Abstract
Multiple myeloma (MM) is an incurable hematological malignancy caused by accumulation of abnormal clonal plasma cells. Despite the recent development of novel therapies, relapse of MM eventually occurs as a result of a remaining population of drug‐resistant myeloma stem cells. Side population (SP) cells show cancer stem cell‐like characteristics in MM; thus, targeting these cells is a promising strategy to completely cure this malignancy. Herein, we showed that SP cells expressed higher levels of enhancer of zeste homolog (EZH) 1 and EZH2, which encode the catalytic subunits of Polycomb repressive complex 2 (PRC2), than non‐SP cells, suggesting that EZH1 as well as EZH2 contributes to the stemness maintenance of the MM cells and that targeting both EZH1/2 is potentially a significant therapeutic approach for eradicating myeloma stem cells. A novel orally bioavailable EZH1/2 dual inhibitor, OR‐S1, effectively eradicated SP cells and had a greater antitumor effect than a selective EZH2 inhibitor in vitro and in vivo, including a unique patient‐derived xenograft model. Moreover, long‐term continuous dosing of OR‐S1 completely cured mice bearing orthotopic xenografts. Additionally, PRC2 directly regulated WNT signaling in MM, and overactivation of this signaling induced by dual inhibition of EZH1/2 eradicated myeloma stem cells and negatively affected tumorigenesis, suggesting that repression of WNT signaling by PRC2 plays an important role in stemness maintenance of MM cells. Our results show the role of EZH1/2 in the maintenance of myeloma stem cells and provide a preclinical rationale for therapeutic application of OR‐S1, leading to significant advances in the treatment of MM.
Keywords: EZH1/2 dual inhibitor, multiple myeloma, myeloma stem cell, PRC2, WNT signaling
1. INTRODUCTION
Multiple myeloma (MM) is the second most common hematological malignancy and is characterized by accumulation of malignant plasma cells within the bone marrow.1 Multiple myeloma is largely incurable and relapse eventually occurs because it is difficult to eradicate all myeloma cells, despite the recent development of novel therapies.2 This incurable feature is mainly attributed to a remaining population of drug‐resistant cancer stem cells.3, 4 Side population (SP) cells are identified in a flow cytometry plot with weak staining of Hoechst 33342 as a result of their high expression of an ATP‐binding cassette (ABC) membrane transporter that exports this dye.5, 6, 7, 8 Side population cells in MM are known to be enriched with myeloma stem cells featured by repopulation, self‐renewal, differentiation, and clonogenicity abilities.9, 10 Therefore, targeting SP cells can be a promising strategy to prevent and treat MM relapse.
Polycomb repressive complex 2 (PRC2), a member of the polycomb group of protein complexes, is an important epigenetic regulator that maintains the “stemness” of embryonic and hematopoietic stem cells.11, 12, 13, 14, 15, 16, 17, 18 Enhancer of zeste homolog 1 and 2 (EZH1/2) are catalytic components of PRC2 that trimethylate histone H3 at lysine 27 (H3K27me3) to repress transcription of its target genes. Mutation or overexpression of EZH2 is associated with tumorigenesis or tumor progression in many cancer types, including MM.10, 19, 20, 21, 22, 23, 24, 25 Indeed, increased silencing of H3K27me3 targets was reported in MM patients at advanced stages of the disease, and the expression pattern of H3K27me3‐marked genes correlates with poor patient survival.21, 26 These results suggest that overexpression of EZH2 is responsible for tumor progression and that EZH2 is a potential therapeutic target in MM. Indeed, selective EZH2 inhibitors have been developed and some of them are currently being investigated in clinical trials against various malignant tumors, including MM.26, 27, 28, 29 Furthermore, upregulation of EZH2 in SP cells has been reported and this suggests that EZH2 has an important role for stem cell maintenance in MM.10
However, it remains unclear whether EZH1, the other catalytic subunit of PRC2, is important to maintain the stemness of MM cells, although EZH1 only partially compensates for loss of EZH2 in stem cell maintenance.30, 31, 32 Our group recently discovered that EZH1 complements EZH2 and that dual inactivation of EZH1/2 depletes quiescent leukemia stem cells to cure acute myeloid leukemia.33 Therefore, we hypothesized that EZH1, in addition to EZH2, is also important for stem cell maintenance in MM and that dual inhibition of EZH1/2 could eradicate myeloma stem cells as seen in acute myeloid leukemia. Here, we used a novel orally bioavailable EZH1/2 dual inhibitor, OR‐S1, which potently inhibits both EZH1 and EZH2.34 This translational tool enabled us to investigate the role of EZH1/2 in myeloma stem cells by analyzing SP cells.
The present study aimed to investigate the function of EZH1/2 in the maintenance of myeloma stem cells and to evaluate whether dual inhibition of EZH1/2 can be an effective therapeutic approach to eradicate myeloma stem cells.
2. MATERIALS AND METHODS
2.1. Compounds
GSK126 was generated as previously described.35 The synthesis and characterization of OR‐S1 (Daiichi Sankyo, Tokyo, Japan) are described in a Patent Cooperation Treaty application (publication number: WO2015/141616).
2.2. In vivo xenograft studies
NOD/ShiJic‐scidJcl (NOD‐SCID) mice were purchased from CLEA Japan (Tokyo, Japan). All animal procedures were undertaken in accordance with the Guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the National Cancer Center (Tokyo, Japan). Each experiment was carried out in a specific pathogen‐free environment at the animal facility of the National Cancer Center according to institutional guidelines. A total of 5 × 106 MM.1S or RPMI8226 cells transduced with pMSCV‐Luc‐neo were suspended in 100 μL of 50% Matrigel prepared in PBS and s.c. inoculated into the left flank of 6‐week‐old female mice. Tumor‐bearing mice were divided into two groups by stratified randomization. Treatment was started 1 and 3 weeks after inoculation of MM.1S and RPMI8226 cells when tumor engraftment was confirmed by bioluminescence imaging, respectively. For s.c. tumors, OR‐S1 was dissolved (0.5% w/v) in sterile methyl cellulose 400 solution (Wako, Osaka, Japan) and given orally (200 or 400 mg/kg per day bid) for 3 weeks. Tumor burden was assessed weekly by serial bioluminescence imaging and measurement of tumor volume. Images were acquired 10 minutes after i.p. injection of d‐Luciferin (Summit Pharmaceuticals, Tokyo, Japan; 150 mg/kg) using an IVIS 100 system (Caliper Life Sciences, Hopkinton, MA, USA). Signals were quantified using Living Image 4.3.1 (Caliper Life Sciences). For the survival assay, 6‐week‐old NOD‐SCID mice were injected with 5 × 106 MM.1S cells by the tail vein. Mice were treated with OR‐S1 orally (200 or 400 mg/kg per day bid) for 21 days from 1 week after transplantation or by continuous dose ad libitum mixed with sterilized pellet food (CRF‐1; Oriental Yeast Co., Tokyo, Japan) from 3 days after transplantation. Mice were killed when treatment was completed, and bone marrow cells were collected for flow cytometric analysis. For the limiting dilution assay, secondary transplantation was carried out by s.c. injecting NOD‐SCID mice with MM.1S cells treated with or without 1 μmol/L OR‐S1 for 72 hours. Mice were inoculated with 1 × 104, 1 × 103, 1 × 102, or 1 × 101 cells (n = 4 mice per group). Tumor burden was assessed weekly for a total of 10 weeks by measurement of the tumor volume.
2.3. Patient‐derived xenograft model
Human MM samples were obtained from patients at Tokyo Medical and Dental University or National Cancer Center Hospital. All patients provided informed consent, and the study was approved by the Institutional Review Board. For primary MM samples obtained from iliac bone marrow, mononuclear cells were purified using a Ficoll‐density gradient centrifugation enrichment protocol and transplanted into 6‐week‐old NOD.Cg‐Prkdcscid Il2rgtm1Sug/Jic (NOG) mice (Central Institute for Experimental Animals, Kawasaki, Japan) by tibial intramedullary injection. Successful engraftment was confirmed by detecting human immunoglobulins in the serum of mice using ELISA (Human IgG ELISA Kit; Bethyl Laboratories, Montgomery, TX, USA), and their levels were measured monthly to evaluate the drug response. Mice were killed when treatment was completed and bone marrow cells were collected for flow cytometric analysis.
2.4. Statistical analyses
Statistical significance was determined using Student's t test or an analysis of variance after testing whether the data were normally distributed. Mantel‐Cox log‐ranked test was used to determine the statistical significance of Kaplan‐Meier survival analysis. Data are expressed as mean values, with error bars representing the standard deviation (SD).
Full methods and any associated references are available in Data S1.
3. RESULTS
3.1. Expression of EZH1 and EZH2 is significantly increased in SP cells
We first examined the proportion of SP cells in human myeloma cell lines based on their ability to efflux Hoechst 33342. In flow cytometric analysis, SP cells weakly stained with Hoechst 33342 were observed in the lower region of the dot plot and were distinct from main population (MP) cells, which were intensely stained with this dye. SP cells were characterized by poor intracellular accumulation of Hoechst 33342 and disappeared following treatment with ABC transporter inhibitors such as verapamil (Figure 1A). We identified SP cells in all MM cell lines tested (1.7%‐8.2%), and verapamil treatment significantly reduced the proportion of these cells (Figure 1B). To investigate the expression profiles of EZH1 and EZH2 in myeloma stem cells, we sorted MM cells into SP and MP fractions. RT‐PCR showed that expression of EZH1 and EZH2 was significantly higher in SP cells than in MP cells (Figure 1C). These results suggest that overexpression of EZH1 and EZH2 is important to maintain the stemness of MM cells, and that EZH1 and EZH2 are both potential therapeutic targets.
Figure 1.
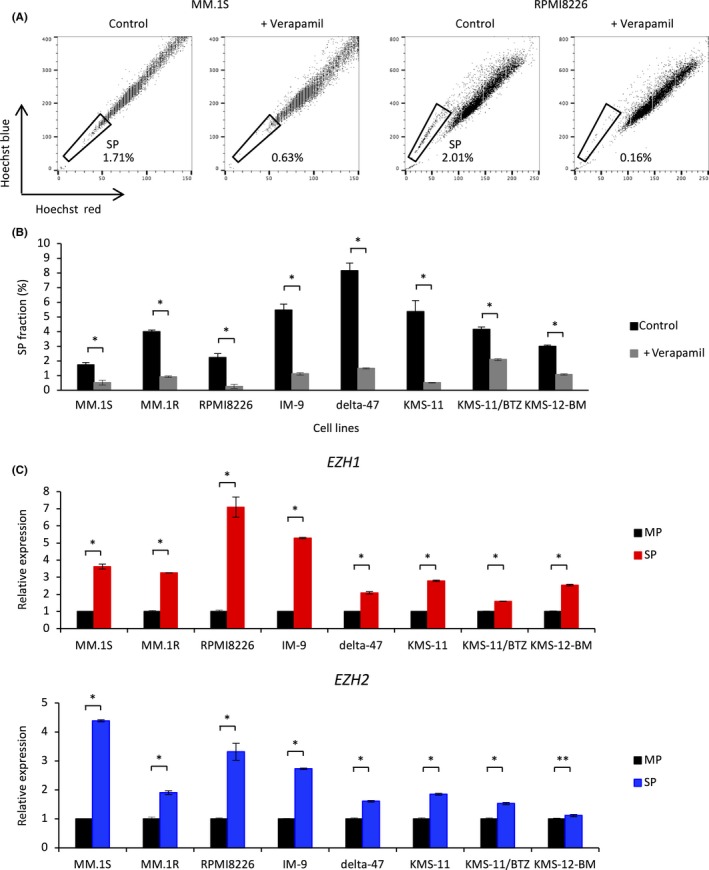
Side population (SP) cells express significantly higher levels of enhancer of zeste homolog 1/2 (EZH1/2) than main population (MP) cells. A, Representative FACS plots of the SP fraction of MM.1S and RPMI8226 cells. Cells were incubated with Hoechst 33342 alone (left) or together with 100 μmol/L verapamil (right). B, Percentage of SP cells in untreated multiple myeloma (MM) cell lines and those treated with 100 μmol/L verapamil. C, qRT‐PCR analysis showing relative expression of EZH1 and EZH2 in SP and MP cells isolated from MM cell lines by FACS. Expression was normalized against that of ACTB. All error bars represent the mean ± SD. *P < .005; **P < .05 (Student's t test)
3.2. OR‐S1 depletes myeloma stem cells and impairs proliferation of all MM cell lines
To investigate the effect of pharmacological inhibition of EZH1/2 on MM, we used a novel orally bioavailable EZH1/2 dual inhibitor, OR‐S1, which is a histone methyltransferase inhibitor with high selectivity for both EZH1 and EZH2 (Figure S1A).34 We first investigated whether OR‐S1 reduces the level of H3K27me3 in MM cells. Western blot analysis showed that the level of H3K27me3 was markedly reduced in OR‐S1‐treated cells (Figure S1B). Flow cytometric analysis showed that treatment with OR‐S1 for 48 hours significantly depleted SP cells in both MM.1S and RPMI8226 cell lines (Figure 2A). In a serial transplantation assay, tumor growth of OR‐S1‐treated MM.1S cells, in which OR‐S1 did not clearly induce apoptosis (Figure S1C), was significantly delayed compared with that of non‐treated MM.1S cells (Figures 2B and S1D). In addition, s.c. tumors formed following transplantation of 1 × 101 non‐treated cells, but not following transplantation of 1 × 102 OR‐S1‐treated cells (Figure 2C). Furthermore, OR‐S1 decreased the frequency of myeloma‐initiating cells from 1/34 to 1/994 (Figure 2D). Multiple myeloma cell lines were also highly sensitive to OR‐S1 in a growth inhibition assay; OR‐S1 inhibited the growth of all MM cell lines tested in a dose‐dependent method, and its half maximal growth inhibition (GI50) values were significantly lower than those of the EZH2‐specific inhibitor GSK126 (Figures 2E and S1E, and Table S1).35 OR‐S1 also suppressed the proliferation of KMS‐11/BTZ cells (GI50 = 3.6 nmol/L), which are resistant to bortezomib as a result of mutation of PSMB5,36 whereas GSK126 did not impair the proliferation of these cells (GI50 = 692.3 nmol/L). These results suggest that OR‐S1 is effective in all MM cell lines and that dual inhibition of EZH1/2 by OR‐S1 eradicates myeloma stem cells.
Figure 2.
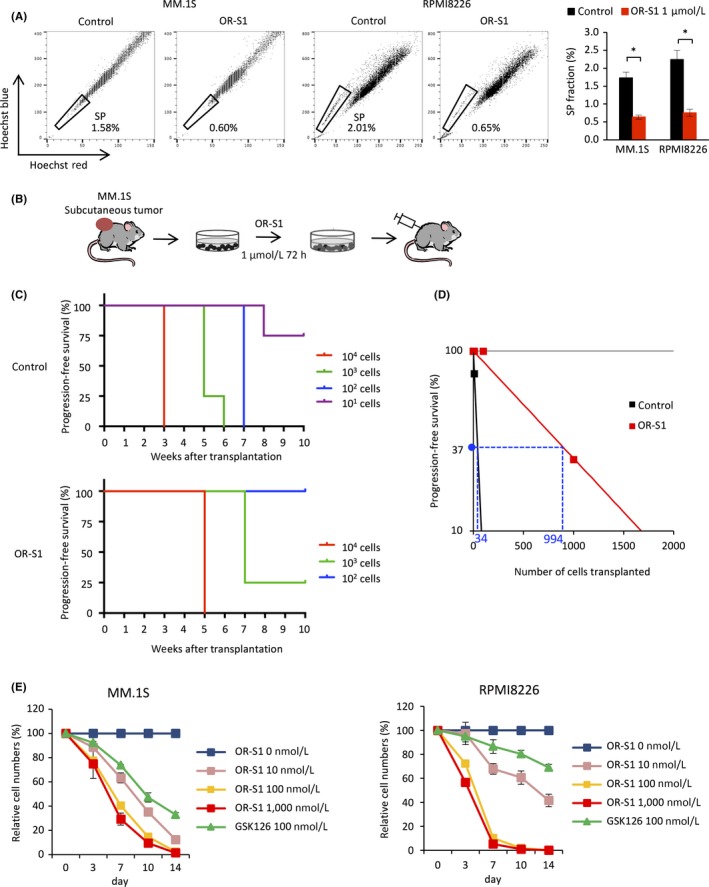
OR‐S1 has a greater antitumor effect than GSK126 in vitro and depletes myeloma stem cells. A, Representative FACS plots of the side population (SP) fraction of MM.1S and RPMI8226 cells. Cells were incubated with Hoechst 33342 alone (left) or together with 1 μmol/L OR‐S1 (right). Bar graph of the SP fraction of cells treated with or without 1 μmol/L OR‐S1 for 48 h is also shown. Error bars represent the mean ± SD. *P < .001 (Student's t test). B, Experimental schema depicting limiting dilution transplantation of MM.1S cells treated with vehicle or 1 μmol/L OR‐S1 for 72 h. Mice were s.c. injected with 1 × 104, 1 × 103, 1 × 102, or 1 × 101 cells (n = 4 mice per group). C, Kaplan‐Meier progression‐free survival curves of mice transplanted with vehicle‐ and OR‐S1‐treated cells (n = 4 mice per group). D, Numbers of myeloma‐initiating cells were determined by limiting dilution transplantation of vehicle‐ and OR‐S1‐treated MM.1S cells. E, Relative proliferation of MM.1S and RPMI8226 cells treated with different concentrations of OR‐S1 and 100 nmol/L GSK126 for the indicated durations. Y‐axis shows the cumulative cell number as a percentage relative to vehicle‐treated cells. Data are expressed as the mean of triplicates (±SD)
3.3. Dual inhibition of EZH1/2 overactivates WNT signaling by reducing the level of H3K27me3
To examine the molecular mechanism by which OR‐S1 regulates the proliferation of MM cells, we carried out RNA sequencing (RNA‐seq) of MM.1S and RPMI8226 cells. OR‐S1 treatment significantly upregulated 421 pathways in both cell lines (Figure 3A). Transcriptional profiles of OR‐S1‐treated MM cell lines were characterized by upregulation of genes related to the WNT, cell cycle, cell differentiation, apoptosis, and RANKL‐RANK pathways (Figures 3B,C and S2A,B). We next carried out ChIP‐sequencing using MM cell lines. This showed that most WNT and FZD loci were marked by H3K27me3 (Figures 3D and S2C). Treatment with 1 μmol/L OR‐S1 for 3 days upregulated many WNT and FZD genes >1.5‐fold, and a peak of H3K27me3 at these loci was detected by model‐based analysis of ChIP‐seq (peak score >10, ±10 kb from transcription start site; Tables S2 and S3). These results suggest that direct crosstalk occurs between PRC2 and WNT signaling, and we therefore focused on this pathway. RT‐PCR confirmed that OR‐S1 treatment markedly increased expression of WNT and FZD family members more than GSK126 treatment (Figure 4A). In addition, western blot analysis showed that non‐phosphorylated (active) β‐catenin was strongly expressed and expression of genes downstream of β‐catenin was significantly upregulated in OR‐S1‐treated cells (Figures 4B and S2D). Furthermore, ChIP‐qPCR analysis showed that OR‐S1 treatment markedly decreased the level of H3K27me3 at most WNT and FZD loci (Figure 4C). These results indicate that PRC2 directly targets WNT signaling and that dual inhibition of EZH1/2 by OR‐S1 activates WNT/β‐catenin signaling.
Figure 3.
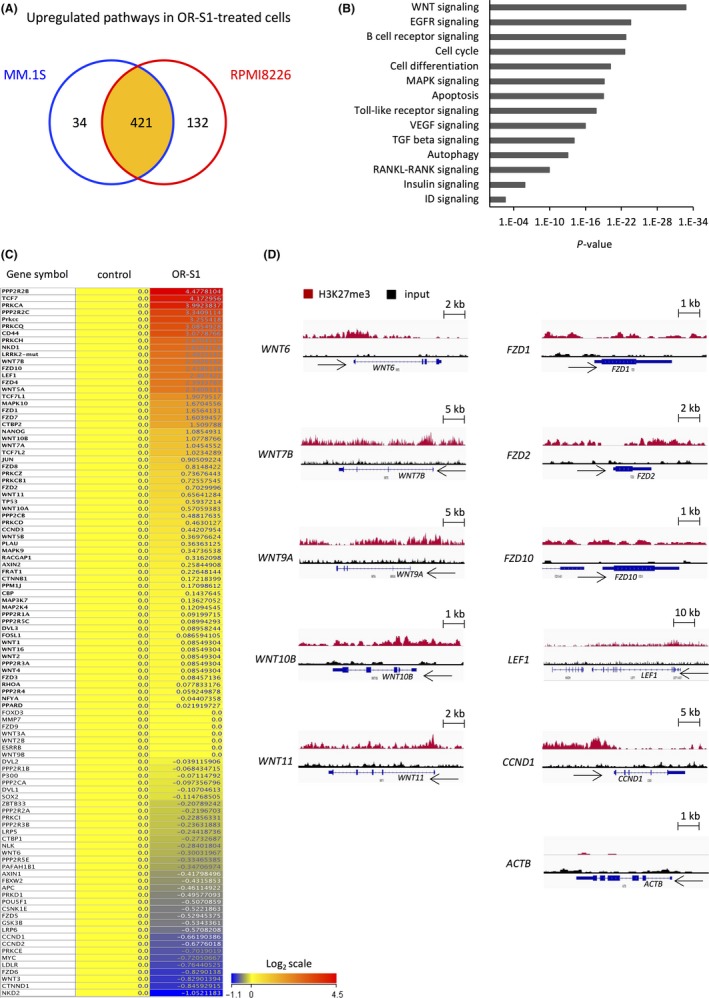
RNA‐seq and ChIP‐seq analysis of OR‐S1‐treated multiple myeloma (MM) cells. A, Schema showing upregulated pathways in OR‐S1‐treated MM.1S and RPMI8226 cells determined by RNA‐seq. A total of 455 and 553 pathways were significantly upregulated in MM.1S and RPMI8226 cells treated with 1 μmol/L OR‐S1 for 72 h, respectively (P < .05), and 421 pathways were upregulated in both OR‐S1‐treated cell lines. B, List of upregulated pathways in OR‐S1‐treated RPMI8226 cells determined by RNA‐seq. C, Heatmap of WNT‐related genes expressed in RPMI8226 cells treated with or without 1 μmol/L OR‐S1 for 72 h (log2 scale). Expression levels were normalized within two replicates of each sample. D, Representative Integrated Genomics Viewer diagrams showing the distribution of ChIP‐seq read densities for H3K27me3 (red) and input (black) at loci of WNT family members in RPMI8226 cells. CCND1 and ACTB loci were used as positive and negative controls, respectively
Figure 4.
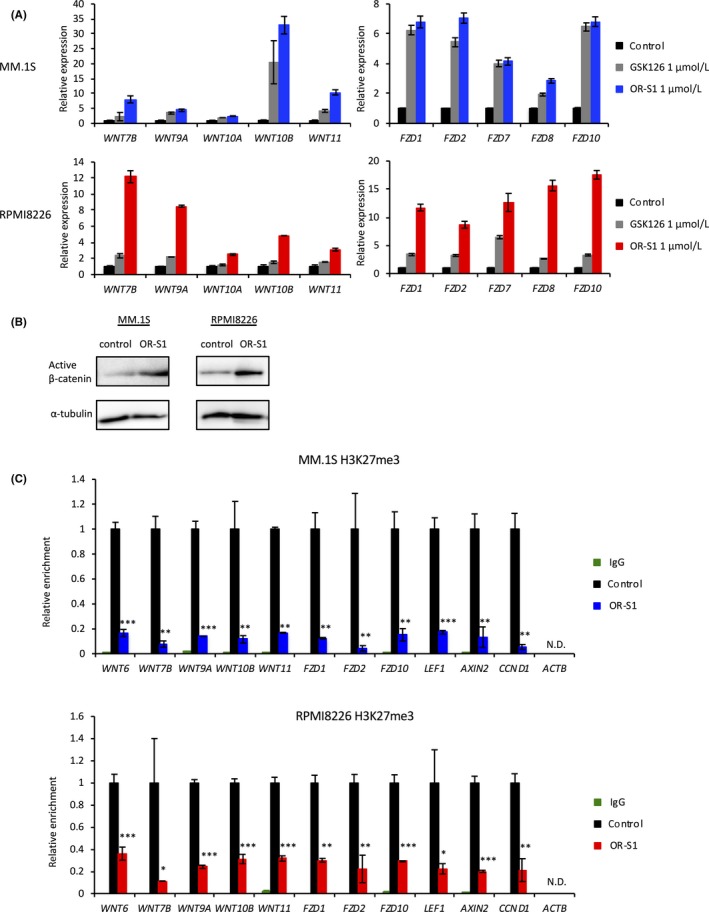
Dual inhibition of enhancer of zeste homolog 1/2 (EZH1/2) overactivates WNT signaling by reducing the level of trimethylated histone H3 at lysine 27 (H3K27me3). A, qRT‐PCR analysis showing relative expression of WNT‐related genes in MM.1S and RPMI8226 cells treated with 1 μmol/L GSK126 or OR‐S1 for 72 h. Y‐axis represents the fold‐change in gene expression after normalization to that of ACTB. Error bars represent the mean ± SD. B, Western blot analysis of non‐phosphorylated (active) β‐catenin in MM.1S and RPMI8226 cells treated with DMSO or 1 μmol/L OR‐S1 for 72 h. α‐Tubulin was used as a loading control. C, ChIP‐qPCR of H3K27me3 at the loci of WNT‐related genes in MM.1S and RPMI8226 cells treated with 1 μmol/L OR‐S1 for 72 h. Level of H3K27me3 was normalized against that of total H3. Background signals in each sample were evaluated by ChIP using rabbit IgG (green). CCND1 and ACTB loci were used as positive and negative controls, respectively. Error bars represent the mean ± SD. *P < .05; **P < .01; ***P < .0001 (Student's t test). N.D., not detected
3.4. Repression of WNT signaling by PRC2 is important to maintain the stemness of MM cells
Increased activation of the WNT/β‐catenin signaling pathway is reportedly related to decreased self‐renewal and differentiation of hematopoietic stem cells.37 Thus, we hypothesized that activation of WNT signaling would perturb the maintenance of myeloma stem cells in a similar way to hematopoietic stem cells, resulting in decreased proliferation of myeloma cells. Therefore, we generated β‐catenin‐overexpressing MM.1S and RPMI8226 cells to examine the effects of WNT signaling activation on MM (Figure 5A). As expected, proliferation of these cells was significantly impaired compared with that of control cells (Figure 5B). In addition, flow cytometric analysis showed that overexpression of β‐catenin significantly depleted SP cells (Figure 5C,D). To examine whether β‐catenin overexpression influences the self‐renewal of myeloma stem cells in vivo, mice were s.c. inoculated with 1 × 104 MM.1S cells retrovirally infected with a construct harboring non‐phosphorylated (active) β‐catenin or an empty vector. Tumor growth was significantly delayed in mice transplanted with β‐catenin‐overexpressing cells compared with mice transplanted with control cells (Figure 5E). Furthermore, to test whether activation of WNT signaling by OR‐S1 is responsible for the impaired proliferation of MM cells, we knocked down β‐catenin in MM cells in vitro using lentiviruses expressing shRNAs. Treatment with β‐catenin‐targeting shRNA1 and 2 (sh1 and sh2, respectively) decreased β‐catenin expression to 15%‐35% of that in control cells (Figure 4F). Cell counts showed that knockdown of β‐catenin using sh1 and sh2 rescued the inhibition of RPMI8226 cell growth induced by OR‐S1 treatment (Figure 5G). GI50 values of OR‐S1 in cells infected with the empty vector, sh1, and sh2 were 7.0, 48.0, and 89.2 nmol/L, respectively (Day 7). These results suggest that overactivation of WNT signaling induced by dual inhibition of EZH1/2 impairs the proliferation of MM cells by disrupting the self‐renewal activity of myeloma stem cells.
Figure 5.
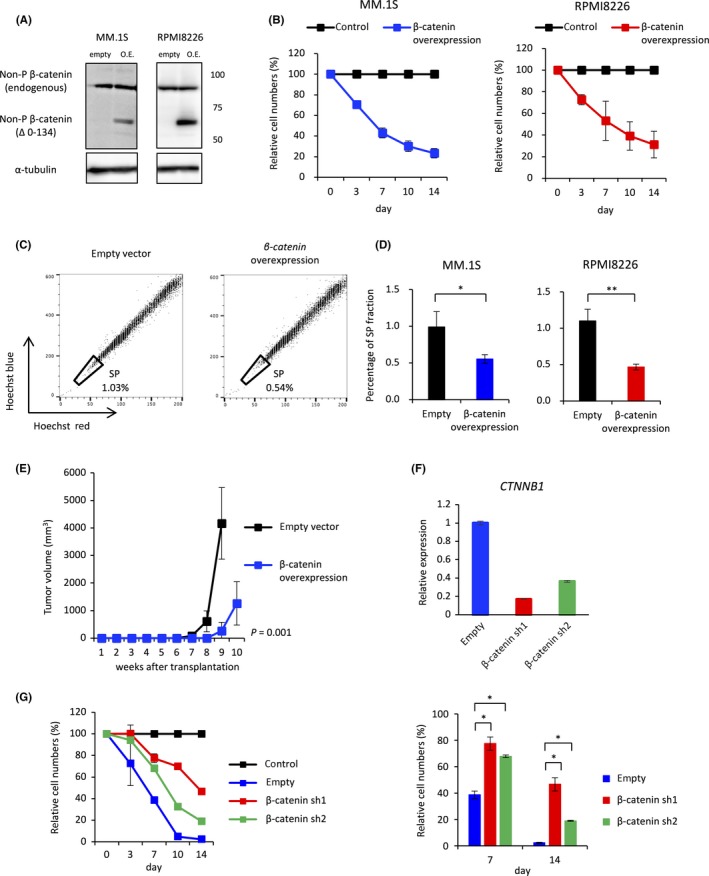
Repression of WNT signaling by Polycomb repressive complex 2 (PRC2) is important to maintain the stemness of multiple myeloma (MM) cells. A, Western blot analysis to confirm overexpression of non‐phosphorylated (active) β‐catenin in MM.1S and RPMI8226 cells. Empty represents cells transduced with pMSCV‐neo. Overexpression (O.E.) represents cells transduced with pMSCV‐β‐catenin (ΔN134)‐neo. B, Relative proliferation of β‐catenin‐overexpressing MM.1S and RPMI8226 cells at the indicated time points. Y‐axis shows cumulative cell number as a percentage relative to control cells. Data are expressed as the mean of triplicates (±SD). C, Representative FACS plots of the side population (SP) fraction of control and β‐catenin‐overexpressing MM.1S cells. D, Bar graphs show the SP fraction of control and β‐catenin‐overexpressing MM.1S and RPMI8226 cells. Error bars represent the mean ± SD. *P < .05 and **P < .01 (Student's t test). E, Tumor volume in mice s.c. transplanted with 1 × 104 MM.1S cells transfected with non‐phosphorylated β‐catenin or the empty vector (n = 4 mice per group). F, qRT‐PCR analysis showing relative expression of β‐catenin (CTNNB1) in RPMI8226 cells infected with lentiviruses harboring β‐catenin‐targeting shRNA or the empty vector. Y‐axis represents the fold‐change in gene expression after normalization against that of ACTB. G, Relative proliferation of β‐catenin‐knockdown (β‐catenin‐sh1 or ‐sh2) RPMI8226 cells treated with 10 nmol/L OR‐S1 for the indicated durations. Y‐axis shows the cumulative cell number as a percentage relative to vehicle‐treated cells. Data are expressed as the mean of triplicates (±SD). *P < .0001 (Student's t test)
3.5. Oral administration of OR‐S1 completely cures MM in mouse xenograft models
Because OR‐S1 inhibited the growth of all MM cell lines tested in vitro, we next examined its effect on these cells in vivo. Oral administration of OR‐S1 to mice bearing MM.1S xenografts significantly impaired s.c. tumor growth in a dose‐dependent way (Figures 6A,B and S3A). Flow cytometric analysis showed that giving OR‐S1 for 2 weeks significantly depleted SP cells in s.c. tumors formed by MM.1S cells (Figure 6C,D). These results suggest that OR‐S1 also eradicated myeloma stem cells in vivo. Furthermore, giving OR‐S1 markedly shrank s.c. tumors in mice with RPMI8226 xenografts (Figure S3B‐D). This effect was sustained until the final follow up even after OR‐S1 dosage was stopped. In contrast, tumors formed by MM.1S cells gradually increased in volume after cessation of OR‐S1 treatment. These different responses are likely due to the fact that RPMI8226 cells showed a high level of apoptosis following OR‐S1 treatment, whereas MM.1S cells did not (Figure S1C). Similarly, the survival assay showed that all OR‐S1‐treated mice with MM.1S orthotopic xenografts eventually died as a result of disease progression, even though oral dosage of OR‐S1 for 3 weeks markedly prolonged their survival time (Figure S3E). Our results showed that myeloma stem cells were specifically sensitive to dual inhibition of EZH1/2; therefore, we hypothesized that continuous dosage of OR‐S1 would cure mice with MM.1S xenografts by significantly impairing the self‐renewal activity of myeloma stem cells. Indeed, giving long‐term continuous OR‐S1 mixed with sterilized pellet food completely cured all mice bearing orthotopic xenografts without eliciting any serious side‐effects (Figure 6E). We confirmed this result by counting minimal residual cells by flow cytometric analysis; this showed that human CD38 (hCD38)+ cells were completely depleted within the bone marrow of OR‐S1‐treated mice (Figure 6F). These results indicate that long‐term continuous administration of OR‐S1 impairs the self‐renewal activity of myeloma stem cells, rendering these cells unable to reconstitute disease and leading to the complete cure of MM.
Figure 6.
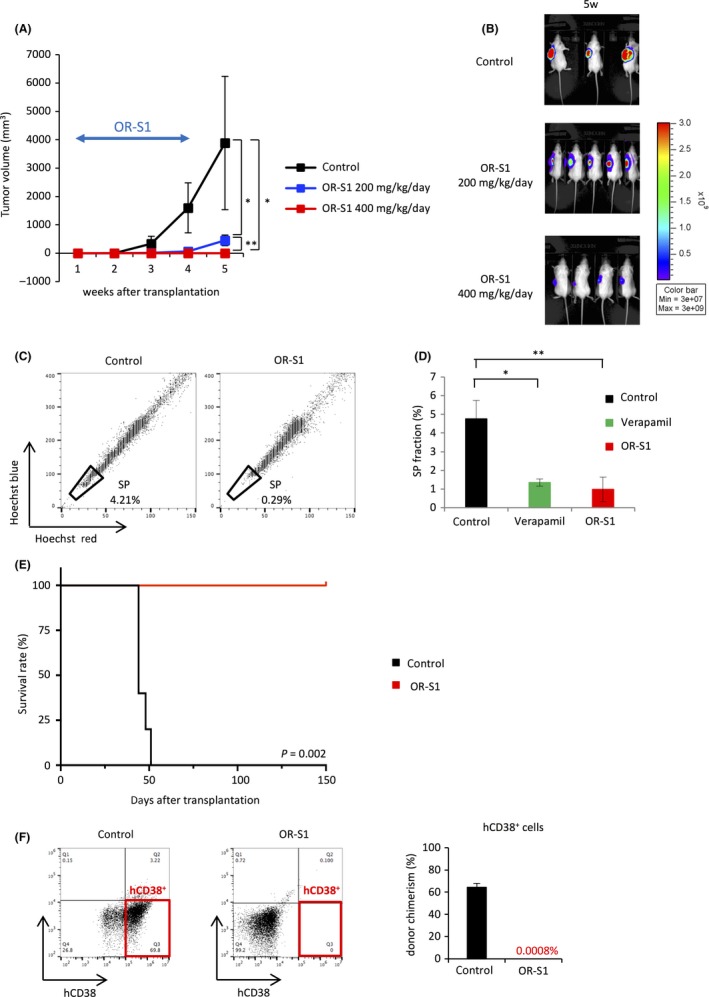
OR‐S1 shows anti‐neoplastic activity in multiple myeloma (MM) xenografts. A, Tumor volume in mice s.c. transplanted with MM.1S‐Luc+ cells. Treatment with OR‐S1 (200 or 400 mg/kg per day orally for 21 d) was started 1 wk after transplantation when tumor engraftment was confirmed by the bioluminescence imaging (n = 7 per group). B, Representative bioluminescence images show tumor burden of MM.1S xenografts at 5 wk after transplantation in mice treated with OR‐S1 (200 or 400 mg/kg per day orally for 21 d) or vehicle. C, Representative FACS plots of the side population (SP) fraction in s.c. tumors formed by MM.1S cells. OR‐S1 (400 mg/kg per day orally) was given for 14 d after the tumors were palpable. D, Bar graph of the SP fraction in control mice incubated with or without 100 μmol/L verapamil and OR‐S1‐treated mice is shown (n = 4 per group). E, Kaplan‐Meier survival curves of mice bearing orthotopic xenografts after i.v. injection of MM.1S cells. OR‐S1 mixed with sterilized pellet food was given continuously from 3 d after transplantation (n = 5 mice per group). F, Representative FACS plots and the bar graph show the percentage of hCD38+ cells within the bone marrow of control and OR‐S1‐treated mice at the final follow up (n = 5 mice per group). All error bars represent the mean ± SD. *P < .01; **P < .001 (Student’s t test)
3.6. OR‐S1 reduces myeloma cells in a patient‐derived xenograft model developed using primary MM samples
It is difficult to establish patient‐derived xenograft (PDX) models by subcutaneous or venous injection of primary MM samples because the bone marrow niche is considered to be crucial for the engraftment or homing of MM cells.38, 39 Thus, we exploited tibial intramedullary injection to develop an orthotopic PDX model using a sample of a relapsed patient pretreated with various drugs, including bortezomib (the patient's background is provided in Table S4). Engraftment was confirmed by detecting human immunoglobulins in the serum of mice, as carried out at the clinical bedside.40, 41 Serum levels of human immunoglobulins were measured monthly to evaluate tumor progression or therapeutic effects (Figure 7A). OR‐S1 was orally given for 2 months after human IgG was clearly detected in serum. OR‐S1 markedly suppressed the increases in human immunoglobulin levels in this PDX model, whereas these levels gradually increased in non‐treated mice (Figure 7B). Flow cytometric analysis showed that the percentage of hCD38+ cells within the bone marrow was markedly reduced in OR‐S1‐treated mice at the final follow up (Figure 7C). Furthermore, RT‐PCR confirmed that OR‐S1 treatment significantly increased expression of WNT and FZD family members in this model, as seen in the MM cell lines (Figure 7D). These results indicate that targeting EZH1/2 with OR‐S1 is also effective against MM in a PDX model.
Figure 7.
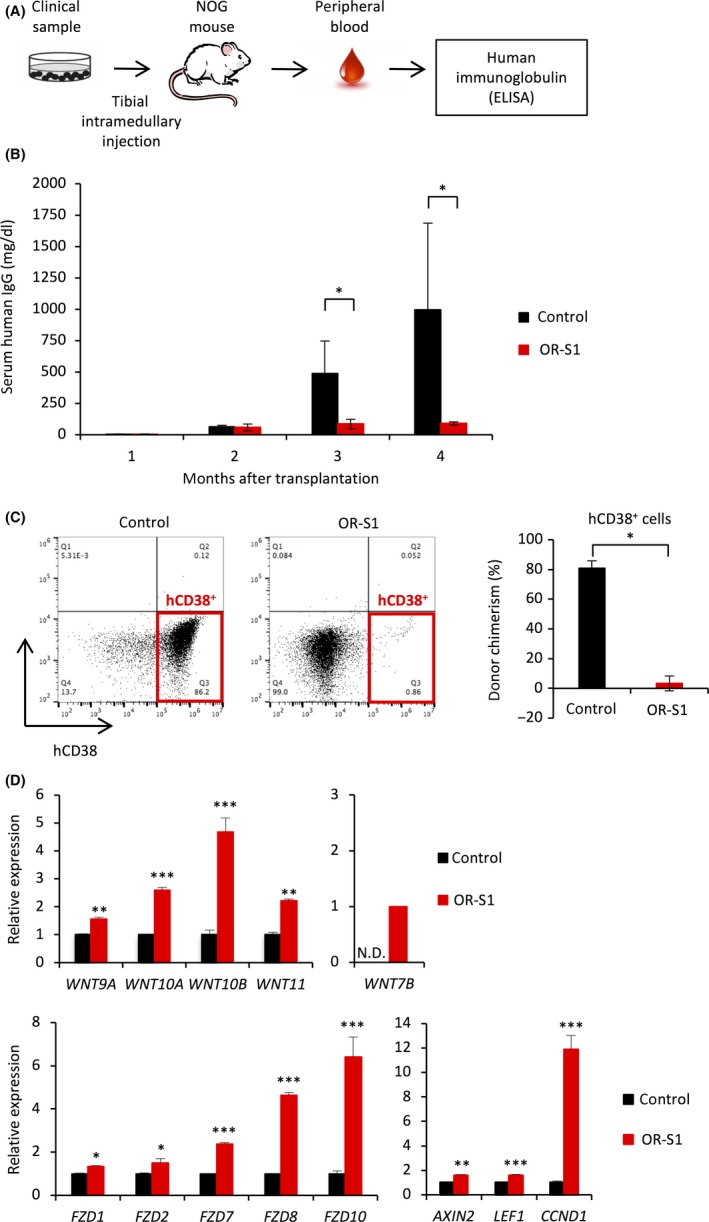
Translational implication of enhancer of zeste homolog 1/2 (EZH1/2) dual inhibition in multiple myeloma (MM). A, Experimental schema showing how successful engraftment was confirmed. Levels of human immunoglobulins in the serum of mice were measured 1 mo after intramedullary injection of clinical samples. NOG, NOD.Cg‐Prkdcscid Il2rgtm1Sug/Jic (Central Institute for Experimental Animals, Kawasaki, Japan). B, Bar graph showing the levels of human IgG in the serum of control and OR‐S1‐treated engrafted mice. Treatment with OR‐S1 (400 mg/kg per day orally for 60 d) was started 2 mo after transplantation, and levels of human IgG were monitored monthly (n = 4 per group). Error bars represent the mean ± SD. *P < .05. C, Representative FACS plots and a bar graph showing the percentage of hCD38+ cells within the bone marrow of control and OR‐S1‐treated mice with patient‐derived xenograft (PDX) at the final follow up. Error bars represent the mean ± SD. *P < .00001 (Student's t test). D, RT‐PCR analysis showing relative expression of WNT‐related genes in myeloma cells in a PDX model. Mice were given 400 mg/kg per day OR‐S1 orally for 3 wk following engraftment of tumors. Y‐axis represents the fold‐change in gene expression after normalization to that of ACTB. Error bars represent the mean ± SD. *P < .05; **P < .01; ***P < .001 (Student's t test)
4. DISCUSSION
This study investigated the role of EZH1/2 in the maintenance of myeloma stem cells by using a novel EZH1/2 dual inhibitor, OR‐S1. Expression of EZH1 and EZH2 was significantly increased in the SP fraction of MM cells, and repression of WNT signaling, a direct target of PRC2, was important to maintain the stemness of myeloma stem cells. Furthermore, OR‐S1 impaired the self‐renewal activity of myeloma stem cells.
Enhancer of zeste homolog 2 has oncogenic activity in MM22 and the expression level of EZH2 is significantly increased in SP cells.10 This suggests that inhibition of EZH2 could be a stem cell‐targeted therapy in MM treatment, because SP cells have stem‐like characteristics in MM.9, 10 However, it has been reported that inhibition of EZH2 alone does not completely disrupt PRC2 function and that EZH1/2 dual inhibition is crucial to treat other hematological malignancies, such as acute myeloid leukemia and malignant lymphoma.33, 42 Although UNC1999, an EZH1/2 dual inhibitor, also inhibits the growth of myeloma cells,43 it is unclear whether EZH1 plays an important role in stem cell maintenance in MM. Here, we showed that the expression of EZH1, as well as EZH2, was significantly increased in the SP fraction of MM cells, suggesting that inhibition of EZH1, in addition to EZH2, is necessary to eradicate myeloma stem cells when targeting PRC2. As expected, dual inhibition of EZH1/2 using OR‐S1 depleted myeloma stem cells in vitro and in vivo. Additionally, giving long‐term continuous OR‐S1 completely cured these mice without eliciting any serious side‐effects, suggesting that myeloma stem cells are specifically susceptible to dual inhibition of EZH1/2 and lose their self‐renewal activity when continuously exposed to OR‐S1. Therefore, continuous administration of OR‐S1 would be an effective treatment option against some types of MM cells that do not show OR‐S1‐induced apoptosis, such as MM.1S cells.
Our results also showed that there is direct crosstalk between PRC2 and WNT signaling in MM, which has also been reported in PRC2‐mediated differentiation of chondrocytes and adipocytes.44, 45 Most WNT and FZD genes were repressed by H3K27me3, and removal of these histone marks by dual inhibition of EZH1/2 resulted in the upregulation of WNT/β‐catenin signaling. Moreover, induced overexpression of β‐catenin impaired the proliferation of MM cells and depleted SP cells in vitro and in vivo, thereby decreasing their self‐renewal activity. These results suggest that repression of WNT signaling catalyzed by PRC2 plays a significant role in stemness maintenance of myeloma stem cells. However, we recognize this finding would be controversial because WNT signaling is often considered to be positively correlated with tumor progression, and several WNT inhibitors have been developed to prevent the proliferation of various types of malignant tumors.46, 47, 48 Thus, we speculate that, although moderately activated WNT signaling helps to maintain the stemness of MM cells, the activity level of this signaling is a determining factor for the reconstitution capacity of myeloma stem cells and then excessive WNT signaling impairs the maintenance of stemness. In fact, our results showed that overexpression of active β‐catenin in MM cells depleted myeloma stem cells and exerted a negative impact on tumorigenesis. We could not fully determine the dosage‐dependent effects of β‐catenin expression level on myeloma stem cells as a result of the technical limitations in controlling the level of β‐catenin expression. However, our speculation is consistent with the previous finding that overactivation of Wnt signaling reduces the self‐renewal activity of hematopoietic stem cells and induces their differentiation.37 Although PRC2 represses various genes to maintain the stemness of several types of stem cells,11, 32, 33 we genetically and pharmacologically showed that WNT signaling is the one of the most significant pathways in MM stemness maintenance and that release of repressed WNT/FZD genes through complete disruption of PRC2 is a crucial molecular mechanism for eradicating myeloma stem cells.
OR‐S1 had a significant antitumor effect in comparison with other established EZH2 or EZH1/2 inhibitors, including the EZH2‐specific inhibitor GSK126,26, 27, 28, 43 and markedly impaired the growth of all MM cells in vitro and in vivo, including a unique PDX model, with single‐agent administration. When compared to other EZH1/2‐targeting compounds, OR‐S1 suppresses the enzymatic activity of EZH1 more potently than UNC1999 and other EZH2 inhibitors.34 This difference would be one of the reasons why OR‐S1 showed better growth inhibition efficacy than other inhibitors. These results indicate that inhibition not only of EZH2 but also of EZH1 is crucial to successfully treat myeloma. Additionally, lower doses of OR‐S1 suppressed the proliferation of KMS‐11/BTZ cells, which are known to be resistant to bortezomib as a result of the mutation of PSMB5.36, 49 This mutation was also recently reported in a bortezomib‐resistant MM patient.50 Although proteasome inhibitors are key drugs in MM therapy, patients eventually develop drug resistance upon prolonged treatment. Thus, OR‐S1 may have significant advantages for the treatment of relapsed MM because its mechanism of action is completely separate from that of proteasome inhibitors. Although PRC2 is known to be an important molecule for stemness maintenance in hematopoietic stem cells, our group previously showed that OR‐S1 does not cause significant myelosuppression even after 4 months of treatment.33 In this study, neither abnormal clinical signs nor significant body weight loss was observed in mice throughout long‐term continuous administration of OR‐S1 for 150 days; therefore, long‐term continuous administration would be a clinically feasible regimen.34, 51 This favorable safety profile has enabled us to move forward to clinical trials as the only clinically applicable EZH1/2 inhibitor;29 subsequently evaluating the effect of OR‐S1 against MM patients would be encouraged as a novel therapeutic option for relapsed MM.
In summary, our results strongly suggest that repression of WNT signaling by EZH1/2 is important to maintain the stemness of MM cells and that dual inhibition of EZH1/2 with OR‐S1 is a promising therapeutic approach to eradicate myeloma stem cells, leading to significant advances in the treatment of MM.
CONFLICTS OF INTEREST
I.K. received a research grant from Daiichi Sankyo, Co., Ltd. D.H. and K.A. are employees of Daiichi Sankyo, Co., Ltd. T.I., A.K., K.I., C.W., and Y.O. are employees of Daiichi Sankyo RD Novare Co., Ltd. The other authors have no conflicts of interest to declare.
Supporting information
ACKNOWLEDGMENTS
The authors would like to thank Yutaka Shima, Emi Takamatsu‐Ichihara, Yukino Machida, and Yukiko Aikawa for expert advice and technical support. This work was supported by JSPS KAKENHI Grant Number JP18K16101 to M. Nakagawa. I. Kitabayashi was funded by Acceleration Transformative Research for Medical Innovation from the Japan Agency for Medical Research and Development and by the National Cancer Center Research and Development Fund.
Nakagawa M, Fujita S, Katsumoto T, et al. Dual inhibition of enhancer of zeste homolog 1/2 overactivates WNT signaling to deplete cancer stem cells in multiple myeloma. Cancer Sci. 2019;110:194–208. 10.1111/cas.13840
REFERENCES
- 1. Braggio E, Kortüm KM, Stewart AK. SnapShot: multiple myeloma. Cancer Cell. 2015;28(5):678‐678.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Greipp PR, Miguel JS, Dune BGM, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23(15):3412‐3420. [DOI] [PubMed] [Google Scholar]
- 3. Donnenberg VS, Donnenberg AD. Multiple drug resistance in cancer revisited: the cancer stem cell hypothesis. J Clin Pharmacol. 2005;45(8):872‐877. [DOI] [PubMed] [Google Scholar]
- 4. Pei XY, Dai Y, Youssefian LE, et al. Cytokinetically quiescent (G0/G1) human multiple myeloma cells are susceptible to simultaneous inhibition of Chk1 and MEK1/2. Blood. 2011;118(19):5189‐5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou S, Schuetz JD, Bunting KD, et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side‐population phenotype. Nat Med. 2001;7(9):1028‐1034. [DOI] [PubMed] [Google Scholar]
- 6. Challen GA, Little MH. A side order of stem cells: the SP phenotype. Stem Cells. 2006;24:3‐12. [DOI] [PubMed] [Google Scholar]
- 7. Wu C, Wei Q, Utomo V, et al. Side population cells isolated from mesenchymal neoplasms have tumor initiating potential. Cancer Res. 2007;67(17):8216‐8222. [DOI] [PubMed] [Google Scholar]
- 8. Wu C, Alman BA. Side population cells in human cancers. Cancer Lett. 2008;268(1):1‐9. [DOI] [PubMed] [Google Scholar]
- 9. Jakubikova J, Adamia S, Kost‐Alimova M, et al. Lenalidomide targets clonogenic side population in multiple myeloma: pathophysiologic and clinical implications. Blood. 2011;117(17):4409‐4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nara M, Teshima K, Watanabe A, et al. Bortezomib reduces the tumorigenicity of multiple myeloma via downregulation of upregulated targets in clonogenic side population cells. PLoS One. 2013;8(3):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xie H, Xu J, Hsu JH, et al. Polycomb repressive complex 2 regulates normal hematopoietic stem cell function in a developmental‐stage‐specific manner. Cell Stem Cell. 2014;14(1):68‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boyer LA, Plath K, Zeitlinger J, et al. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature. 2006;441(7091):349‐353. [DOI] [PubMed] [Google Scholar]
- 13. Lee TI, Jenner RG, Boyer LA, et al. Control of developmental regulators by polycomb in human embryonic stem cells. Cell. 2006;125(2):301‐313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cao R, Zhang Y. SUZ12 is required for both the histone methyltransferase activity and the silencing function of the EED‐EZH2 complex. Mol Cell. 2004;15(1):57‐67. [DOI] [PubMed] [Google Scholar]
- 15. Margueron R, Justin N, Ohno K, et al. Role of the polycomb protein EED in the propagation of repressive histone marks. Nature. 2009;461(7265):762‐767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Czermin B, Melfi R, McCabe D, Seitz V, Imhof A, Pirrotta V. Drosophila enhancer of Zeste/ESC complexes have a histone H3 methyltransferase activity that marks chromosomal Polycomb sites. Cell. 2002;111(2):185‐196. [DOI] [PubMed] [Google Scholar]
- 17. Sparmann A, van Lohuizen M. Polycomb silencers control cell fate, development and cancer. Nat Rev Cancer. 2006;6(11):846‐856. [DOI] [PubMed] [Google Scholar]
- 18. Mills AA. Throwing the cancer switch: reciprocal roles of polycomb and trithorax proteins. Nat Rev Cancer. 2010;10(10):669‐682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xu F, Li X, Wu L, et al. Overexpression of the EZH2, RING1 and BMI1 genes is common in myelodysplastic syndromes: relation to adverse epigenetic alteration and poor prognostic scoring. Ann Hematol. 2011;90(6):643‐653. [DOI] [PubMed] [Google Scholar]
- 20. Fujikawa D, Nakagawa S, Hori M, et al. Polycomb‐dependent epigenetic landscape in adult T‐cell leukemia. Blood. 2016;127(14):1790‐1803. [DOI] [PubMed] [Google Scholar]
- 21. Kalushkova A, Fryknäs M, Lemaire M, et al. Polycomb target genes are silenced in multiple myeloma. PLoS One. 2010;5(7):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Croonquist PA, Van Ness B. The polycomb group protein enhancer of zeste homolog 2 (EZH 2) is an oncogene that influences myeloma cell growth and the mutant ras phenotype. Oncogene. 2005;24(41):6269‐6280. [DOI] [PubMed] [Google Scholar]
- 23. Van Kemenade FJ, Raaphorst FM, Blokzijl T, et al. Coexpression of BMI‐1 and EZH2 polycomb‐group proteins is associated with cycling cells and degree of malignancy in B‐cell non‐Hodgkin lymphoma. Blood. 2001;97(12):3896‐3901. [DOI] [PubMed] [Google Scholar]
- 24. Sasaki D, Imaizumi Y, Hasegawa H, et al. Overexpression of enhancer of zeste homolog 2 with trimethylation of lysine 27 on histone H3 in adult T‐cell leukemia/lymphoma as a target for epigenetic therapy. Haematologica. 2011;96(5):712‐719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim KH, Roberts CWM. Targeting EZH2 in cancer. Nat Med. 2016;22(2):128‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Agarwal P, Alzrigat M, Párraga AA, et al. Genome‐wide profiling of histone H3 lysine 27 and lysine 4 trimethylation in multiple myeloma reveals the importance of Polycomb gene targeting and highlights EZH2 as a potential therapeutic target. Oncotarget. 2016;7(6):6809‐6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zeng D, Liu M, Pan J. Blocking EZH2 methylation transferase activity by GSK126 decreases stem cell‐like myeloma cells. Oncotarget. 2017;8(2):3396‐3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hernando H, Gelato KA, Lesche R, et al. EZH2 inhibition blocks multiple myeloma cell growth through upregulation of epithelial tumor suppressor genes. Mol Cancer Ther. 2016;15(2):287‐298. [DOI] [PubMed] [Google Scholar]
- 29. Nakagawa M, Kitabayashi I. Oncogenic roles of enhancer of zeste homolog 1/2 in hematological malignancies. Cancer Sci. 2018;109(8):2342‐2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shen X, Liu Y, Hsu YJ, et al. EZH1 mediates methylation on histone H3 Lysine 27 and complements EZH2 in maintaining stem cell identity and executing pluripotency. Mol Cell. 2008;32(4):491‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Margueron R, Li G, Sarma K, et al. Ezh1 and Ezh2 maintain repressive chromatin through different mechanisms. Mol Cell. 2008;32(4):503‐518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hidalgo I, Herrera‐Merchan A, Ligos JM, et al. Ezh1 is required for hematopoietic stem cell maintenance and prevents senescence‐like cell cycle arrest. Cell Stem Cell. 2012;11(5):649‐662. [DOI] [PubMed] [Google Scholar]
- 33. Fujita S, Honma D, Adachi N, et al. Dual inhibition of EZH1/2 breaks the quiescence of leukemia stem cells in acute myeloid leukemia. Leukemia. 2018;32(4):855‐864. [DOI] [PubMed] [Google Scholar]
- 34. Honma D, Kanno O, Watanabe J, et al. Novel orally bioavailable EZH1/2 dual inhibitors with greater antitumor efficacy than an EZH2 selective inhibitor. Cancer Sci. 2017;108(10):2069‐2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McCabe MT, Ott HM, Ganji G, et al. EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2‐activating mutations. Nature. 2012;492(7427):108‐112. [DOI] [PubMed] [Google Scholar]
- 36. Ri M, Iida S, Nakashima T, et al. Bortezomib‐resistant myeloma cell lines: a role for mutated PSMB5 in preventing the accumulation of unfolded proteins and fatal ER stress. Leukemia. 2010;24(8):1506‐1512. [DOI] [PubMed] [Google Scholar]
- 37. Luis TC, Naber BAE, Roozen PPC, et al. Canonical wnt signaling regulates hematopoiesis in a dosage‐dependent fashion. Cell Stem Cell. 2011;9(4):345‐356. [DOI] [PubMed] [Google Scholar]
- 38. Gado K, Silva S, Paloczi K, Domjan G, Falus A. Mouse plasmacytoma: an experimental model of human multiple myeloma. Haematologica. 2001;86(3):227‐236. [PubMed] [Google Scholar]
- 39. Groen RWJ, Noort WA, Raymakers RA, et al. Reconstructing the human hematopoietic niche in immunodeficient mice: opportunities for studying primary multiple myeloma. Blood. 2012;120(3):9‐17. [DOI] [PubMed] [Google Scholar]
- 40. Schueler J, Wider D, Klingner K, et al. Intratibial injection of human multiple myeloma cells in NOD/SCID IL‐2Rγ(null) mice mimics human myeloma and serves as a valuable tool for the development of anticancer strategies. PLoS One. 2013;8(11):1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fryer RA, Graham TJ, Smith EM, et al. Characterization of a novel mouse model of multiple myeloma and its use in preclinical therapeutic assessment. PLoS One. 2013;8(2):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xu B, On DM, Ma A, et al. Selective inhibition of EZH2 and EZH1 enzymatic activity by a small molecule suppresses MLL‐ rearranged leukemia. Blood. 2015;125(2):346‐357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rizq O, Mimura N, Oshima M, et al. Dual inhibition of EZH2 and EZH1 sensitizes PRC2‐dependent tumors to proteasome inhibition. Clin Cancer Res. 2017;23(16):4817‐4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mirzamohammadi F, Papaioannou G, Inloes JB, et al. Polycomb repressive complex 2 regulates skeletal growth by suppressing Wnt and TGF‐β signalling. Nat Commun. 2016;7:12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang L, Jin Q, Lee J‐E, Su I‐H, Ge K. Histone H3K27 methyltransferase Ezh2 represses Wnt genes to facilitate adipogenesis. Proc Natl Acad Sci USA. 2010;107(16):7317‐7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14(15):1837‐1851. [PubMed] [Google Scholar]
- 47. Kikuchi A. Tumor formation by genetic mutations in the components of the Wnt signaling pathway. Cancer Sci. 2003;94(3):225‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Le PN, McDermott JD, Jimeno A. Targeting the Wnt pathway in human cancers: therapeutic targeting with a focus on OMP‐54F28. Pharmacol Ther. 2015;146:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Oerlemans R, Franke NE, Assaraf YG, et al. Molecular basis of bortezomib resistance: proteasome subunit 25 (PSMB5) gene mutation and overexpression of PSMB5 protein. Blood. 2008;112(6):2489‐2499. [DOI] [PubMed] [Google Scholar]
- 50. Barrio S, Stühmer T, Teufel E, et al. Parallel evolution of multiple PSMB5 mutations in a myeloma patient treated with bortezomib. Blood. 2016;128(22):Abstract 3282. [Google Scholar]
- 51. Maruyama D, Tobinai K, Makita S, et al. First‐in‐human study of the EZH1/2 dual inhibitor DS‐3201b in patients with relapsed or refractory non‐Hodgkin lymphomas — preliminary results. Blood. 2017;130(Suppl 1):4070 LP‐4070. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


