Abstract
Methylphenidate (MP) is a commonly prescribed psychostimulant for Attention Deficit Hyperactivity Disorder (ADHD). We recently reported behavioral and developmental effects of chronic MP use in healthy rats. The current study investigated how interrupting chronic MP treatment with weekend abstinence altered the behavioral and physiological consequences of chronic MP treatment, and if prolonged abstinence would reverse the observed effects. Male Sprague Dawley rats were assigned to one of three treatment groups: water (W); low dose (LD) MP; and high dose (HD) MP. For 13 weeks, rats had access to drink from a bottle containing 4 mg/kg MP (LD), 30 mg/kg MP (HD) or water (W) for 1 hour, and 10 mg/kg MP (LD), 60 mg/kg MP (HD) or water (W) for the next 7 hours, each week day. During weekends, all animals received only water as well as throughout the 5-week-long abstinence phase, which immediately followed the treatment phase. Throughout the treatment phase, regardless of weekend abstinence, chronic MP resulted in significant decreased food and fluid intake and body weight. Also, HD MP exposure resulted in the following behavioral effects: increased open field and circadian locomotor activity; increased latency to immobility and decreased time spent immobile in the forced swim test; increased center activity in the open field and percent of time spent in an open arm of the elevated-plus-maze; and increased social affiliation and memory in the Crawley’s three chamber sociability test. During the prolonged (5-week) abstinence phase, all these effects were reversed while HD treated rats increased their fluid intake. These results indicated that intermittent brief abstinence periods (weekend’s off-treatment) produced the same behavioral and developmental effects as those previously reported with chronic (7 days/week) MP treatment, but were reversible following a prolonged abstinence period (5 weeks).
Keywords: Methylphenidate, Ritalin, Psychostimulant, Attention Deficit Hyperactivity Disorder, Social Behavior, Anxiety, Depression
1. Introduction
Recently, rapidly increasing rates of non-prescription MP use among healthy individuals has been reported [1]. Specifically, healthy individuals use MP for its cognition-, attention-, and intellectual capacity-enhancing effects, as well as its ability to improve academic performance [2–5]. It is important to understand the implications of such non-prescription chronic MP exposure in healthy individuals.
MP has a wide range of behavioral effects. For instance, in human subjects, MP enhances cognitive performance as reported in individuals with ADHD [3, 6]. MP also reduces impulsivity and inattention, suppresses anxiety- and depressive-like behaviors, and improves social interaction skills and sleep parameters [3, 7–10]. These wide ranging behavioral effects suggest that non-prescription MP use may cause similar effects in healthy subjects, as were recently reported with rats in terms of behavior [11] and development [12]. Moreover, the known side effects of MP administration such as insomnia, stomachache, headache, anorexia, and the potential for abuse due to MP-induced euphoria may pose additional risks for healthy individuals using MP [13]. ADHD and substance abuse has been known to have high co-morbidity, but the effects of non ADHD chronic MP treatment on substance abuse vulnerability is less well understood [14, 15]. Hence, further understanding of the wide range of behavioral and cognitive effects of chronic MP administration in healthy individuals is warranted.
Healthy individuals that use MP primarily for selected benefits may only use it periodically rather than every day. For instance, healthy students may only administer MP to enhance arousal and cognitive ability in order to increase academic performance during the week and only when school is in session [3, 5, 16, 17]. According to [18], college students and students preparing for exams nickname MP and related medication as “steroids for SATs”. In addition, it was reported that doctors and researchers might also use MP to sustain themselves through their long work hours and enhance their ability to concentrate on their patients or tasks [2]. Such individuals may abstain from MP during periods of less demanding cognitive and intellectual activity such as weekends and holidays. Therefore, it is important to understand how interrupting chronic daily MP exposure with abstinence on weekends and an additional long term total abstinence may impact the long-term behavioral effects of MP.
In animal studies, MP is often administered via injection rather than orally as it would be in the clinical setting [19]. The common intravenous, intraperitoneal, and subcutaneous routes of administration in animal models result in significantly different pharmacokinetic properties of MP, including the magnitude and the time-to-peak of serum concentration, the half-life, and the rate of elimination when compared to an oral administration [20]. The dose administered is also critical to the pharmacokinetic and behavioral properties of MP [21]. Putting these factors into consideration, the present study employed an 8-hour voluntary dual-bottle drinking paradigm that we previously introduced to produce clinically relevant pharmacokinetics at various MP doses [22]. Using this experimental paradigm, the present study investigated the behavioral and developmental effects of 13-week-long MP treatment in rats that excluded weekends, and their reversal following a 5-week abstinence period. We hypothesized that during the treatment phase, chronic MP exposure, regardless of weekend abstinence, would produce the same effects as we previously reported [11]. Furthermore, we expected the effects of chronic MP treatment to be generally reversed following prolonged abstinence, as our recent data indicated [23]. The present study examined the behavioral consequences of chronic weekday-only oral MP treatment in rats and revealed that HD MP exposure resulted in increased open field and circadian locomotor activity; increased latency to immobility and decreased time spent immobile in the forced swim test; increased center activity in the open field and percent of time spent in an open arm of the elevated-plus-maze; and increased social affiliation and memory in the Crawley’s three chamber sociability test. During the prolonged (5-week) abstinence phase, all these effects were reversed while HD treated rats increased their fluid intake.
2. Materials and methods
2.1. Animals
Male Sprague-Dawley rats (n=72) were obtained from Taconic Farms (Germantown, NY) at four weeks of age. All rats were allowed 1 week of habituation before starting the MP drinking paradigm. Prior to initiation of MP drinking and behavioral testing, rats were assigned to one of either low-dose MP (4/10 mg/kg MP, n = 24), high-dose MP (30/60 mg/kg MP, n = 24) or a control group receiving plain tap water (n = 23, one rat died prior to initiating of dosing or testing). Rats remained in the same group for the entirety of the experiment. Half of the animals from each group were sacrificed at the end of treatment for purposes of tissue collection and subsequent osteopathic analysis. Rats were single housed in a temperature- and humidity-controlled room on a reverse 12-hour light cycle (lights off 0800h). Rat chow (Teklad, Indianapolis, IN) was provided ad libitum throughout testing. During the 13-weeks MP treatment period and 5-week abstinence period, animals had access to fluids for only 8 hours daily as previously described [22]; LD rats received 4/10 mg/kg MP (first hour/subsequent seven hours of fluid consumption respectively) while HD rats received 30/60 mg/kg MP (first hour/subsequent seven hours, respectively). Animals were single-housed in order to accurately monitor individual fluid and food intake, which were recorded daily and weekly, respectively. Body weight was also recorded daily throughout the experiment. Experiments were conducted in conformity with the National Academy of Science’s Guide for the Care and Use of Laboratory Animals (NAS and NRC, 1996) and approved by the State University at Buffalo’s Institutional Animal Care and Use Committee.
2.2. Experimental Design and Drug Consumption Paradigm
During a 13-week-long treatment phase, rats had limited 8-hour daily access to MP except on Saturday and Sunday. The treatment phase was followed by a 5-week-long abstinence phase of no MP exposure. MP was delivered to rats as methylphenidate hydrochloride (Sigma-Aldrich, St Louis, MO) in water (vehicle). A LD and HD dosing paradigm has been previously reported to produce peak serum concentrations of 8 ng/ml and 30 ng/ml in the LD and HD treated rats (respectively) with concentration of drug in fluid adjusted daily based on prior day’s consumption [22]. This paradigm ensured that dosing was consistent independent of fluid consumption. MP was provided for an 8-hr period (0900h-1700h) during the dark phase (0800h-2000h); fluids were unavailable outside of this window of time. All behavioral tests were conducted during the dark phase between 1100 h and 1700 h while the rats had access to the drug.
2.3. Behavioral Tests
2.3.1. Open field locomotor activity
Locomotor activity was assessed biweekly during treatment weeks 1, 2, 4, 6, 8, 10 and once during abstinence week 5 for 90 min in an open-field chamber as previously described [22]. Rats were tested during the dark phase of the light cycle between 1100 h and 1700 h in an arena (dimensions 40.64 cm × 40.64 cm × 40.64 cm, 2.54 cm beam space and 1.27 cm spatial resolution) that is equipped with a photo beam activity monitoring system (Coulbourn Instruments, Allentown, PA). Open field locomotor activity data was acquired with TruScan v2.0 software and horizontal distance traveled, number of entries and time spent in the vertical plane, and both distance and time spent in the center of the chamber were analyzed.
2.3.2. Circadian Activity
The 24-hour circadian locomotor activity was assessed as previously described [11] during weeks 1, 2, 4, and 8 of the treatment phase and week 4 of the abstinence phase. Circadian locomotor activity, designated by beam breaks, was measured using a photo beam activity monitor attached to the cage top of each animal’s original home cage (50 cm × 25 cm × 30 cm high) (Starr Life Sciences Corp, VitalView software 1.1; Oakmont, Pennsylvania). Throughout the 24-hour experiment, ad libitum access to chow and the 8-hour limited access-drinking paradigm were maintained.
2.3.3. Elevated Plus Maze (EPM)
Anxiety-like behavior was assessed using an elevated plus maze during treatment week 10 and abstinence weeks 2 and 5 as previously described [11]. Briefly, each rat was placed on the center of the elevated plus maze and then allowed to explore the four arms of the maze for 5 minutes. Anxiety was assessed as the proportion of the total test time that was spent in the open arm. The behavior was recorded using D-Link cameras and software (D-Link Corporation Taipei, Taiwan), and subsequently analyzed using TopScan behavior image analysis software (Clever Sys Inc. Reston, Virginia).
2.3.4. Forced Swim Test (FST)
Rats were tested for depressive-like behavioral effects of chronic MP during week 11 of treatment and during week 1 of abstinence using the forced swim test (FST), which is an established and well-validated measure of depressive-like behavior in rats [24]. Buckets measuring 39cm × 27cm (height × diameter) were filled with room temperature water and rats were given a 15-minute habituation session in the apparatus 24 hours prior to the testing session that lasted for five minutes. Testing was recorded with D-Link cameras and software (D-Link Corporation Taipei, Taiwan), and was subsequently analyzed with TopScan software (Clever Sys Inc., Reston, Virginia). Behaviors measured during the five-minute testing period were time spent immobile (velocity <0.05), time spent with high activity (velocity>0.12), and latency to immobility.
2.3.5. Crawley’s three-chamber sociability test (SI)
A social interaction test was performed during week 12 of treatment and during weeks 2 and 5 of abstinence using the Crawley’s three chamber sociability test [25]. In summary, testing was performed in a room illuminated only by red light during the dark cycle between 1100h and 1700h. The test was performed in a social interaction arena consisting of a square unit with three chambers divided by partition, each measuring 20cm by 60 cm. Identical metal cages measuring 21.59cm × 12.7cm × 11.43cm (length × width × height) were located in diametrically opposed corners of the left and right chambers. During a 3-minute habituation session that preceded testing, rats were placed in the center of the arena alone and allowed to explore all chambers. During the test session that commenced 30 minutes after the habituation ended, each test animal was placed in the center of the same arena that now contains a “social stimulus” rat in a smaller cage within the arena. This was the first time the test animal was exposed to the social stimulus rat and allowed to freely explore the arena for five minutes. Test sessions were recorded using D-Link cameras and software (D-Link Corporation Taipei, Taiwan) and the time spent interacting with both cages was rated using the TopScan software (Clever Sys Inc., Reston, Virginia). The discrimination index during the test was calculated as a ratio of interaction time with the social stimulus rat cage to the interaction time with both cages (control cage had no rat inside). Testing chambers were cleaned with a 10% ammonia solution between runs to eliminate possible confounds from odor cues.
2.4. Statistical Analysis
Body weight, as well as food and fluid intake, were analyzed separately during the treatment and abstinence phases using two-way repeated measure ANOVAs, with treatment group and week as the between- and within-subject variables, respectively. This same ANOVA design was used to analyze open field locomotor activity during the treatment phase, circadian activity during the dark phase of the light cycle compared across weeks, and both EPM and SI during the abstinence phase. Hourly circadian activity on week 8 of the treatment phase and week 4 of the abstinence phase were each analyzed using a two-way repeated measure ANOVA with time of the day and treatment group as within- and between-subject variables, respectively. Open field locomotor activity during the abstinence phase, EPM and SI data during the treatment phase, and the FST data separately during the treatment and abstinence phases were each analyzed using a one-way ANOVA with treatment group as the independent variable. The use of Tukey’s HSD for post-hoc analysis is typically only conducted as justified by an overall main effect of treatment or its interaction with time. However, in light of prior findings in our lab showing strong effects of HD MP, but small or negligible effects of LD MP, pair-wise comparisons were conducted in all cases.
3. Results
Consumption effects
Fluid consumption:
During the treatment phase, a two-way repeated measure ANOVA of the weekly average of daily fluid intake revealed significant main effects of treatment [F(2,884)=47.330; p<0.001] and time [F(13, 884)=165.445; p<.001], and a significant interaction between treatment and time [F(26, 884)=19.777; p<0.001]. Tukey’s post hoc analysis revealed that fluid intake was higher in the water treated rats on weeks 7–13 compared to the LD MP treated rats (p<.01), and on weeks 1–13 compared to the HD MP treated rats (p<.05). Additionally, fluid intake was higher in the LD MP treated rats compared to the HD MP treated rats on weeks 2–13 (p<.05; Figure 1). During abstinence, a two-way repeated measure ANOVA revealed significant main effects of treatment [F(2, 128)=5.320; p < .05] and time [F(4,128=16.088; p < 0.001] on fluid intake, but no interaction between treatment and time. Tukey’ s post hoc analysis revealed that fluid intake was higher in the HD MP treated rats compared to the water treated rats on week 14, 15, 17, and 18, and compared to LD MP treated rats on weeks 14–18 (p < 0.01; Figure 1). One-way ANOVA of total fluid consumption throughout each of the treatment and abstinence phases confirmed these effects (Figure 1, insert).
Figure 1:
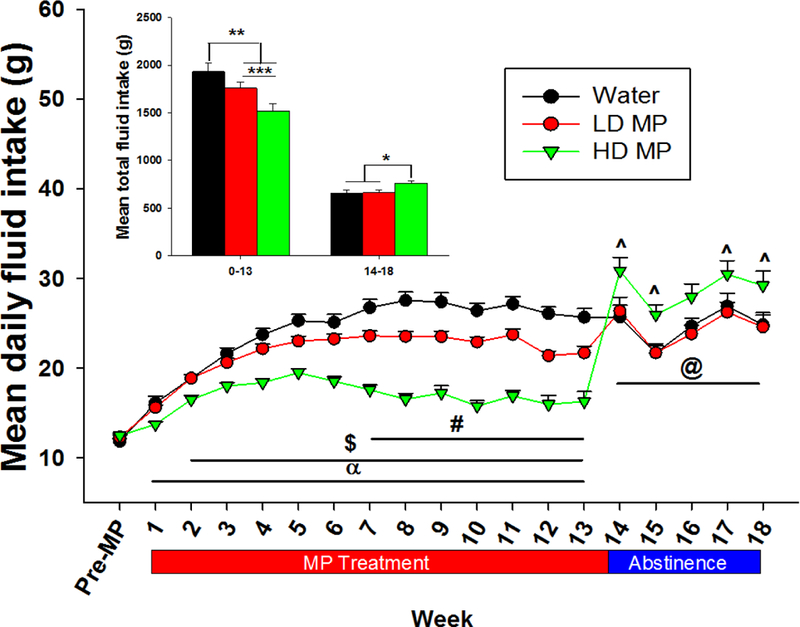
Daily fluid intake presented as weekly Mean + SEM (α - HD > W, p<0.05; $ - HD > LD, p<0.05; # - W > LD, p<0.05; ^ - HD > W, p<0.05; @ - HD > LD, p<0.01). Insert: Total fluid consumption during treatment weeks 0–12 (left) and abstinence weeks 14–17 (right) presented as Mean + SEM (*p<0.05, **p<0.01, ***p<0.001).
Food consumption:
During the treatment phase, a two-way repeated measure ANOVA revealed a significant main effect of time [F(13, 884)=334.849; p<0.001] on food consumption, while the effect of treatment (p=0.134) and the interaction between treatment and time (p=0.031), approached statistical significance. However, a one-way ANOVA of total food consumed throughout treatment phase revealed a significant main effect of treatment [F(2,991)=4.563; p < 0.05]. Tukey’s post-hoc analysis further revealed that the HD treated rats consumed less food than both water and LD treated rats (both p < 0.05; Figure 2). During abstinence, a two-way repeated measure ANOVA revealed a significant main effect of time [F(4, 128)=11.512; p < 0.001] on food intake, while the effect of treatment and the interaction between treatment and time were not significant (Figure 2). A one-way ANOVA of total food consumed revealed that the HD treated rats consumed less food than water and LD treated rats during treatment (p < 0.05) while there was no significant differences between treatment groups during abstinence (Figure 2, insert).
Figure 2:
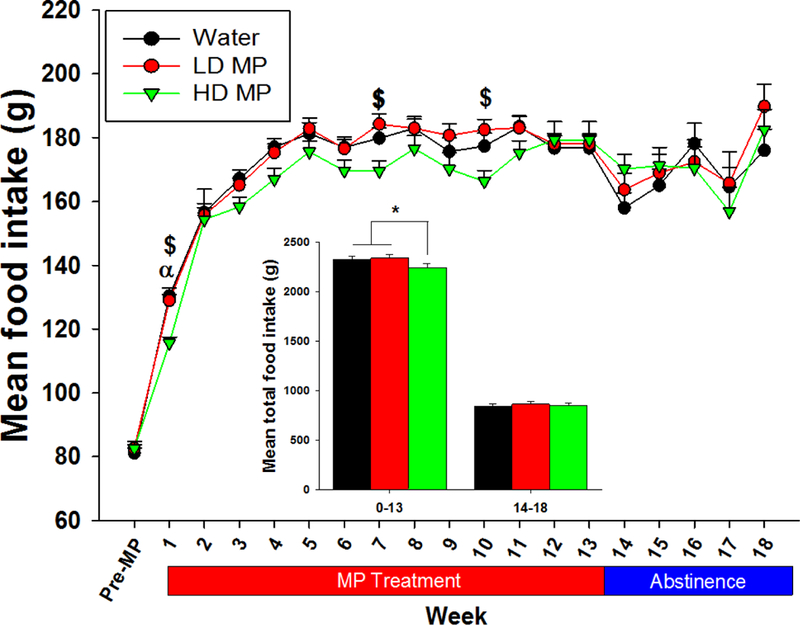
Weekly food intake presented as Mean + SEM (α - HD < W, p<0.05; $ - HD < LD, p<0.05). Insert: Total food consumption during treatment week 0–12 (left) and abstinence weeks 14–17 (right) presented as Mean + SEM (*p<0.05).
Body weight:
During the treatment phase, a two-way repeated measure ANOVA of weekly body weight revealed significant main effects of treatment [F(2, 884)=10.361; p<0.001] and time [F(13, 884)=3985.616; p<0.001], and a significant interaction between treatment and time [F(26, 884)=9.147; p<0.001]. Tukey’s post hoc analysis revealed that the HD treated rats weighed less than both LD and water treated rats throughout weeks 4–13 (p<0.05 for both; Figure 3). During abstinence, a two-way repeated measure ANOVA revealed a main effect of time [F(4, 128)=371.816; p<0.001] and an interaction between treatment and time [F(8, 128)=3.642; p<0.001] on body weight, while the main effect of treatment was not significant. Tukey’s post hoc analysis revealed that the HD treated rats weighed less than both LD and water treated rats at weeks 14 and 15 of abstinence (p<0.05 for both; Figure 3). A one-way ANOVA of percent change in body weight during the treatment phase [F(2, 68)=9.651; p<0.001] revealed a significant effect of treatment. Tukey’s post-hoc analysis further revealed that the HD treated rats had less percent body weight gain compared to both LD and water treated rats. There was no significant effect of treatment during the abstinence phase (Figure 3, insert).
Figure 3:
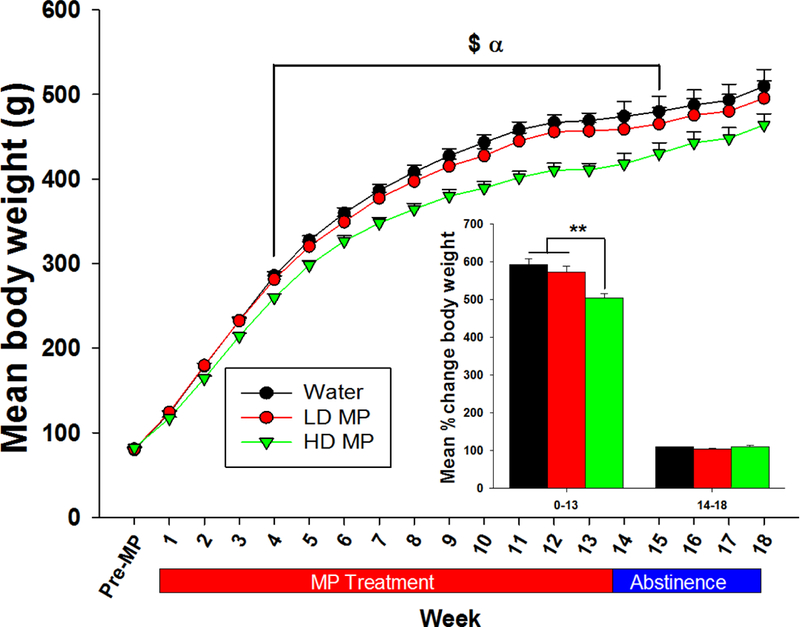
Weekly body weight presented as Mean + SEM (α - HD < W, p<0.05; $ - HD < LD, p<0.05). Insert: Percent change in body weight during treatment (week 0–13) and abstinence (week 14–18) presented as Mean + SEM (**p<0.05).
Behavioral effects
3.2. Open Field.
Horizontal Distance.
During the treatment phase, a two-way repeated measure ANOVA revealed main effects of treatment [F(2, 340)=8.998; p<0.001] and time [F(5,340)=8.134; p<0.001] but no significant interaction between treatment and time. Tukey’ s post hoc analysis revealed that the HD treated rats traveled a greater distance compared to the LD treated rats during weeks 2 through 10 and water treated rats during weeks 6–10. A one-way ANOVA of the area under the curve also revealed a significant effect of treatment [F(2, 68)=4.403; p < 0.05]. During abstinence, a one-way ANOVA revealed no significant effect of treatment (Figure 4A).
Figure 4:
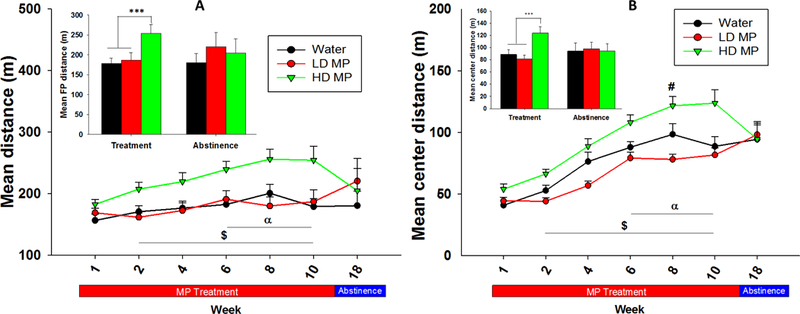
(A) Floor plane distance traveled in the open field presented as Mean + SEM (α - HD > W, p<0.05; $ - HD > LD, p<0.05). Insert: Floor plane distance traveled during treatment (week 10) and abstinence (week 5) presented as Mean + SEM (***p<0.001). Figure 4: (B) Center distance travelled presented as Mean + SEM ($ - HD > LD, p<0.05; α – HD > W, p<0.05). Insert: Center distance travelled during treatment (week 10) and abstinence (week 5) presented as Mean + SEM (***p<0.001).
Center Distance.
A two-way repeated measure ANOVA revealed significant main effects of treatment [F(2, 340)=15.773; p<0.001], time [F(5, 340)=62.281; p<0.001], and an interaction between treatment and time [F(10, 340)=2.113; p < 0.05]. Tukey’s post hoc analysis revealed that the HD treated rats traveled greater center distance compared to the LD treated rats during weeks 2 through 10 and the water treated rats during weeks 6–10. Additionally, the water treated rats had greater center activity compared to the LD treated rats during treatment week 8. A one-way ANOVA of the area under the curve for treatment weeks revealed a significant effect of treatment [F(2, 68)=6.200; p < 0.01]. A one-way ANOVA of center distance traveled during abstinence revealed no significant differences between groups (Figure 4B). Data on other measures of locomotor activity in the open field including velocity, center time, rearing events, and time spent rearing were obtained and analyzed as well (Supplement Figures 1S, 2S, 3S, and 4S).
Circadian Activity.
A two-way repeated measure ANOVA of hourly circadian activity on week 8 of the treatment phase revealed a significant main effect of treatment [F(2, 1496)=20.426; p<0.001], time [F(22, 1496)=137.773; p<0.001], and a significant interaction between treatment and time [F(44, 1496)=9.864; p<0.001]. Tukey’s post hoc analysis revealed that the HD treated rats had greater activity compared to the LD treated rats from 09:00 to 16:00 and compared to the water treated rats from 10:00 to 16:00 during the dark cycle (Figure 5A).
Figure 5:
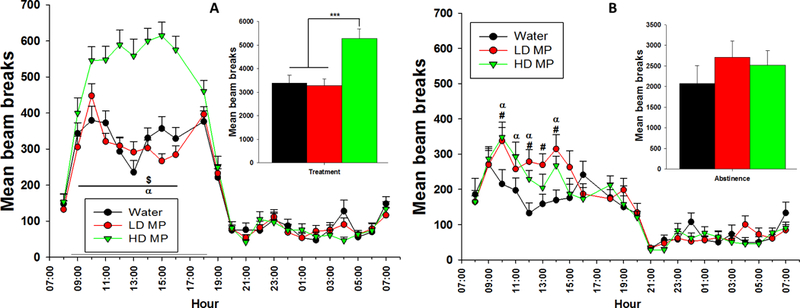
(A) Hourly circadian locomotor activity during week 8 of treatment presented as Mean + SEM ($ - HD > LD, p<0.05; α – HD > W, p<0.05). Insert: Total activity throughout the dark cycle presented as Mean + SEM (***p<0.001). Figure 5: (B) Hourly circadian locomotor activity during week 4 of abstinence presented as Mean + SEM (α – HD > W, p<0.05; # - LD > W, p<0.05). Insert: Total activity throughout the dark cycle presented as Mean + SEM.
A two-way repeated measure ANOVA of hourly circadian activity on week 4 of the abstinence phase revealed a significant main effect of time [F(22, 704)=30.321; p<0.001] and a significant interaction between treatment and time [F(44, 704)=1.638; p < 0.01], while the effect of treatment was not significant (Figure 5B).
The mean activity during the dark phase of the light cycle was averaged for each treatment group during both treatment and abstinence phases and a one-way ANOVA was conducted for each phase of the experiment. During the treatment phase, the one-way ANOVA revealed a main effect of treatment groups [F(2, 281)=19.956; p<0.001] and Tukey’s post hoc analysis revealed that the HD treated rats were more active than both water and LD treated rats (Figure 5A inset). During abstinence, there was no significant main effect of treatment. There were no significant differences between any groups during the light cycle (Figure 5B inset). Circadian activity was measured bi-weekly and analyzed as well (Supplement Figure 5S).
Elevated Plus Maze.
Time in the open arms of the elevated plus maze was calculated as a percent of total time spent in all arms (Figure 6). During week 10 of treatment, a one-way ANOVA revealed a significant main effect of treatment [F(2, 67)=5.151; p < 0.01] on percent of time spent in the open arm. Tukey’s post hoc analysis revealed that the HD treated rats spent more time in the open arm compared to water treated rats (p<.01). For both abstinence weeks that were tested, a two-way repeated measures ANOVA revealed a main effect of time [F(2, 64)=40.978; p<0.001] but neither a significant effect of treatment nor an interaction between treatment and time.
Figure 6:
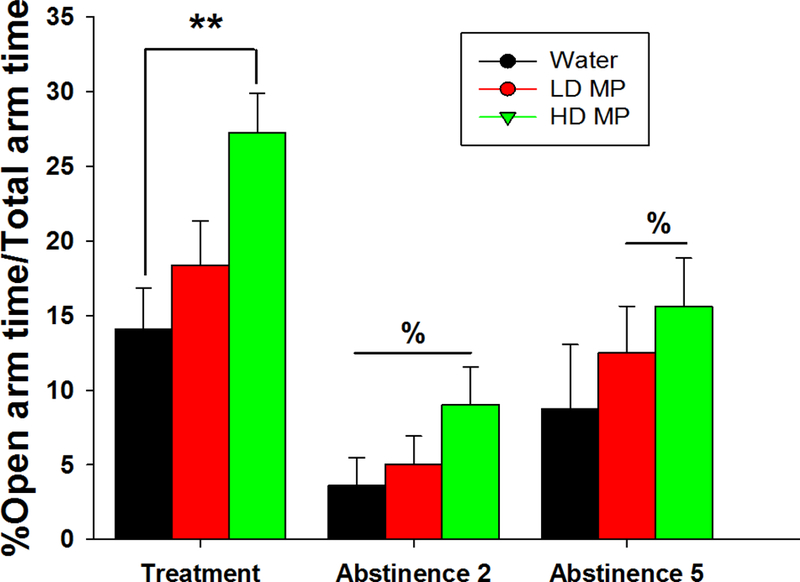
Percentage of total elevated plus maze test time spent in the open arm on treatment week 10 and abstinence week 2 and 5 presented as Mean + SEM (% - significantly different from treatment week, p<0.01; **p<0.01).
Forced Swim Test.
A two-way repeated measure ANOVA of latency to immobility revealed a main effect of time [F(1,32)=5.803; p= 0.022], while the effect of treatment and an interaction between treatment and time were not significant. Tukey’s post-hoc analysis further revealed that the HD treated rats had significantly shorter latency to immobility during the abstinence week compared to the treatment week (p<.01). During the treatment phase, a one-way ANOVA of latency to immobility revealed a significant main effect of treatment [F(2,68)=4.547; p < 0.05]. Tukey’s post-hoc analysis during the treatment phase further revealed that the HD treated rats had greater latency to immobility compared to both water and LD treated rats (p<0.05 for both). A one-way ANOVA during abstinence revealed no main effect of treatment (Figure 7A).
Figure 7:
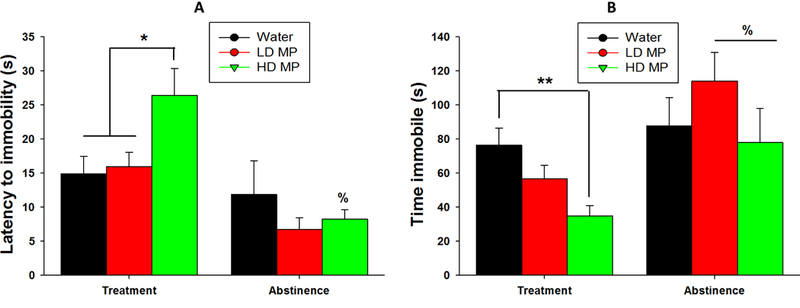
(A) Latency to immobility in the forced swim test presented as Mean + SEM (% - significantly different from treatment week, p<0.01; *p<0.05). (B) Time spent immobile in the forced swim test presented as Mean + SEM (% - significantly different from treatment week, p<0.05; **p<0.01).
A two-way repeated measures ANOVA of time spent immobile revealed a main effect of time [F(1,32)=12.468; p=0.001], while the main effect of treatment was not significant and an interaction between treatment and time approached significance (p=.071). Tukey’s post-hoc analysis revealed that both LD and HD treated rats had greater time spent immobile during abstinence than during treatment (p<.05 for both). During the treatment phase, a one-way ANOVA of time spent immobile revealed a significant main effect of treatment [F(2,68)=6.515; p<.01]. Tukey’s post-hoc analysis revealed HD MP treated rats spent a significantly less time immobile when compared to the water treated rats (p<.01). A one-way ANOVA of time spent immobile during abstinence revealed no significant main effect of treatment (Figure 7B).
Crawley’s three-chamber sociability test.
A two-way repeated measures ANOVA on discrimination index revealed a main effect of time [F(2, 64)=21.572; p<0.001] but not of treatment, and the interaction between treatment and time was not significant. Tukey’s post hoc analysis revealed a decrease in discrimination index during week 2 of abstinence compared to the treatment week for both LD MP treated rats and HD MP treated rats (p<0.05 for both). Also, discrimination index decreased during the fifth week of abstinence compared to the treatment week for all three treatment groups (p<0.05 for all). A one-way ANOVA of the discrimination index during the treatment phase revealed a significant main effect of treatment [F(2, 68)=5.623; p< 0.01]. Tukey’s post hoc analysis revealed that both HD and LD treated rats demonstrated greater discrimination index compared to the water treated rats (p<0.05). During abstinence weeks 2 and 5, a one-way ANOVA on each week, revealed no significant differences between treatment groups (Figure 8).
Figure 8:
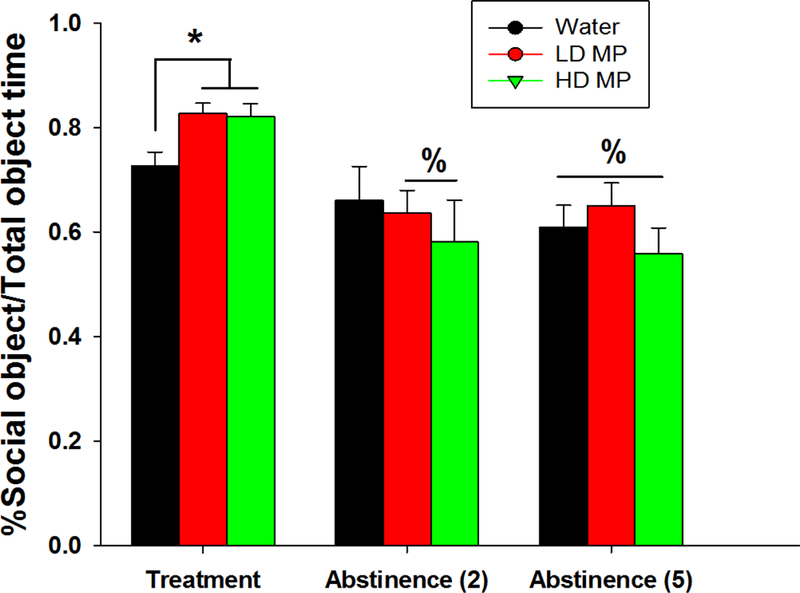
Discrimination index in Crawley’s three-chamber sociability test on treatment week 12 and abstinence week 2 and 5 presented as Mean + SEM (% - significantly different from treatment week, p<0.05; *p<0.05).
4. Discussion
The present study examined how interrupting chronic MP treatment with weekend abstinence periods modulates the behavioral and developmental consequences of MP, and how prolonged abstinence following the chronic exposure phase affects them. We found that chronic HD MP exposure decreased fluid and food intake as well as body weight, increased locomotor activity, exploratory behavior, social affiliation, and decreased anxiety- and depression-like behaviors regardless of weekend abstinence. These effects were generally reversed following prolonged (5 week) abstinence. These findings are important given that not all individuals consume MP seven days a week [2, 26].
Psychostimulants suppress appetite [27, 28] and may lead to body weight reduction following chronic exposure [29]. Indeed, during the treatment phase, we observed decreased fluid intake in both LD and HD treated rats compared to the water treated rats, and decreased food intake and body weight only in the HD treated rats. This is consistent with our prior findings [11] and agrees with a clinical study [26] that found no differences between children with ADHD who received MP for seven days a week and those that received placebo during the weekend. During our 5-week abstinence phase, the effects of HD MP on body weight and food intake were eliminated while the HD treated rats consumed more fluid than both LD and water treated rats suggesting a possible compensation for suppressed fluid intake during treatment. Consistent with our hypothesis, these findings reveal that prolonged, rather than a brief abstinence period from MP is necessary to reverse the behavioral and developmental consequences of chronic MP use that we previously reported [11].
The greater distance traveled and velocity in the open field, as well as higher circadian locomotor activity during the dark phase in our HD group supports existing data showing that MP dose-dependently increases locomotor activity [11, 22, 30]. The elevated locomotor activity recorded is potentially a result of the use of Sprague-Dawley rats. In contrast, the spontaneously hypertensive rat (SHR) is the standard model of the clinical ADHD diagnosis [31]. Response to stimulant medications and other drugs can vary considerably according to genetic differences [32]. Our data suggest the possibility that clinically relevant doses of MP increases some measures of hyperactivity in a non-ADHD model of rat; use of this paradigm in the SHR may yield differing results. This pattern of results, contrary to the effects of stimulants typically reported in an ADHD population, is a recurring finding in experiment and in prior work in our lab and others. Although concerns have been expressed that MP treatment may affect sleep patterns in children diagnosed with ADHD [33, 34], our findings of no MP-related effect on locomotor activity during the light-phase suggest otherwise. Furthermore, while weekend abstinence had no impact on HD MP-related increase in locomotor activity, this effect was significantly attenuated to the levels of the LD and water treated rats after prolonged abstinence from the drug. Therefore, the locomotor activity-related effects of chronic MP exposure were also eliminated following prolonged abstinence.
Increased time spent in the open arms of the elevated plus maze (EPM) [35], and increased activity in the center of an open field chamber [36] are common indicators of anxiolytic behaviors in rodents. We showed greater center activity and increased open arm entries in the HD treated rats in the present study, which supports clinical [7, 37] and preclinical [11, 22] observations of MP-related anxiolytic effects. We believe that analysis of the percentage of total test time rather than the absolute time spent in the open arms controlled for the potential impact of greater locomotor activity on the attenuated anxiety-like behavior that we report in the HD MP-treated group. Moreover, like locomotor activity, food intake and body weight, the anxiolytic effects of MP in the HD rats returned to LD and water treated rats levels following prolonged abstinence but not weekend breaks.
Previous reports of the effects of MP on social interaction have been inconsistent. While some studies reported MP-related increase in social interaction [8, 38], others found decreased social interaction [39]. We previously found no effect [11]. However, our current findings indicate that HD chronic MP exposure, interrupted by weekend abstinence from the drug, increased social interaction. The current findings differ from the previous [11] partly because both studies employed different tests of social interaction. Using the Crawley’s three chamber sociability test in the present study, our findings suggests that chronic MP increases social affiliation and social memory [25], and prolonged abstinence from MP eliminates this effect.
Chronic HD MP exposure with weekend breaks from the drug in the present study also suppressed depressive-like behavior. Previous results have proven inconsistent, with some clinical reports that MP attenuated depressive-like behavior in patients [7, 40, 41]; or no later increased risk for onset of depression-like behavior and cause no increase in risk for a later onset of depression [42]. While preclinical reports have reported that MP alleviates depression-like behavior [43]. During the prolonged abstinence phase, HD MP-related increase in latency to immobility was reduced to water and LD levels, while the time spent immobile by both LD and HD treated rats significantly increased compared to the treatment period. This effect could support the notion that chronic adolescent MP exposure could lead to depressive like behavior in adulthood as previously reported in rats [44].
A limitation of the present study is the isolated housing condition employed. Although this housing condition was necessary to adequately quantify and monitor drug self-administration, this “isolated” environment could have stressed the animals and altered their behavior. To elucidate how isolated housing may have influenced our results, future studies will investigate the impact of chronic MP exposure on group-housed animals.
5. Conclusion
This study revealed that chronic HD MP exposure during the week followed by weekends off the drug decreased food and fluid intake, and body weight, increased locomotor activity, exploratory behavior and social interaction, and attenuated anxiety- and depression-like behaviors in agreement with 7-days/week treatment previously reported [11]. These behavioral effects persisted despite weekend abstinence from MP, but they were generally reversed following prolonged weeks of abstinence from MP. Although the present study was conducted in males, they were consistent with recent data in females (unpublished data). These results suggest that MP chronic treatment (weekdays only) of non ADHD subjects produced significant physiological (body weight) and behavioral effects (consummatory behavior, locomotor activity, anxiety, depression) that were generally reversible with abstinence. Further research will examine this treatment regimen’s impact on neurochemistry and brain functional connectivity as well as skeletal development.
Supplementary Material
Acknowledgements:
We thank the following people for technical support in this project: John Hamilton, Antonio Figueiredo, Matt Marrion, Courtney Lowinger, Macauley Mackintosh, Ashley Oshiro, Meagan Schreiner. This work was supported by the National Institute of Child Health and Human Development [RO1HD70888].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Swanson JM and Volkow ND, Increasing use of stimulants warns of potential abuse. Nature, 2008. 453(7195): p. 586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beyer C, Staunton C, and Moodley K, The implications of methylphenidate use by healthy medical students and doctors in South Africa. BMC Med Ethics, 2014. 15: p. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lakhan SE and Kirchgessner A, Prescription stimulants in individuals with and without attention deficit hyperactivity disorder: misuse, cognitive impact, and adverse effects. Brain Behav, 2012. 2(5): p. 661–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Outram SM, The use of methylphenidate among students: the future of enhancement? J Med Ethics, 2010. 36(4): p. 198–202. [DOI] [PubMed] [Google Scholar]
- 5.Fallah G, Moudi S, Hamidia A, and Bijani A, Stimulant use in medical students and residents requires more careful attention. Caspian J Intern Med, 2018. 9(1): p. 87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pietrzak RH, Mollica CM, Maruff P, and Snyder PJ, Cognitive effects of immediate-release methylphenidate in children with attention-deficit/hyperactivity disorder. Neurosci Biobehav Rev, 2006. 30(8): p. 1225–45. [DOI] [PubMed] [Google Scholar]
- 7.Golubchik P, Rapaport M, and Weizman A, The effect of methylphenidate on anxiety and depression symptoms in patients with Asperger syndrome and comorbid attention deficit/hyperactivity disorder. Int Clin Psychopharmacol, 2017. 32(5): p. 289–293. [DOI] [PubMed] [Google Scholar]
- 8.Jahromi LB, Kasari CL, McCracken JT, Lee LS, Aman MG, McDougle CJ, Scahill L, Tierney E, Arnold LE, Vitiello B, Ritz L, Witwer A, Kustan E, Ghuman J, and Posey DJ, Positive effects of methylphenidate on social communication and self-regulation in children with pervasive developmental disorders and hyperactivity. J Autism Dev Disord, 2009. 39(3): p. 395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koyuncu A, Celebi F, Ertekin E, and Kahn DA, Extended-release Methylphenidate Treatment and Outcomes in Comorbid Social Anxiety Disorder and Attention-deficit/Hyperactivity Disorder: 2 Case Reports. J Psychiatr Pract, 2015. 21(3): p. 225–31. [DOI] [PubMed] [Google Scholar]
- 10.Sunohara GA, Malone MA, Rovet J, Humphries T, Roberts W, and Taylor MJ, Effect of methylphenidate on attention in children with attention deficit hyperactivity disorder (ADHD): ERP evidence. Neuropsychopharmacology, 1999. 21(2): p. 218–28. [DOI] [PubMed] [Google Scholar]
- 11.Robison LS, Michaelos M, Gandhi J, Fricke D, Miao E, Lam CY, Mauceri A, Vitale M, Lee J, Paeng S, Komatsu DE, Hadjiargyrou M, and Thanos PK, Sex Differences in the Physiological and Behavioral Effects of Chronic Oral Methylphenidate Treatment in Rats. Front Behav Neurosci, 2017. 11: p. 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uddin S, Robison LS, Fricke D, Chernoff E, Hadjiargyrou M, Thanos PK, and Komatsu DE, Methylphenidate regulation of osteoclasts in a dose- and sex-dependent manner adversely affects skeletal mechanical integrity. Scientific Reports, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morton WA and Stockton GG, Methylphenidate Abuse and Psychiatric Side Effects. Prim Care Companion J Clin Psychiatry, 2000. 2(5): p. 159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kollins SH, English J, Robinson R, Hallyburton M, and Chrisman AK, Reinforcing and subjective effects of methylphenidate in adults with and without attention deficit hyperactivity disorder (ADHD). Psychopharmacology (Berl), 2009. 204(1): p. 73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kollins SH, MacDonald EK, and Rush CR, Assessing the abuse potential of methylphenidate in nonhuman and human subjects: a review. Pharmacol Biochem Behav, 2001. 68(3): p. 611–27. [DOI] [PubMed] [Google Scholar]
- 16.Wilens TE, Adler LA, Adams J, Sgambati S, Rotrosen J, Sawtelle R, Utzinger L, and Fusillo S, Misuse and Diversion of Stimulants Prescribed for ADHD: A Systematic Review of the Literature. Journal of the American Academy of Child & Adolescent Psychiatry. 47(1): p. 21–31. [DOI] [PubMed] [Google Scholar]
- 17.Mache S, Eickenhorst P, Vitzthum K, Klapp BF, and Groneberg DA, Cognitive-enhancing substance use at German universities: frequency, reasons and gender differences. Wiener Medizinische Wochenschrift, 2012. 162(11): p. 262–271. [DOI] [PubMed] [Google Scholar]
- 18.Svetlov SI, Kobeissy FH, and Gold MS, Performance enhancing, non-prescription use of Ritalin: a comparison with amphetamines and cocaine. J Addict Dis, 2007. 26(4): p. 1–6. [DOI] [PubMed] [Google Scholar]
- 19.Volkow ND and Insel TR, What are the long-term effects of methylphenidate treatment? Biol Psychiatry, 2003. 54(12): p. 1307–9. [DOI] [PubMed] [Google Scholar]
- 20.Kuczenski R and Segal DS, Stimulant actions in rodents: implications for attention-deficit/hyperactivity disorder treatment and potential substance abuse. Biol Psychiatry, 2005. 57(11): p. 1391–6. [DOI] [PubMed] [Google Scholar]
- 21.Gerasimov MR, Franceschi M, Volkow ND, Gifford A, Gatley SJ, Marsteller D, Molina PE, and Dewey SL, Comparison between intraperitoneal and oral methylphenidate administration: A microdialysis and locomotor activity study. J Pharmacol Exp Ther, 2000. 295(1): p. 51–7. [PubMed] [Google Scholar]
- 22.Thanos PK, Robison LS, Steier J, Hwang YF, Cooper T, Swanson JM, Komatsu DE, Hadjiargyrou M, and Volkow ND, A pharmacokinetic model of oral methylphenidate in the rat and effects on behavior. Pharmacol Biochem Behav, 2015. 131: p. 143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin C, Fricke D, Vijayashanthar A, Lowinger C, Koutsomitis D, Popoola D, Hadijargyrou M, Komatsu D, and Thanos PK, Recovery from behavior and developmental effects of chronic oral methylphenidate following an abstinence period. 2018: In press. Pharmacology Biochemistry and Behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walf AA and Frye CA, The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc, 2007. 2(2): p. 322–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaidanovich-Beilin O, Lipina T, Vukobradovic I, Roder J, and Woodgett JR, Assessment of Social Interaction Behaviors. Journal of Visualized Experiments : JoVE, 2011(48): p. 2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martins S, Tramontina S, Polanczyk G, Eizirik M, Swanson JM, and Rohde LA, Weekend holidays during methylphenidate use in ADHD children: a randomized clinical trial. J Child Adolesc Psychopharmacol, 2004. 14(2): p. 195–206. [DOI] [PubMed] [Google Scholar]
- 27.Davis C, Fattore L, Kaplan AS, Carter JC, Levitan RD, and Kennedy JL, The suppression of appetite and food consumption by methylphenidate: the moderating effects of gender and weight status in healthy adults. Int J Neuropsychopharmacol, 2012. 15(2): p. 181–7. [DOI] [PubMed] [Google Scholar]
- 28.Goldfield GS, Lorello C, Cameron J, and Chaput JP, Gender differences in the effects of methylphenidate on energy intake in young adults: a preliminary study. Appl Physiol Nutr Metab, 2011. 36(6): p. 1009–13. [DOI] [PubMed] [Google Scholar]
- 29.Fazelipour S, Jahromy MH, Tootian Z, Kiaei SB, Sheibani MT, and Talaee N, The effect of chronic administration of methylphenidate on morphometric parameters of testes and fertility in male mice. J Reprod Infertil, 2012. 13(4): p. 232–6. [PMC free article] [PubMed] [Google Scholar]
- 30.Algahim MF, Yang PB, Burau KD, Swann AC, and Dafny N, Repetitive ritalin treatment modulates the diurnal activity pattern of young SD male rats. Cent Nerv Syst Agents Med Chem, 2010. 10(3): p. 247–57. [DOI] [PubMed] [Google Scholar]
- 31.Sagvolden T, Behavioral validation of the spontaneously hypertensive rat (SHR) as an animal model of attention-deficit/hyperactivity disorder (AD/HD). Neuroscience & Biobehavioral Reviews, 2000. 24(1): p. 31–39. [DOI] [PubMed] [Google Scholar]
- 32.George FR and Goldberg SR, Genetic differences in responses to cocaine. NIDA Res Monogr, 1988. 88: p. 239–49. [PubMed] [Google Scholar]
- 33.Sangal RB, Owens J, Allen AJ, Sutton V, Schuh K, and Kelsey D, Effects of atomoxetine and methylphenidate on sleep in children with ADHD. Sleep, 2006. 29(12): p. 1573–85. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz G, Amor LB, Grizenko N, Lageix P, Baron C, Boivin DB, and Joober R, Actigraphic monitoring during sleep of children with ADHD on methylphenidate and placebo. J Am Acad Child Adolesc Psychiatry, 2004. 43(10): p. 1276–82. [DOI] [PubMed] [Google Scholar]
- 35.Zhu N, Weedon J, and Dow-Edwards DL, The multifaceted effects of oral administration of methylphenidate in juvenile rats: anxiety, activity, and attention. Eur Neuropsychopharmacol, 2010. 20(4): p. 236–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernandez-Teruel A, Escorihuela RM, Driscoll P, Tobena A, and Battig K, Differential effects of early stimulation and/or perinatal flumazenil treatment in young Roman low- and high-avoidance rats. Psychopharmacology (Berl), 1992. 108(1–2): p. 170–6. [DOI] [PubMed] [Google Scholar]
- 37.Bouffard R, Hechtman L, Minde K, and Iaboni-Kassab F, The efficacy of 2 different dosages of methylphenidate in treating adults with attention-deficit hyperactivity disorder. Can J Psychiatry, 2003. 48(8): p. 546–54. [DOI] [PubMed] [Google Scholar]
- 38.Hara Y, Ago Y, Taruta A, Katashiba K, Hasebe S, Takano E, Onaka Y, Hashimoto H, Matsuda T, and Takuma K, Improvement by methylphenidate and atomoxetine of social interaction deficits and recognition memory impairment in a mouse model of valproic acid-induced autism. Autism Res, 2016. 9(9): p. 926–39. [DOI] [PubMed] [Google Scholar]
- 39.Vanderschuren LJ, Trezza V, Griffioen-Roose S, Schiepers OJ, Van Leeuwen N, De Vries TJ, and Schoffelmeer AN, Methylphenidate disrupts social play behavior in adolescent rats. Neuropsychopharmacology, 2008. 33(12): p. 2946–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hardy SE, Methylphenidate for the treatment of depressive symptoms, including fatigue and apathy, in medically ill older adults and terminally ill adults. Am J Geriatr Pharmacother, 2009. 7(1): p. 34–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kerr CW, Drake J, Milch RA, Brazeau DA, Skretny JA, Brazeau GA, and Donnelly JP, Effects of methylphenidate on fatigue and depression: a randomized, double-blind, placebo-controlled trial. J Pain Symptom Manage, 2012. 43(1): p. 68–77. [DOI] [PubMed] [Google Scholar]
- 42.Chang Z, D’Onofrio BM, Quinn PD, Lichtenstein P, and Larsson H, Medication for Attention-Deficit/Hyperactivity Disorder and Risk for Depression: A Nationwide Longitudinal Cohort Study. Biol Psychiatry, 2016. 80(12): p. 916–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brookshire BR and Jones SR, Chronic methylphenidate administration in mice produces depressive-like behaviors and altered responses to fluoxetine. Synapse, 2012. 66(9): p. 844–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carlezon WA Jr., Mague SD, and Andersen SL, Enduring behavioral effects of early exposure to methylphenidate in rats. Biol Psychiatry, 2003. 54(12): p. 1330–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


