Abstract
Introduction
AIDS‐related deaths in people living with HIV/AIDS have been decreasing in number since the introduction of combination antiretroviral treatment (cART). However, data on recent causes of death in the Asia‐Pacific region are limited. Hence, we analysed and compared AIDS‐related and non‐AIDS–related mortality in high‐ and low‐income settings in the region.
Methods
Patients from the TREAT Asia HIV Observational Database (TAHOD) and Australian HIV Observational Database (AHOD) receiving cART between 1999 and 2017 were included. Causes of death verification were based on review of the standardized Cause of Death (CoDe) form designed by the D:A:D group. Cohorts were grouped as AHOD (all high‐income sites), TAHOD‐high (high/upper‐middle income countries) and TAHOD‐low (lower‐middle income countries). TAHOD sites were split into high/upper‐middle income and lower‐middle income country settings based on World Bank classifications. Competing risk regression was used to analyse factors associated with AIDS and non‐AIDS–related mortality.
Results
Of 10,386 patients, 522 died; 187 from AIDS‐related and 335 from non‐AIDS–related causes. The overall incidence rate of deaths during follow‐up was 0.28 per 100 person‐years (/100 PYS) for AIDS and 0.51/100 PYS for non‐AIDS. Analysis indicated that the incidence rate of non‐AIDS mortality decreased from 0.78/100 PYS to 0.37/100 PYS from year groups 2003 to 2007 to 2013 to 2017 (p < 0.001). Similarly, incidence rates of AIDS‐related deaths decreased from 0.51/100 PYS to 0.09/100 PYS from year groups 2003 to 2007 to 2013 to 2017 (p < 0.001). More recent years of follow‐up were associated with reduced hazard for non‐AIDS mortality (2008 to 2012: aSHR (adjusted sub‐hazard ratio) 0.72, 95% confidence interval (CI) 0.54 to 0.96, p = 0.027; 2013 to 2017: aSHR 0.64, 95% CI 0.47 to 0.87, p = 0.004) compared to years 2003 to 2007. The AHOD cohort had almost twice the hazard of non‐AIDS mortality compared to TAHOD‐low (lower‐middle income sites) (aSHR 1.72, 95% CI, 1.20 to 2.46, p = 0.003); there were no differences between cohorts for AIDS‐related mortality (p = 0.834).
Conclusion
AIDS and non‐AIDS–related mortality rates have decreased over the past years in the Asia‐Pacific region. There is a greater risk for non‐AIDS–associated deaths in the AHOD cohort compared to lower‐middle income settings in TAHOD.
Keywords: cohort studies, risk factors, mortality, Asia‐Pacific, low‐income, high‐income
1. Introduction
Since the introduction of combination antiretroviral treatment (cART), the incidence and major causes of death among individuals with HIV infection have changed substantially 1, 2, 3, 4. Compared to the pre‐cART era, there has been a decrease in death rates attributable to AIDS itself and an increasing proportion of deaths related to non‐AIDS causes 1, 5. The incidence of AIDS‐defining cancers decreased and the incidence of non‐AIDS‐defining cancers increased after the introduction of cART, due to better viral replication control and increased immunity, which led to longer lifespans and therefore an increased chance of developing comorbidities that are more common with age, such as most non‐AIDS–defining cancers 5. According to the Data collection on Adverse events of anti‐HIV Drugs (D:A:D) study, trends over time in all‐cause mortality in people with HIV demonstrated a decrease in AIDS‐related death rates 1. Moreover, the proportion of non‐AIDS cancers increased from 9% in 1999 to 2000 to 23% in 2009 to 2011 1. A Swiss HIV cohort study demonstrated similar results with 84% of deaths between 2005 and 2009 due to non‐AIDS–related causes 6. Improvement in CD4 cell count was accountable for the recent reductions in rates of AIDS‐related deaths 1.
A previous study conducted in the Asia‐Pacific region examined factors predictive of AIDS‐related and non‐AIDS–related mortality, as well as the cumulative incidences of each cause of death before the year 2007: 215 deaths (89 from AIDS, 97 from non‐AIDS and 29 unknown) were identified, immune deficiency defined by lower CD4 cell counts was predictive of increased risk of AIDS‐related and non‐AIDS–related deaths, and older age (≥50 years) predicted non‐AIDS mortality 7. Other studies in the region have identified risk factors for developing AIDS‐defining cancers 8, 9.
We hypothesize that the causes of AIDS‐related and non‐AIDS–related mortality have changed over the past decade as HIV treatment programs in the Asia‐Pacific region have matured, and that rates of AIDS mortality have decreased, followed by a relative increase in non‐AIDS–related mortality. The objective of this study was to investigate the incidence rates of AIDS‐related and non‐AIDS–related deaths, and identify factors associated with different causes of death (COD) across country income levels in the Asia‐Pacific region during the cART era.
2. Methods
2.1. Study population and definitions
This analysis includes both Australian HIV Observational Database (Australian HIV Observational Database) and the TREAT Asia HIV Observational Database (TAHOD) enrolled patients who started cART and attended at least one subsequent follow‐up visit. The AHOD and TAHOD are observational clinical cohort studies of patients with HIV infection in Australia and New Zealand (AHOD) and 12 countries (Cambodia, China and Hong Kong SAR, India, Indonesia, Japan, Malaysia, the Philippines, Singapore, South Korea, Taiwan, Thailand, and Vietnam) in Asia and the Pacific region (TAHOD), whose methods have previously been described 7, 8, 9.
The start date (baseline) for cART was defined as the date patients started their first triple regimen. Those who used mono/dual therapy prior to starting cART were excluded. Cohorts were grouped as AHOD (all high‐income sites), TAHOD‐high (high/upper‐middle income countries) and TAHOD‐low (lower‐middle income countries). TAHOD sites were split into high/upper‐middle income and lower‐middle income settings (there are no low‐income sites in TAHOD) based on World Bank classifications 10. Deaths were classified as AIDS‐related where the death was a direct consequence of any AIDS‐defining condition (as defined by the Centers for Disease Control and Prevention 11). Non‐AIDS deaths were further classified as being related to cardiovascular disease, liver disease, cancer, accidental/overdose/violence, infectious disease, other and unknown 1. Causes of death were based on review of the standardized Cause of Death (CoDe) form designed by the D:A:D group 12.
2.2. Statistical analysis
2.2.1. Proportions and incidence rates of AIDS‐related and non‐AIDS–related mortality
Proportions of AIDS‐related and non‐AIDS–related mortality were analysed descriptively. Calendar years were categorized into ≤2007, 2008 to 2012 and 2013 to 2017. The denominator used to calculate each proportion was the total number of deaths in each year group. Mortality rates for different causes of deaths were also examined. The proportions and mortality rates were plotted across calendar years of follow‐up. Unknown causes of death were plotted as a separate group to allow for visual inspection of the reported cases of non‐AIDS causes of death.
2.2.2. Factors associated with AIDS‐related and non‐AIDS–related mortality
Fine and Grays’ competing risk regression model was used to analyse factors associated with AIDS‐related and non‐AIDS–related mortality. The resulting sub‐hazard ratio (SHR) can be interpreted in a similar way to the hazard ratio. Follow‐up time began from cART initiation and left truncated at cohort entry for those who entered the cohort after starting cART. Left truncation minimizes survival bias by taking into account survival time prior to cohort entry in those who had initiated cART before enrolment into the cohort. The earliest year of follow‐up started in 1999 for AHOD and 2002 for TAHOD. While follow‐up time ended at date of death for patients who experienced mortality, all other patients were censored on their date of last follow‐up. Both AHOD and TAHOD cohorts had their last follow‐up date in year 2017. Other causes of death and loss to follow‐up (LTFU) 13, 14, 15 were defined as competing events. Time‐fixed covariates were age, sex, HIV mode of exposure, and cohort groups. Patients were considered hepatitis B virus (HBV) or hepatitis C virus (HCV) positive if they were ever tested positive for HBV surface antigen or positive for HCV antibody respectively. Covariates which varied with time were diabetes, hypertension, body mass index (BMI), HIV RNA, CD4 cell count and calendar year (≤2002, 2003 to 2007, 2008 to 2012, and 2013 to 2017). These variables were time‐updated from risk time which began at antiretroviral treatment (ART) initiation or cohort entry for those who had initiated cART prior to cohort entry, till the date of last follow‐up for those who were alive and date of death for those who experienced mortality. Time‐updated variables contribute different risk time in different categories. The missing category in the time‐updated variables refers to missing baseline values or patients who never had a test in the entire follow‐up time. Diabetes was defined as documentation of one fasting blood glucose measurement ≥7 mmol/L. Hypertension was not included as blood pressure was not recorded by the AHOD cohort. BMI was defined as underweight (<18.5), normal range (18.5 to 24.9) and overweight (≥25). LTFU was defined as not having been seen in clinic for >12 months without evidence of transfer.
With the exception of cohort groups, covariates in the univariate analysis with p < 0.10 were fitted in the multivariate model using backward stepwise selection process. Covariates with p < 0.05 in the multivariate model were considered significant. A sensitivity analysis was performed by including age as a time‐updated covariate, and adjusting for age, sex and CD4 cell count in the final multivariate model.
Ethics approvals for TAHOD were obtained from the respective local ethics committees of all participating sites, the data management and biostatistical centre at the Kirby Institute (The University of New South Wales (UNSW) Human Research Ethics Committee), and the coordinating centre at TREAT Asia/amfAR. Written informed consent was sought in TAHOD if required by a site's local institutional review board. All TAHOD data transfers are anonymized prior to submission to the Kirby Institute. Ethics approval for AHOD was obtained from the respective local ethics committees of all participating sites and the UNSW Human Research Ethics Committee; written informed consent was obtained from all participants. All data management and statistical analyses were performed using SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA) and Stata software version 14.2 (Stata Corp., College Station, TX, USA).
3. Results
A total of 10,386 patients who had initiated cART and were in follow‐up between years 1999 and 2017 were included in the study. With a median follow‐up time of 6.0 years, 522 patients (155 from AHOD, 208 from TAHOD‐high and 159 from TAHOD‐low) experienced AIDS or non‐AIDS mortality: 187 died from AIDS‐related (incidence rate of 0.28 per 100 person‐years (PYS)) and 335 died from non‐AIDS–related causes (0.51/100 PYS) (Table 1). In AHOD, the mortality rate for AIDS‐related death was 0.15/100 PYS and for non‐AIDS–related death it was 0.66/100 PYS, with a median follow‐up time of 6.3 years. The mortality rates for TAHOD‐high were 0.29/100 PYS for AIDS and 0.42/100 PYS for non‐AIDS deaths with a median follow‐up of 7.1 years, and for TAHOD‐low the mortality rate was 0.40/100 PYS for AIDS and 0.49/100 PYS for non‐AIDS with a median follow‐up of 5.5 years.
Table 1.
AIDS and non‐AIDS causes of death in low and high income settings in the TREAT Asia HIV Observational Database and AHOD cohortsa
| TAHOD, low income (N) | TAHOD, high income (N) | AHOD, high income (N) | All patients | Incidence rate (/100 PYS) (follow‐up time of 66,250 years) | |
|---|---|---|---|---|---|
| Total patients in database | 3240 | 4429 | 2717 | 10,386 | |
| Causes of death categoryb | |||||
| AIDS related | 72 | 86 | 29 | 187 | 0.28 |
| Non‐AIDS related | 87 | 122 | 126 | 335 | 0.51 |
| Cardiovascular disease | 10 | 12 | 19 | 41 | 0.06 |
| Cancer | 2 | 19 | 37 | 58 | 0.09 |
| Liver disease | 9 | 17 | 14 | 40 | 0.06 |
| Accidental/overdose/violence | 9 | 14 | 11 | 34 | 0.05 |
| Infectious disease | 11 | 13 | 6 | 30 | 0.05 |
| Other | 13 | 10 | 9 | 32 | 0.05 |
| Unknown | 33 | 37 | 30 | 100 | 0.15 |
| Total | 159 | 208 | 155 | 522 | 0.79 |
AHOD, Australian HIV Observational Database; TAHOD, TREAT Asia HIV Observational Database.
aCohorts were grouped as AHOD (all high‐income sites), TAHOD‐high (high/upper‐middle income countries) and TAHOD‐low (low‐middle/low income countries). TAHOD sites were split into high/upper‐middle income and low‐middle/low income settings based on World Bank classifications. bCauses of death were based on review of the standardized Cause of Death (CoDe) form designed by the Data collection on Adverse events of Anti‐HIV Drugs (D:A:D) group.
Of the 522 patients who died, the median age in the TAHOD‐low group was 34 years [interquartile range (IQR) 29 to 40], 41 years [IQR 34 to 51] in the TAHOD‐high group and 42 years [IQR 35 to 53] in AHOD. Ninety‐five per cent of patients in AHOD were men compared with 81% and 75% in TAHOD‐high and TAHOD‐low income groups respectively. Heterosexual contact was the primary route of HIV exposure in TAHOD‐high and TAHOD‐low income sites (67%), whereas men who have sex with men (MSM) was the most common HIV exposure in the AHOD sites (70%). Median baseline CD4 cell counts were 61 cells/μL [IQR 20 to 154] in the TAHOD‐low group, 59 cells/μL [IQR 19 to 164] in the TAHOD‐high group and 210 cells/μL [IQR 87 to 350] in AHOD. Baseline HIV RNA were 190,000 copies/mL [IQR 85,138 to 480,000] in the TAHOD‐low group, 140,000 copies/mL [IQR 47,650 to 450,000] in the TAHOD‐high group and 110,000 copies/mL [IQR 25,228 to 460,000] in AHOD. (Table 2)
Table 2.
Patient characteristics at baseline in low and high income settings in the TREAT Asia HIV Observational Database and AHOD cohortsa
| TAHOD, low income (N) | TAHOD, high income (N) | AHOD, high income (N) | p‐valueb | ||||
|---|---|---|---|---|---|---|---|
| n (%) | No. of deaths (%) | n (%) | No. of deaths (%) | n (%) | No. of deaths (%) | ||
| Total | 3240 (100) | 159 (100) | 4429 (100) | 208 (100) | 2717 (100) | 155 (100) | |
| Age (years) | |||||||
| Median (IQR) | 33 (28 to 38) | 34 (29 to 40) | 36 (30 to 42) | 41 (34 to 51) | 38 (31 to 46) | 42 (35 to 53) | <0.001 |
| ≤30 | 1213 (37) | 55 (35) | 1225 (28) | 35 (17) | 573 (21) | 18 (12) | <0.001 |
| 31 to 40 | 1417 (44) | 66 (42) | 1822 (41) | 67 (32) | 1000 (37) | 54 (35) | |
| 41 to 50 | 444 (14) | 23 (14) | 959 (22) | 50 (24) | 713 (26) | 35 (23) | |
| 51+ | 166 (5) | 15 (9) | 423 (10) | 56 (27) | 431 (16) | 48 (31) | |
| Sex | |||||||
| Male | 2172 (67) | 120 (75) | 3195 (72) | 169 (81) | 2475 (91) | 147 (95) | <0.001 |
| Female | 1068 (33) | 39 (25) | 1234 (28) | 39 (19) | 242 (9) | 8 (5) | |
| HIV exposure | |||||||
| Heterosexual | 2314 (71) | 107 (67) | 2522 (57) | 139 (67) | 530 (20) | 18 (12) | <0.001 |
| MSM | 235 (7) | 9 (6) | 1425 (32) | 40 (19) | 1901 (70) | 108 (70) | |
| Injecting drug use | 503 (16) | 31 (19) | 109 (2) | 12 (6) | 152 (6) | 18 (12) | |
| Blood | 15 (1) | 1 (1) | 62 (1) | 5 (92) | 16 (1) | 2 (1) | |
| Bisexual | 79 (2) | 6 (4) | 61 (1) | 4 (2) | 0 (0) | 0 (0) | |
| Other/unknown | 94 (3) | 5 (3) | 250 (6) | 8 (4) | 118 (4) | 9 (6) | |
| CD4 cell count (cells/μL) | |||||||
| Median (IQR) | 124 (42 to 225) | 61 (20 to 154) | 133 (40 to 232) | 59 (19 to 164) | 304 (180 to 460) | 210 (87 to 350) | <0.001 |
| ≤100 | 1199 (37) | 80 (50) | 1575 (36) | 119 (57) | 295 (11) | 35 (23) | <0.001 |
| 101 to 200 | 691 (21) | 32 (20) | 893 (20) | 30 (14) | 324 (12) | 24 (15) | |
| 201 to 350 | 655 (20) | 12 (8) | 931 (21) | 22 (11) | 646 (24) | 32 (21) | |
| 351 to 500 | 104 (3) | 1 (1) | 201 (5) | 10 (5) | 440 (16) | 16 (10) | |
| 501+ | 75 (2) | 1 (1) | 92 (2) | 1 (1) | 430 (16) | 13 (8) | |
| Missing | 516 (16) | 33 (21) | 737 (17) | 26 (13) | 582 (21) | 35 (23) | |
| Viral load (copies/mL) | |||||||
| Median (IQR) | 130,000 (33,500 to 390,000) | 190,000 (85,138 to 480,000) | 89,571 (26,650 to 250,000) | 140,000 (47,650 to 450,000) | 64,702 (14,362 to 180,000) | 110,000 (25,228 to 460,000) | <0.001 |
| ≤400 | 28 (1) | 0 (0) | 120 (3) | 5 (2) | 190 (7) | 7 (5) | <0.001 |
| 401 to 10,000 | 77 (2) | 1 (1) | 278 (6) | 4 (2) | 258 (10) | 15 (10) | |
| 10,001 to 100,000 | 209 (6) | 5 (3) | 1158 (26) | 49 (24) | 926 (34) | 31 (20) | |
| 100,001+ | 392 (12) | 16 (10) | 1348 (30) | 74 (36) | 698 (26) | 54 (35) | |
| Missing | 2534 (78) | 137 (86) | 1525 (34) | 76 (37) | 645 (24) | 48 (31) | |
| HBV co‐infection surface antigen | |||||||
| Negative | 1984 (61) | 91 (57) | 3317 (75) | 137 (66) | 2156 (79) | 122 (79) | <0.001 |
| Positive | 231 (7) | 11 (7) | 379 (9) | 29 (14) | 95 (4) | 10 (6) | |
| Missing | 1025 (32) | 57 (36) | 733 (17) | 42 (20) | 466 (17) | 23 (15) | |
| HCV co‐infection antibody | |||||||
| Negative | 1439 (44) | 57 (36) | 3269 (74) | 136 (65) | 2184 (80) | 118 (76) | <0.001 |
| Positive | 556 (17) | 28 (180 | 307 (7) | 38 (18) | 247 (9) | 25 (16) | |
| Missing | 1245 (38) | 74 (47) | 853 (19) | 34 (16) | 286 (11) | 12 (8) | |
| Diabetes | |||||||
| No | 1121 (34) | 48 (30) | 1162 (26) | 41 (20) | 356 (13) | 6 (4) | <0.001 |
| Yes | 67 (2) | 5 (3) | 29 (1) | 1 (1) | 19 (1) | 2 (1) | |
| Missing | 2052 (63) | 106 (67) | 3238 (73) | 166 (80) | 2717 (86) | 147 (95) | |
| BMI groups | |||||||
| Underweight (<18.5) | 699 (22) | 57 (36) | 499 (11) | 31 (15) | 16 (1) | 0 (0) | <0.001 |
| Normal range (18.5 to 24.9) | 1296 (40) | 41 (26) | 1590 (36) | 74 (36) | 221 (8) | 9 (6) | |
| Overweight (≥25) | 243 (8) | 6 (4) | 316 (7) | 11 (5) | 105 (4) | 4 (3) | |
| Missing | 1002 (31) | 55 (35) | 2024 (46) | 92 (44) | 2375 (87) | 142 (92) | |
| Prior AIDS | |||||||
| No | 1902 (59) | 49 (31) | 2930 (66) | 90 (43) | 2405 (89) | 122 (79) | <0.001 |
| Yes | 1338 (41) | 110 (69) | 1499 (34) | 118 (57) | 312 (11) | 33 (21) | |
| Total lost to follow‐up | 532 (2.98/100 PYS) | 729 (2.49/100 PYS) | 853 (4.45/100 PYS) | ||||
AHOD, Australian HIV Observational Database; BMI, body mass index; HBV, hepatitis B virus; HCV, hepatitis C virus; IQR, interquartile range; MSM, men who have sex with men; No., number; PYS, person‐years; TAHOD, TREAT Asia HIV Observational Database.
aCohorts were grouped as AHOD (all high‐income sites), TAHOD‐high (high/upper‐middle income countries) and TAHOD‐low (lower‐middle income countries). TAHOD sites were split into high/upper‐middle income and lower‐middle income settings based on World Bank classifications. b p‐values are tests for differences in proportions (chi‐squared) or medians (Kruskal‐Wallis test) for total patients across different cohorts. Baseline was defined as the date patients started their first triple regimen.
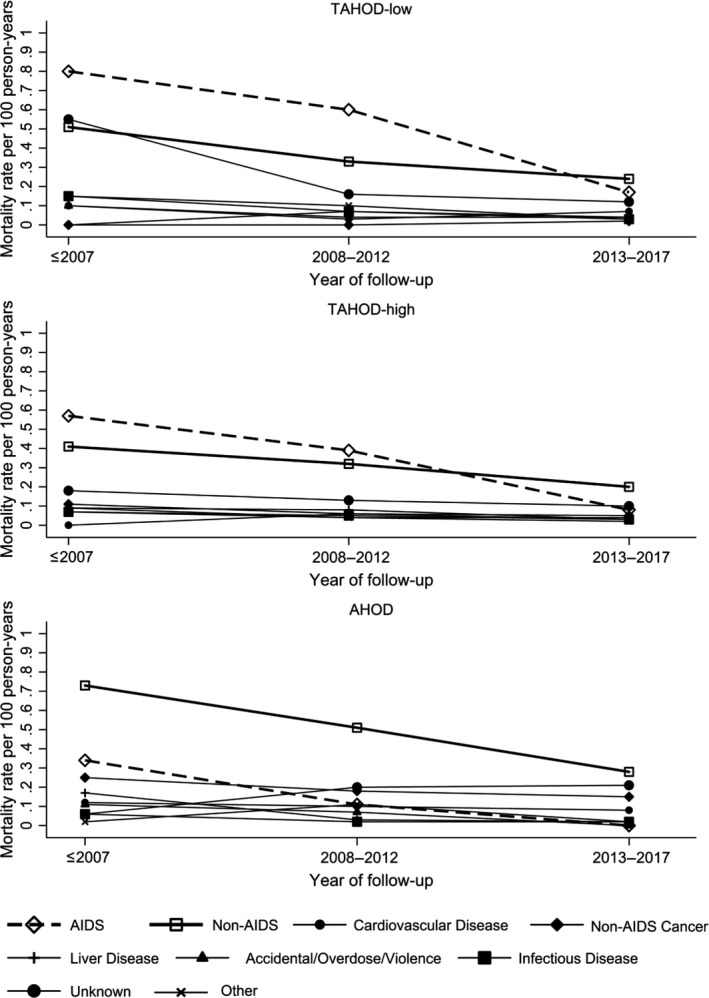
Figure 1 demonstrates the proportions of AIDS‐related, non‐AIDS–related and unknown causes of mortality among those who died. The proportions of AIDS, non‐AIDS and unknown causes of death did not differ significantly across year groups before 2007, 2008 to 2012 and 2013 to 2017 in the TAHOD‐low cohort (p = 0.196). In contrast, the TAHOD‐high and AHOD cohorts demonstrated a significant difference in the proportions of AIDS, non‐AIDS and unknown causes of deaths over all year groups (TAHOD‐high: p = 0.025; AHOD: p < 0.001). Figure 2 shows a plot of mortality rates for different causes of death across calendar years of follow‐up. While AIDS mortality in TAHOD‐low and AHOD cohorts decreased with statistical significance across all year groups (TAHOD‐low: p = 0.001; AHOD: p < 0.001), the decrease across all year groups in TAHOD‐high cohort was not significant (TAHOD‐high: p = 0.169). Non‐AIDS mortality decreased with statistical significance across all year groups in all cohorts (TAHOD‐low: p = 0.010; TAHOD‐high: p = 0.034; AHOD: p = 0.027). Non‐AIDS mortality decreased at a slower rate compared to AIDS‐related deaths in TAHOD‐high (p = 0.039) and AHOD (p = 0.035) cohorts. In the TAHOD‐low cohort, the rate of decrease between the incidence of AIDS mortality and non‐AIDS mortality was not significant (p = 0.071). A greater decrease in the incidence of non‐AIDS mortality was shown in AHOD compared to TAHOD‐high (p = 0.009) and TAHOD‐low (p = 0.026) cohorts.
Figure 1. Proportions of AIDS and non‐AIDS mortality among those who have died.
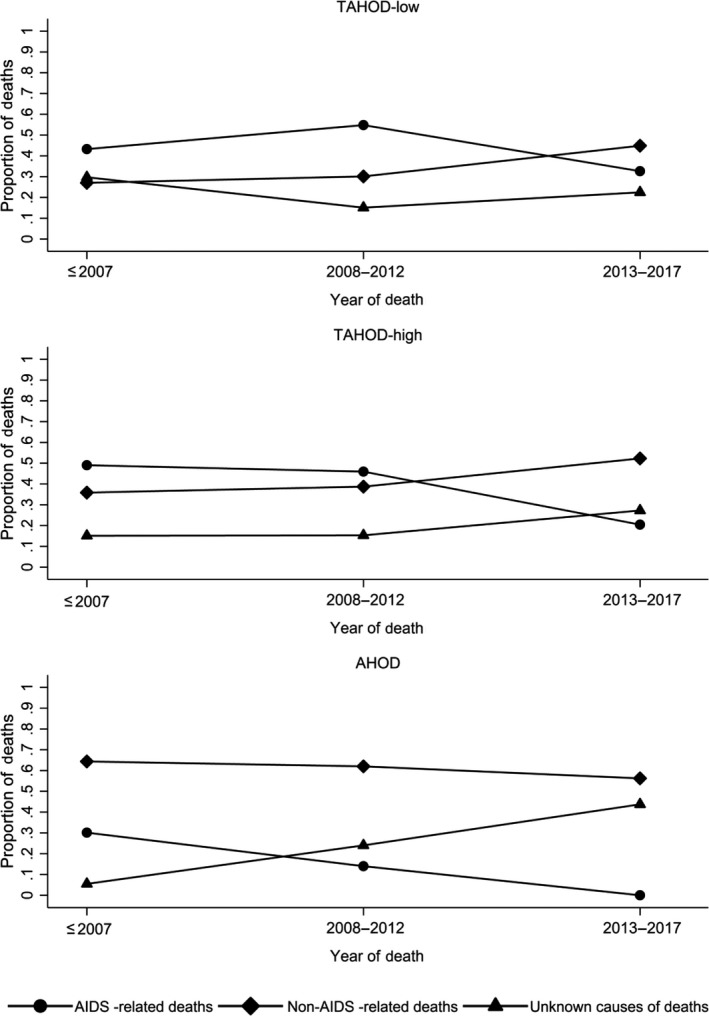
Figure 2. Mortality rates for different causes of death.
The incidence rate of AIDS‐related mortality during follow‐up was 0.28/100 PYS (Table 3). Univariate analysis demonstrated that mode of HIV exposure (p < 0.001), HCV co‐infection (p = 0.027), cohort group (p < 0.001), diabetes (p = 0.014), BMI, CD4, RNA and calendar year (all p < 0.001) were associated with AIDS‐related mortality. In multivariate analysis adjusting for cohort groups, factors associated with AIDS‐related mortality were higher viral load (HIV RNA ≥100,000 copies/ml) compared to HIV RNA ≤400 copies/mL (aSHR = 1.95, 95% CI 1.22 to 3.13, p = 0.006); and high blood glucose (aSHR = 2.56, 95% CI 1.23 to 5.33, p = 0.012). Factors showing protective effects were CD4 count >200 cells/μL (aSHR = 0.04, 95% CI 0.03 to 0.07, p < 0.001) compared to CD4 ≤ 100 cells/μL; and higher BMI (overweight) patients (aSHR = 0.13, 95% CI 0.03 to 0.54, p < 0.005) compared to patients who were in the normal BMI range of 18.5 to 24.9. Calendar year was not statistically significant in the multivariate model; however, incidence rates of AIDS‐related deaths decreased from 0.51/100 PYS to 0.09/100 PYS in year groups from 2003 to 2017.
Table 3.
Factors associated with AIDS mortality in the TREAT Asia HIV Observational Database and AHOD cohortsa
| No. of patients | Follow‐up (years) | No. of deaths | Incidence rate (/100 PYS) | Univariate | Multivariate | |||
|---|---|---|---|---|---|---|---|---|
| SHR (95% CI) | p‐value | aSHR (95% CI) | p‐value | |||||
| Total | 10385 | 66250 | 187 | 0.28 | ||||
| Age (years) | 0.116 | |||||||
| ≤30 | 3011 | 17,628 | 49 | 0.28 | 1 | |||
| 31 to 40 | 4239 | 27,915 | 70 | 0.25 | 0.98 (0.68, 1.41) | 0.905 | ||
| 41 to 50 | 2116 | 14,302 | 40 | 0.28 | 1.11 (0.73, 1.69) | 0.621 | ||
| 51+ | 1020 | 6405 | 28 | 0.44 | 1.65 (1.03, 2.62) | 0.037 | ||
| Sex | 0.357 | |||||||
| Male | 7842 | 50,749 | 137 | 0.27 | 1 | |||
| Female | 2544 | 15,501 | 50 | 0.32 | 1.16 (0.84 to 1.61) | 0.357 | ||
| HIV mode of exposure | <0.001 | |||||||
| Heterosexual contact | 5366 | 33,915 | 114 | 0.34 | 1 | |||
| MSM | 3561 | 24,134 | 36 | 0.15 | 0.47 (0.32, 0.68) | <0.001 | ||
| Injecting drug use | 764 | 3940 | 22 | 0.56 | 1.42 (0.90, 2.24) | 0.134 | ||
| Blood products | 93 | 450 | 3 | 0.67 | 1.55 (0.49, 4.90) | 0.453 | ||
| Bisexual | 140 | 901 | 4 | 0.44 | 1.31 (0.48, 3.55) | 0.601 | ||
| Other/unknown | 462 | 2908 | 8 | 0.28 | 0.81 (0.40, 1.66) | 0.564 | ||
| CD4 (cells/μL) | <0.001 | <0.001 | ||||||
| ≤100 | ‐ | 2215 | 102 | 4.60 | 1 | 1 | ||
| 101 to 200 | ‐ | 4726 | 39 | 0.83 | 0.23 (0.16, 0.34) | <0.001 | 0.31 (0.21, 0.46) | <0.001 |
| >200 | ‐ | 58,513 | 36 | 0.06 | 0.03 (0.02, 0.04) | <0.001 | 0.04 (0.03, 0.07) | <0.001 |
| Missing | ‐ | 795 | 10 | 1.26 | ||||
| Viral load (copies/mL) | <0.001 | 0.019 | ||||||
| ≤400 | ‐ | 50,977 | 51 | 0.10 | 1 | 1 | ||
| 401 to 10,000 | ‐ | 2469 | 12 | 0.49 | 3.42 (1.79, 6.52) | <0.001 | 1.71 (0.87, 3.34) | 0.118 |
| ≥100,001 | ‐ | 4489 | 69 | 1.54 | 8.66 (5.84, 12.84) | <0.001 | 1.95 (1.22, 3.13) | 0.006 |
| Missing | ‐ | 8314 | 55 | 0.66 | ||||
| HBV co‐infection | 0.6085 | |||||||
| Negative | 7457 | 49,623 | 120 | 0.24 | 1 | |||
| Positive | 705 | 4421 | 13 | 0.29 | 1.16 (0.65, 2.06) | 0.608 | ||
| Missing | 2224 | 12,206 | 54 | 0.44 | ||||
| HCV co‐infection | 0.027 | |||||||
| Negative | 6892 | 46,347 | 111 | 0.24 | 1 | |||
| Positive | 1110 | 6525 | 28 | 0.43 | 1.6 (1.05, 2.42) | 0.027 | ||
| Missing | 2384 | 13,377 | 48 | 0.36 | ||||
| Diabetesb | 0.014 | 0.012 | ||||||
| No | ‐ | 39,723 | 70 | 0.18 | 1 | 1 | ||
| Yes | ‐ | 1904 | 8 | 0.42 | 2.52 (1.21, 5.26) | 0.014 | 2.56 (1.23, 5.33) | 0.012 |
| Missing | ‐ | 24,623 | 109 | 0.44 | ||||
| BMI groups | <0.001 | <0.001 | ||||||
| Underweight (<18.5) | ‐ | 4364 | 66 | 1.51 | 5.98 (4.24, 8.44) | <0.001 | 3.33 (2.31, 4.81) | <0.001 |
| Normal range (18.5 to 24.9) | ‐ | 31,289 | 65 | 0.21 | 1 | 1 | ||
| Overweight (≥25) | ‐ | 12,168 | 2 | 0.02 | 0.09 (0.02, 0.37) | 0.001 | 0.13 (0.03, 0.54) | 0.005 |
| Missing | ‐ | 18,429 | 54 | 0.29 | ||||
| Cohort groups | <0.001 | 0.834 | ||||||
| TAHOD‐low | 3240 | 17,833 | 72 | 0.40 | 1 | 1 | ||
| TAHOD‐high | 4429 | 29,242 | 86 | 0.29 | 0.82 (0.60, 1.13) | 0.228 | 0.99 (0.68, 1.44) | 0.953 |
| AHOD | 2717 | 19,175 | 29 | 0.15 | 0.44 (0.29, 0.68) | <0.001 | 1.15 (0.64, 2.07) | 0.629 |
| Calendar year | <0.001 | |||||||
| ≤2002 | ‐ | 2046 | 8 | 0.39 | 0.58 (0.28, 1.21) | 0.148 | ||
| 2003 to 2007 | ‐ | 11,006 | 56 | 0.51 | 1 | |||
| 2008 to 2012 | ‐ | 25,968 | 98 | 0.38 | 0.87 (0.62, 1.21) | 0.414 | ||
| 2013 to 2017 | ‐ | 27,230 | 25 | 0.09 | 0.34 (0.21, 0.54) | <0.001 | ||
Global p‐values were calculated by excluding the missing category. AHOD, Australian HIV Observational Database; aSHR, adjusted sub‐hazard ratio; BMI, body mass index; CI, confidence interval; HBV, hepatitis B virus; HCV, hepatitis C virus; MSM, Men who have sex with men; PYS, person‐years; SHR, sub‐hazard ratio; TAHOD, TREAT Asia HIV Observational Database.
aCohorts were grouped as AHOD (all high‐income sites), TAHOD‐high (high/upper‐middle income countries) and TAHOD‐low (low‐middle/low income countries). TAHOD sites were split into high/upper‐middle income and low‐middle/low income settings based on World Bank classifications. Time‐fixed covariates: Age, Sex, HIV mode of exposure, HBV co‐infection, HCV co‐infection, Cohort Groups; time‐updated covariates: CD4, Viral Load, Diabetes, BMI Groups and Calendar year. bDiabetes was defined as documentation of one fasting blood glucose measurement ≥7 mmol/L.
The incidence rate of non‐AIDs–related mortality during follow‐up was 0.51 per 100 PYS (Table 4). Factors associated with non‐AIDS mortality in the multivariate analysis were: older age (31 to 40 years: aSHR = 1.40, 95% CI 1.02 to 1.93, p = 0.039; 41 to 50 years: aSHR = 1.58, 95% CI 1.10 to 2.27, p = 0.013; and >50 years: aSHR = 4.43, 95% CI 3.15 to 6.25, p < 0.001) compared to age ≤30 years; HBV co‐infection (aSHR = 1.87, 95% CI 1.32 to 2.65, p < 0.001); HCV co‐infection (aSHR = 1.96, 95% CI 1.44 to 2.67, p < 0.001); high blood glucose (aSHR = 1.77, 95% CI 1.09 to 2.86, p = 0.021); and AHOD cohort (aSHR = 1.72, 95% CI 1.20 to 2.46, p = 0.003) compared to TAHOD‐low. Non‐AIDS mortality was less likely in females (aSHR = 0.5, 95% CI 0.35 to 0.70, p < 0.001); higher BMI (overweight: aSHR = 0.69, 95% CI 0.47 to 1.01, p < 0.054) compared to normal weight range (BMI of 18.5 to 24.9); and later calendar years of follow‐up (2008 to 2012: aSHR = 0.72, 95% CI 0.54 to 0.96, p = 0.027; 2013 to 2017: aSHR = 0.64, 95% CI 0.47 to 0.87, p = 0.004) compared to year group 2003 to 2007. Higher CD4 count (101 to 200 cells/μL: aSHR = 0.61, 95% CI 0.42 to 0.90, p = 0.011; >200 cells/μL: aSHR = 0.23, 95% CI 0.17 to 0.33, p < 0.001) compared to CD4 ≤ 100 cells/μL was also associated with lower non‐AIDS mortality. However, since the multivariable models were adjusted for time‐updated CD4 count and viral load, changes over time in the rate of non‐AIDS mortality are not entirely explained by CD4 count and viral load.
Table 4.
Factors associated with non‐AIDS mortality in the TREAT Asia HIV Observational Database and AHODa
| No. patients | Follow‐up (years) | No. of deaths | Incidence rate (/100 PYS) | Univariate | Multivariate | |||
|---|---|---|---|---|---|---|---|---|
| SHR (95% CI) | p‐value | aSHR (95% CI) | p‐value | |||||
| Total | 10,385 | 66,250 | 335 | 0.51 | ||||
| Age (years) | <0.001 | <0.001 | ||||||
| ≤30 | 3011 | 17,628 | 59 | 0.33 | 1 | 1 | ||
| 31 to 40 | 4239 | 27,915 | 117 | 0.42 | 1.31 (0.96, 1.79) | 0.091 | 1.40 (1.02, 1.93) | 0.039 |
| 41 to 50 | 2116 | 14,302 | 68 | 0.48 | 1.51 (1.06, 2.14) | 0.021 | 1.58 (1.10, 2.27) | 0.013 |
| 51+ | 1020 | 6405 | 91 | 1.42 | 4.39 (3.17, 6.08) | <0.001 | 4.43 (3.15, 6.25) | <0.001 |
| Sex | <0.001 | <0.001 | ||||||
| Male | 7842 | 50,749 | 299 | 0.59 | 1 | 1 | ||
| Female | 2544 | 15,501 | 36 | 0.23 | 0.40 (0.28, 0.57) | <0.001 | 0.50 (0.35, 0.70) | <0.001 |
| HIV mode of exposure | 0.005 | |||||||
| Heterosexual contact | 5366 | 33,915 | 150 | 0.44 | 1 | |||
| MSM | 3561 | 24,134 | 121 | 0.50 | 1.13 (0.89, 1.44) | 0.298 | ||
| Injecting drug use | 764 | 3940 | 39 | 0.99 | 1.99 (1.40, 2.83) | <0.001 | ||
| Blood products | 93 | 450 | 5 | 1.11 | 1.98 (0.82, 4.83) | 0.131 | ||
| Bisexual | 140 | 901 | 6 | 0.67 | 1.52 (0.67, 3.46) | 0.313 | ||
| Other/unknown | 462 | 2908 | 14 | 0.48 | 1.06 (0.61, 1.84) | 0.826 | ||
| CD4 (cells/μL) | <0.001 | <0.001 | ||||||
| ≤100 | ‐ | 2215 | 63 | 2.84 | 1 | 1 | ||
| 101 to 200 | ‐ | 4726 | 57 | 1.21 | 0.57 (0.40, 0.83) | 0.003 | 0.61 (0.42, 0.90) | 0.011 |
| >200 | ‐ | 58,513 | 204 | 0.35 | 0.20 (0.15, 0.26) | <0.001 | 0.23 (0.17, 0.33) | <0.001 |
| Missing | ‐ | 795 | 11 | 1.38 | ||||
| Viral load (copies/mL) | <0.001 | |||||||
| ≤400 | ‐ | 50,977 | 204 | 0.40 | 1 | |||
| 401 to 10,000 | ‐ | 2469 | 15 | 0.61 | 1.31 (0.77, 2.22) | 0.313 | ||
| ≥100,001 | ‐ | 4489 | 57 | 1.27 | 2.42 (1.78, 3.29) | <0.001 | ||
| Missing | ‐ | 8314 | 59 | 0.71 | ||||
| HBV co‐infection | <0.001 | <0.001 | ||||||
| Negative | 7457 | 49,623 | 230 | 0.46 | 1 | 1 | ||
| Positive | 705 | 4421 | 37 | 0.84 | 1.80 (1.27, 2.54) | 0.001 | 1.87 (1.32, 2.65) | <0.001 |
| Missing | 2224 | 12,206 | 68 | 0.56 | ||||
| HCV co‐infection | <0.001 | <0.001 | ||||||
| Negative | 6892 | 46,347 | 200 | 0.43 | 1 | 1 | ||
| Positive | 1110 | 6525 | 63 | 0.97 | 2.08 (1.57, 2.76) | <0.001 | 1.96 (1.44, 2.67) | <0.001 |
| Missing | 2384 | 13,377 | 72 | 0.54 | ||||
| Diabetesb | <0.001 | 0.021 | ||||||
| No | ‐ | 39,723 | 164 | 0.41 | 1 | 1 | ||
| Yes | ‐ | 1904 | 19 | 1 | 2.44 (1.51, 3.92) | <0.001 | 1.77 (1.09, 2.86) | 0.021 |
| Missing | ‐ | 24,623 | 152 | 0.62 | ||||
| BMI groups | <0.001 | <0.001 | ||||||
| Underweight (<18.5) | ‐ | 4364 | 56 | 1.28 | 2.69 (1.96, 3.69) | <0.001 | 2.21 (1.56, 3.12) | <0.001 |
| Normal range (18.5 to 24.9) | ‐ | 31,289 | 127 | 0.41 | 1 | 1 | ||
| Overweight (≥25) | ‐ | 12,168 | 36 | 0.30 | 0.75 (0.52, 1.09) | 0.135 | 0.69 (0.47, 1.01) | 0.054 |
| Missing | ‐ | 18,429 | 116 | 0.63 | ||||
| Cohort groups | 0.003 | 0.001 | ||||||
| TAHOD‐low | 3240 | 17,833 | 87 | 0.49 | 1 | 1 | ||
| TAHOD‐high | 4429 | 29,242 | 122 | 0.42 | 0.89 (0.68, 1.18) | 0.421 | 0.99 (0.73, 1.34) | 0.936 |
| AHOD | 2717 | 19,175 | 126 | 0.66 | 1.35 (1.03, 1.78) | 0.029 | 1.72 (1.20, 2.46) | 0.003 |
| Calendar year | <0.001 | 0.028 | ||||||
| ≤2002 | ‐ | 2046 | 13 | 0.64 | 0.77 (0.43, 1.39) | 0.39 | 0.66 (0.36, 1.23) | 0.194 |
| 2003 to 2007 | ‐ | 11,006 | 86 | 0.78 | 1 | 1 | ||
| 2008 to 2012 | ‐ | 25,968 | 136 | 0.52 | 0.68 (0.52, 0.89) | 0.006 | 0.72 (0.54, 0.96) | 0.027 |
| 2013 to 2017 | ‐ | 27,230 | 100 | 0.37 | 0.50 (0.37, 0.67) | <0.001 | 0.64 (0.47, 0.87) | 0.004 |
Global p‐values were calculated by excluding the missing category. AHOD, Australian HIV Observational Database; aSHR, adjusted sub‐hazard ratio; BMI, body mass index; CI, confidence interval; HBV, hepatitis B virus; HCV, hepatitis C virus; MSM, Men who have sex with men; No., number; PYS, person‐years; SHR, sub‐hazard ratio; TAHOD, TREAT Asia HIV Observational Database.
aCohorts were grouped as AHOD (all high‐income sites), TAHOD‐high (high/upper‐middle income countries) and TAHOD‐low (low‐middle/low income countries). TAHOD sites were split into high/upper‐middle income and low‐middle/low income settings based on World Bank classifications. Time‐fixed covariates: age, sex, HIV mode of exposure, HBV co‐infection, HCV co‐infection, cohort groups; time‐updated covariates: CD4, viral load, diabetes, BMI groups and calendar year. bDiabetes was defined as documentation of one fasting blood glucose measurement ≥7 mmol/L.
Tables S1 and S2 show that by including time‐updated age, sex and CD4 in the final multivariate model, there was a similar increasing trend in the hazard for mortality with increasing age, which has now shown significant associations with both AIDS and non‐AIDS mortality. Other risk factors and their effects are similar to those observed in the main analyses.
4. Discussion
In our cross‐regional observational cohorts who received cART, the overall incidence rate of mortality over a median follow‐up time of 6.0 years was 0.28/100 PYS from AIDS‐related and 0.51/100 PYS from non‐AIDS–related causes. Moreover, incidence rates for AIDS‐related and non‐AIDS–related mortality have decreased over the years with the AHOD cohort showing higher hazard for non‐AIDS deaths compared to TAHOD‐low sites.
With the introduction of cART, AIDS‐related death has decreased followed by an increase in non‐AIDS death 4. According to the D:A:D multicohort collaboration data, the mortality rate of all‐cause death was 17.5/1000 PYS in 1999 to 2000 and decreased to 9.1/1000 PYS in 2009 to 2011 1. AIDS‐related deaths demonstrated similar decreases in death rates over the same period from 5.9/1000 PYS to 2.0/1000 PYS 1. In our study, incidence rates of AIDS‐related deaths decreased from 0.51/100 PYS to 0.09/100 PYS from year groups 2003 to 2007 to 2013 to 2017. AIDS‐related COD was the most common COD in the TAHOD cohort in year group ≤2007, and over the years there was a decrease in the overall incidence of AIDS deaths for all cohorts. These findings are thought to be associated with the introduction of effective cART in resource‐limited settings, which slows the decline of CD4 cell counts, extending the time to AIDS‐related death and the total number of AIDS deaths itself 3, 16.
In a Swiss HIV cohort study, AIDS‐related death was highest in 1992 (11.0/100 PYS, 95% CI 9.94 to 12.1) and decreased to 0.211/100 PYS (95% CI 0.122 to 0.363) in 2010 6. Non‐AIDS–related mortality decreased from 1.74/100 PYS (95% CI 1.36 to 2.23) in 1993 to 0.86/100 PYS (95% CI 0.657 to 1.13) in 2010 6. The results of our study also demonstrated a declining trend in non‐AIDS deaths in the later calendar years. Additionally, we found that there has been an increase in the proportion of unknown deaths recorded in the AHOD cohort. This increase in unknown deaths in AHOD was due to delay in reporting of the causes of death by the site. Mortality rates for AIDS and non‐AIDS causes of death have declined over the years, with the incidence rate of non‐AIDS–related death remaining high in AHOD, but declining, compared to other causes of death. Overall, there are higher incidences of non‐AIDS deaths than AIDS‐related mortality in later years in the Asia‐Pacific region, similar to those demonstrated in other regions 1, 3.
HIV‐infected patients are prone to increased risk of cancer, and an estimated 30% to 40% of patients will develop cancer during their lifetime 17. According to the D:A:D multicohort collaboration data, the incidence of non‐AIDS cancers increased from 1.6/1000 PYS (1999 to 2000) to 2.1/1000 PYS (2009 to 2011) 1. As people with HIV have a longer lifespan with potent cART, more patients develop malignancies including cancers not associated with AIDS 18, 19, and this might also explain the higher proportion of non‐AIDS deaths found in our cohorts. However, in this study, mortality rates for non‐AIDS–related malignancies decreased from 0.16/100 PYS in year groups before 2007 to 0.07/100 PYS in year groups 2013 to 2017. Early treatment due to aggressive screening may be accountable for the decrease in non‐AIDS malignancy mortality. Moreover, more detection of cancer due to aggressive screening in the AHOD cohort may be the reason for the higher risk of non‐AIDS–related deaths in the AHOD cohort. The number of cardiovascular–related deaths was also higher in the AHOD cohort. Although there were no differences between cohorts for AIDS‐related mortality, the AHOD cohort was at higher risk of non‐AIDS mortality compared to both TAHOD cohorts with almost twice the hazard of non‐AIDS mortality compared to TAHOD‐low. The non‐AIDS mortality highlights the importance for healthcare professionals to provide more comprehensive healthcare that includes screening for malignancy or cardiovascular disease.
Cardiovascular events associated with lower CD4 cell counts were suggested in both the Strategies for Management of Antiretroviral Therapy (SMART) and D:A:D studies 20, 21. Furthermore, immunodeficiency, identified by lower CD4 cell counts, was also strongly associated with liver‐related non‐AIDS death 22. In a prospective cohort study of HIV‐infected patients diagnosed at the age of 50 years or more, the baseline CD4 cell count was lower and presented shorter survival 23. Moreover, continuing improvements in CD4 cell count over time were attributable to the reduction in AIDS‐related deaths 1. Our findings are similar to results of these studies, demonstrating lower CD4 cell counts and older age as independent risk factors for both AIDS and non‐AIDS mortality. Our study was also comparable to studies conducted throughout South America and the Caribbean, which have shown that low BMI and low haemoglobin were predictive of mortality, especially in the early phases of cART initiation 24. Moreover, one prospective cohort study which followed up 1119 HIV patients for a median number of 8.2 years demonstrated that HIV‐infected persons with food insecurity, categorized by low BMI, were about two times more likely to experience mortality 25. In our study, low BMI categorized as underweight was also a risk factor for AIDS and non‐AIDS mortality, putting emphasis on the need to incorporate nutritional support in HIV treatment programs.
There are several limitations to our study. Information on patient adherence data is not collected in AHOD and it is possible that poor patient compliance or adherence may have been a reason for AIDS death. The delay in reporting in the causes of death in AHOD may lead to incomplete COD ascertainment and underestimation of the AIDS and non‐AIDS causes of death. However, the majority of the unknown causes of death would be traced at a later date. While TAHOD represents a primarily urban referral patient population, crossing from low to high country income categories, it is less representative of national‐level data. There is also the risk of unascertained mortality among those with LTFU.
5. Conclusions
We observed that the rates of AIDS‐related and non‐AIDS‐related mortality in our Asia‐Pacific cohorts have decreased over the past years. Factors associated with increased risk of AIDS‐related mortality were higher viral load, high blood glucose, low CD4 cell count and low BMI. Older age, hepatitis B and C co‐infection, high blood glucose, low CD4 cell count and BMI were associated with increased non‐AIDS death. Blood glucose levels, CD4 cell count and being underweight were risk factors for both categories of deaths. The higher risk for non‐AIDS–associated deaths in the AHOD cohort compared to the lower‐middle income setting in TAHOD may be related to older age and higher per cent of reporting number of deaths. The slower rate in the decline of non‐AIDS deaths and the higher proportions of non‐AIDS deaths still persisting over AIDS‐related deaths demonstrate that longer lifespans contribute to increased risk of developing cancers and other conditions more common with older age, resulting in more deaths from non‐AIDS causes.
Competing interests
None of the authors have competing interests to declare.
Authors’ contributions
JYC contributed to conceptualization and design of the study; IYJ wrote the first draft of the manuscript and interpreted the results; DR contributed to acquisition of data, data analysis and interpretation; IW, CO, MG and IA contributed to editing of the article; all authors have read and approved the final manuscript.
Supporting information
Table S1. Factors associated with AIDS mortality in the TREAT Asia HIV Observational Database and Australian HIV Observational Database cohorts with age as a time‐dependent covariate including age, sex and CD4 in the multivariate modela
Table S2. Factors associated with non‐AIDS mortality in the TREAT Asia HIV Observational Database and Australian HIV Observational Database cohorts with age as a time‐dependent covariate including age, sex and CD4 in the multivariate modela
Acknowledgements
Australian HIV Observational Database contributors include: New South Wales: D Ellis, Plaza Medical Centre, Coffs Harbour; M Bloch, B Gallegher, T Vincent, Holdsworth House Medical Practice, Sydney; D Allen, Holden Street Clinic, Gosford; D Smith, A Rankin, Lismore Sexual Health & AIDS Services, Lismore; D Baker, East Sydney Doctors, Surry Hills; DJ Templeton, CC O'Connor, R Jackson, RPA Sexual Health, Camperdown; K McCallum, Blue Mountains Sexual Health and HIV Clinic, Katoomba; N Ryder, G Sweeney, B Moran, Clinic 468, HNE Sexual Health, Tamworth; A Carr, K Macrae, K Hesse, St Vincent's Hospital, Darlinghurst; R Finlayson, C Tan, LL Phuoc, Taylor Square Private Clinic, Darlinghurst; E Jackson, J Shakeshaft, Nepean Sexual Health and HIV Clinic, Penrith; K Brown, S Idle, JL Little, Illawarra Sexual Health Service, Warrawong; R Varma, H Lu, Sydney Sexual Health Centre, Sydney; D Couldwell, S Eswarappa, Western Sydney Sexual Health Clinic; DE Smith, V Furner, D Smith, Albion Street Centre; S Fernando, Clinic 16 – Royal North Shore Hospital; A Cogle, National Association of People living with HIV/AIDS; C Lawrence, National Aboriginal Community Controlled Health Organisation; B Mulhall, Department of Public Health and Community Medicine, University of Sydney; M Law, K Petoumenos, R Puhr, J Hutchinson, T Dougherty, The Kirby Institute, University of NSW. Northern Territory: M Gunathilake, SD Reekie, Centre for Disease Control, Darwin. Queensland: M O'Sullivan, K Loggie, Gold Coast Sexual Health Clinic, Miami; D Russell, F Bassett, C Roberts, Cairns Sexual Health Service, Cairns; D Sowden, K Taing, P Marshall, Clinic 87, Sunshine Coast‐Wide Bay Health Service District, Nambour; D Orth, D Youds, Gladstone Road Medical Centre, Highgate Hill; D Rowling, N Latch, F Taylor, Sexual Health and HIV Service in Metro North, Brisbane; B Dickson, CaraData. South Australia: W Donohue, O'Brien Street General Practice, Adelaide. M Boyd, University of Adelaide. Victoria: R Moore, S Edwards, S Boyd, Northside Clinic, North Fitzroy; NJ Roth, H Lau, Prahran Market Clinic, South Yarra; T Read, J Silvers, W Zeng, Melbourne Sexual Health Centre, Melbourne; J Hoy, M Giles, K Watson, M Bryant, S Price, The Alfred Hospital, Melbourne; I Woolley, T Korman, Monash Medical Centre, Clayton. Western Australia: D Nolan, A Allen, G Guelfi. Department of Clinical Immunology, Royal Perth Hospital, Perth. New Zealand: G Mills, C Wharry, Waikato District Hospital Hamilton; N Raymond, K Bargh, Wellington Hospital, Wellington. TREAT Asia HIV Observational Database (TAHOD) contributors include: PS Ly* and V Khol, National Center for HIV/AIDS, Dermatology & STDs, Phnom Penh, Cambodia; FJ Zhang* †, HX Zhao and N Han, Beijing Ditan Hospital, Capital Medical University, Beijing, China; MP Lee*, PCK Li, W Lam and YT Chan, Queen Elizabeth Hospital, Hong Kong SAR; N Kumarasamy*, S Saghayam and C Ezhilarasi, Chennai Antiviral Research and Treatment Clinical Research Site (CART CRS), YRGCARE Medical Centre, VHS, Chennai, India; S Pujari*, K Joshi, S Gaikwad and A Chitalikar, Institute of Infectious Diseases, Pune, India; S Sangle*, V Mave and I Marbaniang, BJ Government Medical College and Sassoon General Hospital, Pune, India; TP Merati*, DN Wirawan and F Yuliana, Faculty of Medicine Udayana University & Sanglah Hospital, Bali, Indonesia; E Yunihastuti*, D Imran and A Widhani, Faculty of Medicine Universitas Indonesia – Dr. Cipto Mangunkusumo General Hospital, Jakarta, Indonesia; J Tanuma*, S Oka and T Nishijima, National Center for Global Health and Medicine, Tokyo, Japan; JY Choi*, Na S and JM Kim, Division of Infectious Diseases, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, South Korea; BLH Sim*, YM Gani, and NB Rudi, Hospital Sungai Buloh, Sungai Buloh, Malaysia; A Kamarulzaman*, SF Syed Omar, S Ponnampalavanar and I Azwa, University Malaya Medical Centre, Kuala Lumpur, Malaysia; R Ditangco*, MK Pasayan and ML Mationg, Research Institute for Tropical Medicine, Muntinlupa City, Philippines; WW Wong*, WW Ku and PC Wu, Taipei Veterans General Hospital, Taipei, Taiwan; OT Ng* ‡, PL Lim, LS Lee and Z Ferdous, Tan Tock Seng Hospital, Singapore; A Avihingsanon*, S Gatechompol, P Phanuphak and C Phadungphon, HIV‐NAT/Thai Red Cross AIDS Research Centre, Bangkok, Thailand; S Kiertiburanakul*, A Phuphuakrat, L Chumla and N Sanmeema, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand; R Chaiwarith*, T Sirisanthana, W Kotarathititum and J Praparattanapan, Research Institute for Health Sciences, Chiang Mai, Thailand; S Khusuwan*, P Kantipong and P Kambua, Chiangrai Prachanukroh Hospital, Chiang Rai, Thailand; KV Nguyen*, HV Bui, DTH Nguyen and DT Nguyen, National Hospital for Tropical Diseases, Hanoi, Vietnam; CD Do*, AV Ngo and LT Nguyen, Bach Mai Hospital, Hanoi, Vietnam; AH Sohn*, JL Ross* and B Petersen, TREAT Asia, amfAR – The Foundation for AIDS Research, Bangkok, Thailand; MG Law*, A Jiamsakul* and D Rupasinghe, The Kirby Institute, UNSW Sydney, NSW, Australia. * TAHOD Steering Committee member; † Steering Committee Chair; ‡ co‐Chair.
Funding
The TREAT Asia HIV Observational Database (TAHOD) and the Australian HIV Observational Database (AHOD) are initiatives of TREAT Asia, a programme of amfAR, The Foundation for AIDS Research, with support from the U.S. National Institutes of Health's National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Cancer Institute, the National Institute of Mental Health and the National Institute on Drug Abuse, as part of the International Epidemiology Databases to Evaluate AIDS (IeDEA; U01AI069907). The AHOD is also funded by unconditional grants from Merck Sharp & Dohme; Gilead Sciences; Bristol‐Myers Squibb; Boehringer Ingelheim; Janssen‐Cilag; and ViiV Healthcare. The Kirby Institute is funded by the Australian Government Department of Health, and is affiliated with the Faculty of Medicine, UNSW Sydney. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of any of the governments or institutions mentioned above.
Jung, I. Y. , Rupasinghe, D. , Woolley, I. , O'Connor, C. C. , Giles, M. , Azwa, R. I. S. R. and Choi, J. Y. on behalf of IeDEA Asia‐Pacific . Trends in mortality among ART‐treated HIV‐infected adults in the Asia‐Pacific region between 1999 and 2017: results from the TREAT Asia HIV Observational Database (TAHOD) and Australian HIV Observational Database (AHOD) of IeDEA Asia‐Pacific. J Int AIDS Soc. 2019;22(1): e25219
References
- 1. Smith CJ, Ryom L, Weber R, Morlat P, Pradier C, Reiss P, et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet. 2014;384(9939):241–8. [DOI] [PubMed] [Google Scholar]
- 2. Nakagawa F, Lodwick RK, Smith CJ, Smith R, Cambiano V, Lundgren JD, et al. Projected life expectancy of people with HIV according to timing of diagnosis. AIDS. 2012;26(3):335–43. [DOI] [PubMed] [Google Scholar]
- 3. Murray CJ, Ortblad KF, Guinovart C, Lim SS, Wolock TM, Roberts DA, et al. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9947):1005–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang H, Wolock TM, Carter A, Nguyen G, Kyu HH, Gakidou E, et al. Estimates of global, regional, and national incidence, prevalence, and mortality of HIV, 1980–2015: the Global Burden of Disease Study 2015. Lancet HIV. 2016;3(8):e361–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cobucci RN, Lima PH, de Souza PC, Costa VV, Cornetta Mda C, Fernandes JV, et al. Assessing the impact of HAART on the incidence of defining and non‐defining AIDS cancers among patients with HIV/AIDS: a systematic review. J Infect Public Health. 2015;8(1):1–10. [DOI] [PubMed] [Google Scholar]
- 6. Weber R, Ruppik M, Rickenbach M, Spörri A, Furrer H, Battegay M, et al. Decreasing mortality and changing patterns of causes of death in the Swiss HIV Cohort Study. HIV Med. 2013;14(4):195–207. [DOI] [PubMed] [Google Scholar]
- 7. Falster K, Choi JY, Donovan B, Duncombe C, Mulhall B, Sowden D, et al. AIDS‐related and non‐AIDS‐related mortality in the Asia‐Pacific region in the era of combination antiretroviral treatment. AIDS. 2009;23(17):2323–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Petoumenos K, Hui E, Kumarasamy N, Kerr SJ, Choi JY, Chen YM, et al. Cancers in the TREAT Asia HIV Observational Database (TAHOD): a retrospective analysis of risk factors. J Int AIDS Soc. 2010;13:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Petoumenos K, van Leuwen MT, Vajdic CM, Woolley I, Chuah J, Templeton DJ, et al. Cancer, immunodeficiency and antiretroviral treatment: results from the Australian HIV Observational Database (AHOD). HIV Med. 2013;14(2):77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. The World Bank . Countries and Economies 2015. [cited 2015 Feb 02]. Available from: http://data.worldbank.org/country
- 11. National Center for Infectious Diseases Division of HIV/AIDS , Kenneth G, Castro MD, John W, Ward MD, Laurence Slutsker MD, et al. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. JAMA. 1993;269(6):729–30. [PubMed] [Google Scholar]
- 12. Friis‐Moller N, Sabin CA, Weber R, d'Arminio Monforte A, El‐Sadr WM, Reiss P, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349(21):1993–2003. [DOI] [PubMed] [Google Scholar]
- 13. Zhou J, Tanuma J, Chaiwarith R, Lee CK, Law MG, Kumarasamy N, et al. Loss to followup in HIV‐infected patients from Asia‐Pacific region: results from TAHOD. AIDS Res Treat. 2012;2012:375217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. De La Mata NL, Ly PS, Nguyen KV, Merati TP, Pham TT, Lee MP, et al. Loss to follow‐up trends in HIV‐positive patients receiving antiretroviral treatment in Asia from 2003 to 2013. J Acquir Immune Defic Syndr. 2017;74(5):555–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McManus H, Petoumenos K, Brown K, Baker D, Russell D, Read T, et al. Loss to follow‐up in the Australian HIV observational database. Antivir Ther. 2015;20(7):731–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Collaboration ATC . Causes of death in HIV‐1‐infected patients treated with antiretroviral therapy, 1996–2006: collaborative analysis of 13 HIV cohort studies. Clin Infect Dis. 2010;50(10):1387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Berretta M, Cinelli R, Martellotta F, Spina M, Vaccher E, Tirelli U. Therapeutic approaches to AIDS‐related malignancies. Oncogene. 2003;22(42):6646. [DOI] [PubMed] [Google Scholar]
- 18. Hessol NA, Pipkin S, Schwarcz S, Cress RD, Bacchetti P, Scheer S. The impact of highly active antiretroviral therapy on non‐AIDS‐defining cancers among adults with AIDS. Am J Epidemiol. 2007;165(10):1143–53. [DOI] [PubMed] [Google Scholar]
- 19. Sackoff JE, Hanna DB, Pfeiffer MR, Torian LV. Causes of death among persons with AIDS in the era of highly active antiretroviral therapy: New York City. Ann Intern Med. 2006;145(6):397–406. [DOI] [PubMed] [Google Scholar]
- 20. El‐Sadr WM, Lundgren J, Neaton JD, Gordin F, Abrams D, Arduino RC, et al. CD4+ count‐guided interruption of antiretroviral treatment. N Engl J Med. 2006;355(22):2283–96. [DOI] [PubMed] [Google Scholar]
- 21. Friis‐Moller N, Reiss P, Sabin CA, Weber R, Monforte A, El‐Sadr W, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356(17):1723–35. [DOI] [PubMed] [Google Scholar]
- 22. Weber R, Sabin CA, Friis‐Moller N, Reiss P, El‐Sadr WM, Kirk O, et al. Liver‐related deaths in persons infected with the human immunodeficiency virus: the D: A: D study. Arch Intern Med. 2006;166(15):1632–41. [DOI] [PubMed] [Google Scholar]
- 23. Nogueras M, Navarro G, Antón E, Sala M, Cervantes M, Amengual M, et al. Epidemiological and clinical features, response to HAART, and survival in HIV‐infected patients diagnosed at the age of 50 or more. BMC Infect Dis. 2006;6(1):159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Caribbean T. Mortality during the first year of potent antiretroviral therapy in HIV‐1‐infected patients in 7 sites throughout Latin America and the Caribbean. J Acquir Immune Defic Syndr. 2009; 51(5):615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Weiser SD, Fernandes KA, Brandson EK, Lima VD, Anema A, Bangsberg DR, et al. The association between food insecurity and mortality among HIV‐infected individuals on HAART. J Acquir Immune Defic Syndr. 2009; 52(3):342. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Factors associated with AIDS mortality in the TREAT Asia HIV Observational Database and Australian HIV Observational Database cohorts with age as a time‐dependent covariate including age, sex and CD4 in the multivariate modela
Table S2. Factors associated with non‐AIDS mortality in the TREAT Asia HIV Observational Database and Australian HIV Observational Database cohorts with age as a time‐dependent covariate including age, sex and CD4 in the multivariate modela


