Abstract
Resveratrol was first isolated in 1939 by Takaoka from Veratrum grandiflorum O. Loes. Following this discovery, sporadic descriptive reports appeared in the literature. However, spurred by our seminal paper published nearly 60 years later, resveratrol became a household word and the subject of extensive investigation. Now, in addition to appearing in over 20,000 research papers, resveratrol has inspired monographs, conferences, symposia, patents, chemical derivatives, etc. In addition, dietary supplements are marketed under various tradenames. Once resveratrol was brought to the limelight, early research tended to focus on pharmacological activities related to the cardiovascular system, inflammation, and cancer but, over the years, the horizon greatly expanded. Around 130 human clinical trials have been (or are being) conducted with varying results. This may be due to factors such as disparate doses (ca. 5 to 5,000 mg/day) and variable experimental settings. Further, molecular targets are numerous and a dominant mechanism is elusive or nonexistent. In this context, the compound is overtly promiscuous. Nonetheless, since the safety profile is pristine, and use as a dietary supplement is prevalent, these features are not viewed as detrimental. Given the ongoing history of resveratrol, it is reasonable to advocate for additional development and further clinical investigation. Topical preparations seem especially promising, as do conditions that can respond to anti-inflammatory action and/or direct exposure, such as colon cancer prevention. Although the ultimate fate of resveratrol remains an open question, thus far, the compound has inspired innovative scientific concepts and enhanced public awareness of preventative health care.
Keywords: Mechanism, Discovery, Anti-inflammation, Clinical value, Utilization
INTRODUCTION
Resveratrol (3,4′,5-trihydroxy-trans-stilbene; Fig. 1) was first isolated in 1939 by Takaoka from Veratrum grandiflorum O. Loes (root of the white hellebore) (Takaoka, 1939). It can be speculated the trivial name resveratrol was created as a conjunction based on its chemical structure and the plant source used for isolation: a resorcinol derivative or polyphenol in resin, occurring in Veratrum species, and containing hydroxy groups forming an alcohol. Following the Takaoka report, sporadic papers appeared in the literature, most of which were descriptive in nature (Takaoka, 1939).
Fig. 1.
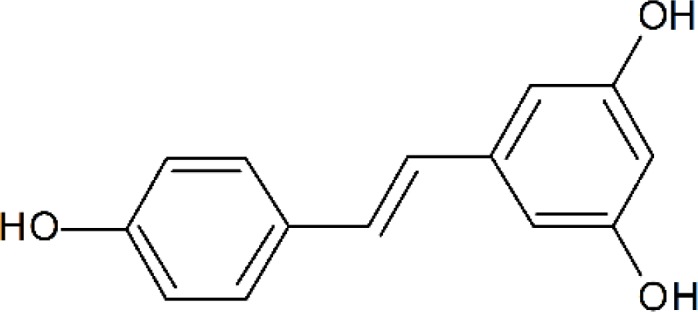
The structure of resveratrol.
As described herein, nearly 60 years later, resveratrol was rediscovered and reported in a seminal paper describing pleiotropic activities related to cancer chemoprevention and other disease states (Jang et al., 1997). Subsequently, the compound has been the subject of intensive investigation. Thousands of manuscripts have appeared in the scientific literature (Fig. 2), monographs have been published (Aggarwal and Shishodia, 2006; Wu and Hsieh, 2018), patents have been issued (Pezzuto et al., 2013), symposia and conferences have been conducted, derivatives and metabolites have been studied (Hoshino et al., 2010), clinical trials have been performed, etc. A search of the word ‘resveratrol’ on Google yields over 7 million results. Numerous commercial products are marketed to the general public with suggestions of life extension as well as a multitude of other health benefits. Nonetheless, at the present time, there is no consensus regarding the usage of resveratrol based on scientific evidence.
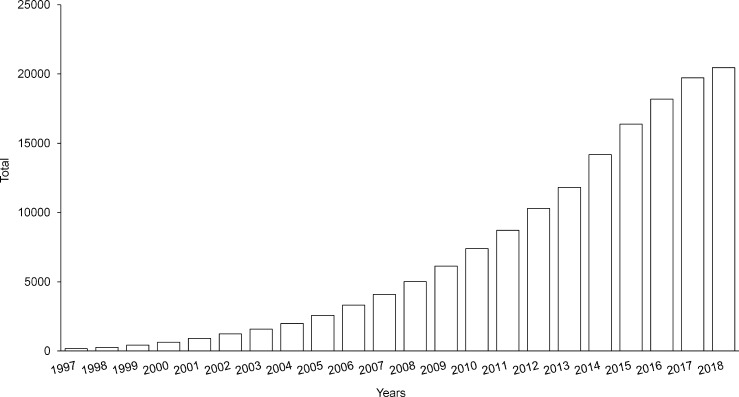
Fig. 2.
Cumulative documents related to resveratrol by year (1997–2018). Using the SciFinder® database, documents were queried using the chemical structure of resveratrol (CAS number 501-36-0). The process yields a total of 20,459 references (accessed September-02-2018).
Interest in resveratrol as a bioactive molecule largely stemmed from the natural occurrence in grapes and grape products, primarily wine, which of course are consumed by humans. Once our report appeared, the sale of grape products containing resveratrol, particularly red wine, significantly increased. In fact, the sale of some grape products containing little or no resveratrol (e.g., grape juice) increased as well. Dietary consumption of resveratrol from natural sources continues to remain of interest. However, most animal, human, and in vitro studies employ concentrations of resveratrol that vastly exceed even the largest quantity that could be rationally ingested by dietary means. For example, daily doses of 5 g of resveratrol have been reported (Fig. 3), whereas a liter of wine contains only low milligram quantities (Guilford and Pezzuto, 2011). Further, based on a perusal of the various dietary supplements available on the open market, nearly all products provide high milligram to gram levels.
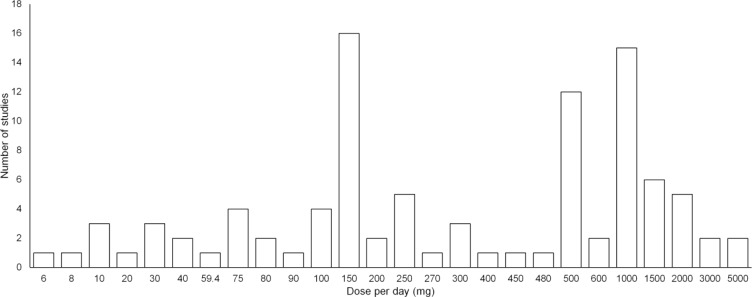
Fig. 3.
Frequency distribution of daily resveratrol dosage administered in human trials. The publications were searched using PubMed (accessed August-28-2018; search term: resveratrol; limit: clinical trials). In total, 79 trials were reported.
The fact that so much literature currently exists concerning resveratrol begs the question of why publish yet another manuscript? Here, the main objective is not to rehash the myriad activities that have already been reported and reviewed. Rather, the objective of this report is to present a perspective of events that have transpired in regard to resveratrol over the past 20 years, and a contemporary view of where things stand concerning the future of resveratrol. In addition, some controversy has been generated with resveratrol, as is often the case with substances receiving such colossal attention, and this will be addressed.
Finally, it has been clear from the outset that resveratrol does not abide by the concept of ‘one drug, one target’. In the case of resveratrol, this dogma simply does not apply. The molecule is incredibly promiscuous, interacting with a host of targets (Pezzuto, 2011). Bearing this in mind, a cursory analysis is presented that depicts a network or web of response that likely exists when resveratrol enters the biological milieu. Many literature reports have focused on single targets or isolated pathways but, realistically, resveratrol has the potential of mediating a response analogous to a biological tsunami.
DISCOVERY THAT SPARKED INTEREST IN RESVERATROL
Natural product cancer chemopreventive agents
Throughout history, natural products have played a dominant role in the treatment of human ailments. The association of salicylates with the willow, and quinine with cinchona, are renowned examples. Similarly, the legendary discovery of penicillin transformed global existence. Traditional remedies, largely based on terrestrial plants, still dominate therapeutic practices throughout the world, and natural products comprise a large portion of current-day pharmaceutical agents, most notably in the areas of antibiotic and cancer therapies.
For the treatment of cancer, early diagnosis and definitive tumor eradication through radiation therapy or surgical resection offer greatest hope. However, when dealing with malignant, metastatic disease, it is generally necessary to resort to chemotherapy. Although the therapeutic indices of cancer chemotherapeutic agents are often poor, many of the most useful agents have resulted from the systematic investigation of nature. Notable examples include taxol, vinblastine, and camptothecin, or derivatives thereof. These structurally unique agents function by novel mechanisms of action; isolation from natural sources is the only plausible method that could have led to their discovery. In addition to terrestrial plants as sources for starting materials, the marine environment (e.g., bathymodiolamides A and B, bryostatin, ecteinascidin 743, kahalalide F, salinosporamide A), microbes (e.g., bleomycin, doxorubicin, staurosporin), and slime molds (e.g., epothilone B) have yielded remarkable cancer chemotherapeutic agents (Cragg and Pezzuto, 2016).
Irrespective of these advances, cancer remains a leading cause of death worldwide. In the United States, for example, cancer is responsible for about one in every four deaths. Given the morbidity and mortality associated with the disease, as well as the significant economic burden, there continues to be a critical need for more effective strategies (Pezzuto, 1997).
Undoubtedly, the prevention of human cancer is highly preferable to treatment. In this sense, the advent of vaccines for the prevention of hepatitis and liver cancer is probably the greatest success, and the more recent development of vaccines for the prevention of cervical cancer offers promise. Cancer chemoprevention, the use of synthetic or natural agents to inhibit, retard, or reverse the process of carcinogenesis, is another important approach for easing this formidable public health burden. In an ideal world, cancer chemoprevention would work as well as vaccines for the prevention of human ailments. Although this has yet to be accomplished, proof-of-principal has been established by seminal clinical trials conducted for the prevention of breast cancer with tamoxifen, and more recently with tamoxifen relatives such as raloxifene, and a separate class of aromatase inhibitors. Agents such as finasteride have shown promise for the prevention of prostate cancer.
Similar to cancer chemotherapeutic agents, natural products play an important role in the field of cancer chemoprevention. Through serendipity or epidemiological observations, dietary phytochemicals such as sulforaphane and phenethyl isothiocyanate (cruciferous vegetables), epigallocatechin-3-gallate (green tea), curcumin (turmeric), sulfur-containing compounds and selenium (the genus Allium), and lycopene (tomatoes) are considered positively for cancer prevention. Some clinical trials have demonstrated promise. Consequently, it is reasonable to search for new natural product cancer chemopreventive agents.
Using the approach of activity-guided fractionation with a battery of in vitro assays, we established a program to monitor the natural product purification process so as to isolate the most active agents in their pure form (Pezzuto et al., 2005). Once purified, the structures of the molecules have been determined using advanced NMR, mass spec and X-ray crystallographic methods. During the course of the project, we discovered active substances from a variety of structural classes such as alkaloids, flavonoids, coumarins, triperpenoids, and withanolides. Some of the compounds have shown promise for clinical trials, such as the rotenoid, deguelin. We also concentrated on the discovery of marine microorganism-based cancer chemopreventive agents. It logically follows that synthetic organic chemistry was an integral component the program, and some semi-synthetic compounds such as 4′-bromoflavone, oxomate (a relative of sulforaphane), and 3-amino-6-(3-aminopropyl)-5,6-dihydro-5,11-dioxo-11H-indeno[1,2-c]isoquinoline have shown promise.
In sum, natural product research is powerful approach for discovering biologically active compounds with unique structures and mechanisms of action. Given the unfathomable diversity of nature, it is reasonable to suggest that chemical leads can be generated that are capable of interacting with most or possibly all therapeutic targets. With the advent of high-throughput screening, a large number of potential starting materials can be readily evaluated, so informed selections can be made for unearthing prototype ligands worthy of further development as therapeutic agents. This is the backdrop leading to the resurrection of resveratrol.
The process of rediscovering and repurposing resveratrol
During the course of the cancer chemopreventive drug discovery project described above, one of the most notable discoveries was the structurally simple stilbene known as resveratrol. Thousands of plant and marine extracts were tested. Some selections were based on literature reports or traditional use; most were randomly selected although factors such as uniqueness (e.g., lack of previous investigation) or endemicity was taken into account. One plant falling into the latter category was acquisition number 46, Cassia quinquangulata Rich. (Leguminosae). Relative to about 1000 extracts tested using an in vitro enzyme assay monitoring oxygen consumption, an extract of this nonedible Peruvian legume was found to mediate appreciable inhibition of cylooxygenase (COX)-1. Inhibitors of cyclooxygenase were of interest for a number of reasons. For example, high levels of COX products (e.g., prostaglandins) can stimulate the growth of tumor cells, COX can bioactivate xenobiotics (e.g., aromatic and heterocyclic amines to carcinogenic products), and COX inhibitors (e.g., nonsteroidal anti-inflammatory drugs; NSAIDs) can reduce the relative risk of colorectal cancer and reduce the frequency and number of premalignant and malignant lesions. The active principle associated with the Cassia extract was found to be resveratrol. The inhibitory activity was decent, compared with indomethacin, the position control NSAID used for the assay.
At this point, from a phytochemical viewpoint, very little enthusiasm was engendered by a well-known simple stilbene that was devoid of structural novelty. Nonetheless, the fact of good inhibitory activity with COX remained, and it was recognized that significance could be heightened due to the presence of resveratrol in edible products, particularly the grape, and of course products derived from grapes, particularly wine. Accordingly, additional tests were performed and, surprisingly, significant activity was observed in every assay that was performed. In that the test panel had been designed to assess the potential of blocking multiple stages of carcinogenesis (i.e., initiation, promotion, progression), and every test showed a positive result, interest was further intensified.
Nevertheless, in spite of promising data obtained with in vitro and ex vivo experiments, it was recognized that some type of animal experiment would be necessary to establish physiological relevance. But, in our case, as is the case with other natural product drug discovery programs, procurement of sufficient material to perform animal work was problematic. Of course at the present time resveratrol is readily available. However, at the time of our discovery, this was not true. Large-scale isolation or synthesis were considered, but given a sense of urgency, and realizing these procedures would be labor and time intensive, other avenues were explored. Thus, it was discovered that Sigma Chemical Company could supply an adequate amount of material, but at a relatively steep price. In spite of the economic burden, a decision was made to move forward and purchase the material. With this in hand, two animal studies were performed: anti-inflammatory activity with the rat paw edema model, and inhibition of mouse skin tumors generated with the two-stage model of carcinogenesis. In both cases, impressive results were obtained, and the studies were found suitable for publication in Science (Jang et al., 1997).
Subsequently, we continued to study mechanistic aspects of resveratrol, including interaction within the arachidonic acid binding site of the enzyme cyclooxygenase through crystallographic analysis, absorption and metabolism of resveratrol, production and testing of a series of resveratrol derivatives capable of demonstrating responses with much greater potency and specificity, cancer chemopreventive activity with additional animal models, etc. Obviously, many others in the scientific community did as well (Fig. 2).
CONTROVERSIAL AND PARADOXICAL SITUATIONS SURROUNDING RESVERATROL
Oral absorption of resveratrol
Shortly after publication of the paper in Science, the work became the subject of a media blitz. The topic was covered worldwide by newspapers, magazines, television and radio. One notable piece appeared on the first page of the New York Times. The article was well-done, but surprising led to controversy. Professor David M. Goldberg from the University of Toronto was quoted as saying “It doesn’t matter how potent a compound is in the test tube. If it doesn’t get into the blood-stream, it won’t have any effect.” In essence, this translates into saying the work might be interesting from an academic point-of-view but basically it is irrelevant. In addition to being shocking, the veracity of this argument was not sound simply based on the structure of resveratrol, plus the animal studies presented in our report. Nonetheless, in order to respond to the criticism in a more definitive manner, a human experiment was conducted post haste. In the study, a human volunteer (the author of this paper), consumed 1 g of resveratrol and collected a 24 h urine sample. Based on LC/MS analysis, resveratrol metabolites were found in the urine sample, unequivocally establishing absorption via the oral route of administration (albeit N=1).
Of course, over the years, many studies have been performed to examine resveratrol absorption, metabolism, excretion, dose-responses, etc. The notion of resveratrol not being absorbed through the alimentary tract following oral administration was simply a ‘red herring.’ Which is not to say the situation is straightforward. For example, achievable serum concentrations are generally many orders of magnitude below the concentrations used in the labyrinth of in vitro studies. Thus, it may be difficult to rationalize the actual relationship of these results with pharmacological relevance since high concentrations of the parent compound are necessary to mediate a response. On the other hand, concentrations of metabolites such as the 3-O-glucuronide may be much higher than the parent compound (Brown et al., 2010), and the mean plasma level of resveratrol itself can be enhanced by processes such as micronization (Carboni, 2013). Also, recent studies have suggested that improvements in resveratrol bioavailability can be realized through combination with other compounds. For example, co-treatment with piperine improved the bioavailability of resveratrol, increased maximum serum concentrations in mice (Johnson et al., 2011a), exerted a synergistic antidepressant-like effect with a mouse model (Huang et al., 2013), and enhanced bioefficacy on cerebral blood flow in human subjects (Wightman et al., 2014). In addition, many other factors come into play, such as enzymatic reconversion of resveratrol metabolites to the parent compound at the site of action (Patel et al., 2013). A definitive therapeutic approach remains moot, but there is no question regarding the absorption of resveratrol following oral consumption.
Estrogenic activity of resveratrol
Another surprising development occurred within a year of publication of our Science paper. A report appeared ostensibly demonstrating that resveratrol was a super estrogenic agonist (Gehm et al., 1997). The technology used was a cell line expressing an estrogen sensitive reporter gene. The authors concluded the results implied resveratrol could mediate beneficial cardiovascular activity in addition to cancer chemopreventive activity. Unfortunately, however, the results were concerning since estrogenic compounds can actual promote some forms of cancer, and there was some implication the structure of resveratrol could bear a resemblance to diethylstilbestrol, a noxious substance known to cause vaginal tumors in girls and women who had been exposed in utero. We thought it best to investigate the matter further.
Using the same reporter cell line, and as similar experimental conditions as conceivable, our group was not able to reproduce the estrogenic response with resveratrol. There is no simple explanation for this discrepancy, but we felt confident with our results (Bhat et al., 2001). Nonetheless, in order to examine the effect in a more physiologically relevant manner, experiments were performed with ovariectomized rats. In this model, studying uterine weight and cell-type distribution, estrogen was found to mediate the anticipated response, but no response was observed with resveratrol (Bhat and Pezzuto, 2002; Pezzuto, 2011). In sum, it does not appear that resveratrol functions as an estrogen.
Nonetheless, as implied by Gehm et al. (1997), there is some evidence that resveratrol can function as a cardiovascular protectant. Resveratrol is reported to protect against atherosclerosis through inhibition of PTEN (Ma et al., 2018); NOX1, MCP, AKT, ERK1/2, FOXO3A (Park et al., 2009); CAV1 (Penumathsa et al., 2008), and ERK1/2 signaling (El-Mowafy et al., 2009), and activation of HO1, eNOS, VEGF (Penumathsa et al., 2008) and pGC/kinase G signaling (El-Mowafy et al., 2009). Its role in the protection against ischemic myocardial injury through inhibition of VEGF, HIF1α (Mukhopadhyay et al., 2012); GSK3β (Goh et al., 2007), and activation of KLF15 (Rogers and Otis, 2017); AKT, p38 (Goh et al., 2007); MAPK signaling (Das et al., 2006) has also been documented. Protection by resveratrol against other cardiovascular conditions through inhibition of p38 MAPK signaling (Mukhopadhyay et al., 2010), and activation of SIRT1, FOXO1, FOXO3A, eNOS (Xia et al., 2013); ERK1/2 (Gracia-Sancho et al., 2010); SIRT1, KLF2, eNOS, TN, CNP, MEK5, MEF2 (Gracia-Sancho et al., 2010); SIRT1, eNOS, PGC1α, NRF1, TFAM, Porin, Complex I-V (Csiszar et al., 2009); NOX1, NOX4 (Schilder et al., 2009); iNOS, VEGF, KDR, and eNOS (Das et al., 2005), has been reported as well.
Antiaging effect of resveratrol
For time immemorial, human beings have been interested in life extension. Stories appear dating from writings in the 5th century BC and became especially prominent in the 16th century when the “Fountain of Youth” was associated with the Spanish explorer Juan Ponce de León. Resveratrol has been and still is touted as an antiaging concoction, based on reports in the scientific literature with mechanistic underpinnings [e.g., inhibition of IL6, TNFα, and activation of β-catenin (Palomera-Avalos et al., 2017); SIRT2 (Pan et al, 2017); YAP1P, TRX2, TRR1, AHP1 (Escote et al., 2012); SIRT1, AMPK, p53, BCL-xL (Cao et al., 2009)], and consequently in the lay literature (Sardi, 2004; Maroon, 2009). The primary driving force for this notion was activation of SIRT1 (Howitz, 2003).
SIRT1 is a nicotinamide adenine dinucleotide (NAD+)-dependent, class III histone deacetylase (HDAC). Studies reporting activation of SIRT1 by resveratrol were touted to promote longevity and well as mimicking caloric restriction. As the story gained momentum, a company by the name of Sirtris Pharmaceuticals was formed with a proprietary formulation of resveratrol as the product (SRT501). Interestingly, this seemed to capture the attention of the pharmaceutical company Glaxo-SmithKline, and led to the purchased Sirtris Pharmaceuticals for the sum $720 million (US) in 2008 (Pollack, 2008).
Remarkably, however, employing NMR, surface plasmon resonance, and isothermal calorimetry, a report appeared definitively illustrating that resveratrol does not lead to activation of SIRT1 with native peptide or full-length protein substrates (Pacholec et al., 2010). Similarly, other compounds previously reported as SIRT1 activators (SIRT1720, SIRT2183 and SIRT1460) were found to be ineffective (Pacholec et al., 2010). Consistent with this, there does not appear to be X-ray diffraction data of a full length of SIRT1 interacting with resveratrol, and some studies have report resveratrol actually inhibits SIRT1 (Buhrmann et al., 2016; Guo et al., 2018).
Thus, in essence, the activation demonstrated in previous reports was an experimental artifact resulting from utilization of SIRT1 with peptide substrate containing a covalently attached fluorophore and not with native peptide or full length protein substrates. Frankly, this seemed intuitively apparent. The response reported to be mediated by resveratrol using an assay based on fluorometric output clearly was not specific. Similar results were observed with a variety of mundane compounds such as flavonoids (Howitz et al., 2003). Should it logically be expected that such a disparate collection of natural products should mediate a response with such ostensibly profound significance? Not likely.
At the time Sirtris Pharmaceuticals was acquired by Glaxo-SmithKline, a phase 2 trial was being conducted in patients with relapsed and or refractory multiple myeloma. The study was terminated noting five patients developed renal failure (Popat et al., 2013). About five years after the $720 million acquisition, in 2013, GlaxoSmithKline closed down Sirtris Pharmaceuticals.
Irrespective of these unusual events, SIRT1 remains one of the most extensively studied targets of resveratrol. It seems that the hype surrounding the direct activation of SIRT1 led to the burst of investment and media frenzy. Therefore, when Pacholec et al. (2010) dampened this notion, it became necessary to explore other avenues that might be credible enough to further propagate the story. Given the promiscuity of resveratrol, and the myriad of legitimate targets the compound affects, it is actually surprising that one of the few targets it does not directly modulate maintains such stealth. Nonetheless, considering the vast interconnected network operating within a living cell, it is obvious that some interrelationship can be construed for essentially any component operating within the milieu. Certainly, SIRT1 should be included somewhere within the tsunami of an intracellular response that can be generated by resveratrol.
One pathway that is obvious to satiate the desire to activate SIRT1 involves activation of AMPK which in turn may activate SIRT1 (Canto et al., 2009; Ruderman et al., 2010). As such, several studies have reported AMPK activation by resveratrol (Cao et al., 2009; Villa-Cuesta et al., 2011; Do et al., 2012; Cho et al., 2014; Tameda et al., 2014; Shrotriya et al., 2015; Wan et al., 2016). As another example, one X-ray crystallographic datum deposited in RCSB PDB (http://www.rcsb.org) (Berman et al., 2000) illustrates a co-crystal structure of SIRT1 in complex with resveratrol and a four-residue acetylated p53 peptide which carries 7-amino-4-methylcoumarin (AMC) as a substrate (Cao et al., 2015). With a resolution of 3.2 Å [R-value (free) of 0.252], these results indicated that resveratrol mediates the interaction between AMC peptide and SIRT1.
In terms of the potential of resveratrol to extend the life of mammals, in a long-term study reported by Miller et al. (2011), resveratrol had no effect on the lifespan of genetically heterogeneous UM-HET3 mice treated from 12 months of age. Presumably, the quest for the “Fountain of Youth” must still continue.
MECANISTIC CONSIDERATIONS REGARDING RESVERATROL
Considering the data presented in our first publication regarding the cancer chemopreventive potential of resveratrol (Jang et al, 1997), it has been clear from the outset that resveratrol does not abide by the concept of ‘one drug, one target’. The molecule is incredibly promiscuous, interacting with a host of targets (Pezzuto, 2011). The action of resveratrol helped to dispel the dogma of specificity being a requirement for having interest in a new drug lead. As evidenced by the large number of manuscripts describing the action of resveratrol (Fig. 2), the characteristic of promiscuity has not held back scientific inquiry. As a result of this large body of work, it is clear the number of molecular targets that are influenced by resveratrol is immense. We have recently cataloged many of the protein targets influenced by resveratrol (Kumar et al., 2018) in the context of neuroprotective effects, cardiovascular protective effects, antidiabetic effects, antiobesity effects, antiaging effects, anticancer activity, and others. Particular emphasis was placed on the rare high affinity target, quinone reductase 2, and sirtuin, since so many reports have appeared regarding the latter.
Many reports in the literature dealing with resveratrol focus on a single target, a group of related targets, or a pathway influenced by an affected target. Given that ‘you find what you seek’, and resveratrol is extraordinarily promiscuous, many individual ‘findings’ have been reported. Naturally, limited data sets are typically presented in any given manuscript, but in a complex biological matrix, other actions are simultaneously mediated, even though the lens of the investigator may not be focused on those responses. In this context, we thought it might be worthwhile to use publicly available drug-target databases to explore a holistic view of targets influenced by resveratrol (Kumar et al., 2018).
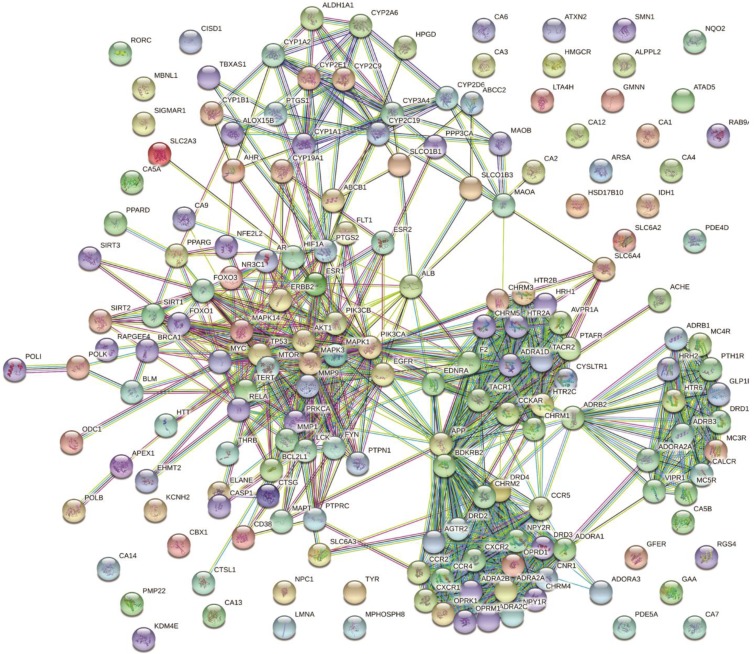
Based on this premise, protein-protein interaction networks found as targets of resveratrol were created. After combing DrugBank, ChEMBL (protein target classes), PubChem, BindingDB and a manual PubMed search (ABCG2_HUMAN, AMY1_HUMAN, ANO1_HUMAN, BACE1_HUMAN, CCR2_ HUMAN, NM3A_HUMAN, DNM3B_HUMAN, G3P_HUMAN, GSTP1_HUMAN, DM1A_HUMAN, TOP2A_HUMAN, VDR_ HUMAN), a resveratrol-target network was constructed using the STRING (Szklarczyk et al., 2017) database (Fig. 4). Based on this analysis, we can infer that resveratrol acts on several pathways, including neuroactive ligand-receptor interaction (5.64e-47), calcium signaling pathway (1.56e-23), pathways in cancer (3.14e-19), nitrogen metabolism (1.29e-18), and serotonergic synapse (1.7e-18), with low false discovery rates (FDR; given in parenthesis). In fact, from other curated/integrated databases, resveratrol showed potent effects on inhibition of amyloid beta, Tau proteins. Therefore, in terms of drug repositioning/repurposing, resveratrol can be a plausible candidate for the treatment of various diseases, with neuronal diseases being especially notable (see below).
Fig. 4.
Protein-protein interactions and plausible target pathways (see text) of resveratrol. Protein-protein interaction networks found as targets of resveratrol were created using STRING. After combing DrugBank, ChEMBL (protein target classes), a resveratrol-target network was constructed using the STRING database.
CLINICAL INVESTRIGATIONS CONDUCTED WITH RESVERATROL
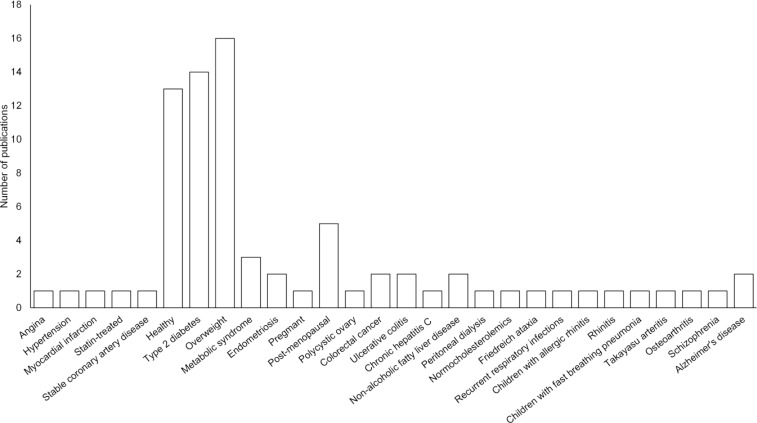
As indicated by a search of PubMed, 79 clinical investigations have been reported with resveratrol (Fig. 5). However, 133 trials are listed at ClinicalTrials.gov (2018a). Most of the studies have been (or are being) conducted in the Europe (55) or the US (50), and others have been (or are being) performed in Canada (8), Mexico (4), the Mideast (4), South America (4), China (3), Southeast Asia (3), Australia (1), and Russia (1). We have recently summarized some of the work performed with animals and human beings (Park and Pezzuto, 2015) as have others (Smoliga et al., 2011). As illustrated in Fig. 5, the majority of clinical trials have been performed with healthy or obese subjects, or individuals with type 2 diabetes.
Fig. 5.
Frequency distribution of resveratrol intervention studies based on the health status of participants. Based on publications that were searched using PubMed (accessed August-28-2018; search term: resveratrol; limit: clinical trials). In total, 79 trials were reported.
Type 2 diabetes (T2DM) is a good example of the dichotomy of results. When administrated for three months at a dose of 250 mg/day, glycemic control and the associated risk factors were improved in patients with T2DM. Resveratrol decreased the mean hemoglobin A1c, systolic blood pressure, low-density lipoprotein cholesterol (LDL-C), total cholesterol, urea nitrogen, and total protein (Bhatt et al., 2012). In another study, wherein T2DM patients were given 1 g/day, resveratrol was reported to decrease systolic blood pressure, fasting blood glucose, hemoglobin A1c, insulin, and insulin resistance. HDL was increased (Movahed et al., 2013). In yet another study, in which patients were give 400 mg two-times per day, resveratrol was reported to have an antioxidant effect. Plasma protein carbonyl content and PBMC O2−· levels were reduced, total antioxidant capacity and total thiol content were increased in plasma, and the expression of Nrf2 and SOD were significantly increased (Seyyedebrahimi et al., 2018). Further, when given at a dose of 50 mg two-times per day, the size of foot ulcers in T2DM patients was reduced (Bashmakov et al., 2014).
One might think results such as those described above might be adequate to recommend the use of resveratrol by T2DM patients. To the contrary, however, as reported by Kjaer et al. (2017) in a study with 66 patients given 1000 or 150 mg of resveratrol per day, treatment did not improve inflammatory status, glucose homeostasis, blood pressure, or hepatic lipid content. In fact, relative to placebo control, the high dose increased total cholesterol, LDL cholesterol, and fructosamine levels. Taking all of this into account, any therapeutic advantage for T2DM patients remains moot.
As noted above, based on a survey of the literature, there appears to be a high probability of resveratrol being of some value for neuronal diseases (FDR, 5.64e-47). To a large extent, this is based on animal models investigating conditions such as neuropathic pain [thermal hyperalgesia (Sharma et al., 2006, 2007; Kumar et al., 2007), cold allodynia (Sharma et al., 2006)], sensory neuropathy [e.g., thermal hypoalgesia with an increase in intraepidermal nerve fiber loss and the mean axonal diameter of myelinated axons of the tibial nerve (Chowdhury et al., 2012], cerebral infarction upon I/R exposure (Prabhakar, 2013), neurodegeneration (Jing et al., 2013), the reduction in motor nerve conduction velocity (Kumar et al., 2007), nerve blood flow (Kumar et al., 2007), DNA damage and apoptosis in sciatic nerve sections (Kumar et al., 2007), memory impairment (Schmatz et al., 2009), anxiety (Damián et al., 2014), and neuroinflammation (astrocytic activation) (Jing et al., 2013). Further studies have been conducted to study effects of resveratrol on depression, epilepsy/seizure, Alzheimer’s disease, Huntington’s disease, Parkinson’s disease, memory function, neuronal damage, etc. (Park and Pezzuto, 2015).
In clinical trials with post-menapausal women, when given a dose of 75 mg/day, it has been suggested that resveratrol can improve brain function by increasing cerebrovascular responsiveness to both hypercapnic and cognitive stimuli, and improve performance of cognitive tasks (verbal memory) and overall cognitive performance (Evans et al., 2017). In another study, with healthy subjects given 250 or 500 mg/day, cerebral blood flow was increased (Kennedy et al., 2010). There is suggestive evidence that evaluating the potential utility of resveratrol in the treatment or prevention of Alzheimer’s disease would be rational (Sawda et al., 2017), and the same likely applies for anther neuronal disorders (Krishnan and Nestler, 2011; Hurley et al., 2014).
Overall, however, at that present time, the consensus of expert opinion does not yet support the use of resveratrol for the treatment or prevention of any human ailment (Vang et al., 2011).
IMMEDIATE PROSPECTS FOR THE CLINICAL USEFULNESS OF RESVERATROL
When considering the potential clinical usefulness of resveratrol, it seems rational to take into account actual blood or tissue levels that can realistically be achieved. In the case of normal dietary consumption, resveratrol intake is frankly minuscule. Thus, most studies involve supplementation, generally through oral administration. However, even following the oral administration of relatively large quantities of resveratrol, average serum concentrations remain in the low to mid nM range. On the other hand, appreciable average plasma concentrations (e.g., 5 μM) of metabolites (resveratrol-3-O-sulfate, resveratrol-4′-O-glucuronide, and resveratrol-3-O-glucuronide) can be readily attained, with resveratrol-3-O-sulfate being dominant (Brown et al., 2010). These metabolites may mediate biological responses similar to the parent molecule (Hoshino et al., 2010) or, alternatively, it is feasible that the metabolites are reconverted to the parent molecule at a target site (Patel et al., 2013). Further, resveratrol and/or resveratrol metabolites may demonstrate a hormetic response (Calabrese et al., 2010; Juhasz et al., 2010).
Irrespective of these considerations, it seems reasonable that high affinity targets warrant greater attention, and a few have been identified (Kumar et al., 2018). Interesting, one of these targets, cyclooxygenase, was the key target described in our seminal report (Jang et al., 1997). More importantly, in the same report, using relatively low doses of resveratrol, we demonstrated anti-inflammatory activity using a rat paw edema model. Inhibition of yet another high affinity target, QR2, may also contribute to an anti-inflammatory effect. Thus, the implications of anti-inflammatory activity are perhaps more profound that commonly recognized. Although other targets such as SIRT have garnered great publicity, even though the response remains a polemic, it appears that anti-inflammation is the common denominator responsible for amelioration of a host of disease states and other beneficial effects. Considering the plethora of disease states that have been touted to be ameliorated by resveratrol, the inflammation theory of disease (Hunter, 2012) may be viewed as a unifying hypothesis for explaining the action of resveratrol.
Taking anti-inflammatory activity into account, as well as the pragmatic issue of having effective concentrations reach the target tissue, the use of resveratrol for the prevention of colon cancer is especially noteworthy. Particularly, positive responses have been reported in many animal studies (Park and Pezzuto, 2015). Moreover, in clinical studies performed by Patel et al. (2010), oral administration of resveratrol produced levels in the human gastrointestinal tract sufficient to elicit anti-carcinogenic effects, and it was concluded that resveratrol merits further clinical evaluation as a potential colorectal cancer chemopreventive agent. Four human trials for the treatment or prevention of colorectal cancer are listed at ClinicalTrials.gov (2018b).
Further, again considering the anti-inflammatory effect of resveratrol, it seems apparent a superlative use of the compound should be the prevention of skin cancer. In our first report describing the cancer chemopreventive potential of resveratrol (Jang et al., 1997), a highly efficacious inhibitory response was observed using the two-stage mouse skin model of carcinogenesis [treatment with 7,12-dimethylbenz[a]anthracene (DMBA) and 12-O-tetradecanoylphorbol-13-acetate (TPA)].
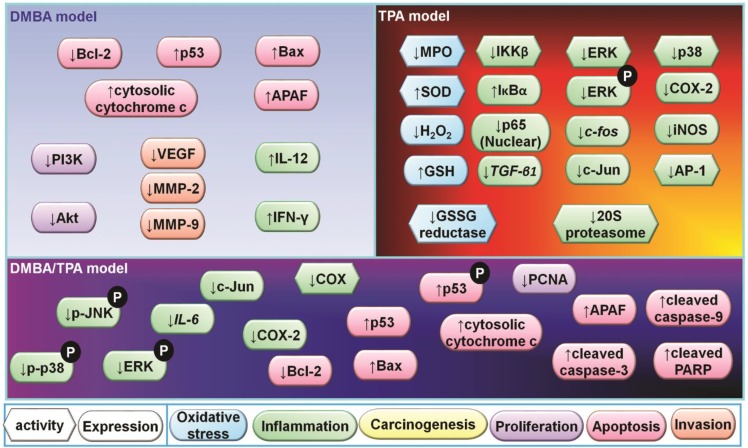
This inhibitory response has subsequently been studied by numerous investigators using a variety of models including DMBA/TPA (Kapadia et al., 2002; Soleas et al., 2002; Kalra et al., 2008; Boily et al., 2009; Kowalczyk et al., 2013), DMBA alone (Szaefer et al., 2008; Roy et al., 2009; Yusuf et al., 2009; Kowalczyk et al., 2010), TPA alone (Jang et al., 1998; Kundu et al., 2004; Cichocki et al., 2008), DMBA/croton oil (Fu et al., 2004), UVB exposure (Afaq et al., 2003; Reagan-Shaw et al., 2004; Aziz et al., 2005; Steele et al., 2005; Kim et al., 2011; Ruggeri et al., 2014), benzo[a]pyrene (BP) (Szaefer et al., 2008), and xenograft (Hao et al., 2013a, 2013b) models. Topical application of resveratrol is the most commonly used route of treatment in skin cancer models. In DMBA/TPA models, resveratrol treatment reduced the incidence (Jang et al., 1997; Kapadia et al., 2002; Soleas et al., 2002; Kalra et al., 2008; Boily et al., 2009), multiplicity (Jang et al., 1997; Kapadia et al., 2002; Kalra et al., 2008; Boily et al., 2009), and tumor volume (Kalra et al., 2008; Boily et al., 2009; Kowalczyk et al., 2013), and delayed the onset of tumorigenesis (Kalra et al., 2008). At biomarker levels, resveratrol induced apoptosis and decreased the expression levels of Bcl-2 while it increased p53 and Bax. In addition, resveratrol enhanced the release of cytochrome c, induced apoptotic protease-activating factor-1 (APAF-1), and cleaved caspase-9,-3, and poly (ADP-ribose) polymerase (PARP) (Kalra et al., 2008). Further, resveratrol decreased cell survival-related proteins including phosphatidylinositol-3-kinase (PI3K) and Akt (Roy et al., 2009), and inflammatory markers including interleukin (IL)-6, cyclooxygenase-2 (COX-2), and c-Jun (Kowalczyk et al., 2013).
With UVB models, resveratrol decreased bi-fold skin thickness (Afaq et al., 2003; Reagan-Shaw et al., 2004), hyperplasia (Reagan-Shaw et al., 2004), infiltration of leukocytes (Reagan-Shaw et al., 2004), and incidence (Aziz et al., 2005), and delayed the onset of tumorigenesis (Aziz et al., 2005). In addition, biomarkers were affected by resveratrol treatment. Activities of ornithine decarboxylase (ODC) (Afaq et al., 2003) and COX (Afaq et al., 2003) and expression levels of ODC (Afaq et al., 2003), proliferating cell nuclear antigen (PCNA) (Reagan-Shaw et al., 2004), cyclin-dependent kinase (CDK)2, CDK6, and cyclin D2 (Reagan-Shaw et al., 2004), mitogen-activated protein kinase kinase (MEK) (Reagan-Shaw et al., 2004), extracellular signal-regulated kinase (ERK) (Reagan-Shaw et al., 2004), survivin, and phosphorylated (p-)survivin were downregulated. On the other hand, the expression of p21 (Reagan-Shaw et al., 2004), p53 (Reagan-Shaw et al., 2004), and Smac/DIABLO (Aziz et al., 2005) was upregulated. Furthermore, resveratrol exerted an antioxidant effect with reduction of H2O2 and lipid peroxidation in the skin (Afaq et al., 2003).
Oral administration of resveratrol also resulted in positive effects, including decreases in the tumor multiplicity and volume, and delay in the onset of tumorigenesis (Kim et al., 2011). The anti-tumor effect of resveratrol was associated with decreased expression levels of TGF-β1 (Kim et al., 2011) and Rictor (Back et al., 2012), and increased expression levels of E-cadherin (Kim et al., 2011).
With a human cutaneous skin squamous carcinoma A431 cell line xenograft model, tumor volume was decreased by resveratrol treatment, along with increased expression levels of p53 and ERK, and decreased levels of survivin. Although ERK is considered as a proliferation and survival protein in general, ERK was also reported to form a complex with p53, leading to an increase in p53 phosphorylation and expression. Also, resveratrol enhanced the activation of caspase-3 (Hao et al., 2013a, 2013b).
In addition, the antitumor effect of resveratrol was reduced with genetically engineered animals including TLR4 deficient C3H/HeJ mice in the DMBA model (Yusuf et al., 2009) and sirtuin 1-null mice in the DMBA/TPA model (Boily et al., 2009). Oral gavage of resveratrol inhibited the growth of a mouse melanoma (B16BL6 cell line) xenograft carried in mice, with decreased expression of Akt (Bhattacharya et al., 2011). In another xenograft model with A2058 human melanoma cells, intratumoral injection of resveratrol reduced tumor volume and this was associated with inhibition of STAT3-DNA methyltransferase 1 (DNMT1) complex formation and the sequential decrease in the methylation of several tumor-suppressor gene promoters (PTPN6, CDKN2A, and SOCS3) (Lee et al., 2012). On the other hand, tumor growth of other melanoma cell lines, including B16M (Asensi et al., 2002), A375 (Niles et al., 2006), and Duke melanoma 738 xenografts in mice, was not attenuated by resveratrol, demonstrating limited potential as an anti-melanoma agent (Osmond et al., 2013). But topical administration of resveratrol reduced UVB-induced hyperpigmentation which is related to melanoma formation, with a decrease in tyrosinase-related protein 2 in male brownish guinea pigs (KIWA:A1) (Lee et al., 2014).
A graphic representation of some of the numerous responses mediated by resveratrol in skin models is depicted in Fig. 6. Obviously, since resveratrol can be applied as a topical, perhaps in conjunction with a sun block, high concentrations can readily be achieved and thereby issues of absorption and metabolism are largely subjugated. Used as a topical in human trials, resveratrol has been reported to improve Acne vulgaris (Fabbrocini et al., 2011) and photoaging (Ayala, 2011; Moyano-Mendez et al., 2014).
Fig. 6.
Graphic depiction of some representative biomarkers that are influenced by resveratrol in various models of skin cancer.
In sum, amelioration of skin issues and diseases by topical application of resveratrol is particularly encouraging. Somewhat logically, resveratrol has been incorporated into a number of cosmetic products (Baxter, 2008; Ndiaye et al., 2011; Ratz-Łyko and Arct, 2018). Perusal of the internet readily reveals numerous commercial products containing resveratrol such as lipsticks, moisturizers, creams, oils, lifts, serums, powders, pencils, etc. Claims such as skin-calming, antimicrobial, skin-protectant, skin-firming, skin-lightening, etc., are common, but most claims focus on an anti-aging effect. Some products attempt to achieve a superior position through special proprietary methods such as the stabilization and micronization of resveratrol. Taking into account the discussion presented above regarding the effects of resveratrol on skin, it is difficult to be overly critical of resveratrol-laden cosmetic products. Nonetheless, it will be comforting when more clinical results are generated and made publically available.
CONCLUDING REMARKS
“For rheumatism, neuralsia, sciatica, lame back, lumbaro, contracted cords, toothache, sprains, swellings, etc. For frost bite, bruises, sore throat, bites of animals, insects and reptiles. Good for man or beast. It gives immediate relieve. It is good for everything…” These claims are from an advertisement produced by the Clark Stanley Snake Oil Lintment Company for his product, snake oil (ca. 1900). The bombastic claims of Clark Stanley are reminiscent of the multitude of advertisements regarding resveratrol that can be found on the internet and typified by the publication Natural News Report #139: “World renowned doctors including Dr. Oz, Dr. Sinclair and Dr. Gruss are recommending resveratrol, the ‘miracle molecule’ saying ‘people can live to 120′; resveratrol may be an incredible preventative weapon against cancer; resveratrol may eradicate brain plaque associated with senility; compound in red wine may fight Alzheimer’s; ‘resveratrol has anti-obesity properties by exerting its effects directly on the fat cells’; resveratrol protects you from head to toe!”
Given that evidence-based responses are sparse and controversial, such claims, innuendo and hyperbole are atrocious. Nonetheless, irrespective of scientific underpinning, as noted above, a company was created and acquired for hundreds of millions of dollars, and dietary supplements currently on the market are estimated to yield about $50 million (US) per year (Bomgardner, 2017). There is even a piece of jewelry available that has been fashioned after the chemical structure of resveratrol. At least in the case of resveratrol-shaped jewelry, true market value can be determined by the weight of gold, silver or platinum.
In terms of the therapeutic effect of the actual compound, at the present time, there is a close parallel to past practice of marketing snake oil. It is highly disconcerting to think consumers could actually believe taking resveratrol will extend their lifespan, reduce their body weight, and fulfill all of the other numerous claims of vibrancy and contentment. But on the positive side, it is generally agreed that the safety profile of resveratrol is pristine (Johnson et al., 2011b). Even at very high daily doses with human beings, e.g., 5 g/day, side effects are mild (Brown et al., 2010). Thus, irrespective of the actual therapeutic efficacy of resveratrol, perhaps real or perceived benefits derived from a placebo effect should not be discounted (Price et al., 2008). In order to realize a placebo effect when working with a ‘medicine’, a placebo, or an ineffective but safe substance functioning as a placebo (in this case, resveratrol), must be administered.
So, in the end, perhaps the most important thing is to abide by ethical rules of modern medicine (primum nil nocere) and, hopefully, at the same time, provide some benefit for humanity. At this time, expert working groups have not be able to make any specific recommendations regarding the health benefits of resveratrol in general (Vang et al., 2011). Nonetheless, as described herein, numerous targets have been investigated, and there is certainly a general perception that some value exists. Perhaps of equal importance, resveratrol has become a household word in the context of a natural product that has the potential of promoting good health. American editorial cartoonist Arthur “Chip” Bok has generated outstanding illustrations depicting subjects such as a customer asking a pharmacist for a case of cabernet since red wine contains resveratrol. Thus, irrespective of the ultimate fate of resveratrol, having enhanced public awareness of the interrelationship of diet and health, and the notion of preventative healthcare, are achievements that have already been accomplished.
Acknowledgments
The author is grateful to Dr. Eun-Jung Park for her invaluable help in accumulating some of the data given in this report, as well as exceptional assistance with the graphics.
Footnotes
CONFLICT OF INTEREST
The author declares no conflict of interest.
REFERENCES
- Afaq F, Adhami VM, Ahmad N. Prevention of short-term ultraviolet B radiation-mediated damages by resveratrol in SKH-1 hairless mice. Toxicol. Appl. Pharmacol. 2003;186:28–37. doi: 10.1016/S0041-008X(02)00014-5. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Shishodia S. S. Resveratrol in Health and Disease. Marcel Dekker, Inc.; New York: 2006. [Google Scholar]
- Asensi M, Medina I, Ortega A, Carretero J, Baño MC, Obrador E, Estrela JM. Inhibition of cancer growth by resveratrol is related to its low bioavailability. Free Radic. Biol. Med. 2002;33:387–398. doi: 10.1016/S0891-5849(02)00911-5. [DOI] [PubMed] [Google Scholar]
- Ayala F. Resveratrol-containing gel for the treatment of acne vulgaris: a single-blind, vehicle-controlled, pilot study. Am. J. Clin. Dermatol. 2011;12:133–141. doi: 10.2165/11530630-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Aziz MH, Reagan-Shaw S, Wu J, Longley BJ, Ahmad N. Chemoprevention of skin cancer by grape constituent resveratrol: relevance to human disease? FASEB J. 2005;19:1193–1195. doi: 10.1096/fj.04-3582fje. [DOI] [PubMed] [Google Scholar]
- Back JH, Zhu Y, Calabro A, Queenan C, Kim AS, Arbesman J, Kim AL. Resveratrol-mediated downregulation of Rictor attenuates autophagic process and suppresses UV-induced skin carcinogenesis. Photochem. Photobiol. 2012;88:1165–1172. doi: 10.1111/j.1751-1097.2012.01097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashmakov YK, Assaad-Khalil SH, Seif MA, Udumyan R, Megallaa M, Rohoma KH, Zeitoun M, Petyaev IM. Resveratrol promotes foot ulcer size reduction in type 2 diabetes patients. ISRN Endocrinol. 2014;2014:816307. doi: 10.1155/2014/816307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter RA. Anti-aging properties of resveratrol: review and report of a potent new antioxidant skin care formulation. J. Cosmet. Dermatol. 2008;7:2–7. doi: 10.1111/j.1473-2165.2008.00354.x. [DOI] [PubMed] [Google Scholar]
- Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. The protein data bank. Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat KPL, Lantvit D, Christov K, Mehta RG, Moon RC, Pezzuto JM. Estrogenic and antiestrogenic properties of resveratrol in mammary tumor models. Cancer Res. 2001;61:7456–7463. [PubMed] [Google Scholar]
- Bhat KP, Pezzuto JM. Cancer chemopreventive activity of resveratrol. Ann. N.Y. Acad. Sci. 2002;957:210–229. doi: 10.1111/j.1749-6632.2002.tb02918.x. [DOI] [PubMed] [Google Scholar]
- Bhatt JK, Thomas S, Nanjan MJ. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr. Res. 2012;32:537–541. doi: 10.1016/j.nutres.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S, Darjatmoko SR, Polans AS. Resveratrol modulates the malignant properties of cutaneous melanoma through changes in the activation and attenuation of the antiapoptotic protooncogenic protein Akt/PKB. Melanoma Res. 2011;21:180–187. doi: 10.1097/CMR.0b013e3283456dfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boily G, He XH, Pearce B, Jardine K, McBurney MW. Sirt1-nullmice develop tumors at normal rates but are poorly protected by resveratrol. Oncogene. 2009;28:2882–2893. doi: 10.1038/onc.2009.147. [DOI] [PubMed] [Google Scholar]
- Bomgardner MM. Reviving resveratrol. Chem. Eng. News. 2017;95:38–39. [Google Scholar]
- Brown VA, Patel KR, Viskaduraki M, Crowell JA, Perloff M, Booth TD, Vasilinin G, Sen A, Schinas AM, Piccirilli G, Brown K, Steward WP, Gescher A. J., Brenner DE. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res. 2010;70:9003–9011. doi: 10.1158/0008-5472.CAN-10-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhrmann C, Shayan P, Popper B, Goel A, Shakibaei M. Sirt1 is required for resveratrol-mediated chemopreventive effects in colorectal cancer cells. Nutrients. 2016;8:145. doi: 10.3390/nu8030145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese EJ, Mattson MP, Calabrese V. Resveratrol commonly displays hormesis: occurrence and biomedical significance. Hum. Exp. Toxicol. 2010;29:980–1015. doi: 10.1177/0960327110383625. [DOI] [PubMed] [Google Scholar]
- Cao C, Lu S, Kivlin R, Wallin B, Card E, Bagdasarian A, Tamakloe T, Wang WJ, Song X, Chu WM, Kouttab N, Xu A, Wan Y. SIRT1 confers protection against UVB- and H2O2-induced cell death via modulation of p53 and JNK in cultured skin keratinocytes. J. Cell. Mol. Med. 2009;13:3632–3643. doi: 10.1111/j.1582-4934.2008.00453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao D, Wang M, Qiu X, Liu D, Jiang H, Yang N, Xu RM. Structural basis for allosteric, substrate-dependent stimulation of SIRT1 activity by resveratrol. Genes Dev. 2015;29:1316–1325. doi: 10.1101/gad.265462.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carboni L. Peripheral biomarkers in animal models of major depressive disorder. Dis. Markers. 2013;35:33–41. doi: 10.1155/2013/284543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KS, Lee EJ, Kwon KJ, Gonzales EL, Kim YB, Cheong JH, Bahn GH, Lee J, Han SH, Kim YT, Shin CY. Resveratrol down-regulates a glutamate-induced tissue plasminogen activator via Erk and AMPK/mTOR pathways in rat primary cortical neurons. Food Funct. 2014;5:951–960. doi: 10.1039/c3fo60397k. [DOI] [PubMed] [Google Scholar]
- Chowdhury SKR, Smith DR, Saleh A, Schapansky J, Marquez A, Gomes S, Akude E, Morrow D, Calcutt NA, Fernyhough P. Impaired adenosine monophosphate-activated protein kinase signaling in dorsal root ganglia neurons is linked to mitochondrial dysfunction and peripheral neuropathy in diabetes. Brain. 2012;135:1751–1766. doi: 10.1093/brain/aws097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ClinicalTrials.gov 2018a Available from: https://clinicaltrials.gov/ct2/results/map?term=resveratrol&map=) [accessed 2018 Sep 4].
- ClinicalTrials.gov 2018b Available from: https://clinicaltrials.gov/ct2/results?cond=colon+cancer&term=resveratrol&cntry=&state=&city=&dist=) [accessed 2018 Oct 3].
- Cichocki M, Paluszczak J, Szaefer H, Piechowiak A, Rimando AM, Baer-Dubowska W. Pterostilbene is equally potent as resveratrol in inhibiting 12-O-tetradecanoylphorbol-13-acetate activated NFkappaB, AP-1, COX-2, and iNOS in mouse epidermis. Mol. Nutr. Food Res. 2008;52:S62–S70. doi: 10.1002/mnfr.200700466. [DOI] [PubMed] [Google Scholar]
- Cragg GM, Pezzuto JM. Natural products as a vital source for the discovery of cancer chemotherapeutic and chemopreventive agents. Med. Princ. Pract. 2016;25(Suppl 2):41–59. doi: 10.1159/000443404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Pinto JT, Ballabh P, Zhang H, Losonczy G, Pearson K, de Cabo R, Pacher P, Zhang C, Ungvari Z. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2009;297:H13–H20. doi: 10.1152/ajpheart.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damián JP, Acosta V, Da Cuña M, Ramírez I, Oddone N, Zambrana A, Bervejillo V, J.C. Benech J. C. Effect of resveratrol on behavioral performance of streptozotocin-induced diabetic mice in anxiety tests. Exp. Anim. 2014;63:277–287. doi: 10.1538/expanim.63.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Alagappan VK, Bagchi D, Sharma HS, Maulik N, Das DK. Coordinated induction of iNOS-VEGF-KDR-eNOS after resveratrol consumption: a potential mechanism for resveratrol preconditioning of the heart. Vascul. Pharmacol. 2005;42:281–289. doi: 10.1016/j.vph.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Das S, Tosaki A, Bagchi D, Maulik N, Das DK. Potentiation of a survival signal in the ischemic heart by resveratrol through p38 mitogen-activated protein kinase/mitogen- and stress-activated protein kinase 1/cAMP response element-binding protein signaling. J. Pharmacol. Exp. Ther. 2006;317:980–988. doi: 10.1124/jpet.105.095133. [DOI] [PubMed] [Google Scholar]
- Do GM, Jung UJ, Park HJ, Kwon EY, Jeon SM, McGregor RA, Choi MS. Resveratrol ameliorates diabetes-related metabolic changes via activation of AMP-activated protein kinase and its downstream targets in db/db mice. Mol. Nutr. Food Res. 2012;56:1282–1291. doi: 10.1002/mnfr.201200067. [DOI] [PubMed] [Google Scholar]
- El-Mowafy AM, Alkhalaf M, Nassar NN. Resveratrol reverses ET-1-evoked mitogenic effects in human coronary arterial cells by activating the kinase-G to inhibit ERK-enzymes. Int. J. Cardiol. 2009;136:263–269. doi: 10.1016/j.ijcard.2008.04.094. [DOI] [PubMed] [Google Scholar]
- Escote X, Miranda M, Menoyo S, Rodriguez-Porrata B, Carmona-Gutierrez D, Jungwirth H, Madeo F, Cordero RR, Mas A, Tinahones F, Clotet J, Vendrell J. Resveratrol induces antioxidant defence via transcription factor Yap1p. Yeast. 2012;29:251–263. doi: 10.1002/yea.2903. [DOI] [PubMed] [Google Scholar]
- Evans HM, Howe PRC, Wong RHX. Effects of resveratrol on cognitive performance, mood and cerebrovascular function in post-menopausal women; a 14-week randomised placebo-controlled intervention trial. Nutrients. 2017;9:E27. doi: 10.3390/nu9010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbrocini G, Staibano S, De Rosa G, Battimiello V, Fardella N, Ilardi G, La Rotonda MI, Longobardi A, Mazzella M, Siano M, Pastore F, De Vita V, Vecchione ML, Ayala F. Resveratrol-containing gel for the treatment of acne vulgaris: a single-blind, vehicle-controlled, pilot study. Am. J. Clin. Dermatol. 2011;12:133–141. doi: 10.2165/11530630-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Fu ZD, Cao Y, Wang KF, Xu SF, Han R. Chemo-preventive effect of resveratrol to cancer. Ai Zheng. 2004;23:869–873. [PubMed] [Google Scholar]
- Gehm BD, McAndrews JM, Chien PY, Jameson JL. Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Proc. Natl. Acad. Sci. U.S.A. 1997;94:14138–14143. doi: 10.1073/pnas.94.25.14138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh SS, Woodman OL, Pepe S, Cao AH, Qin C, Ritchie RH. The red wine antioxidant resveratrol prevents cardiomyocyte injury following ischemia-reperfusion via multiple sites and mechanisms. Antioxid. Redox. Signal. 2007;9:101–113. doi: 10.1089/ars.2007.9.101. [DOI] [PubMed] [Google Scholar]
- Gracia-Sancho J, Villarreal G, Jr., Zhang Y, Garcia-Cardena G. Activation of SIRT1 by resveratrol induces KLF2 expression conferring an endothelial vasoprotective phenotype. Cardiovasc. Res. 2010;85:514–519. doi: 10.1093/cvr/cvp337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilford JM, Pezzuto JM. Wine and health: a review. Am. J. Enol. Vitic. 2011;62:471–486. doi: 10.5344/ajev.2011.11013. [DOI] [Google Scholar]
- Guo D, Xie J, Zhao J, Huang T, Guo X, Song J. Resveratrol protects early brain injury after subarachnoid hemorrhage by activating autophagy and inhibiting apoptosis mediated by the Akt/mTOR pathway. Neuroreport. 2018;29:368–379. doi: 10.1097/WNR.0000000000000975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, Huang W, Liao M, Zhu Y, Liu H, Hao C, Liu G, Zhang G, Feng H, Ning X, Li H, Li Z. The inhibition of resveratrol to human skin squamous cell carcinoma A431 xenografts in nude mice. Fitoterapia. 2013a;86:84–91. doi: 10.1016/j.fitote.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Hao YQ, Huang WX, Feng HX, Zhang GH, Ning XH, Li HG, Hao CG, Li ZH. Study of apoptosis related factors regulatory mechanism of resveratrol to human skin squamous cell carcinoma A431 xenograft in nude mice. Zhonghua Yi Xue Za Zhi. 2013b;93:464–468. [PubMed] [Google Scholar]
- Hoshino J, Park EJ, Kondratyuk TP, Marler L, Pezzuto JM, van Breemen RB, Mo S, Li Y, Cushman M. Selective synthesis and biological evaluation of sulfate-conjugated resveratrol metabolites. J. Med. Chem. 2010;53:5033–5043. doi: 10.1021/jm100274c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Huang W, Chen Z, Wang O, Lin M, Wu S, Yan Q, Wu F, Yu X, Xie X, Li G, Xu Y, Pan J. Piperine potentiates the antidepressant-like effect of trans-resveratrol: involvement of monoaminergic system. Metab. Brain Dis. 2013;28:585–595. doi: 10.1007/s11011-013-9426-y. [DOI] [PubMed] [Google Scholar]
- Hunter P. The inflammation theory of disease. EMBO Rep. 2012;13:968–970. doi: 10.1038/embor.2012.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LL, Akinfiresoye L, Kalejaiye O, Tizabi Y. Anti-depressant effects of resveratrol in an animal model of depression. Behav. Brain Res. 2014;268:1–7. doi: 10.1016/j.bbr.2014.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- Jang M, Pezzuto JM. Effects of resveratrol on 12-O-tet-radecanoylphorbol-13-acetate-induced oxidative events and gene expression in mouse skin. Cancer Lett. 1998;134:81–89. doi: 10.1016/S0304-3835(98)00250-X. [DOI] [PubMed] [Google Scholar]
- Jing YH, Chen KH, Kuo PC, Pao CC, Chen JK. Neurodegeneration in streptozotocin-induced diabetic rats is attenuated by treatment with resveratrol. Neuroendocrinology. 2013;98:116–127. doi: 10.1159/000350435. [DOI] [PubMed] [Google Scholar]
- Johnson JM, Nihal M, Siddiqui IA, Scarlett CO, Bailey HH, Mukhtar H, Ahmad H. Enhancing the bioavailability of resveratrol by combining it with piperine. Mol. Nutr. Food Res. 2011a;55:1169–1176. doi: 10.1002/mnfr.201100117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson WD, Morrissey RLU, sborne AL, Kapetanovic I, Crowell JA, Muzzio M, McCormick DL. Subchronic oral toxicity and cardiovascular safety pharmacology studies of resveratrol, a naturally occurring polyphenol with cancer preventive activity. Food Chem. Toxicol. 2011b;49:3319–3327. doi: 10.1016/j.fct.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhasz B, Mukherjee S, Das DK. Hormetic response of resveratrol against cardioprotection. Exp. Clin. Cardiol. 2010;15:e134–e138. [PMC free article] [PubMed] [Google Scholar]
- Kalra N, Roy P, Prasad S, Shukla Y. Resveratrol induces apoptosis involving mitochondrial pathways in mouse skin tumorigenesis. Life Sci. 2008;82:348–358. doi: 10.1016/j.lfs.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Kapadia GJ, Azuine MA, Tokuda H, Takasaki M, Mukainaka T, Konoshima T, Nishino H. Chemopreventive effect of resveratrol, sesamol, sesame oil and sunflower oil in the Epstein–Barr virus early antigen activation assay and the mouse skin two-stage carcinogenesis. Pharmacol. Res. 2002;45:499–505. doi: 10.1006/phrs.2002.0992. [DOI] [PubMed] [Google Scholar]
- Kennedy DO, Wightman EL, Reay JL, Lietz G, Okello EJ, Wilde A, Haskell CF. Effects of resveratrol on cerebral blood flow variables and cognitive performance in humans: a double-blind, placebo-controlled, crossover investigation. Am. J. Clin. Nutr. 2010;91:1590–1597. doi: 10.3945/ajcn.2009.28641. [DOI] [PubMed] [Google Scholar]
- Kim KH, Back JH, Zhu Y, Arbesman J, Athar M, Kopelovich L, Kim AL, Bickers DR. Resveratrol targets transforming growth factor-β2 signaling to block UV-induced tumor progression. J. Invest. Dermatol. 2011;131:195–202. doi: 10.1038/jid.2010.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaer TN, Ornstrup MJ, Poulsen MM, Stodkilde-Jørgensen H, Jessen N, Jørgensen JOL, Richelsen B, Pedersen SB. No beneficial effects of resveratrol on the metabolic syndrome: a randomized placebo-controlled clinical trial. J. Clin. Endocrinol. Metab. 2017;102:1642–1651. doi: 10.1210/jc.2016-2160. [DOI] [PubMed] [Google Scholar]
- Kowalczyk MC, Kowalczyk P, Tolstykh O, Hanausek M, Walaszek Z, Slaga TJ. Synergistic effects of combined phytochemicals and skin cancer prevention in SENCAR mice. Cancer Prev. Res. 2010;3:170–178. doi: 10.1158/1940-6207.CAPR-09-0196. [DOI] [PubMed] [Google Scholar]
- Kowalczyk MC, Junco JJ, Kowalczyk P, Tolstykh O, Hanausek M, Slaga TJ, Walaszek Z. Effects of combined phytochemicals on skin tumorigenesis in SENCAR mice. Int. J. Oncol. 2013;43:911–918. doi: 10.3892/ijo.2013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. Animal models of depression: molecular perspectives. Curr. Top. Behav. Neurosci. 2011;7:121–147. doi: 10.1007/7854_2010_108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Kaundal RK, Iyer S, Sharma SS. Effects of resveratrol on nerve functions, oxidative stress and DNA fragmentation in experimental diabetic neuropathy. Life Sci. 2007;80:1236–1244. doi: 10.1016/j.lfs.2006.12.036. [DOI] [PubMed] [Google Scholar]
- Kumar A, Park E.-J, Pezzuto JM. Chapter 3. Resveratrol as an activator or inhibitor of enzymes and proteins. In: Wu J. M, Hsieh T.-C, editors. Resveratrol: State-of-the-Art Science and Health Applications. World Scientific Publishing; Singapore: 2018. pp. 55–113. [Google Scholar]
- Kundu JK, Chun KS, Kim SO, Surh YJ. Resveratrol inhibits phorbol ester-induced cyclooxygenase-2 expression in mouse skin: MAPKs and AP-1 as potential molecular targets. Biofactors. 2004;21:33–39. doi: 10.1002/biof.552210108. [DOI] [PubMed] [Google Scholar]
- Lee H, Zhang P, Herrmann A, Yang C, Xin H, Wang Z, Hoon DS, Forman SJ, Jove R, Riggs AD, Yu H. Acetylated STAT3 is crucial for methylation of tumor suppressor gene promoters and inhibition by resveratrol results in demethylation. Proc. Natl. Acad. Sci. U.S.A. 2012;109:7765–7769. doi: 10.1073/pnas.1205132109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TH, Seo JO, Baek SH, Kim SY. Inhibitory effects of resveratrol on melanin synthesis in ultraviolet B-induced pigmentation in Guinea pig skin. Biomol. Ther. (Seoul) 2014;22:35–40. doi: 10.4062/biomolther.2013.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma SC, Zhang HP, Jiao Y, Wang YH, Zhang H, Yang XL, Yang AN, Jiang YD. Homocysteine-induced proliferation of vascular smooth muscle cells occurs via PTEN hypermethylation and is mitigated by resveratrol. Mol. Med. Rep. 2018;17:5312–5319. doi: 10.3892/mmr.2018.8471. [DOI] [PubMed] [Google Scholar]
- Maroon J. The Longevity Factor: How Resveratrol and Red Wine Activate Genes for a Longer and Healthier Life. Atria Paper-back; New York: 2009. [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, Pletcher S, Sharp ZD, Sinclair D, Starnes JW, Wilkinson JE, Nadon NL, Strong R. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J. Gerontol. A Biol. Sci. Med. Sci. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movahed A, Nabipour I, Louis XL, Thandapilly SJ, Yu L, Kalantarhormozi M, Rekabpour SJ, Netticadan T. Anti-hyperglycemic effects of short term resveratrol supplementation in type 2 diabetic patients. Evid. Based Complement. Alternat. Med. 2013;2013:851267. doi: 10.1155/2013/851267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyano-Mendez JR, Fabbrocini G, De Stefano D, Mazzella C, Mayol L, Scognamiglio I, Carnuccio R, Ayala F, La Rotonda MI, De Rosa G. Enhanced antioxidant effect of trans-resveratrol: potential of binary systems with polyethylene glycol and cyclodextrin. Drug Deve. Ind. Pharm. 2014;40:1300–1307. doi: 10.3109/03639045.2013.817416. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay P, Mukherjee S, Ahsan K, Bagchi A, Pacher P, Das DK. Restoration of altered microRNA expression in the ischemic heart with resveratrol. PLoS One. 2010;5:e15705. doi: 10.1371/journal.pone.0015705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay P, Das S, Ahsan MK, Otani H, Das DK. Modulation of microRNA 20b with resveratrol and longevinex is linked with their potent anti-angiogenic action in the ischaemic myocardium and synergistic effects of resveratrol and gamma-tocotrienol. J. Cell. Mol. Med. 2012;16:2504–2517. doi: 10.1111/j.1582-4934.2011.01480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndiaye M, Philippe C, Mukhtar H, Nihal Ahmad N. The grape antioxidant resveratrol for skin disorders: promise, prospects, and challenges. Arch. Biochem. Biophys. 2011;508:164–170. doi: 10.1016/j.abb.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niles RM, Cook CP, Meadows GG, Fu YM, McLaughlin JL, Rankin GO. Resveratrol is rapidly metabolized in athymic (nu/nu) mice and does not inhibit human melanoma xenograft tumor growth. J. Nutr. 2006;136:2542–2546. doi: 10.1093/jn/136.10.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmond GW, Masko EM, Tyler DS, Freedland SJ, Pizzo S. In vitro and in vivo evaluation of resveratrol and 3,5-dihydroxy-4′-acetoxy-trans-stilbene in the treatment of human prostate carcinoma and melanoma. J. Surg. Res. 2013;179:e141–e148. doi: 10.1016/j.jss.2012.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, Flynn D, Garofalo RS, Griffith D, Griffor M, Loulakis P, Pabst B, Qiu X, Stockman B, Thanabal V, Varghese A, Ward J, Withka J, Ahn K. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J. Biol. Chem. 2010;285:8340–8351. doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomera-Avalos V, Grinan-Ferre C, Puigoriol-Ilamola D, Camins A, Sanfeliu C, Canudas AM, Pallas M. Resveratrol protects SAMP8 brain under metabolic stress: focus on mitochondrial function and Wnt pathway. Mol. Neurobiol. 2017;54:1661–1676. doi: 10.1007/s12035-016-9770-0. [DOI] [PubMed] [Google Scholar]
- Pan Y, Zhang H, Zheng Y, Zhou J, Yuan J, Yu Y, Wang J. Resveratrol exerts antioxidant effects by activating SIRT2 to deacetylate Prx1. Biochemistry. 2017;56:6325–6328. doi: 10.1021/acs.biochem.7b00859. [DOI] [PubMed] [Google Scholar]
- Park DW, Baek K, Kim JR, Lee JJ, Ryu SH, Chin BR, Baek SH. Resveratrol inhibits foam cell formation via NADPH oxidase 1-mediated reactive oxygen species and monocyte chemotactic protein-1. Exp. Mol. Med. 2009;41:171–179. doi: 10.3858/emm.2009.41.3.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EJ, Pezzuto JM. The pharmacology of resveratrol in animals and humans. Biochim. Biophys. Acta. 2015;1852:1071–1113. doi: 10.1016/j.bbadis.2015.01.014. [DOI] [PubMed] [Google Scholar]
- Patel KR, Brown VA, Jones DJL, Britton RG, Hemingway D, Miller AS, West KP, Booth TD, Perloff M, Crowell JA, Brenner DE, Steward WP, Gescher AJ, Brown K. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res. 2010;70:7392–7399. doi: 10.1158/0008-5472.CAN-10-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel KR, Andreadi C, Britton RG, Horner-Glister E, Karmokar A, Sale S, Brown VA, Brenner DE, Singh R, Steward WP, Gescher AJ, Brown K. Sulfate metabolites provide an intracellular pool for resveratrol generation and induce autophagy with senescence. Sci. Trans. Med. 2013;5 doi: 10.1126/scitranslmed.3005870. 205ra133. [DOI] [PubMed] [Google Scholar]
- Penumathsa SV, Koneru S, Samuel SM, Maulik G, Bagchi D, Yet SF, Menon VP, Maulik N. Strategic targets to induce neovascularization by resveratrol in hypercholesterolemic rat myocardium: role of caveolin-1, endothelial nitric oxide synthase, hemeoxygenase-1, and vascular endothelial growth factor. Free Radic. Biol. Med. 2008;45:1027–1034. doi: 10.1016/j.freeradbiomed.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzuto JM. Plant-derived anticancer agents. Biochem. Pharmacol. 1997;53:121–133. doi: 10.1016/S0006-2952(96)00654-5. [DOI] [PubMed] [Google Scholar]
- Pezzuto JM, Kosmeder J.W, II P, ark EJ, Lee SK, Cuendet M, Gills J, Bhat K, Grubjesic S, Park H.-S, Mata-Greenwood E, Tan YM, Yu R, Lantvit DD, Kinghorn AD. Characterization of natural product chemopreventive agents. In: Kelloff G.J, Hawk E.T, Sigman C.C, editors. Cancer Chemoprevention, Volume 2: Strategies for Cancer Chemoprevention. Humana Press Inc.; Totowa, New Jersey: 2005. pp. 3–37. [DOI] [Google Scholar]
- Pezzuto JM. The phenomenon of resveratrol: redefining the virtues of promiscuity. Ann. N.Y. Acad. Sci. 2011;1215:123–130. doi: 10.1111/j.1749-6632.2010.05849.x. [DOI] [PubMed] [Google Scholar]
- Pezzuto JM, Kondratyuk TP, Olgas T. Resveratrol derivatives: a patent review (2009–2012). Expert Opin. Ther. Pat. 2013;23:1529–1546. doi: 10.1517/13543776.2013.834888. [DOI] [PubMed] [Google Scholar]
- Pollack A. Glaxo says compound in wine may fight aging ( https://www.nytimes.com/2008/04/23/business/23wine.html) accessed October 3, 2018.
- Popat R, Plesner T, Davies F, Cook G, Cook M, Elliott P, Jacobson E, Gumbleton T, Oakervee H, Cavenagh J. A phase 2 study of SRT501 (resveratrol) with bortezomib for patients with relapsed and or refractory multiple myeloma. Br. J. Haematol. 2013;160:714–717. doi: 10.1111/bjh.12154. [DOI] [PubMed] [Google Scholar]
- Prabhakar O. Cerebroprotective effect of resveratrol through antioxidant and anti-inflammatory effects in diabetic rats. Naunyn Schmiedebergs Arch. Pharmacol. 2013;386:705–710. doi: 10.1007/s00210-013-0871-2. [DOI] [PubMed] [Google Scholar]
- Price DD, Finniss DG, Benedetti F. A comprehensive review of the placebo effect: recent advances and current thought. Ann. Rev. Psychol. 2008;59:565–590. doi: 10.1146/annurev.psych.59.113006.095941. [DOI] [PubMed] [Google Scholar]
- Ratz-Łyko A, Arct J. Resveratrol as an active ingredient for cosmetic and dermatological applications: a review. J. Cosmet. Laser Ther. 2018. [DOI] [PubMed]
- Reagan-Shaw S, Afaq F, Aziz MH, Ahmad N. Modulations of critical cell cycle regulatory events during chemoprevention of ultraviolet B-mediated responses by resveratrol in SKH-1 hair-less mouse skin. Oncogene. 2004;23:5151–5160. doi: 10.1038/sj.onc.1207666. [DOI] [PubMed] [Google Scholar]
- Rogers RG, Otis JS. Resveratrol-mediated expression of KLF15 in the ischemic myocardium is associated with an improved cardiac phenotype. Cardiovasc. Drugs Ther. 2017;31:29–38. doi: 10.1007/s10557-016-6707-9. [DOI] [PubMed] [Google Scholar]
- Roy P, Kalra N, Prasad S, George J, Shukla Y. Chemopreventive potential of resveratrol in mouse skin tumors through regulation of mitochondrial and PI3K/AKT signaling pathways. Pharm. Res. 2009;26:211–217. doi: 10.1007/s11095-008-9723-z. [DOI] [PubMed] [Google Scholar]
- Ruderman NB, Xu XJ, Nelson L, Cacicedo JM, Saha AK, Lan F, Ido Y. AMPK and SIRT1: a long-standing partnership? Am. J. Physiol. Endocrinol. Metab. 2010;298:E751–E760. doi: 10.1152/ajpendo.00745.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri BA, Camp F, Miknyoczki S. Animal models of disease: pre-clinical animal models of cancer and their applications and utility in drug discovery. Biochem. Pharmacol. 2014;87:150–161. doi: 10.1016/j.bcp.2013.06.020. [DOI] [PubMed] [Google Scholar]
- Sardi B. The Anti-Aging Pill. Here and Now Books; San Dimas, CA: 2004. [Google Scholar]
- Sawda C, Moussa C, Turner RS. Resveratrol for Alzheimer’s disease. Ann. N.Y. Acad. Sci. 2017;1403:142–149. doi: 10.1111/nyas.13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilder YD, Heiss EH, Schachner D, Ziegler J, Reznicek G, Sorescu D, Dirsch VM. NADPH oxidases 1 and 4 mediate cellular senescence induced by resveratrol in human endothelial cells. Free Radic. Biol. Med. 2009;46:1598–1606. doi: 10.1016/j.freeradbiomed.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Schmatz R, Mazzanti CM, Spanevello R, Stefanello N, Gutierres J, Corrêa M, da Rosa MM, Rubin MA, Chitolina Schetinger MR, Morsch VM. Resveratrol prevents memory deficits and the increase in acetylcholinesterase activity in streptozotocin-induced diabetic rats. Eur. J. Pharmacol. 2009;610:42–48. doi: 10.1016/j.ejphar.2009.03.032. [DOI] [PubMed] [Google Scholar]
- Seyyedebrahimi S, Khodabandehloo H, Esfahani EN, Meshkani R. The effects of resveratrol on markers of oxidative stress in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled clinical trial. Acta Diabetologica. 2018;55:341–353. doi: 10.1007/s00592-017-1098-3. [DOI] [PubMed] [Google Scholar]
- Sharma S, Kulkarni SK, Chopra K. Resveratrol, a polyphenolic phytoalexin attenuates thermal hyperalgesia and cold allodynia in STZ-induced diabetic rats. Indian J. Exp. Biol. 2006;44:566–569. [PubMed] [Google Scholar]
- Sharma S, Kulkarni SK, Chopra K. Effect of resveratrol, a polyphenolic phytoalexin, on thermal hyperalgesia in a mouse model of diabetic neuropathic pain. Fundam. Clin. Pharmacol. 2007;21:89–94. doi: 10.1111/j.1472-8206.2006.00455.x. [DOI] [PubMed] [Google Scholar]
- Shrotriya S, Tyagi A, Deep G, Orlicky DJ, Wisell J, Wang XJ, Sclafani RA, Agarwal R, Agarwal C. Grape seed extract and resveratrol prevent 4-nitroquinoline 1-oxide induced oral tumorigenesis in mice by modulating AMPK activation and associated biological responses. Mol. Carcinog. 2015;54:291–300. doi: 10.1002/mc.22099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoliga JM, Baur JA, Hausenblas HA. Resveratrol and health-a comprehensive review of human clinical trials. Mol. Nutr. Food Res. 2011;55:1129–1141. doi: 10.1002/mnfr.201100143. [DOI] [PubMed] [Google Scholar]
- Soleas GJ, Grass L, Josephy PD, Goldberg DM, Diamandis EP. A comparison of the anticarcinogenic properties of four red wine polyphenols. Clin. Biochem. 2002;35:119–124. doi: 10.1016/S0009-9120(02)00275-8. [DOI] [PubMed] [Google Scholar]
- Steele VE, Lubet RA, Moon RC. Preclinical Animal Models for the Development of Cancer Chemoprevention Drugs. Humana Press Inc.; Totowa, NJ: 2005. [Google Scholar]
- Szaefer H, Krajka-Kuźniak V, Baer-Dubowska W. The effect of initiating doses of benzo[a]pyrene and 7,12-dimethylbenz[a] anthracene on the expression of PAH activating enzymes and its modulation by plant phenols. Toxicology. 2008;251:28–34. doi: 10.1016/j.tox.2008.07.047. [DOI] [PubMed] [Google Scholar]
- Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, Santos A, Doncheva NT, Roth A, Bork P, Jensen LJ, von Mering C. The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45:D362–D68. doi: 10.1093/nar/gkw937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaoka M. Resveratrol, a new phenolic compound, from Veratrum grandiflorum. J. Chem. Soc. Jpn. 1939;60:1090–1100. [Google Scholar]
- Tameda M, Sugimoto K, Shiraki K, Inagaki Y, Ogura S, Kasai C, Yoneda M, Okamoto R, Yamamoto N, Takei Y, Ito M, Nobori T. Resveratrol sensitizes HepG2 cells to TRAIL-induced apoptosis. Anticancer Drugs. 2014;25:1028–1034. doi: 10.1097/CAD.0000000000000128. [DOI] [PubMed] [Google Scholar]
- Vang O, Ahmad N, Baile CA, Baur JA, Brown K, Csiszar A, Das DK, Delmas D, Gottfried C, Lin HY, Ma QY, Mukhopadhyay P, Nalini N, Pezzuto JM, Richard T, Shukla Y, Surh YJ, Szekeres T, Szkudelski T, Walle T, Wu JM. What is new for an old molecule? Systematic review and recommendations on the use of resveratrol. PLoS ONE. 2011;6:e19881. doi: 10.1371/journal.pone.0019881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa-Cuesta E, Boylan JM, Tatar M, Gruppuso PA. Resveratrol inhibits protein translation in hepatic cells. PLoS ONE. 2011;6:e29513. doi: 10.1371/journal.pone.0029513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan D, Zhou Y, Wang K, Hou Y, Hou R, Ye X. Resveratrol provides neuroprotection by inhibiting phosphodiesterases and regulating the cAMP/AMPK/SIRT1 pathway after stroke in rats. Brain Res. Bull. 2016;121:255–262. doi: 10.1016/j.brainresbull.2016.02.011. [DOI] [PubMed] [Google Scholar]
- Wightman EL, Reay JL, Haskell CF, Williamson G, Dew TP, Kennedy DO. Effects of resveratrol alone or in combinationwith piperine on cerebral blood flow parameters and cognitive performance in human subjects: a randomised, double-blind, placebo-controlled, cross-over investigation. Br. J. Nutr. 2014;112:203–213. doi: 10.1017/S0007114514000737. [DOI] [PubMed] [Google Scholar]
- Wu JM, Hsieh T.-C. Resveratrol: State-of-the-Art Science and Health Applications. World Scientific Publishing; Singapore: 2018. [Google Scholar]
- Xia N, Strand S, Schlufter F, Siuda D, Reifenberg G, Kleinert H, Forstermann U, Li H. Role of SIRT1 and FOXO factors in eNOS transcriptional activation by resveratrol. Nitric Oxide. 2013;32:29–35. doi: 10.1016/j.niox.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Yusuf Y, Nasti TH, Meleth S, Elmets CA. Resveratrol enhances cell-mediated immune response to DMBA through TLR4 and prevents DMBA induced cutaneous carcinogenesis. Mol. Carcinog. 2009;48:713–723. doi: 10.1002/mc.20517. [DOI] [PMC free article] [PubMed] [Google Scholar]