Abstract
Altered expression levels of the long noncoding RNA (lncRNA) nuclear‐enriched abundant transcript 1 (NEAT1) have been reported in different types of cancer. More than half of the NEAT1 studies in cancer have been published within the last 2 years. In this review, we discuss very recent developments and insights into NEAT1 contribution to carcinogenesis. Summarizing the literature, it becomes obvious that NEAT1 is a lncRNA highly de‐/upregulated in a variety of cancer entities, in which it primarily acts as a competing endogenous RNA (ceRNA) which sponges tumor‐suppressive microRNA (miRNA). The sponged miRNA lose their ability to degrade, silence, or hamper translation of their downstream—mostly oncogenic—target transcripts, ultimately promoting carcinogenesis. This role of NEAT1 function in tumorigenesis suggests it may be a prognostic biomarker as well as potential therapeutic target, pending the completion of further studies into the underlying mechanisms.
Keywords: cancer, ceRNA, lncRNA, miRNA, NEAT1
Abbreviations
- ceRNA
competing endogenous RNA
- EMT
epithelial–mesenchymal transition
- IRAlu
inverted repeat Alu elements
- lncRNA
long noncoding RNA
- miRNA/miR
microRNA
- ncRNA
noncoding RNA
- NEAT1
nuclear‐enriched abundant transcript 1
- p54nrb/NoNO
54 kDa nuclear RNA‐ and DNA‐binding protein/Non‐POU domain‐containing octamer‐binding protein
- PSF/SFPQ
PTB‐associated splicing factor/splicing factor proline glutamine rich
- SIM
structured illumination microscopy
1. Introduction
Since results of genome‐wide studies have shown that approximately 70% of the human genome is transcribed into RNA but < 2% is protein‐coding (Consortium, 2012), noncoding RNA (ncRNA) have come more into focus of research (Ling et al., 2015; Nana‐Sinkam and Croce, 2011). Technical progress in sequencing technologies led to the discovery of the huge family of ncRNA. In the last 2 years, extensive research was done in this field and long noncoding RNA (lncRNA), typically > 200 nucleotides in length, have on the one hand been found to be implicated in a variety of biological processes such as gene expression, subcellular architecture, and stabilization of protein complexes (Gutschner et al., 2018; Kung et al., 2013; Reicher et al., 2018; Smolle et al., 2017). On the other hand, studies with lncRNA proved their involvement in physiology and pathophysiology (Chen et al., 2014; Del Vecchio et al., 2018; Schanza et al., 2017; Smolle and Pichler, 2018). Besides mRNA, pseudogenes, and circular RNA, also lncRNA often function as competing endogenous RNA (ceRNA) in a variety of pathologies (Cesana et al., 2011; Hansen et al., 2013; Memczak et al., 2013). ceRNA are transcripts able to regulate each other at the post‐transcriptional level by competing for shared microRNA (miRNA), or more precisely, they act as natural molecular sponge for miRNA (Qi et al., 2015). Consequently, the sponged miRNA lose their ability to degrade, silence, or hamper the translation of their downstream target transcripts (Bartel, 2009). The mechanism underlying the ceRNA hypothesis was first described in 2007 by Franco‐Zorrilla et al. where they reported that the lncRNA induced by phosphate starvation 1 (IPS1) regulates PHO2 protein levels in plants by limiting the availability of miR‐399 and therefore inhibiting the repressive function of miR‐399 on PHO2 mRNA (Franco‐Zorrilla et al., 2007). In the same year, Ebert et al. reported on a new technique controlling endogenous miRNA levels by expressing competitive inhibitors containing multiple, tandem binding sites for specific miRNA in cell systems resulting in a sponging of these miRNA. This group for the first time introduced the term ‘miRNA sponge’ (Ebert et al., 2007). These early studies report on the principle of miRNA target regulation by competitively sequestering miRNA affecting the activity of their targets. Based on these findings, Salmena et al. proposed the hypothesis of ‘ceRNA’ in 2011 stating that RNA transcripts (i.e., messenger RNA, transcribed pseudogenes, or lncRNA) containing microRNA‐response elements (MREs) are able to exert the function as ceRNA de‐repressing the activity of other RNA with similar MREs by competing for the same miRNA in the available miRNA pool (Salmena et al., 2011).
The subject of lncRNA exerting ceRNA function contributing to carcinogenesis is currently under extensive investigation, and many lncRNA have been demonstrated to be molecular sponges for miRNA in several cancer entities, for example, AFAP1‐AS1 in nasopharyngeal carcinoma (Lian et al., 2018) and pancreatic cancer (Chen et al., 2018), FLVCR1‐AS1 in lung cancer (Gao et al., 2018), or TP73‐AS1 in gastric cancer (Ding et al., 2018) to name just a few. Thomson et al. published a detailed review critically discussing the evidence and controversy of miRNA sponges (Thomson and Dinger, 2016).
This review will give an overview of the most recent findings on the lncRNA NEAT1, especially focusing on the consequences of its deregulation and its function as ceRNA in the development of cancer.
2. NEAT1—history, structure, function
Nuclear‐enriched abundant transcript 1 has been discovered in 2007 by Hutchinson et al. as being a lncRNA enriched in the nucleus localized within paraspeckles (Hutchinson et al., 2007) and was found to be essentially needed for paraspeckle integrity (Clemson et al., 2009). Two variants of this lncRNA exist; that is, NEAT1_1 (3.7 kb) and NEAT1_2 (23 kb) encoded by the NEAT1 gene (Sasaki et al., 2009) and transcribed from the multiple endocrine neoplasia locus in human chromosome 11qA (Guru et al., 1997). Paraspeckles are nuclear complexes consisting of proteins, that is, PTB‐associated splicing factor/splicing factor proline glutamine rich (PSF/SFPQ; Prasanth et al., 2005), 54 kDa nuclear RNA‐ and DNA‐binding protein/Non‐POU domain‐containing octamer‐binding protein (p54NRB/NONO) and paraspeckle component 1 (PSPC1; Fox et al., 2002), and the lncRNA NEAT1. Paraspeckles are responsible for regulating gene expression by retaining A‐I‐edited mRNA in the nucleus, whereas unedited RNA are transported into the cytoplasm (Zhang and Carmichael, 2001).
2.1. NEAT1 structure
A combination of immunofluorescence and fluorescent in situ hybridization (FISH) experiments revealed an intensive colocalization of NEAT1 lncRNA with paraspeckle proteins p54nrb/NONO and PSP1 (Clemson et al., 2009), which have earlier been shown to form heterodimers within paraspeckles (Fox et al., 2005). The interaction between NEAT1 and p54nrb/NONO happens through three protein interaction sites localized near the 5′ and 3′ ends of NEAT1 (Murthy and Rangarajan, 2010). Further elegant studies combining FISH (RNA detection) and structured illumination microscopy (SIM) allowed the simultaneous detection of the RNA and protein components of paraspeckles, which was not possible with standard fluorescent microscopy due to resolution limits (Mito et al., 2016). Detailed analyses with the SIM technique uncovered the structural organization of NEAT1 within paraspeckles; that is, the paraspeckle components are arranged in a core‐shell spheroidal structure, whereas the 5′ and 3′ ends of NEAT1 are located at the periphery of the paraspeckles and the central sequence of NEAT1 is localized within the core (West et al., 2016). In contrast to the long NEAT1 isoform, the short isoform is not a major component of paraspeckles but rather localizes to so‐called microspeckles and therefore probably exerting other regulatory functions (Li et al., 2017b). CRISPR/Cas9 deletion experiments provided more explicit information on the functional domains of NEAT1‐dependent paraspeckle organization. Yamazaki et al. provided details of two prerequisites essential for paraspeckle formation, that is, (a) the middle domain of NEAT1 which is sufficient for the formation and (b) the binding of p54nrb/NONO to this middle domain via its NOPS (NONA/paraspeckle) dimerization domain. Due to its architectural role in paraspeckle structure, authors defined NEAT1 as being an architectural RNA (Yamazaki et al., 2018b). By selectively overexpressing NEAT1 with the CRISPR/Cas9 synergistic activation mediator (SAM) system, the same group was able to induce intact paraspeckles with ordered core‐shell structure. This approach allows more detailed studies in the future on the functional role of NEAT1 (Yamazaki et al., 2018a).
2.2. NEAT1 function
As mentioned above, NEAT1 was first discovered with expression array experiments in 2007 in parallel with NEAT2 also known as metastasis‐associated lung adenocarcinoma transcript 1 (MALAT1) as approximately 4‐kb‐long unspliced, polyadenylated nuclear‐restricted noncoding transcript which is broadly expressed with high abundance in ovary, prostate, colon, and pancreas. Already in early studies, the existence of a second > 17‐kb‐long isoform with lower expression rate was suggested. Authors proposed a localization of NEAT1 within paraspeckles which are described as structures on the edges of nuclear speckles (Hutchinson et al., 2007). The Pol II‐transcribed NEAT1 was shown to be essential for paraspeckle integrity since a depletion of NEAT1 eradicates them. Overexpression of NEAT1 increases the amount of paraspeckles within the cells, while PSP1 overexpression does not indicating NEAT1 as the bottleneck for paraspeckle formation. Physiologic implications of NEAT1 have been investigated by several groups where they show that the short isoform of NEAT1 is broadly expressed in a wide range of tissues, whereas the long isoform is expressed in a subpopulation of cells in adult mice with highest abundance in stomach and intestine. Accordingly, intense paraspeckle formation, which crucially depends on the long isoform, is observed in cellular subpopulations of living mice. Since NEAT1 knockout mice are viable and fertile and do not show an apparent phenotype, authors propose that paraspeckles are nonessential subpopulation‐specific nuclear bodies (Nakagawa et al., 2011). NEAT1 is essential for corpus luteum formation and defines fertility in a subpopulation of mice (Nakagawa et al., 2014), is fundamental for mammary gland development and lactation capacity in mice (Standaert et al., 2014), and modulates neuronal excitability in humans (Barry et al., 2017). The functional role of NEAT1 in the cellular context is not fully uncovered yet, but early studies show an involvement in the sequestration of paraspeckle proteins of the drosophila behavior human splicing (DBHS) family, that is, PSP1 and p54nrb/NONO which are implicated in A‐I editing of mRNA. Although NEAT1 itself is not A‐I edited, it is nevertheless retained in the nucleus; therefore, authors propose an architectural role of this lncRNA (Clemson et al., 2009). One component of the PSP1‐p54nrb/NONO‐NEAT1 complex namely p54nrb/NONO is involved in the retention of A‐I‐edited mRNA by dsRNA‐dependent adenosine deaminases (ADARs) preventing the nuclear export of these mRNA containing inverted repeated Alu (IRAlu) elements within the 3′UTR (Chen et al., 2008). In human embryonic stem cells (hESC), mRNA containing IRAlu elements are not as one would expect retained in the nucleus except after they have differentiated. The explanation for this phenomenon is the absence of paraspeckles in undifferentiated hESC because of lacking NEAT1 expression. Upon differentiation, NEAT1 expression is started leading to paraspeckle formation enabling nuclear retention of mRNA and therefore representing an interesting functional role of NEAT1 in the process of differentiation (Chen and Carmichael, 2009). Paraspeckle‐driven nuclear retention is controlled by coactivator‐associated arginine methyltransferase 1 (CARM1) on two levels. On the one hand, CARM1 methylates p54nrb/NONO disabling its binding to mRNA containing IRAlu elements and on the other hand suppresses NEAT1 transcription reducing the amount of paraspeckles. Both events lead to less nuclear retention of mRNA and provide another regulatory mechanism in which NEAT1 is involved (Hu et al., 2015). Another functional involvement of NEAT1 was shown by Imamura et al. and Hirose et al., that is, transcriptional regulation due to NEAT1‐dependent PSF/SFPQ sequestration to paraspeckles. PSF/SFPQ is a repressor of interleukin 8 (IL‐8) transcription. Upon viral infection, NEAT1 expression is induced leading to a NEAT1‐dependent relocation of PSF/SFPQ from the IL‐8 promotor to paraspeckles activating IL‐8 transcription and subsequent stimulating immune response (Imamura et al., 2014). Another example for transcriptional regulation executed by NEAT1‐dependent PSF/SFPQ relocation is the control of RNA‐specific adenosine deaminase B2 (ADARB2) transcription which requires PSF/SFPQ. Upon cellular stress induced with proteasome inhibition, NEAT1 expression is upregulated subsequently sequestering PSF/SFPQ to paraspeckles reversing its binding to the ADARB2 promoter and subsequently leading to reduced ADARB2 transcription (Hirose et al., 2014).
3. NEAT1 in cancer biology
In this section, the involvement of NEAT1 in cancer biology will be discussed. Research concerning this topic before 2017 has been very well reviewed by Lo et al. (2016a) and Yu et al. (2017). The next part will mainly focus on knowledge gained within the last 2 years providing detailed information about the most commonly studied cancer types, that is, nonsmall lung cancer, breast cancer, hepatocellular carcinoma, ovarian cancer, and prostate cancer. Meta‐analyses clearly show that the lncRNA NEAT1 is upregulated in various cancer entities resulting in an unfavorable prognosis as well as a poor overall survival and, thus, these studies conclude that NEAT1 could be a suitable prognostic biomarker candidate for clinicopathological features in cancer pathology (Chen et al., 2017b; Yang et al., 2017a; Zhang et al., 2017c). A recent pan‐cancer study predicts that NEAT1 is the lncRNA which has the most cancer gene targets closely followed by the lncRNA MALAT1, LINC00969, and OIP5‐AS1 (Chiu et al., 2018). Contradictory findings show that NEAT1 is downregulated in some cancers (e.g., acute promyelocytic leukemia (Zeng et al., 2014)) and is a downstream target of the tumor suppressor p53, thus proposing that NEAT1 works as a potential tumor suppressor (Adriaens et al., 2016; Idogawa et al., 2017; Mello et al., 2017).
3.1. Breast cancer
The first evidence that NEAT1 plays a crucial role in breast cancer biology was delivered by Choudhry et al. when they showed that NEAT1 is a direct target of hypoxia‐inducible factor 2 (HIF‐2), which is known to be activated in cancer (Lofstedt et al., 2007). HIF‐2 was shown to transcriptionally regulate NEAT1. Under hypoxia—which leads to HIF‐2 activation—NEAT1 is upregulated and consequently paraspeckle formation is induced, resulting in a nuclear retention of F11R, a factor which was earlier shown to be retained in the nucleus under hypoxic conditions (Ben‐Zvi et al., 2013). Choudhry et al. could prove that F11R retention depends on hypoxia‐induced NEAT1 upregulation. Increased cellular proliferation and clonogenic survival as well as decreased apoptosis of breast cancer cells are the outcome of hypoxia‐induced NEAT1 upregulation (Choudhry et al., 2015).
Since 2016, a lot of research was done on NEAT1 contribution to breast cancer progression and several studies have shown a connection of this lncRNA with different miRNA. MiRNA are small endogenous RNA that regulate gene expression pattern through direct interaction with larger messenger RNA. Ke et al. found a correlation between NEAT1 and mir‐548ar‐3p with fused in sarcoma/translocated in liposarcoma (FUS/TLS). Knockdown of NEAT1 leads to reduced cellular growth and increased apoptosis of breast cancer cell lines. FUS and NEAT1 directly interact, and also, a knockdown of FUS resulted in increased apoptosis. miR‐548 was shown to regulate NEAT1 expression since overexpression of miR‐548 leads to decreased NEAT1 expression, ultimately inducing apoptosis (Ke et al., 2016).
Nuclear‐enriched abundant transcript 1 is also a target of breast cancer susceptibility gene 1 (BRCA1), which is commonly mutated in hereditary cases of breast cancer (Miki et al., 1994). BRCA1 deficiency increases NEAT1 expression and boosts tumorigenicity in vitro and in vivo. NEAT1 negatively regulates miR‐129‐5p by increasing DNA methylation of CpG islands in the miR‐129‐5p gene. Reduced miR‐129‐5p levels result in augmented WNT4 expression activating oncogenic WNT signaling. The authors concluded that the BRCA1/NEAT1/miR‐129‐5p signaling axis contributes to breast cancer tumorigenesis (Lo et al., 2016b).
Recent studies showed that NEAT1 is upregulated in breast cancer cell lines as well as in patient tumor tissue and that this upregulation is associated on the one hand with increased cell growth and proliferation, invasion, promoted epithelial–mesenchymal transition (EMT), reduced apoptosis in breast cancer cell lines and on the other hand with an unfavorable prognosis and overall patient survival, increased tumor size, lymph node metastasis, and cancer aggressiveness in patients (Li et al., 2017c,d; Qian et al., 2017; Zhang et al., 2017b; Zhao et al., 2017). Upregulated NEAT1 expression increases breast cancer cell growth by targeting miR‐101, a miRNA which was shown to negatively correlate with NEAT1 expression levels. miR‐101 targets enhancer of zeste homolog 2 (EZH2), a marker for aggressive breast cancer (Kleer et al., 2003), and an upregulation of miR‐101 results in decreased EZH2 levels. Thus, authors propose that NEAT1 knockdown might repress cancer cell growth via miR‐101‐dependent EZH2 regulation (Qian et al., 2017). As mentioned above, upregulation of NEAT1 promotes EMT, which triggers 5‐fluorouracil (5‐FU) resistance (Wu et al., 2016b). Li et al. showed that NEAT1‐induced EMT as well as chemo‐resistance of breast cancer cells is regulated via miR‐211/HMGA2 axis. miR‐211—known for inhibiting cancer cell migration and invasion (Chen et al., 2017a)—is repressed by upregulated NEAT1 and consequently leading to an upregulation of the EMT inducer high‐mobility group AT‐hook 2 (HMGA2; Wu et al., 2016a). Authors conclude that NEAT1 sponges miR‐211 and thus inhibits its repressor function on HMGA2 (Li et al., 2017d).
Nuclear‐enriched abundant transcript 1 was shown to be responsible for the interaction between forkhead/winged helix transcription factor 3 (FOXN3) and paired amphipathic helix protein 3 (SIN3A) in hormonally dependent breast cancer, forming a complex repressing genes such as trans‐acting T cell‐specific transcription factor 3 (GATA3), which is under normal conditions an EMT repressor (Yan et al., 2010). The NEAT1/FOXN3/SIN3A axis is promoting EMT and is responsible for dissemination and metastasis formation in vivo (Li et al., 2017c).
Jiang et al. detected increased NEAT1 levels in diverse breast cancer cell lines compared to MCF‐10A cells (normal mammary epithelial cells). The upregulation of NEAT1 negatively correlated with miR‐448 expression, which is a known inhibitor of cancer cell growth (Ma et al., 2018). NEAT‐1‐induced sponging of miR‐448 removes the inhibitory effect of this miRNA on ZEB1 (Jiang et al., 2018b), thus upregulating this transcription factor responsible for cancer progression by promoting EMT (Graham et al., 2010).
The reason for all these involved miRNA and different regulatory pathways might be explained in the excellent recent work of Zhou et al. They showed the implication on lncRNA in the four subtypes of breast cancer, that is, (a) basal‐like, (b) HER2+, (c) luminal A, and (d) luminal B. NEAT1 was one of three lncRNA—besides OPI5‐AS1 and AC008124.1—involved in all four subtypes. These lncRNA exert specific roles in the ceRNA network by competing with diverse miRNA, and an aberrant expression could disrupt the network structure. NEAT1 was shown to compete with different RNA within the four breast cancer subtypes and thus exerting diverse regulatory functions on cell activities. For example, in the basal like type NEAT1 competes with TGFB1 influencing vasculogenesis which is the basis for tumorigenesis. In the HER2+ type, NEAT1 competes with LDHA regulating glycolytic processes within cancer cells (Zhou et al., 2018).
3.2. Nonsmall cell lung cancer
In the last 2 years, several studies indicating the involvement of NEAT1 in nonsmall cell lung cancer (NSCLC) were published. All of these studies have in common that they show an upregulation of NEAT1 in cancer tissue as well as in cell lines and that this increase in NEAT1 levels was associated with more lymph node metastasis, higher TNM grades, and a poor overall survival in patients as well as increased proliferation, invasion, and migration in vitro (Jen et al., 2017; Li et al., 2018a; Sun et al., 2017; Wu et al., 2017; Zhang et al., 2017a). Also, in NSCLC NEAT1 is promoting tumorigenesis by regulating diverse molecular pathways. Sun et al. could associate NEAT1 with Wnt/β‐catenin signaling (Sun et al., 2017). The Wnt/β‐catenin axis contribution to breast cancer and nonsmall lung cancer has been shown before, that is, an activation of Wnt/β‐catenin signaling is promoting tumorigenesis (Fu et al., 2016; Xiao et al., 2016). Knockdown of NEAT1 leads to an inhibition of Wnt/β‐catenin consequently resulting in reduced proliferation, invasion, and aggressiveness of NSCLC (Jiang et al., 2018a; Sun et al., 2017). The expression of the transcription factor octamer‐binding transcription factor 4 (Oct4), which has been shown to be upregulated as a stem cell factor in several cancer entities (Villodre et al., 2016), positively correlates with NEAT1 expression in NSCLC patients. Jen et al. propose that Oct4 controls NEAT1 transcription since Oct4 overexpression leads to an increased NEAT1 expression. Rescue experiments in Oct4‐silenced cells show that NEAT1 overexpression is re‐establishing cancer cell proliferation (Jen et al., 2017). Recently, three groups created a connection between NEAT1 and miRNA in NSCLC by showing NEAT1 to be a ceRNA for these miRNA. (a) NEAT1 acts as a sponge for miR‐181a‐5p—high NEAT1 levels inversely correlate with miR‐181a‐5p levels—leading to an upregulation of high‐mobility group box 2 (HMGB2; Li et al., 2018a)—a protein known for being upregulated and being the driver for tumorigenesis in several cancer types (Fu et al., 2018; Kwon et al., 2010). (b) NEAT1 promotes E2F3 expression by competitively binding miR‐377‐3p. The higher E2F3 expression of NEAT1‐induced sponging of miR‐377‐3p levels results in increased proliferation by cell cycle regulation (Zhang et al., 2017a). (c) The group of Wu et al. investigated the impact of the NEAT1/miR‐98‐5p/MAPK6 axis on NSCLC. NEAT1 again is competitively sponging miR‐98‐5p, where high miR‐98‐5p levels are inhibiting cancer cell growth, migration, and invasion on the one hand and reducing mitogen‐activated protein kinase 6 (MAPK6) levels on the other hand. The authors showed that NEAT1 is sponging miR‐98‐5p which in turn upregulates MAPK6. MAPK6 has been found to be upregulated in breast and gastric cancer being responsible for promoted tumorigenesis in these cancer types (Evtimova et al., 2001; Liang et al., 2005).
3.3. Hepatocellular carcinoma
Recent studies elucidating the role of NEAT1 in hepatocellular carcinoma could show an upregulation of this lncRNA in HCC patient tissue and HCC cell lines. A reduction of NEAT1 expression levels by knocking down the gene leads to decreased cell viability, cell growth, migration, invasion, and EMT in vitro as well as to reduced tumor size and metastasis in vivo (Fang et al., 2017; Fu et al., 2017; Tu et al., 2018; Wang et al., 2017c; Zhang et al., 2018). NEAT1 is crucially interplaying with miRNA also in hepatocellular carcinoma acting as a ceRNA and therefore reducing expression of these miRNA in the case of (a) miR‐129‐5p (Fang et al., 2017; Fu et al., 2017), (b) miR‐613 (Wang et al., 2017c), (c) miR‐485 (Zhang et al., 2018), and (d) miR‐139‐5p (Tu et al., 2018). Ad i., Liu et al. proved an involvement of miR‐129‐5p in HCC progression. miR‐129‐5p directly targets valosin‐containing protein (VCP) and IκB, both of which are known contributors to HCC progression. Overexpression of miR‐129‐5p decreased VCP and increased the NFκB inhibitor IκB expression resulting in augmented apoptosis and decreased migration of HCC cells (Liu et al., 2012). Fang et al. expanded this mechanism by showing that NEAT1‐induced sponging of miR‐129‐5p results in increased VCP and decreased IκB levels ultimately activating NFκB pathway promoting tumor progression (Fang et al., 2017). Ad ii., NEAT1 sponging of miR‐613 was discovered in 2017 by Wang Z. et al. One year earlier, the underlying mechanism of the tumor‐suppressive features of this miRNA was discovered by Wang W. et al. This group showed that downregulated miR‐613 leads to an increase of doublecortin‐like kinase 1 (DCLK1). DCLK1 is a microtubule‐associated protein and serves as tumor stem cell marker. This protein is often upregulated in solid tumors and is able to drive tumorigenesis (Wang et al., 2016). To summarize this, upregulated NEAT1 levels in HCC sponge miR‐613 ultimately leading to cancer progression due to upregulation of DCLK1. Ad iii., in case of miR‐485, authors show that this miRNA directly regulates expression levels of signal activator and transducer of transcription 3 (STAT3); that is, overexpression of miR‐485 results in decreased STAT3 expression and vice versa. Increased NEAT1 levels in HCC sponge miR‐485 and increased levels of STAT3 oncogene (Avalle et al., 2017) are the consequence (Zhang et al., 2018). Ad iv., for the interplay between NEAT1 with miR‐139‐5p, authors proved TGF‐β1—commonly upregulated in several cancer types regulating cancer progression and metastasis (Bierie and Moses, 2006)—being a downstream target of this miRNA which is upregulated by NEAT1‐induced sponging of miR‐139‐5p leading to promoted HCC proliferation and invasion (Tu et al., 2018). The recent work of Liu et al. provides information about a contribution of lipolysis in HCC. Adipose triglyceride lipase (ATGL) is upregulated in HCC and is associated with poor prognosis since increased ATGL levels lead to promoted HCC cell growth and colony formation. NEAT1 modulates ATGL expression leading to disrupted lipolysis in HCC cells by augmenting miR‐124‐3p leading to promoted cancer progression (Liu et al., 2018b).
3.4. Ovarian cancer
In ovarian cancer (OC), NEAT1 levels have been reported being upregulated in tissue of OC patients as well as in OC cell lines (An et al., 2017; Chen et al., 2016; Ding et al., 2017; Liu et al., 2018c). High NEAT1 levels correlate with tumor grade, occurrence of metastasis, and an unfavorable prognosis and could therefore be a potential biomarker for OC (Chen et al., 2016). Ding et al. showed that NEAT1 overexpression increases OC cell proliferation and decreases apoptosis by negatively regulating miR‐34a‐5p which in turn loses its repressor function on the antiapoptotic oncogene B‐cell lymphoma‐2 (BCL‐2; Tsujimoto et al., 1985) consequently leading to cancer cell proliferation (Ding et al., 2017). Another study shed more light on paclitaxel‐resistant OC by showing that NEAT1 sponges miR‐194 resulting in an upregulation of the EMT‐associated transcription factor zinc finger E‐box‐binding homeobox 1 (ZEB1; Zhang et al., 2015) leading to chemoresistance of OC cells (An et al., 2017). In the recent work of Liu et al., repression of NEAT1 was proven to inhibit the metastasis‐related gene Rho‐associated coiled‐coil containing protein kinase 1 (ROCK1). NEAT1 and ROCK1 were identified as being targets of the tumor‐suppressive miR‐382‐3p. NEAT1 upregulation is promoting OC progression and metastasis by regulating the ROCK1/miR‐382‐3p axis, or more precisely, NEAT1 promotes ROCK‐mediated formation of metastasis by acting as a ceRNA for miR‐382‐3p in OC (Liu et al., 2018c).
3.5. Prostate cancer
An interaction of NEAT1 with steroid receptor coactivator‐3 (SCR‐3), which is essentially needed for prostate cancer cell proliferation and growth (Zhou et al., 2005) via promoting insulin‐like growth factor receptor 1 (IGFR1) transcription followed by AKT signaling activation in prostate cancer, was observed by Xiong et al. Subsequently, the group was able to prove that NEAT1 increases AKT phosphorylation via the IGFR1 pathway promoting cancer cell growth (Xiong et al., 2018). An increased occurrence of mutations in the NEAT1 promoter was recently reported in patients who underwent androgen deprivation therapy in castration‐resistant prostate cancer (Wedge et al., 2018) highlighting the correlation between NEAT1 upregulation and resistance to androgen receptor antagonists (Chakravarty et al., 2014). A recent study of Li et al. showed that cell division cycle 5‐like protein (CDC5L) is a regulatory target of NEAT1 and that knockdown of NEAT1 results in reduced expression of AGRN, which is a direct target of CDC5L and a interaction partner of transforming growth factor beta 1 (TGFβ1) leading to DNA damage, cell cycle arrest ultimately resulting in decreased cancer cell growth and tumorigenesis in prostate cancer cells (Li et al., 2018b).
3.6. Nasopharyngeal carcinoma
Recent research could draw connections between NEAT1 and nasopharyngeal carcinoma (NPC). Also, in NPC NEAT1 is differentially expressed and its expression influences cancer cell characteristics. Liu et al. and Chen et al. report an upregulation of NEAT1 in cancer tissue and NPC cell lines and showed that knockdown of this lncRNA leads to inhibited proliferation, chemoresistance, and induced apoptosis (Cheng and Guo, 2017; Liu et al., 2018a). Increased NEAT1 levels found in NPC tissue and cells negatively correlated with expression levels of the tumor‐suppressive (Sampson et al., 2007) miRNA let‐7a‐5p resulting in upregulation of the oncogenic (Hrustanovic and Bivona, 2016) Ras‐MAPK‐signaling pathway; thus, authors conclude a contribution of the NEAT1/let‐7a‐5p axis to chemoresistance in NPC by modulation of Ras‐MAPK signaling (Liu et al., 2018a). Chen et al. proved a direct interaction and negative correlation of NEAT1 with miR‐124, a known tumor‐suppressive miRNA (Feng et al., 2015). miR‐124 regulates proliferation and apoptosis via NF‐κB—known to be activated during NPC progression (Sun et al., 2012)—or more precisely, upregulation of NEAT1 results in reduced miR‐124 expression leading to increased NF‐κB signaling in NPC (Cheng and Guo, 2017). Contradictory findings are presented in the work of Wang et al. where they showed (a) a significant downregulation of NEAT1 in NPC patient tissue, (b) that high expression levels are associated with a better survival in patients, and (c) that NEAT1 knockdown boosted migration but had no effect on proliferation (Wang et al., 2017b). Maybe, these contradictory findings are the result of the often very little sample sizes (e.g., microarray data control (n = 3) vs cancer tissue (n = 25)) or the different technical approach. The other groups had significant proliferation changes and proved these data by rescue experiments (Cheng and Guo, 2017; Liu et al., 2018a).
3.7. NEAT1 in other cancer types
A contribution of NEAT1 to cancer progression has recently been investigated in the following cancer types as well, that is, gastric cancer (Tan et al., 2018), osteosarcoma (Hu et al., 2018; Wang et al., 2017a), glioblastoma (Gong et al., 2016; Yang et al., 2017b), oral and esophageal carcinoma (Huang et al., 2018; Li et al., 2017a), clear cell renal carcinoma (Liu et al., 2017), and cervical carcinoma (Han et al., 2018; Wang and Zhu, 2018). Although these cancer entities are completely different regarding their localization, progression, molecular, and cellular features, they have one thing in common, that is, the NEAT1 phenotype namely (a) highly increased NEAT1 levels in tumor tissue and cancer cell lines. In all these different cancer types, upregulation of NEAT1 is associated with tumor stage and progression, metastasis, and an unfavorable patient survival. (b) Knockdown of NEAT1 results in a reduction of cancer cell growth, proliferation, migration, invasion, and an increase in apoptosis in vitro as well as in reduced tumor size and metastasis in vivo. In most cases, NEAT1 acts as ceRNA for a specific miRNA, therefore reducing the expression levels of the respective miRNA and consequently leading to the modulation—that is, mostly upregulation—of known oncogenic proteins (Fig. 1). More precisely, the underlying cellular mechanism is comparable in the above‐mentioned cancer types, though they only differ in the respective miRNA and modulated oncogenes, which are listed in Table 1.
Figure 1.
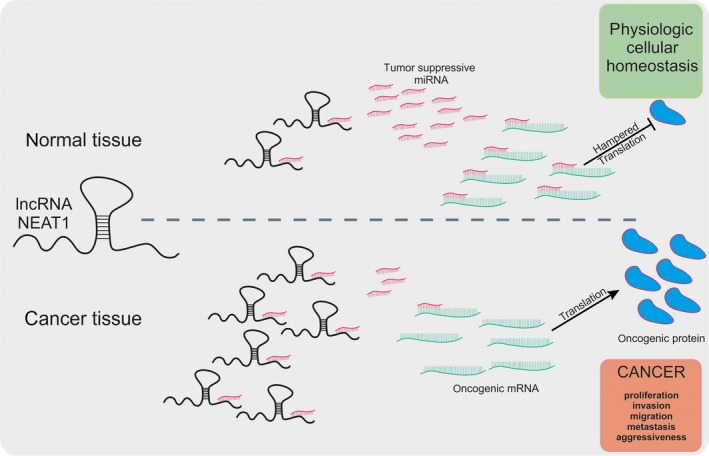
Schematic representation of the consequences of elevated NEAT1 expression levels in the context of cancer. (Upper panel) In normal tissue, NEAT1 expression levels are low; therefore, tumor‐suppressive miRNA are not sponged which enables them binding to oncogenic miRNA resulting in a hampered translation and low levels of oncogenic proteins. (Lower panel) In cancer tissue and cancer cell lines NEAT1 expression levels are high. Tumor‐suppressive miRNA are sponged by NEAT1 resulting in reduced binding of these miRNA to oncogenic mRNA. High numbers of these mRNA are translated to oncogenic proteins and cancer cell proliferation, invasion, migration, etc. are promoted.
Table 1.
Interplay of certain miRNA with NEAT1 in diverse cancer types and the corresponding modulated protein targets (arrows indicate upregulation (↑) or downregulation (↓) of the respective factor). Ca, carcinoma; PBS, predicted binding site; Luc, Luciferase promotor assay; PD, RNA pull‐down assay; bis‐sequ., bisulfite sequencing
| Cancer type | miRNA | 3′–5′ sequence | PBS to NEAT1 | PBS investigated in literature | Experimental method | Target protein | References |
|---|---|---|---|---|---|---|---|
| Nonsmall lung cancer | 181a‐5p | UGAGUGGCUGUCGCAACUUACAA | 4 | 1066 | Luc, PD | ↑HMBG2 | Li et al. (2018a) |
| 377‐3p | UGUUUUCAACGGAAACACACUA | 5 | No details | Luc | ↑E2F3 | Zhang et al. (2017a) | |
| 98‐5p | UUGUUAUGUUGAAUGAUGGAGU | 3 | 4179a | Luc, PD | ↑MAPK6 | Wu et al. (2017) | |
| Breast cancer | 101‐3p | AAGUCAAUAGUGUCAUGACAU | 1 | 12 605 | Luc | ↑EZH2 | Qian et al. (2017) |
| 211‐5p | UCCGCUUCCUACUGUUUCCCUU | 3 | 3209 | Luc | ↑HMGA2 | Li et al. (2017d) | |
| 488 | UACCCUGUAGGAUGUAUACGUU | 2 | 2331a | Luc | ↑ZEB1 | Jiang et al. (2018b) | |
| 548ar‐3p | CGUUUUUAUUGACGUCAAAAU | 0 | 2443 RNA hybrid | qPCR | Ke et al. (2016) | ||
| 129‐5p | CGUUCGGGUCUGGCGUUUUUC | 3 | No details | qPCR, bis‐sequ. | ↑WNT4 | Lo et al. (2016b) | |
| Hepatocellular Ca | 129‐5p | CGUUCGGGUCUGGCGUUUUUC | 3 | 10 197 | PD | ↑VCP, ↓κB | Fu et al. (2017) |
| 613 | CCGUUUCUUCCUUGUAAGGA | 5 | 1863a | Luc | ↑DCLK1 | Wang et al. (2016, 2017c) | |
| 485‐5p | CUUAAGUAGUGCCGGUCGGAGA | 5 | 4456 | Luc | ↑STAT3 | Zhang et al. (2018) | |
| 139‐5p | UGACCUCUGUGCACGUGACAUCU | 2 | 1588a | Luc, PD | ↑TGFβ1 | Tu et al. (2018) | |
| 124‐3p | AACCGUAAGUGGCGCACGGAAU | 3 | 2928 | Luc | ↑ATGL | Liu et al. (2018b) | |
| Ovarian Cancer | 34a‐5p | UGUUGGUCGAUUCUGUGACGGU | 5 | 14 939 | Luc | ↑BCL2 | Ding et al. (2017) |
| 194‐5p | AGGUGUACCUCAACGACAAUGU | 2 | 3639 | Luc | ↑ZEB1 | An et al. (2017) | |
| 382‐3p | UUCACAACAGGCACUUACUAA | 3 | 22 189a | Luc | ↑ROCK | Liu et al. (2018c) | |
| Gastric Cancer | 506‐3p | AGAUGAGUCUUCCCACGGAAU | 3 | 2928 | Luc, PD | ↑STAT3 | Tan et al. (2018) |
| Cervical Ca | 193b‐3p | UCGCCCUGAAACUCCCGGUCAA | 3 | 1991 | Luc, PD | ↑Cyclin D1 | Han et al. (2018) |
| 101 | AAGUCAAUAGUGUCAUGACAU | 1 | 12 605 | Luc | ↑FOS | Wang and Zhu (2018) | |
| Nasopharyngeal Ca | let‐7a‐5p | UUGAUAUGUUGGAUGAUGGAGU | 3 | 14 917 | Luc | ↑Ras‐MAPK | Liu et al. (2018a) |
| 124‐3p | AACCGUAAGUGGCGCACGGAAU | 3 | 3252 | Luc, PD | ↑NFκB | Cheng and Guo (2017) | |
| Oral squamous cell Ca | 129‐5p | CGUUCGGGUCUGGCGUUUUUC | 3 | ‐ | Luc | ↑CTBP2 | Li et al. (2017a) |
| 365‐3p | UAUUCCUAAAAAUCCCCGUAAU | 3 | 1901 | Luc | ↑RGS20 | Huang et al. (2018) | |
| Clear cell renal Ca | 34a‐5p | UGUUGGUCGAUUCUGUGACGGU | 5 | 14 939 | Luc | ↑c‐MET | Liu et al. (2017) |
| Osteosarcoma | 34c‐5p | CGUUAGUCGAUUGAUGUGACGGA | 5 | 14 938 | qPCR | ↑BCL2+ ↑CCND1 | Hu et al. (2018) |
| 194‐5p | AGGUGUACCUCAACGACAAUGU | 2 | 3639 | Luc | Wang et al. (2017a) | ||
| Glioblastoma | 107 | ACUAUCGGGACAUGUUACGACGA | 1 | 1514 | Prediction, functional assays | ↑CDK6 | Yang et al. (2017b) |
| let‐7e‐5p | UUGAUAUGUUGGAGGAUGGAGU | 3 | 14 917,14 737 | Luc | ↑NRAS | Gong et al. (2016) |
Investigated miRNA binding site was not predicted with Starbase database.
4. Conclusion NEAT1 in cancer
In most cancer types, NEAT1 seems to be upregulated in cancer tissue compared to the corresponding noncancerous tissue as well as in the investigated cancer cell lines. High levels of NEAT1 have been shown to be associated with advanced tumor stage and cancer progression, the occurrence of metastasis, and poor patient survival. Knockdown of this lncRNA is associated with inhibition of proliferation, migration, invasion, increased apoptosis as well as decreased tumor size, and fewer metastases. This review highlights the function of NEAT1 as competitive endogenous RNA which is sponging many different miRNA in cancer and consequently leading to the modulation of oncogenic factors driving cancer related processes such as proliferation, invasion, migration, and often promoting epithelial to mesenchymal transition.
Although the NEAT1‐ceRNA hypothesis seems to be of common acceptance, there is one contradiction when regarding the underlying mechanism of miRNA processing, that is, miRNA are transcribed, modified, and processed into hairpin‐shaped pre‐miRNA in the nucleus followed by transport into the cytoplasm via Exportin‐5/Ran‐GTP complex where the hairpin is cut and the mature miRNA strand is incorporated into the RNA‐induced silencing complex (RISC) now able to interact with its target mRNA (Shukla et al., 2011). Based on this fundamental principle, miRNA sponging is supposed to happen in the cytoplasm. This is contradictory to the consistently described nuclear localization of NEAT1 (Clemson et al., 2009; Mito et al., 2016; West et al., 2016; Yamazaki et al., 2018a). As depicted in Table 1, miRNA binding sites on NEAT1 are mostly in the long isoform, strictly locating in nuclear paraspeckles (Nakagawa et al., 2011). Most reports demonstrating ceRNA function of NEAT1 have performed luciferase reporter assays showing a NEAT1‐dependent expression regulation of the respective miRNA or even pull‐down assays proving a direct interaction of NEAT1 with the investigated miRNA (Table 1). In our opinion, there are two possibilities allowing NEAT1 to act as ceRNA (a) NEAT1 is transported into the cytoplasm or (b) miRNA are transported in the nucleus. (a) Several reports demonstrate that lncRNA translocate from the nucleus to the cytoplasm upon cellular stress. In case of the lncRNA FILNC1, it is post‐transcriptionally methylated upon stress induction and subsequently exported into the cytoplasm (Xiao et al., 2017). Stress‐induced lncRNA get 5′‐capped, escape nuclear degradation, and are exported into the cytoplasm (Galipon et al., 2013). Therefore, it could be possible that NEAT1 undergoes post‐transcriptional modification and/or 5′‐capping, and gets exported to the cytoplasm, therefore possibly escaping conventional detection methods. The study of Nishizawa et al. is ruling out this possibility since they show that LINC00152 but not NEAT1 is relocating to the cytoplasm upon cellular stress (Nishizawa et al., 2018). (b) Upon cellular stress, miRNA, siRNA, and oligonucleotides are transported in the nucleus via a stress‐induced response complex (SIRC). Castanotto et al. showed that MALAT1 (also known as NEAT2 which was found in parallel to NEAT1 (Hutchinson et al., 2007) and is—as NEAT1—strictly localizing to the nucleus (Ip and Nakagawa, 2012)) is degraded in the nucleus because miR‐9 is transported there and directly targets MALAT1. Authors propose that the process of stress‐induced miRNA relocation to the nucleus is a universal process (Castanotto et al., 2018) enabling ceRNA function of NEAT1 in theory. If this is the way of ceRNA function of NEAT1 or if there is another explanation of the observed processes, then it still needs to be further investigated, but there are too many reports denying a role of NEAT1 in cancer.
The amount and diversity of involved miRNA, oncogenic factors, and pathways even within one cancer type underline the complexity of this malignant disease. The central molecular role of NEAT1 is an important shared feature in all cancers and indicates to the enormous potential of NEAT1 as a target in cancer therapy. As many authors conclude high levels of NEAT1 in cancer patients could serve as a useful prognostic biomarker. Although extensive research has so far shed light on the implication of NEAT1 in cancer biology and predicts promising potential for the use of this lncRNA in cancer diagnosis, prognosis, and therapy, more studies are needed toward therapeutic interventions.
Author contributions
CK and MP designed the structure of the review; CK and FP performed literature research; CK, FP, and MP wrote the paper.
Conflicts of interest
The authors have no conflicts of interest to declare.
References
- Adriaens C, Standaert L, Barra J, Latil M, Verfaillie A, Kalev P, Boeckx B, Wijnhoven PW, Radaelli E, Vermi W et al (2016) p53 induces formation of NEAT1 lncRNA‐containing paraspeckles that modulate replication stress response and chemosensitivity. Nat Med 22, 861–868. [DOI] [PubMed] [Google Scholar]
- An J, Lv W and Zhang Y (2017) LncRNA NEAT1 contributes to paclitaxel resistance of ovarian cancer cells by regulating ZEB1 expression via miR‐194. Onco Targets Ther 10, 5377–5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avalle L, Camporeale A, Camperi A and Poli V (2017) STAT3 in cancer: a double edged sword. Cytokine 98, 42–50. [DOI] [PubMed] [Google Scholar]
- Barry G, Briggs JA, Hwang DW, Nayler SP, Fortuna PR, Jonkhout N, Dachet F, Maag JL, Mestdagh P, Singh EM et al (2017) The long non‐coding RNA NEAT1 is responsive to neuronal activity and is associated with hyperexcitability states. Sci Rep 7, 40127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136, 215–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben‐Zvi M, Amariglio N, Paret G and Nevo‐Caspi Y (2013) F11R expression upon hypoxia is regulated by RNA editing. PLoS ONE 8, e77702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierie B and Moses HL (2006) Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer 6, 506–520. [DOI] [PubMed] [Google Scholar]
- Castanotto D, Zhang X, Alluin J, Zhang X, Ruger J, Armstrong B, Rossi J, Riggs A and Stein CA (2018) A stress‐induced response complex (SIRC) shuttles miRNAs, siRNAs, and oligonucleotides to the nucleus. Proc Natl Acad Sci U S A 115, E5756–E5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesana M, Cacchiarelli D, Legnini I, Santini T, Sthandier O, Chinappi M, Tramontano A and Bozzoni I (2011) A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell 147, 358–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarty D, Sboner A, Nair SS, Giannopoulou E, Li R, Hennig S, Mosquera JM, Pauwels J, Park K, Kossai M et al (2014) The oestrogen receptor alpha‐regulated lncRNA NEAT1 is a critical modulator of prostate cancer. Nat Commun 5, 5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LL and Carmichael GG (2009) Altered nuclear retention of mRNAs containing inverted repeats in human embryonic stem cells: functional role of a nuclear noncoding RNA. Mol Cell 35, 467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LL, Decerbo JN and Carmichael GG (2008) Alu element‐mediated gene silencing. EMBO J 27, 1694–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Li Q, Zhou Y, Wang X, Zhang Q, Wang Y, Zhuang H, Jiang X and Xiong W (2018) The long coding RNA AFAP1‐AS1 promotes tumor cell growth and invasion in pancreatic cancer through upregulating the IGF1R oncogene via sequestration of miR‐133a. Cell Cycle 17, 1949–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Wang H, Yang P and He ZY (2017b) Prognostic role of long noncoding RNA NEAT1 in various carcinomas: a meta‐analysis. Onco Targets Ther 10, 993–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Xu J, Hong J, Tang R, Zhang X and Fang JY (2014) Long noncoding RNA profiles identify five distinct molecular subtypes of colorectal cancer with clinical relevance. Mol Oncol 8, 1393–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZJ, Zhang Z, Xie BB and Zhang HY (2016) Clinical significance of up‐regulated lncRNA NEAT1 in prognosis of ovarian cancer. Eur Rev Med Pharmacol Sci 20, 3373–3377. [PubMed] [Google Scholar]
- Chen LL, Zhang ZJ, Yi ZB and Li JJ (2017a) MicroRNA‐211‐5p suppresses tumour cell proliferation, invasion, migration and metastasis in triple‐negative breast cancer by directly targeting SETBP1. Br J Cancer 117, 78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng N and Guo Y (2017) Long noncoding RNA NEAT1 promotes nasopharyngeal carcinoma progression through regulation of miR‐124/NF‐kappaB pathway. Onco Targets Ther 10, 5843–5853. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Chiu HS, Somvanshi S, Patel E, Chen TW, Singh VP, Zorman B, Patil SL, Pan Y, Chatterjee SS; Genome Atlas Research Network et al. (2018) Pan‐cancer analysis of lncRNA regulation supports their targeting of cancer genes in each tumor context. Cell Rep 23, 297–312 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhry H, Albukhari A, Morotti M, Haider S, Moralli D, Smythies J, Schodel J, Green CM, Camps C, Buffa F et al (2015) Tumor hypoxia induces nuclear paraspeckle formation through HIF‐2alpha dependent transcriptional activation of NEAT1 leading to cancer cell survival. Oncogene 34, 4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemson CM, Hutchinson JN, Sara SA, Ensminger AW, Fox AH, Chess A and Lawrence JB (2009) An architectural role for a nuclear noncoding RNA: NEAT1 RNA is essential for the structure of paraspeckles. Mol Cell 33, 717–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium, E. P (2012) An integrated encyclopedia of DNA elements in the human genome. Nature 489, 57–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Vecchio F, Lee GH, Hawezi J, Bhome R, Pugh S, Sayan E, Thomas G, Packham G, Primrose J, Pichler M et al (2018) Long non‐coding RNAs within the tumour microenvironment and their role in tumour‐stroma cross‐talk. Cancer Lett 421, 94–102. [DOI] [PubMed] [Google Scholar]
- Ding Z, Lan H, Xu R, Zhou X and Pan Y (2018) LncRNA TP73‐AS1 accelerates tumor progression in gastric cancer through regulating miR‐194‐5p/SDAD1 axis. Pathol Res Pract 214, 1993–1999. [DOI] [PubMed] [Google Scholar]
- Ding N, Wu H, Tao T and Peng E (2017) NEAT1 regulates cell proliferation and apoptosis of ovarian cancer by miR‐34a‐5p/BCL2. Onco Targets Ther 10, 4905–4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert MS, Neilson JR and Sharp PA (2007) MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nat Methods 4, 721–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evtimova V, Schwirzke M, Tarbe N, Burtscher H, Jarsch M, Kaul S and Weidle UH (2001) Identification of breast cancer metastasis‐associated genes by chip technology. Anticancer Res 21, 3799–3806. [PubMed] [Google Scholar]
- Fang L, Sun J, Pan Z, Song Y, Zhong L, Zhang Y, Liu Y, Zheng X and Huang P (2017) Long non‐coding RNA NEAT1 promotes hepatocellular carcinoma cell proliferation through the regulation of miR‐129‐5p‐VCP‐IkappaB. Am J Physiol Gastrointest Liver Physiol 313, G150–G156. [DOI] [PubMed] [Google Scholar]
- Feng T, Xu D, Tu C, Li W, Ning Y, Ding J, Wang S, Yuan L, Xu N, Qian K et al (2015) MiR‐124 inhibits cell proliferation in breast cancer through downregulation of CDK4. Tumour Biol 36, 5987–5997. [DOI] [PubMed] [Google Scholar]
- Fox AH, Bond CS and Lamond AI (2005) P54nrb forms a heterodimer with PSP1 that localizes to paraspeckles in an RNA‐dependent manner. Mol Biol Cell 16, 5304–5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AH, Lam YW, Leung AK, Lyon CE, Andersen J, Mann M and Lamond AI (2002) Paraspeckles: a novel nuclear domain. Curr Biol 12, 13–25. [DOI] [PubMed] [Google Scholar]
- Franco‐Zorrilla JM, Valli A, Todesco M, Mateos I, Puga MI, Rubio‐Somoza I, Leyva A, Weigel D, Garcia JA and Paz‐Ares J (2007) Target mimicry provides a new mechanism for regulation of microRNA activity. Nat Genet 39, 1033–1037. [DOI] [PubMed] [Google Scholar]
- Fu X, Li H, Liu C, Hu B, Li T and Wang Y (2016) Long noncoding RNA AK126698 inhibits proliferation and migration of non‐small cell lung cancer cells by targeting Frizzled‐8 and suppressing Wnt/beta‐catenin signaling pathway. Onco Targets Ther 9, 3815–3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu D, Li J, Wei J, Zhang Z, Luo Y, Tan H and Ren C (2018) HMGB2 is associated with malignancy and regulates Warburg effect by targeting LDHB and FBP1 in breast cancer. Cell Commun Signal 16, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu MC, Yuan LQ, Zhang T, Yan XM, Zhou Y, Xia HL, Wu Y, Xu LX, Cao X and Wang J (2017) Nuclear paraspeckle assembly transcript 1 promotes the metastasis and epithelial‐mesenchymal transition of hepatoblastoma cells by inhibiting miR‐129‐5p. Oncol Lett 14, 5773–5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galipon J, Miki A, Oda A, Inada T and Ohta K (2013) Stress‐induced lncRNAs evade nuclear degradation and enter the translational machinery. Genes Cells 18, 353–368. [DOI] [PubMed] [Google Scholar]
- Gao X, Zhao S, Yang X, Zang S and Yuan X (2018) Long non‐coding RNA FLVCR1‐AS1 contributes to the proliferation and invasion of lung cancer by sponging miR‐573 to upregulate the expression of E2F transcription factor 3. Biochem Biophys Res Commun 505, 931–938. [DOI] [PubMed] [Google Scholar]
- Gong W, Zheng J, Liu X, Ma J, Liu Y and Xue Y (2016) Knockdown of NEAT1 restrained the malignant progression of glioma stem cells by activating microRNA let‐7e. Oncotarget 7, 62208–62223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham TR, Yacoub R, Taliaferro‐Smith L, Osunkoya AO, Odero‐Marah VA, Liu T, Kimbro KS, Sharma D and O'Regan RM (2010) Reciprocal regulation of ZEB1 and AR in triple negative breast cancer cells. Breast Cancer Res Treat 123, 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guru SC, Agarwal SK, Manickam P, Olufemi SE, Crabtree JS, Weisemann JM, Kester MB, Kim YS, Wang Y, Emmert‐Buck MR et al (1997) A transcript map for the 2.8‐Mb region containing the multiple endocrine neoplasia type 1 locus. Genome Res 7, 725–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutschner T, Richtig G, Haemmerle M and Pichler M (2018) From biomarkers to therapeutic targets‐the promises and perils of long non‐coding RNAs in cancer. Cancer Metastasis Rev 37, 83–105. [DOI] [PubMed] [Google Scholar]
- Han D, Wang J and Cheng G (2018) LncRNA NEAT1 enhances the radio‐resistance of cervical cancer via miR‐193b‐3p/CCND1 axis. Oncotarget 9, 2395–2409. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK and Kjems J (2013) Natural RNA circles function as efficient microRNA sponges. Nature 495, 384–388. [DOI] [PubMed] [Google Scholar]
- Hirose T, Virnicchi G, Tanigawa A, Naganuma T, Li R, Kimura H, Yokoi T, Nakagawa S, Benard M, Fox AH et al (2014) NEAT1 long noncoding RNA regulates transcription via protein sequestration within subnuclear bodies. Mol Biol Cell 25, 169–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrustanovic G and Bivona TG (2016) RAS‐MAPK signaling influences the efficacy of ALK‐targeting agents in lung cancer. Mol Cell Oncol 3, e1091061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu SB, Xiang JF, Li X, Xu Y, Xue W, Huang M, Wong CC, Sagum CA, Bedford MT, Yang L et al (2015) Protein arginine methyltransferase CARM1 attenuates the paraspeckle‐mediated nuclear retention of mRNAs containing IRAlus. Genes Dev 29, 630–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Yang Q, Wang L, Wang S, Sun F, Xu D and Jiang J (2018) Knockdown of the oncogene lncRNA NEAT1 restores the availability of miR‐34c and improves the sensitivity to cisplatin in osteosarcoma. Biosci Rep 38, 10.1042/BSR20180375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, He X and Wei XL (2018) lncRNA NEAT1 promotes cell proliferation and invasion by regulating miR365/RGS20 in oral squamous cell carcinoma. Oncol Rep 39, 1948–1956. [DOI] [PubMed] [Google Scholar]
- Hutchinson JN, Ensminger AW, Clemson CM, Lynch CR, Lawrence JB and Chess A (2007) A screen for nuclear transcripts identifies two linked noncoding RNAs associated with SC35 splicing domains. BMC Genom 8, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idogawa M, Ohashi T, Sasaki Y, Nakase H and Tokino T (2017) Long non‐coding RNA NEAT1 is a transcriptional target of p53 and modulates p53‐induced transactivation and tumor‐suppressor function. Int J Cancer 140, 2785–2791. [DOI] [PubMed] [Google Scholar]
- Imamura K, Imamachi N, Akizuki G, Kumakura M, Kawaguchi A, Nagata K, Kato A, Kawaguchi Y, Sato H, Yoneda M et al (2014) Long noncoding RNA NEAT1‐dependent SFPQ relocation from promoter region to paraspeckle mediates IL8 expression upon immune stimuli. Mol Cell 53, 393–406. [DOI] [PubMed] [Google Scholar]
- Ip JY and Nakagawa S (2012) Long non‐coding RNAs in nuclear bodies. Dev Growth Differ 54, 44–54. [DOI] [PubMed] [Google Scholar]
- Jen J, Tang YA, Lu YH, Lin CC, Lai WW and Wang YC (2017) Oct4 transcriptionally regulates the expression of long non‐coding RNAs NEAT1 and MALAT1 to promote lung cancer progression. Mol Cancer 16, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang P, Chen A, Wu X, Zhou M, Ul Haq I, Mariyam Z and Feng Q (2018a) NEAT1 acts as an inducer of cancer stem cell‐like phenotypes in NSCLC by inhibiting EGCG‐upregulated CTR1. J Cell Physiol 233, 4852–4863. [DOI] [PubMed] [Google Scholar]
- Jiang X, Zhou Y, Sun AJ and Xue JL (2018b) NEAT1 contributes to breast cancer progression through modulating miR‐448 and ZEB1. J Cell Physiol 233, 8558–8566. [DOI] [PubMed] [Google Scholar]
- Ke H, Zhao L, Feng X, Xu H, Zou L, Yang Q, Su X, Peng L and Jiao B (2016) NEAT1 is required for survival of breast cancer cells through FUS and miR‐548. Gene Regul Syst Bio 10, 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleer CG, Cao Q, Varambally S, Shen R, Ota I, Tomlins SA, Ghosh D, Sewalt RG, Otte AP, Hayes DF et al (2003) EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc Natl Acad Sci U S A 100, 11606–11611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung JT, Colognori D and Lee JT (2013) Long noncoding RNAs: past, present, and future. Genetics 193, 651–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon JH, Kim J, Park JY, Hong SM, Park CW, Hong SJ, Park SY, Choi YJ, Do IG, Joh JW et al (2010) Overexpression of high‐mobility group box 2 is associated with tumor aggressiveness and prognosis of hepatocellular carcinoma. Clin Cancer Res 16, 5511–5521. [DOI] [PubMed] [Google Scholar]
- Li Y, Chen D, Gao X, Li X and Shi G (2017a) LncRNA NEAT1 regulates cell viability and invasion in esophageal squamous cell carcinoma through the miR‐129/CTBP2 axis. Dis Markers 2017, 5314649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Harvey AR, Hodgetts SI and Fox AH (2017b) Functional dissection of NEAT1 using genome editing reveals substantial localization of the NEAT1_1 isoform outside paraspeckles. RNA 23, 872–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wang S, Li Z, Long X, Guo Z, Zhang G, Zu J, Chen Y and Wen L (2017d) The lncRNA NEAT1 facilitates cell growth and invasion via the miR‐211/HMGA2 axis in breast cancer. Int J Biol Macromol 105, 346–353. [DOI] [PubMed] [Google Scholar]
- Li X, Wang X, Song W, Xu H, Huang R, Wang Y, Zhao W, Xiao Z and Yang X (2018b) Oncogenic properties of NEAT1 in prostate cancer cells depend on the CDC5L‐AGRN transcriptional regulation circuit. Cancer Res 78, 4138–4149. [DOI] [PubMed] [Google Scholar]
- Li S, Yang J, Xia Y, Fan Q and Yang KP (2018a) Long noncoding RNA NEAT1 promotes proliferation and invasion via targeting miR‐181a‐5p in non‐small cell lung cancer. Oncol Res 26, 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Zhang Z, Liu X, Cheng X, Zhang Y, Han X, Zhang Y, Liu S, Yang J, Xu B et al (2017c) The FOXN3‐NEAT1‐SIN3A repressor complex promotes progression of hormonally responsive breast cancer. J Clin Invest 127, 3421–3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian Y, Xiong F, Yang L, Bo H, Gong Z, Wang Y, Wei F, Tang Y, Li X, Liao Q et al (2018) Long noncoding RNA AFAP1‐AS1 acts as a competing endogenous RNA of miR‐423‐5p to facilitate nasopharyngeal carcinoma metastasis through regulating the Rho/Rac pathway. J Exp Clin Cancer Res 37, 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang B, Wang S, Zhu XG, Yu YX, Cui ZR and Yu YZ (2005) Increased expression of mitogen‐activated protein kinase and its upstream regulating signal in human gastric cancer. World J Gastroenterol 11, 623–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling H, Vincent K, Pichler M, Fodde R, Berindan‐Neagoe I, Slack FJ and Calin GA (2015) Junk DNA and the long non‐coding RNA twist in cancer genetics. Oncogene 34, 5003–5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Chen N, Gong Y, Xiao R, Wang W and Pan Z (2017) The long non‐coding RNA NEAT1 enhances epithelial‐to‐mesenchymal transition and chemoresistance via the miR‐34a/c‐Met axis in renal cell carcinoma. Oncotarget 8, 62927–62938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Hei Y, Shu Q, Dong J, Gao Y, Fu H, Zheng X and Yang G (2012) VCP/p97, down‐regulated by microRNA‐129‐5p, could regulate the progression of hepatocellular carcinoma. PLoS ONE 7, e35800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Liang Y, Song R, Yang G, Han J, Lan Y, Pan S, Zhu M, Liu Y, Wang Y et al (2018b) Long non‐coding RNA NEAT1‐modulated abnormal lipolysis via ATGL drives hepatocellular carcinoma proliferation. Mol Cancer 17, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Tai Y and Ma J (2018a) LncRNA NEAT1/let‐7a‐5p axis regulates the cisplatin resistance in nasopharyngeal carcinoma by targeting Rsf‐1 and modulating the Ras‐MAPK pathway. Cancer Biol Ther 19, 534–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang Y, Fu X and Lu Z (2018c) Long non‐coding RNA NEAT1 promoted ovarian cancer cells’ metastasis via regulating of miR‐382‐3p/ROCK1 axial. Cancer Sci 109, 2188–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo PK, Wolfson B and Zhou Q (2016a) Cellular, physiological and pathological aspects of the long non‐coding RNA NEAT1. Front Biol (Beijing) 11, 413–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo PK, Zhang Y, Wolfson B, Gernapudi R, Yao Y, Duru N and Zhou Q (2016b) Dysregulation of the BRCA1/long non‐coding RNA NEAT1 signaling axis contributes to breast tumorigenesis. Oncotarget 7, 65067–65089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofstedt T, Fredlund E, Holmquist‐Mengelbier L, Pietras A, Ovenberger M, Poellinger L and Pahlman S (2007) Hypoxia inducible factor‐2alpha in cancer. Cell Cycle 6, 919–926. [DOI] [PubMed] [Google Scholar]
- Ma P, Ni K, Ke J, Zhang W, Feng Y and Mao Q (2018) miR‐448 inhibits the epithelial‐mesenchymal transition in breast cancer cells by directly targeting the E‐cadherin repressor ZEB1/2. Exp Biol Med (Maywood) 243, 473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello SS, Sinow C, Raj N, Mazur PK, Bieging‐Rolett K, Broz DK, Imam JFC, Vogel H, Wood LD, Sage J et al (2017) Neat1 is a p53‐inducible lincRNA essential for transformation suppression. Genes Dev 31, 1095–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M et al (2013) Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 495, 333–338. [DOI] [PubMed] [Google Scholar]
- Miki Y, Swensen J, Shattuck‐Eidens D, Futreal PA, Harshman K, Tavtigian S, Liu Q, Cochran C, Bennett LM, Ding W et al (1994) A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 266, 66–71. [DOI] [PubMed] [Google Scholar]
- Mito M, Kawaguchi T, Hirose T and Nakagawa S (2016) Simultaneous multicolor detection of RNA and proteins using super‐resolution microscopy. Methods 98, 158–165. [DOI] [PubMed] [Google Scholar]
- Murthy UM and Rangarajan PN (2010) Identification of protein interaction regions of VINC/NEAT1/Men epsilon RNA. FEBS Lett 584, 1531–1535. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Naganuma T, Shioi G and Hirose T (2011) Paraspeckles are subpopulation‐specific nuclear bodies that are not essential in mice. J Cell Biol 193, 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Shimada M, Yanaka K, Mito M, Arai T, Takahashi E, Fujita Y, Fujimori T, Standaert L, Marine JC et al (2014) The lncRNA Neat1 is required for corpus luteum formation and the establishment of pregnancy in a subpopulation of mice. Development 141, 4618–4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nana‐Sinkam SP and Croce CM (2011) Non‐coding RNAs in cancer initiation and progression and as novel biomarkers. Mol Oncol 5, 483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa Y, Konno M, Asai A, Koseki J, Kawamoto K, Miyoshi N, Takahashi H, Nishida N, Haraguchi N, Sakai D et al (2018) Hypoxia stimulates the cytoplasmic localization of oncogenic long noncoding RNA LINC00152 in colorectal cancer. Int J Oncol 52, 453–460. [DOI] [PubMed] [Google Scholar]
- Prasanth KV, Prasanth SG, Xuan Z, Hearn S, Freier SM, Bennett CF, Zhang MQ and Spector DL (2005) Regulating gene expression through RNA nuclear retention. Cell 123, 249–263. [DOI] [PubMed] [Google Scholar]
- Qi X, Zhang DH, Wu N, Xiao JH, Wang X and Ma W (2015) ceRNA in cancer: possible functions and clinical implications. J Med Genet 52, 710–718. [DOI] [PubMed] [Google Scholar]
- Qian K, Liu G, Tang Z, Hu Y, Fang Y, Chen Z and Xu X (2017) The long non‐coding RNA NEAT1 interacted with miR‐101 modulates breast cancer growth by targeting EZH2. Arch Biochem Biophys 615, 1–9. [DOI] [PubMed] [Google Scholar]
- Reicher A, Fosselteder J, Kwong LN and Pichler M (2018) Crosstalk between the Notch signaling pathway and long non‐coding RNAs. Cancer Lett 420, 91–96. [DOI] [PubMed] [Google Scholar]
- Salmena L, Poliseno L, Tay Y, Kats L and Pandolfi PP (2011) A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell 146, 353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson VB, Rong NH, Han J, Yang Q, Aris V, Soteropoulos P, Petrelli NJ, Dunn SP and Krueger LJ (2007) MicroRNA let‐7a down‐regulates MYC and reverts MYC‐induced growth in Burkitt lymphoma cells. Cancer Res 67, 9762–9770. [DOI] [PubMed] [Google Scholar]
- Sasaki YT, Ideue T, Sano M, Mituyama T and Hirose T (2009) MENepsilon/beta noncoding RNAs are essential for structural integrity of nuclear paraspeckles. Proc Natl Acad Sci U S A 106, 2525–2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schanza LM, Seles M, Stotz M, Fosselteder J, Hutterer GC, Pichler M and Stiegelbauer V (2017) MicroRNAs associated with von hippel‐lindau pathway in renal cell carcinoma: a comprehensive review. Int J Mol Sci 18, E2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla GC, Singh J and Barik S (2011) MicroRNAs: processing, maturation, target recognition and regulatory functions. Mol Cell Pharmacol 3, 83–92. [PMC free article] [PubMed] [Google Scholar]
- Smolle MA, Bauernhofer T, Pummer K, Calin GA and Pichler M (2017) Current insights into long non‐coding RNAs (LncRNAs) in prostate cancer. Int J Mol Sci 18, E473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolle MA and Pichler M (2018) The role of long non‐coding RNAs in osteosarcoma. Noncoding RNA 4, E7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standaert L, Adriaens C, Radaelli E, Van Keymeulen A, Blanpain C, Hirose T, Nakagawa S and Marine JC (2014) The long noncoding RNA Neat1 is required for mammary gland development and lactation. RNA 20, 1844–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Guo MM, Han P, Lin JZ, Liang FY, Tan GM, Li HB, Zeng M and Huang XM (2012) Id‐1 and the p65 subunit of NF‐kappaB promote migration of nasopharyngeal carcinoma cells and are correlated with poor prognosis. Carcinogenesis 33, 810–817. [DOI] [PubMed] [Google Scholar]
- Sun SJ, Lin Q, Ma JX, Shi WW, Yang B and Li F (2017) Long non‐coding RNA NEAT1 acts as oncogene in NSCLC by regulating the Wnt signaling pathway. Eur Rev Med Pharmacol Sci 21, 504–510. [PubMed] [Google Scholar]
- Tan HY, Wang C, Liu G and Zhou X (2018) Long noncoding RNA NEAT1‐modulated miR‐506 regulates gastric cancer development through targeting STAT3. J Cell Biochem. 10.1002/jcb.26691 [DOI] [PubMed] [Google Scholar]
- Thomson DW and Dinger ME (2016) Endogenous microRNA sponges: evidence and controversy. Nat Rev Genet 17, 272–283. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y, Gorham J, Cossman J, Jaffe E and Croce CM (1985) The t(14;18) chromosome translocations involved in B‐cell neoplasms result from mistakes in VDJ joining. Science 229, 1390–1393. [DOI] [PubMed] [Google Scholar]
- Tu J, Zhao Z, Xu M, Lu X, Chang L and Ji J (2018) NEAT1 upregulates TGF‐beta1 to induce hepatocellular carcinoma progression by sponging hsa‐mir‐139‐5p. J Cell Physiol 233, 8578–8587. [DOI] [PubMed] [Google Scholar]
- Villodre ES, Kipper FC, Pereira MB and Lenz G (2016) Roles of OCT4 in tumorigenesis, cancer therapy resistance and prognosis. Cancer Treat Rev 51, 1–9. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang C, Chen C, Wu F, Shen P, Zhang P, He G and Li X (2017b) Long non‐coding RNA NEAT1 regulates epithelial membrane protein 2 expression to repress nasopharyngeal carcinoma migration and irradiation‐resistance through miR‐101‐3p as a competing endogenous RNA mechanism. Oncotarget 8, 70156–70171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Yu Y, Fan S and Luo L (2017a) Knockdown of long non‐coding RNA NEAT1 inhibits proliferation and invasion and induces apoptosis of osteosarcoma by inhibiting miR‐194 Expression. Yonsei Med J 58, 1092–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Zhang H, Wang L, Zhang S and Tang M (2016) miR‐613 inhibits the growth and invasiveness of human hepatocellular carcinoma via targeting DCLK1. Biochem Biophys Res Commun 473, 987–992. [DOI] [PubMed] [Google Scholar]
- Wang L and Zhu H (2018) Long noncoding nuclear paraspeckle assembly transcript 1 acts as prognosis biomarker and increases cell growth and invasion in cervical cancer by sequestering microRNA101. Mol Med Rep 17, 2771–2777. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zou Q, Song M and Chen J (2017c) NEAT1 promotes cell proliferation and invasion in hepatocellular carcinoma by negative regulating miR‐613 expression. Biomed Pharmacother 94, 612–618. [DOI] [PubMed] [Google Scholar]
- Wedge DC, Gundem G, Mitchell T, Woodcock DJ, Martincorena I, Ghori M, Zamora J, Butler A, Whitaker H, Kote‐Jarai Z et al (2018) Sequencing of prostate cancers identifies new cancer genes, routes of progression and drug targets. Nat Genet 50, 682–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JA, Mito M, Kurosaka S, Takumi T, Tanegashima C, Chujo T, Yanaka K, Kingston RE, Hirose T, Bond C et al (2016) Structural, super‐resolution microscopy analysis of paraspeckle nuclear body organization. J Cell Biol 214, 817–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Mo Q, Wan X, Dan J and Hu H (2017) NEAT1/has‐mir‐98‐5p/MAPK6 axis is involved in non‐small‐cell lung cancer (NSCLC) development. J Cell Biochem. 10.1002/jcb.26442 [DOI] [PubMed] [Google Scholar]
- Wu Y, Sarkissyan M and Vadgama JV (2016b) Epithelial‐mesenchymal transition and breast cancer. J Clin Med 5, E13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Zhang S, Shan J, Hu Z, Liu X, Chen L, Ren X, Yao L, Sheng H, Li L et al (2016a) Elevated HMGA2 expression is associated with cancer aggressiveness and predicts poor outcome in breast cancer. Cancer Lett 376, 284–292. [DOI] [PubMed] [Google Scholar]
- Xiao ZD, Han L, Lee H, Zhuang L, Zhang Y, Baddour J, Nagrath D, Wood CG, Gu J, Wu X et al (2017) Energy stress‐induced lncRNA FILNC1 represses c‐Myc‐mediated energy metabolism and inhibits renal tumor development. Nat Commun 8, 783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Wu CH and Hu HZ (2016) LncRNA UCA1 promotes epithelial‐mesenchymal transition (EMT) of breast cancer cells via enhancing Wnt/beta‐catenin signaling pathway. Eur Rev Med Pharmacol Sci 20, 2819–2824. [PubMed] [Google Scholar]
- Xiong W, Huang C, Deng H, Jian C, Zen C, Ye K, Zhong Z, Zhao X and Zhu L (2018) Oncogenic non‐coding RNA NEAT1 promotes the prostate cancer cell growth through the SRC3/IGF1R/AKT pathway. Int J Biochem Cell Biol 94, 125–132. [DOI] [PubMed] [Google Scholar]
- Yamazaki T, Fujikawa C, Kubota A, Takahashi A and Hirose T (2018a) CRISPRa‐mediated NEAT1 lncRNA upregulation induces formation of intact paraspeckles. Biochem Biophys Res Commun 504, 218–224. [DOI] [PubMed] [Google Scholar]
- Yamazaki T, Souquere S, Chujo T, Kobelke S, Chong YS, Fox AH, Bond CS, Nakagawa S, Pierron G and Hirose T (2018b) Functional domains of NEAT1 architectural lncRNA induce paraspeckle assembly through phase separation. Mol Cell 70, 1038–1053 e7. [DOI] [PubMed] [Google Scholar]
- Yan W, Cao QJ, Arenas RB, Bentley B and Shao R (2010) GATA3 inhibits breast cancer metastasis through the reversal of epithelial‐mesenchymal transition. J Biol Chem 285, 14042–14051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Li Z, Li Y, Xu R, Wang Y, Tian Y and Chen W (2017a) Long non‐coding RNA NEAT1 overexpression is associated with poor prognosis in cancer patients: a systematic review and meta‐analysis. Oncotarget 8, 2672–2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Xiao Z, Du X, Huang L and Du G (2017b) Silencing of the long non‐coding RNA NEAT1 suppresses glioma stem‐like properties through modulation of the miR‐107/CDK6 pathway. Oncol Rep 37, 555–562. [DOI] [PubMed] [Google Scholar]
- Yu X, Li Z, Zheng H, Chan MT and Wu WK (2017) NEAT1: A novel cancer‐related long non‐coding RNA. Cell Prolif 50, e12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng C, Xu Y, Xu L, Yu X, Cheng J, Yang L, Chen S and Li Y (2014) Inhibition of long non‐coding RNA NEAT1 impairs myeloid differentiation in acute promyelocytic leukemia cells. BMC Cancer 14, 693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z and Carmichael GG (2001) The fate of dsRNA in the nucleus: a p54(nrb)‐containing complex mediates the nuclear retention of promiscuously A‐to‐I edited RNAs. Cell 106, 465–475. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li Y, Dong M and Wu D (2017a) Long non‐coding RNA NEAT1 regulates E2F3 expression by competitively binding to miR‐377 in non‐small cell lung cancer. Oncol Lett 14, 4983–4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lun L, Li H, Wang Q, Lin J, Tian R, Pan H, Zhang H and Chen X (2017c) The value of lncRNA NEAT1 as a prognostic factor for survival of cancer outcome: a meta‐analysis. Sci Rep 7, 13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Sun Y and Ma L (2015) ZEB1: at the crossroads of epithelial‐mesenchymal transition, metastasis and therapy resistance. Cell Cycle 14, 481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Wu WB, Wang ZW and Wang XH (2017b) lncRNA NEAT1 is closely related with progression of breast cancer via promoting proliferation and EMT. Eur Rev Med Pharmacol Sci 21, 1020–1026. [PubMed] [Google Scholar]
- Zhang XN, Zhou J and Lu XJ (2018) The long noncoding RNA NEAT1 contributes to hepatocellular carcinoma development by sponging miR‐485 and enhancing the expression of the STAT3. J Cell Physiol 233, 6733–6741. [DOI] [PubMed] [Google Scholar]
- Zhao D, Zhang Y, Wang N and Yu N (2017) NEAT1 negatively regulates miR‐218 expression and promotes breast cancer progression. Cancer Biomark 20, 247–254. [DOI] [PubMed] [Google Scholar]
- Zhou S, Wang L, Yang Q, Liu H, Meng Q, Jiang L, Wang S and Jiang W (2018) Systematical analysis of lncRNA‐mRNA competing endogenous RNA network in breast cancer subtypes. Breast Cancer Res Treat 169, 267–275. [DOI] [PubMed] [Google Scholar]
- Zhou HJ, Yan J, Luo W, Ayala G, Lin SH, Erdem H, Ittmann M, Tsai SY and Tsai MJ (2005) SRC‐3 is required for prostate cancer cell proliferation and survival. Cancer Res 65, 7976–7983. [DOI] [PubMed] [Google Scholar]


