In this study, we characterize ophthalmic manifestations of antenatal ZIKV exposure in infants who were referred for evaluation during the 2015–2016 Rio de Janeiro outbreak.
Abstract
Video Abstract
OBJECTIVES:
To characterize ophthalmic manifestations of confirmed or suspected antenatal Zika virus (ZIKV) exposure.
METHODS:
Infants with antenatal ZIKV exposure were referred for evaluation during the 2015–2016 Rio de Janeiro outbreak. Mothers with symptomatic ZIKV infection during pregnancy and/or infants with microcephaly or other findings that were suggestive of suspected antenatal exposure were tested with reverse transcriptase polymerase chain reaction (RT-PCR). Complete eye examinations were performed by pediatric ophthalmologists between January 2016 and February 2017. The main outcome measure was eye abnormalities in RT-PCR–positive and suspected (ie, not tested or RT-PCR–negative) antenatal ZIKV cases.
RESULTS:
Of 224 infants, 189 had RT-PCR testing performed. Of 189 patients, 156 had positive RT-PCR results in their blood, urine, and/or placenta. Of 224 infants, 90 had central nervous system (CNS) abnormalities, including microcephaly (62 infants). Eye abnormalities were present in 57 of 224 (25.4%) infants. Optic nerve (44 of 57; 77.2%) and retina abnormalities (37 of 57; 64.9%) were the most common. The group with suspected ZIKV infection (68 infants) had proportionally more eye (36.8% vs 20.5%; P = .022) and CNS abnormalities (68.3% vs 28.1%; P = .008), likely because of referral patterns. Eye abnormalities consistent with ZIKV infection were clinically comparable in both RT-PCR–positive and unconfirmed groups, including 4 RT-PCR–positive infants of 5 without any CNS abnormalities.
CONCLUSIONS:
Similar eye manifestations were identified regardless of laboratory confirmation. Well-appearing infants were also found to have eye abnormalities. Therefore, all infants born after ZIKV outbreaks should be universally screened for eye abnormalities.
What’s Known on This Subject:
Single-case reports and small case series have revealed that the eye can be affected in infants with microcephaly and congenital Zika virus (ZIKV) syndrome.
What This Study Adds:
Eye abnormalities that are consistent with ZIKV infection were clinically comparable in both reverse transcriptase polymerase chain reaction–positive and unconfirmed groups.
During the Zika virus (ZIKV) outbreak of 2015 to 2016, the state of Rio de Janeiro had a slightly delayed ZIKV outbreak compared with the northeastern region of Brazil, providing researchers with the opportunity for prospective testing for ZIKV infection during pregnancy.1 Instituto Fernandes Figueira (IFF) was the main referral center for suspected cases of antenatal ZIKV infection in the state of Rio de Janeiro. Although antenatal infections can have overlapping clinical presentations, the full spectrum of congenital Zika virus syndrome (CZS) has been characterized by 5 unique features: (1) severe microcephaly with partially collapsed skull, (2) thin cerebral cortices with subcortical calcifications, (3) macular scarring and focal pigmentary retinal mottling, (4) congenital contractures, and (5) marked early hypertonia and symptoms of extrapyramidal involvement.2 Nevertheless, infants who are exposed in utero to ZIKV may not have classic CZS because there is a wide spectrum of congenital manifestations, which may range from microcephaly to asymptomatic viral shedding. A high number of pregnant women with suspected ZIKV infection and newborns with clinical suspicion of CZS were referred to IFF for evaluation during the 2015–2016 ZIKV outbreak.
Laboratory confirmation of antenatal ZIKV infection is challenging for several reasons. First, although the incubation period is typically 3 to 14 days,3 most infections are asymptomatic, so patients do not get tested.4 Furthermore, the virus is typically only detectable in blood and urine by nucleic acid assays 3 to 16 days after symptom onset,5 and so a negative test result may not be used to exclude infection.6,7 Interpretation of serologic assays is confounded by cross-reactivity with preexisting dengue antibodies, dengue being a common infection in endemic areas. Therefore, the lack of laboratory confirmation of ZIKV infection is a commonly encountered clinical scenario, and information regarding eye manifestations in this population is needed to guide screening recommendations. Our objective in the current study was to characterize ophthalmic manifestations of ZIKV in infants with suspected in utero ZIKV infection regardless of laboratory confirmation. The comparison of ophthalmic findings between infants with reverse transcriptase polymerase chain reaction (RT-PCR)–positive and unconfirmed case patients who were born during and soon after an outbreak period is used to justify the diagnosis of ZIKV on the basis of typical eye abnormalities in clinical settings where laboratory confirmation of ZIKV infection is not possible. We also performed follow-up eye examinations in a subset of children to determine whether disease progression or reactivation may occur.
Methods
Setting
Patients were examined at IFF, Oswaldo Cruz Foundation, in Rio de Janeiro, Brazil. The hospital is a Ministry of Health referral center for high-risk pregnancies and infectious diseases in infants and children. Pregnancies with suspected ZIKV infection were referred to IFF by the Acute Febrile Illness Service of the National Institute of Infectology, which is a reference site for arboviral infections in the region and is presently conducting a prospective cohort study of maternal ZIKV infection. There were also referrals made from departments of infectious disease and general pediatric services across the city and the state of Rio de Janeiro as well as other governmental and private institutions. Referrals were made for suspected ZIKV cases in pregnancy on the basis of maternal symptoms that are compatible with ZIKV, such as fever, rash, headache, and malaise during pregnancy, and/or for prenatal ultrasound abnormalities that are suspicious for ZIKV infection or in cases of infants born with birth defects that are consistent with congenital ZIKV infection.
Inclusion Criteria
The study population consisted of (1) mothers with positive RT-PCR results during pregnancy (blood, urine, or amniotic fluid) and/or placental tissue at delivery or (2) mothers with clinical suspicion for ZIKV infection during pregnancy (fever, rash, joint pain, malaise, or retrobulbar pain); (3) mothers with fetal ultrasound findings that are suspicious for ZIKV infection; (4) infants with a positive RT-PCR result in their blood, urine, and/or cerebrospinal fluid before leaving the hospital; and (5) infants who were born with a clinical manifestation of congenital ZIKV infection. All subjects were enrolled during or immediately after the ZIKV outbreak period in Brazil between June 2015 and December 2016. The study population was drawn in part from a cohort that is registered on www.clinicaltrials.gov (identifier NCT 03255369).
Exclusion Criteria
Mother-infant pairs with other serologically diagnosed perinatal infectious diseases, genetic disorders, family history of microcephaly, perinatal alcohol abuse, or illicit drug exposures were excluded.
Laboratory Procedures
Laboratory diagnosis of ZIKV infection was conducted after total RNA extraction was performed with the TRIzol Reagent (Thermo Fisher Scientific, Waltham, MA) and RT-PCR was achieved with the QuantiTect Probe polymerase chain reaction kit (Qiagen, Hilden, Germany) with the same primers and cycle times as the standard RT-PCR technique for ZIKV detection.8 In some women who delivered at our institution, amniotic fluid and placental specimens were tested by using RT-PCR. Infants who were born at our institution were tested within 24 hours after birth by using RT-PCR in urine, blood, or cerebrospinal fluid specimens when available.
As part of routine antenatal and/or postnatal care, maternal serum specimens (or infant specimens if maternal testing was not available) were tested for HIV (reverse transcriptase HIV viral load; Abbott Laboratories, Chicago, IL) and syphilis (venereal disease research laboratory test assay and confirmatory treponemal assay if positive).
Systemic Evaluation
For symptomatic pregnant women, the timing of maternal ZIKV infection was defined as the week of gestation that was coincident with symptom onset (first trimester <14 weeks, second trimester 14–25 weeks, and third trimester ≥26 weeks). Detailed demographic, medical, and prenatal history information was collected. Infants underwent comprehensive clinical and neurologic examination by a pediatric infectious disease specialist. Microcephaly was defined as a head circumference z score of <−2 SDs for gestational age and sex.1 Complementary examinations included a transfontanelle ultrasound for all infants and computerized tomography or MRI when further diagnostic clarification was required.
Eye Examination
All infants underwent external eye examination with a portable slit lamp (Kowa Ophthalmic and Medical Equipments, Torrance, CA) and dilated indirect ophthalmoscopy by pediatric ophthalmologists at birth or at presentation and every 3 months subsequently. Increased optic nerve (ON) cupping was defined as a cup/disk ratio of >0.5, and ON hypoplasia was defined as a small ON appearance, which is often associated with the double-ring sign. Pigment mottling was defined as increased hyperpigmentation at the level of the retinal pigment epithelium and retina. Chorioretinal atrophy was characterized as flat, circumscribed lesions of variable depth either confined to the retinal pigment epithelium or involving the choroidal layer. Eye abnormalities were graded by 2 examiners. If there was a discrepancy in grading, findings were discussed with a third, independent reviewer. All examiners were blinded to RT-PCR test results. Eye abnormalities were documented with fundus imaging (RetCam Shuttle; Clarity Medical Systems, Inc, Pleasanton, CA) after pupillary dilation. Interobserver agreement studies were undertaken with 20 digital fundus images on an 8-point scale by 5 ophthalmologists.
Statistical Analysis
Potential associations between RT-PCR results, eye abnormalities, microcephaly, and other central nervous system (CNS) abnormalities were analyzed with Fisher’s exact test. All P values were 2 sided and considered statistically significant if <.05. The analysis was performed by using Stata 14 (Stata Corp, College Station, TX). Interobserver reliability was estimated by using the quadratic-weighted κ statistic with the intraclass correlation coefficient. The following values were adopted for the level of agreement: κ <0.20, very weak; κ between 0.21 and 0.40, weak; κ between 0.41 and 0.60, moderate; κ between 0.61 and 0.80, strong; and κ between 0.81 and 1.0, very strong.
Ethics
IFF and University of California, Los Angeles Institutional Review Board approvals were obtained. Parents or guardians provided written informed consent. In this study, we followed the Declaration of Helsinki, Brazilian Resolution No. 466 (passed on December 12, 2012), and were compliant with the Health Insurance Portability and Accountability Act.
Results
Study Population
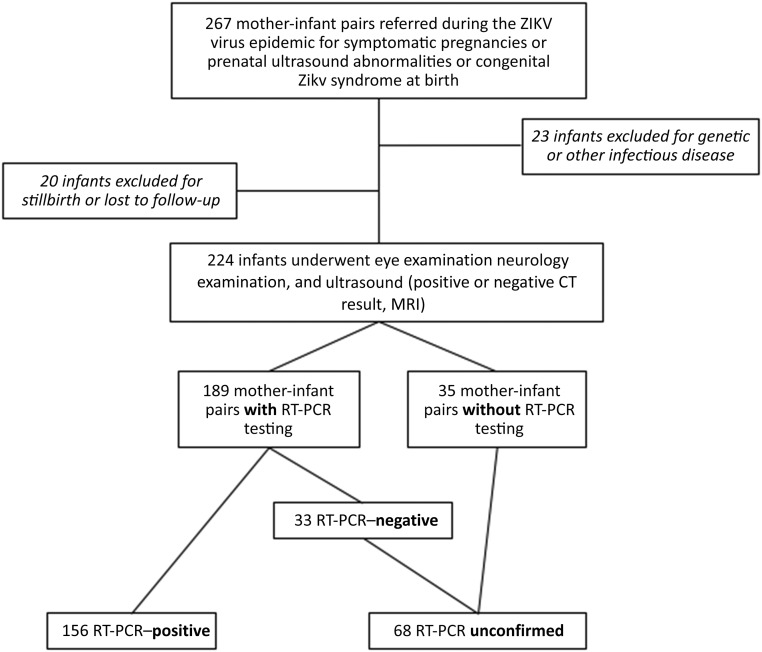
There were 267 infants referred to our institution for suspected in utero ZIKV exposure during or immediately after the outbreak period. Of these, 6 infants were lost to follow-up before having an eye examination. Thirty-seven infants with genetic abnormalities or other infections were excluded, resulting in 224 infants being included in the analysis (Fig 1). There were 196 infants who were examined for the first time during the ZIKV epidemic (until December 2016), and 28 were seen for the first time in 2017. Eye examinations were performed between January 2, 2016, and February 28, 2017. The median age at the time of the first examination was 44 days (interquartile range 12–99 days). Demographic and baseline information is reported in Table 1. Of 224 mother-infant pairs, 94 (42.0%) originated from a longitudinal maternal cohort,9 whereas 112 (50%) were reported on in a previous case series.10
FIGURE 1.
Flowchart of the ZIKV cohort in Rio de Janeiro with excluded patients and RT-PCR results. The RT-PCR unconfirmed group consisted of mother-infant pairs who were not tested and had negative test results. CT, computed tomography.
TABLE 1.
Demographics, Trimester of Onset of Symptoms (if Any), and Physical Examination Findings in Infants With Normal and Abnormal Eye Examination Results
| All Infants (N = 224), n (%) | Abnormal Eye Results (N = 57), n (%) | Normal Eye Results (N = 167), n (%) | P | |
|---|---|---|---|---|
| Born at our institution | 91 (40.6) | 25 (43.9) | 66 (39.5) | .64 |
| Female sex | 110 (49.1) | 30 (52.6) | 80 (47.6) | .54 |
| Asymptomatic pregnancy | 24 (10.7) | 13 (22.8) | 11 (6.6) | <.001 |
| First trimester symptoms | 84 (37.5) | 34 (59.6) | 50 (29.9) | |
| Second trimester symptoms | 78 (34.8) | 9 (15.8) | 69 (41.3) | |
| Third trimester symptoms | 38 (17.0) | 1 (1.8) | 37 (22.2) | |
| Microcephaly, HC <2 SDs | 62 (27.7) | 43 (75.4) | 19 (11.4) | <.001 |
| Any CNS abnormality, including microcephaly | 90 (40.2) | 52 (91.2) | 38(22.8) | <.001 |
| Normal physical examination findings at birth | 134 (60.0) | 5 (8.8) | 129 (77.2) | <.001 |
HC, head circumference.
RT-PCR Testing
Of 224 mother-infant pairs, 189 (84.4%) had RT-PCR testing. There was a total of 156 of 189 (82.5%) RT-PCR–positive mother-infant pairs: 144 were from maternal specimens, and 12 were from infant specimens. The RT-PCR unconfirmed group (n = 68) consisted of 35 untested mother-infants pairs and 33 mother-infant pairs with negative RT-PCR results. Infants in the RT-PCR unconfirmed group were presumed to have antenatal ZIKV exposure on the basis of maternal prenatal symptoms, fetal ultrasound abnormalities, and/or postnatal clinical findings with the exclusion of genetic causes or other antenatal infections, as seen in Table 1.
Eye and CNS Abnormalities
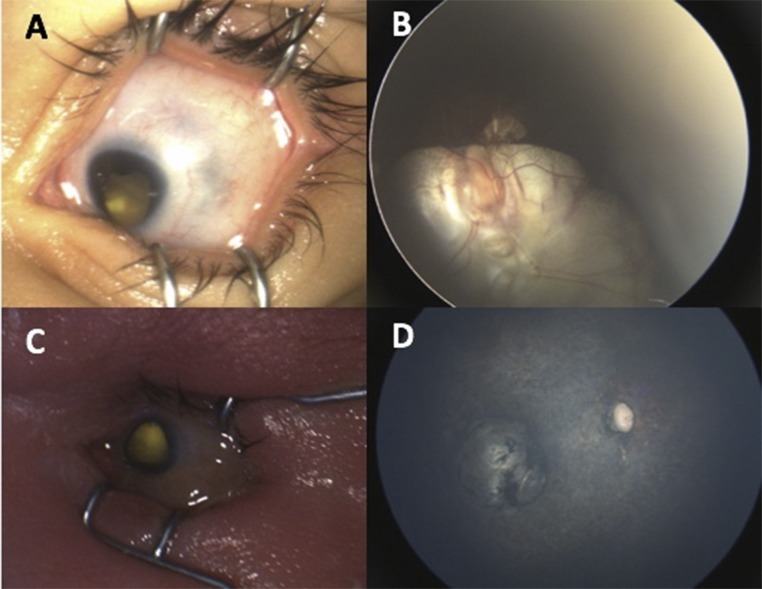
Eye abnormalities were found in 25.4% of all study infants (n = 57 of 224): 34 of 156 (21.8%) of the RT-PCR–positive group and 26 of 68 (38.2%) of the RT-PCR unconfirmed group. ON abnormalities were seen in 44 of 224 infants (19.6%). Retina findings were seen in 37 of 224 infants (16.5%). Specific ON and retina abnormalities are detailed in Table 2. In Fig 2, we show representative, typical ON and retina findings. Uncommon ZIKV eye abnormalities are illustrated in Fig 3.11 These include 1 patient with unilateral microcornea, inferior iris coloboma, and ON coloboma extending into the retina; 1 patient with unilateral microcornea and microphthalmia; and 1 patient with ON atrophy, bilateral severe retinal vessel attenuation, and macular chorioretinal scar or atrophy. The first 2 patients had RT-PCR–positive test results, and although the third patient was RT-PCR unconfirmed, the infant had all the features of CZS. κ values for interobserver agreement ranged from 0.73 to 0.93.
TABLE 2.
Eye Abnormalities in All Infants, RT-PCR–Positive Mother-Infant Pairs, and RT-PCR Unconfirmed Mother-Infant Pairs
| Total ZIKV Cases (N = 224), n (%) | RT-PCR–Positive (N = 156), n (%) | RT-PCR Unconfirmed (N = 68), n (%) | |
|---|---|---|---|
| Any eye abnormality | 57 (25.4) | 32 (20.5) | 25 (36.8) |
| Bilateral disease | 51 (22.8) | 31 (19.9) | 20 (29.4) |
| Any ON abnormality | 44 (19.6) | 26 (16.7) | 18 (26.5) |
| ON cupping and/or pallor | 35 (15.6) | 19 (12.2) | 16 (23.5) |
| ON hypoplasia | 12 (5.4) | 9 (5.7) | 3 (4.4) |
| ON coloboma | 1 (0.5) | 1 (0.6) | 0 (0) |
| Any retina abnormality | 37 (16.5) | 23 (14.7) | 14 (20.6) |
| Chorioretinal atrophy | 22 (9.8) | 15 (9.6) | 7 (10.3) |
| Pigment mottling | 20 (8.9) | 12 (7.7) | 8 (11.8) |
| Colobomatous | 2 (0.9) | 1 (0.6) | 1 (1.5) |
| Severe vessel attenuation | 1 (0.5) | 0 (0) | 1 (1.5) |
| Abnormal eye finding with normal physical examination | 5 (2.2) | 4 (2.6) | 1 (1.5) |
Eye abnormalities were not mutually exclusive.
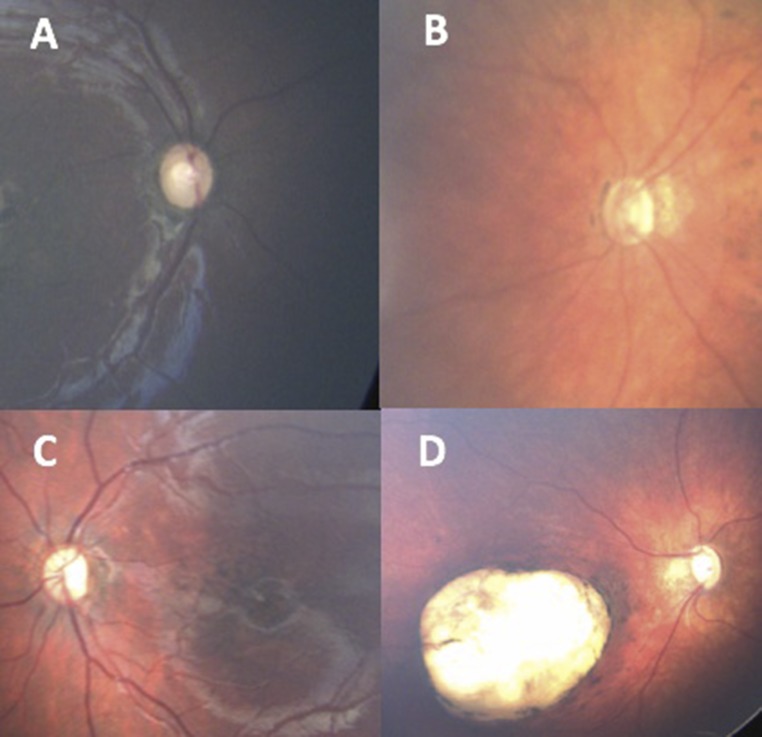
FIGURE 2.
Typical findings in ZIKV. A, Temporal ON pallor. B, ON hypoplasia with double-ring sign. C, Pigment mottling in the macula with ON pallor. D, ON cupping and deep chorioretinal scarring.
FIGURE 3.
Uncommon presentations of ZIKV. A, Eye with microcornea and inferior iris coloboma. B, Large ON coloboma extending into the retina. C, Eye with microcornea and microphthalmia. D, Eye with ON atrophy, severe retinal vessel attenuation, and macular chorioretinal scarring.
Among 224 evaluable infants, 90 (40.2%) had CNS abnormalities, including microcephaly. Eye abnormalities were found in 49 of 90 (54.4%) infants and were correlated with the presence of CNS findings (odds ratio 14.9; 95% confidence interval: 7.3–30.3; P < .0001). RT-PCR test results were positive in 44 of 90 (48.9%) infants with CNS abnormalities. There were 134 children in the cohort without microcephaly or other CNS abnormalities. Of these infants, 5 (3.7%) had eye abnormalities, which further underscores the incidence of eye findings in infants with and without nonocular manifestations of ZIKV infection after antenatal exposure.
Among 224 infants, 116 (51.8%) had 2 eye examinations, 55 (24.6%) had 3 eye examinations, and 27 (12.1%) had 4 eye examinations ∼3 months apart. No ocular lesions revealed signs of activity, change, or progression compared with what was seen in the baseline examination.
Discussion
Antenatal ZIKV infection is known to cause severe ophthalmic impairment in addition to CNS abnormalities, such as microcephaly and delayed neurodevelopment. Ophthalmic manifestations of antenatal ZIKV infection have been reported in single-case reports and small case series, mainly without RT-PCR confirmation of infection. Early reports on eye manifestations of antenatal ZIKV infection originated from cohorts in northeastern Brazil, where the epidemic first began. Current Brazilian Ministry of Health guidelines recommend eye screening for infants with microcephaly only.12,13
We have previously reported ZIKV-related eye manifestations in infants with RT-PCR confirmation.9,10 In the current study, we evaluated whether eye manifestations were different in cases that lacked molecular confirmation of infection, which is a more common clinical scenario because of current challenges with ZIKV diagnostic testing. We were able to have polymerase chain reaction confirmation of antenatal ZIKV infection in 70% of our cohort. The RT-PCR–positive group and the RT-PCR unconfirmed group differed in their frequency of eye abnormalities and CNS abnormalities, with higher incidence of eye findings observed in the suspected, laboratory-unconfirmed group. This difference likely reflects the referral pattern, with more affected infants in the RT-PCR unconfirmed group being referred because of findings that were consistent with in utero ZIKV infection after birth, often outside the window period of RT-PCR positivity. In contrast, most RT-PCR–positive specimens were from specimens of mothers who were referred during the time of symptomatic infection in pregnancy.
Previously published case series in which researchers reported ophthalmic manifestations of ZIKV after antenatal exposure include few subjects and lack laboratory confirmation of infection.11,14–26 Eye abnormalities that are previously described include ON pallor, ON hypoplasia, macular pigment mottling, chorioretinal atrophy, glaucoma, and microphthalmia.10,11,15–21,23–28 Eye abnormalities in our larger cohort are consistent with those in previous reports, the most common features being ON hypoplasia, ON atrophy, macular pigment mottling, and chorioretinal atrophy. Eye abnormalities were essentially identical in both RT-PCR–positive and unconfirmed groups.
In addition to the typical retinal and ON findings described, we also saw 3 infants with less common ophthalmic manifestations of ZIKV (Fig 3).11 Two of these infants had confirmatory maternal RT-PCR infection during pregnancy, and 1 was an RT-PCR unconfirmed case with systemic findings of CZS. Of note, we did not include intraretinal hemorrhages as a manifestation of ZIKV as other groups have done because they have been shown to be present in ∼25% of spontaneous vaginal births and can be attributed to birth trauma.29
Eye abnormalities were identified during the neonatal period at the time of the first eye examination. These lesions persisted and were unchanged over time; 52% of infants had repeat eye examinations, and we found no evidence of worsening, ongoing activity or regression of lesions. Until now, no signs of reactivation have been observed, contrary to what is observed in toxoplasmosis, in which new activity can be detected, usually at the edge of chorioretinal scars.
The pathogenesis of ZIKV eye abnormalities is highly debated and still speculative in humans, and it is not known how the timing of infection affects eye manifestations. Histopathology in 3 of 4 eyes from human fetuses revealed the strongest expression of ZIKV antigen in the iris sphincter.30 Laboratory studies in interferon receptor–deficient mice that were infected postnatally have revealed astrocytic and/or neuronal transport of ZIKV in the brain as well as the virus crossing the blood-brain barrier to affect the retina.31,32 In adult ifnar1−/− mice, systemic inoculation with ZIKV infected all regions of the eye, including the iris, retina, and ON, leading to conjunctivitis, panuveitis, and neuroretinitis.33 Of note, in this same study, eye abnormalities were not detected in ifnar1± fetuses from C57BL/6 ifnar1−/− pregnancies. Another mouse model of ZIKV in which researchers used direct inoculation of ZIKV into the eye was able to manifest severe chorioretinitis similar to what has been noted clinically in infants.34
CNS abnormalities significantly increased the chances of eye abnormalities in our cohort by 15-fold. Nevertheless, we also examined infants who had no apparent clinical findings and were born to mothers with a diagnosis of ZIKV infection during pregnancy. Five infants had eye abnormalities identified in the absence of any CNS findings. This is important to note because current screening guidelines in Brazil only recommend eye examinations in infants with microcephaly. In the United States, Centers for Disease Control and Prevention guidelines used to recommend eye examinations with laboratory confirmation of infection but now recommend eye examinations at the discretion of individual providers. RT-PCR is an expensive assay and is not widely available in Brazil and other Latin American countries, and antibody tests for ZIKV cross-react with dengue,35 contributing to the difficulty often encountered in confirming Zika infection in the laboratory. Given our findings, we strongly recommend universal newborn eye screening in infants with potential antenatal ZIKV exposure, particularly in endemic areas. Although there is no specific treatment of eye abnormalities, low-vision therapy that is initiated earlier in life may result in better long-term outcomes. In addition, eye abnormalities in an otherwise normal infant may be a harbinger of other neurologic issues to come and may aid in a clinical diagnosis of antenatal ZIKV infection.
A limitation is that our results cannot be extrapolated to provide general population incidence data because infants were often referred to our center because of CNS abnormalities, such as microcephaly or other stigmata of in utero ZIKV infection. In addition, we included 94 patients from a prospective symptomatic pregnancy cohort study, which presents another bias toward symptomatic mothers. Nevertheless, with this study, we confirm typical antenatal ZIKV eye abnormalities in the largest series of infants evaluated to date to our knowledge, including both RT-PCR–confirmed and unconfirmed cases. In addition, we had detailed and complete eye examinations performed by experienced pediatric ophthalmologists with consistent documentation and high agreement in lesion identification.
Conclusions
The frequency of RT-PCR–positive results was high, and eye abnormalities were found in one-quarter of the infants in our mother-infant cohort who were referred for evaluation of suspected ZIKV infection. Because universal eye screening for ZIKV endemic areas has not yet been adopted, it is difficult to assess the true frequency of eye abnormalities in ZIKV-exposed infants. We believe all infants with potential ZIKV exposure (ie, all infants born during outbreak periods in endemic areas) should have screening eye examinations regardless of the presence of microcephaly, other CNS abnormalities, or laboratory confirmation of infection. Digital retinal imaging by trained health care providers could help maximize resources, especially in areas where ophthalmologists may not be available for in-person eye examinations. The early identification of eye abnormalities enables low-vision interventions to improve visual function with important repercussions for neurocognitive development.
Glossary
- CNS
central nervous system
- CZS
congenital Zika virus syndrome
- IFF
Instituto Fernandes Figueira
- ON
optic nerve
- RT-PCR
reverse transcriptase polymerase chain reaction
- ZIKV
Zika virus
Footnotes
Dr A.A. Zin conceptualized and designed the study, designed the data collection instruments, collected data, conducted the initial analyses, drafted the initial manuscript, and reviewed and revised the manuscript; Drs Tsui, Gaw, Adachi, Arumugaswami, Nielsen-Saines, and Belfort conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript; Drs Moreira, Rossetto, Vasconcelos, Neves, O.A. Zin, Haefeli, Silveira Filho, Gomes, M. Pone, S. Pone, Pereira, and Brasil designed the data collection instruments, collected data, conducted the initial analyses, and reviewed and revised the manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
This trial includes a cohort that is registered at www.clinicaltrials.gov (identifier NCT 03255369).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by the Brazilian National Council for Scientific and Technological Development (grant 441098/2016-9), the National Institute of Allergy and Infectious Diseases (grant AI AI128697), and the National Eye Institute (grant R21EY028318-01). Dr Tsui was supported in part by a Research to Prevent Blindness unrestricted grant given to the Jules Stein Eye Institute. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Kleber de Oliveira W, Cortez-Escalante J, De Oliveira WT, et al. Increase in reported prevalence of microcephaly in infants born to women living in areas with confirmed Zika virus transmission during the first trimester of pregnancy - Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(9):242–247 [DOI] [PubMed] [Google Scholar]
- 2.Moore CA, Staples JE, Dobyns WB, et al. Characterizing the pattern of anomalies in congenital Zika syndrome for pediatric clinicians. JAMA Pediatr. 2017;171(3):288–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krow-Lucal ER, Biggerstaff BJ, Staples JE. Estimated incubation period for Zika virus disease. Emerg Infect Dis. 2017;23(5):841–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duffy MR, Chen TH, Hancock WT, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360(24):2536–2543 [DOI] [PubMed] [Google Scholar]
- 5.Fontaine A, de Laval F, Belleoud D, Briolant S, Matheus S. Duration of Zika viremia in serum [published online ahead of print March 30, 2018]. Clin Infect Dis. doi: 10.1093/cid/ciy261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lessler J, Ott CT, Carcelen AC, et al. Times to key events in Zika virus infection and implications for blood donation: a systematic review. Bull World Health Organ. 2016;94(11):841–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Musso D, Roche C, Nhan TX, Robin E, Teissier A, Cao-Lormeau VM. Detection of Zika virus in saliva. J Clin Virol. 2015;68:53–55 [DOI] [PubMed] [Google Scholar]
- 8.Lanciotti RS, Kosoy OL, Laven JJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis. 2008;14(8):1232–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brasil P, Pereira JP Jr, Moreira ME, et al. Zika virus infection in pregnant women in Rio de Janeiro. N Engl J Med. 2016;375(24):2321–2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zin AA, Tsui I, Rossetto J, et al. Screening criteria for ophthalmic manifestations of congenital Zika virus infection. JAMA Pediatr. 2017;171(9):847–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Paula Freitas B, Zin A, Ko A, Maia M, Ventura CV, Belfort R Jr. Anterior-segment ocular findings and microphthalmia in congenital Zika syndrome. Ophthalmology. 2017;124(12):1876–1878 [DOI] [PubMed] [Google Scholar]
- 12.Secretaria Municipal de Saúde Prefeitura Do Rio De Janeiro. Available at: http://riocomsaude.rj.gov.br/Publico/MostrarArquivo.aspx?C=xaOb3t7ifYA=. Accessed August 2, 2018
- 13.Brazilian Ministry of Health Preventing and combating Dengue, Chiungunya e Zika. Available at: http://portalarquivos2.saude.gov.br/images/pdf/2016/dezembro/12/orientacoes-integradas-vigilancia-atencao.pdf. Accessed August 2, 2018
- 14.Campos AG, Lira RP, Arantes TE. Optical coherence tomography of macular atrophy associated with microcephaly and presumed intrauterine Zika virus infection. Arq Bras Oftalmol. 2016;79(6):400–401 [DOI] [PubMed] [Google Scholar]
- 15.de Oliveira Dias JR, Ventura CV, Borba PD, et al. Infants with congenital Zika syndrome and ocular findings from São Paulo, Brazil: spread of infection [published online ahead of print January 2, 2017]. Retin Cases Brief Rep. doi: 10.1097/ICB.0000000000000518 [DOI] [PubMed] [Google Scholar]
- 16.de Paula Freitas B, de Oliveira Dias JR, Prazeres J, et al. . Ocular findings in infants with microcephaly associated with presumed Zika virus congenital infection in Salvador, Brazil [published online ahead of print February 9, 2016]. JAMA Ophthalmol. doi: 10.1001/jamaophthalmol.2016.0267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miranda HA II, Costa MC, Frazão MAM, Simão N, Franchischini S, Moshfeghi DM. Expanded spectrum of congenital ocular findings in microcephaly with presumed Zika infection. Ophthalmology. 2016;123(8):1788–1794 [DOI] [PubMed] [Google Scholar]
- 18.Moshfeghi DM, de Miranda HA II, Costa MC. Zika virus, microcephaly, and ocular findings. JAMA Ophthalmol. 2016;134(8):945. [DOI] [PubMed] [Google Scholar]
- 19.Rifkin LM, Duker JS. Use of retinal optical coherence tomography to detect congenital Zika syndrome. JAMA Ophthalmol. 2016;134(12):1427–1428 [DOI] [PubMed] [Google Scholar]
- 20.Ventura CV, Albini TA, Berrocal AM. First locally transmitted Zika virus cases identified in the United States. JAMA Ophthalmol. 2016;134(11):1219–1220 [DOI] [PubMed] [Google Scholar]
- 21.Ventura CV, Maia M, Bravo-Filho V, Góis AL, Belfort R Jr. Zika virus in Brazil and macular atrophy in a child with microcephaly. Lancet. 2016;387(10015):228. [DOI] [PubMed] [Google Scholar]
- 22.Ventura CV, Maia M, Dias N, Ventura LO, Belfort R Jr. Zika: neurological and ocular findings in infant without microcephaly. Lancet. 2016;387(10037):2502. [DOI] [PubMed] [Google Scholar]
- 23.Ventura CV, Maia M, Travassos SB, et al. Risk factors associated with the ophthalmoscopic findings identified in infants with presumed Zika virus congenital infection. JAMA Ophthalmol. 2016;134(8):912–918 [DOI] [PubMed] [Google Scholar]
- 24.Ventura CV, Maia M, Ventura BV, et al. Ophthalmological findings in infants with microcephaly and presumable intra-uterus Zika virus infection. Arq Bras Oftalmol. 2016;79(1):1–3 [DOI] [PubMed] [Google Scholar]
- 25.Ventura CV, Ventura LO, Bravo-Filho V, et al. Optical coherence tomography of retinal lesions in infants with congenital Zika syndrome. JAMA Ophthalmol. 2016;134(12):1420–1427 [DOI] [PubMed] [Google Scholar]
- 26.Yepez JB, Murati FA, Pettito M, et al. ; Johns Hopkins Zika Center . Ophthalmic manifestations of congenital Zika syndrome in Colombia and Venezuela. JAMA Ophthalmol. 2017;135(5):440–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verçosa I, Carneiro P, Verçosa R, et al. . The visual system in infants with microcephaly related to presumed congenital Zika syndrome. J AAPOS. 2017;21(4):300–304.e1 [DOI] [PubMed] [Google Scholar]
- 28.Ventura LO, Ventura CV, Lawrence L, et al. . Visual impairment in children with congenital Zika syndrome. J AAPOS. 2017;21(4):295–299.e2 [DOI] [PubMed] [Google Scholar]
- 29.Watts P, Maguire S, Kwok T, et al. Newborn retinal hemorrhages: a systematic review. J AAPOS. 2013;17(1):70–78 [DOI] [PubMed] [Google Scholar]
- 30.Fernandez MP, Parra Saad E, Ospina Martinez M, et al. . Ocular histopathologic features of congenital Zika syndrome. JAMA Ophthalmol. 2017;135(11):1163–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao Z, Yang M, Azar SR, et al. Viral retinopathy in experimental models of Zika infection. Invest Ophthalmol Vis Sci. 2017;58(10):4355–4365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van den Pol AN, Mao G, Yang Y, Ornaghi S, Davis JN. Zika virus targeting in the developing brain. J Neurosci. 2017;37(8):2161–2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miner JJ, Sene A, Richner JM, et al. Zika virus infection in mice causes panuveitis with shedding of virus in tears. Cell Reports. 2016;16(12):3208–3218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh PK, Guest JM, Kanwar M, et al. Zika virus infects cells lining the blood-retinal barrier and causes chorioretinal atrophy in mouse eyes. JCI Insight. 2017;2(4):e92340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Staples JE, Dziuban EJ, Fischer M, et al. Interim guidelines for the evaluation and testing of infants with possible congenital Zika virus infection - United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(3):63–67 [DOI] [PubMed] [Google Scholar]