Abstract
Leptin is a pro-inflammatory cytokine that plays an important role in energy homeostasis. Emerging evidence suggests that leptin levels are altered in children with autism spectrum disorder (ASD); however this has not been studied prospectively. Rapid growth during infancy and early childhood has been implicated in ASD, but the evidence is inconsistent. Since leptin is involved in growth and is a potential risk factor for ASD, we explored the associations between 1) cord, early childhood leptin and ASD; and 2) birth weight for gestational age, early childhood weight gain and ASD. We also assessed the mediating role of leptin in the relationship between weight gain during infancy and ASD. This study was conducted in a sample of 822 subjects from the Boston Birth Cohort. ASD was defined from diagnostic codes in electronic medical records. Extremely rapid weight gain during infancy was associated with a greater ASD risk and this persisted after adjusting for potential confounders (aOR: 3.11; 95% CI: 1.37, 7.07). Similarly, children that had higher plasma leptin levels, prior to ASD diagnosis, had an increased ASD risk in both unadjusted and adjusted models (aOR: 7.87; 95% CI: 2.06, 30.04). Further, early childhood leptin indirectly mediated the relationship between rapid weight gain and ASD. No associations were found between birth weight for gestational age, cord leptin and risk of ASD. Our findings provide a basis to further explore whether the combination of early life growth pattern and a biomarker such as leptin can predict ASD earlier.
Keywords: Leptin, rapid weight gain in infancy, autism
Lay Summary:
Is early life growth and a biomarker leptin related to ASD risk? To answer this question, we followed 822 children from birth and found that those who gained weight very quickly in infancy, had higher leptin levels in early childhood, had a greater chance of later ASD diagnosis. More research is needed to see if infant’s weight gain pattern along with a biomarker (such as leptin) can be used to identify children with ASD sooner.
Introduction
Autism Spectrum Disorder (ASD) is a neurodevelopmental conditions characterized by impairments in sociability and communication, as well as increased repetitive and/or restrictive behaviors and interests (Jeste, 2015; Leitner, 2014; Murray, 2010; Schaevitz, Berger-Sweeney, & Ricceri, 2014; Zhubi, Cook, Guidotti, & Grayson, 2014). ASD prevalence in the U.S. has increased dramatically since 1996 and now, 1 in 68 children are diagnosed with ASD (Krakowiak et al., 2012; Van Naarden Braun et al., 2015). The precise cause of ASD is largely unknown (Moore, Kneitel, Walker, Gilbert, & Xing, 2012). Numerous studies have demonstrated associations with genetic, environmental, perinatal and immunological risk factors, leading to a hypothesis that ASD likely has a multifactorial pathogenesis or is a common end point of multiple causal pathways (Limperopoulos, 2009; Moore et al., 2012).
Among the perinatal risk factors, preterm birth and small for gestational age (SGA) have been studied extensively in the context of ASD (Joseph et al., 2017; Moore et al., 2012; Padilla et al., 2017; Schieve et al., 2014). SGA is a proxy for intrauterine growth restriction (Hunter et al., 2016) and studies have reported that SGA children are at increased risk of ASD (Lampi et al., 2012; Larsson et al., 2005; Moore et al., 2012); however, the results are inconsistent (Glasson et al., 2004; Larsson et al., 2005; Schendel & Bhasin, 2008). Many children that are small at birth tend to have rapid catch-up growth during early postnatal period (Castanys-Munoz et al., 2017; Ong, 2007). Rapid growth during first year of life has been shown to be associated with ASD (Dementieva et al., 2005; Dissanayake, Bui, Huggins, & Loesch, 2006; Torrey, Dhavale, Lawlor, & Yolken, 2004), although not all studies have confirmed these findings (Courchesne, Carper, & Akshoomoff, 2003; Rommelse et al., 2011). While abnormal birth weight percentiles and rapid early growth have been identified as independent risk factors of ASD, the combined effect of SGA with rapid postnatal weight gain has not been explored. Most studies previously conducted on SGA infants have not accounted for the rate of postnatal growth, and vice versa. Further, the biological mechanisms behind SGA and rapid weight gain and ASD risk have not been clearly elucidated (Sacco et al., 2007; Suren et al., 2013).
Leptin is a peptide hormone that is predominantly secreted by the white adipose tissue and has been studied in the context of fetal growth and early childhood weight gain (Blardi et al., 2010; Karakosta et al., 2011). Studies have found that children with SGA have lower leptin levels possibly reflecting a lower fat mass as a result of intrauterine restricted fetal weight gain (Catov et al., 2007; Harigaya, Nagashima, Nako, & Morikawa, 1997; Koistinen et al., 1997; Pighetti et al., 2003). Metabolically, lower cord blood leptin levels are known to predict rapid weight gain in infancy (Kettaneh et al., 2007; S. Li et al., 2016; Ong et al., 1999; Perng et al., 2016). Beyond leptin’s role in prenatal and postnatal weight gain, this pleiotropic cytokine has been shown to be important in the regulation of the immune system, neurodevelopment including neuron excitability, synaptic plasticity, neural differentiation and promoting migration of neuronal lineage cells to the cortical plate (Ashwood et al., 2008; Blardi et al., 2010; Harvey, 2007; Harvey, Solovyova, & Irving, 2006; Paz-Filho et al., 2008). Emerging evidence suggests that children (2–15 years) with ASD have significantly higher plasma leptin levels than controls (Al-Zaid, Alhader, & Al-Ayadhi, 2014; Ashwood et al., 2008; Blardi et al., 2010; Essa M.M. et al., 2011; Rodrigues et al., 2014). Among a few studies that have researched leptin-ASD association in children, most were done after ASD diagnosis (Ashwood et al., 2008; Blardi et al., 2010), thus unable to assess the temporal relationship. To our knowledge, none of the studies have assessed cord and early childhood leptin levels independently and simultaneously in relation to ASD in a prospective birth cohort.
Despite studies observing cytokine involvement in ASD (Krakowiak et al., 2017; Masi, Glozier, Dale, & Guastella, 2017; Rodrigues et al., 2014; N. Xu, Li, & Zhong, 2015) and the knowledge about the role of fetal and infant growth and ASD (Dementieva et al., 2005; Dissanayake et al., 2006; Torrey et al., 2004), existing studies have not evaluated the potential link between growth and leptin levels related to ASD. We set out to understand whether elevated leptin levels observed in people with ASD are related to rapid weight gain during infancy, and whether leptin has a mechanistic role in explaining the early growth-ASD relationship. Specifically, in this report we sought to explore the relationship between – 1) birth weight for gestational age, weight gain during first year of life and ASD risk; 2) cord and early childhood leptin and ASD risk; and 3) the potential of leptin mediating the relationship between weight gain during first year and ASD risk. We analyzed the longitudinal data from the Boston Birth Cohort (BBC), a predominantly urban low-income minority population.
Methods
Participation and data collection procedure
As illustrated in supplemental figure 1, this study included 822 children from the BBC, who were recruited at birth (between 1998 and 2009), were followed prospectively until 2015 and had a median length of follow-up of 7.5 years (interquartile range: 5.2 – 9.8 years). Mothers of newborns were invited to participate in the study 24–72 hours after birth. Over 90% of those that were approached agreed to participate and were initiated into the study (G. Wang et al., 2014; X. Wang et al., 2002). For every preterm (defined as <37 weeks of gestation) and/or low birth weight baby (defined as <2,500 g), approximately two term and normal birth weight babies (and their mothers) were enrolled in the study (X. Wang et al., 2002). The exclusion criterion for initial enrolment was multiple-gestation pregnancies and newborns with major birth defects. Participants and non-participants did not differ on characteristics such as infant birth weight, maternal ethnicity, or other sociodemographic characteristics (X. Wang et al., 2002). A sub-set of the participants were enrolled in the follow-up study that began in 2003 and children not planning to receive pediatric care at the Boston Medical Center were not included in the postnatal follow-up. There were no major differences in baseline demographic characteristics between those with and without postnatal follow-up (M. Li et al., 2016).
Of 2,932 that were eligible for postnatal follow-up, 2,110 were excluded for the following reasons: 1,589 did not have at least one of the exposure variables (cord leptin, early childhood leptin) and 521 had other competing diagnosis such as ADHD, intellectual disabilities (ID) or other developmental disabilities (DD). Electronic Medical Records (EMR) containing clinicians’ primary and secondary diagnoses using ICD-9 codes were obtained for every postnatal clinical visit since 2003. The study was approved by the Institutional Review Boards of the Johns Hopkins Bloomberg School of Public Health and Boston University Medical Center.
Identification of children with ASD
Based on EMR, children that were ever diagnosed with autism (ICD-9 code 299.00), Asperger syndrome (299.80) and/or pervasive developmental disorder not otherwise specified (299.90) were categorized as having ASD. Neurotypical children were those that were never diagnosed with ASD, ADHD, ID and other DD. When children had concurrent diagnosis, such as ASD and ADHD or ASD and ID or ASD and other DD, they were classified as having ASD. Two separate sensitivity analyses were conducted - 1) using a stringent criteria, defined as having ASD diagnosis on more than two separate occasions in the EMR and one visit to specialists such as behavioral pediatrician, pediatric neurologist or child psychologist; and 2) using a stringent control that additionally excluded children who did not have other competing diagnoses (Conduct disorder (312.0 – 312.9), Emotional disturbances of childhood or adolescence including Oppositional Defiant Disorder (313.0 – 313.9), Congenital Anomalies (740 – 759.9)).
Exposure variables
Birth weight for gestational age was defined as follows: SGA (<10th percentile), appropriate for gestational age (AGA) (≥10th – 90th percentile) and large for gestational age (LGA) (>90th percentile) (G. Wang et al., 2014). Child’s length (<2 years) and height and weight were measured during the well-child visits at the Boston Medical Center. WHO reference values was used to calculate weight-for-age z-scores and was defined as the change in weight-for-age z-scores from birth until the target time-point. Weight-for-age z-score was categorized into the following groups: slow (weight gain z-score <−0.67), on track (−0.67 to 0.67), rapid (>0.67 to 1.28), and extremely rapid (>1.28) (G. Wang, Johnson, et al., 2016).
Umbilical cord blood sample was collected at delivery and non-fasting early childhood venous blood sample were collected during subsequent follow-up visits. The median age of early childhood leptin measurement was 18.4 months (IQR: 10.3–49.2 months). All the blood samples were processed immediately after collection and plasma samples were stored in a freezer at −80°C. Only children with blood samples obtained prior to ASD diagnosis were included in the early childhood biomarker analysis. As mentioned elsewhere, plasma leptin levels were measured in duplicates using a sandwich immunoassay based on flow metric xMAP technology on Luminex 200 machines (Luminex Corp., Austin, TX). The interassay coefficient of variation was 4.5% (G. Wang, Johnson, et al., 2016). Unlikely leptin levels, defined as greater than 3 SD, were observed in 8 and 7 subjects for cord and early childhood leptin, respectively and were re-assigned a value of 3SD (G. Wang, Hu, et al., 2016).
Statistical Analyses
The outcome variable was ASD and major exposure variables were 1) birth weight for gestational age and weight gain during first year of life and 2) cord blood and early childhood leptin levels. Correlation between cord and early childhood leptin was minimal (0.02). Normality of the data was assessed using Shapiro-Wilk test and both cord and early childhood leptin levels were log transformed because of the skewed distribution. Data analyses were performed to compare neurotypical children and those with ASD using chi-square tests for categorical variables and ANOVA for continuous variables. Logistic regression models were applied to estimate the crude and adjusted associations between weight gain during first year of life, log-transformed leptin levels at birth and early childhood (X, independent variables) and ASD (Y, dependent variable). All results are presented as odds ratio. Throughout, we used 2-sided statistical tests with a significance level of 0.05. Data were analyzed using STATA version 13.0 (StataCorp, College Station, TX).
Mediation analysis:
The role of leptin as a potential mediator of the association between weight gain during infancy and ASD risk was examined. KHB command in STATA was used to decompose the total effect of weight gain in infancy into natural direct and indirect effects, mediated by early childhood leptin levels (Breen, Karlson, & Holm, 2013). The total effect (defined as the effect of weight gain during infancy on ASD without the mediating variable early childhood leptin) was decomposed into direct effect (the effect of weight gain during infancy on ASD when controlling for early childhood leptin, the mediator) and indirect effect (the effect of weight gain during infancy on ASD through early childhood leptin, the mediator). The proportion of mediating effect among the total effect was calculated as indirect effect divided by the total effect.
Other covariates
Covariates were selected a priori based on the existing literature, including our own work in the BBC (M. Li et al., 2016; Raghavan et al., 2017; G. Wang et al., 2014; G. Wang, Hu, et al., 2016). Following covariates were adjusted in the analysis: maternal age at delivery, smoking during pregnancy (ever smoked 3 months before pregnancy/during pregnancy vs. not smoked before pregnancy/during pregnancy), parity (not including the index pregnancy), maternal education (high school or less vs. some college or more), maternal pre-pregnancy BMI, maternal diabetes status (defined below), maternal age at delivery, race/ethnicity, child’s sex (female vs. male), gestational age at birth (defined below), year of the baby’s birth (1998–2006 vs. 2007–2013), mode of feeding (defined below), age at which early childhood blood was drawn and follow-up time for each subject.
Maternal diabetes status was classified into the following: 1) no preexisting diabetes mellitus (DM) or gestational diabetes mellitus (GDM), 2) preexisting DM, and 3) GDM. Subjects were categorized as having preexisting DM if any of the following criteria were met before pregnancy: (a) diabetes diagnosis by a physician; (b) received a ICD-9 code of “250.x” or “648.0x”; (c) fasting plasma glucose level ≥126 mg/dl (7.0 mmol/l) or a casual plasma glucose≥200 mg/dl (11.1 mmol/l); (d) treatment with anti-diabetes medicines (oral medicines or insulin). Subjects were categorized as having GDM if there was no evidence of preexisting diabetes (as defined above) and met any of the following criteria: (a) physician diagnoses of GDM; (b) received a ICD code of “648.8x”; (c) fasting plasma glucose level ≥126 mg/dl (7.0 mmol/l) or casual plasma glucose≥200 mg/dl (11.1 mmol/l); (d) two or more of the following plasma glucose values in oral glucose tolerance test (OGTT) were met or exceeded: i) Fasting: 95 mg/dL (5.3 mmol/L); ii) 1 h: 180 mg/dL (10.0 mmol/L); iii) 2 h: 155 mg/dL (8.6 mmol/L); iv) 3-h 140mg/dL (7.8mmol/L); v) treatment with anti-diabetes medicines (oral medicines or insulin) (American Diabetes, 2003, 2016; Organization).
Neonates who were delivered ≥ 37 completed weeks of gestation were categorized as term, while those delivered <34 weeks, and ≥34 but <37 weeks of gestation were considered early and late preterm, respectively. Race/ethnicity was categorized into black, white, Hispanic and Other. Data on mode of feeding was collected from mothers using a standardized questionnaire during a follow-up visit in the first few years of life. Mode of feeding was categorized into the following: 1) formula only, 2) both formula and breastfeeding, and 3) breastfeeding only. When mothers had multiple follow-up visits, data collected during the first visit was used (Hong et al., 2011).
Results
Table 1 describes the characteristics of mothers and children by child’s case status (neurotypical vs. ASD). As expected, previously recognized risk factors were more prevalent among children with ASD compared to neurotypical children, including male sex, advanced maternal age, preterm birth and lower birth weight. Consistent with the literature (Mantzoros et al., 2009; Tome et al., 1997), girls had higher cord and early childhood plasma leptin levels than boys (Supplemental Table 1). Children that were SGA had lower cord leptin levels and were more likely to have extremely rapid weight gain (Supplemental tables 1 and 2). Children with most rapid weight gain during infancy were also likely to have higher early childhood leptin levels, but the latter did not differ by birth weight for gestational age (Supplemental table 1).
Table 1:
Maternal and child characteristics by child’s status (neurotypical vs. ASD) in the BBC
| Neurotypical (n=769) | ASD (n=53) | p value | |
|---|---|---|---|
| Characteristics | |||
| Mothers | |||
| Age at birth (yrs), mean (SD) | 28.21 (6.51) | 30.35 (6.28) | 0.02 |
| Parity (%) | 0.92 | ||
| 0 | 331 (41.74) | 21 (39.62) | |
| 1 or more | 447 (58.13) | 32 (60.38) | |
| Missing | 1 (0.13) | 0 (0.0) | |
| Mother’s education (%) | 0.43 | ||
| High School or less | 498 (64.76) | 31 (58.49) | |
| Some college or more | 266 (34.59) | 21 (39.62) | |
| Missing | 5 (0.65) | 1 (1.89) | |
| Maternal BMI (%) | 0.16 | ||
| Underweight (<18.5) + Normal Weight (≥18.5-<25) | 371 (48.24) | 19 (35.85) | |
| Overweight (25–29.9) | 235 (30.56) | 18 (33.96) | |
| Obesity (≥30) | 163 (21.20) | 16 (30.19) | |
| Diabetes mellitus (%) | 0.19 | ||
| No | 688 (89.47) | 45 (84.91) | |
| Gestational | 53 (6.89) | 3 (5.66) | |
| Pre-gestational diabetes | 27 (3.51) | 5 (9.43) | |
| Missing | 1 (0.13) | 0 (0.0) | |
| Smoking during & 3 months prior to pregnancy (%) | 0.17 | ||
| No | 659 (85.70) | 41 (77.36) | |
| Yes | 105 (13.65) | 12 (22.64) | |
| Missing | 5 (0.65) | 0 (0.00) | |
| Offspring | |||
| Sex (%) | <0.001 | ||
| Male | 327 (42.52) | 39 (73.58) | |
| Female | 442 (57.48) | 14 (26.42) | |
| Race-ethnicity (%) | 0.91 | ||
| Black | 434 (56.44) | 32 (60.38) | |
| White | 46 (5.98) | 4 (7.55) | |
| Hispanic | 185 (24.06) | 11 (20.75) | |
| Other | 99 (12.87) | 6 (11.32) | |
| Missing | 5 (0.65) | 0 (0.0) | |
| Gestational age (%) | 0.007 | ||
| Term | 579 (75.29) | 32 (60.38) | |
| Late preterm (≥34 - <37 weeks) | 126 (16.38) | 10 (18.87) | |
| Early preterm (<34 weeks) | 64 (8.32) | 11 (20.75) | |
| Birthweight (g) | 3009.07 (714.09) | 2782.26 (886.18) | 0.03 |
| Year of birth (%) | 0.70 | ||
| 1998–2006 | 400 (52.02) | 29 (54.72) | |
| 2007–2013 | 369 (46.98) | 24 (45.28) | |
| Birth weight for gestational age (%)a,b | 0.73 | ||
| Appropriate for gestational age (AGA) | 442 (80.07) | 36 (76.60) | |
| Small for gestational age (SGA) | 59 (10.69) | 5 (10.64) | |
| Large for gestational age (LGA) | 51 (9.24) | 6 (12.77) | |
| Weight gain during infancy (%)c, d | 0.06 | ||
| On target | 178 (33.78) | 9 (19.57) | |
| Slow | 65 (12.33) | 5 (10.87) | |
| Rapid weight gain | 89 (16.89) | 6 (13.04) | |
| Extremely rapid weight gain | 195 (37.00) | 26 (56.52) | |
| Cord blood leptin (SD)e | 38.23 (34.01) | 27.37 (19.94) | 0.05 |
| Early childhood leptin (SD)f | 4.22 (5.60) | 5.88 (5.68) | 0.08 |
| Mode of feeding (%) | 0.62 | ||
| Formula | 177 (23.02) | 10 (18.87) | |
| Both | 537 (69.83) | 37 (69.81) | |
| Breastfeeding | 49 (6.37) | 5 (9.43) | |
| Missing | 6 (0.78) | 1 (1.89) |
Fetal growth defined as AGA (≥10th – 90th percentile); SGA (<10th percentile), and LGA (>90th percentile)
n =599 (Neurotypical n=552; ASD n=47)
Weight gain z-scores during the first year of life were defined as the change in weight-for-age z-scores from birth until the target time-point and was categorized into the following groups: slow (weight gain z-score <−0.67), on track (−0.67 to 0.67), rapid (>0.67 to 1.28), and extremely rapid (>1.28)
n=573 (Neurotypical n=527; ASD n=46)
n=655 (Neurotypical n=616; ASD n=39)
n=652 (Neurotypical n=616; ASD n=36)
Birth weight for gestational age and ASD risk
A total of 599 children had birth weight for gestational age data, of which 47 were later diagnosed with ASD (Supplemental Figure 1). Five of these 47 ASD subjects had co-occurring ID. Compared to children with AGA, neither SGA children nor LGA children were at a greater risk of ASD, before or after the adjustment of covariates including maternal age at delivery, parity, smoking status, education, race, maternal BMI, maternal diabetes, child’s sex, follow-up time and gestational age (Table 2).
Table 2:
Association between in-utero growth, weight gain during infancy and ASD risk in children in the BBC
| Total n | ASD n | OR | 95% CI | p value | |
|---|---|---|---|---|---|
| Unadjusted | |||||
| AGA | 478 | 36 | Ref | ||
| SGA | 64 | 5 | 1.04 | 0.39, 2.76 | 0.94 |
| LGA | 57 | 6 | 1.44 | 0.58, 3.59 | 0.43 |
| Model 1 | |||||
| AGA | 478 | 36 | Ref | ||
| SGA | 64 | 5 | 0.81 | 0.28, 2.34 | 0.70 |
| LGA | 57 | 6 | 1.23 | 0.44, 3.47 | 0.69 |
| Model 2 | |||||
| AGA | 478 | 36 | Ref | ||
| SGA | 64 | 5 | 0.86 | 0.29, 2.54 | 0.79 |
| LGA | 57 | 6 | 1.37 | 0.48, 3.91 | 0.55 |
| Unadjusted | |||||
| On target | 187 | 9 | Ref | ||
| Slow | 70 | 5 | 1.52 | 0.49, 4.71 | 0.47 |
| Rapid weight gain | 95 | 6 | 1.33 | 0.46, 3.86 | 0.60 |
| Extremely rapid weight gain | 221 | 26 | 2.64 | 1.20, 5.78 | 0.02 |
| Model 3 | |||||
| On target | 187 | 9 | Ref | ||
| Slow | 70 | 5 | 1.71 | 0.53, 5.52 | 0.37 |
| Rapid weight gain | 95 | 6 | 1.39 | 0.43, 4.44 | 0.58 |
| Extremely rapid weight gain | 221 | 26 | 3.11 | 1.37, 7.07 | 0.007 |
| Model 4 | |||||
| On target | 187 | 9 | Ref | ||
| Slow | 70 | 5 | 1.55 | 0.46, 5.24 | 0.48 |
| Rapid weight gain | 95 | 6 | 1.50 | 0.46, 4.88 | 0.50 |
| Extremely rapid weight gain | 221 | 26 | 3.33 | 1.44, 7.72 | 0.005 |
| Model 5 | |||||
| On target | 187 | 9 | Ref | ||
| Slow | 70 | 5 | 1.74 | 0.54, 5.66 | 0.36 |
| Rapid weight gain | 95 | 6 | 1.32 | 0.41, 4.24 | 0.64 |
| Extremely rapid weight gain | 221 | 26 | 2.08 | 0.84, 5.13 | 0.11 |
| Model 6 | |||||
| On target | 187 | 9 | Ref | ||
| Slow | 70 | 5 | 1.40 | 0.40, 4.86 | 0.59 |
| Over growth | 95 | 6 | 1.22 | 0.36, 4.14 | 0.75 |
| Extremely rapid weight gain | 221 | 26 | 3.33 | 1.40, 7.89 | 0.006 |
In-utero growth defined as Appropriate for gestational age (≥10th – 90th percentile); Small for gestational age (<10th percentile), and Large for gestational age (>90th percentile)
Weight gain during infancy defined as the change in weight-for-age z-scores from birth until the target time-point and was categorized into the following groups: slow (weight gain z-score <−0.67), on track (−0.67 to 0.67), rapid (>0.67 to 1.28), and extremely rapid (>1.28)
Model 1: Adjusted for maternal age at delivery, parity, smoking, education, maternal BMI, maternal diabetes status, race, child’s sex and follow-up time
Model 2: Adjusted for Model 1 + gestational age
Model 3: Adjusted for child’s sex, race, follow-up time and breastfeeding status
Model 4: Adjusted for Model 3 + birth weight for gestational age
Model 5: Adjusted for Model 3 + gestational age
Model 6: Adjusted for Model 3 + maternal age, maternal diabetes status, maternal BMI
Weight gain during infancy and ASD risk
A total of 573 children had weight gain during infancy data, of which 46 were later diagnosed with ASD (Supplemental Figure 1). Five of these 46 subjects had co-occurring ID. Prenatal determinants of weight gain during infancy are presented in Supplemental Table 2, and preterm birth was a major determinant of infant excessive weight gain. When compared to children whose growth was on track based on weight gain z-scores, children that had slow weight gain or rapid weight gain did not have an increased ASD risk (ORslow: 1.52; 95% CI: 0.49, 4.71, ORrapid: 1.33; 95% CI: 0.46, 3.86) (Table 2). However, extremely rapid weight gain during infancy was associated with an increased risk of ASD (ORextremely rapid: 2.64; 95% CI: 1.20, 5.78). This association persisted after adjusting for covariates (child’s sex, race, follow-up time and breastfeeding status) in model 3 (aORextremely rapid: 3.11; 95% CI: 1.37, 7.07). Next, we sequentially added birth weight for gestational age to model 3 covariates and observed a consistent association (Table 2). However, the significance attenuated after adjusting for gestational age in addition to model 3 covariates (aOR: 2.08; 95% CI: 0.84, 5.13). The association did not attenuate after adjusting for prenatal determinants such as maternal BMI, diabetes status and age at the time of delivery, in addition to covariates specified in model 3 (Table 2). Sensitivity analyses using stringent comparators or cases showed a consistent association between birth weight for gestational age, weight gain during infancy and risk of ASD (supplemental tables 3 and 4).
Cord leptin and ASD risk
A total of 655 children had data on cord leptin levels, of which 39 were later diagnosed with ASD (Supplemental Figure 1). Two of these 39 ASD subjects had co-occurring ID. Prenatal and perinatal determinants of cord leptin are presented in Supplemental Table 1. The mean cord leptin levels were 38.23 pg/mL in children with neurotypical development and 27.37 pg/mL in children with ASD (Table 1). As observed in other cohorts (Mantzoros et al., 2009), higher cord blood leptin levels correlated with increase in gestational age. In the unadjusted model, cord leptin levels (stratified into quartiles) were not associated with the risk of ASD (Table 3). Similarly, no associations were observed after adjusting for covariates in models 1 and 2. The relationship between cord leptin and ASD was not altered, irrespective of whether cord leptin was assessed as a continuous or a categorical variable (Supplement table 5).
Table 3:
Association between cord, early childhood plasma leptin levels and ASD risk in children in the BBC
| Total n | ASD n | OR | 95% CI | p value | |
|---|---|---|---|---|---|
| Cord leptin | |||||
| Unadjusted | |||||
| Q1 | 164 | 10 | Ref | ||
| Q2 | 163 | 11 | 1.11 | 0.46, 2.70 | 0.81 |
| Q3 | 164 | 11 | 1.11 | 0.46, 2.68 | 0.82 |
| Q4 | 164 | 7 | 0.69 | 0.25, 1.85 | 0.46 |
| Model 1 | |||||
| Q1 | 164 | 10 | Ref | ||
| Q2 | 163 | 11 | 1.44 | 0.53, 3.87 | 0.48 |
| Q3 | 164 | 11 | 1.76 | 0.66, 4.69 | 0.26 |
| Q4 | 164 | 7 | 0.96 | 0.31, 2.94 | 0.94 |
| Model 2 | |||||
| Q1 | 164 | 10 | Ref | ||
| Q2 | 163 | 11 | 2.01 | 0.68, 5.92 | 0.21 |
| Q3 | 164 | 11 | 2.74 | 0.90, 8.31 | 0.08 |
| Q4 | 164 | 7 | 1.40 | 0.42, 4.66 | 0.58 |
| Early childhood leptin Unadjusted | |||||
| Q1 | 163 | 3 | Ref | ||
| Q2 | 163 | 8 | 2.75 | 0.72, 10.57 | 0.14 |
| Q3 | 163 | 10 | 3.49 | 0.94, 12.91 | 0.06 |
| Q4 | 163 | 15 | 5.41 | 1.53, 19.05 | 0.009 |
| Model 3 | |||||
| Q1 | 163 | 3 | Ref | ||
| Q2 | 163 | 8 | 3.32 | 0.83, 13.37 | 0.09 |
| Q3 | 163 | 10 | 4.61 | 1.17, 18.22 | 0.03 |
| Q4 | 163 | 15 | 7.87 | 2.06, 30.04 | 0.003 |
| Model 4 | |||||
| Q1 | 163 | 3 | Ref | ||
| Q2 | 163 | 8 | 3.30 | 0.81, 13.41 | 0.10 |
| Q3 | 163 | 10 | 4.93 | 1.23, 19.77 | 0.02 |
| Q4 | 163 | 15 | 7.89 | 2.05, 30.44 | 0.003 |
| Model 5 | |||||
| Q1 | 163 | 3 | Ref | ||
| Q2 | 163 | 8 | 3.59 | 0.68, 18.96 | 0.13 |
| Q3 | 163 | 10 | 4.18 | 0.79, 22.19 | 0.09 |
| Q4 | 163 | 15 | 8.41 | 1.69, 41.81 | 0.009 |
| Model 6 | |||||
| Q1 | 163 | 3 | Ref | ||
| Q2 | 163 | 8 | 3.43 | 0.84, 13.94 | 0.09 |
| Q3 | 163 | 10 | 5.09 | 1.28, 20.21 | 0.02 |
| Q4 | 163 | 15 | 7.46 | 1.93, 28.82 | 0.004 |
Model 1: Adjusted for maternal characteristics such as maternal age at delivery, parity, smoking, maternal BMI, maternal diabetes status, education, race, child’s sex and follow-up time
Model 2: Adjusted for Model 1 + gestational age
Model 3: Adjusted for child’s sex, race, age of leptin measurement, follow-up time and breastfeeding status
Model 4: Adjusted for Model 3 + gestational age
Model 5: Adjusted for Model 3 + cord leptin levels
Model 6: Adjusted for Model 3 + maternal age, maternal BMI, maternal diabetes status
Early childhood leptin and ASD risk
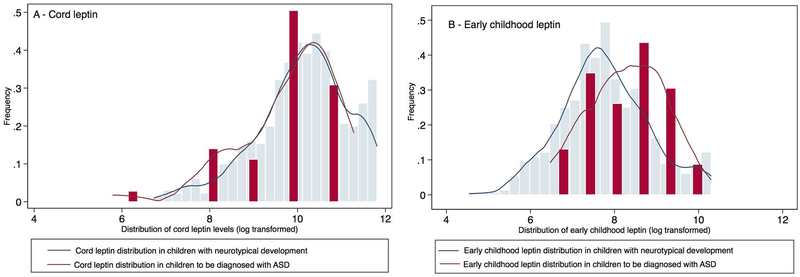
A total of 652 children were included in the analyses, of which 36 were later diagnosed with ASD (Supplemental Figure 1). Four of these 36 ASD subjects had co-occurring ID. Figure 1 provides a distribution of early childhood leptin levels among neurotypical children and those with ASD, showing a shift in distribution towards right for children with ASD. Supplemental figure 2 provides a distribution of when early childhood leptin levels were measured. When compared to children that had the lowest leptin levels (quartile 1), children with highest leptin levels (quartile 4) had an increased ASD risk (OR: 5.41; 95% CI: 1.53, 19.05) (Table 3). The association remained significant after adjusting for covariates (model 3) including child’s sex, race, child’s age when leptin was measured, follow-up time and breastfeeding status (aOR: 7.87; 95% CI: 2.06, 30.04). Further adjusting for gestational age (model 4), cord leptin levels (model 5) or prenatal determinants such as maternal BMI, diabetes status and age at the time of delivery (model 6) did not attenuate the association between early childhood leptin and ASD. Analysis using early childhood plasma leptin levels as a continuous variable or categorical variable yielded consistent findings (Supplement table 5). Sensitivity analyses using stringent controls or cases showed similar trends in association between cord, early childhood leptin and ASD risk (Supplemental tables 6 and 7). Results from alternative analyses using Cox proportional hazard regression for birth weight for gestational age, weight gain during infancy, cord and early childhood leptin, and ASD risk (supplemental tables 8 and 9) were consistent in direction and statistical significance.
Figure 1:
Distribution of cord and early childhood plasma leptin levels (log transformed) in children categorized by ASD status
Early childhood leptin mediating the relationship between weight gain during infancy and ASD risk
A total 476 children had both weight gain during infancy data and early childhood leptin measurements (Table 4). In the unadjusted model that assessed the role of early childhood leptin as a mediator of the relationship between weight gain during infancy and risk of ASD, the total effect of extremely rapid weight gain was statistically significant (OR: 2.80; 95% CI: 1.07, 7.28). Both direct (OR: 2.22; 95% CI: 0.84, 5.87) and indirect effects (OR: 1.26; 95% CI: 0.95, 1.67) were non-significant. In the adjusted model, the total effect attenuated (aOR: 1.80; 95% CI: 0.55, 5.90). However, the indirect effect became significant (aOR: 1.56; 95% CI: 1.01, 2.42), suggesting an indirect-only mediation (Zhao, Lynch, & Chen, 2010) and that the early childhood leptin potentially mediates 76.03% of the total relationship between extreme rapid weight gain and ASD, after adjusting for confounders. Other weight gain categories such as slow and rapid weight gain were not significantly associated with ASD and adjusting for early childhood leptin levels did not alter their association with ASD.
Table 4:
Mediation analysis – Leptin as a mediator in the relationship between weight gain during first year of life and ASD risk
| Total Effect, OR (95% CI) | Direct Effect, OR (95% CI) | Indirect effect, OR (95% CI) | Percentage mediated by early childhood leptin (%) | |
|---|---|---|---|---|
| Unadjusted (Total N=476; ASD=32) | ||||
| On track | Ref | |||
| Slow | 1.92 (0.52, 7.11) | 1.93 (0.52, 7.16) | 0.99 (0.81, 1.22) | |
| Rapid weight gain | 1.39 (0.38, 5.11) | 1.17 (0.32, 4.32) | 1.19 (0.93, 1.52) | |
| Extreme rapid weight gain | 2.80 (1.08, 7.28) | 2.22 (0.84, 5.87) | 1.26 (0.95, 1.67) | |
| Model 1: Adjusted | ||||
| On track | Ref | |||
| Slow | 1.80 (0.44, 7.47) | 1.81 (0.44, 7.49) | 1.00 (0.72, 1.39) | |
| Rapid weight gain | 1.06 (0.23, 4.85) | 0.82 (0.18, 3.78) | 1.29 (0.89, 1.87) | |
| Extreme rapid weight gain | 1.80 (0.55, 5.90) | 1.15 (0.34, 3.88) | 1.56 (1.01, 2.42) | 76.03 |
Model 1: Adjusted for child’s sex, race, breast-feeding category, age of leptin measurement, follow-up time and gestational age
A causal inference framework was used to estimate ORs and 95% CI for total, direct and indirect effects. A logistic regression model was fit using categorical exposure (weight gain during infancy) and continuous mediator (leptin).
Discussion
In this prospective cohort study, our results showed that extremely rapid weight gain during infancy and elevated early childhood leptin levels measured prior to ASD diagnosis were associated with an increased ASD risk in childhood. Our findings are in line with several studies that have reported that rapid weight gain during infancy is a potential indicator of early autism risk (Chawarska et al., 2011; Dissanayake et al., 2006; Sacco et al., 2007; Suren et al., 2013; Torrey et al., 2004). We extend the previous findings and suggest that the association between rapid weight gain and ASD is potentially mediated, at least indirectly, by early childhood plasma leptin. To our knowledge, this is the first study to assess the interrelationships between birth weight, infancy weight gain and leptin in the context of ASD in a prospective birth cohort.
Conceptual Framework of ASD risk factors from prenatal to early childhood:
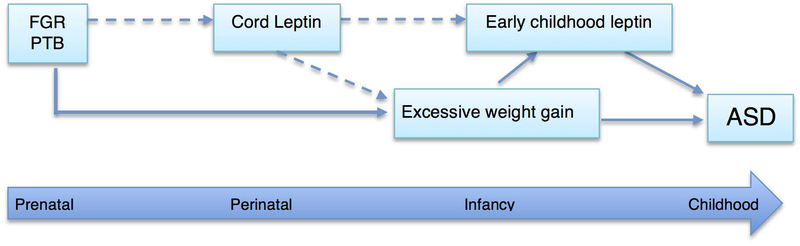
Epidemiological studies and animal models have consistently showed that an adverse in-utero environment may lead to altered programming of tissue structure and function, predisposing to later behavioral problems, learning difficulties, abnormal or delayed cognitive development and other conditions (Van den Bergh, 2011; Vickers, 2007). Sub-optimal prenatal environmental influences could induce permanent fetal adaptations that are beneficial for short-term survival, but increases the vulnerability to later pathogenic environmental stimuli (Krechowec, Vickers, Gertler, & Breier, 2006; Vickers & Sloboda, 2012). As illustrated in Figure 2, based on the findings by us and others, postnatal influences such as extremely rapid weight gain and elevated leptin levels may not be isolated stand-alone occurrences during the first year of life; but could rather be a compensatory event to an adverse prenatal condition or deviation in biological mechanism (Courchesne et al., 2003; Karaolis-Danckert et al., 2008; G. Wang, Johnson, et al., 2016). In support of this argument, we observed that children that were exposed to maternal diabetes or overweight/obesity during pregnancy and those that were SGA or early preterm, were more likely to have extremely rapid weight gain during infancy. Further, in line with the existing evidence (Ong, Ahmed, Emmett, Preece, & Dunger, 2000), low concentrations of cord blood leptin were associated with rapid weight gain during the first year of life suggesting that cord leptin could serve as a signal for catch-up growth. Taken together, it can be inferred that the incongruent prenatal (e.g. SGA) and postnatal milieu (rapid catch up growth) associated with endocrinologic alterations (early childhood leptin levels) may have a negative impact on the brain architecture and circuits, which could predispose an individual to adverse neurobehavioral outcomes (Pylipow et al., 2009; Van den Bergh, 2011). Given this context, we further elaborate our findings and discuss how they compare to the previous studies and provide possible explanations.
Figure 2:
Illustration of Prenatal, perinatal and early childhood determinants of ASD in a life course framework
Many studies, in addition to ours, reported an inconsistent association between fetal growth and ASD (Glasson et al., 2004; Langridge et al., 2013; Larsson et al., 2005; Schendel & Bhasin, 2008). Langridge et al. demonstrated that the percentage of optimal birth weight, a measure of fetal growth (Blair, Liu, de Klerk, & Lawrence, 2005), was not associated with ASD, especially among those with intellectual disability (Langridge et al., 2013). Glasson et al. also showed no association between SGA and ASD (Glasson et al., 2004), while Schnedel et al., noted that the relationship was observed only in girls and not in boys (Schendel & Bhasin, 2008). Similarly, Larrson et al. observed that the association between fetal growth and ASD attenuated after adjusting for other covariates (Larsson et al., 2005). In contrast, a few studies showed that SGA children had elevated risk of ASD (Lampi et al., 2012; Maimburg & Vaeth, 2006; Moore et al., 2012). A possible explanation for the lack of association between fetal growth and ASD is likely due to the heterogeneity in the SGA group related to the timing of onset. Fetal growth restriction may have different clinical manifestation and sequelae depending on whether the onset of growth restriction is early or late during gestation (Dall’Asta, Brunelli, Prefumo, Frusca, & Lees, 2017; Savchev et al., 2014). In our study, considering SGA as a homogenous group could have possibly blurred the association between fetal growth and ASD. We do not have data in the current study to tease apart this association, but can be explored further in future studies. Other potential reasons including methodological differences, lack of control for confounding factors, and sample size variations could explain some of the inconsistencies (Lampi et al., 2012; Schendel & Bhasin, 2008).
Consistent with our findings, several studies have shown that children who are later diagnosed with ASD have accelerated weight gain during infancy and early childhood (Dissanayake et al., 2006; Mraz, Green, Dumont-Mathieu, Makin, & Fein, 2007; Sacco et al., 2007; Torrey et al., 2004). This accelerated weight gain may not be a distinct morphological feature, but is suggestive of a broader autistic phenotype characterized by rapid increase in head circumference, height and weight (Dissanayake et al., 2006; Fukumoto et al., 2008; Mraz et al., 2007; Sacco, Gabriele, & Persico, 2015; Sacco et al., 2007; van Daalen, Swinkels, Dietz, van Engeland, & Buitelaar, 2007). In support of this hypothesis, studies have shown that head circumference is well correlated with weight and height in ASD children(Dementieva et al., 2005; Mraz et al., 2007; Sacco et al., 2007). Rapid increase in head circumference in children with ASD is one of the most consistent findings that many studies have demonstrated (Chawarska et al., 2011; Courchesne et al., 2003; Dementieva et al., 2005; Dissanayake et al., 2006; Fukumoto et al., 2008; Sacco et al., 2015; Sacco et al., 2007). Although we did not analyze head circumferences due to incomplete data, weight has been shown to be the strongest predictor of head circumference during most of infancy (Mraz et al., 2007). Taken together, our findings support the existing evidence that extremely rapid weight gain during infancy is associated with ASD, possibly indicative of an overall growth dysfunction. There are many speculations about why rapid weight gain is observed in children with ASD. Studies have posited that an abnormality in factors (such as metabolism, growth or neurotrophic factors and hormone levels) may predispose an individual to overall accelerated growth as well as ASD (Dissanayake et al., 2006; Fukumoto et al., 2008; Mraz et al., 2007; Torrey et al., 2004).
Our study showed that early childhood leptin levels were altered in children with ASD. While prior studies assessing this relationship were mainly cross-sectional, our prospective study for the first time showed that elevated leptin levels are observed even prior to ASD diagnosis. Considering the role of leptin in neurocognition, elevated leptin and associated leptin resistance during the critical periods of postnatal brain development may have permanent adverse implications (Glavas et al., 2010; Valleau & Sullivan, 2014). While the mechanism behind leptin resistance is still being understood, it is believed to involve reduced transport of leptin to the brain, poor negative feedback mechanism, endoplasmic reticulum stress and an intracellular leptin signaling system that is saturable (Boeke et al., 2013; Glavas et al., 2010; Mantzoros et al., 2011).
Similar to other studies (Dulloo, 2008; Ong et al., 1999), we noted that low cord leptin levels closely reflected birth weight and also predicted the greatest weight gain during infancy. However, cord blood leptin was not associated with ASD. This finding may be intriguing especially in the context that early childhood leptin is associated with ASD; however, our results are consistent with the existing evidence that cord and early childhood leptin may have different roles to play (Boeke et al., 2013; Zhang et al., 2017).
Cord blood leptin is derived primarily from the fetal tissue and is reflective of fetal adiposity (Catov et al., 2007; Hauguel-de Mouzon, Lepercq, & Catalano, 2006; Mellati et al., 2010; Tessier, Ferraro, & Gruslin, 2013). While leptin is detectable in the fetus at around 18 weeks, rapid increase in leptin levels are observed after 34 weeks, in tandem with increase in fetal adipose tissues (Grisaru-Granovsky, Samueloff, & Elstein, 2008; Mellati et al., 2010). Perinatal and neonatal periods are considered to be a window of maximum leptin sensitivity with normal neonates having two to three times higher leptin when compared to adults (Bouret, 2012, 2013; Paz-Filho et al., 2008; Valleau & Sullivan, 2014). Neonatal leptin has a different physiological response and promotes hyperphagia and swallowing activity in newborn and may not inhibit growth, food intake or energy expenditure (Alexe, Syridou, & Petridou, 2006; Bouret & Simerly, 2004; El-Haddad, Desai, Gayle, & Ross, 2004; Vickers & Sloboda, 2012). However, leptin sensitivity declines with age (Boeke et al., 2013; Levin, Dunn-Meynell, & Banks, 2004).
After closure of the critical window, higher leptin does not protect against adiposity and some children even develop leptin tolerance (Boeke et al., 2013). Thus, leptin, once positively associated with birth weight and less adiposity during early childhood (Boeke et al., 2013; Karakosta et al., 2011; Mantzoros et al., 2009) no longer possesses the same effect – demonstrating that the effect of leptin in perinatal period is distinct from that of later life (Cottrell et al., 2009). One study that longitudinally measured cord and early childhood leptin showed that while high cord blood leptin was initially shown to be protective against adiposity, it was subsequently associated with weight gain and adiposity at age 7 (Boeke et al., 2013). These age-specific effects of leptin have been linked to developmental changes in leptin receptor expression – which are widely expressed in the central nervous system starting from mid-gestation (Cottrell, Mercer, & Ozanne, 2010). While these findings are related to adiposity, it is plausible to believe that leptin’s role may be similar with neurocognitive outcomes.
Leptin as a mediator:
After establishing independent associations between ASD and 1) extremely rapid weight gain during infancy, and 2) early childhood leptin, we showed that children with extremely rapid weight gain during infancy had elevated leptin levels. In support of this, animal models that have shown that rapid catch-up growth in early childhood is associated with leptin resistance and this occurs independent of postnatal diet induced obesity (Coupe, Grit, Hulin, Randuineau, & Parnet, 2012; Krechowec et al., 2006; Yura et al., 2005). It has been hypothesized that proinflammatory cytokines may mediate the relationship between rapid postnatal growth and ASD (Pylipow et al., 2009). As a proof of concept, our study was able to demonstrate the mediating effect of leptin in the association between extremely rapid weight gain and ASD. However, in our dataset, cord leptin did not possess any mediating effects unlike early childhood leptin.
Mechanism of leptin in ASD:
Inflammation is a possible mechanism through which leptin may impact the psychopathology of ASD. Leptin, a pro-inflammatory cytokine may play a role in the pathophysiology of conditions such as schizophrenia (Haupt et al., 2005; Stubbs, Wang, Vancampfort, & Miller, 2016) and ASD (Ashwood et al., 2011; Goines & Ashwood, 2013). A variety of independent studies have linked cytokine dysregulation to ASD (Goines & Ashwood, 2013). Cytokines act as immune mediators and their imbalance during development and throughout life can adversely impact neural activity and mediate behavioral aspect of the disorder (Goines & Ashwood, 2013). Inflammatory cytokines are implicated in higher neurological functions such as memory and cognition, in addition to being involved in brain development, synaptic functioning including processes of differentiation, migration, proliferation and impairments in behavior (Tonhajzerova et al., 2015). Thus, abnormal inflammatory activity and imbalance of cytokines during development can adversely impact neural activity and could contribute to behavioral and neurological dysfunction in ASD (Goines & Ashwood, 2013; Tonhajzerova et al., 2015).
Altered leptin levels can also impact brain structure and function. For example, leptin levels are increased at the site of inflammation in the post-mortem brain tissue (Ashwood et al., 2008). Leptin deficient and leptin resistant state is associated with lower brain weight, protein content, reduction in brain myelin, neuronal soma size and several synaptic proteins. Reduced brain weight is observed in animals that lacked leptin signaling (Bouret & Simerly, 2004). A study conducted on autopsy tissues showed that there is a marked increase in leptin levels in anterior cingulate gyrus among those that had ASD (Vargas, Nascimbene, Krishnan, Zimmerman, & Pardo, 2005). In a small subset of patients, genetic correlation was observed between ASD and leptin coding (Bochukova et al., 2010). Leptin is involved in long-term potentiation and long-term depression (Bliss, Collingridge, & Morris, 2014) and dysregulation of this function is implicated in ASD (Bliss et al., 2014). Further, leptin is also known to suppress serotonin synthesis, which is reported in ASD, possibly suggesting another biological pathway through which leptin can be involved in ASD (Valleau & Sullivan, 2014).
In our earlier report in the BBC, we showed that maternal obesity and diabetes was associated with increased risk of ASD in offspring (M. Li et al., 2016). Our results, along with consistent findings across diverse populations (Y. M. Li et al., 2016; Nahum Sacks et al., 2016; Xiang et al., 2015; G. Xu, Jing, Bowers, Liu, & Bao, 2014) raised the possibility of early metabolic dysfunction in the development of ASD. Studies have posited that early life manipulations of leptin in animal models alter susceptibility to subsequent obesity and metabolic disorders (Dulloo, 2008; Vickers & Sloboda, 2012). Periods of hypo- or hyperleptinemia may induce metabolic adaptations, which could be the basis of developmental programming (Vickers & Sloboda, 2012). In this context, the role of leptin as a potential mediator of the developmental programming of ASD (Cottrell et al., 2009) may be a novel proposition for ASD, but requires further investigation. Additional research is warranted on the role of other hormones with leptin opposing action, so as to better understand the metabolic milieu involved in ASD. Similarly, future studies should also examine variants in leptin and leptin receptors to better understand the biological pathways of leptin in ASD.
Limitations and Strengths
Although our study stemmed from a rigorously designed prospective birth cohort, the findings may be tempered due to some limitations. First, case and neurotypical development classification of children was based on EMR data and it is possible that there may be outcome misclassification. However, this misclassification may not be differential given the prospective study design. Second, the relatively small number of cases in our prospective cohort design could have resulted in wide confidence intervals and imprecise estimates. Third, although our models accounted for breastfeeding vs. formula feeding, more research is needed to examine the role of perinatal nutrition and its influences on weight gain, early childhood leptin and ASD. Fourth, we could not directly assess fat mass at the time of leptin measurement and this could have resulted in some residual confounding. Fifth, plasma leptin levels follow a circadian rhythm (Mantzoros et al., 2011) and may be impacted by fasting status; although, the timing of plasma sample collection for leptin measurements was random, the distribution was comparable between ASD and neurotypical groups. Finally, our study population consisted mainly of urban low-income minority populations that were also at high risk for conditions such as SGA and preterm births and thus, the results may not be generalizable to the U.S. population.
Despite these limitations, our study has a number of strengths. This is one of the first longitudinal studies that addressed leptin levels at birth and in early childhood in the context of ASD. Infant weight gain was assessed as part of well-child visit during first year of life, when child’s ASD status was not known. By using a sample of children that have weight gain data as well as data on cord and early childhood leptin, we were uniquely poised to examine the inter-relationship of these variables in the development of ASD.
Conclusion
In the BBC, we showed that extremely rapid weight gain during infancy and elevated leptin levels during early childhood were independently associated with greater ASD risk and early childhood plasma leptin levels at least indirectly mediated the relationship between early childhood weight gain and ASD. Furthermore, the prenatal and postnatal risk factors for ASD are interrelated and act along a continuum from prenatal to postnatal periods. An important implication of these findings is that in addition to prenatal factors, pathogenic processes underlying ASD likely continue during the postnatal period, including infancy and possibly extending to early childhood (Sacco et al., 2007). Even though accelerated weight gain in early life, combined with elevated plasma leptin may not be a unique biomarker for ASD, our preliminary findings provide a basis from which to further explore the relationship between prenatal events, infancy rapid weight gain, leptin and ASD under a life course framework (Courchesne et al., 2003). Additional research is needed to understand if a combination of prenatal and early childhood anthropometric, biological, genetic variables and behavioral signs together can accurately predict ASD sooner. If proven to be useful by future studies, this will provide an opportunity to start intervention earlier thereby potentially halting or mitigating the progression towards ASD (Allely, Gillberg, & Wilson, 2014; Courchesne et al., 2003; Dissanayake et al., 2006).
Supplementary Material
Acknowledgments:
Funding Source:
Grant sponsor: Health Resources and Services Administration (HRSA) of the U.S. Department of Health and Human Services (HHS); Grant number: R40MC27443; UJ2MC31074
Grant sponsor: March of Dimes; Grant number: 20-FY02–56, #21-FY07–605
Grant sponsor: National Institutes of Health (NIH); Grant numbers: R21ES011666, R01HD041702, R21HD066471, U01AI090727, R21AI079872, and R01HD086013
Grant sponsor: Doctoral fellowship to Dr. Ramkripa Raghavan; John and Alice Chenoweth-Pate Fellowship; Grant number: N/A
Footnotes
Conflict of interest: None of the authors have a conflict of interest pertaining to this work.
References
- Al-Zaid FS, Alhader AA, & Al-Ayadhi LY (2014). Altered ghrelin levels in boys with autism: a novel finding associated with hormonal dysregulation. Scientific Reports, 4, 6478. doi: 10.1038/srep06478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexe DM, Syridou G, & Petridou ET (2006). Determinants of early life leptin levels and later life degenerative outcomes. Clin Med Res, 4(4), 326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allely CS, Gillberg C, & Wilson P (2014). Neurobiological abnormalities in the first few years of life in individuals later diagnosed with autism spectrum disorder: a review of recent data. Behav Neurol, 2014, 210780. doi: 10.1155/2014/210780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes A. (2003). Gestational diabetes mellitus. Diabetes Care, 26 Suppl 1, S103–105. [DOI] [PubMed] [Google Scholar]
- American Diabetes A. (2016). 2. Classification and Diagnosis of Diabetes. Diabetes Care, 39 Suppl 1, S13–22. doi: 10.2337/dc16-S005 [DOI] [PubMed] [Google Scholar]
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, & Van de Water J (2011). Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun, 25(1), 40–45. doi: 10.1016/j.bbi.2010.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Kwong C, Hansen R, Hertz-Picciotto I, Croen L, Krakowiak P, et al. (2008). Brief report: plasma leptin levels are elevated in autism: association with early onset phenotype? J Autism Dev Disord, 38(1), 169–175. doi: 10.1007/s10803-006-0353-1 [DOI] [PubMed] [Google Scholar]
- Blair EM, Liu Y, de Klerk NH, & Lawrence DM (2005). Optimal fetal growth for the Caucasian singleton and assessment of appropriateness of fetal growth: an analysis of a total population perinatal database. BMC Pediatr, 5(1), 13. doi: 10.1186/1471-2431-5-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blardi P, de Lalla A, Ceccatelli L, Vanessa G, Auteri A, & Hayek J (2010). Variations of plasma leptin and adiponectin levels in autistic patients. Neurosci Lett, 479(1), 54–57. doi: 10.1016/j.neulet.2010.05.027 [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL, & Morris RG (2014). Synaptic plasticity in health and disease: introduction and overview. Philos Trans R Soc Lond B Biol Sci, 369(1633), 20130129. doi: 10.1098/rstb.2013.0129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochukova EG, Huang N, Keogh J, Henning E, Purmann C, Blaszczyk K, et al. (2010). Large, rare chromosomal deletions associated with severe early-onset obesity. Nature, 463(7281), 666–670. doi: 10.1038/nature08689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeke CE, Mantzoros CS, Hughes MD, L. R.-S. S, Villamor E, Zera CA, et al. (2013). Differential associations of leptin with adiposity across early childhood. Obesity (Silver Spring), 21(7), 1430–1437. doi: 10.1002/oby.20314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret SG (2012). Nutritional programming of hypothalamic development: critical periods and windows of opportunity. Int J Obes Suppl, 2(Suppl 2), S19–24. doi: 10.1038/ijosup.2012.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret SG (2013). Organizational actions of metabolic hormones. Front Neuroendocrinol, 34(1), 18–26. doi: 10.1016/j.yfrne.2013.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret SG, & Simerly RB (2004). Minireview: Leptin and development of hypothalamic feeding circuits. Endocrinology, 145(6), 2621–2626. doi: 10.1210/en.2004-0231 [DOI] [PubMed] [Google Scholar]
- Breen R, Karlson KB, & Holm A (2013). Total, Direct, and Indirect effects in logit and probit models. Sociological Methods & Research, 1–23. [Google Scholar]
- Castanys-Munoz E, Kennedy K, Castaneda-Gutierrez E, Forsyth S, Godfrey KM, Koletzko B, et al. (2017). Systematic review indicates postnatal growth in term infants born small-for-gestational-age being associated with later neurocognitive and metabolic outcomes. Acta Paediatr, 106(8), 1230–1238. doi: 10.1111/apa.13868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catov JM, Patrick TE, Powers RW, Ness RB, Harger G, & Roberts JM (2007). Maternal leptin across pregnancy in women with small-for-gestational-age infants. Am J Obstet Gynecol, 196(6), 558 e551–558. doi: 10.1016/j.ajog.2007.01.032 [DOI] [PubMed] [Google Scholar]
- Chawarska K, Campbell D, Chen L, Shic F, Klin A, & Chang J (2011). Early generalized overgrowth in boys with autism. Archives of General Psychiatry, 68(10), 1021–1031. doi: 10.1001/archgenpsychiatry.2011.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell EC, Cripps RL, Duncan JS, Barrett P, Mercer JG, Herwig A, et al. (2009). Developmental changes in hypothalamic leptin receptor: relationship with the postnatal leptin surge and energy balance neuropeptides in the postnatal rat. Am J Physiol Regul Integr Comp Physiol, 296(3), R631–639. doi: 10.1152/ajpregu.90690.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell EC, Mercer JG, & Ozanne SE (2010). Postnatal development of hypothalamic leptin receptors. Vitam Horm, 82, 201–217. doi: 10.1016/S0083-6729(10)82011-4 [DOI] [PubMed] [Google Scholar]
- Coupe B, Grit I, Hulin P, Randuineau G, & Parnet P (2012). Postnatal growth after intrauterine growth restriction alters central leptin signal and energy homeostasis. PLoS One, 7(1), e30616. doi: 10.1371/journal.pone.0030616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Carper R, & Akshoomoff N (2003). Evidence of brain overgrowth in the first year of life in autism. JAMA, 290(3), 337–344. doi: 10.1001/jama.290.3.337 [DOI] [PubMed] [Google Scholar]
- Dall’Asta A, Brunelli V, Prefumo F, Frusca T, & Lees CC (2017). Early onset fetal growth restriction. Matern Health Neonatol Perinatol, 3, 2. doi: 10.1186/s40748-016-0041-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dementieva YA, Vance DD, Donnelly SL, Elston LA, Wolpert CM, Ravan SA, et al. (2005). Accelerated head growth in early development of individuals with autism. Pediatr Neurol, 32(2), 102–108. doi: 10.1016/j.pediatrneurol.2004.08.005 [DOI] [PubMed] [Google Scholar]
- Dissanayake C, Bui QM, Huggins R, & Loesch DZ (2006). Growth in stature and head circumference in high-functioning autism and Asperger disorder during the first 3 years of life. Dev Psychopathol, 18(2), 381–393. doi: 10.1017/S0954579406060202 [DOI] [PubMed] [Google Scholar]
- Dulloo AG (2008). Thrifty energy metabolism in catch-up growth trajectories to insulin and leptin resistance. Best Pract Res Clin Endocrinol Metab, 22(1), 155–171. doi: 10.1016/j.beem.2007.08.001 [DOI] [PubMed] [Google Scholar]
- El-Haddad MA, Desai M, Gayle D, & Ross MG (2004). In utero development of fetal thirst and appetite: potential for programming. J Soc Gynecol Investig, 11(3), 123–130. doi: 10.1016/j.jsgi.2003.12.001 [DOI] [PubMed] [Google Scholar]
- Essa MM, Braidy N, Al-Sharbati MM, Al-Farsi YM, Ali A, Waly MI, et al. (2011). Elevated plasma leptin levels in autisic children of Sultanate of Oman. International Journal of Biological & Medical Research, 2(3), 803–805. [Google Scholar]
- Fukumoto A, Hashimoto T, Ito H, Nishimura M, Tsuda Y, Miyazaki M, et al. (2008). Growth of head circumference in autistic infants during the first year of life. J Autism Dev Disord, 38(3), 411–418. doi: 10.1007/s10803-007-0405-1 [DOI] [PubMed] [Google Scholar]
- Glasson EJ, Bower C, Petterson B, de Klerk N, Chaney G, & Hallmayer JF (2004). Perinatal factors and the development of autism: a population study. Archives of General Psychiatry, 61(6), 618–627. doi: 10.1001/archpsyc.61.6.618 [DOI] [PubMed] [Google Scholar]
- Glavas MM, Kirigiti MA, Xiao XQ, Enriori PJ, Fisher SK, Evans AE, et al. (2010). Early overnutrition results in early-onset arcuate leptin resistance and increased sensitivity to high-fat diet. Endocrinology, 151(4), 1598–1610. doi: 10.1210/en.2009-1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goines PE, & Ashwood P (2013). Cytokine dysregulation in autism spectrum disorders (ASD): possible role of the environment. Neurotoxicology and Teratology, 36, 67–81. doi: 10.1016/j.ntt.2012.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisaru-Granovsky S, Samueloff A, & Elstein D (2008). The role of leptin in fetal growth: a short review from conception to delivery. Eur J Obstet Gynecol Reprod Biol, 136(2), 146–150. doi: 10.1016/j.ejogrb.2007.06.021 [DOI] [PubMed] [Google Scholar]
- Harigaya A, Nagashima K, Nako Y, & Morikawa A (1997). Relationship between concentration of serum leptin and fetal growth. J Clin Endocrinol Metab, 82(10), 3281–3284. doi: 10.1210/jcem.82.10.4321 [DOI] [PubMed] [Google Scholar]
- Harvey J (2007). Leptin regulation of neuronal excitability and cognitive function. Curr Opin Pharmacol, 7(6), 643–647. doi: 10.1016/j.coph.2007.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey J, Solovyova N, & Irving A (2006). Leptin and its role in hippocampal synaptic plasticity. Prog Lipid Res, 45(5), 369–378. doi: 10.1016/j.plipres.2006.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauguel-de Mouzon S, Lepercq J, & Catalano P (2006). The known and unknown of leptin in pregnancy. American Journal of Obstetrics and Gynecology, 194(6), 1537–1545. doi: 10.1016/j.ajog.2005.06.064 [DOI] [PubMed] [Google Scholar]
- Haupt DW, Luber A, Maeda J, Melson AK, Schweiger JA, & Newcomer JW (2005). Plasma leptin and adiposity during antipsychotic treatment of schizophrenia. Neuropsychopharmacology, 30(1), 184–191. doi: 10.1038/sj.npp.1300563 [DOI] [PubMed] [Google Scholar]
- Hong X, Wang G, Liu X, Kumar R, Tsai HJ, Arguelles L, et al. (2011). Gene polymorphisms, breast-feeding, and development of food sensitization in early childhood. J Allergy Clin Immunol, 128(2), 374–381 e372. doi: 10.1016/j.jaci.2011.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter DS, Hazel SJ, Kind KL, Owens JA, Pitcher JB, & Gatford KL (2016). Programming the brain: Common outcomes and gaps in knowledge from animal studies of IUGR. Physiol Behav, 164(Pt A), 233–248. doi: 10.1016/j.physbeh.2016.06.005 [DOI] [PubMed] [Google Scholar]
- Jeste SS (2015). Neurodevelopmental behavioral and cognitive disorders. Continuum (Minneap Minn), 21(3 Behavioral Neurology and Neuropsychiatry), 690–714. doi: 10.1212/01.CON.0000466661.89908.3c [DOI] [PubMed] [Google Scholar]
- Joseph RM, Korzeniewski SJ, Allred EN, O’Shea TM, Heeren T, Frazier JA, et al. (2017). Extremely low gestational age and very low birthweight for gestational age are risk factors for autism spectrum disorder in a large cohort study of 10-year-old children born at 23–27 weeks’ gestation. Am J Obstet Gynecol, 216(3), 304 e301–304 e316. doi: 10.1016/j.ajog.2016.11.1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakosta P, Chatzi L, Plana E, Margioris A, Castanas E, & Kogevinas M (2011). Leptin levels in cord blood and anthropometric measures at birth: a systematic review and meta-analysis. Paediatric and Perinatal Epidemiology, 25(2), 150–163. doi: 10.1111/j.1365-3016.2010.01163.x [DOI] [PubMed] [Google Scholar]
- Karaolis-Danckert N, Buyken AE, Kulig M, Kroke A, Forster J, Kamin W, et al. (2008). How pre- and postnatal risk factors modify the effect of rapid weight gain in infancy and early childhood on subsequent fat mass development: results from the Multicenter Allergy Study 90. Am J Clin Nutr, 87(5), 1356–1364. [DOI] [PubMed] [Google Scholar]
- Kettaneh A, Heude B, Romon M, Oppert JM, Borys JM, Balkau B, et al. (2007). High plasma leptin predicts an increase in subcutaneous adiposity in children and adults. Eur J Clin Nutr, 61(6), 719–726. doi: 10.1038/sj.ejcn.1602579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koistinen HA, Koivisto VA, Andersson S, Karonen SL, Kontula K, Oksanen L, et al. (1997). Leptin concentration in cord blood correlates with intrauterine growth. J Clin Endocrinol Metab, 82(10), 3328–3330. doi: 10.1210/jcem.82.10.4291 [DOI] [PubMed] [Google Scholar]
- Krakowiak P, Goines PE, Tancredi DJ, Ashwood P, Hansen RL, Hertz-Picciotto I, et al. (2017). Neonatal Cytokine Profiles Associated With Autism Spectrum Disorder. Biol Psychiatry, 81(5), 442–451. doi: 10.1016/j.biopsych.2015.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakowiak P, Walker CK, Bremer AA, Baker AS, Ozonoff S, Hansen RL, et al. (2012). Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics, 129(5), e1121–1128. doi: 10.1542/peds.2011-2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krechowec SO, Vickers M, Gertler A, & Breier BH (2006). Prenatal influences on leptin sensitivity and susceptibility to diet-induced obesity. J Endocrinol, 189(2), 355–363. doi: 10.1677/joe.1.06679 [DOI] [PubMed] [Google Scholar]
- Lampi KM, Lehtonen L, Tran PL, Suominen A, Lehti V, Banerjee PN, et al. (2012). Risk of autism spectrum disorders in low birth weight and small for gestational age infants. J Pediatr, 161(5), 830–836. doi: 10.1016/j.jpeds.2012.04.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langridge AT, Glasson EJ, Nassar N, Jacoby P, Pennell C, Hagan R, et al. (2013). Maternal conditions and perinatal characteristics associated with autism spectrum disorder and intellectual disability. PLoS One, 8(1), e50963. doi: 10.1371/journal.pone.0050963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson HJ, Eaton WW, Madsen KM, Vestergaard M, Olesen AV, Agerbo E, et al. (2005). Risk factors for autism: perinatal factors, parental psychiatric history, and socioeconomic status. Am J Epidemiol, 161(10), 916–925; discussion 926–918. doi: 10.1093/aje/kwi123 [DOI] [PubMed] [Google Scholar]
- Leitner Y (2014). The co-occurrence of autism and attention deficit hyperactivity disorder in children - what do we know? Front Hum Neurosci, 8, 268. doi: 10.3389/fnhum.2014.00268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin BE, Dunn-Meynell AA, & Banks WA (2004). Obesity-prone rats have normal blood-brain barrier transport but defective central leptin signaling before obesity onset. Am J Physiol Regul Integr Comp Physiol, 286(1), R143–150. doi: 10.1152/ajpregu.00393.2003 [DOI] [PubMed] [Google Scholar]
- Li M, Fallin MD, Riley A, Landa R, Walker SO, Silverstein M, et al. (2016). The Association of Maternal Obesity and Diabetes With Autism and Other Developmental Disabilities. Pediatrics, 137(2), 1–10. doi: 10.1542/peds.2015-2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Liu R, Arguelles L, Wang G, Zhang J, Shen X, et al. (2016). Adiposity trajectory and its associations with plasma adipokine levels in children and adolescents-A prospective cohort study. Obesity (Silver Spring), 24(2), 408–416. doi: 10.1002/oby.21378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YM, Ou JJ, Liu L, Zhang D, Zhao JP, & Tang SY (2016). Association Between Maternal Obesity and Autism Spectrum Disorder in Offspring: A Meta-analysis. J Autism Dev Disord, 46(1), 95–102. doi: 10.1007/s10803-015-2549-8 [DOI] [PubMed] [Google Scholar]
- Limperopoulos C (2009). Autism spectrum disorders in survivors of extreme prematurity. Clin Perinatol, 36(4), 791–805, vi. doi: 10.1016/j.clp.2009.07.010 [DOI] [PubMed] [Google Scholar]
- Maimburg RD, & Vaeth M (2006). Perinatal risk factors and infantile autism. Acta Psychiatr Scand, 114(4), 257–264. doi: 10.1111/j.1600-0447.2006.00805.x [DOI] [PubMed] [Google Scholar]
- Mantzoros CS, Magkos F, Brinkoetter M, Sienkiewicz E, Dardeno TA, Kim SY, et al. (2011). Leptin in human physiology and pathophysiology. Am J Physiol Endocrinol Metab, 301(4), E567–584. doi: 10.1152/ajpendo.00315.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantzoros CS, Rifas-Shiman SL, Williams CJ, Fargnoli JL, Kelesidis T, & Gillman MW (2009). Cord blood leptin and adiponectin as predictors of adiposity in children at 3 years of age: a prospective cohort study. Pediatrics, 123(2), 682–689. doi: 10.1542/peds.2008-0343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masi A, Glozier N, Dale R, & Guastella AJ (2017). The Immune System, Cytokines, and Biomarkers in Autism Spectrum Disorder. Neurosci Bull, 33(2), 194–204. doi: 10.1007/s12264-017-0103-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellati AA, Mazloomzadeh S, Anjomshoaa A, Alipour M, Karimi F, Mazloomi S, et al. (2010). Multiple correlations between cord blood leptin concentration and indices of neonatal growth. Arch Med Res, 41(1), 26–32. doi: 10.1016/j.arcmed.2009.12.001 [DOI] [PubMed] [Google Scholar]
- Moore GS, Kneitel AW, Walker CK, Gilbert WM, & Xing G (2012). Autism risk in small- and large-for-gestational-age infants. Am J Obstet Gynecol, 206(4), 314 e311–319. doi: 10.1016/j.ajog.2012.01.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mraz KD, Green J, Dumont-Mathieu T, Makin S, & Fein D (2007). Correlates of head circumference growth in infants later diagnosed with autism spectrum disorders. J Child Neurol, 22(6), 700–713. doi: 10.1177/0883073807304005 [DOI] [PubMed] [Google Scholar]
- Murray MJ (2010). Attention-deficit/Hyperactivity Disorder in the context of Autism spectrum disorders. Curr Psychiatry Rep, 12(5), 382–388. doi: 10.1007/s11920-010-0145-3 [DOI] [PubMed] [Google Scholar]
- Nahum Sacks K, Friger M, Shoham-Vardi I, Abokaf H, Spiegel E, Sergienko R, et al. (2016). Prenatal exposure to gestational diabetes mellitus as an independent risk factor for long-term neuropsychiatric morbidity of the offspring. Am J Obstet Gynecol, 215(3), 380 e381–387. doi: 10.1016/j.ajog.2016.03.030 [DOI] [PubMed] [Google Scholar]
- Ong KK (2007). Catch-up growth in small for gestational age babies: good or bad? Curr Opin Endocrinol Diabetes Obes, 14(1), 30–34. doi: 10.1097/MED.0b013e328013da6c [DOI] [PubMed] [Google Scholar]
- Ong KK, Ahmed ML, Emmett PM, Preece MA, & Dunger DB (2000). Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. BMJ, 320(7240), 967–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong KK, Ahmed ML, Sherriff A, Woods KA, Watts A, Golding J, et al. (1999). Cord blood leptin is associated with size at birth and predicts infancy weight gain in humans. ALSPAC Study Team. Avon Longitudinal Study of Pregnancy and Childhood. J Clin Endocrinol Metab, 84(3), 1145–1148. doi: 10.1210/jcem.84.3.5657 [DOI] [PubMed] [Google Scholar]
- Organization W. H. The ICD-9 classification of mental and behavioural disorders: Clinical descriptions and diagnostic guidelines. Retrieved from http://www.icd9data.com/2015/Volume1/default.htm
- Padilla N, Eklof E, Martensson GE, Bolte S, Lagercrantz H, & Aden U (2017). Poor Brain Growth in Extremely Preterm Neonates Long Before the Onset of Autism Spectrum Disorder Symptoms. Cereb Cortex, 27(2), 1245–1252. doi: 10.1093/cercor/bhv300 [DOI] [PubMed] [Google Scholar]
- Paz-Filho GJ, Babikian T, Asarnow R, Delibasi T, Esposito K, Erol HK, et al. (2008). Leptin replacement improves cognitive development. PLoS One, 3(8), e3098. doi: 10.1371/journal.pone.0003098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perng W, Oken E, Roumeliotaki T, Sood D, Siskos AP, Chalkiadaki G, et al. (2016). Leptin, acylcarnitine metabolites and development of adiposity in the Rhea mother-child cohort in Crete, Greece. Obes Sci Pract, 2(4), 471–476. doi: 10.1002/osp4.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pighetti M, Tommaselli GA, D’Elia A, Di Carlo C, Mariano A, Di Carlo A, et al. (2003). Maternal serum and umbilical cord blood leptin concentrations with fetal growth restriction. Obstet Gynecol, 102(3), 535–543. [DOI] [PubMed] [Google Scholar]
- Pylipow M, Spector LG, Puumala SE, Boys C, Cohen J, & Georgieff MK (2009). Early postnatal weight gain, intellectual performance, and body mass index at 7 years of age in term infants with intrauterine growth restriction. J Pediatr, 154(2), 201–206. doi: 10.1016/j.jpeds.2008.08.015 [DOI] [PubMed] [Google Scholar]
- Raghavan R, Riley AW, Volk H, Caruso D, Hironaka L, Sices L, et al. (2017). Maternal Multivitamin Intake, Plasma Folate and Vitamin B12 Levels and Autism Spectrum Disorder Risk in Offspring. Paediatr Perinat Epidemiol. doi: 10.1111/ppe.12414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues DH, Rocha NP, Sousa LF, Barbosa IG, Kummer A, & Teixeira AL (2014). Changes in adipokine levels in autism spectrum disorders. Neuropsychobiology, 69(1), 6–10. doi: 10.1159/000356234 [DOI] [PubMed] [Google Scholar]
- Rommelse NN, Peters CT, Oosterling IJ, Visser JC, Bons D, van Steijn DJ, et al. (2011). A pilot study of abnormal growth in autism spectrum disorders and other childhood psychiatric disorders. J Autism Dev Disord, 41(1), 44–54. doi: 10.1007/s10803-010-1026-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco R, Gabriele S, & Persico AM (2015). Head circumference and brain size in autism spectrum disorder: A systematic review and meta-analysis. Psychiatry Res, 234(2), 239–251. doi: 10.1016/j.pscychresns.2015.08.016 [DOI] [PubMed] [Google Scholar]
- Sacco R, Militerni R, Frolli A, Bravaccio C, Gritti A, Elia M, et al. (2007). Clinical, morphological, and biochemical correlates of head circumference in autism. Biol Psychiatry, 62(9), 1038–1047. doi: 10.1016/j.biopsych.2007.04.039 [DOI] [PubMed] [Google Scholar]
- Savchev S, Figueras F, Sanz-Cortes M, Cruz-Lemini M, Triunfo S, Botet F, et al. (2014). Evaluation of an optimal gestational age cut-off for the definition of early- and late-onset fetal growth restriction. Fetal Diagn Ther, 36(2), 99–105. doi: 10.1159/000355525 [DOI] [PubMed] [Google Scholar]
- Schaevitz L, Berger-Sweeney J, & Ricceri L (2014). One-carbon metabolism in neurodevelopmental disorders: using broad-based nutraceutics to treat cognitive deficits in complex spectrum disorders. Neuroscience and Biobehavioral Reviews, 46 Pt 2, 270–284. doi: 10.1016/j.neubiorev.2014.04.007 [DOI] [PubMed] [Google Scholar]
- Schendel D, & Bhasin TK (2008). Birth weight and gestational age characteristics of children with autism, including a comparison with other developmental disabilities. Pediatrics, 121(6), 1155–1164. doi: 10.1542/peds.2007-1049 [DOI] [PubMed] [Google Scholar]
- Schieve LA, Tian LH, Baio J, Rankin K, Rosenberg D, Wiggins L, et al. (2014). Population attributable fractions for three perinatal risk factors for autism spectrum disorders, 2002 and 2008 autism and developmental disabilities monitoring network. Ann Epidemiol, 24(4), 260–266. doi: 10.1016/j.annepidem.2013.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs B, Wang AK, Vancampfort D, & Miller BJ (2016). Are leptin levels increased among people with schizophrenia versus controls? A systematic review and comparative meta-analysis. Psychoneuroendocrinology, 63, 144–154. doi: 10.1016/j.psyneuen.2015.09.026 [DOI] [PubMed] [Google Scholar]
- Suren P, Stoltenberg C, Bresnahan M, Hirtz D, Lie KK, Lipkin WI, et al. (2013). Early growth patterns in children with autism. Epidemiology, 24(5), 660–670. doi: 10.1097/EDE.0b013e31829e1d45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier DR, Ferraro ZM, & Gruslin A (2013). Role of leptin in pregnancy: consequences of maternal obesity. Placenta, 34(3), 205–211. doi: 10.1016/j.placenta.2012.11.035 [DOI] [PubMed] [Google Scholar]
- Tome MA, Lage M, Camina JP, Garcia-Mayor RV, Dieguez C, & Casanueva FF (1997). Sex-based differences in serum leptin concentrations from umbilical cord blood at delivery. Eur J Endocrinol, 137(6), 655–658. [DOI] [PubMed] [Google Scholar]
- Tonhajzerova I, Ondrejka I, Mestanik M, Mikolka P, Hrtanek I, Mestanikova A, et al. (2015). Inflammatory Activity in Autism Spectrum Disorder. Adv Exp Med Biol, 861, 93–98. doi: 10.1007/5584_2015_145 [DOI] [PubMed] [Google Scholar]
- Torrey EF, Dhavale D, Lawlor JP, & Yolken RH (2004). Autism and head circumference in the first year of life. Biol Psychiatry, 56(11), 892–894. doi: 10.1016/j.biopsych.2004.09.014 [DOI] [PubMed] [Google Scholar]
- Valleau JC, & Sullivan EL (2014). The impact of leptin on perinatal development and psychopathology. Journal of Chemical Neuroanatomy, 61–62, 221–232. doi: 10.1016/j.jchemneu.2014.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Daalen E, Swinkels SH, Dietz C, van Engeland H, & Buitelaar JK (2007). Body length and head growth in the first year of life in autism. Pediatr Neurol, 37(5), 324–330. doi: 10.1016/j.pediatrneurol.2007.06.006 [DOI] [PubMed] [Google Scholar]
- Van den Bergh BR (2011). Developmental programming of early brain and behaviour development and mental health: a conceptual framework. Dev Med Child Neurol, 53 Suppl 4, 19–23. doi: 10.1111/j.1469-8749.2011.04057.x [DOI] [PubMed] [Google Scholar]
- Van Naarden Braun K, Christensen D, Doernberg N, Schieve L, Rice C, Wiggins L, et al. (2015). Trends in the prevalence of autism spectrum disorder, cerebral palsy, hearing loss, intellectual disability, and vision impairment, metropolitan atlanta, 1991–2010. PloS One, 10(4), e0124120. doi: 10.1371/journal.pone.0124120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, & Pardo CA (2005). Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol, 57(1), 67–81. doi: 10.1002/ana.20315 [DOI] [PubMed] [Google Scholar]
- Vickers MH (2007). Developmental programming and adult obesity: the role of leptin. Curr Opin Endocrinol Diabetes Obes, 14(1), 17–22. doi: 10.1097/MED.0b013e328013da48 [DOI] [PubMed] [Google Scholar]
- Vickers MH, & Sloboda DM (2012). Leptin as mediator of the effects of developmental programming. Best Pract Res Clin Endocrinol Metab, 26(5), 677–687. doi: 10.1016/j.beem.2012.03.005 [DOI] [PubMed] [Google Scholar]
- Wang G, Divall S, Radovick S, Paige D, Ning Y, Chen Z, et al. (2014). Preterm birth and random plasma insulin levels at birth and in early childhood. JAMA, 311(6), 587–596. doi: 10.1001/jama.2014.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Hu FB, Mistry KB, Zhang C, Ren F, Huo Y, et al. (2016). Association Between Maternal Prepregnancy Body Mass Index and Plasma Folate Concentrations With Child Metabolic Health. JAMA Pediatr, 170(8), e160845. doi: 10.1001/jamapediatrics.2016.0845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Johnson S, Gong Y, Polk S, Divall S, Radovick S, et al. (2016). Weight Gain in Infancy and Overweight or Obesity in Childhood across the Gestational Spectrum: a Prospective Birth Cohort Study. Sci Rep, 6, 29867. doi: 10.1038/srep29867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zuckerman B, Pearson C, Kaufman G, Chen C, Wang G, et al. (2002). Maternal cigarette smoking, metabolic gene polymorphism, and infant birth weight. JAMA, 287(2), 195–202. [DOI] [PubMed] [Google Scholar]
- Xiang AH, Wang X, Martinez MP, Walthall JC, Curry ES, Page K, et al. (2015). Association of maternal diabetes with autism in offspring. JAMA, 313(14), 1425–1434. doi: 10.1001/jama.2015.2707 [DOI] [PubMed] [Google Scholar]
- Xu G, Jing J, Bowers K, Liu B, & Bao W (2014). Maternal diabetes and the risk of autism spectrum disorders in the offspring: a systematic review and meta-analysis. J Autism Dev Disord, 44(4), 766–775. doi: 10.1007/s10803-013-1928-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu N, Li X, & Zhong Y (2015). Inflammatory cytokines: potential biomarkers of immunologic dysfunction in autism spectrum disorders. Mediators Inflamm, 2015, 531518. doi: 10.1155/2015/531518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yura S, Itoh H, Sagawa N, Yamamoto H, Masuzaki H, Nakao K, et al. (2005). Role of premature leptin surge in obesity resulting from intrauterine undernutrition. Cell Metab, 1(6), 371–378. doi: 10.1016/j.cmet.2005.05.005 [DOI] [PubMed] [Google Scholar]
- Zhang M, Cheng H, Zhao X, Hou D, Yan Y, Cianflone K, et al. (2017). Leptin and Leptin-to-Adiponectin Ratio Predict Adiposity Gain in Nonobese Children over a Six-Year Period. Child Obes, 13(3), 213–221. doi: 10.1089/chi.2016.0273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Lynch JGJ, & Chen Q (2010). Reconsidering Baron and Kenny: Myths and Truths about Mediation Analysis. The Journal of Consumer Research, 37(2), 197–206. [Google Scholar]
- Zhubi A, Cook EH, Guidotti A, & Grayson DR (2014). Epigenetic mechanisms in autism spectrum disorder. Int Rev Neurobiol, 115, 203–244. doi: 10.1016/B978-0-12-801311-3.00006-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.