Abstract
Background
Arthropod-borne diseases remain a major health-threat for humans and animals worldwide. To estimate the distribution of pathogenic agents and especially Bartonella spp., we conducted tick microbiome analysis and determination of the infection status of wild animals, pets and pet owners in the state of Hesse, Germany.
Results
In total, 189 engorged ticks collected from 163 animals were tested. Selected ticks were analyzed by next generation sequencing (NGS) and confirmatory PCRs, blood specimens of 48 wild animals were analyzed by PCR to confirm pathogen presence and sera of 54 dogs, one cat and 11 dog owners were analyzed by serology. Bartonella spp. were detected in 9.5% of all ticks and in the blood of 17 roe deer. Further data reveal the presence of the human and animal pathogenic species of genera in the family Spirochaetaceae (including Borrelia miyamotoi and Borrelia garinii), Bartonella spp. (mainly Bartonella schoenbuchensis), Rickettsia helvetica, Francisella tularensis and Anaplasma phagocytophilum in ticks. Co-infections with species of several genera were detected in nine ticks. One dog and five dog owners were seropositive for anti-Bartonella henselae-antibodies and one dog had antibodies against Rickettsia conorii.
Conclusions
This study provides a snapshot of pathogens circulating in ticks in central Germany. A broad range of tick-borne pathogens are present in ticks, and especially in wild animals, with possible implications for animal and human health. However, a low incidence of Bartonella spp., especially Bartonella henselae, was detected. The high number of various detected pathogens suggests that ticks might serve as an excellent sentinel to detect and monitor zoonotic human pathogens.
Keywords: Bartonella, Microbiome, Tick, Dog, Roe deer, Nanopore, Illumina, One health
Background
Globalization and climate change both contribute to the spread of infectious diseases often transmitted by insects and arthropod vectors. The monitoring and control of vector-borne diseases is an important task in public health, consolidating medical, veterinary and environmental research to realize potential health threats and to avert and reduce them [1].
Ticks are distributed worldwide and are of special interest in infection epidemiology research as these vectors are able to transmit a broad variety of infectious agents to humans and animals. Hard ticks usually feed three times during their life-cycle and pathogens can be acquired or transmitted during every blood meal. Furthermore, pathogens are transmitted transstadially from larvae to nymphs or from nymphs to adults, or vertically from mother ticks to eggs [2]. For the last two decades, pathogen-harboring-ticks were mainly analyzed by PCR-based applications, e.g. for Borrelia spp. and Bartonella spp. [3–5]. However, these analyses were limited naturally in their spectrum of detectable pathogens. With the availability of next generation sequencing (NGS) technologies, several studies have analyzed the microbiome of ticks, and besides endosymbionts, a large variety of bacterial pathogen DNA has been found (e.g. Francisella spp., Rickettsia spp., Anaplasma spp., Bartonella spp. and Borrelia spp.) without the need to select particular tests in advance [6–12].
Bartonella spp. are typical examples for vector-borne pathogens. These Gram-negative, facultative intracellular bacteria cause long-lasting intraerythrocytic infections in their respective reservoir hosts and are usually transmitted by blood sucking arthropods [13–15]. For example, rodents and bats serve as primary reservoirs for various Bartonella spp., including species with medical relevance for humans [16, 17]. Today, Bartonella henselae is the most common pathogenic representative of the genus Bartonella. Its reservoir host is the cat from which it is transmitted to humans (causing cat-scratch disease and other diseases) and dogs (causing endocarditis, fever of unknown origin and peliosis hepatis) [13, 18–20]. Bartonella schoenbuchensis was isolated first from the blood of wild roe deer in 1999 [21] and it turned out that several ruminant species serve as a reservoir hosts for this particular pathogen [22–29]. In animal reservoir hosts, asymptomatic infections with Bartonella spp. are common, although their pathogenicity remains unclear [30, 31]. Bartonella schoenbuchensis has been suggested to cause deer ked dermatitis in humans [30] and was isolated from a patient with a history of tick bites who suffered from fatigue, muscle pain and fever [32]. Currently, at least 37 Bartonella spp. are known to infect humans and animals [33].
In ticks, the prevalence of B. henselae DNA has been demonstrated to be up to ~40% [3] and, although controversially discussed [34], ticks are suspected to transmit Bartonella spp. [35]. The vector-competence of ticks has been confirmed in a murine infection model [36] and by using an artificial feeding system [37]. Several studies have shown that various tick species harbor several pathogenic bacteria alongside with Bartonella spp. [5, 38, 39], leading to a potential risk of co-infections in humans and animals. Two studies have reported co-infections with Borrelia burgdorferi [not specified, respectively B. burgdorferi (sensu lato)] and B. henselae in humans [40, 41]. As co-infections can result in more severe and irregular courses of disease, studies of the microbiome are a crucial prerequisite to estimate the health threat for humans and animals arising from tick bites and allow broader insights in the epidemiology of tick-borne pathogens.
We investigated the presence of Bartonella spp. and other pathogens in feeding ticks and blood of pets and wild animals in central Germany (federal state of Hesse) by combining NGS and conventional PCRs for pathogen detection. Moreover, we attempted to detect pathogen-specific antibodies in the serum of pets and their owners.
Methods
Sample collection
Ticks and serum from pets were collected by veterinarians located in the state of Hesse, Germany, and tick and blood samples from wild animals were collected by hunters directly after shooting and by employees of the Landesbetrieb Hessisches Landeslabor, Gießen, Germany. All locations are given in Table 1. Blood was collected in EDTA- and serum-tubes and ticks were stored in sterile, DNA-free vials (Eppendorf, Hamburg, Germany) containing 70% DNA-free ethanol. Human blood samples were taken by general practitioners or in the outpatient clinics of the Institute for Medical Microbiology and Infection Control, Frankfurt am Main, Germany. The workflow of all samples is shown in Fig. 1.
Table 1.
Geographical coordinates of hunting sites, veterinary practices and state health authorities in Germany where samples were taken
| Animal species (n) | Location | Geographical coordinates |
|---|---|---|
| Hunting sites | ||
| Boar (n = 1) | Urban forest, Frankfurt am Main | 50°04'19.8"N, 8°40'52.2"E |
| Roe deer (n = 9); boar (n = 3); raccoon (n = 1) | Vogelsberg, Schotten | 50°31'00.8"N, 9°14'30.1"E |
| Roe deer (n = 3) | Hainchen | 50°51'15.912"N, 8°12'57.51"E |
| Roe deer (n = 1) | Koenigstein | 50°10'43.464"N, 8°28'18.876"E |
| Roe deer (n = 1) | Altenhain, Taunus | 50°9'22.428"N, 8°28'14.048"E |
| Roe deer (n = 1) | Hofheim-Wallau | 50°3'43.186"N, 8°22'20.345"E |
| Roe deer (n = 7) | Biedenkopf | 50°54'24.35"N, 8°32'9.55"E |
| Roe deer (n = 6); boar (n = 1) | Neu-Anspach, Gruenwiesenweiher | 50°19'33.8"N, 8°29'38.4"E |
| Roe deer (n = 1) | Buedingen | 50°17'10.667"N, 9°6'40.982"E |
| Roe deer (n = 3) | Biblis / Wattenheim | 49°41'28.0"N, 8°24'24.9"E |
| Roe deer (n = 2) | Gedern | 50°26'54.0"N, 9°14'21.9"E |
| Veterinary practices and government agencies | ||
| Dog (n = 19) | Oberursel | 50°12'16.7"N, 8°35'40.3"E |
| Dog (n = 4) | Hattersheim | 50°04'11.2"N, 8°28'22.1"E |
| Dog (n = 1) | Bad Vilbel | 50°11'13.2"N, 8°44'24.5"E |
| Dog (n = 1); cat (n = 1) | Offenbach | 50°06'14.3"N, 8°45'19.9"E |
| Dog (n = 2) | Bad Homburg | 50°13'11.8"N, 8°38'46.4"E |
| Dog (n = 16) | Frankfurt am Main | 50°07'00.3"N, 8°38'35.7"E |
| Dog (n = 2) | Frankfurt am Main | 50°10'50.5"N, 8°39'37.9"E |
| Dog (n = 1) | Frankfurt am Main | 50°05'11.2"N, 8°35'05.2"E |
| Dog (n = 2) | Hofheim | 50°03'52.7"N, 8°23'15.7"E |
| Dog (n = 3) | Moerfelden-Walldorf | 49°59'43.5"N, 8°34'34.1"E |
| Dog (n = 1) | Dreieich | 50°01'07.8"N, 8°40'23.8"E |
| Dog (n = 1) | Dreieich | 50°01'14.7"N, 8°41'12.3"E |
| Dog (n = 1) | Frankfurt am Main | 50°08'49.0"N, 8°40'00.1"E |
| Roe deer (n = 3); boar (n = 1); red fox (n = 1); wisent (n = 1) | Landesbetrieb Hess, Landeslabor Gießen | 50°34'03.2"N, 8°39'45.2"E |
| Roe deer (n = 2) | Forestry district, Koenigstein | 50°10'43.464"N, 8°28' 18.876"E |
Fig. 1.
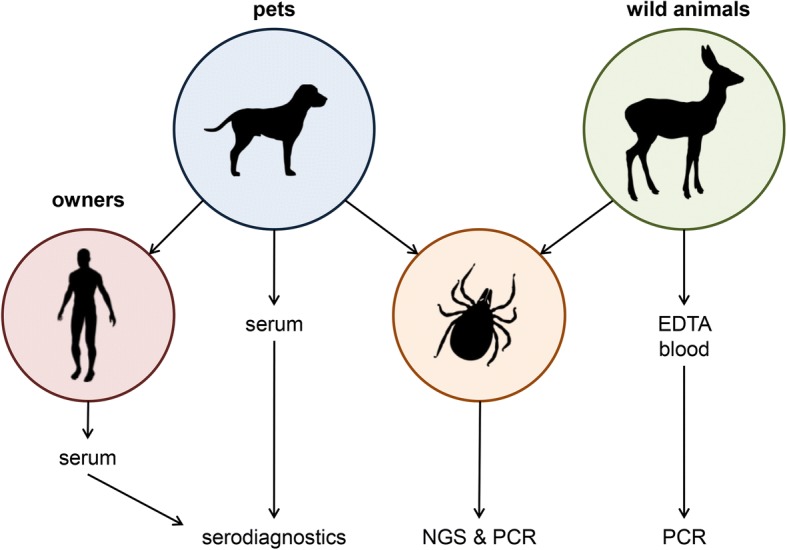
Workflow of all samples. DNA of ticks and animal blood samples was extracted and PCRs for Bartonella-specific genes (16S rDNA, 16S-23S ITS) were conducted (with subsequent Sanger-sequencing of the amplicons). 16S rDNA metagenomics was used for determination of the tick microbiome (confirmed by specific PCRs) revealing the presence of further pathogens. Serum of pets and, if available, of pet owners was analyzed for serological infection markers (antibodies) known to indicate previous infections in regard to the molecular findings from ticks
DNA-extraction from ticks and whole-blood
The laboratories of the Institute for Medical Microbiology and Infection Control at the University Hospital of the Goethe University in Frankfurt (Germany) undergo a strict and externally reviewed quality control management with all required positive and negative controls (laboratory accreditation according to ISO 15189:2014 standards; certificate number D-ML-13102-01-00, valid through January 25th, 2021) and are appointed as National Consiliary Laboratories for Bartonella by the Robert Koch Institute, Berlin, Germany. There was no increase of Bartonella-positive cases during this study; therefore, the possibility of DNA contamination from non-study sources is highly unlikely.
DNA extraction from ticks was conducted as previously described [42]. All ticks were individually removed from tubes with sterile forceps, identified using standard taxonomic keys [43] and washed twice in sterile ethanol and once in sterile water to avoid DNA contamination by environmental microorganisms present on the cuticle of the ticks. Each tick was treated individually to prevent DNA cross-contamination. DNA was extracted by using the QIAamp DNA Mini kit (Qiagen, Hilden, Germany) according to manufacturer’s instructions and eluted in 200 μl elution buffer. Grinding was conducted with disposable sterile mortars and pestles. The extraction procedure was strictly controlled using specific pathogen-free ticks (Insect Services, Berlin, Germany) in which Bartonella spp. were never detected (data not shown). DNA from whole-blood was extracted using the DNeasy Blood and Tissue Kit (Qiagen) and eluted in 200 μl of elution buffer as recommended by the manufacturer.
PCR-detection of pathogen-specific genes by PCR
In this study, we focused on the detection of Bartonella spp. in addition to other pathogens. Therefore, all ticks and EDTA-blood were analyzed for Bartonella spp. DNA using two different PCR methods [42]: 16S ribosomal DNA (rDNA) nested PCR [3, 44] using the Taq DNA Polymerase-Kit (Invitrogen, Schwerte, Germany) and 16S-23S rDNA internal transcribed spacer (ITS) region PCR [45] using the Platinum Taq Polymerase-Kit (Invitrogen). Positive (B. henselae Houston ATCC 49882, 1 ng) and negative (water) intra-assay controls were always included. DNA was amplified in a Biometra T3000 thermocycler (Biometra, Goettingen, Germany). Products were separated on agarose gels, stained with ethidium bromide and visualized under UV light. PCR products were sequenced by a commercial provider (GATC, Konstanz, Germany), analyzed using the Chromas software (Technelysium, v.2.6, South Brisbane, Australia) and compared to Bartonella spp. strains deposited in the NCBI databank using the BLAST online tool for species level identification.
Whenever potentially pathogenic bacterial species were detected by NGS in the microbiome analysis (see below), subsequent PCRs for species determination were conducted from ticks and from EDTA-blood of the animals, if available. Primers for the detection of the Bartonella spp. 16S-rDNA nested PCR and the Bartonella spp. 16S-23S rDNA ITS region PCR, the Rickettsia spp. 23S-5S ITS region [46], the Coxiella burnetii IS1111 region [47], 16S rDNA of Anaplasma spp. [48], the Leptospira spp. LipL32 gene [49] and Borrelia spp. 16S rDNA [50] are given in Table 2. PCRs were conducted employing the standard protocol for the Platinum Taq Polymerase Kit and all PCR-products were sequenced and analyzed. Detection of the ospA gene of Borrelia spp. in ticks and of Francisella tularensis were conducted using LightMix Kits (TIB MOLBIOL, Berlin, Germany) according to the manufacturer’s instructions. Positive controls (each containing 1 ng of DNA) were the following: Rickettsia helvetica (friendly gift of Dr. Dobler, München, Germany), Coxiella burnetii (German laboratory quality assessment trials, INSTAND e.v., Düsseldorf, Germany), Anaplasma phagocytophilum Webster (friendly gift of Prof. von Loewenich, Mainz, Germany), Leptospira interrogans (German Federal Institute for Risk Assessment, Berlin, Germany) and Borrelia miyamotoi HT31 (CDC, Fort Collins, USA).
Table 2.
Targets, primers and amplicon size for the PCR-testing from ticks and EDTA-blood
| Target sequence | Designation | Sequence (5'-3') | Amplicon length (bp) | Reference |
|---|---|---|---|---|
| Bartonella spp. 16S rDNA, 1st round | A-proteo | AGAGTTTGATC(AC)TGGCTCAGA | 1210 | [44] |
| r-Alpha-sh | GTAGCACGTGTGTAGCCCA | |||
| Bartonella spp. 16S rDNA, 2nd round | Bart | CACTCTTTTAGAGTGAGCGGCAA | 990 | [44] |
| r-BH | CCCCCTAGAGTGCCCAACCA | |||
| Bartonella 16S-23S ITS region | 325s | CTTCAGATGATGATCCCAAGCCTTCTGGCG | various Bartonella spp., ~500 bp | [45] |
| 1100as | GAACCGACGACCCCCTGCTTGCAAAGCA | |||
| Bartonella spp. rpoB | prAPT0244 | GATGTGCATCCTACGCATTATGG | 406 | [51] |
| prAPT0245 | AATGGTGCCTCAGCACGTATAAG | |||
| Anaplasma spp. 16S rDNA 1st round | ge3A | CACATGCAAGTCGAACGGATTATTC | 932 | [48] |
| ge10r | TTCCGTTAAGAAGGATCTAATCTCC | |||
| Anaplasma spp. 16S rDNA 2nd round | ge9f | AACGGATTATTCTTTATAGCTTGCT | 546 | [48] |
| ge2 | GGCAGTATTAAAAGCAGCTCCAGG | |||
| C. burnetii IS1111 | CB_S4k | GAAACGGGTGTTGAATTGTTTG | 290 | [47] |
| CB_A2k | ATCACCAATCGCTTCGTCCCGGT | |||
| Rickettsia spp. 23S-5S ITS region | 23S for | GATAGGTCGGGTGTGGAAGCAC | various Rickettsia spp., ~500 bp | [46] |
| 23S rev | GGGATGGGATCGTGTGTTTCAC | |||
| Leptospira spp. LipL32 | LipL32-270F | CGCTGAAATGGGAGTTCGTATGATT | 423 | [49] |
| LipL32-692R | CCAACAGATGCAACGAAAGATCCTTT | |||
| Borrelia spp. 16S rDNA | 16S FW | GGCTTAGAACTAACGCTGGCAGTGC | 552 | [50] |
| 16S RV | CCCTTTACGCCCAATAATCCCGA |
Differentiation of ruminant-associated Bartonella spp. by rpoB-PCR
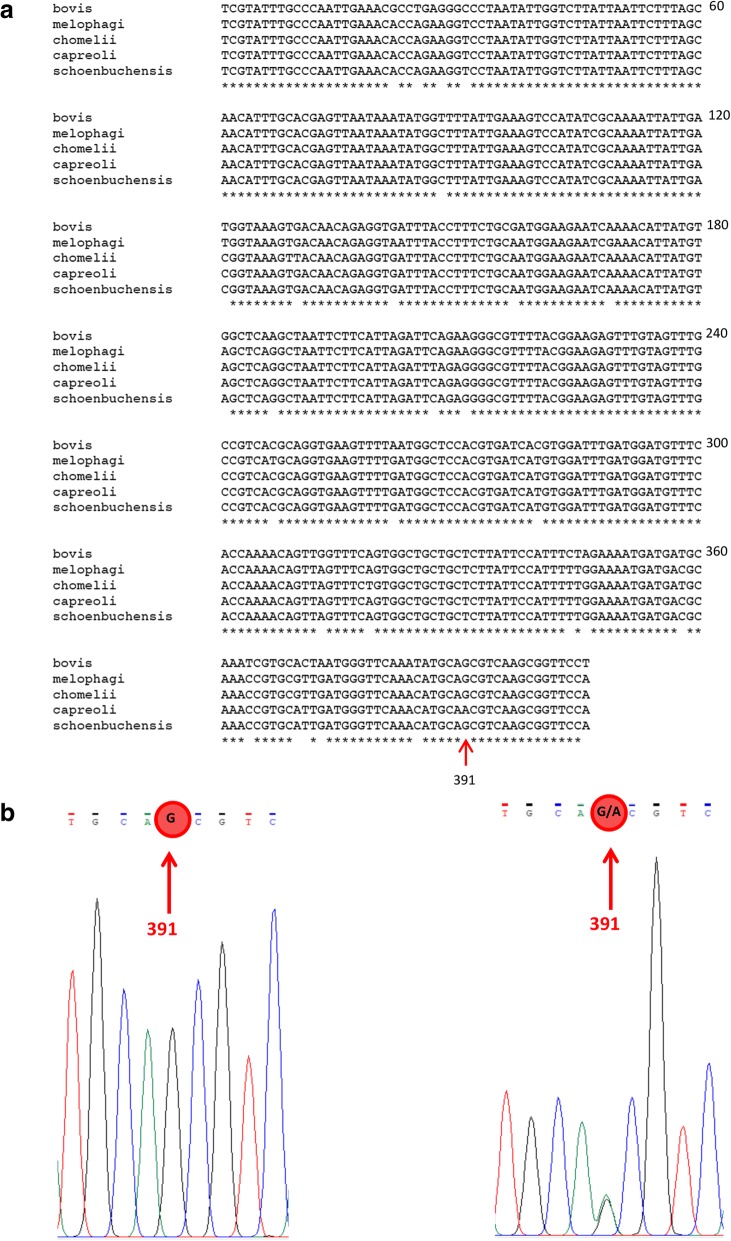
To increase the species-discriminatory power of the rDNA-sequences within the Bartonella ruminant-lineage, a PCR protocol specific for a 406 bp internal fragment of the rpoB gene (β-subunit of the bacterial RNA polymerase) was performed [51]. Bartonella rpoB DNA was amplified by using 5 μl of starting material. Positive (B. henselae Houston ATCC 49882, 0.5 ng) and a negative (water) controls were always included. Primers are given in Table 2. PCR products were sequenced (GATC) and analyzed using Chromas software. To analyze the Bartonella species-discriminatory nucleotide polymorphism of the 403 bp rpoB fragment, the obtained sequences were aligned to sequences of Bartonella schoenbuchensis (type strain R1, AY167409.1), B. capreoli (type strain IBS193, AB290188.1), Bartonella chomelii (type strain A828, AB290189.1), B. bovis (type strain 91-4, AY166581.1) and Bartonella melophagi (type strain K-2C, EF605288.1) using Clone Manager Professional Suite 8 (Scientific and Educational Software, Denver, USA). As shown in Fig. 2, the 406 bp rpoB fragment allows species discrimination of B. chomelii, B. bovis and B. melophagi based on multiple nucleotide positions, as well as species discrimination of B. schoenbuchensis and B. capreoli based on a single nucleotide position (position 391). Co-infections with B. schoenbuchensis and B. capreoli were detected by analyzing double peaks at the discriminatory nucleotides.
Fig. 2.
Discrimination of ruminant-associated Bartonella spp. by SNP-analysis. a Alignment of B. bovis, B. melophagi, B. chomelii, B. capreoli and B. schoenbuchensis. Discriminatory nucleotide positions are on 27 positions. b Left: unibacterial B. schoenbuchensis infection (sequence at the discriminatory nucleotide …TGCAGCGTC…); right: B. schoenbuchensis and B. capreoli-co-infection (sequence at the discriminatory nucleotide …TGCAG/ACGTC…)
Microbiome analysis of ticks using next generation sequencing by Illumina technology
The V4 region of the 16S rRNA gene of each tick was amplified using previously described primers [52]. Amplification was done using the Platinum SuperFi PCR Master Mix (Thermo Fisher Scientifc, Carlsbad, USA). Each PCR reaction was performed in a total of 25 μl of reaction solution comprising 2× SuperFi PCR Master Mix, 1.25 μl of 10 pmol forward and reverse primers and a maximum of 10 μl of DNA per reaction. After mixing the solutions, the following thermocycler conditions were run: 98 °C for 2 min; 25 cycles at 98 °C for 10 s, 55 °C for 10 s and 72 °C for 30 s; and a final extension step at 72 °C for 5 min. The size of PCR products was confirmed on a 2% agarose gel and purified using AMPure XP DNA beads (Beckman Coulter, Brea, USA). The index and adapter ligation PCR was done using a Nextera XT Index Kit v2 Set A and B (Illumina, San Diego, USA) and performed according to the manufacturer’s protocol. PCR conditions were set as follows: 95 °C for 3 min; 8 cycles at 95 °C for 30 s, 55 °C for 30 s and 72 °C for 30 s; and a final extension step at 72 °C for 5 min. Quality and quantity control of purified PCR products were done using a Qubit 2.0 Fluorometer (Thermo Fisher Scientific) and a 2100 Bioanalyzer (Agilent Technologies, Santa Clara, USA). All samples were diluted to the same molarity, pooled together, spiked with an internal control (15% PhiX) and paired-end sequenced on the MiSeq Illumina platform using a flow cell with V2 chemistry (500 cycles). Negative controls (kit and PCR controls) were performed using pure water and elution buffer. In addition, microbial mock communities (Zymo Research, Freiburg, Germany) were run alongside as a standard and as quality control for determining contamination bias of DNA extraction. To ensure the best quality sequencing results, the 16S rDNA nested PCR and the 16S-23S-rDNA-ITS region PCR were always run in parallel to the NGS were compared. Furthermore, whenever human pathogens were detected by NGS, all positive results were confirmed by conventional PCR methods (see above).
16S full length rRNA gene sequencing using Nanopore
In brief, the entire 16S rRNA gene (~ 1.5kb) of selected samples was amplified using the native 16S Barcoding Kit SQK- RAB204 (Nanopore Technologies, Oxford, England). Library preparation and sequencing were done following the manufacturer’s instructions. Each PCR reaction was performed in a total of 50 μl of reaction solution comrpising 14 μl of nuclease-free water, 10 ng of input DNA, 1 μl of barcode and 25 μl of LongAmp Taq 2X Mastermix (New England Biolabs, Frankfurt, Germany). The following PCR protocol was performed: 95 °C for 1 min; 25 cycles at 95 °C for 20 s, 55 °C for 30 s and 65 °C for 2 min, and a final extension step at 72 °C for 5 min. PCR products were purified using 30 μl of AMPure XP DNA beads (Beckman Coulter). Samples were eluted in 10 μl of 10 mM Tris-HCl pH 8.0 with 50 mM NaCl. Quantification of the libraries was performed using a Qubit 2.0 Fluorometer (Thermo Fisher Scientific); subsequently they were pooled together, prepared for loading and eventually sequenced on a R9.4.1 FLO-MIN106 flowcell.
Bioinformatic microbiome analysis workflow
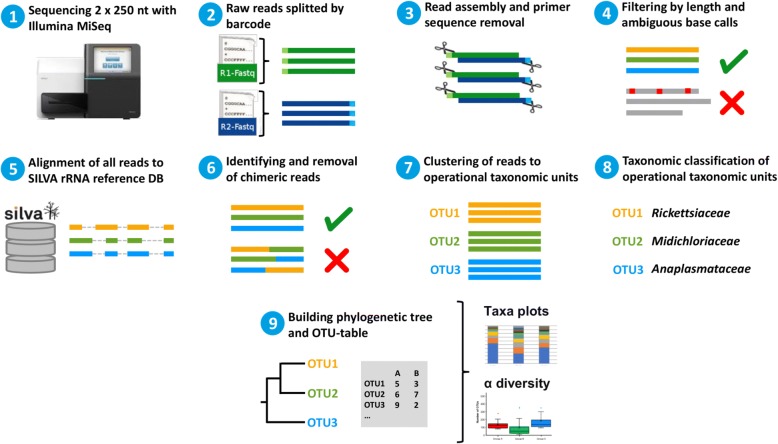
MiSeq Software v.2.6 was used to split the sequences by barcode and to generate the fastq files. The microbiome analysis was done following the MiSeq standard operation procedures [53] using Mothur (v.1.36.1) [54]. Qiime (v.1.9.1) was used for the alpha-diversity calculation and the taxa summary plots [55].
The paired-end reads were joined and the primer sequences were removed. We filtered for the expected amplicon length and removed reads with ambiguous base calls or with homopolymers longer than eight nucleotides. Duplicate sequences were merged. The unique reads were aligned against the SILVA-bases bacterial reference alignment [56]. Nucleotides outside the expected alignment region were trimmed. Reads with a difference of two nucleotides were merged during pre-clustering. Chimeric reads were removed using the Mothur implementation of the uchime algorithm. After chimera removal, taxonomy was assigned and non-bacterial reads were removed. OTUs were created using the cluster split method of Mothur. After clustering, we reassigned the taxonomy to the OTUs. In preparation for the analysis with Qiime, a phylogenetic tree and an OTU table in biom format was created. Alpha-diversity analysis and the taxa summary plots were created using the Qiime core diversity analysis script. The workflow is summarized in Fig. 3. 16S full length rRNA gene sequencing data were analyzed applying the EPI2ME platform with FASTQ 16S (v.3.0.0) from Oxford Nanopore Technologies.
Fig. 3.
Schematic overview of microbiome bioinformatic analysis workflow. The hypervariable V4 region of 16S rDNA from tick samples was sequenced and split by barcode with Illumina MiSeq. Resulting paired-end reads were joined and the primer region was removed. Reads were filtered by amplicon length and aligned to SILVA as the reference database. After removal of chimeras, reads were clustered into operational taxonomic units (OTU) and taxonomically classified. Finally, an OTU-table was created and results were visualized
Serodiagnostics of pets and pet owners
All serum samples were screened for the presence of anti-B. henselae-antibodies by indirect immunofluorescence assay (IIFA) using a B. henselae/B. quintana (IgG) kit (Euroimmun, Luebeck, Germany) according to the manufacturer’s instructions with slight modifications [42]. Pet sera were tested with a 1:100 dilution of Alexa Fluor 488-conjugated AffiniPure Goat Anti-Dog/Cat IgG (Jackson ImmunoResearch laboratories, West Grove, USA) as secondary antibodies. Serum dilutions from 1:20 to 1:2560 were screened for Bartonella dog/cat IgG antibodies. Signals were evaluated as positive when specific fluorescence was detected at a titer of ≥ 1:64 [57] for animals and > 1:80 for humans.
Whenever potentially pathogenic genera were found in ticks by NGS or PCR, the corresponding animal sera and, if available, sera of the respective pet owners were analyzed for antibodies against the most common pathogenic bacterial species of these genera. Animal sera were sent to the veterinarian diagnostic laboratory Laboklin (Bad Kissingen, Germany) for the detection of pet antibodies against Anaplasma phagocytophilum (ELISA; cut-off: ≥ 8 TE), Rickettsia conorii (IIFA) (cut-off: ≥ 1:256), R. rickettsii (IIFA; cut-off: IgG ≥ 1:256), Borrelia spp. (ELISA; cut-off: ≥ 8 units), Leptospira spp. (microagglutination test; cut-off: ≥ 1:400), Coxiella burnetii (IIFA; cut-off: ≥ 1:1:20) and Francisella tularensis (qualitative serum slow agglutination test).
Serodiagnostics of human sera was performed in the diagnostic laboratories of the Institute for Medical Microbiology and Infection Control at the University Hospital of the Goethe University in Frankfurt am Main (Germany) under fully certified conditions (ISO 15189:2014, certificate number D-ML-13102-01-00, valid through January 25th, 2021). The method used for the detection of antibodies against Anaplasma phagocytophilum was IIFA (A. phagocytophilum IFA IgG/IgM (Focus Diagnostics, Cypress, CA, USA; cut-off: IgG ≥ 1:64, IgM ≥ 1:20), for Rickettsia typhi and Rickettsia rickettsii it was IIFA (Rickettsia IFA IgG/IgM Focus Diagnostics; cut-off: ≥ 1:64), for B. burgdorferi (sensu lato) it was ELISA (Enzygnost® Borreliosis/IgM/Lyme link VlsE/ IgG, Siemens, Marburg, Germany; cut-off: ≥ 7 U/ml), for Leptospira spp. it was ELISA (Leptospira IgG/IgM Serion ELISA classic, Serion, Wuerzburg, Germany; cut-off: IgG ≥ 10 U/ml, IgM ≥15 U/ml) and for Coxiella burnetii it was IIFA (C. burnetii IgG/IgM IFA Kit, Fuller Laboratories, Fullerton, CA, USA; cut-off ≥ 1:16). Testing for anti-Francisella tularensis antibodies was conducted at the Institut für Mikrobiologie der Bundeswehr, München, Germany by ELISA (qualitative cut-off OD 0.25).
Results
Sample collection
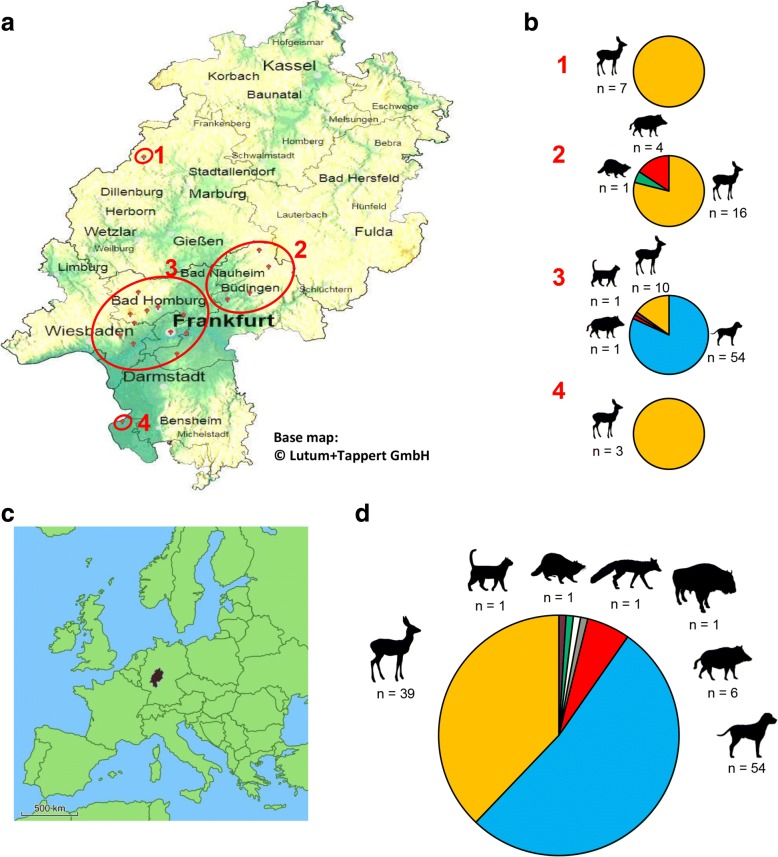
From March to October 2017, 189 engorged ticks from 103 animals were collected at four collection sites in the federal state of Hesse, Germany shown in Fig. 4a. Pet samples comprised samples from 54 dogs (54 dog sera, 84 ticks, 17 whole-blood samples) and one cat (serum, whole-blood and one tick). Pet owner samples comprised 11 sera obtained from dog-owners. The samples of wild animals comprised samples from 39 roe deer (Capreolus capreolus; 39 whole-blood samples and 95 ticks), 6 boar (Sus scrofa; 6 whole-blood samples and 6 ticks), 1 wisent (Bos bonasus; 1 whole-blood sample and 1 tick), 1 raccoon (Procyon lotor; 1 whole-blood sample and 1 tick) and one red fox (Vulpes vulpes; 1 whole-blood sample and 1 tick). The number of sampled animals is summarized in Fig. 4d. Ticks were mostly female adult Ixodes ricinus ticks (identified by standard taxonomic keys). Among the 189 ticks, two were nymphs and 6 were male adults. Two tick samples (1 dog and 1 cat) were identified as Rhipicephalus sanguineus.
Fig. 4.
a Geographical map of the federal state of Hesse (Germany) displaying the locations of feeding-tick collections. The red marks represent the locations where the ticks were collected. From top to bottom, numbers in red: 1, North Hesse; 2, Mid-west Hesse; 3, Greater metropolitan area Frankfurt am Main; 4, South Hesse. The base map was generated using EasyMap 11.0 © Lutum+Tappert DV-Beratung GmbH. b Distribution of sampled ticks and their hosts in relation to their location. From top to bottom: 1, North Hesse; 2, Mid-west Hesse; 3; Greater metropolitan area Frankfurt am Main; 4, South Hesse. c Map of Europe with exact location of Hesse tagged. d Fractions of all animals examined in this study: dogs, roe deer, cat, raccoon, fox, wisent and boars
PCR detection of Bartonella spp.
In addition to the detection of multiple pathogens by microbiome analysis, we focused on the detection of Bartonella spp. since there is a broad spectrum of Bartonella spp. that can lead to severe infections in humans and animals [33]. Bartonella spp. were detected in ticks and full blood of roe deer. DNA of two different Bartonella spp. was found in roe deer blood: B. schoenbuchensis in 10 (25.6%) animals, B. capreoli in 4 (10.3%) animals and a co-infection of both Bartonella spp. was detected in the blood of 3 (7.7%) roe deer. Among the roe deer with blood positive for Bartonella spp., nine did not have Bartonella spp.-positive ticks. DNA of Bartonella spp. was found in 9.5% of all ticks. Bartonella schoenbuchensis DNA was detected in 14 ticks (7.4% of all ticks) from 10 roe deer, B. capreoli DNA was found in one tick (0.5% of all ticks) and B. henselae DNA was detected in three ticks (1.6% of all ticks) of three roe deer. Moreover, Bartonella spp. DNA was found in ticks of three animals whose blood contained no Bartonella spp. DNA.
Microbiome analysis of collected tick samples throughout the state of Hesse
It is widely known that ticks act as vectors for various pathogens including Bartonella spp., Anaplasma spp. and Rickettsia spp., all known to be harmful to humans. Thus, we were interested to analyze and identify the microbial composition of blood-fed ticks from wild animals and pets sampled throughout Hesse. For this, a 16S rRNA gene amplicon-sequencing (V4 region) and bioinformatics analysis workflow was established. Overall, 136 ticks sampled from 97 animals were sequenced on the MiSeq Illumina platform, resulting in a minimum sequencing depth of 5000 reads per sample for further analysis. After performing the initial NGS sequencing of 136 ticks we continued tick collection, resulting in the fact that not all of the total collected ticks (n = 189) underwent microbiome analysis.
The alpha diversity of ticks sampled from wild animals showed a dominant higher number of operational taxonomic units (OTUs) compared to ticks collected from pets, indicating greater species richness in wild animals (Fig. 5). Here, we also observed more outliers and a wider range for the group of wild animals compared to the pet group.
Fig. 5.
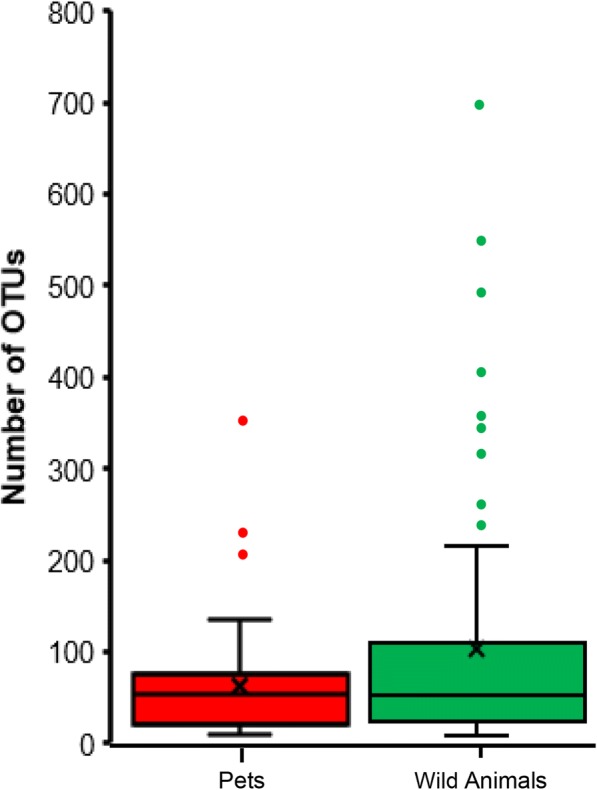
Number of operational taxonomic units (OTUs) in ticks from pets and wild animals at a sampling depth of 5000 reads. Subsampling without replacement was repeated 1000 times and averages reported
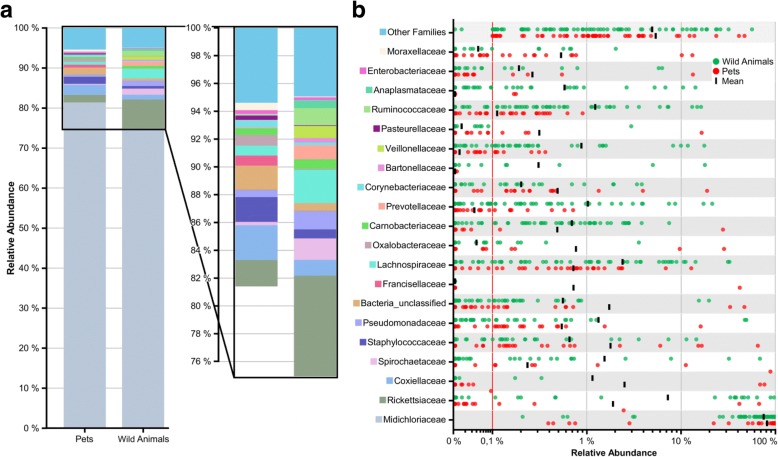
To examine the microbial taxonomic distribution of sampled ticks, cumulative bar charts composed on family level were created. Comparing both groups, we observed that Midichloriaceae, a common tick endosymbiont, is represented as the most dominant OTU for pets (~ 80%) and wild animals (~75%) (Fig. 6a). As seen in Fig. 6b, which represents the variation in relative abundances of the top 20 OTUs between wild animals and pets, the group of wild animals revealed a higher abundance of Rickettsiaceae, Spirochaetaceae, Bartonellaceae and Anaplasmataceae. Other tick-associated OTUs which were found more dominantly represented in ticks sampled from pets included Coxiellaceae and Francisellaceae. Interestingly, ticks of wild animals exhibit a “companion microbiome” as observed in higher abundances of Ruminococcaceae, Carnobacteriaceae and Lachnospiraceae, which regularly colonize the intestine of animals and can be detected in microbiome studies. We also observed the families of Prevotellaceae and Veillonellaceae enriched in wild animals, which are representatives of anaerobic bacteria known to colonize, for example, the oral cavity. A well-known issue of the microbiome analysis is the “kitome” contamination problem, which was extensively reported by several groups [58, 59]. As identification of false positive OTUs generated from DNA kits as well as PCR contaminations are critical issues in the analysis of microbiome data, kit and water controls together with the tick microbiome samples were performed. Here, we identified species of the genera Halomonas and Shewanella as potential kit DNA contaminants which were, however, absent in the top 20 families found in the herein described tick sample microbiome results. These species with low abundance were present in few samples but had no deeper impact in our further analysis. We also identified barcode crosstalk as background noise in barcode controls, but this sequencing-specific artifact did not affect the results of this study.
Fig. 6.
Overview of top 20 bacterial families found in ticks by NGS. a Cumulative bar charts comparing relative family abundances for ticks collected from pets and wild animals. b Variation in relative abundance of each family in tick samples. Red line shows cut-off for noise. Families not in the top 20 by relative abundance are categorized as other families
In order to identify bacteria to the species level, full length sequencing of the 16S rRNA gene using Oxford Nanopore Technology was applied. Three samples revealing a higher percentage of unclassified bacteria (relative abundance > 15%) were selected in addition to two samples indicating the presence of the genus Borrelia. Our results revealed that the previously identified OTU “Bacteria unclassified” was resolved to Spiroplasma ixodetis, another known tick endosymbiont [60]. Borrelia miyamotoi was found in the selected two tick samples formerly by Illumina sequencing and PCR confirmation, and was also identified by Nanopore sequencing to species level.
Confirmation PCRs were conducted for all pathogenic species (Table 2). DNA of Bartonella spp. was detected in four ticks (5.3%) from three roe deer. Three were confirmed to be B. schoenbuchensis by PCR. DNA of Spirochaetaceae was found in 13 ticks (17.1%) from six roe deer, four dogs and one boar. The presence of Borrelia garinii was confirmed in one tick from a dog and that of Borrelia miyamotoi in one tick from another dog and two ticks from two roe deer. Rickettsia DNA was detected in 15 ticks (19.7%) from 10 roe deer, one raccoon, two dogs and one boar. The presence of Rickettsia helvetica was confirmed in nine of those ticks. Coxiella DNA was detected in three ticks (3.9%) from two dogs and one raccoon. Confirmation PCRs for Coxiella burnetii remained negative so, potentially, those Coxiella spp. detected by NGS and not confirmed in Coxiella burnetii-specific PCRs represent Coxiella-like tick endosymbionts [61]. Francisella tularensis DNA was detected in one female adult Rhipicephalus sanguineus tick from a dog. This result was confirmed by real-time PCR. Anaplasma DNA was found in 15 ticks (19.7%) from nine roe deer, one dog and one boar and Anaplasma phagocytophilum was confirmed to be present in 12 of those ticks. Staphylococcus DNA was detected in eight ticks (10.5%) from five dogs, two boars and one raccoon. Table 3 shows a summary of pathogens found by NGS data analysis.
Table 3.
Potentially pathogenic genera found in the microbiome of 76 ticks obtained from 48 animals
| OTU (n) | PCR confirmation (n) | Host species (n) |
|---|---|---|
| Spirochaetaceae (n = 13) | B. garinii (n = 1); B. miyamotoi (n = 3) | Roe deer (n = 6); dog (n = 4); boar (n = 1) |
| Bartonella spp. (n = 4) | B. schoenbuchensis (n = 3) | Roe deer (n = 3) |
| Rickettsia spp. (n = 15) | R. helvetica (n = 9) | Roe deer (n = 10); raccoon (n = 1); dog (n = 2); boar (n = 1) |
| Coxiella spp. (n = 3) | C. burnetii found | Dog (n = 2), raccoon (n = 1) |
| Francisella spp. (n = 1) | F. tularensis (n = 1) | Dog (n = 1) |
| Anaplasma spp. (n = 15) | A. phagocytophilum (n = 12) | Roe deer (n = 10); dog (n = 1); boar (n = 1) |
| Staphylococcus spp. (n = 8) | Not conducted | Dog (n = 5); boar (n = 2); raccoon (n = 1) |
Whenever potentially pathogenic genera were found in the microbiome analysis, animal whole-blood (when available) was also analyzed by PCR. Here, Anaplasma phagocytophilum was detected in the blood of eight roe deer.
Furthermore, co-infections of ticks with various bacteria were also detected. NGS revealed that nine ticks (11.8%) were co-infected with more than one pathogen (Table 4). One tick from a boar contained DNA from Spirochaetaceae, Anaplasma spp. and Staphylococcus spp. In another tick from a raccoon, Rickettsia spp., Coxiella spp. and Staphylococcus spp. DNA were found. Spirochaetaceae, Bartonella spp. and Anaplasma spp. were detected in three ticks from three roe deer. Spirochaetaceae, Rickettsia spp. and Anaplasma spp. were found in one tick from a roe deer. Furthermore, we detected three ticks from three roe deer with co-infections with two pathogens: one tick was co-infected with Bartonella spp. and Anaplasma spp. DNA, another tick was co-infected with Spirochaetaceae and Anaplasma spp. and the third tick was co-infected with Rickettsia spp. and Anaplasma spp. DNA.
Table 4.
Co-infections with various pathogens found in ticks taken from wild animals
| Host species | Spirochaetaceae | Anaplasma spp. | Staphylococcus spp. | Rickettsia spp. | Coxiella spp. | Bartonella spp. |
|---|---|---|---|---|---|---|
| Boar | + | + | + | - | - | - |
| Raccoon | - | - | + | + | + | - |
| Roe deer | + | + | - | - | - | + |
| Roe deer | + | + | - | - | - | + |
| Roe deer | + | + | - | - | - | + |
| Roe deer | + | + | - | + | - | - |
| Roe deer | - | + | - | - | - | + |
| Roe deer | + | + | - | - | - | - |
| Roe deer | - | + | - | + | - | - |
Key: + positive; - negative
Indirect immunofluorescence assay (IIFA) of pets and pet owners
One of 54 dogs (1.9%) was seropositive for anti-B. henselae-antibodies (IgG) (titer: 1:640); in the respective tick, no B. henselae DNA was detected. One dog serum was found to be positive for Rickettsia conorii-antibodies (titer: 1:128); in the respective tick, Rickettsia helvetica DNA was detected. For all other dogs, antibodies against Anaplasma phagocytophilum, Borrelia spp., Leptospira spp., Coxiella burnetii, Francisella tularensis and Rickettsia rickettsii were not detected.
In five dog owners, anti-B. henselae-antibodies (IgG) were found (titers: 4 × 1:160, 1 × 1:320). All of the respective dogs, however, were seronegative, and, moreover, the remaining six dog owners did not show antibodies against B. henselae. No antibodies against Anaplasma phagocytophilum, Rickettsia typhi, Rickettsia rickettsii, Borrelia burgdorferi, Francisella tularensis, Coxiella burnetii or Leptospira spp. were found in the sera of the dog owners whose dogs were infested with ticks harboring a particular pathogen’s DNA.
Discussion
Ticks and tick-borne diseases remain a remarkable health threat for humans and animals in modern days. In this study, seven zoonotic potentially human pathogenic genera (Bartonella spp., Spirochaetaceae, Anaplasma spp., Rickettsia spp., Coxiella spp., Francisella spp. and Staphylococcus spp.) were found in ticks feeding on pets and wild animals in Hesse, Germany, and identified using NGS and classical PCR diagnostics for microbiome profiling.
By conducting a literature search including 19 published studies, Bartonella spp. DNA was formerly detected in about 15% of ticks (reviewed in [62]). In the present study, Bartonella DNA was found to be present in ~10% of all ticks (n = 18 of 189 ticks in total) suggesting a similar prevalence of Bartonella spp. in ticks collected at different locations in the state of Hesse, Germany. DNA of the ruminant-associated B. schoenbuchensis and B. capreoli was detected in 7.4% and 0.5% of the ticks collected from roe deer, respectively. Furthermore, DNA of B. schoenbuchensis (25.6%), B. capreoli (10.3%) and DNA from both Bartonella spp. (7.7%) were found in the blood of roe deer. In ticks collected from nine of those Bartonella-positive animals, no Bartonella spp. was detectable indicating that these negative ticks cleared the infection or that there were not enough pathogens in the ticks to be detected by the applied PCRs. The deer ked (Lipoptena cervi) is suspected to be the main vector for B. schoenbuchensis [22, 23, 30, 63–65] and represents a common ectoparasite of roe deer and other cervids [2] which serve as reservoir hosts for the ruminant-associated B. schoenbuchensis, B. capreoli, B. chomelii and B. bovis [22–29, 66]. It remains unclear to which extent these Bartonella spp. can cause diseases in their reservoir or accidental human hosts but, in general, Bartonella spp. are known to cause chronic asymptomatic infections in their mammalian reservoir hosts [30, 31]. Bartonella schoenbuchensis is suspected to cause unspecific symptoms like muscle pain and fever in humans as it was isolated from one patient with a history of tick bites in which no other causative agent was detected [32]. Bartonella DNA was detected in ticks collected from three animals whose blood samples were free from Bartonella DNA, indicating that the infection might have been acquired elsewhere. This result is in-line with another study where B. henselae DNA-positive and negative ticks were removed from dogs at the same time, with this observation leading to the hypothesis that the DNA-positive ticks had already acquired the B. henselae infection before feeding on these dogs [67].
Several studies suggest that vertical transmission of Bartonella spp. in ticks can occur so that even larvae can possibly transmit these pathogens. Bartonella DNA was detected in unfed adult I. persulcatus [68], in unengorged larvae and nymphs of Dermacentor variabilis and I. scapularis ticks [8], in unfed I. ricinus adults and nymphs [69] and in questing I. ricinus ticks collected from France [5], indicating that these ticks acquired the pathogen by vertical transmission. Further hints arguing for ticks as vectors for Bartonella spp. are co-infections of animals with multiple tick-borne pathogens. Chomel et al. [70] reported, that a dog suffering from B. clarridgeiae endocarditis was not only Bartonella seropositive but also seropositive for Anaplasma phagocytophila which is usually transmitted by ticks [70]. Multiple co-infections with tick-borne pathogens such as Ehrlichia spp., Babesia canis, Bartonella vinsonii and Rickettsia rickettsii were found in dogs which were heavily exposed to ticks [71]. Furthermore, dogs suffering from endocarditis caused by Bartonella spp. were found to have high antibody titers against several tick-borne pathogens (Anaplasma phagocytophilum, Rickettsia rickettsii, Ehrlichia canis and Borrelia burgdorferi [72]). However, the fact that B. henselae was only detected in three ticks, leads to the conclusion that there is a low risk of acquiring B. henselae infections by tick bites, at least in the herein sampled area.
The microbiome analysis presented here reveals that, besides Bartonella spp., six other potentially pathogenic genera were detected by NGS. Spirochaetaceae (17.1%), Rickettsia spp. (19.7%), Coxiella spp. (3.9%), Francisella spp. (1.3%), Anaplasma spp. (19.7%) and Staphylococcus spp. (10.5%) were also identified in ticks. These results coincide with several studies investigating the microbiome of ticks worldwide. Francisella spp., Coxiella spp., Rickettsia spp. and Shigella spp. were detected in two tick species collected from humans in Turkey [6] and Coxiella spp., Rickettsia spp., Anaplasma spp., Ehrlichia spp., Wolbachia spp., Mycobacteria spp., Pseudomonas spp., Staphylococcus spp., Acinetobacter spp., Klebsiella spp. and Leptospira spp. were found in ticks removed from domestic animals in Malaysia [7]. In Indiana, USA, Francisella spp., Rickettsia spp. and Bartonella spp. were detected in ticks removed from small rodents [8], Anaplasma spp., Borrelia spp., Coxiella spp., Ehrlichia spp., Francisella spp. and Rickettsia spp. were found in questing ticks collected by flagging in France [9] and ticks collected from dogs and the environment in France, Senegal and USA showed presence of Rickettsia spp., Coxiella spp. and Bacillus spp. [11]. All these pathogens are known to be transmitted by ticks, occur worldwide and can cause infections in humans. Our results suggest that there is no huge difference between the tick microbiome in warmer regions e.g. Turkey and Malaysia [6, 7] and our sampling sites in Hesse, Germany.
Confirmatory PCRs conducted depending on the NGS results revealed the presence of Borrelia garinii, Borrelia miyamotoi, Rickettsia helvetica, Francisella tularensis and Anaplasma phagocytophilum; these results were widely congruent with those from the NGS approach. All of these bacterial species can cause unspecific febrile illnesses in humans and probably also in pets [73–81]. Furthermore, Anaplasma phagocytophilum was detected in the blood of eight roe deer. Compared to the average prevalence of Borrelia burgdorferi (sensu lato) in questing ticks in Europe (12.3% [82]), the DNA-prevalence in this study is quite low. A possible explanation for this phenomenon could be that, in contrast to most other studies, we examined feeding ticks from different vertebrate species and it has been shown that the complement system of several vertebrates, especially ruminants, can effectively eliminate Lyme disease spirochetes inside the feeding ticks [83, 84].
Nine ticks showed co-infections with two or more pathogens. Co-infections of ticks with different Borrelia spp. or Borrelia spp. and other pathogens such as Anaplasma phagocytophilum or Rickettsia spp. are not rare [85] and they can alter the course of human infection or the response to a certain antimicrobial therapy. Patients with chronic, therapy-resistant Lyme disease showed co-infections with B. henselae leading to the assumption that infections with multiple microorganisms might cause irregular responses to antibiotic therapies [40]. From this, knowledge about co-infections of ticks with various pathogens, even when limited to a relatively small area as in our study, is mandatory to guide for best antimicrobial treatment of patients after tick exposure.
A limitation of our study is the fact that blood samples were taken at the same time point as tick collection was performed. From this, pathogens detected in those ticks might not have induced an actual serological response in infected hosts as ticks feed on dogs normally for only 2–10 days [2]. Furthermore, since we decided to work on feeding ticks, there are two possibilities by nature where the detected bacteria might derive from: (i) from the tick, which can possibly act as a vector and transmit those pathogens to different vertebrates; or (ii) the pathogens were already present in the blood of the host animal and were ingested while feeding.
Anti-Bartonella antibodies can be found in up to 16.1% of healthy blood donors [86]. In five of 11 dog owners investigated herein, anti-B. henselae-IgG antibodies were detected with titers between 1:160 to 1:320, resulting in an antibody prevalence of ~45%. Such relatively low titers might be an indicator for a former infection by Bartonella spp. and the percentage of B. henselae-positive dog owners seems to be elevated. However, it has to be mentioned that this elevated seroprevalence might also be caused by exposure to other Bartonella spp. as exact data on cross-reactivity of human anti-Bartonella antibodies with B. henselae antigen (which is used for serodiagnostics) are not available.
Conclusions
In summary, our data provide an overview of different pathogens circulating in ticks in central Germany (federal state of Hesse) and suggest a low incidence of Bartonella spp. in animals and their ectoparasites. Members of several pathogenic genera were found in ticks and also in wild and domestic animals by PCR and NGS techniques with Illumina short read and Nanopore long read sequencing, and this indicates a potential infection risk for humans and animals. Even though the number of samples used herein was too small to evaluate the epidemiology of different tick-borne diseases, knowledge of the presence of pathogens in ticks might allow to monitor circulating pathogens that could harm humans and animals. Ectoparasite control and an increased attention toward possible tick-borne infection are crucial to the prevention of (or at least early diagnosis of) tick-borne infections in humans and animals.
Acknowledgements
The authors thank Professor Christa Ewers for helpful advice and suggestions, Wibke Ballhorn, Agnes Hillebrecht, Carmen Jung, Rebecca Kaufmann, Heike Podlich, Corinna Sonntag, Kim Strauch and Yael Wiegand for excellent technical assistance, and Dr. Gerhard Dobler (Munich), Professor Dr Silke Fischer (Stuttgart), Professor Friederike von Loewenich (Mainz) and Dr Wolf Splettstößer (Braunschweig, all Germany) for providing PCR protocols and control DNA. Furthermore, we want to thank Olaf Ambros, Kurt Becker, Susanne Bergmann, Dr Wolfgang Blaß, Dr Christine Blendinger, Dr Berthold Dichmann, Dr Tobias Eisenberg, Dr Günther Fenn, Maja Firlé, Dr Detlef Gissel, Dr Olaf Hattenhauer, Ralf Heitmann, Michael Jüngling, Mandy Körner, Sandra Meyer, Peter Nennstiel, Claudia Poth, Annette Schneider, Claus Schnelle, Oskar Schuch and Dr Kirsten Tönnies for their support in sample collection.
Funding
The work of VAJK was partially supported by a grant from the Bayer Animal Health Company, Leverkusen, Germany and by the Robert Koch-Institute, Berlin, Germany (Bartonella consiliary laboratory, 1369-354). Funding parties had no influence on data analysis, data interpretation, or writing of the manuscript. The work of TH was supported by grants of the Deutsche Forschungsgemeinschaft SFB-TR84 project B01 (TRR 84/2 2014) and B08 (TRR 84/3 2018). KK and TH were supported by BMBF CAPSyS project (01ZX16004C). The research in the laboratory of ATP was financially supported by the Academy of Finland (Academy Project, no. 295296, 2016–2020), Sigrid Jusélius Foundation (Junior group leader grant, 2015–2018) and University of Turku, Finland. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Availability of data and materials
Microbiome sequencing data have been submitted to the NCBI Short Read Archive repository under the SRA accession number SRP150503 (https://www.ncbi.nlm.nih.gov/sra/SRP150503).
Abbreviations
- NGS
Next generation sequencing
- rDNA
Ribosomal DNA
- ITS
Internal transcribed spacer
- OTU
Operational taxonomic unit
Authors’ contributions
YR performed sample collection, DNA extraction and PCRs; KK, MW and TH performed next generation sequencing and bioinformatic analysis; AL and AP performed differentiation of ruminant associated Bartonella spp.; YR, KK, MW, PK, AL, AP, TH and VK performed writing of the manuscript. YR and VK planned the experimental studies. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was approved by the ethics committee of the University Hospital Frankfurt (275/16, University Hospital Frankfurt) and by the Regierungspraesidium Darmstadt (V 54-19 c 20/15-FK/Anz. 1003).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yvonne Regier, Email: Yvonne.Regier@kgu.de.
Kassandra Komma, Email: Kassandra.Komma@mikrobio.med.uni-giessen.de.
Markus Weigel, Email: Markus.Weigel@mikrobio.med.uni-giessen.de.
Peter Kraiczy, Email: Kraiczy@em.uni-frankfurt.de.
Arttu Laisi, Email: arttu.h.laisi@utu.fi.
Arto T. Pulliainen, Email: arto.pulliainen@utu.fi
Torsten Hain, Email: Torsten.Hain@mikrobio.med.uni-giessen.de.
Volkhard A. J. Kempf, Email: volkhard.kempf@kgu.de
References
- 1.Stark K, Niedrig M, Biederbick W, Merkert H, Hacker J. Die Auswirkungen des Klimawandels. Welche neuen Infektionskrankheiten und gesundheitlichen Probleme sind zu erwarten? Bundesgesundheitsbl. 2009;52:699–714. doi: 10.1007/s00103-009-0874-9. [DOI] [PubMed] [Google Scholar]
- 2.Deplazes P, Eckert J, Gv S-H, Zahner H. Lehrbuch der Parasitologie für die Tiermedizin, 14.1.1 Ordnung Metastigmata (Ixodida, Zecken) Stuttgart: Enke; 2013. pp. 377–401. [Google Scholar]
- 3.Dietrich F, Schmidgen T, Maggi RG, Richter D, Matuschka F-R, Vonthein R, et al. Prevalence of Bartonella henselae and Borrelia burgdorferi sensu lato DNA in Ixodes ricinus ticks in Europe. Appl Environ Microbiol. 2010;76:1395–1398. doi: 10.1128/AEM.02788-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.C-c C, Hayashidani H, Pusterla N, Kasten RW, Madigan JE, Chomel BB. Investigation of Bartonella infection in ixodid ticks from California. Comp Immunol Microbiol Infect Dis. 2002;25:229–236. doi: 10.1016/S0147-9571(02)00012-7. [DOI] [PubMed] [Google Scholar]
- 5.Halos L, Jamal T, Maillard R, Beugnet F, Le Menach A, Boulouis H-J, Vayssier-Taussat M. Evidence of Bartonella sp. in questing adult and nymphal Ixodes ricinus ticks from France and co-infection with Borrelia burgdorferi sensu lato and Babesia sp. Vet Res. 2005;36:79–87. doi: 10.1051/vetres:2004052. [DOI] [PubMed] [Google Scholar]
- 6.Tekin S, Dowd SE, Davinic M, Bursali A, Keskin A. Pyrosequencing based assessment of bacterial diversity in Turkish Rhipicephalus annulatus and Dermacentor marginatus ticks (Acari: Ixodidae) Parasitol Res. 2017;116:1055–1061. doi: 10.1007/s00436-017-5387-0. [DOI] [PubMed] [Google Scholar]
- 7.Khoo JJ, Chen F, Kho KL, Ahmad SAI, Lim FS, Tan KK, et al. Bacterial community in Haemaphysalis ticks of domesticated animals from the Orang Asli communities in Malaysia. Ticks Tick Borne Dis. 2016;7:929–37. [DOI] [PubMed]
- 8.Rynkiewicz EC, Hemmerich C, Rusch DB, Fuqua C, Clay K. Concordance of bacterial communities of two tick species and blood of their shared rodent host. Mol Ecol. 2015;24:2566–2579. doi: 10.1111/mec.13187. [DOI] [PubMed] [Google Scholar]
- 9.Vayssier-Taussat M, Moutailler S, Michelet L, Devillers E, Bonnet S, Cheval J, et al. Next generation sequencing uncovers unexpected bacterial pathogens in ticks in western Europe. PLoS One. 2013;8:e81439. doi: 10.1371/journal.pone.0081439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Budachetri K, Browning RE, Adamson SW, Dowd SE, Chao CC, Ching W-M, Karim S. An Insight into the microbiome of the Amblyomma maculatum (Acari: Ixodidae) J Med Entomol. 2014;51:119–129. doi: 10.1603/ME12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.René-Martellet M, Minard G, Massot R, Van v T, Valiente Moro C, Chabanne L, Mavingui P. Bacterial microbiota associated with Rhipicephalus sanguineus (s.l.) ticks from France, Senegal and Arizona. Parasit Vectors. 2017;10:416. doi: 10.1186/s13071-017-2352-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varela-Stokes AS, Park SH, Stokes JV, Gavron NA, Lee SI, Moraru GM, Ricke SC. Tick microbial communities within enriched extracts of Amblyomma maculatum. Ticks Tick Borne Dis. 2018;9:798–805. doi: 10.1016/j.ttbdis.2018.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maggi RG, VAJ K, Chomel BB, Breitschwerdt EB. Bartonella. In: Versalovic J, Jorgensen JH, Funke G, Warnock DW, Landry ML, Carroll KC, editors. Manual of Clinical Microbiology, 10th edition. Washington D.C: American Society of Microbiology; 2011.
- 14.Dehio C. Bartonella-host-cell interactions and vascular tumour formation. Nat Rev Microbiol. 2005;3:621–631. doi: 10.1038/nrmicro1209. [DOI] [PubMed] [Google Scholar]
- 15.Mändle T, Einsele H, Schaller M, Neumann D, Vogel W, Autenrieth IB, Kempf VA. Infection of human CD34+ progenitor cells with Bartonella henselae results in intraerythrocytic presence of B. henselae. Blood. 2005;106:1215–1222. doi: 10.1182/blood-2004-12-4670. [DOI] [PubMed] [Google Scholar]
- 16.Veikkolainen V, Vesterinen EJ, Lilley TM, Pulliainen AT. Bats as reservoir hosts of human bacterial pathogen, Bartonella mayotimonensis. Emerging Infect Dis. 2014;20:960–967. doi: 10.3201/eid2006.130956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silaghi C, Pfeffer M, Kiefer D, Kiefer M, Obiegala A. Bartonella, rodents, fleas and ticks: a molecular field study on host-vector-pathogen associations in Saxony, eastern Germany. Microb Ecol. 2016;72:965–74. [DOI] [PubMed]
- 18.Kitchell BE, Fan TM, Kordick D, Breitschwerdt EB, Wollenberg G, Lichtensteiger CA. Peliosis hepatis in a dog infected with Bartonella henselae. J Am Vet Med Assoc. 2000;216:519. doi: 10.2460/javma.2000.216.519. [DOI] [PubMed] [Google Scholar]
- 19.Fenimore A, Varanat M, Maggi R, Schultheiss P, Breitschwerdt E, Lappin MR. Bartonella spp. DNA in cardiac tissues from dogs in Colorado and Wyoming. J Vet Intern Med. 2011;25:613–616. doi: 10.1111/j.1939-1676.2011.0722.x. [DOI] [PubMed] [Google Scholar]
- 20.Drut A, Bublot I, Breitschwerdt EB, Chabanne L, Vayssier-Taussat M, Cadoré J-L. Comparative microbiological features of Bartonella henselae infection in a dog with fever of unknown origin and granulomatous lymphadenitis. Med Microbiol Immunol. 2014;203:85–91. doi: 10.1007/s00430-013-0318-x. [DOI] [PubMed] [Google Scholar]
- 21.Dehio C, Lanz C, Pohl R, Behrens P, Bermond D, Piémont Y, et al. Bartonella schoenbuchii sp. nov., isolated from the blood of wild roe deer. Int J Syst Evol Microbiol. 2001;51:1557–1565. doi: 10.1099/00207713-51-4-1557. [DOI] [PubMed] [Google Scholar]
- 22.Duodu S, Madslien K, Hjelm E, Molin Y, Paziewska-Harris A, Harris PD, et al. Bartonella infections in deer keds (Lipoptena cervi) and moose (Alces alces) in Norway. Appl Environ Microbiol. 2013;79:322–327. doi: 10.1128/AEM.02632-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korhonen EM, Perez VC, Pulliainen AT, Sironen T, Aaltonen K, Kortet R, et al. Molecular detection of Bartonella spp. in deer ked pupae, adult keds and moose blood in Finland. Epidemiol Infect. 2015;143:578–585. doi: 10.1017/S0950268814001411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adamska M. Wild ruminants in the area of the north-western Poland as potential reservoir hosts of Bartonella schoenbuchensis and B. bovis. Acta Parasitol. 2008;53:407. doi: 10.2478/s11686-008-0058-z. [DOI] [Google Scholar]
- 25.Bermond D, Boulouis H-J, Heller R, van Laere G, Monteil H, Chomel BB, et al. Bartonella bovis Bermond et al. sp. nov. and Bartonella capreoli sp. nov., isolated from European ruminants. Int J Syst Evol Microbiol. 2002;52:383–390. doi: 10.1099/00207713-52-2-383. [DOI] [PubMed] [Google Scholar]
- 26.Chang CC, Chomel BB, Kasten RW, Heller RM, Kocan KM, Ueno H, et al. Bartonella spp. isolated from wild and domestic ruminants in North America. Emerging Infect Dis. 2000;6:306–311. doi: 10.3201/eid0603.000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maillard R, Vayssier-Taussat M, Bouillin C, Gandoin C, Halos L, Chomel B, et al. Identification of Bartonella strains isolated from wild and domestic ruminants by a single-step PCR analysis of the 16S-23S intergenic spacer region. Vet Microbiol. 2004;98:63–69. doi: 10.1016/j.vetmic.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 28.Rolain JM, Rousset E, La Scola B, Duquesnel R, Raoult D. Bartonella schoenbuchensis isolated from the blood of a French cow. Ann N Y Acad Sci. 2003;990:236–238. doi: 10.1111/j.1749-6632.2003.tb07370.x. [DOI] [PubMed] [Google Scholar]
- 29.Welc-Falęciak R, Werszko J, Cydzik K, Bajer A, Michalik J, Behnke JM. Co-infection and genetic diversity of tick-borne pathogens in roe deer from Poland. Vector Borne Zoonotic Dis. 2013;13:277–288. doi: 10.1089/vbz.2012.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dehio C, Sauder U, Hiestand R. Isolation of Bartonella schoenbuchensis from Lipoptena cervi, a blood-sucking arthropod causing deer ked dermatitis. J Clin Microbiol. 2004;42:5320–5323. doi: 10.1128/JCM.42.11.5320-5323.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rolain JM, La Scola B, Liang Z, Davoust B, Raoult D. Immunofluorescent detection of intraerythrocytic Bartonella henselae in naturally infected cats. J Clin Microbiol. 2001;39:2978–2980. doi: 10.1128/JCM.39.8.2978-2980.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vayssier-Taussat M, Moutailler S, Féménia F, Raymond P, Croce O, La Scola B, et al. Identification of novel zoonotic activity of Bartonella spp., France. Emerg Infect Dis. 2016;22:457–462. doi: 10.3201/eid2203.150269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Regier Y, Kempf VAJ. 2018. Bartonella-Infektionen bei Haustieren - eine Gefahr in der Tierarztpraxis? Berl Münch Tierärztl Wochenschr. 2017;131:89–101. [Google Scholar]
- 34.Telford SR, Wormser GP. Bartonella spp. transmission by ticks not established. Emerg Infect Dis. 2010;16:379–84. [DOI] [PMC free article] [PubMed]
- 35.Billeter SA, Levy MG, Chomel BB, Breitschwerdt EB. Vector transmission of Bartonella species with emphasis on the potential for tick transmission. Med Vet Entomol. 2008;22:1–15. doi: 10.1111/j.1365-2915.2008.00713.x. [DOI] [PubMed] [Google Scholar]
- 36.Reis C, Cote M, Le Rhun D, Lecuelle B, Levin ML, Vayssier-Taussat M, Bonnet SI. Vector competence of the tick Ixodes ricinus for transmission of Bartonella birtlesii. PLoS Negl Trop Dis. 2011;5:e1186. doi: 10.1371/journal.pntd.0001186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cotté V, Bonnet S, Le Rhun D, Le Naour E, Chauvin A, Boulouis H-J, et al. Transmission of Bartonella henselae by Ixodes ricinus. Emerging Infect Dis. 2008;14:1074–1080. doi: 10.3201/eid1407.071110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adelson ME, Rao R-VS, Tilton RC, Cabets K, Eskow E, Fein L, et al. Prevalence of Borrelia burgdorferi, Bartonella spp., Babesia microti, and Anaplasma phagocytophila in Ixodes scapularis ticks collected in northern New Jersey. J Clin Microbiol. 2004;42:2799–801. [DOI] [PMC free article] [PubMed]
- 39.Holden K, Boothby JT, Kasten RW, Chomel BB. Co-detection of Bartonella henselae, Borrelia burgdorferi, and Anaplasma phagocytophilum in Ixodes pacificus ticks from California, USA. Vector Borne Zoonotic Dis. 2006;6:99–102. doi: 10.1089/vbz.2006.6.99. [DOI] [PubMed] [Google Scholar]
- 40.Eskow E, Rao R-VS, Mordechai E. Concurrent infection of the central nervous system by Borrelia burgdorferi and Bartonella henselae. Arch Neurol. 2001;58:1357–63. [DOI] [PubMed]
- 41.Podsiadly E, Chmielewski T, Tylewska-Wierzbanowska S. Bartonella henselae and Borrelia burgdorferi infections of the central nervous system. Ann N Y Acad of Sci. 2003;990:404–406. doi: 10.1111/j.1749-6632.2003.tb07400.x. [DOI] [PubMed] [Google Scholar]
- 42.Regier Y, Ballhorn W, Kempf VAJ. Molecular detection of Bartonella henselae in 11 Ixodes ricinus ticks extracted from a single cat. Parasit Vectors. 2017;10:105. doi: 10.1186/s13071-017-2042-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arthur D. British Ticks. London: Butterworths; 1963. [Google Scholar]
- 44.Dauga C, Miras I, Grimont PA. Identification of Bartonella henselae and B. quintana 16S rDNA sequences by branch-, genus- and species-specific amplification. J Med Microbiol. 1996;45:192–9. [DOI] [PubMed]
- 45.Cherry NA, Maggi RG, Cannedy AL, Breitschwerdt EB. PCR detection of Bartonella bovis and Bartonella henselae in the blood of beef cattle. Vet Microbiol. 2009;135:308–312. doi: 10.1016/j.vetmic.2008.09.063. [DOI] [PubMed] [Google Scholar]
- 46.Chitimia-Dobler L, Rieß R, Kahl O, Wölfel S, Dobler G, Nava S, Estrada-Peña A. Ixodes inopinatus - occurring also outside the Mediterranean region. Ticks Tick Borne Dis. 2017;9:196–200. doi: 10.1016/j.ttbdis.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 47.Frangoulidis D, Meyer H, Kahlhofer C, Splettstoesser WD. ‘Real-time’ PCR-based detection of Coxiella burnetii using conventional techniques. FEMS Immunol Med Microbiol. 2012;64:134–6. [DOI] [PubMed]
- 48.Massung RF, Slater K, Owens JH, Nicholson WL, Mather TN, Solberg VB, Olson JG. Nested PCR assay for detection of granulocytic ehrlichiae. J Clin Microbiol. 1998;36:1090–1095. doi: 10.1128/jcm.36.4.1090-1095.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levett PN, Morey RE, Galloway RL, Turner DE, Steigerwalt AG, Mayer LW. Detection of pathogenic leptospires by real-time quantitative PCR. J Med Microbiol. 2005;54:45–49. doi: 10.1099/jmm.0.45860-0. [DOI] [PubMed] [Google Scholar]
- 50.Fingerle V, Pritsch M, Wächtler M, Margos G, Ruske S, Jung J, et al. “Candidatus Borrelia kalaharica” detected from a febrile traveller returning to Germany from vacation in southern Africa. PLoS Negl Trop Dis. 2016;10:e0004559. [DOI] [PMC free article] [PubMed]
- 51.Oksi J, Rantala S, Kilpinen S, Silvennoinen R, Vornanen M, Veikkolainen V, et al. Cat scratch disease caused by Bartonella grahamii in an immunocompromised patient. J Clin Microbiol. 2013;51:2781–2784. doi: 10.1128/JCM.00910-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci USA. 2011;108(Suppl. 1):4516–4522. doi: 10.1073/pnas.1000080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.MiSeq SOP - mothur. 2017. https://www.mothur.org/wiki/MiSeq_SOP. Accessed 21 Mar 2018.
- 54.Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol. 2013;79:5112–5120. doi: 10.1128/AEM.01043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, et al. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013;41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Glaus T, Hofmann-Lehmann R, Greene C, Glaus B, Wolfensberger C, Lutz H. Seroprevalence of Bartonella henselae infection and correlation with disease status in cats in Switzerland. J Clin Microbiol. 1997;35:2883–2885. doi: 10.1128/jcm.35.11.2883-2885.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salter SJ, Cox MJ, Turek EM, Calus ST, Cookson WO, Moffatt MF, et al. Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC Biol. 2014;12:87. doi: 10.1186/s12915-014-0087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weiss S, Amir A, Hyde ER, Metcalf JL, Song SJ, Knight R. Tracking down the sources of experimental contamination in microbiome studies. Genome Biol. 2014;15:564. [DOI] [PMC free article] [PubMed]
- 60.Tully JG, Rose DL, Yunker CE, Carle P, Bové JM, Williamson DL, Whitcomb RF. Spiroplasma ixodetis sp. nov., a new species from Ixodes pacificus ticks collected in Oregon. Int J Syst Bacteriol. 1995;45:23–28. doi: 10.1099/00207713-45-1-23. [DOI] [PubMed] [Google Scholar]
- 61.Bonnet SI, Binetruy F, Hernández-Jarguín AM, Duron O. The tick microbiome: why non-pathogenic microorganisms matter in tick biology and pathogen transmission. Front Cell Infect Microbiol. 2017;7:236. doi: 10.3389/fcimb.2017.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Regier Y, O'Rourke F, Kempf VAJ. Bartonella spp. - a chance to establish One Health concepts in veterinary and human medicine. Parasit Vectors. 2016;9:261. doi: 10.1186/s13071-016-1546-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matsumoto K, Berrada ZL, Klinger E, Goethert HK, Telford SR., 3rd Molecular detection of Bartonella schoenbuchensis from ectoparasites of deer in Massachusetts. Vector Borne Zoonotic Dis. 2008;8:549–554. doi: 10.1089/vbz.2007.0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de BA, van Leeuwen AD, Jahfari S, Takken W, Foldvari M, Dremmel L, et al. Vertical transmission of Bartonella schoenbuchensis in Lipoptena cervi. Parasit Vectors. 2015;8:176. doi: 10.1186/s13071-015-0764-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Szewczyk T, Werszko J, Steiner-Bogdaszewska Ż, Jeżewski W, Laskowski Z, Karbowiak G. Molecular detection of Bartonella spp. in deer ked (Lipoptena cervi) in Poland. Parasit Vectors. 2017;10:487. doi: 10.1186/s13071-017-2413-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Engel P, Salzburger W, Liesch M, C-c C, Maruyama S, Lanz C, et al. Parallel evolution of a type IV secretion system in radiating lineages of the host-restricted bacterial pathogen Bartonella. PLoS Genet. 2011;7:e1001296. doi: 10.1371/journal.pgen.1001296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Podsiadly E, Chmielewski T, Sochon E, Tylewska-Wierzbanowska S. Bartonella henselae in Ixodes ricinus ticks removed from dogs. Vector Borne Zoonotic Dis. 2007;7:189–192. doi: 10.1089/vbz.2006.0587. [DOI] [PubMed] [Google Scholar]
- 68.Morozova OV, Cabello FC, Dobrotvorsky AK. Semi-nested PCR detection of Bartonella henselae in Ixodes persulcatus ticks from Western Siberia, Russia. Vector Borne Zoonotic Dis. 2004;4:306–309. doi: 10.1089/vbz.2004.4.306. [DOI] [PubMed] [Google Scholar]
- 69.Hercik K, Hasova V, Janecek J, Branny P. Molecular evidence of Bartonella DNA in ixodid ticks in Czechia. Folia Microbiol (Praha). 2007;52:503–509. doi: 10.1007/BF02932111. [DOI] [PubMed] [Google Scholar]
- 70.Chomel BB, Mac Donald KA, Kasten RW, Chang CC, Wey AC, Foley JE, et al. Aortic valve endocarditis in a dog due to Bartonella clarridgeiae. J Clin Microbiol. 2001;39:3548–3554. doi: 10.1128/JCM.39.10.3548-3554.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kordick SK, Breitschwerdt EB, Hegarty BC, Southwick KL, Colitz CM, Hancock SI, et al. Coinfection with multiple tick-borne pathogens in a Walker Hound kennel in North Carolina. J Clin Microbiol. 1999;37:2631–2638. doi: 10.1128/jcm.37.8.2631-2638.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.MacDonald KA, Chomel BB, Kittleson MD, Kasten RW, Thomas WP, Pesavento P. A prospective study of canine infective endocarditis in northern California (1999–2001): emergence of Bartonella as a prevalent etiologic agent. J Vet Intern Med. 2004;18:56–64. doi: 10.1892/0891-6640(2004)18<56:apsoci>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 73.Chen SM, Dumler JS, Bakken JS, Walker DH. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J Clin Microbiol. 1994;32:589–595. doi: 10.1128/jcm.32.3.589-595.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chirek A, Silaghi C, Pfister K, Kohn B. Granulocytic anaplasmosis in 63 dogs: Clinical signs, laboratory results, therapy and course of disease. J Small Anim Pract. 2018;59:112–120. doi: 10.1111/jsap.12787. [DOI] [PubMed] [Google Scholar]
- 75.Stanek G, Strle F. Lyme disease: European perspective. Infect Dis Clin North Am. 2008;22:327–339. doi: 10.1016/j.idc.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 76.Kybicová K, Schánilec P, Hulínská D, Uherková L, Kurzová Z, Spejchalová S. Detection of Anaplasma phagocytophilum and Borrelia burgdorferi sensu lato in dogs in the Czech Republic. Vector Borne Zoonotic Dis. 2009;9:655–661. doi: 10.1089/vbz.2008.0127. [DOI] [PubMed] [Google Scholar]
- 77.Krause PJ, Fish D, Narasimhan S, Barbour AG. Borrelia miyamotoi infection in nature and in humans. Clin Microbiol Infect. 2015;21:631–639. doi: 10.1016/j.cmi.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maurin M, Gyuranecz M. Tularaemia: clinical aspects in Europe. Lancet Infect Dis. 2016;16:113–124. doi: 10.1016/S1473-3099(15)00355-2. [DOI] [PubMed] [Google Scholar]
- 79.Nordstoga A, Handeland K, Johansen TB, Iversen L, Gavier-Widén D, Mattsson R, et al. Tularaemia in Norwegian dogs. Vet Microbiol. 2014;173:318–322. doi: 10.1016/j.vetmic.2014.06.031. [DOI] [PubMed] [Google Scholar]
- 80.Fournier PE, Grunnenberger F, Jaulhac B, Gastinger G, Raoult D. Evidence of Rickettsia helvetica infection in humans, eastern France. Emerging Infect Dis. 2000;6:389–392. doi: 10.3201/eid0604.000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wächter M, Wölfel S, Pfeffer M, Dobler G, Kohn B, Moritz A, et al. Serological differentiation of antibodies against Rickettsia helvetica, R. raoultii, R. slovaca, R. monacensis and R. felis in dogs from Germany by a micro-immunofluorescent antibody test. Parasit Vectors. 2015;8:126. doi: 10.1186/s13071-015-0745-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Strnad M, Hönig V, Růžek D, Grubhoffer L, Rego ROM. Europe-wide meta-analysis of Borrelia burgdorferi sensu lato prevalence in questing Ixodes ricinus ticks. Appl Environ Microbiol. 2017;83:e00609–e00617. doi: 10.1128/AEM.00609-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kraiczy P. Travelling between two worlds: complement as a gatekeeper for an expanded host range of Lyme disease spirochetes. Vet Sci. 2016;3:E12. doi: 10.3390/vetsci3020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Richter D, Matuschka F-R. Elimination of Lyme disease spirochetes from ticks feeding on domestic ruminants. Appl Environ Microbiol. 2010;76:7650–7652. doi: 10.1128/AEM.01649-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Raileanu C, Moutailler S, Pavel I, Porea D, Mihalca AD, Savuta G, Vayssier-Taussat M. Borrelia diversity and co-infection with other tick borne pathogens in ticks. Front Cell Infect Microbiol. 2017;7:36. doi: 10.3389/fcimb.2017.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McGill S, Wesslen L, Hjelm E, Holmberg M, Auvinen MK, Berggren K, et al. Bartonella spp. seroprevalence in healthy Swedish blood donors. Scand J Infect Dis. 2005;37:723–730. doi: 10.1080/00365540510012152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Microbiome sequencing data have been submitted to the NCBI Short Read Archive repository under the SRA accession number SRP150503 (https://www.ncbi.nlm.nih.gov/sra/SRP150503).