Abstract
Although impaired auditory–phonological processing is the most popular explanation of developmental dyslexia (DD), the literature shows that the combination of several causes rather than a single factor contributes to DD. Functioning of the visual magnocellular–dorsal (MD) pathway, which plays a key role in motion perception, is a much debated, but heavily suspected factor contributing to DD. Here, we employ a comprehensive approach that incorporates all the accepted methods required to test the relationship between the MD pathway dysfunction and DD. The results of 4 experiments show that (1) Motion perception is impaired in children with dyslexia in comparison both with age-match and with reading-level controls; (2) pre-reading visual motion perception—independently from auditory–phonological skill—predicts future reading development, and (3) targeted MD trainings—not involving any auditory–phonological stimulation—leads to improved reading skill in children and adults with DD. Our findings demonstrate, for the first time, a causal relationship between MD deficits and DD, virtually closing a 30-year long debate. Since MD dysfunction can be diagnosed much earlier than reading and language disorders, our findings pave the way for low resource-intensive, early prevention programs that could drastically reduce the incidence of DD.
Keywords: action video games, motion perception, perceptual learning, reading disorders, visual pathways
Introduction
Developmental dyslexia (DD) is the most common neurodevelopmental disorder (representing 80% of all specific learning disorders) and is characterized by severe difficulties in learning to read despite normal intelligence and adequate instruction (American Psychiatric Association 2013). DD leads to a severe cost to society, hampering the higher education of about 10% of children across cultures (Shaywitz et al. 2004).
Although learning to read involves multiple linguistic, visual, and attentional processes, the dominant view is that DD is an impairment in phonological awareness [see Snowling (2001), Goswami (2003), Shaywitz et al. (2004), Vellutino et al. (2004), Ziegler and Goswami (2005), Gabrieli (2009), Peterson and Pennington (2012) for reviews]. Phonological awareness refers to abilities in perceiving and manipulating the sounds of spoken words (Mattingly 1972), and involves not only discriminating speech sounds (Tallal 1980; Goswami et al. 2002; Hornickel and Kraus 2013), but also explicitly acting upon them (Castles and Coltheart 2004; Boets et al. 2013). The dominant hypothesis is that deficits in phonological awareness impair the ability to map speech sounds onto their homologous visual letters (i.e., grapheme–phoneme integration), preventing the attainment of fluent reading (Vellutino et al. 2004).
A key question is whether deficits in phonological awareness are the sole cause of DD. While the relevance of phonological awareness deficits should not be minimized, as many suggest that they are casual to DD (Bradley and Bryant 1983; Snowling 2001; Goswami 2003; Vellutino et al. 2004; Ziegler and Goswami 2005; Peterson and Pennington 2012) and they are correlated with poor input tuning into the regions mediating grapheme–phoneme integration (Blau et al. 2009; Dehaene et al. 2010; Clark et al. 2014; Myers et al. 2014; Krafnick et al. 2014; Thiebaut de Schotten et al. 2014; see for a recent review, Dehaene et al. 2015), there is substantial evidence that other factors are also involved in DD. In fact, multiple neurocognitive domains, such as auditory sensory processing (Tallal 1980; Goswami et al. 2002; Hornickel and Kraus 2013), multisensory selective attention (e.g., Hari and Renvall 2001; Visser et al. 2004; Bosse et al. 2007; Roach and Hogben 2007; Facoetti, Corradi, et al. 2010; Facoetti, Trussardi, et al. 2010; Lallier et al. 2010; Ruffino et al. 2010, 2014; Franceschini et al. 2012, 2013; Zorzi et al. 2012; Ronconi et al. 2014), and motion perception, specifically processed by the magnocellular–dorsal (MD) stream (e.g., Galaburda and Livingstone 1993; Stein and Talcott 1999; Tallal 2004; Kevan and Pammer 2008, 2009; Menghini et al. 2010; Boets et al. 2011; Gori, Cecchini, et al. 2014; Gori et al. 2015), have been widely recognized as correlates of DD [see Walsh (1995), Stein and Walsh (1997), Tallal (2004), Boden and Giaschi (2007), Laycock and Crewther (2008), Vidyasagar and Pammer (2010), Facoetti (2012), Gori and Facoetti (2014, 2015), Norton et al. (2015) for reviews]. This supports the view that DD is a multifactorial disorder characterized by a number of deficits that, in combination, lead to the resulting reading impairment (e.g., Menghini et al. 2010). Consequently, the search for a “unique DD deficit” is likely inadequate to explaining this complex neurodevelopmental disorder (Pennington 2006).
The MD pathway deficit theory is a popular model of DD that suggests that mild dysfunctions in visual motion processing are related to DD (Stein and Walsh 1997; Vidyasagar 1999; Boden and Giaschi 2007; Vidyasagar and Pammer 2010; Facoetti 2012; Gori and Facoetti 2014, 2015). However, after 30 years of scientific research characterized by intense debate around the causal relationship between the MD deficits and DD, this visual theory remains controversial [e.g., Amitay et al. 2002; Sperling et al. 2005, 2006; Olulade et al. 2013; see Goswami (2015) for a recent review].
The MD theory of DD stems from the observation that a high percentage of reading disabled children are impaired in tasks related to the function of visual MD pathway (Stein and Walsh 1997; Livingstone et al. 1991; Vidyasagar 1999; Boden and Giaschi 2007; Vidyasagar and Pammer 2010; Facoetti 2012; Gori and Facoetti 2014, 2015; Stein 2014). The MD pathway originates in the ganglion cells of the retina, passes through the M-layer of the lateral geniculate nucleus, and finally reaches the occipital and parietal cortices (Maunsell and Newsome 1987). The MD stream is considered to be color-blind, and responds well to luminance contrast, low spatial frequencies, high temporal frequencies, and both real and illusory motion (e.g., Livingstone and Hubel 1987; Morrone et al. 2000; Gori and Hamburger 2006; Gori and Yazdanbakhsh 2008; Gori et al. 2010, 2011; Ruzzoli et al. 2011; Yazdanbakhsh and Gori 2011; Gori, Agrillo, et al. 2014; Agrillo et al. 2015).
Much of the evidence supporting the MD deficit theory of DD is related to research on perception of coherent dot motion [CDM; e.g., Cornelissen et al. 1995; Talcott et al. 2000, 2002, 2013; Boets et al. 2011; see Stein (2001, 2014) for reviews], which heavily relies upon processing within the MD pathway (Newsome and Paré 1988). While motion perception is just a single function of the MD pathway, it is the most accepted proxy of MD functioning (e.g., Talcott et al. 2000, 2013; Sperling et al. 2006; Kevan and Pammer 2009; Boets et al. 2011; Olulade et al. 2013). Consistent with the MD deficit theory of DD, individuals with DD and pre-readers at risk for DD show poor performance on CDM tasks compared with typically reading controls (Eden et al. 1996; Kevan and Pammer 2008; Boets et al. 2011), while performing similarly to the controls on tasks, such as those involving color and form (Merigan and Maunsell 1993), preferentially associated with the parvocellular–ventral (PV) pathway (Kevan and Pammer 2009; Gori et al. 2014). It has been reported that up to 75% of dyslexic individuals show visual MD processing deficits (Lovegrove et al. 1986). Moreover, a postmortem study showed that magnocellular neurons of the lateral geniculate nucleus were significantly smaller in individuals with DD than those of normal readers, whereas the parvocellular neurons did not differ between the 2 groups (Livingstone et al. 1991). This finding was recently buttressed by the first in vivo MRI study (Giraldo-Chica et al. 2015), showing a smaller lateral geniculate nucleus volume in a larger sample of individuals with DD compared with controls. Recently, Gori, Cecchini, et al. (2014) and Gori et al. (2015) demonstrated that children with DD showed lower performance in tasks related to visual illusions that are thought to rely upon the MD pathway (i.e., the spatial frequency doubling illusion; Kelly 1966, the rotating-tilted-lines illusion, and the accordion grating illusion, Gori and Hamburger 2006; Gori and Yazdanbakhsh 2008; Gori et al. 2010, 2011, 2013; Yazdanbakhsh and Gori 2011) in comparison with both age and IQ-matched controls and also with reading-level (RL) controls (e.g., younger typically developed children reading at the same level as the DD group). Gori et al. (2015) also reported an association between a genetic variance (the DCDC2-Intron 2 deletion) and MD deficits in both individuals with DD and typical readers. This was confirmed by a replication study employing different MD measures (Cecchini et al. 2015) and is consistent with the DCDC2-Intron 2 deletion being a known genetic risk factor for DD (e.g., Meng et al. 2005; Marino et al. 2011, 2012, 2014; Mascheretti et al. 2013; Riva et al. 2015). Interestingly, the MD deficit in individuals with DD was found also in logographic languages such as Chinese (e.g., Zhao et al. 2014). Furthermore, several neuroimaging studies indicate the involvement of MD pathway regions (e.g., inferior parietal cortex) in reading (e.g., Cohen et al. 2008; see for reviews, Richlan et al. 2009; Richlan 2012).
However, some studies failed to confirm differences in high temporal, low spatial frequency stimulus perception, which are thought to rely upon MD processing, between individuals with DD and controls [e.g., Victor et al. 1993; Johannes et al. 1996; Williams et al. 2003; see Schulte-Körne and Bruder (2010) for a review]. Although there exists a substantial body of evidence that suggests a relationship between MD processing and DD (Stein 2012), the main criticism of MD deficit theory of DD is that MD deficits may not be causal to DD and, instead, could be a consequence of lack of reading experience (Olulade et al. 2013; Goswami 2015) since children with DD read far less than their peers (Cunningham and Stanovich 1997).
In the present study, we employ multiple accepted metrics to establish a causal relationship between MD function and DD (Goswami 2015). These are (1) comparison with RL controls; (2) a prospective, longitudinal approach where MD function is measured in pre-readers and its predictability with future reading development is investigated; and (3) remediation studies, in which MD processes are specifically trained and the subsequent effect on reading is measured. If MD deficit is truly a cause of DD, it is expected that an MD training, even that not involving any concomitant phonological and/or grapheme–phoneme integration, can improve reading abilities in DD (Gori and Facoetti 2014). Here, for the first time, we employ a comprehensive approach incorporating all these methods to test the relationship between the MD pathway dysfunction (as assessed by performance on CDM tasks) and DD.
Experiment 1: Visual Motion Perception in Children with Dyslexia and RL Controls
Materials and Methods
Participants
Visual motion perception was investigated in 15 children diagnosed with DD (American Psychiatric Association 2013; mean age = 11.13 years, SD = 0.74, mean full IQ = 102.73, SD = 7.12). Children with DD were recruited from clinical databases of the Child Psychopathology Unit of the Scientific Institute “E. Medea” (Bosisio Parini, Lecco, Italy), and were diagnosed by professional clinicians according to the following criteria: normal full IQ (≥85), normal or corrected-to-normal vision, absence of attention deficit disorder with hyperactivity or other neurological disorder, and reading performance (errors and/or speed) at least 2 SDs below the age-standardized norm in word, pseudo-words, or text reading. The 2 control groups with typical reading abilities, recruited from 3 schools in Mantova (Italy), comprised 18 chronological-age (CA)-matched children (mean age = 10.78 years, SD = 1.43) and 13 RL-matched children (mean age = 8.46 years, SD = 1.56, significantly different from the DD group age, t(26) = 5.91, P < 0.001). RL children were matched to the DD group for reading abilities, measured by an inefficiency index calculated as a ratio between word reading speed (seconds) and accuracy (rate). The mean inefficiency index did not differ between the DD group and the RL group (t(26) = 0.48, P = 0.63; Table 1).
Table 1.
Chronological age and reading abilities of participants in Experiment 1
| Dyslexics (n =
15) Mean (SD) |
Chronological-age controls
(n = 18) Mean (SD) |
Reading-level controls
(n = 13) Mean (SD) |
|
|---|---|---|---|
| Chronological age (years) | 11.13 (0.74) | 10.78 (1.43) | 8.46 (1.56) |
| Word reading speed and accuracy mean (age-standardized z-score) | −4.07 (2.56) | 0.48 (0.53) | 0.04 (0.42) |
| Word reading inefficiency (speed/accuracy) | 258 (119) | 73 (8) | 234 (186) |
Informed written consent was obtained for each child from their parents and the ethic committee of the University of Padua approved the research protocol. The entire investigation process was conducted according to the principles expressed in the Declaration of Helsinki.
Apparatus, Stimuli, and Procedure
CDM task
Participants were asked to discriminate the direction of dot movement (upward, downward, left, or right; chance level = 0.25), and only response accuracy was collected. There were 4 levels of coherence, randomly intermixed (10%, 20%, 30%, and 40%). The experimental session consisted of 80 trials (20 trials for each coherence level). The CDM display duration was 300 ms. Participants were seated in a dimly lit room in front of a 15-in. CRT monitor placed at a viewing distance of 57 cm (screen resolution 1024 × 768/60 Hz, with 0.3 mm of pixel size). The fixation point was a red dot in the center of the screen. After 500 ms, white dots, subtending a visual angle of 0.08°, appeared on a black background. Dots were contained in a circle of 13° of diameter and their number was approximately 10 deg−2 at each frame (duration = 16.7 ms). The dots density remained constant throughout the trial using the Shadlen–Movshon algorithm with limited lifetime of 3 frames (Britten et al. 1992; Pilly and Seitz 2009). Dots speed was 7 °/s. The procedure was similar to the one adopted by Ronconi et al. (2012).
Data Analysis
Accuracy rates were analyzed with a 4 × 3 mixed-design analysis of variance (ANOVA), with coherence (4 levels: 10%, 20%, 30%, and 40%) as a within-subjects factor and group (DD, CA, and RL) as a between-subjects factor.
Results
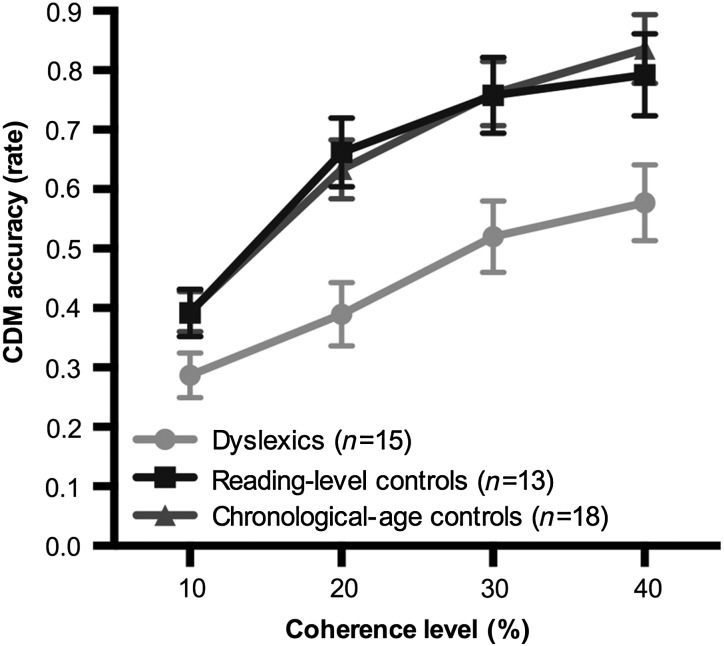
We found a main effect of coherence (F3,129 = 75.82, P < 0.0001). The group main effect was also significant (F2,40 = 6.87, P = .003), showing that the children with DD differed not only from CA controls, but also from the RL controls (Fig. 1).
Figure 1.
CDM accuracy in dyslexics (n = 15), reading-level (n = 13), and chronological-age controls (n = 18).
Experiment 2: Pre-Reading Visual Motion Perception in Future Children with Reading Disorders
Materials and Methods
Participants
Seventy-two 5-year-old children, attending the last year of 6 kindergartens in Northern Italy, took part in the present prospective, longitudinal study. In the Italian school system, formal reading instruction starts in grade 1. Consequently, Italian preschoolers are also pre-readers. We excluded from a larger sample the few children who were able to read at the kindergarten stage and those with an attention–deficit hyperactivity disorder. All children were native Italian speakers without any documented history of brain damage, hearing, or visual deficits. The verbal IQ level was estimated through the standard score in the Similarities subtest of the WPPSI scale (Wechsler 2002). Phonological awareness (errors in a syllabic segmentation task; Marotta et al. 2004) and visuo-attentional processing (errors and time in a visual search task; Franceschini et al. 2012) were also measured in kindergarten (T1). We examined the CDM (Britten et al. 1992; Pilly and Seitz 2009; Ronconi et al. 2012) performance in pre-reader children in T1. The development of reading skills in grade 1 was measured across the next year of compulsory schooling (T2). Each child was assigned to the poor reader group if her/his z-score for averaged speed and accuracy text reading (Sartori et al. 1995) was below −1.5 SDs. All children who did not meet the criterion for inclusion in the poor reader (n = 12) group were assigned to the typical reader (n = 60) group (Table 2).
Table 2.
Chronological age, verbal IQ, phonological awareness, and serial visual search abilities at pre-reading stage (T1) in future (T2) typical readers and poor readers in Experiment 2
| Typical readers (n =
60) Mean (SD) |
Poor readers (n =
12) Mean (SD) |
t (and P) value df = 70 |
|
|---|---|---|---|
| Chronological age (months) | 71.18 (3.41) | 71.17 (3.97) | 0.15 (0.99) |
| Verbal IQ (standard point) | 12.63 (2.79) | 11.25 (2.7) | 1.58 (0.12) |
| Syllabic segmentation (errors/15 items) | 1.02 (1.9) | 3.64 (4.99) | −3.09 (0.003) |
| Visual search (errors/25 targets) | 3.27 (3.19) | 6 (5.71) | −2.33 (0.02) |
| Visual search (s) | 93.03 (30.37) | 122.60 (38.75) | −2.94 (0.004) |
Informed written consent was obtained for each child from their parents and the ethic committee of the University of Padua approved the research protocol.
Apparatus, Stimuli, and Procedure
CDM task
The same CDM task described in Experiment 1 was employed.
Data Analysis
Pre-reading CDM performance was analyzed by a mixed-design ANOVA with 4 (motion coherence levels: 10%, 20%, 30%, and 40%) as a within-subjects factor and group by 2 (groups: typical readers and poor readers) as a between-subjects factor. A linear regression analysis on the entire sample was applied.
Results
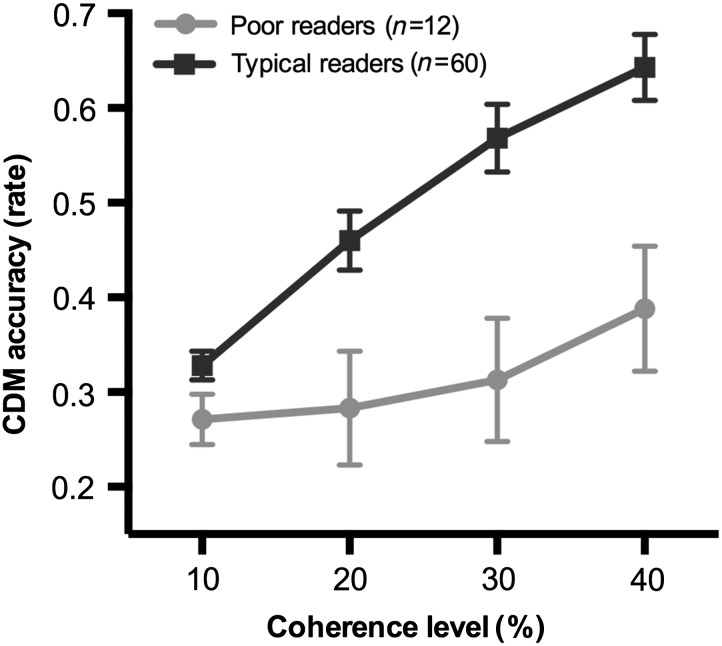
The main effects of motion coherence (F1,70 = 17.15, P < 0.0001) and group (F1,70 = 9.14, P = 0.003) were significant, with overall accuracy in motion discrimination that was decreased for the poor reader relative to the typical reader group (mean = 31.4%, SD = 19.05 vs. mean = 50% SD = 22.46). A significant “motion coherence” by “group” interaction (F3,210 = 6.94, P = 0.0002; Fig. 2) was found.
Figure 2.
CDM accuracy at pre-reading stage (T1) in future poor and typical readers (T2).
Complementing our a priori group classification in poor or typical readers, a linear regression analysis on the entire sample shows that pre-reading MD functioning captures future text reading skills in T2 after controlling for age and verbal IQ (ANOVA F3,71 = 2.96, P = 0.038; r2-change = 0.103, F change(1,68) = 7.889, P = 0.006). The unique variance explained by the pre-reading MD functioning controlling also for the syllabic segmentation task remains unchanged (r2-change = 0.102, F change(1,67) = 7.812, P = 0.007).
Experiment 3: Visual Motion Perception and Reading Skills After Action Video Games Training in Children with DD
Materials and Methods
Participants
Participants were 11 children with DD (mean age = 11.02 years, SD = 1.26, range 9.9–12.9 years). Children with DD were recruited from clinical databases of the “APprendo” clinical center (Padova, Italy) and were diagnosed by professional clinicians according to the criteria described above for Experiment 1. In addition, inexperience with action video games (AVG) was requested to participate in the study. Information about video game experience was collected in interviews of parents during pre-informative briefings before the experimental treatment. All children participated in both non-action video game (NAVG) and AVG treatment in 2 different phases (within-subject experimental design) and did not know the aim of the different treatments.
Informed written consent was obtained for each child from their parents and the ethic committee of the University of Padua approved the research protocol.
Apparatus, Stimuli, and Procedure
Video Game Trainings
The video game procedure for AVG and NAVG was exactly the same as used by Franceschini et al. (2013). Participants were tested 3–5 days before (Baseline: T1) the start of NAVG treatment and re-tested between 1 and 3 days after the end of the NAVG (T2) and AVG treatments (T3) in several tasks described below.
Pre- and Post-Training Tasks
Word text reading
Reading speed and errors in age-standardized prose passages from the Italian clinical test (Sartori et al. 1995) were used to measure natural-context reading. The mean between speed and accuracy z-scores was used to control reading speed–accuracy tradeoff effect.
Pseudo-words reading
Phonological decoding ability was measured using 3 different experimental texts of 46 pseudo-words (100 syllables; Franceschini et al. 2013), counterbalanced in T1, T2, and T3. Speed and accuracy were analyzed using an inefficiency index (time/accuracy) to control reading speed–accuracy tradeoff effect.
Phonological pseudo-word repetition
Forty pseudo-words (2–5 syllables; Bertelli and Bilancia 2006) were presented to children via headphones. Children had to repeat the single pseudo-word just presented. Pseudo-word repetition accuracy was measured.
CDM task
The exact same CDM task described in Experiments 1 and 2 was employed except for the 30% of coherence level that was not used here.
Illusory motion task
We tested illusory motion perception by employing the rotating-tilted-lines Illusion (Gori and Hamburger 2006). The rotating-tilted-lines Illusion represents an appropriate candidate for testing the functioning of this visual pathway and it has served already as a tool for this aim by Gori et al. (2015). Individual curves, representing the performance at the illusory effect task, in which the observers had to report if rotation was perceived, were fitted by a logistic function for each group. The upper bound was set at 1 and the lower bound at y0 = 0, where y = 0 means that the illusory rotation was never perceived, and y = 1 that it was always perceived. The only free parameters of the function were b (the function slope) and t (the 50% threshold). The resulting logistic function (Gori et al. 2008; Giora and Gori 2010; Gori and Spillmann 2010; Ronconi et al. 2012) is:
| (1) |
In this equation, x represents the percentage of contrast increment between the rotating-tilted-lines illusion and the background, and y the relative response frequency.
The rotating-tilted-lines illusion is the simplest pattern able to trigger illusory rotation in the presence of only radial expansion motion on the retina. Illusory motion perception is processed by the V5/MT complex, which is a core, neural station of the MD pathway. Visual stimuli were presented in the center of the computer screen. The experiment was carried out in a dimly lit (luminance of 1.5 cd/m2) and quiet room. Participants were seated 40 cm away from the screen. The fixation point consisted of a black dot displayed at the center of the screen (0.1°). The stimuli were movies where the rotating-tilted-lines illusion at a given contrast contracted and expanded continuously on the screen varying in diameter size in the range of 12.7–14.6° with a speed of 5.33 mm/s. Eleven Michelson contrast values (with a 1% step between each other), ranging from 0% to 10% between rotating-tilted-lines illusion and the background, were used. Before the experiment started, children were familiarized with a 98% contrast rotating-tilted-lines illusion and with an isoluminant colored version watching these patterns contracting and expanding on the screen. All children reported to see rotation motion in the high contrast rotating-tilted-lines illusion and no rotation but only expansion in the isoluminant version. During the experiment, the children performed 2 tasks in the presence of the same stimuli: a detection task and an illusory effect task. For the detection task, in each trial, the children were exposed to 1 of the 10 movies differing in contrast. The participants were required to perform a Yes/No task: He/she had to report if the circle of lines was present or not. Each movie was presented 5 times in a random order. The aim was to obtain a contrast detection threshold in the same condition of the illusory effect task. For the illusory effect task, in each trial, participants were exposed to 1 of the 10 movies differing in contrast. The participant's task was a Yes/No task: He/she had to say if rotation was perceived or not. Participants viewed the stimuli binocularly without time constraints. Each movie was presented 5 times, in a random order.
PV task
The stimuli used in this task were circular isoluminant gratings (7.4°, 40cd/m2) characterized by high spatial frequency (1.4 cycles/deg). The grating was oriented in 1 of 4 possible degrees of rotation (i.e., 40°, 85°, 130°, and 175°; chance level = 0.25). Five levels of colored random isoluminant noise were superimposed to the stimulus ranging from 0 (absence of noise) to 4 (maximum level of noise). This task presents all the characteristics to tap the PV pathway functionality (Kaplan and Shapley 1986) and has served already as a tool for this aim by Gori et al. (2015). Visual stimuli were presented in the center of the computer screen. The experiment was carried out in a dimly lit (luminance of 1.5 cd/m2) and quiet room. Participants were seated 40 cm away from the screen. The fixation point consisted of a cross displayed at the center of the screen (0.5°). Participants viewed each stimulus binocularly. Each trial began with the fixation mark. Participants were instructed to keep their eyes on the fixation mark throughout the duration of the trial. After 500 ms, the stimulus was displayed for 102 ms. Participants were instructed to identify the grating orientation among 4 possible degrees of rotation displayed on the screen until the response was given. Each participant was instructed to use all the time he/she needed to identify the target as accurately as possible, and no feedback was provided. The experimental session consisted of 40 trials (4 directions × 5 noise levels × 2 repetitions).
Data Analysis
To evaluate the effect of video game training on reading abilities, text reading z-score (average of speed and accuracy) and pseudo-word reading inefficiency (speed/accuracy) index score were analyzed with a one-way ANOVA with time (T1, T2, and T3) as a within-subject factor. To evaluate the effect of video game training on short-term phonological memory, we performed an one-way ANOVA on pseudo-word repetition accuracy with time (T1, T2, and T3) as a within-subject factor. We also calculated a standardized index including text reading, pseudo-word reading, and pseudo-word repetition to measure the amount of language-related learning triggered by the 2 video game trainings. To evaluate the effect of video game training on MD pathway functionality, a time (T1, T2, and T3) by coherence (10%, 20%, and 40%) ANOVA was performed on CDM accuracy. Moreover, a time (T1, T2, and T3) by noise (5 levels of noise) ANOVA was performed on the PV task accuracy. For all the ANOVAs, planned comparisons (paired sample t-tests) were used to explore main effects and interactions. Paired sample t-tests were then used to compare thresholds in the rotating-tilted-lines Illusion. To measure the improvement in the video game abilities of the 2 training methods, the dyslexic group was evaluated before session 3 and 9 (before day 3 many children did not reach a game recordable score) on a single mini-game (“Rabbids Just Want to Have Fun” for the AVG and “Bunnies Don't Understand Bowling” for the NAVG training). The z-scores from the video game scores were calculated and analyzed with a repeated-measures ANOVA, in which the 2 within-subject factors were time (performance recorded each of the day of treatment) and type of training (AVG and NAVG).
Results
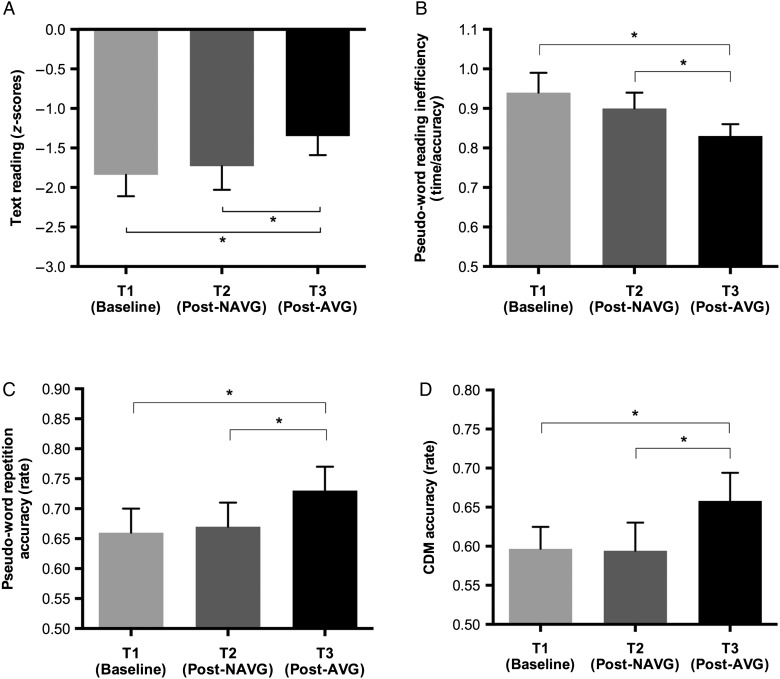
One-way ANOVA on text reading z-score (average of speed and accuracy) with time (T1, T2, and T3) as a within-subject factor showed the main effect of time (F2, 20 = 3.536, P = 0.048). Paired sample t-test showed that improvement in reading abilities was significant after AVG (T1 vs. T3: t(10) = −2.589, P = .027; T2 vs. T3: t(10) = −3.017, P = 0.013) but not after NAVG training (T1 vs. T2: t(10) = −0.041, P = 0.69; Fig. 3A).
Figure 3.
Performance at baseline (T1), post-NAVG (T2) and post-AVG training (T3) in: (A) word text reading ability (z-score); (B) pseudo-word reading ability inefficiency (time/accuracy); (C) pseudo-word repetition accuracy; and (D) CDM accuracy. Error bars denote the standard error of the mean. Asterisks indicate a significant difference with P < 0.05.
The one-way ANOVA on pseudo-word reading inefficiency index (speed/accuracy) with time (T1, T2, and T3) as a within-subject factor showed a main effect of time (F2, 20 = 3.989, P = 0.035). Again, paired sample t-test showed that improvement in phonological decoding abilities was significant after AVG (T1 vs. T3: t(10) = 2.559, P = 0.028; T2 vs. T3: t(10) = 2.391, P = 0.038) but not after NAVG training (T1 vs. T2: t(10) = 0.917, P = 0.381; Fig. 3B; see Table 3 for details in reading changes). An one-way ANOVA on pseudo-word repetition accuracy with time (T1, T2, and T3) as a within-subject factor showed that the main effect approached the significance (F2,20 = 3.379, P = 0.054). Paired sample t-test showed that improvements in phonological abilities were significant after AVG (T1 vs. T3: t(10) = −2.385, P = 0.019; T2 vs. T3: t(10) = −1.883, P = 0.044) but not after NAVG training (T1 vs. T2: t(10) = −0.635, P = 0.27; Fig. 3C). The standardized index language-related learning including text reading, pseudo-word reading, and pseudo-word repetition was analyzed. Language-related learning was not significantly different from zero after NAVG training (T2–T1 learning = 0.19 and SD = 0.5, t(10) = 1.261, P = 0.24), whereas it was significantly different from zero after AVG training (T3–T1 learning = 0.67 and SD = 0.45, t(10) = 4.906) as well as T3–T2 (learning = 0.48 and SD = 0.38; t(10) = 4.174, P = 0.002 and 0.001).
Table 3.
Word text and pseudo-word reading skills (speed and accuracy) at 3 times of training: baseline (T1), post-NAVG (T2), and post-AVG training (T3)
| Baseline (T1) Mean (SD) |
Post-NAVG (T2) Mean (SD) |
Post-AVG (T3) Mean (SD) |
|
|---|---|---|---|
| Word text reading average of speed and accuracy (age-standardized z-score) | −1.84 (0.9) | −1.74 (1.02) | −1.35 (0.81) |
| Word text reading speed (age-standardized z-score) | −1.80 (0.5) | −1.48 (0.67) | −1.26 (0.66) |
| Word text reading accuracy (age-standardized z-score) | −1.87 (1.66) | −1.99 (2.22) | −1.45 (1.97) |
| Pseudo-word reading inefficiency (time/accuracy) | 0.94 (0.15) | 0.90 (0.14) | 0.83 (0.11) |
| Pseudo-word reading speed (s) | 85.14 (11.55) | 80.2 (17.69) | 73.86 (16.99) |
| Pseudo-word reading accuracy (errors) | 9.09 (7.03) | 10.91 (13.19) | 11.18 (14.28) |
The ANOVA on CDM accuracy showed a main effects for coherence (F2,20 = 120.6, P < 0.0001) and time (F1,10 = 7.1, P = 0.024); paired sample t-test showed that improvement in CDM performance was significant after AVG (T1 vs. T3: t(10) = −2.665, P = 0.024; T2 vs. T3: t(10) = −2.289, P = 0.045), but not after NAVG training (T1 vs. T2: t(10) = 0.077, P = 0.941; Fig. 3D).
On the other hand, the ANOVA on the PV task accuracy showed only a significant main effect for noise level (F4,40 = 55.18, P < 0.0001), without a main effect of time (F < 1) nor an significant interaction (F < 1). PV task accuracy did not significantly change across the 3 times of training (mean accuracies: T1 = 0.70 and SD = 0.10, T2 = 0.71 and SD = 0.11, and T3 = 0.69 and SD = 0.10).
The specificity of the MD pathway is confirmed by the results of the illusory motion perception task. All 11 children with dyslexia reached the 100% of detection for the stimulus at 1% Michelson's contrast at each time point (T1, T2, and T3), showing that the contrast detection in this specific condition was not impaired. However, the 50% mean threshold for the illusory effect task was decreased only after AVG training (T2 = 5.27% and SD = 3.089 and T3 = 3.79%, SD = 2.535; paired sample t-test, t(10) = 1.997, P = 0.038). An ANOVA on time (performance recorded each day of treatment) and type of training (AVG and NAVG) showed significant main effects both for time (F1,10 = 185.74, P < 0.0001) and type of training (F1,10 = 44.09, P < 0.0001), without an interaction (F < 1). Paired sample t-tests revealed a significant improvement (i.e., mean z-score for day 3 vs. 9) in both AVG (from 0.62 SD = 0.11 to −1.3 SD = 0.26; t(10) = 6.54, P < 0.0001) and NAVG players (from 1.6 SD = 0.23 to −0.57 SD = 0.1; t(10) = 8.05, P < 0.0001).
Experiment 4: Reading Skills After MD Perceptual Learning Training in Adults with Dyslexia
Materials and Methods
Participants
Twenty-nine adult students (mean age = 22, age range 20–28 years), selected from a larger sample of about 300 students in the University of Padua, participated to a newly developed perceptual learning remediation approach (Sotiropoulos et al. 2011; Gori and Facoetti 2014) based on an adaptive version of the CDM task designed to train the MD pathway (Seitz et al. 2006). Participants received course credit for their participation. Eighteen students were diagnosed with DD (American Psychiatric Association 2013) according to the criteria described above for Experiments 1 and 3. They were randomly assigned to 3 different training groups (no-training, active-training, or MD-training). Their word text reading disabilities (Judica and De Luca 2005) measured before the training did not differ significantly (no-training: z-score mean = −2.424, SD = 0.986; active-training: z-score mean = −2.301, SD = 0.849; MD-training: z-score mean = −1.985, SD = 0.369; independent sample t-test all Ps > 0.314). The active-training control group (DD-Active) played card games on a personal computer for the same amount of time of the MD-training group. The other 11 participants were typical readers and were randomly assigned to the no-training and MD-training group. Phonological processing (i.e., phoneme blending task) in dyslexics of the MD-training group was significantly impaired relative to the control participants (independent sample t(15) = −2.22, P = 0.021), but not relative to the no-training or the active-training dyslexic groups (all Ps > 0.13).
Informed written consent was obtained for each participant and the ethic committee of the University of Padua approved the research protocol.
Apparatus, Stimuli, and Procedure
MD Trainings
Task-relevant and -irrelevant perceptual learning were used for the training. The only information of a direct comparison between the effectiveness of task-relevant and -irrelevant perceptual learning is about acoustic learning, and the results showed that they are both effective at the same level (Seitz et al. 2010). The duration of each training was 7.5 h, and the order of the 2 training was counterbalanced among participants.
Task-relevant perceptual learning training
The task was to decide which of the 2 displays in sequence contained coherent motion. The 2 displays were identical to that described for the pre- and post-test, but in one display the dots were moving all randomly, while in the other coherence level was controlled with a 3-down/1-up staircase with a step-size of 95% of the current coherence level. The participant's task was to indicate by pressing a key which of the 2 displays contains motion. The interval between the 2 displays was 500 ms. Each session lasted 45 min for 800 trials.
Task-irrelevant perceptual learning training
Here, we used the procedure of Seitz and Watanabe (2003, 2009) in which learning is found for motion stimuli paired with targets of a rapid serial visual presentation task (Seitz and Watanabe 2003). In these training sessions, participants were asked to perform a foveal rapid serial visual presentation character identification task to find a 2 target numbers among 6 letter distractors.
For each participant, 1 of the 8 possible motion directions was always paired with the target (numbers), whereas the other motion directions were randomly associated with the distractors (letters). Each training session contained 400 trials of the rapid serial visual presentation task and lasted 45 min. In these training sessions, participants were asked to perform a foveal rapid serial visual presentation character identification task to find a 2 target numbers among 6 letter distractors. Letter and number stimuli subtended 0.75° of visual angle. At the end of the trial (after 8 characters), subjects had to type, in order of presentation, the identity of the 2 numeric targets inside 4000 ms. No feedback was given. Each character in a sequence was presented for 300 ms with 200 ms interval between consecutive characters. In the periphery, an annulus of moving dots with the same characteristics of the one used in the pre- and post-training was presented with 5% of coherence motion that changed for every character presented in the rapid serial visual presentation task. The contrast between the characters and the background changed in function of the participant's performance due to an adaptive staircase.
Pre- and Post-Training Tasks
CDM test
Participants were asked to discriminate the direction of dot movement (8 directions). There were 2 levels of coherence, randomly intermixed (5% and 10%). Participants were seated in a dimly lit room in front of a 17-in. CRT monitor placed at a viewing distance of 40 cm (screen resolution 1024 × 768/80 Hz, with 0.3 mm of pixel size). The fixation point was a red dot in the center of the screen. After 500 ms, white dots, subtending a visual angle of 0.06° appeared on a black background. Dots were contained in a circle of 12.84° of diameter, and their density remained constant throughout the trial and was 16.7 dots per deg2/s. For each set, the probability that a dot moved in a specific direction as opposed to randomly is given by the coherence value. Dots speed was 12 °/s. The CDM display duration was 400 ms and each dot has a lifetime of 3 frames (105). The experimental session consisted of 576 trials (288 trials for both coherence levels and 36 for each of direction).
Word text reading task
Reading speed and errors in age-standardized prose passages from the Italian clinical test (Judica and De Luca 2005) were used to measure natural-context reading. The mean between speed and accuracy z-scores was used to control reading speed–accuracy tradeoff effect. Two different tests were used before (T1) and after (T2) the training to control for test–retest effect. To control for possible differences in the difficulty of the 2 reading tests, we considered as a baseline the difference in performance (T2–T1) of participants in the no-training groups.
Peripheral target perception task
The peripheral target perception task measured the ability of detecting a target in the peripheral vision (e.g., Ronconi, Gori, Ruffino, Molteni, et al. 2013; Ronconi et al. forthcoming) The experiment was conducted in a quiet and dimly lit room. Participants were seated 40 cm from an LCD screen (17 inch, 75 Hz). All stimuli were middle gray, displayed on a black background. The fixation point consisted of a cross (0.5°) presented in the center of the screen. The target stimulus was a dot of 0.5° which could appear at 1 of the 2 possible distances from the fixation point on the horizontal axis (i.e., 2° and 12°). The target was randomly presented in the left and in the right visual hemifield. Participants were instructed to keep their eyes on the fixation point throughout the duration of the trial. Each trial started with the onset of the fixation point. After 20, 50, or 500 ms (interstimulus interval, ISI), the target was displayed for 20 ms. Participants were instructed to press the spacebar on the keyboard as fast as possible at the target onset, and the computer recorded reaction times. Responses were recorded within 2 s from the stimulus onset. If any response was given, participants were advised with an 800-Hz sound played for 500 ms. Catch trials, in which the stimulus was not presented and the participant did not have to respond, were intermixed with response trials. The experimental session consisted of 54 randomized trials (8 repetitions for each ISI and eccentricity, and 6 catch trials).
Temporal attention task
The temporal attention task measured the ability of rapidly engages the attentional resources on a target before the appearance of a distractor (e.g., Facoetti et al. 2008; Ruffino et al. 2010; Dispaldro et al. 2013; Ronconi et al. 2013). The experimental environment was the same as described above for the peripheral visual perception task. Each trial began with the onset of the fixation mark (0.5°, 600 ms of duration) on a blue background. Participants were instructed to keep their eyes on the fixation mark throughout the duration of the trial. Two conditions, a “baseline” and a “masked” condition, were randomly presented to each participant. In the baseline condition, a single target (low spatial frequency circular grating filtered with a low-pass filter; diameter = 6.8°) was displayed for a duration of 100 ms. The target flickered at 60 Hz and was oriented in 4 possible orientations. The target was composed by black and white bands gradually soften, and a grayscale random-level noise was added to avoid a ceiling effect. In the baseline condition, after the target offset there was a 500-ms blank screen. In the masked condition, a mask composed by 4 circles (6.8°) positioned in the upper-right, upper-left, lower-right, and lower-left positions relative to the target was displayed for 500 ms. Participants were asked to indicate the perceived target orientation among the 4 possibilities (chance level = 0.25). Only response accuracy was collected and no feedback was provided. The entire experimental session consisted of 48 trials (i.e., 24 for baseline and 24 for masked condition).
Data Analysis
Text reading abilities were evaluated with a 3 × 2 mixed-design ANOVA with the group (DD–MD, DD-Active, and Control-MD) as a between-subjects factor and time (pre- and post-training) as a within-subjects factor.
To measure the training-induced improvements in the peripheral target perception task, we performed a 2 × 3 × 2 mixed-design ANOVA on mean reaction times. The within-subject factors were: time (pre- and post-training), ISI (20, 50, and 500 ms), and eccentricity (2° and 12°). The between-subjects factor was the training group (active-training vs. MD-training) of adults with DD.
To measure the training-induced improvements in the temporal attention task, we used an index in which the target accuracy difference between the baseline and the masked condition was calculated in T1 (pre-trainings) and T2 (post- trainings). Independent and paired sample t-tests were then used to compare performance of the difference training groups of individuals with DD.
To determine the predictive relationships between visuo-attentional and reading improvements, we performed a one-step, multiple regression analysis. The dependent variable was the word text reading improvements, and the predictors were visuo-attentional improvements.
Results
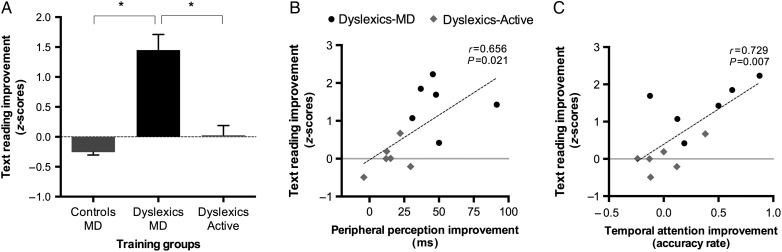
ANOVA on text reading ability revealed a significant group by time interaction (F2,15 = 6.964, P = 0.007). After training, a dramatic increase in word text reading (+1.45 z-score, average of speed and accuracy) was found only in the MD-training group (DD–MD vs. DD-Active-training, t(10) = 4.656, P = 0.001; DD–MD vs. Control-MD, t(10) = 6.446, P = 0.0001; Control-MD vs. DD-Active-training, t(10) = 1.703, P = 0.119; see Table 4 for details and Fig. 4A).
Table 4.
Word text reading improvements (T2–T1) for averaged speed/accuracy, speed, and accuracy in each trained group
| Reading improvements after treatments
(T2–T1): z-scores (SD) |
|||
|---|---|---|---|
| Dyslexics treated with MD
training Mean (SD) |
Dyslexics treated with active
training Mean (SD) |
Control group with MD training Mean (SD) |
|
| Word text reading mean between speed and accuracy | 1.451 (0.637) | 0.028 (0.391) | −0.256 (0.119) |
| Word text reading speed | 2.095 (0.788) | 0.721 (0.883) | −0.675 (0.491) |
| Word test reading accuracy | 0.806 (0.958) | −0.663 (1.134) | 0.162 (0.363) |
Note: Values are expressed in age-standardized z-scores.
DD–MD: dyslexics that underwent MD training; DD-Active: dyslexics that underwent a control active training; Control-MD: normal readers that underwent MD training
Figure 4.
(A) Reading improvement after the MD and active-trainings in the adults with (DD) and without (C) dyslexia. Error bars denote the standard error of the mean. Correlations between reading and peripheral target detection improvements (B), and between reading and temporal attention improvements (C) in trained adults with dyslexia (n = 12).
Peripheral Target Perception Task
A repeated-measures ANOVA on mean reaction times was performed. The ANOVA revealed main effects of time (F1,10 = 15.73, P = 0.003) and eccentricity (F1,10 = 16.95, P = 0.002). A significant “time” by “eccentricity” by “group” interaction emerged (F1,10 = 5.062, P = 0.048), revealing that only peripheral target perception (12°) was accelerated in the MD-training dyslexics group between T1 and T2 (paired sample t(5) = 5.78, P = 0.002) and with greater extent relative to the active-training dyslexics group (independent sample t(10) = 2.71, P = 0.022; Fig. 4B).
Temporal Attention Task
To measure the training-induced improvement in temporal attention, we used an index in which the target accuracy difference between the baseline and the masked condition was calculated in T1 (pre-trainings) and T2 (post-trainings). We found that the T1–T2 improvement in the DD–MD-training group differed significantly relative to those found in the active-training group (independent sample t-test: t(10) = −1.81, P = 0.033), and that temporal attention index (baseline-masking condition) was significantly improved from T1 to T2 only in the DD–MD-training group (paired sample t-test: t(5) = −1.88, P = 0.03; active-training group = P > 0.05; Fig. 4C).
The improvements in peripheral target perception and temporal attention (i.e., visuo-attentional improvements) correlated with those in word text reading skills on the entire sample of trained adults with dyslexia (n = 12; r(12) = 0.656, P = 0.021 and r(12) = 0.729, P = 0.007, respectively; Fig. 4B,C). We performed a one-step, multiple regression analysis. The visuo-attentional enhancements accounted for 63% of the variance of reading improvement (F2,9 = 7.53, P = 0.012).
Discussion
The causal role of the MD pathway deficit in DD is at the center of one of the most relevant debates in the last 30 years of neuroscience literature [e.g., Lovegrove et al. 1980; Livingstone et al. 1991; Victor et al. 1993; Olulade et al. 2013; see Goswami (2015) for a recent review]. Although the association between a mild MD deficit and DD has been consistently observed [see Walsh (1995), Stein and Walsh (1997), Vidyasagar (1999), Hari and Renvall (2001), Boden and Giaschi (2007), Laycock and Crewther (2008), Schulte-Körne and Bruder (2010), Vidyasagar and Pammer (2010), Stein (2012, 2014), Gori and Facoetti (2014, 2015), Goswami (2015) for reviews], the lack of studies employing causal experimental designs led to debate regarding the relationship between MD processing deficits and reading disorders (Goswami 2015).
In Experiment 1, we found a motion perception deficit in an unselected sample of children with DD both in comparison with age-matched and with younger typically reading children. These results strongly point in the direction of the causal role of MD pathway deficits in dyslexia. Motion is a primary feature processed by the MD pathway. Since the RL control group presents the same reading abilities of the DD group, these results could be the first step in research aimed at delineating the causal factors in reading difficulties. Consequently, these findings can rule out that the MD deficit is caused by poor reading skills (Goswami 2015). However, experimental designs using prospective-longitudinal and training studies are necessary to clearly establish a causal link between the MD deficit and DD.
Thus, in Experiment 2, we demonstrated that future poor readers were already impaired in motion processing at the pre-reading stage, showing reduced benefit from an increasing dot motion coherence level in comparison with future typical readers. These findings confirm that pre-readers at risk for DD and future poor readers are less sensitive than typically reading controls to motion displays (Kevan and Pammer 2008; Boets et al. 2011). Moreover, our findings provide powerful evidence that MD functioning in preschoolers predicts future reading acquisition independently from phonological awareness, according to a multifactorial probabilistic model for the etiology of DD (e.g., Pennington 2006; Menghini et al. 2010).
In Experiment 3, we found that both visual motion perception and reading skills were specifically improved after AVG training in children with DD. However, children similarly improved their video game abilities during the 2 video game treatments, indicating similar engagement for the 2 kinds of video games (AVG and NAVG). AVGs share an extraordinary speed in terms of transient events and moving objects, a high degree of perceptual and motor load, and an emphasis on peripheral processing. All these visual characteristics are processed by the MD stream; consequently, the AVG treatment is mainly tapping into the MD pathway.
In addition, we found significant improvement in auditory–phonological abilities after AVG treatment, showing how MD functioning and attentional improvements can also affect phonological skills. These data confirmed the cross- and multisensory effects of the AVG training (Green et al. 2010; Franceschini et al. 2013). It is important to note that in our study, we tested specifically the visual modality. However, we did not demonstrate that the causal relationship between MD and reading is exclusively related to visual mechanism. The theory, known as the temporal processing hypothesis, is the multisensory (i.e., visual and auditory) version of the MD theory of DD, and suggests that children with DD have specific deficits in processing rapidly presented or brief sensory stimuli in either the visual or auditory modalities [see Farmer and Klein (1995) and Hari and Renvall (2001) for reviews]. Chiefly, the MD temporal hypothesis explicitly claims that phonological decoding deficits in individuals with DD could arise from impairments in sensory processing of visual and auditory dynamic stimuli (e.g., Witton et al. 1998; Facoetti, Trussardi, et al. 2010; Facoetti, Corradi, et al. 2010; Vidyasagar 2013). Future studies about the role of the auditory MD pathway and reading seem to be necessary in order to call for a causal link between them.
Since CDM and PV tasks both presented signals embedded in noise, our findings that only CDM performance improved after AVG training rule out the possibility that improvement in the CDM task could be simply explained by improved noise exclusion mechanisms (Sperling et al. 2005, 2006). The specific effect of AVG training on the MD pathway is confirmed also by improved illusory motion perception which is an accepted proxy of the MD functionality that is not related to perceptual noise exclusion (Gori et al. 2015). These results not only expand on previous findings, but also indicate that the underlying neural substrate of the AVG training may be the MD pathway.
Although the AVG training presents important advantages to the development of specific trainings for DD, because of the appealing task that encourages compliance, on the other hand, the complicated task involved in the commercial video game makes it difficult to isolate the core mechanisms of how this type of training impacts DD. Consequently, a training that is based on a task known to rely upon the MD pathway is necessary to further establish a causal role of the MD deficit in DD.
In Experiment 4, we demonstrated that training of the MD pathway based on a CDM perceptual learning procedure drastically improved the reading skills in adults with DD. Thus, improvements in the MD pathway functioning directly translate to better reading skills. Interestingly, the MD pathway training also increased both peripheral visual perception and temporal mechanism of visual attention. Moreover, the training-induced perceptual and attentional changes explained a large quote of variance of the reading performance gain of the individuals with DD.
Our study is based on Italian language: A shallow language in comparison with other languages such English. One may argue that our results could not be easily generalized to other languages because of the high level of transparency of Italian. In transparent languages, it is possible that the phonological deficit could be less relevant for DD in comparison with more opaque ones. However, there are solid reasons why the difference in the deepness among languages cannot be crucial for the generalization of our results (Gori and Facoetti 2013). To clarify that we would like to specify that the cognitive mechanisms controlled by the MD pathway precede the orthographic-to-phonological mapping (e.g., Pammer et al. 2004, 2005, 2006). Reading depends on accurate visual analysis of the stimulus prior to the complex integration of orthographic and phonological information. The MD pathway provides a mechanism for the early selection of features in space (e.g., Vidyasagar 1998). According to Vidyasagar (1998), the MD pathway identifies and selects relevant regions in space to be then passed onto the ventral pathway. In other words, the MD pathway guides the ventral pathway. Thus, a deficit the MD pathway function can have a cascade effect on all the successive cognitive processes. An MD deficit is a peripheral deficit by definition and the dyslexia characterized by peripheral deficits is often found irrespectively of different degrees of language deepness. Importantly, several studies demonstrated that the core phonological decoding deficit in individuals with DD in different languages could partially arise from impairments in dynamic sensory processing of visual and auditory stimuli (e.g., Witton et al. 1998; Hari and Renvall 2001; Geiger et al. 2008; Facoetti, Trussardi, et al. 2010; Vidyasagar and Pammer 2010; Stein 2012, 2014; Gori et al. 2015). Given this it is likely that a training of MD will be beneficial to individuals with DD regardless the DD subtypes and the deepness of the language.
In summary, our findings, consistently across methods, demonstrate a causal role of the MD pathway deficit in DD. This comprehensive multimethod approach provides important evidence to the long-lasting debate about the MD theory. We suggest that the unsuccessful search for a single cause for DD makes the identification of other causes than phonological awareness of utmost importance to the development of more efficient remediation and prevention programs. The fact that the MD deficits can be tested in pre-readers (even at the infant level) paves the way for more effective DD remediation and prevention programs.
Authors' Contributions
S.G., A.S., and A.F. designed the experiments; S.G., A.S., L.R., S.F., and A.F. performed the data analyses and wrote the paper. L.R. and S.F. performed the experiments.
Funding
This work was funded with grants by the CARIPARO Foundation (“Borse di Dottorato CARIPARO 2009” and “Progetti di Eccellenza CARIPARO 2012–2014 rep. no. 1873/2012” to S.G., L.R., S.F., and A.F.) by the University of Padua (“Senior Post Doc Researcher 2014–2016” to S.G., “Young Researcher 2014” to L.R.; Progetto di Ateneo 2011; 2014 to A.F. and S.F.).
Notes
We thank Sara Bertoni for help in collecting data of the Experiments 1 and 3, Milena Ruffino and Katia Pedrolli for the Experiment 2, Francesca Noce and Concetta Cataudella (“APprendo” Clinical center of Padova) for the Experiment 3, and Julia R. Duggan for her helpful comments on the manuscript. Conflict of Interest: None declared.
Footnotes
Some errors in the text have been corrected.
References
- Agrillo C, Gori S, Beran MJ. 2015. Do rhesus monkeys (Macaca mulatta) perceive illusory motion. Anim Cogn. 18:895–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association, editors. 2013. Diagnostic and Statistical Manual of Mental Disorders. 5th ed Washington, DC: American Psychiatric Association. [Google Scholar]
- Amitay S, Ben-Yehudah G, Banai K, Ahissar M. 2002. Disabled readers suffer from visual and auditory impairments but not from a specific magnocellular deficit. Brain. 125:2272–2285. [DOI] [PubMed] [Google Scholar]
- Bertelli B, Bilancia G. 2006. Batteria per la Valutazione dell'Attenzione Uditiva e della Memoria di Lavoro Fonologica nell'Età Evolutiva. Firenze: Organizzazioni Speciali. [Google Scholar]
- Blau V, van Atteveldt N, Ekkebus M, Goebel R, Blomert L. 2009. Reduced neural integration of letters and speech sounds links phonological and reading deficits in adult dyslexia. Curr Biol. 24:503–508. [DOI] [PubMed] [Google Scholar]
- Boden C, Giaschi D. 2007. M-stream deficits and reading-related visual processes in developmental dyslexia. Psychol Bull. 133:346–366. [DOI] [PubMed] [Google Scholar]
- Boets B, Op de Beeck HP, Vandermosten M, Scott SK, Gillebert CR, Mantini D, Bulthé J, Sunaert S, Wouters J, Ghesquière P. 2013. Intact but less accessible phonetic representations in adults with dyslexia. Science. 3426163:1251–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boets B, Vandermosten M, Cornelissen P, Wouters J, Ghesquière P. 2011. Coherent motion sensitivity and reading development in the transition from prereading to reading stage. Child Dev. 82:854–869. [DOI] [PubMed] [Google Scholar]
- Bosse ML, Tainturier MJ, Valdois S. 2007. Developmental dyslexia: the visual attention span deficit hypothesis. Cognition. 104:198–230. [DOI] [PubMed] [Google Scholar]
- Bradley L, Bryant P. 1983. Categorizing sounds and learning to read: a causal connection. Nature. 301:419–421. [Google Scholar]
- Britten KH, Shadlen MN, Newsome WT, Movshon JA. 1992. The analysis of visual motion: a comparison of neuronal and psychophysical performance. J Neurosci. 12:4745–4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castles A, Coltheart M. 2004. Is there a causal link from phonological awareness to success in learning to read. Cognition. 91:77–111. [DOI] [PubMed] [Google Scholar]
- Cecchini GM, Marino C, Mascheretti S, Perani D, Morrone MC. 2015. Strong motion deficits in dyslexia associated with DCDC2 gene alteration. J Neurosci. 35:8059–8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark KA, Clark KA, Helland T, Specht K, Narr KL, Manis FR, Toga AW et al. 2014. Neuroanatomical precursors of dyslexia identified from pre-reading through to age 11. Brain. 137:3136–3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L, Dehaene S, Vinckier F, Jobert A, Montavont A. 2008. Reading normal and degraded words: contribution of the dorsal and ventral visual pathways. Neuroimage. 40:353–366. [DOI] [PubMed] [Google Scholar]
- Cornelissen P, Richardson A, Mason A, Fowler S, Stein J. 1995. Contrast sensitivity and coherent motion detection measured at photopic luminance levels in dyslexics and controls. Vision Res. 35:1483–1494. [DOI] [PubMed] [Google Scholar]
- Cunningham AE, Stanovich KE. 1997. Early reading acquisition and its relation to reading experience and ability 10 years later. Dev Psychol. 33:934–945. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Cohen L, Morais J, Kolinsky R. 2015. Illiterate to literate: behavioural and cerebral changes induced by reading acquisition. Nat Rev Neurosci. 16:234–244. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Pegado F, Braga LW, Ventura P, Nunes Filho G, Jobert A et al. 2010. How learning to read changes the cortical networks for vision and language. Science. 330:1359–1364. [DOI] [PubMed] [Google Scholar]
- Dispaldro M, Leonard LB, Corradi N, Ruffino M, Bronte T, Facoetti A. 2013. Visual attentional engagement deficits in children with specific language impairment and their role in real-time language processing. Cortex. 49:2126–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden G, VanMeter JW, Rumsey JM, Maisog JM, Woods RP, Zeffiro TA. 1996. Abnormal processing of visual motion in dyslexia revealed by functional neuroimaging. Neuron. 21:279–282. [DOI] [PubMed] [Google Scholar]
- Facoetti A. 2012. Spatial attention disorders in developmental dyslexia: towards the prevention of reading acquisition deficits. In: Stein J, Kapoula Z, editors. Visual aspect of dyslexia. Oxford, UK: Oxford University Press; pp. 123–136. [Google Scholar]
- Facoetti A, Corradi N, Ruffino M, Gori S, Zorzi M. 2010. Visual spatial attention and speech segmentation are both impaired in preschoolers at familial risk for developmental dyslexia. Dyslexia. 16:226–239. [DOI] [PubMed] [Google Scholar]
- Facoetti A, Ruffino M, Peru A, Paganoni P, Chelazzi L. 2008. Sluggish engagement and disengagement of non-spatial attention in dyslexic children. Cortex. 44:1221–1233. [DOI] [PubMed] [Google Scholar]
- Facoetti A, Trussardi AN, Ruffino M, Lorusso ML, Cattaneo C, Galli R et al. 2010. Multisensory spatial attention deficits are predictive of phonological decoding skills in developmental dyslexia. J Cogn Neurosci. 22:1011–1025. [DOI] [PubMed] [Google Scholar]
- Farmer ME, Klein RM. 1995. The evidence for a temporal processing deficit linked to dyslexia: A review. Psychon Bull Rev. 2(4):460–493. [DOI] [PubMed] [Google Scholar]
- Franceschini S, Gori S, Ruffino M, Pedrolli K, Facoetti A. 2012. A causal link between visual spatial attention and reading acquisition. Curr Biol. 22:814–819. [DOI] [PubMed] [Google Scholar]
- Franceschini S, Gori S, Ruffino M, Viola S, Molteni M, Facoetti A. 2013. Action video games make dyslexic children read better. Curr Biol. 23:462–466. [DOI] [PubMed] [Google Scholar]
- Gabrieli JD. 2009. Dyslexia: a new synergy between education and cognitive neuroscience. Science. 325:280–283. [DOI] [PubMed] [Google Scholar]
- Galaburda A, Livingstone M. 1993. Evidence for a magnocellular defect in developmental dyslexia. Ann N Y Acad Sci. 682:70–82. [DOI] [PubMed] [Google Scholar]
- Geiger G, Cattaneo C, Galli R, Pozzoli U, Lorusso ML, Facoetti A, Molteni M. 2008. Wide and diffuse perceptual modes characterize dyslexics in vision and audition. Perception. 37:1745–1764. [DOI] [PubMed] [Google Scholar]
- Giora E, Gori S. 2010. The perceptual expansion of a filled area depends on textural characteristics. Vis Res. 50:2466–2475. [DOI] [PubMed] [Google Scholar]
- Giraldo-Chica M, Hegarty JP II, Schneider KA. 2015. Morphological differences in the lateral geniculate nucleus associated with dyslexia. Neuroimage Clin. 20:830–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gori S, Agrillo C, Dadda M, Bisazza A. 2014. Do fish perceive illusory motion. Sci Rep. 4:6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gori S, Cecchini P, Bigoni A, Molteni M, Facoetti A. 2014. Magnocellular-dorsal pathway and sub-lexical route in developmental dyslexia. Front Hum Neurosci. 8:460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gori S, Facoetti A. 2013. Is the language transparency really that relevant for the outcome of the action video games training. Curr Biol. 23; http://www.cell.com/current-biology/abstract/S0960-9822(13)00258-3#Comments Comment on the Dispatch by Bavelier et al. Curr Biol. 23 R282-R283. [Google Scholar]
- Gori S, Facoetti A. 2015. How the visual aspects can be crucial in reading acquisition: the intriguing case of crowding and developmental dyslexia. J Vis. 15:8. [DOI] [PubMed] [Google Scholar]
- Gori S, Facoetti A. 2014. Perceptual learning as a possible new approach for remediation and prevention of developmental dyslexia. Vis Res. 99:78–87. [DOI] [PubMed] [Google Scholar]
- Gori S, Giora E, Pedersini R. 2008. Perceptual multistability in figure-ground segregation using motion stimuli. Acta Psychol. 129:399–409. [DOI] [PubMed] [Google Scholar]
- Gori S, Giora E, Stubbs DA. 2010. Perceptual compromise between apparent and veridical motion indices: the Unchained-Dots illusion. Perception. 39:863–866. [DOI] [PubMed] [Google Scholar]
- Gori S, Giora E, Yazdanbakhsh A, Mingolla E. 2011. A new motion illusion based on competition between two kinds of motion processing units: the accordion grating. Neural Netw. 24:1082–1092. [DOI] [PubMed] [Google Scholar]
- Gori S, Giora E, Yazdanbakhsh A, Mingolla E. 2013. The novelty of the accordion-grating. Neural Netw. 39:52. [DOI] [PubMed] [Google Scholar]
- Gori S, Hamburger K. 2006. A new motion illusion: the Rotating-Tilted-Lines illusion. Perception. 356:853–885. [DOI] [PubMed] [Google Scholar]
- Gori S, Mascheretti S, Giora E, Ronconi L, Ruffino M, Quadrelli E et al. 2015. The DCDC2 Intron 2 deletion impairs illusory motion perception unveiling the selective role of magnocellular-dorsal stream in reading (dis)ability. Cereb Cortex. 25:1685–1695. [DOI] [PubMed] [Google Scholar]
- Gori S, Spillmann L. 2010. Detection vs. grouping thresholds for elements differing in spacing, size and luminance. An alternative approach towards the psychophysics of Gestalten. Vis Res 50:1194–1202. [DOI] [PubMed] [Google Scholar]
- Gori S, Yazdanbakhsh A. 2008. The riddle of the Rotating-Tilted-Lines illusion. Perception. 37:631–635. [DOI] [PubMed] [Google Scholar]
- Goswami U. 2015. Sensory theories of developmental dyslexia: three challenges for research. Nat Rev Neurosci. 16:43–54. [DOI] [PubMed] [Google Scholar]
- Goswami U. 2003. Why theories about developmental dyslexia require developmental designs. Trends Cogn Sci 7:534–540. [DOI] [PubMed] [Google Scholar]
- Goswami U, Thomson J, Richardson U, Stainthorp R, Hughes D, Rosen S et al. 2002. Amplitude envelope onsets and developmental dyslexia: a new hypothesis. Proc Natl Acad Sci USA. 99:10911–10916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CS, Pouget A, Bavelier D. 2010. Improved probabilistic inference as a general learning mechanism with action video games. Curr Biol. 20:1573–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari R, Renvall H. 2001. Impaired processing of rapid stimulus sequences in dyslexia. Trends Cogn Sci. 5:525–532. [DOI] [PubMed] [Google Scholar]
- Hornickel J, Kraus N. 2013. Unstable representation of sound: a biological marker of dyslexia. J Neurosci. 33:3500–3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannes S, Kussmaul CL, Münte TF, Mangun GR. 1996. Developmental dyslexia: passive visual stimulation provides no evidence for a magnocellular processing defect. Neuropsychologia. 34:1123–1127. [DOI] [PubMed] [Google Scholar]
- Judica A, De Luca M. 2005. Prova di velocità di lettura di brani per la Scuola Media Superiore. Italy: Fondazione Santa Lucia. [Google Scholar]
- Kaplan E, Shapley RM. 1986. The primate retina contains two types of ganglion cells, with high and low contrast sensitivity. Proc Natl Acad Sci USA. 83:2755–2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DH. 1966. Frequency doubling in visual responses. J Opt Soc Am. 56:1628–1632. [Google Scholar]
- Kevan A, Pammer K. 2009. Predicting early reading skills from pre-reading measures of dorsal stream functioning. Neuropsychologia. 47:3174–3181. [DOI] [PubMed] [Google Scholar]
- Kevan A, Pammer K. 2008. Visual processing deficits in preliterate children at familial risk for dyslexia. Vis Res. 48:2835–2839. [DOI] [PubMed] [Google Scholar]
- Krafnick AJ, Flowers DL, Luetje MM, Napoliello EM, Eden GF. 2014. An investigation into the origin of anatomical differences in dyslexia. J Neurosci. 34:901–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallier M, Donnadieu S, Valdois S. 2010. Visual attentional blink in dyslexic children: parameterizing the deficit. Vision Res. 50:1855–1861. [DOI] [PubMed] [Google Scholar]
- Laycock R, Crewther SG. 2008. Towards an understanding of the role of the “magnocellular advantage” in fluent reading. Neurosci Biobehav Rev. 32:1494–1506. [DOI] [PubMed] [Google Scholar]
- Livingstone MS, Hubel DH. 1987. Psychophysical evidence for separate channels for the perception of form, color, movement, and depth. J Neurosci. 7:3416–3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone MS, Rosen GD, Drislane FW, Galaburda AM. 1991. Physiological and anatomical evidence for a magnocellular defect in developmental dyslexia. Proc Natl Acad Sci USA. 8818:7943–7947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovegrove W, Martin F, Slaghuis WA. 1986. Theoretical and experimental case for residual deficit in specific reading disability. Cogn Neuropsychol. 3:225–267. [Google Scholar]
- Lovegrove WJ, Bowling A, Badcock D, Blackwood M. 1980. Specific reading disability: differences in contrast sensitivity as a function of spatial frequency. Science. 210:439–440. [DOI] [PubMed] [Google Scholar]
- Marino C, Mascheretti S, Riva V, Cattaneo F, Rigoletto C, Rusconi M et al. 2011. Pleiotropic effects of DCDC2 and DYX1C1 genes on language and mathematics traits in nuclear families of developmental dyslexia. Behav Genet. 4:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino C, Meng H, Mascheretti S, Rusconi M, Cope N, Giorda R et al. 2012. DCDC2 genetic variants and susceptibility to developmental dyslexia. Psychiatr Genet. 22:25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino C, Scifo P, Della Rosa PA, Mascheretti S, Facoetti A, Lorusso ML et al. 2014. The DCDC2/intron 2 deletion and white matter disorganization: focus on developmental dyslexia. Cortex. 57:227–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marotta L, Ronchetti C, Trasciani M, Vicari S. 2004. CMF: Valutazione Delle Competenze Meta Fonologiche. Italy: Erickson Trento. [Google Scholar]
- Mascheretti S, Bureau A, Battaglia M, Simone D, Quadrelli E, Croteau J et al. 2013. An assessment of gene-by-environment interactions in developmental dyslexia-related phenotypes. Genes Brain Behav. 12:47–55. [DOI] [PubMed] [Google Scholar]
- Mattingly IG. 1972. Speech cues and sign stimuli. Am Sci. 603:327–337. [PubMed] [Google Scholar]
- Maunsell JH, Newsome WT. 1987. Visual processing in monkey extrastriate cortex. Annu Rev Neurosci. 10:363–401. [DOI] [PubMed] [Google Scholar]
- Meng H, Smith SD, Hager K, Held M, Liu J, Olson RK et al. 2005. DCDC2 is associated with reading disability and modulates neuronal development in the brain. Proc Natl Acad Sci USA. 102:17053–17058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menghini D, Finzi A, Benassi M, Bolzani R, Facoetti A, Giovagnoli S et al. 2010. Different underlying neurocognitive deficits in developmental dyslexia: a comparative study. Neuropsychologia. 48:863–872. [DOI] [PubMed] [Google Scholar]
- Merigan WH, Maunsell JH. 1993. How parallel are the primate visual pathways. Annu Rev Neurosci. 16:369–402. [DOI] [PubMed] [Google Scholar]
- Morrone MC, Tosetti M, Montanaro D, Fiorentini A, Cioni G, Burr DC. 2000. A cortical area that responds specifically to optic flow, revealed by fMRI. Nat Neurosci. 3:1322–1328. [DOI] [PubMed] [Google Scholar]
- Myers CA, Vandermosten M, Farris EA, Hancock R, Gimenez P, Black JM, Casto B, Drahos M, Tumber M, Hendren RL et al. 2014. White matter morphometric changes uniquely predict children's reading acquisition. Psychol Sci. 2510:1870–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome WT, Paré EB. 1988. A selective impairment of motion perception following lesions of the middle temporal visual area (MT). J Neurosci. 8(6):2201–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton ES, Beach SD, Gabrieli J. 2015. Neurobiology of dyslexia. Curr Opin Neurobiol. 30:73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olulade OA, Napoliello EM, Eden GF. 2013. Abnormal visual motion processing is not a cause of dyslexia. Neuron. 79:180–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pammer K, Hansen P, Holliday I, Cornelissen P. 2006. Attentional shifting and the role of the dorsal pathway in visual word recognition. Neuropsychologia. 44:2926–2936. [DOI] [PubMed] [Google Scholar]
- Pammer K, Lavis R, Cooper C, Hansen PC, Cornelissen PL. 2005. Symbol-string sensitivity and adult performance in lexical decision. Brain Lang. 94(3):278–296. [DOI] [PubMed] [Google Scholar]
- Pammer K, Lavis R, Hansen P, Cornelissen PL. 2004. Symbol-string sensitivity and children's reading. Brain Lang. 89:601–610. [DOI] [PubMed] [Google Scholar]
- Pennington BF. 2006. From single to multiple deficit models of developmental disorders. Cognition. 101:385–413. [DOI] [PubMed] [Google Scholar]
- Peterson RL, Pennington BF. 2012. Developmental dyslexia. Lancet. 26:1997–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilly PK, Seitz AR. 2009. What a difference a parameter makes: a psychophysical comparison of random dot motion algorithms. Vis Res. 49:1599–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F. 2012. Developmental dyslexia: dysfunction of a left hemisphere reading network. Front Hum Neurosci. 6:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, Wimmer H. 2009. Functional abnormalities in the dyslexic brain: a quantitative meta-analysis of neuroimaging studies. Hum Brain Mapp. 30:3299–3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva V, Marino C, Giorda R, Molteni M, Nobile M. 2015. The role of DCDC2 genetic variants and low socioeconomic status in vulnerability to attention problems. Eur Child Adolesc Psychiatry. 24:309–318. [DOI] [PubMed] [Google Scholar]
- Roach NW, Hogben JH. 2007. Impaired filtering of behaviourally irrelevant visual information in dyslexia. Brain. 130:771–785. [DOI] [PubMed] [Google Scholar]
- Ronconi L, Basso D, Gori S, Facoetti A. 2014. TMS on right frontal eye fields induces an inflexible focus of attention. Cereb Cortex. 24:396–402. [DOI] [PubMed] [Google Scholar]
- Ronconi L, Franchin L, Valenza E, Gori S, Facoetti A Forthcoming. The attentional “zoom-lens” in 8-month-old infants. Dev Sci. 10.1111/desc.12288. [DOI] [PubMed] [Google Scholar]
- Ronconi L, Gori S, Giora E, Ruffino M, Molteni M, Facoetti A. 2013. Deeper attentional masking by lateral objects in children with autism. Brain Cogn. 82:213–218. [DOI] [PubMed] [Google Scholar]
- Ronconi L, Gori S, Ruffino M, Franceschini S, Urbani B, Molteni M et al. 2012. Decreased coherent motion discrimination in autism spectrum disorder: the role of attentional zoom-out deficit. PLoS ONE. 7:e49019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronconi L, Gori S, Ruffino M, Molteni M, Facoetti A. 2013. Zoom-out attentional impairment in children with autism spectrum disorder. Cortex. 49:1025–1033. [DOI] [PubMed] [Google Scholar]
- Ruffino M, Gori S, Boccardi D, Molteni M, Facoetti A. 2014. Spatial and temporal attention in developmental dyslexia. Front Hum Neurosci. 8:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffino M, Trussardi AN, Gori S, Finzi A, Giovagnoli S, Menghini D et al. 2010. Attentional engagement deficits in dyslexic children. Neuropsychologia. 48:3793–3801. [DOI] [PubMed] [Google Scholar]
- Ruzzoli M, Gori S, Pavan A, Pirulli C, Marzi CA, Miniussi C et al. 2011. The neural basis of the Enigma illusion: a transcranial magnetic stimulation study. Neuropsychologia. 4:3648–3655. [DOI] [PubMed] [Google Scholar]
- Sartori G, Job R, Tressoldi PE. 1995. Batteria per la valutazione della dislessia e della disortografia evolutiva. Firenze: Organizzazioni Speciali. [Google Scholar]
- Schulte-Körne G, Bruder J. 2010. Clinical neurophysiology of visual and auditory processing in dyslexia: a review. Clin Neurophysiol. 121:1794–1809. [DOI] [PubMed] [Google Scholar]
- Seitz AR, Nanez JE, Holloway SR, Watanabe T. 2006. Perceptual learning of motion leads to faster flicker perception. PLoS ONE. 1:e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz AR, Protopapas A, Tsushima Y, Vlahou EL, Gori S, Grossberg S et al. 2010. Unattended exposure to components of speech sounds yields same benefits as explicit auditory training. Cognition. 115:435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz AR, Watanabe T. 2009. The phenomenon of task-irrelevant perceptual learning. Vis Res. 49:2604–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz AR, Watanabe T. 2003. Psychophysics: is subliminal learning really passive. Nature. 422:36. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Blachman BA, Pugh KR, Fulbright RK, Skudlarski P et al. 2004. Development of left occipitotemporal systems for skilled reading in children after a phonologically-based intervention. Biol Psychiatry. 55:926–933. [DOI] [PubMed] [Google Scholar]
- Snowling MJ. 2001. From language to reading and dyslexia. Dyslexia. 7:37–46. [DOI] [PubMed] [Google Scholar]
- Sotiropoulos G, Seitz AR, Series P. 2011. Changing expectations about speed alters perceived motion direction. Curr Biol. 21:R883–R884. [DOI] [PubMed] [Google Scholar]
- Sperling AJ, Lu ZL, Manis FR, Seidenberg MS. 2005. Deficits in perceptual noise exclusion in developmental dyslexia. Nat Neurosci. 87:862–863. [DOI] [PubMed] [Google Scholar]
- Sperling AJ, Lu ZL, Manis FR, Seidenberg MS. 2006. Motion-perception deficits and reading impairment: it's the noise, not the motion. Psychol Sci. 1712:1047–1053. [DOI] [PubMed] [Google Scholar]
- Stein J. 2014. Dyslexia: the role of vision and visual attention. Curr Dev Disord Rep. 1:267–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein J. 2001. The magnocellular theory of developmental dyslexia. Dyslexia. 7:12–36. [DOI] [PubMed] [Google Scholar]
- Stein J. 2012. Visual contributions to reading difficulties: the magnocellular theory. In: Stein J, Kapoula Z, editors. Visual aspect of dyslexia. Oxford, UK: Oxford University Press; pp. 171–197. [Google Scholar]
- Stein J, Walsh V. 1997. To see but not to read; the magnocellular theory of dyslexia. Trends Neurosci. 20:147–152. [DOI] [PubMed] [Google Scholar]
- Stein JF, Talcott JB. 1999. Impaired neuronal timing in developmental dyslexia—the magnocellular hypothesis. Dyslexia. 5:59–78. [Google Scholar]
- Talcott JB, Witton C, Hebb GS, Stoodley CJ, Westwood EA, France SJ et al. 2002. On the relationship between dynamic visual and auditory processing and literacy skills; results from a large primary-school study. Dyslexia. 8:204–225. [DOI] [PubMed] [Google Scholar]
- Talcott JB, Witton C, McLean MF, Hansen PC, Rees A, Green GG et al. 2000. Dynamic sensory sensitivity and children's word decoding skills. Proc Natl Acad Sci USA. 97:2952–2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talcott JB, Witton C, Stein JF. 2013. Probing the neurocognitive trajectories of children's reading skills. Neuropsychologia. 51:472–481. [DOI] [PubMed] [Google Scholar]
- Tallal P. 2004. Improving language and literacy is a matter of time. Nat Rev Neurosci 5:721–728. [DOI] [PubMed] [Google Scholar]
- Tallal P. 1980. Language disabilities in children: a perceptual or linguistic deficit. J Pediatr Psychol. 5:127–140. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Cohen L, Amemiya E, Braga LW, Dehaene S. 2014. Learning to read improves the structure of the arcuate fasciculus. Cereb Cortex. 24:989–995. [DOI] [PubMed] [Google Scholar]
- Vellutino FR, Fletcher JM, Snowling MJ, Scanlon DM. 2004. Specific reading disability (dyslexia): what have we learned in the past four decades. J Child Psychol Psychiatry. 45:2–40. [DOI] [PubMed] [Google Scholar]
- Victor JD, Conte MM, Burton L, Nass RD. 1993. Visual evoked potentials in dyslexics and normals: failure to find a difference in transient or steady-state responses. Vis Neurosci. 10:939–946. [DOI] [PubMed] [Google Scholar]
- Vidyasagar TR. 1998. Gating of neuronal responses in macaque primary visual cortex by an attentional spotlight. Neuroreport. 9(9):1947–1952. [DOI] [PubMed] [Google Scholar]
- Vidyasagar TR. 1999. A neuronal model of attentional spotlight: parietal guiding the temporal. Brain Res Brain Res Rev. 30:66–76. [DOI] [PubMed] [Google Scholar]
- Vidyasagar TR. 2013. Reading into neuronal oscillations in the visual system: implications for developmental dyslexia. Front Hum Neurosci. 7:811 10.3389/fnhum.2013.00811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidyasagar TR, Pammer K. 2010. Dyslexia: a deficit in visuo-spatial attention, not in phonological processing. Trends Cogn Sci. 14:57–63. [DOI] [PubMed] [Google Scholar]
- Visser TA, Boden C, Giaschi DE. 2004. Children with dyslexia: evidence for visual attention deficits in perception of rapid sequences of objects. Vision Res. 44:2521–2535. [DOI] [PubMed] [Google Scholar]
- Walsh V. 1995. Dyslexia. Reading between the laminae. Curr Biol. 5:1216–1217. [DOI] [PubMed] [Google Scholar]
- Wechsler D. 2002. WIPPSI-R. Wechsler Preschool and Primary Scale of Intelligence. Italian ed Firenze: Organizzazioni Speciali. [Google Scholar]
- Williams MJ, Stuart GW, Castles A, McAnally KI. 2003. Contrast sensitivity in subgroups of developmental dyslexia. Vis Res. 43:467–477. [DOI] [PubMed] [Google Scholar]
- Witton C, Talcott JB, Hansen PC, Richardson AJ, Griffiths TD, Rees A et al. 1998. Sensitivity to dynamic auditory and visual stimuli predicts nonword reading ability in both dyslexic and normal readers. Curr Biol. 8:791–797. [DOI] [PubMed] [Google Scholar]
- Yazdanbakhsh A, Gori S. 2011. Mathematical analysis of the Accordion Grating illusion: a differential geometry approach to introduce the 3D aperture problem. Neural Netw. 24:1093–1101. [DOI] [PubMed] [Google Scholar]
- Zhao J, Qian Y, Bi HY, Coltheart M. 2014. The visual magnocellular-dorsal dysfunction in Chinese children with developmental dyslexia impedes Chinese character recognition. Sci Rep. 4:7068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler JC, Goswami U. 2005. Reading acquisition, developmental dyslexia, and skilled reading across languages: a psycholinguistic grain size theory. Psychol Bull. 131:3–29. [DOI] [PubMed] [Google Scholar]
- Zorzi M, Barbiero C, Facoetti A, Lonciari I, Carrozzi M, Montico M et al. 2012. Extra spacing letter improves reading in dyslexia. Proc Natl Acad Sci USA. 109:11455–11459. [DOI] [PMC free article] [PubMed] [Google Scholar]