Abstract
Purpose:
That the malignant clone of Waldenström’s macroglobulinemia (WM) demonstrates significant intraclonal heterogeneity with respect to plasmacytoid differentiation indicates the mechanistic complexity of tumorigenesis and progression. Identification of WM genes by comparing different stages of B cells may provide novel druggable targets.
Experimental Design:
The gene expression signatures of CD19+ B cells (BC) and CD138+ plasma cells (PC) from 19 patients with WM were compared to those of BC from peripheral blood and tonsil and to those of PC from the marrow of healthy (N-PC) and multiple myeloma donors (MM-PC), as well as tonsil (T-PC). The flow cytometry and immunofluorescence were used to examine T-cell marker expression on WM tumor cells.
Results:
Consistent with defective differentiation, both BC and PC from WM cases expressed abnormal differentiation markers. Sets of 55 and 46 genes were differentially expressed in WM-BC and WM-PC, respectively; and 40 genes uniquely dysregulated in WM samples were identified. Dysregulated genes included cytokines, growth factor receptors, and oncogenes not previously implicated in WM or other plasma cell dyscrasias. Interestingly, strong upregulation of both IL6 and IL6R was confirmed. Supervised cluster analysis of PC revealed that marrow-derived WM-PC was either MM-PC-like or T-PC-like, but not N-PC-like. The aberrant expression of T cell markers was confirmed at the protein level in WM-BC.
Conclusions:
We showed that comparative microarray profiles allowed to gain more comprehensive insights into the biology of WM. The data presented here have implications for the development of novel therapies, such as targeting aberrant T-cell markers in WM.
Keywords: Waldenström’s macroglobulinemia, gene expression profiling, T cell marker, multiple myeloma, immunohistochemistry, Translational Cancer Mechanisms, Therapy
Introduction
Waldenström’s macroglobulinemia (WM) is a rare chronic lymphoproliferative disorder characterized by immunoglobulin M (IgM) paraproteinemia and bone marrow infiltration by lymphoplasmacytoid cells (1,2). The World Health Organization (WHO) classification defined it as one of the subtypes of Non-Hodgkin B lymphoma (NHL) (3). Although WM itself is clinically heterogeneous, ranging from largely asymptomatic disease to the presence of symptoms related to monoclonal IgM deposition and tissue infiltration (4,5), the major clinical manifestations include cytopenia, paraprotein-related cryoglobulinemia, cold agglutinin syndrome, demyelinating neuropathy, and symptomatic hyperviscosity (6).
Unlike the related gammopathies, multiple myeloma (MM) and non-IgM monoclonal gammopathy of undetermined significance (MGUS), WM is characterized by a conspicuous absence of DNA ploidy or involving the immunoglobulin heavy chain locus (IgH) 14q translocations (7). Deletion of chromosome 6q, seen in approximately 30% of cases, represents the most recurrent genetic lesion identified (8), but the clinicopathogenetic consequences of this abnormality are not yet understood. Recently, a major breakthrough was that Treon et al discovered highly recurrent MYD88 L265P (92%) and CXCR4 (30 ~ 40%) somatic mutations in WM using whole-genome sequencing (9). Similar to the WM, the IgM MGUS, the precursor of WM (10), shows a high frequency of MYD88 mutation but much less structural variants (11). Although a few papers using global gene expression profiling (GEP) or deep-sequencing have been reported and gained insight into the enigmatic genetics of this disease, a precision targeting of these mutations and signaling pathways are still challenging in WM clinical treatment.
WM is characterized immunophenotypically as consisting of cells expressing markers of mature B cells with varying degrees of plasma cell differentiation (12). Given this heterogeneity, we purified both lymphoid and plasmacytoid components from WM bone marrow and compared the expression profiles of these cells with those of normal peripheral blood B cells, tonsil B cells, tonsil plasma cells, bone marrow plasma cells, and MM plasma cells. Here, we report on the molecular signatures of WM in the context of both normal B cells and plasma cells, and MM. Our discovery that T cell antigens express in WM-BC may explain well for drug resistance and develop a novel treatment strategy in WM.
Materials and methods
Samples
De-identified clinical bone marrow aspirates were obtained from WM patients at the University of Arkansas for Medical Sciences (UAMS), USA, and the Institute of Hematology & Blood Diseases Hospital, Chinese Academy of Medical Science & Peking Union Medical College, Tianjin, China. Studies were approved by the Institutional Review Boards at each institution. Informed consent was obtained in accordance with the Declaration of Helsinki. Mononuclear cells were obtained from bone marrow aspirates from 19 patients with a newly diagnosis of WM. Expression of CD20 and CD138 on lymphoid cells was examined by flow cytometry to determine the dominant phenotype of the tumor cell population and the choice of antibody for cell enrichment. Tumor cells expressing B-cell (CD19 or CD20) or plasma cell (CD138) markers were isolated with the use of immunomagnetic beads according to the manufacturer’s guidelines (Miltenyi Biotec, Auburn, CA). WM-BC and WM-PC purities of >90% homogeneity were confirmed via two-color flow cytometry using CD38+/CD45– and CD20+/CD45+ staining criteria, respectively (Becton Dickinson, San Jose, CA). Cell isolation with immunomagnetic beads and analysis of the other cell types used in this study have been previously reported (13). Of the 19 cases analyzed, both CD19 and CD138 cells were isolated from 2 cases by separating the sample as two parts, only CD19 cells from 10 cases, and only CD138 cells from 7 cases. The sample cohort studied consisted of CD19-selected peripheral blood B cells (PB-BC; n = 7), tonsil B cells (T-BC; n = 7), bone marrow B cells from WM (WM-BC; n = 12), tonsil plasma cells (T-PC; n = 9), bone marrow plasma cells from healthy donors (N-PC; n = 10), WM plasma cells (WM-PC, n = 10), and MM plasma cells (MM-PC; n = 10) (14). Tonsils were obtained from patients undergoing tonsillectomy for chronic tonsillitis (14).
RNA purification and microarray hybridization and analysis
Detailed protocols for RNA purification, cDNA synthesis, cRNA preparation, and hybridization to the Human Genome U95Av2 GeneChip microarray (Affymetrix, Santa Clara, CA) have been previously described (13–15). The Gene Expression Omnibus database accession number performed in this paper is GSE9656.
Data processing
All data used in our analyses were derived from Affymetrix Microarray Suite 5.0 output files. Expression values are notated (i) as a signal representing the difference between the intensities of the sequence-specific perfect match and the mismatch probe set, or (ii) as a signal indicating a “present,” “marginal,” or “absent” call as determined by the GeneChip 5.0 algorithm. Gene arrays were scaled to an average signal of 1,500 and then analyzed independently. Signal calls were transformed by the log base 2.
Gene expression data analysis
The statistical software package SPSS 12.0 (SPSS Inc.) and the significance analysis of microarrays (SAM) method were used to analyze the data (16). Genes were selected for analysis on the basis of detection and false discovery rate. In each comparison, genes having “present” detection calls in more than one-third of the samples in the overexpressed gene group were retained for statistical analysis. For two-class and multiclass supervised analyses, the SAM method was used with sample-label permutations to evaluate statistical significance.
Hierarchical clustering of average linkage with the centered correlation metric was employed (17).
The modified “spike” method was used to define unique, highly overexpressed genes in WM (13). After the elimination of control genes and the filtering out of genes with a maximum signal of <500 across all samples, the median expression value (log base 2) of each gene across all samples, including PB-BC, T-BC or T-PC, N-PC, WM-BC or WM-PC, or MM-PC, was determined. The value of the ith gene was called medgene (i). The ith gene was called a “spike” gene if it had at least three (>14%) sample expression values >2.8+ medgene (i) in WM. All genes having low to undetectable expression in the all-normal controls and MM were defined as specifically expressed genes in WM.
Flow cytometry assay for T cell antigens in WM-BC
Mononuclear cells were obtained from bone marrow aspirates from 5 newly diagnosed WM patients. About 0.5 ~ 1×107 mononuclear cells were suspended in 100μl of flow cytometry buffer and WM-B cells were gated with double positive CD45 and CD19. The strong CD45+CD19+ cells were further analyzed the expression of CD3 and CD8 antigens by flow cytometry to determine T-cell marker expression. All analyses were performed on an LSRII flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA).
Immunofluorescence staining for CD19 and CD3 antigens on WM-BC
Fluorescence-activated cell sorting (FACS) were performed to sort CD45+CD19+ cells from above 5 WM samples. Cytospin slides were prepared and immunofluorescence was carried out using mouse anti-human CD3 (1:500) and rabbit anti-human CD19 (1:50) antibodies (Novocastra Lab Ltd). Cell nucleus was stained with DAPI on WM-BC. Normal CD3+ and CD19+ cells were isolated from peripheral blood of healthy donors as positive controls.
Results
Expression of B cell markers and transcription factors in WM
WM morphology is marked by small lymphocytes with a variable degree of plasma cell maturation with an immunophenotype of CD19+/CD20+/CD5–/CD10–/Ig+/sIgM++ and distinct histopathology (18–20). Gene expression levels for CD45, CD20, CD79B, CD52, CD19, CD22, CD83, and CD72 showed no differences in comparisons between WM-BC, PB-BC, and T-BC (Supplemental Table 1). Expression levels of CD5, CD23, and CD1, which are high in chronic lymphocytic leukemia, showed a low intensity in WM-BC (data not shown). Interestingly, although selected with CD19, the plasma cell–associated markers CD138, CD38, and CD27 were significantly higher in WM-BC than in other B-cell samples (Fig. 1A). Similarly, expression levels of CD45, CD19, CD20 were significantly higher in WM-PC than in other plasma cell samples, whereas plasma cell antigens CD38 and CD138 were significantly lower (Fig. 1A). CD27, a soluble protein increased in WM serum and linked to hemoglobin levels in WM patients (21,22), was expressed at similar high levels in WM-PC, T-PC, and N-PC, while this gene was expressed at a much lower level in MM-PC.
Fig. 1. Distinct expression patterns of B-cell markers and transcription factors in WM.
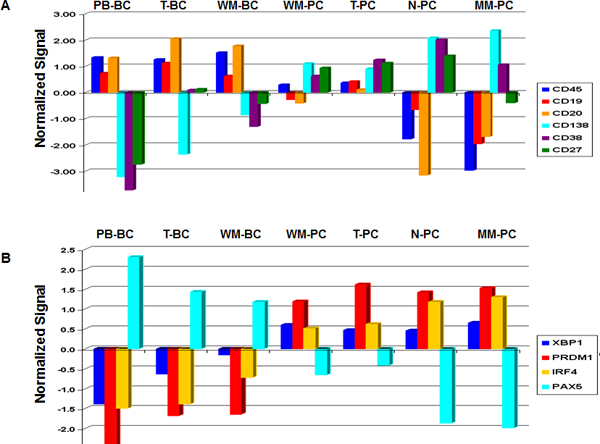
Samples included 7 PB-BC, 7 T-BC, 12 WM-BC, 9 WM-PC, 9 T-PCs, 10 N-PC, and 11 MM-PC and are distributed along the x axis; the normalized signal is plotted on the y axis. A, B-cell markers for CD45, CD19, CD20, CD138, CD38, and CD27; the expression of normalized signal appears in blue, red, yellow, cyan, purple, and green, respectively. B, Transcription factors for XBP1, PRDM1, IRF4, and PAX5; the expression of normalized signal appears in blue, red, yellow, and cyan, respectively.
Plasma cell differentiation is controlled by the differential activation and inactivation of several transcription factors (23). The interferon regulatory protein 4 (IRF4) and X-box-binding protein 1 (XBP1) genes are activated, while PAX5 and BCL6 are inactivated. XBP1 showed low levels in WM-BC but a gradual increase in expression from PB-BC to T-BC to WM-BC and similar high levels in the plasma cell samples. PRDM1 expression was lowest in PB-BC; expression was higher and at similar levels in T-BC and WM-BC. PAX5 expression was lowest in WM-PC, showing similar expression levels to those of T-PC, N-PC, and MM-PC. IRF4 was expressed at higher levels in WM-BC than in other B-cell samples, but its expression was higher still in WM-PC, whose expression level was similar to that of T-PC; N-PC and MM-PC expressed IRF4 at still higher levels. BSAP/PAX5 showed a progressive loss in expression from PB-BC to T-BC to WM-BC. While much lower than the B-cell samples, expression levels of both WM-PC and T-PC were similar and much higher than those observed in N-PC and MM-PC (Fig. 1B).
Distinct gene expression profiles in B-cells and plasma cells from WM cases
In an attempt to better define molecular signatures of WM-BC, we performed an unsupervised hierarchical cluster analysis of 6,504 genes exhibiting the greatest variation across all B-cell samples (Standard Deviation S.D. >0.6) (Fig. 2A). This produced a sample dendrogram with two major branches, one containing the PB-BC cases and the other the T-BC and WM-BC cases on two sub-branches. These data showed that WM-BC have a unique gene expression signature relative to other normal B-cell populations that were also selected based on the expression of CD19. A similar strategy was employed to evaluate global differences in expression patterns in the plasma cell samples (Fig. 2B). In this case, the unsupervised cluster analysis produced two major branches one containing the T-PC and the other the bone marrow–derived N-PC and MM-PC. Unlike with the WM-BC, the WM-PC failed to segregate into a separate sub-branch; instead, these samples were either dispersed within the T-PC cases (n = 5) or the MM-PC cases (n = 4). These data show that although derived from the bone marrow, CD138-selected PC from WM are not normal and have molecular features of immature plasma cells (consistent with data in Fig. 1) or MM. The clinical implications of these results are not known; however, they may indicate that at least two types of WM exist and a different therapeutic strategy may be needed for each type.
Fig. 2. Unsupervised hierarchical cluster analysis of WM cases and their controls.
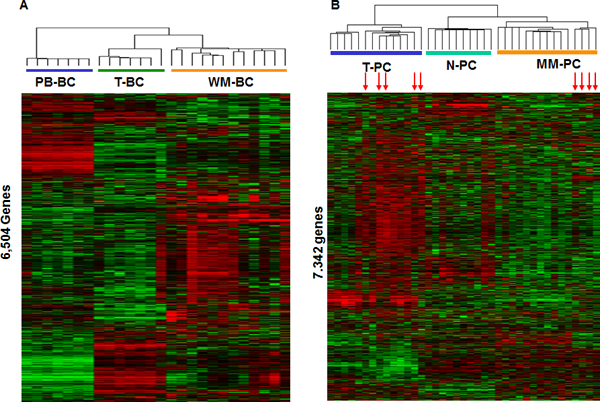
A, Two-way cluster analysis of all 12 WM-BC, 7 PB-BC, and 7 T-BC cases sorted with CD19/CD20 based on the expression of 6,504 genes. Mean-centered gene expression is depicted by a normalized-signal pseudocolor scale. Red and green indicate the genes overexpressed and underexpressed, respectively. Each row represents a gene, and each column a tissue sample. B, Cluster analysis of all 9 WM-PC, 9 T-PC, 10 N-PC, and 11 MM-PC cases sorted with CD138 based on the expression of 7,870 genes.
Comparative gene expression profiling of WM-BC, PB-BC, and T-BC populations
To identify genes uniquely expressed in WM-BC, we applied SAM analysis with a 0.1% false discovery rate to the B-cell samples. This analysis revealed 55 genes that were differentially expressed by greater than 2-fold in WM-BC, including 31 upregulated and 24 downregulated genes common to the WM-BC samples (Supplemental Table 2). A colorgram of the gene expression patterns of these 55 genes in the B-cell samples clearly demonstrated significant differences in expression across the samples (Fig. 3A). Genes exhibiting greater than 10-fold overexpression in WM-BC included the chemokine receptor CCR2, the proto-oncogene MYB, and the early B-cell genes IGLL1, RAG1, and RAG2. Although not as uniformly overexpressed, the T-cell gene fibrinogen-like protein 2 (FGL2) was highly overexpressed in a subset of WM cases.
Fig. 3. Delineation of the top significantly expressed genes distinguishing WM cases from the controls.
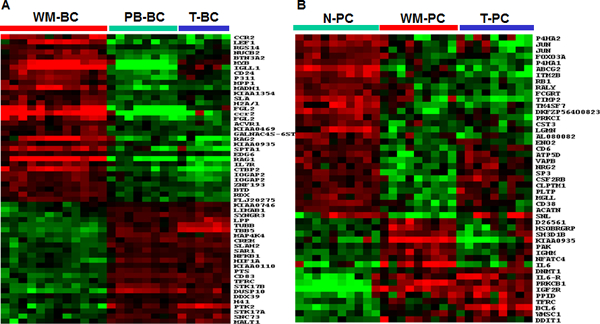
A, Expression of the 55 top significantly expressed genes in WM-BC samples. The WM-BC-defined clusters for tumor B-cells (horizontal red bar), PB-BC (horizontal green bar), and T-BC (horizontal blue bar) are indicated. B, Expression of the 46 significantly expressed genes in WM-PC. The WM-PC-defined clusters for tumor plasma cells (horizontal red bar), N-PC (horizontal green bar), and T-PC (horizontal blue bar) are indicated. The clustering analysis illustrates that the expression pattern of WM-PC is more similar to that of T-PC than N-PC.
Gene expression profiles of WM-PC
An identical SAM analysis of WM-PC, T-PC, and N-PC revealed 242 differentially expressed genes, 46 of which were differentially expressed by more than 2-fold (Table 2). As would be expected, the IGHM gene was uniquely overexpressed in WM-PC. Most notably, while IL6R was overexpressed in T-PC and WM-PC, WM-PC also significantly overexpressed the IL-6 gene. These data suggest that hyperactivation of both the ligand and the receptor of this important plasma cell survival-signaling cascade may play a pathological role in WM. Protein kinase C, beta 1 (PRKCB1), which plays a major role in early B-cell survival and function, was also upregulated. Among other transcripts overexpressed in WM-PC were genes involved in cell cycle regulation (PAK2 and DDIT1); the transcription activator (NFATC4); the transcription repressor proto-oncogene (BCL6), which normally functions to block B-cell terminal differentiation; the gene for DNA methyltransferase 1 (DNMT1); WHSC1/MMSET, which is aberrantly activated in MM by the t(4;14)(p16;q32) translocation; the insulin-like growth factor receptor (IGF2R); an iron transporter (TRFC); and a few genes of unknown function. Of the 30 underexpressed genes, the cytoskeleton-related gene for actin-bindling protein (SNL) was the most significantly underexpressed gene (4.9-fold); two other cytoskeleton-related genes, P4HA1 and P4HA2, were also included in this group. Three metabolism-related genes, including PLTP, MGLL, and ENO2, were underexpressed 2.1–3.4-fold. Also significantly underexpressed in WM-PC were the transcription factors FOXO3A and SP3; the cell adhesion genes TM4SF7 and CD6; the transporter-related genes ABCG2, VAPB, ATP5D, and ACATN; the metalloproteinase gene TIMP2; an amyloid-related gene ITM2B; and the autoantigen gene RALY.
To further define the differentially expressed genes in WM-PC, the 46 most significantly differentially expressed genes relative to N-PC were further analyzed by examination of their expression levels in T-PC. Genes encoding B-cell and plasma cell markers, including CD45, CD20, CD79B, CD52w, CD138, and CD38, that were found to be up or downregulated in WM-PC relative to N-PC were not differentially expressed in a comparison between WM-PC and T-PC (data not shown). Only 17 (37%) of 46 genes were found to be significantly differentially expressed in the comparison between WM-PC and T-PC. This finding suggests that although derived from marrow, WM-PC are more similar to T-PC than N-PC, a feature consistent with data presented in Fig. 3B.
To explore the relationship between malignant plasma cells from two related gammopathies, we compared the gene expression profiles of WM-PC and MM-PC. Among the 46 genes differentially expression in WM-PC relative to N-PC, 17 (37%) were also identified as dysregulated in MM. Twenty-nine genes (63%) showed no changes in WM-PC relative to MM-PC (Supplemental Table 3). The commonly upregulated genes included two cytokine genes (IGF2R and IL6R) and a cell cycle–related gene (DDIT1). The commonly downregulated genes included adhesion- and cytoskeleton-related genes (SNL, TM4SF7, and CD6). These genes may be more important for tumor cell proliferation and survival. Interestingly, genes encoding some B-cell CD markers (Supplemental Table 1) were identified as significantly overexpressed in WM-PC relative to MM-PC. Examples include CD45 (9.6-fold), CD20 (2.4-fold), CD79B (3.0-fold), CD52w (15.6-fold), CD19 (3.2-fold), and CD22 (2.8-fold). These findings suggest that WM-PC, although CD138+ and expressing other markers consistent with plasma cell differentiation, continue the expression of genes associated with earlier B-cell development. It is also noteworthy that no transcription factor associated with plasma cell differentiation, including XBP1, BLIMP1/PRDF1, and IRF4, was found to be significantly differentially expressed in the comparison between WM-PC and MM-PC.
Identification of genes specifically expressed in WM cells
MM is characterized by “spiked” gene expression patterns for many genes in unique subsets of patients. Expression of CCND3, CCND1, MAF, MAFB, and FGFR3/MMSET are undetectable in normal bone marrow plasma cells, and low (CCND1 only) to undetectable in most MM cases. However, as a result of chromosomal translocations between these genes and the IGH locus, these genes exhibit a characteristic “spiked” pattern in specific subsets of tumors. Although WM has been shown to lack translocations involving the IGH locus (24), we nevertheless applied to WM samples a method used to identify spiked gene expression in MM. This strategy was employed to identify genes that may not be uniformly overexpressed across the entire group but might nevertheless be highly overexpressed in subsets of patients and therefore may point to subtypes of disease. Table 1 lists 40 genes with expression values >2.8+ medgene (i) (log 2 of the signal) in at least three WM cases. PDGFRA, encoding receptor tyrosine kinase, was upregulated in 15 of 21 WM samples and expressed at very high levels (Affymetrix signal >10,000) in 9 of 21 samples. CD24, SOX4, MYB, and WAV, mapping to chromosome 6, represented the largest group of genes mapping to one chromosome. Several signaling-related genes were in this list, including IL7R, SCYA4, SCYA5, CX3CR, HCK, and PTGER2 (a prostaglandin E2 receptor and COX2 downstream gene). Also represented were Annexin 1 (ANNX1), a cell surface receptor linked to caspase-2 apoptosis and calcium metabolism, and TYROBP, which encodes tyrosine kinase–binding protein, which acts as a docking site for Syk or ZAP-70 protein tyrosine kinases in natural killer cells (25).
Table 1.
Genes uniquely overexpressed in WM cells
| Probe set | Location | Symbol | n*/N (21) |
|---|---|---|---|
| 37363_at | 8p22 | KIAA0429 | 16 |
| 1988_at | 4q11-q13 | PDGFRA | 15 |
| 39710_at | 5 | P311 | 14 |
| 266_s_at | 6q21 | CD24 | 13 |
| 35780_at | 2 | KIAA0657 | 13 |
| 2045_s_at | 20q11-q12 | HCK | 12 |
| 40742_at | 20q11-q12 | HCK | 12 |
| 1077_at | 11p13 | RAG1 | 12 |
| 39610_at | 17q21-q22 | HOXB2 | 11 |
| 38514_at | 22q11.23 | IGLL1 | 11 |
| 36674_at | 17q21 | SCYA4 | 10 |
| 33131_at | 6p22.3 | SOX4 | 10 |
| 36859_at | 5q23-q31 | NME5 | 10 |
| 33244_at | 7p15.3 | CHN2 | 9 |
| 2042_s_at | 6q22-q23 | MYB | 8 |
| 38937_at | 15q | AP3B2 | 8 |
| 40780_at | 10q26.13 | CTBP2 | 8 |
| 34168_at | 10q23-q24 | DNTT | 8 |
| 39119_s_at | 16P13.3 | NK4 | 6 |
| 36227_at | 5p13 | IL7R | 6 |
| 36293_at | 3p21 | TYMSTR | 6 |
| 1106_s_at | 14q11.2 | TCRA | 6 |
| 828_at | 5p13.1 | PTGER2 | 6 |
| 37190_at | 6q21-q22 | WAVE | 6 |
| 37403_at | 9q12-q21.2 | ANX1 | 5 |
| 40738_at | 1p13 | CD2 | 5 |
| 40646_at | 3p21.3 | CX3CR1 | 5 |
| 40757_at | 5q11-q12 | GZMA | 5 |
| 36280_at | 5q11-q12 | GZMK | 5 |
| 1403_s_at | 17q11.2 | SCYA5 | 5 |
| 32264_at | 19p13.3 | GZMM | 5 |
| 37078_at | 1q22-q23 | CD3Z | 5 |
| 1405_i_at | 17q11.2 | SCYA5 | 5 |
| 38147_at | Xq25-q26 | SH2D1A | 5 |
| 39226_at | 11q23 | CD3G | 5 |
| 38319_at | 11q23 | CD3D | 5 |
| 40699_at | 2p12 | CD8A | 5 |
| 39260_at | 1 | SLC16A4 | 4 |
| 37835_at | 1q22-q23 | CD1C | 3 |
| 38363_at | 19q13 | TYROBP | 3 |
* Spike sample number.
Consistent with the finding of overexpression of the T-cell marker Fgl2 in WM-BC (see Fig. 3A), this analysis revealed that a panel of T-cell-related genes were overexpressed in a subset of WM cases. These genes included TCRA, CD2, CD3D, CD3G, CD3Z, and CD8A. WM cell expression of T-cell markers was also accompanied by expression of cytotoxic T lymphocyte–associated serine esterase 3 (GZMA), granzyme 3 (GZMK), granzyme (GZMM), a cell adhesion gene (NK4), and cytokines (IL7R and SCYA5). In seven patients (34%), genes associated with overexpression of T-cell markers were identified as being coactivated in WM-BC (Fig. 4A).
Fig. 4. Aberrant T cell marker expression in WM-B cells.
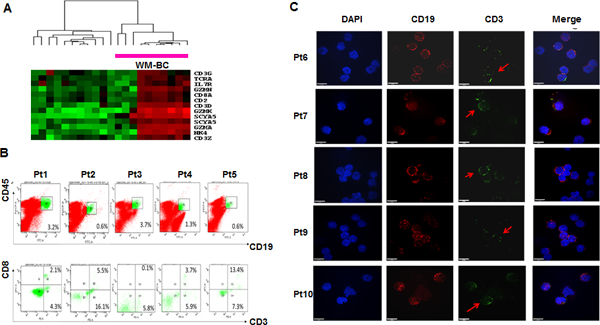
A, A two dimensional hierarchical cluster of 12 WM-BC and 9 WM-PC samples clustered based on the correlation of experimental expression signatures of 13 probe sets representing T-cell markers or related genes. The clustering was presented graphically as a colored image. Along the vertical axis, the analyzed genes were arranged as ordered by the similar patterns of expression. Experimental samples were arranged the same way along the horizontal axis; those with the similar patterns of expression across all genes were adjacent to each other. The samples labelled the Red-bar were derived from 7 WB-BC samples. B, Cell analyses of T-cell marker expression in WM-BC samples. Two-color dot plots of flow cytometry analysis showed the population of CD19 and CD45 double stain cells derived from bone marrow aspirates of WM patients, the strong CD19 and CD45 positive B cells were labelled with green color (Top panel); Two-color dot plots presented dual antibody analyses of CD3 and CD8 in CD19+CD45+ enriched WM-B cells (green-labelled in the top panel), about 4.49% (0.1 ~ 13.4%) CD3+CD8+ cells were found in the CD19+CD45+ WM-B cells (Bottom panel). C, Immunofluorescence confirms CD3 expression on CD19-positive WM-B cells. The CD19+CD45+ WM-B cells sorted out from above 5 WM patients were also performed CD19 (red) and CD3 (green) double stains by immunofluorescence; the yellow color indicated the merged signals. DAPI (blue color) was used to stain cell nuclei.
Validate the expression of T-cell antigens in WM-BC
To validate if T cell markers identified by GEP are truly expressed on WM-BC, flow cytometry and immunofluorescence were used to detect the expression of CD3 and/or CD8 antigens on CD19+CD45+ cells collected from WM patient samples. Five WM patient samples were collected from bone marrow aspirate and determined CD19+CD20+ tumor cells (Supplemental Table 4). As shown in Fig. 4B, the strong CD19+CD45+ cells were sorted by fluorescence-activated cell sorting (FACS) and further analyzed for CD3 and CD8 expression. The results clearly showed both CD3 and CD8 to be expressed in subsets of cells (mean: 4.9%; range: 0.1 ~ 13.4%) within in the CD19+CD45+ tumor cells. We also examined CD3 expression in CD19+ WM cells from the same five patient samples using immunofluorescence. Both anti-CD3 and anti-CD19 antibodies were tested in the normal T cells and B cells respectively (Supplemental Fig. 1) representing positive controls. Double staining with CD3 and CD19 confirmed that a subset of CD19+ cells expressed CD3 (Fig. 4C).
Discussion
WM is a distinct clinical entity characterized by IgM monoclonal gammopathy and infiltration of the bone marrow by lymphoplasmacytoid tumor cells. The expression profiles of WM compared with other B cell malignant as well as normal peripheral-blood B cells and bone marrow plasma cells have recently been published (26,27). However, none of them identified WM related genes differentially expressed during different stage BC development. In this study, we show that comparative microarray profiling of CD19-enriched tonsil B cells, peripheral-blood B cells as well as CD138-enriched PCs from tonsil and bone marrow allowed to gain more comprehensive insights into the biology of WM. GEP studies in this type of lymphoma are challenging, as the malignant clone demonstrates significant intraclonal heterogeneity with respect to plasmacytoid differentiation. WM is a low-grade B-cell or plasma cell tumor. Evidence has accumulated, indicating that malignant B cells display a variety of physiologic activities and requirements possessed by normal cells at similar stages of differentiation (24). We show that WM-PCs differ from other plasma cells in the overexpression of B-cell markers, such as CD45, CD20, CD79B, and CD52. Likewise, WM-BCs exhibit much higher expression levels of the plasma cell markers CD138, CD38, and CD63. The high expression of BCR in both WM-BC and WM-PC indicates that antigen stimulation may be important to maintain tumor cell viability and the immature status of WM-PC. In line with these results, WM-PC cases show a similar gene expression pattern to that of immature T-PC. Unfortunately, we could not collect age-matched normal bone marrow B cells as a control in this study, which will be performed in the future.
Several of the genes identified as overexpressed in WM-BC or WM-PC in this study may provide novel insights into WM disease biology, as well as potential targets for therapeutic intervention. For example, this analysis shows that the oncogene MYB is commonly dysregulated in WM, with a greater than 100-fold increase in expression over normal counterparts. MYB activation, in particular, may be important, as activation of this gene has been shown to induce plasmacytoid lymphosarcomas in mice and B-cell lymphomas in chickens (28,29). Therefore, further study is needed to understand the basis and consequences of MYB dysregulation in WM. BCL6 is another candidate oncogene that may be dysregulated in these tumors. Dysregulation of BCL6, an important transcription repressor that blocks plasma cell differentiation (30), may also be linked to the observed overexpression of a IGLL1, which has been studied for its BCL6-rearranging properties in lymphoma (31). Another candidate oncogene is BLIMP1/PRDMI, which encodes a zinc-finger transcription factor that drives the maturation of B lymphocytes to immunoglobulin-secreting plasma cells in vitro and in vivo (32,33). Importantly, the dysregulation of oncogenes such as BCL6 may also be related to prognosis for WM, as in other lymphomas (34). Consistent with previous reports, the src family (HCK) and the gp130 cytokine family (IL6 and IL6R) were significantly overexpressed in WM, HCK, belongs to the src family of tyrosine kinases, which has also been implicated in B-cell proliferation and malignancies, as well as a direct target of BTK inhibitor (35,36). IL-6 is known to play a critical role in normal and malignant plasma cell growth and survival (26,27,37). The upregulation of both IL-6 and its receptor on WM cells creates the possibility of an autocrine signaling cascade in this disease. Interestingly, It was reported that the IL6/IL6R signaling pathway could enhance HCK kinase activity including MM (38,39).
This analysis also identified dysregulation of key genes in several signaling pathways not previously implicated in WM. These genes include the protein kinase C family (PRKCB1) and the receptor tyrosine kinases (PDGFRA). Protein kinase C-β plays a critical role in signaling through the B-cell receptor and in early B-cell survival. Upregulation of PRKCB1 in WM as observed in this study, along with data from other mature B-cell tumors (40), suggests dysregulation of this pathway in mature B-cell/plasma cell malignancies as well. Indeed, overexpression of PRKCB1 represents a poor prognostic signature in large B-cell lymphomas in one study (34). PDGFRA, encoding receptor tyrosine kinase, was found to be upregulated in most WM patients. PDGFRA has previously been reported to enhance medulloblastoma migration and activation of downstream Ras/MAPK pathway (41).
Although the expression of T-cell markers has been considered to be exclusive to the T-cell linage and has not been found on normal B cells, a panel of T-cell markers and cytotoxic T lymphocyte–associated genes, including TCRA, CD2, CD3D, CD3G, CD3Z, CD8A, GZMA, GZMK, and GZMM, were found to be differentially upregulated in a subset of WM patients. To our knowledge, this is the first indication that T-cell markers are involved in WM. Other genes involved in T-cell biology, namely TYROBP, FGL2, IL7R, and SCYA5, were also identified in this study as being overexpressed in WM, which suggests that WM cells may be responsive to cytokine signaling through T-cell receptors. The expression of p56lck, one of the first molecules to be activated downstream of the T-cell receptor in CD4+ and CD8+ thymocytes (42) was highly correlated with CD8A, suggesting that functional CD8A signaling and possibly other T cell–specific stimuli may affect WM biology. The increased expression of T cell markers exists only in a small subset of B cells of WM, suggesting this population may have de-differentiated during tumor development. It will be interesting to examine whether the CD19+CD3+ WM cells have “stemness” features, such as self-renewal and increased drug resistance in the future. If this is true, targeting T-cell signaling in WM may eliminate drug resistant stem cell-like tumor cells. This discovery is not unique in WM-BC, since aberrant T cell marker expression was also reported in plasmablastic transformation of plasma cell myeloma, chronic lymphocytic leukemia (CLL), diffuse large B cell lymphoma (DLBCL), and ALK-positive large B cell lymphoma (43–47).
The data presented here have implications for the development of novel therapies for WM. For example, inhibitors of signaling pathways found to be dysregulated in WM (protein kinase C-β and src kinases) are being developed and should be considered. Imatinib, targeting PDGFRA, or other similar therapeutic agents may prove useful. Approaches to inhibiting IL-6 signaling may also be of value in WM. N-acetylcysteine (NAC) targeting IL7R signaling pathways has demonstrated therapeutic efficacy in T-ALL (48,49). We believe that these therapeutic approaches are best tested in the context of patients with known abnormalities in the respective signaling pathways. Further studies of single cell sequencing in WM may improve understanding of disease heterogeneity and optimal application of targeted therapies.
Supplementary Material
Translation Relevance.
We discovered and validated that T-cell antigens are expressed on WM B cells. The increased expression of T cell markers was observed only in a small subset of B cells of WM, suggesting this population may have de-differentiated during tumor development. It will be interesting to examine in future studies whether the CD19+CD3+ WM cells have indeed “stemness” features, such as self-renewal and increased drug resistance. Our data may have implications for the development of novel therapies for WM. For example, the expression of p56lck, one of the first molecules to be activated downstream of the T-cell receptor in CD4+ and CD8+ thymocytes was highly correlated with IL7R, suggesting that functional IL7R signaling and possibly other T cell-specific stimuli may affect WM biology. Intriguingly, targeting of IL7R signaling pathways with N-acetylcysteine (NAC) has demonstrated therapeutic efficacy in T cell acute lymphoblastic leukemia (T-ALL) by disrupting IL7R homodimerization.
Acknowledgments
This work was supported by National Institutes of Health grants CA55819 (J.D.S., F.Z., G.T., B.B.), the Leukemia & Lymphoma Society TRP (6549–18), the Multiple Myeloma Research Foundation (F.Z.), the NIH Lymphoma Spore grant P50 CA097274, Cancer Center Support Grant NIH number P30 CA086862; institutional start-up funds from the Department of Internal Medicine, Carver College of Medicine, University of Iowa (to F. Zhan); Cancer Center Support Grant NIH number P30 CA086862, and the National Natural Science Foundation of China (81570181 M.H.& 81630007 L.Q.).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Dimopoulos MA, Panayiotidis P, Moulopoulos LA, Sfikakis P, Dalakas M. Waldenstrom’s macroglobulinemia: clinical features, complications, and management. J Clin Oncol 2000;18(1):214–26 doi 10.1200/JCO.2000.18.1.214. [DOI] [PubMed] [Google Scholar]
- 2.Dimopoulos MA, Alexanian R. Waldenstrom’s macroglobulinemia. Blood 1994;83(6):1452–9. [PubMed] [Google Scholar]
- 3.Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016;127(20):2375–90 doi 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin P, Medeiros LJ. Lymphoplasmacytic lymphoma/waldenstrom macroglobulinemia: an evolving concept. Adv Anat Pathol 2005;12(5):246–55. [DOI] [PubMed] [Google Scholar]
- 5.Kriangkum J, Taylor BJ, Strachan E, Mant MJ, Reiman T, Belch AR, et al. Impaired class switch recombination (CSR) in Waldenstrom macroglobulinemia (WM) despite apparently normal CSR machinery. Blood 2006;107(7):2920–7 doi 10.1182/blood-2005-09-3613. [DOI] [PubMed] [Google Scholar]
- 6.Herrinton LJ, Weiss NS. Incidence of Waldenstrom’s macroglobulinemia. Blood 1993;82(10):3148–50. [PubMed] [Google Scholar]
- 7.Schop RF, Van Wier SA, Xu R, Ghobrial I, Ahmann GJ, Greipp PR, et al. 6q deletion discriminates Waldenstrom macroglobulinemia from IgM monoclonal gammopathy of undetermined significance. Cancer Genet Cytogenet 2006;169(2):150–3 doi S0165-4608(06)00259-7[pii]10.1016/j.cancergencyto.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Ocio EM, Schop RF, Gonzalez B, Van Wier SA, Hernandez-Rivas JM, Gutierrez NC, et al. 6q deletion in Waldenstrom macroglobulinemia is associated with features of adverse prognosis. Br J Haematol 2007;136(1):80–6 doi 10.1111/j.1365-2141.2006.06389.x. [DOI] [PubMed] [Google Scholar]
- 9.Treon SP, Xu L, Yang G, Zhou Y, Liu X, Cao Y, et al. MYD88 L265P somatic mutation in Waldenstrom’s macroglobulinemia. N Engl J Med 2012;367(9):826–33 doi 10.1056/NEJMoa1200710. [DOI] [PubMed] [Google Scholar]
- 10.Kyle RA, Therneau TM, Rajkumar SV, Remstein ED, Offord JR, Larson DR, et al. Long-term follow-up of IgM monoclonal gammopathy of undetermined significance. Blood 2003;102(10):3759–64 doi 10.1182/blood-2003-03-0801. [DOI] [PubMed] [Google Scholar]
- 11.Paiva B, Corchete LA, Vidriales MB, Garcia-Sanz R, Perez JJ, Aires-Mejia I, et al. The cellular origin and malignant transformation of Waldenstrom macroglobulinemia. Blood 2015;125(15):2370–80 doi 10.1182/blood-2014-09-602565. [DOI] [PubMed] [Google Scholar]
- 12.Hunter ZR, Branagan AR, Manning R, Patterson CJ, Santos DD, Tournilhac O, et al. CD5, CD10, and CD23 expression in Waldenstrom’s macroglobulinemia. Clin Lymphoma 2005;5(4):246–9. [DOI] [PubMed] [Google Scholar]
- 13.Zhan F, Hardin J, Kordsmeier B, Bumm K, Zheng M, Tian E, et al. Global gene expression profiling of multiple myeloma, monoclonal gammopathy of undetermined significance, and normal bone marrow plasma cells. Blood 2002;99(5):1745–57. [DOI] [PubMed] [Google Scholar]
- 14.Zhan F, Tian E, Bumm K, Smith R, Barlogie B, Shaughnessy J, Jr. Gene expression profiling of human plasma cell differentiation and classification of multiple myeloma based on similarities to distinct stages of late-stage B-cell development. Blood 2003;101(3):1128–40 doi 10.1182/blood-2002-06-1737. [DOI] [PubMed] [Google Scholar]
- 15.Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med 2003;349(26):2483–94 doi 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- 16.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A 2001;98(9):5116–21 doi 10.1073/pnas.091062498091062498[pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A 1998;95(25):14863–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonewald L, Virella G, Wang AC. Evidence for the biclonal nature of a Waldenstrom’s macroglobulinemia. Clin Chim Acta 1985;146(1):53–63. [DOI] [PubMed] [Google Scholar]
- 19.Pangalis GA, Angelopoulou MK, Vassilakopoulos TP, Siakantaris MP, Kittas C. B-chronic lymphocytic leukemia, small lymphocytic lymphoma, and lymphoplasmacytic lymphoma, including Waldenstrom’s macroglobulinemia: a clinical, morphologic, and biologic spectrum of similar disorders. Semin Hematol 1999;36(2):104–14. [PubMed] [Google Scholar]
- 20.Schwonzen M, Pohl C, Steinmetz T, Seckler W, Vetten B, Thiele J, et al. Immunophenotyping of low-grade B-cell lymphoma in blood and bone marrow: poor correlation between immunophenotype and cytological/histological classification. Br J Haematol 1993;83(2):232–9. [DOI] [PubMed] [Google Scholar]
- 21.Ho AW, Hatjiharissi E, Ciccarelli BT, Branagan AR, Hunter ZR, Leleu X, et al. CD27-CD70 interactions in the pathogenesis of Waldenstrom macroglobulinemia. Blood 2008;112(12):4683–9 doi 10.1182/blood-2007-04-084525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vos JM, Tsakmaklis N, Patterson CJ, Meid K, Castillo JJ, Brodsky P, et al. CXCL13 levels are elevated in patients with Waldenstrom macroglobulinemia, and are predictive of major response to ibrutinib. Haematologica 2017;102(11):e452–e5 doi 10.3324/haematol.2017.172627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calame KL. Plasma cells: finding new light at the end of B cell development. Nat Immunol 2001;2(12):1103–8 doi 10.1038/ni1201-1103. [DOI] [PubMed] [Google Scholar]
- 24.Schop RF, Fonseca R. Genetics and cytogenetics of Waldenstrom’s macroglobulinemia. Semin Oncol 2003;30(2):142–5 doi 10.1053/sonc.2003.50075. [DOI] [PubMed] [Google Scholar]
- 25.Sjolin H, Tomasello E, Mousavi-Jazi M, Bartolazzi A, Karre K, Vivier E, et al. Pivotal role of KARAP/DAP12 adaptor molecule in the natural killer cell-mediated resistance to murine cytomegalovirus infection. J Exp Med 2002;195(7):825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chng WJ, Schop RF, Price-Troska T, Ghobrial I, Kay N, Jelinek DF, et al. Gene-expression profiling of Waldenstrom macroglobulinemia reveals a phenotype more similar to chronic lymphocytic leukemia than multiple myeloma. Blood 2006;108(8):2755–63 doi 10.1182/blood-2006-02-005488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gutierrez NC, Ocio EM, de Las Rivas J, Maiso P, Delgado M, Ferminan E, et al. Gene expression profiling of B lymphocytes and plasma cells from Waldenstrom’s macroglobulinemia: comparison with expression patterns of the same cell counterparts from chronic lymphocytic leukemia, multiple myeloma and normal individuals. Leukemia 2007;21(3):541–9 doi 10.1038/sj.leu.2404520. [DOI] [PubMed] [Google Scholar]
- 28.Kanter MR, Smith RE, Hayward WS. Rapid induction of B-cell lymphomas: insertional activation of c-myb by avian leukosis virus. J Virol 1988;62(4):1423–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen-Ong GL, Potter M, Mushinski JF, Lavu S, Reddy EP. Activation of the c-myb locus by viral insertional mutagenesis in plasmacytoid lymphosarcomas. Science 1984;226(4678):1077–80. [DOI] [PubMed] [Google Scholar]
- 30.Verkoczy LK, Guinn B, Berinstein NL. Characterization of the human B cell RAG-associated gene, hBRAG, as a B cell receptor signal-enhancing glycoprotein dimer that associates with phosphorylated proteins in resting B cells. J Biol Chem 2000;275(28):20967–79 doi 10.1074/jbc.M001866200. [DOI] [PubMed] [Google Scholar]
- 31.Chaganti SR, Rao PH, Chen W, Dyomin V, Jhanwar SC, Parsa NZ, et al. Deregulation of BCL6 in non-Hodgkin lymphoma by insertion of IGH sequences in complex translocations involving band 3q27. Genes Chromosomes Cancer 1998;23(4):328–36. [DOI] [PubMed] [Google Scholar]
- 32.Turner CA Jr., Mack DH, Davis MM. Blimp-1, a novel zinc finger-containing protein that can drive the maturation of B lymphocytes into immunoglobulin-secreting cells. Cell 1994;77(2):297–306. [DOI] [PubMed] [Google Scholar]
- 33.Angelin-Duclos C, Cattoretti G, Lin KI, Calame K. Commitment of B lymphocytes to a plasma cell fate is associated with Blimp-1 expression in vivo. J Immunol 2000;165(10):5462–71. [DOI] [PubMed] [Google Scholar]
- 34.Lossos IS, Czerwinski DK, Alizadeh AA, Wechser MA, Tibshirani R, Botstein D, et al. Prediction of survival in diffuse large-B-cell lymphoma based on the expression of six genes. N Engl J Med 2004;350(18):1828–37 doi 10.1056/NEJMoa032520. [DOI] [PubMed] [Google Scholar]
- 35.Baumgartner M, Angelisova P, Setterblad N, Mooney N, Werling D, Horejsi V, et al. Constitutive exclusion of Csk from Hck-positive membrane microdomains permits Src kinase-dependent proliferation of Theileria-transformed B lymphocytes. Blood 2003;101(5):1874–81 doi 10.1182/blood-2002-02-0456. [DOI] [PubMed] [Google Scholar]
- 36.Yang G, Buhrlage SJ, Tan L, Liu X, Chen J, Xu L, et al. HCK is a survival determinant transactivated by mutated MYD88, and a direct target of ibrutinib. Blood 2016;127(25):3237–52 doi 10.1182/blood-2016-01-695098. [DOI] [PubMed] [Google Scholar]
- 37.Kobayashi M, Miyagishima T, Imamura M, Maeda S, Gotohda Y, Iwasaki H, et al. Establishment and characterization of a plasma cell leukaemia cell line dependent for growth on IL-6 and a bi-phenotypic subclone dependent upon both IL-3 and IL-6. Br J Haematol 1991;78(2):217–21. [DOI] [PubMed] [Google Scholar]
- 38.Ernst M, Oates A, Dunn AR. Gp130-mediated signal transduction in embryonic stem cells involves activation of Jak and Ras/mitogen-activated protein kinase pathways. J Biol Chem 1996;271(47):30136–43. [DOI] [PubMed] [Google Scholar]
- 39.Abdollahi P, Vandsemb EN, Hjort MA, Misund K, Holien T, Sponaas AM, et al. Src Family Kinases Are Regulated in Multiple Myeloma Cells by Phosphatase of Regenerating Liver-3. Mol Cancer Res 2017;15(1):69–77 doi 10.1158/1541-7786.MCR-16-0212. [DOI] [PubMed] [Google Scholar]
- 40.Chiaretti S, Li X, Gentleman R, Vitale A, Wang KS, Mandelli F, et al. Gene expression profiles of B-lineage adult acute lymphocytic leukemia reveal genetic patterns that identify lineage derivation and distinct mechanisms of transformation. Clin Cancer Res 2005;11(20):7209–19 doi 10.1158/1078-0432.CCR-04-2165. [DOI] [PubMed] [Google Scholar]
- 41.MacDonald TJ, Brown KM, LaFleur B, Peterson K, Lawlor C, Chen Y, et al. Expression profiling of medulloblastoma: PDGFRA and the RAS/MAPK pathway as therapeutic targets for metastatic disease. Nat Genet 2001;29(2):143–52 doi 10.1038/ng731. [DOI] [PubMed] [Google Scholar]
- 42.Mulligan SP, Dao LP, Francis SE, Thomas ME, Gibson J, Cole-Sinclair MF, et al. B-cell chronic lymphocytic leukaemia with CD8 expression: report of 10 cases and immunochemical analysis of the CD8 antigen. Br J Haematol 1998;103(1):157–62. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki Y, Yoshida T, Wang G, Aoki T, Katayama T, Miyamoto S, et al. Incidence and clinical significance of aberrant T-cell marker expression on diffuse large B-cell lymphoma cells. Acta Haematol 2013;130(4):230–7 doi 10.1159/000348550. [DOI] [PubMed] [Google Scholar]
- 44.Tsuyama N, Ennishi D, Yokoyama M, Baba S, Asaka R, Mishima Y, et al. Clinical and prognostic significance of aberrant T-cell marker expression in 225 cases of de novo diffuse large B-cell lymphoma and 276 cases of other B-cell lymphomas. Oncotarget 2017;8(20):33487–500 doi 10.18632/oncotarget.16532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaleem Z, White G, Zutter MM. Aberrant expression of T-cell-associated antigens on B-cell non-Hodgkin lymphomas. Am J Clin Pathol 2001;115(3):396–403 doi 10.1309/EHMY-7WU1-UYE7-EE99. [DOI] [PubMed] [Google Scholar]
- 46.Pan Z, Hu S, Li M, Zhou Y, Kim YS, Reddy V, et al. ALK-positive Large B-cell Lymphoma: A Clinicopathologic Study of 26 Cases With Review of Additional 108 Cases in the Literature. Am J Surg Pathol 2017;41(1):25–38 doi 10.1097/PAS.0000000000000753. [DOI] [PubMed] [Google Scholar]
- 47.Mishra P, Kakri S, Gujral S. Plasmablastic transformation of plasma cell myeloma with heterotropic expression of CD3 and CD4: a case report. Acta Clin Belg 2017;72(4):250–3 doi 10.1080/17843286.2016.1201629. [DOI] [PubMed] [Google Scholar]
- 48.Mansour MR, Reed C, Eisenberg AR, Tseng JC, Twizere JC, Daakour S, et al. Targeting oncogenic interleukin-7 receptor signalling with N-acetylcysteine in T cell acute lymphoblastic leukaemia. Br J Haematol 2015;168(2):230–8 doi 10.1111/bjh.13115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cramer SD, Aplan PD, Durum SK. Therapeutic targeting of IL-7Ralpha signaling pathways in ALL treatment. Blood 2016;128(4):473–8 doi 10.1182/blood-2016-03-679209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


