Abstract
Adrenocortical carcinoma (ACC) is an aggressive form of cancer that originates in the cortex of the adrenal gland; the incidence of ACC is 1.5 to 2 cases per million people per year. ACCs are rare and mostly sporadic. A small proportion of ACC cases are associated with hereditary cancer syndromes. Here, we present a case of ACC with a pathogenic heterozygous germline deletion in CHEK2 (c.1100delC). This is, to our knowledge, the first report of a patient with ACC associated with a CHEK2 germline deletion.
Keywords: adrenocortical carcinoma, CHEK2, germline
Adrenocortical carcinoma (ACC) is a rare endocrine malignancy, with an incidence of 1.5 to 2 cases per million per year. The overall prognosis is extremely poor; the 5-year staging-dependent survival ranges from 81% for patients with stage I disease to 13% for those with stage [1]. The average survival from the time of diagnosis is 14.5 months [2]. There are few effective treatment options [3]. Approximately 50% of patients diagnosed with ACC have additional complications, such as Cushing syndrome [4, 5]. Therefore, the management of ACC usually requires a multidisciplinary therapeutic approach, especially for recurrent and metastatic disease [6]. Approximately 3% to 5% of ACCs are associated with hereditary cancer syndromes, including Li-Fraumeni syndrome, familial adenomatous polyposis, Lynch syndrome, and Beckwith-Wiedemann syndrome, and in <1% of ACCs are associated with multiple endocrine neoplasia type 1 syndromes [3, 7]. In addition, ACC has been reported in patients with neurofibromatosis type 1 [8, 9], hereditary nonpolyposis colorectal cancer [10–12], and succinate dehydrogenase pathogenic mutations [13].
The CHEK2 gene encodes a cell-cycle checkpoint kinase 2 protein, or CHK2. CHK2 plays an important role in cell-cycle regulation through interactions with other cancer susceptibility genes (e.g., ATM, p53, BRCA1, BRCA2). When activated by ATM, it subsequently phosphorylates CDC25A, CDC25C, TP53, and BRCA1 [14]. As a result, it acts as a tumor suppressor in response to DNA damage and also functions as a genetic risk modifier of other cancer susceptibility genes [15, 16]. It is hypothesized that the clinical spectrum of cancers associated with CHEK2 mutations may reflect their relative contribution toward the function of TP53 and/or BRCA1 [17, 18]. There are multiple CHEK2 variants that have been associated with various sporadic cancers or familial hereditary syndromes, including large deletion of exon 9 and 10 [19, 20], 1100delC [21], missense mutations affecting the forkhead and kinase domains [22], G190A [9], A751T [23], IVS2+1G-A, and I157T [24]. However, to our knowledge, a germline CHEK2 defect has never been reported in association with ACC. Here, we report a case of a 48-year-old woman diagnosed initially with Cushing syndrome and later with ACC, and whom was found to be a carrier of a pathogenic heterozygous deletion in CHEK2 (namely, c.1100delC).
1. Case report
A 48-year-old woman initially came to Mary Washington Hospital in December 2010 with blurry vision, fatigue, weight gain, muscle weakness, hair loss, increased abdominal girth, abdominal striae, and indurated swelling on the back of her neck (Fig. 1). Subsequently, she was found to have an elevated morning cortisol level of 33.4 μg/dL at 8:40 am, a 24-hour urine free cortisol level of 498 μg/24 h (normal range, 10 to34 μg/24 h), and an adrenocorticotropic hormone level <5.0 μg/dL (normal range, 5.0 to 25.0 μg/dL). The patient’s salivary cortisol level was elevated at 0.99 μg/dL (normal range, <0.01 to 0.09 μg/dL). The clinical phenotype and the biochemical findings confirmed the diagnosis of Cushing syndrome.
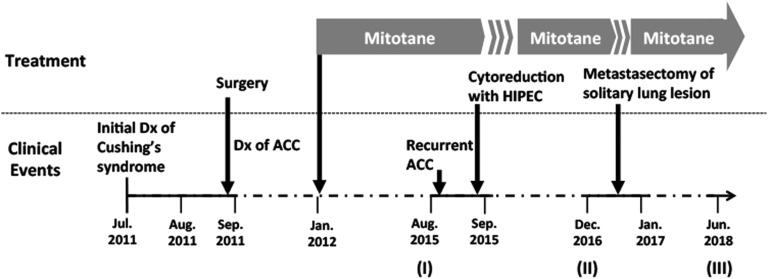
Figure 1.
Clinical course. Dx, diagnosis; HIPEC, hyperthermic intraperitoneal chemotherapy.
A CT scan of the abdomen and pelvis revealed an 8 × 6 × 9–cm left adrenal mass with areas of necrosis and soft tissue extending from the left adrenal vein to the level of the inferior vena cava. The patient underwent left adrenalectomy 24 August 2011, after biochemical workup excluded pheochromocytoma. After surgical resection, pathologic evaluation confirmed a high-grade ACC. The tumor was encapsulated with an MIB-1 proliferation index in 15% of the neoplastic cells, without invasion into vasculature, and was staged as a European Network for the Study of Adrenal Tumor stage II. The patient was discharged home with a replacement dose of hydrocortisone after surgery, which was discontinued after 2 months.
Two months after surgery, the patient was referred to the National Institutes of Health for further management of her cancer. She received adjuvant mitotane therapy starting at 2 g/d, increasing by 500 mg every 7 to 10 days to a total dose of 4 g/d; mitotane was well tolerated with no serious adverse effects. She also received the replacement doses of hydrocortisone and fludrocortisone and was closely monitored radiologically and biochemically every 3 months.
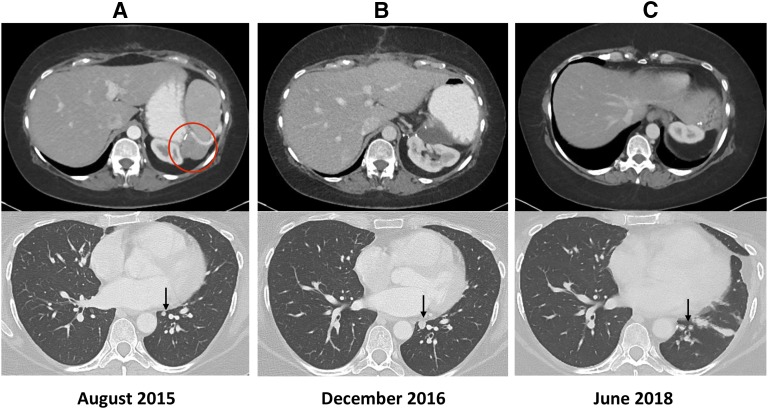
Unfortunately, 4 years later, a recurrence developed, identified by fluorodeoxyglucose–positron emission tomography scan (3 × 3 cm; Fig. 2, panel I), in the prior surgical bed. The patient was then treated with extensive surgery (including exploratory laparotomy, extensive lysis of adhesions, splenectomy, partial gastrectomy, and distal pancreatectomy en bloc with adrenal bed mass, partial omentectomy, diaphragmatic repair, and bilateral oophorectomy) and hyperthermic intraperitoneal chemotherapy with 450 mg of cisplatin. Surveillance CT scans of the chest indicated a left lower-lung nodule that grew slowly from 3 × 3 mm to 8 × 7 mm (Fig. 2, panels I and II). Subsequently, the patient underwent a left-side thoracoscopic procedure to remove the solitary lesion in the left lower lobe of the lung; ACC metastases were confirmed (Fig. 3). After this procedure, there was no evidence of disease (Fig. 2, panel III). She is currently receiving mitotane without significant adverse effects.
Figure 2.
Radiologic response after the treatments. (A) Upper panel: Recurrent ACC on the surgical bed, indicated with circle. Lower panel: Subcentimeter lung lesion indicated at arrow. (B) Upper panel: Postsurgical change in the original recurrent site. Lower panel: enlarged lung lesion indicated at arrow. (C) Representative image showing no evidence of disease and postsurgical change.
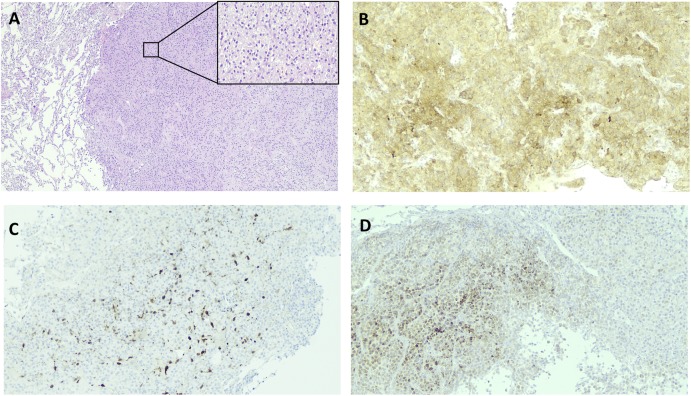
Figure 3.
Pathologic evidence of ACC metastases to lung. (A) Representative hematoxylin and eosin staining of resected lung lesion, indicated on the right; normal lung parenchyma is evident on the left (magnification, ×4). The enlarged section shows sheets of polygonal tumor cells with granular to clear cytoplasm, some nuclear pleomorphism, and very low mitotic rate (magnification, ×10). (B) Representative immunohistochemical (IHC) staining showing diffuse inhibin expression (magnification, ×10). (C) Representative IHC staining showing a patchy area positive for MELAN-A protein expression (magnification, ×10). (D) Representative IHC staining showing S-100 protein expression in tumor tissue (magnification, ×10).
Because of the early onset of ACC (<50 years of age), a saliva sample was sent to Invitae (San Francisco, CA) for a hereditary cancer panel. The following genes were evaluated for sequence changes and exonic deletions or duplications: AKT1, ATM, BRCA1, BRCA2, BRIP1, CDC73, CDH1, CHEK2, DICER1, PECAM, FAM175A, FANCC, MLH1, MRE11A, MSH2, MSH6, MUTYH, NBN, NF1, PALB2, PIK3CA, PMS2, POLD1, PTEN, RAD50, RAD51C, RAD51D, RINT1, SDHB, SDHD, SMARCA4, STK11, TP53, and XRCC2. The results revealed a heterozygous deletion (c.1100delC) of the CHEK2 gene as the only positive finding. No tumor sample was submitted for somatic mutation test.
The patient’s family history was positive for cancer; her maternal grandmother died at age 70 years of colon cancer (age at diagnosis is unknown). Both of the patient’s parents are alive and in good general health; her father had a basal cell carcinoma diagnosed at age 50 years. The proband is G6P5; all children are in good general health. One daughter was tested for the CHEK2 mutation and was negative. Genetic counseling was provided; consanguinity was denied.
2. Discussion
Here, we report an ACC case with a heterozygous deletion of the CHEK2 gene (c.1100delC). The c.1100delC at exon 11 of the CHEK2 mRNA leads to a frameshift of codon 367 and subsequently creates a premature translational stop signal as p.Thr367Metfs*15 that is expected to result in an absent or disrupted CHK2 protein.
It is unclear if this mutation is linked to this patient’s ACC tumorigenesis. However, variants with loss of function in CHEK2 are known to be pathogenic for other cancer types [18, 21]. In a large meta-analysis of 16 studies, including 26,488 patients and 27,402 control subjects, women with heterozygosity of this variant had a relative risk for familial breast cancer of 4.8 (95% CI, 3.3 to 7.2). The cumulative risk at age 70 years was 37% (95% CI, 26% to 56%), compared to 7.8% for the average population of white women [25]. Although a study reports that the c.1100delC variant contributes an ∼10-fold increased risk of breast cancer in males [18], the results have yet to be validated in others [26, 27]. Patients with a history of familial breast cancer who carry the c.1100delC variant and wild-type BRCA1/2 have a considerably higher incidence of the 1-bp deletion [28]. Moreover, more patients with hereditary breast cancer with colorectal cancer carry the c.1100delC variant than do patients with breast cancer but without colorectal cancer (18% vs 4%, respectively). Nevertheless, the c.1100delC appeared to synergistically act with at least an unknown vulnerability gene and was not the major predisposing factor for the HBCC presentation [29]. Interestingly, c.1100delC carriers not only had more frequent female breast cancer in their first- or second-degree relatives, they had a higher occurrence of contralateral breast cancer and poorer distant metastasis-free survival [30]. It has been suggested c.1100delC is an adverse prognostic indicator. Nevertheless, c.1100delC mutation was found in 14 (0.8%) of 1864 Polish men with prostate cancer, in 3 of 249 Polish men (1.2%) with familial prostate cancer, and in 12 (0.2%) of 5496 healthy control subjects (OR, 5.6). In a study [31] in which effort was made to confine the incidence of cancer to those other than breast cancer from 11,116 families with a history of non-BRCA1/2 breast cancer, c.1100delC mutation primarily was associated with breast cancer but also slightly with increased overall risk of other cancers. In this study, the ACC cases may have been underreported [31]. Findings indicate c.1100delC is a founder mutation for the aforementioned cancers [19]. To our knowledge, ours is the first report of an association between ACC and a germline defect in CHEK2.
Inactivation of the TP53 pathway is an established feature of human cancers, with nearly all cancers evolving a system to evade this essential tumor-suppressive mechanism. Although inactivation of TP53 through mutations or deletions of the TP53 locus directly leads to development of various human cancers, there are many other molecular alterations that can functionally attenuate the pathway [32]. Indeed, CHEK2 arrests the cell cycle via several mechanisms under the circumstance of DNA double-strand damage. One mechanism is phosphorylation of TP53 by CHEK2, which stabilizes and activates TP53 [17, 33]. Therefore, CHEK2 is crucial for cell-cycle regulation, and its abnormal expression could lead to cancer independent of TP53 mutation status [34]. More studies are needed to further clarify the role of CHEK2 on ACC tumorigenesis.
This ACC case shows that c.1100delC of CHEK2 may be linked to ACC tumorigenesis. Therefore, at least the TP53 and CHEK2 gene analyses will be informative to all patients with ACC who have a negative family history of other inherited conditions [20, 35]. The European Society of Endocrinology recommends basic clinical genetic evaluation to explore any evidence of hereditary predisposition for adult patients with ACC [36]. Specialized genetics counseling should be pursued to understand the pros and cons of genetic testing. The European Society of Endocrinology currently has no definite recommendation for somatic mutation testing of tumors [36]. If genetic testing is performed, post-test genetic counseling should follow. The finding of a germline CHEK2 mutation may entail a clinical surveillance for patients with asymptomatic nonadrenal neoplasms.
3. Conclusions
In conclusion, we report a case showing ACC tumorigenesis is associated with a germline CHEK2 mutation. The patient’s clinical presentation and negative family history were inconstant with the typical CHEK2-associated phenotype. Additional studies are needed to determine a causative role; no management recommendations can be done at this time, though close clinical surveillance will be helpful.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- ACC
adrenocortical carcinoma
- CHK2
checkpoint kinase 2
References and Notes
- 1. Fassnacht M, Kroiss M, Allolio B. Update in adrenocortical carcinoma. J Clin Endocrinol Metab. 2013;98(12):4551–4564. [DOI] [PubMed] [Google Scholar]
- 2. Paragliola RM, Torino F, Papi G, Locantore P, Pontecorvi A, Corsello SM. Role of mitotane in adrenocortical carcinoma - review and state of the art. Eur Endocrinol. 2018;14(2):62–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Else T, Kim AC, Sabolch A, Raymond VM, Kandathil A, Caoili EM, Jolly S, Miller BS, Giordano TJ, Hammer GD. Adrenocortical carcinoma. Endocr Rev. 2014;35(2):282–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ayala-Ramirez M, Jasim S, Feng L, Ejaz S, Deniz F, Busaidy N, Waguespack SG, Naing A, Sircar K, Wood CG, Pagliaro L, Jimenez C, Vassilopoulou-Sellin R, Habra MA. Adrenocortical carcinoma: clinical outcomes and prognosis of 330 patients at a tertiary care center. Eur J Endocrinol. 2013;169(6):891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Terzolo M, Daffara F, Ardito A, Zaggia B, Basile V, Ferrari L, Berruti A. Management of adrenal cancer: a 2013 update. J Endocrinol Invest. 2014;37(3):207–217. [DOI] [PubMed] [Google Scholar]
- 6. Terzolo M, Berruti A. Adjunctive treatment of adrenocortical carcinoma. Curr Opin Endocrinol Diabetes Obes. 2008;15(3):221–226. [DOI] [PubMed] [Google Scholar]
- 7. Mazzuco TL, Durand J, Chapman A, Crespigio J, Bourdeau I. Genetic aspects of adrenocortical tumours and hyperplasias. Clin Endocrinol (Oxf). 2012;77(1):1–10. [DOI] [PubMed] [Google Scholar]
- 8. Li Q, Zhao F, Ju Y. Germline mutation of CHEK2 in neurofibromatosis 1 and 2: two case reports. Medicine (Baltimore). 2018;97(23):e10894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang X, Teer JK, Tousignant RN, Levin AM, Boulware D, Chitale DA, Shaw BM, Chen Z, Zhang Y, Blakeley JO, Acosta MT, Messiaen LM, Korf BR, Tainsky MA. Breast cancer risk and germline genomic profiling of women with neurofibromatosis type 1 who developed breast cancer. Genes Chromosomes Cancer. 2018;57(1):19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Berends MJ, Cats A, Hollema H, Karrenbeld A, Beentjes JA, Sijmons RH, Mensink RG, Hofstra RM, Verschueren RC, Kleibeuker JH. Adrenocortical adenocarcinoma in an MSH2 carrier: coincidence or causal relation? Hum Pathol. 2000;31(12):1522–1527. [DOI] [PubMed] [Google Scholar]
- 11. Broaddus RR, Lynch PM, Lu KH, Luthra R, Michelson SJ. Unusual tumors associated with the hereditary nonpolyposis colorectal cancer syndrome. Mod Pathol. 2004;17(8):981–989. [DOI] [PubMed] [Google Scholar]
- 12. Medina-Arana V, Delgado L, González L, Bravo A, Díaz H, Salido E, Riverol D, González-Aguilera JJ, Fernández-Peralta AM. Adrenocortical carcinoma, an unusual extracolonic tumor associated with Lynch II syndrome. Fam Cancer. 2011;10(2):265–271. [DOI] [PubMed] [Google Scholar]
- 13. Else T, Lerario AM, Everett J, Haymon L, Wham D, Mullane M, Wilson TL, Rainville I, Rana H, Worth AJ, Snyder NW, Blair IA, McKay R, Kilbridge K, Hammer G, Barletta J, Vaidya A. Adrenocortical carcinoma and succinate dehydrogenase gene mutations: an observational case series. Eur J Endocrinol. 2017;177(5):439–444. [DOI] [PubMed] [Google Scholar]
- 14. Jalilvand M, Oloomi M, Najafipour R, Alizadeh SA, Saki N, Rad FS, Shekari M. An association study between CHEK2 gene mutations and susceptibility to breast cancer. Comp Clin Pathol. 2017;26(4):837–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nevanlinna H, Bartek J. The CHEK2 gene and inherited breast cancer susceptibility. Oncogene. 2006;25(43):5912–5919. [DOI] [PubMed] [Google Scholar]
- 16. Serrano-Fernández P, Debniak T, Górski B, Bogdanova N, Dörk T, Cybulski C, Huzarski T, Byrski T, Gronwald J, Wokołorczyk D, Narod SA, Lubiński J. Synergistic interaction of variants in CHEK2 and BRCA2 on breast cancer risk. Breast Cancer Res Treat. 2009;117(1):161–165. [DOI] [PubMed] [Google Scholar]
- 17. Chehab NH, Malikzay A, Appel M, Halazonetis TD. Chk2/hCds1 functions as a DNA damage checkpoint in G(1) by stabilizing p53. Genes Dev. 2000;14(3):278–288. [PMC free article] [PubMed] [Google Scholar]
- 18. Meijers-Heijboer H, van den Ouweland A, Klijn J, Wasielewski M, de Snoo A, Oldenburg R, Hollestelle A, Houben M, Crepin E, van Veghel-Plandsoen M, Elstrodt F, van Duijn C, Bartels C, Meijers C, Schutte M, McGuffog L, Thompson D, Easton D, Sodha N, Seal S, Barfoot R, Mangion J, Chang-Claude J, Eccles D, Eeles R, Evans DG, Houlston R, Murday V, Narod S, Peretz T, Peto J, Phelan C, Zhang HX, Szabo C, Devilee P, Goldgar D, Futreal PA, Nathanson KL, Weber B, Rahman N, Stratton MR; CHEK2-Breast Cancer Consortium . Low-penetrance susceptibility to breast cancer due to CHEK2(*)1100delC in noncarriers of BRCA1 or BRCA2 mutations. Nat Genet. 2002;31(1):55–59. [DOI] [PubMed] [Google Scholar]
- 19. Cybulski C, Wokołorczyk D, Huzarski T, Byrski T, Gronwald J, Górski B, Debniak T, Masojć B, Jakubowska A, Gliniewicz B, Sikorski A, Stawicka M, Godlewski D, Kwias Z, Antczak A, Krajka K, Lauer W, Sosnowski M, Sikorska-Radek P, Bar K, Klijer R, Zdrojowy R, Małkiewicz B, Borkowski A, Borkowski T, Szwiec M, Narod SA, Lubiński J. A large germline deletion in the Chek2 kinase gene is associated with an increased risk of prostate cancer. J Med Genet. 2006;43(11):863–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Herrmann LJ, Heinze B, Fassnacht M, Willenberg HS, Quinkler M, Reisch N, Zink M, Allolio B, Hahner S. TP53 germline mutations in adult patients with adrenocortical carcinoma. J Clin Endocrinol Metab. 2012;97(3):E476–E485. [DOI] [PubMed] [Google Scholar]
- 21. Bell DW, Varley JM, Szydlo TE, Kang DH, Wahrer DC, Shannon KE, Lubratovich M, Verselis SJ, Isselbacher KJ, Fraumeni JF, Birch JM, Li FP, Garber JE, Haber DA. Heterozygous germ line hCHK2 mutations in Li-Fraumeni syndrome. Science. 1999;286(5449):2528–2531. [DOI] [PubMed] [Google Scholar]
- 22. Miller CW, Ikezoe T, Krug U, Hofmann WK, Tavor S, Vegesna V, Tsukasaki K, Takeuchi S, Koeffler HP. Mutations of the CHK2 gene are found in some osteosarcomas, but are rare in breast, lung, and ovarian tumors. Genes Chromosomes Cancer. 2002;33(1):17–21. [DOI] [PubMed] [Google Scholar]
- 23. Dong X, Wang L, Taniguchi K, Wang X, Cunningham JM, McDonnell SK, Qian C, Marks AF, Slager SL, Peterson BJ, Smith DI, Cheville JC, Blute ML, Jacobsen SJ, Schaid DJ, Tindall DJ, Thibodeau SN, Liu W. Mutations in CHEK2 associated with prostate cancer risk. Am J Hum Genet. 2003;72(2):270–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cybulski C, Górski B, Huzarski T, Masojć B, Mierzejewski M, Debniak T, Teodorczyk U, Byrski T, Gronwald J, Matyjasik J, Zlowocka E, Lenner M, Grabowska E, Nej K, Castaneda J, Medrek K, Szymańska A, Szymańska J, Kurzawski G, Suchy J, Oszurek O, Witek A, Narod SA, Lubiński J. CHEK2 is a multiorgan cancer susceptibility gene. Am J Hum Genet. 2004;75(6):1131–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Weischer M, Bojesen SE, Ellervik C, Tybjaerg-Hansen A, Nordestgaard BG. CHEK2*1100delC genotyping for clinical assessment of breast cancer risk: meta-analyses of 26,000 patient cases and 27,000 controls. J Clin Oncol. 2008;26(4):542–548. [DOI] [PubMed] [Google Scholar]
- 26. Syrjäkoski K, Kuukasjärvi T, Auvinen A, Kallioniemi OP. CHEK2 1100delC is not a risk factor for male breast cancer population. Int J Cancer. 2004;108(3):475–476. [DOI] [PubMed] [Google Scholar]
- 27. Sodha N, Wilson C, Bullock SL, Phillimore H, Houlston RS, Eeles RA. Analysis of familial male breast cancer for germline mutations in CHEK2. Cancer Lett. 2004;215(2):187–189. [DOI] [PubMed] [Google Scholar]
- 28. Vahteristo P, Bartkova J, Eerola H, Syrjäkoski K, Ojala S, Kilpivaara O, Tamminen A, Kononen J, Aittomäki K, Heikkilä P, Holli K, Blomqvist C, Bartek J, Kallioniemi OP, Nevanlinna H. A CHEK2 genetic variant contributing to a substantial fraction of familial breast cancer. Am J Hum Genet. 2002;71(2):432–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Meijers-Heijboer H, Wijnen J, Vasen H, Wasielewski M, Wagner A, Hollestelle A, Elstrodt F, van den Bos R, de Snoo A, Fat GT, Brekelmans C, Jagmohan S, Franken P, Verkuijlen P, van den Ouweland A, Chapman P, Tops C, Möslein G, Burn J, Lynch H, Klijn J, Fodde R, Schutte M. The CHEK2 1100delC mutation identifies families with a hereditary breast and colorectal cancer phenotype. Am J Hum Genet. 2003;72(5):1308–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. de Bock GH, Schutte M, Krol-Warmerdam EM, Seynaeve C, Blom J, Brekelmans CT, Meijers-Heijboer H, van Asperen CJ, Cornelisse CJ, Devilee P, Tollenaar RA, Klijn JG. Tumour characteristics and prognosis of breast cancer patients carrying the germline CHEK2*1100delC variant. J Med Genet. 2004;41(10):731–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thompson D, Seal S, Schutte M, McGuffog L, Barfoot R, Renwick A, Eeles R, Sodha N, Houlston R, Shanley S, Klijn J, Wasielewski M, Chang-Claude J, Futreal PA, Weber BL, Nathanson KL, Stratton M, Meijers-Heijboer H, Rahman N, Easton DF. A multicenter study of cancer incidence in CHEK2 1100delC mutation carriers. Cancer Epidemiol Biomarkers Prev. 2006;15(12):2542–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hainaut P, Pfeifer GP. Somatic TP53 mutations in the era of genome sequencing. Cold Spring Harb Perspect Med. 2016;6(11):a026179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hirao A, Kong YY, Matsuoka S, Wakeham A, Ruland J, Yoshida H, Liu D, Elledge SJ, Mak TW. DNA damage-induced activation of p53 by the checkpoint kinase Chk2. Science. 2000;287(5459):1824–1827. [DOI] [PubMed] [Google Scholar]
- 34. Ruijs MW, Broeks A, Menko FH, Ausems MG, Wagner A, Oldenburg R, Meijers-Heijboer H, van’t Veer LJ, Verhoef S. The contribution of CHEK2 to the TP53-negative Li-Fraumeni phenotype. Hered Cancer Clin Pract. 2009;7(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Raymond VM, Else T, Everett JN, Long JM, Gruber SB, Hammer GD. Prevalence of germline TP53 mutations in a prospective series of unselected patients with adrenocortical carcinoma. J Clin Endocrinol Metab. 2013;98(1):E119–E125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fassnacht M, Dekkers OM, Else T, Baudin E, Berruti A, de Krijger R, Haak HR, Mihai R, Assie G, Terzolo M. European Society of Endocrinology Clinical Practice Guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2018;179(4):G1–G46. [DOI] [PubMed] [Google Scholar]