Supplemental Digital Content is available in the text
Keywords: allergic rhinitis, apnea hypopnea index, body mass index, Epworth Sleep Scale score, meta-analysis, obstructive sleep apnea
Abstract
Background:
The co-existence of allergic rhinitis (AR) and obstructive sleep apnea (OSA) is a common phenomenon in clinical practice. AR has long been considered a risk factor for OSA. However, the relationship is not completely clear. Therefore, we conducted a meta-analysis to evaluate the prevalence of AR in sleep-disordered breathing (SDB) /OSA and their relationship.
Methods:
A comprehensive literature search was performed in PubMed/Medline, Google Scholar, Wiley Online Library, EMBASE, and Web of Science. Data were analyzed and pooled to estimate effect size (ES) /odds ratio (OR) with 95% confidence intervals (95%CI). Heterogeneity was quantified and evaluated by chi-squared-based Q-test and I2 test, with P < .05 and I2 > 50% indicating evidence of heterogeneity.
Results:
44 studies contained 6086 participants were included in this meta-analysis. For adults, the prevalence of AR was 22.8 (95% CI, 15.0–30.6) % in SDB and 35.2 (95% CI, 25.6–44.7) % in OSA. In children with SDB and OSA, the prevalence of AR was 40.8 (95% CI, 24.3–57.2) %, and 45.2 (95% CI, 25.4–65.0) % respectively. The odds ratios of prevalence of the SDB pediatric patients with AR was 2.12 (95%CI, 1.75, 2.57; P < .0001) times higher than that of non-SDB pediatric patients. There were no significant differences between OSA adults with or without AR in BMI (Body Mass Index), neck circumference, apnea hypopnea index (AHI) and epworth sleep scale score (ESS).
Conclusion:
The prevalence of AR in OSA/SDB is considerably high and children with SDB suffering from a higher incidence of AR than non-SDB. OSA adults accompanied with AR do not have any influences on sleep parameters.
1. Introduction
Obstructive sleep apnea (OSA) is the most common sleep-disordered breathing (SDB). The prevalence of OSA in healthy children and adults was as high as 1% to 5% and 3.5% to 20.4%, respectively, and it was even higher for SDB.[1–4] OSA is characterized by prolonged partial upper airway obstruction and/or intermittent complete obstruction. It disrupts normal ventilation and patterns during sleep.[1] Moreover, these breathing disorders may increase the risks of complications of cardiovascular, neurocognitive, and metabolic morbidities.[5] Allergic diseases, allergic rhinitis (AR), asthma, and eczema are common among individuals. AR is a very common disease that affects 10% to 40% of the global population.[6] With the increasing exposure to allergens and pollutants, the prevalence of AR has increased over the past few decades.[7] Since it usually leads to nasal obstruction and increased upper airway resistance, AR has long been recognized as a risk factor of OSA in previous studies.[8–10] Some articles have reviewed the association between AR and SDB in children. However, no meta-analysis was obtained from databases. Therefore, in view of this scenario, we conducted a meta-analysis to explore and summarize the prevalence and association between OSA and AR in order to gain a deeper insight of these 2 diseases.
2. Materials and methods
We performed this meta-analysis in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.[11]
2.1. Literature search
In accordance with the PRISMA guidelines, we identified relevant research articles through a systematic review of scientific databases (PubMed /Medline, Google Scholar, Wiley Online Library, Embase, and Web of Science). The MeSH and keywords used in different logical combinations and phrases were: allergic rhinitis, allergic rhino conjunctivitis, hay fever, nasal allergy, OSA, apnea, hypopnea, Epworth Sleepiness Scale (ESS), apnea-hypopnea index (AHI), SDB, sleep-associated breathing disorder, sleep-related disordered breathing, snoring. The search encompassed original research papers published by July 1, 2017 in online journals in English language.
2.2. Inclusion and exclusion criteria
Inclusion criterion was clinical or epidemiological studies which examined the relationship between AR and sleep disorders and reported the prevalence of AR in OSA or SDB patients. Studies were excluded from the meta-analysis if reported only the sleep quality measures other than OSA or SDB, or provided qualitative information only. Other studies that were excluded include review article, conference abstract, article not published in English, animal study, case report, article with no abstract/full text available.
2.3. Data extraction
The following data were extracted from each eligible study: participants’ demographic and clinical characteristics, the prevalence of AR in OSA (diagnosed with sleep studies according to guidelines),[1,12] and SDB (one or more abnormal/difficulty breathing during sleep and/or gas exchange patterns during sleep including habitual snoring 3 or more times per week) patients, body mass index (BMI), neck circumference, AHI and ESS score, and other relevant information were obtained from the selected research articles of the respective studies and organized on data sheets. To ensure the quality of the meta-analysis, all eligible publications were reviewed by 2 researchers independently according to the standardized approach and later cross checked the work of each other. The final selection of a study for inclusion in the meta-analysis was reached in consensus.
2.4. Statistical analyses
Random effects meta-analyses were performed with STATA 12.0 (Stata Inc. Texas) to achieve overall effect sizes of the prevalence of AR in OSA and SDB patients and to achieve a summary estimate of the odds ratio of the prevalence of AR between OSA/SDB and non-OSA/SDB patients observed in the individual studies. The significance of differences in BMI, neck circumference, AHI, and ESS between OSA/SDB patients with and without AR were carried out by STATA 12.0 with under random effects model. Heterogeneity was quantified and evaluated by the chi-squared-based Q-test and I2 test, with P < .05 and I2 > 50% indicating evidence of heterogeneity.
3. Results
Data were acquired from 44 studies [8,13–56] (6086 patients) which fulfilled the eligibility criteria (Fig. 1). Important characteristics of the included studies are presented in Table 1. Average age of adult SDB patients was 47.97 ± 4.00 years and 7.73 ± 3.34 years for SDB children. Proportion of males in this sample population was 68.55 ± 20.85% in adults and 62.09 ± 12.17% in children. In adult SDB and OSA patients, the prevalence of AR was 22.8 (95%CI, 15.0–30.6)%, and 35.2 (95%CI, 25.6–44.7)%, respectively (Fig. 2 A). In children SDB and OSA patients, the prevalence of AR was 40.8 (95%CI, 24.3–57.2)% and 45.2 (95%CI, 25.4–65.0)%, respectively (Fig. 2 B). The overall prevalence of AR (in SDB and OSA) was 41.6 (95%CI, 23.1–60.1)% in Asia, and 33.5 (95%CI, 25.3–41.6)% in other continents (Europe, Oceania, and America, Fig. S1). Pooling analysis of odds ratios observed in the children's studies showed that the prevalence of the AR was 2.12 (95%CI, 1.75, 2.57; P<.0001) times higher in SDB patients than in non-SDB patients (Fig. 3A). However, in adult studies, the prevalence of AR showed no significant difference between SDB/OSA patients and non-SDB/non-OSA patients (P = .082; P = .078, Fig. 3B). In adults, there was no significant difference between OSA with AR and OSA without AR in BMI (mean difference: −0.19 [95%CI, −0.45–0.07]; P = .149), neck circumference (mean difference: 0.16 [95%CI, −0.11–0.42]; P = .245), AHI (mean difference: −0.52 [95%CI, −1.79–0.74]; P = .416), or ESS (mean difference: 0.21 [95%CI, −0.15–0.58]; P = .246, Fig. S2).
Figure 1.
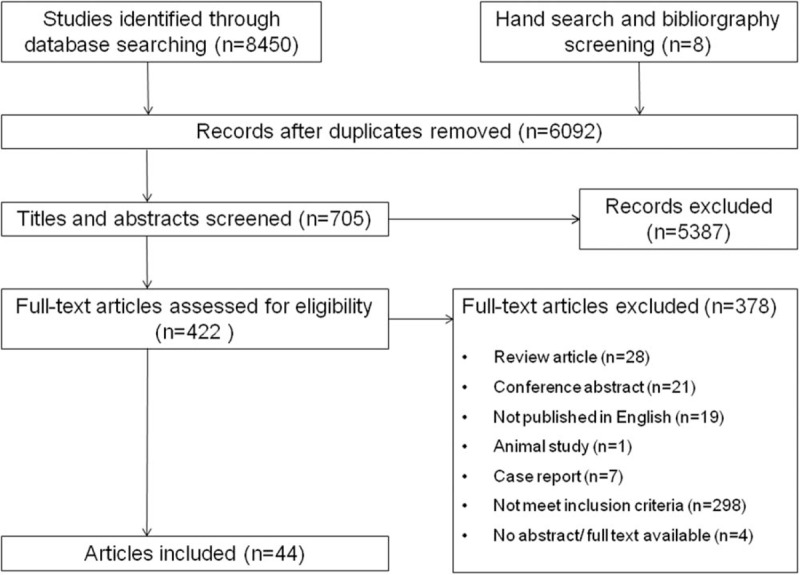
Flowchart of study screening and selection process.
Table 1.
Characteristics of the included studies.
Figure 2.
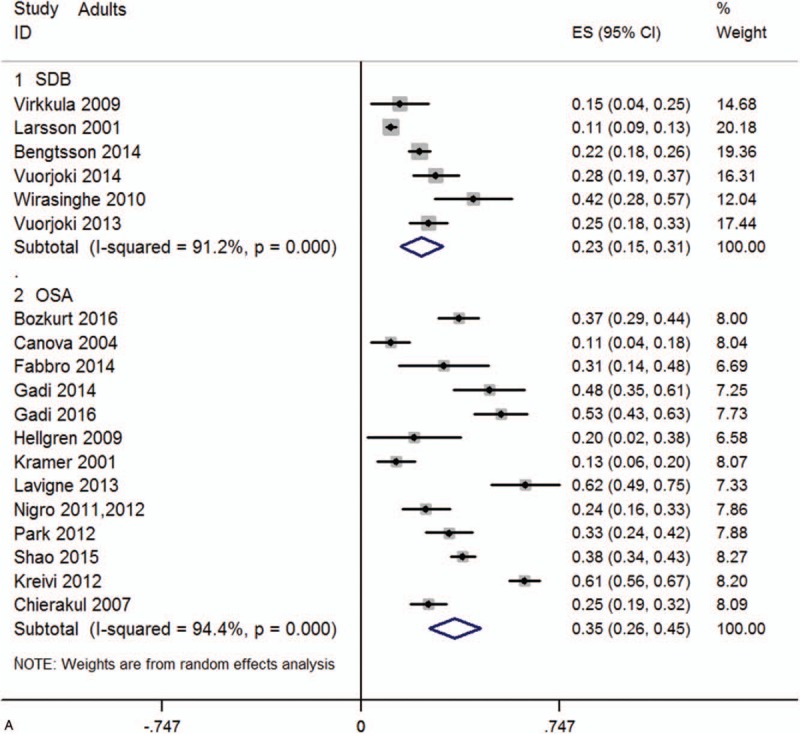
(A) Forest graph showing the percent prevalence of AR in SDB and OSA children. (B) Forest graph showing the percent prevalence of AR in SDB and OSA adults. AR= allergic rhinitis, OSA= obstructive sleep apnea, SDB= sleep-disordered breathing.
Figure 2 (Continued).
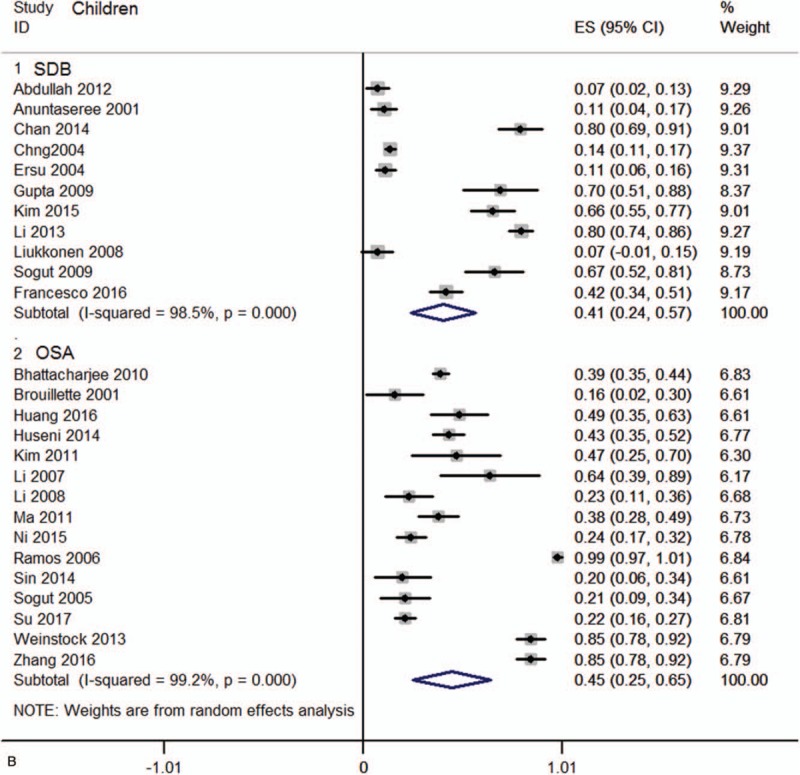
(A) Forest graph showing the percent prevalence of AR in SDB and OSA children. (B) Forest graph showing the percent prevalence of AR in SDB and OSA adults. AR= allergic rhinitis, OSA= obstructive sleep apnea, SDB= sleep-disordered breathing.
Figure 3.
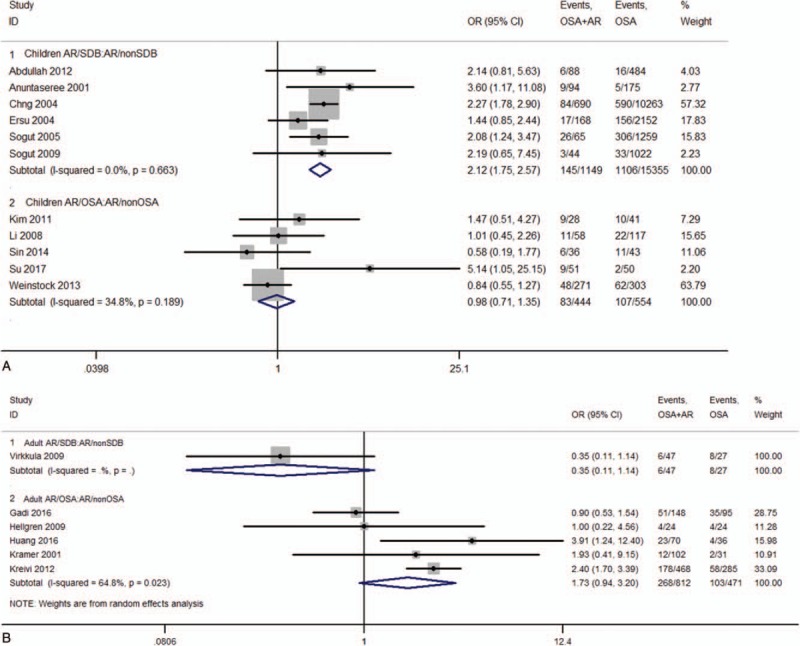
Forest graph showing the meta-analysis of odds ratios reported in individual studies with regard to the odds of prevalence of AR in SDB/OSA and non-SDB/OSA [(A) Children, (B) Adults]. AR= allergic rhinitis, OSA= obstructive sleep apnea, SDB= sleep-disordered breathing.
4. Discussion
Nasal obstruction had long been considered as one of the leading risk facts for the upper airway obstruction during the sleep. Further, some clinical studies had found that patients with nasal congestion caused by AR were more susceptible to disturbed sleep.[57–59] Rhinorrhea, nasal blockage, or congestion always led to stuffy nose, which were the most complained symptoms of AR patients.[60] The nasal obstruction may gradually increase, resulting in daytime fatigue, sleepiness, and performance decrements. In addition, symptoms brought by AR such as apnea and snoring were also considered to be risk factors for sleep-disordered breathing events and contributed to the development of OSA.[61] In addition, some studies had demonstrated that several chemical mediators and inflammatory cytokines play interaction roles between AR and OSA, including histamine, cysteinyl leukotrienes (cysLTs), interleukin-1β(IL-1β), and interleukin-1 (IL-4) and so on.[58,62] Given the impact of AR to OSA, it seemed logical to investigate their relationship which may help understand overlapped subjects.[62]
In this meta-analysis, we reported that the prevalence of children diagnosed with AR is 2.12 times higher in SDB patients than that of non-SDB patients. However, we did not find this tendency in adults, due to immaturity of immune system in children and the studies’ bias. AR was one of the most common chronic diseases in children. Children's immune system developed around the age of 2-year old. During this time, they were more likely to acquire Type I hypersensitivity which reflecting more T helper 2 lymphocyte (Th2) and consequently immunoglobulin E (IgE) driven response to allergen exposure.[63] Adenotonsillar hypertrophy (AH) was a common comorbidity of pediatric AR, it was reported that 92.6% of AR children also suffered AH .[61] Furthermore, AH is the main cause of OSAS for children aged 3 to 6, yet adults patients were often caused by obesity.[64] This meta-analysis showed no significant difference in the prevalence of AR in OSA and non-OSA patients (children and adults). This would lead to widely underdiagnosis of OSA in group of patients in clinical practice.[65,66] Although weight and neck circumference had been shown to be good predictors for OSA in epidemiologic studies, our study suggested that there was not enough evidence that OSA adults patients coexist AR were related to individuals’ BMI, neck circumference, ESS, and AHI. Additionally, Francesco and Alvarez[24] reported that AR is not an aggravating factor regarding the severity of AHI in children. The relationship between the OSA and AR is remaining a long-standing controversy. In traditional view, AR was considered as a potential risk factor for OSA. However, Kramer et al[33] revealed that AR did not influence sleeping parameters of OSA. They found no statistically significant difference in sleeping behavior or polysomnography (PSG) parameters between AR and non-AR patients. Recent studies had also revealed AR only had effect on symptoms, but did not affect PSG results for OSA patients nor belong to risk factors for OSA.[15,17] Similarly, a demographic study conducted in OSA children reported that allergic rhinitis did not contribute to sleep disordered breathing in Australian.[67] Intranasal corticosteroids (INCS) were generally considered as the most effective agent in relieving nasal symptoms of allergic rhinitis. Therefore, it was believed that the effectiveness of INCS in relieving nasal congestion may have a positive effect on SDB. A randomized, controlled trial of OSA children aged 6 to 18 years old demonstrated that intranasal mometasone furoate effectively improved obstructive apnea hypopnea index and oxygen desaturation index.[20] Beyond that, Lavigne et al[36] found that INCS not only reduced upper airway inflammation but also improved OSA morbidity in patients with concomitant AR. A meta-analysis showed that patients receiving INCS had a better effect on decreasing the AHI; however, with limited evidences.[68] In general, continuous positive airway pressure (CPAP) was the preferred therapy for OSA, whereas, INCS did not alleviate nasal symptoms during CPAP treatment in OSA patients.[69] Hence, further research should be performed on INCS in these 2 entities.
In conclusion, patients with AR were more likely to become habitual snorers, and had increasing risk of SDB and the SDB children suffered from a higher incidence of AR. Physicians may need a comprehensive understanding of the overlapping disorders before making a reasonable therapeutic strategy.
5. Conclusion
This meta-analysis revealed that the prevalence of AR in adult SDB/OSA patients was 23%, and 35%, and in children SDB/OSA patients 41%, and 45%, respectively. The odds of having AR were 2.12 times higher in SDB than that of non-SDB children patients (significantly). However, there was no significant difference between OSA patients and those who suffered AR and OSA in neck circumference simultaneously BMI, AHI, or ESS. Whether AR is a risk event of OSA need further consideration, meanwhile, patients with SDB should be cautiously focused especially accompanied with nasal symptoms.
Author contributions
Conceptualization: Yuan Cao, Qiao Li.
Data curation: Yuan Cao, Liyu Zhang.
Formal analysis: Yuan Cao, Ying Yang.
Funding acquisition: Liyu Zhang, Sancheng Cao.
Writing – original draft: Shuang Wu.
Writing – review & editing: Qiao Li.
Qiao Li: 0000-0002-2575-7005.
QIAO LI orcid: 0000-0002-2575-7005.
Supplementary Material
Footnotes
Abbreviations: AH = adenotonsillar hypertrophy, AHI = apnea hypopnea index, AR= allergic rhinitis, BMI = body mass index, CI = confidence intervals, CPAP = continuous positive airway pressure, cysLTs = cysteinyl leukotriene, ES= estimate effect size, ESS = Epworth Sleep Scale Score, IgE = immunoglobulin E, IL-1β= interleukin-1β, IL-4 = interleukin-1, INCS = intranasal corticosteroids, OR = odds ratio, OSA= obstructive sleep apnea, PRISRM = Preferred Reporting Items for Systematic Reviews and Meta-analysis, PSG = polysomnography, SDB= sleep-disordered breathing, Th2 = T helper 2 lymphocyte.
For this type of study formal consent is not required.
Ethical approval and informed consent were not suitable for this study.
Funding: This work was funded by Shannxi Key Science and Technology of Social Development Program 2016SF-036, Xi’an Science and Technology program SF1510 (4).
Conflict of interest: All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or nonfinancial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Supplemental Digital Content is available for this article.
References
- [1].Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 2012;130:576–84. [DOI] [PubMed] [Google Scholar]
- [2].Sharma SK, Ahluwalia G. Epidemiology of adult obstructive sleep apnoea syndrome in India. Indian J Med Res 2010;131:171–5. [PubMed] [Google Scholar]
- [3].Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med 2015;3:310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Moreira GA, Pradella-Hallinan M. Sleepiness in children: an update. Sleep Med Clin 2017;12:407–13. [DOI] [PubMed] [Google Scholar]
- [5].Carberry JC, Amatoury J, Eckert DJ. Personalized management approach for OSA. Chest 2018;153:744–55. [DOI] [PubMed] [Google Scholar]
- [6].Brozek JL, Bousquet J, Agache I, et al. Allergic rhinitis and its impact on asthma (ARIA) guidelines—2016 revision. J Allergy Clin Immunol 2017;140:950–8. [DOI] [PubMed] [Google Scholar]
- [7].Stewart MG. Identification and management of undiagnosed and undertreated allergic rhinitis in adults and children. Clin Exp Allergy 2008;38:751–60. [DOI] [PubMed] [Google Scholar]
- [8].Ersu R, Arman AR, Save D, et al. Prevalence of snoring and symptoms of sleep-disordered breathing in primary school children in Istanbul. Chest 2004;126:19–24. [DOI] [PubMed] [Google Scholar]
- [9].Lin SY, Melvin TA, Boss EF, et al. The association between allergic rhinitis and sleep-disordered breathing in children: a systematic review. Int Forum Allergy Rhinol 2013;3:504–9. [DOI] [PubMed] [Google Scholar]
- [10].Muliol J, Maurer M, Bousquet J. Sleep and allergic rhinitis. J Investig Allergol Clin Immunol 2008;18:415–9. [PubMed] [Google Scholar]
- [11].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009;62:e1–34. [DOI] [PubMed] [Google Scholar]
- [12].Chesson AL, Jr, Berry RB, Pack A. Practice parameters for the use of portable monitoring devices in the investigation of suspected obstructive sleep apnea in adults. Sleep 2003;26:907–13. [DOI] [PubMed] [Google Scholar]
- [13].Fadzil Abdullah AA, Jamalludin AR, Norrashidah AW, et al. Prevalence of sleep disordered breathing symptoms among Malay school children in a primary school in Malaysia. Med J Malaysia 2012;67:181–5. [PubMed] [Google Scholar]
- [14].Anuntaseree W, Rookkapan K, Kuasirikul S, et al. Snoring and obstructive sleep apnea in Thai school-age children: prevalence and predisposing factors. Pediatr Pulmonol 2001;32:222–7. [DOI] [PubMed] [Google Scholar]
- [15].Bengtsson C, Jonsson L, Holmstrom M, et al. Impact of nasal obstruction on sleep quality: a community-based study of women. Eur Arch Otorhinolaryngol 2015;272:97–103. [DOI] [PubMed] [Google Scholar]
- [16].Bhattacharjee R, Kheirandish-Gozal L, Spruyt K, et al. Adenotonsillectomy outcomes in treatment of obstructive sleep apnea in children: a multicenter retrospective study. Am J Respir Crit Care Med 2010;182:676–83. [DOI] [PubMed] [Google Scholar]
- [17].Bozkurt B, Serife Ugur K, Karamanli H, et al. Polysomnographic findings in persistent allergic rhinitis. Sleep Breath 2017;21:255–61. [DOI] [PubMed] [Google Scholar]
- [18].Brouillette RT, Manoukian JJ, Ducharme FM, et al. Efficacy of fluticasone nasal spray for pediatric obstructive sleep apnea. J Pediatr 2001;138:838–44. [DOI] [PubMed] [Google Scholar]
- [19].Canova CR, Downs SH, Knoblauch A, et al. Increased prevalence of perennial allergic rhinitis in patients with obstructive sleep apnea. Respiration 2004;71:138–43. [DOI] [PubMed] [Google Scholar]
- [20].Chan CC, Au CT, Lam HS, et al. Intranasal corticosteroids for mild childhood obstructive sleep apnea—a randomized, placebo-controlled study. Sleep Med 2015;16:358–63. [DOI] [PubMed] [Google Scholar]
- [21].Chierakul N, Chaipattarapol C, Ruttanaumpawan P, et al. Comparison of clinical and polysomnographic characteristics of non-obese and obese patients with obstructive sleep apnea. J Med Assoc Thai 2007;90suppl 2:48–53. [PubMed] [Google Scholar]
- [22].Chng SY, Goh DY, Wang XS, et al. Snoring and atopic disease: a strong association. Pediatr Pulmonol 2004;38:210–6. [DOI] [PubMed] [Google Scholar]
- [23].Dal-Fabbro C, Garbuio S, D’Almeida V, et al. Mandibular advancement device and CPAP upon cardiovascular parameters in OSA. Sleep Breath 2014;18:749–59. [DOI] [PubMed] [Google Scholar]
- [24].Di Francesco RC, Alvarez J. Allergic rhinitis affects the duration of rapid eye movement sleep in children with sleep-disordered breathing without sleep apnea. Int Forum Allergy Rhinol 2016;6:465–71. [DOI] [PubMed] [Google Scholar]
- [25].Gadi GU, Albar MH, Fida R, et al. The frequency of allergic rhinitis among obstructive sleep apnea patients: a hospital-based, cross-sectional study. J King Abdulaziz Univ 2014;21:21–59. [Google Scholar]
- [26].Gadi G, Wali S, Koshak E, et al. The prevalence of allergic rhinitis and atopic markers in obstructive sleep apnea. J Epidemiol Glob Health 2017;7:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gupta N, Emre U, Kearney S, et al. Allergic rhinitis and inner-city children—is there a relationship to sleep-disordered breathing? J Allergy Clin Immunol 2007;119:S154. [Google Scholar]
- [28].Hellgren J, Yee BJ, Dungan G, et al. Altered positional regulation of nasal patency in patients with obstructive sleep apnoea syndrome. Eur Arch Otorhinolaryngol 2009;266:83–7. [DOI] [PubMed] [Google Scholar]
- [29].Huang YS, Guilleminault C, Hwang FM, et al. Inflammatory cytokines in pediatric obstructive sleep apnea. Medicine (Baltimore) 2016;95:e4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Huseni S, Gutierrez MJ, Rodriguez-Martinez CE, et al. The link between rhinitis and rapid-eye-movement sleep breathing disturbances in children with obstructive sleep apnea. Am J Rhinol Allergy 2014;28:e56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kim HY, Dhong HJ, Lee JK, et al. Sleep quality and effects of position on sleep apnea in East Asian children. Auris Nasus Larynx 2011;38:228–32. [DOI] [PubMed] [Google Scholar]
- [32].Kim DK, Han DH. Impact of allergic rhinitis on quality of life after adenotonsillectomy for pediatric sleep-disordered breathing. Int Forum Allergy Rhinol 2015;5:741–6. [DOI] [PubMed] [Google Scholar]
- [33].Kramer MF, De La Chaux R, Dreher A, et al. Allergic rhinitis does not constitute a risk factor for obstructive sleep apnea syndrome. Acta Otolaryngol 2001;121:494–9. [PubMed] [Google Scholar]
- [34].Kreivi HR, Virkkula P, Lehto JT, et al. Upper airway symptoms in primary snoring and in sleep apnea. Acta Otolaryngol 2012;132:510–8. [DOI] [PubMed] [Google Scholar]
- [35].Larsson LG, Lindberg A, Franklin KA, et al. Symptoms related to obstructive sleep apnoea are common in subjects with asthma, chronic bronchitis and rhinitis in a general population. Respir Med 2001;95:423–9. [DOI] [PubMed] [Google Scholar]
- [36].Lavigne F, Petrof BJ, Johnson JR, et al. Effect of topical corticosteroids on allergic airway inflammation and disease severity in obstructive sleep apnoea. Clin Exp Allergy 2013;43:1124–33. [DOI] [PubMed] [Google Scholar]
- [37].Li AM, Hung E, Tsang T, et al. Induced sputum inflammatory measures correlate with disease severity in children with obstructive sleep apnoea. Thorax 2007;62:75–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Li AM, Lam HS, Chan MH, et al. Inflammatory cytokines and childhood obstructive sleep apnoea. Ann Acad Med Singapore 2008;37:649–54. [PubMed] [Google Scholar]
- [39].Li AM, Zhu Y, Au CT, et al. Natural history of primary snoring in school-aged children: a 4-year follow-up study. Chest 2013;143:729–35. [DOI] [PubMed] [Google Scholar]
- [40].Liukkonen K, Virkkula P, Aronen ET, et al. All snoring is not adenoids in young children. Int J Pediatr Otorhinolaryngol 2008;72:879–84. [DOI] [PubMed] [Google Scholar]
- [41].Ma AL, Lam YY, Wong SF, et al. Risk factors for post-operative complications in Chinese children with tonsillectomy and adenoidectomy for obstructive sleep apnea syndrome. Sleep Breath 2012;16:909–11. [DOI] [PubMed] [Google Scholar]
- [42].Ni K, Zhao L, Wu J, et al. Th17/Treg balance in children with obstructive sleep apnea syndrome and the relationship with allergic rhinitis. Int J Pediatr Otorhinolaryngol 2015;79:1448–54. [DOI] [PubMed] [Google Scholar]
- [43].Nigro CA, Dibur E, Aimaretti S, et al. Comparison of the automatic analysis versus the manual scoring from ApneaLink device for the diagnosis of obstructive sleep apnoea syndrome. Sleep Breath 2011;15:679–86. [DOI] [PubMed] [Google Scholar]
- [44].Park CE, Shin SY, Lee KH, et al. The effect of allergic rhinitis on the degree of stress, fatigue and quality of life in OSA patients. Eur Arch Otorhinolaryngol 2012;269:2061–4. [DOI] [PubMed] [Google Scholar]
- [45].Ramos RT, da Cunha Daltro CH, Gregorio PB, et al. OSAS in children: clinical and polysomnographic respiratory profile. Braz J Otorhinolaryngol 2006;72:355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Shao C, Jiang JB, Wu HC, et al. Clinical assessment and polysomnographic study of sleep apnea in a Chinese population of snorers. J Zhejiang Univ Sci B 2015;16:215–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Sin S, Wootton DM, McDonough JM, et al. Anterior nasal resistance in obese children with obstructive sleep apnea syndrome. Laryngoscope 2014;124:2640–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sogut A, Altin R, Uzun L, et al. Prevalence of obstructive sleep apnea syndrome and associated symptoms in 3–11-year-old Turkish children. Pediatr Pulmonol 2005;39:251–6. [DOI] [PubMed] [Google Scholar]
- [49].Sogut A, Yilmaz O, Dinc G, et al. Prevalence of habitual snoring and symptoms of sleep-disordered breathing in adolescents. Int J Pediatr Otorhinolaryngol 2009;73:1769–73. [DOI] [PubMed] [Google Scholar]
- [50].Su MS, Xu L, Xu K, et al. Association of T lymphocyte immune imbalance and IL-10 gene polymorphism with the risk of obstructive sleep apnea in children with obesity. Sleep Breath 2017;21:929–37. [DOI] [PubMed] [Google Scholar]
- [51].Virkkula P, Maasilta P, Hytonen M, et al. Nasal obstruction and sleep-disordered breathing: the effect of supine body position on nasal measurements in snorers. Acta Otolaryngol 2003;123:648–54. [DOI] [PubMed] [Google Scholar]
- [52].Vuorjoki-Ranta T-R, Lobbezoo F, Tuomilehto H, et al. Mandibular advancement devices in the treatment of obstructive sleep apnea and snoring in community dental care: A pilot study on self-reported sleep quality. Health 2013;5:1–5. [Google Scholar]
- [53].Vuorjoki-Ranta T-R, Lobbezoo F, Tuomilehto H, et al. Mandibular advancement device therapy in obstructive sleep apnea and snoring in community dental care: two-year follow-up study on self-reported sleep quality, side effects, and compliance. J Sleep Disorders Ther 2014;3:180. [Google Scholar]
- [54].Weinstock TG, Rosen CL, Marcus CL, et al. Predictors of obstructive sleep apnea severity in adenotonsillectomy candidates. Sleep 2014;37:261–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Wirasinghe C, Godevithanage S, Nakandala SC, et al. The use of overnight pulse oximetry for obstructive sleep apnoea in a resource poor setting in Sri Lanka. J Ceylon Coll Physicians 2011;41:61–6. [Google Scholar]
- [56].Zhang J, Zhao J, Chen M, et al. Airway resistance and allergic sensitization in children with obstructive sleep apnea hypopnea syndrome. Pediatr Pulmonol 2016;51:426–30. [DOI] [PubMed] [Google Scholar]
- [57].McColley SA, Carroll JL, Curtis S, et al. High prevalence of allergic sensitization in children with habitual snoring and obstructive sleep apnea. Chest 1997;111:170–3. [DOI] [PubMed] [Google Scholar]
- [58].Young T, Finn L, Kim H. Nasal obstruction as a risk factor for sleep-disordered breathing. The University of Wisconsin Sleep and Respiratory Research Group. J Allergy Clin Immunol 1997;99:S757–62. [DOI] [PubMed] [Google Scholar]
- [59].Young T, Finn L, Palta M. Chronic nasal congestion at night is a risk factor for snoring in a population-based cohort study. Arch Intern Med 2001;161:1514–9. [DOI] [PubMed] [Google Scholar]
- [60].Craig TJ, McCann JL, Gurevich F, et al. The correlation between allergic rhinitis and sleep disturbance. J Allergy Clin Immunol 2004;114:S139–45. [DOI] [PubMed] [Google Scholar]
- [61].Said SA, McHembe MD, Chalya PL, et al. Allergic rhinitis and its associated co-morbidities at Bugando Medical Centre in Northwestern Tanzania; a prospective review of 190 cases. BMC Ear Nose Throat Disord 2012;12:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Chirakalwasan N, Ruxrungtham K. The linkage of allergic rhinitis and obstructive sleep apnea. Asian Pac J Allergy Immunol 2014;32:276–86. [PubMed] [Google Scholar]
- [63].Sih T, Mion O. Allergic rhinitis in the child and associated comorbidities. Pediatr Allergy Immunol 2010;21:e107–13. [DOI] [PubMed] [Google Scholar]
- [64].Greenfeld M, Tauman R, DeRowe A, et al. Obstructive sleep apnea syndrome due to adenotonsillar hypertrophy in infants. Int J Pediatr Otorhinolaryngol 2003;67:1055–60. [DOI] [PubMed] [Google Scholar]
- [65].Jennum P, Ibsen R, Kjellberg J. Morbidity and mortality in children with obstructive sleep apnoea: a controlled national study. Thorax 2013;68:949–54. [DOI] [PubMed] [Google Scholar]
- [66].Kapur V, Strohl KP, Redline S, et al. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath 2002;6:49–54. [DOI] [PubMed] [Google Scholar]
- [67].Tamanyan K, Walter LM, Davey MJ, et al. Risk factors for obstructive sleep apnoea in Australian children. J Paediatr Child Health 2016;52:512–7. [DOI] [PubMed] [Google Scholar]
- [68].Liu HT, Lin YC, Kuan YC, et al. Intranasal corticosteroid therapy in the treatment of obstructive sleep apnea: A meta-analysis of randomized controlled trials. Am J Rhinol Allergy 2016;30:215–21. [DOI] [PubMed] [Google Scholar]
- [69].Charakorn N, Hirunwiwatkul P, Chirakalwasan N, et al. The effects of topical nasal steroids on continuous positive airway pressure compliance in patients with obstructive sleep apnea: a systematic review and meta-analysis. Sleep Breath 2017;21:3–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


