Abstract
Lipid droplets are dynamic organelles that store neutral lipids during times of energy excess and serve as an energy reservoir during deprivation. Many prevalent metabolic diseases, such as the metabolic syndrome or obesity, often result in abnormal lipid accumulation in lipid droplets in the liver, also called hepatic steatosis. Obesity-related steatosis, or NAFLD in particular, is a major public health concern worldwide and is frequently associated with insulin resistance and type 2 diabetes mellitus. Here, we review the latest insights into the biology of lipid droplets and their role in maintaining lipid homeostasis in the liver. We also offer a perspective of liver diseases that feature lipid accumulation in these lipid storage organelles, which include NAFLD and viral hepatitis. Although clinical applications of this knowledge are just beginning, we highlight new opportunities for identifying molecular targets for treating hepatic steatosis and steatohepatitis.
The liver is a central and major integrator of metabolism, and a properly functioning liver is essential to health. A variety of conditions result in abnormal lipid accumulation in the liver, socalled hepatic steatosis. Obesityrelated steatosis or NAFLD is the hepatic manifestation of the metabolic syndrome and represents a huge public health problem. The estimated prevalence of NAFLD in the general population in North America alone is 20–30%, whereas the prevalence in adults with morbid obesity is 75–92%, and 60–70% in patients with type 2 diabetes mellitus1. China is poised for a major epidemic of hepatic steatosis and its ensuing complications, given the increasing incidence of obesity and the metabolic syndrome in that country2. More than 28% of Chinese men and 27% of Chinese women are overweight or obese3. Patients with hepatic steatosis are at risk of serious complications, including progression to steato hepatitis, fibrosis, cirrhosis, liver failure and hepato cellular carcinoma4,5. Currently, the only effective treatment option for NAFLD is weight loss; there are no pharmaceutical treatments approved5, although a number are in development6. The net result is that NAFLD is the second leading liver disease among adults awaiting liver transplantation in the USA alone and is predicted to become the leading cause7. One could expect this trend to be repeated in other countries.
The excess lipids in hepatic steatosis are primarily neutral lipids, such as triglycerides and cholesterol esters. In hepatocytes and other liver cells (for example, hepatic stellate cells (HSCs) and Kupffer cells), neutral lipids are stored in dynamic organelles called lipid droplets (LDs). During the past decade, new insights have emerged with respect to LD biology and, in particular, their protein composition, lifecycle in cells, and how abnormalities in LD biology contribute to disease. Here, we review the basic biology of LDs that underlies hepatic steatosis and diseases that result in lipid accumulation in the liver. We do not comprehensively review hepatic steatosis or its progression (for this aspect, see other reviews elsewhere4,8,9), but instead offer a view of hepatic steatosis from the perspective of the basic biology of lipid storage in cells. For the purposes of this Review, we focus mainly on LD biology in hepatocytes.
Hepatic lipid metabolism
The liver is a central hub for lipid metabolism, with uptake, esterification, oxidation and secretion of fatty acids (FAs) all occurring in hepatocytes. FAs in the liver originate from the diet (15–30% of the liver FA pool), de novo lipogenesis (up to 30% of liver FA pools during feeding) and recycling of FAs released from adipose tissue during fasting10.
Dietary triglycerides are broken down into FAs in the intestinal lumen and taken up by the enterocyte. There, the FAs can be temporarily stored in lipid droplets, or packaged as triglyceride or cholesterol ester in chylomicrons for secretion11. The chylomicrons deliver triglyceride to peripheral tissues, and the remaining remnant particles are delivered to the liver12. Their uptake into hepatocytes is not completely understood, but it is mediated by the LDL receptor and LDL receptor–related protein (LRP)13–15. Chylomicrons also transport dietary retinol that has been esterified in the intestine16. These retinyl esters are then hydrolysed to free retinol in hepatocytes, and most are transferred to HSCs for storage17,18. Retinoids bound to retinolbinding protein can also be taken up directly by HSCs from the circulation18.
During the fed state, the liver has a crucial role in storing excess carbohydrates as lipid via de novo lipogenesis. Essentially, twocarbon units (acetylCoA) derived from glycolysis are used to synthesize longchain FAs in the cytoplasm. This process is regulated by transcriptional factors, such as sterol regulatory element binding protein 1 (SREBP1)19, carbohydrate response element binding protein (ChREBP)20 and liver × receptors20,21. These transcription factors are activated by insulin, enabling the expression of lipogenic genes, such as acetylcoenzyme A carboxylase and fatty acid synthase. FAs derived from de novo lipogenesis can be esterified in a series of enzymatic reactions, culminating in formation of triglyceride, most likely primarily by diacylglycerol Oacyltransferase (DGAT)2 (REFS 22–24) and stored in LDs.
The liver also has a major role in distributing lipids to other organs5. FAs in the liver can be converted to triglyceride and cholesterol ester to be secreted as verylowdensity lipoprotein (VLDL) particles25. The composition and biogenesis of LDs and VLDLs are different. VLDL formation begins with the synthesis of apolipoproteinB100 (apoB100)25 and is followed by two stages of lipidation26,27. During translation, apoB100 is initially lipidated by microsomal triglyceride transfer protein28, which helps transfer neutral and polar lipids to apoB100 to form preVLDL or a nascent lipo protein29. The endoplasmic reticulum (ER) luminal enzyme carboxyl esterase triacylglycerol lipase (also known as CES3 in mouse, CES1 in human) has been implicated in mobilizing triglyceride for secretion as part of VLDL30. Cell death activator CIDEB (CIDEB), highly expressed in the liver, localizes to the ER membrane and has been proposed to function in the initial lipidation process by associating with LDs and apoB100 (REF. 31). This function is further supported by the phenotype of Cidebknockout mice, which have circulating VLDLs with reduced triglyceride content31. The final maturation and lipidation of the nascent lipoprotein particle to VLDL are thought to occur elsewhere, possibly along the secretory pathway32–34. The mechanism by which VLDL is transported out of the liver remains mostly unclear, but apoB100 exits the ER in COPII vesicles34, and VLDL particles are eventually secreted in the plasma to be taken up by other organs for energy25. VLDL export from the ER is mediated by TANGO1 and TALI (Mia2–cTAGE5 fusion) that enable the fusion of ER–Golgi intermediate compartment membrane to ER exit sites necessary for the subsequent export of VLDL particles35,36.
Imbalances in hepatic lipid metabolism
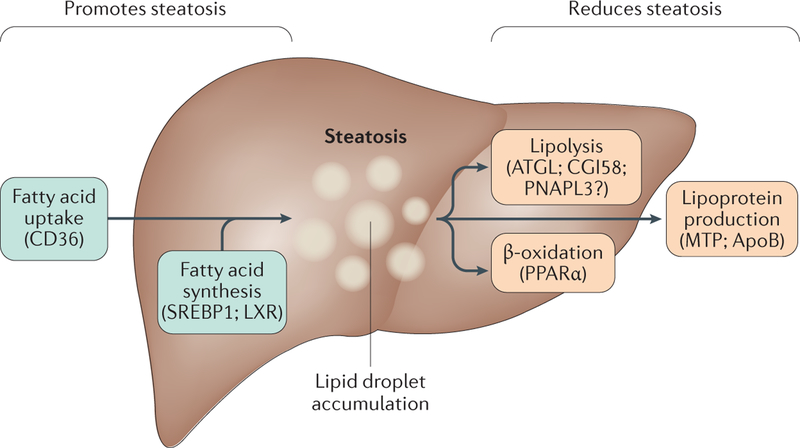
Imbalances of the processes that maintain the normal homeostasis of lipid metabolism in the liver can lead to nonphysiological accumulation of triglyceride in hepatocytes, or steatosis5. Conceptually, excess triglyceride storage in LDs results from different processes: increased triglyceride synthesis combined with LD biogenesis or growth; decreased LD catabolism (including decreased fatty acid oxidation); or impaired triglyceride or VLDL secretion (FIG. 1).
Figure 1 |. Hepatic steatosis results from an imbalance in lipid storage and lipolysis or secretion.
Hepatic steatosis can result from different processes: increased fatty acid (FA) uptake, de novo lipogenesis and triglyceride synthesis combined with lipid droplet (LD) biogenesis or growth; decreased LD catabolism (including decreased fatty acid oxidation); or impaired triglyceride or very-low-density lipoprotein (VLDL) secretion. Factors associated with these processes are listed in the figures, those that upregulate steatosis (green boxes) and those that downregulate steatosis (orange boxes).
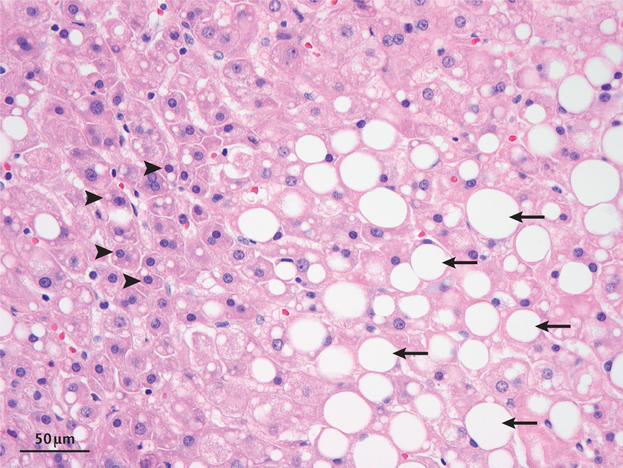
Historically, steatosis has been classified pathologically as microvesicular steatosis (accumulation of small fat droplets with preserved cellular architecture) and macrovesicular steatosis (the formation of larger droplets that displaces the nucleus)37. In both of these situations, the LDs in hepatocytes are much larger (several microns in diameter) than the LDs normally found in the cytoplasm of most other culture cells (between 0.5–2 μm) (T.C.W. and R.V.F., unpublished observations) (FIG. 2). The mechanisms for generating larger LDs are discussed later. The factors promoting progression from simple steatosis to steatohepatitis, fibrosis and cirrhosis in the setting of NAFLD are less clear, but two large longitudinal studies showed that fibrosis is the strongest predictor of liverrelated complications and diseasespecific mortality38,39. Fibrosis is related to activation of HSCs, leading to secretion of extracellular matrix, and this process is tied to lipid metabolism4,18.
Figure 2 |. Histopathology of NAFLD showing areas of macrosteatosis and microsteatosis.
A haematoxylin and eosin-stained tissue sample from a human showing hepatocytes exhibiting microvesicular (arrowheads) and macrovesicular (arrows) steatosis. Hepatocytes with microvesicular steatosis have abnormal accumulation of lipid with preserved cellular architecture, including a non-displaced nucleus, whereas hepatocytes with macrovesicular steatosis have one large droplet that displaces the nucleus. We thank S. Alexandrescu at Boston Children’s Hospital, USA, for this image.
Cell biology of lipid droplets
The basic biology of LDs is only now being unravelled. Thus, there is still a large disconnect between our understanding of LD biology and how this knowledge relates to NAFLD disease progression and the potential for therapeutics. Here we review the basic cell biology of LDs, which is beginning to reveal how these organelles relate to NAFLD pathophysiology.
Today, we know that LDs are highly dynamic organelles with specific cellular machinery regulating each aspect of their biology. That was not always the case. LDs were first described by Richard Altmann and E. B. Wilson in 1890 and 1896, respectively, as fat droplets inside cells40,41. Because LDs were assumed to be inert forms of fat storage, investigation of their biology was limited for much of the next century. This aspect changed, however, in 1991 when Dean Londos and his laboratory discovered that LDs contained specific proteins, the most abundant of which he named perilipin42. It became apparent that perilipin regulates major aspects of lipid metabolism and LD cell biology, and a new view of LD biology began to emerge.
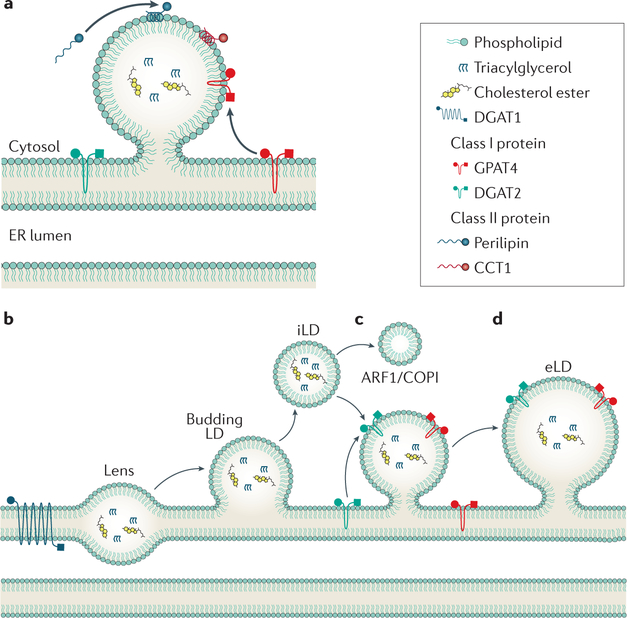
LDs are composed of a core of neutral lipids that is surrounded by a phospholipid monolayer and specific proteins that bind to their surfaces (FIG. 3a). The presence of LDs converts cells to emulsions, in which LDs are the dispersed, hydrophobic phase and the cytosol is the aqueous, continuous phase43. Depending on the type of cell and its metabolic state, the oil phase consists mainly of triglycerides and cholesterol esters, but other neutral lipids, such as ether lipids and waxes, are also found in LDs44. For example, the LDs of HSCs contain retinyl esters comprising ~70% of total body retinoid content17. At the interface of neutral lipids with water, molecules of both sides interact with molecules that are part of the other, nonpreferred phase. The energy penalty due to these unfavourable interactions is called surface tension and drives oil and water mixtures to evolve to minimize the surface area between phases43. To prevent the coalescence of droplets, cells coat the hydrophobic core of LDs with a surface monolayer of phospholipids44, which act as surfactants and stabilize the dispersed particles. In particular, phosphatidylcholine, because of its ability to reduce surface tension, seems to have a key role in LD biology45. Other phospholipids are also important in stabilizing LDs, and the LD phospholipid surface and ER membrane lipid composition seem to be similar46.
Figure 3 |. Lipid droplet formation and expansion.
a | Lipid droplets (LDs) consist of a neutral lipid core surrounded by a phospholipid monolayer. Proteins access the LD surface by relocalizing from the ER bilayer (class I) or from the cytosol (class II). b | LD formation begins with neutral lipid synthesis. The lipids accumulate in the ER bilayer to form a lens. c | Eventually, the bilayer deforms and causes the droplet to bud forming an initial LD (iLD). d | COPI can bud nano-LDs from the iLD, resulting in increased surface tension and reconnection of the iLD with the ER. This contact allows class I proteins to access the droplet, including GPAT4 and DGAT2. These enzymes are involved in triglyceride synthesis and result in LD growth, forming an expanding LD.
Across different cell types, the size and number of LDs differ considerably. Nearly all cells seem to form small droplets (300–800 nm diameter) that are designated initial LDs (iLDs). Numbers of iLDs in cells range from four to eight in yeast cells up to hundreds in cultured mammalian cells44. Later in LD formation, most cells convert some iLDs to larger socalled expanding LDs (eLDs, >1 μm diameter), giving rise to two distinct LD types within cells47. Specific cell types, such as adipocytes and hepatocytes, have even larger LDs (socalled giant LDs, up to tens of microns in diameter).
Neutral lipid synthesis and LD formation
Neutral lipids of LDs are synthesized by enzymes that reside primarily in the ER, and triglycerides are the neutral lipid most often found in hepatic steatosis5,48. Triglyceride synthesis is catalysed by DGAT1 and DGAT2 (REFS 49,50). Although both DGAT enzymes synthesize triglyceride, the enzymes are unrelated by sequence and seem to have distinct substrate specificities and subcellular localizations48. Current evidence suggests that DGAT1, an ER enzyme, is responsible for esterifying excess fatty acids that otherwise would cause toxicity to the ER membrane51. DGAT2, by contrast, seems to be more closely linked to de novo lipogenesis and to have a primary role in triglyceride storage22–24. Studies of selective DGAT1 and DGAT2 chemical inhibitors lend support to these respective functions. Two studies in HepG2 cells show that DGAT1 catalyses the reesterification of triglycerides via utilization of exogenously supplied FAs, whereas DGAT2 has a minor role in reesterification, but mediates esterification of newly synthesized FAs23,24. Both DGAT enzymes have emerged as drug targets (discussed later).
In addition to triglyceride, hepatocytes have a large capacity to synthesize and store cholesterol esters. Cholesterol ester synthesis is catalysed by the acyl CoA:cholesterol acyltransferase (ACAT) enzymes, ACAT1 and ACAT2 (also known as sterol Oacyltransferase 1 and 2, respectively)52–55. Both ACAT1 and ACAT2 are expressed in human hepatocytes, although their relative contributions to cholesterol ester synthesis in the human liver are debated. In immunodepletion experiments, ACAT1 has been reported to be responsible for as much as 90% of cholesterol ester synthesis in adult human liver, with ACAT2 accounting for most of ACAT activity in fetal human liver56,57. However, ACAT2knockout mice have almost no ACAT activity in the liver58, and another study investigating adult human liver samples treated with ACAT2 inhibitor shows that ACAT2 accounted for >50% of ACAT activity59. The relative contribution of ACAT enzymes to liver cholesterol esters in humans, therefore, remains uncertain.
How neutral lipids, such as triglyceride, are packaged into LDs is beginning to be determined60–62. A current model posits that neutral lipids accumulate within the ER bilayer due to synthesis by enzymes, such as DGAT1 or ACAT1 or ACAT2 (REFS 60,63) (FIG. 3b). Once a critical concentration of neutral lipids is reached, there is phase separation to generate lipid lenses, and these grow and deform the ER bilayer membrane until they reach a critical size that triggers budding of iLDs into the cytoplasm60 (FIG. 3c). At some point during this reaction, LDs acquire a number of specific proteins, such as perilipins64, which target the organelles from the cytoplasm.
Although it is now generally accepted that the ER is the site of LD formation65–68, many questions remain that have not been directly addressed. What bio physical processes mediate the formation of lipid lenses? At what size do LDs bud off into the cytoplasm? Do they really bud off, or do LDs remain connected with the ER, essentially forming a subdomain of this organelle? In addition, a number of proteins, including seipin, encoded by the lipodystrophy gene BSCL2, and fat storage inducing transmembrane protein 2 (FITM2, also known as FIT2) have been implicated in LD formation69–71. Seipin is required for early steps of LD formation72–74 and has been shown in multiple cell lines to be involved in the maturation of nascent LDs to mature iLDs73. FITM2 has been implicated in the budding of LDs toward the cytosol68. However, the molecular functions of seipin and FITM2 with respect to LD formation remain largely unclear.
LD growth
Once iLDs form, a subset of them can be converted to eLDs47, which grow to larger sizes by localized triglyceride synthesis (FIG. 3d). This process is mediated by a class of triglyceride synthesis enzymes that relocalize from the ER to the surfaces of this LD population47. Among the enzymes that relocalize from the ER to LDs are glycerol3phosphate acyltransferase 4 (GPAT4) and DGAT2, which catalyse the first and last steps of triglycer ide synthesis, respectively. Interestingly, some triglyceride synthesis enzymes, such as DGAT1, always remain in the ER. DGAT1 contains multiple membranespanning domains with large ER luminal domains that anchor the protein in the ER75. By contrast, GPAT4 and maybe DGAT2 have most of their protein mass in the cytoplasm and are anchored by a membraneembedded segment that does not seem to span the entire bilayer and might adopt a hairpin conformation47. This topology, lacking ER luminal domains, is compatible with localization to either the ER bilayer or the LD surface monolayer76,77.
Protein targeting to LDs
The targeting of GPAT4, and probably other enzymes of the eLD pathway, occurs through membrane bridges between the ER and LDs, which provide physical continuity between the surfaces of the organelles47,65. How these bridges are established and maintained is unclear, but the ADP ribosylation factor 1 (ARF1)–COPI vesiclegenerating machinery has a crucial role78–80. When members of the ARF1–COPI machinery are depleted from cells, GPAT4 and other proteins fail to target to eLDs, and as a consequence, LDs remain a uniform size, failing to expand78. Current evidence suggests a model in which ARF1–COPI proteins perform this function not by generating transport carriers to the LD (analogous to its function in ER–Golgi trafficking), but by directly modulating the LD surface. In this model, ARF1–COPI buds off nanovesicles from the LD surface, removing mostly phospholipids, resulting in increased LD surface tension43. This increase in surface tension destabilizes the LD, enabling it to fuse with the ER and establishing a bridge (FIG. 3d). In addition to this targeting pathway from the ER, a number of proteins access LDs directly from the cytoplasm. How specificity of this targeting (versus targeting of other membranes) is achieved is poorly understood currently81.
LD proteins
The protein composition of LDs varies among cell types. For liver tissue and hepatocytes, several studies reported on the LD proteome82–85. Those proteomic experiments were performed on the LD fraction and led to identification of a large number of proteins. Only a few candidates were found in common between these studies, such as PLIN2 and PLIN3, suggesting many nonspecific proteins were identified due to variation in LD purification protocols and contamination with other organelles. Interestingly, PLIN2 and PLIN3 proteins are upregulated in the setting of NAFLD (discussed later). LD surface proteins are not limited to perilipins and neutral lipid synthesis enzymes. Indeed, many proteomic studies performed in nonliver samples and in various organisms show that LDs display a diverse set of proteins at their surface84. Among these are members of the CIDE family of proteins involved in LD growth86 and enzymes involved in breakdown of neutral lipids, such as adipose triglyceride lipases87.
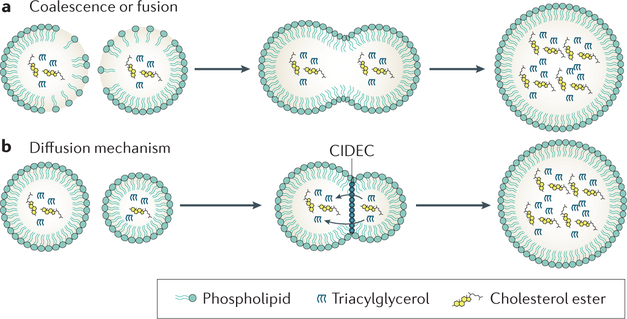
Giant LD formation
LDs in hepatocytes and adipocytes are often quite large (for example, >1–2 μm diameter) and are called giant or supersized LDs. These droplets arise from one of two processes. They can form by coalescence (for example, when there is insufficient phosphatidylcholine to cover the LD surface to lower surface tension to maintain a metastable state), or through proteinmediated, facilitated diffusion of neutral lipids from one LD to another43 (FIG. 4). In this ripening process, the neutral lipids diffuse from the smaller to the larger LD, owing to a higher LaPlace in smaller LDs. The CIDE family of proteins seem to play a unique part in mediating this mechanism of LD growth88. Cell death activator CIDEA (CIDEA) and cell death activator CIDEC (CIDEC, also known as fatspecific protein 27 or FSP27), for example, are found at LD–LD contact sites, especially in adipocytes, and promote exchange of lipids between LDs88. CIDEB has also been proposed to mediate lipid transfer in the process of VLDL lipidation31,86.
Figure 4 |. Giant lipid droplet formation.
Giant lipid droplets (LDs) form in one of two ways. a | Coalescence, which occurs for example during relative phosphatidylcholine deficiency, results in rapid fusion of two LDs. b | In a process called ripening, neutral lipids are transferred by the slower process of diffusion with a net transfer from smaller to larger LDs. Ripening seems to be facilitated by proteins such as CIDEC (also known as FSP27).
LD consumption
FAs can be mobilized from the LD neutral lipid pool for metabolic fuel. This step occurs either through the action of lipases that act directly on the LD surface or by delivering LDs to lysosomes where lysosomal acid lipase hydrolyses lipids (FIG. 5).
Figure 5 |. Lipid droplet consumption.
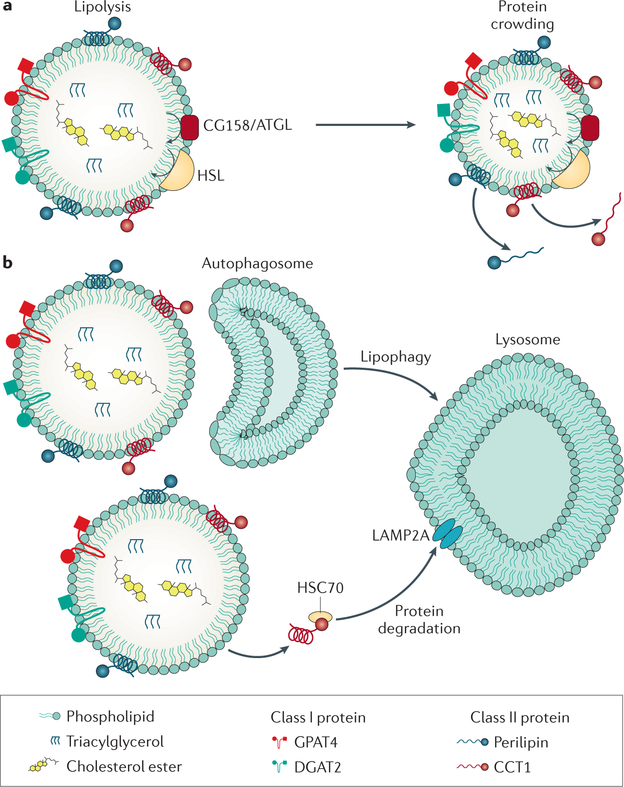
a | Lipid droplets (LDs) can be degraded by lipolysis. As the surface of the LD shrinks, there is protein crowding and some proteins, especially class II proteins, fall off. b | Other proteins are removed from the LD surface and brought to the lysosome by chaperone-mediated autophagy. Small LDs or parts of an LD can be engulfed by a membrane bilayer to form autophagosomes that can be delivered to the lysosome for degradation.
LD lipases.
Our current understanding about lipase action on LDs is derived chiefly from work in adipocytes. Patatinlike phospholipase domain containing (PNPLA)2 (also known as ATGL) catalyses the first step of triglyceride hydrolysis on the LD surface to generate diacylglycerol and free FA87. Diacylglycerol is then cleaved into monoacylglycerol and FA by hormonesensitive lipase, which also has broad substrate specificity (hydrolysing triglyceride, monoacylglycerol, and cholesterol ester)89. Monoacylglycerol is finally cleaved into glycerol and FA by monoglyceride lipase90,91.
ATGL, hormonesensitive lipase and mono glyceride lipase are all expressed in liver, albeit at lower levels than in adipocytes92, and whether these enzymes constitute the main enzymes for hepatic lipolysis remains uncertain. Hepatic ATGL overexpression increases FA oxidation and decreases triglyceride amounts93. Liverspecific ATGLknockout mice accumulate triglycerides in the liver, indicating a major role for this enzyme in hepatic lipid homeostasis94. On the other hand, other lipases might function in liver triglyceride metabolism. In particular, PNPLA3 is localized at LDs and shares 50% homology with ATGL95. In contrast to other lipases, PNPLA3 is upregulated by increased insulin levels and the lipogenic transcription factor SREBP1c96. In addition, PNPLA3 might be involved in the production of substrates for reesterification of FAs during lipolysis97. The physiological role of PNPLA3 is not completely understood, but a polymorphism in the protein is the predominant genetic risk factor for hepatic steatosis (discussed later).
A major role for triglyceride hydrolysis in liver is to provide substrates for VLDL assembly and secretion. Surprisingly, overexpression or knockdown of ATGL or hormonesensitive lipase does not affect hepatic VLDL secretion93–98, suggesting that another lipase is involved in lipolysis for this purpose92. The chief candidate for providing substrates for VLDL secretion is the ER luminal triacylglycerol lipase30, which is highly expressed in liver99. How a luminal lipase would access LD substrates is currently unclear. An attractive hypothesis is that it acts at the ER–LD membrane bridges, in which the ER lumen would have access to the cores of connected LDs to mobilize precursors.
Lipophagy.
In addition to lipolysis, lipids can be mobilized from LDs by lipophagy. During this process, small LDs are thought to be engulfed by a membrane bilayer with the activated LC3II protein to form an autophagosome that can be delivered to the lysosome100,101. Lipophagy of LDs during conditions of cell starvation seems to be required to maintain the flow of FAs to mitochondria for respiration102. Whether lipophagy has a prominent role during normal energy fluctuations remains uncertain. Even if lipophagy has a minor role in normal dietary conditions, it might be important in limiting lipid accumulation during transient dietary lipid overload.
Protein removal from LDs
When lipases digest the core of LDs, the LDs shrink in size, and as a consequence, the LD surface also shrinks103. What happens to the proteins on the LD surface under these conditions is not fully understood, but at least two pathways have been suggested: proteins might fall off due to macromolecular crowding103 or be selectively extracted by chaperonemediated autophagy, a type of autophagy that targets proteins for lysosomal degradation via chaperones104. At times of lipid deprivation and lipolysis, the protein composition of the LDs changes due to crowding and competition for binding to the LD surface103. In general, proteins with amphipathic helices that target the LD from the cytosol are more likely to be displaced from the shrinking surface, whereas proteins with more hydrophobic domains that localize to the LDs via ER bridges remain on the LD surface103. During chaperonemediated autophagy, proteins are thought to be recognized by the heat shock cognate 70 kDa protein, brought to the lysosomes, and recognized by lysosomeassociated membrane protein 2A (LAMP2A) for uptake and degradation104. Chaperonemediated autophagy can degrade LDassociated proteins, such as perilipin 2 and perilipin 3, which might be necessary for lipases and autophagic factors to access the cores of LDs104.
Lipid droplets in NAFLD
Hepatic steatosis and LD formation
One factor leading to steatosis in hepatocytes is increased substrate availability for LD formation. Notably, obesity is often accompanied by insulin resistance, which increases FA release from adipocytes105. FAs from peripheral adipose stores are estimated to contribute to ~60% of the triglycerides stored in hepatocytes in patients with NAFLD106. In the case of alcoholic fatty liver disease (AFLD), chronic alcohol exposure in mice stimulates adipose tissue lipolysis with increased activity of ATGL and hormonesensitive lipase. These excessive free FAs can then be taken up by the liver, providing substrate for triglyceride synthesis107. In mammals, increased hepatic mRNA levels of several FAbinding proteins promoting FA uptake into hepatocytes (including FABP4, FABP5 and CD36) have been associated with fatty liver disease108–110.
The contribution of de novo lipogenesis to hepatic triglyceride production in healthy individuals is fairly small and accounts for ~5% of triglyceride incorporated into secreted VLDL105. By contrast, the contrib ution of de novo lipogenesis in patients with NAFLD is ~25%105. Despite insulin resistance associated with the metabolic syndrome, insulin paradoxically continues to stimulate de novo lipogenesis in patients with NAFLD via the SREBP pathway110. In the case of AFLD, ethanolfed mice show increased de novo lipogenesis, probably through increased expression of SREBP1 in the liver111.
The formation of very large LDs in hepatocytes is the hallmark of steatosis. These large LDs in steatosis can arise from LDlocalized triglyceride synthesis47 or from one of two known ‘fusion’ processes: ripening or coalescence. Ripening occurs when the contents of one LD diffuse into another LD43. A member of the CIDE family of proteins, CIDEC, promotes LD growth by this mechanism88 and potentially contributes to giant LD formation112,113. By contrast, coalescence represents true fusion of LD organelles, which occurs more quickly114. Under normal conditions, coalescence of LDs is rare because phosphatidylcholine on the surface of LDs acts as a surface tensionlowering surfactant45. Coalescence of LDs and progression of NAFLD are more likely to occur in mouse hepatocytes if the phosphatidylcholine:phosphatidylethanolamine ratio of the LD membrane is decreased115. This feature has been confirmed in Drosophila cells in which knockdown of the ratelimiting enzyme in phosphatidylcholine synthesis, CTP:phosphocholine cytidylyltransferase, results in cells with fewer phosphatidylcholines and larger LDs45. Adequate levels of phosphatidylcholine are, therefore, required to prevent coalescence of LDs, and phosphatidylcholine levels are maintained in part by sensing of phosphatidylcholine deficiency. Moreover, livers of mice with NAFLD are often markedly deficient in phosphatidylcholine116, and phosphatidylcholine supplementation improves liver weight and serum transaminases levels in a mouse model of NAFLD117.
In addition to its role in preventing LD coalescence, normal phosphatidylcholine levels are required for VLDL secretion from hepatocytes118. Moreover, lysophosphatidylcholine acyltransferase 3 (LPCAT3) has a critical role in promoting VLDL production119. Mice lacking LPCAT3 in the liver have decreased amounts of arachidonoyl phosphatidylcholine in liver membrane fractions and VLDL particles. Despite normal morphology of the ER, the Golgi apparatus and mitochondria, these mice secrete less triglyceride from the liver and do so in smaller VLDL particles. When challenged with a highsucrose diet, they accumulate lipid droplets in the liver, as altered triglyceride secretion can lead to steatosis119.
Increased LD formation can also contribute to hepatic steatosis, which occurs in a variety of circumstances. Activation of cell stress pathways generally increases the numbers of forming LDs in cells120–122. In mice, obesity due to genetic mutations or a highfat diet leads to the formation of massive amounts of LDs in hepatocytes. These processes are often accompanied by activation of ER stress123, a known activator of a lipogenic gene expression program in liver124. ER stress additionally probably triggers inflammatory responses, further promoting insulin resistance and lipogenesis125.
Impaired LD consumption
Impaired mobilization of triglyceride and other neutral lipids (for example, from impaired lipolysis, lipophagy or FA oxidation) can also contribute to hepatic steatosis. Liverspecific ATGLknockout mice display hepatic steatosis94. Moreover, comparative gene identification58 (CGI58, also known as ABHD5) activates ATGL126, and deficiency of liver CGI58 in mice leads to steatohepatitis and fibrosis127. In humans, genetic mutations leading to deficiencies in ATGL or CGI58 are rare and lead to neutral lipid storage disease with myopathy or icthyosis (Chanarin–Dorfman syndrome), respectively128,129. Both diseases lead to abnormal lipid accumulation in most tissues, including the liver130,131, and in the case of CGI58 deficiency, progression to cirrhosis and liver failure130–132. Furthermore, a role for CGI58 in diacylglycerol and triacylglycerol turnover independent of ATGL has been reported in mouse liver133. If CGI58 also performs such a function in humans, it could explain the higher risk of developing hepatic steatosis associated with CGI58 mutation than with ATGL mutation134.
Studies have linked alterations in autophagy to hepatic lipid homeostasis. Ablation or inhibition of lipophagy promotes steatosis in cultured hepatocytes100. Moreover, adenovirus expression of Atg7, an autophagy gene, resulted in the reduction of hepatic triglyceride content and serum insulin levels in obese mice135. However, whether autophagic failure is a cause or a consequence of hepatic steatosis is still debated. Although the details are beyond the scope of this Review, autophagy might have an important role in fibrogenesis. HSCs seem to have increased levels of autophagy during their activation, and inhibition of autophagy inhibits their activation136,137.
FA oxidation occurs in the liver during fasting or intense physical activity and alterations in FA oxidation have also been linked to NAFLD37. Mitochondria are the site of FA oxidation, and several reports show that LDs interact physically with mitochondria138,139. Proteins associated with LDs, such as PLIN2 and PLIN5, can alter FA oxidation140,141. PLIN5 is predominantly expressed in highly oxidative tissues, such as muscle and fasted liver, and promotes LD–mitochondria interaction139. In muscle, PLIN5 has been proposed to promote lipid storage and limit FA oxidation to protect against lipotoxic effects of high FA release, but this lipoprotective role of PLIN5 in liver has yet to be established142. By using labelled FAs, a study demonstrated that FAs are transferred from LD to mitochondria during starvation, and alteration of this process redistributes FA to neighbouring cells102. Even if it remains to be demonstrated in a context of NAFLD development, decreased LD–mitochondria interaction might favour hepatic steatosis.
LD‑associated proteins and steatosis
Numerous LDassociated proteins have been implicated in the development of hepatic steatosis. Among these, perilipins and CIDE proteins are the most studied. PLIN1 is normally found in adipocytes and coats large LDs143. PLIN1 interacts with CIDEC and mediates exchange of lipids between droplets144. PLIN1 becomes expressed in the liver during NAFLD145 and could have a similar role in hepatocytes as it does in adipose tissue. PLIN1 also suppresses lipolysis by interacting with CGI58 and, therefore, might lead to increased steatosis by this mechanism146. PLIN2 is the most upregulated perilipin in rodents and humans with NAFLD145 and promotes triglyceride accumulation and inhibits FA oxidation140,147. Plin2knockdown mice are protected from steatosis induced by a highfat diet, although this reduction in steatosis was associated with an increase in the expression of fibrogenesis genes147,148. Plin2 expressed in an adenoviral vector in rat hepatocytes is linked to decreased lipidrich VLDL production and secretion140, suggesting that PLIN2 is a potential negative regulator of VLDL lipidation. In summary, PLIN2 activity results in decreased lipid secretion from hepatocytes and accumulation of triglycerides, leading to steatosis. PLIN3 and PLIN5 are also increased in livers of mice with steatosis143. PLIN5 binds ATGL and an ATGL activator, CGI58 (also known as ABHD5), in the livers of mice with acute hepatic steatosis, providing a link between PLIN5 and lipolysis. However, the probable complex mechanism of regulating triglyceride metabolism has yet to be elucidated149. As with NAFLD, the perilipins have been implicated in AFLD. PLIN1 probably has a similar role in the formation of larger LDs150. PLIN2 is the major LD protein associated with AFLD and is required for ethanolfed mice to develop steatosis151. AFLD and other LDassociated proteins have been reviewed in detail elsewhere143,150.
The CIDE proteins are also an area of active research with regard to hepatic steatosis. CIDEA and CIDEC expression in the liver is upregulated in obese mice and associated with increased triglyceride storage in hepatocytes86,152. Thus, the CIDE family of proteins might have a similar role in hepatocytes as they do in adipocytes, promoting larger LDs and steatosis. CIDEB has a role in VLDL lipidation and how this process might relate to hepatic disease is under investigation31.
Steatosis and insulin resistance
Insulin resistance often accompanies hepatic steatosis153, but the causality behind this relationship is poorly understood (reviewed elsewhere9,154,155). Hyperinsulinaemia in insulin resistance drives hepatic steatosis, a process termed ‘selective insulin resistance’ because insulin continues to drive lipogenesis via the SREBP1 pathway154,156 but fails to suppress gluconeogenesis.
Whether the accumulation of a specific type of lipid is sufficient and necessary for causing insulin resistance is unclear. Considerable evidence in multiple mouse models suggests that hepatic triglyceride accumulation per se does not lead to insulin resistance154. In fact, overexpression of DGAT in the liver, promoting triglyceride synthesis, does not result in insulin resistance157. In addition, whereas ATGL or CGI58 deficiency leads to increased hepatic triglyceride content, patients with ATGL deficiency have preserved wholebody insulin sensitivity131, and CGI58knockdown mice are more sensitive to insulin158.
Other lipids, such as diacylglycerols and ceramides, have been implicated in insulin resistance because they can activate protein kinase C (PKC) isoforms159,160 that can interfere with insulin signalling154. In some contexts, they might well promote insulin resistance. However, in other cases, increased levels of diacylglycerol have been associated with normal hepatic insulin sensitivity157,161. In addition, mice with hepatic DGAT2 overexpression have increased levels of diacylglycerol and ceramides in the liver, but maintained normal insulin sensitivity in some but not all studies157,162. Buildup of a particular species of diacylglycerol (such as selected species of sn‑1,2 diacylglycerol) or diacylglycerol accumulation in specific cellular locations, may predispose to insulin resistance due to activation of protein kinase C163,164, but further studies are necessary to clarify this issue.
The relationship of LDs to hepatic insulin resistance is unclear, but, in general, LDs probably protect against lipotoxicity by storing lipids as triglycerides and protecting the ER from lipotoxic insults. Furthermore, when LD triglycerides are hydrolysed by ATGL, sn1,3 and sn‑2,3 diacylglycerol are generated, and DGAT2 in the ER and at LD surfaces preferentially reesterifies sn‑1,3 diacylglycerol to triglyceride165. This finding suggests that LDderived diacylglycerol is unlikely to activate PKC unless it undergoes isomerization to sn1,2 diacylglycerol.
Genetic risk factors for NAFLD
Genomewide association studies identified a mutation in the ATGL homologue PNPLA3 (also known as adiponutrin) as a risk factor for hepatic steatosis prevalence and progression166–169. This mutation is a single nucleotide polymorphism (rs738409 C>G) encoding an isoleucinetomethionine variant at amino acid position 148 (Ile148Met) of the 481amino acid PNPLA3 protein166–168. This variant predisposes patients to the spectrum of NAFLD, progression of AFLD, as well as fibrogenesis associated with HCV infection170. The mechanism by which the Ile148Met variant leads to increased hepatic fat content is a subject of debate and remains unclear. Recombinant Ile148Met protein was initially shown to have decreased glycerolipid hydrolase activity, suggesting that the variant might result in decreased triglyceride hydrolysis at LDs95,171,172. However, mice with PNPLA3 deficiency do not have steatosis173,174, implying that the variant does not result in simple loss of lipase function. In addition, overexpression of the Ile148Met variant in mouse liver resulted in hepatic steato sis175, which would not be expected if the protein acted solely as a lipase. PNPLA3 has lysophosphatidic acid acyltransferase (LPAAT) activity when expressed in bacteria and, therefore, could be involved in the triglyceride synthesis pathway176. However, this activity was not observed in mouse liver expressing wildtype or mutant protein175. Moreover, PNPLA3Ile148Met knockin mice challenged with a highsucrose diet had increased hepatic steatosis and 40fold higher levels of PNPLA3 on LDs without changes in mRNA levels177, again suggesting a gain of function rather than a loss. These authors entertained the possibility that PNPLA3 Ile148Met coats the LD sequestering CGI58, decreasing lipolysis, but their experiments had mixed results with respect to testing this hypothesis177. An alternative possibility is that, because the Ile148Met variant is found at increased abundance in LDs177, it might affect the LD protein composition indirectly by crowding out other proteins103, and altered composition might adversely affect the capacity for lipolysis.
The involvement of PNPLA3 in hepatic steatosis has been linked to its retinyl ester lipase activity in HSCs. Retinol is released from HSCs during the progression to a fibrotic state and is associated with a loss of HSCassociated LDs18,178. Interestingly, this activity is affected in the Ile148Met variant. Moreover, PNPLA3 over expression reduces LD content in HSCs in contrast to the Ile148Met variant, suggesting a lossoffunction mutation179. Even if PNPLA3 does not have retinylester lipase activity in hepatocytes, this study opens new avenues on the role played by PNPLA3 in different liver cell types during steatosis development. At present, the precise role of PNPLA3 in hepatic steatosis and disease progression remains elusive and is an area of active research.
The next most significant genetic factor pre disposing patients to increased hepatic triglyceride content and elevated serum alanine aminotransferase is the transmembrane 6 superfamily member 2 (TM6SF2) rs58542926 C>T variant encoding Glu167Lys180,181. The TM6SF2 protein has 351 amino acids with multiple predicted transmembrane domains and localizes to the ER and ER–Golgi intermediate compartment in human liver cells182. When the human Glu167Lys variant is expressed in hepatoma cells, mRNA levels are similar to that of the wildtype, but protein levels are decreased by ~50%180. TM6SF2 siRNA inhibition in human hepatic cell lines results in decreased secretion of triglyceriderich lipoprotein particles and increased LD content182. In mice, hepatic knockdown of Tm6sf2 resulted in increased hepatic triglyceride content and low circulating levels of cholesterol and triglyceride levels, consistent with a defect in VLDL secretion180. In Tm6sf2knockdown mice fed a highsucrose diet, LDs were larger and more numerous180. Although another study did not demonstrate a hepatic phenotype in mice when Tm6sf2 was overexpressed or knocked down, the authors suggested that transiently altering expression is probably different from a genetic variant in humans181.
Yet another genomewide association study revealed that the rs641738 C>T variant in the locus coding for the membranebound lysophospholipid acyltransferase 7 (MBOAT7) is linked to an increased risk of alcoholrelated cirrhosis183. This variant was also associated with an increased risk of fibrosis184. MBOAT7 is an enzyme involved in the transfer of FAs between phospholipids and lysophospholipids, but the precise role of MBOAT7 in liver disease development remains unclear.
LDs in HCV infection
About half of patients chronically infected with HCV have steatosis, and the prevalence of steatosis is as high as 75% in patients with HCV genotype 3a infection185. Patients with HCV infection and >30% of hepatocytes with steatosis are more likely to have fibrosis, suggesting that patients with steatosis might represent a subgroup at increased risk of disease progression185. The development of steatosis in this population seems to be mediated by altered triglyceride lipolysis186. The role of LDs in the HCV life cycle and pathology have been extensively reviewed elsewhere187–190. HCV core protein represents the first 191 amino acids of the polyprotein and provides an anchor to the ER membrane. After two cleavages at the Nterminus signal peptide, the core protein is produced and has access to the LD via the D2 domain191,192. This domain has two amphipathic αhelices that enable attachment of the core protein to LDs192. Overexpression of the core protein alone is sufficient to cause hepatic steatosis in mice193. The association between HCV core protein and LDs depends on DGAT1 (REFS 186,191), which also traffics the nonstructural protein NS5A to the LD and facilitates the NS5A and core protein interaction194. This finding suggests that HCV core accesses LDs from the ER during formation, although this process has not been formally demonstrated experimentally. Whether HCV core requires ARF1–COPI proteins to traffic to LDs, such as some ERLD proteins, is unclear78. DGAT1dependent localization of core to the LD also seems necessary for the steatogenic properties of HCV191; core protein expression results in increased triglyceride content in wildtype mouse liver, but not Dgat1knockout mouse liver186. Nonetheless, DGAT1 activity is not increased in core infected livers, implying that increased triglyceride synthesis is not a cause of steatosis186. Instead, the mechanism for HCVcoreinduced steatosis seems to involve decreased triglyceride turnover, an effect that depends on HCV core protein localization to the LD186. Furthermore, the effect of HCV core protein on lipolysis seems to be mediated by inhibition of ATGL through an unclear mechanism, while enhancing ATGL interaction with CGI58. It is speculated that ATGL cannot access the TG in LDs surrounded by HCV core protein195.
LD‑associated therapeutics
At present, the most effective treatment for NAFLD is to reduce body fat by weight loss and exercise, and there are no approved pharmacological therapies for NASH. As the underlying causes for the progression from simple steatosis to steatohepatitis and fibrosis and/or cirrhosis remains unclear, treatments could be designed to decrease lipid accumulation in the liver or to prevent the progression towards more advanced inflammatory disease. A consensus from a joint workshop with the Association for the Study of Liver Diseases and the FDA published in 2015 suggested outcome measures for studies investigating therapeutics that focus on the reversal of steatohepatitis with no progression to advanced fibrosis, or development of cirrhosis196. We suggest that therapeutic strategies might also include treatment or resolution of steatosis, analogous to the concept of lowering lipids to prevent atherosclerosis. Of note, the resolution of steatohepatitis almost never occurs without improvement in steatosis196. Here, we therefore focus on potential targets that reduce steatosis as it relates to triglyceride storage in LDs (FIG. 6).
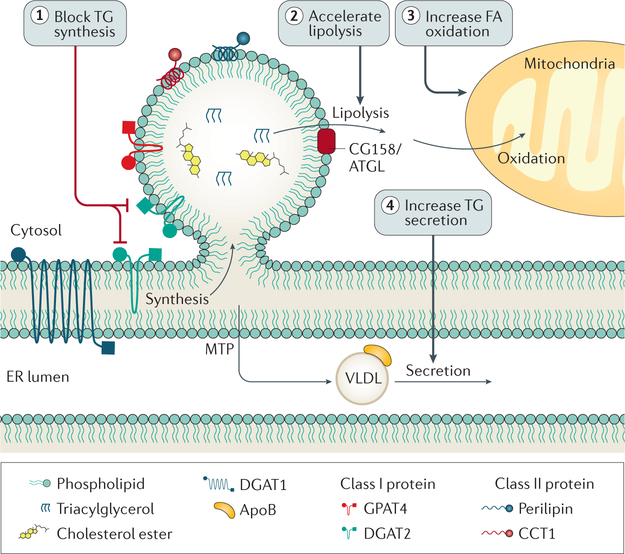
Figure 6 |. Possible therapeutic targets to decrease hepatic steatosis.
Interventions designed to decrease triglyceride (TG) synthesis, increase lipolysis, increase fatty acid (FA) oxidation, or increase very-low-density lipoprotein (VLDL) secretion could decrease hepatic TG content. ER, endoplasmic reticulum.
An initial thought to improve hepatic lipid accumulation would be to decrease triglyceride synthesis, which could be achieved by targeting the triglyceride synthesis enzymes, DGAT1 or DGAT2. DGAT1 inhibition decreases liver triglyceride content in mice challenged with highfat diet197, but, unfortunately, DGAT1 inhibitors administered to humans resulted in intolerable gastro intestinal adverse effects, most notably diarrhoea198. DGAT2 also seems a viable target, especially given its link to de novo lipogenesis22 and eLDs47. Human DGAT2 inhibitor studies have not been reported, but the role of DGAT2 in hepatic steatosis has been studied in mice. One study used antisense oligonucleotides to decrease hepatic DGAT2 expression in obese mice on a highfat diet. Reductions in DGAT2 expression resulted in decreased steatosis and improved hyperlipidaemia199. Another study used DGAT2 antisense oligonucleotides in obese mice on a methionine and cholinedeficient diet (used to model NASH), which again decreased hepatic lipid content, but resulted in increased liver damage200. Similar results were found by inhibiting acylCoA desaturase (also known as stearoylCoA desaturase), a key enzyme involved fatty desaturation, pointing to a key role of triglyceride formation and storage in LDs as protective in hepatocytes by buffering them from the potential lipotoxic effects of free FAs201. This finding highlights the complexity in choosing therapeutic targets, and the importance of studying the roles of DGAT1 and DGAT2, in steatosis and steatohepatitis.
Further understanding of the roles of LD formation, growth and catabolism as they relate to disease progression might provide additional opportunities for intervention. Particular proteins of interest include seipin, implicated in LD formation70,73, GPAT4, important for LD growth47, and CIDEC, involved in LD growth via ripening. Understanding the role of Ile148Met PNPLA3 variant, which seems to be a gainoffunction mutation, is paramount. As this mutation represents the most prevalent genetic risk factor for steatosis and disease progression, targeting this protein stands to benefit a large number of people. Strategies to increase lipolysis, possibly by increasing fat oxidation, are attractive avenues to lower hepatic lipid levels. Increasing secretion of triglycerides, although logical, might be problematic, as this strategy might be complicated by increases in atherogenic apoBcontaining lipoproteins. In fact, any strategy to reduce triglyceride synthesis or storage needs to be evaluated to ensure that it does not trigger a lipotoxic stress pathway and generate nonsalutary effects.
Conclusions
The latest discoveries have shed light on LD biology and the delicate balance of liver fat in human health and disease. Despite our growing knowledge and the burden of hepatic steatosis in multiple disease states, in particular NAFLD, there is a lack of treatment options beyond lifestyle changes and liver transplantation. Therein lies an opportunity to translate our growing understanding of LD regulation to develop therapies that target the process of abnormal lipid accumulation and progression of liver disease. The first step in accelerating this process is to address some key unanswered questions concerning the biology of LDs in the liver. First, there remain uncertainties about mechanisms for triglyceride packaging into newly formed LDs, LD budding and contact of eLDs with the ER during times of growth. Second, a better understanding of lipolysis in the liver is needed, since alterations in lipolysis can contribute greatly to NAFLD. Third, more studies are needed to define the molecular processes that lead to VLDL lipidation and secretion. The contribution of lipids from iLDs and eLDs for secretion and what proteins might mobilize these neutral lipids are open to discovery. A better understanding of these processes will ultimately lead to the design of molecular therapies aimed at decreasing NAFLD and its sequelae.
Key points.
Depending on the body’s needs, the liver utilizes lipids to generate metabolic energy, secretes them as lipoproteins, or packages them for storage
Unbalanced lipid storage and utilization result in supraphysiological triglyceride accumulation in hepatocytes, known as hepatic steatosis
Hepatic lipids accumulate in organelles known as cytoplasmic lipid droplets
Our expanding knowledge of lipid droplets and their associated protein machinery provide opportunities for molecular-based approaches for treating nonalcoholic steatosis and steatohepatitis
Acknowledgements
We thank S. Alexandrescu for the pathology images in Figure 2, and G. Howard for editorial assistance. R.V.F. acknowledges support from NIH National Institute of General Medical Sciences R01 GM099844, NIH National Institute of Diabetes and Digestive and Kidney Diseases R01 DK056084 and R01 DK101579. T.C.W. acknowledges support from NIH National Institute of General Medical Sciences R01 GM097194. T.C.W. is an investigator of the Howard Hughes Medical Institute. M.B. is supported by a fellowship from the Jane Coffin Childs Foundation.
Footnotes
Competing interests statement
The authors declare no competing interests.
References
- 1.Fazel Y, Koenig AB, Sayiner M, Goodman ZD & Younossi ZM Epidemiology and natural history of non-alcoholic fatty liver disease. Metab. Clin. Exp 65, 1017–1025 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Li R et al. Prevalence of metabolic syndrome in mainland China: a meta-analysis of published studies. BMC Public Health 16, 296 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng M et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384, 746 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haas JT, Francque S & Staels B Pathophysiology and mechanisms of nonalcoholic fatty liver disease. Annu. Rev. Physiol 78, 181–205 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Kawano Y & Cohen DE Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J. Gastroenterol 48, 434–441 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hardy T, Anstee QM & Day CP Nonalcoholic fatty liver disease: new treatments. Curr. Opin. Gastroenterol 31, 175–183 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong RJ et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 148, 547–555 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Buzzetti E, Pinzani M & Tsochatzis EA The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metab. Clin. Exp 65, 1038–1048 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Cohen JC, Horton JD & Hobbs HH Human fatty liver disease: old questions and new insights. Science 332, 1519–1523 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mashek DG Hepatic fatty acid trafficking: multiple forks in the road. Adv. Nutr 4, 697–710 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hussain MM Intestinal lipid absorption and lipoprotein formation. Curr. Opin. Lipidol 25, 200–206 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nestel PJ, Havel RJ & Bezman A Sites of initial removal of chylomicron triglyceride fatty acids from the blood. J. Clin. Invest 41, 1915–1921 (1962). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldstein JL & Brown MS Binding and degradation of low density lipoproteins by cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J. Biol. Chem 249, 5153–5162 (1974). [PubMed] [Google Scholar]
- 14.Hussain MM et al. Clearance of chylomicron remnants by the low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor. J. Biol. Chem 266, 13936–13940 (1991). [PubMed] [Google Scholar]
- 15.Rohlmann A, Gotthardt M, Hammer RE & Herz J Inducible inactivation of hepatic LRP gene by cre-mediated recombination confirms role of LRP in clearance of chylomicron remnants. J. Clin. Invest 101, 689–695 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrison EH & Hussain MM Mechanisms involved in the intestinal digestion and absorption of dietary vitamin A. J. Nutr 131, 1405–1408 (2001). [DOI] [PubMed] [Google Scholar]
- 17.Blaner WS et al. Hepatic stellate cell lipid droplets: a specialized lipid droplet for retinoid storage. Biochim. Biophys. Acta 1791, 467–473 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedman SL Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol. Rev 88, 125–172 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horton JD, Goldstein JL & Brown MS SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Invest 109, 1125–1131 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Viscarra J, Kim S-J & Sul HS Transcriptional regulation of hepatic lipogenesis. Nat. Rev. Mol. Cell Biol 16, 678–689 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tontonoz P & Mangelsdorf DJ Liver × receptor signaling pathways in cardiovascular disease. Mol. Endocrinol 17, 985–993 (2003). [DOI] [PubMed] [Google Scholar]
- 22.Villanueva CJ et al. Specific role for acyl CoA:Diacylglycerol acyltransferase 1 (Dgat1) in hepatic steatosis due to exogenous fatty acids. Hepatology 50, 434–442 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wurie HR, Buckett L & Zammit VA Diacylglycerol acyltransferase 2 acts upstream of diacylglycerol acyltransferase 1 and utilizes nascent diglycerides and de novo synthesized fatty acids in HepG2 cells. FEBS J. 279, 3033–3047 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Qi J et al. The use of stable isotope-labeled glycerol and oleic acid to differentiate the hepatic functions of DGAT1 and −2. J. Lipid Res 53, 1106–1116 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tiwari S & Siddiqi SA Intracellular trafficking and secretion of VLDL. Arterioscler. Thromb. Vasc. Biol 32, 1079–1086 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alexander CA, Hamilton RL & Havel RJ Subcellular localization of B apoprotein of plasma lipoproteins in rat liver. J. Cell Biol 69, 241–263 (1976). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boren J, Rustaeus S & Olofsson SO Studies on the assembly of apolipoprotein B-100- and B-48-containing very low density lipoproteins in McA-RH7777 cells. J. Biol. Chem 269, 25879–25888 (1994). [PubMed] [Google Scholar]
- 28.Wetterau JR & Zilversmit DB A triglyceride and cholesteryl ester transfer protein associated with liver microsomes. J. Biol. Chem 259, 10863–10866 (1984). [PubMed] [Google Scholar]
- 29.Ginsberg HN & Fisher EA The ever-expanding role of degradation in the regulation of apolipoprotein B metabolism. J. Lipid Res 50 (Suppl.), S162–S166 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lehner R & Verger R Purification and characterization of a porcine liver microsomal triacylglycerol hydrolase. Biochemistry 36, 1861–1868 (1997). [DOI] [PubMed] [Google Scholar]
- 31.Ye J et al. Cideb, an ER- and lipid droplet-associated protein, mediates VLDL lipidation and maturation by interacting with apolipoprotein B. Cell Metab. 9, 177–190 (2009). [DOI] [PubMed] [Google Scholar]
- 32.Bamberger MJ & Lane MD Possible role of the Golgi apparatus in the assembly of very low density lipoprotein. Proc. Natl Acad. Sci. USA 87, 2390–2394 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higgins JA Evidence that during very low density lipoprotein assembly in rat hepatocytes most of the triacylglycerol and phospholipid are packaged with apolipoprotein B in the Golgi complex. FEBS Lett. 232, 405–408 (1988). [DOI] [PubMed] [Google Scholar]
- 34.Gusarova V, Brodsky JL & Fisher EA Apolipoprotein B100 exit from the endoplasmic reticulum (ER) is COPII-dependent, and its lipidation to very low density lipoprotein occurs post-ER. J. Biol. Chem 278, 48051–48058 (2003). [DOI] [PubMed] [Google Scholar]
- 35.Santos AJM, Nogueira C, Ortega-Bellido M & Malhotra V TANGO1 and Mia2/cTAGE5 (TALI) cooperate to export bulky pre-chylomicrons/VLDLs from the endoplasmic reticulum. J. Cell Biol 213, 343–354 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pitman JL, Bonnet DJ, Curtiss LK & Gekakis N Reduced cholesterol and triglycerides in mice with a mutation in Mia2, a liver protein that localizes to ER exit sites. J. Lipid Res 52, 1775–1786 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei Y, Rector S, Thyfault JP & Ibdah JA Nonalcoholic fatty liver disease and mitochondrial dysfunction. World J. Gastroenterol 14, 193 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ekstedt M et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 61, 1547–1554 (2015). [DOI] [PubMed] [Google Scholar]
- 39.Angulo P et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 149, 389–397.e10 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Altman R Die Elementarorganismen und Ihre Beziehungen zu den Zellen (in German) (Veit, 1890).
- 41.Wilson E The Cell in Development and Inheritance (Macmillan, 1896).
- 42.Greenberg AS et al. Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J. Biol. Chem 266, 11341–11346 (1991). [PubMed] [Google Scholar]
- 43.Thiam AR, Farese RV Jr & Walther, T. C. The biophysics and cell biology of lipid droplets. Nat. Rev. Mol. Cell Biol 14, 775–786 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walther TC & Farese RV Lipid droplets and cellular lipid metabolism. Annu. Rev. Biochem 81, 687–714 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krahmer N et al. Phosphatidylcholine synthesis for lipid droplet expansion is mediated by localized activation of CTP:phosphocholine cytidylyltransferase. Cell Metab. 14, 504–515 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bartz R et al. Lipidomics reveals that adiposomes store ether lipids and mediate phospholipid traffic. J. Lipid Res 48, 837–847 (2007). [DOI] [PubMed] [Google Scholar]
- 47.Wilfling F et al. Triacylglycerol synthesis enzymes mediate lipid droplet growth by relocalizing from the ER to lipid droplets. Dev. Cell 24, 384–399 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yen C-LE, Stone SJ, Koliwad S, Harris C & Farese RV Thematic review series: glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J. Lipid Res 49, 2283–2301 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cases S et al. Identification of a gene encoding an acyl CoA:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proc. Natl Acad. Sci. USA 95, 13018–13023 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cases S et al. Cloning of DGAT2, a second mammalian diacylglycerol acyltransferase, and related family members. J. Biol. Chem 276, 38870–38876 (2001). [DOI] [PubMed] [Google Scholar]
- 51.Listenberger LL et al. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc. Natl Acad. Sci. USA 100, 3077–3082 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chang CC, Huh HY, Cadigan KM & Chang TY Molecular cloning and functional expression of human acyl-coenzyme A:cholesterol acyltransferase cDNA in mutant Chinese hamster ovary cells. J. Biol. Chem 268, 20747–20755 (1993). [PubMed] [Google Scholar]
- 53.Anderson RA et al. Identification of a form of Acyl-CoA:cholesterol acyltransferase specific to liver and intestine in nonhuman primates. J. Biol. Chem 273, 26747–26754 (1998). [DOI] [PubMed] [Google Scholar]
- 54.Cases S et al. ACAT-2, a second mammalian Acyl-CoA:cholesterol acyltransferase. Its cloning, expression, and characterization. J. Biol. Chem 273, 26755–26764 (1998). [DOI] [PubMed] [Google Scholar]
- 55.Oelkers P, Behari A, Cromley D, Billheimer JT & Sturley SL Characterization of two human genes encoding acyl coenzyme A:cholesterol acyltransferase-related enzymes. J. Biol. Chem 273, 26765–26771 (1998). [DOI] [PubMed] [Google Scholar]
- 56.Lee O, Chang CCY, Lee W & Chang T-Y Immunodepletion experiments suggest that acylcoenzyme A:cholesterol acyltransferase-1 (ACAT-1) protein plays a major catalytic role in adult human liver, adrenal gland, macrophages, and kidney, but not in intestines. J. Lipid Res 39, 1722–1727 (1998). [PubMed] [Google Scholar]
- 57.Chang CC et al. Immunological quantitation and localization of ACAT-1 and ACAT-2 in human liver and small intestine. J. Biol. Chem 275, 28083–28092 (2000). [DOI] [PubMed] [Google Scholar]
- 58.Buhman KK et al. Resistance to diet-induced hypercholesterolemia and gallstone formation in ACAT2-deficient mice. Nat. Med 6, 1341–1347 (2000). [DOI] [PubMed] [Google Scholar]
- 59.Parini P et al. ACAT2 is localized to hepatocytes and is the major cholesterol-esterifying enzyme in human liver. Circulation 110, 2017–2023 (2004). [DOI] [PubMed] [Google Scholar]
- 60.Wilfling F, Haas JT, Walther TC & Farese RV Lipid droplet biogenesis. Curr. Opin. Cell Biol 29, 39–45 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thiam AR & Forêt L The physics of lipid droplet nucleation, growth and budding. Biochim. Biophys. Acta 1861, 715–722 (2016). [DOI] [PubMed] [Google Scholar]
- 62.Tan JSY, Seow CJP, Goh VJ & Silver DL Recent advances in understanding proteins involved in lipid droplet formation, growth and fusion. J. Genet. Genomics 41, 251–259 (2014). [DOI] [PubMed] [Google Scholar]
- 63.Chang TY, Chang CC, Lu X & Lin S Catalysis of ACAT may be completed within the plane of the membrane: a working hypothesis. J. Lipid Res 42, 1933–1938 (2001). [PubMed] [Google Scholar]
- 64.Brasaemle DL Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J. Lipid Res 48, 2547–2559 (2007). [DOI] [PubMed] [Google Scholar]
- 65.Jacquier N et al. Lipid droplets are functionally connected to the endoplasmic reticulum in Saccharomyces cerevisiae. J. Cell Sci 124, 2424–2437 (2011). [DOI] [PubMed] [Google Scholar]
- 66.Jacquier N, Mishra S, Choudhary V & Schneiter R Expression of oleosin and perilipins in yeast promotes formation of lipid droplets from the endoplasmic reticulum. J. Cell Sci 126, 5198–5209 (2013). [DOI] [PubMed] [Google Scholar]
- 67.Kassan A et al. Acyl-CoA synthetase 3 promotes lipid droplet biogenesis in ER microdomains. J. Cell Biol 203, 985–1001 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Choudhary V, Ojha N, Golden A & Prinz WA A conserved family of proteins facilitates nascent lipid droplet budding from the ER. J. Cell Biol 211, 261–271 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fei W et al. Fld1p, a functional homologue of human seipin, regulates the size of lipid droplets in yeast. J. Cell Biol 180, 473–482 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Szymanski KM et al. The lipodystrophy protein seipin is found at endoplasmic reticulum lipid droplet junctions and is important for droplet morphology. Proc. Natl Acad. Sci. USA 104, 20890–20895 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kadereit B et al. Evolutionarily conserved gene family important for fat storage. Proc. Natl Acad. Sci. USA 105, 94–99 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cartwright BR et al. Seipin performs dissectible functions in promoting lipid droplet biogenesis and regulating droplet morphology. Mol. Biol. Cell 26, 726–739 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang H et al. Seipin is required for converting nascent to mature lipid droplets. eLife 5, e16582 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grippa A et al. The seipin complex Fld1/Ldb16 stabilizes ER-lipid droplet contact sites. J. Cell Biol 211, 829–844 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McFie PJ, Stone SL, Banman SL & Stone SJ Topological orientation of acyl-CoA:diacylglycerol acyltransferase-1 (DGAT1) and identification of a putative active site histidine and the role of the n terminus in dimer/tetramer formation. J. Biol. Chem 285, 37377–37387 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stone SJ et al. The endoplasmic reticulum enzyme DGAT2 is found in mitochondria-associated membranes and has a mitochondrial targeting signal that promotes its association with mitochondria. J. Biol. Chem 284, 5352–5361 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kuerschner L, Moessinger C & Thiele C Imaging of lipid biosynthesis: how a neutral lipid enters lipid droplets. Traffic 9, 338–352 (2008). [DOI] [PubMed] [Google Scholar]
- 78.Wilfling F et al. Arf1/COPI machinery acts directly on lipid droplets and enables their connection to the ER for protein targeting. eLife 3, e01607 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Beller M et al. COPI complex is a regulator of lipid homeostasis. PLoS Biol. 6, e292 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guo Y et al. Functional genomic screen reveals genes involved in lipid-droplet formation and utilization. Nature 453, 657–661 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kory N, Farese RV & Walther TC Targeting fat: mechanisms of protein localization to lipid droplets. Trends Cell Biol. 26, 535–546 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu CC et al. Proteomics reveal a link between the endoplasmic reticulum and lipid secretory mechanisms in mammary epithelial cells. Electrophoresis 21, 3470–3482 (2000). [DOI] [PubMed] [Google Scholar]
- 83.Turró S et al. Identification and characterization of associated with lipid droplet protein 1: a novel membrane-associated protein that resides on hepatic lipid droplets. Traffic 7, 1254–1269 (2006). [DOI] [PubMed] [Google Scholar]
- 84.Fujimoto Y et al. Identification of major proteins in the lipid droplet-enriched fraction isolated from the human hepatocyte cell line HuH7. Biochim. Biophys. Acta 1644, 47–59 (2004). [DOI] [PubMed] [Google Scholar]
- 85.Sato S et al. Proteomic profiling of lipid droplet proteins in hepatoma cell lines expressing hepatitis C virus core protein. J. Biochem 139, 921–930 (2006). [DOI] [PubMed] [Google Scholar]
- 86.Xu L, Zhou L & Li P CIDE proteins and lipid metabolism. Arterioscler. Thromb. Vasc. Biol 32, 1094–1098 (2012). [DOI] [PubMed] [Google Scholar]
- 87.Zimmermann R et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 306, 1383–1386 (2004). [DOI] [PubMed] [Google Scholar]
- 88.Gong J et al. Fsp27 promotes lipid droplet growth by lipid exchange and transfer at lipid droplet contact sites. J. Cell Biol 195, 953–963 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fredrikson G, Stralfors P, Nilsson NO & Belfrage P Hormone-sensitive lipase of rat adipose tissue. Purification and some properties. J. Biol. Chem 256, 6311–6320 (1981). [PubMed] [Google Scholar]
- 90.Vaughn M, Berger J & Steinberg D Hormone-sensitive lipase and monoglyceride lipase activities in adipose tissue. J. Biol. Chem 239, 401–409 (1964). [PubMed] [Google Scholar]
- 91.Tornqvist H & Belfrage P Purification and some properties of a monoacylglycerol-hydrolyzing enzyme of rat adipose tissue. J. Biol. Chem 251, 813–819 (1976). [PubMed] [Google Scholar]
- 92.Quiroga AD & Lehner R Liver triacylglycerol lipases. Biochim. Biophys. Acta 1821, 762–769 (2012). [DOI] [PubMed] [Google Scholar]
- 93.Reid BN et al. Hepatic overexpression of hormone-sensitive lipase and adipose triglyceride lipase promotes fatty acid oxidation, stimulates direct release of free fatty acids, and ameliorates steatosis. J. Biol. Chem 283, 13087–13099 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu JW et al. Deficiency of liver adipose triglyceride lipase in mice causes progressive hepatic steatosis. Hepatology 54, 122–132 (2011). [DOI] [PubMed] [Google Scholar]
- 95.He S et al. A sequence variation (I148M) in PNPLA3 associated with nonalcoholic fatty liver disease disrupts triglyceride hydrolysis. J. Biol. Chem 285, 6706–6715 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Qiao A et al. Mouse patatin-like phospholipase domain-containing 3 influences systemic lipid and glucose homeostasis. Hepatology 54, 509–521 (2011). [DOI] [PubMed] [Google Scholar]
- 97.Ruhanen H et al. PNPLA3 mediates hepatocyte triacylglycerol remodeling. J. Lipid Res 55, 739–746 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ong KT, Mashek MT, Bu SY, Greenberg AS & Mashek DG Adipose triglyceride lipase is a major hepatic lipase that regulates triacylglycerol turnover and fatty acid signaling and partitioning. Hepatology 53, 116–126 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dolinsky VW, Sipione S, Lehner R & Vance DE The cloning and expression of a murine triacylglycerol hydrolase cDNA and the structure of its corresponding gene. Biochim. Biophys. Acta 1532, 162–172 (2001). [DOI] [PubMed] [Google Scholar]
- 100.Singh R et al. Autophagy regulates lipid metabolism. Nature 458, 1131–1135 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Singh R & Cuervo AM Lipophagy: connecting autophagy and lipid metabolism. Int. J. Cell Biol 2012, 282041 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rambold AS, Cohen S & Lippincott-Schwartz J Fatty acid trafficking in starved cells: regulation by lipid droplet lipolysis, autophagy, and mitochondrial fusion dynamics. Dev. Cell 32, 678–692 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kory N, Thiam AR, Farese RV & Walther TC Protein crowding is a determinant of lipid droplet protein composition. Dev. Cell 34, 351–363 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kaushik S & Cuervo AM Degradation of lipid droplet-associated proteins by chaperone-mediated autophagy facilitates lipolysis. Nat. Cell Biol 17, 759–770 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fabbrini E, Sullivan S & Klein S Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology 51, 679–689 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Donnelly KL et al. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Invest 115, 1343–1351 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhong W et al. Chronic alcohol exposure stimulates adipose tissue lipolysis in mice: role of reverse triglyceride transport in the pathogenesis of alcoholic steatosis. Am. J. Pathol 180, 998–1007 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Westerbacka J et al. Genes involved in fatty acid partitioning and binding, lipolysis, monocyte/macrophage recruitment, and inflammation are overexpressed in the human fatty liver of insulin-resistant subjects. Diabetes 56, 2759–2765 (2007). [DOI] [PubMed] [Google Scholar]
- 109.Miquilena-Colina ME et al. Hepatic fatty acid translocase CD36 upregulation is associated with insulin resistance, hyperinsulinaemia and increased steatosis in non-alcoholic steatohepatitis and chronic hepatitis C. Gut 60, 1394–1402 (2011). [DOI] [PubMed] [Google Scholar]
- 110.Greco D et al. Gene expression in human NAFLD. Am. J. Physiol. Gastrointest. Liver Physiol 294, 1281–1287 (2008). [DOI] [PubMed] [Google Scholar]
- 111.Ji C & Kaplowitz N Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology 124, 1488–1499 (2003). [DOI] [PubMed] [Google Scholar]
- 112.Xu X, Park JG, So JS & Lee AH Transcriptional activation of Fsp27 by the liver-enriched transcription factor CREBH promotes lipid droplet growth and hepatic steatosis. Hepatology 61, 857–869 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Langhi C & Baldán Á CIDEC/FSP27 is regulated by peroxisome proliferator-activated receptor alpha and plays a critical role in fasting-and diet-induced hepatosteatosis. Hepatology 61, 1227–1238 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Murphy S, Martin S & Parton RG Quantitative analysis of lipid droplet fusion: inefficient steady state fusion but rapid stimulation by chemical fusogens. PLoS ONE 5, e15030 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ling J, Chaba T, Zhu L-F, Jacobs RL & Vance DE Hepatic ratio of phosphatidylcholine to phosphatidylethanolamine predicts survival after partial hepatectomy in mice. Hepatology 55, 1094–1102 (2012). [DOI] [PubMed] [Google Scholar]
- 116.Niebergall LJ, Jacobs RL, Chaba T & Vance DE Phosphatidylcholine protects against steatosis in mice but not non-alcoholic steatohepatitis. Biochim. Biophys. Acta 1811, 1177–1185 (2011). [DOI] [PubMed] [Google Scholar]
- 117.Lee HS et al. Beneficial effects of phosphatidylcholine on high-fat diet-induced obesity, hyperlipidemia and fatty liver in mice. Life Sci. 118, 7–14 (2014). [DOI] [PubMed] [Google Scholar]
- 118.Yao ZM & Vance DE The active synthesis of phosphatidylcholine is required for very low density lipoprotein secretion from rat hepatocytes. J. Biol. Chem 263, 2998–3004 (1988). [PubMed] [Google Scholar]
- 119.Rong X et al. Lpcat3-dependent production of arachidonoyl phospholipids is a key determinant of triglyceride secretion. eLife 4, e6557 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fei W, Wang H, Fu X, Bielby C & Yang H Conditions of endoplasmic reticulum stress stimulate lipid droplet formation in Saccharomyces cerevisiae. Biochem. J 424, 61–67 (2009). [DOI] [PubMed] [Google Scholar]
- 121.Yamamoto K et al. Induction of liver steatosis and lipid droplet formation in ATF6alpha-knockout mice burdened with pharmacological endoplasmic reticulum stress. Mol. Biol. Cell 21, 2975–2986 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lee J-S, Mendez R, Heng HH, Yang Z-Q & Zhang K Pharmacological ER stress promotes hepatic lipogenesis and lipid droplet formation. Am. J. Transl Res 4, 102–113 (2012). [PMC free article] [PubMed] [Google Scholar]
- 123.Özcan U et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306, 457–461 (2004). [DOI] [PubMed] [Google Scholar]
- 124.Lee A-H, Scapa EF, Cohen DE & Glimcher LH Regulation of hepatic lipogenesis by the transcription factor XBP1. Science 320, 1492–1496 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gregor MF & Hotamisligil GS Inflammatory mechanisms in obesity. Annu. Rev. Immunol 29, 415–445 (2011). [DOI] [PubMed] [Google Scholar]
- 126.Lass A et al. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metab. 3, 309–319 (2006). [DOI] [PubMed] [Google Scholar]
- 127.Guo F et al. Deficiency of liver comparative gene identification-58 causes steatohepatitis and fibrosis in mice. J. Lipid Res 54, 2109–2120 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fischer J et al. The gene encoding adipose triglyceride lipase (PNPLA2) is mutated in neutral lipid storage disease with myopathy. Nat. Genet 39, 28–30 (2006). [DOI] [PubMed] [Google Scholar]
- 129.Wu JW et al. Inborn errors of cytoplasmic triglyceride metabolism. J. Inherit. Metab. Dis 38, 85–98 (2015). [DOI] [PubMed] [Google Scholar]
- 130.Mitra S, Samanta M, Sarkar M & Chatterjee S Dorfman-Chanarin syndrome: a rare neutral lipid storage disease. Indian J. Pathol. Microbiol 53, 799–801 (2010). [DOI] [PubMed] [Google Scholar]
- 131.Natali A et al. Metabolic consequences of adipose triglyceride lipase deficiency in humans: an in vivo study in patients with neutral lipid storage disease with myopathy. J. Clin. Endocrinol. Metab 98, E1540–E1548 (2013). [DOI] [PubMed] [Google Scholar]
- 132.Cakmak E et al. Steatohepatitis and liver cirrhosis in Chanarin-Dorfman syndrome with a new ABDH5 mutation. Clin. Res. Hepatol. Gastroenterol 36, e34–e37 (2012). [DOI] [PubMed] [Google Scholar]
- 133.Lord CC et al. Regulation of hepatic triacylglycerol metabolism by CGI-58 does not require ATGL co-activation. Cell Rep. 16, 939–949 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schweiger M, Lass A, Zimmermann R, Eichmann TO & Zechner R Neutral lipid storage disease: genetic disorders caused by mutations in adipose triglyceride lipase/PNPLA2 or CGI-58/ABHD5. Am. J. Physiol. Endocrinol. Metab 297, E289–E296 (2009). [DOI] [PubMed] [Google Scholar]
- 135.Yang L, Li P, Fu S, Calay ES & Hotamisligil GS Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab. 11, 467–478 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Thoen LFR et al. A role for autophagy during hepatic stellate cell activation. J. Hepatol 55, 1353–1360 (2011). [DOI] [PubMed] [Google Scholar]
- 137.Hernández Gea V et al. Autophagy releases lipid that promotes fibrogenesis by activated hepatic stellate cells in mice and in human tissues. Gastroenterology 142, 938–946 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Blanchette-Mackie EJ & Scow RO Movement of lipolytic products to mitochondria in brown adipose tissue of young rats: an electron microscope study. J. Lipid Res 24, 229–244 (1983). [PubMed] [Google Scholar]
- 139.Wang H et al. Perilipin 5, a lipid droplet-associated protein, provides physical and metabolic linkage to mitochondria. J. Lipid Res 52, 2159–2168 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Magnusson B et al. Adipocyte differentiation-related protein promotes fatty acid storage in cytosolic triglycerides and inhibits secretion of very low-density lipoproteins. Arterioscler. Thromb. Vasc. Biol 26, 1566–1571 (2006). [DOI] [PubMed] [Google Scholar]
- 141.Kimmel AR & Sztalryd C Perilipin 5, a lipid droplet protein adapted to mitochondrial energy utilization. Curr. Opin. Lipidol 25, 110–117 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kimmel AR & Sztalryd C The perilipins: major cytosolic lipid droplet-associated proteins and their roles in cellular lipid storage, mobilization, and systemic homeostasis. Annu. Rev. Nutr 36, 471–509 (2016). [DOI] [PubMed] [Google Scholar]
- 143.Carr RM & Ahima RS Pathophysiology of lipid droplet proteins in liver diseases. Exp. Cell Res 340, 187–192 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sun Z et al. Perilipin1 promotes unilocular lipid droplet formation through the activation of Fsp27 in adipocytes. Nat. Commun 4, 1594 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Straub BK, Stoeffel P, Heid H, Zimbelmann R & Schirmacher P Differential pattern of lipid droplet-associated proteins and de novo perilipin expression in hepatocyte steatogenesis. Hepatology 47, 1936–1946 (2008). [DOI] [PubMed] [Google Scholar]
- 146.Yamaguchi T et al. CGI-58 facilitates lipolysis on lipid droplets but is not involved in the vesiculation of lipid droplets caused by hormonal stimulation. J. Lipid Res 48, 1078–1089 (2007). [DOI] [PubMed] [Google Scholar]
- 147.Imai Y et al. Reduction of hepatosteatosis and lipid levels by an adipose differentiation-related protein antisense oligonucleotide. Gastroenterology 132, 1947–1954 (2007). [DOI] [PubMed] [Google Scholar]
- 148.Imai Y et al. Effects of perilipin 2 antisense oligonucleotide treatment on hepatic lipid metabolism and gene expression. Physiol. Genomics 44, 1125–1131 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Granneman JG, Moore H, Mottillo EP & Zhu Z Interactions of perilipin-5 (Plin5) with adipose triglyceride lipase. J. Biol. Chem 286, 5126–5135 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ikura Y & Caldwell SH Lipid droplet-associated proteins in alcoholic liver disease: a potential linkage with hepatocellular damage. Int. J. Clin. Exp. Pathol 8, 8699–8708 (2015). [PMC free article] [PubMed] [Google Scholar]
- 151.Carr RM, Peralta G, Yin X & Ahima RS Absence of perilipin 2 prevents hepatic steatosis, glucose intolerance and ceramide accumulation in alcohol-fed mice. PLoS ONE 9, e97118 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Matsusue K et al. Hepatic steatosis in leptin-deficient mice is promoted by the PPARgamma target gene Fsp27. Cell Metab. 7, 302–311 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shulman GI Cellular mechanisms of insulin resistance. J. Clin. Invest 106, 171–176 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Farese RV, Zechner R, Newgard CB & Walther TC The problem of establishing relationships between hepatic steatosis and hepatic insulin resistance. Cell Metab. 15, 570–573 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kumashiro N et al. Cellular mechanism of insulin resistance in nonalcoholic fatty liver disease. Proc. Natl Acad. Sci. USA 108, 16381–16385 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Brown MS & Goldstein JL Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 7, 95–96 (2008). [DOI] [PubMed] [Google Scholar]
- 157.Monetti M et al. Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab. 6, 69–78 (2007). [DOI] [PubMed] [Google Scholar]
- 158.Brown JM et al. CGI-58 knockdown in mice causes hepatic steatosis but prevents diet-induced obesity and glucose intolerance. J. Lipid Res 51, 3306–3315 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Samuel VT, Petersen KF & Shulman GI Lipid-induced insulin resistance: unravelling the mechanism. Lancet 375, 2267–2277 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Summers SA Sphingolipids and insulin resistance: the five Ws. Curr. Opin. Lipidol 21, 128–135 (2010). [DOI] [PubMed] [Google Scholar]
- 161.Minehira K et al. Blocking VLDL secretion causes hepatic steatosis but does not affect peripheral lipid stores or insulin sensitivity in mice. J. Lipid Res 49, 2038–2044 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Jornayvaz FR et al. Hepatic insulin resistance in mice with hepatic overexpression of diacylglycerol acyltransferase 2. Proc. Natl Acad. Sci. USA 108, 5748–5752 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Boni LT & Rando RR The nature of protein kinase C activation by physically defined phospholipid vesicles and diacylglycerols. J. Biol. Chem 260, 10819–10825 (1985). [PubMed] [Google Scholar]
- 164.Griffin ME et al. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes 48, 1270–1274 (1999). [DOI] [PubMed] [Google Scholar]
- 165.Eichmann TO et al. Studies on the substrate and stereo/regioselectivity of adipose triglyceride lipase, hormone-sensitive lipase, and diacylglycerol-O-acyltransferases. J. Biol. Chem 287, 41446–41457 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Romeo S et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet 40, 1461–1465 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yuan X et al. Population-based genome-wide association studies reveal six loci influencing plasma levels of liver enzymes. Am. J. Hum. Genet 83, 520–528 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dongiovanni P et al. PNPLA3 I148M polymorphism and progressive liver disease. World J. Gastroenterol 19, 6969–6978 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Valenti L et al. Homozygosity for the patatin-like phospholipase-3/adiponutrin I148M polymorphism influences liver fibrosis in patients with nonalcoholic fatty liver disease. Hepatology 51, 1209–1217 (2010). [DOI] [PubMed] [Google Scholar]
- 170.Macaluso FS, Maida M & Petta S Genetic background in nonalcoholic fatty liver disease: a comprehensive review. World J. Gastroenterol 21, 11088–11111 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Huang Y, Cohen JC & Hobbs HH Expression and characterization of a PNPLA3 protein isoform (I148M) associated with nonalcoholic fatty liver disease. J. Biol. Chem 286, 37085–37093 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Pingitore P et al. Recombinant PNPLA3 protein shows triglyceride hydrolase activity and its I148M mutation results in loss of function. Biochim. Biophys. Acta 1841, 574–580 (2014). [DOI] [PubMed] [Google Scholar]
- 173.Basantani MK et al. Pnpla3/Adiponutrin deficiency in mice does not contribute to fatty liver disease or metabolic syndrome. J. Lipid Res 52, 318–329 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chen W, Chang B, Li L & Chan L Patatin-like phospholipase domain-containing 3/adiponutrin deficiency in mice is not associated with fatty liver disease. Hepatology 52, 1134–1142 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Li JZ et al. Chronic overexpression of PNPLA3I148M in mouse liver causes hepatic steatosis. J. Clin. Invest 122, 4130–4144 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kumari M et al. Adiponutrin functions as a nutritionally regulated lysophosphatidic acid acyltransferase. Cell Metab. 15, 691–702 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Smagris E et al. Pnpla3I148M knockin mice accumulate PNPLA3 on lipid droplets and develop hepatic steatosis. Hepatology 61, 108–118 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Friedman SL, Wei S & Blaner WS Retinol release by activated rat hepatic lipocytes: regulation by Kupffer cell-conditioned medium and PDGF. Am. J. Physiol. Gastrointest. Liver Physiol 264, G947–G952 (1993). [DOI] [PubMed] [Google Scholar]
- 179.Pirazzi C et al. PNPLA3 has retinyl-palmitate lipase activity in human hepatic stellate cells. Hum. Mol. Genet 23, 4077–4085 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kozlitina J et al. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet 46, 352–356 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Holmen OL et al. Systematic evaluation of coding variation identifies a candidate causal variant in TM6SF2 influencing total cholesterol and myocardial infarction risk. Nat. Genet 46, 345–351 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mahdessian H et al. TM6SF2 is a regulator of liver fat metabolism influencing triglyceride secretion and hepatic lipid droplet content. Proc. Natl Acad. Sci. USA 111, 8913–8918 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Buch S et al. A genome-wide association study confirms PNPLA3 and identifies TM6SF2 and MBOAT7 as risk loci for alcohol-related cirrhosis. Nat. Genet 47, 1443–1448 (2015). [DOI] [PubMed] [Google Scholar]
- 184.Mancina RM et al. The MBOAT7-TMC4 variant rs641738 increases risk of nonalcoholic fatty liver disease in individuals of European descent. Gastroenterology 150, 1219–1230.e6 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Adinolfi LE et al. Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology 33, 1358–1364 (2001). [DOI] [PubMed] [Google Scholar]
- 186.Harris C, Herker E, Farese RV & Ott M Hepatitis C virus core protein decreases lipid droplet turnover: a mechanism for core-induced steatosis. J. Biol. Chem 286, 42615–42625 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Filipe A & McLauchlan J Hepatitis C virus and lipid droplets: finding a niche. Trends Mol. Med 21, 34–42 (2015). [DOI] [PubMed] [Google Scholar]
- 188.Roingeard P & Melo RCN Lipid droplet hijacking by intracellular pathogens. Cell. Microbiol 19, e12688 (2017). [DOI] [PubMed] [Google Scholar]
- 189.Camus G, Vogt DA, Kondratowicz AS & Ott M Lipid droplets and viral infections. Methods Cell Biol. 116, 167–190 (2013). [DOI] [PubMed] [Google Scholar]
- 190.Herker E & Ott M Unique ties between hepatitis C virus replication and intracellular lipids. Trends Endocrinol. Metab 22, 241–248 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Herker E et al. Efficient hepatitis C virus particle formation requires diacylglycerol acyltransferase-1. Nat. Med 16, 1295–1298 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Boulant S et al. Structural determinants that target the hepatitis C virus core protein to lipid droplets. J. Biol. Chem 281, 22236–22247 (2006). [DOI] [PubMed] [Google Scholar]
- 193.Moriya K et al. Hepatitis C virus core protein induces hepatic steatosis in transgenic mice. J. Gen. Virol 78, 1527–1531 (1997). [DOI] [PubMed] [Google Scholar]
- 194.Camus G et al. Diacylglycerol acyltransferase-1 localizes hepatitis C virus NS5A protein to lipid droplets and enhances NS5A interaction with the viral capsid core. J. Biol. Chem 288, 9915–9923 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Camus G et al. The hepatitis C virus core protein inhibits adipose triglyceride lipase (ATGL)-mediated lipid mobilization and enhances the ATGL interaction with comparative gene identification 58 (CGI-58) and lipid droplets. J. Biol. Chem 289, 35770–35780 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sanyal AJ et al. Challenges and opportunities in drug and biomarker development for nonalcoholic steatohepatitis: findings and recommendations from an American Association for the Study of Liver Diseases — U.S. Food and Drug Administration Joint Workshop. Hepatology 61, 1392–1405 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Yamamoto T, Yamaguchi H, Miki H & Shimada M Coenzyme a: diacylglycerol acyltransferase 1 inhibitor ameliorates obesity, liver steatosis, and lipid metabolism abnormality in KKA y mice fed high-fat or high-carbohydrate diets. Eur. J. Pharmacol 640, 243–249 (2010). [DOI] [PubMed] [Google Scholar]
- 198.Denison H et al. Diacylglycerol acyltransferase 1 inhibition with AZD7687 alters lipid handling and hormone secretion in the gut with intolerable side effects: a randomized clinical trial. Diabetes Obes. Metab 16, 334–343 (2014). [DOI] [PubMed] [Google Scholar]
- 199.Yu XX et al. Antisense oligonucleotide reduction of DGAT2 expression improves hepatic steatosis and hyperlipidemia in obese mice. Hepatology 42, 362–371 (2005). [DOI] [PubMed] [Google Scholar]
- 200.Yamaguchi K et al. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology 45, 1366–1374 (2007). [DOI] [PubMed] [Google Scholar]
- 201.Brown JM & Rudel LL Stearoyl-coenzyme A desaturase 1 inhibition and the metabolic syndrome: considerations for future drug discovery. Curr. Opin. Lipidol 21, 192–197 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]