Abstract
Objective:
Several published studies have investigated the association between the −308G/A (rs1800629) polymorphism in the tumor necrosis factor-α (TNF-α) gene and the risk of dilated cardiomyopathy (DCM). However, the TNF-α gene polymorphism has a controversial role in the pathogenesis of DCM among different populations. In the present study, a meta-analysis was performed to resolve this inconsistency.
Methods:
Potentially eligible papers reporting an association between the TNF-α rs1800629 polymorphism and DCM susceptibility were searched in 4 databases including PubMed, EMBASE, Chinese Biomedical Database (CBM), and the Cochrane Library up to April 1, 2018. The odds ratio (OR) with its 95% confidence interval (CI) was used to estimate the strength of the associations. Subgroup analysis based on the ethnicity, studies with or without ischemic and valvular DCM was conducted. Publication bias detection was conducted using Begg test.
Results:
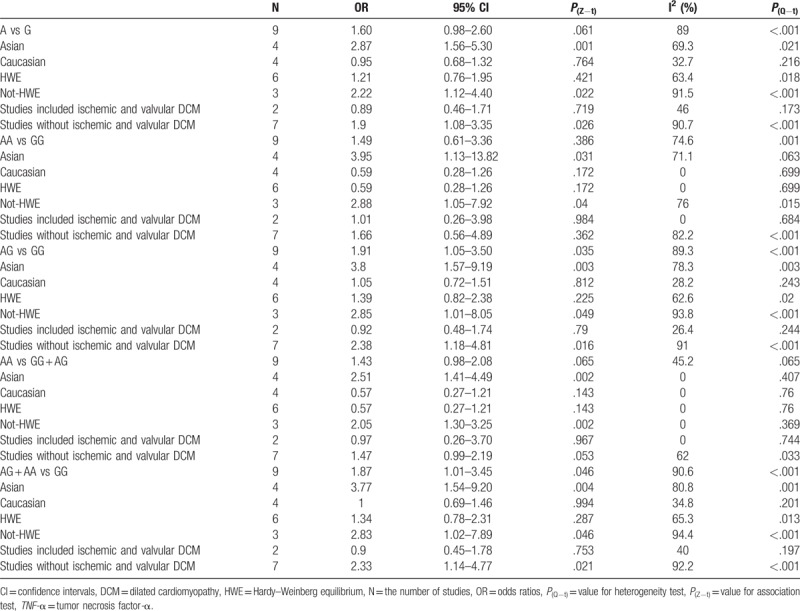
Nine papers detailing case-control studies were included, reporting a total of 1339 DCM cases and 1677 healthy controls. The meta-analysis results indicated that TNF-α rs1800629 was associated with increased DCM susceptibility in the populations studied under the heterozygous model (AG vs GG: OR = 1.91, 95% CI = 1.05−3.50, P = .035) and dominant model (AG + AA vs GG: OR = 1.87, 95% CI = 1.01−3.45, P = .046). In the subgroup analysis for ethnicity, rs1800629 polymorphism was significantly associated with the susceptibility of DCM for Asians under the 5 models (A vs G: OR = 2.87, 95% CI = 1.56−5.30, P = .001; AA vs GG: OR = 3.95, 95% CI = 1.13−13.82, P = 0.031; AG vs GG: OR = 3.8, 95% CI = 1.57−9.19, P = .003; AA vs GG + AG: OR = 2.51, 95% CI = 1.41−4.49, P = .002; AG + AA vs GG: OR = 3.77, 95% CI = 1.54−9.20, P = .004).
Conclusion:
There may be a moderate association between TNF-α rs1800629 polymorphism and DCM susceptibility in the whole populations studied; however, TNF-α rs1800629 polymorphism was significantly associated with the susceptibility of DCM for Asians, which indicates that such associations may be different between ethnicities.
Keywords: dilated cardiomyopathy, polymorphism, rs1800629, tumor necrosis factor-α
1. Introduction
DCM is a leading cause of end-stage heart failure with characteristic of ventricular dilatation and diminished contraction of the left or both the ventricles.[1,2] Despite of the advancement in medical and surgical therapies, DCM remains a major cause of cardiac morbidity and death because of congestive heart failure or arrhythmias and is a leading indication for heart transplantation.[3] The etiopathogenesis of DCM is multifactorial and varied clinical conditions can result in phenotype of DCM; however, the precise etiology of DCM remains uncertain. The etiology of DCM is idiopathic in about 50% of the patients,[4] and among them, 20% to 25% of cases had a familial origin.[5] These facts raised the hypothesis that gene factors might contribute to the disease pathogenesis.[4] It is known to all that genetic and environmental factors contribute to the pathogenesis of cardiac disease.[6]
Inflammation is regarded as one of the commonest mechanisms in cardiomyopathy and many cardiovascular diseases.[7]TNF-α is a pro-inflammatory cytokine possessed pleiotropic biological effects. The rs1800629 polymorphism is located in the promoter region of the TNF-α gene, the 308th nucleotide before the transcription initiation, and the polymorphism present as adenine mononucleotide (A) substituting for guanosine monophosphate (G). It was reported that TNF-α involved in pathogenesis of myocardial infarction, atherosclerosis, and chronic heart failure.[8] Elevated TNF-α levels have been confirmed in patients with DCM compared with healthy controls, which indicated its role in the disease pathogenesis.[9]
The human TNF gene is located in 6p21.3. The −308 G/A rs1800629 polymorphism has been confirmed to affect TNF-α transcriptional activity.[10] Recently, several studies explored the association between TNF-α gene polymorphism and DCM, which yielded controversial conclusions because of limited sample sizes and ethnic variations.[6,11–14] Therefore, we employed a meta-analysis of various studies involving more subjects to investigate the relationship between rs1800629 polymorphism in TNF-α and DCM susceptibility.
2. Materials and methods
2.1. Search strategy
The meta-analysis was performed according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.[15] Two authors independently searched the database of PubMed, EMBASE, CBM, and the Cochrane Library up to April 1, 2018. The following terms were used: (dilated cardiomyopathy or DCM or congestive cardiomyopathies) and (tumor necrosis factor-α or tumor necrosis factor alpha or TNF-α or TNF alpha) and (polymorphism or single nucleotide polymorphism (SNP) or mutation or variation or Genotype or Alleles). There were no language restrictions in the searching process. Publications listed in the references included were also hand reviewed carefully to find potentially relevant articles. Disagreements concerning the inclusion of a particular study were resolved by consensus. Approval of the research ethics committee was not needed in this study.
2.2. Inclusion and exclusion criteria
Studies were included in this meta-analysis if they met all the following criteria:
-
(1)
Research designed as case–control studies for humans;
-
(2)
studies evaluated the relationship between the TNF-α rs1800629 polymorphism and DCM susceptibility;
-
(3)
sufficient data available regarding the total number of cases and controls, especially the number of cases and controls with G/G, G/A, and A/A genotypes; and
-
(4)
at least 2 comparison groups (DCM group and control group).
The exclusion criteria were:
-
(1)
Studies without control group, or were case reports, editorials, review articles, or comments;
-
(2)
duplicated publications;
-
(3)
without detailed genotyping data; and
-
(4)
animal studies or basic experimental studies.
2.3. Data extraction
Two experienced authors independently extracted the data from each included study according to a preset data extraction form. The following information from the included studies was extracted: First author's surname; publication year; country in which the study was performed; ethnicity; genotyping method; diagnosis criteria of DCM; total number in the case and control groups, as well as the number of cases and controls with G/G, G/A, and A/A genotypes; whether studies included ischemic and valvular DCM; and the P value used to calculate the Hardy–Weinberg equilibrium (HWE).
2.4. Quality assessment
Two investigators independently carried out a quality assessment of each study based on the Newcastle-Ottawa Scale (NOS),[16] which is one of the most used rating systems to assess the quality of observational studies in a meta-analysis. The system included 3 broad aspects: Case and control selection, exposure, and comparability. Each aspect consists of 4, 2, and 3 items respectively. This scale contains 9 items and each item scores a value of 1 with 9 scores in total. A NOS score of 6 stars or above would be classified as high-quality study.
2.5. Statistical analysis
The association between TNF-α rs1800629 polymorphism and DCM risk was estimated by calculating the pooled odds ratio (OR) along with the 95% confidence interval (CI) in the allele model, the homozygous genetic model, the recessive model, the dominant model, and the heterozygous genetic model. The pooled ORs were measured using the Z test. The HWE for each study was tested using the χ2 test and P >.05 was considered to be consistent with HWE. The ORs were pooled using either the fixed effects model or the random-effects model, as previously described.[17] A P value less than .05 were classified as a statistically significant difference. Heterogeneity across the studies was determined using the Q test and the I2 statistical tests, with I2 <50% indicating little difference. To explore the influence of a single study on the overall results, sensitivity analysis was also carried out to confirm the stability of the results under all genetic models. Begg's funnel plot test was used to assess possible publication bias, with P <.05 being considered to present statistical significance. All statistical analyses were conducted with STATA 12.0 software (StataCorp, College Station, TX).
3. Results
3.1. Characteristics of the included studies
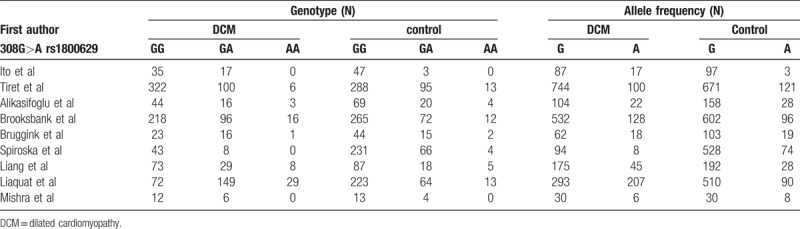
The specific flow chart of the study selection process is shown in Figure 1. The initial search of electronic databases yielded 79 papers based on the selection strategy. We also identified 1 paper through other sources. After removing duplicate articles, 43 papers remained. Then titles and abstracts were further screened and 22 articles obviously unrelated were excluded. After the careful assessment of eligibility using full-text, an additional 12 studies were excluded, including 6 case reports or systematic reviews, 3 articles presented irrelevant data, 2 studies without control groups, and 1 basic experimental study. Finally, 9 original articles were identified that met the inclusion criteria for the meta-analysis.[1,4,6,7,11,12,14,18,19] The detailed characteristics of the 9 included studies are shown in Table 1. Four studies were performed in Asians, 4 in Caucasians, and 1 in South Africa. Two studies included ischemic and valvular DCM,[7,12] while the remained studies without ischemic and valvular DCM.[1,4,6,11,14,18,19] The frequencies of each genotype and allele, with their HWE values, are presented in Table 2. The analyzed SNP was within the HWE in 6 studies,[1,4,7,11,12,14] while in the other 3 studies, the polymorphism was not consistent with the HWE.[6,18,19] The NOS results suggested that the included studies had scores ranging from 6 to 8, with an average of 7.44, which indicated that the methodological quality of the 9 included studies was generally reliable (Table 1).
Figure 1.
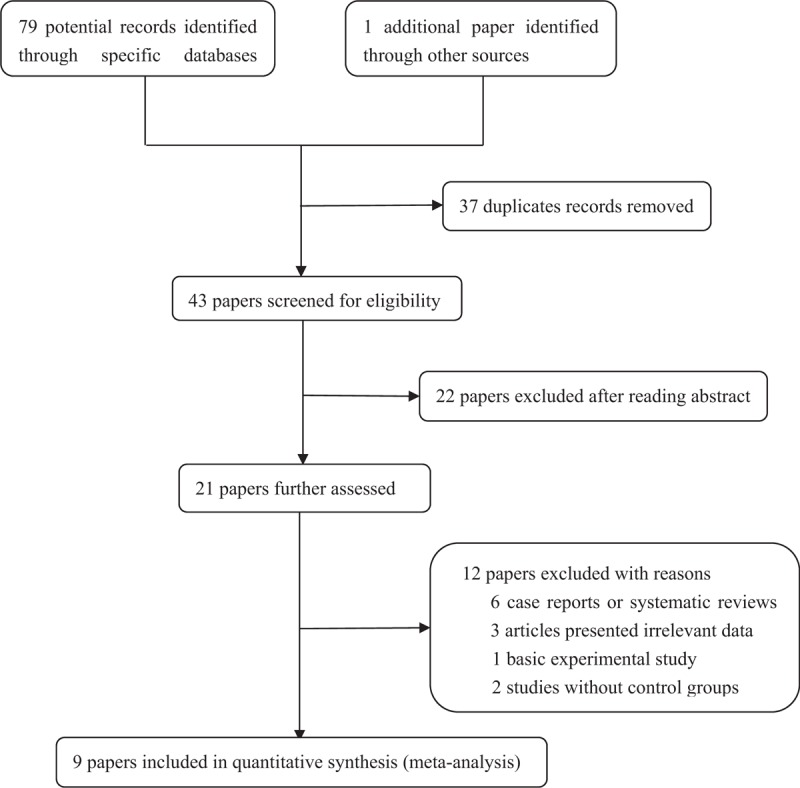
chart of the study selection process.
Table 1.
Characteristics of the studies included in the meta-analysis.
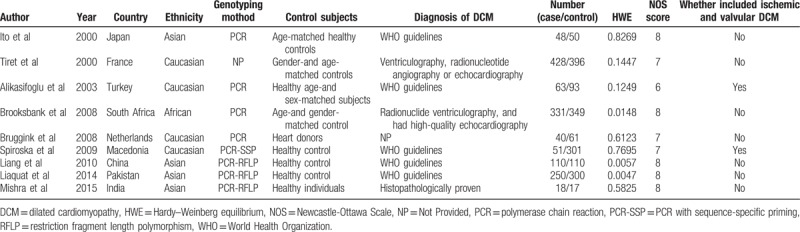
Table 2.
Polymorphisms genotype distribution and allele frequency in dilated cardiomyopathy and controls.
3.2. Main meta-analysis results
In total, 9 case-control papers provided data for 1339 DCM cases and 1677 healthy controls were included. Overall, the main results indicated that TNF-α rs1800629 was associated with increased DCM susceptibility in the populations studied under the heterozygous model (AG vs GG: OR = 1.91, 95% CI = 1.05−3.50, P = .035) and dominant model (AG + AA vs GG: OR = 1.87, 95% CI = 1.01−3.45, P = .046). The forest plot of the pooled ORs of the association of TNF-α rs1800629 with DCM susceptibility under the dominant model is shown in Figure 2. In order to explore the potential association among ethnicities, whether studies consistent with the HWE, and whether studies included ischemic and valvular DCM, a stratified analysis was conducted. In the subgroup analysis for ethnicity, rs1800629 polymorphism was significantly associated with the susceptibility of DCM for Asians under the 5 models (A vs G: OR = 2.87, 95% CI = 1.56−5.30, P = .001; AA vs GG: OR = 3.95, 95% CI = 1.13−13.82, P = .031; AG vs GG: OR = 3.8, 95% CI = 1.57−9.19, P = .003; AA vs GG + AG: OR = 2.51, 95% CI = 1.41−4.49, P = .002; AG + AA vs GG: OR = 3.77, 95% CI = 1.54−9.20, P = .004) (Fig. 3). The main results of the meta-analysis were listed in Table 3.
Figure 2.
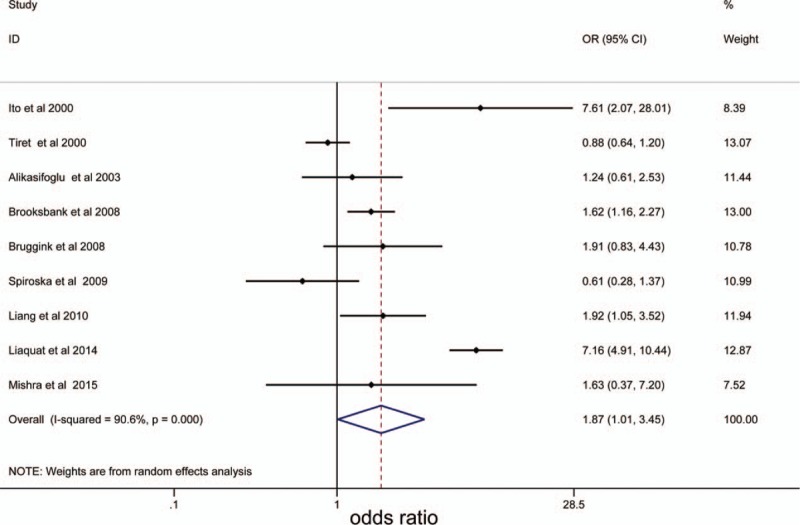
The forest plot of the pooled ORs of the association of TNF-α rs1800629 with dilated cardiomyopathy susceptibility under the dominant model. OR = odds ratios, TNF-α = tumor necrosis factor-α.
Figure 3.

Subgroup analysis by ethnicity between rs1800629 polymorphism and the susceptibility of DCM for Asians under homozygous model (A), allele model (B), dominant model (C), recessive model (D), and heterozygous model (E). DCM = dilated cardiomyopathy.
Table 3.
Meta-analysis of the association between rs1800629 polymorphism in TNF-α gene and dilated cardiomyopathy susceptibility under 5 models.
3.3. Sensitivity analysis and meta-regression analysis
Sequential omission of single-study was utilized to conduct the sensitivity analysis in 5 models. The pooled OR and 95% CI showed no significant quantitative changes, indicating the pooled results of the meta-analysis were robust and reliable (data not shown). After 2 studies included ischemic and valvular DCM was excluded, the main results did not change the statistical significance under 5 genetic models. So we have no enough reason to exclude the 2 studies. Apparent heterogeneity was detected in multiple comparison models even we performed subgroup analysis. In a meta-analysis, it is important to make clear the potential possible sources of heterogeneity since the source of heterogeneity may have an effect on the interpretation of the results. Therefore, we further conducted a meta-regression analysis to explore potential sources of heterogeneity. The study publication year, ethnicity, genotyping method, diagnosis of DCM, number of cases and controls, P value of HWE, and the NOS scores of studies were regarded as the potential confounding factors. However, the meta-regression fail to identify the source of heterogeneity in all allelic models (all P > .05).
3.4. Publication bias
Publication bias was explored with Begg test and no evident publication bias was identified. Publication bias under the dominant model suggested that the result is statistically robust (P = .881, Fig. 4).
Figure 4.
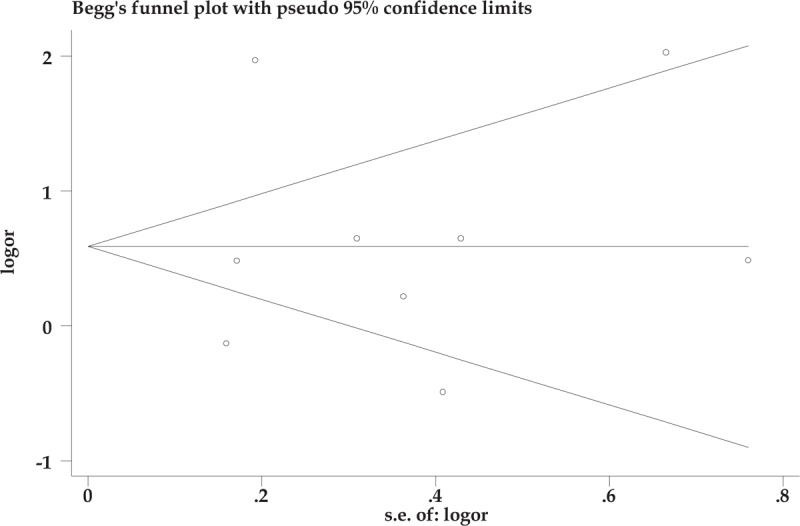
Publication bias under the dominant model.
4. Discussion
DCM is a cardiac muscle disease characterized by reduced left ventricular systolic function.[20] Myocardial inflammation is regarded as the most common potential mechanism in the pathogenesis of DCM in which inflammation cytokines may play a vital role.[21,22]TNF-α is produced in the heart by numerous cells including cardiomyocytes.[23–25] It has been revealed that TNF-α is up-regulated in the myocardium and plays a vital role in the physiology of end-stage heart failure, including DCM.[26] Many studies have revealed associations of TNF-α gene polymorphisms at position −308 with susceptibility to DCM. However, knowledge about the genetics risk factors for DCM remains inconclusive. High frequency of TNF-α allele A was reported in DCM.[11] It was reported that TNF-α allele A (−308) was over-expressed in patients who suffered from end-stage non-ischemic myocardial dysfunction.[27] Previous studies failed to confirm the association between TNF-α rs1800629 polymorphisms and DCM. A previous study, which mainly included patients with class II or III according to the New York Heart Association criteria, performed in Turkey concluded that TNF-α polymorphism was not associated with the presence of DCM.[12] A study from Japan revealed an association between TNF-α polymorphism and idiopathic DCM.[11] While in another study GG genotype of TNF-α rs1800629 showed a protective effect against DCM.[28] There may be several reasons account for the difference between the previous studies. The first reason involved in the different ethnic origins. The etiology pathogenesis of DCM may be diverse among countries or races. Previous meta-analysis using dominant genetic model revealed an OR with 1.42 (95% CI: 1.05−1.93, P = .02) in overall population, which concluded that rs1800629 might be associated with the risk of DCM. However, they failed to perform a subgroup analysis and comparison.[13] Whether there was a racial/ethnic difference still unclear. While in the present study, we found that TNF-α rs1800629 polymorphism was significantly associated with the increased susceptibility of DCM in Asians, while this association was not found in Caucasians. The second reason may be due to differences involved in underlying diseases of the included studies. For example, the study from Japan included only patients with idiopathic DCM,[11] while other studies contained patients with congestive heart failure due to ischemic heart disease, cardiomyopathy, and valvular DCM.[7,12] Another possible reason is that end-stage heart failure patients missed the chance to attend to the hospital as a result of high mortality and therefore failed to perform the TNF-α polymorphism screening.[12] It is revealed that low levels of TNF-α presented a cytoprotective effect in the heart,[29] while high levels of TNF-α may regulate several detrimental effects on the heart, which involved a decreased cardiac contractility, cardiac dilatation, and cardiomyocyte apoptosis.[30,31] Elevated circulating levels of soluble TNF-α receptors and increased plasma levels of TNF-α have been observed in DCM patients compared with normal hearts.[32] All these findings support that TNF may act as an important mediator of cardiac inflammation developing to DCM.
A single study lacks sufficient statistical power to confirm the relationship between TNF-α rs1800629 and the risk of DCM because of the small sample size. To explore the uncertain association between these 2 entities, we performed a meta-analysis in a large sample size to verify the associations between TNF-α rs1800629 functional polymorphism and DCM susceptibility. The results demonstrated that TNF-α rs1800629 polymorphism may be moderately associated with DCM susceptibility in the whole populations studied. While, TNF-α rs1800629 polymorphism was significantly associated with the susceptibility of DCM in Asians, which indicates that such associations may be different between ethnicities.
However, some limitations should be considered in interpreting the results. First, some confounding factors addressed across different studies, such as age, family history, gender, and heart function classification (from class II to class IV), may influence the reliability of the results. Second, significant heterogeneity was detected even we performed subgroup analysis, and various potential factors accounted for the heterogeneity, including the basic characteristics of the study population and the study design. Third, 3 studies were not in agreement with HWE even the pooled ORs were not materially altered in the sensitivity analysis. Last, owing to the limited studies in certain subgroup, some conclusions should be interpreted with caution in subgroup analysis.
5. Conclusion
In summary, our meta-analysis based on 9 case–control studies concluded that TNF-α rs1800629 polymorphism was moderately associated with DCM susceptibility in the whole populations studied. TNF-α rs1800629 polymorphism was significantly associated with the susceptibility of DCM for Asians, which indicates that such associations may be different between ethnicities. Given the limited number of ethnic groups and sample size, more evidence from larger epidemiological studies is further needed to validate these results.
Author contributions
Yongdong Zhang and Bingshuang Liu designed this study; Yanhong Cao collected and collated data; Linlin Xin and Ningning Gao extracted and confirmed the data; Yongdong Zhang and Ningning Gao analyzed data; Yongdong Zhang and Ningning Gao wrote the manuscript; Bingshuang Liu edited the manuscript.
Conceptualization: Yongdong Zhang, Bingshuang Liu.
Formal analysis: Yanhong Cao, Ningning Gao.
Investigation: Linlin Xin, Ningning Gao.
Methodology: Yanhong Cao, Ningning Gao.
Supervision: Yanhong Cao, Linlin Xin, Bingshuang Liu.
Validation: Ningning Gao, Bingshuang Liu.
Writing – original draft: Yongdong Zhang.
Writing – review & editing: Bingshuang Liu.
Footnotes
Abbreviations: CI = confidence interval, DCM = dilated cardiomyopathy, HWE = Hardy–Weinberg equilibrium, NOS = Newcastle-Ottawa Scale, OR = odds ratio, SNP =single nucleotide polymorphism, TNF-α = tumor necrosis factor-α.
The authors have no conflicts of interest to disclose.
References
- [1].Bruggink AH, van Oosterhout MF, De Jonge N, et al. TNFalpha in patients with end-stage heart failure on medical therapy or supported by a left ventricular assist device. Transpl Immunol 2008;19:64–8. [DOI] [PubMed] [Google Scholar]
- [2].Tavares PS, Rocon-Albuquerque R, Jr, Leite-Moreira AF. Innate immune receptor activation in viral myocarditis: pathophysiologic implications. Rev Port Cardiol 2010;29:57–78. [PubMed] [Google Scholar]
- [3].Grimm W, Christ M, Bach J, et al. Noninvasive arrhythmia risk stratification in idiopathic dilated cardiomyopathy: results of the Marburg cardiomyopathy study. Circulation 2003;108:2883–91. [DOI] [PubMed] [Google Scholar]
- [4].Tiret L, Mallet C, Poirier O, et al. Lack of association between polymorphisms of eight candidate genes and idiopathic dilated cardiomyopathy: the CARDIGENE study. J Am Coll Cardiol 2000;35:29–35. [DOI] [PubMed] [Google Scholar]
- [5].Keeling PJ, Gang Y, Smith G, et al. Familial dilated cardiomyopathy in the United Kingdom. Br Heart J 1995;73:417–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Liaquat A, Asifa GZ, Zeenat A, et al. Polymorphisms of tumor necrosis factor-alpha and interleukin-6 gene and C-reactive protein profiles in patients with idiopathic dilated cardiomyopathy. Ann Saudi Med 2014;34:407–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Spiroska V, Kedev S, Antov S, et al. Association between 22 cytokine gene polymorphisms and dilated cardiomyopathy in Macedonian patients. Kardiol Pol 2009;67:1237–47. [PubMed] [Google Scholar]
- [8].Yamada T, Matsumori A, Sasayama S. Therapeutic effect of anti-tumor necrosis factor-alpha antibody on the murine model of viral myocarditis induced by encephalomyocarditis virus. Circulation 1994;89:846–51. [DOI] [PubMed] [Google Scholar]
- [9].Baena A, Leung JY, Sullivan AD, et al. TNF-alpha promoter single nucleotide polymorphisms are markers of human ancestry. Genes Immun 2002;3:482–7. [DOI] [PubMed] [Google Scholar]
- [10].Wilson AG, Symons JA, McDowell TL, et al. Effects of a polymorphism in the human tumor necrosis factor alpha promoter on transcriptional activation. Proc Nat Acad Sci USA 1997;94:3195–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ito M, Takahashi H, Fuse K, et al. Polymorphisms of tumor necrosis factor-alpha and interleukin-10 genes in Japanese patients with idiopathic dilated cardiomyopathy. Jpn Heart J 2000;41:183–91. [DOI] [PubMed] [Google Scholar]
- [12].Alikasifoglu M, Tokgozoglu L, Acil T, et al. Tumor necrosis factor-alpha polymorphism in Turkish patients with dilated cardiomyopathy. Eur J Heart Fail 2003;5:161–3. [DOI] [PubMed] [Google Scholar]
- [13].Luo R, Li X, Fan X, et al. Association of tumor necrosis factor-alpha gene G-308A polymorphism with dilated cardiomyopathy: a meta-analysis. DNA Cell Biol 2013;32:130–7. [DOI] [PubMed] [Google Scholar]
- [14].Mishra B, Sharma M, Sarkar S, et al. Tumour necrosis factor-alpha promoter polymorphism and its association with viral dilated cardiomyopathy in Indian population: a pilot study. Indian J Med Microbiol 2015;33:16–20. [DOI] [PubMed] [Google Scholar]
- [15].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clin Res ed) 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [17].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [18].Brooksbank R, Badenhorst D, Sliwa K, et al. The G-308A polymorphism of the TNF-alpha gene does not predict changes in cardiac function in response to medical therapy for idiopathic dilated cardiomyopathy. Cardiovasc J Africa 2008;19:254–8. [PMC free article] [PubMed] [Google Scholar]
- [19].Liang WB, Lv ML, Su XW, et al. Association of tumor necrosis factor gene polymorphisms with susceptibility to dilated cardiomyopathy in a Han Chinese population. DNA Cell Biol 2010;29:625–8. [DOI] [PubMed] [Google Scholar]
- [20].Nugent AW, Daubeney PE, Chondros P, et al. The epidemiology of childhood cardiomyopathy in Australia. New Engl J Med 2003;348:1639–46. [DOI] [PubMed] [Google Scholar]
- [21].Adamopoulos S, Kolokathis F, Gkouziouta A, et al. Cytokine gene polymorphisms are associated with markers of disease severity and prognosis in patients with idiopathic dilated cardiomyopathy. Cytokine 2011;54:68–73. [DOI] [PubMed] [Google Scholar]
- [22].Yndestad A, Damas JK, Oie E, et al. Systemic inflammation in heart failure—the whys and wherefores. Heart Fail Rev 2006;11:83–92. [DOI] [PubMed] [Google Scholar]
- [23].Doyama K, Fujiwara H, Fukumoto M, et al. Tumour necrosis factor is expressed in cardiac tissues of patients with heart failure. Int J Cardiol 1996;54:217–25. [DOI] [PubMed] [Google Scholar]
- [24].Torre-Amione G, Kapadia S, Lee J, et al. Tumor necrosis factor-alpha and tumor necrosis factor receptors in the failing human heart. Circulation 1996;93:704–11. [DOI] [PubMed] [Google Scholar]
- [25].Satoh M, Nakamura M, Tamura G, et al. Inducible nitric oxide synthase and tumor necrosis factor-alpha in myocardium in human dilated cardiomyopathy. J Am Coll Cardiol 1997;29:716–24. [DOI] [PubMed] [Google Scholar]
- [26].Ferrari R. Tumor necrosis factor in CHF: a double facet cytokine. Cardiovasc Res 1998;37:554–9. [DOI] [PubMed] [Google Scholar]
- [27].Densem CG, Hutchinson IV, Yonan N, et al. Tumour necrosis factor alpha gene polymorphism: a predisposing factor to non-ischaemic myocardial dysfunction. Heart (Br Cardiac Soc) 2002;87:153–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Balci SO, Col-Araz N, Baspinar O, et al. Cytokine gene polymorphisms in childhood dilated cardiomyopathy: interferon- gamma, tumor necrosis factor-alpha and transforming growth factor—beta 1 genes are associated with the disease in Turkish patients. Iran J Pediatr 2013;23:603–4. [PMC free article] [PubMed] [Google Scholar]
- [29].Kurrelmeyer KM, Michael LH, Baumgarten G, et al. Endogenous tumor necrosis factor protects the adult cardiac myocyte against ischemic-induced apoptosis in a murine model of acute myocardial infarction. Proc Nat Acad Sci USA 2000;97:5456–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bozkurt B, Kribbs SB, Clubb FJ, Jr, et al. Pathophysiologically relevant concentrations of tumor necrosis factor-alpha promote progressive left ventricular dysfunction and remodeling in rats. Circulation 1998;97:1382–91. [DOI] [PubMed] [Google Scholar]
- [31].Irwin MW, Mak S, Mann DL, et al. Tissue expression and immunolocalization of tumor necrosis factor-alpha in postinfarction dysfunctional myocardium. Circulation 1999;99:1492–8. [DOI] [PubMed] [Google Scholar]
- [32].Monden Y, Kubota T, Inoue T, et al. Tumor necrosis factor-alpha is toxic via receptor 1 and protective via receptor 2 in a murine model of myocardial infarction. Am J Physiol Heart Circ Physiol 2007;293:H743–753. [DOI] [PubMed] [Google Scholar]


