Abstract
Due to its complex pathogenesis, the prevention and therapization of Alzheimer’s disease (AD) remains a serious challenge. Crocin, the main compound isolated from Crocus sativus L., demonstrates various pharmacological activities including anti-apoptotic properties. The present study investigated the neuroprotective effect of crocin and the underlying mechanisms. In l-glutamate-damaged HT22 cells, 3-h crocin pretreatment strongly enhanced the HT22 cell viability, reduced the apoptotic rate, mitigated mitochondrial dysfunction, suppressed intracellular reactive oxygen species (ROS) accumulation and Ca2+ overload compared with untreated cells. Additionally, crocin significantly decreased the expression levels of Bax, Bad and cleaved caspase-3 and increased the expression levels of B-cell lymphoma-extra large, phosphorylated (P-) protein kinase B and P-mammalian target of rapamycin compared with untreated cells. In mice with AD induced by d-galactose and aluminum trichloride, crocin substantially improved the cognition and memory abilities of the mice as measured by their coordination of movement in an open field test, and reduced their escape time in the Morris water maze test compared with untreated mice. Biochemical analysis confirmed that crocin was able to reduce the Aβ1-42 content in the mouse brains, increase the levels of glutathione peroxidase, superoxide dismutase, acetylcholine and choline acetyltransferase, and reduce the levels of ROS and acetylcholinesterase in the serum, cerebral cortex and hypothalamus compared with untreated mice. Immunohistochemical analysis demonstrated that crocin reduced Aβ1-42 deposition in the hippocampus of the brains of treated mice compared with untreated mice. In conclusion, crocin demonstrates good prospects in the treatment of AD through the oxidative stress-associated apoptosis signaling pathway.
Key words: Alzheimer’s disease, crocin, oxidative stress, apoptosis, cholinergic neurotransmitter
Introduction
Alzheimer’s disease (AD), a progressive degenerative disease of the nervous system, is characterized by a substantial decline in cognitive function and poor prognosis. Intracellular neurofibrillary tangles and extracellular senile plaques, particularly aggregated amyloid-β titanium and hyper-phosphorylated tau, are considered typical markers of AD histopathology (1). Although the exact molecular mechanism of neurodegeneration in AD remains unclear, through basic and clinical studies (2,3), oxidative stress and neuronal apoptosis have been reported to serve a pivotal function in the process of AD. Oxidative stress, resulting in an imbalance of anti-oxidation and pro-oxidation, may promote amyloid β (Aβ) aggregation accompanied by the over-accumulation of inflammatory reactive oxygen species (ROS) (4). Additionally, hyper-levels of ROS tend to enhance the dissipation of the mitochondrial transmembrane potential (MMP). In a short feedback loop, the increased permeability of mitochondria further enhances the over-releasing of ROS and other factors, including cytochrome c, into the cytoplasm, which triggers the cell apoptosis program (5,6). Abnormally high levels of neurons damaged by glutamate, a central-nervous neurotransmitter, are closely associated with ROS accumulation (7).
The global impact of AD is rapidly expanding (8), as >40 million patients globally have been reported, which is expected to increase to 70 million by 2030 (8). Therefore, the prevention and therapization of AD is an urgent challenge. Unfortunately, clinical trials of AD drugs have a high failure rate, and the majority of them exert various adverse effects including loss of appetite, gastrointestinal discomfort, difficulty sleeping and muscle spasms (9). Herbal compounds have been considered as potential agents for the prevention of AD (10,11). Crocin, the main component of Crocus sativus L. extract, is a yellow carotenoid that has been reported to exert anti-inflammatory (12), anti-depressant (13), memory improvement (14) and anti-apoptotic properties (15). The structure of crocin is presented in Fig. 1. In PC12 cells, crocin prevents cell apoptosis via increasing glutathione synthesis (16); meanwhile, in aging Wistar rats, crocin enhances their memory abilities, mainly through its anti-oxidant activities (17). In rats administered tramadol hydrochloride, crocin improves their learning and reduces memory impairment (18). However, no systematic study of the effects of crocin on AD, and its underlying mechanisms, has been reported.
Figure 1.
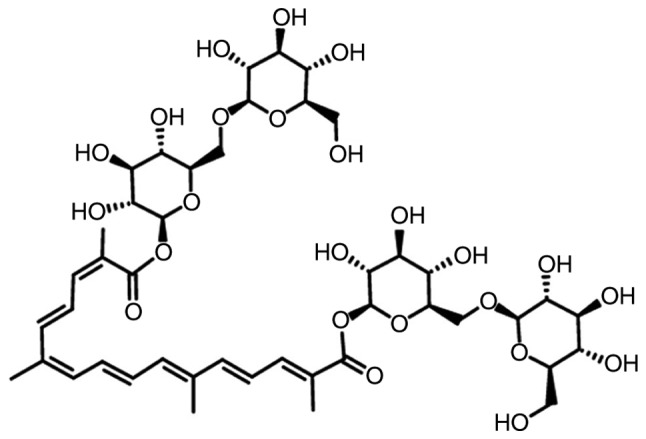
Structural formula of crocin.
In the present study, the neuroprotective effects of crocin against AD were investigated in l-glutamate (L-Glu)-induced HT22 apoptotic cells, and in aluminum trichloride (AlCl3) and d-galactose (d-gal)-induced AD mice. The present study aimed to provide experimental evidence that crocin may be a candidate agent for the adjuvant treatment of AD.
Materials and methods
Cell culture
HT22 cells (cat. no. 337709; BeNa Culture Collection, Beijing, China), a mouse hippocampal neuronal cell line, were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum, 1% 100 µg/ml streptomycin and 100 units/ml penicillin (all Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C in a 5% CO2 incubator to provide a humidified atmosphere (Thermo Fisher Scientific, Inc.).
MTT assay
HT22 cells cultured in 96-well plates, were pretreated with crocin (cat. no. B21336; purity ≥98%; Shanghai Yuanye Biotechnology Co., Ltd., Shanghai, China) at doses of 0.5, 1, 2 and 4 µM for 3 h at 37°C and then incubated with 25 mM l-Glu for another 24 h at 37°C. The control cells were not treated with 25 mM l-Glu and crocin, and incubated for 24 h. A total of 5 mg/ml MTT (Merck KGaA, Darmstadt, Germany) was added for 4 h at 37°C, and then 100 µl dimethyl sulfoxide (Merck KGaA, Darmstadt, Germany) was added. Absorbance was analyzed using a SynergyTM4 Microplate Reader at 490 nm (BioTek Instruments, Inc., Winooski, VT, USA).
Cell apoptosis assay
HT22 cells cultured in 6-well plates were pretreated with crocin at doses of 0.5 and 2 µM for 3 h at 37°C and then incubated with 25 mM l-Glu for 24 h at 37°C. Collect cells and resuspend in 100 µl 1% FBS, then incubated with Annexin V & Dead Cell Reagent (cat. no. 4700-1485, 100 tests/bottle) for 20 min at 25°C in the dark, and detected using the Muse™ Cell Analyzer (EMD Millipore, Billerica, MA, USA).
MMP, intracellular ROS and Ca2+ measurement
HT22 cells were pretreated with crocin at doses of 0.5 and 2 µM for 3 h and then incubated with 25 mM l-Glu for 12 h. Cells were further incubated with 2 µM 5,5′,6,6′-TetrAchloro-1,1′,3,3′-tetraethyl-imidacarbocyanine iodide staining (EMD Millipore, Billerica, MA, USA), 10 µM 2′-7′-dichlorodihydrofluorescein diacetate (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) or 2 µM Fluo-4-AM (Molecular Probes; Thermo Fisher Scientific, Inc.) for 20 min at 25°C in the dark to investigate the changes in MMP and the levels of intracellular of ROS and Ca2+, respectively. All fluorochrome were dissolve in serum-free DMEM. Subsequent to washing, the fluorescence intensities were detected using fluorescence microscopy (CCD camera, TE2000; Nikon Corporation, Tokyo, Japan).
Western blot analysis
HT22 cells were pretreated with crocin at doses of 0.5 and 2 µM for 3 h and then incubated with 25 mM l-GLU for 24 h. Cells were lysed for 0.5 h at 0°C with radio immunoprecipitation assay buffer containing 2% phenylmethanesulfonyl fluoride (Sigma-Aldrich; Merck KGaA) and 1% protease inhibitor cocktail (Sigma-Aldrich; Merck KGaA). Subsequent to the detection of the protein concentration using the BCA kit (Merck KGaA) 40 µg lysates were separated using 12% dodecyl sulfate, sodium salt poly-vinylidene fluoride membranes (0.45 µm; Merck KGaA). The membranes were blocked with 5% bovine serum albumin for 2 h at 4°C, and then incubated with primary antibodies as follows: B-cell lymphoma-extra large (Bcl-xL; 26 kDa; cat. no. ab32370), Bcl2 associated X, apoptosis regulator (Bax; 21 kDa; cat. no. ab32503), Bcl2 associated agonist of cell death (Bad; 23 kDa; cat. no. ab32445), cleaved caspase-3 (32 kDa; cat. no. ab2302), phosphorylated (P-) protein kinase B (Akt; phospho S473; 60 kDa S473; cat. no. ab18206), total (T)-Akt (60 kDa; cat. no. ab106693), P-mammalian target of rapamycin (mTOR; phospho S2448; 289 kDa S2448; cat. no. ab109268), T-mTOR (289 kDa; cat. no. ab83495) and GAPDH (36 kDa; cat. no. ab181602), which were purchased from Abcam (Cambridge, MA, USA) at 4°C overnight at a dilution of 1:2,000. Subsequent to three washes with TBST (consists of 500 ml TBS and 0.5 ml Tween-20), TBS (20X) is composed of 48.4 g Tris, 584 g NaCl and 900 ml distilled water (Beijing Chemical Works Beijing, China), the membranes were incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit immunoglobulin G antibody (cat. no. SH-0032; Bejing Dingguo Changsheng Biotechnology Co., Ltd., Beijing, China) at 4°C for 4 h at a dilution of 1:2,000. An enhanced chemiluminesence kit (Merck KGaA) was used to visualize the protein bands under an imaging system (BioSpectrum600). Quantitation of the results was accomplished by ImageJ (×64) 1.48u software (National Institutes of Health, Bethesda, MD, USA).
AD mice model establishment and administration
The experiment was ethically approved by the Institution Animal Ethics Committee of Jilin University (Changchun, China; license no. 20160409). A total of 100 BALB/c mice (6-8 weeks; 18-20 g) were maintained at a standard 12:12 h light/dark cycle at 23±1°C. The mice were fed autoclaved standard chow ad libitum.
As described in a previous study (19), a total of 60 mice were randomly intraperitoneally injected with 120 mg/kg d-gal (Sigma-Aldrich; Merck KGaA) and intragastrically administrated with 20 mg/kg AlCl3 (Sigma-Aldrich; Merck KGaA) once a day for 8 weeks, as the process of the establishment of the AD mice model. From the 5th week onwards, crocin-treated mice were intragastrically treated with 5 or 20 mg/kg crocin everyday (n=20/group); meanwhile, model mice were intragastrically administrated with saline (n=20). A total of 20 mice injected intraperitoneal and intragastrical with saline for 8 weeks served as the control group. Another 20 mice were intraperitoneally and intragastrically injected with saline throughout the whole experiment and intragastric treatment with 20 mg/kg crocin from the 5th week, which served as the crocin single treated mice group. If any mouse exhibited endpoint signs, including rapidly reduced bodyweight (loss of >20% bodyweight), they were euthanized using an intraperitoneal injection of 200 mg/kg pentobarbital. During the whole experiment period, the maximum body weight loss was <8.3% amongst all experimental mice.
Animal behavioral detection Morris water maze test
As described in a previous study (19), a circular pool filled with 10 cm (depth) water (22-24°C) containing 1 l milk was used to perform the water maze test. A total of 5 days prior to the form test, mice were trained for 5 min per day. The latency from the immersion of each mouse into the pool until they escaped onto the hidden platform was recorded. On the test day, mice were subjected to the pool in the same quadrant to identify the platform, and the time spent to locate the platform within 120 sec was recorded.
Open field test
A soundproof dark box was set with a bottom area of 30×30 cm, with a central region of 15×15 cm as the central area, and the remaining area as the surrounding area. On the test day, mice were placed in the fixed central position in the box, and their moving track and the time of exploring the central area within 5 min were observed and recorded.
Sample collection and biochemical detection
Blood of all experimental mice were collected from a caudal vein subsequent to the behavioral tests. All mice were euthanized by an intraperitoneal injection of 200 mg/kg pentobarbital. Following this, the brains and hypothalamus were immediately collected. The cerebral cortex, separated from the brains (n=20/group) and the hypothalamus (n=20/group) were homogenized in ice-cold phosphate buffered saline (PBS).
Subsequent to detecting the protein concentration using a BCA assay kit (Merck KGaA), the levels of acetyl-choline (Ach; cat. no. CK-E20536), acetylcholinesterase (AchE; cat. no. CK-E93899), choline acetyltransferase (ChAT; cat. no. CK-E94456), super oxidase dismutase (SOD; cat. no. CK-E20348), glutathione peroxidase (GSH-Px; cat. no. CK-E92669) and ROS (cat. no. CK-E91516) in serum, the cerebral cortex and the hypothalamus, and Aβ1-42 (cat. no. CK-E94157) in the serum and cerebral cortex were detected using enzyme-linked immunosorbent assay kits according to the manufacturers protocols (Shanghai Yuanye Biotechnology Co., Ltd.).
Histological examination
The fixed whole brain hemisphere, kidney and spleen were washed, gradient dehydration using 30, 50, 70, 80, 95 and 100% ethanol in sequence and finally embedded in paraffin at 25°C for 12 h, which were further sliced into 4 µm sections. Then, a hydrating gradient using 100, 95, 80, 70 and 50% ethanol, and distilled water were applied in sequence and sections were stained using hematoxylin-eosin (H&E) as previously described (20).
Immunohistochemistry
Brain tissues (n=10/group) were fixed with 4% formalin solution at 25°C for 24 h, gradient dehydration using 30, 50, 70, 80, 95 and 100% ethanol in sequence, washed in xylene and embedded in paraffin, and then sliced into 5 µm-thick sections. Slides were dewaxed, hydrated and boiled for 10 min in 10 mM sodium citrate buffer (pH 6) and cooled at 25°C for 30 min. Subsequent to incubation for 10 min in 3% hydrogen peroxide, sections were blocked with normal 10% goat serum for 30 min at 25°C and then incubated overnight at 4°C with primary antibodies against Aβ (1:200; cat. no. ab32136; Abcam, Cambridge, UK). Subsequent to washing with PBS, the sections were incubated with horseradish peroxidase-conjugated anti-rabbit secondary antibody (cat. no. sc-3836; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) for 1 h at 25°C and then incubated with streptavidin-organism HRP complex (Shanghai BestBio Science, China) for 60 min at 25°C. The peroxidase conjugate was counterstained using 5% diaminobenzidine tetrahydrochloride solution as the chromogen and hematoxylin at 25°C for 5 min. Immunoperoxidase staining of Aβ was observed using light microscopy (magnification ×100; Olympus Corporation, Tokyo, Japan).
Statistical analysis
Data were expressed as the mean ± the standard error of the mean. One-way analysis of variance followed by post-hoc multiple comparisons (Dunn’s test) was used to determine statistical significance using SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Crocin protected HT22 cells against l-Glu-induced mitochondrial apoptosis
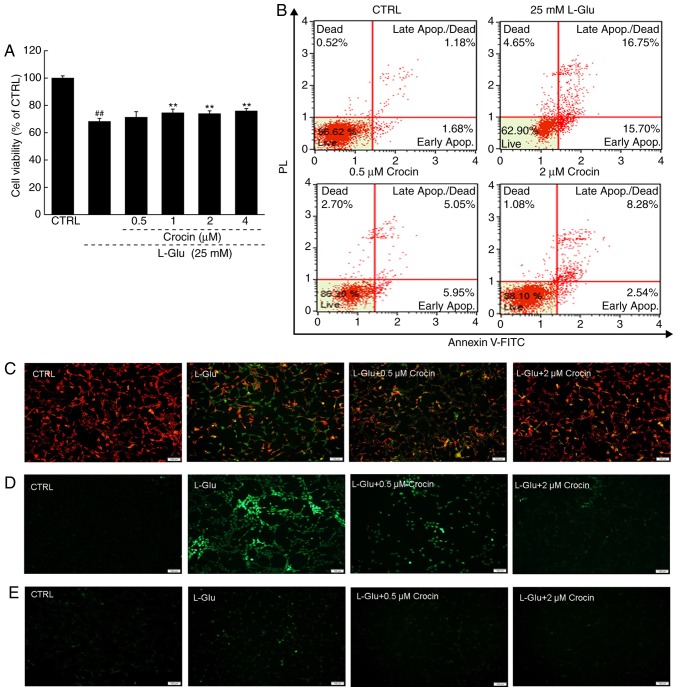
Crocin significantly increased cell viability in the cells exposed to l-Glu for 24 h compared with cells exposed to l-Glu alone (P<0.01; Fig. 2A). Among the HT22 cells exposed to l-Glu for 24 h, 32.5% were revealed to be apoptotic; in contrast, 3 h pre-incubation with crocin reduced the apoptosis rate to 10.8% (Fig. 2B).
Figure 2.
Crocin protected HT22 cells against l-Glu induced mitochondrial apoptosis. (A) Crocin enhanced cell viability in HT22 cells exposed to l-Glu for 24 h (n=10). ##P<0.01 vs. CTRL. **P<0.01 vs. l-Glu-treated cells. (B) Crocin reduced the apoptosis rate in HT22 cells exposed to l-Glu for 24 h identified by analysis by Annexin V-FITC/PI staining (n=10). (C) Crocin restored l-Glu-induced MMP dissipation following 12 h co-incubation (n=10; scale bar, 100 µm). (D) Crocin decreased the over-accumulation of ROS induced by 12 h l-Glu exposure (n=10; scale bar, 100 µm). (E) Crocin prevented intracellular Ca2+ overload induced by 12 h l-Glu exposure (n=10; scale bar, 100 µm). l-Glu, l -Glutamate; CTRL, control; FITC, fluorescein isothiocyanate; PI, propidium iodide; ROS, reactive oxygen species.
An imbalance in MMP characterizes the early stage of mitochondrial injury (21). Compared with the l-Glu-damaged HT22 cells subsequent to 12 h of exposure, 3 h of pre-incubation with crocin strongly restored the MMP dissipation, as indicated by the increased red fluorescence intensity and reduced green fluorescence intensity (Fig. 2C).
Crocin also strongly suppressed the hyper-levels of intracellular ROS compared with the l-Glu-damaged HT22 cells, as indicated by the reduced green fluorescence intensity (Fig. 2D); and intracellular ROS serves as an important mediator of cell damage (22).
The overload of Ca2+ caused by l-Glu is another factor reportedly associated with mitochondrial function (23). In the 12 h l-Glu-exposed HT22 cells, crocin strongly inhibited the overload of Ca2+ compared with the l-Glu-damaged HT22 cells, as indicated by the reduced green fluorescence intensity (Fig. 2E).
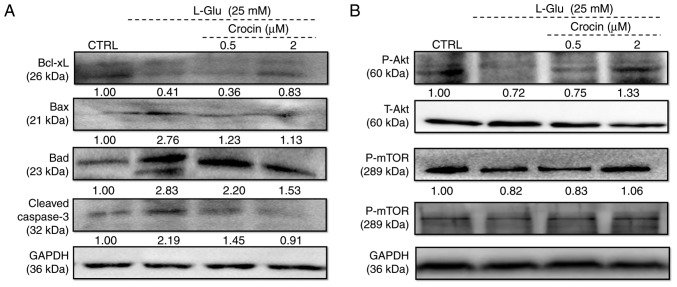
The expression of Bcl-2 family members directly influence mitochondrial function (24). In l-Glu-damaged HT22 cells, the low expression of Bcl-xL and high expression of Bax, Bad and cleaved caspase-3 compared with control cells were identified (Fig. 3A). Comparatively, 3 h of pre-incubation with crocin substantially reversed these changes to anti- and pro-apoptotic protein levels, and even restored them to their standard levels (Fig. 3A).
Figure 3.
Crocin regulated the expression of anti- and pro-apoptotic proteins in HT22 cells exposed to l-Glu for 24 h. Expression of (A) Bcl-xL, Bax, Bad, cleaved caspase-3, (B) P-Akt, T-Akt, P-mTOR and T-mTOR were examined. Crocin increased the expression of Bcl-xL, P-Akt and P-mTOR, and reduced the expression of Bax, Bad and cleaved caspase-3. Quantification data were normalized using GAPDH and corresponding total proteins, and the mean fold of band intensity compared with the CTRL group was noted (n=6). l-Glu, l-glutamate; CTRL, control; Bcl-xL, B-cell lymphoma-extra large; Bax, Bcl2 associated X, apoptosis regulator; Bad, Bcl2 associated agonist of cell death; P-, phosphorylated; T-, total; Akt, protein kinase B; mTOR, mechanistic target of rapamycin.
Akt/mTOR signaling is involved in the pro-survival and anti-apoptosis of neuronal protection (25). Following 24 h of exposure in HT22 cells, 25 mM of l-Glu substantially reduced the phosphorylation of Akt and mTOR compared with the control cells (Fig. 3B), and these reductions were markedly reversed by 3 h of pre-incubation of crocin (Fig. 3B).
Crocin improved cognitive abilities of AD mice, and reduced Aβ deposition in their brains
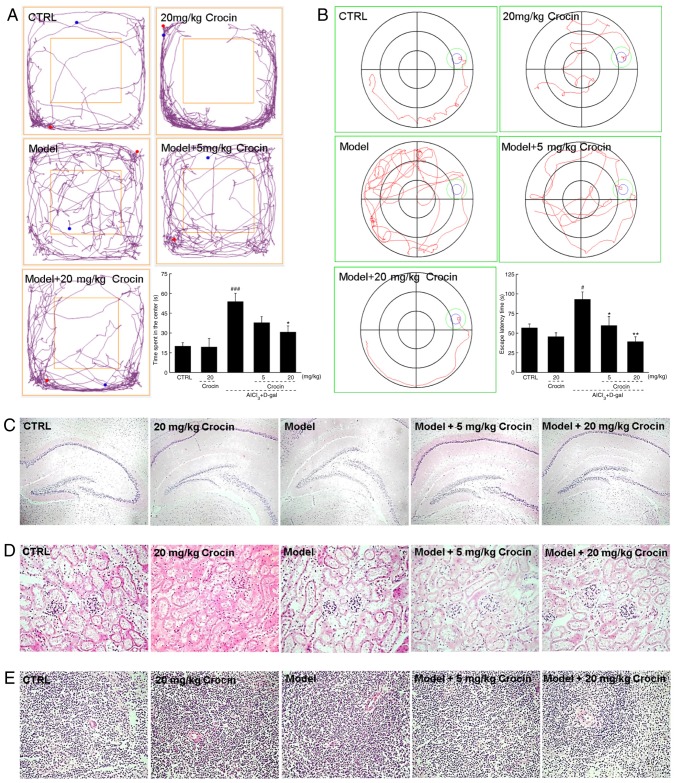
In the present study, mice with AD induced by AlCl3 and d-gal administration were studied to investigate the beneficial effects of crocin in animal models. In the open field test, the AD mice demonstrated significantly more chaotic movements, without purpose, around the central field area compared with the control group (P<0.001; Fig. 4A). The most notable feature of AD is memory loss, with diminishing spatial discernment. The Morris water maze, an useful tool for the evaluation of anti-AD agents, has been widely used to assess learning and memory abilities (26). Herein, in the Morris water maze test, the AD mice required significantly more time to locate the platform hidden in the water, and demonstrated chaotic movements compared with the control group (P<0.05; Fig. 4B). The crocin-treated mice circulated around the periphery significantly more compared with the center in the open field test (P<0.05; Fig. 4A), and required significantly less time to locate the platform in the Morris water maze test (P<0.05; Fig. 4B) compared with the non-treated AD mice. Crocin-alone treatment caused no significant changes to healthy mice behaviors in the open field (Fig. 4A) and Morris water maze tests (Fig. 4B).
Figure 4.
Crocin improves AD-like behaviors in AlCl3 and d-gal developed AD mice. Compared with non-treated AD mice, crocin administration reduced (A) the time spent in the center of the open-yield test, and (B) the time spent locating the platform in Morris water maze. Data expressed as mean ± the standard error of the mean (n=20). #P<0.05 and ###P<0.001 vs. control mice, *P<0.05 and **P<0.01 vs. AD mice. (C) No substantial changes in brain tissue were noted amongst all experimental mice detected by H&E staining (magnification, ×40; n=10). No substantial changes in the (D) kidney and (E) spleen were noted amongst all experimental mice detected by H&E staining (magnification, ×200; n=10). AD, Alzheimer’s disease; AlCl3, aluminum trichloride; d-gal, d-galactose; CTRL, control; H&E, haemotoxylin and eosin.
The data obtained from H&E staining demonstrated that crocin displayed no adverse effects on the brains (Fig. 4C), kidneys (Fig. 4D) or spleens of the mice (Fig. 4E).
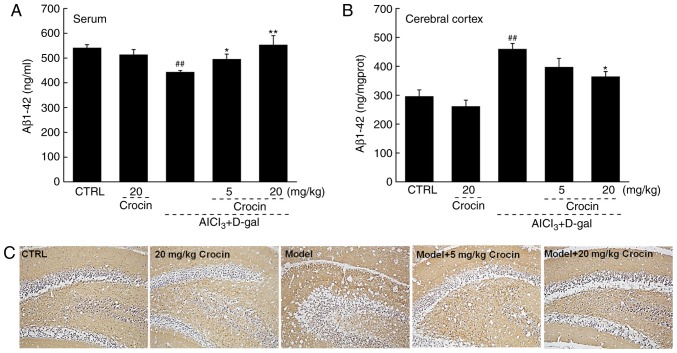
Aβ is the principal constituent of neuritic plaques, which have been proposed, mainly on genetic grounds, to be central to the pathogenesis of this form of dementia (27). In the AD mice, significantly lower levels of Aβ1-42 in serum and significantly higher levels of Aβ1-42 in the cerebral cortex were observed compared with the control (P<0.01; Fig. 5A and B). Comparatively, crocin resulted in a >24.8% increment in the serum levels of Aβ1-42 (P<0.05; Fig. 5A), and a >20.8% reduction in the cerebral cortex levels of Aβ1-42 compared with the AD untreated mice (P<0.05; Fig. 5B). The results of the immunohistochemical assay revealed that the deposition of Aβ1-42 in the hippocampal area was strongly suppressed following 4 weeks of crocin administration (Fig. 5C).
Figure 5.
Crocin reduced Aβ1-42 deposition in brains of AlCl3 and d-gal development AD mice. Compared with non-treated AD mice, 28 days of crocin administration enhanced (A) the content of Aβ1-42 in the serum, and reduced (B) the content of Aβ1-42 in the cerebral cortex analyzing using an enzyme-linked immunosorbent assay. Data expressed as mean ± the standard error of the mean (n=10). ##P<0.01 vs. control mice, *P<0.05 and **P<0.01 vs. AD mice. (C) Crocin reduced the deposition of Aβ1-42 in the hippocampus area detected by immunohistochemistry (magnification, ×100; n=10). AD, Alzheimer’s disease; AlCl3, aluminum trichloride; d-gal, d-galactose; CTRL, control; Aβ, amyloid β.
Crocin regulated the levels of cholinergic neurotransmitters in the serum, cerebral cortex and hypothalamus of AD mice
The characteristic memory impairment of AD is closely associated with the lack of cholinergic neurotransmitters (28). Significantly lower levels of Ach and ChAT and higher levels of AchE were observed in the serum, cerebral cortex and hypothalamus of the AD mice compared with the control groups, indicating injury to the cholinergic system in the AD mice (P<0.05; Table I). Four-week crocin administration signficantly restored the pathological alterations of the levels of Ach, AchE and ChAT in the serum, cerebral cortex and hypothalamus of the AD mice, suggesting the ability of crocin to mitigate cholinergic dysfunction (P<0.05; Table I).
Table I.
Crocin regulated the levels of Ach, AchE and ChAT in the serum, cerebral cortex and hypothalamus.
| Control | Crocin (20 mg/kg) | AlCl3 + d-gal | Crocin (mg/kg)
|
||
|---|---|---|---|---|---|
| 5 | 20 | ||||
| Serum | |||||
| Ach (µg/ml) | 911.9±19.9 | 1057.0±24.4b | 768.8±13.4b | 826.3±15.7 | 1022.5±35.4f |
| AchE (nmol/l) | 138.9±2.2 | 150.9±6.0 | 179.3±5.0b | 121.8±8.2e | 113.2±8.5e |
| ChAT (pmol/l) | 284.7±10.6 | 283.8±8.6 | 233.2±3.9b | 273.5±9.1d | 281.6±2.9e |
| Cerebral cortex | |||||
| Ach (µg/mgprot) | 735.7±56.4 | 741.8±22.1 | 509.6±8.4a | 629.7±61.8 | 743.2±50.5d |
| AchE (nmol/gprot) | 56.6±4.7 | 55.9±1.8 | 105.1±4.8c | 97.7±5.4 | 40.6±2.3f |
| ChAT (pmol/gprot) | 229.3±15.6 | 207.2±11.3 | 167.6±3.3a | 252.2±25.3d | 221.6±1.5 |
| Hypothalamus | |||||
| Ach (µg/mgprot) | 262.7±13.0 | 292.0±27.1 | 161.3±15.1c | 210.1±10.2 | 239.2±16.0d |
| AchE (nmol/gprot) | 53.4±3.1 | 55.9±1.8 | 77.5±4.8b | 60.4±1.6d | 55.7±3.8e |
| ChAT (pmol/gprot) | 144.3±5.4 | 165.8±6.5 | 80.9±6.5b | 109.6±3.3e | 115.0±5.2e |
Data are expressed as the mean ± the standard error of the mean (n=10).
P<0.05,
P<0.01 and
P<0.001 vs. control mice.
P<0.05,
P<0.01 and
P<0.001 vs. AD mice. Ach, acetylcholine; AchE, acetylcho-linesterase; ChAT choline acetyltransferase; AlCl3, aluminum trichloride; d-gal, d-galactose.
Crocin regulated the levels of pro- and anti-oxidative factors in the serum, cerebral cortex and hypothalamus of AD mice
The brain is sensitive to oxidative stress, and hyper-levels of ROS may arise in response to β-amyloid precursor proteins and mitochondrial DNA, resulting in neuronal apoptosis (29). In the AD-induced mice, the levels of ROS were increased, and the levels of SOD and GSH-Px were decreased significantly in the serum, cerebral cortex and hypothalamus compared with the control mice (P<0.05; Table II). Encouragingly, crocin significantly suppressed ROS levels, and enhanced the SOD and GSH-Px levels in the serum, cerebral cortex and hypothalamus of the AD mice compared with the AD alone mice (P<0.05; Table II).
Table II.
Crocin regulated the levels of ROS, SOD and GSH-Px in the serum, cerebral cortex and hypothalamus.
| Control | Crocin (20 mg/kg) | AlCl3 + d-gal | Crocin (mg/kg)
|
||
|---|---|---|---|---|---|
| 5 | 20 | ||||
| Serum | |||||
| ROS (U/ml) | 450.8±4.5 | 475.5±6.3 | 540.6±5.9b | 532.5±6.5 | 491.3±18.0d |
| SOD (U/ml) | 331.4±7.5 | 317.5±13.4 | 277.5±5.0b | 309.2±7.7d | 336.3±13.7e |
| GSH-Px (U/ml) | 780.0±21.4 | 756.3±17.7 | 632.8±20.8b | 685±14.9 | 775±24.4e |
| Cerebral cortex | |||||
| ROS (U/mgprot) | 286.3±24.8 | 323.8±2.1 | 402.5±23.8c | 389.2±35.3 | 303.3±7.3e |
| SOD (U/mgprot) | 175.3±13.5 | 162.0±16.8 | 128.8±5.3a | 166.1±13.9d | 195.7±19.2e |
| GSH-Px (U/mgprot) | 322.4±20.9 | 282.2±12.0 | 269.2±1.9a | 358.2±28.0d | 412.7±36.5e |
| Hypothalamus | |||||
| ROS (U/mgprot) | 126.0±11.8 | 138.8±8.9 | 176.6±12.6b | 144.2±9.9d | 131.9±8.2d |
| SOD (U/mgprot) | 132.7±10.9 | 118.6±4.3 | 68.2±3.7c | 92.6±6.5e | 107.4±9.8e |
| GSH-Px (U/mgprot) | 212.8±20.7 | 220.1±7.7 | 121.9±10.2b | 199.0±18.6d | 211.4±15.9e |
Data are expressed as the mean ± the standard error of the mean (n=10).
P<0.05,
P<0.01 and
P<0.001 vs. control mice.
P<0.05 and
P<0.01 vs. AD mice. ROS, reactive oxygen species; SOD, superoxide dismutase; GSH-Px, glutathione peroxidase; AlCl3, aluminum trichloride; d-gal, d-galactose.
Discussion
Although AD is the most prevalent neurodegenerative disease, affecting over 47 million people globally, no satisfactory treatment has been reported so far (30). Neuronal apoptosis is believed to be one of the key events precipitating AD (31). In the present study, crocin mitigated cell apoptosis and mitochondrial dysfunction in HT22 cells induced by l-Glu. A rise in extracellular glutamate concentration may result in neuronal cell death by means of oxidative stress, which is associated with mitochondrial disorder and ROS accumulation (32). Mitochondria are involved in the apoptosis and regulation of intracellular Ca2+ homeostasis (33). The overload of intracellular Ca2+ results in mitochondrial depolarization, which eventually intensifies oxidative stress-induced cell apoptosis (34,35). ROS, produced by aerobic cells during metabolism, is the foremost cause of oxidative stress (36), resulting in neuronal degeneration and cellular damage in AD (37). Cumulative oxygen damage may cause mitochondrial dysfunction and simultaneously increase ROS production (31). In the present study, crocin inhibited the high expression of cleaved caspase-3, Bad and Bax, and upregulated the low expression of Bcl-xL in l-Glu-exposed HT22 cells. Bcl-2 family proteins are directly responsible for mitochondrial apoptosis, and their ratio is considered to be a biomarker indicating mitochondrial function (37,38). Consistent with a previous study, upregulated Bax and Bad expression accelerates cell apoptosis by increasing mitochondrial permeability; meanwhile, enhanced levels of Bcl-xL help to inhibit cell apoptosis (38). Conversely, MMP dissipation caused by oxidative damage sparks caspase-3, which is cleaved, eventually resulting in apoptosis (39). It has been previously demonstrated that glycyrrhizic acid and Sparassis crispa polysaccharides exert neuroprotective effects on differentiated PC12 cells, protecting those cells against the toxicity of l-Glu by adjusting their MMP levels (40,41). Taken together, the evidence demonstrates that crocin protects HT22 cells against l-Glu-induced apoptosis mainly through modulating mitochondrial apoptotic changes.
Furthermore, crocin upregulated the phosphorylation levels of Akt and mTOR in 24-h l-Glu-exposed cells. Akt serves a central role during apoptosis, and helps to regulate the levels of Bcl-2 family members in AD to exert a neuro-protective effect (30). mTOR, a serine/threonine kinase, is highly conserved in evolution (42), and may be phosphorylated by activated Akt. mTOR is responsible for regulating cell metabolism via the mitochondrial-associated pathway (43). As reported, the activation of Akt signaling may be controlled by ROS levels (44). These data suggest that Akt/mTOR signaling is involved, at least partially, in crocin-mediated neuroprotection of l-Glu-damaged HT22 cells.
Mice with AD, induced by chronic treatment with AlCl3 and d-gal, exhibited AD-like behaviors in the present study, particularly cognitive disorders and dysmnesia, which were strongly improved by crocin treatment. The AD-like behaviors associated with brain damage are mainly caused by oxidative stress (45,46), cholinergic system impairment (47,48) and the aggregation of Aβ (49). Abnormally high levels of intracellular ROS accelerate AD progression (29); meanwhile, SOD and GSH-Px, representative endogenous antioxidants, have been reported as the first-line defense against oxidative stress (50). Encouragingly, crocin enhanced the levels of SOD and GSH-Px, and reduced the levels of ROS in the serum, cerebral cortex and hypothalamus of the AD model mice. Aβ plaque deposition in the brain is closely associated with AD (51), and may trigger a series of cascade reactions including mitochondrial dysfunction and oxidative stress (30,37). Aβ is normally cleared from the brain into the periphery, while changes in its production or clearance may result in its accumulation in the brain, accompanied by reduced peripheral levels and the clinicopathological manifestations of AD (52). Herein, crocin modulated the concentrations of Aβ1-42 in the serum and the brain. Its anti-oxidative activities were key to crocin’s ability to improve the cognitive competence of the experimental AD mice.
The central cholinergic system of patients with AD is severely damaged (53). As a major modulator of learning and memory abilities, Ach is dynamically controlled by the terminating enzyme AchE and the synthesizing enzyme ChAT (54). As the level of AchE increases, the cognitive function of the nervous system gradually decreases (28). ChAT is the most suitable factor for monitoring cholinergic neurons (55). Oxidative stress may enhance the expression of AchE (56), which helps to promote the formation of Aβ and its aggregation into Aβ plaques (57). The cholinergic system in AD models induced by Aβ aggregation is severely damaged (58). Altogether, the present data have revealed that crocin regulates the levels of Ach, AchE and ChAT in the serum, cerebral cortex and hypothalamus of AD mice, suggesting the importance of cholinergic function.
The neuroprotective effects of crocin were successfully confirmed in l-Glu-damaged HT22 cells, and the ability of crocin to improve memory abilities and cognitive functions was verified in AlCl3 and d-gal-induced AD mice. For the first time to the best of our knowledge, the present study verified the roles of oxidative stress-mediated apoptosis and cholinergic functions in the neuroprotective mechanism of crocin.
Acknowledgments
Not applicable.
Abbreviations
- Ach
acetylcholine
- AchE
acetylcholinesterase
- Akt
protein kinase B
- AlC13
aluminum trichloride
- Aβ
amyloid β
- Bcl-xL
B-cell lymphoma-extra large
- ChAT
choline acetyltransferase
- d-gal
d-galactose
- DMEM
Dulbecco’s modified Eagle’s medium
- Fluo-4,AM
1-[2-Amino-5-(2,7-difluoro-6-hydroxy-3-oxo-9-xanthenyl) phenoxy]-2-(2-amino-5-methylphenoxy) ethane-N,N,N′,N′-tetraacetic acid, pentaacetoxymethyl ester
- GSH-Px
glutathione peroxidase
- HRP
horseradish peroxidase
- HT22
Hippocampal neuronal cell line
- l-Glu
l-glutamate
- MMP
mitochondrial transmembrane potential
- mTOR
mammalian target of rapamycin
- MTT
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TBS
t-butyldimethylsilyl
- TBST
TBS + Tween
Funding
The present study is supported by the Science Foundation in Jilin Province of China (grant no. 20180101098JC) and the Special Projects of Cooperation between Jilin University and Jilin Province in China (grant no. SXGJSF2017-1).
Availability of data and materials
All data generated and analyzed during the present study are included in this published article.
Authors’ contributions
DW and XCh designed the experiments. CW, XCa, WH, ZL and FK performed the experiments. CW, XCa and WH processed data. DW and CW wrote the paper. DW and XCh revised the paper.
Ethics approval and consent to participate
Institution Animal Ethics Committee of Jilin University approved the experimental protocol (approval no. 20160409).
Patient consent for publication
Not applicable.
Competing interests
The authors have declared that there are no competing interests.
References
- 1.Holtzman DM, Morris JC, Goate AM. Alzheimer’s disease: The challenge of the second century. Sci Transl Med. 2011;3:77sr71. doi: 10.1126/scitranslmed.3002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Z, Zhong C. Oxidative stress in Alzheimer’s disease. Neurosci Bull. 2014;30:271–281. doi: 10.1007/s12264-013-1423-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mines MA, Beurel E, Jope RS. Regulation of cell survival mechanisms in Alzheimer’s disease by glycogen synthase kinase-3. Int J Alzheimers Dis. 2011;2011:861072. doi: 10.4061/2011/861072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daulatzai MA. Cerebral hypoperfusion and glucose hypo-metabolism: Key pathophysiological modulators promote neurodegeneration, cognitive impairment, and Alzheimer’s disease. J Neurosci Res. 2017;95:943–972. doi: 10.1002/jnr.23777. [DOI] [PubMed] [Google Scholar]
- 5.Tyagi N, Ovechkin AV, Lominadze D, Moshal KS, Tyagi SC. Mitochondrial mechanism of microvascular endothelial cells apoptosis in hyperhomocysteinemia. J Cell Biochem. 2006;98:1150–1162. doi: 10.1002/jcb.20837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu X, Wang J, Lu C, Zhu C, Qian B, Li Z, Liu C, Shao J, Yan J. The role of lysosomes in BDE 47-mediated activation of mitochondrial apoptotic pathway in HepG2 cells. Chemosphere. 2015;124:10–21. doi: 10.1016/j.chemosphere.2014.10.054. [DOI] [PubMed] [Google Scholar]
- 7.Luo P, Fei F, Zhang L, Qu Y, Fei Z. The role of glutamate receptors in traumatic brain injury: Implications for postsynaptic density in pathophysiology. Brain Res Bull. 2011;85:313–320. doi: 10.1016/j.brainresbull.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 8.McDade E, Bateman RJ. Stop alzheimer’s before it starts. Nature. 2017;547:153–155. doi: 10.1038/547153a. [DOI] [PubMed] [Google Scholar]
- 9.Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 10.Jesky R, Hailong C. Are herbal compounds the next frontier for alleviating learning and memory impairments? An integrative look at memory, dementia and the promising therapeutics of traditional chinese medicines. Phytother Res. 2011;25:1105–1118. doi: 10.1002/ptr.3388. [DOI] [PubMed] [Google Scholar]
- 11.Man SC, Chan KW, Lu JH, Durairajan SS, Liu LF, Li M. Systematic review on the efficacy and safety of herbal medicines for vascular dementia. Evid Based Complement Alternat Med. 2012;2012:426215. doi: 10.1155/2012/426215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ochiai T, Soeda S, Ohno S, Tanaka H, Shoyama Y, Shimeno H. Crocin prevents the death of PC-12 cells through sphingomyelinase-ceramide signaling by increasing glutathione synthesis. Neurochem Int. 2004;44:321–330. doi: 10.1016/S0197-0186(03)00174-8. [DOI] [PubMed] [Google Scholar]
- 13.Vahdati Hassani F, Naseri V, Razavi BM, Mehri S, Abnous K, Hosseinzadeh H. Antidepressant effects of crocin and its effects on transcript and protein levels of CREB, BDNF, and VGF in rat hippocampus. Daru. 2014;22:16–25. doi: 10.1186/2008-2231-22-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Essa MM, Vijayan RK, Castellano-Gonzalez G, Memon MA, Braidy N, Guillemin GJ. Neuroprotective effect of natural products against Alzheimer’s disease. Neurochem Res. 2012;37:1829–1842. doi: 10.1007/s11064-012-0799-9. [DOI] [PubMed] [Google Scholar]
- 15.Qi Y, Chen L, Zhang L, Liu WB, Chen XY, Yang XG. Crocin prevents retinal ischaemia/reperfusion injury-induced apoptosis in retinal ganglion cells through the PI3K/AKT signalling pathway. Exp Eye Res. 2013;107:44–51. doi: 10.1016/j.exer.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Ochiai T, Ohno S, Soeda S, Tanaka H, Shoyama Y, Shimeno H. Crocin prevents the death of rat pheochromyctoma (PC-12) cells by its antioxidant effects stronger than those of alpha-tocopherol. Neurosci Lett. 2004;362:61–64. doi: 10.1016/j.neulet.2004.02.067. [DOI] [PubMed] [Google Scholar]
- 17.Heidari S, Mehri S, Hosseinzadeh H. Memory enhancement and protective effects of crocin against d-galactose aging model in the hippocampus of Wistar rats. Iran J Basic Med Sci. 2017;20:1250–1259. doi: 10.22038/IJBMS.2017.9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baghishani F, Mohammadipour A, Hosseinzadeh H, Hosseini M, Ebrahimzadeh-Bideskan A. The effects of tramadol administration on hippocampal cell apoptosis, learning and memory in adult rats and neuroprotective effects of crocin. Metab Brain Dis. 2018;33:907–916. doi: 10.1007/s11011-018-0194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang D, Li S, Chen J, Liu L, Zhu X. The effects of astilbin on cognitive impairments in a transgenic mouse model of Alzheimer’s disease. Cell Mol Neurobiol. 2017;37:695–706. doi: 10.1007/s10571-016-0405-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X, Chen Y, Cai G, Li X, Wang D. Carnosic acid induces apoptosis of hepatocellular carcinoma cells via ROS-mediated mitochondrial pathway. Chem Biol Interact. 2017;277:91–100. doi: 10.1016/j.cbi.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Ravindran S, Swaminathan K, Ramesh A, Kurian GA. Nicorandil attenuates neuronal mitochondrial dysfunction and oxidative stress associated with murine model of vascular calcification. Acta Neurobiol Exp (Wars) 2017;77:57–67. doi: 10.21307/ane-2017-036. [DOI] [PubMed] [Google Scholar]
- 22.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Kritis AA, Stamoula EG, Paniskaki KA, Vavilis TD. Researching glutamate-induced cytotoxicity in different cell lines: A comparative/collective analysis/study. Front Cell Neurosci. 2015;9:91. doi: 10.3389/fncel.2015.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peña-Blanco A, García-Sáez AJ. Bax, Bak and beyond-mitochondrial performance in apoptosis. FEBS J. 2018;285:416–431. doi: 10.1111/febs.14186. [DOI] [PubMed] [Google Scholar]
- 25.Heras-Sandoval D, Pérez-Rojas JM, Hernández-Damián J, Pedraza-Chaverri J. The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell Signal. 2014;26:2694–2701. doi: 10.1016/j.cellsig.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 26.Brandeis R, Brandys Y, Yehuda S. The use of the Morris Water Maze in the study of memory and learning. Int J Neurosci. 1989;48:29–69. doi: 10.3109/00207458909002151. [DOI] [PubMed] [Google Scholar]
- 27.Butterfield DA, Drake J, Pocernich C, Castegna A. Evidence of oxidative damage in Alzheimer’s disease brain: Central role for amyloid beta-peptide. Trends Mol Med. 2001;7:548–554. doi: 10.1016/S1471-4914(01)02173-6. [DOI] [PubMed] [Google Scholar]
- 28.Yamini P, Ray RS, Chopra K. Vitamin D3 attenuates cognitive deficits and neuroinflammatory responses in ICV-STZ induced sporadic Alzheimer’s disease. Inflammopharmacology. 2018;26:39–55. doi: 10.1007/s10787-017-0372-x. [DOI] [PubMed] [Google Scholar]
- 29.Tönnies E, Trushina E. Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J Alzheimers Dis. 2017;57:1105–1121. doi: 10.3233/JAD-161088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu T, Niu C, Zhang X, Dong M. β-Ecdysterone protects SH-SY5Y cells against beta-amyloid-induced apoptosis via c-Jun N-terminal kinase- and Akt-associated complementary pathways. Lab Invest. 2018;98:489–499. doi: 10.1038/s41374-017-0009-0. [DOI] [PubMed] [Google Scholar]
- 31.Koh CH, Whiteman M, Li QX, Halliwell B, Jenner AM, Wong BS, Laughton KM, Wenk M, Masters CL, Beart PM, et al. Chronic exposure to U18666A is associated with oxidative stress in cultured murine cortical neurons. J Neurochem. 2006;98:1278–1289. doi: 10.1111/j.1471-4159.2006.03958.x. [DOI] [PubMed] [Google Scholar]
- 32.Prasansuklab A, Meemon K, Sobhon P, Tencomnao T. Ethanolic extract of Streblus asper leaves protects against glutamate-induced toxicity in HT22 hippocampal neuronal cells and extends lifespan of Caenorhabditis elegans. BMC Complement Altern Med. 2017;17:551. doi: 10.1186/s12906-017-2050-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hung CH, Cheng SS, Cheung YT, Wuwongse S, Zhang NQ, Ho YS, Lee SM, Chang RC. A reciprocal relationship between reactive oxygen species and mitochondrial dynamics in neurodegeneration. Redox Biol. 2018;14:7–19. doi: 10.1016/j.redox.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park HS, Cho HS, Kim TW. Physical exercise promotes memory capability by enhancing hippocampal mitochondrial functions and inhibiting apoptosis in obesity-induced insulin resistance by high fat diet. Metab Brain Dis. 2018;33:283–292. doi: 10.1007/s11011-017-0160-8. [DOI] [PubMed] [Google Scholar]
- 35.Xu D, Peng Y. Apolipoprotein E 4 triggers multiple pathway-mediated Ca2+ overload, causes CaMK II phosphorylation abnormity and aggravates oxidative stress caused cerebral cortical neuron damage. Eur Rev Med Pharmaco. 2017;21:5717–5728. doi: 10.26355/eurrev_201712_14018. [DOI] [PubMed] [Google Scholar]
- 36.Fan LF, He PY, Peng YC, Du QH Ma YJ, Jin JX, Xu HZ, Li JR, Wang ZJ, Cao SL, et al. Mdivi-1 ameliorates early brain injury after subarachnoid hemorrhage via the suppression of inflammation-related blood-brain barrier disruption and endoplasmic reticulum stress-based apoptosis. Free Radic Biol Med. 2017;112:336–349. doi: 10.1016/j.freeradbiomed.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 37.Tong YN, Bai L, Gong R, Chuan JL, Duan XM, Zhu YX. Shikonin protects PC12 cells against β-amyloid peptide-induced cell injury through antioxidant and antiapoptotic activities. Sci Rep. 2018;8:26. doi: 10.1038/s41598-017-18058-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X, Wu J, Yu C, Tang Y, Liu J, Chen H, Jin B, Mei Q, Cao S, Qin D. Lychee seed saponins improve cognitive function and prevent neuronal injury via inhibiting neuronal apoptosis in a rat model of Alzheimer’s disease. Nutrients. 2017;9:E105. doi: 10.3390/nu9020105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen JX, Yan SD. Pathogenic role of mitochondrial [correction of mitochondral] amyloid-beta peptide. Expert Rev Neurother. 2007;7:1517–1525. doi: 10.1586/14737175.7.11.1517. [DOI] [PubMed] [Google Scholar]
- 40.Wang D, Guo TQ, Wang ZY, Lu JH, Liu DP, Meng QF, Xie J, Zhang XL, Liu Y, Teng LS. ERKs and mitochondria-related pathways are essential for glycyrrhizic acid-mediated neuropro-tection against glutamate-induced toxicity in differentiated PC12 cells. Braz J Med Biol Res. 2014;47:773–779. doi: 10.1590/1414-431X20143760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu S, Wang D, Zhang J, Du M, Cheng Y, Liu Y, Zhang N, Wang D, Wu Y. Mitochondria related pathway is essential for polysaccharides purified from Sparassis crispa mediated neuro-protection against glutamate-induced toxicity in differentiated PC12 cells. Int J Mol Sci. 2016;17:E133. doi: 10.3390/ijms17020133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pourtalebi Jahromi L, Sasanipour Z, Azadi A. Promising horizon to alleviate Alzeheimer’s disease pathological hallmarks via inhibiting mTOR signaling pathway: A new application for a commonplace analgesic. Med Hypotheses. 2018;110:120–124. doi: 10.1016/j.mehy.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 43.Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang J, Wang X, Vikash V, Ye Q, Wu D, Liu Y, Dong W. ROS and ROS-mediated cellular signaling. Oxid Med Cell Longev. 2016;2016:4350965. doi: 10.1155/2016/4350965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu J, Zheng YL, Luo L, Wu DM, Sun DX, Feng YJ. Quercetin reverses d-galactose induced neurotoxicity in mouse brain. Behav Brain Res. 2006;171:251–260. doi: 10.1016/j.bbr.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 46.Praticò D, Uryu K, Sung S, Tang S, Trojanowski JQ, Lee VM. Aluminum modulates brain amyloidosis through oxidative stress in APP transgenic mice. FASEB J. 2002;16:1138–1140. doi: 10.1096/fj.02-0012fje. [DOI] [PubMed] [Google Scholar]
- 47.Xiao F, Li XG, Zhang XY, Hou JD, Lin LF, Gao Q, Luo HM. Combined administration of d-galactose and aluminium induces Alzheimer-like lesions in brain. Neurosci Bull. 2011;27:143–155. doi: 10.1007/s12264-011-1028-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumar A, Dogra S, Prakash A. Protective effect of curcumin (Curcuma longa), against aluminium toxicity: Possible behavioral and biochemical alterations in rats. Behav Brain Res. 2009;205:384–390. doi: 10.1016/j.bbr.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 49.Banks WA, Niehoff ML, Drago D, Zatta P. Aluminum complexing enhances amyloid beta protein penetration of blood-brain barrier. Brain Res. 2006;1116:215–221. doi: 10.1016/j.brainres.2006.07.112. [DOI] [PubMed] [Google Scholar]
- 50.Islam MT. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol Res. 2017;39:73–82. doi: 10.1080/01616412.2016.1251711. [DOI] [PubMed] [Google Scholar]
- 51.Dey A, Bhattacharya R, Mukherjee A, Pandey DK. Natural products against Alzheimer’s disease: Pharmaco-therapeutics and biotechnological interventions. Biotechnol Adv. 2017;35:178–216. doi: 10.1016/j.biotechadv.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 52.Fei M, Jianghua W, Rujuan M, Wei Z, Qian W. The relationship of plasma Abeta levels to dementia in aging individuals with mild cognitive impairment. J Neurol Sci. 2011;305:92–96. doi: 10.1016/j.jns.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 53.Karthivashan G, Ganesan P, Park SY, Kim JS, Choi DK. Therapeutic strategies and nano-drug delivery applications in management of ageing Alzheimer’s disease. Drug Deliv. 2018;25:307–320. doi: 10.1080/10717544.2018.1428243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferreira-Vieira TH, Guimaraes IM, Silva FR, Ribeiro FM. Alzheimer’s disease: Targeting the Cholinergic System. Curr Neuropharmacol. 2016;14:101–115. doi: 10.2174/1570159X13666150716165726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fu AL, Li Q, Dong ZH, Huang SJ, Wang YX, Sun MJ. Alternative therapy of Alzheimer’s disease via supplementation with choline acetyltransferase. Neurosci Lett. 2004;368:258–262. doi: 10.1016/j.neulet.2004.05.116. [DOI] [PubMed] [Google Scholar]
- 56.Zhang JY, Jiang H, Gao W, Wu J, Peng K, Shi YF, Zhang XJ. The JNK/AP1/ATF2 pathway is involved in H2O2-induced acetylcholinesterase expression during apoptosis. Cell Mol Life Sci. 2008;65:1435–1445. doi: 10.1007/s00018-008-8047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lushchekina SV, Kots ED, Novichkova DA, Petrov KA, Masson P. Role of Acetylcholinesterase in β-Amyloid aggregation studied by accelerated molecular dynamics. Bionanoscience. 2017;7:396–402. doi: 10.1007/s12668-016-0375-x. [DOI] [Google Scholar]
- 58.Nitta A, Itoh A, Hasegawa T, Nabeshima T. beta-Amyloid protein-induced Alzheimer’s disease animal model. Neurosci Lett. 1994;170:63–66. doi: 10.1016/0304-3940(94)90239-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated and analyzed during the present study are included in this published article.