Abstract
Aims/Introduction
There is controversy as to whether hyperuricemia is an independent risk factor for cardiometabolic diseases. The serum level of uric acid is affected by a wide variety of factors involved in its production and excretion. In contrast, evidence has accumulated that locally‐ and systemically‐activated xanthine oxidase (XO), a rate‐limiting enzyme for production of uric acid, is linked to metabolic derangement in humans and rodents. We therefore explored the clinical implication of plasma XO activity in patients with type 2 diabetes mellitus and metabolic syndrome (MetS).
Materials and Methods
We enrolled 60 patients with type 2 diabetes mellitus and MetS. MetS was defined according to the 2005 International Diabetes Federation guidelines. Plasma XO activity was measured by highly‐sensitive fluorometric assay measuring the conversion of pterin to isoxanthopterin, and explored associations between the value of plasma XO activity and metabolic parameters.
Results
The value of plasma XO activity was correlated with indices of insulin resistance and the level of circulating liver transaminases. In contrast, the level of serum uric acid was not correlated with indices of insulin resistance. The value of plasma XO activity was not correlated with the serum uric acid level.
Conclusions
Plasma XO activity correlates with indices of insulin resistance and liver dysfunction in Japanese patients with type 2 diabetes mellitus and MetS. Through assessing the plasma XO activity, patients showing normal levels of serum uric acid with higher activity of XO can be screened, thereby possibly providing a clue to uncovering metabolic risks in type 2 diabetes mellitus and MetS patients.
Keywords: Insulin resistance, Uric acid, Xanthine oxidase
Introduction
It has long been recognized that hyperuricemia is a potential risk for a variety of cardiometabolic diseases1, 2. In contrast, some previous reports showed that hyperuricemia is not an independent risk, but just a surrogate marker for them3, 4. Collectively, there is still controversy as to whether hyperuricemia is an independent risk factor for cardiometabolic diseases. In contrast, it has been shown that oxidative stress produced by xanthine oxidoreductase (XOR) is related to vascular endothelial dysfunction in humans5 and rodents6. XOR is also a key enzyme in the production of uric acid (UA), one of the most powerful circulating anti‐oxidants7. The gene for XOR is initially transcribed as xanthine dehydrogenase (XDH), which controls purine metabolism mainly in the liver and intestine in humans8. Previous studies showed that the majority of XOR protein is derived from the liver throughout the body in humans8, and noticeably, when the liver is suffers from hypoxia or inflammation, XDH is released into the circulation and is subsequently converted into xanthine oxidase (XO) by proteases in the peripheral vasculature9. Importantly, XO locally produces a considerable amount of reactive oxygen species (ROS), such as H2O2 and O2 − 8, 10.
It was reported that XO activity in the vasculature is elevated considerably in patients with coronary artery diseases11. Furthermore, multiple regression analyses showed that the value of plasma XO activity is associated with cardiovascular events and its future risk in patients with chronic heart failure12. It has also been suggested that local XO activities in a variety of organs and tissues are likely to elevate under a line of stimuli including hypoxia, inflammatory cytokines and glucocorticoids10, 13. Therefore, there is a possibility that local XO activities in a variety of organs or tissues would be elevated in patients with type 2 diabetes and obesity syndrome. However, little is known about plasma XO activities in the pathophysiology of insulin resistance and obesity in humans. In this context, here we explored the clinical implication of plasma XO activity in Japanese patients with type 2 diabetes mellitus and metabolic syndrome (MetS).
Methods
Participants
The present study was approved by the institutional ethical committee (approval number: 909, institutional ethical committee of University of the Ryukyus on 2 October 2015). Written informed consent was obtained from all participants. A line of studies was carried out in accordance with the Helsinki Declaration.
We enrolled 50 patients with type 2 diabetes mellitus and MetS, and five lean patients without diabetes who did not have hyperuricemia, who were primarily diagnosed as essential hypertension, primary aldosteronism and non‐functional benign pituitary tumor, respectively. Patients were hospitalized at or visited the outpatient clinics of the Ryukyu University Hospital, Okinawa, Japan. In the present study, MetS was defined according to the 2005 International Diabetes Federation guidelines14. The present study was carried out between May 2016 and December 2017. We consecutively measured plasma XO activity in the patients recruited, and explored possible associations between the value of plasma XO activity and a variety of metabolic parameters. Insulin and oral drugs for hyperuricemia were not used for all patients.
Sample preparation for assessing XO activity in plasma
Blood was collected into 5‐mL tubes containing heparin from participants in a fasting state early in the morning. Blood collection tubes were purchased from TERUMO Corporation (Venoject II vacuum blood collection tube; Tokyo, Japan). Samples were centrifuged at 1,500 g for 15 min at 4°C for isolation of plasma. Centrifuge was carried out within 1 h after blood collection to avoid the leak of hypoxanthine from erythrocytes into plasma. Supernatants were subsequently transferred to new tubes and were pooled at −80°C as plasma samples.
Measurement of plasma XO activity
Plasma XO activity was determined using a fluorometric assay measuring the conversion of pterin to isoxanthopterin with a considerably high sensitivity15. Briefly, the enzyme reaction was initiated by mixing 50 μL of plasma with 50 μL of 0.05 mol/L Tris‐HCl buffer (pH 9.0) containing 100 μmol/L pterin and 1% dimethylsulfoxide. For the blank sample, 0.05 mol/L Tris‐HCl buffer (pH 9.0) was used instead of plasma. After incubation for 3 h at 37°C, 100 μL of 4% HClO4 was added to stop the reaction. The resulting mixture was vigorously shaken and centrifuged at 15,000 g for 10 min at room temperature. Then, 150 μL of the supernatant was neutralized with 6 μL of 5 mol/L K2CO3 and centrifuged at 15,000 g for 10 min at room temperature; 10 μL of supernatant was subjected fluorometric analysis.
Isoxanthopterin concentration in each sample was measured by high‐performance liquid chromatography with a fluorescence detector. The high‐performance liquid chromatography system was Nexera X2 (Shimadzu, Kyoto, Japan) and fluorescence detector RF‐20Axs (Shimadzu) using a YMC‐Triart C18 3‐μm column (YMC, Kyoto, Japan). The mobile phase was 20 mmol/L potassium phosphate buffer, the flow‐rate was 1.0 mL/min, and the excitation and emission wavelengths were 345 and 410 nm, respectively. For standard solution, 10–2,000 nmol/L of isoxanthopterin solution diluted with 0.1 N NaOH or 0.05 mol/L Tris‐HCl buffer (pH 9.0) was used. The area under the curve in the plasma sample and blank sample was calculated, and the concentration was calibrated by standard linear regression. Isoxanthopterin concentration produced by plasma was given by subtracting the concentration of the sample from that of the blank. Plasma XO activity was expressed as pmol isoxanthopterin/min/mL (pmol IXP/min/mL).
Assessment of possible diurnal variation in plasma XO activity
To examine the possible changes in plasma XO activity throughout the day, we enrolled five lean patients without diabetes who did not have hyperuricemia, who were primarily diagnosed as essential hypertension, primary aldosteronism and non‐functional benign pituitary tumor, respectively. Plasma XO activity was measured at the time‐points of 08.00, 15.00 and 23.00 hours, and 08.00 hours the next day.
Assessment of plasma XO activity before and after the meal intake or 1 h of mild aerobic exercise
To examine the variability of plasma XO activity by having meals or doing exercise, we newly enrolled 10 patients who were not receiving insulin or oral drugs for hyperuricemia. Plasma XO activity was measured before/after breakfast and before/after lunch (n = 5), as well as before/after ~1 h of mild aerobic exercise in another group (n = 5). All patients were provided standardized hospital diets three times a day (25–30 kcal/kg ideal bodyweight/day). Five patients underwent mild exercise therapies for 1 h after lunch, including slow pace walking and stretching under the supervision of specialized medical doctors. The heart rate of participants was strictly controlled to <120 b.p.m. during the course of exercise.
Measurement of metabolic parameters
Anthropometric variables were measured in the standing position. Body mass index (BMI) was calculated as weight (in kg) divided by the square of height in meters. Serum biochemical variables including aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ‐glutamyltransferase, triglyceride, high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, creatinine, fasting blood glucose and immunoreactive insulin (IRI) were measured with a conventional automated analyzer. High‐sensitivity C‐reactive protein (hs‐CRP) was measured by using nephelometry, a latex particle enhanced immunoassay (Behring Nephelometer II; Dade Behring Marburg GmbH, Marburg, Germany). High‐molecular weight adiponectin was measured by electro‐chemiluminescent enzyme immunoassay (Lumipulse Presto II, Fujirebio Inc., Tokyo, Japan) at SRL Inc. (Tokyo, Japan).
Multiple regression analyses to explore possible independent factors for directly influencing the plasma XO activity
A multiple linear regression analysis with plasma XO activity as a dependent variable, and sex (female = 1, male = 0), age, BMI, glycated hemoglobin (HbA1c), UA, estimated glomerular filtration rate (eGFR), natural logarithm (ln) AST, ln ALT, fasting IRI and homeostatic model assessment for insulin resistance (HOMA‐IR) as explanatory variables was carried out by using the least squared estimation. Because the serum value of AST and ALT, as well as the plasma value of XO activity, did not show a normal distribution, each value was converted logarithmically (ln AST, ln ALT and ln plasma XO activity, respectively), and subjected to a series of multiple regression analyses. In all the models developed (Table 2; model 1, 2 and 3), ln plasma XO activity was included as a dependent variable, and sex, age, BMI, HbA1c, UA and eGFR as common explanatory variables. We then carried out analyses by using ln AST, ln ALT and fasting IRI as independent variables (Table 2, model 1) and ln AST, ln ALT and HOMA‐IR as independent variables (Table 2, model 2), as well as HOMA‐IR as an independent variable (Table 2, model 3).
Statistical analysis
Data are expressed as mean ± standard error of the mean or mean ± standard deviation, where applicable. For a line of analyses, plasma XO activity, AST and ALT were natural logarithm transformed (ln) to normalize the skewed distribution. Statistical calculations for significant differences were carried out using the Wilcoxon signed‐rank test, Wilcoxon rank sum test and Spearman's rank correlation coefficients. Significance was accepted at P < 0.05. Statistical analyses were carried out using standard software packages (JMP version 12; SAS Institute Inc., Cary, NC, USA).
Results
Clinical characteristics of participants
The clinical characteristics of participants (n = 50) in analyses of plasma XO activity in relation to metabolic parameters RE summarized in Table 1. Their mean age was 55 ± 14 years and mean BMI was 29.5 ± 6.2 kg/m2. The numbers of patients who had used antidiabetic drugs were 12 (sulfonylureas or glinides), 26 (dipeptidyl peptidase‐4 inhibitors), 25 (metformin), four (α‐glucosidase inhibitors), four (thiazolidines) and three (sodium–glucose cotransporter 2 inhibitors).
Table 1.
Clinical characteristics of the study participants
| n (women:men) | 50 (24:26) |
| Age (years) | 55 ± 14 (22–79) |
| BMI (kg/m2) | 29.5 ± 6.2 (16.1–53.5) |
| HbA1c (%) | 7.8 ± 1.7 (5.4–12.3) |
| FPG (mg/dL) | 132 ± 34 (77–241) |
| FIRI (μIU/mL) | 11.1 ± 6.9 (1.8–34.2) |
| HOMA‐IR | 3.4 ± 2.1 (0.66–9.71) |
| γ‐GTP (IU/L) | 42 ± 29 (10–161) |
| Cre (mg/dL) | 0.7 ± 0.2 (0.4–1.2) |
| eGFR (mL/min/1.73 m2) | 85 ± 23 (40–138) |
| TG (mg/dL) | 172 ± 81 (59–417) |
| HDL‐C (mg/dL) | 45 ± 11 (23–73) |
| LDL‐C (mg/dL) | 108 ± 33 (41–168) |
| UA, women + men (mg/dL) | 5.8 ± 1.5 (2.4–9.2) |
| UA, women (mg/dL) | 5.6 ± 1.7 (2.8–9.2) |
| UA, men (mg/dL) | 6.0 ± 1.3 (2.4–7.9) |
| ln AST (IU/L) | 3.1 ± 0.50 |
| ln ALT (IU/L) | 3.3 ± 0.62 |
| ln XO activity, women + men (pmol IXP/min/mL) | −0.54 ± 1.1 |
| ln XO activity, women (pmol IXP/min/mL) | −0.40 ± 1.2 |
| ln XO activity, men (pmol IXP/min/mL) | −0.67 ± 1.0 |
Data are expressed as mean ± standard deviation (range) for normally distributed values and median (range) for non‐normally distributed values. ALT, aspartate aminotransferase; AST, alanine aminotransferase; BMI, body mass index; Cre, creatinine; eGFR, estimated glomerular filtration rate; FIRI, fasting immunoreactive insulin; FPG, fasting plasma glucose; γ‐GTP, γ‐glutamyltransferase; HbA1c, glycated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; HOMA‐IR, homeostatic model assessment for insulin resistance; IRI, immunoreactive insulin; IXP, isoxanthopterin; LDL‐C, low‐density lipoprotein cholesterol; ln, natural logarithm; TG, triglyceride; UA, uric acid; XO, xanthine oxidase.
Characterization of XO activity in plasma
The value of plasma XO activity was distributed across a wide range (0.05–5.40 pmol IXP/min/mL), with roughly 100‐fold variations from minimum to maximum levels in patients with type 2 diabetes mellitus and MetS (Figure 1). It is recognized that plasma XO activities in humans show considerably low values compared with those in rodents16, 17, 18, and therefore, a highly sensitive method to precisely detect the XO activities is required. In the present study, we measured XO activities using highly sensitive fluorometric assay, so we could detect the XO activities in all samples. We confirmed that the XO activities measured were completely blocked by febuxostat, which is a potent, selective XO inhibitor, thereby showing that we could determine the value of XO activities with high sensitivity and specificity.
Figure 1.
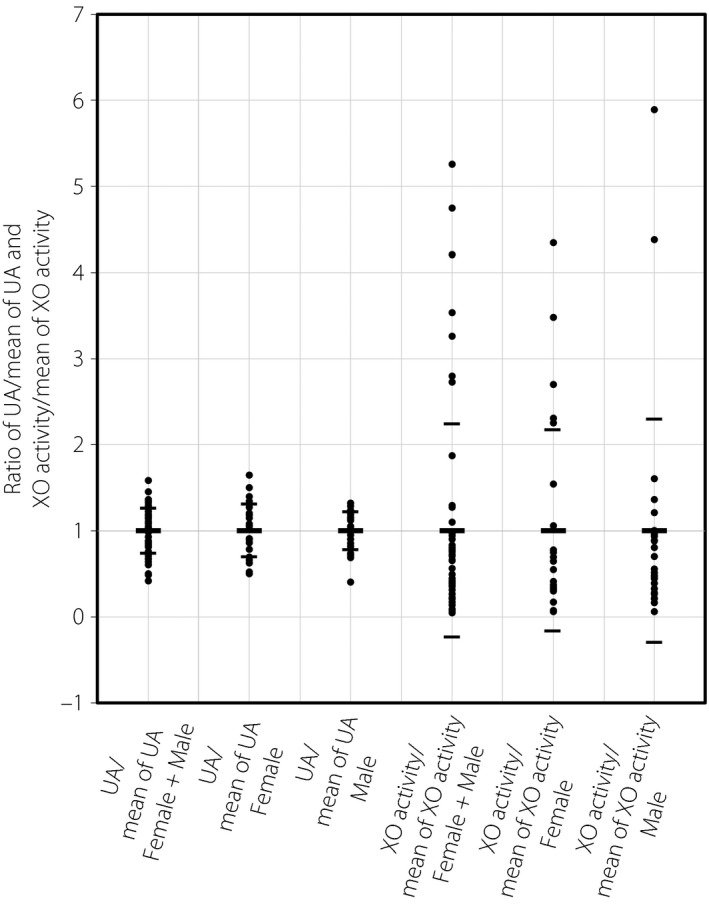
Distribution of the values of plasma xanthine oxidase (XO) activity and serum uric acid (UA) in patients with type 2 diabetes mellitus and metabolic syndrome. “UA/mean of UA” indicates the ratio of serum UA value vs the mean of serum UA value. “XO activity/mean of XO activity” indicates the ratio of plasma XO activity value vs the mean of plasma XO activity value. Small bars in each column show the respective range of standard deviations.
In contrast, the values of serum UA were distributed in a relatively narrow range (2.4–9.2 mg/dL), with roughly fourfold variations from minimum to maximum levels (Figure 1; Table 1). Consistent with a previous report1, the mean value of serum UA in men (6.0 mg/dL) showed a trend of being higher than that in women (5.6 mg/dL; Figure 1, left side; Table 1). Notably, there was no statistical difference in plasma XO activity between men and women (Figure 1, right side; Table 1).
Value of plasma XO activity throughout the day, before/after meals or mild aerobic exercise in lean patients without diabetes
To examine whether plasma XO activity shows diurnal variation, we measured plasma XO activity at 08.00, 15.00 and 23.00 hours, and 08.00 hours the next day in five lean patients without diabetes. There were no statistical changes in the value of plasma XO activity among the four time‐points (Figure 2a).
Figure 2.
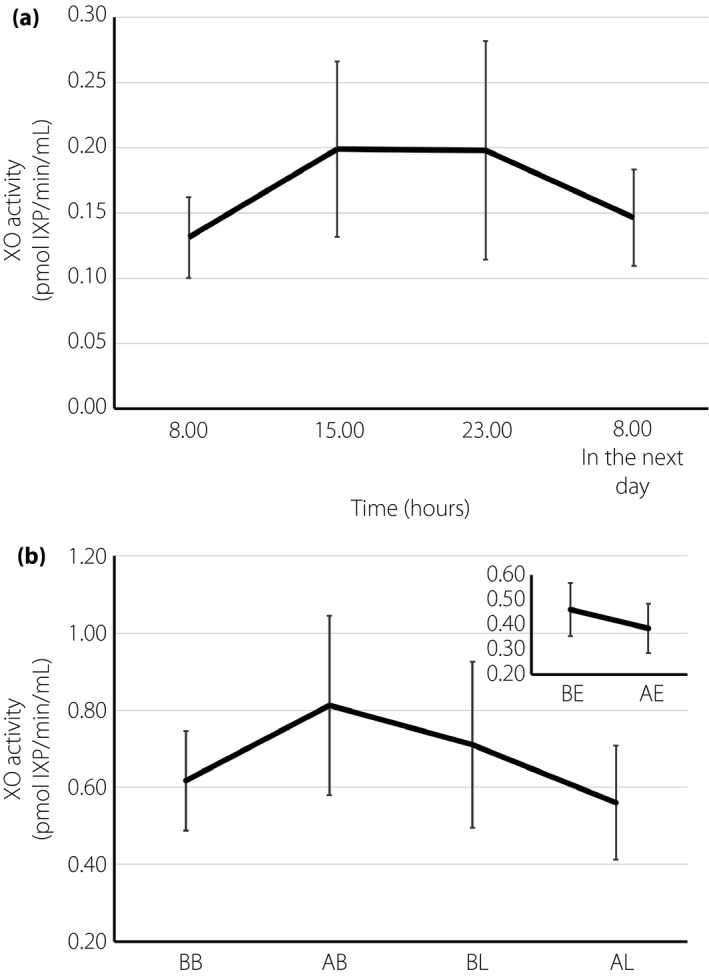
The value of plasma xanthine oxidase (XO) activity did not show apparent diurnal variation and was not altered by meals or aerobic mild exercise in lean patients without diabetes. (a) In lean patients without diabetes, plasma XO activity was measured at the point of 08.00, 15.00 and 23.00 hours, and 08.00 hours in the next day (n = 5). (b) In patients now being treated with insulin or oral drugs for hyperuricemia, plasma XO activities were measured before/after breakfast and before/after lunch (large graph, n = 5), as well as before/after ~1 h of mild aerobic exercise in another group (small graph, n = 5). Data are expressed as mean ± standard error of the mean, and were analyzed by Wilcoxon signed‐rank tests. BB, before breakfast; AB, after breakfast; BL, before lunch; AL, after lunch; BE, before exercise; AE, after exercise; IXP, isoxanthopterin.
To examine whether plasma XO activity was affected by meals or exercise, we measured plasma XO activity before/after breakfast or lunch, as well as before/after 1 h of mild aerobic exercise. There were no statistical changes in plasma XO activities between before and after the meals (breakfast, lunch) or mild aerobic exercise (Figure 2b).
Value of plasma XO activity in patients with type 2 diabetes mellitus and MetS
We assessed plasma XO activity in patients with type 2 diabetes mellitus and MetS, in comparison with lean patients without diabetes. Plasma XO activity in patients with type 2 diabetes mellitus and MetS (n = 50, median value 0.55 pmol IXP/min/mL) was significantly higher than that in lean patients without diabetes (n = 5, median value 0.13 pmol IXP/min/mL, P = 0.003). We also determined the plasma XO activity in patients with type 2 diabetes mellitus and in patients without type 2 diabetes mellitus but with MetS. Plasma XO activity showed no statistical difference between patients with type 2 diabetes mellitus (n = 43, median value 0.46 pmol IXP/min/mL) and patients without type 2 diabetes mellitus but with MetS (n = 7, median value 0.83 pmol IXP/min/mL, P = 0.146).
Correlations between the value of logarithmic plasma XO activity and metabolic parameters
Correlations between the value of ln plasma XO activity and that in a line of metabolic markers are shown in Figures 3, 4, 5, 6. Importantly, there was no correlation between ln XO activity and serum UA (Figure 3a). Serum UA level also showed no correlations with parameters of insulin resistance (Figure 3b,c). Noticeably, the level of ln XO activity was strongly correlated with fasting IRI and HOMA‐IR (Figure 4e,f). In addition, the level of ln XO activity was weakly but significantly correlated with BMI (Figure 4b). In contrast, ln XO activity was not correlated with age, HbA1c and FPG (Figure 4a,c,d).
Figure 3.
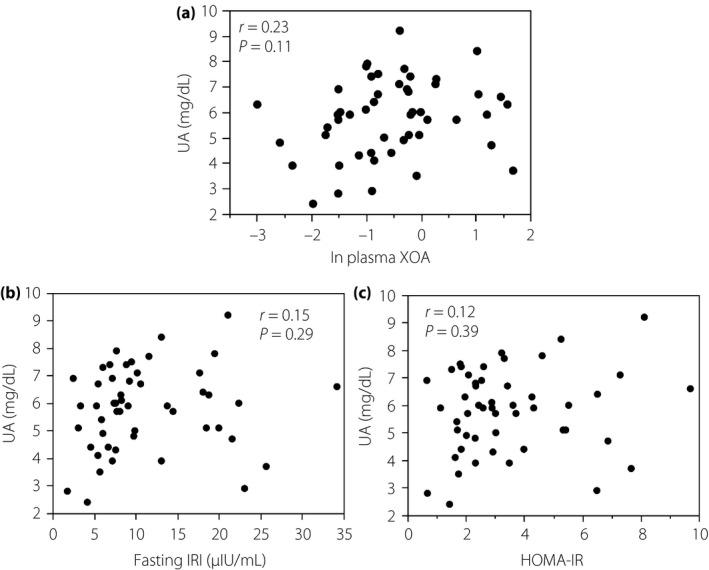
Serum uric acid (UA) level did not correlate with (a) plasma xanthine oxidase activity (XOA), (b) immunoreactive insulin (IRI) and (c) homeostatic model assessment for insulin resistance (HOMA‐IR) in patients with type 2 diabetes mellitus and metabolic syndrome. Data were analyzed by Spearman's rank correlation coefficients. ln, natural logarithm.
Figure 4.
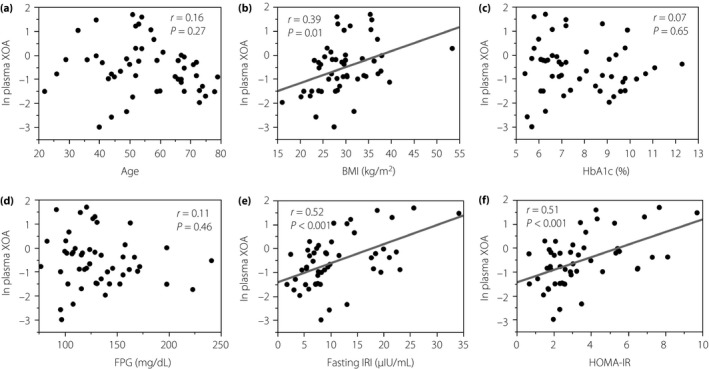
Plasma xanthine oxidase activity (XOA) showed positive correlations with indices of insulin resistance in patients with type 2 diabetes mellitus and metabolic syndrome. Data were analyzed by Spearman's rank correlation coefficients. BMI, body mass index; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; HOMA‐IR, homeostatic model assessment for insulin resistance; IRI, immunoreactive insulin; ln, natural logarithm.
Figure 5.
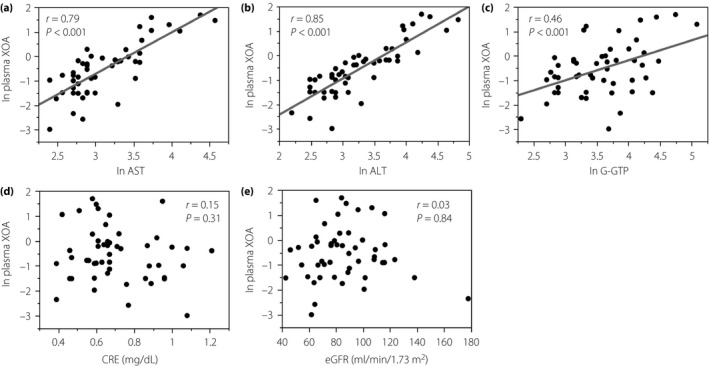
Correlations between natural logarithm (ln) plasma xanthine oxidase activity (XOA) and indices of liver dysfunction or parameters closely related to circulating uric acid level in patients with type 2 diabetes mellitus and metabolic syndrome. Data were analyzed by Spearman's rank correlation coefficients. AST, alanine aminotransferase; ALT, aspartate aminotransferase; CRE, creatinine; eGFR, estimated glomerular filtration rate; G‐GTP, ?‐glutamyltransferase; ln, natural logarithm.
Figure 6.
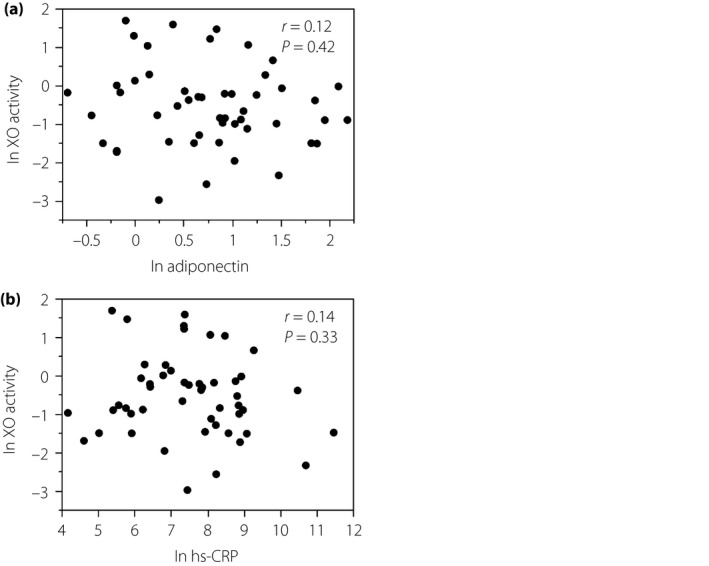
Correlations between natural logarithm (ln) plasma xanthine oxidase (XO) activity and (a) ln adiponectin or (b) ln high‐sensitivity C‐reactive protein (hs‐CRP) in patients with type 2 diabetes mellitus and metabolic syndrome. Data were analyzed by Spearman's rank correlation coefficients.
To examine whether indices of insulin resistance would be affected by receiving antidiabetic drugs in the patients studied, we assessed the correlation between ln XO activity and indices of insulin resistance in participants not taking metformins or thiazolidines, or sodium–glucose cotransporter 2 inhibitors (n = 19). In these participants, plasma XO activities were also correlated with indices of insulin resistance (fasting IRI r = 0.74, P < 0.001; HOMA‐IR r = 0.67, P = 0.001, respectively). In contrast, there was no statistical difference in the value of HOMA‐IR between patients treated with sulfonylureas or glinides (n = 12) and other patients (n = 38; P = 0.220).
Notably, the level of ln XO activity was strongly correlated with that of ln AST and ln ALT (Figure 5a,b), and was also weakly but significantly correlated with the Fibrosis‐4 score19, one of the representative markers for hepatic fibrosis (r = 0.32, P < 0.001). The level of plasma XO activity was also significantly correlated with that of ln γ‐glutamyltransferase (Figure 5c), whereas it was not correlated with creatinine and eGFR (Figure 5d,e), both of which are known to be associated with serum UA in patients with chronic kidney diseases.
To explore if ln XO activity would be associated with parameters of inflammation, we assessed serum levels of hs‐CRP and adiponectin, both of which are representative biomarkers for systemic inflammation (Figure 6). As serum levels of hs‐CRP and adiponectin did not show a normal distribution, they were converted logarithmically (ln hs‐CRP and ln adiponectin, respectively). Consequently, there were no significant relationships between ln XO activity and ln adiponectin (Figure 6a), as well as between ln XO activity and ln hs‐CRP (Figure 6b).
Multiple regression analyses to explore possible independent factors for directly influencing the plasma XO activity
To explore possible independent factors for directly influencing the plasma XO activity in patients with type 2 diabetes mellitus and MetS, we carried out multiple regression analyses (Table 2). As serum values of AST, ALT and plasma XO activity did not show a normal distribution, they were converted logarithmically (ln AST, ln ALT and ln plasma XO activity). In a series of analyses, ln XO activity was used as a dependent variable.
Table 2.
Multiple regression analysis with natural logarithm xanthine oxidase activity as a dependent variable and metabolic parameters as explanatory variables
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| Adjusted r 2 = 0.747 | Adjusted r 2 = 0.737 | Adjusted r 2 = 0.254 | ||||
| P < 0.001 | P < 0.001 | P = 0.006 | ||||
| β | P | β | P | β | P | |
| Sex | −0.051 | 0.582 | −0.046 | 0.613 | −0.163 | 0.281 |
| Age | 0.045 | 0.662 | 0.043 | 0.677 | −0.009 | 0.575 |
| BMI | 0.180 | 0.052 | 0.185 | 0.045 | 0.297 | 0.056 |
| HbA1c | 0.108 | 0.212 | 0.080 | 0.375 | −0.014 | 0.921 |
| UA | 0.071 | 0.416 | 0.066 | 0.451 | 0.050 | 0.736 |
| eGFR | 0.036 | 0.694 | 0.037 | 0.690 | −0.021 | 0.893 |
| ln AST | 0.188 | 0.314 | 0.171 | 0.371 | ||
| ln ALT | 0.614 | 0.001 | 0.628 | 0.001 | ||
| FIRI | 0.106 | 0.284 | ||||
| HOMA‐IR | 0.105 | 0.276 | 0.469 | 0.002 | ||
Multiple linear regression analysis with natural logarithm (ln) xanthine oxidase activity as a dependent variable, and sex (female = 1, male = 0), age, body mass index (BMI), glycated hemoglobin (HbA1c), uric acid (UA), estimated glomerular filtration rate (eGFR), ln alanine aminotransferase (AST), ln aspartate aminotransferase (ALT), fasting immunoreactive insulin (FIRI) and homeostatic model assessment for insulin resistance (HOMA‐IR) as explanatory variables was carried out by using the least squares estimation. In all the models developed (model 1, 2 and 3), plasma xanthine oxidase activity was included as a dependent variable, and sex, age, BMI, HbA1c, UA and eGFR as common explanatory variables. We then carried out analyses by using ln AST, ln ALT, and FIRI as independent variables (model 1), and ln AST, ln ALT and HOMA‐IR as independent variables (model 2), as well as HOMA‐IR as an independent variable (model 3). β, standard partial regression coefficient.
When values of age, BMI, sex, HbA1c, UA, eGFR, ln AST, ln ALT and fasting IRI were used as explanatory variables, the value of ln ALT was an independent strong factor for determining plasma XO activity (Table 2, model 1). When values of age, BMI, sex, HbA1c, UA, eGFR, ln AST, ln ALT and HOMA‐IR were used as explanatory variables, the value of ln ALT was also an independent strong factor for determining the plasma XO activity (Table 2, model 2). Furthermore, when values of age, BMI, sex, HbA1c, UA, eGFR and HOMA‐IR were used as explanatory variables without a line of parameters for liver functions including AST, ALT and γ‐glutamyltransferase, the value of HOMA‐IR was an independent factor for determining the plasma XO activity (Table 2, model 3). These data suggest that the values of ALT and HOMA index, a clinical marker for hepatic insulin resistance, might be independent factors for directly influencing the plasma XO activity in patients with type 2 diabetes mellitus and MetS.
Discussion
The major finding of the present study was that the value of plasma XO activity was significantly correlated with indices of insulin resistance and liver dysfunction in a small number of Japanese patients with type 2 diabetes mellitus and MetS. In contrast, the level of serum UA was not correlated with indices of insulin resistance. Importantly, the value of plasma XO activity was not correlated with the serum UA level. The multiple regression analyses further suggested that the value of plasma XO activity would be influenced by liver dysfunction (ALT) and hepatic insulin resistance (HOMA‐IR).
It is widely accepted that hyperinsulinemia followed by systemic insulin resistance causes elevation of serum UA levels through enhancement of renal proximal tubular sodium reabsorption20. Furthermore, insulin resistance in the liver, commonly seen in type 2 diabetes mellitus and MetS, alternatively activates the pentose phosphate pathway, resulting in activation of a de novo synthesis pathway of UA21. Importantly, the circulating level of UA is influenced by a variety of factors, including dehydration, purine‐ or fructose‐rich foods, alcohol and urinary sugar‐associated excretion of urate22. In this context, it might be critical to identify patients showing normal levels of serum UA with higher activity of XO, thereby uncovering hidden risks for type 2 diabetes mellitus and MetS.
In the present study, we showed that the value of plasma XO activity was correlated with that in liver transaminases (AST, ALT) in patients with type 2 diabetes mellitus and MetS. Such a positive correlation between plasma XO activity and liver dysfunction is consistent with the notion that leakage of XDH into the circulation and subsequent conversion of XDH to XO by proteases in the vasculature is exaggerated by hypoxia23, ischemia/reperfusion24, inflammation with tissue damage9 and viral infection25 in the liver. The present findings are also in accordance with the notion that XO is activated by hypoxia, inflammation and animal fat13.
A recent study in young and healthy individuals demonstrated that plasma XO activity was shown to correlate with BMI, indices of insulin resistance (quantitative insulin sensitivity check index) and serum levels of UA26. In contrast, the present study showed that plasma XO activity correlates with insulin resistance and dysfunction of the liver and BMI, but not with serum UA in middle‐aged (mean age 55 ± 14) patients with established type 2 diabetes mellitus and MetS. Although the mechanism whereby such a difference could occur between two studies is not fully understood, the average of age and severity of metabolic diseases would interfere with the results. To clarify this issue, further extensive studies are warranted. Also, in a previous report, plasma XO activity in patients with familial combined hyperlipidemia was correlated with indices of insulin resistance (HOMA‐IR), UA, high‐density lipoprotein cholesterol and inflammatory markers (nuclear factor‐κB, interleukin‐6, hs‐CRP)27. To our knowledge, the present study might be the first to show that the value of plasma XO activity is correlated with indices of insulin resistance and liver dysfunction independently of UA in patients with type 2 diabetes mellitus and MetS.
Importantly, the sensitivity and specificity of the methods to determine the level of plasma XO activity would also interfere with the results, because plasma XO activities in humans show a considerably low value compared with those in rodents16, 17, 18. It should also be noted that almost all of the previous reports on plasma XO activity measured UA or its stable isotope produced by XO, and estimated the value as an enzymatic activity of XO. However, such a method resulted in relatively low sensitivities28. Even in recent reports, considerable numbers of plasma samples were below the detectable range in assays of XO activity29, 30. In other assays, the production of ROS was measured for assessing plasma XO activity31. However, ROS was produced by oxidases other than XO, and was eliminated by anti‐oxidant enzymes, such as superoxide dismutase. It is therefore difficult to precisely measure the XO activity when ROS is used. In the present study, we measured XO activities using considerably high sensitivity fluorometric assay, thereby precisely estimating the plasma XO activities in all samples. We also confirmed that the XO activities measured were completely blocked by febuxostat, which is a potent, selective XO inhibitor. Taken together, in the present study, we were successful in determining the value of XO activities distributed in a very wide range.
We acknowledge that there were some limitations in the present study. First, due to the small sample size studied, the present study is defined as a pilot, exploratory study. Second, such a clinical, observational work as the current study could not clarify the direct mechanistic link between the elevation of plasma XO activity and pathophysiology of insulin resistance and/or liver dysfunction. However, previous works in rats showed that oxidative stress provoked by administration of xanthine and XO caused the elevation of plasma XO activity and insulin resistance32, suggesting a causal relationship between the two events. Regarding liver dysfunction, the elevation of plasma XO activity is a reflection of liver damage, such as inflammation or hypoxia, which is sometimes related with the pathophysiology of type 2 diabetes and obesity syndrome. In this context, further studies are warranted to clarify the possible pathophysiological relationship between the elevation of plasma XO activity and insulin resistance and/or liver dysfunction in humans. Third, participants diagnosed as type 2 diabetes mellitus and MetS had taken some medications including oral antidiabetic drugs. This would fluctuate the value of metabolic markers to a certain extent. For example, a previous report showed that plasma XO activity and thiobarbituric acid reactive substances were decreased after patients with type 2 diabetes mellitus took metformin33. In the present study, however, there was no statistical difference of plasma XO activities between participants taking metformin and not taking metformin (data not shown). Furthermore, in patients not treated with metformin, thiazolidinedione or sodium–glucose cotransporter 2 inhibitors, plasma XO activities were also significantly correlated with indices of insulin resistance (fasting IRI r = 0.80, P < 0.001; HOMA‐IR r = 0.67, P < 0.001, respectively). These results raise a possibility that the current medication of oral antidiabetic drugs would not strongly influence plasma XO activities in patients with type 2 diabetes mellitus and Mets, but further prospective studies are warranted to test this hypothesis.
In summary, in the present pilot, exploratory study, the value of plasma XO activity was significantly correlated with indices of insulin resistance and liver dysfunction in Japanese patients with type 2 diabetes mellitus and MetS. Plasma XO activity shows no apparent diurnal variations and no significant changes after meals or mild aerobic exercise. Through assessing plasma XO activities precisely in clinics, patients showing normal levels of serum UA with higher activity of XO can be screened, thereby possibly providing a clue to uncover hidden risks for type 2 diabetes mellitus and MetS.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
This work was supported in part by Grants‐in‐Aid from the Japan Society for the Promotion of Science (JSPS; KAKENHI grant numbers 15K19520 and 24591338), Council for Science, Technology and Innovation (CSTI), Cross‐ministerial Strategic Innovation Promotion Program (SIP), “Technologies for Creating Next‐Generation Agriculture, Forestry and Fisheries”, Lotte Foundation, Japan Foundation for Applied Enzymology, New Energy and Industrial Technology Development Organization (NEDO), Project for Formation of Life Science Network (Pharmaceutical field), the Promotion Project of Medical Clustering of Okinawa prefecture and a grant from Okinawa Prefecture for Promotion of Advanced Medicine. We thank Y Murayama and T Ikematsu for excellent technical help. We are also grateful to M Hirata, I Asato, H Kaneshiro, T Uema and C Noguchi for excellent secretarial assistance.
J Diabetes Investig 2019; 10: 94–103
References
- 1. Levine W, Dyer AR, Shekelle RB, et al Serum uric acid and 11.5‐year mortality of middle‐aged women: findings of the Chicago Heart Association Detection Project in Industry. J Clin Epidemiol 1989; 42: 257–267. [DOI] [PubMed] [Google Scholar]
- 2. Fang J, Alderman MH. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow‐up study, 1971‐1992. National Health and Nutrition Examination Survey. JAMA 2000; 283: 2404–2410. [DOI] [PubMed] [Google Scholar]
- 3. Culleton BF, Larson MG, Kannel WB, et al Serum uric acid and risk for cardiovascular disease and death: the Framingham Heart Study. Ann Intern Med 1999; 131: 7–13. [DOI] [PubMed] [Google Scholar]
- 4. Moriarity JT, Folsom AR, Iribarren C, et al Serum uric acid and risk of coronary heart disease: Atherosclerosis Risk in Communities (ARIC) Study. Ann Epidemiol 2000; 10: 136–143. [DOI] [PubMed] [Google Scholar]
- 5. Battelli MG, Polito L, Bolognesi A. Xanthine oxidoreductase in atherosclerosis pathogenesis: not only oxidative stress. Atherosclerosis 2014; 237: 562–567. [DOI] [PubMed] [Google Scholar]
- 6. White CR, Darley‐Usmar V, Berrington WR, et al Circulating plasma xanthine oxidase contributes to vascular dysfunction in hypercholesterolemic rabbits. Proc Natl Acad Sci U S A 1996; 93: 8745–8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Martin HM, Hancock JT, Salisbury V, et al Role of xanthine oxidoreductase as an antimicrobial agent. Infect Immun 2004; 72: 4933–4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kelley EE. Dispelling dogma and misconceptions regarding the most pharmacologically targetable source of reactive species in inflammatory disease, xanthine oxidoreductase. Arch Toxicol 2015; 89: 1193–1207. [DOI] [PubMed] [Google Scholar]
- 9. Houston M, Estevez A, Chumley P, et al Binding of xanthine oxidase to vascular endothelium. Kinetic characterization and oxidative impairment of nitric oxide‐dependent signaling. J Biol Chem 1999; 274: 4985–4994. [DOI] [PubMed] [Google Scholar]
- 10. Berry CE, Hare JM. Xanthine oxidoreductase and cardiovascular disease: molecular mechanisms and pathophysiological implications. J Physiol 2004; 555: 589–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Spiekermann S, Landmesser U, Dikalov S, et al Electron spin resonance characterization of vascular xanthine and NAD (P) H oxidase activity in patients with coronary artery disease: relation to endothelium‐dependent vasodilation. Circulation 2003; 107: 1383–1389. [DOI] [PubMed] [Google Scholar]
- 12. Otaki Y, Watanabe T, Kinoshita D, et al Association of plasma xanthine oxidoreductase activity with severity and clinical outcome in patients with chronic heart failure. Int J Cardiol 2017; 228: 151–157. [DOI] [PubMed] [Google Scholar]
- 13. Tsushima Y, Nishizawa H, Tochino Y, et al Uric acid secretion from adipose tissue and its increase in obesity. J Biol Chem 2013; 288: 27138–27149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alberti KG, Zimmet P, Shaw J, et al The metabolic syndrome‐a new worldwide definition. Lancet 2005; 366: 1059–1062. [DOI] [PubMed] [Google Scholar]
- 15. Beckman JS, Parks DA, Pearson JD, et al A sensitive fluorometric assay for measuring xanthine dehydrogenase and oxidase in tissues. Free Radic Biol Med 1989; 6: 607–615. [DOI] [PubMed] [Google Scholar]
- 16. Wajner M, Harkness RA. Distribution of xanthine dehydrogenase and oxidase activities in human and rabbit tissues. Biochim Biophys Acta 1989; 991: 79–84. [DOI] [PubMed] [Google Scholar]
- 17. Muxfeldt M, Schaper W. The activity of xanthine oxidase in heart of pigs, guinea pigs, rabbits, rats, and humans. Basic Res Cardiol 1987; 82: 486–492. [DOI] [PubMed] [Google Scholar]
- 18. Abadeh S, Killacky J, Benboubetra M, et al Purification and partial characterization of xanthine oxidase from human milk. Biochim Biophys Acta 1992; 1117: 25–32. [DOI] [PubMed] [Google Scholar]
- 19. Shah AG, Lydecker A, Murray K, et al Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009; 7: 1104–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Facchini F, Chen YD, Hollenbeck CB, et al Relationship between resistance to insulin‐mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. JAMA 1991; 266: 3008–3011. [PubMed] [Google Scholar]
- 21. Leyva F, Wingrove CS, Godsland IF, et al The glycolytic pathway to coronary heart disease: a hypothesis. Metabolism 1998; 47: 657–662. [DOI] [PubMed] [Google Scholar]
- 22. Yamamoto T, Moriwaki Y, Takahashi S. Effect of ethanol on metabolism of purine bases (hypoxanthine, xanthine, and uric acid). Clin Chim Acta 2005; 356: 35–57. [DOI] [PubMed] [Google Scholar]
- 23. Aslan M, Ryan TM, Adler B, et al Oxygen radical inhibition of nitric oxide‐dependent vascular function in sickle cell disease. Proc Natl Acad Sci U S A 2001; 98: 15215–15220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tan S, Gelman S, Wheat JK, et al Circulating xanthine oxidase in human ischemia reperfusion. South Med J 1995; 88: 479–482. [DOI] [PubMed] [Google Scholar]
- 25. Wolko K, Krawczyński J. Diagnostic value of determination of serum xanthine oxidase activity in acute viral hepatitis. Mater Med Pol 1974; 6: 95–98. [PubMed] [Google Scholar]
- 26. Washio KW, Kusunoki Y, Murase T, et al Xanthine oxidoreductase activity is correlated with insulin resistance and subclinical inflammation in young humans. Metabolism 2017; 70: 51–56. [DOI] [PubMed] [Google Scholar]
- 27. Martinez‐Hervas S, Real JT, Ivorra C, et al Increased plasma xanthine oxidase activity is related to nuclear factor kappa beta activation and inflammatory markers in familial combined hyperlipidemia. Nutr Metab Cardiovasc Dis 2010; 20: 734–739. [DOI] [PubMed] [Google Scholar]
- 28. Kizaki H, Sakurada T. Simple micro‐assay methods for enzymes of purine metabolism. J Lab Clin Med 1977; 89: 1135–1144. [PubMed] [Google Scholar]
- 29. Murase T, Nampei M, Oka M, et al A highly sensitive assay of human plasma xanthine oxidoreductase activity using stable isotope‐labeled xanthine and LC/TQMS. J Chromatogr B Analyt Technol Biomed Life Sci 2016; 1039: 51–58. [DOI] [PubMed] [Google Scholar]
- 30. Fujimura Y, Yamauchi Y, Murase T, et al Relationship between plasma xanthine oxidoreductase activity and left ventricular ejection fraction and hypertrophy among cardiac patients. PLoS ONE 2017; 12: e01826993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Real JT, Martínez‐Hervás S, García‐García AB, et al Circulating mononuclear cells nuclear factor‐kappa B activity, plasma xanthine oxidase, and low grade inflammatory markers in adult patients with familial hypercholesterolaemia. Eur J Clin Invest 2010; 40: 89–94. [DOI] [PubMed] [Google Scholar]
- 32. Salim S, Asghar M, Chugh G, et al Oxidative stress: a potential recipe for anxiety, hypertension and insulin resistance. Brain Res 2010; 1359: 178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cosić V, Antić S, Pesić M, et al Monotherapy with metformin: does it improve hypoxia in type 2 diabetic patients? Clin Chem Lab Med 2001; 39: 818–821. [DOI] [PubMed] [Google Scholar]


