Abstract
The effect of early rehabilitation on the outcome of patients with chronic obstructive pulmonary disease (COPD) and acute respiratory failure (ARF) in intensive care units (ICUs) remains unclear. We examined the effect of early rehabilitation on the outcomes of COPD patients requiring mechanical ventilation (MV) in the ICU. This retrospective, observational, case–control study was conducted in a medical center with a 19-bed ICU. The records of all 105 ICU patients with COPD and ARF who required MV from January to December 2011 were examined. The outcomes (MV duration, rates of successful weaning and survival, lengths of ICU and hospital stays, and medical costs) were recorded and analyzed. During the study period, 35 patients with COPD underwent early rehabilitation in the ICU and 70 demographically and clinically matched patients with similar COPD stage, cause of intubation, type of respiratory failure, and levels of disease severity who had not undergone early rehabilitation in the ICU were selected as comparative controls. Multiple regression analysis showed that early rehabilitation was significantly negatively associated with MV duration. Early rehabilitation for COPD patients in the ICU with ARF shortened the duration of their MV.
Keywords: Early rehabilitation, mechanical ventilation, intensive care unit, chronic obstructive pulmonary disease
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by progressive airway limitation, which can cause dyspnea, chronic cough, sputum production, wheezing, fatigue, and hypoxemia. Acute exacerbation, a common complication of COPD, sometimes requires mechanical ventilation (MV) in an intensive care unit (ICU). Pulmonary rehabilitation as a nonpharmacological therapy has recently been added to the standard of care for COPD patients1,2 because accumulating evidence supports its effectiveness.3–5 Several studies1–3,5–7 have reported that pulmonary rehabilitation therapy reduced dyspnea, improved exercise capacity, improved quality of life (QoL), and reduced the lengths of hospital stays. Even for patients with a recent exacerbation of COPD,4,8–10 pulmonary rehabilitation has been proved an effective and safe intervention that reduced hospital admissions and mortality and improved health-related quality of life (HRQoL). All these studies focus on patients with the most severe form of COPD.8–11 We previously reported12 that the early mobilization of MV patients in the ICU shortened MV durations and ICU stays. However, early pulmonary rehabilitation for patients with acute exacerbations COPD, which causes respiratory failure and requires invasive MV in the ICU, is rare. Therefore, we evaluated the effects of this early rehabilitation on the outcomes of COPD patients with acute respiratory failure (ARF) that required MV in the ICU. We collected data on MV duration, rates of successful weaning and survival, lengths of ICU and hospital stays, and medical costs as outcome measurements and analyzed them.
Methods
Setting and patients
The study was done in a medical center with a 19-bed medical ICU. We retrospectively reviewed the medical records of all COPD patients who required an invasive MV using an endotracheal tube for 48 hours and who had been prepared for a scheduled extubation between January 1 and December 31, 2011. Each patient was assessed for their readiness for extubation using the following criteria: hemodynamic stability and recovery from the precipitating illness; and respiratory criteria. Respiratory therapists evaluated patients daily using these criteria for weaning. The intensivists in charge decided whether to extubate.
A multidisciplinary team—a critical care nurse, a nursing assistant, a respiratory therapist, and a physical therapist—was set up to initiate the rehabilitation program within 72 hours of MV in hemodynamically and respiratory stable patients, that is, they did not need a vasopressor, or else they had been using <60% FiO2 since early 2011. The pulmonary rehabilitation therapy component included dyspnea-relieving strategies, muscle training for upper and lower extremities and inspiratory muscles, and sputum clearance techniques. The rehabilitation protocol included two stages: stage I: passive extremity movement for unconscious patients; and stage II: active extremity movement and interaction with the physical therapist for conscious patients who were able to respond to simple commands in a sitting position on the bed. Physiotherapy was provided twice daily 5 days per week. During the study period, rehabilitation was at the discretion of an attending physician, and rehabilitation was initiated only after physicians had consulted the physical therapist. Therefore, the patients who had not been treated with invasive MV using an endotracheal tube for 48 hours did not undergo additional physiotherapy.
Data collection
Demographic and clinical information of all included patients was collected. The COPD stage was classified using the outcome of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) (http://goldcopd.org/) of each patient’s pulmonary function test 3 months before this admission. Outcomes—duration of MV, lengths of ICU and hospital stays, and medical costs—were recorded. The data were retrospectively collected and then analyzed; therefore, informed consent was specifically waived and the study was approved by the Institutional Review Board of Chi Mei Medical Center (IRB #: 10603-009).
Definitions of causes of respiratory failure and successful extubation
Causes of respiratory failure were classified as the pulmonary system (upper airway obstruction, acute respiratory distress syndrome, COPD, pneumonia, malignant effusion, lobar collapse, asthma attack), cardiovascular system (congestive heart failure, pericarditis, cardiomyopathy, acute myocardial infarction, endocarditis), neurological system (status epilepticus, stroke), renal system (acute renal failure), gastrointestinal system, and others, as previously described.12 Successful extubation was defined as not reintubated within 48 hours after extubation.
Statistical analysis
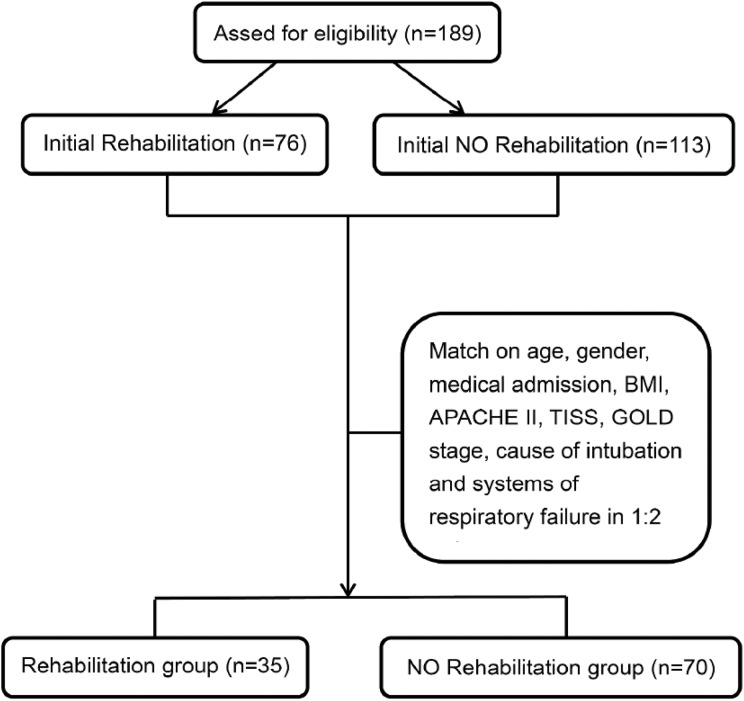
The medical records of 189 COPD patients with respiratory failure were initially reviewed. They were assigned to one of two groups: patients who had undergone rehabilitation therapy (n = 76) and patients who had not (n = 113) (Figure 1). To decrease selection bias, we matched age, gender, medical admission; body mass index (BMI), Acute Physiology and Chronic Health Evaluation (APACHE) II, Therapeutic Intervention Scoring System (TISS), and GOLD stage scores; cause of intubation and systems of respiratory failure in a 1:2 ratio (35 rehabilitation patients and 70 nonrehabilitation patients). Descriptive and inferential statistics were then analyzed and presented as mean ± standard deviation (SD) for continuous variables and as frequencies (%) for categorical variables for the two groups. The differences in demographic, clinical, and laboratory characteristics between the groups were examined using independent samples t tests. The associations between the variables and patient characteristics were analyzed using chi-square or Fisher exact test. Finally, to examine the association between the rehabilitation therapy and clinical outcomes (MV, lengths of ICU and hospital stays, and medical costs), we used multiple regression analysis and adjusted for the confounding effects of demographic, clinical, and laboratory variables significantly related to outcomes. SPSS 19.0 for Windows was used for all statistical analyses.
Figure 1.
Study algorithm.
Results
Demographic characteristics of patients
We matched—based on age, gender, medical admission, BMI, APACHE II, TISS, GOLD stage, cause of intubation, and systems of respiratory failure (Table 1)—35 patients who had undergone early rehabilitation with 70 controls who had not. Most patients (101 (96.2%)) came from Internal Medicine; mean BMI was 22.1 ± 5.3 kg/m2; mean APACHE II scores were 19.2 ± 6.2; GOLD stages were: 1 (n = 3 (2.9%)), 2 (n = 24 (22.9%)), 3 (n = 50 (47.6%)), and 4 (n = 28 (26.7%)). The mean number of comorbidities was 0.9 ± 0.8. Diabetes was the most common, cancer was the second, and coronary artery disease was the third. The largest number of patients (n = 51 (48.6%)) was postoperatively put on MV (intubated), and the second largest was intubated because of pneumonia (n = 28 (26.7%)). The respiratory system was the most common cause of respiratory failure, and the cardiovascular system was the second most common. The mean MV duration was 152.5 ± 129.3 hours, and the mean lengths of stay were 8.1 ± 7.8 days in the ICU and 22.9 ± 21.5 days in the hospital.
Table 1.
Baseline characteristics of planned extubation in COPD patients with respiratory failure.a
| Groups: Variables | Total (n = 105) | Rehabilitation (n = 35) | No rehabilitation (n = 70) | p |
|---|---|---|---|---|
| Age (years) | 74.8 ± 0.6 | 74.9 ± 10.5 | 74.7 ± 10.7 | 0.912 |
| Age ≥ 65 years | 88 (83.8) | 30 (85.7) | 58 (82.9) | 0.708 |
| Male | 81 (77.01) | 28 (80.0) | 53 (75.7) | 0.622 |
| Medical admission | 101 (96.2) | 34 (97.1) | 67 (95.7) | 1.000 |
| BMI | 221 ± 5.3 | 21.4 ± 4.5 | 22.4 ± 5.6 | 0.325 |
| APACHE II | 19.2 ± 6.2 | 17.6 ± 5.6 | 20.0 ± 6.4 | 0.061 |
| TISS scale | 23.9 ± 6.5 | 23.1 ± 6.6 | 24.4 ± 6.4 | 0.361 |
| COPD stage | ||||
| GOLD 1 | 3 (2.9) | 1 (2.9) | 2 (2.9) | 1.000 |
| GOLD 2 | 24 (22.9) | 7 (20.0) | 17 (24.3) | 0.622 |
| GOLD 3 | 50 (47.6) | 17 (48.6) | 33 (47.1) | 0.890 |
| GOLD 4 | 28 (26.7) | 10 (28.6) | 18 (25.7) | 0.755 |
| Comorbidity | 0.9 ± 0.8 | 1.3 ± 0.9 | 0.8 ± 0.7 | 0.002 |
| Coronary artery disease | 24 (22.9) | 11 (31.4) | 13 (18.6) | 0.139 |
| Uremia | 2 (1.9) | 0 (0) | 2 (2.9) | 0.551 |
| Liver cirrhosis | 2 (1.9) | 0 (0) | 2 (2.9) | 0.551 |
| Diabetes | 26 (24.8) | 14 (40.0) | 12 (17.1) | 0.011 |
| Stroke | 19 (18.1) | 8 (22.9) | 11 (15.7) | 0.370 |
| Cancer | 24 (22.9) | 11 (31.4) | 13 (18.6) | 0.139 |
| Cause of intubation | ||||
| Hypoventilation | 3 (2.9) | 1 (2.9) | 2 (2.9) | 1.000 |
| Upper airway obstruction | 5 (4.8) | 2 (5.7) | 3 (4.3) | 1.000 |
| Pneumonia | 28 (26.7) | 7 (20.0) | 21 (30.0) | 0.275 |
| Cardiogenic edema | 7 (6.7) | 2 (5.7) | 5 (7.1) | 1.000 |
| Extrapulmonary shock | 11 (10.5) | 4 (11.4) | 7 (10.0) | 1.000 |
| Postoperative | 51 (48.6) | 19 (54.3) | 32 (45.7) | 0.407 |
| Systems of respiratory failure | ||||
| Pulmonary | 81 (77.1) | 25 (71.4) | 56 (80.0) | 0.324 |
| Cardiovascular | 9 (8.6) | 4 (11.4) | 5 (7.1) | 0.477 |
| Neurogenic | 3 (2.9) | 2 (5.7) | 1 (1.4) | 0.257 |
| Renal | 4 (3.8) | 1 (2.9) | 3 (4.3) | 1.000 |
| Gastrointestinal | 4 (3.8) | 2 (5.7) | 2 (2.9) | 0.599 |
| Othersb | 4 (3.8) | 1 (2.9) | 3 (4.3) | 1.000 |
COPD: chronic obstructive pulmonary disease; GOLD: Global Initiative for Chronic Obstructive Lung Disease; BMI: body mass index; APACHE: Acute Physiology and Chronic Health Evaluation; TISS: Therapeutic Intervention Scoring System.
a Continuous variables: mean ± standard deviation; nominal variables: number (%); COPD stage using the GOLD (http://goldcopd.org/) classification from each patient’s pulmonary function test 3 months before this admission
b Bone fracture, oral surgery, and nasopharyngeal surgery.
Patients who had undergone early rehabilitation had more comorbidities (1.3 ± 0.9 vs. 0.8 ± 0.7, p = 0.002) and a higher mean hemoglobin level (12.0 ± 2.2 vs. 11.0 ± 2.0, p = 0.018) than did patients who had not undergone rehabilitation therapy (Tables 1 and 2). Most of their laboratory findings and respiratory parameters were not significantly different.
Table 2.
Vital signs and laboratory data before and after planned extubation in COPD patients with respiratory failure.
| Groups: Variables | Total (n = 105) | Rehabilitation (n = 35) | No rehabilitation (n = 70) | p |
|---|---|---|---|---|
| Hemoglobin (g/dL) | 11.3 ± 2.1 | 12.0 ± 2.2 | 11.0 ± 2.0 | 0.018 |
| Hematocrit (%) | 35.1 ± 7.8 | 37.4 ± 7.3 | 33.9 ± 7.8 | 0.029 |
| Blood urea nitrogen (mg/dL) | 28.3 ± 26.9 | 31.2 ± 36.1 | 26.9 ± 21.1 | 0.450 |
| Creatinine (mg/dL) | 1.8 ± 1.9 | 1.8 ± 1.9 | 1.8 ± 2.0 | 0.881 |
| Sodium (mmol/L) | 137.5 ± 7.6 | 137.4 ± 7.5 | 137.6 ± 7.7 | 0.913 |
| Potassium (mmol/L) | 3.9 ± 0.5 | 3.9 ± 0.6 | 3.9 ± 0.5 | 0.788 |
| Calcium (mg/dL) | 7.9 ± 0.7 | 8.0 ± 0.5 | 7.9 ± 0.8 | 0.447 |
| Phosphate (mg/dL) | 3.51 ± 0.87 | 3.6 ± 0.8 | 3.5 ± 0.9 | 0.569 |
| Albumin (g/dL) | 2.72 ± 0.42 | 2.83 ± 0.49 | 2.7 ± 0.37 | 0.058 |
| Heart rate change | −0.1 ± 0.3 | −0.1 ± 0.3 | −0.1 ± 0.2 | 0.432 |
| Before extubation | 110.0 ± 24.0 | 107.6 ± 25.0 | 111.2 ± 23.6 | 0.477 |
| After extubation | 101.8 ± 21.7 | 97.7 ± 21.7 | 103.9 ± 21.5 | 0.167 |
| Mean artery pressure change (%) | −0.2 ± 0.3 | −0.2 ± 0.3 | –0.2 ± 0.3 | 0.739 |
| Before extubation | 103.1 ± 23.4 | 106.5 ± 21.8 | 101.4 ± 24.2 | 0.292 |
| After extubation | 88.3 ± 17.5 | 92.6 ± 17.2 | 86.2 ± 17.3 | 0.075 |
| Respiratory rate change (%) | −0.4 ± 0.5 | −0.3 ± 0.6 | −0.4 ± 0.5 | 0.745 |
| Before extubation | 24.6 ± 6.5 | 23.5 ± 5.7 | 25.2 ± 6.8 | 0.196 |
| After extubation | 19.4 ± 5.7 | 19.0 ± 5.0 | 19.5 ± 6.0 | 0.664 |
| PH change (%) | 0.01 ± 0.02 | 0.01 ± 0.01 | 0.01 ± 0.02 | 0.066 |
| Before extubation | 7.29 ± 0.14 | 7.31 ± 0.08 | 7.29 ± 0.16 | 0.285 |
| After extubation | 7.38 ± 0.09 | 7.37 ± 0.07 | 7.39 ± 0.10 | 0.530 |
| PaCO2 change (%) | −0.4 ± 0.6 | –0.2 ± 0.3 | –0.4 ± 0.7 | 0.119 |
| Before extubation | 64.1 ± 26.7 | 61.0 ± 20.0 | 65.7 ± 29.5 | 0.396 |
| After extubation | 47.8 ± 13.2 | 50.0 ± 13.4 | 46.7 ± 13.1 | 0.233 |
| PaO2 change (%) | −0.2 ± 1.0 | –0.3 ± 0.9 | –0.2 ± 1.1 | 0.679 |
| Before extubation | 121.2 ± 76.2 | 127.4 ± 66.2 | 118.1 ± 81.0 | 0.556 |
| After extubation | 128.7 ± 83.5 | 131.4 ± 94.0 | 127.4 ± 78.4 | 0.817 |
| PaO2/FiO2 change (%) | −0.2 ± 1.1 | −0.2 ± 0.7 | −0.3 ± 1.2 | 0.699 |
| Before extubation | 244.7 ± 73.5 | 265.2 ± 62.8 | 235.9 ± 76.9 | 0.054 |
| After extubation | 260.5 ± 125.8 | 267.5 ± 101.4 | 256.9 ± 136.9 | 0.687 |
COPD: chronic obstructive pulmonary disease.
a Continuous variables: mean ± standard deviation; nominal variables: number (%).
Outcome analyses
Outcome analyses showed that the early rehabilitation group had a higher survival rate and a higher successful extubation rate, a shorter MV duration, shorter ICU and hospital stays, and lower medical costs (Table 3). Moreover, a multiple regression model controlled for age, sex, APACHE II and TISS, COPD stage, hemoglobin and albumin levels, comorbidities, and pulmonary system-induced respiratory failure showed that early mobilization was significantly negatively associated with MV duration, but not significantly associated with lengths of ICU and hospital stays, or with medical costs (Table 4).
Table 3.
Clinical outcomes of the groups.a
| Groups: Variables | Total (n = 105) | Rehabilitation (n = 35) | No rehabilitation (n = 70) | p |
|---|---|---|---|---|
| 28-Day survival | 97 (92.4) | 33 (94.3) | 64 (91.4) | 0.716 |
| Survival at discharge | 90 (85.7) | 31 (88.6) | 59 (84.3) | 0.554 |
| Successful extubation | 93 (88.6) | 33 (94.3) | 60 (85.7) | 0.329 |
| MV duration (hours) | 152.5 ± 129.3 | 137.3 ± 136.9 | 160.1 ± 125.7 | 0.396 |
| ICU stays (days) | 8.1 ± 7.8 | 5.8 ± 6.1 | 9.2 ± 8.3 | 0.033 |
| Hospital stays (days) | 22.9 ± 21.5 | 17.9 ± 14.6 | 25.4 ± 24.0 | 0.095 |
| Medical costs (×NT$10,000) | 20.3 ± 19.7 | 15.2 ± 13.6 | 22.9 ± 21.7 | 0.058 |
COPD: chronic obstructive pulmonary disease; MV: mechanical ventilation; ICU: intensive care unit; NT$: New Taiwan dollars (US$1 = NT$30).
a Continuous variables: mean ± standard deviation; nominal variables: number (%).
Table 4.
Multiple regression model: the predictors of MV, ICU stays, hospital stays, and medical costs after planned extubation in COPD patients with respiratory failure.
| Variables | Mechanical ventilation (hours) | ICU stays (days) | Hospital stays (days) | Medical costs (×NT$10,000) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | t | p | 95.0% CI | β | SE | t | p | 95.0% CI | β | SE | t | p | 95.0% CI | β | SE | t | p | 95.0% CI | |||||
| Rehabilitation therapy | −.188 | 24.327 | 2.115 | 0.037 | −99.754 | −3.137 | −0.001 | 1.361 | −0.008 | 0.994 | −2.713 | 2.693 | −0.058 | 4.622 | −0.568 | 0.572 | −11.802 | 6.554 | −0.041 | 3.962 | −0.429 | 0.669 | −9.568 | 6.169 |
| Age | −0.027 | 1.066 | −0.308 | 0.759 | −2.446 | 1.789 | 0.090 | 0.060 | 1.110 | 0.270 | −0.052 | 0.185 | −.039 | 0.203 | −.388 | 0.699 | −.481 | 0.324 | 0.079 | 0.174 | 0.840 | 0.403 | −.199 | 0.491 |
| Gender | −0.088 | 25.401 | −1.064 | 0.290 | −77.465 | 23.417 | −0.033 | 1.421 | −0.422 | 0.674 | −3.422 | 2.222 | 0.038 | 4.826 | 0.397 | 0.692 | −7.668 | 11.498 | 0.052 | 4.137 | 0.582 | 0.562 | −5.808 | 10.623 |
| APACHE II Score | −0.117 | 2.360 | −1.035 | 0.304 | −7.129 | 2.245 | 0.128 | 0.132 | 1.222 | 0.225 | −0.101 | 0.424 | 0.110 | 0.448 | 0.854 | 0.395 | −.507 | 1.273 | 0.132 | 0.384 | 1.092 | 0.277 | −.343 | 1.183 |
| TISS | 0.062 | 1.868 | 0.668 | 0.506 | −2.461 | 4.959 | −0.005 | 0.105 | −0.058 | 0.954 | −0.214 | 0.202 | 0.103 | 0.355 | 0.967 | 0.336 | −0.362 | 1.048 | 0.137 | 0.304 | 1.372 | 0.174 | −.187 | 1.022 |
| Hemoglobin | −0.300 | 5.706 | −3.188 | 0.002 | −29.522 | −6.859 | −0.156 | 0.319 | −1.781 | 0.078 | −1.203 | 0.065 | −.140 | 1.084 | −1.307 | 0.194 | −3.570 | 0.736 | −.146 | 0.929 | −1.454 | 0.149 | −3.197 | 0.494 |
| Albumin | −0.089 | 26.254 | −1.047 | 0.298 | −79.629 | 24.640 | −0.033 | 1.469 | −0.411 | 0.682 | −3.521 | 2.313 | −0.026 | 4.988 | −0.266 | 0.791 | −11.232 | 8.578 | −.028 | 4.276 | −.310 | 0.757 | −9.818 | 7.165 |
| Comorbidity | −.198 | 14.089 | −2.294 | 0.024 | −60.307 | −4.349 | −0.076 | 0.788 | −.951 | 0.344 | −2.315 | 0.816 | 0.074 | 2.677 | 0.750 | 0.455 | −3.309 | 7.323 | −.016 | 2.295 | −.175 | 0.861 | −4.959 | 4.155 |
| Pulmonary cause of respiratory failure | 0.277 | 25.561 | 3.319 | 0.001 | 34.081 | 135.598 | 0.110 | 1.430 | 1.413 | 0.161 | −0.819 | 4.861 | 0.220 | 4.856 | 2.308 | 0.023 | 1.566 | 20.853 | 0.113 | 4.163 | 1.262 | 0.210 | −3.015 | 13.520 |
MV: mechanical ventilation; ICU: intensive care unit; COPD: chronic obstructive pulmonary disease; APACHE: Acute Physiology and Chronic Health Evaluation.
Discussion
In this 1-year study, we examined the effect of early rehabilitation, with which COPD patients with ARF were treated in a medical ICU within the first 3 days of MV. We found that the duration of MV was significantly shorter in patients treated with early rehabilitation than in matched controls who were not. In one randomized controlled study,8 airflow obstruction and dyspnea were attenuated and exercise capacity increased in patients who had been admitted with exacerbated COPD and had undergone early rehabilitation therapy compared with patients who had not undergone early rehabilitation therapy. Puhan et al.10 reported that rehabilitation therapy had moderate-to-large positive effects on HRQoL and exercise capacity in patients with ARF. Therefore, our findings are consistent with those of other studies3,8,10 and indicate that early rehabilitation for COPD patients with ARF is beneficial. However, our findings are different from one recent RCT,11 which reported that early rehabilitation during hospital admission for ARF did not reduce the risk of readmission or improve patients’ recovery of physical function. These differences between our study and this trial might be because of different patients, methods, and outcome measurements. All our patients had ARF, required MV, and underwent early rehabilitation in the ICU, and we focused on short-term outcomes such as MV duration and length of hospital stay. In the cited RCT,11 not all were COPD patients, the setting was not an ICU, and the outcomes of readmission and physical performance were measured, not the outcomes of early rehabilitation. Thus, our findings differ.
In summary, we found that early rehabilitation (within the first 3 days of MV) was beneficial for COPD patients with ARF and in a medical ICU. Although the early rehabilitation therapy programs in our study and others might differ in some details, this kind of intervention can result in better outcomes for COPD patients in the ICU. Most important, as we previously reported,12 early rehabilitation therapy should be encouraged for critically ill patients with COPD if the health-care facility can afford this treatment.
Several plausible mechanisms support these benefits of early rehabilitation. COPD comorbid with ARF might cause an ICU patient to be physical inactive, and severe physical inactivity reduces muscle protein synthesis, lean mass, and leg muscle strength.13–15 In addition, ARF is associated with increased systemic inflammation (higher levels of C-reactive protein, interleukin (IL)-6, IL-8, tumor necrosis factor-α, leptin, endothelin-1, and fibrinogen).16–19 Moreover, systemic inflammation contributes to muscle dysfunction.20 One randomized trial,21 however, reported that a quadriceps resistance training program increased isometric quadriceps force by 10% and that muscle biopsies confirmed the benefit of the training on the anabolic–catabolic balance. Two other studies22,23 also reported that rehabilitation therapy had a positive effect on quadriceps strength and exercise tolerance in patients with COPD comorbid with ARF.
Limitations
This study has some limitations. First, all findings are based on our experience in a single center; thus, they might not be generalizable to all ICUs. Second, we did not assess the safety or feasibility of early rehabilitation therapy, but patient medical records showed no specific adverse effects during or after the rehabilitation therapy. Third, we did not measure changes in physical function before and after rehabilitation therapy to provide more objective evidence to confirm our findings. Fourth, this study was retrospective and could neither be randomized nor blindly evaluated. Some patients did not undergo early rehabilitation because their in-charge physician was unaware of or unwilling to use (or both) the therapy. However, because most patient profiles were as matched as possible between the rehabilitation and no-rehabilitation groups, confounding factors should be minimal in the present study. Prospective clinical trials are required to confirm our findings. Finally, we assessed only the short-term effects in this study, and we did not check the prevalence of ICU-acquired weakness. Post-discharge studies of the long-term effects of early rehabilitation are warranted, especially on the prevalence and recovery from ICU-acquired weakness.
Conclusions
Early rehabilitation for patients in the ICU with COPD comorbid with ARF shortened the duration of their MV.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Chin-Ming Chen  https://orcid.org/0000-0001-6751-280X
https://orcid.org/0000-0001-6751-280X
References
- 1. Nici L, ZuWallack R. Chronic obstructive pulmonary disease-evolving concepts in treatment: advances in pulmonary rehabilitation. Semin Respir Crit Care Med 2015; 36: 567–574. [DOI] [PubMed] [Google Scholar]
- 2. Nici L, Lareau S, ZuWallack R. Pulmonary rehabilitation in the treatment of chronic obstructive pulmonary disease. Am Fam Phys 2010; 82: 655–660. [PubMed] [Google Scholar]
- 3. Burtin C, Langer D, van Remoortel H, et al. Physical activity counselling during pulmonary rehabilitation in patients with COPD: a randomised controlled trial. PLoS One 2015; 10(12): e0144989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tang CY, Taylor NF, Blackstock FC. Chest physiotherapy for patients admitted to hospital with an acute exacerbation of chronic obstructive pulmonary disease (COPD): a systematic review. Physiotherapy 2010; 96: 1–13. [DOI] [PubMed] [Google Scholar]
- 5. McCarthy B, Casey D, Devane D, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2015; 2: Cd003793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Langer D, Hendriks E, Burtin C, et al. A clinical practice guideline for physiotherapists treating patients with chronic obstructive pulmonary disease based on a systematic review of available evidence. Clin Rehabil 2009; 23: 445–462. [DOI] [PubMed] [Google Scholar]
- 7. Ries AL. Pulmonary rehabilitation: summary of an evidence-based guideline. Respir Care 2008; 53: 1203–1207. [PubMed] [Google Scholar]
- 8. Eaton T, Young P, Fergusson W, et al. Does early pulmonary rehabilitation reduce acute health-care utilization in COPD patients admitted with an exacerbation? A randomized controlled study. Respirology 2009; 14: 230–238. [DOI] [PubMed] [Google Scholar]
- 9. Ko FW, Dai DL, Ngai J, et al. Effect of early pulmonary rehabilitation on health care utilization and health status in patients hospitalized with acute exacerbations of COPD. Respirology 2011; 16: 617–624. [DOI] [PubMed] [Google Scholar]
- 10. Puhan MA, Gimeno-Santos E, Cates CJ, et al. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2016; 12: Cd005305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Greening NJ, Williams JE, Hussain SF, et al. An early rehabilitation intervention to enhance recovery during hospital admission for an exacerbation of chronic respiratory disease: randomised controlled trial. BMJ 2014; 349: g4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lai CC, Chou W, Chan KS, et al. Early mobilization teduces furation of mechanical ventilation and intensive care unit dtay in patients with acute respiratory failure. Arch Phys Med Rehabil 2017; 98: 931–939. [DOI] [PubMed] [Google Scholar]
- 13. Kortebein P, Ferrando A, Lombeida J, et al. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA 2007; 297: 1772–1774. [DOI] [PubMed] [Google Scholar]
- 14. Spruit MA, Gosselink R, Troosters T, et al. Muscle force during an acute exacerbation in hospitalised patients with COPD and its relationship with CXCL8 and IGF-I. Thorax 2003; 58: 752–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pitta F, Troosters T, Probst VS, et al. Physical activity and hospitalization for exacerbation of COPD. Chest 2006; 129: 536–544. [DOI] [PubMed] [Google Scholar]
- 16. Wouters EF, Groenewegen KH, Dentener MA, et al. Systemic inflammation in chronic obstructive pulmonary disease: the role of exacerbations. Proc Am Thorac Soc 2007; 4: 626–634. [DOI] [PubMed] [Google Scholar]
- 17. Hurst JR, Donaldson GC, Perera WR, et al. Use of plasma biomarkers at exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006; 174: 867–874. [DOI] [PubMed] [Google Scholar]
- 18. Markoulaki D, Kostikas K, Papatheodorou G, et al. Hemoglobin, erythropoietin and systemic inflammation in exacerbations of chronic obstructive pulmonary disease. Eur J Int Med 2011; 22: 103–107. [DOI] [PubMed] [Google Scholar]
- 19. Wedzicha JA, Seemungal TA, MacCallum PK, et al. Acute exacerbations of chronic obstructive pulmonary disease are accompanied by elevations of plasma fibrinogen and serum IL-6 levels. Thromb Haemost 2000; 84: 210–215. [PubMed] [Google Scholar]
- 20. Vermeeren MA, Schols AM, Wouters EF. Effects of an acute exacerbation on nutritional and metabolic profile of patients with COPD. Eur Respir J 1997; 10: 2264–2269. [DOI] [PubMed] [Google Scholar]
- 21. Troosters T, Probst VS, Crul T, et al. Resistance training prevents deterioration in quadriceps muscle function during acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010; 181: 1072–1077. [DOI] [PubMed] [Google Scholar]
- 22. Seymour JM, Moore L, Jolley CJ, et al. Outpatient pulmonary rehabilitation following acute exacerbations of COPD. Thorax 2010; 65: 423–428. [DOI] [PubMed] [Google Scholar]
- 23. Murphy N, Bell C, Costello RW. Extending a home from hospital care programme for COPD exacerbations to include pulmonary rehabilitation. Respir Med 2005; 99: 1297–1302. [DOI] [PubMed] [Google Scholar]