxicology. Hum. Exp. Toxicol. 31, 236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J., Oliveri A., Levin E. D. (2013). Zebrafish model systems for developmental neurobehavioral toxicology. Birth Defects Res. C Embryo Today 99, 14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behl M., Hsieh J. H., Shafer T. J., Mundy W. R., Rice J. R., Boyd W. A., Freedman J. H., Hunter E. S. 3rd, Jarema K. A., Padilla S., et al. (2015). Use of alternative assays to identify and prioritize organophosphorus flame retardants for potential developmental and neurotoxicity. Neurotoxicol. Teratol. 52, 181–193. [DOI] [PubMed] [Google Scholar]
- Carney S. A., Prasch A. L., Heideman W., Peterson R. E. (2006). Understanding dioxin developmental toxicity using the zebrafish model. Birth Defects Res. A Clin. Mol. Teratol. 76, 7–18. [DOI] [PubMed] [Google Scholar]
- Chen X., Huang C., Wang X., Chen J., Bai C., Chen Y., Chen X., Dong Q., Yang D. (2012). BDE-47 disrupts axonal growth and motor behavior in developing zebrafish. Aquat. Toxicol. 120-121, 35–44. [DOI] [PubMed] [Google Scholar]
- Collins F. S., Gray G. M., Bucher J. R. (2008). Toxicology. Transforming environmental health protection. Science 319, 906–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowden J., Padnos B., Hunter D., MacPhail R., Jensen K., Padilla S. (2012). Developmental exposure to valproate and ethanol alters locomotor activity and retino-tectal projection area in zebrafish embryos. Reprod. Toxicol. 33, 165–173. [DOI] [PubMed] [Google Scholar]
- Emran F., Rihel J., Dowling J. E. (2008). A behavioral assay to measure responsiveness of zebrafish to changes in light intensities. J. Visual. Exp. 20, 923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank C. L., Brown J. P., Wallace K., Wambaugh J. F., Shah I., Shafer T. J. (2018). Defining toxicological tipping points in neuronal network development. Toxicol. Appl. Pharmacol. 354, 81–93. [DOI] [PubMed] [Google Scholar]
- Garcia G. R., Bugel S. M., Truong L., Spagnoli S., Tanguay R. L. (2018). AHR2 required for normal behavioral responses and proper development of the skeletal and reproductive systems in zebrafish. PLoS One 13, e0193484.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia G. R., Noyes P. D., Tanguay R. L. (2016). Advancements in zebrafish applications for 21st century toxicology. Pharmacol. Ther. 161, 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George S., Xia T., Rallo R., Zhao Y., Ji Z., Lin S., Wang X., Zhang H., France B., Schoenfeld D., et al. (2011). Use of a high-throughput screening approach coupled with in vivo zebrafish embryo screening to develop hazard ranking for engineered nanomaterials. ACS Nano 5, 1805–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert S. F., Barresi M. J. F. (2016). Developmental Biology, 11th ed Sinauer Associates, Sunderland, MA. [Google Scholar]
- Gurvich N., Berman M. G., Wittner B. S., Gentleman R. C., Klein P. S., Green J. B. (2005). Association of valproate-induced teratogenesis with histone deacetylase inhibition in vivo. FASEB J. 19, 1166–1168. [DOI] [PubMed] [Google Scholar]
- Hagstrom D., Truong L., Zhang S., Tanguay R., Collins E. S. (2018). Comparative analysis of zebrafish and planarian model systems for developmental neurotoxicity screens using an 87-compound library. Toxicol Sci. 167, 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper C., Lawrence C. (2011). The Laboratory Zebrafish. CRC Press, Boca Raton, FL. [Google Scholar]
- Herrmann K. (1993). Effects of the anticonvulsant drug valproic acid and related substances on the early development of the zebrafish (Brachydanio rerio). Toxicol. In Vitro 7, 41–54. [DOI] [PubMed] [Google Scholar]
- Howe K., Clark M. D., Torroja C. F., Torrance J., Berthelot C., Muffato M., Collins J. E., Humphray S., McLaren K., Matthews L., et al. (2013). The zebrafish reference genome sequence and its relationship to the human genome. Nature 496, 498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg J. S., Jia Y., Field L., Ridnour L. A., Sparatore A., Del Soldato P., Sowers A. L., Yeh G. C., Moody T. W., Wink D. A., et al. (2007). Modulation of angiogenesis by dithiolethione-modified NSAIDs and valproic acid. Br. J. Pharmacol. 151, 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y., Liu Z., Peng T., Fu Z. (2015). The toxicity of chlorpyrifos on the early life stage of zebrafish: A survey on the endpoints at development, locomotor behavior, oxidative stress and immunotoxicity. Fish Shellfish Immunol. 43, 405–414. [DOI] [PubMed] [Google Scholar]
- Kim S., Bolton E. E., Bryant S. H. (2012). Effects of multiple conformers per compound upon 3-D similarity search and bioassay data analysis. J. Cheminform. 4, 28.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B., Schilling T. F. (1995). Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253–310. [DOI] [PubMed] [Google Scholar]
- Lanham K. A., Peterson R. E., Heideman W. (2012). Sensitivity to dioxin decreases as zebrafish mature. Toxicol. Sci. 127, 360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein P., Silbergeld E., Locke P., Goldberg A. M. (2005). In vitro and other alternative approaches to developmental neurotoxicity testing (DNT). Environ. Toxicol. Pharmacol. 19, 735–744. [DOI] [PubMed] [Google Scholar]
- Li X., Jia S., Wang S., Wang Y., Meng A. (2009). Mta3-NuRD complex is a master regulator for initiation of primitive hematopoiesis in vertebrate embryos. Blood 114, 5464–5472. [DOI] [PubMed] [Google Scholar]
- Liu Y., Ma P., Cassidy P. A., Carmer R., Zhang G., Venkatraman P., Brown S. A., Pang C. P., Zhong W., Zhang M., Leung Y. F. (2017). Statistical analysis of zebrafish locomotor behaviour by generalized linear mixed models. Scientific Reports 71, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Zhang Q., Li S., Mi P., Chen D., Zhao X., Feng X. (2018). Developmental toxicity and neurotoxicity of synthetic organic insecticides in zebrafish (Danio rerio): A comparative study of deltamethrin, acephate, and thiamethoxam. Chemosphere 199, 16–25. [DOI] [PubMed] [Google Scholar]
- MacPhail R. C., Brooks J., Hunter D. L., Padnos B., Irons T. D., Padilla S. (2009). Locomotion in larval zebrafish: Influence of time of day, lighting and ethanol. Neurotoxicology 30, 52–58. [DOI] [PubMed] [Google Scholar]
- Marani D., Macchi G., Pagano M. (1995). Lead precipitation in the presence of sulfate and carbonate - Testing of thermodynamic predictions. Water Res. 29, 1085–1092. [Google Scholar]
- Miller G. W., Chandrasekaran V., Yaghoobi B., Lein P. J. (2018). Opportunities and challenges for using the zebrafish to study neuronal connectivity as an endpoint of developmental neurotoxicity. Neurotoxicology 67, 102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy W. R., Padilla S., Breier J. M., Crofton K. M., Gilbert M. E., Herr D. W., Jensen K. F., Radio N. M., Raffaele K. C., Schumacher K., et al. (2015). Expanding the test set: Chemicals with potential to disrupt mammalian brain development. Neurotoxicol. Teratol. 52, 25–35. [DOI] [PubMed] [Google Scholar]
- Noyes P. D., Haggard D. E., Gonnerman G. D., Tanguay R. L. (2015). Advanced morphological - Behavioral test platform reveals neurodevelopmental defects in embryonic zebrafish exposed to comprehensive suite of halogenated and organophosphate flame retardants. Toxicol. Sci. 145, 177–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares C. I., Field J. A., Simonich M., Tanguay R. L., Sierra-Alvarez R. (2016). Arsenic (III, V), indium (III), and gallium (III) toxicity to zebrafish embryos using a high-throughput multi-endpoint in vivo developmental and behavioral assay. Chemosphere 148, 361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasch A. L., Teraoka H., Carney S. A., Dong W., Hiraga T., Stegeman J. J., Heideman W., Peterson R. E. (2003). Aryl hydrocarbon receptor 2 mediates 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin developmental toxicity in zebrafish. Toxicol. Sci. 76, 138–150. [DOI] [PubMed] [Google Scholar]
- Rand M. D. (2010). Drosophotoxicology: The growing potential for Drosophila in neurotoxicology. Neurotoxicol. Teratol. 32, 74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrı´guez F., López J. C., Vargas J. P., Broglio C., Gómez Y., Salas C. (2002a). Spatial memory and hippocampal pallium through vertebrate evolution: Insights from reptiles and teleost fish. Brain Res. Bull. 57, 499–503. [DOI] [PubMed] [Google Scholar]
- Rodriguez F., Lopez J. C., Vargas J. P., Gomez Y., Broglio C., Salas C. (2002b). Conservation of spatial memory function in the pallial forebrain of reptiles and ray-finned fishes. J. Neurosci. 22, 2894–2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selderslaghs I. W., Van Rompay A. R., De Coen W., Witters H. E. (2009). Development of a screening assay to identify teratogenic and embryotoxic chemicals using the zebrafish embryo. Reprod. Toxicol. 28, 308–320. [DOI] [PubMed] [Google Scholar]
- Sun L., Xu W., Peng T., Chen H., Ren L., Tan H., Xiao D., Qian H., Fu Z. (2016). Developmental exposure of zebrafish larvae to organophosphate flame retardants causes neurotoxicity. Neurotoxicol. Teratol. 55, 16–22. [DOI] [PubMed] [Google Scholar]
- Truong L., Harper S. L., Tanguay R. L. (2011). Evaluation of embryotoxicity using the zebrafish model. Methods Mol. Biol. 691, 271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong L., Reif D. M., St Mary L., Geier M. C., Truong H. D., Tanguay R. L. (2014). Multidimensional in vivo hazard assessment using zebrafish. Toxicol. Sci. 137, 212–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. ZFIN. (2000). The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish Danio (Brachydanio) rerio, 4th ed ZFIN, Eugene, OR. [Google Scholar]
- Wullimann M. F., Mueller T. (2004). Teleostean and mammalian forebrains contrasted: Evidence from genes to behavior. J. Comp. Neurol. 475, 143–162. [DOI] [PubMed] [Google Scholar]
- Zellner D., Padnos B., Hunter D. L., MacPhail R. C., Padilla S. (2011). Rearing conditions differentially affect the locomotor behavior of larval zebrafish, but not their response to valproate-induced developmental neurotoxicity. Neurotoxicol. Teratol. 33, 674–679. [DOI] [PubMed] [Google Scholar]
- Zhang S., Hagstrom D., Hayes P., Graham A., Collins E. S. (2018). Multi-behavioral endpoint testing of an 87-chemical compound library in freshwater planarians. Toxicol. Sci. 167, 26–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Xu J., Kuang X., Li S., Li X., Chen D., Zhao X., Feng X. (2017). Biological impacts of glyphosate on morphology, embryo biomechanics and larval behavior in zebrafish (Danio rerio). Chemosphere 181, 270–280. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.