Abstract
Background and aims:
Tumor necrosis factor receptor type 1 (TNFR1) is associated with kidney disease and mortality risk in various populations. Whether or not kidney function mediates mortality risk is unknown. We evaluated associations of TNFR1 levels with measures of kidney function, cardiovascular events, and mortality in a population of veterans with stable ischemic heart disease.
Methods:
TNFR1 was measured from baseline serum samples in the Heart and Soul Study; elevated levels were defined by the highest quartile (Q4, > 3.4 ng/mL). We evaluated associations of high TNFR1 with baseline estimated glomerular filtration rate (eGFR) and urine albumin to creatinine ratio (ACR) and with longitudinal changes in eGFR (rapid loss), as well as with incident myocardial infarction (MI), heart failure hospitalizations (HF), and mortality over a median follow-up time of 8.9 years. Covariates included demographics and comorbid conditions.
Results:
Among 985 participants who had TNFR1 measurements, median TNFR1 was 2.33 ng/ml (IQR 1.8–3.1). Relative to Q1, Q4 had higher risk of eGFR < 60 ml/min/1.73 m2 (RR 11.71 [95% CI 5.46, 25.11]); ACR ≥ 30 mg/g (2.44 [1.15, 5.19]); and rapid loss in kidney function (2.10 [1.12, 3.92]). Although TNFR1 Q4 was associated with MI, HF, and mortality after demographic adjustment, there were no associations in fully-adjusted models (1.04 [0.44, 2.49]; 1.02 [0.48, 2.15]; 1.42 [0.88, 2.28] respectively).
Conclusions:
Levels of TNFR1 are associated longitudinally with kidney function decline but not with MI, HF or mortality risk after adjustment. Kidney disease may mediate the risk of MI, HF, and mortality associated with TNFR1.
Keywords: atherosclerosis, kidney disease, inflammation, albuminuria
Introduction
Inflammation is a possible mechanism to explain the association between atherosclerosis and mortality risk. Tumor necrosis factor receptors 1 (TNFR1) and 2 (TNFR2) are cell membrane-bound receptors involved in apoptosis, inflammation, and immune host defense.1 The soluble TNF receptors are present in the sera of healthy individuals, and elevated levels occur in a variety of pathologic states including sepsis and autoimmune disorders.1 Circulating TNFR1 levels have been found to be associated with mortality risk and heart failure (HF) among individuals with acute myocardial infarction,2 while levels are also elevated in individuals at higher risk of developing HF.3 Tumor necrosis factor alpha (TNFα), the main ligand for TNFR1 and TNFR2, is a pro-inflammatory cytokine produced by activated monocytes4 and is responsible for a normal immune response. Shedding of TNFRs is induced by TNFα, as well as by other cytokines including interleukin-6, TFR-beta, and interferon gamma.1 In type 2 diabetics, concentrations of TNFR1 and TNFR2 are independently associated with kidney function decline and ESRD5 and are also associated with early glomerular structural lesions on biopsy.6 Associations of TNFRs and cardiovascular and mortality risk outcomes in individuals with kidney disease are not clearly established. Furthermore, the extent to which the association between TNFR1 and poor cardiovascular outcomes is explained by reduced kidney function is not known.
Previous studies evaluating TNFR1 and cardiovascular outcomes were conducted in patients with predominantly normal kidney function,3 while studies evaluating kidney outcomes did not evaluate cardiovascular events.7 This study evaluated circulating tumor necrosis factor receptor type 1 (TNFR1) as a risk factor for prevalent CKD and kidney function decline and risk of myocardial infarction, heart failure, and all-cause mortality in the Heart and Soul study, a population of individuals with stable ischemic heart disease and a range of kidney functions measured at multiple time points, who were followed for cardiovascular events. Due to prior studies showing the strong associations between TNFR1 and kidney function, we hypothesized that associations with cardiovascular outcomes would be mediated by worse kidney function, assessed by estimated glomerular filtration rate (eGFR) and albuminuria (ACR), and that TNFR1 would be associated with more accelerated kidney function loss.
Materials and methods
Study population
The Heart and Soul Study was a prospective cohort study designed to investigate the effects of psychosocial factors on health outcomes in patients with stable ischemic heart disease (IHD).8 Participants were eligible if they had a history of myocardial infarction; angiographic evidence of ≥50% stenosis in ≥1 coronary vessels; evidence of exercise-induced ischemia by treadmill ECG or stress nuclear perfusion imaging; or a history of coronary revascularization. Participants were excluded if they were unable to walk one block, had an acute coronary syndrome within the previous six months, or were likely to move out of the area within three years.
Heart and Soul Study data collection
1024 subjects were recruited from 12 outpatient clinics in the San Francisco Bay Area between 9/2000 and 12/2002. This cohort included 549 (54%) with a history of myocardial infarction, 237 (23%) with a history of revascularization but not myocardial infarction, and 238 (23%) with a diagnosis of coronary disease documented by their physician, based on a positive angiogram or treadmill test in over 98% of cases. After enrollment, a complete medical history, extensive questionnaires, and an exercise treadmill test with baseline and stress echocardiograms were completed in all participants. Serum samples were obtained after a 12-hour fast in the morning, prior to stress test, and frozen at −80° C. Of 1024 participants, 39 were excluded from this analysis because serum was not available to perform the TNFR1 assay, yielding 985 participants. After five years, all surviving participants were invited to return for a repeat examination. More than 80% of surviving participants (N= 627) completed the 5-year follow-up examination (between 9/2005 and 12/2007). Institutional Review Boards at each site approved this study protocol. All participants provided written informed consent.
Measurement of TNF-alpha receptor 1
A multiplexed bead-based immunoassay on microtiter plates was used to measure serum TNFR1 levels. The TNFR1 antibody (Alere, San Diego, CA) was conjugated to modified paramagnetic Luminex beads (Radix Biosolutions) and antigens were biotinylated. Fluorescent signals were generated using Streptavidin-R-Phycoerythrin (SA-RPE: Prozyme PJ31S) and read on a Luminex LX200 reader. Each antigen was spiked into a calibration matrix in order to gravimetrically construct an 8-point calibration curve. The antigen concentrations were calculated using a standard curve determined by fitting a five parameter logistic function to the signals obtained for the 8-point calibration curves (Alere, San Diego, CA). Each sample was assayed in duplicate, and the TNFR1 level was calculated as the average of two measurements. The limits of detection for this assay are 0.021 to 74 ng/mL. In our study, the intra-assay and inter-assay coefficients of variation were 12% and 13% at low concentrations (1.3 ng/ml) of TNFR1 and 15% and 16% at high concentrations (30.33 ng/ml) of TNFR1.
Primary outcomes: kidney function
Baseline serum samples were used to measure serum creatinine (measured by the rate Jaffe method (mg/dl)) and cystatin C (measured by a BNII nephelometer (Dade Behring Inc., Deerfield, IL, USA) with a particle-enhanced immunonephelometric assay (N Latex Cystatin C; Dade Behring, Inc.)).9 Estimated glomerular filtration rate (eGFR) was determined by the combined creatinine-cystatin C equation.10 Baseline reduced eGFR was defined as an eGFR<60 ml/min/1.73 m2 based on KDIGO definitions.11
Urine albumin and creatinine were measured in a 24-hour urine collection at baseline and at the 5-year follow-up visit. The method for collection was as follows: participants were given a 3-L collection jug for urine and were asked to save all urine between the end of their intake appointment and the time when a research technician recovered the urine at the participant’s home 24 hours later. Participants were instructed to keep the urine collections refrigerated at all times. The research technician arrived at the patient’s home to retrieve the sample 24 hours after the timed collection was initiated, to avoid over- or under-collection. If participants reported missing any urine or if the collections were less than 1 or more than 3 liters in volume, then collections were repeated. If participants were unable to collect all urine for any reason or had urinary incontinence, then no data were recorded. A urine albumin to creatinine ratio (ACR) was calculated in mg/g from the 24-hour sample. Baseline albuminuria was defined as urine ACR ≥ 30 mg/g based on KDIGO definitions.11
Rapid kidney function loss was defined as a change in eGFR of greater than 3% per year as in our prior work.12 Annualized percent change in eGFR was calculated from the difference between baseline and 5-year follow-up eGFR.
Primary outcomes: cardiovascular events
Interval hospitalizations or mortality risks were assessed by annual telephone interviews conducted with each participant or their proxy. Medical records, electrocardiograms, mortality risk certificates, autopsy, and coroner’s reports were obtained as supporting documentation of any reported events. Two independent and blinded reviewers adjudicated all events. In the event of disagreement, the adjudicators conferred, reconsidered their classification, and requested consultation from a third blinded adjudicator.
Our cardiovascular outcomes included myocardial infarction, hospital admission for congestive heart failure, and all-cause mortality. Myocardial infarction was defined using standard diagnostic criteria.13 Heart failure was defined as hospitalization for incident heart failure or exacerbation using detailed duplicate chart reivew.14 Mortality risks were verified by mortality risk certificates. Methods have been described in greater detail previously.15
Statistical analysis
Participants were divided into quartiles of TNFR1 levels. TNFR1 levels were normally distributed in the population studied. Baseline participant characteristics across quartiles were compared using analysis of variance (ANOVA) for continuous variables and χ2 test for dichotomous variables. We evaluated cross-sectional associations with baseline kidney function and with longitudinal rapid kidney function loss using Poisson regression. We compared rates of the outcomes of MI, HF, and mortality between quartile 4 versus quartile 1 using multivariable Poisson regression models. For all regression models, adjustment variables included demographic characteristics (age, sex, race); lifestyle characteristics (smoking, BMI); and comorbid conditions (history of hypertension, diabetes, MI, HF, ACEI/ARB use, betablocker use, HDL, triglycerides, hemoglobin A1c, LVEF, METs). Covariates were selected based on evaluation of known confounders of atherosclerosis and kidney disease. In analyses of rapid kidney function loss, we adjusted for the baseline value and additionally adjusted for ACR in the final model. We also performed subgroup analyses by gender, age, and baseline kidney function. In analyses of cardiovascular outcomes and mortality risk, we followed demographic-adjusted models with additional adjustment for eGFR and then adjusted for comorbid conditions. We used Statistical Analysis Software (version 9.2; SAS Institute Inc., Cary, NC) and STATA (version 12.0; Statacorp LP, College Station, TX) for all analyses. We performed sensitivity analyses censoring cardiovascular and mortality outcomes at five years, based on the follow-up time available for kidney function measures.
Results
Participant characteristics
Among 985 individuals with serum measurements of TNFR1 at baseline, mean age was 66.7 (± 11) years, 81% were men, 60% were white, 70% had hypertension, and 26% had diabetes. Mean eGFR at baseline was 70.6 (± 22.2) ml/min/1.73 m2 and the prevalence of eGFR < 60 ml/min/1.73 m2 at baseline was 31%. Individuals in the highest quartile of TNFR1 were more likely to be older, male, and white; to have hypertension, diabetes, and heart failure; to have much lower baseline eGFR and higher baseline ACR; and to have modestly lower HDL levels and higher triglycerides and glycosylated hemoglobin (Table 1).
Table 1.
Baseline characteristics by quartile of TNFR1 (N=985).
| Q1 (n=247) 0.21–1.86 ng/ml |
Q2 (n=246) 1.87–2.47 ng/ml |
Q3 (n=246) 2.48–3.40 ng/ml |
Q4 (n=246) 3.41–38.5 ng/ml |
p-valuea | |
|---|---|---|---|---|---|
| Demographic factors | |||||
| Age (years) | 62 (54, 69) | 66 (59, 73) | 69 (62, 76) | 73 (64, 79) | <0.001 |
| White race | 109 (44%) | 142 (58%) | 182 (74%) | 160 (65%) | <0.001 |
| Male | 186 (76%) | 200 (81%) | 213 (86%) | 203 (83%) | 0.024 |
| BMI (kg/m2) | 27.2 (24.4, 30.2) | 27.4 (24.7, 31.3) | 28.0 (25.3, 31.3) | 28.2 (24.9, 31.6) | 0.110 |
| Never smoker | 64 (26%) | 78 (32%) | 83 (34%) | 74 (30%) | 0.305 |
| Clinical history | |||||
| Hypertension | 162 (66%) | 168 (68%) | 165 (67%) | 197 (80%) | 0.002 |
| Diabetes mellitus | 44 (18%) | 62 (25%) | 66 (27%) | 86 (35%) | <0.001 |
| Myocardial infarction | 125 (51%) | 128 (52%) | 124 (51%) | 148 (61%) | 0.090 |
| Heart failure | 28 (11%) | 41 (17%) | 41 (17%) | 63 (26%) | <0.001 |
| COPD/Asthma | 38 (16%) | 39 (16%) | 39 (16%) | 40 (16%) | 0.997 |
| Medication use | |||||
| Aspirin | 187 (76%) | 190 (77%) | 199 (81%) | 186 (76%) | 0.545 |
| Statins | 148 (60%) | 165 (67%) | 160 (65%) | 159 (65%) | 0.443 |
| ACE inhibitor / ARB | 94 (38%) | 124 (50%) | 141 (57%) | 146 (59%) | <0.001 |
| Beta blocker | 127 (52%) | 140 (57%) | 143 (58%) | 156 (63%) | 0.071 |
| Metabolic markers | |||||
| HDL cholesterol (mg/dl) | 46 (37, 56) | 43 (37, 54) | 43 (36, 52) | 41 (34, 50) | 0.001 |
| LDL cholesterol (mg/dl) | 98 (85, 125) | 99 (84, 123) | 102 (82, 121) | 97 (79, 116) | 0.178 |
| C-reactive protein (mg/L) | 2.9 (1.3, 6.5) | 2.8 (1.4, 6.3) | 3.9 (1.8, 8.9) | 5.0 (2.2, 15.5) | <0.001 |
| Triglycerides (mg/dl) | 99 (68, 137) | 111 (73, 170) | 112 (75, 159) | 126 (78, 188) | 0.002 |
| Glycosylated hemoglobin (%) | 5.5 (5.1, 5.8) | 5.7 (5.3, 6.1) | 5.7 (5.4, 6.4) | 5.8 (5.5, 6.5) | <0.001 |
| Kidney function | |||||
| eGFR (ml/min/1.73 m2) | 89.2 (77.9, 99) | 78.3 (66.1, 87.3) | 67.3 (57.7, 76.8) | 47.3 (36.1, 59.2) | <0.001 |
| ACR (mg/g) | 7.1 (3.9, 12.7) | 8.1 (4.1, 15.0) | 8.8 (5.1, 17.5) | 14.3 (7.4, 67.8) | <0.001 |
| Cardiac function | |||||
| LV ejection fraction (%) | 65 (60, 69) | 64 (59, 68) | 63 (57, 68) | 63 (57, 67) | 0.017 |
| Treadmill exercise capacity (METs) | 8 (7, 10) | 8 (6, 10) | 7 (5, 10) | 5 (4, 7) | <0.001 |
Values reported as n (%), mean (SD), or median (IQR).
p-value is by χ2 test for categorical variables and Kruskal-Wallis test for continuous variables.
COPD: Chronic Obstructive Pulmonary Disease; ACE: angiotensin converting enzyme; ARB: angiotensin receptor blocker; HDL: high-density lipoprotein; LDL: low-density lipoprotein; eGFR: estimated glomerular filtration rate; LV: left ventricular; METs: Metabolic Equivalents.
Associations of TNFR1 with baseline reduced eGFR and albuminuria
TNFR1 was strongly associated with eGFR <60 ml/min/1.73 m2 at baseline. After adjustment for demographics and comorbid conditions, the highest quartile of TNFR1 had a nearly 12-fold increased odds of eGFR <60 ml/min/1.73 m2 (Table 2A). The highest quartile of TNFR1 also had a 3-fold increased odds of baseline urine ACR ≥30 mg/g, after adjustment for demographics and comorbid conditions (Table 2A). These associations were attenuated but remained statistically significant after additional adjustment for eGFR (Model 3b).
Table 2.
Associations between baseline quartiles of TNFR1 and CKD assessed by eGFR and ACR (A) and rapid loss (>3% per year) in kidney function (B).
| (A) CKD outcomes | Q1 | Q2 OR (95% CI) |
Q3 OR (95% CI) |
Q4 OR (95% CI) |
|
|---|---|---|---|---|---|
| eGFR < 60 ml/min/1.73 m2 | |||||
| Model 1 (n=980) | Ref | 2.39 (1.23, 4.64) | 5.70 (3.09, 10.53) | 13.24 (7.28, 24.09) | |
| Model 2 (n=881) | Ref | 1.92 (0.99, 3.72) | 4.48 (2.41, 8.33) | 9.29 (5.03, 17.15) | |
| Model 3a (n=833) | Ref | 2.64 (1.20, 5.83) | 6.05 (2.83, 12.93) | 11.71 (5.46, 25.11) | |
| ACR ≥ 30 mg/g | |||||
| Model 1 (n=922) | Ref | 2.05 (1.13, 3.72) | 2.69 (1.48, 4.89) | 6.11 (3.57, 10.46) | |
| Model 2 (n=837) | Ref | 1.68 (0.88, 3.22) | 1.99 (1.02, 3.87) | 3.17 (1.64, 6.14) | |
| Model 3b (n=833) | Ref | 1.67 (0.85, 3.29) | 1.82 (0.90, 3.66) | 2.44 (1.15, 5.19) | |
| Model 1: Adjusted for demographic factors (age, sex, race) | |||||
| Model 2: Model 1 + comorbid conditions (smoking, BMI, history of hypertension, diabetes, MI, HF, ACEI/ARB use, beta-blocker use, HDL, triglycerides, hemoglobin A1c, LVEF, METs) | |||||
| Model 3a: Model 2 + ACR | |||||
| Model 3b: Model 2+ eGFR | |||||
| (B) Rapid loss | Q1 | Q2 OR (95% CI) |
Q3 OR (95% CI) |
Q4 OR (95% CI) |
|
|---|---|---|---|---|---|
| Model 1 (n=627) | Ref | 1.28 (0.74, 2.23) | 1.48 (0.82, 2.68) | 3.43 (1.95, 6.04) | |
| Model 2 (n=577) | Ref | 1.14 (0.65, 2.01) | 1.22 (0.68, 2.19) | 2.27 (1.26, 4.10) | |
| Model 3 (n=550) | Ref | 1.17 (0.65, 2.11) | 1.17 (0.64, 2.14) | 2.10 (1.12, 3.92) |
Model 1: Adjusted for demographic factors (age, sex, race) + baseline eGFR
Model 2: Model 1 + comorbid conditions (smoking, BMI, history of hypertension, diabetes, MI, HF, ACEI/ARB use, beta-blocker use, HDL, triglycerides, hemoglobin A1c, LVEF, METs).
Model 3: Model 2 + baseline ACR.
Associations of TNFR1 with rapid kidney function loss
TNFR1 was strongly associated with a rapid loss of kidney function of >3% per year, after adjustment for demographics and baseline eGFR (Table 2B). After adjustment for comorbidities, these associations were somewhat attenuated, but remained strong at approximately 2-fold (Model 2). Adjustment for ACR did not substantially affect these associations (Model 3).
Associations of TNFR1 with kidney function outcomes by subgroup
There were no significant differences between TNFR1 and kidney function outcomes by subgroup, with the exception of age. Age less than the median age of 67 years was associated with a significantly larger effect size for eGFR < 60 ml/min/1.73 m2 than age greater than 67 years (p for interaction 0.0004).
Associations with myocardial infarction, heart failure, and all-cause mortality
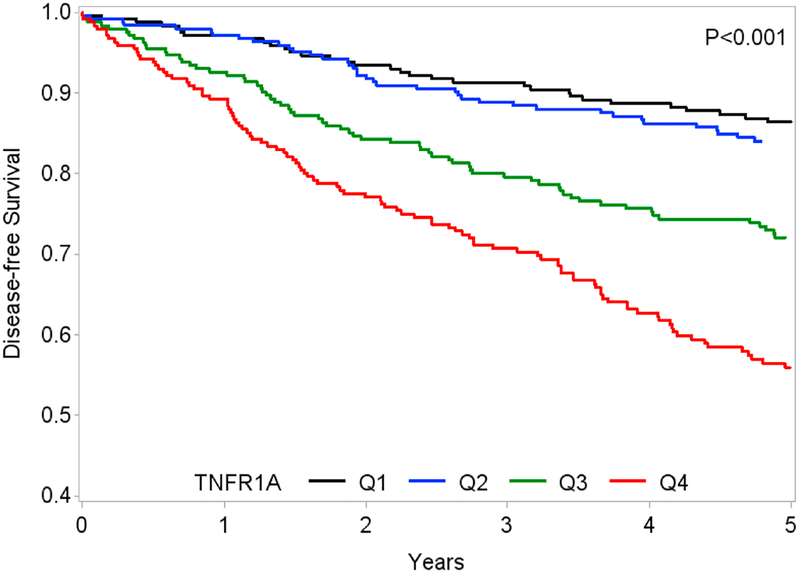
There were 116 MI events in the cohort overall. Fig. 1 shows survival free of all three outcomes (MI, HF, and mortality risk) by quartile of TNFR1. In demographic-adjusted models, the highest quartile of TNFR1 was associated with a nearly 4-fold increased risk of MI (Table 3). After adjustment for baseline eGFR, this association was attenuated, but remained significant (Model 2). After further adjustment for clinical characteristics, this association was completely attenuated (Model 3). In demographic-adjusted models, the highest quartile of TNFR1 was associated with a greater than 4-fold increased risk of HF (Table 3). Adjustment by eGFR alone largely attenuated this association (Model 2). Adjustment for clinical characteristics further attenuated this association (Model 3). The highest quartile of TNFR1 was associated with a 2.5-fold increased risk of mortality in models adjusted for demographics and clinical characteristics (Table 3). Adjustment for eGFR alone attenuated this somewhat association but it retained statistical significance (Model 2), while further adjustment for comorbid conditions attenuated the association almost completely (Model 3). A sensitivity analysis, censoring at 5 years to correspond with the measurements of kidney function, showed similar results (Supplemental Table 1). The mortality result was qualitatively stronger and almost reached significance even after adjustment for eGFR.
Figure 1.
Associations of TNFR1 with disease-free survival, defined by absence of MI, HF, and death during follow-up.
Table 3.
Associations between TNFR1 and cardiovascular outcomes and mortality risk.
| Q1 Ref |
Q2 IRR (95% CI) |
Q3 IRR (95% CI) |
Q4 IRR (95% CI) |
||
|---|---|---|---|---|---|
| Myocardial infarction | Model 1 | 1 | 1.62 (0.82, 3.22) | 2.46 (1.27, 4.77) | 3.70 (1.94, 7.06) |
| (N=116) | Model 2 | 1.41 (0.7, 2.82) | 1.91 (0.96, 3.81) | 2.25 (1.05, 4.84) | |
| Model 3 | 1 | 1.06 (0.50, 2.22) | 1.49 (0.71, 3.11) | 1.04 (0.44, 2.49) | |
| Heart failure | Model 1 | 1 | 1.07 (0.57, 2.01) | 2.19 (1.24, 3.85) | 4.57 (2.67, 7.82) |
| (N=164) | Model 2 | 0.84 (0.44, 1.58) | 1.39 (0.77, 2.51) | 1.88 (0.99, 3.56) | |
| Model 3 | 1 | 0.53 (0.25, 1.12) | 1.00 (0.51, 1.96) | 1.02 (0.48, 2.15) | |
| All-cause mortality | Model 1 | 1 | 1.01 (0.69, 1.46) | 1.55 (1.09, 2.20) | 2.57 (1.84, 3.59) |
| (N=359) | Model 2 | 0.94 (0.64, 1.37) | 1.31 (0.90, 1.89) | 1.78 (1.18, 2.68) | |
| Model 3 | 1 | 0.92 (0.59, 1.41) | 1.18 (0.77, 1.80) | 1.42 (0.88, 2.28) |
Model 1: Adjusted for demographic factors (age, sex, race).
Model 2: Model 1+ baseline eGFR.
Model 3: Model 1 + baseline eGFR + comorbid conditions (smoking, BMI, history of hypertension, diabetes, MI, HF, ACEI/ARB use, beta-blocker use, HDL, triglycerides, hemoglobin A1c, LVEF, METs) + baseline ACR.
Discussion
In a cohort of individuals with known ischemic heart disease, we found that higher levels of TNFR1 were associated with increased risk of myocardial infarction, heart failure, and mortality risk in demographic adjusted models, but these relationships were attenuated by adjustment for eGFR and comorbidities associated with cardiovascular disease. TNFR1 had very strong associations with measures of kidney function, including baseline eGFR and ACR. The reason for the heightened risk of these associations among those aged less than 67 years is uncertain. The associations of TNFR1 with rapid kidney function loss may also suggest a mechanism by which TNFR1 is associated with adverse cardiovascular events, as worsening kidney function over time may accelerate onset of MI and HF.
This is the first study to our knowledge to examine the relationship between TNFR1 and cardiovascular events and mortality risk in individuals with established ischemic heart disease and a range of kidney function. Our findings extend the strong associations of TNFRs with kidney disease progression described by Niewczas in diabetic patients from the Joslin Clinic, which were independent of baseline eGFR or ACR as well as other markers of inflammation.7 Our findings differ from those of Valgimigli et al., who found that TNFR1 levels were associated with HF and mortality risk independently of cardiac comorbidities. Their study included a smaller population (N=184) with a single diagnosis of ST-elevation MI, which may have represented a more homogeneous population than ours. The renal function in this group is not clearly stated.2 In another study of TNFR1 in a cohort of comparable size, adjustment for eGFR also attenuated the association between TNFR1 and cardiovascular mortality risk,16 suggesting that eGFR was a major mediator of the association between TNFR1 and cardiovascular mortality risk in that cohort.
Our findings are of interest for several reasons. First, TNFR1 appears to be a more robust marker of kidney function than of cardiovascular outcomes. This may be because of a direct mechanism of TNFR1 causing damage to endothelial fenestration in the glomerular basement membrane or expansion of the mesangium,6 while associations with cardiovascular outcomes are mediated by other pathways less directly affected by TNFR1. The high cardiovascular risk of individuals with CKD is attributed to many pathways including inflammation,17 and even a modest reduction in kidney function is associated with greater cardiovascular risk.18 The mechanisms of increased cardiovascular risk in CKD are not fully understood.
Our study has several strengths. The Heart and Soul Study is a unique cohort in which the relationship between TNFR1 and kidney and cardiovascular outcomes is examined. This cohort has robust measurements of TNFR1 and repeated measures of eGFR and ACR in 985 individuals, as well as adjudicated MI, HF, and mortality risk outcomes in all patients. Estimated GFR was measured using both creatinine and cystatin C.10 Moreover, our samples were all measured in concert, minimizing risks of drift among biomarker samples.
Our study also has several limitations. First, TNFR1 was only measured in baseline serum samples and we do not have measurements of TNFR2, another circulating TNF receptor that binds TNFα. The coefficients of variation of biomarker measurement were slightly higher than in other studies,2 although the storage conditions were comparable to those of other studies.2, 5, 17 The optimal storage conditions for TNFR1 are not established, but we would expect that any degradation over time would bias our results toward the null. We did not measure TNFα levels in concert with TNFR1, although previous studies found no associations between TNFα and kidney disease progression7 and the half-life of TNFRs is longer than that of TNFα, making its measurement more feasible. We did not have power to detect associations with incident CKD or other measures of kidney disease and also had inadequate power to stratify by race. Finally, there were relatively fewer women in this cohort.
In conclusion, elevated TNFR1 may indicate increased risk of rapid kidney function loss in individuals with known atherosclerotic disease. TNFR1 is not associated with increased risk of cardiovascular events or mortality risk independently of renal function. Further examination of the biology of this cytokine in individuals with CKD and heart disease may explain the associations of TNFR1 with mortality risk observed in other populations.
Supplementary Material
Highlights.
Tumor necrosis factor receptor 1 (TNFR1) is a cell membrane-bound receptor involved in apoptosis, inflammation, and immune host defense.
TNFR1 is associated with kidney disease and mortality risk in various populations.
Our study found that higher levels of TNFR1 are associated with kidney function decline over time, but not with cardiovascular disease events including myocardial infarction and heart failure or with mortality.
Kidney disease may mediate the risk of cardiovascular disease events and mortality associated with TNFR1.
Acknowledgments
The authors thank the participants in the Heart and Soul Study.
Financial support
This work was supported by Meyeon Park’s NIH/NIDDK K23 DK099238 and Doris Duke Charitable Foundation Clinical Scientist Development Award. M.S. is supported by R01 AG034853–04, 5R01AG027002–06, and 5R01DK087961–02. The Heart and Soul Study was funded by the Department of Veteran Affairs (Epidemiology Merit Review Program), Washington, DC; grant R01 HL-079235 from the National Heart, Lung, and Blood Institute, Bethesda, MD; the Robert Wood Johnson Foundation (Generalist Physician Faculty Scholars Program), Princeton, NJ; the American Federation for Aging Research (Paul Beeson Faculty Scholars in Aging Research Program), New York, NY; and the Ischemia Research and Education Foundation, South San Francisco, CA.
Abbreviations:
- TNFR1
tumor necrosis factor alpha receptor type 1
- eGFR
estimated glomerular filtration rate
- CKD
chronic kidney disease
- ACR
albumin to creatinine ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declared they do not have anything to disclose regarding conflict of interest with respect to this manuscript.
References
- 1.Aderka D The potential biological and clinical significance of the soluble tumor necrosis factor receptors. Cytokine & Growth Factor Reviews. 1996;7:231–240. [DOI] [PubMed] [Google Scholar]
- 2.Valgimigli M, Ceconi C, Malagutti P, Merli E, Soukhomovskaia O, Francolini G, Cicchitelli G, Olivares A, Parrinello G, Percoco G, Guardigli G, Mele D, Pirani R and Ferrari R. Tumor Necrosis Factor-α Receptor 1 Is a Major Predictor of Mortality and New-Onset Heart Failure in Patients With Acute Myocardial Infarction. The Cytokine-Activation and Long-Term Prognosis in Myocardial Infarction (CALPHA) Study. 2005;111:863–870. [DOI] [PubMed] [Google Scholar]
- 3.Vasan RS, Sullivan LM, Roubenoff R, Dinarello CA, Harris T, Benjamin EJ, Sawyer DB, Levy D, Wilson PWF and D’Agostino RB. Inflammatory Markers and Risk of Heart Failure in Elderly Subjects Without Prior Myocardial Infarction. The Framingham Heart Study. 2003;107:1486–1491. [DOI] [PubMed] [Google Scholar]
- 4.Aggarwal BB, Eessalu TE and Hass PE. Characterization of receptors for human tumour necrosis factor and their regulation by gamma-interferon. Nature. 1985;318:665–7. [DOI] [PubMed] [Google Scholar]
- 5.Pavkov ME, Nelson RG, Knowler WC, Cheng Y, Krolewski AS and Niewczas MA. Elevation of circulating TNF receptors 1 and 2 increases the risk of end-stage renal disease in American Indians with type 2 diabetes. Kidney Int. 2015;87:812–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pavkov ME, Weil EJ, Fufaa GD, Nelson RG, Lemley KV, Knowler WC, Niewczas MA and Krolewski AS. Tumor necrosis factor receptors 1 and 2 are associated with early glomerular lesions in type 2 diabetes. Kidney Int. 2016;89:226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niewczas MA, Gohda T, Skupien J, Smiles AM, Walker WH, Rosetti F, Cullere X, Eckfeldt JH, Doria A, Mayadas TN, Warram JH and Krolewski AS. Circulating TNF Receptors 1 and 2 Predict ESRD in Type 2 Diabetes. Journal of the American Society of Nephrology. 2012;23:507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whooley MA, de Jonge P, Vittinghoff E, Otte C, Moos R, Carney RM, Ali S, Dowray S, Na B, Feldman MD, Schiller NB and Browner WS. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. Jama. 2008;300:2379–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erlandsen EJ, Randers E and Kristensen JH. Evaluation of the Dade Behring N Latex Cystatin C assay on the Dade Behring Nephelometer II System. Scand J Clin Lab Invest. 1999;59:1–8. [DOI] [PubMed] [Google Scholar]
- 10.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J and Levey AS. Estimating Glomerular Filtration Rate from Serum Creatinine and Cystatin C. New England Journal of Medicine. 2012;367:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G and National Kidney F. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Annals of Internal Medicine. 2003;139:137–147. [DOI] [PubMed] [Google Scholar]
- 12.Peralta CA, Vittinghoff E, Bansal N, Jacobs D, Muntner P Jr., Kestenbaum B, Lewis C, Siscovick D, Kramer H, Shlipak M and Bibbins-Domingo K. Trajectories of kidney function decline in young black and white adults with preserved GFR: results from the Coronary Artery Risk Development in Young Adults (CARDIA) study. Am J Kidney Dis. 2013;62:261–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luepker RV, Apple FS, Christenson RH, Crow RS, Fortmann SP, Goff D, Goldberg RJ, Hand MM, Jaffe AS, Julian DG, Levy D, Manolio T, Mendis S, Mensah G, Pajak A, Prineas RJ, Reddy KS, Roger VL, Rosamond WD, Shahar E, Sharrett AR, Sorlie P, Tunstall-Pedoe H, Prevention ACoEa, Committee AS, Prevention WHFCoEa, Prevention ESoCWGoEa, Prevention CfDCa and National Heart Ln, and Blood Institute. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108:2543–9. [DOI] [PubMed] [Google Scholar]
- 14.Bibbins-Domingo K, Gupta R, Na B, Wu AH, Schiller NB and Whooley MA. N-terminal fragment of the prohormone brain-type natriuretic peptide (NT-proBNP), cardiovascular events, and mortality in patients with stable coronary heart disease. JAMA. 2007;297:169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bibbins-Domingo K, Gupta R, Na B, Wu AH, Schiller NB and Whooley MA. N-terminal fragment of the prohormone brain-type natriuretic peptide (NT-proBNP), cardiovascular events, and mortality in patients with stable coronary heart disease. JAMA : the journal of the American Medical Association. 2007;297:169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carlsson AC, Juhlin CC, Larsson TE, Larsson A, Ingelsson E, Sundstrom J, Lind L and Arnlov J. Soluble tumor necrosis factor receptor 1 (sTNFR1) is associated with increased total mortality due to cancer and cardiovascular causes - findings from two community based cohorts of elderly. Atherosclerosis. 2014;237:236–42. [DOI] [PubMed] [Google Scholar]
- 17.Amdur RL, Feldman HI, Gupta J, Yang W, Kanetsky P, Shlipak M, Rahman M, Lash JP, Townsend RR, Ojo A, Roy-Chaudhury A, Go AS, Joffe M, He J, Balakrishnan VS, Kimmel PL, Kusek JW, Raj DS and Investigators tCS. Inflammation and Progression of CKD: The CRIC Study. Clinical Journal of the American Society of Nephrology. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Go AS, Chertow GM, Fan D, McCulloch CE and Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. The New England journal of medicine. 2004;351:1296–1305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.