Abstract
A new tetraphenylethylene (TPE) functionalized 1,4-dihydropyrrolo[3,2-b]pyrrole derivative (APPTPECN) was synthesized with obvious aggregation-induced emission (AIE) active by simple synthetic method. APPTPECN exhibited reversible mechanofluorochromic (MFC) behavior. The powder X-ray diffraction (PXRD) and scanning electron microscopy (SEM) investigations exhibited that the MFC nature is originated through a conversion from the microcrystalline to amorphous phase under the stimulus of external force. The results obtained would be of major help in understanding the MFC mechanism and designing new MFC materials. Compound APPTPECN has the potential possibility to employ in rewritable data storage and is of assistance in the rational design of smart luminescent materials.
Keywords: 1,4-dihydropyrrolo[3,2-b]pyrrole derivative; aggregation-induced emission (AIE); reversible mechanochromism behavior; rewritable data storage
1. Introduction
Fluorescent mechanofluorochromic (MFC) is a phenomenon where solid and liquid crystalline materials change their photoluminescence properties upon applying mechanical stimulation, such as grinding, ball-milling, and crushing [1,2,3,4,5,6,7]. Fluorescence emission and color change could often recover by another stimulation or more, such as heating, organic solvent vapor, and light [8,9,10,11,12,13,14,15,16]. Mechanofluorochromic materials as a kind of “smart material’’ have its potential applications in mechanosensors, fluorescence switches, and data storage so on [17,18,19,20,21,22,23,24,25,26]. Recently, rationally controlling the design of molecular mechanofluorochromic behavior is still a huge challenging, two main problems restrict the development of this kind of materials: the lack of deep understanding for the structure-property relationship of such materials and the fluorescent quenching effect in the solid state [27,28,29,30,31]. It is exciting that a major breakthrough came from an unusual aggregation-induced emission (AIE) phenomenon in the study of 1-methyl-1,2,3,4,5-pentaphenylsilole [32]. Since then, AIE concept has been extensively used to design efficient solid-state fluorescent materials and MFC materials because of highly twisted backbone structures bearing rotatable units [33,34,35]. Also, the twisted molecular conformations which not only enable them to emit strong fluorescence in the aggregated state by weakening intermolecular close stacking and intense π-π interactions, but also easily lead to the formation of MFC properties by changing the molecular packing modes upon pressure [36,37,38]. Herein, the synthesis of novel effective solid-state luminogens is still a hot topic for the researchers.
In order to obtain excellent MFC materials with strong fluorescence emission in the solid state and aggregated state, an artful design of the molecule was important for tuning molecular MFC properties. Typically, 1,4-dihydropyrrolo[3,2-b]pyrroles (PPs), a class of 10 π-electron aromatic dihydroheteropentalenes, were discovered by Hemetsberger and Knittel in 1972 [39]. Importantly, Gryko and co-workers developed a one-step synthetic method to acquire tetraaryl-pyrrolo[3,2-b]pyrroles in 2013 [40]. Moreover, these compounds have received increasing attention because PPs have a lot of advantages including high thermal, photostability, good absorptivity, bright fluorescence and high quantum yields [41,42,43]. Meanwhile, molecules and the modifications of TPE have been proved an efficient way to construct new aggregation-induced emission (AIE) luminogens with high solid-state efficiency [44,45,46,47,48,49].
Based on the above considerations, we present a simple method to discover MFC compound of novel 1,4-dihydropyrrolo[3,2-b]pyrroles derivative containing TPE unit. We have been trying our best knowledge to grow crystal to explain the phenomenon why the material fluorescence enhanced after grinding, unfortunately, we still unable to successfully cultivate crystal. APPTPECN showed strong AIE property. Also, the obtained APPTPECN exhibited reversible MFC behaviors. It was shown that interconversion between the microcrystalline and amorphous phase, and the MFC mechanism was proved by PXRD and SEM.
2. Results and Discussion
Synthesis of the APPCN and APPTPECN
The synthesis of APPCN was carried out using one-step method and compound APPTPECN was synthesized using a classical Knoevenagel condensation reaction. The chemical structures and synthetic route of the compounds are shown in Scheme 1. The detailed procedure is listed as follows.
Scheme 1.
Synthetic route of APPCN and APPTPECN.
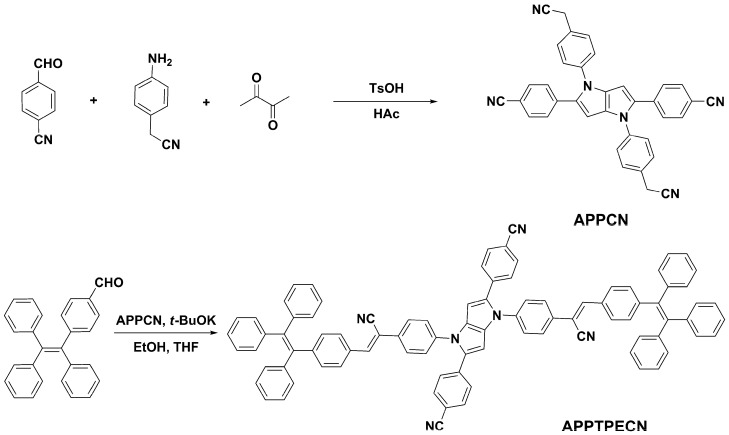
Considering the miscibility of DMF and H2O, for the purpose of checking whether APPTPECN is AIE-active, we used a standard approach to investigate UV-vis absorption and FL spectra. Concentrations of APPTPECN were kept at 1.0 × 10−5 mol L−1 in DMF and water mixture solution. For UV-vis absorption spectra, obviously, with the increasing water fraction, the solubility of the compound gradually decreased and the molecules gradually shifted from the free state to the aggregation state, forming the molecular aggregated particles. Meanwhile, the level-off tail suggested that the aggregates have been formed in mixtures (Figure 1a). DLS experiment showed the formation of aggregates with average hydrodynamic diameter of 265 nm (polydispersity index = 0.342) (Figure S6). For FL spectra, APPTPECN showed very weak visible light excited by 365 nm UV light in pure DMF because of the intramolecular rotation of tetraphenylethylene core. FL intensity remarkably increases from 30% to 99% water fraction and reaches its peak at 90% (Figure 1b–d). Also, there is a significant red-shift of APPTPECN fluorescence wavelength. The bright yellow-green light emitting of APPTPECN (located at 532 nm) was observed for the aggregates, which was about 30 times higher than that in the pure DMF, likely due to the intramolecular rotation restriction of tetraphenylethene unit [32,50]. These results indicated a typical AIE activity that the non-radiative processes were restrained because of the restriction of intramolecular rotation and intramolecular interaction [51,52,53,54].
Figure 1.
The UV-vis absorption (a) and FL spectra (b) of APPTPECN in DMF/water mixtures with different water volume fractions; the effect of the water volume fraction on the maximum emission intensity (c); optical photographs recorded under 365 nm UV irradiation with various fractions of water (d).
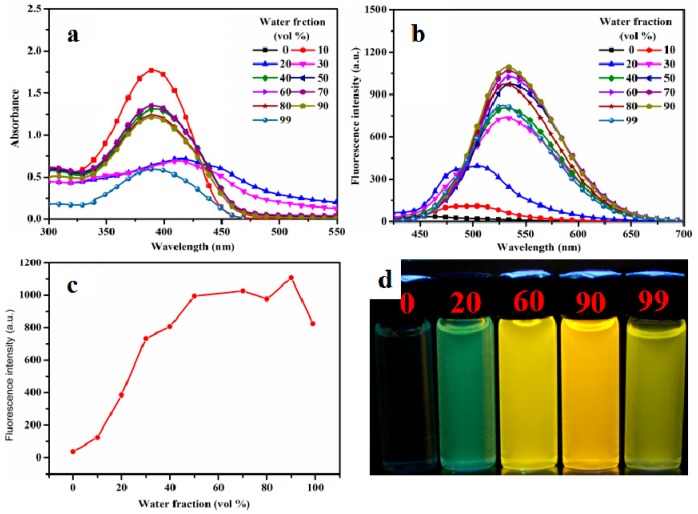
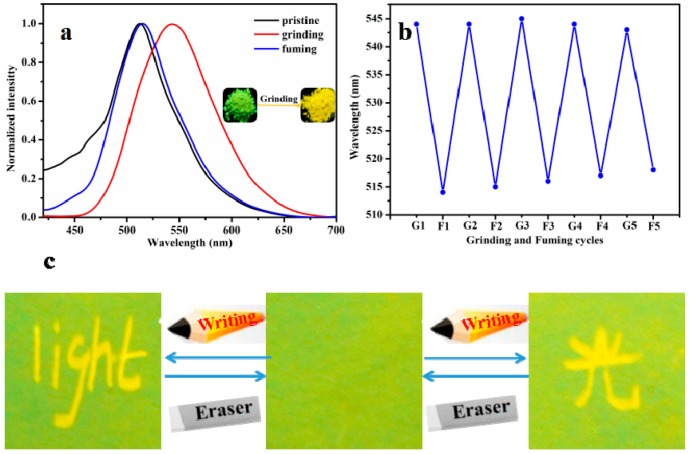
FL spectra was utilized to investigate the MFC behavior of APPTPECN in the solid state. As shown in Figure 2a, The pristine solid APPTPECN emitted green fluorescence in 512 nm (ΦF = 14%, τ < 0.1 ns, Figure S5). Interestingly, APPTPECN exhibited an obvious red-shift upon grinding (34 nm) yellow emission (λem = 546 nm, ΦF = 22%) with a long lifetime (τ = 1.15 ns, Figure S7). Higher ΦF values indicated a higher average excited state lifetime compared to the pristine sample. This result is worth noting that only a few materials have been reported to display higher fluorescence quantum yield for the pristine sample than that for the grinding sample in the field of smart materials [55,56,57,58]. Additionally, we take insight to determine the self-reversible mechanofluorochromic fluorescence conversion by alternating grinding and EtOH fuming, the fluorescence emission and colour of grinding powder could be nearly converted numerous times between the green and yellow emissions (Figure 2b). It was possible that an external mechanical force resulted in a change in the molecular morphology, indicative an outstanding reversibility of the MFC process. Owing to the excellent mechanofluorochromic properties of the smart fluorescent materials. We further explore the practical application, a simple and portable ink-free rewritable paper were conducted on Figure 2c, the pristine sample was dissolved in CH2Cl2 (DCM), then a Whatman filter paper was immersed into the solvent for approximately 30 min. Afterwards, Whatman filter paper was taken out and dried at room temperature. Intriguingly, they were very clear and discernible under 365 nm irradiation and filter paper emitted green emission. When the APPTPECN-loaded paper was crushed, the fluorescence emission significantly red-shift. The letter “light” can be erased after the “paper” was exposed to DCM vapor. Furthermore, a Chinese letter “light” was easily written in the Whatman filter paper again. As a result, the material has great potential for application in ink-free rewritable paper.
Figure 2.
FL spectra of APPTPECN as pristine, grinding and fuming samples (a); Repeated switching of the solid-state fluorescence of APPTPECN (b) by repeated grinding and fuming cycles; (c) Photos of the luminescence writing/erasing process on the APPTPECN-loaded paper under a 365 nm UV lamp.
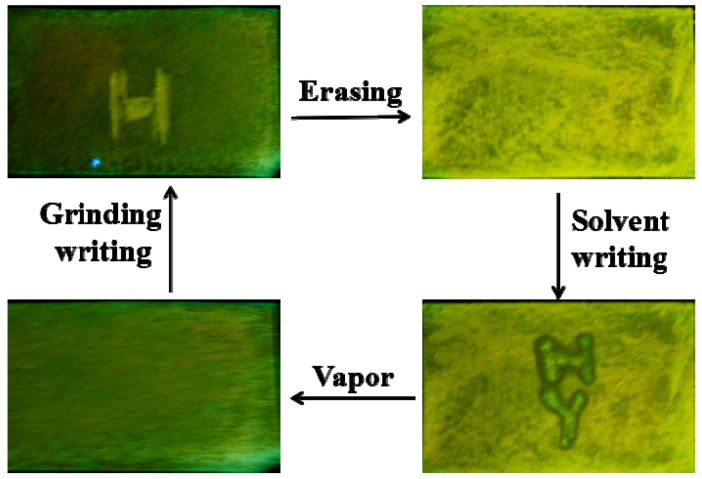
The excellent rewritable paper further promotes us explore the application of rewritable data storage, as shown in Figure 3, compared to green background, the preparation of Whatman filter paper was crushed and appear yellow ‘H’ letter. Then, a green background is easily also converted into a yellow background with crushing. Next, ‘H’ and ‘Y’ letters were written using a specially made ‘pen’ with DCM vapor as ‘ink’, which exhibiting green fluorescence emission due to the grinding sample back to the original state after DCM treatment. Last, exposure of the Whatman filter paper to DCM vapor can readily erase the fluorescent letters and return to original green fluorescence background. Herein, compound APPTPECN is employed in rewritable data storage and is of assistance in the rational design of smart luminescent materials.
Figure 3.
Photographs of the writing/erasing cycle under UV light lamp.
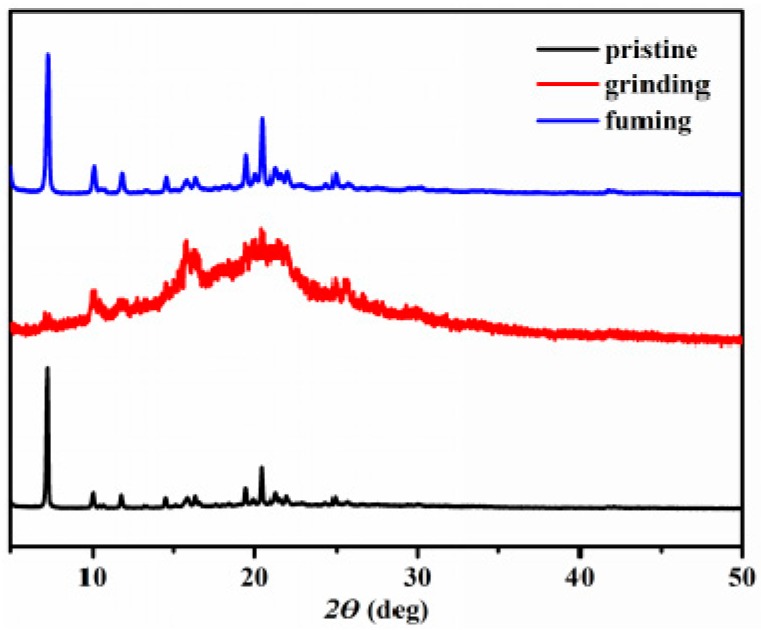
To further understand and evaluate the origin and MFC properties in the powder state, PXRD was investigated for the sample before and after grinding. As shown in Figure 4, the diffraction curves of the pristine sample revealed many sharp and intense reflections, indicative of the well-ordered crystalline structures. In contrast, after grinding, the diffraction peaks nearly disappeared and only some diffuse, broad and weak band were observed, exhibiting disordered molecular packing or amorphous states. The process implied that grinding led to a microcrystalline to amorphous phase conversion of solids. After exposing the grinding sample to EtOH vapor, the sharp and intense diffraction peaks reappeared. Therefore, it was manifested APPTPECN has the reversible mechanofluorochromic behavior.
Figure 4.
PXRD patterns of pristine, grinding and fuming samples of APPTPECN.
The aggregation morphology of APPTPECN before and after grinding were investigated by scanning electron microscopy (SEM) investigations. As performed in Figure 4, the APPTPECN exhibited an analogous “magnesium” structure (Figure 5a), whereas the irregular and rough aggregates were observed for the grinding APPTPECN (Figure 5b). When the grinding sample was fumed by EtOH, it essentially reverted to its original microcrystalline state (Figure 5c). It is further proved that a microcrystalline to amorphous phase conversion of solid after grinding.
Figure 5.
SEM image of pristine sample (a); grinding sample (b) and fuming sample (c).
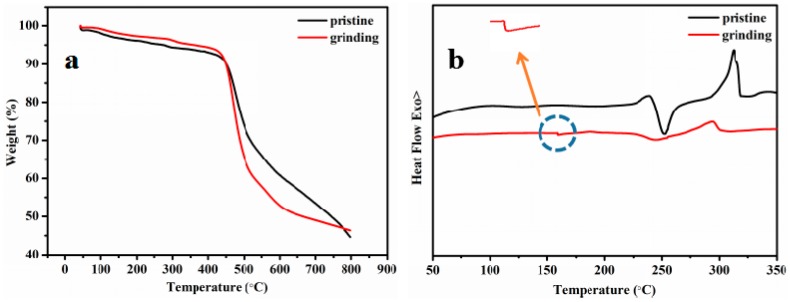
TG analysis curves revealed that the degradation temperature (Td) of 5% weight loss of APPTPECN before and after grinding are 438 °C, 440 °C, respectively (Figure 6a). This result revealed that APPTPECN has an excellent thermal stability. In addition, DSC thermograms demonstrated that a new exothermic peak at 159 °C was observed for grinding sample. This exothermic peak could be attributed to the cold-crystallization transition of the amorphous APPTPECN caused by mechanical grinding (Figure 6b). Hence, PXRD, SEM and DSC experiments verified that phase transformation between the microcrystalline and amorphous states should be responsible for the MFC behavior.
Figure 6.
TGA curves (a) and DSC curves of APPTPECN in different states (b).
3. Materials and Methods
3.1. Measurements and Instrument
All of the chemicals and solvents were purchased from Darui of Shanghai without further purification. 1H NMR (400 and 600 MHz) spectra was registered in a Bruker Avance with CDCl3 as the solvent and tetramethylsilane (TMS) as the internal standard. The UV-vis absorption spectra was measured in a TU-1901 spectrometer (Purkinje General Instrument Co., Ltd. Beijing, China). The fluorescence spectra were recorded on a Hitachi FL-7000 (Shanghai, China). The absolute solid-state fluorescence quantum yield in the solid state was conducted on a HORIBA FluoroMax-4 spectrofluorometer (Irvine, CA, USA) with an integrating sphere. The powder X-ray diffraction (PXRD) patterns were performed on an MXP18AHF (Hefei, China). The thermogravimetry (TG) analysis using TGA5500 and differential scanning calorimetry (DSC) was measured at a heating rate of 10 °C·min−1 under a N2 atmosphere using DSC apparatus (METTLER82le/400 (Zurich, Switzerland)).
3.2. Synthesis
3.2.1. The Generally Synthetic Procedure of Compound 2,6-di(4-Cyanomethylphenyl)-1,5-di(4-cyanophenyl)-1,4-dihydropyrrolo[3,2-b]pyrrole (APPCN)
4-Aminobenzyl cyanide (1.0 g, 7.6 mmol), p-cyanobenzaldehyde (886 mg, 7.6 mmol) and p-toluenesulfonic acid (45 mg, 757 μmol) were dissolve in 10 mL glacial acetic acid, the mixture were heated to 90 °C, keeping the temperature for 1 h, Then, followed by addition of 2,3-Butanedione (651 mg, 3.8 mmol), the resulting mixture was stirred at 90 °C for 3 h. The reaction was cooled to ambient temperature. Then, the crude product was purified by washing with ethyl acetate. Yellow solid, 700 mg. Yield 35%. 1H NMR (400 MHz, DMSO-d6): δ (ppm): 4.12 (s, 2H), 6.76 (s, 1H), 7.34 (d, J = 7.44 Hz, 2H), 7.38 (d, J = 7.44 Hz, 2H), 7.46 (d, J = 7.76 Hz, 2H), 7.73 (d, J = 7.48 Hz, 2H). FT-IR (KBr, cm−1): 3061, 2939, 2224, 1600, 1514, 1457, 1420, 1366, 1177, 1140, 843. MALDI-TOF: m/z = 539.02 ([M + H]+), calcd for C36H22N6+ = 539.12([M + H]+).
3.2.2. The Generally Synthetic Procedure of Compound APPTPECN
A solution of 4-(1, 2, 2-triphenylvinyl)benzaldehyde (294 mg, 0.82 mmol) in EtOH-THF (10 mL, v/v = 1:1.5) was heated to reflux, then, APPCN (200 mg, 0.37 mmol) and t-BuOK (167 mg, 1.5 mmol) were added into solution, stirred 5 h. Then, The reaction was cooled to room temperature and filtration. The target compound was purified by washing with heated EtOH. Yellow solid, 272 mg, Yield: 60%. 1H NMR (600 MHz, CDCl3): δ (ppm): 6.54 (s, 1H), 7.04–7.14 (m, 17H), 7.31–7.32 (m, 4H), 7.43 (s, 1H), 7.54 (d, J = 7.62 Hz, 2H), 7.67–7.70 (m, 4H). 13C NMR (150 MHz, CDCl3): δ (ppm): 146.92, 143.27, 143.12, 142.57, 142.28, 139.94, 139.64, 137.22, 135.17, 133.02, 132.23, 132.01, 131.35, 131.33, 131.27, 128.83, 128.07, 127.91, 127.89, 127.70, 127.10, 126.94, 128.78, 125.54, 118.84, 117.91, 109.81, 109.38, 97.07, FT-IR (KBr, cm−1): 3077, 3045, 2219, 1594, 1511, 1440, 1389, 1168, 1072, 835. MALDI-TOF: m/z = 1223.30 ([M + H]+), calcd for C86H60N6+ = 1223.37 ([M + H]+).
4. Conclusions
In this work, a novel molecular design of 1,4-dihydropyrrolo[3,2-b]pyrrole derivative (APPTPECN) containing TPE was synthesized, The incorporation of TPE unit in the backbone endows the APPTPECN an obvious aggregation-induced emission (AIE) behaviour. Effective MFC behavior with the red-shifts of 34 nm in FL spectra upon grinding is observed and indicative of reversible performance. Moreover, compound APPTPECN has the potential possibility to employ rewritable data storage and is of assistance in the rational design of smart luminescent materials. The present study provides valuable information for designing new AIE MFC materials with desired properties.
Acknowledgments
We thank Mengyi Zhou, Lin Kong for their assistance in the characterization of the compounds.
Supplementary Materials
The following are available online. Figure S1. 1H NMR spectrum of APPCN. Figure S2. MALDI-TOF spectrum of APPCN. Figure S3. 1H NMR spectrum of APPTPECN. Figure S4. 13C NMR spectrum of APPTPECN. Figure S5. MALDI-TOF spectrum of APPTPECN. Figure S6. DLS plot of APPTPECN. Figure S7. Fluorescence decay curves for solid-state of APPTPECN before and after grinding.
Author Contributions
Writer of this manuscript (Y.M.), Study of the optical properties (Y.Z.), FRET Studies (L.K.), Supervision (J.Y.).
Funding
This work was supported by the National Natural Science Foundation of China (51673001, 51432001), the Educational Commission of Anhui Province of China (KJ2014ZD02).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds APPCN and APPTPECN are not available
References
- 1.Teng M.J., Jia X.R., Yang S., Chen X.F., Wei Y. Reversible tuning luminescent color and emission intensity: A dipeptide-based light-emitting materials. Adv. Mater. 2012;24:1255–1261. doi: 10.1002/adma.201104592. [DOI] [PubMed] [Google Scholar]
- 2.Chi G., Zhang X.Q., Xu B.J., Zhou X., Ma C.P., Zhang Y., Liu S.W., Xu J.R. Recent advances in organic mechanofluorochromic materials. Chem. Soc. Rev. 2012;41:3878–3896. doi: 10.1039/c2cs35016e. [DOI] [PubMed] [Google Scholar]
- 3.Zhang X.Q., Chi Z.G., Zhang Y., Liu S.W., Xu J.R. Recent advances in mechanochromic luminescent metal complexes. J. Mater. Chem. C. 2013;1:3376–3390. doi: 10.1039/c3tc30316k. [DOI] [Google Scholar]
- 4.Sagara Y., Yamane S., Mitani M., Weder C., Kato T. Mechanoresponsive luminescent molecular assemblies: An emerging class of materials. Adv. Mater. 2016;28:1073–1095. doi: 10.1002/adma.201502589. [DOI] [PubMed] [Google Scholar]
- 5.Reus C., Baumgartner T. Stimuli-responsive chromism in organophosphorus chemistry. Dalton Trans. 2016;45:1850–1855. doi: 10.1039/C5DT02758F. [DOI] [PubMed] [Google Scholar]
- 6.Wiggins K.M., Brantleya J.N., Bie-lawski C.W. Methods for activating and characterizing mechanically responsive polymers. Chem. Soc. Rev. 2013;42:7130–7147. doi: 10.1039/c3cs35493h. [DOI] [PubMed] [Google Scholar]
- 7.Zhao J., Chi Z.H., Zhang Y., Mao Z., Yang Z.Y., Ubba E., Chi Z.G. Recent progress in the mechanofluorochromism of cyanoethylene derivatives with aggregation-induced emission. J. Mater. Chem. C. 2018;6:6327–6353. doi: 10.1039/C8TC01648H. [DOI] [Google Scholar]
- 8.Zhang G.Q., Lu J.W., Sabat M., Fraser C.L. Polymorphism and reversible mechanochromic luminescence for solid-State difluoroboron avobenzone. J. Am. Chem. Soc. 2010;132:2160–2162. doi: 10.1021/ja9097719. [DOI] [PubMed] [Google Scholar]
- 9.Gundu S., Kim M., Mergu N., Son Y.A. AIE-active and reversible mechanochromic tetraphenylethene-tetradiphenylacrylonitrile hybrid luminogens with re-writable optical data storage application. Dyes Pigm. 2017;146:7–13. doi: 10.1016/j.dyepig.2017.06.043. [DOI] [Google Scholar]
- 10.Han J., Zang Y., Yu Z., He Y., Guo P. Synthesis, photochromism and morphology changes of a new asymmetric fluorescent quaterthiophene. Tetrahedron Lett. 2015;56:5213–5217. doi: 10.1016/j.tetlet.2015.07.067. [DOI] [Google Scholar]
- 11.Safin D.A., Bolte M., Garcia Y. Solid-state photochromism and thermochromism of N-salicylidene pyrene derivatives. CrystEngComm. 2014;16:8786–8793. doi: 10.1039/C4CE01325E. [DOI] [Google Scholar]
- 12.Yang X.D., Chen C., Zhang Y.J., Cai L.X., Zhang J. The effects of molecular configuration on the photochromism and decolorization of two bipyridinium-based coordination polymers. Inorg. Chem. Commun. 2015;60:122–125. doi: 10.1016/j.inoche.2015.07.022. [DOI] [Google Scholar]
- 13.Jadhav T., Dhokale B., Misra R. Effect of the cyano group on solid state photophysical behavior of tetraphenylethene substituted benzothiadiazoles. J. Mater. Chem. C. 2015;3:9063–9068. doi: 10.1039/C5TC01871D. [DOI] [Google Scholar]
- 14.Liu C., He W., Shi G., Luo H.Y., Zhang S., Chi Z.G. Synthesis and properties of a new class of aggregation-induced enhanced emission compounds: Intense blue light emitting triphenylethylene derivatives. Dyes Pigm. 2015;112:154–161. doi: 10.1016/j.dyepig.2014.07.002. [DOI] [Google Scholar]
- 15.Niu C.X., You Y., Zhao L., He D.C., Na N., Ouyang J. Solvatochromism, reversible chromism and self-assembly effects of heteroatom-assisted aggregation-induced enhanced emission (AIEE) compounds. Chem. Euro. J. 2015;21:13983–13990. doi: 10.1002/chem.201501902. [DOI] [PubMed] [Google Scholar]
- 16.Lee M.H., Lee H., Chang M.J., Kim H.S., Kang C., Kim J.S. A fluorescent probe for the Fe3+ ion pool in endoplasmic reticulum in liver cells. Dyes Pigm. 2016;130:245–250. doi: 10.1016/j.dyepig.2016.03.032. [DOI] [Google Scholar]
- 17.Davis D.A., Hamilton A., Yang J., Cremar L.D., Van Gough D., Potisek S.L., Ong M.T., Braun P.V., Martínez T.J., White S.R., et al. Force-induced activation of covalent bonds in mechanoresponsive polymeric materials. Nature. 2009;459:68–72. doi: 10.1038/nature07970. [DOI] [PubMed] [Google Scholar]
- 18.Sagara Y., Kato T. Mechanically induced luminescence changes in molecular assemblies. Nat. Chem. 2009;1:605–610. doi: 10.1038/nchem.411. [DOI] [PubMed] [Google Scholar]
- 19.Kundu P.K., Olsen G.L., Kiss V., Klajn R. Nanoporous frameworks exhibiting multiple stimuli responsiveness. Nat. Commun. 2014;5:3588–3590. doi: 10.1038/ncomms4588. [DOI] [PubMed] [Google Scholar]
- 20.Sun H., Liu S., Lin W., Zhang K.Y., Lv W., Huang X., Huo F., Yang H., Jenkins G., Zhao Q., et al. Smart responsive phosphorescent materials for data recording and security protection. Nat. Commun. 2014;5 doi: 10.1038/ncomms4601. [DOI] [PubMed] [Google Scholar]
- 21.Yuan W.Z., Tan Y.Q., Gong Y.Y., Lu P., Lam J.W.Y., Shen X.Y., Feng C.F., Sung H.H.Y., Lu Y.W., Williams I.D., et al. Synergy between twisted conformation and effective intermolecular interactions: Strategy for efficient mechanochromic luminogens with high contrast. Adv. Mater. 2013;25:2837–2843. doi: 10.1002/adma.201205043. [DOI] [PubMed] [Google Scholar]
- 22.Gong Y.Y., Tan Y.Q., Liu J., Lu P., Feng C.F., Yuan W.Z., Lu Y.W., Sun J.Z., He G.F., Zhang Y.M. Twisted D-π-A solid emitters: Efficient emission and high contrast mechanochromism. Chem. Commun. 2013;49:4009–4011. doi: 10.1039/c3cc39243k. [DOI] [PubMed] [Google Scholar]
- 23.Feng C., Wang K., Xu Y., Liu L., Zou B., Lu P. Unique piezochromic fluorescence behavior of organic crystal of carbazole-substituted CNDSB. Chem. Commun. 2016;52:3836–3839. doi: 10.1039/C5CC09152G. [DOI] [PubMed] [Google Scholar]
- 24.Sagara Y., Kubo K., Nakamura T., Tamaoki N., Weder C. Temperature-dependent mechanochromic behavior of mechanoresponsive luminescent compounds. Chem. Mater. 2017;29:1273–1278. doi: 10.1021/acs.chemmater.6b04720. [DOI] [Google Scholar]
- 25.Zhang Y.J., Sun J.W., Lv X.J., Ouyang M., Cao F., Pan G.X., Pan L.P., Fan G.B., Yu W.W., He C., et al. Heating and mechanical force-induced “turn on” fluorescence of cyanostilbene derivative with H-type stacking. Cryst. Eng. Comm. 2013;15:8998–9002. doi: 10.1039/c3ce41221k. [DOI] [Google Scholar]
- 26.Shen Y.B., Chen P., Liu J.X., Ding J.P., Xue P.C. Effects of electron donor on luminescence and mechanochromism of D-π-A benzothiazole derivatives. Dyes Pigm. 2018;150:354–362. doi: 10.1016/j.dyepig.2017.12.034. [DOI] [Google Scholar]
- 27.Ito S., Taguchi T., Yamada T., Ubukata T., Yamaguchi Y., Asami M. Indolylbenzothiadiazoles with varying substituents on the indole ring: A systematic study on the self-recovering mechanochromic luminescence. RSC Adv. 2017;7:16953–16962. doi: 10.1039/C7RA01006K. [DOI] [Google Scholar]
- 28.Sun H.W., Zhang Y., Yan W., Chen W.X., Lan Q., Liu S.W., Jiang L., Chi Z.G., Chen X.D., Xu J.R. A novel ultrasound-sensitive mechanofluorochromic AIE-compound with remarkable blue-shifting and enhanced emission. J. Mater. Chem. C. 2014;2:5812–5817. doi: 10.1039/C4TC00553H. [DOI] [Google Scholar]
- 29.Zhang Y.Y., Li H.F., Zhang G.B., Xu X.Y., Kong L., Tao X.T., Tian Y.P., Yang J.X. Aggregation-induced emission enhancement and mechanofluorochromic properties of α-cyanostilbene functionalized tetraphenyl imidazole derivatives. J. Mater. Chem. C. 2016;4:2971–2978. doi: 10.1039/C5TC03348A. [DOI] [Google Scholar]
- 30.Ma C.P., Zhang X.Q., Yang Y., Ma Z.Y., Tang L.T., Wu Y.J., Liu H.L., Rui X.J., Wei Y. Halogen effect on mechanofluorochromic properties of alkyl phenothiazinyl phenylacrylonitrile derivatives. Dyes Pigm. 2016;129:141–148. doi: 10.1016/j.dyepig.2016.02.028. [DOI] [Google Scholar]
- 31.Zhang X.Q., Ma Z.Y., Liu M.Y., Zhang X.Y., Jia X.R., Wei Y. A new organic far-red mechanofluorochromic compound derived from cyano-substituted diarylethene. Tetrahedron. 2013;69:10552–10557. doi: 10.1016/j.tet.2013.10.066. [DOI] [Google Scholar]
- 32.Luo J.D., Xie Z.L., Lam J.W.Y., Cheng L., Chen H.Y., Qiu C.F., Kwok H., Zhan X.W., Liu Y.Q., Tang B.Z. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001;21:1740–1741. doi: 10.1039/b105159h. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y.H., Xu D.F., Gao H.Z., Wang Y., Liu X.L., Han A.X., Zhang C., Zhang L. Twisted donor-acceptor cruciform luminophores possessing substituent-dependent properties of aggregation-induced emission and mechanofluorochromism. J. Phys. Chem. C. 2018;122:2297–2306. doi: 10.1021/acs.jpcc.7b11368. [DOI] [Google Scholar]
- 34.Ekbote A., Jadhav T., Misra R. T-shaped donor-acceptor-donor type tetraphenylethylene substituted quinoxaline derivatives: Aggregation-induced emission and mechanochromism. New J. Chem. 2017;41:9346–9353. doi: 10.1039/C7NJ01531C. [DOI] [Google Scholar]
- 35.Jiang Y., Gindre D., Allain M., Liu P., Cabanetos C., Roncali J. A mechanofluorochromic push-pull small molecule with aggregation-controlled linear and nonlinear optical properties. Adv. Mater. 2015;27:4285–4289. doi: 10.1002/adma.201501697. [DOI] [PubMed] [Google Scholar]
- 36.Zhao F., Chen Z., Liu G., Fan C.B., Pu S.Z. Tetraphenylethene-based highly emissive fluorescent molecules with aggregation-induced emission (AIE) and various mechanofluorochromic characteristics. Tetrahedron Lett. 2018;59:836–840. doi: 10.1016/j.tetlet.2018.01.051. [DOI] [Google Scholar]
- 37.Ohtani S., Gon M., Tanaka K., Chujo Y. A flexible, fused, azomethine-Boron complex: Thermochromic luminescence and thermosalient behavior in structural transitions between crystalline polymorphs. Chem. Eur. J. 2017;23:11827–11833. doi: 10.1002/chem.201702309. [DOI] [PubMed] [Google Scholar]
- 38.Xue P.C., Yang Z.C., Chen P. Hiding and revealing information using the mechanochromic system of a 2,5-dicarbazolesubstituted terephthalate derivative. J. Mater. Chem. C. 2018;6:4994–5000. doi: 10.1039/C8TC01379A. [DOI] [Google Scholar]
- 39.Hemetsberger H., Knittel D. Synthese und Thermolyse von α-Azidoacrylestern. Monatsh. Chem. 1972;103:194–204. doi: 10.1007/BF00912944. [DOI] [Google Scholar]
- 40.Janiga A., Glodkowska-Mrowka E., Stokłosa T., Gryko D.T. Synthesis and optical properties of tetraaryl-1,4-dihydropyrrolo[3,2-b]pyrroles. Asian J. Org. Chem. 2013;2:411–415. doi: 10.1002/ajoc.201200201. [DOI] [Google Scholar]
- 41.Janiga A., Gryko D.T. 1,4-Dihydropyrrolo[3,2-b]pyrrole and its π-expanded analogues. Chem. Asian J. 2014;9:3036–3045. doi: 10.1002/asia.201402367. [DOI] [PubMed] [Google Scholar]
- 42.Sadowski B., Hassanein K., Ventura B., Gryko D.T. Tetraphenylethylenepyrrolo[3,2-b]pyrrole hybrids as solid-state emitters: The role of substitution pattern. Org. Lett. 2018;20:3183–3186. doi: 10.1021/acs.orglett.8b01011. [DOI] [PubMed] [Google Scholar]
- 43.Peng Z., Ji Y.C., Huang Z.H., Huang B., Shi J.B., Dong Y.P. A strategy for the molecular design of aggregation-induced emission units further modified by substituents. Mater. Chem. Front. 2018;2:1175–1183. doi: 10.1039/C8QM00096D. [DOI] [Google Scholar]
- 44.Chan C.Y.K., Lam J.W.Y., Zhao Z.J., Chen S.M., Lu P., Sung H.H.Y. Aggregation-induced emission, mechanochromism and blue electroluminescence of carbazole and triphenylamine-substituted ethenes. J. Mater. Chem. C. 2014;2:4320–4327. doi: 10.1039/C4TC00097H. [DOI] [Google Scholar]
- 45.Jadhav T., Choi J.M., Lee J.Y., Dhokale B., Misra R. Non-doped blue organic light emitting devices based on tetraphenylethylene-π-imidazole derivatives. Org. Electron. 2016;37:448–452. doi: 10.1016/j.orgel.2016.07.009. [DOI] [Google Scholar]
- 46.Jadhav T., Dhokale B., Patil Y., Mobin S.M., Misra R. Multi-stimuli responsive donor-acceptor tetraphenylethylene substituted benzothiadiazoles. J. Phys. Chem. C. 2016;120:24030–24040. doi: 10.1021/acs.jpcc.6b09015. [DOI] [Google Scholar]
- 47.Zhang T.F., Zhang R., Zhao Y., Ni Z.H. A new series of N-substituted tetraphenylethene-based benzimidazoles: Aggregation-induced emission, fast-reversible mechanochromism and blue electroluminescence. Dyes Pigm. 2018;148:276–285. doi: 10.1016/j.dyepig.2017.09.018. [DOI] [Google Scholar]
- 48.Ramachandran E., Dhamodharan R. Tetrakis(trialkylsilylethynylphenyl)ethenes: Mechanofluorochromism arising from steric considerations with an unusual crystal structure. J. Mater. Chem. C. 2017;5:10469–10476. doi: 10.1039/C7TC03211K. [DOI] [Google Scholar]
- 49.Jadhav T., Choi J.M., Shinde J., Lee J.Y., Misra R. Mechanochromism and electroluminescence in positional isomers of tetraphenylethylene substituted phenanthroimidazoles. J. Mater. Chem. C. 2017;5:6014–6020. doi: 10.1039/C7TC00950J. [DOI] [Google Scholar]
- 50.Jadhav T., Dhokale B., Mobin S.M., Misra R. Mechanochromism and aggregation induced emission in benzothiazole substituted tetraphenylethylenes: A structure function correlation. RSC Adv. 2015;5:29878–29884. doi: 10.1039/C5RA04881H. [DOI] [Google Scholar]
- 51.Yan X.Z., Wang M., Cook T.R., Zhang M.M., Saha M.L., Zhou Z.X., Li X.P., Huang F.H., Stang P.J. Light-emitting superstructures with anion effect: Coordination drivenself-assembly of pure tetraphenylethylene metallacycles and metallacages. J. Am. Chem. Soc. 2016;138:4580–4588. doi: 10.1021/jacs.6b00846. [DOI] [PubMed] [Google Scholar]
- 52.Han T., Deng H.Q., Qiu Z.J., Zhao Z., Zhang H.K., Zou H., Leung N.L.C., Shan G.G., Elsegood M.R.J., Lam J.W.Y., Tang B.Z. Facile multicomponent polymerizations toward unconventional luminescent polymers with readily openable small heterocycles. J. Am. Chem. Soc. 2018;140:5588–5598. doi: 10.1021/jacs.8b01991. [DOI] [PubMed] [Google Scholar]
- 53.Mehar N., Panda S., Kumar S., Lyer P.K. Aldehyde group driven aggregation-induced enhanced emission in naphthalimides and its application for ultradetection of hydrazine on multiple platforms. Chem. Sci. 2018;9:3978–3985. doi: 10.1039/C8SC00643A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang C., Feng H., Huang Y.Y., Qian Z.S. Reversible luminescent nanoswitches based on aggregation-induced emission enhancement of silver nanoclusters for luminescence turn-on assay of inorganic pyrophosphatase activity. Anal. Chem. 2017;89:4994–5002. doi: 10.1021/acs.analchem.7b00319. [DOI] [PubMed] [Google Scholar]
- 55.Dou C.D., Han L., Zhao S.S., Zhang H.Y., Wang Y. Multi-stimuli-responsive fluorescence switching of a donor-acceptor π-conjugated compound. J. Phys. Chem. Lett. 2011;2:666–670. doi: 10.1021/jz200140c. [DOI] [Google Scholar]
- 56.Wang Y., Zhang T., Yu B.H., Fang X.F., Su X., Zhang Y.M., Zhang T., Yang B., Li M.J., Zhang S.X.A. Full-color tunable mechanofluorochromism and excitation-dependent emissions of single-arm extended tetraphenylethylenes. J. Mater. Chem. C. 2015;3:12328–12334. doi: 10.1039/C5TC02623G. [DOI] [Google Scholar]
- 57.Liu L.Y., Wen T., Fu W.Q., Liu M., Chen S.M., Zhang J. Structure-dependent mechanochromism of two Ag (I) imidazolate chains. CrystEngComm. 2016;18:218–221. doi: 10.1039/C5CE01852H. [DOI] [Google Scholar]
- 58.Sudhakar P., Neenar K.K., Thilagar P. H-Bond assisted mechanoluminescence of borylated aryl amines: Tunable emission and polymorphism. J. Mater. Chem. C. 2017;5:6537–6546. doi: 10.1039/C7TC01676J. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.