Abstract
Despite substantial progress in oncology, lung cancer remains the number one malignancy in terms of both incidence and mortality rates, and there thus remains an urgent need for new therapeutic alternatives. MicroRNA (miRNA) have an important role in cancer initiation and progression due to their capacity to interfere with transcriptional signaling and regulate key cellular processes. miR‐181a and miR‐181b (miR‐181a/b), which are located on chromosomes 1 and 9, are pathologically expressed in the tumor tissue and plasma of patients diagnosed with lung cancer. The miR‐181a/b regulatory mechanisms are sophisticated and are directly related to different target genes. In recent years, an ever‐increasing number of studies have focused on the biological relevance of miR‐181a/b in key cellular processes. In this paper, we aim to discuss the challenging experimental data related to miR‐181a/b and their potential use for the development of new therapeutic approaches in lung cancer. We will further present the ongoing issues regarding the regulation of their multiple target genes, and their potential use as biomarkers and therapeutic targets in this deadly malignancy.
Keywords: lung cancer, miR‐181a/b, therapy
Abbreviations
- A549/cis
A549 cell line resistant to cisplatin
- ADC
adenocarcinoma
- AMO‐miR‐181
antimiR‐181a oligonucleotides
- EMT
epithelial‐to‐mesenchymal transition
- IL
interleukin
- LNA
locked nucleic acid
- lncRNA
long ncRNA
- LSCC
lung squamous cell carcinoma
- MAK
mitogen activated protein kinases
- miRNA
microRNA
- ncRNA
non‐coding RNA
- NF‐κB
nuclear factor kappa beta
- N
normal tissue
- NSCLC
non‐small cell lung cancer
- SCLC
small cell lung cancer
- siRNA
small interfering RNA
- TGFβR1
transforming growth factor β receptor 1
- TGFβR2
transforming growth factor β receptor 2
- TGFβ
transforming growth factor β
- TNM
tumor
node
metastases
- T
tumor tissue
1. Introduction
Lung cancer is the most frequent cause of death for patients diagnosed with cancer worldwide, and is responsible for approximately 18.4% of the total cancer deaths in both sexes (Bray et al., 2018; Didkowska et al., 2016). The mortality and incidence ratios in both developed and developing countries (Bray et al., 2018) are affected by the presence of various risk factors, the efficiency of the diagnostic methods, and/or the treatment accessibility (Bray et al., 2018; Choi et al., 2017). There are two histological subtypes of lung cancer: small cell lung cancer (SCLC, 15%) and non‐SCLC (NSCLC, 85%) (Herbst et al., 2018; Liu et al., 2016). The most common NSCLC subtypes are lung adenocarcinoma (ADC) and squamous cell carcinoma (SCC; Herbst et al., 2018).
Lung cancer progression is dependent on the tumor microenvironment and is caused by different factors (tobacco smoke remains the most relevant, along with asbestos, arsenic, inorganic arsenic compounds, and ionizing radiation; Field and Withers, 2012) which affect the clinical phenotype, the development of bone and brain metastases, and the response to therapy (Herbst et al., 2018; Popper, 2016). One major clinical issue is the lack of early diagnostic and prognostic markers, together with the absence of specific treatment targets. Therefore, advanced forms of disease are usually unresponsive to chemotherapy and only 10–15% of patients have a survival rate of over 5 years. The use of checkpoint inhibitors improved the response rate and survival of some lung cancer patients (Aguiar et al., 2017; Jain et al., 2018; Thungappa et al., 2017). It is therefore of great interest at the present time to explore new targeted therapeutic alternatives or adjuvant systems.
Non‐coding RNAs (ncRNAs) are classified based on their size as small ncRNA (< 200 nucleotides) and long ncRNA (lncRNA, > 200 nucleotides). The main representatives of small ncRNA are microRNAs (miRNAs), small interfering RNAs (siRNAs), Piwi‐interacting RNAs, and small nucleolar RNAs (snRNAs). There is growing evidence that deregulated ncRNA have an important function in the onset and progression of lung cancer (Berindan‐Neagoe et al., 2014; Catana et al., 2015), contributing to disease prognosis as well, and regulating the response to therapy (Braicu et al., 2014; Pan et al., 2017; Volinia et al., 2006).
MiRNA are short non‐coding transcripts approximately 22 nucleotides in length (Braicu et al., 2014; Calin and Croce, 2006; Redis et al., 2012; Strmsek and Kunej, 2015). By directly binding RNA from the messenger RNA and ncRNA categories, the function of a wide range of genes can be regulated through degradation of the RNA or inhibition of the translational processes (Braicu et al., 2015; Catana et al., 2015, 2017; Cipolla et al., 2018; Irimie et al., 2017a). An essential mechanistic feature of these transcripts relates to the partial complementarity to their target genes (Cipolla et al., 2018); therefore, a miRNA transcript can target multiple RNAs and a specific RNA can be regulated by several miRNAs (Berindan‐Neagoe and Calin, 2014; Berindan‐Neagoe et al., 2017; Braicu et al., 2014; Calin and Croce, 2006; Pop‐Bica et al., 2017; Sonea et al., 2018). MiRNAs have significant roles in all fundamental biological processes (cell differentiation or proliferation, apoptosis, cell cycle progression, invasion/distant metastasis and immune responses) (Eastlack and Alahari, 2015; Munker and Calin, 2011).
Alterations in miRNA expression levels are related to cancer pathogenesis (Chira et al., 2018; Sevignani et al., 2007). Generally, the transcripts with a reduced expression level have a tumor role, whereas overexpressed transcripts support oncogenesis (Berindan‐Neagoe et al., 2014; Catana et al., 2015; Irimie et al., 2017a; Munker and Calin, 2011). Moreover, due to their high stability, miRNAs can be found in different biological fluids either as free circulating molecules or incorporated in extracellular vesicles (e.g. exosomes). Variations of miRNAs levels in liquid biopsies are important minimally invasive diagnostic/prognostic tools and also therapeutic targets (e.g. exosome depletion; Gulei et al., 2018b; Pop‐Bica et al., 2018).
Currently, an increasing number of studies focus on experimental modulation of some miRNAs that are altered in different tumors to restore their normal expression level (miRNA inhibition or replacement; Berindan‐Neagoe et al., 2014; Braicu et al., 2014; Munker and Calin, 2011; Redis et al., 2012; Shah et al., 2016). In this review, we present an outline of recent studies on common and specific functions of miR‐181a and miR‐181b in lung cancers. A particular focus is on understanding the role of miR‐181a/b in lung cancer biology in order to facilitate the development of novel therapies based on miRNA modulation.
The miR‐181 family is highly conserved in different species (Yang et al., 2014). This family contains four mature members, of which miR‐181a and miR‐181b are located on chromosomes 1 and 9, and miR‐181c and miR‐181d are clustered on chromosome 19 (Yang et al., 2017). As a consequence of genome duplication, miRNA‐181a as well as miR‐181b, have duplicate copies in the human genome, and can for this reason be derived from different precursors (Fig. 1).
Figure 1.
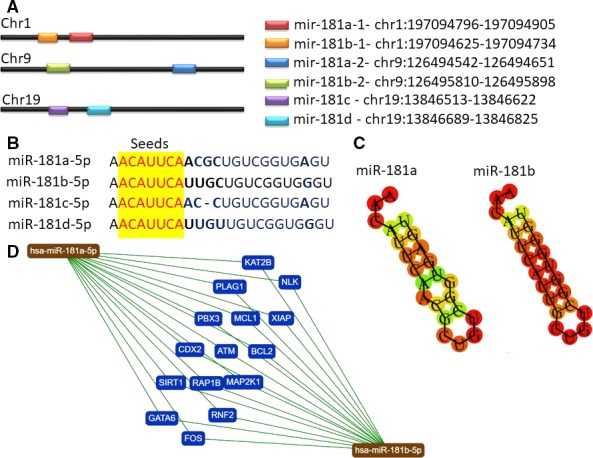
Localization, sequence and targets of the miR‐181 family members. (A) Chromosomal location of the members of the miR‐181 family and their sequence; genomic localization of miR‐181 family members was done using UCSC genome browser (https://genome.ucsc.edu). (B) Mir‐181 sequences containing the seed region (yellow) that is common for all transcripts. (C) MiR‐181a and miR‐181b common validated target genes according to miRtargetLink human database (https://ccb-web.cs.uni-saarland.de/mirtargetlink/).
miR‐181a and miR‐181b play diverse roles in regulating key aspects of cellular growth, development, angiogenesis, invasion, and metastasis in a wide range of solid tumors, including lung cancers (Xu et al., 2015). In this malignancy, the expression levels of miR‐181a and miR‐181b are decreased, indicating that depletion of these transcripts may facilitate lung tumorigenesis or disease progression, and activate drug resistance mechanisms (Cao et al., 2017; Cipolla et al., 2018; Liu et al., 2016; Shukla, 2018; Yang et al., 2013a). The downregulated profile of miR‐181a/b in cancer can be related to the methylation status, as proven in colorectal cancer (Shi et al., 2018), but no information regarding possible epigenetic regulation of the other two transcripts (miR‐181c/d) exists at this moment. Most of the research on lung cancer has centered on miR‐181a/b, probably with the expectation that the other members (miR‐181c and miR‐181d) have similar functions due to their identical seed sequences.
2. miR‐181a/b expression levels in lung cancer
miR‐181a/b are associated with a wide range of tumor and non‐tumor pathologies (metabolic disorders, neurodegenerative or infectious diseases, cardiovascular pathologies) (An et al., 2017; Sun et al., 2014a). These two transcripts can have dual roles, depending on different target genes or their mutational status (Seoudi et al., 2012). Overexpression was observed in breast (Bisso et al., 2013; Liu et al., 2017), ovarian (Lee et al., 2012; Li et al., 2016b; Parikh et al., 2014; Xia and Gao, 2014) and cervical cancer (Chen et al., 2014; Ke et al., 2013; Xu et al., 2016), whereas miR‐181a/b are generally downregulated in lung cancer (Cao et al., 2017; Cinegaglia et al., 2016; Huang et al., 2015; Liu et al., 2016; Ma et al., 2015; Tian et al., 2016; Wang et al., 2015a; Yang et al., 2013a) and glioblastoma (Ayala‐Ortega et al., 2016; He et al., 2016; Lakomy et al., 2011; Shi et al., 2008; Slaby et al., 2010; Sun et al., 2014b; Zhang et al., 2012; Zhi et al., 2014). Moreover, the expression levels of these transcripts are often cell type‐specific and, in some cases, transitory or consistent with the degree of cell differentiation (Chu et al., 2015; Zhang et al., 2017b).
The expression levels of these two transcripts in lung cancer are related to clinico‐pathological characteristics (Table 1) (Gao et al., 2010; Ma et al., 2015). Decreased expression levels were confirmed in a meta‐analysis study on NSCLC, further correlated with patient survival rate (Pop‐Bica et al., 2018). miR‐181a/b are associated not only with an unfavorable survival but also with TNM staging (Gao et al., 2010; Liu et al., 2016; Wang et al., 2015a; Yang et al., 2013a). MiR‐181b expression alone is related to overall survival (OS) and disease‐free survival (DFS) for NSCLC (Wang et al., 2015a; Yang et al., 2013a). Distant metastases are important factors in patient prognoses and are one of the main reasons for the failure of NSCLC treatment. MiR‐181a/b can be used as prognostic markers or as therapeutic targets for limitation of the spread of lung cancer, based on their direct regulation of metastasis (Wang et al., 2015a; Yang et al., 2013a).
Table 1.
Summary of the relative expression of miR‐181a and miR‐181b in tissue and other biological specimens from patients diagnosed with lung cancer. N, normal tissue; T, tumor tissue; FC, fold change; ↓, downregulation; ↑, upregulation; ns, not statistically significant; AUC, area under the curve for the ROC (receiver operating characteristic)
| Type of lung cancer | Expression level | Biological specimens and approach used for evaluation | Relevant finding of the study | Reference |
|---|---|---|---|---|
| NSCLC | ↓miR‐181a | 8 paired samples for microarray; 47 matched paired samples for qRT‐PCR | Microarray data FC: 0.42; qRT‐PCR FC: 0.54; a correlation with low miR‐181a, high clinical stage and lymph node positive leads to poor prognosis of NSCLC | Gao et al. (2010) |
| NSCLC | ↓miR‐181b | 35 patients with NSCLC and 24 normal tissues | ↓miR‐181a in T versus N; FC: 0.3 ± 0.05, P ≤ 0.05 | Cao et al. (2017) |
| NSCLC | ↓miR‐181a | 22 paired tissues | ↓miR‐181a in T and cell lines; FC: 0.5 ± 0.2 | Wang et al. (2017a) |
| NSCLC | ↓miR‐181b | 126 paired tissues | FC for miR‐181b N: 5.9 ± 0.9, T: 2.5 ± 0.7, P ≤ 0.01; ↓miR‐181b associated with unfavorable prognostic | Yang et al. (2013a) |
| NSCLC | ↓miR‐181b | 62 paired tissues | FC for miR‐181b N: 2 ± 1, T: 5.5 ± 0.5, P ≤ 0.01; ↓miR‐181b associated with TNM and metastases, inhibits metastasis by downregulation of HMGB1 | Liu et al. (2016) |
| NSCLC | ↓miR‐181b | 27 match paired tissues | ↓miR‐181b in T: 0.2978 ± 0.03, N 1.202 ± 0.06 | Huang et al. (2015) |
| NSCLC | ↓miR‐181b | 20 NSCLC patients sensitive to therapy; 18 NSCLC patients’ non‐responders to therapy | ↓miR‐181b in resistant to therapy cases; FC 0.8 ± 2.2 for sensitive (n = 20); 4.1 ± 1.8 for resistant (n = 20) | Wang et al. (2015a) |
| Stage I NSCLC | ↑miR‐181b | Profiling study: 46 patients stage I NSCLC and 42 healthy control; qRT‐PCR validation: 20 NSCLC stage I and 30 healthy control | ↑miR‐181a upregulated NSCLC versus healthy controls; exosomal miR‐181a a specific biomarker for ADC | Jin et al. (2017) |
| Lung ADC | ↓miR‐181a | miRNA‐Seq for 7 paired samples; qRT‐PCR in 22 LA and 12 normal lung tissues | miRNA‐Seq: logFC for miR‐181a‐1 T: −1.1444 (P‐value 0.0006); logFC for miR‐181a‐2 T: −1.1145 (P‐value 0.0002); qRT‐PCR: logFC for miR‐181a‐1: −0.862 (P‐value 0.140); logFC for miR‐181a: 0.862 (P‐value 0.140) | Cinegaglia et al. (2016) |
| LSCC | ↑miR‐181b | Profiling NGS Illumina: 9 LSCC and 9 ADC paired samples; qRT‐PCR validation 18 paired tissue and plasma | ↑miR‐181b‐5p upregulated in tissue and plasma LSCC | Tian et al. (2016) |
| LSCC | miR‐181a |
23 paired LSC 102 male LSCC Patients plasma and 101 healthy controls; 16 LSCC plasma and 16 healthy controls plasma |
↓ miR‐181a in T versus N ↑miR‐181a in LSCC plasma versus healthy controls, FC: 3.04, AUC: 0.731; ns in LSCC plasma versus healthy controls plasma exosomes |
Shan et al. (2018) |
Despite a general downregulated profile for MiR‐181a/b, some studies report a different trend. MiR‐181a was found upregulated in an NSCLC model of Gefitinib‐resistant cells when compared with the sensitive counterparts. The same pattern was observed in the plasma samples of patients with acquired Gefitinib resistance compared with the levels measured before Gefitinib treatment from the same patients. A negative correlation between miR‐181a and GAS7 was identified in NSCLC tumors; moreover, increased GAS7 expression is associated with improved patient survival. These data sustain the role of miR‐181a/GAS7 axis in controlling Gefitinib resistance, an axis that could become a therapeutic target in these patients (Ping et al., 2018).
In an integrative analysis focused on the altered miRNA pattern in lung cancer, the authors found that miR‐181a/b/c were all downregulated in ADC samples (Cinegaglia et al., 2016). An unpaired analysis of 17 lung ADC tumors and seven normal tissue samples identified 11 statistically significant, differentially expressed miRNA transcripts, including underexpressed miR‐181b‐1 and miR‐181b‐2. Meanwhile, paired sample analysis demonstrated 22 statistically significant miRNAs, eight transcripts with a reduced expression level (including miR‐181a‐1 and miR‐181a‐2), and 14 transcripts with an increased level (including miR‐181c). In a database comprising 1491 lung ADC and 441 normal tissues, 13 overexpressed transcripts were identified, including miR‐181b, miR‐181c, and three downregulated transcripts (miR‐181a, miR‐574, and miR‐1247; Cinegaglia et al., 2016).
Lung squamous cell carcinoma (LSCC) displays a differential expression level of miR‐181a between plasma and tissue; a miRNA pattern for male LSCC patients from the TCGA dataset revealed a downregulation of this transcript which was further validated in another patient cohort of 23 paired samples of LSCC. The same study showed an overexpression of miR‐181a in plasma, but when independently analyzing only the exosomal fraction, the results were not statistically significant (Shan et al., 2018). Contradictory data showed that miR‐181b is overexpressed in tumor tissue and plasma in LSCC patients in a profiling study on nine LSCC (paired samples) and nine ADC (paired samples), followed by a validation on 18 LSCC paired tissue and plasma samples (Tian et al., 2016). Such data can also be explained by the limited number of samples analyzed, indicating that larger studies are essential.
All in all, the expression/function of miR‐181a/b in lung cancer is not always consistent between studies, prompting the dual or context‐dependent role of these transcripts. In addition, there is growing evidence from clinical studies that these miRNAs can act as biomarkers, but it might be more relevant to consider not only their absolute expression levels but also the balance between the expression of miRNA and targeted genes.
3. miR‐181a and miR‐181b mediate lung cancer hallmarks
Recent studies demonstrate that MiR‐181a/b are involved in the regulation of lung cancer hallmarks (Hanahan and Weinberg, 2011). MiR‐181a/b are downregulated in most of the studies and target important genes involved in the regulation of cell proliferation, evasion of growth suppression or resistance to cell death, as well as replicative immortality. These miRNAs also interfere with pathways involved in tumor angiogenesis, invasion, metastasis, and drug sensitivity/resistance in lung cancer (Fig. 2). Another less studied aspect is the connection with energy metabolism in lung cancer (Chu et al., 2015; Li et al., 2013).
Figure 2.
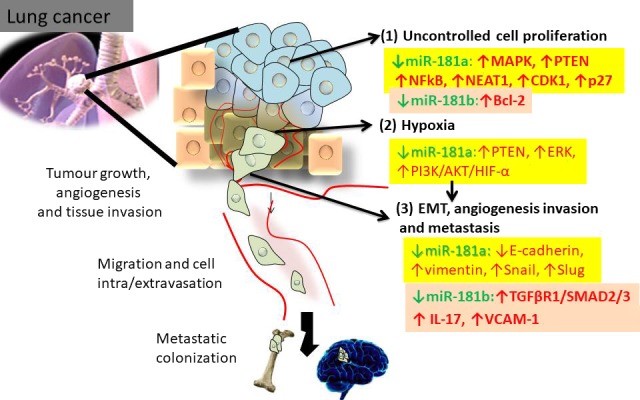
Relevant mechanistic insights connected with miR‐181a/b in lung cancer. (1) MiR‐181a/b target key genes involved in the regulation (1) of cell proliferation; (2) intra‐tumor hypoxia; (3) EMT, tumor angiogenesis, invasion, and distant metastasis.
3.1. miR‐181a/b inhibit proliferative signaling in lung cancer
miR‐181a/b are involved in mechanisms related to proliferation and growth signals (Shi et al., 2017). A549 cells transfected with miR‐181a‐5p mimic have a decreased cell proliferation and migration rate compared with the control counterparts, the effect being mediated in part by targeting of K‐RAS (Ma et al., 2015) and MAPK activity (He et al., 2015). Exposure of lung cancer cells (H226 and H460 cells) to interleukin (IL)‐17 decreases miR‐181a levels and upregulates VCAM‐1 expression, a direct target. Administration of miR‐181a attenuates cell proliferation and migration rates, demonstrating the therapeutic potential of the IL‐17/miR‐181a/VCAM‐1 axis (Wang et al., 2015a). Contradictory data were shown in a study where treatment of A549 cells with anti‐miR‐181a oligonucleotides (AMO‐miR‐181a) increased the apoptosis rate and lead to S‐phase cell (Fei et al., 2008).
3.2. miR‐181a/b target key genes involved in evasion of growth suppression or resistance to cell death, and replicative immortality
miR‐181b is downregulated in A549/cis (cisplatin resitant) compared with A549 cells, suppression that mediates drug‐resistant mechanisms and migratory features. Replacement strategies decreased cell proliferation, enhanced the sensitivity of the cells to cisplatin, and impaired the migratory phenotype in both in vitro and in vivo models. Transforming growth factor receptor 1 (TGFβR1) is a direct target of miR‐181b; moreover miR‐181b mimic administration decreased c‐Myc and Cyclin D1 and upregulated p27, results that overlap with those obtained by siRNA‐TGFβR1 transfection (Wang et al., 2015a). MiR‐181a contributes to cell cycle arrest by the upregulation of the cell cycle inhibitor p27Kip1 (Galluzzi et al., 2010).
Restoration of miR‐181a expression is connected with inhibition of cyclin B1 and D1 expression in NSCLC cells and direct targeting of CDK1 (Shi et al., 2017). MiR‐181a targets apoptotic genes such as Bcl‐2 in acute lung injury (Li et al., 2016a) and also in lung cancer (Huang et al., 2015). MiR‐181a activates the apoptotic signaling cascade by a p53 tumor‐suppressor independent mechanism (Galluzzi et al., 2010).
3.3. miR‐181a/b are important regulators of angiogenesis, invasion, and metastasis
Hypoxia is a frequent event in malignant solid tumors and is further connected to the activation of angiogenesis (Choudhry and Harris, 2018). A hypoxic environment in lung cancer promotes invasion and metastasis through activation of MAPK signaling and macrophage polarization (Zhang et al., 2014), two mechanisms that are connected with miR‐181a/b expression (Bi et al., 2016; Wang et al., 2017a; Yang et al., 2013b; Zhang et al., 2013). An important aspect of metastasis consists in activation of epithelial‐to‐mesenchymal transition (EMT), which enables cells to migrate and populate secondary sites (Expósito‐Villén et al., 2018; Gulei et al., 2017, 2018a; Tudoran et al., 2012). In solid tumors, including lung cancer, transformed cells that undergo EMT lose epithelial features and acquire mesenchymal features (Expósito‐Villén et al., 2018; Zhang et al., 2017a). This phenotype undergoes self‐renewal and presents an increased capacity for adaptation to diverse environments, while favoring invasion and migration. TGFβ and its receptors (TGFβR1 and TGFβR2) have an important role not only in the regulation of cell fate (cell proliferation and apoptosis; Zhang et al., 2017a) but, more importantly, in the regulation of EMT, being concomitantly modulated by miR‐181a/b. MiR‐181a is present in breast cancer as a TGFβ‐regulated ‘metastamir’ (Taylor et al., 2013) that activates and promotes invasive and metastatic processes (Ionescu et al., 2014; Parikh et al., 2014). MiR‐181a is a promoter of TGFβ‐mediated EMT in ovarian cancer.
Studies showed a connection between miR‐181b with TGFβ signaling and PI3K/AKT signaling (Wang et al., 2015a). The miR‐181b/PI3K/AKT signaling pathway is a fundamental axis not only for the regulation of cell proliferation but also for the EMT and metastasis in lung cancer (Fumarola et al., 2014; Zhao et al., 2018).
3.4. miR‐181a/b has the capacity to avoid immune destruction
The tumor microenvironment (TME), consisting of stroma and extracellular matrix elements as well as immune cells, has an important role in lung cancer progression and invasion, migration, and metastasis (Quail and Joyce, 2013; Wang et al., 2017b). The presence of tumor‐associated macrophages under the immunosuppressive M2 phenotype disturbs the tumor microenvironment and sustains disease advancement (Guo et al., 2016; Zhang et al., 2013). MiR‐181a was observed to have a higher level in M2 than in M1 phenotype (Bi et al., 2016). MiR‐181a regulates the M2 macrophage‐mediated migration and invasion capacity of tumor cells (Bi et al., 2016). M2 macrophages infiltrate the tumor tissue, sustained by the release of cytokines/chemokines (Zhang et al., 2017a), with a possible localization in lung tumor hypoxic regions, where miR‐181a can play important roles (Zhang et al., 2014). MiR‐181a has the capacity to regulate the activity of CD8+ T cell influx, and the downregulation of multiple phosphatases by miR‐181a leads to a reduction in T cell receptor signaling. Therefore, miR‐181a is actively involved in the pro‐tumorigenic symbiotic role between tumor cells and tumor microenvironment effectors (Rupaimoole et al., 2016).
3.5. miR‐181a/b‐related therapeutic strategy in lung cancer
The main issue regarding miRNA therapy consists of the development of efficient delivery systems. The principal pharmaceutical formulations for miRNA delivery are liposomes, polymeric nanoparticles, and viral systems (Irimie et al., 2017b; Jurj et al., 2017; Tomuleasa et al., 2014). There are clinical safety concerns regarding viral delivery systems; among the non‐viral systems, the most promising are represented by liposomes (Chen et al., 2016; Yang, 2015).
Oncogenic miRNA are generally restored to their homeostatic level through different types of molecules: AMO (anti‐miRNA oligonucleotide) or antagomiR (Simonson and Das, 2015), locked nucleic acid (LNA) (Stein et al., 2010), miRNA sponges (Ebert and Sharp, 2010), and miRNA masks and circRNA (circular RNA) (Greene et al., 2017) (Table 2).
Table 2.
The main characteristics of various systems used for miRNA therapy
| Therapeutic strategy | Delivery system | Characteristic | Mechanism | Reference | |
|---|---|---|---|---|---|
| miRNA inhibition therapy (miRNA with oncogenic role) | AMO (Anti‐miRNA oligonucleotides) or antagomiRs | Chemically modified for direct delivery; peptide liked delivery for receptor targeting | Short, synthetic, single‐stranded oligodeoxynucleotides | miRNA degradation by direct binding to target transcript and recycling the antagomir sequence | Rinaldi and Wood (2018), Simonson and Das (2015) |
| LNA | Naked delivery | Monocatenare sequences, some modification for increasing the specificity | Inhibition of miRNA by direct binding to seed region | Stein et al. (2010) | |
| miRNA sponges or decoys | Viral construct encoding multiple miRNA‐binding sites downstream of a promoter | Single‐stranded 23 nt RNA molecules complementary to the targeted miRNA that have been modified to increase the stability of the RNA and protect it from degradation | Block miRNA role by inhibition the binding to their targets | Ebert and Sharp (2010) | |
| miRNA masks | Liposomal delivery | Short single stranded RNA, 2′‐O‐methyl‐modified | To complement the miRISC binding sites in the 3′ UTR of the target mRNA; mRNA specificity | Wang et al. (2015b) | |
| circRNA (circular RNA) | Liposomal delivery | RNA structure from 3′ end of a downstream exon has been backspliced to the 5′ end of an upstream exon, displayed as continuous RNA loop | miRNA sponge inhibiting activity | Greene et al. (2017) | |
| miRNA replacement therapy (miRNA with tumor suppressor role) | miRNA mimics | Liposomal delivery as mature miRNA, miRNA‐mimics, precursors | Mature miRNA sequence, can be chemical modified for increasing the stability | ‘Mimic’ the role of endogenous miRNA, restore its loss of function as a tumor suppressor | Wang et al. (2015b) |
| miRNA vectors | Viral construct encoding miRNA sequence | miRNA cassettes cloned into any site of different destination vectors designed | Restoration of the target miRNA by direct genomic integration | Chira et al. (2015), Wang et al. (2015b) | |
Regulation of lung cancer TME can be considered the ‘Achilles heel’ of therapy success (Mittal et al., 2016), where miR‐181a/b can be an important player. MiR‐181a can be involved in limitation of lung cancer spread, as it was demonstrated to have critical EMT regulatory targets (He et al., 2015). Therefore, miR‐181 might be used as a direct or indirect therapeutic target, not only for the effects on the tumor, but also to regulate the immune response effectors that favor EMT or interact with TME, thus affecting the response to therapy (Parikh et al., 2014; Ye et al., 2018).
MiR‐181a/b therapy in lung cancer generally implies replacement strategies (miR‐181a/b mimics or miR‐181a/b vectors) for restoring the normal expression level. An important number of studies use miR‐181a/b inhibitors for mechanistic studies or because of different expression signatures in cell‐specific contexts. Most of the studies investigating the biological significance of miR‐181a/b transcripts use commercial miR‐181a/b mimics, and, as delivery systems, the commercially available liposomes (e.g. Lipofectamine 2000) (Cao et al., 2017; Fei et al., 2008; Huang et al., 2015; Ma et al., 2015; Wang et al., 2015a).
Recently, there has been important progress in the development of nanoparticle‐based therapies which represent a promising approach (Anselmo and Mitragotri, 2016), as can be observed by the high number of recently preclinical studies using liposomal delivery for miR‐181a/b (Table 3). This remains to be validated in clinical trials.
Table 3.
Cell culture‐based studies examples for the evaluation of the therapeutic efficacy in lung cancer. N/A, data not available; ↓, downregulated; ↑, upregulated; FC, fold change; NF‐κB, nuclear factor kappa beta
| Pathology | In vitro systems | Therapeutic approaches/Delivery system | FC in lung cancer cells | Observation | Reference |
|---|---|---|---|---|---|
| NSCLC | H226 and H460 | miR‐181a mimic and inhibitor (50 nM)/Lipofectamine 2000 | N/A | miR‐181a overexpression reduce cell proliferation and migration via VCAM‐1, NFκB and IL‐17 | Cao et al. (2017) |
| NSCLC | A549 cells | AMO‐miR‐181a/Lipofectamine 2000 (lipofectin: oligonucleotides 2.5 : 1) | N/A | AMO‐miR‐181a reduces cell proliferation by activation of apoptosis and S‐phase cell cycle arrest | Fei et al. (2008) |
| NSCLC | H23 and H522 cells | miR‐181b mimic/Luciferase reporter vector and Lipofectamine 2000 |
Relative luciferases intensity for 95 ± 10 NC, 38 ± 8, in H23 WT HMGB1 cell; 100 ± 10 NC, 90 ± 5, in H23 WT HMGB1 cell Relative luciferase intensity for 95 ± 10 NC, 56 ± 5, in H522 WT HMGB1 cell; 100 ± 10 NC, 90 ± 5, in H522 WT HMGB1 cell |
HMGB1 is a direct target gene of miR‐181b in NSCLC; miR‐181b inhibits cell migration and invasion in NSCLC | Liu et al. (2016) |
| NSCLC | A549 | miR‐181a mimic/inhibitor (150 nm)/Luciferase reporter vector and Lipofectamine 2000 |
H23: 0.25 ± 0.01 H1299: 0.3 ± 0.01 A549: 0.6 ± 0.05; HCC827 0.7 ± 0.1 95‐D: 0.7 ± 0.2 and SPCA‐1 0.85 ± 0.1 versus bronchial epithelial cell line (BEAS‐2B) |
miR‐181a mimic reduced cell proliferation and colony formation, cell migration; target Kras | Ma et al. (2015) |
| NSCLC | A549, A549/PTX and A549/cis | 50 pmol of miR‐181a inhibitor mimic/Lipofectamine 2000 |
↑miR‐181a in A549/PTX: 16 ± 1 A549/cis: 16 ± 1 than A549: 1 ± 0.2 |
miR‐181a targets PTEN; miR‐181a inhibitor reduces cell migration, invasion and expression of EMT‐associated genes; miR‐181a inhibitor sensitizes cancer cells to chemotherapy | Li et al. (2015) |
| NSCLCa | A549, H226, H460, SW‐900, HBE | MiR‐181 mimic and inhibitor/Lipofectamine 2000 | ↓miR‐181 in A549, H460, H358, and H1299 was about 18.10, 10.85, 7.08, and 16.98%, versus normal human bronchial epithelial cell line HBE | miR‐181 mimic leads to the inhibition of cell proliferation, migration, and invasion and promotes cell apoptosis; miR‐181 targets Bcl‐2, being involved in apoptosis regulation | Huang et al. (2015) |
| NSCLC | A549, A549/cis and H1650 | miR‐181 inhibitor/mimic and negative control/Lipofectamine 2000 |
↓miR‐181b in insensitive to therapy; Relative expression level in HBE: 70.39, A549/cis: 1 A549: 3.11, H1650: 5.94 |
miR‐181b enhances chemosensitivity of NSCLC cells to Cisplatin; miR‐181b attenuates migration and invasion, modulates EMT; TGFβR1 has a critical role in miR‐181b‐mediated cell growth, chemosensitivity to cisplatin and metastasis of NSCLC cells | Wang et al. (2015a) |
| NSCLC | NSCLC A549, H1650, H1975, and HCC827, HCT116cells | pre‐miR‐181a and anti‐miR‐181a/Oligofectamine, HiPerFect (GFP)‐Bax–coding plasmid |
↓miR‐181 tumor cell lines ↑ in cisplatin resistant cells |
Pre‐miR‐181a modulated mitochondrial/post‐mitochondrial steps of the intrinsic pathway of apoptosis and potentiate the effect of cisplatin, carboplatin and Oxaliplatin | Galluzzi et al. (2010) |
| NSCLC | A549, and A549/cis | Mature miR‐181a/b/c/d mimic (100 nm)/Lipofectamine 2000 | ↓miR‐181b in A549cis than A549 | Bcl‐2 is targeted by mature miR‐181s; miR‐181b modulates multidrug resistance by inhibiting Bcl‐2 and sensitizes cells to apoptosis | Zhu et al. (2010) |
| NSCLC | PC‐9, PC‐14 and PC‐9/cis and PC‐14/cis |
miR‐181a mimic and inhibitor/RNAiMax |
↑ miR‐181a/b/c/d in A549cis than A549 | miR‐181 inhibition has minimal effects on resistance to therapy | Pouliot et al. (2013) |
| NSCLC | PC9, NSCLC cell line A549 and A549/cis | miR‐181a mimic and inhibitor/Lipofectamine 2000 | ↓miR‐181a in A549 (FC 0.5), A A549/cis (FC 0.2), A A549/PTX (FC 0.3), H299 (FC: 0.8), H299/cis (FC: 0.5), H299/PTX (FC: 0.6) | SNHG12 is ↑ and miR‐181a is ↓ in NSCLC tissues and cell lines; SNHG12 regulates MAPK/Slug pathway by sponging effect of miR‐181a | Wang et al. (2017a) |
| NSCLC | HBE, A549, A549/cis H1650, H1650 | miR‐181a mimic and inhibitor/Lipofectamine 2000 | HBE FC: 70.39, A549/cis FC: 1, A549 FC: 3.11, H1650 FC: 5.94 | miR‐181b mimic reduced proliferation, enhanced chemosensitivity to cisplatin, attenuated migration and metastatic rate | Wang et al. (2015a) |
Not specified miRNA type.
4. Implication of miR‐181a/b in lung cancer drug resistance
Chemoresistance is frequently observed in most lung cancer subtypes (Li et al., 2015; Shanker et al., 2010). Deciphering the molecular basis of drug resistance will lead to more effective treatments (Shanker et al., 2010). Knowledge‐based improvements in the field of predictive biomarkers for personalized treatment that rely on combining novel agents focused on resistance pathways with standard chemotherapy, might lead to the development of therapeutic designs capable of overcoming chemoresistance. The restoration of the miR‐181a/b expression level can be considered an important adjuvant strategy in lung cancer therapy for the prevention of drug resistance, as demonstrated by the large number of translational studies (Li et al., 2015, 2016a; Niu et al., 2016; Wang et al., 2017a). Nevertheless, we should not underestimate the important role of the immune system effectors and other host cells within the organism microenvironment.
Despite the increased interest in non‐cytotoxic targeted agents, systemic chemotherapy (Docetaxel, Gemcitabine, Irinotecan, Paclitaxel, Pemetrexed, and Vinorelbine) along with some targeted agents (Bevacizumab, Erlotinib, and Gefitinib) remain the pillar of therapy for lung cancer (Kim, 2016). Recent studies showed an increased use and clinical activity for the immune checkpoint inhibitors in lung cancer therapy. Understanding the regulatory mechanisms of PD‐L1 has become one of the biggest challenges for further improving therapeutic efficacy (Smolle et al., 2017). MiR‐181a targets the ubiquitin ligases Cbl‐b and c‐Cbl; these two factors are negatively correlated with PD‐L1 expression in tissue samples from NSCLC patients and are proved to inhibit PD‐L1 in vitro through inactivation of ERK, STAT, and AKT signaling (Wang et al., 2018). Restoration of miR‐181a along with anti‐PD‐1/PD‐L1 might potentiate the therapeutic efficacy in lung cancer (Smolle et al., 2017), this being a research direction for future investigations.
miR‐181b overexpression inhibits cell proliferation and increases the sensitivity of lung cancer cells to DDP, attenuating at the same time the metastatic characteristics of the NSCLC cells (Wang et al., 2015a). The activity of miR‐181a/b is complex and is regulated at diverse levels (Lang et al., 2017; Wang et al., 2017a). MiR‐181a is sponged by small nucleolar RNA host gene 12 (SNHG12), a lncRNA that is overexpressed in lung cancer and inversely correlated with miR‐181a levels. Silencing of the lncRNA resulted in increased expression of the miRNA together with suppression of MAPK1 and MAP2K1 mediated by the high levels of miR‐181a achieved. The experimentally modified regulatory axis has further effects upon increased drug‐induced apoptosis in lung cancer cells (Fu et al., 2018; Wang et al., 2017a).
MicroRNA are involved in signal transduction, connected with drug metabolism and resistance, with potential use in personalized therapy (Gong et al., 2014). MiR‐181a and miR‐181b can also be used to increase the sensitivity to chemotherapeutic agents in lung cancer. MiR‐181a/PTEN is a novel regulatory circuit that mediates EMT in drug‐resistant lung ADC cells (Li et al., 2015). Lung cancer cells with acquired resistance to paclitaxel and cisplatin present a differential profile for miR‐181a with respect to their sensitive counterparts. Concomitantly, PTEN is reduced in these drug‐resistant models and is validated as a direct target of miR‐181a. Modulation of miR‐181a may become a promising strategy to prevent resistance to the main chemotherapeutics, in spite of the fact that some studies show minimal effects of miR‐181a/b on cisplatin‐resistant cells (Li et al., 2015; Pouliot et al., 2013). It is important to underline the necessity for further studies to show whether miR‐181 family members are capable of preventing the activation of chemoresistance mechanisms. The importance of the TME in chemotherapy efficiency is limited to in vitro studies or to immunocompromised mice models, decreasing the true translational value of miR‐181a modulation.
One major mechanism related to drug resistance is the malfunctioning of apoptosis pathways and the activation of complex compensatory pathways (Braicu et al., 2013, 2014; Pileczki et al., 2012). Studies link the overexpression of the proapoptotic gene Bcl‐2 with the downregulated profile of miR‐181b in multi‐drug‐resistant lung cancer cells; after validation of direct inhibition of miR‐181b on Bcl‐2, replacement therapies showed significant improvement in terms of cell sensitivity to chemotherapeutic agents (Zhu et al., 2010). The transfection with mimic sequences showed a significant reduction in cell proliferation in A549/cis cells treated with vincristine, 5‐fluorouracil, cisplatin, and etoposide, but not mitomycin C (Zhu et al., 2010). Another study focused on assessing the impact of miR‐181b in modulating chemoresistance, evaluated the expression of the transcript in HBE cells (normal lung epithelial cell line), as well as in A549, H1650, and A549/DDP lung cancer cell lines (Wang et al., 2015a). qRT‐PCR showed that miR‐181b is downregulated in A549/DDP cells compared with sensitive cancer cells and significantly increased in HBE normal cell lines compared with all three malignant models (Wang et al., 2015a). Functional studies of miR‐181b upregulation showed that the proliferation and migration rates was significantly decreased and sensitivity to the treatment was restored. Moreover, TGFβR1 was validated as a direct target of miR‐181b, where siRNA inhibition of the receptor gene showed similar results as miR‐181b overexpression (Wang et al., 2015a).
miR‐181a is related to Gefitinib resistance in lung cancer through an increased expression profile compared with the sensitive models and direct targeting of GAS7; GAS7 is involved in the regulation of AKT/ERK pathways and EMT markers and is downregulated in plasma from Gefitinib‐resistant patients (Ping et al., 2018). These findings indicate that restoring the expression of miR‐181a/b in lung cancer may play a critical role in fighting chemoresistance.
miR‐181a/b have the capacity to modulate drug resistance mechanisms in cancer cell lines, but the data remain inconsistent and need to be validated further in animal models. Taken together, all preclinical studies underline the therapeutic potential of these transcripts in the regulation of drug resistance. To be able to exploit these findings fully, it is mandatory to study this mechanism in the context of the complex TME.
5. Conclusions
Research performed in recent years demonstrates a wide range of novel functions for miR‐181a and miR‐181b in lung cancer. These studies reveal an important number of mechanisms that have clinical relevance but, at the same time, there are many issues related to the utility of miR‐181a/b in lung cancer management. One aim would be to build a global network that would integrate and interconnect the effects of all types of cells that constitute the TME with the mutational status of the genes that take part in the altered mechanisms. Deciphering this will lead to new and unexpected insights that will contribute to the development of novel and more efficient therapies for lung cancer.
miR‐181b is generally downregulated in lung cancer, and the reduced expression leads to an unfavorable prognosis in most of the cases. Therapeutic targeting of miR‐181a/b may be achieved at multiple levels, as shown by the preclinical studies, but at this moment there are no clinical trials of this. Additional studies are required to confirm the role of these two transcripts as biomarkers or therapeutic targets able to promote a less aggressive disease. MiR‐181a/b regulate structural and cellular elements involved in cell proliferation, as well as cell plasticity and adaptive programs that favor lung cancer invasion and migration.
The recently described role of miR‐181a/b in prevention of drug resistance by restoring the physiological expression levels, is an example of a sophisticated mechanism of action, which further underlines the fact that the expression level of a miRNA is not enough to propose it as a biomarker or therapeutic target. Consequently, this needs to be supported by additional functional studies of a specific phenotype able to prevent resistance to therapy or limit the spread of lung cancer. The biological role of miR‐181a/b needs to be studied in more detail, and the studies should not be limited to a simple exploration of the expression level but should be associated with complex characterization of genomic, transcriptomic, or epigenetic portraits.
Author contributions
CB wrote the manuscript, DG and LR acquired the data, RC and EK drafted and revised the manuscript, AJ prepared the figures. GAC and IB‐N designed the project. All the authors contributed to the writing of the manuscript and approved the final version.
Conflicts of interest
The authors have no conflicts of interest to declare.
Acknowledgements
Work in Dr. Berindan‐Neagoe's laboratory was supported by a POC Grant, Competitively Operational Program, 2014–2020, no. 35/01.09.2016, MySMIS 103375 (CANTEMIR). Dr. Calin is the Felix L. Haas Endowed Professor in Basic Science. Work in Dr. Calin's laboratory is supported by National Institutes of Health (NIH/NCATS) grant UH3TR00943‐01 through the NIH Common Fund, Office of Strategic Coordination (OSC), NCI grants 1R01 CA182905‐01 and 1R01CA222007‐01A1, an NIGMS 1R01GM122775‐01 grant, a U54 grant #CA096297/CA096300 – UPR/MDACC Partnership for Excellence in Cancer Research 2016 Pilot Project, a Team DOD (CA160445P1) grant, a Chronic Lymphocytic Leukemia Moonshot Flagship project, a Sister Institution Network Fund (SINF) 2017 grant, and the Estate of C. G. Johnson, Jr.
References
- Aguiar PN Jr, De Mello RA, Barreto CMN, Perry LA, Penny‐Dimri J, Tadokoro H and Lopes GL Jr (2017) Immune checkpoint inhibitors for advanced non‐small cell lung cancer: emerging sequencing for new treatment targets. ESMO Open 2, e000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An TH, He QW, Xia YP, Chen SC, Baral S, Mao L, Jin HJ, Li YN, Wang MD, Chen JG et al (2017) MiR‐181b antagonizes atherosclerotic plaque vulnerability through modulating macrophage polarization by directly targeting Notch1. Mol Neurobiol 54, 6329–6341. [DOI] [PubMed] [Google Scholar]
- Anselmo AC and Mitragotri S (2016) Nanoparticles in the clinic. Bioeng Transl Med 1, 10–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala‐Ortega E, Arzate‐Mejia R, Perez‐Molina R, Gonzalez‐Buendia E, Meier K, Guerrero G and Recillas‐Targa F (2016) Epigenetic silencing of miR‐181c by DNA methylation in glioblastoma cell lines. BMC Cancer 16, 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berindan‐Neagoe I, Braicu C, Gulei D, Tomuleasa C and Calin GA (2017) Noncoding RNA in lung cancer angiogenesis In Physiologic and Pathologic Angiogenesis – Signaling Mechanisms and Targeted Therapy (Simionescu D. and Simionescu A, eds), Ch. 14. Rijeka: InTech. [Google Scholar]
- Berindan‐Neagoe I and Calin GA (2014) Molecular pathways: microRNA, cancer cells, and microenvironment. Clin Cancer Res 20, 6247–6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berindan‐Neagoe I, Monroig PC, Pasculli B and Calin GA (2014) MicroRNAome genome: a treasure for cancer diagnosis and therapy. CA Cancer J Clin 64, 311–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi J, Zeng X, Zhao L, Wei Q, Yu L, Wang X, Yu Z, Cao Y, Shan F and Wei M (2016) miR‐181a induces macrophage polarized to M2 phenotype and promotes M2 macrophage‐mediated tumor cell metastasis by targeting KLF6 and C/EBPalpha. Mol Ther Nucleic Acids 5, e368. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bisso A, Faleschini M, Zampa F, Capaci V, De Santa J, Santarpia L, Piazza S, Cappelletti V, Daidone M, Agami R et al (2013) Oncogenic miR‐181a/b affect the DNA damage response in aggressive breast cancer. Cell Cycle 12, 1679–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braicu C, Catana C, Calin GA and Berindan‐Neagoe I (2014) NCRNA combined therapy as future treatment option for cancer. Curr Pharm Des 20, 6565–6574. [DOI] [PubMed] [Google Scholar]
- Braicu C, Cojocneanu‐Petric R, Chira S, Truta A, Floares A, Petrut B, Achimas‐Cadariu P and Berindan‐Neagoe I (2015) Clinical and pathological implications of miRNA in bladder cancer. Int J Nanomed 10, 791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braicu C, Pileczki V, Irimie A and Berindan‐Neagoe I (2013) p53siRNA therapy reduces cell proliferation, migration and induces apoptosis in triple negative breast cancer cells. Mol Cell Biochem 381, 61–68. [DOI] [PubMed] [Google Scholar]
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA and Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68, 394–424. [DOI] [PubMed] [Google Scholar]
- Calin GA and Croce CM (2006) Genomics of chronic lymphocytic leukemia microRNA as new players with clinical significance. Semin Oncol 33, 167–173. [DOI] [PubMed] [Google Scholar]
- Cao Y, Zhao D, Li P, Wang L, Qiao B, Qin X, Li L and Wang Y (2017) MicroRNA‐181a‐5p impedes IL‐17‐induced nonsmall cell lung cancer proliferation and migration through targeting VCAM‐1. Cell Physiol Biochem 42, 346–356. [DOI] [PubMed] [Google Scholar]
- Catana CS, Calin GA and Berindan‐Neagoe I (2015) Inflamma‐miRs in aging and breast cancer: are they reliable players? Front Med 2, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catana CS, Pichler M, Giannelli G, Mader RM and Berindan‐Neagoe I (2017) Non‐coding RNA, the Trojan horse in two‐way communication between tumor and stroma in colorectal and hepatocellular carcinoma. Oncotarget 8, 29519–29534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Ke G, Han D, Liang S, Yang G and Wu X (2014) MicroRNA‐181a enhances the chemoresistance of human cervical squamous cell carcinoma to cisplatin by targeting PRKCD. Exp Cell Res 320, 12–20. [DOI] [PubMed] [Google Scholar]
- Chen J, Guo Z, Tian H and Chen X (2016) Production and clinical development of nanoparticles for gene delivery. Mol Ther Methods Clin Dev 3, 16023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chira S, Jackson CS, Oprea I, Ozturk F, Pepper MS, Diaconu I, Braicu C, Raduly L‐Z, Calin GA and Berindan‐Neagoe I (2015) Progresses towards safe and efficient gene therapy vectors. Oncotarget 6, 30675–30703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chira S, Raduly L, Braicu C, Jurj A, Cojocneanu‐Petric R, Pop L, Pileczki V, Ionescu C and Berindan‐Neagoe I (2018) Premature senescence activation in DLD‐1 colorectal cancer cells through adjuvant therapy to induce a miRNA profile modulating cellular death. Exp Ther Med 16, 1241–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi E, Lee S, Nhung BC, Suh M, Park B, Jun JK and Choi KS (2017) Cancer mortality‐to‐incidence ratio as an indicator of cancer management outcomes in Organization for Economic Cooperation and Development countries. Epidemiol Health 39, e2017006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhry H and Harris AL (2018) Advances in hypoxia‐inducible factor biology. Cell Metab 27, 281–298. [DOI] [PubMed] [Google Scholar]
- Chu B, Wu T, Miao L, Mei Y and Wu M (2015) MiR‐181a regulates lipid metabolism via IDH1. Sci Rep 5, 8801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinegaglia NC, Andrade SCS, Tokar T, Pinheiro M, Severino FE, Oliveira RA, Hasimoto EN, Cataneo DC, Cataneo AJM, Defaveri J et al (2016) Integrative transcriptome analysis identifies deregulated microRNA‐transcription factor networks in lung adenocarcinoma. Oncotarget 7, 28920–28934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolla G, de Oliveira J, Salviano‐Silva A, Lobo‐Alves S, Lemos D, Oliveira L, Jucoski T, Mathias C, Pedroso G, Zambalde E et al (2018) Long non‐coding RNA in multifactorial diseases: another layer of complexity. Noncoding RNA 4, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didkowska J, Wojciechowska U, Mańczuk M and Łobaszewski J (2016) Lung cancer epidemiology: contemporary and future challenges worldwide. Ann Transl Med 4, 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastlack SC and Alahari SK (2015) MicroRNA and breast cancer: understanding pathogenesis, improving management. Noncoding RNA 1, 17–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert MS and Sharp PA (2010) MicroRNA sponges: progress and possibilities. RNA 16, 2043–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Expósito‐Villén A, E Aránega A and Franco D (2018) Functional role of non‐coding RNA during epithelial‐to‐mesenchymal transition. Noncoding RNA 4, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei J, Lan F, Guo M, Li Y and Liu Y (2008) Inhibitory effects of anti‐miRNA oligonucleotides (AMOs) on A549 cell growth. J Drug Target 16, 688–693. [DOI] [PubMed] [Google Scholar]
- Field RW and Withers BL (2012) Occupational and environmental causes of lung cancer. Clin Chest Med 33, 681–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Zhang S, Wang D and Wang J (2018) Icotinib enhances lung cancer cell radiosensitivity in vitro and in vivo by inhibiting MAPK/ERK and AKT activation. Clin Exp Pharmacol Physiol 10.1111/1440-1681.12966. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Fumarola C, Bonelli MA, Petronini PG and Alfieri RR (2014) Targeting PI3K/AKT/mTOR pathway in non small cell lung cancer. Biochem Pharmacol 90, 197–207. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Morselli E, Vitale I, Kepp O, Senovilla L, Criollo A, Servant N, Paccard C, Hupe P, Robert T et al (2010) miR‐181a and miR‐630 regulate cisplatin‐induced cancer cell death. Can Res 70, 1793–1803. [DOI] [PubMed] [Google Scholar]
- Gao W, Yu Y, Cao H, Shen H, Li X, Pan S and Shu Y (2010) Deregulated expression of miR‐21, miR‐143 and miR‐181a in non small cell lung cancer is related to clinicopathologic characteristics or patient prognosis. Biomed Pharmacother 64, 399–408. [DOI] [PubMed] [Google Scholar]
- Gong Z, Yang J, Li J, Yang L, Le Y, Wang S and Lin HK (2014) Novel insights into the role of microRNA in lung cancer resistance to treatment and targeted therapy. Curr Cancer Drug Targets 14, 241–258. [DOI] [PubMed] [Google Scholar]
- Greene J, Baird A‐M, Brady L, Lim M, Gray SG, McDermott R and Finn SP (2017) Circular RNA: biogenesis, function and role in human diseases. Front Mol Biosci 4, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulei D, Mehterov N, Ling H, Stanta G, Braicu C and Berindan‐Neagoe I (2017) The ‘good‐cop bad‐cop’ TGF‐beta role in breast cancer modulated by non‐coding RNA. Biochem Biophys Acta 1861, 1661–1675. [DOI] [PubMed] [Google Scholar]
- Gulei D, Magdo L, Jurj A, Raduly L, Cojocneanu‐Petric R, Moldovan A, Moldovan C, Florea A, Pasca S, Pop LA et al (2018a) The silent healer: miR‐205‐5p upregulation inhibits epithelial to mesenchymal transition in colon cancer cells by indirectly upregulating E‐cadherin expression. Cell Death Dis 9, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulei D, Petrut B, Tigu AB, Onaciu A, Fischer‐Fodor E, Atanasov AG, Ionescu C and Berindan‐Neagoe I (2018b) Exosomes at a glance – common nominators for cancer hallmarks and novel diagnosis tools. Crit Rev Biochem Mol Biol 53, 564–577. [DOI] [PubMed] [Google Scholar]
- Guo X, Xue H, Shao Q, Wang J, Guo X, Chen X, Zhang J, Xu S, Li T, Zhang P et al (2016) Hypoxia promotes glioma‐associated macrophage infiltration via periostin and subsequent M2 polarization by upregulating TGF‐beta and M‐CSFR. Oncotarget 7, 80521–80542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D and Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144, 646–674. [DOI] [PubMed] [Google Scholar]
- He X, Liu Z, Peng Y and Yu C (2016) MicroRNA‐181c inhibits glioblastoma cell invasion, migration and mesenchymal transition by targeting TGF‐beta pathway. Biochem Biophys Res Comm 469, 1041–1048. [DOI] [PubMed] [Google Scholar]
- He S, Zeng S, Zhou ZW, He ZX and Zhou SF (2015) Hsa‐microRNA‐181a is a regulator of a number of cancer genes and a biomarker for endometrial carcinoma in patients: a bioinformatic and clinical study and the therapeutic implication. Drug Des Devel Ther 9, 1103–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst RS, Morgensztern D and Boshoff C (2018) The biology and management of non‐small cell lung cancer. Nature 553, 446–454. [DOI] [PubMed] [Google Scholar]
- Huang P, Ye B, Yang Y, Shi J and Zhao H (2015) MicroRNA‐181 functions as a tumor suppressor in non‐small cell lung cancer (NSCLC) by targeting Bcl‐2. Tumour Biol 36, 3381–3387. [DOI] [PubMed] [Google Scholar]
- Ionescu C, Braicu C, Chiorean R, Cojocneanu Petric R, Neagoe E, Pop L, Chira S and Berindan‐Neagoe I (2014) TIMP‐1 expression in human colorectal cancer is associated with SMAD3 gene expression levels: a pilot study. J Gastrointestin Liver Dis 23, 413–418. [DOI] [PubMed] [Google Scholar]
- Irimie AI, Braicu C, Sonea L, Zimta AA, Cojocneanu‐Petric R, Tonchev K, Mehterov N, Diudea D, Buduru S and Berindan‐Neagoe I (2017a) A looking‐glass of non‐coding RNA in oral cancer. Int J Mol Sci 18, pii: E2620. 10.3390/ijms18122620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irimie AI, Sonea L, Jurj A, Mehterov N, Zimta AA, Budisan L, Braicu C and Berindan‐Neagoe I (2017b) Future trends and emerging issues for nanodelivery systems in oral and oropharyngeal cancer. Int J Nanomed 12, 4593–4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain P, Jain C and Velcheti V (2018) Role of immune‐checkpoint inhibitors in lung cancer. Ther Adv Respir Dis 12, 1753465817750075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Chen Y, Chen H, Fei S, Chen D, Cai X, Liu L, Lin B, Su H, Zhao L et al (2017) Evaluation of tumor‐derived exosomal miRNA as potential diagnostic biomarkers for early‐stage non‐small cell lung cancer using next‐generation sequencing. Clin Cancer Res 23, 5311–5319. [DOI] [PubMed] [Google Scholar]
- Jurj A, Braicu C, Pop LA, Tomuleasa C, Gherman CD and Berindan‐Neagoe I (2017) The new era of nanotechnology, an alternative to change cancer treatment. Drug Des Devel Ther 11, 2871–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke G, Liang L, Yang JM, Huang X, Han D, Huang S, Zhao Y, Zha R, He X and Wu X (2013) MiR‐181a confers resistance of cervical cancer to radiation therapy through targeting the pro‐apoptotic PRKCD gene. Oncogene 32, 3019–3027. [DOI] [PubMed] [Google Scholar]
- Kim ES (2016) Chemotherapy resistance in lung cancer. Adv Exp Med Biol 893, 189–209. [DOI] [PubMed] [Google Scholar]
- Lakomy R, Sana J, Hankeova S, Fadrus P, Kren L, Lzicarova E, Svoboda M, Dolezelova H, Smrcka M, Vyzula R et al (2011) MiR‐195, miR‐196b, miR‐181c, miR‐21 expression levels and O‐6‐methylguanine‐DNA methyltransferase methylation status are associated with clinical outcome in glioblastoma patients. Cancer Sci 102, 2186–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang Y, Kong X, He C, Wang F, Liu B, Zhang S, Ning J, Zhu K and Xu S (2017) Musashi1 promotes non‐small cell lung carcinoma malignancy and chemoresistance via activating the Akt signaling pathway. Cell Physiol Biochem 44, 455–466. [DOI] [PubMed] [Google Scholar]
- Lee H, Park CS, Deftereos G, Morihara J, Stern JE, Hawes SE, Swisher E, Kiviat NB and Feng Q (2012) MicroRNA expression in ovarian carcinoma and its correlation with clinicopathological features. World J Surg Oncol 10, 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Chen X, Guan L, Qi Q, Shu G, Jiang Q, Yuan L, Xi Q and Zhang Y (2013) MiRNA‐181a regulates adipogenesis by targeting tumor necrosis factor‐alpha (TNF‐alpha) in the porcine model. PLoS One 8, e71568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zhang P, Sun X, Sun Y, Shi C, Liu H and Liu X (2015) MicroRNA‐181a regulates epithelial‐mesenchymal transition by targeting PTEN in drug‐resistant lung adenocarcinoma cells. Int J Oncol 47, 1379–1392. [DOI] [PubMed] [Google Scholar]
- Li W, Qiu X, Jiang H, Han Y, Wei D and Liu J (2016a) Downregulation of miR‐181a protects mice from LPS‐induced acute lung injury by targeting Bcl‐2. Biomed Pharmacother 84, 1375–1382. [DOI] [PubMed] [Google Scholar]
- Li L, Xu QH, Dong YH, Li GX, Yang L, Wang LW and Li HY (2016b) MiR‐181a upregulation is associated with epithelial‐to‐mesenchymal transition (EMT) and multidrug resistance (MDR) of ovarian cancer cells. Eur Rev Med Pharmacol Sci 20, 2004–2010. [PubMed] [Google Scholar]
- Liu Y, Hu X, Xia D and Zhang S (2016) MicroRNA‐181b is downregulated in non‐small cell lung cancer and inhibits cell motility by directly targeting HMGB1. Oncol Lett 12, 4181–4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Xie F, Gao A, Zhang R, Zhang L, Xiao Z, Hu Q, Huang W, Huang Q, Lin B et al (2017) SOX2 regulates multiple malignant processes of breast cancer development through the SOX2/miR‐181a‐5p, miR‐30e‐5p/TUSC3 axis. Mol Cancer 16, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Qiu X, Wang D, Li Y, Zhang B, Yuan T, Wei J, Zhao B, Zhao X, Lou J et al (2015) MiR‐181a‐5p inhibits cell proliferation and migration by targeting Kras in non‐small cell lung cancer A549 cells. Acta Biochim Biophys Sin 47, 630–638. [DOI] [PubMed] [Google Scholar]
- Mittal V, El Rayes T, Narula N, McGraw TE, Altorki NK and Barcellos‐Hoff MH (2016) The microenvironment of lung cancer and therapeutic implications. Adv Exp Med Biol 890, 75–110. [DOI] [PubMed] [Google Scholar]
- Munker R and Calin GA (2011) MicroRNA profiling in cancer. Clin Sci (Lond) 121, 141–158. [DOI] [PubMed] [Google Scholar]
- Niu J, Xue A, Chi Y, Xue J, Wang W, Zhao Z, Fan M, Yang CH, Shao ZM, Pfeffer LM et al (2016) Induction of miRNA‐181a by genotoxic treatments promotes chemotherapeutic resistance and metastasis in breast cancer. Oncogene 35, 1302–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J‐Y, Sun C‐C, Bi Z‐Y, Chen Z‐L, Li S‐J, Li Q‐Q, Wang Y‐X, Bi Y‐Y and Li D‐J (2017) miR‐206/133b cluster: a weapon against lung cancer? Mol Ther Nucleic Acids 8, 442–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh A, Lee C, Joseph P, Marchini S, Baccarini A, Kolev V, Romualdi C, Fruscio R, Shah H, Wang F et al (2014) microRNA‐181a has a critical role in ovarian cancer progression through the regulation of the epithelial‐mesenchymal transition. Nat Commun 5, 2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pileczki V, Braicu C, Gherman CD and Berindan‐Neagoe I (2012) TNF‐alpha gene knockout in triple negative breast cancer cell line induces apoptosis. Int J Mol Sci 14, 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping W, Gao Y, Fan X, Li W, Deng Y and Fu X (2018) MiR‐181a contributes gefitinib resistance in non‐small cell lung cancer cells by targeting GAS7. Biochem Biophys Res Comm 495, 2482–2489. [DOI] [PubMed] [Google Scholar]
- Pop‐Bica C, Gulei D, Cojocneanu‐Petric R, Braicu C, Petrut B and Berindan‐Neagoe I (2017) Understanding the role of non‐coding RNA in bladder cancer: from dark matter to valuable therapeutic targets. Int J Mol Sci 18, pii: E1514 10.3390/ijms18071514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pop‐Bica C, Pintea S, Cojocneanu‐Petric R, Del Sal G, Piazza S, Wu ZH, Alencar AJ, Lossos IS, Berindan‐Neagoe I and Calin GA (2018) MiR‐181 family‐specific behavior in different cancers: a meta‐analysis view. Cancer Metastasis Rev 37, 17–32. [DOI] [PubMed] [Google Scholar]
- Popper HH (2016) Progression and metastasis of lung cancer. Cancer Metastasis Rev 35, 75–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouliot LM, Shen D‐W, Suzuki T, Hall MD and Gottesman MM (2013) Contributions of microRNA dysregulation to cisplatin resistance in adenocarcinoma cells. Exp Cell Res 319, 566–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail DF and Joyce JA (2013) Microenvironmental regulation of tumor progression and metastasis. Nat Med 19, 1423–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redis RS, Berindan‐Neagoe I, Pop VI and Calin GA (2012) Non‐coding RNA as theranostics in human cancers. J Cell Biochem 113, 1451–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi C and Wood MJA (2018) Antisense oligonucleotides: the next frontier for treatment of neurological disorders. Nat Rev Neurol 14, 9–21. [DOI] [PubMed] [Google Scholar]
- Rupaimoole R, Calin GA, Lopez‐Berestein G and Sood AK (2016) MicroRNA deregulation in cancer cells and the tumor microenvironment. Cancer Discov 6, 235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seoudi AM, Lashine YA and Abdelaziz AI (2012) MicroRNA‐181a – a tale of discrepancies. Expert Rev Mol Med 14, e5. [DOI] [PubMed] [Google Scholar]
- Sevignani C, Calin GA, Nnadi SC, Shimizu M, Davuluri RV, Hyslop T, Demant P, Croce CM and Siracusa LD (2007) MicroRNA genes are frequently located near mouse cancer susceptibility loci. Proc Natl Acad Sci U S A 104, 8017–8022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah MY, Ferrajoli A, Sood AK, Lopez‐Berestein G and Calin GA (2016) microRNA therapeutics in cancer – an emerging concept. EBioMedicine 12, 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan X, Zhang H, Zhang L, Zhou X, Wang T, Zhang J, Shu Y, Zhu W, Wen W and Liu P (2018) Identification of four plasma microRNA as potential biomarkers in the diagnosis of male lung squamous cell carcinoma patients in China. Cancer Med 7, 2370–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanker M, Willcutts D, Roth JA and Ramesh R (2010) Drug resistance in lung cancer. Lung Cancer (Auckl) 1, 23–36. [PMC free article] [PubMed] [Google Scholar]
- Shi L, Cheng Z, Zhang J, Li R, Zhao P, Fu Z and You Y (2008) hsa‐mir‐181a and hsa‐mir‐181b function as tumor suppressors in human glioma cells. Brain Res 1236, 185–193. [DOI] [PubMed] [Google Scholar]
- Shi Q, Zhou Z, Ye N, Chen Q, Zheng X and Fang M (2017) MiR‐181a inhibits non‐small cell lung cancer cell proliferation by targeting CDK1. Cancer Biomark 20, 539–546. [DOI] [PubMed] [Google Scholar]
- Shi L, Li X, Wu Z, Li X, Nie J, Guo M, Mei Q and Han W (2018) DNA methylation‐mediated repression of miR‐181a/135a/302c expression promotes the microsatellite‐unstable colorectal cancer development and 5‐FU resistance via targeting PLAG1. J Genet Genomics 45, 205–214. [DOI] [PubMed] [Google Scholar]
- Shukla S (2018) Unravelling the long non‐coding RNA profile of undifferentiated large cell lung carcinoma. Noncoding RNA 4, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonson B and Das S (2015) MicroRNA therapeutics: the next magic bullet? Mini Rev Med Chem 15, 467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaby O, Lakomy R, Fadrus P, Hrstka R, Kren L, Lzicarova E, Smrcka M, Svoboda M, Dolezalova H, Novakova J et al (2010) MicroRNA‐181 family predicts response to concomitant chemoradiotherapy with temozolomide in glioblastoma patients. Neoplasma 57, 264–269. [DOI] [PubMed] [Google Scholar]
- Smolle MA, Calin HN, Pichler M and Calin GA (2017) Noncoding RNA and immune checkpoints‐clinical implications as cancer therapeutics. FEBS J 284, 1952–1966. [DOI] [PubMed] [Google Scholar]
- Sonea L, Buse M, Gulei D, Onaciu A, Simon I, Braicu C and Berindan‐Neagoe I (2018) Decoding the emerging patterns exhibited in non‐coding RNA characteristic of lung cancer with regard to their clinical significance. Curr Genomics 19, 258–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein CA, Hansen JB, Lai J, Wu S, Voskresenskiy A, Høg A, Worm J, Hedtjärn M, Souleimanian N, Miller P et al (2010) Efficient gene silencing by delivery of locked nucleic acid antisense oligonucleotides, unassisted by transfection reagents. Nucleic Acids Res 38, e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strmsek Z and Kunej T (2015) MicroRNA silencing by DNA methylation in human cancer: a literature analysis. Noncoding RNA 1, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Sit A and Feinberg MW (2014a) Role of miR‐181 family in regulating vascular inflammation and immunity. Trends Cardiovasc Med 24, 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YC, Wang J, Guo CC, Sai K, Wang J, Chen FR, Yang QY, Chen YS, Wang J, To TS et al (2014b) MiR‐181b sensitizes glioma cells to teniposide by targeting MDM2. BMC Cancer 14, 611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MA, Sossey‐Alaoui K, Thompson CL, Danielpour D and Schiemann WP (2013) TGF‐β upregulates miR‐181a expression to promote breast cancer metastasis. J Clin Investig 123, 150–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thungappa S, Ferri J, Caglevic C, Passiglia F, Raez L and Rolfo C (2017) Immune checkpoint inhibitors in lung cancer: the holy grail has not yet been found. ESMO Open 2, e000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian F, Shen Y, Chen Z, Li R, Lu J and Ge Q (2016) Aberrant miR‐181b‐5p and miR‐486‐5p expression in serum and tissue of non‐small cell lung cancer. Gene 591, 338–343. [DOI] [PubMed] [Google Scholar]
- Tomuleasa C, Braicu C, Irimie A, Craciun L and Berindan‐Neagoe I (2014) Nanopharmacology in translational hematology and oncology. Int J Nanomed 9, 3465–3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudoran O, Soritau O, Balacescu O, Balacescu L, Braicu C, Rus M, Gherman C, Virag P, Irimie F and Berindan‐Neagoe I (2012) Early transcriptional pattern of angiogenesis induced by EGCG treatment in cervical tumour cells. J Cell Mol Med 16, 520–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M et al (2006) A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A 103, 2257–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Chen X, Meng Q, Jing H, Lu H, Yang Y, Cai L and Zhao Y (2015a) MiR‐181b regulates cisplatin chemosensitivity and metastasis by targeting TGFbetaR1/Smad signaling pathway in NSCLC. Sci Rep 5, 17618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Jiang Y, Peng H, Chen Y, Zhu P and Huang Y (2015b) Recent progress in microRNA delivery for cancer therapy by non‐viral synthetic vectors. Adv Drug Deliv Rev 81, 142–160. [DOI] [PubMed] [Google Scholar]
- Wang P, Chen D, Ma H and Li Y (2017a) LncRNA SNHG12 contributes to multidrug resistance through activating the MAPK/Slug pathway by sponging miR‐181a in non‐small cell lung cancer. Oncotarget 8, 84086–84101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Zhao J, Zhang L, Wei F, Lian Y, Wu Y, Gong Z, Zhang S, Zhou J, Cao K et al (2017b) Role of tumor microenvironment in tumorigenesis. J Cancer 8, 761–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Xu L, Che X, Li C, Xu L, Hou K, Fan Y, Wen T, Qu X and Liu Y (2018) E3 ubiquitin ligases Cbl‐b and c‐Cbl downregulate PD‐L1 in EGFR wild‐type non‐small cell lung cancer. FEBS Lett 592, 621–630. [DOI] [PubMed] [Google Scholar]
- Xia Y and Gao Y (2014) MicroRNA‐181b promotes ovarian cancer cell growth and invasion by targeting LATS2. Biochem Biophys Res Commun 447, 446–451. [DOI] [PubMed] [Google Scholar]
- Xu L‐J, Ouyang Y‐B, Xiong X, Stary CM and Giffard RG (2015) Post‐stroke treatment with miR‐181 antagomir reduces injury and improves long‐term behavioral recovery in mice after focal cerebral ischemia. Exp Neurol 264, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Zhu J, Hu C, Song H and Li Y (2016) Inhibition of microRNA‐181a may suppress proliferation and invasion and promote apoptosis of cervical cancer cells through the PTEN/Akt/FOXO1 pathway. J Physiol Biochem 72, 721–732. [DOI] [PubMed] [Google Scholar]
- Yang N (2015) An overview of viral and nonviral delivery systems for microRNA. Int J Pharm Investig 5, 179–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Liu H, Wang H and Sun Y (2013a) Down‐regulation of microRNA‐181b is a potential prognostic marker of non‐small cell lung cancer. Pathol Res Pract 209, 490–494. [DOI] [PubMed] [Google Scholar]
- Yang Y, Zhang R, Xia F, Zou T, Huang A, Xiong S and Zhang J (2013b) LPS converts Gr‐1+CD115+ myeloid‐derived suppressor cells from M2 to M1 via P38 MAPK. Exp Cell Res 319, 1774–1783. [DOI] [PubMed] [Google Scholar]
- Yang Z, Wan X, Gu Z, Zhang H, Yang X, He L, Miao R, Zhong Y and Zhao H (2014) Evolution of the mir‐181 microRNA family. Comput Biol Med 52, 82–87. [DOI] [PubMed] [Google Scholar]
- Yang C, Tabatabaei SN, Ruan X and Hardy P (2017) The dual regulatory role of MiR‐181a in breast cancer. Cell Physiol Biochem 44, 843–856. [DOI] [PubMed] [Google Scholar]
- Ye Z, Li G, Kim C, Hu B, Jadhav RR, Weyand CM and Goronzy JJ (2018) Regulation of miR‐181a expression in T cell aging. Nat Commun 9, 3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Zhang J, Hoadley K, Kushwaha D, Ramakrishnan V, Li S, Kang C, You Y, Jiang C, Song SW et al (2012) miR‐181d: a predictive glioblastoma biomarker that downregulates MGMT expression. Neuro Oncol 14, 712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Cao J, Ma S, Dong R, Meng W, Ying M, Weng Q, Chen Z, Ma J, Fang Q et al (2014) Tumor hypoxia enhances non‐small cell lung cancer metastasis by selectively promoting macrophage M2 polarization through the activation of ERK signaling. Oncotarget 5, 9664–9677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Che D, Yang F, Chi C, Meng H, Shen J, Qi L, Liu F, Lv L, Li Y et al (2017a) Tumor‐associated macrophages promote tumor metastasis via the TGF‐beta/SOX9 axis in non‐small cell lung cancer. Oncotarget 8, 99801–99815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S‐F, Chen J‐C, Zhang J and Xu J‐G (2017b) miR‐181a involves in the hippocampus‐dependent memory formation via targeting PRKAA1. Sci Rep 7, 8480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang M, Zhong M, Suo Q and Lv K (2013) Expression profiles of miRNA in polarized macrophages. Int J Mol Med 31, 797–802. [DOI] [PubMed] [Google Scholar]
- Zhao W, Sun Q, Yu Z, Mao S, Jin Y, Li J, Jiang Z, Zhang Y, Chen M, Chen P et al (2018) MiR‐320a‐3p/ELF3 axis regulates cell metastasis and invasion in non‐small cell lung cancer via PI3K/Akt pathway. Gene 670, 31–37. [DOI] [PubMed] [Google Scholar]
- Zhi F, Wang Q, Deng D, Shao N, Wang R, Xue L, Wang S, Xia X and Yang Y (2014) MiR‐181b‐5p downregulates NOVA1 to suppress proliferation, migration and invasion and promote apoptosis in astrocytoma. PLoS One 9, e109124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Shan X, Wang T, Shu Y and Liu P (2010) miR‐181b modulates multidrug resistance by targeting BCL2 in human cancer cell lines. Int J Cancer 127, 2520–2529. [DOI] [PubMed] [Google Scholar]


