Abstract
Bioactive lipid mediators derived from n-3 and −6 fatty acids are known to modulate leukocytes. Metabolic transformation of essential fatty acids to endogenous bioactive molecules plays a major role in human health. Here we tested the potential of substrates; linoleic acid (LA) and docosahexaenoic acid (DHA) and their bioactive products; Resolvin-D1(RvD1) and 12-S-hydroxyeicosatetraenoic acids(HETE) to modulate macrophage plasticity and cardiac fibroblast phenotype in presence or absence of lipid metabolizing enzyme 12/15-lipoxygenase(LOX). Peritoneal macrophages and cardiac fibroblasts were isolated from wild-type (C57BL/6J) and 12/15LOX−/− mice and treated with DHA, LA, 12(S)-HETE), RvD1 for 4,8,12 and 24 h. LA, DHA, 12(S)-HETE and RvD1 elicited mRNA expression of pro-inflammatory markers Tnf-α, IL-6, Ccl2 and IL-1β in WT and in 12/15LOX−/− macrophages at early time-point (4h). Bioactive immunoresolvent RvD1 lowered the levels of Tnf-α, IL-6, and IL-1β at 24 h time-point. Both DHA and RvD1 stimulated the proresolving markers such as Arg-1, Ym-1, and Mrc-1 in WT macrophage. RvD1 induced proresolving phenotype Arg-1 expression in both WT 12/15LOX−/− macrophages even in presence of 12(S)-HETE. RvD1 peaked 5LOX expression in both WT and 12/15LOX−/− at 24 h time-point compared with DHA. RvD1 diminished COX-2 but upregulated 5LOX expression in fibroblast compared with DHA. In summary, the feed-forward enzymatic interaction with fatty acids substrates and direct mediators (RvD1 and 12(S)-HETE) are responsive in determining macrophages phenotype and cardiac fibroblast plasticity. Particularly, macrophages and fibroblast phenotypes are responsive to milieu and RvD1 governs the milieu-dependent chemokine signaling in presence or absence of 12/15LOX enzyme to resolve inflammation.
Keywords: fatty acids, fibroblast, inflammation, macrophages, resolvin D1
Introduction
The importance of differential fatty acids is well-established in many aspects of healthy immune system of human physiology and cardiovascular health (Patterson et al., 2012); (Calder, 2017; Halade et al., 2016; Halade et al., 2017; Kain et al., 2017; Lopez et al., 2015; Yates et al., 2011) Essential fatty acids are transformed using the enzyme lipoxygenases (LOXs) to form number of endogenous bioactive mediators. Respective bioactive lipid mediators are derived from the metabolic transformation of long-chain polyunsaturated fatty acids (n-3 and n-6) in response to injury, infection, stress, or exercise. Fatty acids-derived mediators are immune responsive and help to initiate and repress inflammation depending upon the microenvironment at the site of injury (Serhan et al., 2008b) (Halade and Kain, 2017; Kain et al., 2014). The balance of essential fatty acids, such as linoleic acid (LA), arachidonic acid (AA), and the conditionally essential fatty acids such as docosahexaenoic (DHA), and eicosapentaenoic acid (EPA), are essential for human health and development (Zivkovic et al., 2011). Fatty acids are an integral component of membrane biology, intracellular ion signaling, bioenergetics, energy consumption, and metabolism (Patterson et al., 2012). Higher content of n-6 fatty acids rather than that of n-3 fatty acid is the common feature of the western diet. Thus, increased consumption of western diet altered the optimal balance of n-6 : n-3 ratio which is around 2:1, (Simopoulos, 2001) (Anderson and Ma, 2009) and now by new estimates, it has increased to the range of 20: 1 (Simopoulos, 2000). An increased intake omega-6 fatty acid (linoleic acid)-enriched diet stimulates the production of pro-inflammatory products, such as AA (arachidonic acid), which is subject to oxygenation by 12/15LOX leading to incidences of diseases involving inflammatory processes. The diverse array of the metabolic substrate, are utilized by 5-, 12-, and 15LOX enzymes which insert molecular oxygen into unconjugated double-bond systems via a mechanism that involves hydrogen abstraction (Schneider et al., 2007). Linoleic acid (LA) gets desaturated and elongates to AA by Δ6 desaturase and further catalyzed by 12/15LOX, that forms range of bioactive mediators such as 12(S)- and 15(S)-hydroxyeicosatetraenoic acids (HETE) known as eicosanoids in injury setting including myocardial infarction (Lee and Blair, 2009; Lopez et al., 2015). Macrophages specifically peritoneal macrophages are rich source of eicosanoids (12S)-HETE and 15(S)-HETE derived from the metabolism of arachidonic acid and play important role in acute inflammation (Scott et al., 1980). Acute inflammation is a tightly regulated process monitored by mononuclear phagocyte system which includes monocytes, macrophages, neutrophils and surrounded by collagen secreting myofibroblast. Macrophages are essential for the initiation, maintenance, and resolution of inflammation and are activated and deactivated during inflammatory process (Elhelu, 1983) (Fujiwara and Kobayashi, 2005) and collagen secreting myofibroblast to cover dead tissue area. During this process, several cellular networks interact via a large number of molecules, such as neuropeptide, lipid mediators, fibroblast, cytokines and growth factors through leukocytes, particularly macrophages. To execute and regulate inflammatory response not only immune cells but resident structural cells such as fibroblast also produces many products such as growth factors and lipid mediators (Jordana et al., 1994). As fibroblast is the major component of extracellular matrix (ECM) providing structural tissue integrity, having powerful inductive effects (Jordana et al., 1994) (Rog-Zielinska et al., 2016). Matrix associated proteins influence the shape, movement, and state of activation of inflammatory cells in the tissue. Thus, fibroblasts being major producers of ECM proteins are linked with the regulation of inflammatory response (Rog-Zielinska et al., 2016; Van Linthout et al., 2014).
LOXs, specifically 12/15LOX and 5LOX, metabolize n-6 and n-3 fatty acids to produce bioactive lipid mediators (Kain et al., 2014). Oxygenation step of fatty acids are preceded by a LOX-mediated pathway, which converts DHA to D-series resolvins (RvD1–6). Immune responsive resolvin D1 (RvD1, 7S,8R,17S-trihydroxy-4Z,9E,11E,13Z,15E,19Z-docosahexaenoic acid) is a potent anti-inflammatory molecule and is pro-resolving in action (Saito et al., 2015). Functionally, RvD1 inhibits neutrophil infiltration and promotes efferocytosis in several disease models, and also promotes the alternative macrophage phenotype (Kain et al., 2015) (Hsiao et al., 2013) (Lee et al., 2013). In the presented report, we determine the macrophage and fibroblast chemokine signaling of fatty acids substrate and specific lipid mediators in the absence and presence of 12/15LOX enzyme. Further, we expanded the link between 12/15LOX enzyme and fatty acids substrate nexus indicating both n-3 and n-6 derived lipid mediators govern the macrophage phenotype and cardiac fibroblast chemokine signaling.
Material and methods
Animal care and compliance
8–12-week-old C57BL/6 (wild-type; WT; stock number 000664) and 12/15LOX null mice from a C57BL/6 genetic background (12/15LOX−/−; stock number 002778) were obtained from the Jackson laboratory (Bar Harbor, Maine, USA) and were maintained at constant temperature (19.8–22.2°C). The mice were given free access to water and standard chow diet. All animals were maintained on a 12-h light, 12-h dark cycle. All protocols involving animals conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (revised 2015) and was approved by the Animal Care and Use Committee at the University of Alabama at Birmingham.
Materials
The following reagents were used in the experiments described below: docosahexaenoic acid (DHA), linoleic Acid (LA), 12(S)-hydroxyeicosatetraenoic acid (12(S)-HETE) and resolvin D1 (RvD1) (Cayman chemicals, Ann Arbor, Michigan). Additional materials used included Collagenase II (Worthington Biochemical, Lakewood, NJ), DNase I (AppliChem, Ottoweg Darmstadt), RPMI 1640, and DMEM/F12 media fetal bovine serum (FBS) (Thermo Fischer Scientific, Grand Island, NY) Bovine serum albumin (BSA; Fraction V; Sigma Aldrich St Louis, MO) and radio-immunoprecipitation assay (RIPA) lysis buffer (Sigma Aldrich St Louis, MO). The protease inhibitor cocktail was purchased from Roche GmbH, Germany.
Isolation of peritoneal macrophages
Peritoneal macrophages were isolated from WT and 12/15LOX−/− mice as previously described (Ma et al., 2013). Briefly, the peritoneal cavity was lavage twice with 10 mL of ice-cold, RPMI 1640 media with 10% FBS) and 1% antibiotics (Thermo Fischer Scientific, Grand Island, NY). The recovered media was centrifuged at 250 x g for 10 min. The cell pellet was resuspended in 6 mL of RPMI 1640 media. The cells were plated in 6-well plate (1×106 cells/ well), incubated at 37°C overnight to allow the cells to adhere, and subsequently washed with fresh media to remove any unattached cells.
Isolation of cardiac fibroblast
Cardiac fibroblasts from WT and 12/15LOX−/− mice were isolated by enzymatic digestion with 600 U/mL collagenase II and 60 U/mL DNase I. Cells at passage 2 were plated in 6-well plates (5×104 cells/well) and allowed to attach at 37°C overnight, then washed using DMEM/F12 media with 10% FBS and 1% antibiotics to remove unattached cells.
Macrophages and fibroblast treatment
Isolated macrophages and fibroblast from WT and 12/15LOX−/− mice were plated in 6 well plate overnight before treatments. LA, DHA, 12(S)-HETE, and RvD1 was conjugated with BSA at a maximal concentration of 0.1%. Cultured macrophages were treated with DHA (50μM), LA (200μM), 12(S)-HETE (100nM) and RvD1 (10ng/ml) for 4 h and 24 h. Similarly, cultured fibroblast was treated with DHA (50μM), LA (200μM), 12(S)-HETE (100nM) and RvD1 (10ng/ml) for 4, 8, 12 and 24 h. 0.1% BSA is used as a vehicle control (VC) and cells without any treatment are used as control (C).
Measurements of macrophage phenotype using Real-Time quantitative PCR
For qPCR, reverse transcription was performed with 2.5μg of total RNA using the SuperScript® VILO cDNA Synthesis Kit (Invitrogen, CA, USA). Quantitative PCR for Tnf-α, IL-6, ccl2, IL-1β, Arg-1, Mrc-1 and Ym-1 genes was performed using TaqMan probes (Applied Biosystems, CA, USA) on a master cycler (ABI, 7900HT, Grand Island, NY). The mRNA expression was normalized with the reference genes (β-Actin). The results were reported as 2-ΔCt (ΔΔCt) values. All the experiments were performed in triplicates.
Preparation of protein lysates
Cultured macrophages and fibroblast protein lysates were prepared using RIPA lysis buffer and protease inhibitor cocktail. Briefly, the medium was removed from cells and washed with cold 1x PBS. Cells were gently scraped in 1X cold PBS and centrifuged at 4oC for 2 min at 14000 rpm. The supernatant was discarded, and the cell pellet was resuspended in RIPA lysis with a 1X protease inhibitor and incubated on ice for 20 min. The solution was then centrifuged for 20 min at 4oC at 14000 rpm. The supernatant was collected in fresh tubes and snap frozen until further use. Protein concentration in each sample was determined using 1X Bradford protein assay kit according to the manufacturer’s instructions (Bio-Rad, Hercules, CA, USA) with bovine serum albumin (BSA-2mg/ml) as a standard.
Immunoblotting
Immunoblotting was used to quantify the protein levels in peritoneal macrophages and cardiac fibroblast. The samples were resolved on criterion XT bis-tris 4–12% 18 well (Bio-Rad Inc. Hercules, California) gel and MOPs Buffer (Bio-Rad, Hercules, California). The kaleidoscope precision plus standard (Bio-Rad) was used to determine the molecular weight of the protein for immunoblotting, 10–15 μg of protein lysate per sample was denatured and resolved by using criterion XT bis-tris 4–12% 18-gel (Bio-Rad I, Hercules, California.) gel in MOPs Buffer (Bio-Rad)., then transferred to a nitrocellulose membrane (Bio-Rad, Hercules, California), and blocked with 5% nonfat milk. The membrane was probed with COX-2 (1:1000) and 5LOX (1:200) overnight at 4˚C, followed by secondary antibody (Bio-Rad, Hercules, California). The proteins were detected using the Femto chemiluminescence detection system (Pierce Chemical, Rockford, IL). Densitometry was performed using Image J software (NIH, USA).
Immunofluorescence
The peritoneal macrophages were plated in a 12-well plate (1×106 cells/ well) on coverslips, incubated at 37°C overnight to allow the cells to adhere, and subsequently washed with fresh media to remove any unattached cells. The cells were treated with DHA (50μM and RvD1 (10ng/ml) for 24 h for Tnf-α expression. The cells were treated with 12(S)-HETE (100μM and RvD1 (10ng/ml) for 4 h for Arg-1 and F4/80 expression. Isolated cardiac fibroblast was plated on Millicell® cell culture inserts (Millipore). Cardiac fibroblast was differentiated into myofibroblast by treating them to 15ng/ml Tgf-β (15ng/ml) and co-incubated with 12(S)-HETE for 18 hr. Cells (peritoneal macrophages or cardiac fibroblast) were fixed using 4% PFA (paraformaldehyde), permeabilized using 0.1% Triton and blocked for 1hr in 10% goat serum. Peritoneal macrophages were subsequently incubated with anti-TNF-α- antibody, Arg-1 and F4/80 (Abcam, Cambridge, MA) overnight and probed with an Alexa-488, Alexa-55 secondary antibody (Molecular probe), each for 60 min at room temperature. Cardiac fibroblast was probed with anti-smooth muscle actin-α (SMA-α) antibody (Sigma Aldrich St Louis, MO) overnight and further probed with Alexa-55 secondary antibody for 1 hr. The nucleus in both cell types was stained using Hoechst (molecular probe). Cells were mounted using anti-fade mounting media (Thermo Fisher Scientific, Grand Island, NY) and then visualized and photographed using Nikon A1 high-speed laser confocal microscope.
Statistical analysis
Data are expressed as mean ±SEM. Statistical analyses were performed using GraphPad Prism 5. Two way-analysis of variance (ANOVA) was for multiple comparisons. For 2 groups comparison, student-t-test (unpaired) was applied and p<0.05 was considered as statistically significant.
Results
RvD1 possesses high potential than DHA in limiting pro-inflammatory cytokines in the absence of 12/15LOX
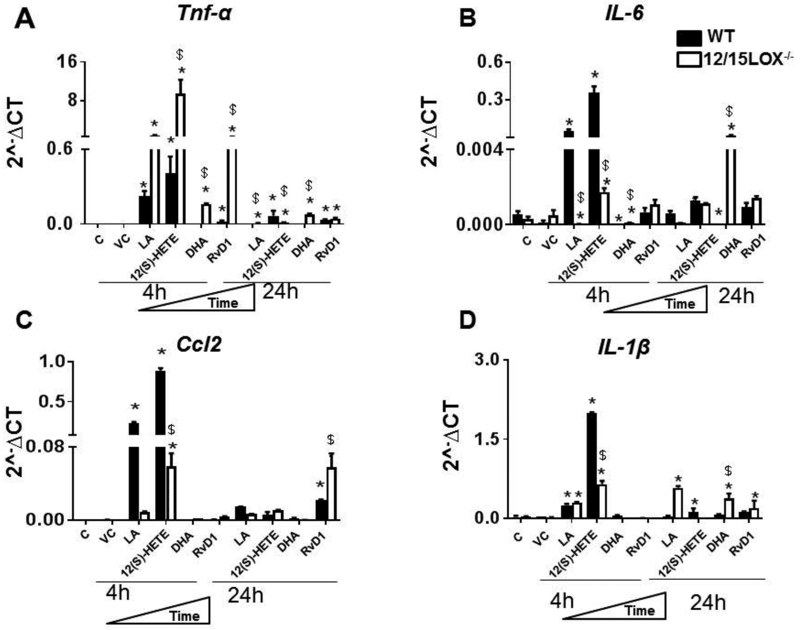
This study aimed to investigate whether fatty acids substrate (LA compared to DHA) or respective immune responsive metabolite (12(S)-HETE compared to RvD1) are inducer or repressor of the macrophage phenotype (proinflammatory or proresolving). After co-culturing macrophages with substrate (LA or DHA) and respective metabolites (12(S)-HETE or RvD1) secreted Tnf-α, IL-6, Ccl2 and IL-1β cytokines in both WT and 12/15 LOX-/−. In comparison to control cells, there was increase in Tnf-α, IL-6, Ccl2 and IL-1β at 4 h and 24 h after treatment with LA, 12(S)-HETE, DHA, or RvD1 (Figure 1 A-D). Pro-inflammatory cytokine levels such as Tnf-α, IL-6, and IL-1β remained elevated with LA and 12(S)-HETE treatment in WT and 12/15LOX-/ - at 24h, but DHA and RvD1 lowered Tnf-α, IL-6 and IL-1β at 24 h in WT macrophages (Figures 1A-D). The levels of Ccl2 expression in macrophages remained elevated in RvD1 treated macrophages for both WT and 12/15LOX-/−. RvD1 induced lower level of Tnf-α expression compared with DHA in WT macrophages provided in supplementary figure 1. The RvD1 limited stimulation of Tnf-α, IL-6, and IL-1β in 12/15LOX−/− macrophages compared with DHA. Our results indicated that specific substrate (LA vs DHA) and substrate-derived product (RvD1 and 12(S)-HETE) are essential in determining the macrophage phenotype in time-dependent manner. RvD1 inhibited the prolonged inflammatory cytokines in the absence of 12/15LOX enzyme while DHA has limited long-term effect to control prolonged inflammatory phase in the absence of 12/15LOX.
Figure1: RvD1 effectively control pro-inflammatory macrophages phenotypes in the absence of 12/15LOX, compared to DHA.
WT and 12/15LOX−/− peritoneal macrophages treated with LA (200μ), 12(S)-HETE (100nM), DHA(50μM) and RvD1(10ng/ml) for 4h and 24 h. mRNA expression of classical M1 markers A. Tnf-α B. IL-6 C. Ccl2 D. IL-1β. Expression levels are normalized to HPRT-1. *p<0.05 vs. untreated cells, $p<0.05 vs. treated WT cells. Results are presented as mean±SEM. Data are representative of a n=3 independent experiments.
RvD1 polarized macrophages towards resolving phenotype than DHA and LA
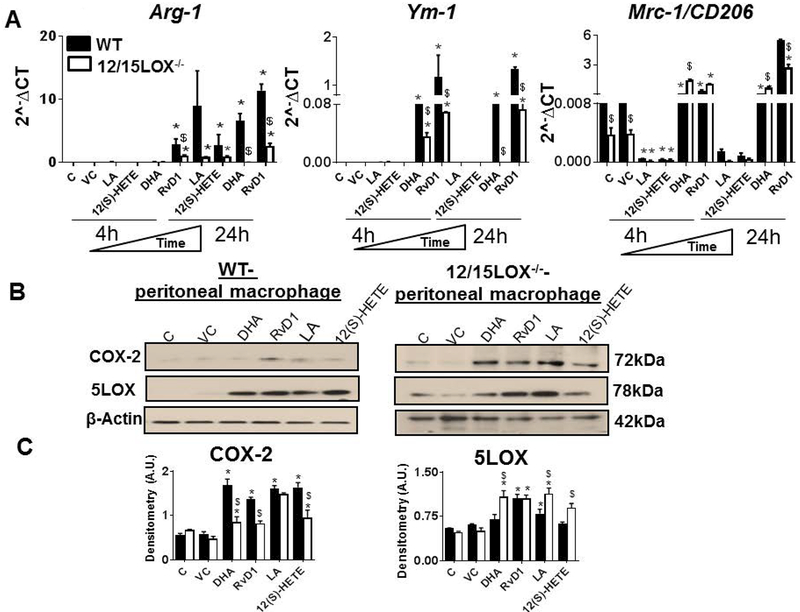
Tissue specific macrophage diversity and macrophage role in acute versus chronic inflammation is active area of investigation (Bronte and Pittet, 2013). For simplicity, pro-resolving macrophage phenotype referred as M2/alternative or resolving macrophages. Our previous, in-vivo study of cardiac healing has shown that the M2 macrophages are essential in controlling the inflammation as they are reparative in action and inhibits their M1 counterparts (Kain et al., 2015; Ma et al., 2013). In-vivo, the concept of M1 and M2 is more heterogenous and complex. Thus we applied in-vitro approach to understand the role of 12/15LOX and substrate-metabolite nexus. For this, we isolated peritoneal macrophages and treated them with substrates (LA and DHA) and metabolites (12(S)-HETE and RvD1). From above results, at 24 h time point, RvD1- but not the DHA was effective in shortening the pro-inflammatory phase. We further evaluated the effect of LA, 12(S)-HETE, DHA and RvD1 on the pro-resolving macrophage markers Arg-1, Ym-1 and Mrc-1/CD206 (Figure 2A). Both LA and 12(S)-HETE had limited effect of pro-resolving macrophages markers such as Ym-1 and Mrc-1, in WT and 12/15LOX−/− at 4 h and 24 h time-point. LA and 12(S)-HETE treated 12/15LOX−/− macrophages lowered expression of Arg-1 compared with WT macrophages at 24 h time point. Both DHA and RvD1 induced higher levels of Arg-1, Ym-1 and Mrc-1 expression in WT macrophages indicating pro-resolving phenotype at 24 h. LA showed the minimal effect on macrophage phenotype in presence or absence of 12/15LOX enzyme. Of note, RvD1 was able to induce pro-resolving phenotype in both WT and 12/15LOX−/− macrophages. These results indicate that both DHA and DHA-derived RvD1 were able to induce the macrophages towards pro-resolving phenotype.
Figure 2: RvD1 is potent than DHA in polarizing macrophages towards reparative phenotype.
WT and 12/15LOX−/− peritoneal macrophages treated with LA (200μM), 12(S)-HETE (100nM), DHA (50μM) and RvD1 (10ng/ml) for 4h and 24 h. mRNA expression of alternative M2 markers A Arg-1, Ym-1, Mrc-1/CD206. Expression levels are normalized to HPRT-1. B WT and 12/15LOX−/− peritoneal macrophages treated with DHA(50μM), RvD1(10ng/ml), LA(200μM), and 12(S)-HETE(100nM) for 24h. Immunoblot representing COX-2 and 5LOX expression. CDensitometric analysis of COX-2 and 5LOX in LA, 12(S)-HETE, DHA and RvD1 treated peritoneal macrophages at 4 h and 24 h.*p<0.05 vs untreated cells, $p<0.05 vs. treated WT cells. Results are presented as mean±SEM. Data are representative of a n=3 independent experiments.
RvD1 induced 5LOX expression and stabilized COX-2 in peritoneal macrophages
Since, immune responsive RvD1 displayed a higher capacity than DHA to polarize macrophages towards the reparative phenotype, we further determined, the effect of RvD1 and DHA on the 5LOX and COX-2 expression. Both LA and 12(S)-HETE were used as negative and positive controls respectively. Our results showed that LA, 12(S)-HETE, RvD1, and DHA were able to induce COX-2 expression in both WT and 12/15LOX−/− macrophages. However, no significant difference was observed in COX-2 expression with respective treatments (Figures 2B and Figures 2C). RvD1 treatment displayed 2.1 fold increase in 5LOX expression compared with DHA, 12(S)-HETE, and LA in WT macrophages at 24 h. Treatment with RvD1 induced higher expression of 5LOX in 12/15LOX−/− macrophages compared with WT. However, there was no difference in the 5LOX expression levels in macrophages treated with LA, 12(S)-HETE, RvD1 and DHA (Figures 2B and Figures 2C). The data indicates RvD1 potentially stabilizes macrophages towards in reparative mode.
12(S)-HETE stimulated early induction of COX-2 and 5LOX in cardiac fibroblast in the absence of 12/15LOX
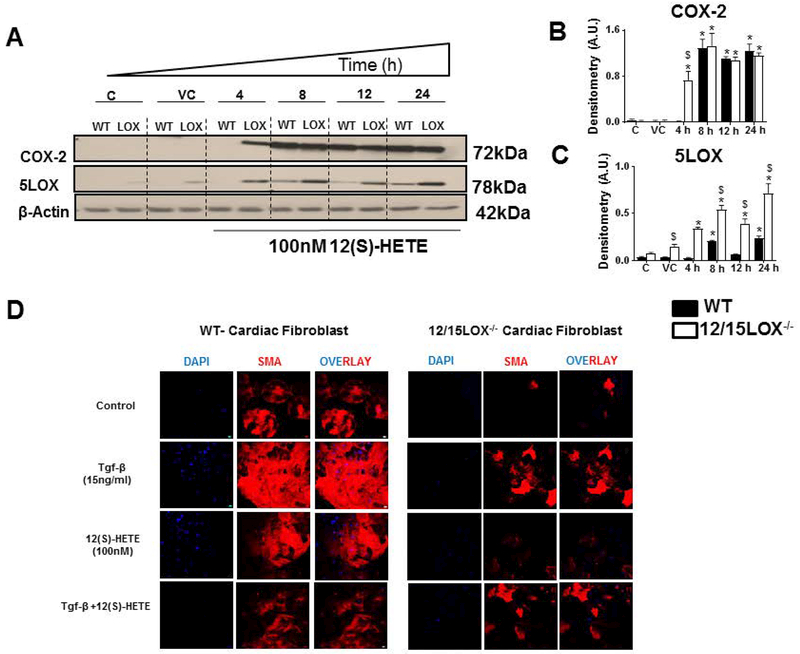
Induction of COX-2 gene in response to 12(S)-HETE has been well demonstrated in different cell types and is responsible for forming the proinflammatory microenvironment (Sun et al., 2015). We further evaluated the impact of 12(S)-HETE on both pro-inflammatory COX-2 and pro-resolving 5LOX in WT and 12/15LOX−/− cardiac fibroblast. 12(S)-HETE induce COX-2 expression in WT and 12/15LOX−/− cardiac fibroblast, however, 12/15LOX−/− cardiac fibroblast displayed early induction of COX-2 at 4h time point compared with WT-cardiac fibroblast (Figures 3A and Figures 3B).In the absence of 12/15LOX, cardiac fibroblast displayed higher expression 5LOX compared with WT cardiac fibroblast that was increased in a time-dependent manner. The expression of 5LOX reached to its peak (~4.1 fold) at 24 h of time-point in 12/15LOX−/− cardiac fibroblast compared with WT cardiac fibroblast (Figures 3A and Figures 3C). 12/15LOX and respective metabolite plays important in fibrosis. Since, presence of 12(S)-HETE activates inflammatory signal, we further delineated impact of 12(S)-HETE on myofibroblast differentiation in presence and absence of 12/15LOX. We induced myofibroblast trans-differentiation using tgf-β (15ng/ml) in cardiac fibroblast isolated from WT and 12/15LOX−/− mice. Differentiation of fibroblast to myofibroblast phenotype was characterized by expression of smooth muscle actin (SMA) (Rohr, 2009). Control WT and 12/15LOX−/− cardiac fibroblast displayed showed lower expression of SMA. 12/15-LOX−/− cardiac fibroblast showed limited tgf-β induced trans-differentiation of myofibroblast indicated by decrease in SMA expression Treatment with 12(S)-HETE induced SMA expression in both WT- and 12/15LOX−/− -cardiac fibroblast. However, 12/15LOX−/− cardiac fibroblast displayed lower level of SMA compared with WT. Combined treatment of tgf-β and 12(S)-HETE in cardiac fibroblast limited SMA expression in both WT and 12/15LOX−/− mice. Interestingly, overall level of SMA was lower is 12/15LOX−/− cardiac fibroblast (Figure 3D). Thus, in-vitro data using naïve fibroblast confirms that 12/15LOX deletion limits fibroblast to myofibroblast transition that supports our in-vivo findings in cardiac remodeling (Kain et al., 2018).
Figure 3: 12(S)-HETE promotes early induction of COX-2 and 5LOX in cardiac fibroblast and trans-differentiation of fibroblast to myofibroblast.
Cardiac fibroblast treated with 12(S)-HETE (100nM) for 4, 8, 12 and 24 h. A Immunoblot representing COX-2 and 5LOX expression in WT and 12/15LOX−/− cardiac fibroblast treated with in 12(S)-HETE. B Densitometric analysis of COX-2 levels. C Densitometric analysis of 5LOX levels. *p<0.05 vs untreated cells, $p<0.05 vs. treated WT cells. Results are presented as mean ± SEM. Data are representative of a n=3 independent experiments. D Representative immunofluorescence images representing α-SMA expression (Red) and Hoechst (blue) in cardiac fibroblast isolated from WT and 12/15LOX−/− with and without treatment of Tgf-β (15ng/ml) and 12(S)-HETE (100nM) for 18 h. Data are representative of n=3 independent experiments.
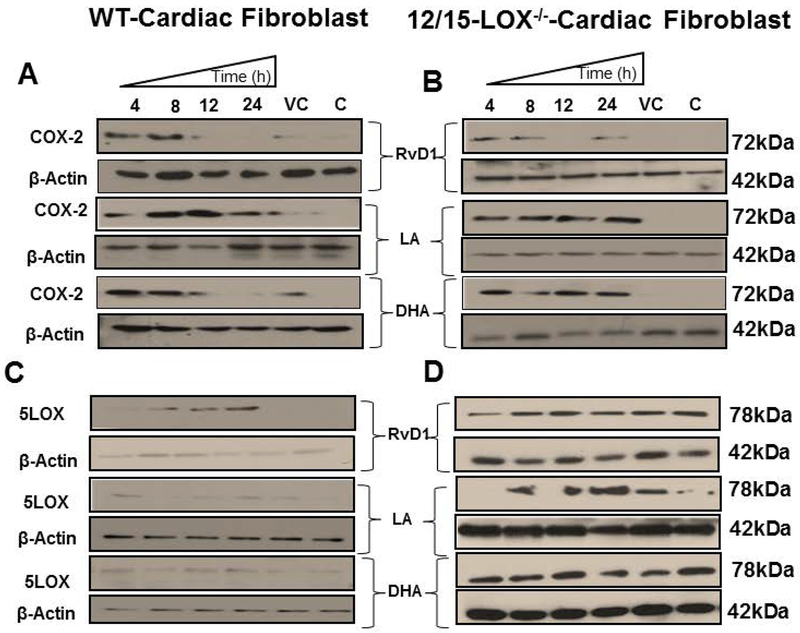
RvD1 but not DHA diminished expression of COX-2 in 12/15LOX−/− cardiac fibroblast
Inflammation and fibrosis are consecutive and inter-related conditions with many overlapping mechanisms. Cardiac fibroblasts activation is an essential step in maintaining the extracellular matrix in the normal heart and their coordination with macrophages is essential in response to injury to reduce inappropriate fibrotic response (Rog-Zielinska et al., 2016). Thus, we further elucidated how substrates; LA and DHA or respective products; 12(S)-HETE and RvD1 impacts COX-2 expression in cardiac fibroblast. We treated cardiac fibroblast with RvD1, LA or DHA in a time-dependent manner for 4, 8, 12 and 24 h. RvD1 treated WT and 12/15LOX−/− cardiac fibroblast displayed an early induction of COX-2, which diminished at 12 h and 24 h of time point (Figures 4A and Figures 4B, upper panel). However, the COX-2 was re-induced in 12/15LOX−/− cardiac fibroblast at 24 h (Figure 4B, upper right panel). LA treated cardiac fibroblast displayed stable COX-2 expression in WT and 12/15LOX−/− cardiac fibroblast from 4 h to 24 h (Figure 4A and Figure 4B, middle panel). DHA treated cardiac fibroblast also showed early induction of COX-2 in both WT and 12/15LOX−/−, but was diminished at 24 h in WT cardiac fibroblast. The COX-2 was continuously expressed in 12/15LOX−/−cardiac fibroblast (Figures 4A and Figures 4B, lower panel). The results displayed that RvD1 modulated the COX-2 expression in time-dependent manner, indicating diversified feed-forward loop interactions in the microenvironment.
Figure 4: Prolonged exposure of RvD1 limited COX-2 and 5LOX expression in cardiac fibroblast compared with DHA and LA.
Immunoblot representing A COX-2 expression in WT-cardiac fibroblast B COX-2 expression in 12/15LOX−/−-cardiac fibroblast C 5LOX expression in WT-cardiac fibroblast D 5LOX expression in 12/15LOX−/−-cardiac fibroblast treated with in RvD1(10ng/ml), LA(200μM), and DHA (50μM) at 4 h, 8 h,12 h and 24 h. Data are representative of a n=3 independent experiments.
RvD1 but not DHA induced expression of 5LOX in cardiac fibroblast
To investigate, whether RvD1 and DHA can induce 5LOX expression in cardiac fibroblast in similar manner to the peritoneal macrophages, we treated cardiac fibroblast with RvD1, LA or DHA in a time-dependent manner. 5LOX was induced in RvD1 treated fibroblast, in a time-dependent manner and reached to its peak expression at 24 h in WT (Figure 4C, upper left panel). Both LA and DHA failed to induce 5LOX in WT-CF (Figure 4C, middle and lower left panel). 5LOX was basally expressed in 12/15LOX−/− cardiac fibroblast compared with WT cardiac fibroblast. The 5LOX expression was not impacted in 12/15LOX−/− cardiac fibroblast treated with RvD1, LA, or DHA (Figure 4D). Overall data indicated RvD1 influenced 5LOX in presence of 12/15LOX enzyme.
RvD1 stimulates expression of proresolving Arg-1 in combination with 12(S)-HETE in macrophages
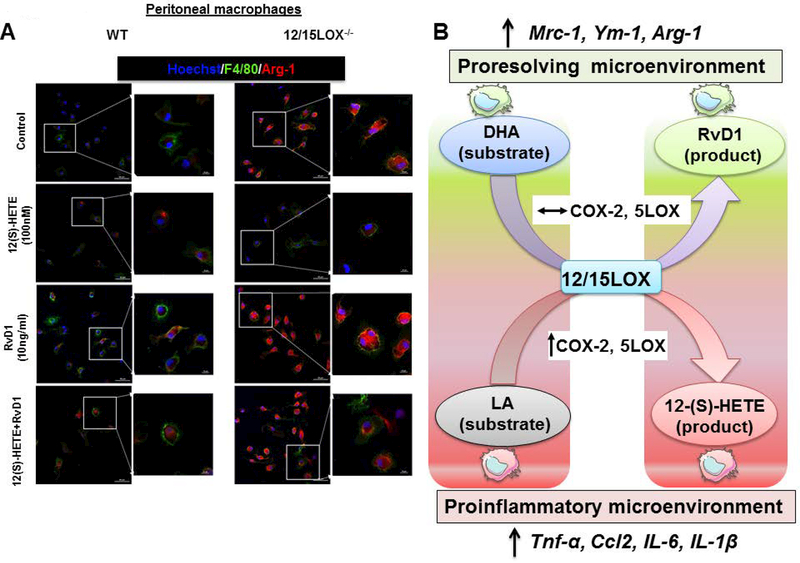
Despite the presence of detectable IL-6, TNF-α, IL-1β or Ccl2 for 4 h treatment of RvD1 in peritoneal macrophages either in presence or absence of 12/15LOX, we noted that the Arg-1 mRNA was higher in macrophages treated with RvD1. Therefore, we next determined how the combination of 12(S)-HETE with RvD1 will impact Arg-1 expression. To evaluate former, naïve peritoneal macrophages were isolated from WT and 12/15LOX−/− mice and were treated independently with RvD1, 12(S)-HETE and combination of RvD1, 12(S)-HETE. The change in expression of Arg-1 (red) along with F4/80 (green) was monitored over time by immunofluorescence. Untreated 12/15LOX−/− macrophages displayed higher basal expression of Arg-1 compared to WT control macrophages (Figure 5A, first panel). In contrast 12(S)-HETE treated WT macrophages, did not displayed any change in Arg-1 expression. There was a decrease in Arg-1 expression in 12/15LOX−/− macrophages treated with 12(S)-HETE compared to 12/15LOX−/− untreated macrophages (Figure 5A, second panel). Interestingly, RvD1 treatments lead to increase in expression of Arg-1 as well as of F4/80 in both WT and 12/15LOX−/− macrophages (Figure 5A, third panel). Combination of 12(S)-HETE and RvD1 consistently expressed Arg-1 among both WT and 12/15LOX−/− macrophages in vitro (Figure 5A, fourth panel). Together, our data demonstrate that RvD1 stimulates Arg-1 expression independent of 12/15LOX leading to alternative activation of primary macrophages.
Figure 5: RvD1 stimulates Arg-1 expression in peritoneal macrophages independent of 12/15LOX-/.
A -Representative immunofluorescence images representing Arg-1 (red), F4/80 (green) expression and Hoechst (blue) in peritoneal macrophages isolated from WT and 12/15LOX−/− mice treated with 12(S)-HETE (100nM), RvD1 (10ng/ml) and combination of 12(S)-HETE and RvD1. Data are representative of n=2 independent experiments. B Figure illustrating the enzymatic interaction of fatty acids and respective products determine the macrophage phenotype and diversified inflammation-resolution milieu.
Discussion
Non-resolving and overactive inflammation is the major driver of cardiac disease pathology and impaired leukocyte directed cellular and molecular healing (Halade et al., 2018; Kain et al., 2018 ; Kain et al., 2014; Lopez et al., 2015). Further, cellular membrane integrity with an optimum balance of fatty acids is important for many physiological and pathological events (Mills et al., 2005). Both n-6 (LA) and n-3 fatty acids (DHA) act as metabolic substrates for fat busting lipoxygenases enzymes which results in the production of series of bioactive lipid mediators (Calder, 2010). LOX-derived bioactive mediators and eicosanoids have a diverse role in modulating cellular function and phenotypes depending on the fatty acids substrate and microenvironment (Buckley et al., 2014). The present study evaluated the impact of the substrate such as DHA or LA and their metabolic product such as RvD1 or 12(S)-HETE, on macrophage plasticity and fibroblast phenotype in presence and absence of enzyme 12/15LOX that are critical in immune responsive process. The major outcomes are; 1) n-3 product RvD1 is more potent than its substrate DHA in limiting proinflammatory macrophages phentopye and promoting pro-resolving macrophages phenotype in the absence of 12/15LOX; 2) RvD1 but not DHA stabilized the expression of 5LOX in both macrophages and cardiac fibroblast, and 4) 12(S)-HETE leads to early induction of COX-2 and 5LOX and led to myofibroblast transdifferentian in cardiac fibroblast in the absence of 12/15LOX (Figure 5B).
Macrophages are essential effector cells which are involved in wound healing and tissue regeneration. Macrophages are major producer of TGF-β, considered the most significant pro-fibrotic agent involved fibrotic response. The macrophages play a complex role in fibrosis via interacting with fibroblast by producing chemokines and cytokines (Van Linthout et al., 2014). Further, incessant production of lipid mediator or cytokines from macrophages creates resolving or non-resolving chronic inflammatory milieu leading to disease pathology (Halade et al., 2018; Kain et al., 2018; Kain et al., 2014). Several inhibitors are synthesized to inhibit the inflammatory cytokines to treat several non-resolving inflammatory disease pathology or chronic inflammation (Chung et al., 2003) (Listing et al., 2008). Both RvD1 and DHA are known to inhibit the expression of inflammatory cytokines in macrophages and in many animal models (Liu et al., 2012) (Kain et al., 2015) (Ali et al., 2016). Fatty acid busting 12/15LOX enzyme utilizes DHA as a substrate and metabolizes into the several bioactive products. These bioactive products serve in important anti-inflammatory and pro-resolving effects, including airway and glomerular inflammation as well as atherosclerosis (Zivkovic et al., 2011) (Nordgren et al., 2014) (Chang and Deckelbaum, 2013). Exposure to DHA inhibits the production of proinflammatory cytokines and chemokines such as IL-6, Tnf-α, Ccl-2, and IL-1β in macrophages. The genetic deletion of 12/15LOX reduces the long-term potency of DHA to inhibit proinflammatory cytokines and chemokines in macrophages. However, the RvD1 inhibited proinflammatory cytokines in presence well as absence of 12/15LOX indicating that the RvD1 is more active than DHA in modulating macrophage phenotype towards proresolving phenotype but 12(S)-HETE displayed early proinflammatory trend, in both the presence and absence of 12/15LOX. Fatty acids substrate and respective metabolites have diverse cellular targets in the healing response, including leukocytes, endothelial cells, fibroblasts, platelets, and smooth muscle cells (Serhan et al., 2008a).
Homeostatic level, LA or DHA are the substrates of membrane integrity, and LOX-interaction modulates cytokine production in cells and tissues indicating their direct role in creating the microenvironment (Nathan and Sporn, 1991). Substrate-enzyme interaction defines the microenvironment post-injury having both contextual positive and negative impacts and depends on where and when the bioactive mediators are produced in the biological system (Dalli and Serhan, 2016). Thus, cytokines are diversified depending upon the chemokine signal they programs cells constitutively, or incidentally. Incidental programming includes inflammatory response, healing, and repair. Similar to cytokines, lipid mediators or classical eicosanoids are the class of chemical molecules which are similar in function to cytokines and widely as “chemokines in context.” The immune responsive bioactive molecules like RvD1 belongs to similar category possessing pro-resolving actions different than anti-inflammatory and are considered to work as endogenous “decelerating signals” for inflammation by polarizing macrophages toward the pro-resolving phenotype (M2) (Kain et al., 2015) (Kain et al., 2014) (Kang and Lee, 2016). In the current study, we evaluated the impact of substrate DHA and bioactive RvD1, LA and 12(S)-HETE in determining macrophages phenotype. Both DHA and RvD1 caused a significant increase in expression of Arg-1, Ym-1, and Mrc-1 indicating pro-resolving macrophage phenotypic profile. The treatment of 12(S)-HETE did not express any pro-resolving phenotype in macrophages, indicating that 12(S)-HETE is more efficient in advancing towards proinflammatory milieu. In the absence of 12/15LOX, DHA lose properties to polarize macrophages towards pro-resolving phenotype; however, it did not impact RvD1 potential. Taken together, our data demonstrate that the pro-resolving bioactive RvD1 modulates macrophage and fibroblast culture milieu towards resolving phenotype in the absence of 12/15LOX enzyme. In contrast, based on provided in-vitro results, the pro-resolving properties of substrate DHA are more dependent on the availability of enzyme 12/15LOX.
Eicosanoids are generated by three separate enzyme families, lipoxygenases (LOX), cyclooxygenases (COX) and cytochrome P450 (CYP), which catalyze lipid peroxidation in a stereo- and regio-specific manner (Hammond and O’Donnell, 2012). Among them, lipid busting enzymes 5LOX and COX-2 are the potent biocatalyst for the generation of lipid mediators leukotrienes and prostaglandins respectively (Poeckel and Funk, 2010) (Ricciotti and FitzGerald, 2011). Fatty acid (n-6)-derived leukotrienes and prostaglandins are essential to initiate a physiological acute inflammatory response which successfully resolve and repair tissue damage. Both DHA and RvD1 were able to induce COX-2 and 5LOX expression in macrophages and fibroblast. In the absence of 12/15LOX, RvD1 reduced the COX-2 expression in cardiac fibroblast in time-dependent manner, but DHA had limited potential. Similarly, 12(S)-HETE displayed the early induction of COX-2 and 5LOX in the absence of 12/15LOX which remained persistent at later time points. Our results showed that RvD1 potentially modulate both 5LOX and COX-2 expression depending on the cell type while DHA had minimal ability to do so, indicating the contextual nature of substrate-COX/LOX interaction.
The anti-inflammatory and pro-resolving actions of substrate DHA and RvD1 are well demonstrated in in-vitro, ex-vivo and in-vivo for different disease pathology (Ali et al., 2016; Chen et al., 2005; Halade et al., 2010; Kain et al., 2015; Saito et al., 2015; Vasconcelos et al., 2015). Our study highlighted the proresolving properties of the bioactive metabolic product RvD1 are different from its substrate molecule DHA in modulating the macrophages phenotype. Further, our study also provides an important insight that like cytokines, RvD1 is immuno-resolvent and acts contextually. RvD1 possess potent immunomodulating properties and is a potential candidate for therapeutics in inflammatory diseases.
Supplementary Material
Immunofluorescence image showing TNF-α expression (green) merge with Hoechst (blue) in DHA (50μM) and RVD1(10ng/ml) treated peritoneal macrophages for 24 h. Data is representative of n=2 independent experiments.
Acknowledgement
Authors acknowledge the support from the National Institutes of Health [AT006704], [HL132989] and Pittman Scholar Award to G.V.H., American Heart Association postdoctoral fellowship [POST31000008] to V. K.
List of abbreviations
- 12/15LOX
12/15lipoxygenase
- 5LOX
5lipoxygenase
- AA
arachidonic acid
- COX-2
cyclooxygenase-2
- DHA
docosahexaenoic acid
- LA
linoleic acid
- RvD1
resolvin D1
- 12(S)-HETE
hydroperoxyeicosatetraenoic acid
Footnotes
Conflict of interest
There is nothing to disclose as a conflict of interest for this manuscript.
References
- Ali M, Heyob K, Rogers LK. 2016. DHA Suppresses Primary Macrophage Inflammatory Responses via Notch 1/ Jagged 1 Signaling. Sci Rep 6:22276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BM, Ma DW. 2009. Are all n-3 polyunsaturated fatty acids created equal? Lipids Health Dis 8:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronte V, Pittet MJ. 2013. The spleen in local and systemic regulation of immunity. Immunity 39(5):806–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley CD, Gilroy DW, Serhan CN. 2014. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity 40(3):315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder PC. 2010. Omega-3 fatty acids and inflammatory processes. Nutrients 2(3):355–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder PC. 2017. Omega-3 fatty acids and inflammatory processes: from molecules to man. Biochem Soc Trans 45(5):1105–1115. [DOI] [PubMed] [Google Scholar]
- Chang CL, Deckelbaum RJ. 2013. Omega-3 fatty acids: mechanisms underlying ‘protective effects’ in atherosclerosis. Curr Opin Lipidol 24(4):345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Esselman WJ, Jump DB, Busik JV. 2005. Anti-inflammatory effect of docosahexaenoic acid on cytokine-induced adhesion molecule expression in human retinal vascular endothelial cells. Invest Ophthalmol Vis Sci 46(11):4342–4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT, Anti TNFTACHFI. 2003. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation 107(25):3133–3140. [DOI] [PubMed] [Google Scholar]
- Dalli J, Serhan C. 2016. Macrophage Proresolving Mediators-the When and Where. Microbiol Spectr 4(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhelu MA. 1983. The role of macrophages in immunology. J Natl Med Assoc 75(3):314–317. [PMC free article] [PubMed] [Google Scholar]
- Fujiwara N, Kobayashi K. 2005. Macrophages in inflammation. Curr Drug Targets Inflamm Allergy 4(3):281–286. [DOI] [PubMed] [Google Scholar]
- Halade GV, Kain V. 2017. Obesity and Cardiometabolic Defects in Heart Failure Pathology. Compr Physiol 7(4):1463–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halade GV, Kain V, Black LM, Prabhu SD, Ingle KA. 2016. Aging dysregulates D- and E-series resolvins to modulate cardiosplenic and cardiorenal network following myocardial infarction. Aging (Albany NY) 8(11):2611–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halade GV, Kain V, Ingle KA, Prabhu SD. 2017. Interaction of 12/15-lipoxygenase with fatty acids alters the leukocyte kinetics leading to improved postmyocardial infarction healing. Am J Physiol Heart Circ Physiol 313(1):H89–H102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halade GV, Norris PC, Kain V, Serhan CN, Ingle KA. 2018. Splenic leukocytes define the resolution of inflammation in heart failure. Science Signaling 11(520). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halade GV, Rahman MM, Bhattacharya A, Barnes JL, Chandrasekar B, Fernandes G. 2010. Docosahexaenoic acid-enriched fish oil attenuates kidney disease and prolongs median and maximal life span of autoimmune lupus-prone mice. J Immunol 184(9):5280–5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond VJ, O’Donnell VB. 2012. Esterified eicosanoids: Generation, characterization and function. Biochimica et Biophysica Acta (BBA) - Biomembranes 1818(10):2403–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao HM, Sapinoro RE, Thatcher TH, Croasdell A, Levy EP, Fulton RA, Olsen KC, Pollock SJ, Serhan CN, Phipps RP, Sime PJ. 2013. A novel anti-inflammatory and pro-resolving role for resolvin D1 in acute cigarette smoke-induced lung inflammation. PLoS One 8(3):e58258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordana M, Sarnstrand B, Sime PJ, Ramis I. 1994. Immune-inflammatory functions of fibroblasts. Eur Respir J 7(12):2212–2222. [DOI] [PubMed] [Google Scholar]
- Kain V, Ingle KA, Colas RA, Dalli J, Prabhu SD, Serhan CN, Joshi M, Halade GV. 2015. Resolvin D1 activates the inflammation resolving response at splenic and ventricular site following myocardial infarction leading to improved ventricular function. J Mol Cell Cardiol 84:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kain V, Ingle KA, Kabarowski J, Barnes S, Limdi NA, Prabhu SD, Halade GV. 2018. Genetic deletion of 12/15 lipoxygenase promotes effective resolution of inflammation following myocardial infarction. Journal of Molecular and Cellular Cardiology 118:70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kain V, Ingle KA, Kachman M, Baum H, Shanmugam G, Rajasekaran NS, Young ME, Halade GV. 2017. Excess Omega-6 Fatty Acids Influx in Aging Drives Metabolic Dysregulation, Electrocardiographic Alterations and Low-grade Chronic Inflammation. Am J Physiol Heart Circ Physiol:ajpheart002972017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kain V, Prabhu SD, Halade GV. 2014. Inflammation revisited: inflammation versus resolution of inflammation following myocardial infarction. Basic Res Cardiol 109(6):444. [DOI] [PubMed] [Google Scholar]
- Kang JW, Lee SM. 2016. Resolvin D1 protects the liver from ischemia/reperfusion injury by enhancing M2 macrophage polarization and efferocytosis. Biochim Biophys Acta 1861(9 Pt A):1025–1035. [DOI] [PubMed] [Google Scholar]
- Lee H- N, Kundu JK, Cha Y- N, Surh Y- J. 2013. Resolvin D1 stimulates efferocytosis through p50/p50-mediated suppression of tumor necrosis factor-α expression. Journal of Cell Science 126(17):4037–4047. [DOI] [PubMed] [Google Scholar]
- Lee SH, Blair IA. 2009. Targeted chiral lipidomics analysis of bioactive eicosanoid lipids in cellular systems. BMB Rep 42(7):401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listing J, Strangfeld A, Kekow J, Schneider M, Kapelle A, Wassenberg S, Zink A. 2008. Does tumor necrosis factor alpha inhibition promote or prevent heart failure in patients with rheumatoid arthritis? Arthritis Rheum 58(3):667–677. [DOI] [PubMed] [Google Scholar]
- Liu G, Fiala M, Mizwicki MT, Sayre J, Magpantay L, Siani A, Mahanian M, Chattopadhyay M, La Cava A, Wiedau-Pazos M. 2012. Neuronal phagocytosis by inflammatory macrophages in ALS spinal cord: inhibition of inflammation by resolvin D1. Am J Neurodegener Dis 1(1):60–74. [PMC free article] [PubMed] [Google Scholar]
- Lopez EF, Kabarowski JH, Ingle KA, Kain V, Barnes S, Crossman DK, Lindsey ML, Halade GV. 2015. Obesity superimposed on aging magnifies inflammation and delays the resolving response after myocardial infarction. Am J Physiol Heart Circ Physiol 308(4):H269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Halade GV, Zhang J, Ramirez TA, Levin D, Voorhees A, Jin YF, Han HC, Manicone AM, Lindsey ML. 2013. Matrix metalloproteinase-28 deletion exacerbates cardiac dysfunction and rupture after myocardial infarction in mice by inhibiting M2 macrophage activation. Circ Res 112(4):675–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills SC, Windsor AC, Knight SC. 2005. The potential interactions between polyunsaturated fatty acids and colonic inflammatory processes. Clin Exp Immunol 142(2):216–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C, Sporn M. 1991. Cytokines in context. J Cell Biol 113(5):981–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordgren TM, Friemel TD, Heires AJ, Poole JA, Wyatt TA, Romberger DJ. 2014. The omega-3 fatty acid docosahexaenoic acid attenuates organic dust-induced airway inflammation. Nutrients 6(12):5434–5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson E, Wall R, Fitzgerald GF, Ross RP, Stanton C. 2012. Health implications of high dietary omega-6 polyunsaturated Fatty acids. J Nutr Metab 2012:539426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeckel D, Funk CD. 2010. The 5-lipoxygenase/leukotriene pathway in preclinical models of cardiovascular disease. Cardiovascular Research 86(2):243–253. [DOI] [PubMed] [Google Scholar]
- Ricciotti E, FitzGerald GA. 2011. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol 31(5):986–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rog-Zielinska EA, Norris RA, Kohl P, Markwald R. 2016. The Living Scar--Cardiac Fibroblasts and the Injured Heart. Trends Mol Med 22(2):99–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr S 2009. Myofibroblasts in diseased hearts: new players in cardiac arrhythmias? Heart Rhythm 6(6):848–856. [DOI] [PubMed] [Google Scholar]
- Saito T, Hasegawa-Moriyama M, Kurimoto T, Yamada T, Inada E, Kanmura Y. 2015. Resolution of Inflammation by Resolvin D1 Is Essential for Peroxisome Proliferator-activated Receptor-gamma-mediated Analgesia during Postincisional Pain Development in Type 2 Diabetes. Anesthesiology 123(6):1420–1434. [DOI] [PubMed] [Google Scholar]
- Schneider C, Pratt DA, Porter NA, Brash AR. 2007. Control of oxygenation in lipoxygenase and cyclooxygenase catalysis. Chem Biol 14(5):473–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott WA, Zrike JM, Hamill AL, Kempe J, Cohn ZA. 1980. Regulation of arachidonic acid metabolites in macrophages. J Exp Med 152(2):324–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Chiang N, Van Dyke TE. 2008a. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol 8(5):349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Yacoubian S, Yang R. 2008b. Anti-inflammatory and proresolving lipid mediators. Annu Rev Pathol 3:279–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simopoulos AP. 2000. Human requirement for N-3 polyunsaturated fatty acids. Poult Sci 79(7):961–970. [DOI] [PubMed] [Google Scholar]
- Simopoulos AP. 2001. n-3 fatty acids and human health: defining strategies for public policy. Lipids 36 Suppl:S83–89. [DOI] [PubMed] [Google Scholar]
- Sun L, Xu YW, Han J, Liang H, Wang N, Cheng Y. 2015. 12/15-Lipoxygenase metabolites of arachidonic acid activate PPARgamma: a possible neuroprotective effect in ischemic brain. J Lipid Res 56(3):502–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Linthout S, Miteva K, Tschöpe C. 2014. Crosstalk between fibroblasts and inflammatory cells. Cardiovascular Research 102(2):258–269. [DOI] [PubMed] [Google Scholar]
- Vasconcelos DP, Costa M, Amaral IF, Barbosa MA, Aguas AP, Barbosa JN. 2015. Modulation of the inflammatory response to chitosan through M2 macrophage polarization using pro-resolution mediators. Biomaterials 37:116–123. [DOI] [PubMed] [Google Scholar]
- Yates CM, Tull SP, Madden J, Calder PC, Grimble RF, Nash GB, Rainger GE. 2011. Docosahexaenoic acid inhibits the adhesion of flowing neutrophils to cytokine stimulated human umbilical vein endothelial cells. J Nutr 141(7):1331–1334. [DOI] [PubMed] [Google Scholar]
- Zivkovic AM, Telis N, German JB, Hammock BD. 2011. Dietary omega-3 fatty acids aid in the modulation of inflammation and metabolic health. Calif Agric (Berkeley) 65(3):106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Immunofluorescence image showing TNF-α expression (green) merge with Hoechst (blue) in DHA (50μM) and RVD1(10ng/ml) treated peritoneal macrophages for 24 h. Data is representative of n=2 independent experiments.